- 1BSN Centre for Nano-materials and Displays, B.M.S. College of Engineering, Bengaluru, India
- 2Centre for Nano and Soft Matter Sciences, Bengaluru, India
- 3Department of Chemistry, Jnana Bharathi Campus, Bangalore University, Bengaluru, India
- 4Department of Chemistry, CHRIST (Deemed to be University), Bengaluru, India
In this paper, we report the photoresponsive behavior of hockey stick-shaped mesogens bearing azo wing with different terminal alkoxy chains at one terminal end. Except for the compound E16, which exhibits SmC along with nematic phase, all other mentioned compounds exhibit nematic phase alone. Influence of chain length on the photophysical properties were investigated using UV-Vis spectroscopy. It is observed here that influence of chain length is negligible on thermal back relaxation time. Spectroscopic investigation with variable intensities of UV light studies reveals that reverse cis-trans isomerization process was inversely proportional to the intensity of illuminated light. The present study also reveals that the structure-property relationship plays a dominant role on shape anisotropic structures. A spectroscopic study of the solid sample using guest-host mixture was also carried out and the compilation of results forecast these mesogens as ideal candidates for optical storage devices.
Introduction
The chromophore moiety, such as azobenzene, is an interesting functional group owing to their reversible photoisomerization between the more stable trans to metastable cis isomers upon irradiation with UV or visible light. This property of photoisomerization could bring about important changes in properties of the azobenzene chromophore, such as a large change in the molecular shape anisotropy and dipole moment. These changes could be imparted to liquid crystals when azobenzene is a part of their structure and even makes the mesogens more interesting and drives towards the applications [1–7]. Also, it is reported that photoisomerization effects are large in the case of liquid crystalline systems composed of azo substituted molecules when compared to azo-dye-doped systems [8–12]; azo-substituted LCs use light as a powerful external stimulus to control the molecular geometry and this subtle change is capitalized upon in optical storage technology. The aforesaid azobenzene undergo trans (E) to cis (Z) isomerization when illuminated with UV light of an appropriate wavelength and the isomers could switch from one state to the other with a particular wavelength of light corresponding to the energy gap of π—π* or n—π* transition. Upon illuminating with UV light (∼360 nm) the energetically, more stable trans form of -N=N- linkage gets converted into a cis form. The cis form of -N=N- linkage can be converted back to the trans form either by illuminating with visible light (∼450 nm) or in the “dark” by a process known as thermal back relaxation. This photoisomerization can bring about the phase transition in liquid crystalline system due to the occurrence of ordering and disordering during this process. The time taken for the phase transitions to occur via this isomerization of the photoactive (azobenzene) behavior is significant from an application point of view. The time required for the conversion of trans to cis is faster since it can be controlled using UV intensity; however, the long thermal back relaxation is essential for optical storage devices [13–17].
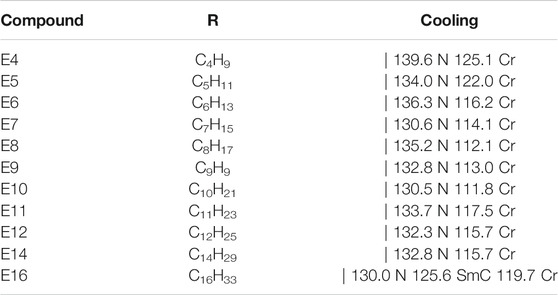
The present investigation is focused on the photo-switching studies on a series of hockey stick-shaped (E) liquid crystalline azo compounds previously reported by us [18]. These compounds exhibit nematic mesophases. The liquid crystalline system that exhibits nematic–isotropic (N–I) phase transitions is one of the simplest phase transitions to be brought about by photoisomerization of the photoactive chromophore and thus it is well studied in such systems. The lowering of the transition temperature, TN—I, could induce an isothermal N–I transition. The photochemically induced transition is promising in order to develop an optical image storage system and their optical rewritable ability [19–25]. Hockey stick-shaped mesogens often exhibit unusual mesomorphic properties due to their frustration between bent-core and linear shape [26–28]. The molecular structure of the HSLCs (E) is shown in Figure 1 and their complete synthesis and spectral characterization are reported [18].
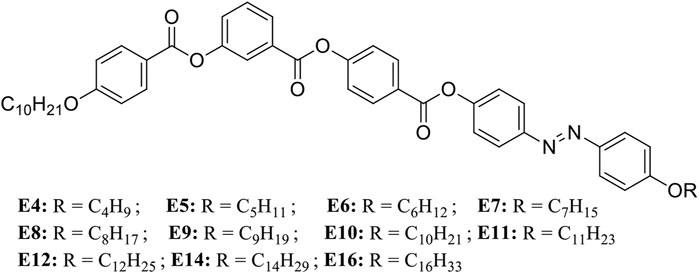
FIGURE 1. Molecular structure of the HSLCs (E) compounds [18].
