- 1Ultrasound Elasticity Imaging Laboratory, Department of Biomedical Engineering, Columbia University, New York, NY, United States
- 2Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy
- 3Athinoula A. Martinos Center for Biomedical Imaging, Harvard Medical School, Charlestown, MA, United States
Focused ultrasound (FUS) neuromodulation has shown that mechanical waves can interact with cell membranes and mechanosensitive ion channels, causing changes in neuronal activity. However, the thorough understanding of the mechanisms involved in these interactions are hindered by different experimental conditions for a variety of animal scales and models. While the lack of complete understanding of FUS neuromodulation mechanisms does not impede benefiting from the current known advantages and potential of this technique, a precise characterization of its mechanisms of action and their dependence on experimental setup (e.g., tuning acoustic parameters and characterizing safety ranges) has the potential to exponentially improve its efficacy as well as spatial and functional selectivity. This could potentially reach the cell type specificity typical of other, more invasive techniques, e.g., opto- and chemogenetics or at least orientation-specific selectivity afforded by transcranial magnetic stimulation. Here, the mechanisms and their potential overlap are reviewed along with discussions on the potential insights into mechanisms that magnetic resonance imaging sequences along with a multimodal stimulation approach involving electrical, magnetic, chemical, light, and mechanical stimuli can provide.
Introduction
The ability to probe spatially specific brain regions enable understanding brain functioning and connectivity. In turn, this can unlock a wealth of potential investigative and therapeutic applications. Focused ultrasound (FUS) has been proven capable of eliciting excitatory and inhibitory effects non-invasively and locally in the central nervous system (CNS) and peripheral nervous system (PNS), depending on the adopted pulsing regime [1]. Several studies have demonstrated the elicitation of motor responses in rodents obtained from the FUS stimulation of cortical brain regions [2–6]. Furthermore, the capability of FUS to reach deeper brain structures (which is one of the major challenges of other current- or voltage-controlled neuromodulation techniques) can provide access to subcortical areas of the brain. For example, the stimulation of deep-seated structures such as locus coeruleus and superior colliculus caused pupil dilation and eyeball movements in mice [6]. Also, FUS stimulation of the putamen induced improvements in speed and accuracy of visual–motor tasks in non-human primates (NHPs) [7]. In humans, targeting the head of the caudate resulted in hemodynamic responses visible through functional magnetic resonance imaging (fMRI) [8] and stimulating the thalamus induced changes in somatosensory evoked responses [9].
The mechanisms proposed to explain the FUS neuromodulation effects are based on multiple hypotheses on how ultrasound interferes with depolarization through mechanical deformation of the cell membrane. In addition, experimental evidences have shown that ultrasound can activate mechanosensitive ion channels in neurons [10–13] and other brain cell types like astrocytes [14], providing additional avenues for FUS to interfere with the membrane potential. Despite the advances provided by in vitro, ex vivo, and in vivo experiments, the high variability in experimental conditions and setups, as well as partially conflicting results, has led to somewhat contradictory interpretations and a variety of possible hypotheses about underlying physiological mechanisms, which may be acting concurrently in a dynamic interplay every time FUS is applied. Moreover, most current animal experiments are performed under anesthesia. The interaction of pharmacological sedation with FUS neuromodulation is not entirely understood and may partially obfuscate the interpretation of a number of FUS neuromodulation experiments [15].
The use of MRI can provide insights into brain structure and activity and hence support FUS-based neuromodulation through targeting, safety evaluation, and the evaluation of brain function and mechanisms. In this context, multimodal stimulation coupled with neuroelectric or MRI may present a better opportunity to understanding of the multiple factors that play a role in neuron functioning as well as how FUS interferes with it.
In this review, the proposed mechanisms for ultrasound neuromodulation and interactions of FUS with tissue are revisited, and current contradictory findings are discussed in light of varying experimental conditions and anesthesia effects. Finally, the potential of multimodal stimulation and the use of MRI is discussed as a promising future avenue for spatiotemporally selective, non-invasive neuromodulation.
Mechanisms of Ultrasound Neuromodulation
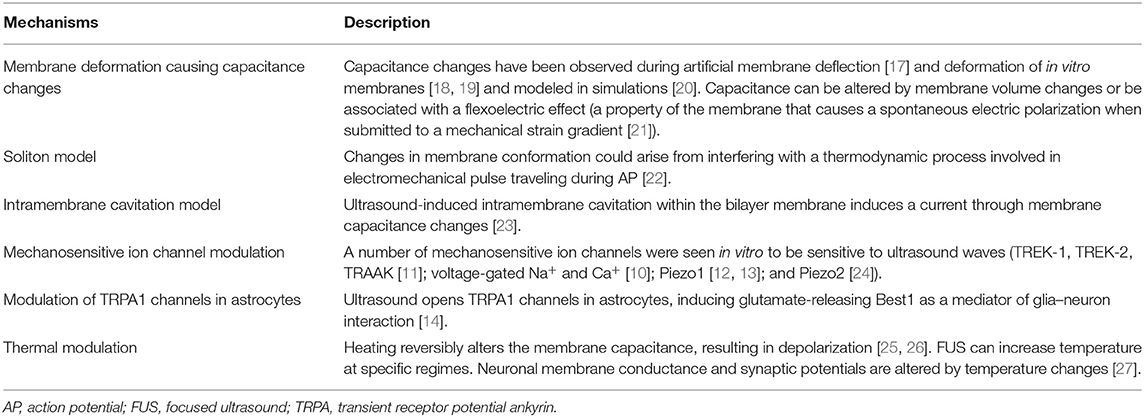
Ultrasound propagation in biological tissue is characterized by vibrational waves traveling with frequencies above the hearing range (>20 kHz). In the compressional phase, ultrasound displaces tissue particles and fluid molecules, generating an elastic restoring force. As the tissue and fluids return to their normal configurations, molecules experience a rarefaction phase. During this process, waves propagate through the tissue, giving rise to an acoustic radiation force (ARF) where part of the energy is stored in the tissue in the form of elastic deformation, and part is dissipated as heat due to viscous frictional forces. When acoustic wave flow experiences opposition due to acoustic impedance discontinuities, parts of the wave are transmitted, reflected, and refracted. Both scattering and heating dissipation are frequency-dependent, where energy deposition in the medium occurs through absorption. The scattered waves can be subsequently partially absorbed and partially re-scattered multiple times. Other effects during the rarefaction phase can occur, such as cavitation (nucleation) [16], which has a higher probability of occurring at higher pressures and lower frequencies. Potential mechanisms for ultrasound neuromodulation are associated with changes in membrane potential due to ultrasound-induced neuronal membrane deformation and the activation of mechanosensitive channels (Table 1) (see Jerusalem et al. [15] for a review). In this context, both theoretical and experimental studies have proposed that mechanical deformations induced by strain gradients produce a membrane polarization, giving rise to a flexoelectric effect [21, 28]. A study using a model lipid bilayer membrane demonstrated that the displacement of the membrane caused by the ARF results in changes in the membrane area and its capacitance, which in turn creates capacitive currents measured with voltage-clamp techniques [17]. Another recent study evoked neuronal calcium responses obtained from local mechanical indentation delivered by a piston