
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 12 March 2025
Sec. Experimental Pharmacology and Drug Discovery
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1549808
DR5, a receptor with the highest affinity for TRAIL under physiological conditions, selectively induces apoptosis in specific target cells such as tumor and aberrant immune cells, while minimally affecting normal cells. The TRAIL-DR5 signaling pathway is a crucial regulatory mechanism when the body responds to various exogenous interference factors, including viruses, chemicals, and radiation. This pathway plays a vital role in maintaining physiological homeostasis and in the pathological development of various diseases. Different modulations of DR5, such as upregulation, activation, and antagonism, hold significant potential for therapeutic applications in tumors, cardiovascular diseases, autoimmune diseases, viral infections, and radiation injuries. This article provides an overview of the current research progress on DR5, including the status and prospects of its clinical applications.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, is expressed in most human cells and primarily induces apoptosis in various cancer cell lines without harming normal cells. Its receptors include death receptor (DR) 4, DR5, death decoy receptor (DcR) I, DcR2, and the soluble receptor osteoprotegerin. DR5, under physiological conditions, demonstrates the strongest affinity for TRAIL. The TRAIL-DR5 signaling pathway is a major regulatory pathway when the body responds to diverse exogenous stimuli, playing an essential role in both physiological homeostasis and disease development. Studies have shown that DR5 protein expression is significantly upregulated in various disease-target organs. Through the modulation of DR5 expression and the intervention with DR5 activators or antagonists, significant therapeutic potential has been demonstrated for treating tumors, cardiovascular diseases, autoimmune diseases, severe viral infections, and radiation injuries. DR5 has emerged as a focal point for clinical disease treatment research. This paper reviews the basic features, main physiological functions, and significant current research progress on DR5, including the status and prospects of its clinical application research.
Known by multiple names including TRAIL-R2, TNFRSF10B, CD262, Apo2, Killer/Ly98, TRICK2A, and TRICKB, DR5 is a type I transmembrane protein. It consists of a signal peptide, an extracellular domain, a transmembrane domain, and an intracellular domain. The full-length DR5 cDNA is 1,146 bp, encoding 381 amino acids (Mert and Sanlioglu, 2017; Min et al., 2019). Gene transcription of DR5 occurs at 8q21.3, with a total DNA sequence length of 49,055 base pairs and a DR5 transcript length of 4,154 nucleotides (Mert and Sanlioglu, 2017). The DR5 mRNA 3′-UTR region encompasses 2,538 nucleotides, constituting more than half of the entire transcript.
Although DR5 and DR4, another major activating TRAIL apoptosis receptor, have relatively high homology (Chaudhary et al., 1997; Wiley et al., 1995) in the cysteine-rich domain and the death domain, the distribution and physiological functions of these two receptors in normal tissues and tumor tissues are significantly different. DR4 is distributed and highly expressed in many immune-related tissues as well as some specific types of tumor cells, while DR5 is widely distributed in normal tissue cells at very low level but highly expressed in many different types of tumor cells (Surget et al., 2012; de Miguel et al., 2016). It was well accepted that TRAIL (Pollack et al., 2001) can transmit apoptotic signals by activating either the apoptotic receptor DR4 or DR5, however, although both the two receptors are highly expressed on the surface of a variety of tumor cells, the relative effects and mechanisms of DR4 or DR5 on apoptosis of different tumor cells, as well as the specific association between the level of receptor expression and the activation of molecular responses by DR4 or DR5 has not yet been clarified (Humphreys and Halpern, 2008), which are probably not solely determined by their surface expression but may be influenced by intracellular apoptotic regulators.
MacFarlane et al. had revealed that TRAIL signals to apoptosis were predominantly transmitted via DR4 in chronic lymphocytic leukemia cells as well as pancreatic carcinoma cells (Stadel et al., 2010; MacFarlane et al., 2005a; Natoni et al., 2007; MacFarlane et al., 2005b), Micheau et al. reported that DR4 as a master player of apoptosis induced by TRAIL and ER stress (Dufour et al., 2016). Meanwhile, more studies had shown that DR5 probably played a major role in the initiation of apoptosis (Ichikawa et al., 2001; Nahacka et al., 2018) and showed better potential for antitumor drug development, for the basis phenomenon that DR5 exhibits high levels of expression in plenty types of cancer cell lines while expressed very low expression in normal tissues, which indicates the potential safety advantage for tumor targeted therapy, and for that DR5 contains the highest affinity to TRAIL at the optimal human body temperature of 37°C (Truneh et al., 2000). Kelley et al. (2005) conducted apoptosis experiments comparing TRAIL variants bound solely to DR4 or DR5, and revealed that lung, colon, and breast cancer cell lines show similarity in the membrane surface expression levels of DR4 and DR5 and exhibit more significant sensitivity to specific mutants of DR5 (Kelley et al., 2005). And the preferential agonistic DR5 antibody reactivity of ovarian, colon, and renal cell carcinoma cell lines is closely related to their high surface DR5 expression levels (Zeng et al., 2006; Nawrocki et al., 2007; Saulle et al., 2007; Marini et al., 2006). These findings emphasize the need to identify TRAIL receptor subtypes that can preferentially or precisely signal apoptosis in a given type of cancer.
DR5 is expressed across a variety of normal human tissues such as the heart, lungs, thymus, liver, kidneys, colon, small intestine, ovaries, prostate, testes, and skeletal muscles with very low levels. In addition, it has been confirmed that (Wu, 2009) DR5 is generally expressed at extremely higher levels than in normal tissues in a variety of tumor cell types, including breast, endometrial, cervical, ovarian, pancreatic, hepatocellular, and rectal cancers. DR5 expression is most common in bone sarcomas (e.g., Ewing’s sarcoma, osteosarcoma, and chondrosarcoma) as well as hematological tumors such as myeloma (Surget et al., 2012; Picarda et al., 2010; Chen W. et al., 2021; Newton, 2023). Subbiah et al. (Newton, 2023) reported that the DR5 agonist INBRX-109 showed encouraging antitumor activity and a favorable safety profile in patients with unresectable/metastatic chondrosarcoma in a phase I study. Pishas et al. (2013). Evaluated the efficacy of drozitumab, a human monoclonal agonistic antibody against DR5, as a novel therapeutic avenue for the targeted treatment of bone and soft tissue sarcomas. Because DR5 is highly expressed on the cell surface of primary osteosarcoma and soft tissue sarcoma (Gamie et al., 2024), targeting DR5 in combination with other antitumor agents has become a promising strategy for the treatment of bone tumors and soft tissue sarcomas.
Various mechanisms have been reported for DR5 upregulation, including CHOP [since CHOP acts as a dimer with other C/EBP proteins, it may form a heterodimer with C/EBPb on the DR5 promoter (Ubeda et al., 1999)]; Activation of ERK [ERK 1/2 and RSK 2 signaling leads to ATF 4 activation, which in turn promotes CHOP induction and subsequent DR 5 expression (Oh et al., 2010)]; p53 [p53 has been shown to directly transactivate the DR5 gene (Takimoto and El-Deiry, 2000)]; JNK [JNK has been shown to activate CHOP by binding to the AP-1 binding site in the CHOP promoter region, which then upregulates DR5 expression (Ubeda et al., 1999)]; Sp1 [Activated Sp1 binds the TATA-minor promoter of the DR5 gene, which contains two Sp1 binding sites spanning regions 198 to 116. Sp1 binding is important for basal transcription of DR5 (Yoshidaa et al., 2001)]; NF-κB [the p65 subunit of NF-κB was also found to be able to increase DR5 expression by binding to the first intronic region of the DR5 gene (Chen et al., 2008)]; YY1 [The transcriptional repressor YY 1 negatively regulates DR5 transcription and expression by binding to putative DNA binding sites (804–794 bp) in the DR5 promoter (Yoshidaa et al., 2001)].
Many studies had demonstrated a significantly more important role of DR5 up-expression in promoting tumor cells apoptosis than other TRAIL receptors such as DR4. Surget et al. highlighted (Surget et al., 2012) that p53 selectively enhances the sensitivity of multiple myeloma cells to apoptosis through the modulation of DR5 but not DR4. Yang et al. (2012) demonstrated that apoptosis was induced in different hepatocellular carcinoma cell lines including Hep3B, Huh-7, and HepG2, through a combination of TRAIL and 5,7-dimethoxyflavone (DMF), with a dose-responsive augmentation of DR5 protein levels, while DR4 levels were unaffected, underscoring the pivotal role of DR5 upregulation in boosting the sensitivity to TRAIL-induced apoptosis in these hepatocellular carcinoma cell lines. Horinaka et al. (2012) reported that aclarubicin (ACR), in conjunction with TRAIL, synergistically promoted apoptosis in Jurkat cells from acute lymphoblastic leukemia and A549 lung adenocarcinoma cells by significantly increasing DR5 expression. Zhou et al. (2013) investigated the impact of zingiber officinale (casticin) on H157 tumor cells apoptosis and documented a marked elevation in DR5 expression while DR4 expression remained stable. Sakai et al. (Todo et al., 2013) discovered that ibuprofen amplified TRAIL-induced apoptosis in HCT116 tumor cells by promoting DR5 expression at both the gene and protein levels while without affecting DR4 expression. Chen et al. (2012) showed that ROS-dependent and CHOP- regulated DR5 expression played a critical role in IOA synergistic enhancement of TRAIL-induced apoptosis in HepG2 cells. Kim et al. (2008) demonstrated that rosiglitazone enhanced TRAIL-induced apoptosis in a variety of cancer cells through ROS-mediated upregulation of DR5 and downregulation of c-FLIPs. Moon et al. (2013) showed that apiacein A (VA) triggered TRAIL-induced apoptosis by generating ROS in response to eIF2α/CHOP-dependent DR5 induction. Taniguchi et al. (2008) showed that in SW 480 colon cancer cells, baicalein upregulated CHOP expression, which subsequently induced DR5 expression and restored sensitivity to TRAIL-induced apoptosis while baicalein increased DR5 transcription in a reactive oxygen species (ROS)-dependent manner in T-cell leukemia Jurkat cells and prostate cancer cell lines PC 3 and DU 145.
