
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys., 21 January 2021
Sec. Medical Physics and Imaging
Volume 8 - 2020 | https://doi.org/10.3389/fphy.2020.617582
This article is part of the Research TopicInnovative Developments in Multi-Modality ElastographyView all 20 articles
 Damian R. Sowinski1*
Damian R. Sowinski1* Matthew D. J. McGarry1
Matthew D. J. McGarry1 Elijah E. W. Van Houten2
Elijah E. W. Van Houten2 Scott Gordon-Wylie1
Scott Gordon-Wylie1 John B Weaver1,3,4
John B Weaver1,3,4 Keith D. Paulsen1,3,5
Keith D. Paulsen1,3,5Magnetic Resonance Elastography allows noninvasive visualization of tissue mechanical properties by measuring the displacements resulting from applied stresses, and fitting a mechanical model. Poroelasticity naturally lends itself to describing tissue - a biphasic medium, consisting of both solid and fluid components. This article reviews the theory of poroelasticity, and shows that the spatial distribution of hydraulic permeability, the ease with which the solid matrix permits the flow of fluid under a pressure gradient, can be faithfully reconstructed without spatial priors in simulated environments. The paper describes an in-house MRE computational platform - a multi-mesh, finite element poroelastic solver coupled to an artificial epistemic agent capable of running Bayesian inference to reconstruct inhomogenous model mechanical property images from measured displacement fields. Building on prior work, the domain of convergence for inference is explored, showing that hydraulic permeabilities over several orders of magnitude can be reconstructed given very little prior knowledge of the true spatial distribution.
In the past two decades magnetic resonance elastography (MRE) has emerged as an imaging modality that extends the capabilities of standard MRI, giving clinicians and researchers unprecedented access to tissue properties in vivo. As it develops rapidly, MRE promises clinicians information akin to the data surgeons acquire through manual palpation - material properties such as stiffness - but noninvasively and quantitatively, with applications in diagnosis and monitoring of diseases. Connecting macroscopic mechanical response to microscopic cellular organization of tissue, MRE opens up new avenues for assessing the etiology of tissue pathology. The modality has been implemented to varying degrees of success on skeletal muscle [1–9], breast [10–17], liver [18–24], and brain [25, 26].
Elastography dates back to Gierke et al’s 1952 paper, Physics of Vibrations in Living Tissue - one can discern tissue properties by observing a tissue’s response to vibration [27]. MRE is multidisciplinary at its core, requiring expertise in magnetic resonance imaging, biophysical modelling/theoretical mechanics, and numerical simulations/machine learning. Several reviews are available [28–33].
The purpose of the current paper is to explore a poroelastic model of tissue, and convey novel results on the simultaneous imaging of both shear stiffness and hydraulic permeability in silico. These results extend prior studies on poroelastic parameter estimation [34–37], and quantify the domain of valid inference. We show that convergence to the correct in-silico property values is possible even when prior knowledge is off by several orders of magnitude. These results are a foundation for ongoing in vitro and in vivo work, giving reasonable bounds for noiseless data.
This paper is organized as follows: Section 2 covers the theoretical details of our tissue model and how inference is accomplished. It also details some of the numerical considerations that have been implemented in our in-house MRE computational platform. Section 3 details the setup of our simulations - inference of one and two properties from in-silico data generated by solving Newton’s Laws for a poroelastic medium. Single and dual property contrast conditions necessary for fidelitous inference are examined. In Section 4 we discuss the results from our simulations and conclude with planned future directions for in vitro and in vivo application.
Throughout, we use coordinate independent tensor notation to emphasize the physical underpinnings of expressions, but, when required to use coordinates, follow the Einstein summation convention where repeated indices in terms are summed over. Indices are drawn from the middle of the Latin alphabet,
We denote the standard tensor product with a
The index gymnastics in evaluating such expressions can get pretty messy, which is why we stick to coordinate independent notation as much as possible. The transpose acts on second rank basis tensors as
Biological tissue is a complex medium, the result of almost four billion years of continual evolutionary pressure and the whims of chance. At the microscopic level, the variety of cellular structures in animals would require a vastly more complicated set of modelling assumptions than continuum mechanics can provide. However, tracing over these microscopic degrees of freedom, an effective theory of tissue should have more in common with a coupled solid-fluid description than one consisting of a single continuum. Such a model already exists, developed extensively during the last century by the geophysics community - poroelasticity.
Poroelasticity is an effective mechanical model describing the continuum as a coarse grained biphasic medium consisting of an elastic matrix coupled to a fluid. The former is not simply-connected, but has a connected pore space with a tortuous topology - it is this pore space that the fluid moves through. The coupled dynamics of solid and fluid result in a much richer phenomenology than single phase continuum models. Figure 1 shows a schematic of a porous structure.
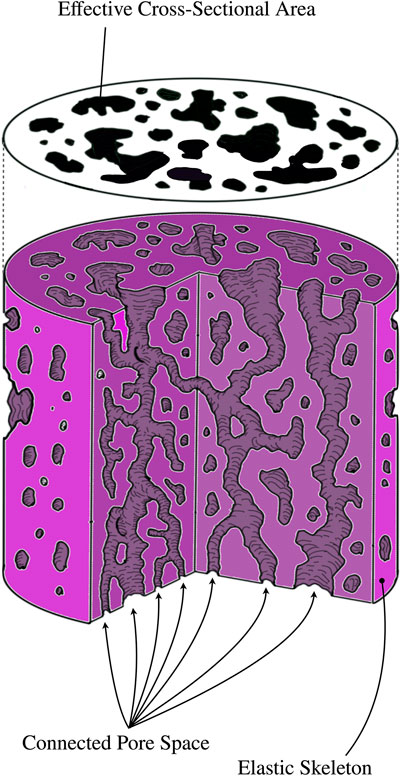
FIGURE 1. The model of a porous material consists of an elastic matrix - the solid - and a connected pore space. Pores whose connected boundaries are homeomorphic (topologically identical) to spheres are not a part of the connected pore space, as nothing can flow through them. The connected pore space is filled with a fluid, whose interactions with the walls of the elastic skeleton provide a much richer phenomenology than elastic/viscoelastic models of materials. When a porous substance is intersected with a plane, the cross section, whose area is A, will have holes in it. The effective cross-sectional area,
The theory of poroelasticity has its roots in the empirical work of Henry Philibert Gaspard Darcy, a water engineer tasked in 1856 with evaluating the many elaborate public fountains within the city of Dijon, France. Darcy, who had been working as a water engineer for nearly three decades, was pivoting towards pure research, and used this as an opportunity to write his 600 page magnum opus Les Fontaines Publiques de la Ville de Dijon, wherein he describes the sand column experiments pictured in Figure 2 [38]. By examining the flow of water through sand in cylindrical pipes, Darcy discovered a linear proportionality between the rate of fluid volume leaving the bottom of a pipe,
where L is the length of the pipe, and A its cross-sectional area. Hydraulic conductivity is the name given to the constant of proportionality, K, and for water flowing through sand was measured to range from
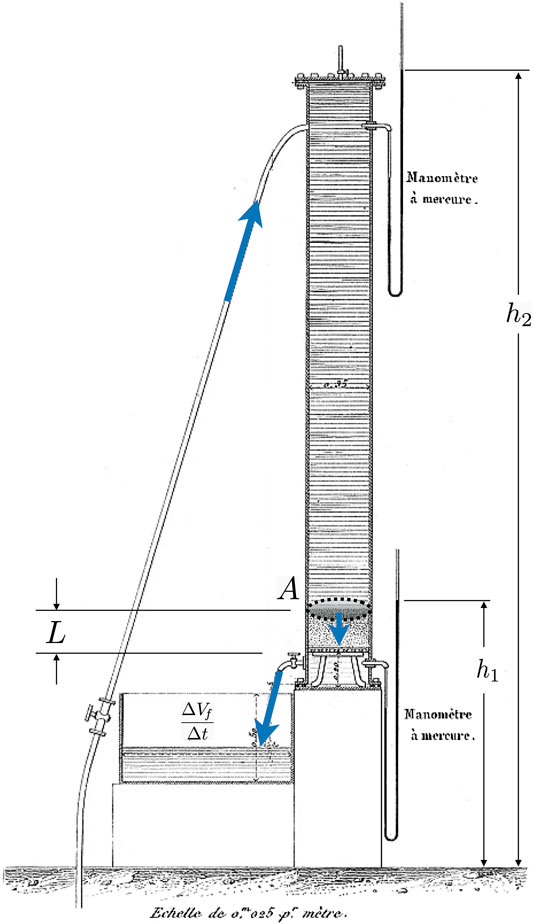
FIGURE 2. The setup of one of the sand column experiments, from plate 24, Figure 3 of Darcy’s 1856 Les Fontaines Publiques de la Ville de Dijon [38]. Annotations have been added to clarify the origin of the physical quantities appearing in Eq. (1). The piezometric heads
A modern view of Eq. (1) utilizes the instantaneous rate of fluid volume moving through a cylinder at constant velocity,
where two clarifications are in order. (1) The LHS is the Darcy velocity (or filtration velocity),
For an isotropic medium, such as Darcy’s sand column,
Karl von Terzaghi, a mechanical engineer, laid out the dynamics of structure consolidation on soil in a series of papers [45–47] between 1922 and 1925, extending Darcy’s insights to become the father of soil mechanics. He incorporated the dynamics of soil in response to the fluid by introducing an effective stress tensor,
Here
Though revolutionary in application, Terzaghi’s assumptions were ill suited for generality. Thermodynamically speaking, his model contains the pair of dynamical variables
is conjugate to the former, where
Maurice Antony Biot, an applied physicist, shed light on the correct coupling in 1941 by introducing [51] the variation in relative fluid content,
where
Here, we see the fluid source,
