
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 24 February 2025
Sec. Pharmacology of Ion Channels and Channelopathies
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1537095
This article is part of the Research Topic Epithelial Transport: from fluid movement toward system organization View all 3 articles
 Simon Y. Graeber1,2,3*
Simon Y. Graeber1,2,3* Olaf Sommerburg4,5
Olaf Sommerburg4,5 Yin Yu4,5
Yin Yu4,5 Julian Berges4,5
Julian Berges4,5 Stephanie Hirtz5
Stephanie Hirtz5 Heike Scheuermann5
Heike Scheuermann5 Jasmin Berger1,2,3
Jasmin Berger1,2,3 Julia Duerr1,2,3
Julia Duerr1,2,3 Marcus A. Mall1,2,3
Marcus A. Mall1,2,3Objective: Intestinal current measurement (ICM) provides a sensitive bioassay for assessment of cystic fibrosis transmembrane conductance regulator (CFTR) function in rectal biopsies ex vivo and is used as a diagnostic tool for cystic fibrosis (CF). Furthermore, ICM was shown to be sensitive to detect pharmacological rescue of CFTR function by CFTR modulators in people with CF carrying responsive CFTR mutations. Results from clinical trials of CFTR modulators across age groups indicate that CFTR function in the sweat duct may be age-dependent with children reaching higher levels than adults. However, little is known about age dependency of CFTR function in the intestinal epithelium.
Methods: We investigated CFTR-mediated chloride secretion in rectal biopsies from 258 people without CF and 72 people with pancreatic-insufficient CF from 1 month to 68 years of age. Change in transepithelial short-circuit current in response to cyclic adenosine monophosphate (cAMP)-mediated (100 μM IBMX, 1 µM forskolin, basolateral) and cholinergic (100 μM carbachol, basolateral) stimulation was assessed as a readout for CFTR function using perfused micro-Ussing chambers. Furthermore, quantitative real-time PCR of CFTR and morphometric analysis of epithelial cells lining the crypts and surface of the rectal mucosa were performed to assess regulation at the levels of gene expression and epithelial cell densities.
Results: We found that CFTR-mediated chloride secretion across rectal tissues, as determined from cAMP-mediated as well as cholinergic chloride-secretory responses was highest during infancy and early childhood and declined with age in people without CF (both P < 0.001). Although, there was no difference in cAMP-mediated currents in people with CF, potassium-secretory responses induced by cholinergic stimulation were also reduced with increasing age. Transcript analyses showed that CFTR mRNA expression was slightly increased with increasing age in people without CF (P < 0.05). Morphometric analyses demonstrated that CFTR expressing colonocytes at the crypt base were decreased with age (P < 0.05). A secondary analysis of the ICM data of our previous studies on the effects of lumacaftor/ivacaftor on CFTR function in F508del -homozygous people with CF aged 12 years and older and 2–11 year old children showed correlations of the change in cAMP-mediated and cholinergic chloride secretory response with the age of people with CF (P < 0.01 and P < 0.05, respectively).
Conclusion: These results demonstrate that CFTR function in the rectal epithelium is reduced with increasing age and indicate that this change is likely due to a decline in the number of secretory colonocytes at the crypt base. These findings suggest that differences in CFTR expressing cells may explain increased functional responses to CFTR modulator therapies in children compared to adult people with CF.
Cystic fibrosis (CF) is a hereditary disorder caused by mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene, which encodes a chloride and bicarbonate channel crucial for maintaining the balance of ion and water transport across epithelial surfaces (Mall et al., 2024; Saint-Criq and Gray, 2017). Key target organs of CF are the lungs, the pancreas and the intestine (Grasemann and Ratjen, 2023). In the airways, CFTR dysfunction leads to impaired anion (chloride and bicarbonate) secretion and enhanced sodium absorption through the epithelial sodium channel (ENaC), resulting in hyperconcentrated and highly visco-elastic mucus (Boucher, 2019; Mall et al., 1998a). This abnormal mucus causes chronic airway infection and inflammation leading to progressive structural lung damage (Boucher, 2007). In the pancreas, CFTR is important for chloride and bicarbonate secretion in the pancreatic ducts. CFTR dysfunction causes hyperconcentration of pancreatic secretions and plugging of the ducts, leading to a backlog of digestive enzymes and auto-digestion of pancreatic tissue, which in turn causes severe pancreatitis and fibrosis with exocrine pancreatic insufficiency already present in ∼85% of infants with CF (Wilschanski and Novak, 2013; Ramsey and Galante, 2024). In the intestine, CFTR plays a key role in the regulation of cAMP-regulated chloride and fluid secretion essential for hydration of the mucus layer and lubrication of the intestinal surface (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Mall et al., 1999). CFTR mediated chloride secretion in the intestine can be stimulated by forskolin via an increase in intracellular cAMP concentration (Figure 1) (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002). Chloride secretion can be further increased by carbachol, a cholinergic agonist that activates calcium-regulated potassium channels increasing the driving force for apical chloride secretion (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002). In CF, impaired chloride secretion leads to dehydration/hyperconcentration of intestinal mucus which can lead to severe bowel obstruction that can manifest as meconium ileus after birth or severe constipation leading to distal intestinal obstruction syndrome (DIOS) in older patients (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Mall et al., 1999; Abraham and Taylor, 2017; Geibel, 2005). Beyond CF, as a key regulator of intestinal fluid homeostasis, CFTR, is also implicated in other intestinal disorders including secretory diarrhea, chronic constipation and colorectal cancer (CRC) (Thiagarajah et al., 2015; Chang et al., 2023; Spelier et al., 2024; Shi et al., 2021).
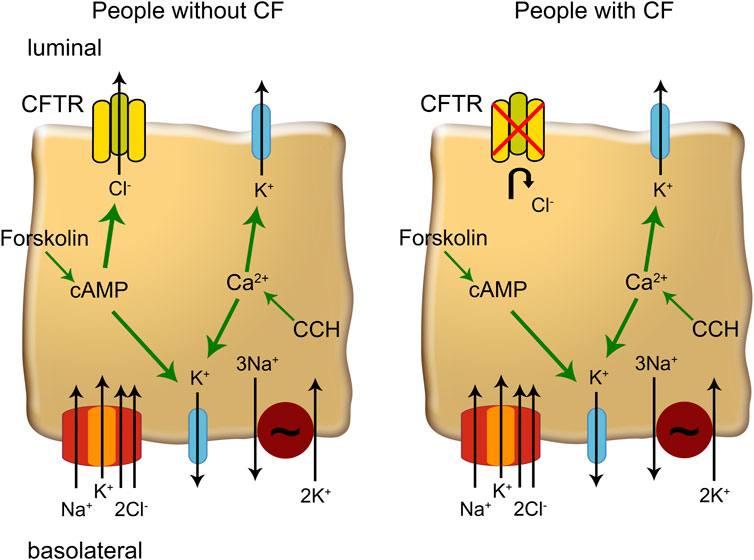
Figure 1. The mechanisms of ion transport in colonic enterocytes. Forskolin increases cytosolic cAMP which enhances CFTR (cystic fibrosis transmembrane conductance regulator)-conductance and increases basolateral K+ conductance. Carbachol (CCH) increases cytosolic Ca2+ concentration that activates K+ channels in the luminal and basolateral membrane, increasing the driving force for chloride secretion via CFTR. In people with CF, CFTR channels cannot be activated and Ca2+ mobilizing agonists enhance K+ secretion.