Experimental
Photoswitching studies were performed by UV-Vis spectrophotometer using a Ocean Optics HR-2000+. The samples were prepared in chloroform solution at fixed concentration 1 × 10−5 molL−1. Quartz cuvette (optical length; 1 cm) is used for the measurement of the UV-Vis spectra. The blank tests were performed in chloroform solution at room temperature (27 ± 1°C). Then the prepared sample was taken in cuvette and placed into the sample holder, which was irradiated with UV light at about 1 mW/cm2 intensity. The Omnicure series 2000 UV was used for illumination along with 365 nm UV filter and heat filter was equipped between the sample and the source to avoid heat radiation from the source. The intensity was calibrated using UV meter (UV513AB) and adjusted for 1mW/cm2. The time dependent absorption spectra of HSLCs were recorded at time zero and at different intervals of irradiation time until photosationary state (PSS) was achieved. After PSS, the thermal back relaxation time as a function of time was determined by keeping the sample in dark. The photoconversion efficiency for trans-cis isomerization at PSS were determined by using Eq. (1).
where CE is conversion efficiency amd A(to), and A(t∞) is absorbance before and after UV illumination, respectively [29].
The first-order kinetics for thermal back relaxation of cis-tans isomerization were measured using Eq. (2).
where At, A0, and A∞ are the absorbance with respect to peak wavelength at time t, time zero, and infinite time respectively. τ is relaxation time of cis isomer [30].
The solid cell has been fabricated from the series of HSLCs using precoated ITO glass substrates. The cleaned ITO glass substrate was coated with polyimide solution and rubbed unidirectionally using a rayon cloth. The thickness of cell was maintained about 5 μm using glass spacers. The guest-host mixture was prepared by physical mixing where 5 wt% of HSLCs and 95 wt% of room temperature nematic liquid crystal E7 (now onwards called as NLC) was mixed and then the cell was filled by capillary action using guest-host mixture. The liquid crystal mixture aligns along the rubbing direction. The spectroscopic investigation was carried out at room temperature by placing a previously prepared solid cell in sample holder between detector and source. The 1 mW/cm2 intensity of UV light was used for illumination at different intervals of time.
Results and Discussions
Mesophase Characterization
It is important to know the mesophase properties of the liquid crystalline compounds. It is not necessary that all the photosensitive compounds should exhibit liquid crystalline properties. One can see from Figure 1 that when–N=N–linkage is away from the central phenyl ring, we get mesomorphicity in all the mentioned compounds here, unlike in the case when–N=N–linkage was directly attached to the central phenyl ring [18] where one gets non-mesomorphic nature. Table 1 shows the phase transition temperatures and liquid crystalline mesophases for all the mentioned compounds in this study. One can also note that all the compounds exhibit nematic phase except for the compound E16, which exhibits the SmC phase along with the nematic phase. To understand further, we took polarizing optical microscopy (POM) textures for the representative compounds, mainly on E8 and E16, which exhibits nematic phases. Figure 2 represents POM textures of the compound E8 1) and E16 2) while cooling from the isotropic phase. It is evident from the figure that the characteristic nematic phase is present in both of them. A detailed investigation on mesomorphic effect can be found in our earlier work [18].
FIGURE 2. Polarizing Optical Microscopy texture of compounds E8 and E16 on cooling from isotropic liquid under the crossed polarizers: one can see N mesophase at 128°C for compound E-8 (A); N mesophase at 127°C for compound E-16 (B). Reproduced the part of the figure from [18].
Photoswitching Studies
The photoswitching studies of HSLCs were performed on quartz cuvette using chloroform as the solvent. These hockey-stick shaped mesogens bearing azo wing at one terminal with constant C10 chain at another end were investigated for their photosensitive behavior. The azo wing has a different alkoxy chain at terminal and another end attached to center core through ester linkage. The photoswitching properties of these compounds were examined by UV light illumination at different time intervals. The wavelength of the UV light used was ∼365 nm and the intensity used was ∼1 mW/cm2.
A heat filter was inserted between the UV source and the sample to avoid any heat radiation arising from the source. Figures 3A–K represents the UV absorption spectra as a function of time for the respective compounds E4-E16, which was evaluated using UV-Vis spectrophotometer in solution. Figure 3L represents the peak absorbance data obtained from 3a-3k and is represented with respect to illumination time. The strong absorption band at ∼ 351 nm in the UV region, which corresponds to the symmetry, allowed π-π* transition; n-π* weak transition band, meanwhile, appears in the visible region (λ ∼ 450 nm). All the compounds show similar absorption spectra and there was also no significant changes in the photosaturation time (PSS). One can see from the figure that all HSLCs took ∼60 s to photo saturate. The maximum absorption peak gradually falls down at wavelength ∼351 nm while UV light illumination is ON and intensity of the lower band at ∼ 450 nm slightly increases. Tacitly, these changes indicate transformation of energetically stable trans isomer to unstable cis isomer during suitable UV light illumination. Whereas with thermal back relaxation, which occurs when UV light is turned off, energetically unstable cis converts to trans configuration without any external influence, which could be utilized in optical storage devices.
