
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys., 22 July 2021
Sec. Biophysics
Volume 9 - 2021 | https://doi.org/10.3389/fphy.2021.704878
This article is part of the Research TopicViscoelasticity: From Individual Cell Behavior to Collective Tissue RemodelingView all 11 articles
The vertex model is a useful mathematical model to describe the dynamics of epithelial cell sheets. However, existing vertex models do not distinguish contraction forces on the cell boundary from adhesion between cells, employing a single parameter to express both. In this paper, we introduce the rest length of the cell boundary and its dynamics into the existing vertex model, giving a novel formulation of the model that treats separately the contraction force and the strength of adhesion between cells. We apply this vertex model to the phenomenon of compartment boundary in the fruit fly pupa, recapturing the observation that increasing the strength of adhesion between cells straightens the compartment boundary, even though contraction forces at cell boundaries remain unchanged. We also discuss possibilities of the novel vertex models by considering the stretching of a cell sheet by external forces.
During embryonic development, epithelial cells form a monolayer sheet that covers the entire embryo. Cells comprising the sheet move drastically, like an active viscoelastic fluid, while maintaining their attachment to adjacent cells. This spontaneous movement of epithelial cells is considered a driving force for morphogenesis of multicellular organisms. Understanding the mechanism of the movement from not only a molecular but also a mechanical point of view is a challenging problem in morphogenesis. Although the molecular mechanism of the movement has come to be relatively well understood [1], its mechanical mechanism is still an ongoing problem.
To approach the mechanical mechanism of the dynamics of the epithelial sheet, a cell-based mathematical model, the vertex model, is often used [2, 3]. In this model, each epithelial cell in the sheet is expressed by a polygon, and the cell configuration within the sheet is completely specified by the positions of the vertices of the polygons. The vertex model can describe various aspects of the epithelial sheet at the cellular level, including mechanical forces generated by each cell and the planar polarities of cells [2, 3]. Indeed, by using the vertex model, important behaviors of the epithelial sheet, such as elongation, bending, and unidirectional movement of the sheet, have been explained from not only a biological but also a mechanical viewpoint [4–7].
Although the existing vertex model is well able to describe important properties of epithelial cell sheets, certain modifications are necessary in order to more precisely describe cell sheet dynamics. One important consideration is the lack of distinction between the contraction forces acting on the cell boundaries and the adhesion between cells. The existing vertex models consider the contraction forces and the strength of adhesion together and express the strengths of these two factors using a single parameter [8, 9]. However, biologically, contraction and adhesion are regulated by different molecules. For example, contraction forces are generated by actomyosin networks beneath the plasma membrane, whereas adhesion between cells is accomplished by adhesion molecules such as cadherin. Hence, to make the vertex model more useful and to more precisely describe epithelial cell sheet dynamics, it is preferable to modify the existing model to separately treat the forces of contraction and adhesion at the cell boundaries.
In this paper, we provide a novel formulation of the vertex model that introduces a phenomenological variable corresponding to the rest length of a cell boundary. This formulation allows us to treat separately the contraction forces acting on cell boundaries and the effects of adhesion between cells. The vertex model presented here is in accordance with and an extension of the existing vertex model. As an application of the model presented in this paper, we consider a phenomenon observed in the anterior-posterior (AP) compartment boundary in the Drosophila pupa [10, 11], in which the AP compartment boundary is straightened not only by an increase in contraction force at this boundary but also by an increase in the strength of adhesion between cells in the posterior region. While it has been demonstrated that the increase in contraction forces at the AP compartment boundary straightens the boundary [10], it has not yet been demonstrated whether the increase in adhesion between posterior cells does likewise. We use the vertex model presented here to show that the increase in adhesion between cells in the posterior region does straighten the AP compartment boundary and explain why the increase in adhesion straightens the boundary. As a second application of the new vertex model, we focus on stretching of the epithelial sheet by an external force. This application illustrates the difference in cell remodeling behavior between existing vertex models and our new model and compares the results predicted by the models with those observed experimentally.
As in existing vertex models, cells comprising an epithelial sheet are represented by polygons. The mechanical forces generated by the cells are expressed by the potential function
where
In the previous vertex models [2, 7, 9], there is another term in
The total mechanical force acting on vertex
holds for all vertices
Next, we consider the time evolution equation for
where
where
As stated above, the quantity
As an application of this new vertex model, we treat the phenomenon of compartment boundary straightening in the fruit fly pupa [15]. In this phenomenon, two types of epithelial cells, anterior (A) cells and posterior (P) cells, form two domains in an epithelial sheet, and the two cell domains meet at a boundary called the compartment boundary. For pupal development to progress correctly, the compartment boundary must undergo sufficient straightening. A mechanism that has been considered for the straightening of the compartment boundary is a strengthening of the contraction force on the compartment boundary, which shortens and straightens the compartment boundary. This scenario has been confirmed using the previous vertex model [10]. Recently, however, another mechanism for straightening of the compartment boundary was experimentally demonstrated [11], in which this boundary is straightened by an increase in the strength of adhesion between P cells, with the contraction force on the compartment boundary remaining unchanged. To restore this phenomenon, we used the new vertex model to try to understand why and how an increase in adhesion between P cells straightens the compartment boundary.
