
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 12 February 2025
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1535621
Atrial fibrillation (AF) causes a heavy socio-economic burden on healthcare systems around the globe. Identification of new preventive, diagnostic, and treatment methods is imperative. In recent years, special attention has been paid to microRNAs (miRNAs) as potential regulators of AF pathogenesis. Through post-transcriptional regulation of genes, miRNAs have been shown to play crucial roles in AF-related structural and electrical atrial remodeling. Altered expression of different miRNAs has been related to proarrhythmic changes in the duration of action potentials and atrial fibrosis. In clinical studies, miRNA changes have been associated with AF, whereas in experimental studies miRNA manipulation has emerged as a potential therapeutic approach. It would appear that, with the advent of miRNAs, we may have found the Holy Grail, and that efficient and personalized AF therapy may be one step away. Yet, the clinical relevance of miRNA evaluation and manipulation remains questionable. Studies have identified numerous miRNAs associated with AF, but none of them have shown sufficient specificity for AF. MicroRNAs are not gene-specific but regulate the expression of a myriad of genes. Cardiac and non-cardiac off-target effects may thus occur following miRNA manipulation. A Pandora’s box might thus have opened with the advent of these sophisticated molecules. In this paper, we provide a critical analysis of the clinical and experimental, epidemiological and mechanistic data linking miRNAs to AF, we discuss the most promising miRNA therapeutic approaches, we emphasize a number of questions that remain to be answered, and we identify hotspots for future research.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting ≈33 million patients worldwide, and is associated with considerable morbidity, mortality and, quality of life impairment (Kornej et al., 2020). Numerous risk factors have been associated with the occurrence and progression of AF. Among them, heart failure, diabetes, hypertension, obesity, and structural and ischemic heart disease are strong predictors of AF (Kornej et al., 2020). Several biomarkers, such as C-reactive protein, cardiac troponins, B-type natriuretic peptide, and components of the renin-angiotensin-aldosterone system (RAAS) have also been associated with AF (Kornej et al., 2020). However, none of these risk factors or biomarkers have proven enough reliability in predicting future AF and none of them are specific for AF. Multimarker strategies and risk scores have also been proposed as potential approaches for AF prediction. However, none of them have managed to prove their efficacy in clinical trials (Olivia et al., 2019). Moreover, AF can also occur in individuals devoid of overt risk factors for AF (Olivia et al., 2019; Fan et al., 2019).
MicroRNAs (miRNAs) are short non-coding RNAs that play critical roles in post-transcriptional regulation of gene expression (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014). Several miRNAs have been associated with cardiac pathologies via regulation of genes involved in inflammation, myocardial remodeling, apoptosis, and cardiac ions dynamics (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014). Recent research has demonstrated an association between different cardiac and circulating miRNAs and atrial structural, electrical, and autonomic remodeling, suggesting that they could serve as biomarkers for AF diagnostic and/or prediction, as well as potential therapeutic targets for AF (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014). If confirmed, these hypotheses could radically change the AF landscape. However, none of those miRNAs are specific for AF (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014) and to date, no study has assessed the therapeutic potential of miRNA manipulation for AF in clinical settings.
This article aims to provide a critical analysis of the most relevant clinical and experimental, epidemiological and mechanistic data linking miRNAs to AF and to discuss the potential role of miRNAs as biomarkers for diagnosis, prognosis and monitoring of AF, as well as therapeutic targets in AF. We also emphasize several questions that remain to be answered and we identify hotspots for future research. To highlight the role of miRNAs in AF, the miRNAs that provide a scientifically comprehensive overview and have practical importance in advancing the management and understanding of AF were selected. miRNAs supported by strong evidence were prioritized, highlighting those extensively documented in both experimental and clinical studies as having significant roles in AF pathophysiology. In addition, the clinical applicability of these miRNAs was considered, focusing on those miRNAs that have demonstrated potential as diagnostic markers, prognostic tools, or therapeutic targets in clinical settings.