In Terzaghi’s analysis, bulk volume change is driven by rearrangements of the pore space and flow of the fluid. Biot realized that this physics may be true for nearly incompressible sand grains, but for a softer elastic medium one should also take into account the contribution of solid compressibility to the bulk volume change. An applied isotropic load can now change the volume of the bulk by altering the volume of the solid skeleton due to the fluid pushing back - for reasonably small volume changes the effective support of the fluid with regards to the bulk is then less than the fluid pressure. Biot argued that the correct effective stress should be a modification of Eq. (3),
with the Biot effective stress coefficient, α, a function of the drained effective bulk modulus and the unjacketed solid bulk modulus, K and
To clarify, experimentally the unjacketed bulk modulus is measured in undrained conditions wherein a sample is submerged in fluid; the fluid pressure is then allowed to vary and the volume change of the sample is measured. A jacketed test, on the other hand, is done under drained conditions wherein a dry sample is wrapped in a membrane and connected to the atmosphere via a small hole; a load is then applied under constant atmospheric pressure, and the volume change measured. In aerated soil
Examining the effective potential can further illuminate the role of α - it is the dimensionless coupling between the volumetric strain of the skeleton and the variation in relative fluid content. Since it and ζ are both dimensionless, the Biot modulus, M, must be introduced to describe the energy density coming from the relative fluid content and its coupling to the elastic matrix, allowing the quadratic potential energy density for a linear poroelastic medium to be written as
where
Combining Eq. (10) and Eq. (11) recovers the Biot decomposition, Eq. (7). These expressions clarify the interpretation of M - it is the change in pore pressure caused by a variation in water content under the constraint of no solid dilation,
Biot, and others, explored the physical meaning of both α and M in a number of manuscripts [53, 60, 61]. The effective potential, Eq. (9), can also be constructed beginning at microscopic scales to justify the expression through homogenization theory, coarse graining, or mixed micro-macro formulations [41–43, 62–64].
We now use the effective stress and pressure to write the coupled equations of motion for the effective medium and relative fluid flow components in the absence of external stresses. The equations of motion can be derived via the principle of least action [42, 44, 62], however that would take us too far afield, so we introduce them qualitatively. Momentum conservation for the effective medium reads
where the LHS is the total change in inertia of both solid and fluid. If we define the effective density as
Similarly, momentum conservation for the fluid reads
The LHS here is the change in fluid inertia; however a mass density,
Note the inverse dependence on hydraulic permeability - the more easily the solid permits fluid flow the less dissipation occurs. Using the definition of the filtration velocity, Eq. (14), can be written as a generalization of Darcy’s Law, Eq. (2),
which now includes the inertial forces coming from the motion of the solid skeleton, as well as the fluid’s interactions with the pore space. Accordingly, we drop the subscript on the solid displacement,
The system of Eqs. (6, 13, 14) together with the constitutive relations Eqs. 10, 11 are a more suitable continuum model of tissues containing a mobile interstitial fluid component than standard viscoelastic continua. Since MRE subjects tissue to oscillatory driving conditions at constant frequency, the system can be simplified by Fourier transforming into frequency space - linearity assures us that distinct temporal modes evolve independently. The driving frequency, ω, and all of its harmonics,
The decomposition acts formally by replacing
together with the transformed constitutive relations. Here, we have introduced the poroelastic β factor, a key ingredient in differentiating poroelastic and viscoelastic dynamics. In a static and constant gravitational field we of course have that
We want to connect the MR images of tissue to this model, so we assume that the MRI phase contrast displacement measurements acquired by MRE trace the motion of the elastic skeleton. Since fluid gives a strong MR signal, this assumption relies on there being significant amounts of fluid in the disconnected pore space, acting as part of the bulk. Since fluid pressure within tissue is critical for homeostasis, we should reduce our system of equations further by eliminating all variables except the pair
After some lengthy algebra, one finds a vector and scalar family of equations for each harmonic,
Up to this point we have not imposed assumptions on the spatial distribution of the elastic moduli in the constitutive relations, or the hydraulic permeability. We do so now by assuming that the underlying solid and fluid are nearly incompressible relative to the bulk, namely that
The vector equation shares some resemblance to viscoelasticity - the main differences being the presence of the complex
Analytical solutions of the equations of motion exist for highly idealized geometries [41, 44, 62]; in the context of classical clinical MRE such idealizations do not exist, hence computational methods are required to find approximate numerical solutions [69]. We use a finite element forward solver, described in detail elsewhere [35, 37]. It exploits multiple meshes - displacement fields can be supported on either tetrahedral or hexahedral element meshes, while material properties, such as the shear modulus or hydraulic permeability, are supported on separate hexahedral meshes [70]. Supporting each property on its own mesh provides versatility. It decouples the resolution of the unknown properties being inferred from the mesh resolution of the (
In MRE we observe the displacement field of the tissue being examined with little or no knowledge of the underlying mechanical properties of the tissue. This inverse problem of using the observation to infer the properties, is ideal for the application of a powerful epistemological tool known as Bayesian inference.
The Bayesian interpretation of probability brings with it many tools, some of which have started to be used by the MRE community [71, 72]. The fundamental idea behind it is simple and intuitive - observation carries with it information, and information leads to a reduction in uncertainty [73–77]. Beliefs evolve in order to reduce uncertainty, thereby maximizing the fitness of a cognizant being within an environment that is sufficiently complex to be indistinguishable from one behaving pseudorandomly. Note that, in a Bayesian setting, belief/plausibility are synonymous with probability, and in what follows the terms will be used interchangeably [78, 79].
Applying these ideas to MRE, let us fix a particular mechanical model of tissue so that the forward problem is specified. The space of all possible material property fields is denoted
The posterior and prior are connected via bayes’ theorem
where the quantity multiplying the prior is the likelihood of the observation given a model. Note that observations that are more likely in the context of a set of material properties,
Specifying a poroelastic model with parameters
MRE data acquisition images steady state vibration fields using phase sensitive MRI sequences [28, 83–87]. Displacements are measured at a number of times across the harmonic motion cycle, which are processed into a sequence of displacements at each voxel and the fundamental mode of the time series is computed to give
With
with the
where the
There are, of course, multiple ways to attack this particular question, but they all have in common the theme of sliding down the cost surface. If the cost surface is convex, such a slide will eventually get us to the most plausible set of parameters. The idea can be broken down as follows: Let’s say that we are at some arbitrary model
Our MRE platform has several of these methods implemented - descent direction can be computed via Gauss-Newton, Conjugate Gradient, or Quasi-Newton updates [37]. Travel distance is modulated by a binary line search once the descent direction is found. Computation of gradients depends not only on the log likelihood, but also on prior information content - the regularizer. Multiple regularization terms have been implemented to take into account prior knowledge on material property limits, their spatial distribution, and smoothness [35, 91, 92].