To do this, we set up the situation where a cell sheet consists of two types of cells, A cells (red) and P cells (blue) (Figure 1A). We refer to the boundary between the A and P cells as the compartment boundary in this model. As the initial state (t = 0) of the cell sheet, we took the equilibrium state obtained under the condition in which both A and P cells had the same parameters. Then at t = 0, we changed the parameters of interest and observed the length difference (
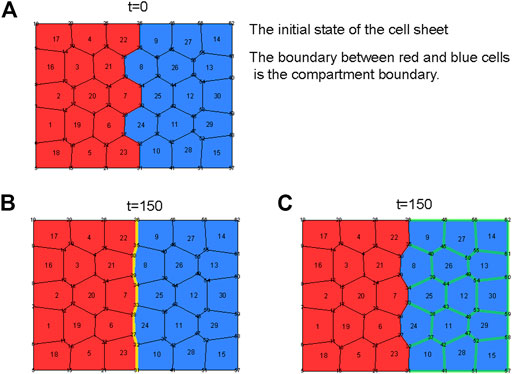
FIGURE 1. (A) The initial state of the cell sheet. Red and blue cells are anterior and posterior cells, respectively. At the initial state of the sheet, both cells have the same parameters. At time t = 0, some parameters are changed. The compartment boundary is the boundary between red and blue cells. (B) The final state of the cell sheet when
Next, to investigate the effects on
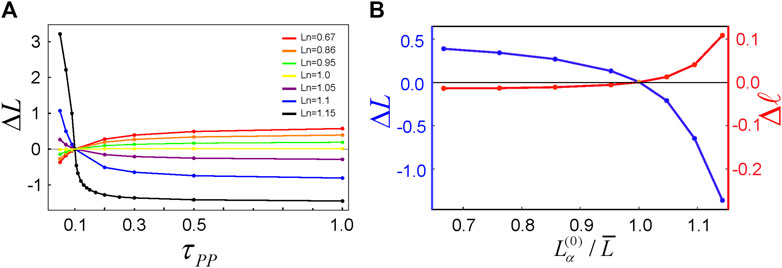
FIGURE 2. (A) The length difference (
Here a question may arise. Why does the increase in
Let us consider a single cell whose dynamics obey Eqs. 1–4 and whose shape is kept rectangular, in which the state of the cell is specified only by the quantities characterizing the vertical and horizontal boundaries of the cell. Let us denote the lengths of vertical and horizontal boundaries of the cell by
The force balance equations at each boundary are given by
In our model,
where
Although we can analytically solve Eqs. 6, 8 for the variables,
It should be noted here that
While the above analysis is restricted to a case in which the cell shape is rectangular, the relation between
In this section, we consider the stretch of a cell sheet by external forces. In the previous vertex models, when the cell sheet is stretched greatly enough by external forces, the sheet necessarily undergoes cell remodeling (Figures 3A,B; Supplementary Movie 3; the formulation of the previous vertex model is given in Supplementary Appendix 1). This behavior originates in two properties of the previous vertex model: 1) cell shape tends to be round, due to the quadratic term (
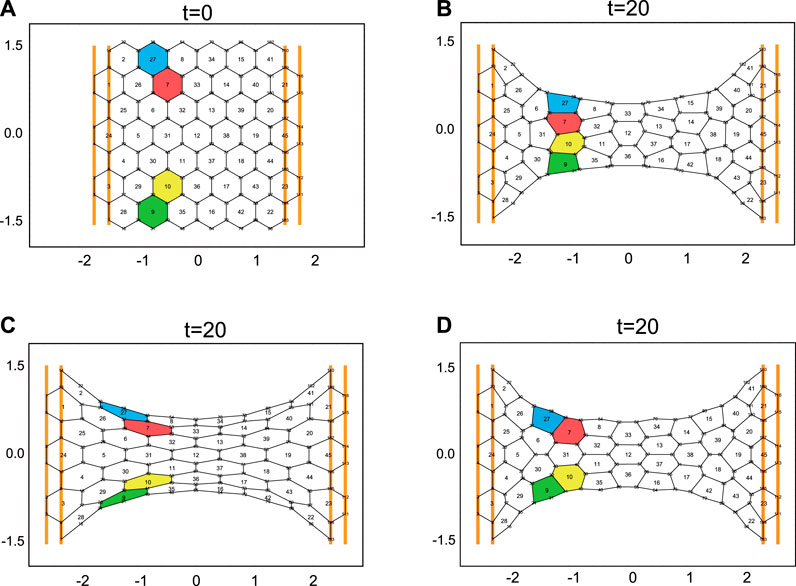
FIGURE 3. (A) The initial configuration of the cell sheet. At time t = 0, the cell sheet begins to be stretched by external forces, which are represented by the orange bars. At these bars, the cell boundaries are fixed, and the bars are shifted with time (for the movement of the bars see Supplementary Movies 3–5). (B) The final cell configuration of the sheet stretched by external forces. The cell sheet dynamics are implemented by the previous vertex model (see Supplementary Appendix 1). The time evolution equation for
