
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Phys., 11 March 2021
Sec. Medical Physics and Imaging
Volume 9 - 2021 | https://doi.org/10.3389/fphy.2021.617445
This article is part of the Research TopicInnovative Developments in Multi-Modality ElastographyView all 20 articles
Shear wave elastography (SWE) relies on the generation and tracking of coherent shear waves to image the tissue's shear elasticity. Recent technological developments have allowed SWE to be implemented in commercial ultrasound and magnetic resonance imaging systems, quickly becoming a new imaging modality in medicine and biology. However, coherent shear wave tracking sets a limitation to SWE because it either requires ultrafast frame rates (of up to 20 kHz), or alternatively, a phase-lock synchronization between shear wave-source and imaging device. Moreover, there are many applications where coherent shear wave tracking is not possible because scattered waves from tissue’s inhomogeneities, waves coming from muscular activity, heart beating or external vibrations interfere with the coherent shear wave. To overcome these limitations, several authors developed an alternative approach to extract the shear elasticity of tissues from a complex elastic wavefield. To control the wavefield, this approach relies on the analogy between time reversal and seismic noise cross-correlation. By cross-correlating the elastic field at different positions, which can be interpreted as a time reversal experiment performed in the computer, shear waves are virtually focused on any point of the imaging plane. Then, different independent methods can be used to image the shear elasticity, for example, tracking the coherent shear wave as it focuses, measuring the focus size or simply evaluating the amplitude at the focusing point. The main advantage of this approach is its compatibility with low imaging rates modalities, which has led to innovative developments and new challenges in the field of multi-modality elastography. The goal of this short review is to cover the major developments in wave-physics involving shear elasticity imaging using a complex elastic wavefield and its latest applications including slow imaging rate modalities and passive shear elasticity imaging based on physiological noise correlation.
The goal of Shear Wave Elastography (SWE) is to measure the tissue’s shear elasticity μ (i.e. elasticity). To this end, SWE relies on shear wave propagation inside soft tissues, since under the assumption of a purely elastic isotropic medium, the shear wave speed
In SWE, the shear wave speed is usually estimated from the coherent or ballistic shear wave propagation. However, there are many applications where coherent shear wave tracking is not possible due to the interference of scattered waves coming from tissue boundaries and internal inhomogeneities. Moreover, additional waves generated by muscular activity, heart beating or other sources of vibrations may interfere with the coherent shear wave resulting in a complex elastic wavefield. Directional filtering has been proposed to solve this issue [4, 5]. However, other researchers focused in developing alternative approaches to measure μ (or equivalently
Recently, inspired by seismic noise correlation [22, 23] and time reversal [24] a novel method [12, 25, 26] to extract the shear elasticity of tissue from a complex elastic field was developed. The first step is to record the complex wavefield generated by random internal or external sources. Then, by cross-correlating the elastic field at different positions, which can be interpreted as a time reversal experiment in the computer [27], shear waves are virtually focused on any point of the imaging plane. From the computed time reversal field there are different independent approaches to image the shear elasticity [12–15, 18], for example, tracking the coherent shear wave as it focus; measuring the focus size which is directly linked to the shear wavelength (
The pioneering work in this field was done at the Laboratorio de Acústica Ultrasonora (LAU) in Montevideo, Uruguay in collaboration with Stefan Catheline, who was at the LAU for a 2 year mission. The work of Catheline et al. [25] presented the observation of a time reversal experiment using shear waves in a tissue-mimicking phantom for the first time. In [25] it was shown that, contrary to the scalar field case (e.g., in fluids), for an elastic field in the bulk of a soft tissue, the focus is no more isotropic. Instead, it has an ellipse-like shape leading to a direction dependent Rayleigh criterion. Then, Benech et al. [12] established numerically that the focus width at −6 dB was approximately equal to one shear wavelength. This approach was termed the focus size (or width) method and was used to measure the elasticity of a tissue mimicking phantom. This method was shown to be independent of the source kind, shape, and time excitation function. This robustness regarding the shear wave source allowed envisioning its application to passive elastography. Alternatively, in [12], the phantom’s elasticity was measured by tracking the phase of the coherent shear wave as it focused back to the source (i.e. the phase method). Both methods (phase and focus size), were also used by Brum et al. [26] to measure the elasticity of a tissue mimicking phantom and cheese using surface waves. Finally, the feasibility to conduct 2D elasticity imaging in vivo using passive elastography was demonstrated by Gallot et al. [13] in the human liver and belly muscle. To image the shear wave speed they used an ultrafast ultrasound scanner along with the phase and focus size methods. The main advantage of the focus size method is its compatibility with low imaging rates modalities. This was first shown in [12] and then used in [13] to conduct a shear wavelength tomography of a two-layered tissue-mimicking phantom. In Catheline et al. [14] it was further demonstrated that the loss of time and/or spatial coherence of the recorded wavefield is not an obstacle for a wavelength tomography, which led to new passive elastography experiments using optical coherence tomography (OCT) [21] and MRI [20]. Finally, in [15] an analytical expression that allowed converting the wavelength tomography into shear elasticity was derived. The compatibility with low imaging rates modalities has led to innovative developments and new challenges in the field of multi-modality elastography. Particularly, it has boost the concept of passive elastography to new applications involving ultrafast and ultraslow imaging modalities like ultrasound, optical methods (digital holography or OCT) and MRI.