Meanwhile, it should be specially addressed that the cellular localization regulation mechanism of DR5 is very complex and has not been fully clarified (Mert and Sanlioglu, 2017; Ren et al., 2004), the DR5 localization on the cell membrane is the prerequisite for its initiation of apoptosis, that is, only when upregulation of DR5 occurs on the cell surface is it directly associated with its pro-apoptotic effect. Haselmann et al. reported that DR5 had a dual but opposite function, that is, when bound by TRAIL on the cell surface, it can induce apoptotic cell death, but once inside the nucleus, it promotes cell survival and/or proliferation (Haselmann et al., 2014). Liu et al. showed that the isolation of esophageal cancer cells (EC9706) did not affect total DR5 protein levels in the cells, but provided a relocation of DR5 to the cell surface (Liu et al., 2009). The localization regulation of DR5 is regulated by multiple levels such as post-translational modification, vesicle transport and stress signal, and its dynamic distribution directly determine the selection and initiation of pro-apoptotic or pro-proliferative functions. In-depth understanding of the localization mechanism of DR5 can provide theoretical basis for the development of novel therapies targeting tumor apoptosis pathways, especially in the field of overcoming drug resistance and precision medicine.
Mediating the classic TRAIL apoptosis signaling pathway is the core biological function of DR5 (Yang, 2012; Cotter and Al-Rubeai, 1995; Bock and Riley, 2023; Moon et al., 2023). Recent research into the complex cell death regulation mechanisms has also indicated that DR5 might also be involved in the regulation of other cell death pathways such as necroptosis (Aikawa et al., 2023; Hayashi et al., 2021; Holler et al., 2000) and autophagy (Das et al., 2017; Hu et al., 2019)-dependent cell death. Although no direct studies have yet demonstrated a link between DR5 and the regulation of pyroptosis, existing research (Fritsch et al., 2019; Orning et al., 2018; Sarhan et al., 2018) suggests that caspase-8, a key downstream molecule of the TRAIL/DR5 signaling pathway, can induce pyroptosis by cleaving GSDMD into its active form. This indirectly implies that DR5 may also play a role in the pyroptosis signaling pathway. Furthermore, our team recently found that DR5 antagonists can effectively mitigate pyroptosis-related damage in intestinal tissue cells induced by high doses of gamma radiation, both in vitro and in vivo (unpublished data). Figure 1 displayed the schematic drawing of the cell death regulatory signaling pathways that DR5 may be involved in according to the existing literature and consequent speculation.
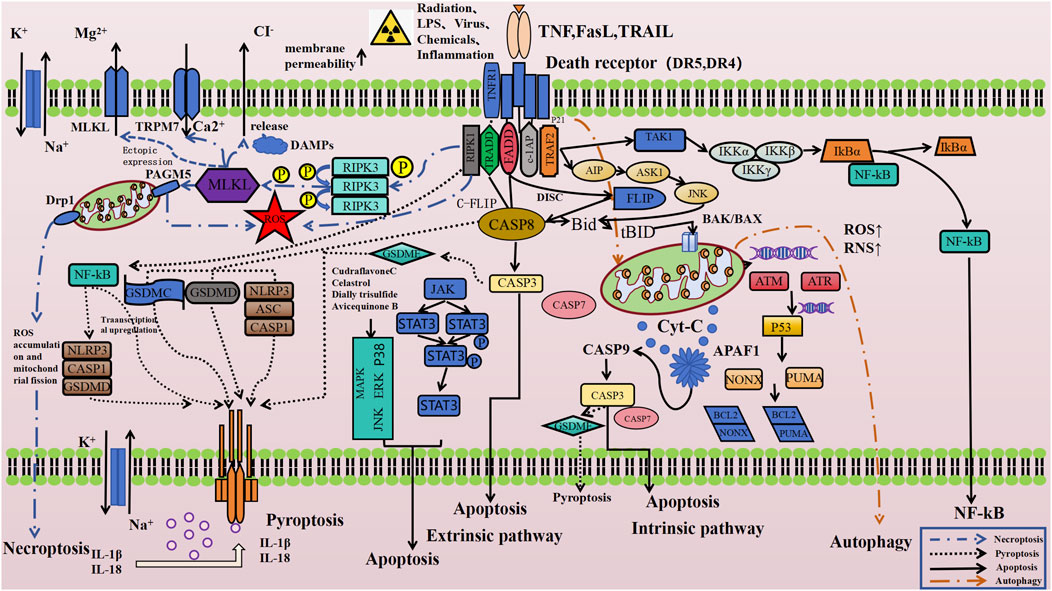
Figure 1. TRAIL-DR5 related cell death regulation signaling pathway (Das et al., 2017; Hu et al., 2019; Gielecińska et al., 2023; Tong et al., 2022; Peng F. et al., 2022; Sanz et al., 2023; Bertheloot et al., 2021; De Meyer et al., 2024; Hadian and Stockwell, 2023; Chen X. et al., 2021; Bedoui et al., 2020; Vandenabeele et al., 2023; Yuan and Ofengeim, 2024; Newton et al., 2021; Hou et al., 2020). Created with BioRender.com, accessed on 10 October 2024.
In addition to regulating cell death, DR5 also plays key regulatory roles in a variety of physiological and pathological processes, including proliferation promotion (Belyanskaya et al., 2008; Vilimanovich and Bumbasirevic, 2008; Ishimura et al., 2006; Wang et al., 2013; Secchiero et al., 2008; Secchiero et al., 2004; Trauzold et al., 2006; Von Karstedt et al., 2017; Schneider et al., 1997; Muhlenbeck et al., 1998; Lee et al., 2002; Milani et al., 2003; Choo et al., 2006; Song and Lee, 2008; Xu et al., 2010; Liu et al., 2024), inflammation (Ehrhardt et al., 2003), tissue regeneration (Vilimanovich and Bumbasirevic, 2008), immune regulation (Ishimura et al., 2006), anti-tumor (Secchiero et al., 2008; Secchiero et al., 2004), maintenance of body development and homeostasis (Guo et al., 2005; Voigt et al., 2014; Wang, 2014), etc., and intersects with a variety of other signaling pathways to form a complex regulatory network. Table 1 summarizes the main biological functions of DR5.
Research on agonists targeting DR5 predominantly focuses on oncology due to DR5’s overexpression in various tumor cells and rare expression in normal tissues, establishing DR5 as a significant target for tumor therapy. Additionally, DR5 agonists are investigated in autoimmune diseases, liver fibrosis, and other conditions. Current studies on DR5 agonists are summarized in Table 2.
Compared to DR5 agonists, research into DR5 antagonists commenced later. However, as studies on DR5-mediated cell signaling pathways deepened. The researchers (Leng et al., 2014) found that DR5 is over-activated in response to external stimuli leading to excessive cell death and impaired function in target organs and tissues, which points to a new direction in exploring the development and pathological mechanisms of various clinical diseases. Currently, the focus on DR5 antagonists has intensified, showing significant promise for treating conditions associated with DR5 hyperactivation, such as severe viral infections (Peng H. et al., 2022), inflammation, ischemia-reperfusion injury (Xiaochun et al., 2022; Zhang, 2018; Liu, 2018), and autoimmune diseases. Our team’s studies have also shown that high-dose γ-rays (Zhao et al., 2023) significantly increase DR5 expression in vital organs and tissues, and administering DR5 antagonist interventions notably improved survival rates and organ function recovery in animals with acute radiation sickness. Research and development efforts for DR5 antagonists currently encompass the screening of small molecule compounds, antibody structures, and peptide sequence optimization. Current studies targeting DR5 antagonists are outlined in Table 3.
With advancing research, the TRAIL-DR5 pathway has been recognized for its crucial physiological functions in maintaining normal physiological homeostasis and growth. A growing body of evidence suggests that it plays an important role in the onset and progression of a variety of diseases, with the specific mechanisms described in Figure 2. External stimuli can significantly upregulate DR5 protein expression, activating the TRAIL-DR5 signaling pathway and triggering apoptosis. While this activation is a key regulatory mechanism for homeostasis, it may also cause excessive death of functional cells in target organs in some instances. Numerous studies have explored the efficacy and safety of targeting DR5 in various diseases using activation or antagonism strategies, analyzing potential challenges and future research directions.
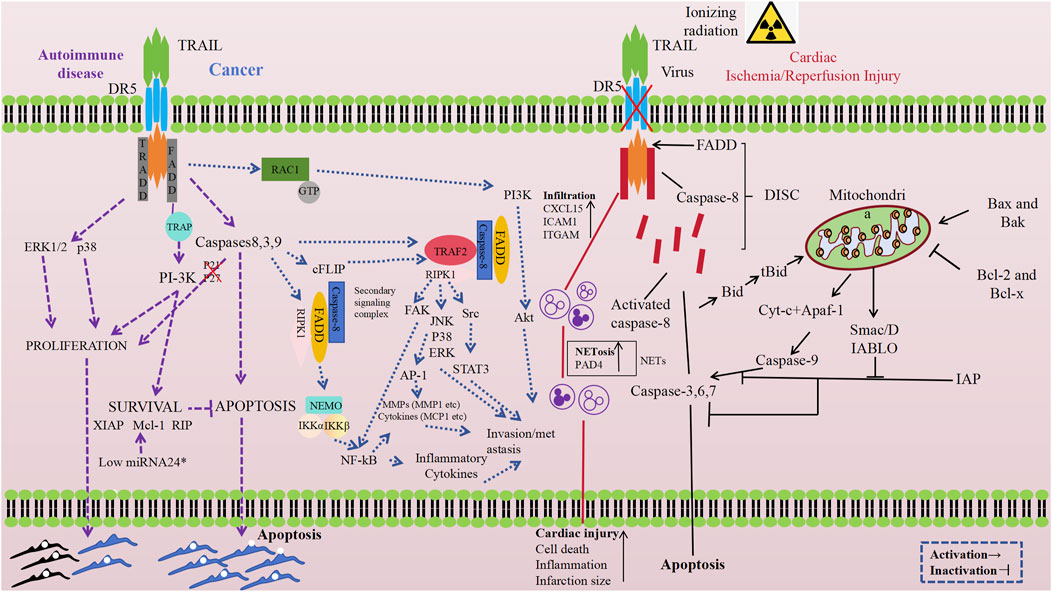
Figure 2. Schematic representation of DR5 antagonists or agonists effects in different diseases (Peng H. et al., 2022; Zhang, 2018; Collison et al., 2009; Oh and Sun, 2021; Audo et al., 2013; Wang et al., 2024). Created with BioRender.com, accessed on 10 October 2024.