The pathophysiological basis of AF involves complex mechanisms (Figure 1) (Șerban et al., 2019; Scridon et al., 2018). The biogenesis and mechanism of action of miRNAs (Figure 2) and the potential role of miRNAs as biomarkers and therapeutic targets in cardiovascular diseases have been intensively studied over the past years (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014).
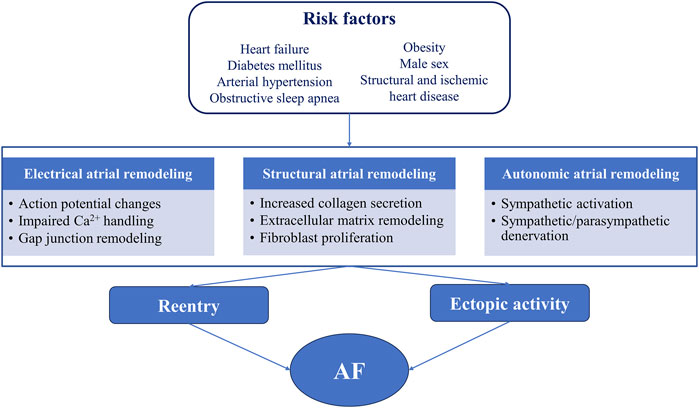
Figure 1. Relationship between common risk factors, atrial proarrhythmic remodeling, and atrial fibrillation AF, atrial fibrillation.
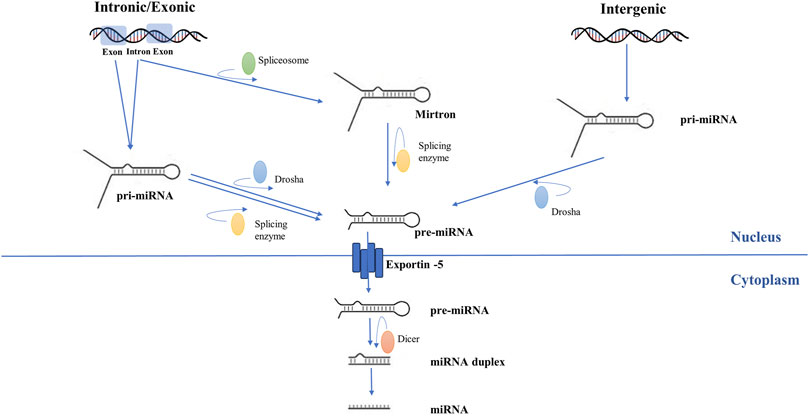
Figure 2. Schematic representation of microRNA biogenesis. Genomic DNA is transcribed into pri-miRNA by RNA polymerase II. Subsequently, endonucleotide cleavage of the pri-miRNA generates a miRNA precursor (pre-miRNA). For intergenic miRNAs, this process is carried out exclusively by ribonuclease 3. For intronic and exonic miRNAs, pre-miRNA formation involves further modulation by splicing enzymes. Pre-miRNA is exported to the cytoplasm. Dicer enzyme processes pre-miRNA and transforms it into duplex miRNA with two arms, of which one will become a mature miRNA and the other will be eliminated.
In AF, miRNAs have been associated with changes in the expression of genes involved in cardiac function and atrial tissue remodeling (Figure 3). Experimental and clinical studies identified several miRNAs that are upregulated or downregulated in AF (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014). Changes in miRNA levels were observed not only in the atrial tissue but also in the peripheral blood, offering the possibility of using them as readily available biomarkers for the prediction and/or diagnosis of AF (Yang et al., 2007; Soeki et al., 2016; Li et al., 2014). In addition, the causal link between AF and certain miRNAs suggests that manipulation of miRNA expression could represent a novel therapeutic strategy in AF (Yang et al., 2007).