In this context, the goal of this short review is to cover the major developments in wave-physics involving elasticity imaging using time reversal and noise correlation of shear waves together with its latest applications. These include time reversal physics and near field effects of shear waves in soft tissues [15, 28, 29], passive brain elasticity imaging using MRI [20], cornea elasticity imaging using OCT [21], thyroid [14] and breast [18] elasticity imaging using low frame rate ultrasound scanners, liver elasticity imaging [13, 30], passive muscle elasticity assessment [11, 19] and most recently cellular elasticity imaging [31].
Time reversal was first proposed by Mathias Fink [24] and is based on the time-reversal invariance of the wave equation in a lossless medium. Time-reversal focusing is a two-step process. In a first step, the direct wave scene is recorded: the impulse response of a point source placed in
where
In practice, an ideal time-reversal cavity as described above is not necessary and can be replaced by a time-reversal mirror using reverberation and/or a multiple scattering medium [33]. For elastography applications one can take advantage of multiple sources, reverberation and diffusion to conduct a time reversal experiment. Specifically, the step where the time reversed field is sent back into the medium (i.e., the second step in a time reversal experiment) is replaced by a virtual time-reversal experiment based on spatial reciprocity and cross-correlation of the wavefield [12].
Usually the imaging systems used in elastography (e.g., ultrasound, OCT or MRI) allow recording at least one component of the elastic wavefield within a given region of interest (ROI). Let this component be
If the field is diffuse, Eq. 2 allows the retrieval of the Green’s function [34]:
The analogy between time reversal and cross-correlation follows directly from comparing Eqs. 1, 3: the time derivative of the correlation field is proportional to the time-reversal field. The proportionality constant in Eq. 3 will depend on the propagation equation, medium and source properties, propagation regime, etc. For instance, in the case of a visco-elastic medium with homogeneously distributed white noise sources Gouedard et al. derived Eq. 3 with the factor
where
Equations 1–4 are the point of departure of many inversion methods developed during these past years to image μ from the correlation field interpreted as a time-reversal experiment. Figure 1 illustrates the main steps involved in an cross-correlation based elastography experiment: from the wavefield acquisition (Figure 1A) to the final shear elasticity image (Figure 1D). The estimation of μ by cross-correlation requires the presence of a diffuse field. In practice, the way that elastography methods have found to create such field is the use of multiple uncorrelated sources in time and space. The complexity of the field comes from the interference of the direct and reflected waves created by the different type of sources: external (active methods), internal (passive elastography) or both.