DR5 has been extensively explored as a novel drug target in oncology, primarily inducing apoptosis in tumor cells, and several DR5 agonists have reached clinical trials (Voigt et al., 2014; Classic, 2018a; Herbst et al., 2010; Ashkenazi, 2008; Ashkenazi and Herbst, 2008; Ashkenazi et al., 2008; Ashkenazi et al., 1999; Pollack et al., 2001; Lawrence et al., 2001; Qin et al., 2001; Burvenich et al., 2016; Dominguez et al., 2017), however, due to the phenomenon of drug resistance caused by tumor immune escape, insufficient drug delivery efficiency, and low targeting efficiency or low receptor cross-linking efficiency, the tumor targeting therapy of DR5 agonists has encountered many difficulties. At present, the research on the mechanism of drug resistance (Kim et al., 2018; Gupta et al., 2013) of DR5 agonists has attracted much attention, and the future research on DR5 agonists as tumor targeted therapy will focus on the novel DR5 agonists design (Schneider et al., 2010; Li et al., 2024), combination therapy (Zheng et al., 2023; Casagrande Raffi et al., 2024) and new delivery system development, etc., aiming to improve targeted efficacy and safety.
For example, Casagrande Raffi et al. (2024) suggested that salinomycin may be effective when used in combination with age-delaying cancer therapies. The combination of a death receptor 5 agonist antibody and Salinomycin is a potent anti-aging drug cocktail and the combination triggers immune destruction of senescent cancer cells mediated by natural killer cells and CD 8 + T cells with the involvement of interleukin-18. Li et al. (2024) prepared a coupling agent containing multiple copies of DR5-targeting peptide (P-cDR5), which significantly improved DR5 aggregation and effectively induced apoptosis. Combining P-cDR5 with the histone deacetylase inhibitor valproic acid further enhances apoptosis-inducing efficacy by increasing Caspase-8 and activating the exogenous apoptotic pathway, while destabilizing mitochondrial membranes and increasing the sensitivity of TRAIL-resistant cells. These findings suggest that ligating multiple cDR5 peptides onto flexible water-soluble polymer carriers not only overcomes the limitations of previous designs, but also provides new ideas for the treatment of drug-resistant cancers.
DR5 agonists play a role in rheumatoid arthritis primarily through their regulatory effects on apoptosis, immunomodulation, and inflammatory responses. I-Tsu et al. (Chyuan et al., 2018) observed that DR5 activation reduced joint inflammation and destruction in a mouse model of rheumatoid arthritis. Jin et al. (2010) demonstrated that activating DR5 could induce apoptosis in synoviocytes and inflammatory cells, reducing the production of inflammatory mediators. Although DR5 agonists show potential in RA, their specific mechanisms require further investigation. Additionally, genotypic and phenotypic variations among patients may affect responses to DR5 agonists. Future research will focus on developing DR5 agonists to promote apoptosis in inflammatory cells, thereby diminishing inflammation. Furthermore, combining DR5 agonists with other anti-inflammatory or immunomodulatory drugs, such as DMARDs (Singh, 2022) or biologics, could enhance therapeutic outcomes.
DR5 agonists regulate apoptosis and immunomodulation in inflammatory bowel disease. Zhu et al. (2014) found that DR5 activation mitigated intestinal inflammation and damage in a mouse model of IBD. In vitro, DR5 agonists reduced the release of inflammatory mediators by inducing apoptosis in inflammatory and intestinal epithelial cells (Kuo et al., 2019). However, the ability of DR5 to induce apoptosis in inflammatory cells does not preclude these cells from evading immune surveillance due to complex immune escape and response mechanisms. Future research is directed toward developing novel DR5 agonists that promote apoptosis in inflammatory cells to reduce inflammation. Therapeutic strategies combining DR5 agonists with other anti-inflammatory agents, such as glucocorticoids, immunosuppressants, or biologics, are also being explored to enhance therapeutic effects.
DR5 agonists regulate apoptosis and immunomodulation in SLE, (Hilliard et al., 2001; Song et al., 2000; Ichikawa et al., 2003), with Lamhamedi-Cherradi SE et al. (Lamhamedi-Cherradi et al., 2003; Liu et al., 2003) finding that DR5 activation reduced inflammation and organ damage in a mouse model of SLE. In vitro studies show that activation of DR5 can induce apoptosis in immune cells, thereby reducing inflammatory mediator production (Crowder et al., 2011). Although DR5 agonists show promise for treating SLE, no large-scale clinical trials have yet been conducted, and issues such as adverse effects and immune escape persist. Further studies is necessary to uncover the roles and influencing factors of DR5 in SLE and to examine the potential benefits of combining it with other anti-inflammatory agents to boost therapeutic effectiveness.
Excessive cardiac cell death, a primary pathological feature of myocardial infarction (MI), can be substantially alleviated by inhibiting TRAIL with DR5 antagonists, such as sDR5-Fc fusion proteins, which have been shown to improve outcomes following myocardial infarction (MI). Wang Y. et al. (2020) noted increased levels of both DR5 and TRAIL in MI contexts, with a corresponding reduction in cardiomyocyte death and inflammation upon blocking TRAIL. Similarly, Cui et al. (2010) observed that blocking the TRAIL-DR5 interaction with a soluble DR5 antagonist decreased ischemic cell death following global cerebral ischemia, suggesting a potential neuroprotective strategy for ischemic stroke through inhibition of the TRAIL-DR5 system. Future research will focus on developing novel DR5 antagonists with improved drug properties and considering co-administration with other cardiovascular therapeutics to enhance efficacy.
Research into the use of DR5 as a therapeutic target for viral hepatitis presents mixed outcomes. Mundt et al. (2003) demonstrated that apoptosis in virally infected hepatocytes is facilitated by the TRAIL-DR5 pathway, enabling the selective elimination of virus-infected hepatocytes while sparing normal cells, proposing DR5 agonists as a viable treatment for viral hepatitis. In contrast, Liu et al. (2007) reported that an sDR5 antagonist could reduce liver damage by blocking TRAIL-induced apoptosis in HBV-infected hepatocytes, underscoring the complexity and crucial role of the TRAIL-DR5 system in the pathogenesis of viral hepatitis. These findings necessitate further research to elucidate TRAIL-DR5 regulatory mechanisms in viral hepatitis, distinguish between the expression levels in virus-infected versus normal cells, and ensure the safety and efficacy of both DR5 antagonists and agonists.
Liver fibrosis is closely related to DR5, an apoptosis factor receptor predominantly expressed on the surface of activated hepatic stellate cells (HSCs), which are central to the development of liver fibrosis. Studies have shown that anti-DR5 antibodies induce apoptosis in activated HSCs, exhibiting anti-fibrotic effects and presenting a potential therapeutic approach for liver fibrosis (You et al., 2021). Furthermore, TRAIL, a member of the TNF family, interacts with HSCs during both progression and reversal stages of liver fibrosis. This interaction, coupled with increased DR5 expression on HSCs, inhibits collagen formation and extracellular matrix (ECM) deposition, mitigating liver fibrosis (Tao et al., 2021). Additional research indicates that exogenous TRAIL induces apoptosis in activated HSC-T6 cells, potentially through upregulated DR5 and mitochondrial Bax expression (Gao et al., 2010). These insights highlight the pivotal role of DR5 in liver fibrosis, suggesting that modulation of DR5 expression and function could be a strategy to control or reverse hepatic fibrosis. However, the mechanisms by which DR5 operates and its potential clinical applications in liver fibrosis are still largely unexplored; future studies should aim to detail the specific regulatory mechanisms of TRAIL-DR5 in liver fibrosis and the differential expression between fibrotic and healthy liver cells to improve the safety and effectiveness of DR5-targeted therapies.
The potential of DR5 antagonists in treating acute radiation damage has recently begun to be investigated. Our team’s previous research (Zhao et al., 2023) had shown that antagonizing DR5 significantly enhanced survival rates in an acute radiation sickness mouse model, reduced tissue damage as well as inflammatory responses, and inhibited the excessive apoptosis of functional cells. And our further study indicated that DR5 antagonist efficiently inhibited the enterocytes excessive pyroptosis caused by large dose of γ-radiation (unpublished data), which indicated the great potential of DR5 antagonist as radiation damage protection candidate. However, the comprehensive cellular regulatory mechanisms of DR5 under biological damage effects caused by radiation require further investigation. Future studies should focus on the regulatory mechanism of cell death, designing novel DR5 antagonists, optimizing their metabolic properties in vivo and addressing the key safety concerns, etc.
In conclusion, although the exploration of DR5 in disease treatment is nascent, its potential therapeutic benefits and broad applicability are promising. Future research should develop diverse, precise, and combinatorial therapeutic strategies based on an in-depth understanding of DR5’s biological activity and regulatory mechanisms, to extend its application across various disease treatments.
XQ: Writing–original draft, Writing–review and editing. SG: Data curation, Writing–review and editing. ZM: Data curation, Writing–review and editing. HG: Data curation, Investigation, Writing–review and editing. ZW: Data curation, Writing–review and editing. YS: Data curation, Writing–review and editing. SL: Data curation, Writing–review and editing. GD: Data curation, Writing–review and editing. RG: Data curation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work financially supported by Beijing Science and Technology Program (No. 1916315ZD00900105).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, C., Totpal, K., Lawrence, D., Marsters, S., Pitti, R., Yee, S., et al. (2008). Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death and Differ. 15 (4), 751–761. doi:10.1038/sj.cdd.4402306
Aikawa, A., Kozako, T., Kato, N., Ohsugi, T., and Honda, S. I. (2023). Anti-tumor activity of 5-aminoimidazole-4-carboxamide riboside with AMPK-independent cell death in human adult T-cell leukemia/lymphoma. Eur. J. Pharmacol. 961, 176180. doi:10.1016/j.ejphar.2023.176180
Ashkenazi, A. (2008). Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 7 (12), 1001–1012. doi:10.1038/nrd2637
Ashkenazi, A., and Herbst, R. S. (2008). To kill a tumor cell: the potential of proapoptotic receptor agonists. J. Clin. investigation 118 (6), 1979–1990. doi:10.1172/JCI34359
Ashkenazi, A., Holland, P., and Eckhardt, S. G. (2008). Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor–related apoptosis-inducing ligand (rhApo2L/TRAIL). J. Clin. Oncol. 26 (21), 3621–3630. doi:10.1200/JCO.2007.15.7198
Ashkenazi, A., Pai, R. C., Fong, S., Leung, S., Lawrence, D. A., Marsters, S. A., et al. (1999). Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. investigation 104 (2), 155–162. doi:10.1172/JCI6926
Audo, R., Combe, B., Hahne, M., and Morel, J. (2013). The two directions of TNF-related apoptosis-inducing ligand in rheumatoid arthritis. Cytokine 63 (2), 81–90. doi:10.1016/j.cyto.2013.04.011
Author Anonymous (2025a). Eponemin (circularly permuted TRAIL) - drug target: DR4 x DR5_Investigational indication: multiple MyelomaPatented clinical development.