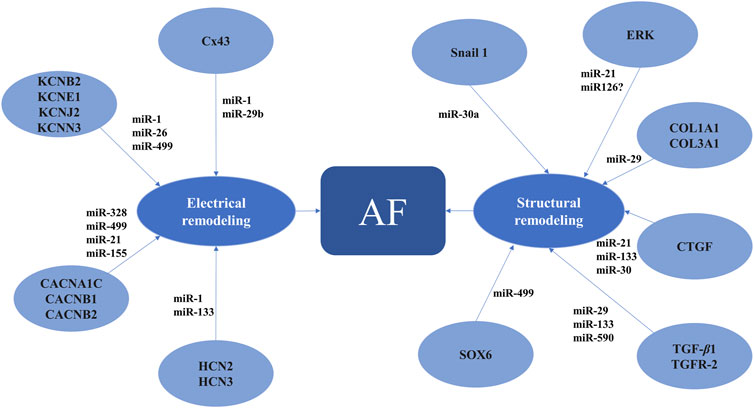
Figure 3. MicroRNAs involved in proarrhythmic atrial electrical and structural remodeling AF, atrial fibrillation; CACNA1C, calcium voltage-gated channel subunit alpha1 C; CACNB1, calcium voltage-gated channel auxiliary subunit beta1; CACNB2, calcium voltage-gated channel auxiliary subunit beta2; COL1A1, collagen type I alpha1 chain; COL3A1, collagen type III alpha1 chain; CTGF, connective tissue growth factor; Cx43, connexin 43; ERK, extracellular regulated mitogen-activated protein kinase; HCN2, 3, hyperpolarization activated cyclic nucleotide gated potassium and sodium channel 2, 3; KCNB2, potassium voltage-gated channel subfamily B member 2; KCNE1, potassium voltage-gated channel subfamily E regulatory subunit 1; KCNJ2, potassium inwardly rectifying channel subfamily J member 2; KCNN3, potassium calcium-activated channel subfamily N member 3; SOX6, SRY-box transcription factor 6; TGF-β1, transforming growth factor beta1; TGFR-2, transforming growth factor beta receptor 2.
The first studies that demonstrated the role of miR-1 in cardiac electrophysiology were performed in the setting of cardiac ischemia, where miR-1 overexpression was shown to contribute to cardiac arrhythmogenesis (Yang et al., 2007). In experimental studies, ventricular miR-1 overexpression increased spontaneous Ca2+ release from the sarcoplasmic reticulum, decreased inward Ca2+ current amplitude, and increased RyR2 activity, leading to Ca2+ handling abnormalities, intracellular Ca2+ overload, and arrhythmias (Terentyev et al., 2009). These changes were accompanied by post-transcriptional repression of KCNJ2 (Yang et al., 2007). Decreased conductance induced by miR-1 overexpression was attributed to post-transcriptional repression of GJA1, encoding for connexin 43 (Cx43), a key component of gap junctions, which are essential for intercellular conduction in the ventricles (Yang et al., 2007). Meanwhile, miR-1 knockdown reduced ischemia-induced arrhythmias, suggesting the potential of miR-1 manipulation as antiarrhythmic strategy (Yang et al., 2007). Considering the importance of Ca2+, GJA1/Cx43, and KCNJ2/Kir2.1/IK1 in atrial electrophysiology, subsequent studies focused on the possible involvement of miR-1 in AF.
In patients with AF, plasma levels of miR-1 were higher in the left atrium than in the pulmonary veins, suggesting that local left atrial production of miR-1 might be involved in proarrhythmic atrial remodeling (Soeki et al., 2016). However, the exact changes that occur in miR-1 in AF remain controversial. Left atrial miR-1 levels were significantly higher in patients who developed post-coronary bypass AF than in those who did not, but this was not reflected in the peripheral blood (Tsoporis et al., 2018). On the contrary, in the study by Girmatsion et al., atrial miR-1 expression was reduced by ≈ 86% in patients with persistent AF compared to sinus rhythm controls (Girmatsion et al., 2009). A progressive decrease in miR-1 expression with advancing age (Li et al., 2015) may explain at least some of these discrepancies.