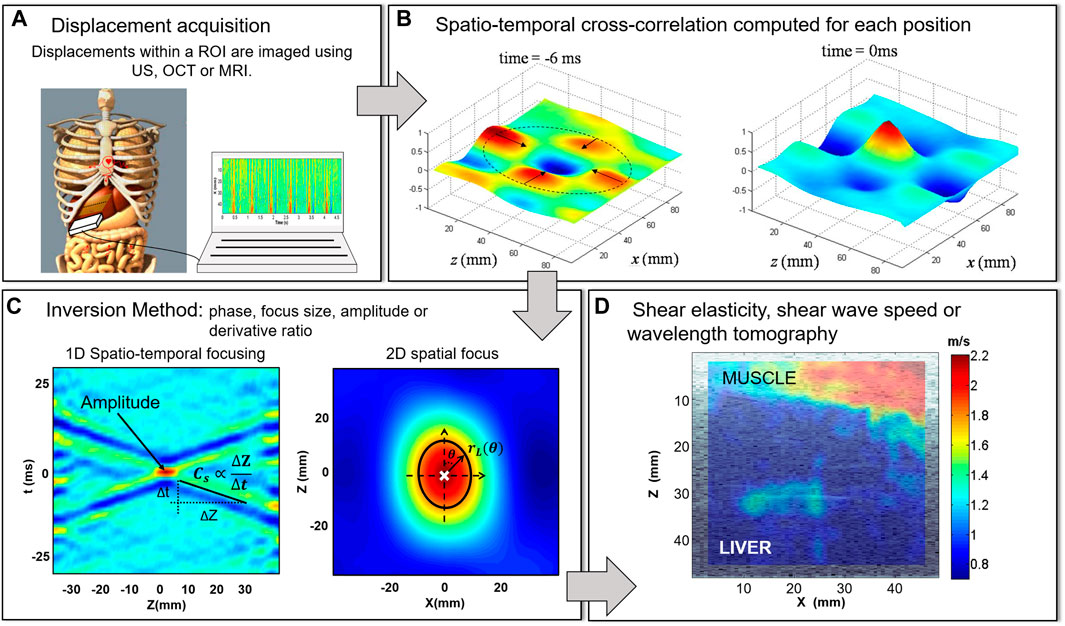
FIGURE 1. Schemmatic representation of the main steps involved in an elastography experiment based on noise correlation and time reversal (A) The first step consists in recording at least one component of the complex elastic wavefield. As an example, we present the acquisition of the physiological noise in the liver using an ultrasound (US) scanner as in [13]. (B) Then, the spatio-temporal cross-correlation field
Cross-correlation methods allow to reconstruct a refocusing wavefield from an apparently random and disorganized wavefield. Figure 1B shows two snapshots of the cross-correlation field
From the correlation field there are different independent inversion methods to image μ or equivalently cs (Figure 1C), e.g. tracking the coherent shear wave as it focus (phase method), measuring
The phase method consists in measuring the phase velocity
where
An alternative inversion method that has shown to be compatible with low imaging rates modalities is the focus size method. In a time reversal experiment with shear waves, the size of the focus is limited by diffraction to dimensions comparable to the shear wavelength. As a first approximation, one can consider that the size of the focus
In [28], by substituting the free space Green’s function for elastodynamics [39] on the right hand side of Eq. 4, the following expression relating the focus size
where
Equation 6 can be used to retrieve
One advantage of the focus size method is that the time and spatial coherence are decoupled in Eq. 6. Thus, it is still possible to retrieve
The focus size method discussed above makes use of the right-hand-side of Eq. 4. However, if the temporal frequency is unknown, as in the case of ultraslow experiments, it is not possible to convert the focus size into shear wave speed. To solve this issue, in the work of Rabin et al. [18] they chose to work out the left-hand-side of Eq. 4. As a result a quantitative expression relating the vibration amplitude at the focus (i.e., autocorrelation value) with the shear elasticity was derived. To this end, the traction
where
This method was first proposed by Catheline et al. [14] and was further developed in [29] by taking into account the near field effects in the vicinity of the focusing point. This method relies on the proportionality between Eqs. 1, 2 in the presence of a diffuse field and is valid for any field obeying a wave equation. If
In an ideal isotropic diffuse filed, plane wave decomposition at a given frequency allows to use the following approximations for the spatial and temporal derivatives:
Or equivalently, for the case of ultraslow experiments the local shear wavelength can be estimated as:
In [14] the proportionality constants for Eqs. 8, 9 were assumed to be 1 and
In this section the main advantages and drawbacks of the inversion methods presented above will be discussed. All of them rely on the presence of a diffuse field. Consequently, there is no need to control or to know the position of the shear wave source(s) nor the direction of its applied force. Moreover, reflected waves do not pose a problem as in a standard SWE experiment. This approach is advantageous in tissues where is difficult to isolate a coherent shear wave propagation from its reflections, for example, in tissues with complex boundary shape or containing several inhomogeneities. Here the more complex the wavefield the better, since waves arriving from all directions are required to improve the Green’s function retrieval from the cross-correlation. In addition, the specific shape of the shear wave sources is not relevant either.
Paradoxically, the need of a diffuse field is also the main drawback of these methods. The hypothesis of an homogeneous repartition of sources (i.e. isotropic shear wave distribution within the ROI) is needed for the exact Green’s function retrieval through cross-correlation [35]. However, this is difficult to achieve in practice. First, wave attenuation may set a limit to the diffusion process. To minimize the effects of attenuation many authors used multiple sources in their experiments [18, 29]. Second, in an internal organ, waves usually come from a given region inside/outside this organ. This may be due to the limited access when using external sources or because physiological noise comes from a preferred region within the body. For example, in the work of Gallot et al. [13] it was noted that the physiological noise recorded in the liver was highly directive coming mainly from the heart region. As result only one branch of the cross-correlation evolution used in the phase method (left panel of Figure 1C) was observed. This directivity of the wavefield can lead to biases in the reconstructed shear wave speed tomography. Nevertheless, some strategies are envisaged to overcome this drawback. One of them is to use a passive inverse filter [40] which allows an optimal spatial redistribution of the incoming energy. Another strategy is to enhance the isotropic distribution shear waves by using multiple sources. This was done in Rabin et al. [18] for the breast and in Ormachea et al. [17] in the liver by including multiple active vibration sources in the patient’s clinical bed. The use of multiple sources may also be advantageous to increase and control the frequency content of the diffuse field. The knowledge of the shear wave excitation frequency allows the shear wavelength tomography conducted with ultraslow imaging modalities to be converted into shear elasticity.