Author Anonymous (2025b). Aponermin_ _aponermin- target: DR5_ indication: multiple myeloma- clinical patent approved.
Bedoui, S., Herold, M. J., and Strasser, A. (2020). Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. cell Biol. 21 (11), 678–695. doi:10.1038/s41580-020-0270-8
Belyanskaya, L. L., Ziogas, A., Hopkins-Donaldson, S., Kurtz, S., Simon, H. U., Stahel, R., et al. (2008). TRAIL-induced survival and proliferation of SCLC cells is mediated by ERK and dependent on TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer 60 (3), 355–365. doi:10.1016/j.lungcan.2007.11.005
Bertheloot, D., Latz, E., and Franklin, B. S. (2021). Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell. and Mol. Immunol. 18 (5), 1106–1121. doi:10.1038/s41423-020-00630-3
Bock, F. J., and Riley, J. S. (2023). When cell death goes wrong: inflammatory outcomes of failed apoptosis and mitotic cell death. Cell Death Differ. 30 (2), 293–303. doi:10.1038/s41418-022-01082-0
Brünker, P., Wartha, K., Friess, T., Grau-Richards, S., Waldhauer, I., Koller, C. F., et al. (2016). RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers FAP-dependent, avidity-driven DR5 hyperclustering and tumor cell apoptosis. Mol. cancer Ther. 15 (5), 946–957. doi:10.1158/1535-7163.MCT-15-0647
Burvenich, I. J., Lee, F. T., Guo, N., Gan, H. K., Rigopoulos, A., Parslow, A. C., et al. (2016). In vitro and in vivo evaluation of 89zr-DS-8273a as a theranostic for anti-death receptor 5 therapy. Theranostics 6 (12), 2225–2234. doi:10.7150/thno.16260
Carter, P. J., and Rajpal, A. (2022). Designing antibodies as therapeutics. Cell 185 (15), 2789–2805. doi:10.1016/j.cell.2022.05.029
Casagrande Raffi, G., Chen, J., Feng, X., Chen, Z., Lieftink, C., Deng, S., et al. (2024). An antibiotic that mediates immune destruction of senescent cancer cells. Proc. Natl. Acad. Sci. 121 (52), e2417724121. doi:10.1073/pnas.2417724121
Chaudhary, P. M., Eby, M., Jasmin, A., Bookwalter, A., Murray, J., and Hood, L. (1997). Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7 (6), 821–830. doi:10.1016/s1074-7613(00)80400-8
Chen, C. Y., Yiin, S. J., Hsu, J. L., Wang, W. C., Lin, S. C., and Chern, C. L. (2012). Isoobtusilactone A sensitizes human hepatoma Hep G2 cells to TRAIL-induced apoptosis via ROS and CHOP-mediated up-regulation of DR5. J. Agric. food Chem. 60 (13), 3533–3539. doi:10.1021/jf2051224
Chen, J. J., Chou, C. W., Chang, Y. F., and Chen, C. C. (2008). Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: involvement of NF-κB and reactive oxygen species-mediated p53 activation. J. Immunol. 180 (12), 8030–8039. doi:10.4049/jimmunol.180.12.8030
Chen, Q., Yan, D., Zhang, Q., Zhang, G., Xia, M., Li, J., et al. (2020). Treatment of acetaminophen-induced liver failure by blocking the death checkpoint protein TRAIL. Biochimica Biophysica Acta (BBA)-Molecular Basis Dis. 1866 (1), 165583. doi:10.1016/j.bbadis.2019.165583
Chen, W., Xia, Z., Fang, B., Fu, C., Li, W., Yang, L., et al. (2021a). P-192: circularly permuted TRAIL (CPT) combined with Thalidomide and Dexamethasone in patients with relapsed/refractory Multiple Myeloma: a randomized, double-blind, placebo-controlled phase 3 study. Clin. Lymphoma Myeloma Leukemia 21, S143. doi:10.1016/S2152-2650(21)02319-3
Chen, X., Zeh, H. J., Kang, R., Kroemer, G., and Tang, D. (2021b). Cell death in pancreatic cancer: from pathogenesis to therapy. Nat. Rev. Gastroenterology and hepatology 18 (11), 804–823. doi:10.1038/s41575-021-00486-6
Choo, M. K., Kawasaki, N., Singhirunnusorn, P., Koizumi, K., Sato, S., Akira, S., et al. (2006). Blockade of transforming growth factor-beta-activated kinase 1 activity enhances TRAIL-induced apoptosis through activation of a caspase cascade. Mol. cancer Ther. 5 (12), 2970–2976. doi:10.1158/1535-7163.MCT-06-0379
Chyuan, I. T., Tsai, H. F., Liao, H. J., Wu, C. S., and Hsu, P. N. (2018). An apoptosis-independent role of TRAIL in suppressing joint inflammation and inhibiting T-cell activation in inflammatory arthritis. Cell. and Mol. Immunol. 15 (9), 846–857. doi:10.1038/cmi.2017.2
Classic (2018a). Cilinical Trials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT02983006 (Accessed January 18, 2025).
Classic (2018b). Cilinical Trials.gov. Available online at: https://classic.clinicaltrials.gov/ct2/show/NCT04553692 (Accessed January 18, 2025).
Collison, A., Foster, P. S., and Mattes, J. (2009). Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin. Exp. Pharmacol. Physiology 36 (11), 1049–1053. doi:10.1111/j.1440-1681.2009.05258.x
Cotter, T. G., and Al-Rubeai, M. (1995). Cell death (apoptosis) in cell culture systems. Trends Biotechnol. 13 (4), 150–155. doi:10.1016/S0167-7799(00)88926-X
Crowder, R. N., Zhao, H., Chatham, W. W., Zhou, T., and Carter, R. H. (2011). B lymphocytes are resistant to death receptor 5-induced apoptosis. Clin. Immunol. 139 (1), 21–31. doi:10.1016/j.clim.2010.12.006
Cui, M., Wang, L., Liang, X., Ma, X., Liu, Y., Yang, M., et al. (2010). Blocking TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal damage after transient global cerebral ischemia. Neurobiol. Dis. 39 (2), 138–147. doi:10.1016/j.nbd.2010.03.018
Das, S., Nayak, A., Siddharth, S., Nayak, D., Narayan, S., and Kundu, C. N. (2017). TRAIL enhances quinacrine-mediated apoptosis in breast cancer cells through induction of autophagy via modulation of p21 and DR5 interactions. Cell. Oncol. 40, 593–607. doi:10.1007/s13402-017-0347-3
De Meyer, G. R., Zurek, M., Puylaert, P., and Martinet, W. (2024). Programmed death of macrophages in atherosclerosis: mechanisms and therapeutic targets. Nat. Rev. Cardiol. 21 (5), 312–325. doi:10.1038/s41569-023-00957-0
de Miguel, D., Lemke, J., Anel, A., Walczak, H., and Martinez-Lostao, L. (2016). Onto better TRAILs for cancer treatment. Cell Death and Differ. 23 (5), 733–747. doi:10.1038/cdd.2015.174
Di Cristofano, F., George, A., Tajiknia, V., Ghandali, M., Wu, L., Zhang, Y., et al. (2023). Therapeutic targeting of TRAIL death receptors. Biochem. Soc. Trans. 51 (1), 57–70. doi:10.1042/BST20220098
Dominguez, G. A., Condamine, T., Mony, S., Hashimoto, A., Wang, F., Liu, Q., et al. (2017). Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin. Cancer Res. 23 (12), 2942–2950. doi:10.1158/1078-0432.CCR-16-1784
Dufour, F., Rattier, T., Constantinescu, A. A., Zischler, L., Morlé, A., Mabrouk, H. B., et al. (2016). TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 8 (6), 9974–9985. doi:10.18632/oncotarget.14285
Ehrhardt, H., Fulda, S., Schmid, I., Hiscott, J., Debatin, K. M., and Jeremias, I. (2003). TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene 22 (25), 3842–3852. doi:10.1038/sj.onc.1206520
Eng, J. W. L., Mace, T. A., Sharma, R., Twum, D. Y., Peng, P., Gibbs, J. F., et al. (2016). Pancreatic cancer stem cells in patient pancreatic xenografts are sensitive to drozitumab, an agonistic antibody against DR5. J. Immunother. cancer 4, 33–11. doi:10.1186/s40425-016-0136-y
Fancy, R. M., Kim, H., Napier, T., Buchsbaum, D. J., Zinn, K. R., and Song, Y. (2018). Calmodulin antagonist enhances DR5-mediated apoptotic signaling in TRA-8 resistant triple negative breast cancer cells. J. Cell. Biochem. 119 (7), 6216–6230. doi:10.1002/jcb.26848
Forero, A., Bendell, J. C., Kumar, P., Janisch, L., Rosen, M., Wang, Q., et al. (2017). First-in-human study of the antibody DR5 agonist DS-8273a in patients with advanced solid tumors. Investig. New Drugs 35, 298–306. doi:10.1007/s10637-016-0420-1
Forero-Torres, A., Infante, J. R., Waterhouse, D., Wong, L., Vickers, S., Arrowsmith, E., et al. (2013). Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2 (6), 925–932. doi:10.1002/cam4.137
Fritsch, M., Günther, S. D., Schwarzer, R., Albert, M. C., Schorn, F., Werthenbach, J. P., et al. (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575 (7784), 683–687. doi:10.1038/s41586-019-1770-6
Gamie, Z., Krippner-Heidenreich, A., Gerrand, C., and Rankin, K. S. (2024). Targeting death receptor 5 (DR5) for the imaging and treatment of primary bone and soft tissue tumors: an update of the literature. Front. Mol. Biosci. 11, 1384795. doi:10.3389/fmolb.2024.1384795
Ganten, T. M., Sykora, J., Koschny, R., Batke, E., Aulmann, S., Mansmann, U., et al. (2009). Prognostic significance of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor expression in patients with breast cancer. J. Mol. Med. 87, 995–1007. doi:10.1007/s00109-009-0510-z
Gao, J., Zhao, Y. H., Kang, P., Liang, M., and Li, S. C. (2010). TRAIL induces apoptosis and mechanism of HSC-T6 in rat hepatic stellate cell line. J. J. Clin. Hepatobiliary Dis. 26 (03), 300–303.
