- 1CNOOC Research Institute Co, Ltd., Beijing, China
- 2School of Petroleum Engineering, Yangtze University, Wuhan, China
- 3Hubei Key Laboratory of Oil and Gas Drilling and Production Engineering (Yangtze University), Wuhan, China
The performance of the temporary plug is a key factor in determining the success of loss-circulation control and temporary plug diversion fracturing. Due to the complexity of geomechanics and working conditions, current commonly used temporary plug agents face problems such as low plug strength and efficiency, large filtration losses due to failure to form filter cakes, and slow degradation affecting the recovery of fracture conductivity. A novel idea for the development of a novel water-soluble polymer plug for fracking is proposed, namely, low-to middle-molecule weight + reinforced chain rigidity + supramolecular aggregation. Using sodium bisulfite and potassium sulfate as initiators, AA, AM, and AMPS as grafting monomers, and SM as hydrophobic functional monomers, the AM-AA-AMPS-SM copolymer was prepared by polymerization. The developed new temporary plugging agent was completely degraded at 70°C for 5–8 h by carrying out experimental evaluation tests, such as water absorption expansion rate, swelling kinetics, density, post-dissolution viscosity, strength of the temporary plugging agent and post-degradation conductivity. After degradation, the viscosity of the solution is 2.5–3.6 mPa s with good fluidity and no gel remnants. The density of the temporary plug material is about 1.14 g/cm3. The absorption expansion rate was 25.8 g/g. The pressure is 60.1 MPa when the thickness of the granular temporary plug is 0.4 cm. Under experimental conditions, the fracture conductivity was found to be 69–123 D*cm at a closing pressure of 30 MPa after degradation of the temporary plug. The test results demonstrate that the new temporary plug agent, with its high plug strength, temperature-controlled degradation, reflux stability and effective self-support after degradation, can meet the requirements of drilling plug and temporary plug fracturing technologies.
1 Introduction
In the drilling fluid plugging and temporary plug diversion fracturing techniques, the performance of the temporary plug is a critical factor in determining the success of plugging and temporary plug diversion fracturing. Presently, commonly used temporary capping agents in temporary capping and fracturing techniques include: solid-sphere temporary capping agents, which are characterized by high strength and small deformation, but suffer from easy detachment, low capping efficiency, and inability to degrade on their own. The disadvantages of suspended temporary plug agents are difficulty in forming large plug strengths due to turbulence and borehole deformation, low plug efficiency, inability to form filter cakes, and high filter loss. The disadvantage of underground cross-linked temporary plugs is that small doses don’t achieve the required plug pressure, while large doses cause new damage to the reservoir. Although it can form filter cakes, the underground reaction is unstable and does not reach the required strength. The conventional surface cross-linked particle temporary plugging agent has the characteristics of easy injection and high plugging strength [1–6].
At present, it is mostly used in the process of temporary plugging and fracturing of oil wells, which is water-soluble polymer (gel forming agent) + inorganic or organic compounds (crosslinking agent) composed of gel type plugging agent [7–9]. After fracturing, the granular diverter slowly degrades under the action of water or oil, restoring the fracture conductivity. However, in low and ultra-low permeability natural gas wells, and especially in non-water producing natural gas wells undergoing diversion fracturing, this plug has the problem of difficult degradation, affecting the effect of diversion fracturing and even leading to invalid fracturing. In addition, another problem with the above-mentioned temporary plug is that the temporary plug portion of the fracture can easily close after the degradation of the temporary plug, resulting in a reduction in fracture conductivity and a reduction in oil and gas well production.
Tian M. et al. [10] studied a new heat and salt resistant water plugging and profile control agent. Based on AM (alkaline monomer), copolymer of 2-EHA (salt tolerant monomer) and VTEOS (temperature tolerant monomer) was introduced, which greatly improved the temperature and salt resistance. Wang C. et al. [11] developed a new heat-resistant plugging agent HTG using unsaturated amide monomer as the main agent, graft copolymerization and non-ionic fillers. The results show that HTG has strong salt and heat resistance, and maintains good gelling strength after being placed at 200°C for 72 h. HTG has a high plugging pressure. Xu et al. [12] established a flow regime-based gas apparent permeability model in high-pressure tight sandstone reservoirs, depending on bridging molecular kinetics, gas transport mechanisms, and apparent permeability. Their model was well validated against simulation and experimental data. Li et al. [13] rewritten the mass balance for determining the adsorbed amount, and two particular concepts, an “apparent excess adsorption” and an “actual excess adsorption,” were considered.
Zhang C. et al. [14] studied a new high-strength gel ABP system for fractured reservoirs with high salinity and low permeability. The temperature resistance, salt resistance and plugging characteristics of the gel system were studied at 29,500 mg/L salinity. The results showed that the crosslinking time was shortened and the gel strength was increased with increasing temperature. The core test results show that the ABP system has good plugging characteristics, and the plugging rate is more than 99%. Zhao T. H. et al. [15] studied a new type Poly (AM/O-MMT) composite by solution polymerization method, and X-ray diffraction analysis showed that the composite formed a completely stripped structure. Poly (AM/OMMT) showed lower water absorption rate and better stability in 95°C distilled water and NaCl solution. From saturation to 90 days, the water absorption base did not change. Poly (AM/O-MMT) has better water plugging and profile control ability in high temperature (95°C) and high salinity (20% NaCl) environment. Xu et al. [16, 17] established a simulation model with periodic lean zones to analyze the effects of these lean zones on SAGD performance, which investigated the effects of vertical distribution, horizontal spacing and sizes, and spatial relationship with SAGD horizontal wells.