in cultured rat cortical and hippocampal neurons [18], giving experimental evidences that neurons are sensitive to mechanical stress. Also, Muratore et al. [19] have shown that ARF can deform the cell membrane. Intriguingly, other theoretical studies have proposed that the rarefactional phase of ultrasound waves can pull apart the two membrane lipid leaflets, leading the formation of bubbles in the intramembrane space, which in turn induces currents by modulating membrane capacitance in an oscillatory manner [23, 29]. However, an ex vivo study has shown that micron-scale tissue displacements consistent with ARF generation triggered spiking activity that remained unchanged to a broad acoustic frequency range (0.5–43 MHz), hence excluding a potential cavitation-related effect at least in an ex vivo setting [30]. Moreover, a new theory known as the soliton model proposes that the action potential (AP) involves an adiabatic process, where a mechanical pulse propagates in phase with an electrical pulse along the axon [22]. The reversed pathway could mean that deformations of the neuronal membrane induced by the ARF could potentially both annihilate or enhance axonal electrophysiology [20]. Also, for specific regimes (high pulse repetition frequency, high duty cycle, high pressure), ultrasound may increase temperature and alter the electrical capacitance of the plasma membrane [25], as demonstrated through light-induced temperature increase [26]. Interestingly, a behavioral study using mutants C. elegans model demonstrated that knocking out mechanosensitive ion channels abolishes neuronal responses to mechanical stimulation, while knocking out thermosensitive ion channels kept responses unaffected [31]. In this context, Thompson et al. [27] have demonstrated a temperature dependence of neuronal membrane conductance and synaptic potentials, while recent studies have shown that ultrasound can directly drive a number of mechanosensitive ion channels (K+ channel family TREK-1, TREK-2, and TRAAK [11], voltage-gated Na+ and Ca+ [10], and piezo-type mechanosensitive channel Piezo1 [12, 13] and Piezo2 [24]) as well as indirectly control neuronal responses via modulation of transient receptor potential ankyrin 1 (TRPA1) channels in astrocytes with glutamate-releasing bestrophin-1 (Best1) as a mediator of glia–neuron interaction [14]. Therefore, it is highly likely that, depending on the pulse regime, different combinations of partially overlapping mechanisms would concur to the final result of the interaction between ultrasound and the cell membrane.
EX VIVO/IN VITRO vs. IN VIVO
Despite the advances provided by ex vivo and in vitro studies, contradictory results regarding the absence [30] or presence [32, 33] of cavitation and its role [23] in ultrasound neuromodulation have been reported. These conflicts may potentially be due to differences in experimental conditions. For instance, the oxygenation process inherent to culturing cells may introduce bubbles in in vitro preparations [32]. Furthermore, in vivo translation of in vitro and ex vivo results is hampered by differences in a number of parameters and effects such as cavitation threshold, the rapid cooling effects associated with brain perfusion [34], the contribution of different cells to the neuromodulatory effect [14] and skull-related effects such as attenuation due to absorption and scattering, and shear wave from mode conversion [35]. Indirect confounding effects may also include activation through auditory pathways [36, 37]. Nevertheless, all ultrasound neuromodulation studies have demonstrated that the paradigm of framing neural activity within and electromagnetic perspective is too simplistic, confirming that ultrasound neuromodulation studies can be of great aid in all applications requiring fast and painless interference of brain function, both in investigative and in therapeutic contexts.
IN VIVO Studies-Anesthesia Effects
Anesthesia effects have long represented a major confounding factor in neuromodulation studies. It has been shown that motor-evoked potentials induced by electrical stimulation are suppressed by isoflurane in a dose-dependent manner [38]. Similarly, ketamine blocks cortical neuron activity, which suppresses ultrasound-elicited motor responses [39]. In this context, in FUS neuromodulation studies, the isoflurane dose was reduced down to 0.1%, which corresponds to operating on a semi-awake animal [4]. However, some experiments have reported auditory artifacts and audible buzzing sounds generated by the ultrasound transducer, which may affect experiments in animals [36, 37], as well as in humans [40–42]. Therefore, the use of low-level anesthesia to maintain animal semi alert requires careful considerations in the setup and techniques such as signal smoothing [43] to avoid confounds. From deep brain stimulation (DBS) studies, it is known that anesthesia affects the spontaneous background firing and the neuronal spike activity patterns, as well as potentiates the inhibitory actions of gamma-aminobutyric acid (GABA) and causes a global depression in neuronal discharge, among other effects [44, 45]. In a repetitive transcranial magnetic stimulation (rTMS) study in rats, isoflurane, dexmedetomidine, and propofol caused significant different effects on functional connectivity, particularly between the sensorimotor cortex and thalamus [46]. In general, as reviewed by Jerusalem et al. [15], anesthetics lead to unconsciousness, immobility, amnesia, and analgesia without a complete understanding of the mechanisms underlying loss of consciousness and depth of anesthesia, which is mirrored by even more partial insights into the implication of sedation and deep anesthesia in humans [47] to the extent that anesthesia itself can be considered an instrument to explore the neural substrates of cognitive processes [48]. Importantly, several hypotheses about how anesthetic drugs modulate membrane excitability overlap with potential mechanisms of FUS neuromodulation. These include membrane deformation, changes in the thermodynamic properties of the membrane, and bubble formation. Therefore, awake studies are needed for a more precise characterization of the neural underpinning of FUS neuromodulation.
MRI
MRI can help advance neuromodulation technologies in a number of ways [49]. Importantly, MRI and ultrasound neuromodulation share similar spatial resolutions, which lies in the order of millimeters or submillimeters. MRI resolution depends on several factors, including magnetic field strength [50–52], coil performance, and subsequent imaging gradients [53, 54]. High magnetic field strengths from 3 to 7 T for humans [55] and above 7 T for preclinical studies [56], dedicated multi-transmit head coils [57, 58], and strong imaging gradients up to 100 mT/m for human scanners and 1,000 mT/m for preclinical systems [59–61] can provide spatial resolutions ranging from 1 to 2 mm3 to submillimeter (depending on imaging modality) for human and animals studies, respectively [60, 62].