The development of CFTR modulators in recent years presents the first therapy to treat the basic defect of CF (Graeber and Mall, 2023). CFTR modulators aim to correct defective CFTR protein, either by improving its folding and trafficking to the cell membrane (e.g., elexacaftor, lumacaftor, tezacaftor) or by enhancing its gating function (e.g., ivacaftor) (Mall et al., 2024). The triple combination therapy elexacaftor/tezacaftor/ivacaftor (ETI) has shown remarkable clinical efficacy in people with CF and at least one F508del-CFTR allele as well as a range of other CFTR mutations (Middleton et al., 2019; Heijerman et al., 2019; Burgel et al., 2024). Real world observational studies showed that ETI therapy improves CFTR function to 40%–50% of normal CFTR activity in the intestinal epithelium and leads to a substantial improvement in lung function, lung ventilation, mucus plugging in the airways as well as airway dysbiosis and inflammation (Nichols et al., 2023; Graeber et al., 2022a; Graeber et al., 2022b; Schaupp et al., 2023; Stahl et al., 2024a). Interestingly, sweat chloride concentration measurements as a biomarker of CFTR function suggests an age dependent effect of ETI. In studies in 2–5 year old children homozygous for F508del, approximately 60% of children achieved sweat chloride levels below 30 mmol/L (Goralski et al., 2023), whereas the mean sweat chloride concentration in adolescent and adult people with CF was 48.0 mmol/L after ETI therapy (Heijerman et al., 2019). Similarly, the CFTR dual combination lumacaftor/ivacaftor reduced sweat chloride concentration by 32 mmol/L in F508del homozygous children aged 2–5 years and only 18 mmol/L in adolescents and adults (McNamara et al., 2019; Stahl et al., 2024b; Graeber et al., 2018). In addition, lumacaftor/ivacaftor restored CFTR function in the rectal epithelium to approximately 30% of normal CFTR activity in F508del homozygous children aged 2–11 years (Berges et al., 2023), whereas in adolescents and adults, functional improvement was more modest, in the range of 10%–20% (Graeber et al., 2018). These findings suggest that younger people with CF may have a greater potential for CFTR rescue, however, the mechanisms underlying this age-dependent response are currently unknown.
Intestinal current measurement (ICM) was developed as a sensitive technique to assess CFTR-mediated chloride transport in the intestinal epithelium ex vivo (De Jonge et al., 2004; Hirtz et al., 2004; Sousa et al., 2012; Veeze et al., 1991; Mall et al., 1998b; Veeze et al., 1994; Mall et al., 2000a). By measuring the change in transepithelial short-circuit current in response to cyclic adenosine monophosphate (cAMP)-mediated as well as cholinergic stimulation, ICM provides a direct readout of CFTR function in the intestinal epithelium (Figure 1). Early studies using Ussing chamber experiments on rectal tissues were pioneering in the field of CF research providing valuable insights into the pathophysiology of CFTR dysfunction (Mall et al., 1999; Veeze et al., 1991; Mall et al., 1998b; Veeze et al., 1994; Mall et al., 2000a; Mall et al., 2002; Mall et al., 2004a; Mall et al., 2000b; Roth et al., 2011). Further, ICM was established as a diagnostic tool and is used to determine the effects of CFTR modulator therapies on CFTR function (Graeber et al., 2018; De Jonge et al., 2004; Hirtz et al., 2004; Sousa et al., 2012; Clancy et al., 2013; Graeber et al., 2015). Despite significant advances in understanding CFTR function across different epithelial tissues, there is still limited knowledge about the age dependency of CFTR function.
The primary objective of this study was, therefore, to investigate whether CFTR function in the intestinal epithelium exhibits age-dependent variability. To achieve this, we conducted a comprehensive analysis of CFTR-mediated chloride secretion in rectal biopsies from 258 people without CF and 72 people with CF, ranging in age from 1 month to 68 years. Additionally, we performed quantitative real-time PCR to assess the expression of CFTR and conducted morphometric analyses of the crypts in the intestinal epithelium to determine whether structural changes in the epithelium could explain differences in CFTR function across age groups. To test the hypothesis that the response to CFTR modulator therapy is age-dependent, we performed a secondary analysis of our previous studies on the effects of lumacaftor/ivacaftor on CFTR function in different age groups (Graeber et al., 2018; Berges et al., 2023).
This retrospective study was approved by the Ethical Committees at the University Hospitals of Heidelberg and Freiburg and the Charité - Universitätsmedizin Berlin. Written informed consent was obtained from all participants included in the study, their parents or legal guardians. ICM was performed in 258 people without CF and 72 people with pancreatic-insufficient CF between 1997 and 2022. The diagnosis of CF was established by clinical symptoms characteristic of CF, increased sweat chloride concentrations (≥60 mmol/L) and/or detection of two disease-causing CFTR mutations. People with CF did not receive any CFTR modulator therapy at the time of the rectal biopsy. People without CF had a sweat chloride concentration below 60 mmol/L and the diagnosis of CF was excluded by a CF physician. People without CF and people with CF were grouped in different age groups according to the American Academy of Pediatrics (Hagan et al., 2017). The correlation of the response to CFTR modulator therapy with lumacaftor/ivacaftor and age was performed as a secondary analysis of our previous studies on the effects of lumacaftor/ivacaftor on CFTR function in 49 F508del homozygous people with CF aged 12 years and older (Graeber et al., 2018) and 12 children aged 2–11 years (Berges et al., 2023).
ICM was performed as previously described (Graeber et al., 2022a; Graeber et al., 2018; Hirtz et al., 2004; Graeber et al., 2015; Mall et al., 2004b). In brief, superficial biopsies of the rectal mucosa (∼2–3 mm in diameter) were collected by endoscopic forceps biopsy and immediately stored in ice cold tissue medium (medium 199 containing Hank’s salts, L-glutamine and 25 mmol/L HEPES complemented with 5 mmol/L glycine and 0.5 mmol/L Sodium-DL-β-hydroxybutyrate). Rectal biopsy specimens were mounted in perfused micro-Ussing chambers (open area ∼0.95 mm2). The luminal and basolateral surfaces of the epithelium were perfused continuously with a bath solution of the following composition (mmol/L): 145 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 5 D-glucose, 1 MgCl2, and 1.3 calcium gluconate, pH 7.4, at 37°C. Experiments were performed under open-circuit conditions. Values for the transepithelial voltage (Vte) were referenced to the serosal surface of the epithelium. Transepithelial resistance (Rte) was determined by applying intermittent (1 s) current pulses (ΔI = 0.5 µA). The equivalent short-circuit current (Isc) was calculated according to Ohm’s law from Vte and Rte (Isc = Vte/Rte) after appropriate correction for fluid resistance. The resistance of the rectal epithelium did not change with age (r = 0.000, P = 0.815; Supplementary Figure S1).
Rectal tissues were equilibrated for 40 min in the presence of amiloride (10 μmol/L, luminal) to block electrogenic sodium absorption and indomethacin (10 μmol/L, basolateral) to inhibit prostaglandin E2 synthesis and endogenous cAMP formation. 3-Isobutyl-1-methylxanthine (IBMX) and forskolin (100 μmol/L and 1 μmol/L, basolateral) were added to obtain maximal cAMP-mediated activation of CFTR as previously described (Figure 1) (Graeber et al., 2022a; Graeber et al., 2018; Hirtz et al., 2004; Graeber et al., 2015; Mall et al., 2004b). To increase the driving force for chloride secretion by CFTR, we determined the responses to carbachol (100 μmol/L, basolateral) after stimulation with IBMX/forskolin. The concentrations used for forskolin and carbachol were based on previous studies assessing a dose-response curve to result in maximal activation of Isc (Strohmeier et al., 1995; McNamara et al., 1999; Kerr et al., 1995). To control for sample-to-sample variability, bioelectric measurements were performed on 2–5 biopsy specimens per individual, and data were averaged to obtain a single value for each individual. Indomethacin, amiloride, IBMX, forskolin, and carbachol were all obtained from Sigma-Aldrich (Taufkirchen, Germany).
Rectal biopsies were stored in RNAlater (Invitrogen, Darmstadt, Germany), total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed into cDNA using Superscript III (Invitrogen, Darmstadt, Germany). Quantitative RT-PCR for CFTR and GAPDH was performed on an Applied Biosystems 7,500 Real Time PCR System using TaqMan universal PCR master mix and inventoried TaqMan gene expression assays according to the manufacturer’s instructions (Applied Biosystems, Darmstadt, Germany). Relative fold changes in target gene expression were calculated from the efficiency of the PCR reaction and the crossing point deviation between samples from the two age groups, and determined by normalization to expression of the reference gene GAPDH, as previously described (Mall et al., 2008; Zhou et al., 2008).
Rectal tissues were embedded in O.C.T. (Sakura Finetek Europe, Umkirch, Germany) and stored at −80°C until further processing. Thin sections (6–8 µm) of frozen rectal tissues were cut and mounted on glass slides. Sections were fixed in 10% buffered formalin for 30 min at room temperature and subsequently stained with hematoxylin and eosin. The length of nine crypts from at least three different sections of the biopsies was measured. Only crypts with a luminal opening and reaching to the serosa were selected for measurements. The total number of cells was determined by counting the number of hematoxylin positive nuclei. Goblet cells were defined by absence of staining and non-goblet cells were calculated by subtracting the number of goblet cells from the number of total cells.