The polymer gel system has fluidity and low viscosity fluid state before gelation. In the reaction process, under the action of crosslinking agent, the linear polymer molecular chains are cross-linked at multiple positions, thus forming a gel with spatial network structure [18–21]. Colloids exhibit partial solid properties, with certain viscoelasticity, strength and bearing capacity. Crosslinking agents can usually be divided into two categories, one is the inorganic crosslinking agent based on metal ions such as chromium ions and aluminum ions; The other is organic crosslinking agent based on phenolic resin and other organic substances. Organic crosslinkers and polymer molecules are mostly connected in the form of covalent bonds, resulting in better gel stability and generally higher temperature resistance. Research and development of new crosslinking agents, using organic/inorganic crosslinking agents and other methods can effectively improve the temperature resistance of the gel system. Nasr-El-Din H A et al. [22] studied a gel type plugging agent with silicate/urea system, which can form gel over 70°C. The results showed that the maximum concentration of NaCl tolerated by sodium silicate/urea solution was 3%, while the maximum concentration of CaCl2 was 0.08%. Prajakta et al. [23] combined different activators with colloidal nano silica to prepare a non-toxic and environmentally friendly gel system with a gel formation time of 4–6 h at 200℉ and 1 h at 300℉. The formation time of the system can be controlled by adjusting the pH. At 200℉, the formation time is less than 1 h in the pH 5-7 range and 2–8 h in the 8–10 range, which can meet the actual construction requirements. Guang Y. et al. [24] experimentally studied three kinds of polymer gel temporary plugging agents with different strengths. The core experiment shows that the polymer gels with different strength have good formation sealing property and erosion resistance. The formation sealing rate is increased by 90% after water erosion of 40 PV. Ayman et al. [25] synthesized a zirconia/graphene nanocomposite material and reacted it with low molecular weight polyacrylamide as a crosslinking agent to prepare a new gel system that can be used for high temperature water plugging. Adewunmi A et al. [26] studied and synthesized the CFA (fly ash) content (mass fraction 0.5%, 1%, and 2%) of pure polyacrylamide PAM/PEI hydrogel and PAM/PEi-CFA hydrogel (PEI as the crosslinking agent). Thermograying analysis results show that the decomposition rate of PAM/PEICFA3 is less than 3% at 200°C, which may be due to the formation of strong chemical bonds between PAM/PEI and alumina and silica, which greatly improves the temperature resistance of hydrogels. Liu Y. et al. [27] studied a series of chemical gel plugging agents with temperature and salt resistance, using the low phase pair particle mass polymer {P (AM-coAMPS)} as the polymerization monomer and phenolic resin (linear) as the crosslinking agent. The results show that the gel system can meet the diversity of water plugging requirements under different geological conditions by adjusting the dosage of each component. The gel system was tested by TG and DSC. It was proved that the gel system had good temperature resistance. Yang H. et al. [28] have poor performance under high salinity because of the rapid dehydration shrinkage of conventional polymer gels. Amphiphilic polymer gels have been studied by cross-linking the salt-tolerant amphiphilic polymer APP4 with organic chromium, with very low dehydration shrinkage at high salinity (80,000 mg/L NaCl) and temperature (85°C).
Water-soluble polymer gel particles have attracted a lot of attention due to their excellent hydrophilicity and have been widely used in agriculture, food, cosmetics, petrochemicals and other fields. Due to its unique swelling and dissolving properties, it can be used as a temporary plug or diverter for fracturing. The study of functional monomers and polymers forming composite systems has attracted much attention in recent years. After extensive preliminary experimental studies, we have prepared a novel water-soluble polymer temporary plug by polymerization in aqueous solution and optimized the preparation process conditions.
2 Preparation of water-soluble polymer temporary plugging agent
2.1 Design technical ideas
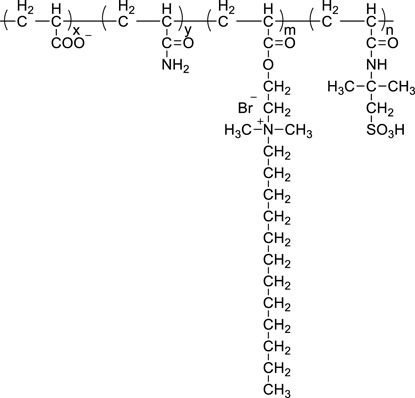
A new technical idea to develop a new water-soluble polymer plugging agent for fracturing is: medium and low molecular weight + reinforced chain rigidity + supramolecular aggregation.
(1) medium and low molecular weight. The main purpose of reducing the molecular weight of polymer is easy to process, easy to degrade and reduce the viscosity of the degraded solution.
(2) Enhance the chain rigidity. The enhancement of chain rigidity has two purposes: 1) to enhance the resistance of copolymer to mechanical degradation during the granulation process. 2) To enhance the chain rigidity and make the polymer chain more entangled, so as to control the dissolution time, especially by introducing different amounts of -SO32- or -COO - to the rigid groups used to increase the chain rigidity, so as to increase the electrostatic repulsion within and between the polymer chains, so as to control the dissolution time of the temporary stopper to a certain extent.
(3) supramolecular aggregation. Although the molecular weight of the polymer is reduced, the dissociation time cannot be reduced or even increased. Clearly, it is no longer practical to rely solely on the entanglement of a single HPAM molecular chain, but must rely more on the interactions between the polymer chains. Hydrophobic groups with specific structures are introduced to achieve hydrophobic association and entanglement between the molecules and thus polymer molecules form supramolecular aggregates. The aggregates are not easily disentangled before entering the fracture pore, and have good hydrophilicity, which favors dispersion in a dispersive solution. Once inside the pore, it begins to swell and form a plug. As the fluid and the fracturing fluid continue to migrate, the swelling system formed by the polymer temporary plug begins to dissolve and eventually disappears and the channel reopens.
2.2 Experimental materials and method
Acrylamide AM, acrylic acid AA, 2-acrylamide-2-methylpropane sulfonic acid AMPS, SM functional monomer (self-made), Na2CO3, urea, sodium bisulfite, potassium persulfate.
The temporary plug is prepared by solution polymerization, and the experimental workflow is as follows:
(1) Under water bath cooling, add 0.3 Kg of acrylamide (AM) and 0.2 Kg of acrylic acid (AA) into a 5 L three-port reaction flask equipped with an agitator, thermometer and reflux condenser, and then add 80 g of water, 0.2 g of 2-acrylamide-2-methylpropane sulfonic acid (AMPS), 0.8 g of SM functional monomer, and 12 g of urea.
(2) Start the agitator and add 46.5 g of sodium carbonate after stirring evenly for 1 h.
(3) 0.014 g of sodium bicarbonate and 0.028 g of potassium bicarbonate were added to initiate polymerization to obtain the product.
(4) After the reaction, pour the obtained mixture onto the container, spread it into a cake with a thickness of about 3 mm, after natural drying, cut it into blocks with a particle size of 3–6 mm, and bake it to constant weight in a 100°C oven to obtain a dry block, which is the finished product.
3 Experimental evaluation results and discussion
3.1 Infrared spectrum analysis
The structure of the synthesized temporary plug material was studied with the Perkin-Elmer 325 FI-IR infrared spectrometer, KBr plate, with measurements in the 400–4,000 cm−1 range. It shows the IR spectra of polymer temporary plug agents synthesized in the laboratory. As can be seen in Figure 1, the absorption peak at 3,423 cm−1 is attributed to the stretching vibration of the O-H bond, indicating the presence of the cross-linking functional group -OH-. The absorption peak at 2,932.7 cm−1 is attributed to stretching vibrations of saturated C-H bonds in the polymer, and shifts towards higher wavenumbers as long carbon chains are introduced; The absorption peak at 1,642.8 cm−1 belongs to the stretching vibration of the C = O bond. The absorption peak near 1,543.1 cm−1 belongs to the bending vibration of the N-H bond in the secondary amide; the absorption peak at 1,451, 1,401 cm−1 belongs to the internal bending vibration of the saturated C-H plane in the polymer, indicating that AA and AM are polymerized. Absorption peaks near 1,335 cm−1 and 1,162 cm−1 in the fingerprint region belong to stretching vibrations of the C=O bonds in the carboxyl group, which proves the presence of carboxylates. The absorption peak near 1,240 cm−1 belongs to −SO3, which justifies the introduction of the AMPS monomer. The absorption peak around 1,195 cm−1 is attributed to asymmetric stretching vibrations of the C-O-C-bond. The absorption peak around 672 cm− 1 was attributed to the vibration of the C-Br bond, demonstrating the introduction of the SM monomer. In summary, the presence of the characteristic absorption peaks of AA, AM, AMPS and SM in the FTIR spectra of the products indicates that the synthesized products are the target products.