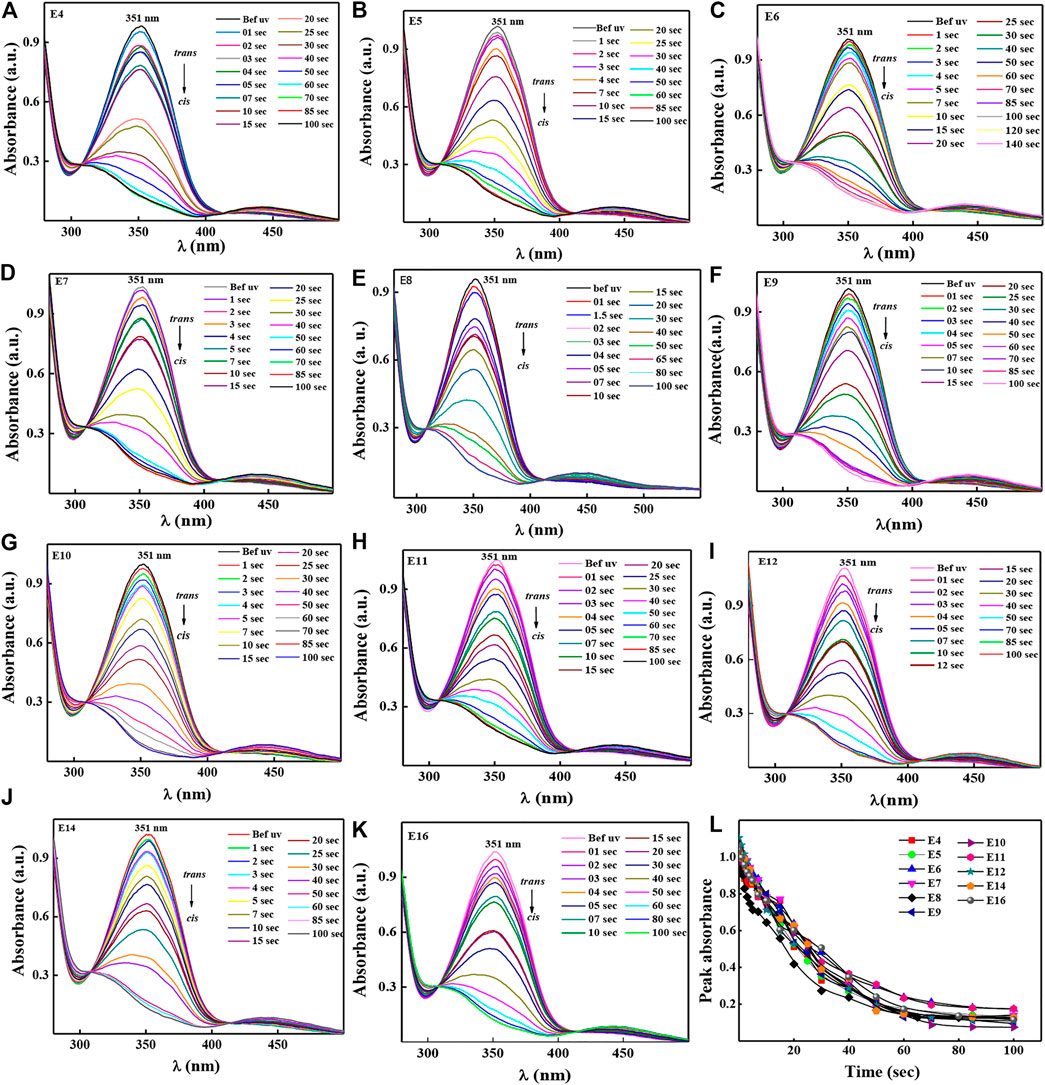
FIGURE 3. Time dependent absorption spectra for the compound E4-E16 represented as Panel 3A–K by illuminating UV light of wavelength ∼365 nm and the intensity of ∼1 mW/cm2. Peak absorbance were calculated using the data obtained from a-k and plotted as a function of time in Panel 3L.
The photoactive behavior of hockey-stick compounds was determined using Eq. 1 and photoconversion efficiency was given in Table 2. The photoconversion efficiency of all investigated compounds in solution state exhibits good E-Z conversion upon UV illumination. In PSS, more than 85% of trans isomers exchanged to unstable cis isomer at ∼70 s of UV irradiation time.
After reaching the PSS, molecules will start to reverse back to their original trans configuration since PSS is an unstable state. The process to relax back with temperature as the only parameter is known as the thermal back relaxation. One can also bring back the system to its original configuration quickly by shining the wavelength of light ∼450 nm.
Since the ideal situation is to get longer thermal back relaxation time, one must not use any external sources other than the temperature to return it to the original configuration. The higher the thermal back relaxation, the better the device is. Figures 4A–K represents the thermal back relaxation process after the system attains the photo saturation level. Peak absorbance as a function of back relaxation time is plotted using Figures 4A–K data and is shown in figure 4L. One can see from this figure that almost all the compounds relax back to their original trans configuration within 150 min.