The exact mechanisms by which miR-1 changes lead to atrial electrical remodeling and AF are also incompletely elucidated. In patients with persistent AF, reduced atrial miR-1 levels were associated with an increase in atrial KCNJ2, Kir2.1, and, IK1 density (Girmatsion et al., 2009). Reduced miR-1 levels were also accompanied by increased HCN2 and HCN4 channels expression in elderly patients with AF (Li et al., 2015). Although miR-1-dependent gene regulation seems to be involved in Ca2+ homeostasis regulation in the ventricles (Terentyev et al., 2009), evidence of this regulatory mechanism extending to atrial fibrillation remains unestablished. The potential of miR-1 manipulation as a therapeutic target in AF was tested in a model of programmed atrial electrical stimulation, where right atrial tachypacing led to miR-1 upregulation and KCNE1 and KCNB2 downregulation (Jia et al., 2013). Contrary to expectations, these were accompanied by an increase in IKs activity and a decrease in atrial efective refractory period (AERP) duration (Jia et al., 2013). Additional miR-1 upregulation led to further amplification of these molecular and electrical changes. In contrast, administration of locked nucleic acids-based antimiR-1 prolonged the AERP and reduced AF susceptibility and duration in rabbits, supporting the potential role of miR-1 as a therapeutic target in AF (Jia et al., 2013).
miR-1 expression is not limited, however, to tissues directly involved in AF, and altered miR-1 expression has also been reported in many other cardiac and non-cardiac diseases (Yang et al., 2007; Ma et al., 2020; De Gonzalo-Calvo et al., 2017; Sugiyama et al., 2020). Thus, miR-1 manipulation to prevent AF could have off-target effects, whereas the potential use of miR-1 as a biomarker for AF, as proposed in several studies (Yang et al., 2007; Soeki et al., 2016), is considerable affected by its low specificity (Li et al., 2014). In addition, various physiological and pathological conditions make miR-1 expression unstable over time.
In a study evaluating peripheral, pulmonary vein, and left atrial appendage plasma levels of miR-328 in patients with and without AF, no significant differences were observed in miR-328 plasma levels between patients with AF and those in sinus rhythm. However, intracardiac levels of miR-328 were increased in AF patients compared to their peripheral and pulmonary vein plasma levels, suggesting that altered left atrial miR-328 expression is involved in atrial remodeling (Soeki et al., 2016). We have recently shown in a rat model of AF a strong association between increased miR-328 levels and AF, suggesting that miR-328 could emerge as a promising biomarker for AF diagnosis (Scridon et al., 2023). Moreover, in that model, the increase in miR-328 preceded the appearance of the arrhythmia, suggesting that dynamic miR-328 monitoring may predict future AF. Similar associations between increased miR-328 levels and AF were also reported in clinical studies (Huang et al., 2022).
Using a computational prediction algorithm, Lu et al. identified CACNA1C and CACNB1, encoding the α1C- and β1-subunits of ICa-L, respectively, as potential targets for miR-328 (Lu et al., 2010). This was further confirmed by Western blot and luciferase activity assay (Lu et al., 2010). The authors subsequently demonstrated that miR-328 overexpression increased AF susceptibility in dogs and mice, probably due to decreased ICa-L and AERP shortening (Lu et al., 2010). Contrarily, antagomiR-328 reversed those changes and decreased AF susceptibility (Lu et al., 2010).