3.2 SEM characterization results
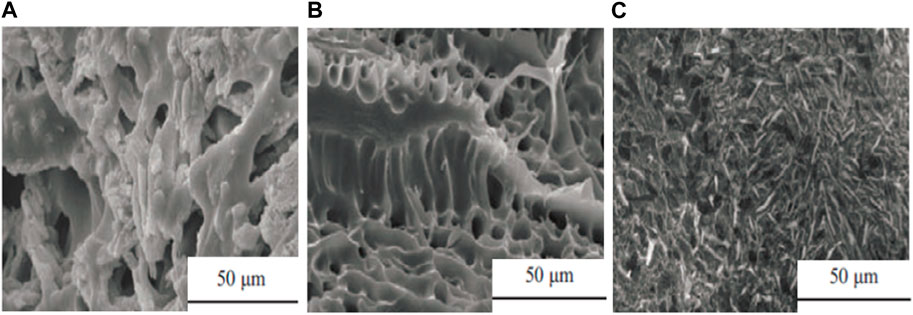
Figure 2A shows SEM photos of the AM-AA polymer, and Figures 2B, C show SEM photos of the new copolymer temporary plug at SM functional monomer doses of 0.8 g and 1.6 g, respectively. As can be seen from Figure 2A, both AM-AA and the novel copolymer have rich 3D network structures. In particular, the AM-AA network has an irregular distribution and a rough surface, while the new copolymer has a regular distribution of network channels and a smooth surface. This is because the introduction of SM functional monomers adds hydrophobic segments to the original molecular chain, which under hydrophobic association form regular 3D network channels, providing copolymers with a large amount of water absorption and retention space. As can be seen in Figures 2B, C, the SEM morphology of the copolymer shows a needle-like array distribution as the functional monomeric SM is increased. This is because an increase in the amount of functional monomeric SM greatly increases the number of hydrophobic segments in the copolymer molecular chain, which aggregate under hydrophobic association to form these needle-like arrays, thus increasing the copolymer contact area.
3.3 Effect of monomer ratio on dissolution rate
Determination of solubility: weigh a small dry sample of a certain mass, put it into 100 mL 0.9% NaCl solution, keep it warm in a 70°C water bath, and record the time (DT) when the sample completely disappears and dissolves.
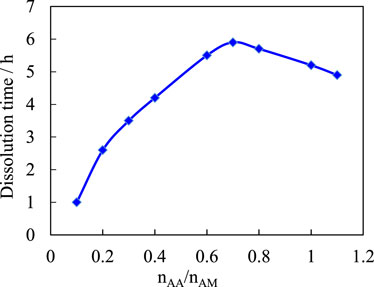
Figure 3 shows the effect of the molar ratio (nAA/nAM) of monomer acrylic acid (AA) and acrylamide (AM) on the solubility of the sample. The total number of AM monomers is kept constant. As the amount of acrylic increases, the sample dissociation time gradually increases, reaching a maximum at nAA/nAM = 0.7. As the number of acrylic increases, the degree of cross-linking of the polymer gradually increases and the dissolution rate of the composite gradually slows down, then its dissolution time increases. When the amount of acrylic acid is small, the amount of carboxylate ions in the complex is small. This is because the molecular chains are relatively stretched due to chelation competition, the degree of cross-linking is also small, water easily enters the complex network, and the complex rapidly swells and dissolves. As the quantity of acrylic increases, the transition from low to high coordination number occurs, the degree of cross-linking of the complex increases, and the network becomes more contractive. In this way, the entry of water becomes difficult, the number of coordination bonds that need to be destroyed in the dissociation of the complex increases, and the dissociation rate of the complex is correspondingly slowed down. When the amount of acrylic acid is increased to a certain extent, the molecular chains of the complex become elongated and the water molecules become more accessible, while the dissolution rate increases slightly. As for the dissociation time of the compound, it decreases after reaching its maximum because the hydrophilicity of acrylic is stronger than that of acrylamide, and water molecules are more likely to enter the network with a higher acrylic content. Comparing the requirements for the dissociation time of the temporary plug, nAA/nAM = 0.7 was determined.
3.4 Effect of neutralization degree of acrylic acid on dissolution rate
Keep the dosage of other raw materials unchanged, change the dosage of sodium carbonate to change the neutralization degree (ND) of acrylic acid, and study the influence of the neutralization degree (ND) of acrylic acid on the solubility of copolymer. Figure 4 shows the effect of neutralization degree (ND) of acrylic acid on the solubility of polymer.
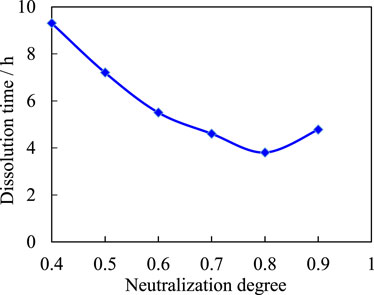
Figure 4 shows that the dissociation rate of copolymers increases with the neutralization degree of acrylic, with a minimum occurring when the neutralization degree of acrylic reaches 80 percent. This is because when the degree of neutralization is low, the concentration of AA is high, the activity is high, the polymerization rate is fast, and it readily self-polymerizes to form a polymer with a high degree of cross-linking, which reduces the dissolution rate of the product. As the degree of neutralization continues to increase, it not only slows down the reaction and reduces the degree of cross-linking, but also increases the ionic group of sodium carboxylate, which increases the osmotic pressure inside the interconnecting network. Increasing the degree of neutralization also increases the osmotic pressure between the polymer and the water, which promotes polymer swelling and accelerates the dissolution rate of the products. However, if the neutralization of the system is too high, the carboxylic acid content increases and the coordination form is dominated by intermolecular coordination. As the degree of cross-linking of the complex increases, the network also becomes more contractive and access to water becomes difficult. The coordination bonds that need to be destroyed in the dissociation of the complex are also increased and the dissociation rate of the complex is correspondingly slowed down. The optimal neutralization of acrylic was determined to be 70% by comparing the required dissociation time of the temporary plug.
3.5 Effect of initiating dose on dissolution rate
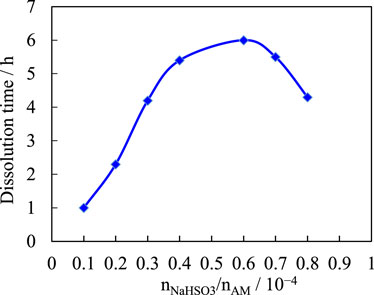
The dosage of the other ingredients was kept constant, the dosage of the initiator sodium bisulfite was varied, and the effect of the initiator dosage on the dissolution rate was studied. Figure 5 shows the effect of the amount of initiator (nNaHSO3/ nAM) on the solubility of the sample.