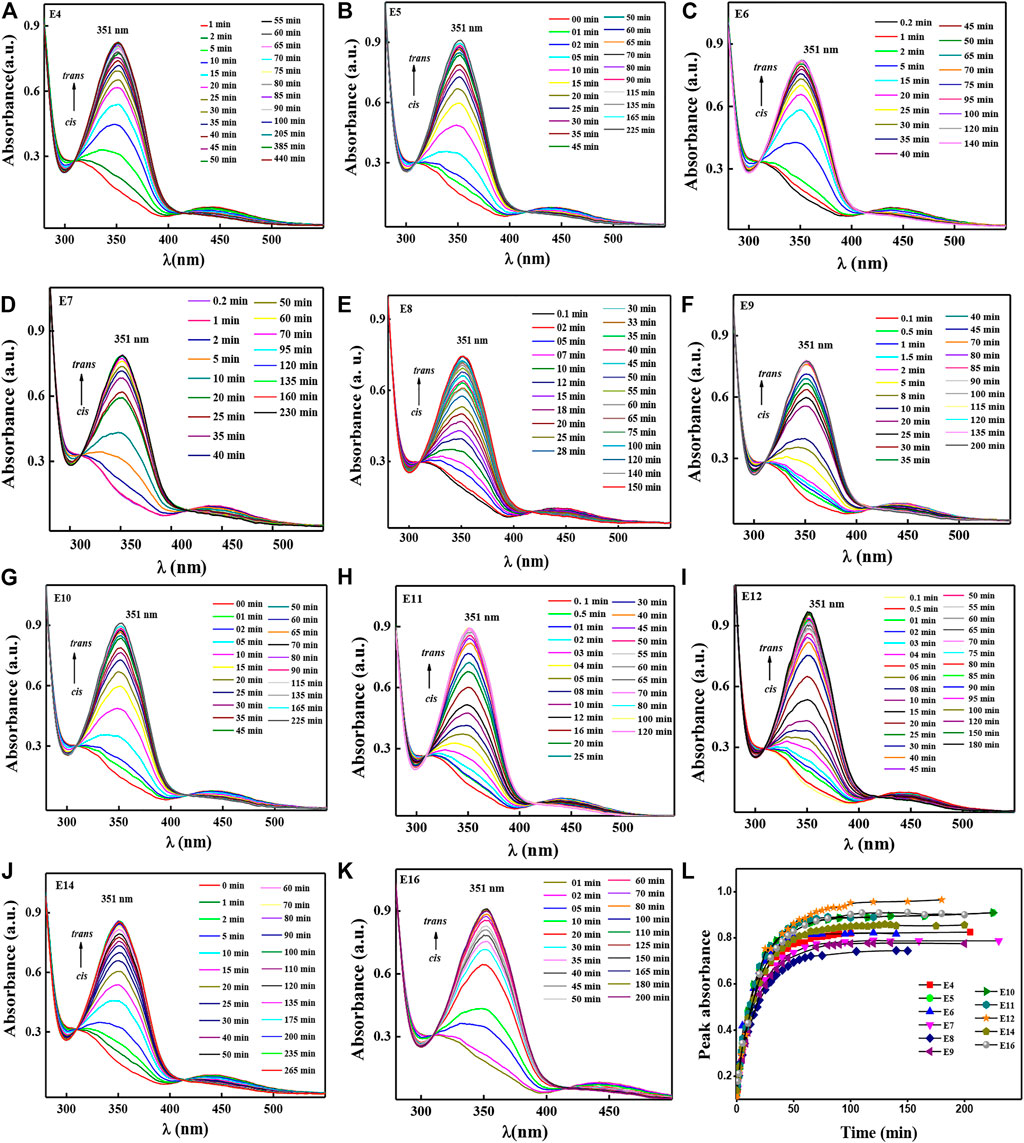
FIGURE 4. Thermal back relaxation data for the compounds E4-E16, as represented as Panel 4A–K. Peak absorbance as a function of thermal back relaxation time for all HSLCs were given in Panel 4L.
As photo conversion efficiency is mainly dictated by the intensity since it is a controllable parameter, we did the variation of intensity vs time to understand the influence of intensity on photosaturation time. Figure 5A shows the influence of intensity on photo saturation time for compound E12. One can see that, photo saturation time is inversely proportional to the intensity of UV light. As the intensity of UV light increases, photo saturation time decreases and vice versa. The compound E12 took 70.6 s to reach its photoequilibrium state at 1 mW/cm2 intensity, whereas, it takes 8 s at 10 mW/cm2. Hence, one can easily notice the influence photoactive azo derivatives with respect to the intensity.
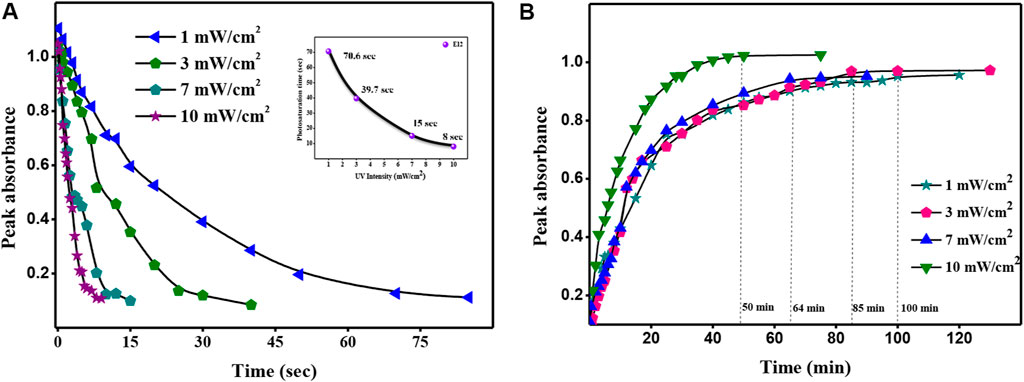
FIGURE 5. (A) Photosaturation time versus UV intensity plot for E–Z isomerization and (B) Thermal back relaxation or Z–E isomerization of compound E12 with different intensities.