Data were analyzed using GraphPad Prism 9.5.1 (GraphPad Software, San Diego, CA, United States of America) and SigmaPlot 12.5 (Grafiti LLC Palo Alto, CA, United States of America). Data are presented as mean and standard error of the mean (SEM) and were tested by Student’s t-test, Mann-Whitney Rank Sum test or one-way ANOVA with Dunn’s post hoc test as appropriate. Correlations were assessed using and Spearman correlation coefficient. P < 0.05 was accepted to indicate statistical significance.
To study the age-dependency of CFTR-dependent chloride secretion in native human rectal epithelia, we performed ICM in 258 people without CF and 72 people with CF with an age ranging from 1 month to 68 years. In infants and preschool children, we observed a greater response to IBMX/forskolin (cAMP-induced short-circuit current (Isc)) and carbachol compared to adults without CF (Figures 2A,B). This age dependency in people without CF was especially observed during childhood and adolescence with decrease over time for cAMP-induced response (r = −0.502, P < 0.001, Figure 2C) and carbachol-induced response (r = −0.456, P < 0.001, Figure 2D). By categorizing people without CF in age groups, we observed a reduction in cAMP- and carbachol-induced responses across age ranges (Figures 2E,F). cAMP-induced responses in infants and preschool children (0–4 years) and school children (5–10 years) without CF were higher compared to adolescents (11–21 years) and adults (≥22 years) (each P < 0.05, Figure 2E). In addition, cAMP-induced responses in adults was smaller compared to adolescents without CF (P < 0.05, Figure 2E). Similarly, Carbachol-induced responses in infants and preschool children (0–4 years) without CF were higher compared to school-age children (5–10 years), adolescents (11–21 years) and adults (≥22 years) (each P < 0.05, Figure 2F). Furthermore, carbachol-induced responses in adults was smaller compared to adolescents as well as school -age children without CF (both P < 0.05, Figure 2F). In people with CF, cAMP- and carbachol-induced negative Isc responses reflect potassium secretion (Figures 3A,B) (Kunzelmann and Mall, 2002; Mall et al., 2000a; Mall et al., 2004b). We observed a weak correlation between cAMP- induced Isc and age (r = 0.266, P < 0.05; Figure 3C), but cAMP-induced responses were overall small and did not differ across age groups (Figure 3E). Carbachol-induced potassium secretory responses decreased with age in people with CF (r = 0.525, P < 0.01; Figure 3D). Adolescents (11–21 years) with CF had lower carbachol-induced responses compared to infants and preschool children (0–4 years) and adults (≥22 years) exhibited lower carbachol-induced responses compared to infants and preschool (0–4 years) as well as school-age children (5–10 years) (all P < 0.05, Figure 3F).
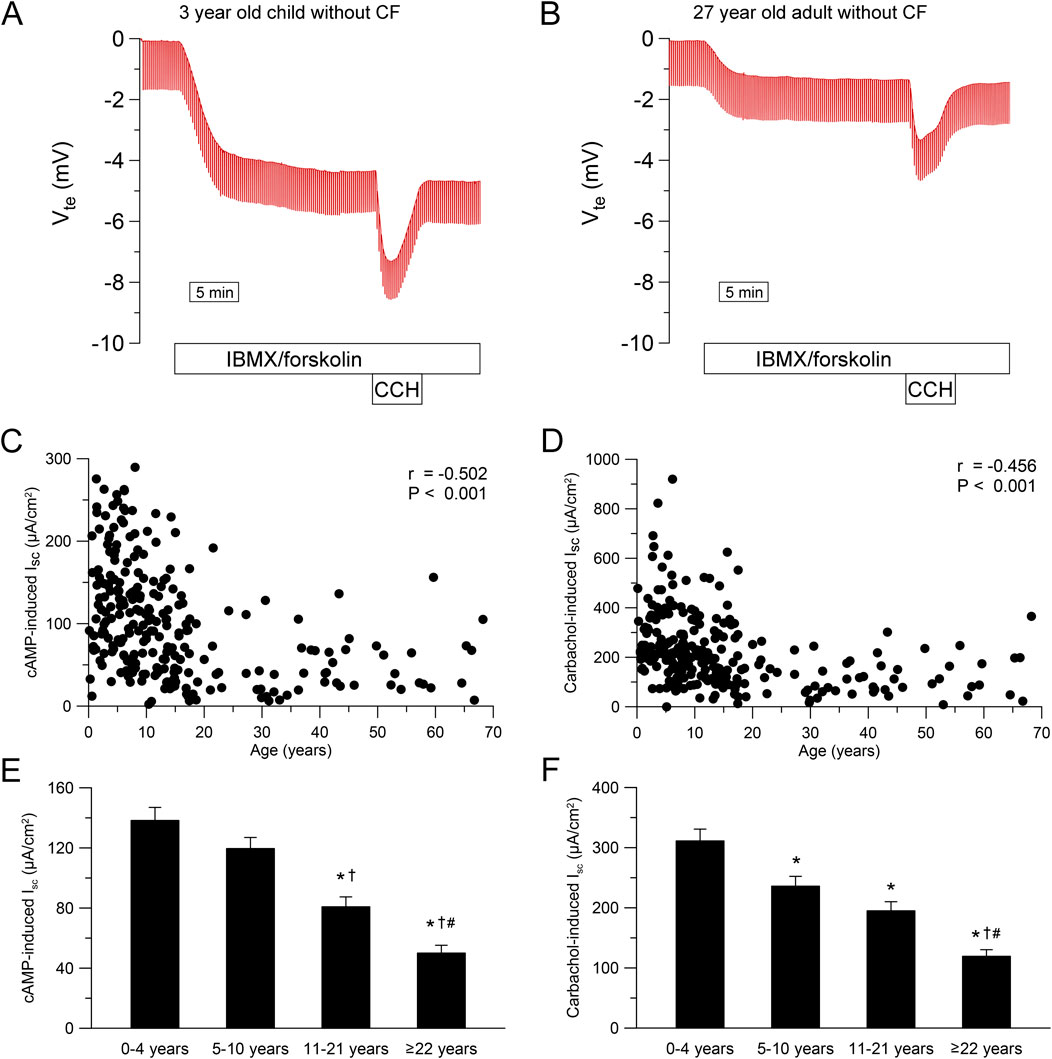
Figure 2. Chloride secretion in human rectal tissue is age-dependent. (A, B) Original recordings of the effects of cAMP-dependent (100 μmol/L IBMX and 1 μmol/L forskolin, basolateral) and cholinergic (100 μmol/L carbachol, basolateral) activation on Vte and Rte in rectal tissue from (A) A 3 year old and (B) A 27 year old person without CF. (C, D) Summary of the effects of (C) cAMP-induced (IBMX/forskolin) and (D) Carbachol-induced short-circuit current (Isc) in rectal tissues from people without CF. (E, F) Summary of the effects of (E) cAMP-induced and (F) carbachol-induced short-circuit currents in rectal tissues from people without CF in different age groups. Experiments were performed in the presence of indomethacin and amiloride. n = 258; *, P < 0.05 vs. 0–4 years, †, P < 0.05 vs. 5–10 years, #, P < 0.05 vs. 11–21 years.