On the other side, the lateral resolution (L) of ultrasound neuromodulation for a concave transducer can be characterized as L = 1.4λF/A, where λ is the wavelength (equal the ratio of the speed of sound in the medium and the ultrasound frequency), F is the focal length, and A is the aperture size (F/A is also known as the f-number). However, the frequency dependence of the ultrasound attenuation factor, mainly influenced by the skull, imposes a trade-off in the frequency choice. The attenuation factor is given by α0fn, where α0 is a temperature-dependent attenuation factor at 1 MHz, f is the ultrasound frequency, and n lies in the range of 0.9–2.1 for the human skull bone and 1.05–1.1 for brain [63]. Typically, ultrasound neuromodulation delivers submillimetric to millimetric resolution that employs frequencies in the kHz range for humans (i.e., f = 250 kHz, L = 7 mm [64]) and NHPs (i.e., f = 320 kHz, L = 5 mm [64]) and kHz to MHz range for rodents (i.e., f = 1.9 MHz, L = 1 mm [6]; f = 5 MHz, L = 0.29 mm [65]).
Motion-sensitizing gradients can detect phase shifts in MR data that encode brain tissue displacements caused by FUS application [66, 67]. This specific MRI technique, called magnetic resonance-acoustic radiation force imaging (MR-ARFI), has been shown to be safe despite the need for high-intensity FUS pulses to displace tissue [68]. Currently, just like in transcranial magnetic stimulation (TMS, which employs pulsed magnetic fields to induce eddy currents in the brain) or transcranial direct current stimulation (tDCS), neuromodulation studies rely on numerical simulations to perform targeting. However, a confirmation of tissue engagement through MR-ARFI would be highly desirable, especially for small brain structures. In this context, targeting accuracy can be improved by using low-frequency ranges and normal incidence angles [69] both minimizing FUS beam distortions and by adopting neuronavigation systems based on MR images [70].
MR phase-difference images can also be used for temperature monitoring during FUS [71, 72] in order to avoid artifacts that would arise from local temperature measurements based on thermocouples [73, 74]. While no significant temperature elevation has been detected in low-intensity neuromodulation protocols [75], higher intensity protocols [6] may cause physiologically relevant temperature elevations [74, 76], and monitoring temperature may provide insights into FUS neuromodulation mechanisms. Other MRI modalities, such as T2-weighted and T2*-weighted imaging, can provide safety evaluation such as the detection of potential hemorrhages and edema formation [77]. Also, T2-weighted fluid-attenuated inversion recovery (FLAIR) can provide safety assessment with better differentiation between cerebrospinal fluid (CSF) and abnormal tissue [78]. In addition, diffusion-weighted imaging (DWI) is highly sensitive to both reversible and irreversible changes in brain microstructure [79]. Moreover, in order to reveal intentional [7] or unintentional breakdown in the blood–brain barrier in the context of neuromodulation, T2- or T1-weighted MR images can be used to evaluate T2 or T1 contrast agent deposition in brain tissue after ultrasound application [80–82]. Finally, fMRI has been used in NHP to identify brain areas to be modulated [64] or to reveal the extent and connectivity of spatial changes in hemodynamic responses caused by FUS [8, 83–87].
Other Neuromodulation Techniques: Multimodal Stimulation
In general, among the numerous techniques available for neuromodulation, keeping more and more of the neurophysiology under experimental control goes hand in hand with an increase in invasiveness and biotechnological constraints. For example, neuronal activity of specific neuronal populations could be reversibly silenced by genetic approaches [88] while FUS would probe specific brain structures. This could potentially reveal the spatial and temporal scales of the different mechanisms of action, the contribution of FUS neuromodulation in different brain cells, and the contribution of defined projection pathways to neuronal network dynamics and animal behavior. On the non-invasive side, which is more immediately translatable to humans, combining magnetic and ultrasound stimuli is capable of enhancing the effect of FUS [89]. It has been proposed that ions in motion under a static magnetic field could be subjected to a Lorentz force, giving rise to electric currents that would contribute to the neuromodulatory effect of FUS [89, 90]. For example, in humans, concurrent FUS and TMS applied to the primary motor cortex (M1) attenuated motor evoked potential amplitude, reduced intracortical facilitation, and slightly shortened (10 μs) the response time in visual tasks [42]. Notably, FUS parameters can span a range of values that has been shown capable of inducing mechanical [91] or thermal effects to obtain excitatory or inhibitory effects on mice sciatic nerve [92]. In general, the ability of FUS to probe spatially specific brain regions enables understanding of, e.g., brain functioning and connectivity in non-invasive and spatially selective manner, with little or no cell type specificity. In this respect, FUS is somewhat similar to TMS, although it may offer better focusing of deeper structures (at least with a single coil) [93, 94]. Interestingly, TMS and FUS still share the large potential for non-invasive brain enhancing and silencing, as well as the lack of a thorough understanding of the mechanisms of action underlying the diversity of effects observed throughout the literature, which may include involuntary cell type specificity, axonal stimulation [95], uncontrolled/uncontrollable activation at different loci of the neuron, distributed stimulation peaks [96], and complex interplay of modulating inhibitory and excitatory synaptic potentials [97]. In this context, current-controlled “priming” techniques such as tDCS can be used in conjunction with time-localized TMS [98] (or possibly FUS) to modify the underlying neuronal activity substrate and possibly enhance specificity.
Conversely, techniques such as optogenetics [99] and chemogenetics/pharmacogenetics [100] can provide cell type-specific, selectively inhibitory, excitatory or combined control of neuronal activity by expressing light-sensitive ion channels called opsins, which can be either excitatory (e.g., channelrhodopsin) or inhibitory (e.g., halorhodopsin). The specificity [100] may be selectively activated [101] with light at different frequencies allowing a virtually infinite combination of stimuli, which can open/close ion channels with extremely high frequencies (up to 30 Hz). The drawbacks of such techniques lie both in practical challenges, e.g., the implantation of fiber optics for stimulation (which may interfere with behavioral experiments and limit human translational potential) and the high spatial selectivity (200 μm) of light delivery (which, interestingly, may not suffice to inhibit the function of a particular brain region and hence examine its function) and in neurobiological effects such the need to genetically modify the organisms to achieve cell type specificity, non-physiological hyperpolarization (which in turn can generate rebound phenomena), and in the potential generation of antidromic potentials (which may blur the physiological significance of the stimulation). While the first set of constraints may be partially solved by pharmacogenetic approaches (which employ chemical stimuli to activate the opsins and hence eliminate the surgical requirements and the need for constant stimulation when envisaging future treatment strategies in humans), the second may not. This calls for a new generation of combined biotechnological and physical neuromodulation techniques in order to achieve successful translation to the human context, especially in the therapeutic and clinical trial arena.
Interestingly, novel paradigms have been proposed involving the combination of genetic approaches with either magnetic or ultrasound stimulation. In magnetogenetics, thermosensitive, and mechano-sensitive ion channels (typically transient receptor potential vanilloid class receptors TRPV, which are selective calcium Ca2+ transporters) are genetically engineered to be tightly coupled to the iron storage protein ferritin (or another paramagnetic protein), so that they can be activated by external magnetic fields [102]. In sonogenetics, through a similar approach, it has been demonstrated that neuron-specific misexpression of TRP-4 (a pore-forming subunit of a mechanotransduction channel) can sensitize neurons to ultrasound stimuli with detectable behavioral outputs [103]. It appears, therefore, evident that combined multimodal strategies are the principal future avenues for tailoring neuromodulation intervention to an application-specific and possibly patient-specific context within a precision medicine paradigm.