We also studied the influence of intensity with respect to the back relaxation time. As expected, the longer thermal back relaxation time was observed for lower UV intensity illuminated samples; higher intensities of UV light shined samples showed the shortest time. The observed back relaxation spectra show different thermal back relaxation times such as 100, 85, 64, and 50 min in solution at room temperature with respect to different intensities (Figure 5B). Concurrently, the observed results agreed with the previous results [16]. Therefore, intensity has a profound influence on the thermal back relaxation time. For any optical storage devices, lower intensity is ideal since it is not only cost effective but also user friendly.
Kinetic Studies
It is obligatory to determine the first-order kinetic plot for trans-cis-trans photoisomerization [29]. The first-order plot for unimolecular thermal cis-trans isomerisation obeys Eq. (2). Figure 6 shows the first-order plot which was studied by furnishing the obtained experimental data to Eq. (2) at room temperature. The indicated time region in the graph obeys first order, later, it is slightly deviated from first order to second order due to experimental conditions (since this experiment is carried out in solution). The deviation was observed from E10 to E16, which bears a higher number of the carbon atom in alkoxy chain at one end of HSLCs. As the carbon atoms increase in alkoxy group at one end of HSLCs, the thermal back relaxation slows down due to its bulkier nature. These results suggest that lower alkoxy chain bearing HSLCs obey first order, whereas the higher the number of caron atom present in alkoxy chain (more than 10 carbon atoms) the more deviation will be shown from first order to second order with respect to molecular structure [30].
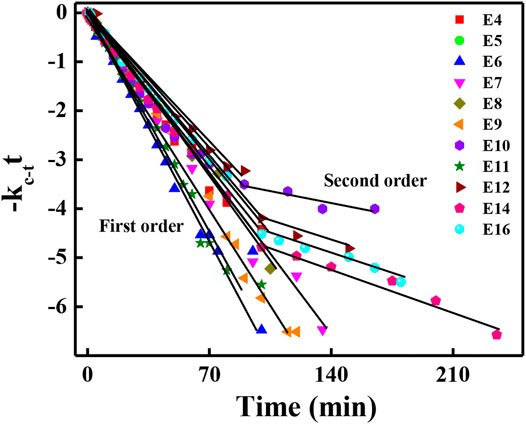
FIGURE 6. First-order plot as a function of time for cis-trans (Z-E) thermal isomerization of all investigated compounds.
Reason Behind the Phenomena
Although the exact reason for such a shorter thermal back relaxation is not clear we speculate the following. The HSLCs, in their trans configuration, are more anisotropic. But as soon as UV light of wavelength ∼365 nm is shined on the system, a kink is observed near to azobenzene moiety due to trans-cis conformational change in molecules. Apart from this, the rest of the molecules remain in the same state where not much change in shape anisotropy takes place (Figure 7). Because of this, guest azo molecules are not creating any hindrance for the liquid crystal host molecules to return from cis to trans configuration in a real device.
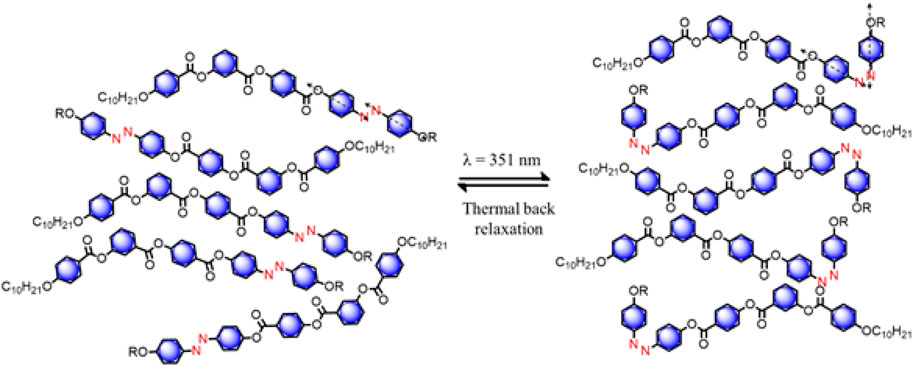
FIGURE 7. Schematic depiction of photoisomerization of HSLCs (E) with UV-Visible illumination, trans geometry isomerized into cis at room temperature (26°C).
Optical Storage Device Studies
It is ideal to check the liquid crystal device characteristics in the actual device to see the potential of the said material. Compound E12 is chosen for this purpose since almost all the compounds exhibit the same mesomorphic behavior. Liquid crystal guest-host mixture is prepared using NLC as the host and E12 as the guest in the ratio 95:5. The freshly prepared guest-host mixture was then filled in the solid cell through capillary action and placed solid cell in the sample holder. Thickness of the cell was maintained at ∼5 μm. The UV light intensity of 1 mW/cm2 was illuminated on solid cell with different intervals of time. The time dependent absorption spectra for UV illumination were recorded (Figure 8A) until photosaturation state took place. Then, absorption spectra of thermal back relaxation were recorded as shown in Figure 8B. The maximum peak absorbance values are noted from Figures 8A,B and the corresponding peak absorbance graph (Figures 8C,D) was plotted as a function of time. From these results, we can infer that trans isomer reaches its photostationary state was 100 s during UV light illumination and returns to its original state within 5.3 h at room temperature.