To manage the computational resources required for multiple solutions of the 3D finite element forward problem required for inference, an efficient zoning strategy has been developed [93, 94] that breaks up the domain of interest into smaller overlapping zones. Measured displacements on the boundary voxels of zones provide Dirichlet boundary conditions, leading to a similar inference problem on a smaller scale. Computational complexity in our MRE-suite for the finite element solution of Eqs. (23) and (24) on a cubic zone of side length L scales as
Convergence of any combination of the above methods is tricky to gauge due to the immense dimensionality of
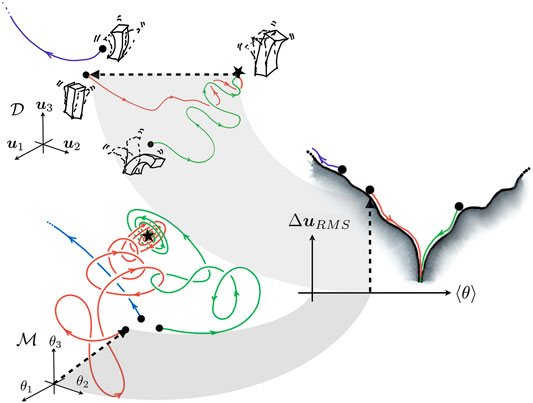
FIGURE 3. A geometric view of the cost surface’s lower dimensional representation. The trajectories in
We use our MRE computational platform - a finite element code capable of simulating displacement fields in poroelastic materials, and an artificial epistemic agent running Bayesian inference on measured displacement fields to extract poroelastic properties. The former situation is referred to as the forward problem, MREf, while the latter the inverse problem, MREi.
The following tests examine MREi’s ability to reconstruct spatial maps of both the hydraulic permeability, k, and the real shear modulus, μ. First we examined our code’s baseline ability to infer distributions of a single property at a time. Next we considered the more complex requirement of inferring both material property distributions simultaneously. Simulated data were generated via MREf, followed by an exploration of the parameter space to see how initial conditions affect material property inference and find a range of starting conditions which converge to the known ground truth.
Reconstructing the shear modulus or the hydraulic permeability one at a time was tested through configurations depicted in Figure 4(10 Hz) and Figure 7(1 Hz) - a long rectangular block containing two conical inclusions. For both frequencies we tested two distinct scenarios. In the first scenario, inclusions were assigned shear modulus contrast. Relative to the background shear modulus
FIGURE 4. (A) Boundary conditions on the top/sides(I) and bottom(
Referencing Figure 4 and Figure 7, we chose
We report results the results for both frequencies,
Material property values used in MREf are summarized in Table 1. Bulk density was chosen to be similar to brain tissue, while fluid density and dynamic viscosity were selected to fall in the range of cerebrospinal fluid [96–100]. The apparent density is a coarse grained effective quantity coming from the interactions between the fluid and the pore space, and we chose a value based on prior studies, noting that a low sensitivity of results to
Inference uses a coarser material property mesh in order to ensure enough constraints to solve the equations of motion. A side effect of the forward and inverse meshes not being identical is that there is no zero error solution. Here we have a 2.0 mm cubic lattice mesh, with 1700 vertices and 1296 volume elements.
We explore the question of how close to the true mean our initial values have to be in order to ensure convergence by examining ranges spanning
These expressions are, of course, only approximate for a discrete mesh - deviations on the order of 1% were observed for our constructed meshes. Since these values are so close to the background fields due to the tiny volume occupied by the inclusions, we considered an inversion successful if the measured mean property fell near the region between the background and mean property values.
We initialize inference with a homogeneous material property distribution of either