However, miR-328 expression is not changed only in AF. Down- or upregulation of miR-328 has been reported in a series of primary tumors and metastases (Wang et al., 2023). Altering miR-328 levels may thus disrupt other physiological functions and lead to unwanted effects, potentially increasing the risk of neoplasia (Wang et al., 2023).
miR-499 was overexpressed in the atrial tissue of patients with AF, compared to patients in sinus rhythm (Ling et al., 2017). In those patients, miR-499 overexpression was linked to reduced expression of small conductance Ca2+-activated potassium channels (SK3), an association that appears to be attributable to a direct interaction between miR-499 and KCNN3 (Ling et al., 2017). The expression of the CACNB2-controlled L-type calcium channel β-subunit was reduced in the atria of patients with long-term persistent AF, while inhibition of miR-499a significantly enhanced the expression of CACNB2 in cultured atrial cells (Ling et al., 2017). In addition, by targeting CACNA1C, miR-499 could modify the expression of the L-type Ca2+ channel Cav1.2 and reduce the activity of ICa-L (Ling et al., 2017).
Although miR-499 manipulation emerged as a target for atrial electrical reverse-remodeling, this strategy is far from perfect, since miR-499 is also modified in other pathologies, including systemic lupus erythematosus or stroke (Wang M. et al., 2019; Jia et al., 2020; Banach et al., 2017).
Reduced atrial expression of miR-26 was highlighted in experimental models and in AF patients (Luo et al., 2013). Meanwhile, AF ablation seemed to restore miR-26 plasma levels (Dai et al., 2022). In vitro and in vivo studies demonstrated miR-26 involvement in regulation of KCNJ2 and, via increased activity of IK1, in action potential duration (APD) shortening (Luo et al., 2013). miR-26 knockdown increased AF propensity, while miR-26 overexpression reduced AF susceptibility, suggesting a potential therapeutic role for miR-26 manipulation (Luo et al., 2013).
Studies have shown that miR-26 is involved in normal tissue growth and development and up- or downregulation of miR-26 was associated with oncogenic or tumor-suppressive genes in various tumor types (Gao and Liu, 2011). Interventions for miR-26 upregulation to decrease AF susceptibility (Luo et al., 2013) could increase the risk of glioma (Gao and Liu, 2011), whereas miR-26 involvement in tissue growth and development may affect its accuracy as stable and specific biomarkers for AF.
In an experimental model, atrial-specific upregulation of miR-31 led to neuronal nitric oxide synthase (nNOS) depletion via dystrophin translation repression, contributing to APD changes and AF inducibility in mice (Reilly et al., 2016). In contrast, miR-31 inhibition restored APD and increased dystrophin expression and nNOS content in atrial myocytes from AF patients. In patients with AF, upregulation of miR-31 seemed to be limited to the atrial myocardium (Reilly et al., 2016). Thus, miR-31 modulation may be a safer approach for AF therapy.
miR-21 overexpression has been associated with decreased ICa-L activity via decreased expression of CACNA1C and CACNB2 58 (Barana et al., 2014). Enhanced miR-21 transcription is likely to result from nuclear factor κB pathway activation, which reflects an increased inflammatory status that is commonly seen in AF (Barana et al., 2014). Recent research has linked low miR-29b levels with decreased Cx43 expression, suggesting that this miRNA may play a role in atrial proarrhythmic electrical remodeling (Lv et al., 2021). With advancing age, the expression of miR-133 appears to decrease, simultaneously with an increase in HCN2 and HCN4 expression (Li et al., 2015), which may contribute to an increase in AF susceptibility. Whether these changes are causally linked or represent independent responses to aging and AF remains unclear, highlighting the need for additional studies to elucidate these relationships. Increased miR-155 levels were also seen in the cardiomyocytes of patients with AF, and this was accompanied by a decrease in CACNA1C expression (Wang et al., 2021). Transfection of miR-155 in human induced pluripotent stem cells-derived atrial cardiomyocytes reduced ICa-L activity, while in mice with miR-155 overexpression APD decreased and there was an increase in AF vulnerability (Wang et al., 2021). Meanwhile, inhibition of miR-155 reversed the AF phenotype and the related electrical remodeling (Wang et al., 2021).
miR-21 is expressed in fibroblasts and is involved in cardiac hypertrophy by increasing ERK pathway activity via inhibition of protein sprouty homologue 1 (Spry1) (Thum et al., 2008). Considering the association between ERK activation and cardiac fibrosis (Thum et al., 2008), these results suggest that miR-21 could be involved in AF-related structural remodeling. Indeed, studies have shown a 2.5-fold higher left atrial expression of miR-21 in patients with AF compared to sinus rhythm controls (Adam et al., 2012). However, miR-21 is also expressed in non-atrial tissues and increased miR-21 expression has been observed in several other cardiac and non-cardiac diseases (Bautista-Sánchez et al., 2020; Yan et al., 2020; Bai and Bian, 2022; Liu et al., 2010).