Discussion
In this review, we have summarized potential mechanisms underlying the neural substrates of FUS neuromodulation and outlined conflicting hypotheses of the current literature. Similar to what has been shown for TMS, it is our opinion that apparent contradictions observed in some experimental and modeling studies could be resulted mainly due to variability from different experimental conditions in vitro, ex vivo, and in vivo applications and that they could be reconciled by detailed standardization and translation studies. In turn, this would allow drawing more informed conclusions on the FUS neuromodulation mechanisms. Additionally, the lack of a complete understanding of anesthesia effects on neurons encourages further awake FUS neuromodulation studies, which with the aid of MRI in assessing brain activity, targeting, and safety, will provide a clearer picture of both the neurophysiological underpinnings and of the potential translational applications of FUS, whether alone or in a multimodal context.
A number of experimental evidences show that the AP involves an electromechanical process and that the deformation of tissues induced by the ARF plays a crucial role in neuromodulation through potential capacitance change modulation or a flexoelectric effect triggering. Another possibility is that FUS could cause a neuronal membrane deformation capable of interfering with membrane electrical depolarization by mechanical coupling with the endogenous mechanical waves (soliton) associated with APs. In addition, ultrasound propagation can deform tissues elastically while the pulse energy is lost through heating due to viscous frictional forces. Whether the thermal effect is detectable or important in the context of neuromodulation will depend on the temperature level that is reached, neuronal sensitivity to temperature transients, tissue diffusion, and perfusion capability. In the soliton model, the membrane temperature is a crucial factor, and it should be noted that the membrane melting point is slightly below physiological temperature. Therefore, small temperature elevations caused by viscous frictional forces during ultrasound propagation may cause an interference with electromechanical membrane physiology. Most studies have consistently strived to avoid thermal effects from FUS effects, which is important to separate ablative from non-ablative effects. However, the mechanical and low-temperature increase generated by FUS could also potentially improve the neuromodulatory effects [104]. In this context, animal experiments based on sedation or anesthesia need to take thermal effects into account as mild hypothermia is common during deep sedation [105].
While the lack of a complete understanding of the FUS neuromodulation mechanisms does not currently impede reaping potential benefits in a more application-driven context, it is reasonable to expect that a better mechanistic understanding will immediately reverberate onto the applicability and efficacy of FUS-based neuromodulation. Importantly, as the technology continues to gain ground and acceptance, safety must remain a prime concern. Therefore, overcoming current limitations in both target confirmation and safety monitoring through the human skull is imperative. Techniques such as mapping of cavitation, temperature, and displacement will ensure a successful clinical translation of ultrasound neuromodulation, at the same time providing more control over the acoustic parameters. This will allow employing precisely determined mechanism combinations for achieving targeted neuronal excitation and inhibition. While more and more studies are being planned, several investigations have already demonstrated the existence of a wide range of safe parameters [106–108].
While fMRI is undoubtedly the gold standard for functional brain imaging, other techniques can provide complementary information on brain function. Recent studies have combined fMRI and optical imaging to show that ultrasound neuromodulation induces cerebral hemodynamic changes in different animals at variable peak latencies: mice ~2.5 s [109, 110], rabbits ~3.2 s [111], and NHPs ~6.5 s [86]. Furthermore, a technique termed functional ultrasound (fUS) is capable of detecting transient changes in blood volume [112], and it has been demonstrated capable of providing deep brain functional images with high spatial resolution (from 50 to 200 μm) [113] and temporal resolution of <1 s [114]. In addition, fUS features high sensitivity and portability, which enable awake experiments with freely moving subjects. The development of 2D transducer arrays [115], capable of generating images and steerable, highly focused beams, potentially with multifrequency capability [116], may facilitate human fUS during the ultrasound neuromodulation.
Crucially, while in vitro and ex vivo studies are necessary for understanding mechanisms, in vivo brain activity studies are essential to gather mesoscale and macroscale information about the effect of FUS on brain functioning. In small animals, experiments in ultra-high-field (UHF) MRI (7–21 T) can provide higher signal-to-noise and contrast-to-noise ratios as well as in the case of fMRI increased susceptibility [60, 117]. In turn, this will unlock more in-depth insights into how the intact brain works and into the available windows in interfering with its activity in a non-invasive or minimally invasive manner, accelerating the translation toward human applications and especially the empowerment of clinical trials. This may include applications to neurological diseases like epilepsy and chronic pains, psychiatry [Obsessive-compulsive disorder (OCD), pharmacoresistant depression, agoraphobia], as well as fostering neuronal plasticity in, e.g., rehabilitation or slowing the progression of degenerative brain diseases. Especially in this latter context, multimodal stimulation (as electrical, magnetic, chemical, light, mechanical), possibly coupled with a state-of-the-art monitoring tool like UHF MRI for non-invasive techniques and calcium imaging [118], may enable simultaneous, multi-scale, brain structure- or cell type-specific silencing or excitation, allowing the exploration of both brain-wide pathways and specific cognitive, emotional, and pathological mechanisms. This can provide a significant step change in keeping more and more neurophysiological aspects under experimental control and hence ultimately approaching the neurobiological goal of neuromodulation in a more precise, targeted, painless, and direct manner.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