In this paper, we provided a novel formulation of the vertex model that separately treats the contraction force on the cell boundary and the strength of adhesion between cells, by considering the resting length of the cell boundary and its dynamics. We applied this vertex model to understanding the straightening of the compartment boundary observed in the fruit fly pupa and showed that the model recaptures compartment boundary straightening in response to an increase in strength of adhesion between P cells. We also used this model to examine the stretching of a cell sheet by external forces and gained insights into cell remodeling resulting from the stretch. This model has the potential to clarify points that were ambiguous in the previous vertex model. One such point is the frictional force exerted on the vertex. In the previous model, the equation for time evolution of vertex positions is obtained by assuming that total mechanical force on the vertex and frictional force on the vertex are balanced. However, the meaning and origin of the frictional force on the vertex had not yet been well discussed. The present vertex model has the potential to explain the origin and meaning of the frictional force between vertices. Indeed, as mentioned in Application 2: The Response of the Cell Sheet When Stretched by External Forces, changes in
Recently, it has been reported that cell intercalation (cell remodeling) in the cell sheet is related to endocytosis at the cell boundary of epithelial cells [12, 14]. In our model, the effect of endocytosis frequency at the cell boundary is represented by
As demonstrated in Figure 3C, the cell sheet described by the present vertex model does not necessarily undergo cell intercalation even when the cells are largely deformed by external forces. Similar behaviors of epithelia are sometimes observed in experiments. A representative example of this is the defect in the formation of the tracheal system in the fruit fly embryo [19]. In the control case of the tracheal system, the tube consisting of epithelial cells undergoes cell intercalation and elongates along the long axis of the tube, during which the tip cells of the tube keep pulling the stalk cells toward the direction of the tip cells. The pulling forces of the tip cells were considered to a dominant factor for cell intercalation in the tube. However, expression of some molecules (e.g., Spalt) inhibits cell intercalation, and tube elongation stops at a certain length, even though the tip cells continue to pull the stalk cells [19]. This experimental result implies that for cell intercalation proceeding external forces on the cell sheet are not sufficient and other factors are necessary. We might be able to consider the factors necessary for cell intercalation through the notion of
In the morphological study of multicellular organisms, it becomes more important to investigate responses of the cell sheet to external mechanical perturbations [17]; thus, more detailed research on this issue using cell-based mathematical models, such as vertex models, is expected.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KS and DU designed the concept of the paper. KS created the model, and performed its numerical simulations and analyses. KS and DU wrote the paper. KS and DU contributed to the review and approval of the paper for publication.