The main advantage of the phase method is a direct estimation of
The focus size method relies in the relation between the focus size and the shear wavelength given in Eq. 6. Again, this relation is valid in the presence of a diffuse field where the Green’s function can be retrieved from cross-correlation. However, since the method uses an average value in all directions, the lack of diffusivity is not as critical as in the phase method. In addition, the size is measured around the focusing point where the signal to noise ratio is highest. Thus, the method is robust in the presence of noise. Finally, the spatial coherence is shown to be independent of the time acquisition rate. As a consequence, this method is compatible with low frame rate imaging modalities like standard ultrasound, MRI or OCT. However, at ultraslow frame rates, only the shear wavelength can be estimated because the frequency information is lost.
The amplitude method is based on the relationship between the peak of autocorrelation function and the local shear elasticity as expressed in Eq. 7. This relationship overcomes the need of knowing the frequency of the field to quantitatively measure the shear elasticity. Thus, it is fully compatible with low frame rate imaging modalities. Nevertheless, Eq. 7 was derived from the free space Green’s function. Therefore, its validity is limited in the presence of inclusions where scattering must be considered. Thus, the spatial resolution of this method as well as its quantitative value near internal boundaries or inhomogeneities needs further revision.
Strictly, the derivative ratio method does not require the presence of a diffuse field since the governing equations of this method are valid for any field obeying the wave equation. This is a great advantage compared to the other methods. However, the lack of diffusivity makes the result dependent on the specific orientation between the direction of observation and the preferred direction of the incoming energy. This method is compatible with low frame rate imaging modalities where the shear wavelength is imaged.
Viscosity is an inherent property to any biological tissue that may influence elastography methods based on noise correlation and time reversal. Although viscosity measurements were early integrated into magnetic resonance elastography [41], how to incorporate and quantify viscosity is still a matter of debate among the ultrasound-based SWE community [42]. Wave attenuation is known to mitigate the focusing quality in time reversal and cross-correlation based methods [43]. However, since spatial reciprocity remains valid even in the presence of attenuation, the best signal-to-noize ratio will be found on the focusing point at the refocusing time, i.e. time reversal acts as a spatio-temporal matched filter [44]. Consequently, in a dissipative medium the refocusing is likely to emerge from the reverberant field allowing the focus size estimation. In the work of Benech et al. [12] the influence of viscosity in the phase and focus size methods was evaluated experimentally in agarose phantoms with different Q-factors. In [12] it was found that for high Q-factors (where several reflections take place) the field can be considered as being diffuse and the mechanical properties may be accurately retrieved through both methods. Contrary, for low Q-factors the focus size method provides a more robust estimation. Moreover, as demonstrated by the numerous works and applications cited along this review, cross-correlation elastography experiments were not hindered by viscosity. Nevertheless, future works should aim to include viscosity while solving the inverse problem through the phase, focus size, amplitude or derivative ratio method.
The compatibility with low imaging rates modalities has led to innovative developments and new challenges in the field of multi-modality elastography. Particularly, the use of diffuse and complex wavefields has boost the concept of passive elastography to new applications involving ultrafast and ultraslow imaging modalities like ultrasound, optical methods (e.g., digital holography or OCT) and MRI.
The work of Sabra et al. [11] was the first proof of concept in the field of passive elastography based on noise correlation. In [11] they used sixteen miniature skin-mounted accelerometer placed along the vastus lateralis to measure muscular noise in the 40–55 Hz frequency band. The shear wave phase velocity dispersion was estimated from the correlation field by using a Morlet wavelet transform and by computing the slope of the shear wave arrival time for increasing sensor separation distances. The muscle’s shear elasticity and viscosity were retrieved by fitting a Voigt model to the phase velocity dispersion curve. They found that elasticity and viscosity increased with muscle load. In the work of Brum et al. [26] they also used surface sensors to record the reverberated elastic field generated by a shaker applied at the medium’s surface. In this work they used the phase and the focus size methods to measure the elasticity of a hard and a soft gelatin-based phantoms and cheese. Experiments performed in cheese allowed envision applications in the food industry, for example, evaluating the cheese ripening time.