Garcia-Martinez, J. M., Wernitznig, A., Rinnenthal, J., Impagnatiello, M. A., Hilberg, F., Giragossian, C., et al. (2019). Abstract 2051: BI 905711, a novel CDH17-targeting TRAILR2 agonist, effectively triggers tumor cell apoptosis and tumor regressions selectively in CDH17-positive colorectal cancer models. Cancer Res. 79 (13_Suppl. ment), 2051. doi:10.1158/1538-7445.AM2019-2051
Gielecińska, A., Kciuk, M., Yahya, E. B., Ainane, T., Mujwar, S., and Kontek, R. (2023). Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochimica Biophysica Acta (BBA)-Reviews Cancer 1878, 189024. doi:10.1016/j.bbcan.2023.189024
Greer, Y. E., Gilbert, S. F., Gril, B., Narwal, R., Peacock Brooks, D. L., Tice, D. A., et al. (2019). MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer. Breast Cancer Res. 21, 27–17. doi:10.1186/s13058-019-1116-1
Guo, Y., Chen, C., Zheng, Y., Zhang, J., Tao, X., Liu, S., et al. (2005). A novel anti-human DR5 monoclonal antibody with tumoricidal activity induces caspase-dependent and caspase-independent cell death. J. Biol. Chem. 280 (51), 41940–41952. doi:10.1074/jbc.M503621200
Gupta, S. C., Francis, S. K., Nair, M. S., Mo, Y. Y., and Aggarwal, B. B. (2013). Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J. Biol. Chem. 288 (45), 32343–32356. doi:10.1074/jbc.M113.455188
Hadian, K., and Stockwell, B. R. (2023). The therapeutic potential of targeting regulated non-apoptotic cell death. Nat. Rev. Drug Discov. 22 (9), 723–742. doi:10.1038/s41573-023-00749-8
Han, J., Machado, A. A., Mahendra, M., Daniele, J. R., Bristow, C. A., Huang, J. K. L., et al. (2021). Abstract 985: BI 905711 selectively induces apoptosis and anti-tumor response in TRAILR2/CDH17- expressing pancreatic cancer models. Cancer Res. 81 (13_Suppl. ment), 985. doi:10.1158/1538-7445.AM2021-985
Harding, J. J., Hofheinz, R. D., Elez, E., Kuboki, Y., Rasco, D. W., Cecchini, M., et al. (2023). “A phase Ia/b first-in-human, open-label, multicenter study of BI 905711, a bispecific TRAILR2 agonist,” in Patients with advanced gastrointestinal cancers. doi:10.1200/JCO.2023.41.4_suppl.115
Haselmann, V., Kurz, A., Bertsch, U., Hübner, S., Olempska–Müller, M., Fritsch, J., et al. (2014). Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 146 (1), 278–290. doi:10.1053/j.gastro.2013.10.009
Hayashi, Y., Suzuki, H., Nakajima, W., Uehara, I., Tanimura, A., Himeda, T., et al. (2021). Virus-infection in cochlear supporting cells induces audiosensory receptor hair cell death by TRAIL-induced necroptosis. Plos one 16 (11), e0260443. doi:10.1371/journal.pone.0260443
Herbst, R. S., Eckhardt, S. G., Kurzrock, R., Ebbinghaus, S., O'Dwyer, P. J., Gordon, M. S., et al. (2010). Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 28 (17), 2839–2846. doi:10.1200/JCO.2009.25.1991
Hilliard, B., Wilmen, A., Seidel, C., Liu, T. S. T., Göke, R., and Chen, Y. (2001). Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J. Immunol. 166 (2), 1314–1319. doi:10.4049/jimmunol.166.2.1314
Holler, N., Zaru, R., Micheau, O., Thome, M., Attinger, A., Valitutti, S., et al. (2000). Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1 (6), 489–495. doi:10.1038/82732
Horinaka, M., Yoshida, T., Nakata, S., Shiraishi, T., Tomosugi, M., Yoshikawa, S., et al. (2012). Aclarubicin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis through death receptor 5 upregulation. Cancer Sci. 103 (2), 282–287. doi:10.1111/j.1349-7006.2011.02150.x
Hou, J., Zhao, R., Xia, W., Chang, C. W., You, Y., Hsu, J. M., et al. (2020). PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. cell Biol. 22 (10), 1264–1275. doi:10.1038/s41556-020-0575-z
Hu, L., Wang, Y., Chen, Z., Fu, L., Wang, S., Zhang, X., et al. (2019). Hsp90 inhibitor SNX-2112 enhances TRAIL-Induced apoptosis of human cervical cancer cells via the ROS-mediated JNK-p53-autophagy-DR5 pathway. Oxidative Med. Cell. Longev. 2019 (1), 9675450. doi:10.1155/2019/9675450
Huang, N., Tang, K., and Chen, W. (2023). New progress of TRAIL in the treatment of HCC. J. Acta Med. Sin. 36 (05), 179–185. doi:10.19296/j.cnki.1008-2409.2023-05-034
Huang, Y., Liang, B., Jiang, Q., Chen, C., Yang, K., Li, C., et al. (2014). Tumoricidal activity of combining the agonistic DR5 antibody D-6 with cisplatin in C30 cisplatin-resistant ovarian cancer in vitro and in vivo. Mol. Med. Rep. 10 (1), 183–190. doi:10.3892/mmr.2014.2193
Humphreys, R. C., and Halpern, W. (2008). Trail receptors: targets for cancer therapy. Adv. Exp. Med. Biol. 615, 127–158. doi:10.1007/978-1-4020-6554-5_7
Ichikawa, K., Liu, W., Fleck, M., Zhang, H., Zhao, L., Ohtsuka, T., et al. (2003). TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J. Immunol. 171 (2), 1061–1069. doi:10.4049/jimmunol.171.2.1061
Ichikawa, K., Liu, W., Zhao, L., Wang, Z., Liu, D., Ohtsuka, T., et al. (2001). Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7 (8), 954–960. doi:10.1038/91000
Ishimura, N., Isomoto, H., Bronk, S. F., and Gores, G. J. (2006). Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am. J. Physiology-Gastrointestinal Liver Physiology 290 (1), G129–G136. doi:10.1152/ajpgi.00242.2005
Jazirehi, A. R., Kurdistani, S. K., and Economou, J. S. (2014). Histone deacetylase inhibitor sensitizes apoptosis-resistant melanomas to cytotoxic human T lymphocytes through regulation of TRAIL/DR5 pathway. J. Immunol. 192 (8), 3981–3989. doi:10.4049/jimmunol.1302532
Jin, C. H., Chae, S. Y., Kim, T. H., Yang, H. K., Lee, E. Y., Song, Y. W., et al. (2010). Effect of tumor necrosis factor-related apoptosis-inducing ligand on the reduction of joint inflammation in experimental rheumatoid arthritis. J. Pharmacol. Exp. Ther. 332 (3), 858–865. doi:10.1124/jpet.109.159517
Kang, Z., Chen, J. J., Yu, Y., Li, B., Sun, S. Y., Zhang, B., et al. (2011). Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin. Cancer Res. 17 (10), 3181–3192. doi:10.1158/1078-0432.CCR-10-2874
Kaplan-Lefko, P. J., Graves, J. D., Zoog, S. J., Pan, Y., Wall, J., Branstetter, D. G., et al. (2010). Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol. and Ther. 9 (8), 618–631. doi:10.4161/cbt.9.8.11264
Kelley, R. F., Totpal, K., Lindstrom, S. H., Mathieu, M., Billeci, K., DeForge, L., et al. (2005). Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J. Biol. Chem. 280 (3), 2205–2212. doi:10.1074/jbc.M410660200
Kelley, S. K., Harris, L. A., Xie, D., DeForge, L., Totpal, K., Bussiere, J., et al. (2001). Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299 (1), 31–38. doi:10.1016/s0022-3565(24)29298-3
Kim, S. L., Min, I. S., Park, Y. R., Lee, S. T., and Kim, S. W. (2018). Lipocalin 2 inversely regulates TRAIL sensitivity through p38 MAPK-mediated DR5 regulation in colorectal cancer Corrigendum in/10.3892/ijo. 2019.4748. Int. J. Oncol. 53 (6), 2789–2799. doi:10.3892/ijo.2019.4748
Kim, Y. H., Jung, E. M., Lee, T. J., Kim, S. H., Choi, Y. H., Park, J. W., et al. (2008). Rosiglitazone promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic. Biol. Med. 44 (6), 1055–1068. doi:10.1016/j.freeradbiomed.2007.12.001
Koyama, M., Sowa, Y., Horinaka, M., Goda, A. E., Fujiwara, J., and Sakai, T. (2014). Peroxisome proliferator-activated receptor γ ligand troglitazone and TRAIL synergistically induce apoptosis. Oncol. Rep. 31 (2), 947–954. doi:10.3892/or.2013.2868
Kuo, W. T., Shen, L., Zuo, L., Shashikanth, N., Ong, M. L. D. M., Wu, L., et al. (2019). Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology 157 (5), 1323–1337. doi:10.1053/j.gastro.2019.07.058
Lamhamedi-Cherradi, S. E., Zheng, S. J., Maguschak, K. A., Peschon, J., and Chen, Y. H. (2003). Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat. Immunol. 4 (3), 255–260. doi:10.1038/ni894
Lawrence, D., Shahrokh, Z., Marsters, S., Achilles, K., Shih, D., Mounho, B., et al. (2001). Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat. Med. 7 (4), 383–385. doi:10.1038/86397
Lee, M. W., Park, S. C., Yang, Y. G., Yim, S. O., Chae, H. S., Bach, J. H., et al. (2002). The involvement of reactive oxygen species (ROS) and p38 mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Lett. 512 (1-3), 313–318. doi:10.1016/s0014-5793(02)02225-1
Lei, G., Xu, M., Xu, Z., Lu, C., and Tan, S. (2018). Combination of novel DR5 targeting agonistic scFv antibody TR2-3 with cisplatin shows enhanced synergistic antitumor activity in vitro and in vivo. Biomed. and Pharmacother. 98, 271–279. doi:10.1016/j.biopha.2017.12.033
Leithner, K., Stacher, E., Wurm, R., Ploner, F., Quehenberger, F., Wohlkoenig, C., et al. (2009). Nuclear and cytoplasmic death receptor 5 as prognostic factors in patients with non-small cell lung cancer treated with chemotherapy. Lung cancer 65 (1), 98–104. doi:10.1016/j.lungcan.2008.10.015
Leng, X., Zhang, Q., Chen, Z., and Wang, D. (2014). Blocking TRAIL-DR5 signaling with soluble DR5 alleviates acute kidney injury in a severely burned mouse model. Int. J. Clin. Exp. Pathology 7 (6), 3460–3468.