As the amount of initiator is gradually increased, the dissolution rate of the sample is accelerated. This is because the number of initiators is small, the decomposition rate of the initiators is low, the amount of radicals is small, the polymerization reaction is slow, the low-polymer content in the polymer is large, and the dissolution rate of the complex is fast. As the number of initiators increases, the polymerization reaction speed is gradually accelerated, the low-polymer content of the polymer is gradually reduced, the chelating and cross-linking effects of the polymer are gradually enhanced, and the dissociation rate of the complex is increased. Increasing the number of initiators further, the molecular weight of the polymer will gradually decrease, the cross-linking network will contain more terminal groups, the solubility of the polymer will increase, and thus the dissolution rate will be accelerated. Compare the requirements for the dissolution time of temporary plugging agent, and determine that nNaHSO3/nAM = 0.45 × 10−4.
3.6 Effect of reaction time on dissolution rate
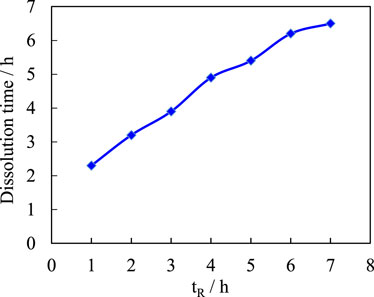
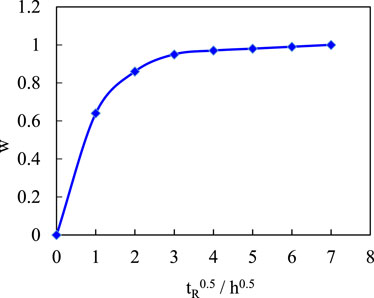
The amount of other ingredients was kept constant, the reaction time was varied, and the effect of the reaction time on the dissociation rate of the copolymer was studied. Figure 6 shows the effect of reaction time (tR) on the dissolution rate of copolymer.
As the reaction time is extended, the polymer dissociation time gradually increases and eventually reaches a certain value. This is caused by a change in the polymerization of the initiator. The reaction time is short, the polymerization reaction is not balanced, and the structure of the compound is stable. There are high cross-linking regions with high cross-linking and low cross-linking regions with low cross-linking. Although high cross-linking regions are unfavorable for water entry, the presence of low cross-linking regions accelerates water entry. At the same time, due to the staggered and random distribution of the high and low cross-linking regions in the polymer, the effective distance of water entering the high cross-linking region is shortened, and finally the speed of water entering the high cross-linking region is accelerated, so the dissolution rate of the composite is fast. As the reaction time is extended, the coordination gradually reaches the most reasonable distributional state due to the gradual reduction of the residual monomers, and the degree of cross-linking increases compared to recombination with short reaction times. In this way, the polymer network structure is uniformly distributed, effectively blocking the entry of water, and the swelling and dissolution rate of the compound is slowed down. A reaction time of 3 h is more reasonable compared to the requirement of a dissociation time for the temporary plug.
3.7 Effect of the amount of functional monomer SM on the dissolution rate
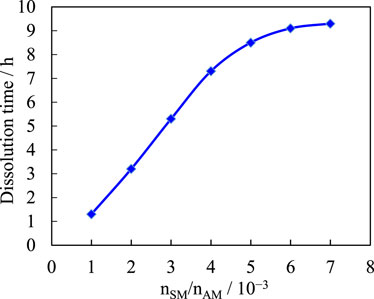
The functional monomer SM is varied while the other raw materials are kept constant, and the effect of the functional monomer SM on the dissociation rate of the copolymer is studied. Figure 7 shows the effect of the functional monomer SM on the dissociation rate of the copolymer. Figure 7 shows that the amount of functional monomer SM has a significant effect on the solubility of the copolymer temporary plug. Within the experimental range, as the functional monomeric SM content increases, the dissociation rate of the sample in water slows down significantly and the water absorption by the copolymer decreases significantly. This is because an increase in the hydrophobic functional monomeric SM content increases the physical cross-linking point of the copolymer, resulting in higher copolymer strength, lower water absorption, and lower dissolution rate. Compared with the requirements for the dissolution time of temporary plugging agent, the functional monomer SM ratio is optimized as 3 × 10−3. Through experimental optimization, the optimum conditions of various influencing factors obtained are as follows: monomer ratio nAA/nAM = 0.7, the optimum neutralization degree of acrylic acid is 70%, initiator ratio nNaHSO3/nAM = 0.45 × 10−4. Reaction time 3 h, functional monomer SM ratio 3 × 10−3.
4 Performance evaluation of new temporary plugging agent
4.1 Determination of water absorption expansion
A sample of a certain quality is accurately weighed by placing it in a 200-g nylon bag, sealing the mouth of the bag and weighing it. The sample was placed in the absorbable solution at a constant temperature of 30°C, sampled at regular intervals, removed free water from the surface, and then weighed. This is repeated several times until the mass is constant or has a steady downward trend. Calculate the water absorption expansion rate (Qs, g/g) according to Formula 1.
Where, m0 is the initial sample mass, g; m1 is the total mass of the initial nylon bag and sample, g; m2 is the total mass of nylon bag and sample after absorbing water for a period of time, g.
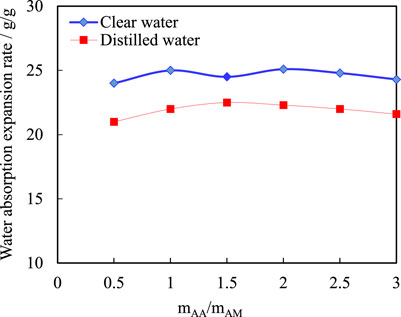
See Figure 8 for the water absorption expansion of copolymers. As can be seen from Figure 8, copolymers with different monomer ratios have a higher water absorption expansion rate in tap water than in distilled water, with a maximum water absorption rate of 25.8 g/g. This is because the hydrophobic interaction between the copolymer segments takes place between molecules, making the curled molecular chain extend and form a large spatial network - dynamic physical cross-linking network structure through the hydrophobic interaction, and the rich needle-like array on the surface increases the contact between the polymer and electrolyte ions such as Na+ and Cl− in the solution, which enhances the hydrophobic association, so the water absorption capacity is also enhanced. The monomer ratios of AM and AA don’t have a significant effect on their water absorption, which is due to the fact that the variation of water absorption not only increases with the number of hydrophilic functional groups, but is also limited by the degree of cross-linking of the polymers. When the degree of cross-linking is large, the density of polymer cross-linking points is large, the space formed by molecular chain swelling is small, and the volume of water contained is also small, so when the water absorption exceeds its tolerance limit, The water absorption capacity of the polymer tends to be stable without increasing.
4.2 Determination of swelling kinetics
The change in the water absorption of the polymer particles at different times allows to measure the water absorption and swelling process of the polymer with the same specific measurement procedure as in Section 4.1. According to Formula 1, the water absorption (Qt) of polymer at time t can be calculated, and the ratio of water absorption of polymer at time t to water absorption at swelling equilibrium (w) is:
Where, Qe is the water absorption of polymer after swelling equilibrium, g/g.