Later, the work of Gallot et al. [13] set a milestone regarding passive elasticity imaging inside the human body. To this end they used an ultrafast ultrasound scanner to record the natural displacements in the human liver and belly muscle. To conduct the shear elasticity imaging they used the focus size method which has shown to be more robust for low signal to noise ratio. To retrieve the shear wave speed, the focus size at -6 dB was assumed to be half a shear wavelength as in the case of fluids. Nevertheless, a good agreement was obtained between the shear elasticity image and its corresponding echographic image with shear velocity values close to the ones reported by other studies. This idea was taken up in the work of Catheline et al. [14] were they used the derivative ratio method and its compatibility with low frame rate imaging modalities to conduct the first experimental in vivo demonstration of passive shear wave speed imaging using an ultrasound scanner working at a conventional frame rate. The experiment was done on the thyroid of a healthy volunteer and the ultrasonic probe was hand held during the acquisition of 800 frames at 25 Hz. Breathing and moving was prohibited during the 32 s long acquisition.
The compatibility with low rate imaging modalities boosted several innovative applications involving standard commercial ultrasound scanners, OCT and MRI systems for elasticity imaging. In the work of Zorgani et al. the derivative ratio method was used to realize a passive shear wavelength tomography in the brain using MRI [20]. The experimental validation of the sequence and method was first conducted in a calibrated tissue mimicking phantom. Then, the proof of concept was demonstrated in vivo in the brain of two healthy volunteers. Compared to other magnetic elastography techniques, this approach does not need any synchronization with the shear wave source. Later, in the work of Nguyen et al. a low frame rate spectral-domain OCT system was used to demonstrate the feasibility of a passive shear wavelength tomography on the eye of an anesthetized rat [21]. The results were cross-validated with an active elastography experiment at ultrafast frame rate. But it was not until the work of Rabin et al. [18] that quantitative elasticity imaging using ultraslow imaging systems was achieved. In their work they used the amplitude method to image in vivo the shear elasticity of healthy and tumorous breast. The B-mode images from a conventional ultrasound scanner (frame rate 30–50 Hz) were used to measure the diffuse displacement field. Since in the breast the signal to noise ratio of the physiological noise as well as the sensitivity of the algorithm used to measure the displacements are low, in this work they used an array of mechanical shakers to create the diffuse field.
Recently as in [18], many authors decided to use one or multiple shakers to generate a controlled diffuse field. In this way the signal to noise ratio inside the region of interest can be increased and the frequency content of the field can be controlled [16, 30, 31, 45]. For example, in Reverberant Shear Wave Elastography (R-SWE) a narrowband diffuse field is generated with several mechanical actuators, then, the shear wavelength is derived from the spatial autocorrelation function [16, 17, 46, 47]. Regarding the application of diffuse field interpreted within the frame of noise correlation and time reversal in the work Grasland et al. [31] a 15 kHz vibrating pipette was used to create a complex reverberated elastic field inside a cell. This novel approach was termed “cell quake elastography.” The displacement field inside the cell was imaged at 200 kHz using a microscope together with a high speed camera and the shear wavelength was estimated from the curvature of the focusing point. Elasticity images allowed identifying different cell zones (e.g. zona pellucida, cytoplasm and nucleus) in isolated and multiple cells configurations. Moreover, in [31] it was shown that elasticity decreases when the cell cytoskeleton was disrupted with cytochalasin B. This technique allow shear wave elastography at microscopic scale, opening a new research field in mechanobiology cellular properties. Moreover, in the work of Barrere et al. [30] the feasibility of monitoring high-intensity focused ultrasound (HIFU) treatments in the liver using the derivative ratio method was investigated. To this end, bovine livers were heated up to 80°C using a planar HIFU transducer and the displacements generated by a mechanical shaker were imaged with a high-frame-rate ultrasound imaging device. The formation of ablated tissue was monitored and evidenced by a tissue stiffening like in [48]. More recently, in the work of Marmin et al. [49] digital holography was used to capture a diffuse shear wave field induced by a piezoelectric actuator with a sensitivity of up to 10 nm. A shear wavelength tomography was conducted in agarose phantoms and ex vivo pork liver. Digital holography allow envisioning contact-less passive elasticity imaging with high sensitivity and spatial resolution [29]. Finally, it is important to mention that the wave-physics and algorithms developed in the frame of noise correlation and time reversal elastography are being applied in other fields of research. For example, Hillers et al. [50] analyzed seismic data with the focus size method to image the San Jacinto fault zone.