The inversion used zones with 10% overlap. Zones were large enough that approximately 500 non-boundary nodes existed in each zone, with about 60 nodes in each zone overlapping with other zones. Gaussian smoothing regularization (spatial filtering) was applied, with the smoothing radius shrinking from 5 to 0.5 mm over the first 50 iterations and held constant for the remaining iterations. A conjugate gradient descent method was used for iterative inference on each zone. During the first 20 global iterations, each zone finds a single CG direction, and the distance moved is computed through a single iteration of a secant linesearch. The next 30 iterations use two of each, and the remaining use three.
Results of inference are summarized for the 10 Hz data in Figure 5 for μ and Figure 6 for k. For the 1 Hz data, the shear modulus results are shown in Figure 8 and the hydraulic permeability in Figure 9.
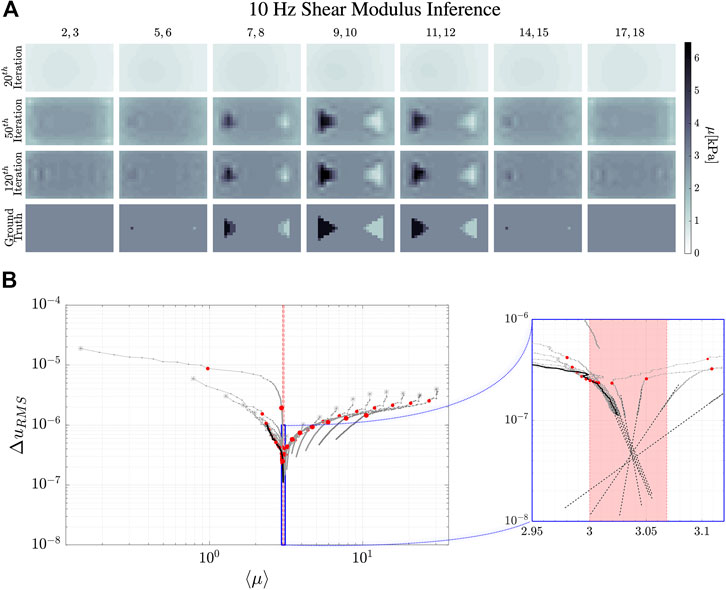
FIGURE 5. Reconstruction evolution of the shear modulus in the 10 Hz simulation. (A) shows the reconstructed images of the shear modulus distribution inside the simulated phantom. The column images are averages of the numbered
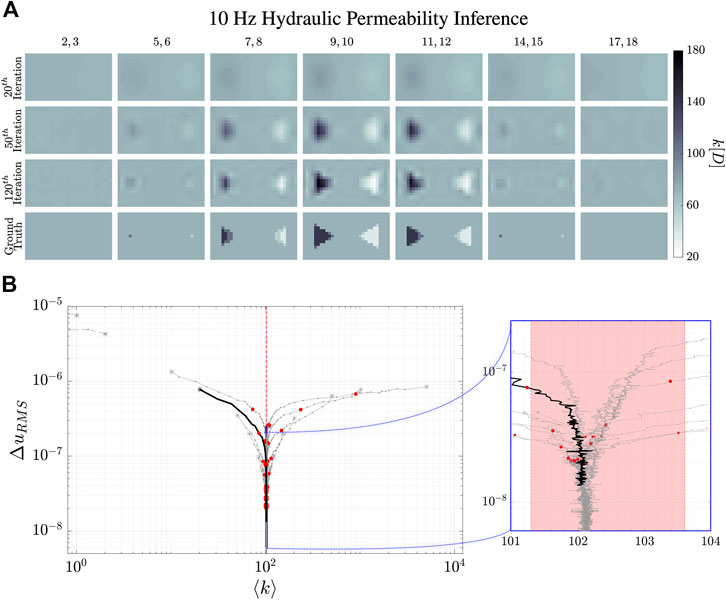
FIGURE 6. Reconstruction evolution of the hydraulic permeability, k, at 10 Hz. (A) shows the reconstructed images of the hydraulic permeability distribution. The column images are the averages of the numbered
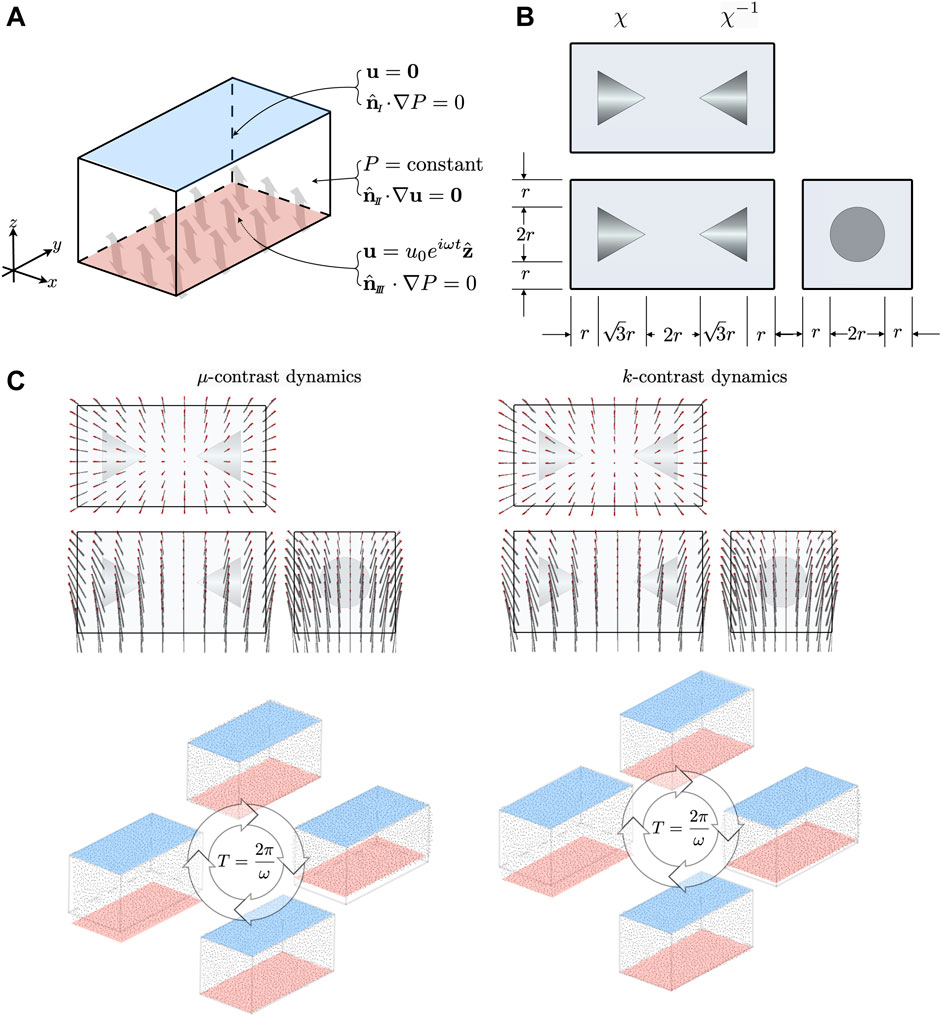
FIGURE 7. (A) Boundary conditions on the top(I), side(
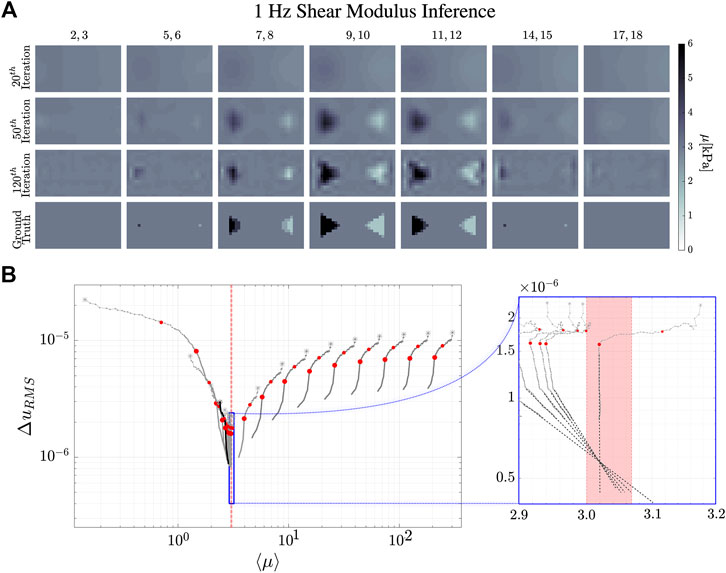
FIGURE 8. Reconstruction evolution of the shear modulus, μ, at 1 Hz. (A) shows the reconstructed images of the shear modulus distribution inside the simulated phantom. The column images are the averages of the numbered
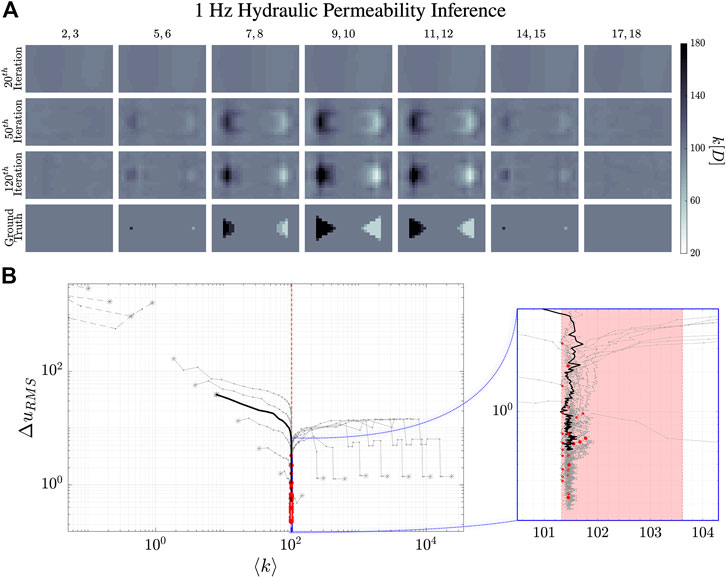
FIGURE 9. Reconstruction evolution of the hydraulic permeability, k, at 1 Hz. (A) shows the reconstructed images of the hydraulic permeability distribution. The column images are the averages of the numbered
MREf simulation of combined shear modulus and hydraulic permeability contrast involved the configuration depicted in Figure 10 - a square block containing eight inclusions. The inclusions are spherically symmetric, and have material property multipliers of either
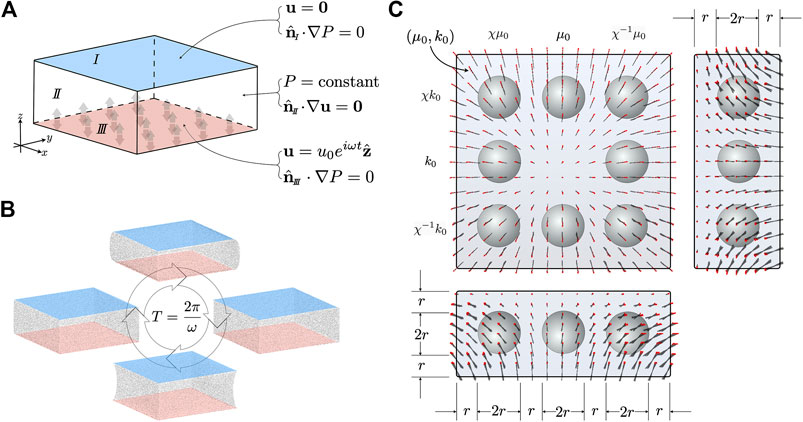
FIGURE 10. (A) Boundary conditions on the top(I), side(
Referring to Figure 10, We chose
Boundary conditions were imposed to simulate the top face of the block being held fixed, while the bottom face was vibrated in the vertical direction,
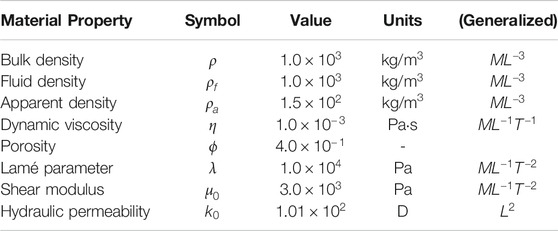
TABLE 1. The first six material properties were used in both MREf and MREi. The last two are background values used only in MREf. Inclusions have multipliers relative to background values as discussed in the text. In MREi, the last two properties, shear modulus and hydraulic permeability, are inferred not specified.
MREi deployed a coarser material property mesh than MREf - a 2.5 mm cubic mesh with 1792 vertices and 1350 elements. Each inclusion sampled 12–13 material mesh nodes.
We examined the effects of initial conditions on reconstruction fidelity by applying 20 different homogeneous initial conditions around the mean values, with the shear modulus ranging between
Once again, these were approximate values on the discrete mesh, but fell within
The first 10 global iterations used a single CG direction and a single secant line search for computing total distance traversed in parameter space while the next 30 applied two of each, and the final 250 invoked three of each. The first 50 iterations used Gaussian smoothing regularization (spatial filtering) on both properties, with the Gaussian standard deviation shrinking linearly from 5 to 0.5 mm. If the change in the cost fell below
Evolution of image reconstruction is summarized in Figure 11 and the lower dimensional representation of the inference trajectory is in Figure 12.