Li, J., Arnold, J., Sima, M., Al Faruque, H., Galang, J., Hu-Lieskovan, S., et al. (2024). Combination of multivalent DR5 receptor clustering agonists and histone deacetylase inhibitors for treatment of colon cancer. J. Control. Release 376, 1014–1024. doi:10.1016/j.jconrel.2024.10.062
Li, J., Hsu, H. C., Yang, P., Wu, Q., Li, H., Edgington, L. E., et al. (2012). Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis and Rheumatism 64 (4), 1098–1109. doi:10.1002/art.33423
Lim, B., Scicchitano, A., Beachler, C., Gusani, N., Sarwani, N., Yang, Z., et al. (2013). FOLFIRI plus dulanermin (rhApo2L/TRAIL) in a patient with BRAF-mutant metastatic colon cancer. Cancer Biol. and Ther. 14 (8), 711–719. doi:10.4161/cbt.25310
Liu, G. C., Zhang, J., Liu, S. G., Gao, R., Long, Z. F., Tao, K., et al. (2009). Detachment of esophageal carcinoma cells from extracellular matrix causes relocalization of death receptor 5 and apoptosis. World J. Gastroenterology WJG 15 (7), 836–844. doi:10.3748/wjg.15.836
Liu, J., Liu, K., Wang, Y., Shi, Z., Xu, R., Zhang, Y., et al. (2024). Death receptor 5 is required for intestinal stem cell activity during intestinal epithelial renewal at homoeostasis. Cell Death and Dis. 15 (1), 27. doi:10.1038/s41419-023-06409-4
Liu, M. (2018). Study on the role and mechanism of macrophages in sDR5-Fc attenuating cardiac ischemia-reperfusion (I/R) injury. Henan: Henan University.
Liu, Y. G., Liu, S. X., Liang, X. H., Zhang, Q., Gao, L. F., Han, L. H., et al. (2007). Blockade of TRAIL pathway ameliorates HBV-induced hepatocyte apoptosis in an acute hepatitis model. Biochem. biophysical Res. Commun. 352 (2), 329–334. doi:10.1016/j.bbrc.2006.11.024
Liu, Z., Xu, X., Hsu, H. C., Tousson, A., Yang, P. A., Wu, Q., et al. (2003). CII-DC-AdTRAIL cell gene therapy inhibits infiltration of CII-reactive T cells and CII-induced arthritis. J. Clin. investigation 112 (9), 1332–1341. doi:10.1172/JCI19209
LoRusso, P., Ratain, M. J., Doi, T., Rasco, D. W., de Jonge, M. J., Moreno, V., et al. (2022). Eftozanermin alfa (ABBV-621) monotherapy in patients with previously treated solid tumors: findings of a phase 1, first-in-human study. Investig. New Drugs 40 (4), 762–772. doi:10.1007/s10637-022-01247-1
MacFarlane, M., Inoue, S., Kohlhaas, S. L., Majid, A., Harper, N., Kennedy, D. B. J., et al. (2005a). Chronic lymphocytic leukemic cells exhibit apoptotic signaling via TRAIL-R1. Cell Death and Differ. 12 (7), 773–782. doi:10.1038/sj.cdd.4401649
MacFarlane, M., Kohlhaas, S. L., Sutcliffe, M. J., Dyer, M. J., and Cohen, G. M. (2005b). TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 65 (24), 11265–11270. doi:10.1158/0008-5472.CAN-05-2801
Maddipatla, S., Hernandez-Ilizaliturri, F. J., Knight, J., and Czuczman, M. S. (2007). Augmented antitumor activity against B-cell lymphoma by a combination of monoclonal antibodies targeting TRAIL-R1 and CD20. Clin. Cancer Res. 13 (15), 4556–4564. doi:10.1158/1078-0432.CCR-07-0680
Maduro, J. H., Noordhuis, M. G., ten Hoor, K. A., Pras, E., Arts, H. J., Eijsink, J. J., et al. (2009). The prognostic value of TRAIL and its death receptors in cervical cancer. Int. J. Radiat. Oncology* Biology* Phys. 75 (1), 203–211. doi:10.1016/j.ijrobp.2009.03.071
Marini, P., Denzinger, S., Schiller, D., Kauder, S., Welz, S., Humphreys, R., et al. (2006). Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene 25 (37), 5145–5154. doi:10.1038/sj.onc.1209516
Marsters, S. A., Pitti, R. M., Donahue, C. J., Ruppert, S., Bauer, K. D., and Ashkenazi, A. (1996). Activation of apoptosis by Apo-2 ligand is independent of FADD but blocked by CrmA. Curr. Biol. 6 (6), 750–752. doi:10.1016/s0960-9822(09)00456-4
McCarthy, M. M., Sznol, M., DiVito, K. A., Camp, R. L., Rimm, D. L., and Kluger, H. M. (2005). Evaluating the expression and prognostic value of TRAIL-R1 and TRAIL-R2 in breast cancer. Clin. Cancer Res. 11 (14), 5188–5194. doi:10.1158/1078-0432.CCR-05-0158
Mert, U., and Sanlioglu, A. D. (2017). Intracellular localization of DR5 and related regulatory pathways as a mechanism of resistance to TRAIL in cancer. Cell. Mol. Life Sci. 74, 245–255. doi:10.1007/s00018-016-2321-z
Milani, D., Zauli, G., Rimondi, E., Celeghini, C., Marmiroli, S., Narducci, P., et al. (2003). Tumour necrosis factor-related apoptosis-inducing ligand sequentially activates pro-survival and pro-apoptotic pathways in SK-N-MC neuronal cells. J. Neurochem. 86 (1), 126–135. doi:10.1046/j.1471-4159.2003.01805.x
Milutinovic, S., Kashyap, A. K., Yanagi, T., Wimer, C., Zhou, S., O'Neil, R., et al. (2016). Dual agonist surrobody simultaneously activates death receptors DR4 and DR5 to induce cancer cell death. Mol. cancer Ther. 15 (1), 114–124. doi:10.1158/1535-7163.MCT-15-0400
Min, K. J., Woo, S. M., Shahriyar, S. A., and Kwon, T. K. (2019). Elucidation for modulation of death receptor (DR) 5 to strengthen apoptotic signals in cancer cells. Archives pharmacal Res. 42, 88–100. doi:10.1007/s12272-018-01103-y
Moon, B., Yang, S., Moon, H., Lee, J., and Park, D. (2023). After cell death: the molecular machinery of efferocytosis. Exp. and Mol. Med. 55 (8), 1644–1651. doi:10.1038/s12276-023-01070-5
Moon, D. O., Asami, Y., Long, H., Jang, J. H., Bae, E. Y., Kim, B. Y., et al. (2013). Verrucarin A sensitizes TRAIL-induced apoptosis via the upregulation of DR5 in an eIF2α/CHOP-dependent manner. Toxicol. vitro 27 (1), 257–263. doi:10.1016/j.tiv.2012.09.001
Muhlenbeck, F., Haas, E., Schwenzer, R., Schubert, G., Grell, M., Smith, C., et al. (1998). TRAIL/Apo2L activates c-Jun NH2-terminal kinase (JNK) via caspase-dependent and caspase-independent pathways. J. Biol. Chem. 273 (49), 33091–33098. doi:10.1074/jbc.273.49.33091
Mundt, B., Kühnel, F., Zender, L., Paul, Y., Tillmann, H., Trautwein, C., et al. (2003). Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 17 (1), 94–96. doi:10.1096/fj.02-0537fje
Nahacka, Z., Svadlenka, J., Peterka, M., Ksandrova, M., Benesova, S., Neuzil, J., et al. (2018). TRAIL induces apoptosis but not necroptosis in colorectal and pancreatic cancer cells preferentially via the TRAIL-R2/DR5 receptor. Biochimica Biophysica Acta (BBA)-Molecular Cell Res. 1865 (3), 522–531. doi:10.1016/j.bbamcr.2017.12.006
Natoni, A., MacFarlane, M., Inoue, S., Walewska, R., Majid, A., Knee, D., et al. (2007). TRAIL signals to apoptosis in chronic lymphocytic leukaemia cells primarily through TRAIL-R1 whereas cross-linked agonistic TRAIL-R2 antibodies facilitate signalling via TRAIL-R2. Br. J. Haematol. 139 (4), 568–577. doi:10.1111/j.1365-2141.2007.06852.x
Nawrocki, S. T., Carew, J. S., Douglas, L., Cleveland, J. L., Humphreys, R., and Houghton, J. A. (2007). Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res. 67 (14), 6987–6994. doi:10.1158/0008-5472.CAN-07-0812
Newton, K., Dixit, V. M., and Kayagaki, N. (2021). Dying cells fan the flames of inflammation. Science 374 (6571), 1076–1080. doi:10.1126/science.abi5934
Newton, W. (2023). Clinicaltrialsarena. Available online at: https://www.clinicaltrialsarena.com/news/newsinhbirx-fda-lifts-partial-hold-chondrosarcoma/ (Accessed January 18, 2025).