Figure 9 shows that during the initial swelling process, the polymer particles exhibit a faster rate of water absorption and the swelling curve deviates from the ideal exponential change. As the swelling time continues to lengthen, the water absorption rate decreases and the swelling rate decreases. This variation is mainly related to the size of the copolymer sample particles and the structure of the copolymer cross-linking network. The swelling kinetics curves of copolymers show that copolymers have a fast water absorption rate and can reach swelling equilibrium in a short time.
4.3 Density
(1) True density (particle density)
Five samples of 20–30 each were randomly selected from the product, their length, width and height were measured in the same way, the mean value was calculated and their mean volume was calculated. The sample was then weighed and the density of the sample was calculated using the density definition formula. The average density is: ρ = 1.15 g/cm3.
(2) Bulk density
Randomly select 3 samples from the product, each containing 20–30 capsules, and weigh them. The bulk density of the sample was determined using the discharge volume method using tetrahydrofuran as the measuring medium. The average density is: ρ = 1.15 g/cm3.
The above experimental data show that the prepared crack steering control agent has uniform particle shape and density ρ = 1.14 g/cm3.
4.4 Determination of viscosity after dissolution
The solubility of the temporary plug is related to whether the temporary plug can effectively plug fractures and percolation channels, and whether it can achieve self-plugging. If the solubility of the temporary plugging agent is too good, the temporary plugging agent is completely dissolved before reaching the predetermined plugging area and cannot play a plugging role, or the effective plugging time isn’t long enough to meet the construction requirements of the shift to re-fracturing shielding temporary plugging. If the solubility of the temporary plugging agent is too poor, it will not be able to remove the plug by itself or the plugging removal cycle will be very long after plugging, which will not only cause damage to the reservoir, but also have a certain impact on the increase of oil and gas productivity. If a temporary plug agent has poor solubility at low temperatures and good solubility at high temperatures, it can effectively plug fractures and percolation channels temporarily during the construction process. Upon completion of construction, the temperature in the near-well zone rises and returns to the formation temperature. At this point, the temporary plugging agent rapidly dissolves to achieve self-plugging.
The water solubility and high temporary plug strength of water-soluble temporary plug are the key technical difficulties of water-soluble temporary plug. Water-soluble temporary plugging agent generally consists of inorganic salts (slightly soluble in water at normal temperature, and the solubility gradually increases with the increase of temperature and solid-liquid ratio), organic acids (a mixture of organic acids and organic acid salts, with a high softening point and a small change in solubility with temperature) and other additives. The solubility of temporary plugging agent was tested at 30°C, 60°C, and 80°C. Experimental materials: temporary plugging agent, tap water. Experimental equipment: thermostatic water bath, electronic stirrer, electronic balance (minimum graduation 0.001 g), measuring cylinder, rubber tip dropper, glass rod.
Experimental steps:
1) Accurately weigh 3 parts of temporary plugging agent with electronic balance, 8 g each;
2) Use a measuring cylinder to measure 100 mL of tap water and put it into three 250 mL beakers;
3) Adjust the temperature of the three water bath pots to 30°C, 60°C, and 80°C, put the beaker containing tap water in the thermostatic water bath pot and connect the electronic stirrer. When the temperature is stable, stir into the beaker the temporary blocking agent and dissolve;
4) Observe the dissolution of temporary plugging agent in tap water at 0.5 h, 1 h, 3 h, 5 h and 8 h respectively, pass the insoluble substances through the 60-mesh screen, dry and weigh, and calculate the degradation ratio;
5) The liquid viscosity after the degradation of temporary plugging agent was measured by DV-II rotary Brinell viscometer (Brookfield Company, United States) at room temperature and 6r/min.
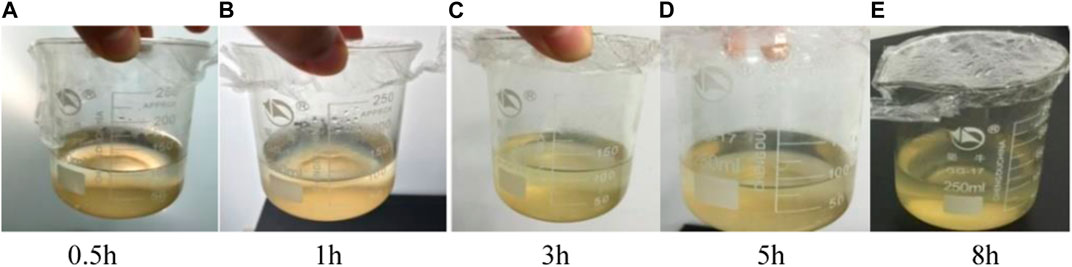
See Figures 10A–E for the dissolution effect of copolymer particles at 60°C.
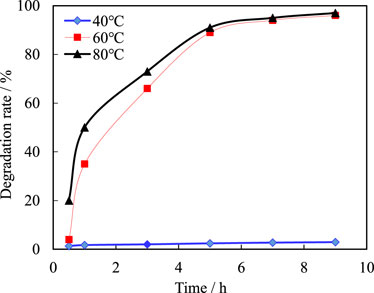
See Figure 11 for the degradation ratio of the new temporary plug at different temperatures and times. It can be seen from Figure 12 that the degradation rate of temporary plugging agent is about 1%–4% at 30°C, which is difficult to degrade. After the temperature rises to 60°C and 80°C, the temporary plugging agent degrades rapidly in the early stage, and the degradation rate reaches more than 90% in about 5 h, and can be completely degraded in 5 h and 8 h. At the same time, the experimental results show that the higher the temperature, the faster the degradation of the temporary plugging agent.
4.5 Strength of temporary plugging agent
The strength of the colloids formed after the injection of the temporary plug into the formation must be higher than the pressure value at the fracture of the pay formation to ensure the reorientation of the new fracture during re-fracture. At 60°C, the plugging strength of temporary plugging agent was studied by using artificial fracture core.
Experimental materials: New water-soluble temporary plug particles. Experimental equipment: electronic stirrer, electronic balance (minimum graduation 0.001 g), beaker, glass rod, natural core, core displacement device.
Experimental steps:
1) Cut the core with a diameter of D ≈ 2.5 cm axially for standby;
2) After drying the core, weigh the dry core mass M1;
3) Put the core into the formation water suction bottle and filter for 8 h. When the core is sufficiently saturated with water, the formation water on the surface of the core is drained off with a clean rag, and the mass M2 is weighed after the core has saturated with water;
4) (M2 − M1)/ρw Effective pore volume V of core obtained from formation water;
5) Add an appropriate amount of temporary plugging agent into the tap water, and fully mix for 5 min at 1,500 rpm;
6) Add appropriate thickness of mixed temporary plugging agent between two cores;
7) Put the core into the core holder, add a confining pressure of 3.0 MPa, place it in the thermostat at the temperature of the reservoir, use the core displacement device (Figure 12), and drive the formation water forward at a flow rate of 0.1 mL/min until the pressure is stable, and record the pressure value;
8) With the extension of displacement time, the temporary plugging agent gradually ages in the core, and continues to inject formation water into the core at a flow rate of 0.1 mL/min, constantly observe the pressure change, record the pressure value at the time of breakthrough, and continue to displace until the pressure is stable, and record the stable pressure.