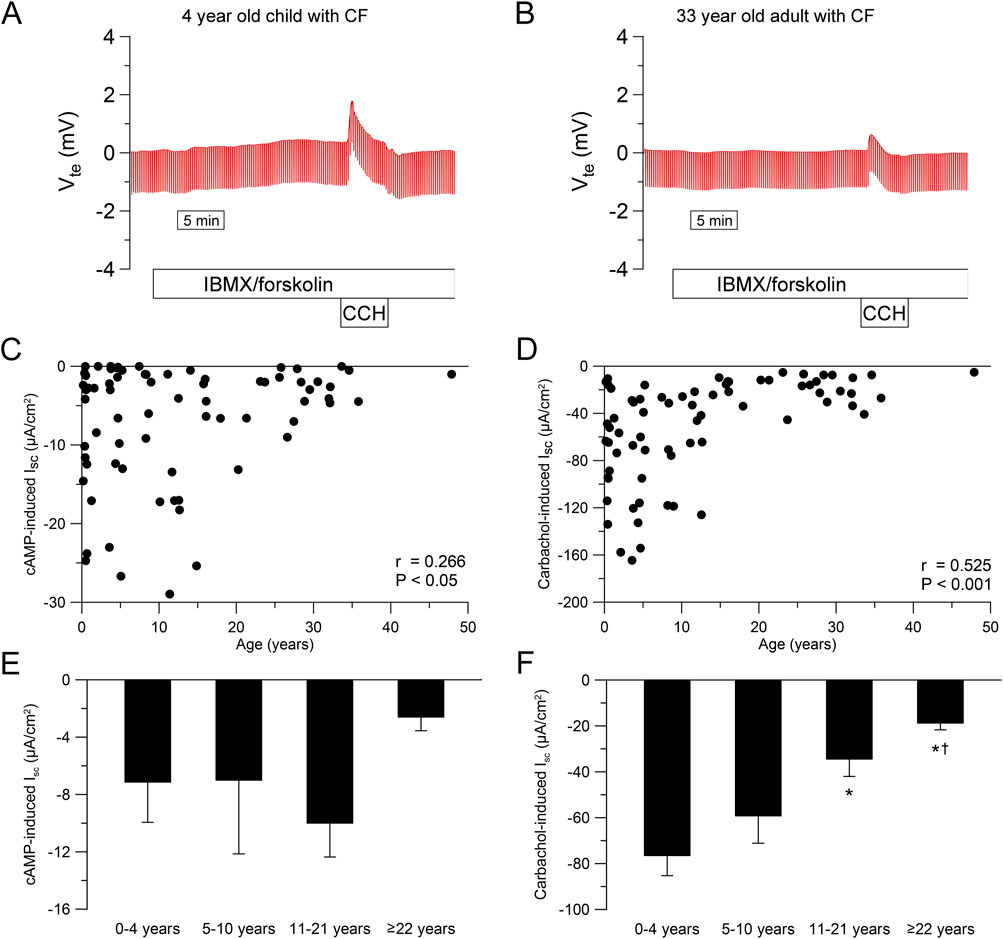
Figure 3. Ion transport in cystic fibrosis rectal tissue in different age groups. (A, B) Original recordings of the effects of cAMP-dependent (100 μmol/L IBMX and 1 μmol/L forskolin, basolateral) and cholinergic (100 μmol/L carbachol, basolateral) activation on Vte and Rte in rectal tissue from (A) A 4 year old and (B) a 33 year old person with CF. (C, D) Summary of the effects of (C) cAMP-induced (IBMX/forskolin) and (D) Carbachol-induced short-circuit current (Isc) in rectal tissues from people with CF. (E, F) Summary of the effects of (E) cAMP-induced and (F) carbachol-induced short-circuit currents in rectal tissues from people with CF in different age groups. Experiments were performed in the presence of indomethacin and amiloride. n = 72; *, P < 0.05 vs. 0–4 years, †, P < 0.05 vs. 5–10 years.
Next, we determined the effect of aging on mRNA transcript levels of CFTR in rectal tissues by quantitative real-time PCR. The expression level of CFTR mRNA was higher in adults (≥22 years) compared to infants and preschool children without CF (≤4 years) (P < 0.05; Supplementary Figure S2).
To investigate age-dependent differences in epithelia cell type composition of the rectal epithelium, we examined the crypt morphology in H&E stained sections of rectal biopsies from infants and preschool children (≤4 years) and adults (≥22 years) without CF (Figure 4A). There was a lower number of non-goblet cells in the whole crypt of adults compared to infants and preschool children (P < 0.05), but no difference was observed in the number of total cells and goblet cells (Figure 4B). Since CFTR was shown to be mostly expressed in non-goblet cells at the crypt base (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Ecke et al., 1996; Linley et al., 2014), we performed a regional sub analysis investigating the upper and the lower half of the crypt. There was no difference in the number of any cell type in the upper half of the crypt (Figure 4C). However, in the lower half of the crypt, the number of goblet cells was increased and the number of non-goblet cells was decreased in adult compared to infants and preschool children without CF (both P < 0.05), whereas no change in the total number of cells was observed (Figure 4D).
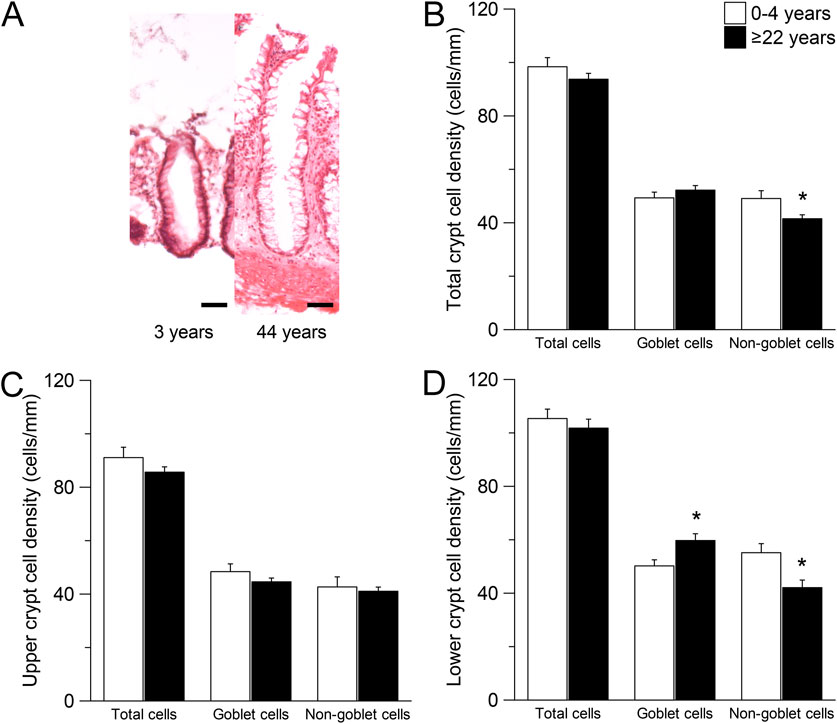
Figure 4. Morphometric analyses of the intestinal crypts in children compared to adults. (A) Morphology of crypts in rectal biopsies of people without CF with the age of 3 years and 44 years. Sections were stained with hematoxylin and eosin (H&E). Scale bars = 50 µm. (B–D) Total cell, goblet cell and non-goblet cell counts from (B) The total crypt (C) the upper crypt half and (D) The lower crypt half of people without CF aged 0–4 years and ≥22 years. n = 9; *, P = 0.05.
To test the hypothesis that the response to CFTR modulator therapy is age-dependent, we performed a secondary analysis of the ICM data of our previous studies on the effects of lumacaftor/ivacaftor on CFTR function in F508del homozygous people with CF aged 12 years and older (Graeber et al., 2018) and 2–11 year old children (Berges et al., 2023). The change in cAMP-induced Isc after initiation of lumacaftor/ivacaftor compared to baseline correlated with the age of people with CF (r = −0.333, P < 0.01; Figure 5A). Similarly, the change in carbachol-induced Isc after initiation of lumacaftor/ivacaftor decreased with age (r = −0.277, P < 0.05; Figure 5B)
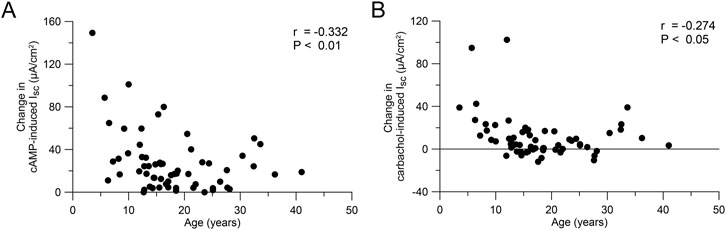
Figure 5. Response to CFTR modulator therapy is age-dependent. (A, B) Change in cAMP-induced (A) and carbachol-induced (B) short circuit current in rectal tissue of people with CF on lumacaftor-ivacaftor therapy compared to baseline (n = 61). Data was reanalyzed from Graeber et al. (2018); Berges et al. (2023).