HK and EK were supported in part by National Institutes of Health (NIH) under award numbers R01CA228275 and R01EB027576, and the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) under award number HR0011-15-2-0054. This study received funding from SoundStim Therapeutics and Google X. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO. Ultrasound neuromodulation: a review of results, mechanisms safety. Ultrasound Med Biol. 45:1509–36. doi: 10.1016/j.ultrasmedbio.2018.12.015
2. Yoo SS, Kim H, Min BK, Franck E, Park S. Transcranial focused ultrasound to the thalamus alters anesthesia time in rats. Neuroreport. (2011) 22:783–7. doi: 10.1097/WNR.0b013e32834b2957
3. Younan Y, Deffieux T, Larrat B, Fink M, Tanter M, Aubry J-F. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med Phys. (2013) 40:082902. doi: 10.1118/1.4812423
4. King RL, Brown JR, Newsome WT, Pauly KB. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med Biol. (2013) 39:312–31. doi: 10.1016/j.ultrasmedbio.2012.09.009
5. Kim H, Chiu A, Lee SD, Fischer K, Yoo S-S. Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters. Brain Stimul. (2014) 5:181–204. doi: 10.1016/j.brs.2014.06.011
6. Kamimura HA, Wang S, Chen H, Wang Q, Aurup C, Acosta C, et al. Focused ultrasound neuromodulation of cortical subcortical brain structures using 1. 9 MHz. Med Phys. (2016) 43:5730–5. doi: 10.1118/1.4963208
7. Downs ME, Teichert T, Buch A, Karakatsani ME, Sierra C, Chen S, et al. Toward a cognitive neural prosthesis using focused ultrasound. Front Neurosci. (2017) 11:607. doi: 10.3389/fnins.2017.00607
8. Ai L, Mueller JK, Grant A, Eryaman Y, Legon W. Transcranial focused ultrasound for BOLD fMRI signal modulation in humans 2016. Conf IEEE Eng Med Biol Soc. (2016) 2016:1758–61. doi: 10.1109/EMBC.2016.7591057
9. Legon W, Ai L, Bansal P, Mueller JK. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum Brain Mapp. (2018) 39:1995–2006. doi: 10.1002/hbm.23981
10. Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE. (2008) 3:e3511. doi: 10.1371/journal.pone.0003511
11. Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J, et al. Ultrasound modulates ion channel currents. Sci Rep. (2016) 6:24170. doi: 10.1038/srep24170
12. Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M. Activation of Piezo1 but not NaV1. 2 channels by ultrasound at 43 MHz. Ultrasound Med Biol. (2018) 44:1217–32. doi: 10.1016/j.ultrasmedbio.2017.12.020
13. Qiu Z, Guo J, Kala S, Zhu J, Xian Q, Qiu W, et al. The mechanosensitive ion channel piezo1 significantly mediates in vitro ultrasonic stimulation of neurons. Science. (2019) 21:448–57. doi: 10.1016/j.isci.2019.10.037
14. Oh S-J, Lee JM, Kim H-B, Lee J, Han S, Bae JY, et al. Ultrasonic neuromodulation via astrocytic TRPA1. Curr Biol. (2019) 29:3386–401. e8. doi: 10.1016/j.cub.2019.08.021
15. Jerusalem A, Al-Rekabi Z, Chen H, Ercole A, Malboubi M, Tamayo-Elizalde M, et al. Electrophysiological-mechanical coupling in the neuronal membrane its role in ultrasound neuromodulation general anaesthesia. Acta Biomater. (2019) 97:116–40. doi: 10.1016/j.actbio.2019.07.041
16. Leighton T. Editor. The Acoustic Bubble. London: Academic Press. (1994). p. 640. doi: 10.1016/B978-0-12-441920-9.50002-X
17. Prieto ML, Oralkan Ö, Khuri-Yakub BT, Maduke MC. Dynamic response of model lipid membranes to ultrasonic radiation force. PLoS ONE. (2013) 8:e77115. doi: 10.1371/journal.pone.0077115
18. Gaub BM, Kasuba KC, Mace E, Strittmatter T, Laskowski PR, Geissler SA, et al. Neurons differentiate magnitude location of mechanical stimuli. Proc Natl Acad Sci USA. (2019) 117:848–56. doi: 10.1073/pnas.1909933117
19. Muratore R, LaManna J, Szulman E, Andrew Kalisz MS, Lamprecht M, Melissa Simon MS, et al. Bioeffective ultrasound at very low doses: reversible manipulation of neuronal cell morphology function in vitro. AIP Conf Proc. (2009) 1113:25–9. doi: 10.1063/1.3131426
20. Chen H, Garcia-Gonzalez D, Jérusalem A. Computational model of the mechanoelectrophysiological coupling in axons with application to neuromodulation. Phys Rev. (2019) 99:032406. doi: 10.1103/PhysRevE.99.032406
21. Petrov AG. Electricity mechanics of biomembrane systems: flexoelectricity in living membranes. Anal Chim Acta. (2006) 568:108. doi: 10.1016/j.aca.2006.01.108
22. Heimburg T, Jackson AD. On soliton propagation in biomembranes nerves. Pro Natl Acad Sci USA. (2005) 102:9790–5. doi: 10.1073/pnas.0503823102
23. Plaksin M, Shoham S, Kimmel E. Intramembrane cavitation as a predictive bio-piezoelectric mechanism for ultrasonic brain stimulation. Phys Rev X. (2014) 4:011004. doi: 10.1103/PhysRevX.4.011004
24. Baba Y, Hoffman BU, Lee SA, Konofagou EE, Lumpkin E. A Focused ultrasound excites action potential firing in mammalian peripheral neurons in intact tissue. Proc Natl Acad Sci U.S.A. (under review).
25. Plaksin M, Shapira E, Kimmel E, Shoham S. Thermal transients excite neurons through universal intramembranemechanoelectrical effects. Phys Rev X. (2018) 8:11043. doi: 10.1103/PhysRevX.8.011043
26. Shapiro MG, Homma K, Villarreal S, Richter C-P Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. (2012) 3:736. doi: 10.1038/ncomms1742
27. Thompson S, Masukawa L, Prince D. Temperature dependence of intrinsic membrane properties synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. (1985) 5:817–24. doi: 10.1523/JNEUROSCI.05-03-00817.1985
28. Petrov AG, Miller BA, Hristova K, Usherwood PN. Flexoelectric effects in model native membarnes containing ion channels. EurBiophys J. (1993) 22:BF00180263. doi: 10.1007/BF00180263
29. Lemaire T, Neufeld E, Kuster N, Micera S. Understanding ultrasound neuromodulation using a computationally efficient interpretable model of intramembrane cavitation. J Neural Eng. (2019) 16:046007. doi: 10.1088/1741-2552/ab1685