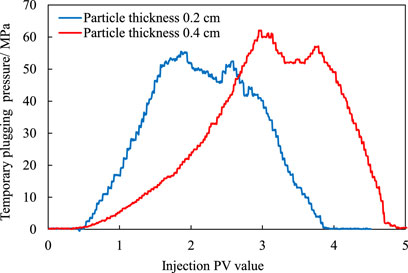
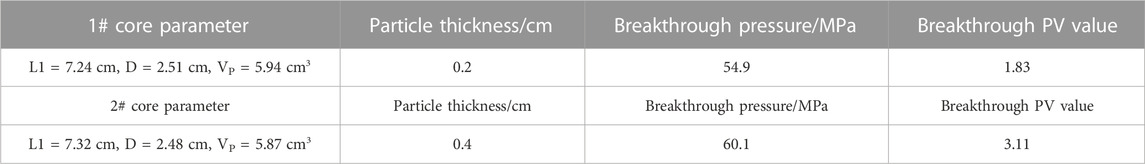
It can be seen from Figure 13 and Table 1 that in the early stages, the pressure increases significantly with the increase of the injected fluid, reaching the maximum pressure value, and the fluid breaks through the temporary plug, allowing the pressure decrease to gradually equalize. The break pressure for particles with thicknesses of 0.2 cm and 0.4 cm is 54.9 MPa and 60.1 MPa, respectively. This indicates that the temporary plug has the ability to seal the original fracture and cause the new fracture to deviate from the direction of maximum principal stress.
Then, with the extension of the displacement time at 70°C, the temporary plugging material gradually ages and loses its temporary plugging effect, resulting in a significant decrease in the injected fluid pressure. The number of breakthrough PV is between 1.2 and 1.5 PV, and the time is between 65 and 84 min. The aging failure time is shorter than the quiescent time and is mainly due to the incomplete degradation of the temporary plug at the time of rupture, but the partial temporary plug failure is caused by the high displacement pressure at the reservoir temperature. Later, as the injection time increases, the process of aging and degradation of the temporary plug continues until the pressure is stabilized. At the same time, the temporary plug pressure of the granular temporary plug is strongly dependent on the amount of the plug. The larger the amount, the higher the temporary plug pressure.
4.6 Preparation of coated temporary plugging agent and test of conductivity after degradation
After the copolymer is synthesized and before cooling, it is mixed with 20–40 mesh ceramics at 9:1, 8:2, and 7:3 and then pelletized to test the conductivity of the particle-temporary plug after degradation.
Experimental material: A novel water-soluble coated temporary plug particle, which is granulated after mixing with a ceramic proppant. Experimental equipment: electronic stirrer, electronic balance (minimum graduation 0.001 g), beaker, glass rod, natural core, core displacement device.
Experimental steps:
1) Cut the core with a diameter of D ≈ 2.5 cm axially for standby;
2) After drying the core, weigh the dry core mass M1;
3) Put the core into the formation water suction bottle and filter for 8 h. When the core is sufficiently saturated with water, the formation water on the surface of the core is drained off with a clean rag, and the mass M2 is weighed after the core has saturated with water;
4) (M2 − M1)/ρw Effective pore volume V of core obtained from formation water;
5) Add an appropriate amount of temporary plugging agent into the tap water, and fully mix for 5 min at 1,500 rpm;
6) Add 0.3 cm thick mixed coated temporary plugging agent between cores;
7) Put the core into the core holder, add a confining pressure of 30 MPa, and place it in the thermostat at the temperature of the reservoir. Use the core displacement device (Figure 12) to drive the formation water forward at a flow rate of 2 mL/min. When saturated, let it stand for 5 h to dissolve the temporary plug;
8) As time goes on, the temporary plugging agent gradually ages in the core, continues to inject formation water into the core at a flow rate of 2 mL/min, constantly observes the pressure change, records the pressure value at the time of breakthrough, and continues to displace until the pressure is stable, and records the stable pressure.
9) After washing the rock sample, change the experimental conditions to conduct another group of experiments.
Data processing:
According to Darcy’s law, the conductivity of supporting fractures is measured, and its calculation formula is:
Where: Kfw is the conductivity of supporting fracture, μm2·cm. μ is the working fluid viscosity, mPa·s. Q is the flow, cm3/min. Δp is the pressure difference, kPa.
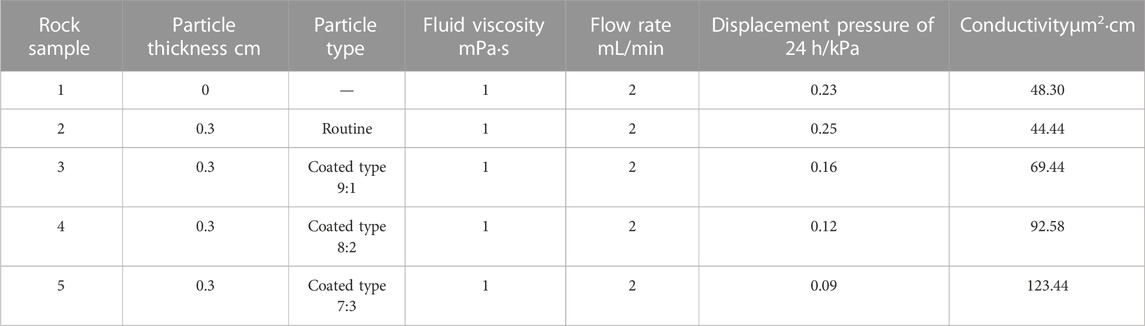
The results of the post-degenerate conductivity test are given in Table 2. Since the same rock sample was used, the data for each group is comparable. When the displacement fluid uses clean water (viscosity is 1 mPa s) and flow rate is 2 mL/min, the displacement pressure of blank rock sample is 0.23 kPa, and the conductivity is about 48.30 calculated by formula 2-3 μm2 cm. For rock samples using conventional temporary plugging agent, the particle temporary plugging agent is dissolved after 24 h, and the conductivity is 44.44 μm2 cm, which is lower than that of the blank sample. It has been analyzed that part of the temporary plug is not completely degraded, which reduces the fracture conductivity to some extent. Change the coated temporary plugging agent with different proportions (9:1, 8:2, 7:3), and the core displacement pressure will be greatly reduced by 0.16, 0.12, and 0.09 MPa after 24 h, and the calculated conductivity is about 69 μm2 cm, 93 μm2 cm, and 123 μm2 cm respectively, both of which have been greatly improved. It shows that the ceramics after the dissociation of the temporary plug act as a local support and significantly increase the fracture conductivity, reflecting that the developed coated temporary plug is in line with the original design intent and achieves the design purpose.