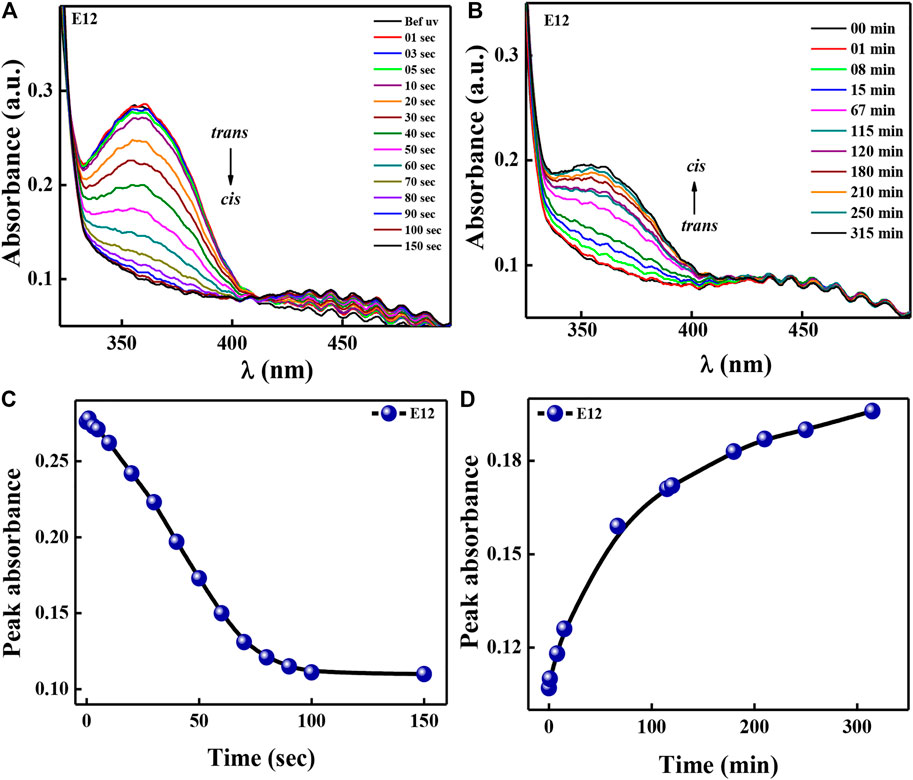
FIGURE 8. Spectral investigation for HSLCs in a solid cell configuration during UV illumination (A) and thermal back relaxation (B). Peak absorbance plot (C,D) was plotted by extracting absorbance values at peak wavelength from fig (A,B).
To see the potential of the said material, an optical storage device was constructed by using guest-host mixture. Since all the compounds exhibit similar characteristics, we chose E12 as the representative compound. Previously prepared LC cells were filled with guest-host mixture where E12 acted as a guest and NLC acted as a host material. Suitable masks were kept above the cell and UV intensity of 5 mW/cm2 was shined for 10 min. One can see the clean optical storage device under the crossed polarizers (Figure 9) with dark and bright states. The dark region corresponds to the illuminated area where the system changes from nematic state to isotropic and the bright region corresponds to the masked area where the system remains in the nematic state.
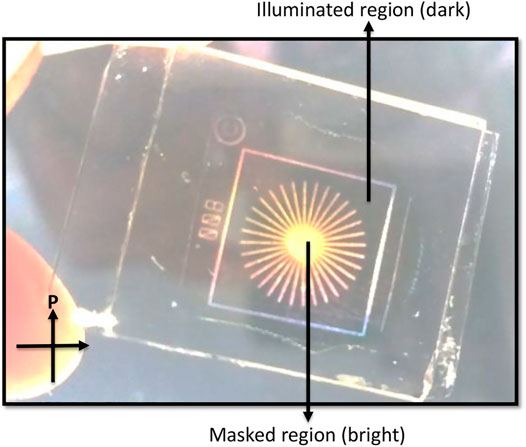
FIGURE 9. Optical storage device showing good contrast between dark and bright states using the guest-host effect under the crossed polarizers. Here compound E12 acts as a guest and NLC acts as a host molecule. Intensity of the light used is 5 mW/cm2 and illuminated for 10 min.
More investigations on changing the different functional groups and azo substitutions at different places in the structure are needed to understand more about the structure property relationships.