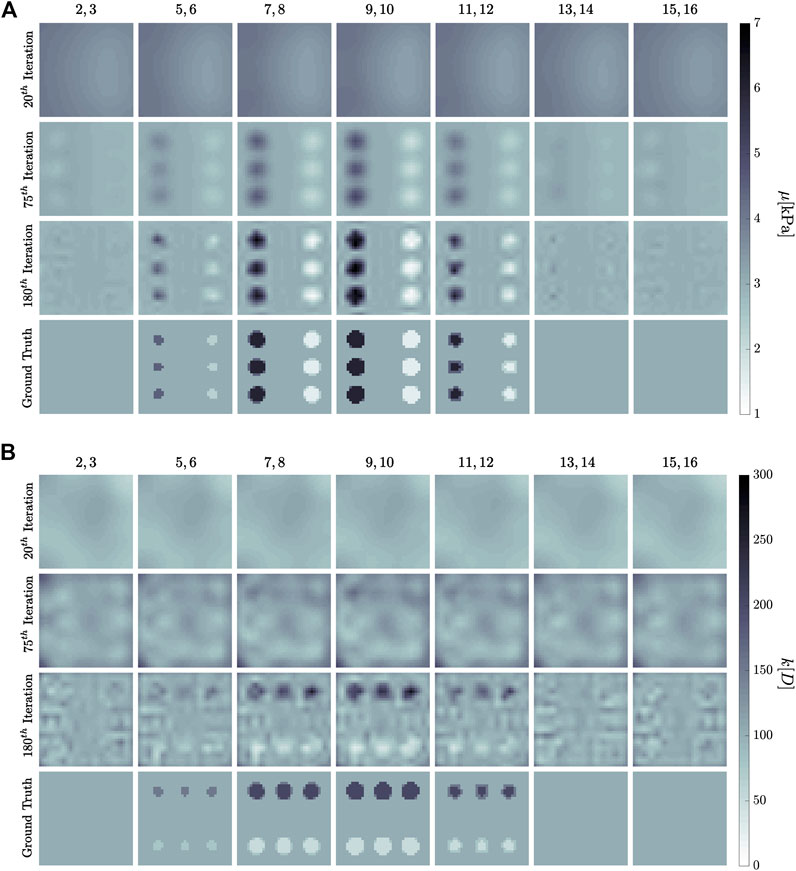
FIGURE 11. Simultaneous inference evolution of both (A) shear modulus, μ, and (B) hydraulic permeability, k, in the 1 Hz experiment setup in Figure 10. The former is measured in kilo-Pascal, while the latter in Darcy. Columns are averages of the numbered
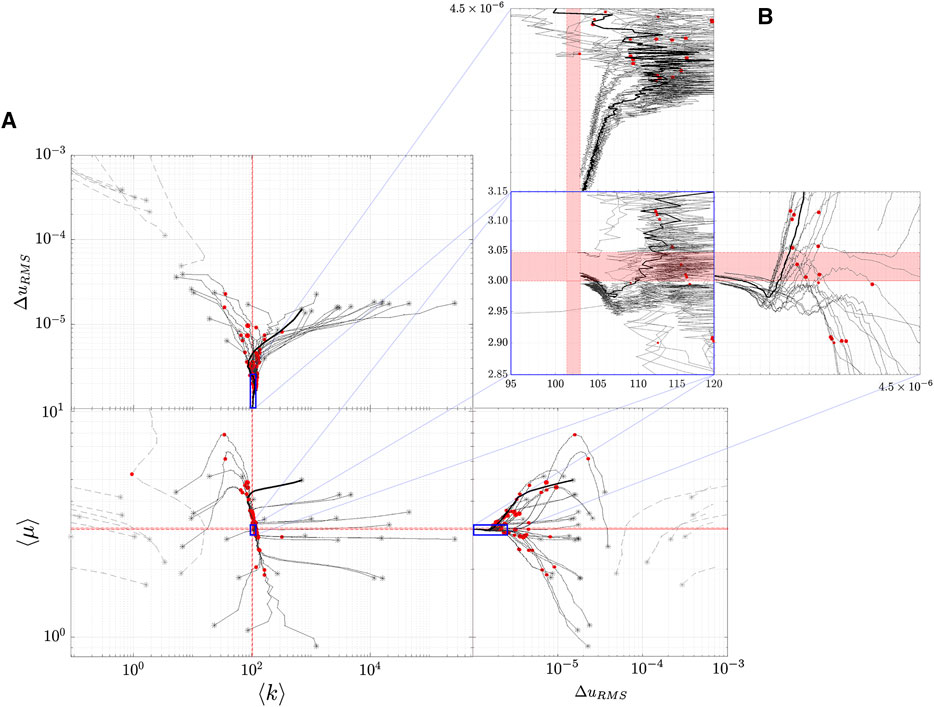
FIGURE 12. Lower dimensional representation of the inference trajectories. Note that because two properties are being reconstructed, the lower dimensional representation is 2-dimensional. This plot conveys the trajectories through an orthographic projection. (A) shows a large region of
The dynamical evolution of our MREi code for single property inference and image reconstruction is displayed in Figure 6 and Figure 7 for
The
Projecting onto the mean field error surface, hydraulic permeability approaches the correct mean field much earlier than shear modulus. Initial values do not affect the late stage reconstruction of the former when they are
The results of the simultaneous property convergence appear in Figure 11 and Figure 12. The former shows the fidelity of image reconstruction, while the latter inference trajectories as material properties are updated at each global iteration. Interestingly, the hydraulic permeability enters its critical strip well before the shear modulus. Mean value convergence, however, is not a strong indicator of image fidelity. The shear modulus inclusion geometry is discernible by the 75th iteration, whereas nearly twice as many iterations are required before hydraulic permeability inclusion geometry begins to resemble ground truth. Furthermore, dependency between the two properties can be observed near the edges of the simulation - hydraulic permeability reconstruction has some vertical patterning that resembles the shear inclusions.
Interestingly, we find that the k-reconstruction trajectories always approach the critical strip from above. Inference tends to overestimate hydraulic permeability with several iterations, and then drives it down. As in the case of single property inference, k initial conditions have a lower limit of about
Prior work [36, 107] has performed similar dual property reconstructions. These results, however, had MREi initial conditions very close to ground truth values, utilized the same property mesh for MREi as used for MREf, and required large amount of regularized smoothing. Our work shows that the first two assumptions can be discarded, and the third relaxed significantly, while maintaining high fidelity for image reconstruction.
There seems to be some confusion in the literature between the terms hydraulic conductivity and hydraulic permeability, with many authors using the two interchangeably. We focused early in Section 2 on making a clear distinction between the two in order to emphasize the specific contributions to fluid flow coming from the viscosity of the fluid and the geometry of the solid. Cerebrospinal fluid has a viscosity ranging between
Our motivation for simulated values of porosity and hydraulic permeability rests on a general lack of knowledge by the scientific community of in vivo values of these parameters. In vitro experiments tend to find small values for both, which we hypothesize is due to pore collapse in sampled tissue. It is easy to imagine that once tissue is removed from the body, the pressure change and unjacketing of the sample results in fluid being expelled. The drop in pore pressure is such that the stresses in the elastic matrix are incapable of supporting the pore space under the influence of gravity, leading to collapse. Once pores are closed, surface to surface cellular interactions may prevent the process from being reversible. Even simple models like the Carman-Kozeny [110, 111] relationship between hydraulic permeability and porosity,
The shear modulus, on the other hand, is much better understood, with far less variance in the literature. In viscoelastic models, white matter typically falls in the range of
The introduction of
One might wonder what happens when we attempt to infer more properties. Unfortunately, as the number of properties increases, the coarseness of the material meshes must also increase. This is simply due to matching displacement constraints coming from MR measurements to the property degrees of freedom in the equations of motion. Theoretical considerations aside, our numerical suite has a much harder time converging with the addition of an extra property to infer - a significant contraction of the domain of convergence being observed. We believe that this is a numerical issue, not a theoretical one, and an active research direction within our group.