4.7 Compatibility evaluation of temporary plugging agent and fracturing fluid
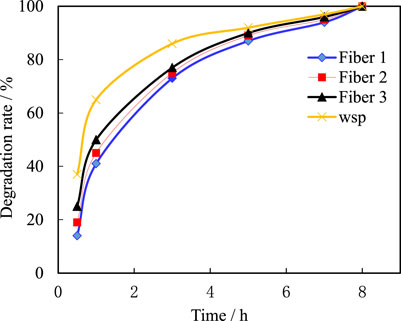
The compatibility of temporary plugging agent and fracturing fluid to a certain extent reflects the controllability of temporary plugging fracturing construction and the damage to the formation. When the compatibility of the two is poor, it is easy to affect the cross-linking of the fracturing fluid, affect construction, or cause precipitation in the formation, damaging the formation. From the experimental results, it can be seen that the addition of particle temporary plugging agent does not affect the gel breaking of guar gum fracturing fluid, and the dissolution of temporary plugging agent in the fracturing fluid has no or micro precipitation, indicating that the temporary plugging agent has good compatibility with the fracturing fluid. At 80°C, after being placed for 3 h, the fiber changes into a colloidal block shape in the gel breaker, and is stirred to break in a lump shape. The gel breaker is thoroughly broken, and the viscosity of the gel breaker is equivalent to that of water. Under the same conditions, the particulate temporary plugging agent is almost completely dissolved after being placed for 3 h. The degradation of the four temporary plugging materials over time at 80 °C after gel breaking is shown in Figure 14.
It can be seen from Figure 14 that, similar to the degradation process in water, the four temporary plugging agents degrade rapidly in the early stage of the gel breaker, with a degradation rate of over 90% within 3 h, and can be completely degraded within 5–8 h (According to the pH test results, guanidine glue and gel breaker are weakly alkaline, which is conducive to the degradation of acidic fibers.); At the same time, it was found that at 80°C, the degradation of particulate temporary plugging agent was faster than that of fibrous materials. In order to appropriately reduce the degradation rate of particles, diesel immersion treatment measures can be taken.
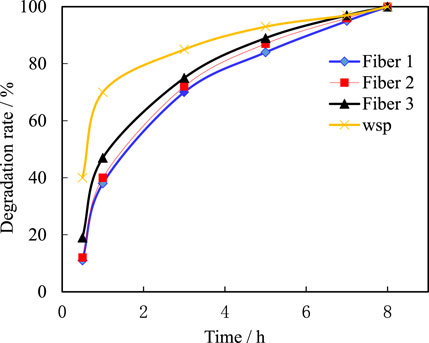
4.8 Time of complete dissolution of plugging agent under formation conditions
The formation temperature in the work area is about 80 °C. When the gel breaker containing four temporary plugging agents is heated to 80 °C, the degradation rates of the four temporary plugging materials at different times in the gel breaker are shown in Figure 15. It can be seen from the Figure 15 that the temporary plugging agent degrades rapidly in the early stage of the gel breaker, with a degradation rate of over 90% within 3 h, and can be completely degraded within 5–8 h.
5 Conclusion
(1) A new idea for the development of a new water-soluble fracturing temporary plugging agent is proposed: medium and low molecular weight + enhanced chain rigidity + supramolecular aggregation. The AM-AA-AMPS-SM copolymer temporary plug is prepared by polymerization with sodium bisulfite and potassium sulfate as initiators, AA, AM and AMPS as grafting monomers, and SM as hydrophobic functional monomers. The FTIR and SEM results indicate that the synthesized product is an AM-AA-AMPS-SM copolymer.
(2) Through experimental optimization, the optimum conditions of various influencing factors are as follows: monomer ratio nAA/nAM = 0.7, the optimum neutralization degree of acrylic acid is 70%, initiator ratio nNaHSO3/nAM = 0.45 × 10−4. Reaction time 3 h, functional monomer SM ratio 3 × 10−3.
(3) The test results of water absorption expansion rate, swelling kinetics, density, viscosity after dissolution, strength of temporary plugging agent and conductivity after degradation of the new temporary plugging agent show that it is completely degraded at 70°C for 5–8 h, and the solution viscosity after degradation is 2.5–3.6 mPa s, with good fluidity and no gel residue. The density of the temporary plug material is approximately 1.14 g/cm3. The water absorption expansion rate reached 25.8 g/g. The pressure is 60.1 MPa when the particle is 0.4 cm thick as a temporary plug. Under experimental conditions, the fracture conductivity at a closure pressure of 30 MPa after degradation of the temporary plug is 69–123 D*cm, which meets the technical requirements for temporary plug and diversion fracturing.
(4) The significant advantage of the new polymer is that the dissolution time and temperature in water can be controlled by the addition of functional monomers, and the addition of functional monomers can improve the interface performance between the solution and the proppant, contributing to the formation of coated temporary plugging agents. The new temporary fracture plugging agent has the characteristics of high plugging strength, temperature-controlled degradation in water, stable reflux, and effective self-supporting after degradation, which helps to ensure the success rate of temporary plugging fracturing and significantly improve the effect of temporary plugging fracturing.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
GW and ZC: conceptualization, funding acquisition, project administration, resources, writing—original draft and software. YH: data curation, formal analysis, methodology. GW and SL: writing—original draft, and writing—review and editing. XK: project administration, resources. JZ: investigation, methodology, software, and visualization. XX and JW: conceptualization, funding acquisition, methodology. XK: investigation, methodology, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Research on offshore large-scale fracturing engineering technology (KJGG2022-0704), Key parameter characterization and dessert area evaluation technology of coalbed methane geological engineering (KJGG2022-1001), Research on Inter-well Disturbance Integral Fracturing Technology of Coalbed Gas (2021-YXKJ-010).”
Acknowledgments
The authors are grateful for reviewers and editors for their careful review of this manuscript.
Conflict of interest
GW, ZC, AZ, JZ, YH, XX, and JW were employed by CNOOC Research Institute Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang X, Shen H, Sun J, Kaihe L, Liu J, Dong X, et al. Nanoscale laponite as A potential shale inhibitor in water-based drilling fluid for stabilization of wellbore stability and mechanism study. Acs Appl Mater Inter (2018) 10(39):33252–9. doi:10.1021/acsami.8b11419
2. Chang X, Sun J, Xu Z, Zhang F, Wang J, Kaihe L, et al. A novel nano-ligninbased amphoteric copolymer as fluid-loss reducer in water-based drilling fluids. Colloids Surf A Physicochemical Eng Aspects (2019) 538:123979. doi:10.1016/j.colsurfa.2019.123979
3. Huang X, Sun J, Lv K, Liu J, Shen H, Zhang F. Application of core-shell structural acrylic resin/nano-sio2 composite in water based drilling fluid to plug shale pores. J Nat Gas Sci Eng (2018) 55:418–25. doi:10.1016/j.jngse.2018.05.023
4. An YX, Jiang GC, Agent P, Ge QY. Plugging agent of shale base on Nano flexible polymer. Appl Mech Mater (2016) 4254(1670):15–9. doi:10.4028/www.scientific.net/amm.835.15
5. Lu Z. Progress and prospect study on temporary plugging agent for diverting fracturing. Sci Tech Eng (2020) 20(31):12691–701.