Oh, Y. T., Liu, X., Yue, P., Kang, S., Chen, J., Taunton, J., et al. (2010). ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J. Biol. Chem. 285 (53), 41310–41319. doi:10.1074/jbc.M110.153775
Oh, Y. T., and Sun, S. Y. (2021). Regulation of cancer metastasis by TRAIL/death receptor signaling. Biomolecules 11 (4), 499. doi:10.3390/biom11040499
Orning, P., Weng, D., Starheim, K., Ratner, D., Best, Z., Lee, B., et al. (2018). Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 362 (6418), 1064–1069. doi:10.1126/science.aau2818
Overdijk, M. B., Cecchini, M., Strumane, K., Brandhorst, M., Lingnau, A., Parren, P. W., et al. (2019). Abstract C025: HexaBody-DR5/DR5 (GEN1029) shows potent preclinical antitumor activity in a variety of patient-derived xenograft (PDX) tumor models. Mol. Cancer Ther. 18 (12_Suppl. ment), C025. doi:10.1158/1535-7163.TARG-19-C025
Papadopoulos, K. P., Isaacs, R., Bilic, S., Kentsch, K., Huet, H. A., Hofmann, M., et al. (2015). Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother. Pharmacol. 75, 887–895. doi:10.1007/s00280-015-2712-0
Peng, F., Liao, M., Qin, R., Zhu, S., Peng, C., Fu, L., et al. (2022a). Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Target. Ther. 7 (1), 286. doi:10.1038/s41392-022-01110-y
Peng, H., Zheng, B., Yang, S., Du, J., Cao, L., Liu, L., et al. (2022b). A soluble DR5-Fc chimeric protein attenuates inflammatory responses induced by coronavirus MHV-A59 and SARS-CoV-2. J. Med. Virology 94 (11), 5574–5581. doi:10.1002/jmv.28021
Phillips, D. C., Buchanan, F. G., Cheng, D., Solomon, L. R., Xiao, Y., Xue, J., et al. (2021). Hexavalent TRAIL fusion protein eftozanermin alfa optimally clusters apoptosis-inducing TRAIL receptors to induce on-target antitumor activity in solid tumors. Cancer Res. 81 (12), 3402–3414. doi:10.1158/0008-5472.CAN-20-2178
Picarda, G., Lamoureux, F., Geffroy, L., Delepine, P., Montier, T., Laud, K., et al. (2010). Preclinical evidence that use of TRAIL in Ewing's sarcoma and osteosarcoma therapy inhibits tumor growth, prevents osteolysis, and increases animal survival. Clin. Cancer Res. 16 (8), 2363–2374. doi:10.1158/1078-0432.CCR-09-1779
Pishas, K. I., Neuhaus, S. J., Clayer, M. T., Adwal, A., Brown, M. P., Evdokiou, A., et al. (2013). Pre-activation of the p53 pathway through Nutlin-3a sensitises sarcomas to drozitumab therapy. Oncol. Rep. 30 (1), 471–477. doi:10.3892/or.2013.2454
Plummer, R., Attard, G., Pacey, S., Li, L., Razak, A., Perrett, R., et al. (2007). Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin. cancer Res. 13 (20), 6187–6194. doi:10.1158/1078-0432.CCR-07-0950
Pollack, I. F., Erff, M., and Ashkenazi, A. (2001). Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin. cancer Res. 7 (5), 1362–1369.
Qin, J. Z., Chaturvedi, V., Bonish, B., and Nickoloff, B. J. (2001). Avoiding premature apoptosis of normal epidermal cells. Nat. Med. 7 (4), 385–386. doi:10.1038/86401
Rajeshkumar, N. V., Rasheed, Z. A., García-García, E., López-Ríos, F., Fujiwara, K., Matsui, W. H., et al. (2010). A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol. cancer Ther. 9 (9), 2582–2592. doi:10.1158/1535-7163.MCT-10-0370
Ren, Y. G., Wagner, K. W., Knee, D. A., Aza-Blanc, P., Nasoff, M., and Deveraux, Q. L. (2004). Differential regulation of the TRAIL death receptors DR4 and DR5 by the signal recognition particle. Mol. Biol. cell 15 (11), 5064–5074. doi:10.1091/mbc.e04-03-0184
Sanz, A. B., Sanchez-Niño, M. D., Ramos, A. M., and Ortiz, A. (2023). Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 19 (5), 281–299. doi:10.1038/s41581-023-00694-0
Sarhan, J., Liu, B. C., Muendlein, H. I., Li, P., Nilson, R., Tang, A. Y., et al. (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. 115 (46), E10888-E10897–E10897. doi:10.1073/pnas.1809548115
Saulle, E., Petronelli, A., Pasquini, L., Petrucci, E., Mariani, G., Biffoni, M., et al. (2007). Proteasome inhibitors sensitize ovarian cancer cells to TRAIL induced apoptosis. Apoptosis 12, 635–655. doi:10.1007/s10495-006-0025-9
Schneider, B., Münkel, S., Krippner-Heidenreich, A., Grunwald, I., Wels, W. S., Wajant, H., et al. (2010). Potent antitumoral activity of TRAIL through generation of tumor-targeted single-chain fusion proteins. Cell death and Dis. 1 (8), e68. doi:10.1038/cddis.2010.45
Schneider, P., Thome, M., Burns, K., Bodmer, J. L., Hofmann, K., Kataoka, T., et al. (1997). TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7 (6), 831–836. doi:10.1016/s1074-7613(00)80401-x
Secchiero, P., Melloni, E., Corallini, F., Beltrami, A. P., Alviano, F., Milani, D., et al. (2008). Tumor necrosis factor-related apoptosis-inducing ligand promotes migration of human bone marrow multipotent stromal cells. Stem Cells 26 (11), 2955–2963. doi:10.1634/stemcells.2008-0512
Secchiero, P., Zerbinati, C., Rimondi, E., Corallini, F., Milani, D., Grill, V., et al. (2004). TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell. Mol. Life Sci. 61, 1965–1974. doi:10.1007/s00018-004-4197-6
Sheridan, J. P., Marsters, S. A., Pitti, R. M., Gurney, A., Skubatch, M., Baldwin, D., et al. (1997). Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277 (5327), 818–821. doi:10.1126/science.277.5327.818
Singh, J. A. (2022). Treatment guidelines in rheumatoid arthritis. Rheumatic Dis. Clin. N. Am. 48 (3), 679–689. doi:10.1016/j.rdc.2022.03.005
Song, J. J., and Lee, Y. J. (2008). Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell. Signal. 20 (5), 892–906. doi:10.1016/j.cellsig.2008.01.001
Song, K., Chen, Y., Göke, R., Wilmen, A., Seidel, C., Göke, A., et al. (2000). Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J. Exp. Med. 191 (7), 1095–1104. doi:10.1084/jem.191.7.1095
Soria, J. C., Márk, Z., Zatloukal, P., Szima, B., Albert, I., Juhász, E., et al. (2011). Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non–small-cell lung cancer. J. Clin. Oncol. 29 (33), 4442–4451. doi:10.1200/JCO.2011.37.2623
Soria, J. C., Smit, E., Khayat, D., Besse, B., Yang, X., Hsu, C. P., et al. (2010). Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non–small-cell lung cancer. J. Clin. Oncol. 28 (9), 1527–1533. doi:10.1200/JCO.2009.25.4847
Soto-Gamez, A., Wang, Y., Zhou, X., Seras, L., Quax, W., and Demaria, M. (2022). Enhanced extrinsic apoptosis of therapy-induced senescent cancer cells using a death receptor 5 (DR5) selective agonist. Cancer Lett. 525, 67–75. doi:10.1016/j.canlet.2021.10.038
Spierings, D. C., de Vries, E. G., Timens, W., Groen, H. J., Boezen, H. M., and de Jong, S. (2003). Expression of TRAIL and TRAIL death receptors in stage III non-small cell lung cancer tumors. Clin. cancer Res. 9 (9), 3397–3405.
Stadel, D., Mohr, A., Ref, C., MacFarlane, M., Zhou, S., Humphreys, R., et al. (2010). TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin. cancer Res. 16 (23), 5734–5749. doi:10.1158/1078-0432.CCR-10-0985
Subbiah, V., Brown, R. E., Buryanek, J., Trent, J., Ashkenazi, A., Herbst, R., et al. (2012). Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol. cancer Ther. 11 (11), 2541–2546. doi:10.1158/1535-7163.MCT-12-0358
Subbiah, V., Chawla, S. P., Conley, A. P., Wilky, B. A., Tolcher, A., Lakhani, N. J., et al. (2023). Preclinical characterization and phase I trial results of INBRX-109, a third-generation, recombinant, humanized, death receptor 5 agonist antibody, in chondrosarcoma. Clin. Cancer Res. 29 (16), 2988–3003. doi:10.1158/1078-0432.CCR-23-0974
Surget, S., Chiron, D., Gomez-Bougie, P., Descamps, G., Ménoret, E., Bataille, R., et al. (2012). Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells. Cancer Res. 72 (17), 4562–4573. doi:10.1158/0008-5472.CAN-12-0487
Szallasi, A., Cortright, D. N., Blum, C. A., and Eid, S. R. (2007). The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6 (5), 357–372. doi:10.1038/nrd2280
Takimoto, R., and El-Deiry, W. S. (2000). Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene 19 (14), 1735–1743. doi:10.1038/sj.onc.1203489
Taniguchi, H., Yoshida, T., Horinaka, M., Yasuda, T., Goda, A. E., Konishi, M., et al. (2008). Baicalein overcomes tumor necrosis factor–related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 68 (21), 8918–8927. doi:10.1158/0008-5472.CAN-08-1120
Tao, S., Li, Q., Chen, Y., Fan, Y., Guo, D., Zhai, B., et al. (2021). Effects of hepatic macrophage-hepatic stellate cell interaction on the occurrence and reversal of hepatic fibrosis. J . Life Sci. 33 (03), 363–373. doi:10.13376/j.cbls/2021040
Thon, L., Mathieu, S., Kabelitz, D., and Adam, D. (2006). The murine TRAIL receptor signals caspase-independent cell death through ceramide. Exp. cell Res. 312 (19), 3808–3821. doi:10.1016/j.yexcr.2006.08.017
Todo, M., Horinaka, M., Tomosugi, M., Tanaka, R., Ikawa, H., Sowa, Y., et al. (2013). Ibuprofen enhances TRAIL-induced apoptosis through DR5 upregulation. Oncol. Rep. 30 (5), 2379–2384. doi:10.3892/or.2013.2713