Conclusion
We report on the photoactive behavior of HSLCs with variable alkoxy chain at terminal end. All the HSLCs exhibit nematic phase at shorter intervals except for E16 which exhibits SmC as well. All HSLCs exhibit the similar cis-trans isomerization behavior due to similar rigid center cores. The trans (E)—cis (Z) isomerization occurs at around 70 s and thermally reversible cis (Z) to trans (E) isomerization in solution occurs ∼150 min. In solid cell, photosaturation time was about 100 s, whereas it took ∼5.3 h to reach its original state at room temperature. Spectroscopic investigation with variable intensities of UV light studies reveals that reverse cis-trans isomerization process was inversely proportional to the intensity of illuminated light. The prevailing tuning of the intensity of UV light shows promise in thermal back relaxation time.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
MM synthesized the compounds and BS did the photoswitching measurements. Both MM and BS wrote part of the manuscript. GS analyzed the results and edited the manuscript. GH conceived the idea and edited the manuscript. VP analyzed the results and edited the manuscript. All authors have approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank CHRIST (deemed to be University) for supporting our research work.
References
1. Lutfor MR, Hegde G, Kumar S, Tschierske C, Chigrinov VG. Synthesis and Characterization of Bent-Shaped Azobenzene Monomers: Guest-Host Effects in Liquid Crystals with Azo Dyes for Optical Image Storage Devices. Opt Mater (2009) 32(1):176–83. doi:10.1016/j.optmat.2009.07.006
2. García-Amorós J, Velasco D. Recent Advances towards Azobenzene-Based Light-Driven Real-Time Information-Transmitting Materials. Beilstein J Org Chem (2012) 8(1):1003–17. doi:10.3762/bjoc.8.113
3. Sun J, Chigrinov V. Effect of Azo Dye Layer on Rewriting Speed of Optical Rewritable E-Paper. Mol Crystals Liquid Crystals (2012) 561(1):1–7. doi:10.1080/15421406.2012.686690
4. Alaasar M. Azobenzene-containing Bent-Core Liquid Crystals: an Overview. Liquid Crystals (2016) 43(13-15):2208–43. doi:10.1080/02678292.2016.1175676
5. Hegde G, Fernandes PZ, Yuvaraj AR, Mei GS, Rahman L, Yusoff MM. Light Sensitive Molecule for Photonic Devices. Macromol Symp (2015) 353:115–20. doi:10.1002/masy.201550315
6. Bisoyi HK, Li Q. Light-driven Liquid Crystalline Materials: from Photo-Induced Phase Transitions and Property Modulations to Applications. Chem Rev (2016) 116(24):15089–166. doi:10.1021/acs.chemrev.6b00415
7. Xiang Y, Jing H, Chen H, Zhang J, Ding X, Li J. Light-driven Rotation of Gratings Formed by Electroconvection Patterns in Cholesteric Liquid Crystals. J Mol Liquids (2021) 337(1):116366. doi:10.1016/j.molliq.2021.116366
8. Ikeda T, Sasaki T, Ichimura K. Photochemical Switching of Polarization in Ferroelectric Liquid-crystal Films. Nature (1993) 361(6411):428–30. doi:10.1038/361428a0
9. Sasaki T, Ikeda T, Ichimura K. Photochemical Control of Properties of Ferroelectric Liquid Crystals. Photochemical Flip of Polarization. J Am Chem Soc (1994) 116(2):625–8. doi:10.1021/ja00081a024
10. Ikeda T, Tsutsumi O. Optical Switching and Image Storage by Means of Azobenzene Liquid-crystal Films. Science (1995) 268(5219):1873–5. doi:10.1126/science.268.5219.1873
11. Ikeda T, Tsutsumi O, Wu Y. Optical Switching and Image Storage by Means of Photochromic Liquid crystalsMolecular Crystals and Liquid Crystals Science and Technology. Mol Crystals Liquid Crystals Sci Techn Section A. Mol Crystals Liquid Crystals (2000) 347(1):1–13. doi:10.1080/10587250008024824
12. Prasad V, Jákli A. Achiral Bent-Core Azo Compounds: Observation of Photoinduced Effects in an Antiferroelectric Tilted Smectic Mesophase. Liquid crystals (2004) 31(4):473–9. doi:10.1080/02678290410001660002
13. Jákli A, Prasad V, Shankar Rao DS, Liao G, Jánossy I. Light-induced Changes of Optical and Electrical Properties in Bent-Core Azo Compounds. Phys Rev E (2005) 71(2):021709. doi:10.1103/PhysRevE.71.021709
14. Sunil BN, Behera PK, Achalkumar AS, Shanker G, Hegde G. Influence of Inter- and Intramolecular H-Bonding on the Mesomorphic and Photoswitching Behaviour of (E)-4-((4-(hexyloxy)phenyl)diazenyl)-N-phenyl Benzamides. RSC Adv (2020) 10(34):20222–30. doi:10.1039/d0ra03024d