Throughout this short review we covered the major developments in wave-physics involving elasticity imaging using cross-correlation of a complex elastic wavefield along with its latest applications. The main advantage of this approach is its compatibility with low imaging rate modalities which has boosted the concept of passive elasticity imaging. Compared to standard SWE with an active shear wave source, a passive approach is a smart solution when shear wave generation is difficult (e.g. in well protected organs as the brain) or even dangerous, as in the case of the cornea. The various works cited along this review demonstrate the significant potential of cross-correlation elastography to become a new imaging tool in the field of multi-modality elastography. Nevertheless, it is important that future works seek to incorporate the mechanical properties inherent to tissue such as viscosity and anisotropy into the inversion methods. Moreover, cross-correlation elastography will clearly benefit from three dimensional and multiple component measurements of elastic wavefields, now possible with MRI or new 3D ultrasound imaging technologies. Over the past thirty years, different technological advances allowed SWE to be incorporated in commercial ultrasound and MRI systems, becoming a new imaging modality in clinics. Cross-correlation elastography is a very recent approach that has demonstrated its feasibility in different medical applications, however its clinical significance still needs to be demonstrated. Therefore, future works should attempt to translate this approach into clinics by proving its reproducibility, repeatability, diagnostic value and its ability to improve in vivo results. If succeeded, we believe that cross-correlation elastography will certainly become a novel multi-modality imaging tool in clinics.
JB conceived the manuscript. JB and NB wrote the manuscript. JB, NB, TG, and CN revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors acknowledge the support of PEDECIBA-Física, Uruguay; Comisión Sectorial de Investigación Científica (CSIC), Universidad de la República, Uruguay; and Agencia Nacional de Investigación e Innovación (ANII), Uruguay.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Nenadic IZ, Urban MW, Greenleaf JF, Gennisson JL, Bernal M, Tanter M. Ultrasound elastography for biomedical applications and medicine. Hoboken, NJ: John Wiley & Sons. (2019).
2. Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics (2017) 7:1303. doi:10.7150/thno.18650
3. Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat (2010) 23:497–511. doi:10.1002/ca.21006
4. Deffieux T, Gennisson JL, Bercoff J, Tanter M. On the effects of reflected waves in transient shear wave elastography. IEEE Trans Ultrason Ferroelectr Freq Control (2011) 58:2032–5. doi:10.1109/TUFFC.2011.2052
5. Manduca A, Lake DS, Kruse SA, Ehman RL. Spatio-temporal directional filtering for improved inversion of mr elastography images. Med Image Anal (2003) 7:465–73. doi:10.1016/s1361-8415(03)00038-0
6. Salcudean SE, French D, Bachmann S, Zahiri-Azar R, Wen X, Morris WJ. Viscoelasticity modeling of the prostate region using vibro-elastography. Med Image Comput Comput Assist Interv (2006) 9:389–96. doi:10.1007/11866565_48
7. Gallichan D, Robson MD, Bartsch A, Miller KL. Tremr: table-resonance elastography with mr. Magn Reson Med (2009) 62:815–21. doi:10.1002/mrm.22046
8. Kanai H, Sato M, Koiwa Y, Chubachi N. Transcutaneous measurement and spectrum analysis of heart wall vibrations. IEEE Trans Ultrason Ferroelect Freq Contr (1996) 43:791–810. doi:10.1109/58.535480
9. Konofagou EE, D'hooge J, Ophir J. Myocardial elastography--a feasibility study in vivo. Ultrasound Med Biol (2002) 28:475–82. doi:10.1016/s0301-5629(02)00488-x
10. Sunagawa K, Kanai H. Measurement of shear wave propagation and investigation of estimation of shear viscoelasticity for tissue characterization of the arterial wall. J Med Ultrason (2001) (2005) 32:39–47. doi:10.1007/s10396-005-0034-2