Toffoli, B., Tonon, F., Tisato, V., Zauli, G., Secchiero, P., Fabris, B., et al. (2021). TRAIL/DR5 pathway promotes AKT phosphorylation, skeletal muscle differentiation, and glucose uptake. Cell death and Dis. 12 (12), 1089. doi:10.1038/s41419-021-04383-3
Tong, X., Tang, R., Xiao, M., Xu, J., Wang, W., Zhang, B., et al. (2022). Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. and Oncol. 15 (1), 174. doi:10.1186/s13045-022-01392-3
Trauzold, A., Siegmund, D., Schniewind, B., Sipos, B., Egberts, J., Zorenkov, D., et al. (2006). TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 25 (56), 7434–7439. doi:10.1038/sj.onc.1209719
Truneh, A., Sharma, S., Silverman, C., Khandekar, S., Reddy, M. P., Deen, K. C., et al. (2000). Temperature-sensitive differential affinity of TRAIL for its receptors: DR5 is the highest affinity receptor. J. Biol. Chem. 275 (30), 23319–23325. doi:10.1074/jbc.m910438199
Ubeda, M., Vallejo, M., and Habener, J. F. (1999). CHOP enhancement of gene transcription by interactions with Jun/Fos AP-1 complex proteins. Mol. Cell. Biol. 19 (11), 7589–7599. doi:10.1128/MCB.19.11.7589
Vandenabeele, P., Bultynck, G., and Savvides, S. N. (2023). Pore-forming proteins as drivers of membrane permeabilization in cell death pathways. Nat. Rev. Mol. Cell Biol. 24 (5), 312–333. doi:10.1038/s41580-022-00564-w
van der Horst, H. J., Gelderloos, A. T., Chamuleau, M. E., Breij, E. C., Zweegman, S., Nijhof, I. S., et al. (2021). Potent preclinical activity of HexaBody-DR5/DR5 in relapsed and/or refractory multiple myeloma. Blood Adv. 5 (8), 2165–2172. doi:10.1182/bloodadvances.2020003731
Vilimanovich, U., and Bumbasirevic, V. (2008). TRAIL induces proliferation of human glioma cells by c-FLIP L-mediated activation of ERK1/2. Cell. Mol. life Sci. 65, 814–826. doi:10.1007/s00018-008-7513-8
Voigt, S., Philipp, S., Davarnia, P., Winoto-Morbach, S., Röder, C., Arenz, C., et al. (2014). TRAIL-induced programmed necrosis as a novel approach to eliminate tumor cells. BMC cancer 14, 74–14. doi:10.1186/1471-2407-14-74
Von Karstedt, S., Montinaro, A., and Walczak, H. (2017). Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 17 (6), 352–366. doi:10.1038/nrc.2017.28
Wang, B. T., Kothambawala, T., Wang, L., Matthew, T. J., Calhoun, S. E., Saini, A. K., et al. (2021b). Multimeric anti-DR5 IgM agonist antibody IGM-8444 is a potent inducer of cancer cell apoptosis and synergizes with chemotherapy and BCL-2 inhibitor ABT-199. Mol. cancer Ther. 20 (12), 2483–2494. doi:10.1158/1535-7163.MCT-20-1132
Wang, D., Liu, D., Gao, J., Liu, M., Liu, S., Jiang, M., et al. (2013). TRAIL-induced miR-146a expression suppresses CXCR 4-mediated human breast cancer migration. FEBS J. 280 (14), 3340–3353. doi:10.1111/febs.12323
Wang, M. X., Devine, C., Segaran, N., and Ganeshan, D. (2021a). Current update on molecular cytogenetics, diagnosis and management of gastrointestinal stromal tumors. World J. gastroenterology 27 (41), 7125–7133. doi:10.3748/wjg.v27.i41.7125
Wang, X. (2014). Total synthesis of several stone pine alkaloids and development of DR5 small molecule agonists. Tianjin: Tianjin University.
Wang, X., Xie, R., Zhao, D., Wang, G., Zhang, L., Shi, W., et al. (2024). Blocking the TRAIL-DR5 pathway reduces cardiac ischemia–reperfusion injury by decreasing neutrophil infiltration and neutrophil extracellular traps formation. Cardiovasc. Drugs Ther., 1–17. doi:10.1007/s10557-024-07591-z
Wang, Y., Zhang, H., Wang, Z., Wei, Y., Wang, M., Liu, M., et al. (2020b). Blocking the death checkpoint protein TRAIL improves cardiac function after myocardial infarction in monkeys, pigs, and rats. Sci. Transl. Med. 12 (540), eaaw3172. doi:10.1126/scitranslmed.aaw3172
Wang, Y. Y., Lee, K. T., Lim, M. C., and Choi, J. H. (2020a). TRPV1 antagonist DWP05195 induces ER stress-dependent apoptosis through the ROS-p38-CHOP pathway in human ovarian cancer cells. Cancers 12 (6), 1702. doi:10.3390/cancers12061702
Wiley, S. R., Schooley, K., Smolak, P. J., Din, W. S., Huang, C. P., Nicholl, J. K., et al. (1995). Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3 (6), 673–682. doi:10.1016/1074-7613(95)90057-8
Wilson, N. S., Yang, A., Yang, B., Couto, S., Stern, H., Gogineni, A., et al. (2012). Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell 22 (1), 80–90. doi:10.1016/j.ccr.2012.05.014
Wu, G. S. (2009). TRAIL as a target in anti-cancer therapy. Cancer Lett. 285 (1), 1–5. doi:10.1016/j.canlet.2009.02.029
Xiaochun, W. A. N., Li, J., Chen, Q., and Zhang, Q. (2022). U.S. Patent No. 11. Washington, DC: U.S. Patent and Trademark Office, 220–533.
Xu, J., Zhou, J. Y., Wei, W. Z., and Wu, G. S. (2010). Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PloS one 5 (4), e10226. doi:10.1371/journal.pone.0010226
Yang, A., Wilson, N. S., and Ashkenazi, A. (2010). Proapoptotic DR4 and DR5 signaling in cancer cells: toward clinical translation. Curr. Opin. cell Biol. 22 (6), 837–844. doi:10.1016/j.ceb.2010.08.001
Yang, J. (2012). Experimental study on the effect of polyene-loaded paclitaxel-targeted lipid microbubbles combined with ultrasound-targeted microbubble rupture technology on hepatocellular carcinoma and its mechanism. Chongqing: Chongqing Medical University.
Yang, J. F., Cao, J. G., Tian, L., and Liu, F. (2012). 5, 7-Dimethoxyflavone sensitizes TRAIL-induced apoptosis through DR5 upregulation in hepatocellular carcinoma cells. Cancer Chemother. Pharmacol. 69, 195–206. doi:10.1007/s00280-011-1686-9
Yoshidaa, T., Maedaa, A., Tania, N., and Sakaia, T. (2001). Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene1. FEBS Lett. 25342 (1), 5. doi:10.1016/s0014-5793(01)02947-7
You, D. G., Kim, C. H., Kwon, S., Um, W., Oh, B. H., An, J. Y., et al. (2021). An anti-DR5 antibody-curcumin conjugate for the enhanced clearance of activated hepatic stellate cells. Int. J. Biol. Macromol. 192, 1231–1239. doi:10.1016/j.ijbiomac.2021.09.176
Yu, H. J., Jung, J. Y., Jeong, J. H., Cho, S. D., and Lee, J. S. (2015). Induction of apoptosis by parthenolide in human oral cancer cell lines and tumor xenografts. Oral Oncol. 51 (6), 602–609. doi:10.1016/j.oraloncology.2015.03.003
Yuan, J., and Ofengeim, D. (2024). A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 25 (5), 379–395. doi:10.1038/s41580-023-00689-6
Zeng, Y., Wu, X. X., Fiscella, M., Shimada, O., Humphreys, R., Albert, V., et al. (2006). Monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells in vitro and inhibits tumor growth in vivo. Int. J. Oncol. 28 (2), 421–430. doi:10.3892/ijo.28.2.421
Zhang, S., Zheng, C., Zhu, W., Xiong, P., Zhou, D., Huang, C., et al. (2019). A novel anti-DR5 antibody-drug conjugate possesses a high-potential therapeutic efficacy for leukemia and solid tumors. Theranostics 9 (18), 5412–5423. doi:10.7150/thno.33598
Zhang, Z. K. (2018). sDR5-Fc inhibits neutrophil infiltration and activation to attenuate myocardial ischemia-reperfusion injury. Henan: Henan University.
Zhao, D., Yang, L., Han, P., Zhang, H., Wang, F., Meng, Z., et al. (2023). Blocking TRAIL-DR5 signaling pathway with soluble death receptor 5 fusion protein mitigates radiation-induced injury. Front. Pharmacol. 14, 1171293. doi:10.3389/fphar.2023.1171293
Zhao, X., Wen, F., Ren, Y., Sun, W., Liang, H., Zhao, G., et al. (2016). The study on YM155-sensitized lexatumumab-induced apoptosis in hepatocellular carcinoma cells. J. Chin. Clin. Oncol. 21 (05), 385–389.
Zheng, C., Zhou, D., Li, W., Duan, Y., Xu, M., Liu, J., et al. (2023). Therapeutic efficacy of a MMAE-based anti-DR5 drug conjugate Oba01 in preclinical models of pancreatic cancer. Cell Death and Dis. 14 (4), 295. doi:10.1038/s41419-023-05820-1
Zhou, Y., Peng, Y., Mao, Q. Q., Li, X., Chen, M. W., Su, J., et al. (2013). Casticin induces caspase-mediated apoptosis via activation of mitochondrial pathway and upregulation of DR5 in human lung cancer cells. Asian Pac. J. Trop. Med. 6 (5), 372–378. doi:10.1016/S1995-7645(13)60041-3
Zhu, J., Chen, L., Shi, J., Liu, S., Liu, Y., and Zheng, D. (2014). TRAIL receptor deficiency sensitizes mice to dextran sodium sulphate-induced colitis and colitis-associated carcinogenesis. Immunology 141 (2), 211–221. doi:10.1111/imm.12181
Keywords: death receptor 5, TRAIL-DR5 signaling pathway, DR5 agonist, DR5 antagonist, tumors, cardiovascular disease, autoimmune diseases, radiation damage protection
Citation: Qiao X, Guo S, Meng Z, Gan H, Wu Z, Sun Y, Liu S, Dou G and Gu R (2025) Advances in the study of death receptor 5. Front. Pharmacol. 16:1549808. doi: 10.3389/fphar.2025.1549808
Received: 22 December 2024; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Hua Li, Air Force Medical University, ChinaReviewed by:
Santanu Maji, Virginia Commonwealth University, United StatesCopyright © 2025 Qiao, Guo, Meng, Gan, Wu, Sun, Liu, Dou and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruolan Gu, gurl311@126.com; Guifang Dou, dougf@bmi.ac.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.