15. Yuvaraj AR, Lutfor Rahman M, Mohd Yusoff M. Synthesis and Light Induced Characteristics of Siloxane Substituted Azobenzene: An Application for Optical Storage Device. Int J Spectrosc (2016) 9:4715230. doi:10.1155/2016/4715230S
16. Gan SM, Pearl ZF, Yuvaraj AR, Lutfor MR, Gurumurthy H. Polarity Dependent Photoisomerization of Ether Substituted Azodyes: Synthesis and Photoswitching Behavior. Spectrochimica Acta A: Mol Biomol Spectrosc (2015) 149:875–80. doi:10.1016/j.saa.2015.05.027
17. Sidharth SN, Yuvaraj AR, Hui TJ, Sarojini BK, Mashitah MY, Hegde G. Light Induced Properties of Chalcones Correlated with Molecular Structure and Photophysical Properties for Permanent Optical Storage Device. Amr (2014) 1033-1034:1149–53. doi:10.4028/www.scientific.net/amr.1033-1034.1149
18. Monika M, Prasad V, Nagaveni NG. Hockey Stick-Shaped Azo Compounds: Effect of Linkage Groups and Their Direction of Linking on Mesomorphic Properties. Liquid Crystals (2015) 42(10):1490–505. doi:10.1080/02678292.2015.1066889
19. Prasad SK, Nair GG, Rao DSS. Photoinduced Phase Transitions. Liquid Crystals (2009) 36(6-7):705–16. doi:10.1080/02678290902755572
20. Sunil BN, Srinatha MK, Shanker G, Hegde G, Alaasar M, Tschierske C. Effective Tuning of Optical Storage Devices Using Photosensitive Bent-Core Liquid Crystals. J Mol Liquids (2020) 304:112719. doi:10.1016/j.molliq.2020.112719
21. Hegde RS, Sunil BN, Hegde G, Prasad V. Influence of Alkyl and Alkoxy Groups on Photoresponsive Behaviour of Bent-Core Azo Mesogens: Synthesis, Mesomorphic and Photoswitching Properties. J Mol Liquids (2020) 309:113091. doi:10.1016/j.molliq.2020.113091
22. Krishna Prasad S, Nair GG, Hegde G, Sandhya KL, Shankar Rao DS, Lobo CV. Photoinduced Effects in Nematic Liquid Crystals. Phase Transitions (2005) 78(6):443–55. doi:10.1080/01411590500185690
23. Cigl M, Bubnov A, Kašpar M, Hampl F, Hamplová V, Pacherová O. Photosensitive Chiral Self-Assembling Materials: Significant Effects of Small Lateral Substituents. J Mater Chem C (2016) 4(23):5326–33. doi:10.1039/C6TC01103A
24. Begum N, Kaur S, Xiang Y, Yin H, Mohiuddin G, Rao NVS. Photoswitchable Bent-Core Nematic Liquid Crystals with Methylated Azobenzene Wing Exhibiting Optic-Field-Enhanced Fréedericksz Transition Effect. J Phys Chem C (2019) 124(1):874–85. doi:10.1021/acs.jpcc.9b09326
25. Amrutha AS, Achalkumar AS, Li Q. Light-Driven Phase Transitions in Liquid Crystals and Their Applications. Photoactive Funct Soft Mater Preparation (2019) 227–83. doi:10.1002/9783527816774.ch7
26. Sathyanarayana P, Radhika S, Sadashiva BK, Dhara S. Structure-property Correlation of a Hockey Stick-Shaped Compound Exhibiting N-SmA-SmCa Phase Transitions. Soft Matter (2012) 8(7):2322–7. doi:10.1039/c2sm06767f
27. Chakraborty A, Das MK, Das B, Baumeister U, Weissflog W. Optical, Dielectric and Visco-Elastic Properties of a Few Hockey Stick-Shaped Liquid Crystals with a Lateral Methyl Group. J Mater Chem C (2013) 1(44):7418–29. doi:10.1039/c3tc31565g
28. Choi E-J, Park K-M, Kim D-Y, Jeong K-U, Lee J-H. Pseudo-rodlike Molecules with Hockey-Stick-Shaped Mesogen. Liquid Crystals (2016) 43(11):1597–605. 1. doi:10.1080/02678292.2016.1189004
29. Sunil BN, Yam WS, Hegde G. Photoresponsive Behavior of Hydrophilic/hydrophobic-Based Novel Azobenzene Mesogens: Synthesis, Characterization and Their Application in Optical Storage Devices. RSC Adv (2019) 9(69):40588–606. doi:10.1039/C9RA08211E
30. Turlapati S, B. N. S, Vishwakarma VK, Achalkumar AS, Hegde G. Influence of Lateral Methyl/chloro Substituents on the Liquid Crystalline and Photoswitching Behaviour of Bent-Core Mesogens Bearing Azobenzene Wings: Synthesis and Characterization. New J Chem (2020) 44(15):5731–8. doi:10.1039/C9NJ06237H
Keywords: azobenzene, photoisomerization, optical storage devices, thermal back relaxation, hockey stick
Citation: Sunil BN, Monika M, Shanker G, Hegde G and Prasad V (2021) Evaluation of Photoswitching Properties for Hockey Stick-Shaped Mesogens Bearing Azo Benzene Moieties. Front. Phys. 9:728632. doi: 10.3389/fphy.2021.728632
Received: 21 June 2021; Accepted: 21 September 2021;
Published: 20 October 2021.
Edited by:
Xuejin Li, Zhejiang University, ChinaCopyright © 2021 Sunil, Monika, Shanker, Hegde and Prasad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gurumurthy Hegde, bXVydGh5aGVnZGVAZ21haWwuY29t; Veena Prasad, dmVlbmFAY2Vucy5yZXMuaW4=
 B. N Sunil1
B. N Sunil1 Govindaswamy Shanker
Govindaswamy Shanker Gurumurthy Hegde
Gurumurthy Hegde