To our knowledge, this is the first study assessing CFTR function in the rectal epithelium across different age groups. Our data show that CFTR-dependent chloride transport in people without CF decreases with age, particularly in childhood and adolescence (Figure 2). Additionally, we observed reduced potassium secretion with increasing age in people with CF (Figure 3). As a decrease in potassium secretion increases the net current, the age dependent decline in chloride transport could be slightly underestimated. Interestingly, we observed an increase in CFTR mRNA levels in older compared to younger people without CF (Supplementary Figure S2). We hypothesize that the increase in CFTR mRNA is caused by a feedback mechanism trying to compensate for the functional decline. However, the sensitivity of the whole tissue PCR is unclear as CFTR levels in the colon are lower compared to other parts of the intestine (Busslinger et al., 2021; Burclaff et al., 2022; Elmentaite et al., 2021). This further suggests that the observed functional decline is not due to reduced transcription with age but may result from tissue remodeling over time. Enterocytes in the crypt base have been described as the major contributors to cAMP-mediated chloride secretion in the colon (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Ecke et al., 1996; Linley et al., 2014). We demonstrate that morphological changes with a reduced number of non-goblet cells especially in the lower crypt are present in older people without CF (Figure 4). These changes may reflect age-related epithelial remodeling, which has been implicated in other studies studying the colon (Tran and Greenwood-Van Meerveld, 2013) and may explain the age-dependent decrease in CFTR-dependent chloride secretion. In addition to morphological changes in the crypt epithelium, the age-dependent decrease in CFTR-dependent chloride secretion may also be associated with age-related alterations in the efficiency of CFTR biogenesis, including folding and trafficking or degradation of CFTR proteins, as well as changes in the cellular CFTR regulation. To the best of our knowledge, there is no clinical evidence regarding altered intestinal secretory function under healthy conditions with increasing age. This lack of evidence may be attributed to compensation mechanisms, such as reduced efficiency of water and electrolyte absorption. Nonetheless, the reduced maximal capacity for cAMP-dependent chloride secretion in older individuals may also remain sufficient to maintain normal intestinal function under physiological conditions but may lower the threshold for the development of pathological conditions, as discussed below.
Clinical trials as well as real world observational studies consistently demonstrated larger effects of CFTR modulator therapy on CFTR function in children compared to adolescents and adults with CF (Middleton et al., 2019; Heijerman et al., 2019; Goralski et al., 2023; McNamara et al., 2019; Stahl et al., 2024b; Graeber et al., 2018; Berges et al., 2023; Boyle et al., 2014; Stahl et al., 2023; Nichols et al., 2022; Mall et al., 2022). Therapy with ETI and lumacaftor/ivacaftor leads to more pronounced sweat chloride reductions in children compared to adults with CF (Middleton et al., 2019; Heijerman et al., 2019; Goralski et al., 2023; McNamara et al., 2019; Stahl et al., 2024b; Graeber et al., 2018). Furthermore, our secondary analysis of previous studies assessing the effects of lumacaftor/ivacaftor in different age groups of F508del homozygous people with CF (Graeber et al., 2018; Berges et al., 2023) supports an age dependent decrease of functional restoration (Figure 5). Of note, pharmacokinetic profiles of CFTR modulators in children were generally consistent with those observed in older patients (Goralski et al., 2023; McNamara et al., 2019; Zemanick et al., 2021). Therefore, our observation of higher baseline CFTR function in younger people may provide a mechanistic basis for the age-dependent response to CFTR modulators and suggests a greater potential of functional restoration in younger age groups (Goralski et al., 2023; McNamara et al., 2019; Stahl et al., 2024b; Berges et al., 2023; Stahl et al., 2023; Mall et al., 2022). Interestingly, a recent study of bulk and single-cell sequencing data from lung epithelium also suggests an age-dependent decline in CFTR function in the lungs (Corcoran et al., 2024). Our findings support the importance of initiating CFTR modulator therapy early in life to maximize long-term therapeutic efficacy, as younger patients may benefit from rescue of higher levels of CFTR function and less structural epithelial remodeling and organ damage, two factors that may facilitate restoration of epithelial homeostasis, as recently supported by single cell RNA sequencing studies of nasal epithelial cells from children with CF who initiated ETI therapy (Loske et al., 2024).
Our findings may also have implications for understanding the role of CFTR in secretory diarrhea, the third leading cause of death in children under 5 years (WHO, 2024; Hartman et al., 2023). CFTR-mediated chloride and water secretion are critical in maintaining intestinal fluid homeostasis (Mall et al., 1998a; Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Abraham and Taylor, 2017; Geibel, 2005). Enterotoxins activate CFTR channels to drive excessive chloride-driven fluid secretion, leading to severe dehydration and electrolyte imbalances (Thiagarajah et al., 2015). The higher CFTR function in infants and pre-school children may aggravate these pathologies contributing to an increased volume loss and morbidity and mortality in this age group. Conversely, older people with diminished CFTR function may experience less severe fluid loss and symptoms during acute intestinal infections, but may conversely be more prone to develop constipation. While chronic constipation has a complex, multifactorial etiology, CFTR plays a pivotal role as a regulator of intestinal ion and fluid balance and serves as a therapeutic target in constipation management (Greger et al., 1997; Greger, 2000; Kunzelmann and Mall, 2002; Black and Ford, 2018). In people with CF, reduced chloride secretion results in an increased susceptibility of constipation and severe complications such as DIOS (Kunzelmann and Mall, 2002; Abraham and Taylor, 2017). The age-related decline in CFTR function observed in our study may therefore contribute to the higher prevalence of constipation observed in older people (Choung et al., 2007).
Furthermore, CFTR dysfunction in the intestinal epithelium has been linked to a higher susceptibility for CRC (Spelier et al., 2024), the third most common cancer with high mortality (Bray et al., 2024). Epidemiological studies suggest that people with CF have a 5 times greater risk of developing CRC compared to the general population (Birch et al., 2023). Interestingly, also people who are only carriers of CFTR mutations have a higher probability of developing CRC suggesting that even minimal CFTR dysfunction may contribute to the complex multifactorial pathophysiology of CRC (Shi et al., 2021). The elevated risk of CRC in people with CF is not yet completely understood but CFTR dysfunction in the intestine is known to be associated dysbiosis of the gut microbiome and chronic inflammation, two factors that have been associated with the development of intestinal cancer (Li et al., 2024; Munn, 2017). Furthermore, several studies suggest that CFTR itself functions as a tumor suppression gene (Than et al., 2017; Amaral et al., 2020; Liu et al., 2020; Scott et al., 2023). Our findings of an age-dependent decline in CFTR function in people without CF may therefore contribute to an increased risk of developing CRC in older people as the majority of CRC patients are diagnosed after the age of 65 years (Siegel et al., 2023).
This study also has some limitations: The cross-sectional design of our study limits the ability to track longitudinal changes in CFTR function and epithelial morphology in individuals, which would provide more detailed insights into the progression of age-related changes. Further, we assessed CFTR function in rectal tissue only and it is unknown if the observed changes with age are tissue-specific and how CFTR function decreases with age in other organs in which CFTR plays important roles in health and disease, especially the lungs. Finally, potential confounding factors such as diet, comorbidities, and prior treatment history were not explicitly controlled for in this study, which may influence the observed age-dependent changes in CFTR function and crypt morphology. Future studies addressing these limitations, including longitudinal analyses and investigations in other tissues will be important to confirm these findings.
This study is the first to demonstrate an age-dependent decline in CFTR-mediated chloride transport in the intestinal epithelium, identifying morphological changes in the crypt epithelium as a potential mechanism. These results provide a mechanistic basis for age-dependent differences in CFTR modulator efficacy and offer new perspectives on the pathophysiology of diseases such as secretory diarrhea and chronic constipation. Our findings suggest that early initiation of CFTR modulator therapies may yield the greatest therapeutic potential for people with CF.
The datasets presented in this article are not readily available because Publication or accessibility of patient-related data beyond what is represented above is not permitted due to local data protection regulations and ethics guidelines. Requests to access the datasets should be directed to c2ltb24uZ3JhZWJlckBjaGFyaXRlLmRl.
The studies involving humans were approved by the ethical committee of the Albert-Ludwigs-University Freiburg, Freiburg, Germany; the ethical committee of the medical faculy of the University Heidelberg, Heidelberg, Germany and the ethical committee of the Charité–Universitätsmedizin Berlin, Berlin, Germany. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants and/or legal guardians/next of kin.