As we move forward with in vitro and in vivo testing, our in silico results give us a good picture at how ignorant we can be when guessing our background values. Hydraulic permeability reconstruction for noiseless data is robust to initial conditions ranging from an order of magnitude below, to
Being able to image multiple poroelastic parameters will be a boon for preclinical diagnosis. Getting our computational suite to work in vivo will allow us to apply poroelasticity to the brain, where both cerebro-spinal fluid and blood play an active roll in homeostasis. The robustness under a wide range of initial conditions at low frequencies bodes well for using intrinsic actuation via the cardiac cycle. Tumors tend to have shear moduli between one and two orders of magnitude higher than surrounding tissue - these will be as visible to poroelastic parameter reconstruction as they are for viscoelasticity. The movement of fluid in the brain is critical in several venues. It is a mixture blood movement and interstitial fluid movement over the scale of a fraction of a voxel. We expect the dominant scale of fluid flow observed in MRE will be between that of perfusion which observes macroscopic bulk blood flow over a fraction of a meter and that of diffusion which observes microscopic fluid flow on a cellular scale. Observing fluid flow in this scale has potential to complement diffusion in characterizing edema and dementia. We postulate this scale of fluid flow will also illuminate tissue viability before diffusion becomes relevant allowing us to better identify and characterize reversible tissue damage. It might also provide additional information about functional changes secondary to brain activity.
It is an exciting time to watch as MRE enters it’s third decade of application. As interdisciplinary as its roots are, it is no surprise that with maturity the field is digging deeper into the fertile soil of its disciplinary constituents. Poroelasticity has been one of the crowning theoretical achievements of the geophysics community, and like every good idea, is ripe for cross-pollination. Perhaps it is no surprise that a model developed to describe rocks and soils is finding use in describing living tissue when in 1510 Leonardo da Vinci penned [115], into his Codex Leicester, that The Earth is a living body. Its flesh is the soil, its bones are the strata of rock, its cartilage is the tufa, its blood is the underground streams, the reservoir of blood around its heart is the ocean, the systole and diastole of the blood in the arteries and veins appear on the Earth as the rising and sinking of the oceans.
Simulations were performed using an in-house FORTRAN MRE Computational Platform written and developed over the past decade by group members. Both forward and inverse problems were computed on the Discovery Cluster, a 3000+ core Linux cluster at Dartmouth College administered by Research Computing. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The raw data supporting the conclusions of this article will be made available under reasonable request by the corresponding author, without undue reservation.
DS ran the simulations, did the data analysis, and wrote the manuscript with input and guidance from MM, SG, JW, and KP. MM and EH wrote the FEM code used for the simulations.
This work was supported by the National Institute for Health Research Grant (No. R01EB027577-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Research Computing at Dartmouth for their ever present ability with troubleshooting our cluster usage, and wish Susan Schwarz an adventurous retirement.
1. Dresner MA, Rose GH, Rossman PJ, Muthupillai R, Manduca A, Ehman RL. Magnetic resonance elastography of skeletal muscle. J Magn Reson Imaging (2001) 13:269–76. doi:10.1002/1522-2586(200102)13:2<269::AID-JMRI1039>3.0.CO;2-1
2. Sack I, Bernarding J, Braun J. Analysis of wave patterns in mr elastography of skeletal muscle using coupled harmonic oscillator simulations. Magn Reson Imaging (2002) 20:95–104. doi:10.1016/S0730-725X(02)00474-5
3. Bensamoun SF, Ringleb SI, Littrell L, Chen Q, Brennan M, Ehman RL, et al. Determination of thigh muscle stiffness using magnetic resonance elastography. J Magn Reson Imaging (2006) 23:242–7. doi:10.1002/jmri.20487
4. Ringleb SI, Bensamoun SF, Chen Q, Manduca A, An KN, Ehman RL. Applications of magnetic resonance elastography to healthy and pathologic skeletal muscle. J Magn Reson Imaging (2007) 25:301–9. doi:10.1002/jmri.20817
5. Chen Q, Manduca A, An K. Characterization of skeletal muscle elasticity using magnetic resonance elastography. Biomedical Applications of Vibration and Acoustics in Imaging and characterizations. New York: ASME Press (2008). doi:10.1115/1.802731.ch8
6. Klatt D, Papazoglou S, Braun J, Sack I. Viscoelasticity-based MR elastography of skeletal muscle. Phys Med Biol (2010) 55:6445. doi:10.1088/0031-9155/55/21/007
7. Aarabon N, Kozinc A, Podrekar N. Using shear-wave elastography in skeletal muscle: a repeatability and reproducibility study on biceps femoris muscle. PLoS One (2019) 14:1–13. doi:10.1371/journal.pone.0222008
8. Yin L, Lu R, Cao W, Zhang L, Li W, Sun H, et al. Three-dimensional shear wave elastography of skeletal muscle: preliminary study. J Ultrasound Med (2018) 37:2053–62. doi:10.1002/jum.14559
9. Nelissen JL, Sinkus R, Nicolay K, Nederveen AJ, Oomens CWJ, Strijkers GJ. Magnetic resonance elastography of skeletal muscle deep tissue injury. NMR Biomed (2019) 32:e4087. doi:10.1002/nbm.4087. E4087 NBM-18-0096.R2
10. McKnight AL, Kugel JL, Rossman PJ, Manduca A, Hartmann LC, Ehman RL. MR elastography of breast cancer: preliminary results. AJR Am J Roentgenol (2002) 178:1411–7. doi:10.2214/ajr.178.6.1781411
11. Lorenzen J, Sinkus R, Lorenzen M, Dargatz M, Leussler C, Röschmann P, et al. MR elastography of the breast:preliminary clinical results. Rofo (2002) 174:830–4. doi:10.1055/s-2002-32690
12. Van Houten EE, Doyley MM, Kennedy FE, Weaver JB, Paulsen KD. Initial in vivo experience with steady-state subzone-based MR elastography of the human breast. J Magn Reson Imaging (2003) 17:72–85. doi:10.1002/jmri.10232
13. Xydeas T, Siegmann K, Sinkus R, Krainick-Strobel U, Miller S, Claussen CD. Magnetic resonance elastography of the breast: correlation of signal intensity data with viscoelastic properties. Invest Radiol (2005) 40:412–20. doi:10.1097/01.rli.0000166940.72971.4a
14. Asbach P, Klatt D, Hamhaber U, Braun J, Somasundaram R, Hamm B, et al. Assessment of liver viscoelasticity using multifrequency mr elastography. Magn Reson Med (2008) 60:373–9. doi:10.1002/mrm.21636
15. Hawley JR, Kalra P, Mo X, Raterman B, Yee LD, Kolipaka A. Quantification of breast stiffness using MR elastography at 3 Tesla with a soft sternal driver: a reproducibility study. J Magn Reson Imaging (2017) 45:1379–84. doi:10.1002/jmri.25511
16. Bohte AE, Nelissen JL, Runge JH, Holub O, Lambert SA, de Graaf L, et al. Breast magnetic resonance elastography: a review of clinical work and future perspectives. NMR Biomed (2018) 31:e3932. doi:10.1002/nbm.3932
17. Balleyguier C, Lakhdar A, Dunant A, Mathieu M, Delaloge S, Sinkus R. Value of whole breast magnetic resonance elastography added to mri for lesion characterization. NMR Biomed (2018) 31:e3795. doi:10.1002/nbm.3795
18. Rouvière O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, et al. MR elastography of the liver: preliminary results. Radiology (2006) 240:440–8. doi:10.1148/radiol.2402050606
19. Yin M, Woollard J, Wang X, Torres VE, Harris PC, Ward CJ, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med (2007) 58:346–53. doi:10.1002/mrm.21286
20. Motosugi U, Ichikawa T, Sano K, Sou H, Muhi A, Koshiishi T, et al. Magnetic resonance elastography of the liver: preliminary results and estimation of inter-rater reliability. Jpn J Radiol (2010) 28:623–7. doi:10.1007/s11604-010-0478-1
21. Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging (2013) 37:544–55. doi:10.1002/jmri.23731
22. Chen J, Yin M, Glaser KJ, Talwalkar JA, Ehman RL. MR elastography of liver disease: state of the art. Appl Radiol (2013) 42:5–12.
23. Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology (2018) 286:738–63. doi:10.1148/radiol.2018170601
24. Hoodeshenas S, Yin M, Venkatesh SK. Magnetic resonance elastography of liver: current update. Top Magn Reson Imaging (2018) 27:319–33. doi:10.1097/RMR.0000000000000177
25. Xu L, Lin Y, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of the human brain: a preliminary study. Acta Radiol (2007) 48:112–5. doi:10.1080/02841850601026401
26. Kruse SA, Rose GH, Glaser KJ, Manduca A, Felmlee JP, Jack CR, et al. Magnetic resonance elastography of the brain. Neuroimage (2008) 39:231–7. doi:10.1016/j.neuroimage.2007.08.030
27. Gierke HE, Oestreicher HL, Franke EK, Parrack HO, Wittern WW. Physics of vibrations in living tissues. J Appl Physiol (1952) 4:886–900. doi:10.1152/jappl.1952.4.12.886
28. Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal (2001) 5:237–54. doi:10.1016/s1361-8415(00)00039-6
29. Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat (2010) 23:497–511. doi:10.1002/ca.21006
30. Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. AN overview OF elastography - an emerging branch OF medical imaging. Curr Med Imaging Rev (2011) 7:255–82. doi:10.2174/157340511798038684
31. Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and mr elastography for assessing liver fibrosis: Part 1, principles and techniques. AJR Am J Roentgenol (2015) 205:22–32. doi:10.2214/AJR.15.14552
32. Low G, Kruse SA, Lomas DJ. General review of magnetic resonance elastography. World J Radiol (2016) 8:59–72. doi:10.4329/wjr.v8.i1.59
33. Bayly PV, Garbow JR. Pre-clinical mr elastography: principles, techniques, and applications. J Magn Reson (2018) 291:73–83. doi:10.1016/j.jmr.2018.01.004
34. Perriñez P, Marra S, Kennedy F, Paulsen K. “3D finite element solution to the dynamic poroelasticity problem for use in MR elastography,” in Medical imaging 2007: physiology, function, and structure from medical images. Editors A Manduca, and XP Hu, Washington: International Society for Optics and Photonics (SPIE) (2007), 6511, 402–12. doi:10.1117/12.709786
35. Perriñez PR, Kennedy FE, Van Houten EE, Weaver JB, Paulsen KD. Magnetic resonance poroelastography: an algorithm for estimating the mechanical properties of fluid-saturated soft tissues. IEEE Trans Med Imaging (2010) 29:746–55. doi:10.1109/TMI.2009.2035309
36. Pattison AJ, McGarry M, Weaver JB, Paulsen KD. Spatially-resolved hydraulic conductivity estimation via poroelastic magnetic resonance elastography. IEEE Trans Med Imaging (2014) 33:1373–80. doi:10.1109/TMI.2014.2311456
37. Tan L, McGarry MD, Van Houten EE, Ji M, Solamen L, Weaver JB, et al. Gradient-based optimization for poroelastic and viscoelastic MR elastography. IEEE Trans Med Imaging (2017) 36:236–50. doi:10.1109/TMI.2016.2604568
38. Darcy H. Les Fontaines publiques de la ville de Dijon. Exposition et application des principes à suivre et des formules à employer dans les questions de distribution d’eau, etc (Paris: V. Dalamont) (1856).
39. Brown G. Henry Darcy and the making of a law. Water Resour Res (2002) 38 11.1–11.12. doi:10.1029/2001WR000727
42. Lopatnikov S, Cheng A. Macroscopic Lagrangian formulation of poroelasticity with porosity dynamics. J Mech Phys Solid (2004) 52:2801–39. 10.1016/j.jmps.2004.05.005
43. Bear J, Cheng A. Modeling groundwater flow and contaminant transport. Dordrecht: Springer, Vol. 23 (2010), 834. doi:10.1007/978-1-4020-6682-5
44. Cheng A. Poroelasticity. Theory and applications of transport in porous media. New York: Springer International Publishing (2016).
47. Terzaghi K. Theoretical soil mechanics. New Jersey: John Wiley & Sons Incorporated (1943). doi:10.1002/9780470172766
49. Wilson K. Renormalization group and critical phenomena. I. Renormalization group and the Kadanoff scaling picture. Physical review B (1971) 4:3174.
50. Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, De Vries AH. The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B (2007) 111:7812–24. doi:10.1021/jp071097f
51. Biot M. General theory of three-dimensional consolidation. J Appl Phys (1941) 12:155–64. doi:10.1063/1.1712886
52. Detournay E, Cheng A. Fundamentals of poroelasticity. Analysis and design methods. Netherlands: Elsevier (1993), 113–71.
54. Bishop A. The influence of an undrained change in stress on the pore pressure in porous media of low compressibility. Geotechnique (1973) 23:435–42.
55. Plona T. Observation of a second bulk compressional wave in a porous medium at ultrasonic frequencies. Appl Phys Lett (1980) 36:259–61. doi:10.1063/1.91445
56. Biot M. Theory of elasticity and consolidation for a porous anisotropic solid. J Appl Phys (1955) 26:182–5. doi:10.1063/1.1721956
57. Biot M. Theory of propagation of elastic waves in a fluid-saturated porous solid. i. low-frequency range. J Acoust Soc Am (1956a) 28:168–78. doi:10.1121/1.1908239
58. Biot M. Theory of propagation of elastic waves in a fluid-saturated porous solid. ii. higher frequency range. J Acoust Soc Am (1956b) 28:179–91. doi:10.1121/1.1908241
59. Biot M. Nonlinear and semilinear rheology of porous solids. J Geophys Res (1973) 78:4924–37. doi:10.1029/JB078i023p04924
60. Biot M. General solutions of the equations of elasticity and consolidation for a porous material. J Appl Mech (1956c) 78:91–6.
61. Biot M, Willis D. The elastic coefficients of the theory of consolidation. J Appl Mech (1957) 24:594–601.
62. Santos J. Introduction to the theory of poroelasticity. Indiana: Purdue Mathematics Department (2014).
63. Nordbotten J, Celia M, Dahle H, Hassanizadeh S. Interpretation of macroscale variables in Darcy’s law. Water resources research (2007) 43:18. doi:10.1029/2006WR005018
64. Apostolakis G, Dargush G. Mixed variational principles for dynamic response of thermoelastic and poroelastic continua. Int J Solid Struct (2013) 50:642–50. doi:10.1016/j.ijsolstr.2012.10.021
65. Bear J, Bachmat Y. Introduction to modeling of transport phenomena in porous media. Berlin/Heidelberg: Springer Science & Business Media, Vol. 4 (1990).
67. Bonnet G, Auriault J. Dynamics of saturated and deformable porous media. Physics of finely divided matter. Berlin/Heidelberg: Springer (1985), 306–16.
68. Perriñez PR, Kennedy FE, Van Houten EE, Weaver JB, aulsen KD. Modeling of soft poroelastic tissue in time-harmonic mr elastography. IEEE Trans Biomed Eng (2008) 56:598–608. doi:10.1109/TBME.2008.2009928
69. Lynch D. Numerical partial differential equations for environmental scientists and engineers: a first practical course. Berlin/Heidelberg: Springer Science & Business Media (2004).
70. McGarry MD, Van Houten EE, Johnson CL, Georgiadis JG, Sutton BP, Weaver JB, et al. Multiresolution mr elastography using nonlinear inversion. Med Phys (2012) 39:6388–96. doi:10.1118/1.4754649
71. Franck IM, Koutsourelakis P. Sparse variational bayesian approximations for nonlinear inverse problems: applications in nonlinear elastography. Comput Methods Appl Mech Eng (2016) 299:215–44. doi:10.1016/j.cma.2015.10.015
72. Jiang Y, Qian S. Bayesian approach for recovering piecewise constant viscoelasticity from mre data. Acta Mathematicae Applicatae Sinica, English Series (2020) 36:223–36. doi:10.1007/s10255-020-0922-7
74. Jaynes E. Probability theory: the logic of science. Cambridge: Cambridge University Press (2003).
75. Terenin A, Draper D. Cox’s theorem and the jaynesian interpretation of probability. arXiv preprint arXiv:1507.06597 (2015).
76. Sowinski D. Complexity and stability for epistemic agents: the foundations and phenomenology of configurational entropy. New Hampshire: Dartmouth College (2016).
77. Gleiser M, Sowinski D. How we make sense of the world: information, map-making, and the scientific narrative. The Map and the Territory. New York: Springer (2018), 141–63.