6. Song L, Zhang H, Wang M, et al. Experimental investigation into temporary acidizing of fractured Carbonate reservoirs in Sichuan Basin. Chem Eng Oil Gas (2021) 50(3):90–5.
7. Pu J, Geng J, Han P, Bai B. Preparation and salt-insensitive behavior study of swellable, Cr3+ -embedded microgels for water management. J Mol Liquids (2019) 273:551–8. doi:10.1016/j.molliq.2018.10.070
8. Hasankhani GM, Madani M, Esmaeilzadeh F, Mowla D. Experimental investigation of asphaltene-augmented gel polymer performance for water shut-off and enhancing oil recovery in fractured oil reservoirs. J Mol Liquids (2019) 275:654–66. doi:10.1016/j.molliq.2018.11.012
9. Salehi MB, Soleimanian M, Moghadam AM. Examination of disproportionate permeability reduction mechanism on rupture of hydrogels performance. Colloids Surf A: Physicochemical Eng Aspects (2019) 560:1–8. doi:10.1016/j.colsurfa.2018.09.085
10. Tian M, Xu YJ. The preparation and characterization of AM/2-EHA/VTEOS copolymer as profile control agent. Advanced Materials Research. Trans Tech Publications Ltd (2013) 602:732–8.
11. Wang C, Liu H, Zheng Q, Liu Y, Dong X, Hong C. A new high-temperature gel for profile control in heavy oil reservoirs. J Energ Resour Tech (2016) 138(2). doi:10.1115/1.4031706
12. Xu J, Chen Z, Wu K, Li R, Liu X, Zhan J. On the flow regime model for fast estimation of tight sandstone gas apparent permeability in high-pressure reservoirs. Energy Sourc A: Recovery, Utilization, Environ Effects (2019) 2019:1–12. doi:10.1080/15567036.2019.1687625
13. Li J, Wu K, Chen Z, Wang K, Luo J, Xu J, et al. On the negative excess isotherms for methane adsorption at high pressure: Modeling and experiment. SPE J (2019) 24(06):2504–25. doi:10.2118/197045-pa
14. Zhang C, Qu G, Song G. Formulation development of high strength gel system and evaluation on profile control performance for high salinity and low permeability fractured reservoir. Int J Anal Chem (2017) 2017:1–9. doi:10.1155/2017/2319457
15. Zhao TH, Xing JY, Pu WF, Dong ZM, Yuan CD, Peng GF, et al. Synthesis and property evaluation of a novel polyacrylamide-montmorillonite composite for water shutoff and profile control in high salinity reservoirs. Polym Composites (2018) 39(2):368–76. doi:10.1002/pc.23945
16. Xu J, Chen Z, Dong X, Zhou W. Effects of lean zones on steam-assisted gravity drainage performance. Energies (2017) 10(4):471. doi:10.3390/en10040471
17. Xu J, Chen Z, Cao J, Li R (2014). Numerical study of the effects of lean zones on SAGD performance in periodically heterogeneous media. In SPE Heavy Oil Conference-Canada. Adelaide, Australia, 10-12 June 2014.
18. Ahmed HS, Mohamad O, Samir MT. A cross link polymer sealant for curing severe lost circulation events in fractured limestone formations. In: SPE Asia Pacific Oil & Gas Conference and Exhibition; 14-16 October 2014; Adelaide, Australia (2014).
19. El-Karsani KS, Al-Muntasheri GA, Hussein IA. Polymer systems for water shutoff and profile modification: A review over the last decade. Spe J (2014) 19(01):135–49. doi:10.2118/163100-pa
20. ElKarsani KSM, Al-Muntasheri GA, Sultan AS, Hussein IA. Performance of PAM/PEI gel system for water shut-off in high temperature reservoirs: Laboratory study. J Appl Polym Sci (2015) 132(17):685–92. doi:10.1002/app.41869
21. Zhang X, Zhang S, Li L, Wu R, Liu D, Wu J, et al. High-temperature-resistant polymer gel system with metal–organic mixed cross-linking agents. J Appl Polym Sci (2015) 132(29):757–64. doi:10.1002/app.42261
22. Nasr-El-Din HA, Taylor K. Evaluation of sodium silicate/urea gels used for water shut-off treatments, Evaluation of sodium silicate/urea gels used for water shut-off treatments. J Pet Sci Eng (2005) 48(3):141–60. doi:10.1016/j.petrol.2005.06.010
23. Prajakta P, Rajendra K. Environmentally acceptable composition comprising nanomaterials for plugging and sealing subterranean formation. In: SPE Paper 154917 presented at the SPE International Oilfield Nanotechnology Conference; June 12–14, 2012; Noordvijk, The Netherlands (2012).
24. Yang G, Zhang J, Xue XS. Development and evaluation of salt-resisting polymer gel profile control agent[C]//Advanced Materials Research. Trans Tech Publications Ltd (2013) 781:426–30.
25. Almoshin AM, Alsharaeh E, Fathima A, Bataweel M. A novel polymer nanocomposite graphene based gel for high temperature water shutoff applications. In: SPE Kingdom of Saudi Arabia annual technical symposium and exhibition; 23-26 April 2018; Dammam, Saudi Arabia (2018).
26. Adewunmi A, Ismail S, Sultan AS. Crosslinked polyacrylamide composite hydrogels impregnated with fly ash: Synthesis, characterization and their application as fractures sealant for high water producing zones in oil and gas wells, characterization and their application as fractures sealant for high water producing zones in oil and gas wells. J Polym Environ (2018) 26(8):3294–306. doi:10.1007/s10924-018-1204-9
27. Liu Y, Jiao B, Chen D. Synthesis of phenolic resin/P(Am-Co-amps) temporary plugging agent and their properties of salt tolerance. High Temperature Oilfield Chem (2018) 35:427–32.
Keywords: water soluble, temporary plugging agent, plugging, diversion fracturing, polymer, degradable
Citation: Wu G, Chen Z, Zhang A, Zhou J, Hou Y, Xie X, Wu J, Kong X and Li S (2023) Experimental studies on the performance evaluation of water-soluble polymers used as temporary plugging agents. Front. Phys. 11:1174268. doi: 10.3389/fphy.2023.1174268
Received: 26 February 2023; Accepted: 05 May 2023;
Published: 12 May 2023.
Edited by:
Chengyuan Xu, Southwest Petroleum University, ChinaReviewed by:
Jinze Xu, University of Calgary, CanadaNanlin Zhang, Zhejiang University, China
Jiajia Bai, Changzhou University, China
Copyright © 2023 Wu, Chen, Zhang, Zhou, Hou, Xie, Wu, Kong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengrong Chen, Y2hlbnpocjhAY25vb2MuY29tLmNu; Xiangwei Kong, a29uZ3h3X3lhbmd0emVAMTYzLmNvbQ==
 Guangai Wu
Guangai Wu Zhengrong Chen1*
Zhengrong Chen1* Jianshu Wu
Jianshu Wu Song Li
Song Li