30. Menz MD, Ye P, Firouzi K, Nikoozadeh A, Pauly KB, Khuri-Yakub P, et al. Radiation force as a physical mechanism for ultrasonic neurostimulation of the ex vivo retina. J Neurosci. (2019) 39:6251–64. doi: 10.1523/JNEUROSCI.2394-18.2019
31. Kubanek J, Shukla P, Das A, Baccus SA, Goodman MB. Ultrasound elicits behavioral responses through mechanical effects on neurons ion channels in a simple nervous system. J Neurosci. (2018) 38:3081–91. doi: 10.1523/JNEUROSCI.1458-17.2018
32. Wright CJ, Rothwell J, Saffari N. Ultrasonic stimulation of peripheral nervous tissue: an investigation into mechanisms. J Phys Conf Ser. (2015) 581:012003. doi: 10.1088/1742-6596/581/1/012003
33. Wright CJ, Haqshenas SR, Rothwell J, Saffari N. Unmyelinated peripheral nerves can be stimulated in vitro using pulsed ultrasound. Ultrasound Med Biol. (2017) 43:2269–83. doi: 10.1016/j.ultrasmedbio.2017.05.008
34. Wang H, Kim M, Normoyle KP, Llano D. Thermal regulation of the brain-an anatomical physiological review for clinical neuroscientists Front Neurosci. (2016) 9:528. doi: 10.3389/fnins.2015.00528
35. Pinton G, Aubry J-F, Bossy E, Muller M, Pernot M, Tanter M. Attenuation, scattering, absorption of ultrasound in the skull bone. Med Phys. (2011) 39:299–307. doi: 10.1118/1.3668316
36. Sato T, Shapiro MG, Tsao DY. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron. (2018) 98:1031–41. e5. doi: 10.1016/j.neuron.2018.05.009
37. Guo H, Hamilton M, Offutt SJ, Gloeckner CD, Li T, Kim Y, et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron. (2018) 98:1020–30. e4. doi: 10.1016/j.neuron.2018.04.036
38. Kawaguchi M, Shimizu K, Furuya H, Sakamoto T, Ohnishi H, Karasawa J. Effect of isoflurane on motor-evoked potentials induced by direct electrical stimulation of the exposed motor cortex with single, double, triple stimuli in rats. Anesthesiology. (1996) 85:1176–83. doi: 10.1097/00000542-199611000-00027
39. Han S. Kim M. Kim H, Shin H, Youn I. Ketamine inhibits ultrasound stimulation-induced neuromodulation by blocking cortical neuron activity. Ultrasound Med Biol. (2018) 44:635–46. doi: 10.1016/j.ultrasmedbio.2017.11.008
40. Mueller J, Legon W, Opitz A, Sato TF, Tyler WJ. Transcranial focused ultrasound modulates intrinsic evoked eeg dynamics. Brain Stimul. (2014) 7:900–8. doi: 10.1016/j.brs.2014.08.008
41. Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. (2014) 17:322–9. doi: 10.1038/nn.3620
42. Legon W, Bansal P, Tyshynsky R, Ai L, Mueller JK. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Sci Rep. (2018) 8:10007. doi: 10.1038/s41598-018-28320-1
43. Mohammadjavadi M, Ye PP, Xia A, Brown J, Popelka G, Pauly KB. Elimination of peripheral auditory pathway activation does not affect motor responses from ultrasound neuromodulation. Brain Stimul. (2019) 12:901–10. doi: 10.1016/j.brs.2019.03.005
44. Chakrabarti R, Ghazanwy M, Tewari A. Anesthetic challenges for deep brain stimulation: a systematic approach. N Am J Med Sci. (2014) 6:359. doi: 10.4103/1947-2714.139281
45. Grant R, Gruenbaum SE, Gerrard J. Anaesthesia for deep brain stimulation. Curr Opin Anaesthesiol. (2015) 28:505–10. doi: 10.1097/ACO.0000000000000230
46. Boonzaier J, van Tilborg GAF, Straathof M, Petrov PI, van Heijningen CL, van Vliet G, et al. Differential outcomes of rTMS anesthesia effects on functional connectivity in the rat brain. Brain Stimul. (2017) 10:418. doi: 10.1016/j.brs.2017.01.241
47. Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KFK, et al. Electroencephalogram signatures of loss recovery of consciousness from propofol. Proc Natl Acad Sci USA. (2013) 110:E1142–51. doi: 10.1073/pnas.1221180110
48. Bonhomme V, Staquet C, Montupil J, Defresne A, Kirsch M, Martial C, et al. General Anesthesia: a probe to explore consciousness. Front Syst Neurosci. (2019) 2013:13–36. doi: 10.3389/fnsys.2019.00036
49. Downar J, Davis KD. Magnetic resonance imaging in neuromodulation. In: Hamani C, Holtzheimer P, Lozano AM, Mayberg H, editors. Neuromodulation in Psychiatry. West Sussex: John Wiley & Sons, Ltd (2015). p. 49–79. doi: 10.1002/9781118801086.ch4
50. Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, et al. Pros cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. (2018) 109:1–50. doi: 10.1016/j.pnmrs.2018.06.001
51. Galante A, Sinibaldi R, Conti A, De Luca C, Catallo N, Sebastiani P, et al. Fast room temperature very low field-magnetic resonance imaging system compatible with magneto encephalo graphy environment. PLoS ONE. (2015) 10:e0142701. doi: 10.1371/journal.pone.0142701
52. Guidotti R, Sinibaldi R, De Luca C, Conti A, Ilmoniemi RJ, Zevenhoven KCJ, et al. Optimized 3D co-registration of ultra-low-field high-field magnetic resonance images. PLoS ONE. (2018) 13:e0193890. doi: 10.1371/journal.pone.0193890
53. Ibrahim TS, Kangarlu A, Chakeress DW. Design performance issues of rf coils utilized in ultra high field MRI: experimental numerical evaluations. IEEE Trans Biomed Eng. (2005). 52:1278–84. doi: 10.1109/TBME.2005.847564
54. Mangrum W, Hoang QB, Amrhein TJ, Duncan SM, Maxfield CM, Merkle E, et al. Duke Review of MRI Principles: Case Review Series E-Book. Philadelphia, PA: Elsevier Health Sciences (2012).
55. Olman CA, Yacoub E. High-field fMRI for human applications: an overview of spatial resolution signal specificity. Open Neuroimag J. (2011) 5:74–89. doi: 10.2174/1874440001105010074
56. Moser D, Zadicario E, Schiff G, Jeanmonod D. MR-guided focused ultrasound technique in functional neurosurgery: targeting accuracy. J Ther Ultrasound. (2013) 1:1–10. doi: 10.1186/2050-5736-1-17
57. Sengupta S, Roebroeck A, Kemper VG, Poser BA, Zimmermann J, Goebel R, et al. A specialized multi-transmit head coil for high resolution fMRI of the human visual cortex at 7T. PLoS ONE. (2016) 11:e0165418. doi: 10.1371/journal.pone.0165418
58. Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil: are there relevant improvements for functional imaging? J Magn Reson Imaging. (2016) 34:173–83. doi: 10.1002/jmri.22614
59. Silva AC, Merkle H. Hardware considerations for functional magnetic resonance imaging Concepts. Magn Reson. (2015) 16A:35–49. doi: 10.1002/cmr.a.10052
60. Ciobanu L, Solomon E, Pyatigorskaya N, Roussel T, Le Bihan D, Frydman L. fMRI contrast at high and ultrahigh magnetic fields: insight from complementary methods. Neuroimage. (2003) 113:37–43. doi: 10.1016/j.neuroimage.2015.03.018