This work was supported by Global Station for Soft Matter at Hokkaido University (KS), the Cooperative Research Program of “NJRC Mater. and Dev.” (KS), and MEXT/JSPS KAKENHI Grant Nos. 17H02939 (KS and DU), 20K03871 (KS), 18H01135 (KS), 17K07402 (DU), and 21K06144 (DU).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Y. Ishimoto, M. Nishikawa, T. Shibata, E. Kuranaga, S. Okuda, T. Taguchi, and S. Hayashi for valuable comments and discussions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphy.2021.704878/full#supplementary-material
1. Friedl P, Gilmour D. Collective Cell Migration in Morphogenesis, Regeneration and Cancer. Nat Rev Mol Cel BiolJul (2009) 10(7):445–57. doi:10.1038/nrm2720
2. Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex Models of Epithelial Morphogenesis. Biophysical J (2014) 106(11):2291–304. doi:10.1016/j.bpj.2013.11.4498
3. Alt S, Ganguly P, Salbreux G. Vertex Models: from Cell Mechanics to Tissue Morphogenesis. Phil Trans R Soc B (2017) 372(1720):20150520. doi:10.1098/rstb.2015.0520
4. Rauzi M, Verant P, Lecuit T, Lenne P-F. Nature and Anisotropy of Cortical Forces Orienting Drosophila Tissue Morphogenesis. Nat Cel Biol. (2008) 10:1401–10. doi:10.1038/ncb1798
5. Hočevar Brezavšček A, Rauzi M, Leptin M, Ziherl P. A Model of Epithelial Invagination Driven by Collective Mechanics of Identical Cells. Biophys J (2012) 103(5):1069–77. doi:10.1016/j.bpj.2012.07.018
6. Okuda S, Inoue Y, Watanabe T, Adachi T. Coupling Intercellular Molecular Signalling with Multicellular Deformation for Simulating Three-Dimensional Tissue Morphogenesis. Interf Focus. (2015) 5:20140095. doi:10.1098/rsfs.2014.0095
7. Sato K, Hiraiwa T, Shibata T. Cell Chirality Induces Collective Cell Migration in Epithelial Sheets. Phys Rev Lett (2015) 115:188102. doi:10.1103/physrevlett.115.188102
8. Nagai T, Honda H. A Dynamic Cell Model for the Formation of Epithelial Tissues. Philosophical Mag B (2001) 81:699–719. doi:10.1080/13642810108205772
9. Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F. The Influence of Cell Mechanics, Cell-Cell Interactions, and Proliferation on Epithelial Packing. Curr Biol (2007) 17:2095–104. doi:10.1016/j.cub.2007.11.049
10. Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, et al. Increased Cell Bond Tension Governs Cell Sorting at the Drosophila Anteroposterior Compartment Boundary. Curr Biol (2009) 19:1950–5. doi:10.1016/j.cub.2009.10.021
11. Iijima N, Sato K, Kuranaga E, Umetsu D. Differential Cell Adhesion Implemented by Drosophila Toll Corrects Local Distortions of the Anterior-Posterior Compartment Boundary. Nat Commun (2020) 11(1):6320. doi:10.1038/s41467-020-20118-y
12. Blackie L, Tozluoglu M, Trylinski M, Walther RF, Schweisguth F, Mao Y, et al. A Combination of Notch Signaling, Preferential Adhesion and Endocytosis Induces a Slow Mode of Cell Intercalation in the Drosophila Retina. Development 148. (2021). p. 197301. doi:10.1242/dev.197301
13. Kaplan J, Moskowitz M. Studies on the Turnover of Plasma Membranes in Cultured Mammalian Cells. Biochim Biophys Acta (Bba) - Biomembranes (1975) 389(2):306–13. doi:10.1016/0005-2736(75)90323-5
14. Levayer R, Pelissier-Monier A, Lecuit T. Spatial Regulation of Dia and Myosin-II by RhoGEF2 Controls Initiation of E-Cadherin Endocytosis during Epithelial Morphogenesis. Nat Cel Biol (2011) 13(5):529–40. doi:10.1038/ncb2224May
16. Liang X, Michael M, Gomez G. Measurement of Mechanical Tension at Cell-Cell Junctions Using Two-Photon Laser Ablation. Bio-protocol (2016) 6(24):e2068. doi:10.21769/BioProtoc.2068
17. Duda M, Kirkland NJ, Khalilgharibi N, Tozluoglu M, Yuen AC, Carpi N, et al. Polarization of Myosin II Refines Tissue Material Properties to Buffer Mechanical Stress. Dev Cel (2019) 48(2):245–60. doi:10.1016/j.devcel.2018.12.020
18. Iyer KV, Piscitello-Gómez R, Paijmans J, Jülicher F, Eaton S. Epithelial Viscoelasticity Is Regulated by Mechanosensitive E-Cadherin Turnover. Curr Biol (2019) 29(4):578–91. doi:10.1016/j.cub.2019.01.021
Keywords: epithelial cells, mathematical model, resting length, contraction force, adhesion strength, turnover rate, cell intercalation
Citation: Sato K and Umetsu D (2021) A Novel Cell Vertex Model Formulation that Distinguishes the Strength of Contraction Forces and Adhesion at Cell Boundaries. Front. Phys. 9:704878. doi: 10.3389/fphy.2021.704878
Received: 04 May 2021; Accepted: 06 July 2021;
Published: 22 July 2021.
Edited by:
Karine Guevorkian, UMR168 Unite physico-chimie Curie (PCC), FranceReviewed by:
Carles Blanch-Mercader, UMR168 Unite physico-chimie Curie (PCC), FranceCopyright © 2021 Sato and Umetsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katsuhiko Sato, a2F0c3VoaWtvX3NhdG9AZXMuaG9rdWRhaS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.