11. Sabra KG, Conti S, Roux P, Kuperman WA. Passive in vivo elastography from skeletal muscle noise. Appl Phys Lett (2007) 90:194101. doi:10.1063/1.2737358
12. Benech N, Catheline S, Brum J, Gallot T, Negreira CA. 1-d elasticity assessment in soft solids from shear wave correlation: the time-reversal approach. IEEE Trans Ultrason Ferroelectr Freq Control (2009) 56:2400–10. doi:10.1109/TUFFC.2009.1328
13. Gallot T, Catheline S, Roux P, Brum J, Benech N, Negreira C. Passive elastography: shear-wave tomography from physiological-noise correlation in soft tissues. IEEE Trans Ultrason Ferroelectr Freq Control (2011) 58:1122–6. doi:10.1109/TUFFC.2011.1920
14. Catheline S, Souchon R, Rupin M, Brum J, Dinh AH, Chapelon J-Y. Tomography from diffuse waves: passive shear wave imaging using low frame rate scanners. Appl Phys Lett (2013) 103:014101. doi:10.1063/1.4812515
15. Brum J, Catheline S, Benech N, Negreira C. Quantitative shear elasticity imaging from a complex elastic wavefield in soft solids with application to passive elastography. IEEE Trans Ultrason Ferroelectr Freq Control (2015) 62:673–85. doi:10.1109/TUFFC.2014.006965
16. Parker KJ, Ormachea J, Zvietcovich F, Castaneda B. Reverberant shear wave fields and estimation of tissue properties. Phys Med Biol (2017) 62:1046. doi:10.1088/1361-6560/aa5201
17. Ormachea J, Parker KJ, Barr RG. An initial study of complete 2d shear wave dispersion images using a reverberant shear wave field. Phys Med Biol (2019) 64:145009. doi:10.1088/1361-6560/ab2778
18. Rabin C, Benech N. Quantitative breast elastography from b-mode images. Med Phys (2019) 46:3001–12. doi:10.1002/mp.13537
19. Sabra KG, Archer A. Tomographic elastography of contracting skeletal muscles from their natural vibrations. Appl Phys Lett (2009) 95:203701. doi:10.1063/1.3254834
20. Zorgani A, Souchon R, Dinh AH, Chapelon JY, Ménager JM, Lounis S, et al. Brain palpation from physiological vibrations using mri. Proc Natl Acad Sci USA (2015) 112:12917–21. doi:10.1073/pnas.1509895112
21. Nguyen TM, Zorgani A, Lescanne M, Boccara C, Fink M, Catheline S. Diffuse shear wave imaging: toward passive elastography using low-frame rate spectral-domain optical coherence tomography. J Biomed Opt (2016) 21:126013. doi:10.1117/1.JBO.21.12.126013
22. Campillo M, Paul A. Long-range correlations in the diffuse seismic coda. Science (2003) 299:547–9. doi:10.1126/science.1078551
23. Shapiro NM, Campillo M, Stehly L, Ritzwoller MH. High-resolution surface-wave tomography from ambient seismic noise. Science (2005) 307:1615–8. doi:10.1126/science.1108339
24. Fink M. Time reversal of ultrasonic fields. i. basic principles. IEEE Trans Ultrason Ferroelectr Freq Control (1992) 39:555–66. doi:10.1109/58.156174
25. Catheline S, Benech N, Brum J, Negreira C. Time reversal of elastic waves in soft solids. Phys Rev Lett (2008) 100:064301. doi:10.1103/PhysRevLett.100.064301
26. Brum J, Catheline S, Benech N, Negreira C. Shear elasticity estimation from surface wave: the time reversal approach. J Acoust Soc Am (2008) 124:3377–80. doi:10.1121/1.2998769
27. Ing RK, Quieffin N, Catheline S, Fink M. In solid localization of finger impacts using acoustic time-reversal process. Appl Phys Lett (2005) 87:204104. doi:10.1063/1.2130720
28. Benech N, Brum J, Catheline S, Gallot T, Negreira C. Near-field effects in Green's function retrieval from cross-correlation of elastic fields: experimental study with application to elastography. J Acoust Soc Am (2013) 133:2755–66. doi:10.1121/1.4795771
29. Zemzemi C, Zorgani A, Daunizeau L, Belabhar S, Souchon R, Catheline S. Super-resolution limit of shear-wave elastography. Epl (2020) 129:34002. doi:10.1209/0295-5075/129/34002
30. Barrere V, Melodelima D, Catheline S, Giammarinaro B. Imaging of thermal effects during high-intensity ultrasound treatment in liver by passive elastography: a preliminary feasibility in vitro study. Ultrasound Med Biol (2020) 45:1968-1977. doi:10.1016/j.ultrasmedbio.2020.03.019
31. Grasland-Mongrain P, Zorgani A, Nakagawa S, Bernard S, Paim LG, Fitzharris G, et al. Ultrafast imaging of cell elasticity with optical microelastography. Proc Natl Acad Sci USA (2018) 115:861–6. doi:10.1073/pnas.1713395115