61. Dickson SL, Mercer JG. Neuroendocrinology of Appetite. Chichester: John Wiley & Sons, Ltd. (2016). doi: 10.1002/9781118839317
62. Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the human connectome project. Neuroimage. (2013) 80:144–68. doi: 10.1016/j.neuroimage.2013.05.039
63. Cobbold R SC. Foundations of Biomedical Ultrasound. New York, NY: Oxford university press (2015)
64. Lee W, Kim H, Jung Y, Song I-U, Chung YA, Yoo S-S. Image-guided transcranial focused ultrasound stimulates human primary somatosensory cortex. Sci Rep. (2006) 5:8743. doi: 10.1038/srep08743
65. Li GF, Zhao HX, Zhou H, Yan F, Wang JY, Xu CX, et al. Improved anatomical specificity of non-invasive neuro-stimulation by high frequency (5 mhz) ultrasound. Sci Rep. (2016) 6:24738. doi: 10.1038/srep24738
66. McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys. (2008) 35:3748–58. doi: 10.1118/1.2956712
67. Larrat B, Pernot M, Aubry J-F, Dervishi E, Sinkus R, Seilhean D, et al. MR-guided transcranial brain HIFU in small animal models. Phys Med Biol. (2010) 55:365–88. doi: 10.1088/0031-9155/55/2/003
68. Phipps MA, Jonathan SV, Yang P-F, Chaplin V, Chen LM, Grissom WA, et al. Considerations for ultrasound exposure during transcranial MR acoustic radiation force imaging. Sci Rep. (2019) 9:16235. doi: 10.1038/s41598-019-52443-8
69. Karakatsani ME, Samiotaki G, Downs ME, Ferrera VP, Konofagou EE. Targeting effects on the volume gray-to-white-matter ratio of the focused-ultrasound induced blood-brain barrier opening in non-human primates in vivo. EEE Trans Ultrason Ferroelectr Freq Control. (2018) 64:798–810. doi: 10.1186/2050-5736-3-S1-P22
70. Wu S-Y, Aurup C, Sanchez CS, Grondin J, Zheng W, Kamimura H, et al. Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound real-time acoustic mapping. Sci Rep. (2015) 8:7978. doi: 10.1038/s41598-018-25904-9
71. De Poorter J. Noninvasive MRI thermometry with the proton resonance frequency method: study of susceptibility effects. Magn Reson Med. (1995) 34:359–67. doi: 10.1002/mrm.1910340313
72. Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. (1995) 34:814–23. doi: 10.1002/mrm.1910340606
73. Morris H, Rivens I, Shaw A, Ter Haar G. Investigation of the viscous heating artefact arising from the use of thermocouples in a focused ultrasound field. Phys Med Biol. (2008) 53 4759–76. doi: 10.1088/0031-9155/53/17/020
74. Kamimura HAS, Aurup C, Bendau EV, Saharkhiz N, Kim MG, Konofagou EE. Iterative curve fitting of the bioheat transfer equation for thermocouple-based temperature estimation in vitro in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. (2020) 67:70–80. doi: 10.1109/TUFFC.2019.2940375
75. Dallapiazza RF, Timbie KF, Holmberg S, Gatesman J, Lopes MB, Price RJ, et al. Noninvasive neuromodulation thalamic mapping with low-intensity focused ultrasound. J. Neurosurg. (2018) 128:875–84. doi: 10.3171/2016.11.JNS16976
76. Constans C, Mateo P, Tanter M, Aubry J-F. Potential impact of thermal effects during ultrasonic neurostimulation: retrospective numerical estimation of temperature elevation in seven rodent setups. Phys Med Biol. (2017) 63:025003. doi: 10.1088/1361-6560/aaa15c
77. Ho M-L, Rojas R, Eisenberg RL. Cerebral edema. Am J Roentgenol. (2012) 199:W258–73. doi: 10.2214/AJR.11.8081
78. Lee EK, Lee EJ, Kim S, Lee YS. Importance of contrast-enhanced fluid-attenuated inversion recovery magnetic resonance imaging in various intracranial pathologic conditions Korean. J Radiol. (2016) 17:127–41. doi: 10.3348/kjr.2016.17.1.127
79. Kamiya K, Hori M, Irie R, Miyajima M, Nakajima M, Kamagata K, et al. Diffusion imaging of reversible irreversible microstructural changes within the corticospinal tract in idiopathic normal pressure hydrocephalus. NeuroImage Clin. (2017) 14:663–71. doi: 10.1016/j.nicl.2017.03.003
80. Hynynen K, McDannold N, Vykhodtseva N, Jolesz F. A noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. (2001) 220:640–6. doi: 10.1148/radiol.2202001804
81. Moon HW, Fung PY, Whipp SC, Isaacson RE. Effects of age ambient temperature on the responses of infant mice to heat-stable enterotoxin of Escherichia coli: assay modifications. Infect Immun. (1978) 20:36–9. doi: 10.1128/IAI.20.1.36-39.1978
82. Conti A, Magnin R, Gerstenmayer M, Tsapis N, Dumont E, Tillement O, et al. Empirical theoretical characterization of the diffusion process of different gadolinium-based nanoparticles within the brain tissue after ultrasound-induced permeabilization of the blood-brain barrier contrast media. Mol Imaging. (2019) 2019:1–13. doi: 10.1155/2019/6341545
83. Legon W, Rowlands A, Opitz A, Sato TF, Tyler WJ. Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG fMRI. PLoS ONE. (2012) 7:e51177. doi: 10.1371/journal.pone.0051177
84. Lee W, Kim H-C, Jung Y, Chung YA, Song I-U, Lee J-H, et al. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci Rep. (2016) 6:34026. doi: 10.1038/srep34026
85. Ai L, Bansal P, Mueller JK, Legon W. Effects of transcranial focused ultrasound on human primary motor cortex using 7T fMRI: a pilot study. BMC Neurosci. (2018) 19:56. doi: 10.1186/s12868-018-0456-6
86. Yang P-F, Phipps MA, Newton AT, Chaplin V, Gore JC, Caskey CF, et al. Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance detection. Sci Rep. (2018) 8:7993. doi: 10.1038/s41598-018-26287-7
87. Verhagen L, Gallea C, Folloni D, Constans C, Jensen DE, Ahnine H, et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife. (2019) 8: e40541. doi: 10.7554/eLife.40541
88. Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. Silencing neurons: tools, applications, experimental constraints. Neuron. (2017) 95:504–29. doi: 10.1016/j.neuron.2017.06.050
89. Yuan Y. Pang N. Chen YD, Wang Y, Li XL. Theoretical analysis of the effects of transcranial magneto-acoustical stimulation on neuronal firing rhythm Ca2+ concentration with chay neuron model. Biomed Phys Eng Exp. (2017) 3:055006. doi: 10.1088/2057-1976/aa84c8
90. Yuan Y, Chen Y, Li X. Theoretical analysis of transcranial magneto-acoustical stimulation with hodgkin-huxley neuron model. Front Comput Neurosci. (2016) 10:35. doi: 10.3389/fncom.2016.00035