SYG: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Supervision, Visualization, Writing–original draft, Writing–review and editing. OS: Formal Analysis, Supervision, Writing–review and editing. YY: Data curation, Formal Analysis, Writing–review and editing. JuB: Data curation, Formal Analysis, Visualization, Writing–review and editing. SH: Data curation, Formal Analysis, Investigation, Writing–review and editing. HS: Data curation, Formal Analysis, Investigation, Writing–review and editing. JaB: Data curation, Formal Analysis, Visualization, Writing–review and editing. JD: Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing–review and editing. MAM: Conceptualization, Formal Analysis, Funding acquisition, Resources, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported grants from the German Research Foundation (SFB 1449 - 431232613), the German Federal Ministry of Education and Research (82DZL009C1 and 01GL2401A to M.A.M.) and structural funding from Mukoviszidose Institut gGmbH, Bonn, the research and development arm of the German Cystic Fibrosis Association Mukoviszidose e.V.
The authors thank participants for their contribution to this study.
SYG reports grants from Mukoviszidose e.V. (German CF Foundation) and Vertex Pharmaceuticals Incorporated outside the submitted work, with payments made to institution; personal fees for advisory board participation from Chiesi GmbH and Vertex Pharmaceuticals Incorporated; lecture honoraria and honoraria for a CME module from Vertex Pharmaceuticals Incorporated. OS reports grants from Vertex Pharmaceuticals Incorporated outside the submitted work, with payments made to institution; lecture honoraria from Teva GmbH and Vertex Pharmaceuticals Incorporated. YY reports grants from Mukoviszidose e.V. (German CF Foundation). MAM reports grants from the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), and an independent medical grant from Vertex Pharmaceuticals, with payments made to the institution; personal fees for advisory board participation or consulting from Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Splisense, and Vertex Pharmaceuticals; lecture honoraria from Vertex Pharmaceuticals; and travel support from Boehringer Ingelheim and Vertex Pharmaceuticals; and is unpaid Associate Editor of the European Respiratory Journal and Fellow of the European Respiratory Society (FERS).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1537095/full#supplementary-material
Abraham, J. M., and Taylor, C. J. (2017). Cystic Fibrosis and disorders of the large intestine: DIOS, constipation, and colorectal cancer. J. Cyst. Fibros. 16 (Suppl. 2), S40–S9. doi:10.1016/j.jcf.2017.06.013
Amaral, M. D., Quaresma, M. C., and Pankonien, I. (2020). What role does CFTR play in development, differentiation, regeneration and cancer? Int. J. Mol. Sci. 21 (9), 3133. doi:10.3390/ijms21093133
Berges, J., Graeber, S. Y., Hammerling, S., Yu, Y., Krumpelmann, A., Stahl, M., et al. (2023). Effects of lumacaftor-ivacaftor therapy on cystic fibrosis transmembrane conductance regulator function in F508del homozygous patients with cystic fibrosis aged 2-11 years. Front. Pharmacol. 14, 1188051. doi:10.3389/fphar.2023.1188051
Birch, R. J., Peckham, D., Wood, H. M., Quirke, P., Konstant-Hambling, R., Brownlee, K., et al. (2023). The risk of colorectal cancer in individuals with mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene: an English population-based study. J. Cyst. Fibros. 22 (3), 499–504. doi:10.1016/j.jcf.2022.10.001
Black, C. J., and Ford, A. C. (2018). Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med. J. Aust. 209 (2), 86–91. doi:10.5694/mja18.00241
Boucher, R. C. (2007). Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170. doi:10.1146/annurev.med.58.071905.105316
Boucher, R. C. (2019). Muco-obstructive lung diseases. N. Engl. J. Med. 380 (20), 1941–1953. doi:10.1056/NEJMra1813799
Boyle, M. P., Bell, S. C., Konstan, M. W., McColley, S. A., Rowe, S. M., Rietschel, E., et al. (2014). A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir. Med. 2 (7), 527–538. doi:10.1016/S2213-2600(14)70132-8
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Burclaff, J., Bliton, R. J., Breau, K. A., Ok, M. T., Gomez-Martinez, I., Ranek, J. S., et al. (2022). A proximal-to-distal survey of healthy adult human small intestine and colon epithelium by single-cell transcriptomics. Cell Mol. Gastroenterol. Hepatol. 13 (5), 1554–1589. doi:10.1016/j.jcmgh.2022.02.007
Burgel, P. R., Sermet-Gaudelus, I., Girodon, E., Durieu, I., Houdouin, V., Audousset, C., et al. (2024). The expanded French compassionate programme for elexacaftor-tezacaftor-ivacaftor use in people with cystic fibrosis without a F508del CFTR variant: a real-world study. Lancet Respir. Med. 12 (11), 888–900. doi:10.1016/S2213-2600(24)00208-X
Busslinger, G. A., Weusten, B. L. A., Bogte, A., Begthel, H., Brosens, L. A. A., and Clevers, H. (2021). Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 34 (10), 108819. doi:10.1016/j.celrep.2021.108819
Chang, L., Chey, W. D., Imdad, A., Almario, C. V., Bharucha, A. E., Diem, S., et al. (2023). American gastroenterological association-American college of gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Gastroenterology 164 (7), 1086–1106. doi:10.1053/j.gastro.2023.03.214
Choung, R. S., Locke, G. R., Schleck, C. D., Zinsmeister, A. R., and Talley, N. J. (2007). Cumulative incidence of chronic constipation: a population-based study 1988-2003. Aliment. Pharmacol. Ther. 26 (11-12), 1521–1528. doi:10.1111/j.1365-2036.2007.03540.x
Clancy, J. P., Szczesniak, R. D., Ashlock, M. A., Ernst, S. E., Fan, L., Hornick, D. B., et al. (2013). Multicenter intestinal current measurements in rectal biopsies from CF and non-CF subjects to monitor CFTR function. PLoS One 8 (9), e73905. doi:10.1371/journal.pone.0073905
Corcoran, T. E., Broerman, M. J., Kliment, C. R., and Lo, C. (2024). CFTR expression decreases with age in several airway cell types. Sci. Rep. 14 (1), 28832. doi:10.1038/s41598-024-80108-8
De Jonge, H. R., Ballmann, M., Veeze, H., Bronsveld, I., Stanke, F., Tummler, B., et al. (2004). Ex vivo CF diagnosis by intestinal current measurements (ICM) in small aperture, circulating Ussing chambers. J. Cyst. Fibros. 3 (Suppl. 2), 159–163. doi:10.1016/j.jcf.2004.05.034
Ecke, D., Bleich, M., and Greger, R. (1996). Crypt base cells show forskolin-induced Cl-secretion but no cation inward conductance. Pflugers Arch. 431 (3), 427–434. doi:10.1007/BF02207282
Elmentaite, R., Kumasaka, N., Roberts, K., Fleming, A., Dann, E., King, H. W., et al. (2021). Cells of the human intestinal tract mapped across space and time. Nature 597 (7875), 250–255. doi:10.1038/s41586-021-03852-1
Geibel, J. P. (2005). Secretion and absorption by colonic crypts. Annu. Rev. Physiol. 67, 471–490. doi:10.1146/annurev.physiol.67.031103.153530
Goralski, J. L., Hoppe, J. E., Mall, M. A., McColley, S. A., McKone, E., Ramsey, B., et al. (2023). Phase 3 Open-Label Clinical Trial of Elexacaftor/Tezacaftor/Ivacaftor in Children Aged 2-5 Years with Cystic Fibrosis and at Least One F508del Allele. Am. J. Respir. Crit. Care Med. 208 (1), 59–67. doi:10.1164/rccm.202301-0084OC
Graeber, S. Y., Dopfer, C., Naehrlich, L., Gyulumyan, L., Scheuermann, H., Hirtz, S., et al. (2018). Effects of Lumacaftor-Ivacaftor Therapy on Cystic Fibrosis Transmembrane Conductance Regulator Function in Phe508del Homozygous Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 197 (11), 1433–1442. doi:10.1164/rccm.201710-1983OC
Graeber, S. Y., Hug, M. J., Sommerburg, O., Hirtz, S., Hentschel, J., Heinzmann, A., et al. (2015). Intestinal current measurements detect activation of mutant CFTR in patients with cystic fibrosis with the G551D mutation treated with ivacaftor. Am. J. Respir. Crit. Care Med. 192 (10), 1252–1255. doi:10.1164/rccm.201507-1271LE
Graeber, S. Y., and Mall, M. A. (2023). The future of cystic fibrosis treatment: from disease mechanisms to novel therapeutic approaches. Lancet 402 (10408), 1185–1198. doi:10.1016/S0140-6736(23)01608-2
Graeber, S. Y., Renz, D. M., Stahl, M., Pallenberg, S. T., Sommerburg, O., Naehrlich, L., et al. (2022b). Effects of Elexacaftor/Tezacaftor/Ivacaftor Therapy on Lung Clearance Index and Magnetic Resonance Imaging in Patients with Cystic Fibrosis and One or Two F508del Alleles. Am. J. Respir. Crit. Care Med. 206 (3), 311–320. doi:10.1164/rccm.202201-0219OC
Graeber, S. Y., Vitzthum, C., Pallenberg, S. T., Naehrlich, L., Stahl, M., Rohrbach, A., et al. (2022a). Effects of Elexacaftor/Tezacaftor/Ivacaftor Therapy on CFTR Function in Patients with Cystic Fibrosis and One or Two F508del Alleles. Am. J. Respir. Crit. Care Med. 205 (5), 540–549. doi:10.1164/rccm.202110-2249OC
Grasemann, H., and Ratjen, F. (2023). Cystic fibrosis. N. Engl. J. Med. 389 (18), 1693–1707. doi:10.1056/NEJMra2216474
Greger, R. (2000). Role of CFTR in the colon. Annu. Rev. Physiol. 62, 467–491. doi:10.1146/annurev.physiol.62.1.467
Greger, R., Bleich, M., Leipziger, J., Ecke, D., Mall, M., and Kunzelmann, K. (1997). Regulation of ion transport in colonic crypts. Physiology 12 (2), 62–66. doi:10.1152/physiologyonline.1997.12.2.62
Hagan, J. F., Shaw, J. S., and Duncan, P. M. (2017). “Bright futures: guidelines for health supervision of infants,” in Children, and adolescents: pocket guide. 4th ed. Elk Grove Village, IL, USA: American Academy of Pediatrics.