32. Cassereau D, Fink M. Time-reversal of ultrasonic fields. iii. theory of the closed time-reversal cavity. IEEE Trans Ultrason Ferroelectr Freq Control (1992) 39:579–92. doi:10.1109/58.156176
33. Fink M. Time reversal in acoustics. Contemp Phys (1996) 37:95–109. doi:10.1080/00107519608230338
34. Gouédard P, Roux P, Campillo M, Verdel A. Convergence of the two-point correlation function toward the Green's function in the context of a seismic-prospecting data set. Geophysics (2008) 73:V47–V53. doi:10.1190/1.2985822
35. Gouédard P, Stehly L, Brenguier F, Campillo M, Colin de Verdière Y, Larose E, et al. Cross-correlation of random fields: mathematical approach and applications. Geophys Prospect (2008) 56:375–93. doi:10.1111/j.1365-2478.2007.00684.x
36. Roux P, Sabra KG, Kuperman WA, Roux A. Ambient noise cross correlation in free space: theoretical approach. J Acoust Soc Am (2005) 117:79–84. doi:10.1121/1.1830673
37. Sánchez-Sesma FJ, Pérez-Ruiz JA, Luzón F, Campillo M, Rodríguez-Castellanos A. Diffuse fields in dynamic elasticity. Wave motion (2008) 45:641–54. doi:10.1016/j.wavemoti.2007.07.005
38. Snieder R, Wapenaar K, Wegler U. Unified Green's function retrieval by cross-correlation; connection with energy principles. Phys Rev E Stat Nonlin Soft Matter Phys (2007) 75:036103. doi:10.1103/PhysRevE.75.036103
39. Richards PG, Aki K. Quantitative seismology: theory and methods. San Francisco, CA: W. H. Freeman and Co. (1980). doi:10.1002/gj.3350160110
40. Gallot T, Catheline S, Roux P, Campillo M. A passive inverse filter for Green's function retrieval. J Acoust Soc Am (2012) 131:EL21–EL27. doi:10.1121/1.3665397
41. Sinkus R, Tanter M, Xydeas T, Catheline S, Bercoff J, Fink M. Viscoelastic shear properties of in vivo breast lesions measured by mr elastography. Magn Reson Imaging (2005) 23:159–65. doi:10.1016/j.mri.2004.11.060
42. Palmeri M, Nightingale K, Fielding S, Rouze N, Deng Y, Lynch T, et al. Rsna qiba ultrasound shear wave speed phase ii phantom study in viscoelastic media. IEEE Int Ultrason Symp (IUS) (IEEE) (2015) 1–4.
43. Zhu T. Viscoelastic time-reversal imaging. Geophysics (2015) 80:A45–50. doi:10.1190/geo2014-0327.1
44. Tanter M, Thomas JL, Fink M. Time reversal and the inverse filter. J Acoust Soc Am (2000) 108:223–34. doi:10.1121/1.429459
45. Beuve S, Kritly L, Callé S, Remenieras JP. Diffuse shear wave spectroscopy for soft tissue viscoelastic characterization. Ultrasonics 110 (2021) 106239. doi:10.1016/j.ultras.2020.106239
46. Ormachea J, Castaneda B, Parker KJ. Shear wave speed estimation using reverberant shear wave fields: implementation and feasibility studies. Ultrasound Med Biol (2018) 44:963–77. doi:10.1016/j.ultrasmedbio.2018.01.011
47. Zvietcovich F, Pongchalee P, Meemon P, Rolland JP, Parker KJ. Reverberant 3d optical coherence elastography maps the elasticity of individual corneal layers. Nat Commun (2019) 10:4895–13. doi:10.1038/s41467-019-12803-4
48. Benech N, Negreira CA. Monitoring heat-induced changes in soft tissues with 1d transient elastography. Phys Med Biol (2010) 55:1753. doi:10.1088/0031-9155/55/6/014
49. Marmin A, Catheline S, Nahas A. Full-field passive elastography using digital holography. Opt Lett (2020) 45:2965–8. doi:10.1364/OL.388327
Keywords: ultrasound, shear wave, elasticity imaging, near field effect, passive elastography
Citation: Brum J, Benech N, Gallot T and Negreira C (2021) Shear Wave Elastography Based on Noise Correlation and Time Reversal. Front. Phys. 9:617445. doi: 10.3389/fphy.2021.617445
Received: 14 October 2020; Accepted: 20 January 2021;
Published: 11 March 2021.
Edited by:
Francesco Moscato, Medical University of Vienna, AustriaReviewed by:
Stefan Catheline, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2021 Brum, Benech, Gallot and Negreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Brum, amJydW1AZmlzaWNhLmVkdS51eQ==; Nicolás Benech, bmJlbmVjaEBmaXNpY2EuZWR1LnV5
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.