78. Van Horn KS. Constructing a logic of plausible inference: a guide to cox’s theorem. Int J Approx Reason (2003) 34:3–24. doi:10.1016/S0888-613X(03)00051-3
79. Caticha A. Lectures on probability, entropy, and statistical physics. arXiv preprint arXiv:0808.0012 (2008).
80. Gelman A, Simpson D, Betancourt M. The prior can often only be understood in the context of the likelihood. Entropy (2017) 19:555. doi:10.3390/e19100555
81. Caticha A, Preuss R. Maximum entropy and bayesian data analysis: entropic prior distributions. Phys Rev E Stat Nonlin Soft Matter Phys (2004) 70:046127. doi:10.1103/PhysRevE.70.046127
82. Shannon C. A mathematical theory of communication. Bell system technical journal (1948) 27:379–423.
83. Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (1995) 269:1854–7. doi:10.1126/science.7569924
84. Sinkus R, Tanter M, Catheline S, Lorenzen J, Kuhl C, Sondermann E, et al. Imaging anisotropic and viscous properties of breast tissue by magnetic resonance-elastography. Magn Reson Med (2005) 53:372–87. doi:10.1002/mrm.20355
85. Sack I, Beierbach B, Hamhaber U, Klatt D, Braun J. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR Biomed (2008) 21:265–71. doi:10.1002/nbm.1189
86. Johnson CL, McGarry MD, Van Houten EE, Weaver JB, Paulsen KD, Sutton BP, et al. Magnetic resonance elastography of the brain using multishot spiral readouts with self-navigated motion correction. Magn Reson Med (2013a) 70:404–12. doi:10.1002/mrm.24473
87. Johnson CL, Holtrop JL, McGarry MD, Weaver JB, Paulsen KD, Georgiadis JG, et al. 3d multislab, multishot acquisition for fast, whole-brain mr elastography with high signal-to-noise efficiency. Magn Reson Med (2014) 71:477–85. doi:10.1002/mrm.25065
88. Wang H, Weaver JB, Doyley MM, Kennedy FE, Paulsen KD. Optimized motion estimation for mre data with reduced motion encodes. Phys Med Biol (2008) 53:2181. doi:10.1088/0031-9155/53/8/012
89. Wang H. Optimizing motion encoding and reconstruction in magnetic resonance elastography. New Hampshire: Dartmouth College (2008).
90. Weaver JB, Pattison AJ, McGarry MD, Perreard IM, Swienckowski JG, Eskey CJ, et al. Brain mechanical property measurement using MRE with intrinsic activation. Phys Med Biol (2012) 57:7275–87. doi:10.1088/0031-9155/57/22/7275
91. McGarry M, Johnson CL, Sutton BP, Van Houten EE, Georgiadis JG, Weaver JB, et al. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE Trans Med Imaging (2013) 32:1901–9. doi:10.1109/TMI.2013.2268978
92. Johnson CL, McGarry MD, Gharibans AA, Weaver JB, Paulsen KD, Wang H, et al. Local mechanical properties of white matter structures in the human brain. Neuroimage (2013b) 79:145–52. doi:10.1016/j.neuroimage.2013.04.089
93. Van Houten EE, Paulsen KD, Miga MI, Kennedy FE, Weaver JB. An overlapping subzone technique for mr-based elastic property reconstruction. Magn Reson Med 42 (1999) 779–86. doi:10.1002/(sici)1522-2594(199910)42:4<779::aid-mrm21>3.0.co;2-z
94. Van Houten EE, Miga MI, Weaver JB, Kennedy FE, Paulsen KD. Three-dimensional subzone-based reconstruction algorithm for mr elastography. Magn Reson Med (2001) 45:827–37. doi:10.1002/mrm.1111
95. McGarry M. Rayleigh damped magnetic resonance elastograpy. New Zealand: University of Canterbury. Mechanical Engineering (2008).
96. Barber TW, Brockway JA, Higgins LS. The density of tissues in and about the head. Acta Neurol Scand (1970) 46:85–92. doi:10.1111/j.1600-0404.1970.tb05606.x
97. Cala LA, Thickbroom GW, Black JL, Collins DW, Mastaglia FL. Brain density and cerebrospinal fluid space size: ct of normal volunteers. AJNR Am J Neuroradiol (1981) 2:41–7.
98. DiResta GR, Lee J, Lau N, Ali F, Galicich JH, Arbit E. Measurement of brain tissue density using pycnometry. Acta Neurochir Suppl (1990) 51:34–6. doi:10.1007/978-3-7091-9115-6_12
99. Bloomfield IG, Johnston IH, Bilston LE. Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr Neurosurg (1998) 28:246–51. doi:10.1159/000028659
100. Yatsushiro S, Sunohara S, Atsumi H, Matsumae M, Kuroda K. Visualization and characterization of cerebrospinal fluid motion based on magnetic resonance imaging. Hydrocephalus: Water on the Brain (2018) 9:73302. doi:10.5772/intechopen.73302
101. Venton J, Bouyagoub S, Harris P, Phillips G. “Deriving spinal cord permeability and porosity using diffusion-weighted MRI data,” in Poromechanics VI (Sixth Biot Conference on Poromechanics), Virginia: American Society of Civil Engineers, Paris, France, July 9‐13, 2017. Sponsored by École des Ponts ParisTech; Instutut Français des Sciences et Technologies des Transports, de l'Aménagement, et des Réseaux (IFSTTAR); Centre National de la Recherche Scientifique (CNRS); Agence Nationale de la Recherche (ANR); and the Engineering Mechanics Institute of ASCE. (2017), 1451–7. doi:10.1061/9780784480779.180
102. Cheng S, Bilston LE. Unconfined compression of white matter. J Biomech (2007) 40:117–24. doi:10.1016/j.jbiomech.2005.11.004
103. Chen X, Sarntinoranont M. Biphasic finite element model of solute transport for direct infusion into nervous tissue. Ann Biomed Eng (2007) 35:2145–58. doi:10.1007/s10439-007-9371-1
104. Støverud K. Modeling convection-enhanced delivery into brain tissue using information from magnetic resonance imaging. Master’s thesis. Netherlands: Utrecht University (2009).
105. Ray L, Heys J. Fluid flow and mass transport in brain tissue. Fluids (2019) 4:196. doi:10.3390/fluids4040196
106. McGarry M, Houten EV, Solamen L, Gordon-Wylie S, Weaver J, Paulsen K. Uniqueness of poroelastic and viscoelastic nonlinear inversion MR elastography at low frequencies. Phys Med Biol (2019) 64:075006. doi:10.1088/1361-6560/ab0a7d
107. McGarry MD, Johnson CL, Sutton BP, Georgiadis JG, Van Houten EE, Pattison AJ, et al. Suitability of poroelastic and viscoelastic mechanical models for high and low frequency MR elastography. Med Phys (2015) 42:947–57. doi:10.1118/1.4905048
108. Schmid-Schönbein H, Wells RE. Rheological properties of human erythrocytes and their influence upon the “anomalous” viscosity of blood. Ergeb Physiol (1971) 63:146–219. doi:10.1007/BFb0047743
109. Gokturk HS, Demir M, Ozturk NA, Unler GK, Kulaksizoglu S, Kozanoglu I, et al. The role of ascitic fluid viscosity in the differential diagnosis of ascites. Can J Gastroenterol (2010) 24:255–9. doi:10.1155/2010/896786
110. Carman P. Fluid flow through granular beds. Chem Eng Res Des (1937) 15:S32–S48. doi:10.1016/S0263-8762(97)80003-2
111. Kozeny J. Uber kapillare leitung der wasser in boden. Royal Academy of Science, Vienna, Proc. Class I (1927) 136:271–306.
112. Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater (2015) 46:318–30. doi:10.1016/j.jmbbm.2015.02.024
113. Sinkus R, Siegmann K, Xydeas T, Tanter M, Claussen C, Fink M. Mr elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced mr mammography. Magn Reson Med (2007) 58:1135–44. doi:10.1002/mrm.21404
114. Tan L, McGarry MDJ, Van Houten EEW, Ji M, Solamen L, Zeng W, et al. A numerical framework for interstitial fluid pressure imaging in poroelastic mre. Plos one (2017) 12:e0178521. doi:10.1371/journal.pone.0178521
Keywords: Elastography, MRI, Biophysics, Biomechanics, Bayesian Inference, Effective Field Theory, Continuum Mechanics, Porous Materials
Citation: Sowinski DR, McGarry MDJ, Van Houten EEW, Gordon-Wylie S, Weaver JB and Paulsen KD (2021) Poroelasticity as a Model of Soft Tissue Structure: Hydraulic Permeability Reconstruction for Magnetic Resonance Elastography in Silico. Front. Phys. 8:617582. doi: 10.3389/fphy.2020.617582
Received: 15 October 2020; Accepted: 04 December 2020;
Published: 21 January 2021.
Edited by:
Jing Guo, Charité – Universitätsmedizin Berlin, GermanyReviewed by:
Sebastian Hirsch, Charité – Universitätsmedizin Berlin, GermanyCopyright © 2021 Sowinski, McGarry, Van Houten, Gordon-Wylie, Weaver and Paulsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Damian R. Sowinski, RGFtaWFuLlNvd2luc2tpQERhcnRtb3V0aC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.