Hartman, R. M., Cohen, A. L., Antoni, S., Mwenda, J., Weldegebriel, G., Biey, J., et al. (2023). Risk factors for mortality among children younger than age 5 Years with severe diarrhea in low- and middle-income countries: findings from the world health organization-coordinated global rotavirus and pediatric diarrhea surveillance networks. Clin. Infect. Dis. 76 (3), e1047–e1053. doi:10.1093/cid/ciac561
Heijerman, H. G. M., McKone, E. F., Downey, D. G., Van Braeckel, E., Rowe, S. M., Tullis, E., et al. (2019). Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 394 (10212), 1940–1948. doi:10.1016/S0140-6736(19)32597-8
Hirtz, S., Gonska, T., Seydewitz, H. H., Thomas, J., Greiner, P., Kuehr, J., et al. (2004). CFTR Cl-channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology 127 (4), 1085–1095. doi:10.1053/j.gastro.2004.07.006
Kerr, P. M., Hillier, K., Wallis, R. M., and Garland, C. J. (1995). Characterization of muscarinic receptors mediating contractions of circular and longitudinal muscle of human isolated colon. Br. J. Pharmacol. 115 (8), 1518–1524. doi:10.1111/j.1476-5381.1995.tb16645.x
Kunzelmann, K., and Mall, M. (2002). Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 82 (1), 245–289. doi:10.1152/physrev.00026.2001
Li, Y., Peng, J., and Meng, X. (2024). Gut bacteria, host immunity, and colorectal cancer: from pathogenesis to therapy. Eur. J. Immunol. 54 (10), e2451022. doi:10.1002/eji.202451022
Linley, J., Loganathan, A., Kopanati, S., Sandle, G. I., and Hunter, M. (2014). Evidence that two distinct crypt cell types secrete chloride and potassium in human colon. Gut 63 (3), 472–479. doi:10.1136/gutjnl-2013-304695
Liu, C., Song, C., Li, J., and Sun, Q. (2020). CFTR functions as a tumor suppressor and is regulated by DNA methylation in colorectal cancer. Cancer Manag. Res. 12, 4261–4270. doi:10.2147/CMAR.S248539
Loske, J., Voller, M., Lukassen, S., Stahl, M., Thurmann, L., Seegebarth, A., et al. (2024). Pharmacological improvement of cystic fibrosis transmembrane conductance regulator function rescues airway epithelial homeostasis and host defense in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 209 (11), 1338–1350. doi:10.1164/rccm.202310-1836OC
Mall, M., Bleich, M., Greger, R., Schreiber, R., and Kunzelmann, K. (1998a). The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J. Clin. Invest. 102 (1), 15–21. doi:10.1172/JCI2729
Mall, M., Bleich, M., Kuehr, J., Brandis, M., Greger, R., and Kunzelmann, K. (1999). CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am. J. Physiology-Gastrointestinal Liver Physiology 277 (3), G709–G716. doi:10.1152/ajpgi.1999.277.3.G709
Mall, M., Bleich, M., Schurlein, M., Kuhr, J., Seydewitz, H. H., Brandis, M., et al. (1998b). Cholinergic ion secretion in human colon requires coactivation by cAMP. Am. J. Physiol. 275 (6), G1274–G1281. doi:10.1152/ajpgi.1998.275.6.G1274
Mall, M., Gonska, T., Thomas, J., Hirtz, S., Schreiber, R., and Kunzelmann, K. (2002). Activation of ion secretion via proteinase-activated receptor-2 in human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 282 (2), G200–G210. doi:10.1152/ajpgi.00137.2001
Mall, M., Hirtz, S., Gonska, T., and Kunzelmann, K. (2004b). Assessment of CFTR function in rectal biopsies for the diagnosis of cystic fibrosis. J. Cyst. Fibros. 3 (Suppl. 2), 165–169. doi:10.1016/j.jcf.2004.05.035
Mall, M., Kreda, S. M., Mengos, A., Jensen, T. J., Hirtz, S., Seydewitz, H. H., et al. (2004a). The DeltaF508 mutation results in loss of CFTR function and mature protein in native human colon. Gastroenterology 126 (1), 32–41. doi:10.1053/j.gastro.2003.10.049
Mall, M., Wissner, A., Seydewitz, H. H., Hubner, M., Kuehr, J., Brandis, M., et al. (2000b). Effect of genistein on native epithelial tissue from normal individuals and CF patients and on ion channels expressed in Xenopus oocytes. Br. J. Pharmacol. 130 (8), 1884–1892. doi:10.1038/sj.bjp.0703520
Mall, M., Wissner, A., Seydewitz, H. H., Kuehr, J., Brandis, M., Greger, R., et al. (2000a). Defective cholinergic Cl(-) secretion and detection of K(+) secretion in rectal biopsies from cystic fibrosis patients. Am. J. Physiol. Gastrointest. Liver Physiol. 278 (4), G617–G624. doi:10.1152/ajpgi.2000.278.4.G617
Mall, M. A., Brugha, R., Gartner, S., Legg, J., Moeller, A., Mondejar-Lopez, P., et al. (2022). Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 Through 11 Years of Age with Cystic Fibrosis Heterozygous for F508del and a Minimal Function Mutation: A Phase 3b, Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med. 206 (11), 1361–1369. doi:10.1164/rccm.202202-0392OC
Mall, M. A., Burgel, P. R., Castellani, C., Davies, J. C., Salathe, M., and Taylor-Cousar, J. L. (2024). Cystic fibrosis. Nat. Rev. Dis. Prim. 10 (1), 53. doi:10.1038/s41572-024-00538-6
Mall, M. A., Harkema, J. R., Trojanek, J. B., Treis, D., Livraghi, A., Schubert, S., et al. (2008). Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am. J. Respir. Crit. Care Med. 177 (7), 730–742. doi:10.1164/rccm.200708-1233OC
McNamara, B., Winter, D. C., Cuffe, J. E., O'Sullivan, G. C., and Harvey, B. J. (1999). Basolateral K+ channel involvement in forskolin-activated chloride secretion in human colon. J. Physiol. 519 (Pt 1), 251–260. doi:10.1111/j.1469-7793.1999.0251o.x
McNamara, J. J., McColley, S. A., Marigowda, G., Liu, F., Tian, S., Owen, C. A., et al. (2019). Safety, pharmacokinetics, and pharmacodynamics of lumacaftor and ivacaftor combination therapy in children aged 2-5 years with cystic fibrosis homozygous for F508del-CFTR: an open-label phase 3 study. Lancet Respir. Med. 7 (4), 325–335. doi:10.1016/S2213-2600(18)30460-0
Middleton, P. G., Mall, M. A., Drevinek, P., Lands, L. C., McKone, E. F., Polineni, D., et al. (2019). Elexacaftor-Tezacaftor-Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 381 (19), 1809–1819. doi:10.1056/NEJMoa1908639
Munn, L. L. (2017). Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 9 (2). doi:10.1002/wsbm.1370
Nichols, D. P., Morgan, S. J., Skalland, M., Vo, A. T., Van Dalfsen, J. M., Singh, S. B., et al. (2023). Pharmacologic improvement of CFTR function rapidly decreases sputum pathogen density, but lung infections generally persist. J. Clin. Invest 133 (10), e167957. doi:10.1172/JCI167957