91. Downs ME, Lee SA, Yang G, Kim S, Wang Q, Konofagou EE. Non-invasive peripheral nerve stimulation via focused ultrasound in vivo. Phys Med Biol. (2018) 63:035011. doi: 10.1088/1361-6560/aa9fc2
92. Kim MG, Kamimura HA, Lee SA, Aurup C, Kwon N, Konofagou EE. Image-guided focused ultrasound modulates electrically evoked motor neuronal activity in the mouse peripheral nervous system in vivo. J Neural Eng. (2020) 17:026026. doi: 10.1088/1741-2552/ab6be6
93. Goetz SM, Deng Z-D. The development modelling of devices paradigms for transcranial magnetic stimulation. Int Rev Psychiatry. (2020) 29:115–45. doi: 10.1080/09540261.2017.1305949
94. Heller L, van Hulsteyn DB. Brain stimulation using electromagnetic sources: theoretical aspects. Biophys J. (1992) 63:129–38. doi: 10.1016/S0006-3495(92)81587-4
95. Aberra AS, Wang B, Grill WM, Peterchev AV. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. (2020) 13:175–89. doi: 10.1016/j.brs.2019.10.002
96. Toschi N, Welt T, Guerrisi M, Keck ME. Transcranial magnetic stimulation in heterogeneous brain tissue: clinical impact on focality, reproducibility true sham stimulation J Psychiatr Res. (2009) 43:255–64. doi: 10.1016/j.jpsychires.2008.04.008
97. Esser SK, Hill SL, Tononi G. Modeling the effects of transcranial magnetic stimulation on cortical circuits. J Neurophysiol. (2005) 94:622–39. doi: 10.1152/jn.01230.2004
98. Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics back. Nat Neurosci. (2013) 16:838–44. doi: 10.1038/nn.3422
99. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. (2005) 8:1263–8. doi: 10.1038/nn1525
100. Jiang J, Cui H, Rahmouni K. Optogeneticspharmacogenetics: principles applications. Am J Physiol Integr Comp Physiol. (2017) 313:R633–45. doi: 10.1152/ajpregu.00091.2017
101. Spangler SM, Bruchas MR. Optogenetic approaches for dissecting neuromodulation GPCR signaling in neural circuits. Curr Opin Pharmacol. (2017) 32:56–70. doi: 10.1016/j.coph.2016.11.001
102. Barbic M. Possible magneto-mechanical magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife. (2019) 8:e45807. doi: 10.7554/eLife.45807
103. Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat Commun. (2015) 6:8264. doi: 10.1038/ncomms9264
104. Darrow DP, O'Brien P, Richner TJ, Netoff TI, Ebbini ES. Reversible neuroinhibition by focused ultrasound is mediated by a thermal mechanism. Brain Stimul. (2019) 12:1439–47. doi: 10.1016/j.brs.2019.07.015
105. Díaz M, Becker DE. Thermoregulation: physiological clinical considerations during sedation general anesthesia. Anesth Prog. (2010) 57:25–33. doi: 10.2344/0003-3006-57.1.25
106. Pasquinelli C, Hanson LG, Siebner HR, Lee HJ, Thielscher A. Safety of transcranial focused ultrasound stimulation: a systematic review of the state of knowledge from both human animal studies. Brain Stimul. (2019) 12:1367–80. doi: 10.1016/j.brs.2019.07.024
107. Legon W, Bansal P, Ai L, Mueller JK, Meekins G, Gillick B. Safety of transcranial focused ultrasound for human neuro modulation. bioRxiv. (2018) 2018:314856. doi: 10.1101/314856
108. Legon W, Adams S, Bansal P, Patel PD, Hobbs L, Ai L, et al. A retrospective qualitative report of symptoms and safety from transcranial focused ultrasound for neuromodulation in humans. Sci Rep. (2020) 10:5573. doi: 10.1038/s41598-020-62265-8
109. Kim E, Anguluan E, Kim JG. Monitoring cerebral hemodynamic change during transcranial ultrasound stimulation using optical intrinsic signal imaging. Sci Rep. (2017) 7:13148. doi: 10.1038/s41598-017-13572-0
110. Yuan Y, Wang Z, Liu M, Shoham S. Cortical hemodynamic responses induced by low-intensity transcranial ultrasound stimulation of mouse cortex. Neuroimage. (2020) 211:116597. doi: 10.1016/j.neuroimage.2020.116597
111. Yoo S-S, Bystritsky A, Lee J-H, Zhang Y, Fischer K, Min B-K, et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. (2011) 56:1267–75. doi: 10.1016/j.neuroimage.2011.02.058
112. Macé E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M. Functional ultrasound imaging of the brain. Nat Methods. (2011) 8:662–4. doi: 10.1038/nmeth.1641
113. Mace E, Montaldo G, Osmanski B-F, Cohen I, Fink M, Tanter M. Functional ultrasound imaging of the brain: theory basic principles. IEEE Trans Ultrason Ferroelectr Freq Control. (2013) 60:492–506. doi: 10.1109/TUFFC.2013.2592
114. Deffieux T, Demene C, Pernot M, Tanter M. Functional ultrasound neuroimaging: a review of the preclinical clinical state of the art. Curr Opin Neurobiol. (2018) 50:128–35. doi: 10.1016/j.conb.2018.02.001
115. Kamimura HA, Urban MW, Carneiro AA, Fatemi M, Alizad A. Vibro-acoustography beam formation with reconfigurable arrays. IEEE Trans Ultrason Ferroelectr Freq Control. (2012) 59:1421–31. doi: 10.1109/TUFFC.2012.2343
116. Constans C, Deffieux T, Pouget P, Tanter M, Aubry J-F. A 200-1380-kHz quadrifrequency focused ultrasound transducer for neurostimulation in rodents primates: transcranial in vitro calibration numerical study of the influence of skull cavity. IEEE Trans Ultrason Ferroelectr. Freq Control. (2017) 64:717–24. doi: 10.1109/TUFFC.2017.2651648
117. Ugurbil K. Magnetic resonance imaging at ultrahigh fields. IEEE Trans Biomed Eng. (2014) 61:1364–79. doi: 10.1109/TBME.2014.2313619
Keywords: central nervous system, focused ultrasound, magnetic resonance imaging, peripheral nervous system, therapeutic ultrasound, ultrasound neuromodulation
Citation: Kamimura HAS, Conti A, Toschi N and Konofagou EE (2020) Ultrasound Neuromodulation: Mechanisms and the Potential of Multimodal Stimulation for Neuronal Function Assessment. Front. Phys. 8:150. doi: 10.3389/fphy.2020.00150
Received: 03 February 2020; Accepted: 15 April 2020;
Published: 26 May 2020.
Edited by:
Federico Giove, Centro Fermi - Museo storico della fisica e Centro studi e ricerche Enrico Fermi, ItalyReviewed by:
Tommaso Gili, IMT School for Advanced Studies Lucca, ItalySimon Auguste Lambert, Université Claude Bernard Lyon 1, France
Copyright © 2020 Kamimura, Conti, Toschi and Konofagou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hermes A. S. Kamimura, a2FtaW11cmEuaGVybWVzQGNvbHVtYmlhLmVkdQ==
 Hermes A. S. Kamimura
Hermes A. S. Kamimura Allegra Conti
Allegra Conti Nicola Toschi
Nicola Toschi Elisa E. Konofagou
Elisa E. Konofagou