Nichols, D. P., Paynter, A. C., Heltshe, S. L., Donaldson, S. H., Frederick, C. A., Freedman, S. D., et al. (2022). Clinical effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: a clinical trial. Am. J. Respir. Crit. Care Med. 205 (5), 529–539. doi:10.1164/rccm.202108-1986OC
Ramsey, M. L., and Galante, G. J. (2024). Pancreas and pancreatitis: exocrine pancreatic insufficiency. Pediatr. Pulmonol. 59 (Suppl. 1), S44–S52. doi:10.1002/ppul.27013
Roth, E. K., Hirtz, S., Duerr, J., Wenning, D., Eichler, I., Seydewitz, H. H., et al. (2011). The K+ channel opener 1-EBIO potentiates residual function of mutant CFTR in rectal biopsies from cystic fibrosis patients. PLoS One 6 (8), e24445. doi:10.1371/journal.pone.0024445
Saint-Criq, V., and Gray, M. A. (2017). Role of CFTR in epithelial physiology. Cell Mol. Life Sci. 74 (1), 93–115. doi:10.1007/s00018-016-2391-y
Schaupp, L., Addante, A., Voller, M., Fentker, K., Kuppe, A., Bardua, M., et al. (2023). Longitudinal effects of elexacaftor/tezacaftor/ivacaftor on sputum viscoelastic properties, airway infection and inflammation in patients with cystic fibrosis. Eur. Respir. J. 62 (2), 2202153. doi:10.1183/13993003.02153-2022
Scott, P., Wang, S., Onyeaghala, G., Pankratz, N., Starr, T., and Prizment, A. E. (2023). Lower expression of CFTR is associated with higher mortality in a meta-analysis of individuals with colorectal cancer. Cancers (Basel) 15 (3), 989. doi:10.3390/cancers15030989
Shi, Z., Wei, J., Na, R., Resurreccion, W. K., Zheng, S. L., Hulick, P. J., et al. (2021). Cystic fibrosis F508del carriers and cancer risk: Results from the UK Biobank. Int. J. Cancer 148 (7), 1658–1664. doi:10.1002/ijc.33431
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A., and Jemal, A. (2023). Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73 (3), 233–254. doi:10.3322/caac.21772
Sousa, M., Servidoni, M. F., Vinagre, A. M., Ramalho, A. S., Bonadia, L. C., Felicio, V., et al. (2012). Measurements of CFTR-mediated Cl-secretion in human rectal biopsies constitute a robust biomarker for Cystic Fibrosis diagnosis and prognosis. PLoS One 7 (10), e47708. doi:10.1371/journal.pone.0047708
Spelier, S., Derksen, S., Hofland, R., Beekman, J. M., and Yetkin-Arik, B. (2024). CFTR and colorectal cancer susceptibility: an urgent need for further studies. Trends Cancer 10 (10), 876–879. doi:10.1016/j.trecan.2024.07.006
Stahl, M., Dohna, M., Graeber, S. Y., Sommerburg, O., Renz, D. M., Pallenberg, S. T., et al. (2024a). Impact of elexacaftor/tezacaftor/ivacaftor therapy on lung clearance index and magnetic resonance imaging in children with cystic fibrosis and one or two F508del alleles. Eur. Respir. J. 64 (3), 2400004. doi:10.1183/13993003.00004-2024
Stahl, M., Roehmel, J., Eichinger, M., Doellinger, F., Naehrlich, L., Kopp, M. V., et al. (2023). Effects of lumacaftor/ivacaftor on cystic fibrosis disease progression in children 2 through 5 Years of age homozygous for F508del-CFTR: a phase 2 placebo-controlled clinical trial. Ann. Am. Thorac. Soc. 20 (8), 1144–1155. doi:10.1513/AnnalsATS.202208-684OC
Stahl, M., Roehmel, J., Eichinger, M., Doellinger, F., Naehrlich, L., Kopp, M. V., et al. (2024b). Long-term impact of lumacaftor/ivacaftor treatment on cystic fibrosis disease progression in children 2-5 Years of age homozygous for F508del-CFTR: a phase 2, open-label clinical trial. Ann. Am. Thorac. Soc. 21 (11), 1550–1559. doi:10.1513/AnnalsATS.202402-201OC
Strohmeier, G. R., Reppert, S. M., Lencer, W. I., and Madara, J. L. (1995). The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Biol. Chem. 270 (5), 2387–2394. doi:10.1074/jbc.270.5.2387
Than, B. L. N., Linnekamp, J. F., Starr, T. K., Largaespada, D. A., Rod, A., Zhang, Y., et al. (2017). CFTR is a tumor suppressor gene in murine and human intestinal cancer. Oncogene 36 (24), 3504. doi:10.1038/onc.2017.3
Thiagarajah, J. R., Donowitz, M., and Verkman, A. S. (2015). Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12 (8), 446–457. doi:10.1038/nrgastro.2015.111
Tran, L., and Greenwood-Van Meerveld, B. (2013). Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. A Biol. Sci. Med. Sci. 68 (9), 1045–1056. doi:10.1093/gerona/glt106
Veeze, H. J., Halley, D. J., Bijman, J., de Jongste, J. C., de Jonge, H. R., and Sinaasappel, M. (1994). Determinants of mild clinical symptoms in cystic fibrosis patients. Residual chloride secretion measured in rectal biopsies in relation to the genotype. J. Clin. Invest 93 (2), 461–466. doi:10.1172/JCI116993
Veeze, H. J., Sinaasappel, M., Bijman, J., Bouquet, J., and de Jonge, H. R. (1991). Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology 101 (2), 398–403. doi:10.1016/0016-5085(91)90017-f
WHO (2024). Diarrhoeal disease. Available at: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease.
Wilschanski, M., and Novak, I. (2013). The cystic fibrosis of exocrine pancreas. Cold Spring Harb. Perspect. Med. 3 (5), a009746. doi:10.1101/cshperspect.a009746
Zemanick, E. T., Taylor-Cousar, J. L., Davies, J., Gibson, R. L., Mall, M. A., McKone, E. F., et al. (2021). A Phase 3 Open-Label Study of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 through 11 Years of Age with Cystic Fibrosis and at Least One F508del Allele. Am. J. Respir. Crit. Care Med. 203 (12), 1522–1532. doi:10.1164/rccm.202102-0509OC
Keywords: CFTR, intestinal current measurement, rectal epithelium, age-dependency, CFTR modulator therapy, secretory diarrhea
Citation: Graeber SY, Sommerburg O, Yu Y, Berges J, Hirtz S, Scheuermann H, Berger J, Duerr J and Mall MA (2025) Intestinal current measurement detects age-dependent differences in CFTR function in rectal epithelium. Front. Pharmacol. 16:1537095. doi: 10.3389/fphar.2025.1537095
Received: 29 November 2024; Accepted: 28 January 2025;
Published: 24 February 2025.
Edited by:
Michael Gray, Newcastle University, United KingdomReviewed by:
Damien Samways, Clarkson University, United StatesCopyright © 2025 Graeber, Sommerburg, Yu, Berges, Hirtz, Scheuermann, Berger, Duerr and Mall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon Y. Graeber, c2ltb24uZ3JhZWJlckBjaGFyaXRlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.