The mechanisms by which miR-21 promotes AF are related to both electrical and structural atrial remodeling. Clinical studies demonstrated an association between extensive areas of low voltage in the left atrium of patients with AF and increased expression of miR-21 (Zhou et al., 2018). Experimental studies in mice with heart failure also associated atrial fibrosis and AF susceptibility with increased miR-21 expression (Cardin et al., 2012). Increased miR-21 levels were associated with increased amounts of atrial collagen and reduced expression of Spry1, connective tissue growth factor (CTGF), lysyl oxidase, and Rac1-GTPase, all of which have implications in atrial fibrosis (Adam et al., 2012). miR-21 could also play a role in atrial structural remodeling by activating the PI3K signaling pathway (Zhang et al., 2018). Meanwhile, miR-21 knockdown prevented left atrial structural remodeling, reduced fibrosis and AF persistence (Cardin et al., 2012), and doxycycline attenuated atrial remodeling by decreasing miR-21 and modulating miR-21-dependent pathways (Zhang et al., 2018). However, considering the involvement of miR-21 in many other pathologies, including cancers (Bautista-Sánchez et al., 2020), its use as a selective therapeutic target is difficult and requires careful study.
In patients with AF, atrial levels of miR-29b were found to be substantially lower compared to those of patients in sinus rhythm (Dawson et al., 2013). Unlike other miRNAs, miR-29 exerts antifibrotic effects (Van Rooij et al., 2008). miR-29 targets multiple extracellular matrix genes, including COL1A1, COL3A1, and fibrillin, and is involved in reducing cardiac (including atrial) fibrosis (Van Rooij et al., 2008). The decrease in miR-29b expression induced an increase in atrial expression of COL1A1 and in the amount of atrial collagen, confirming the antifibrotic effect of miR-29b (Van Rooij et al., 2008). In an atrial fibrosis model, administration of angiotensin II increased the amount of fibrotic tissue, reduced the expression of miR-29b, and increased atrial PDGF-B expression, while miR-29b overexpression reduced the degree of fibrosis, probably via miR-29b-mediated PDGF-B expression reduction (Lv et al., 2021). Another experimental study linked the antifibrotic effect of miR-29b to targeting transforming growth factor-β (TGF-β) receptor type-1 (TGFβRΙ) and inhibiting the Smad-2/3 pathway (Xinyuan et al., 2022).
However, miR-29 seems to also play a role in renal and pulmonary fibrosis (Cushing et al., 2011; Horita et al., 2021), which may affect its specificity as a biomarker for AF (Cushing et al., 2011; Horita et al., 2021; Malpeli et al., 2018; Xu et al., 2014). In addition, miR-29 downregulation has been reported in various tumors and autoimmune and hematological disorders (Horita et al., 2021; Xu et al., 2014).
In patients with AF, miR-126 levels were significantly lower than in patients in sinus rhythm (Wei et al., 2015). miR-126 overexpression has been shown to suppress Spred1 expression, increase ERK activity in primary bone marrow cells, and increase cytokine production (Ishizaki et al., 2011). However, it remains to be investigated whether these mechanisms are also active at the cardiac level.
In an AF model based on nicotine administration and rapid pacing, the two interventions induced atrial fibrosis via TGF-β1 and TGF-β receptor type-2 (TGFBR2), in parallel with a significant reduction in the expressions of miR-133 and miR-590, which target TGF-β1 and TGFBR2 (Shan et al., 2009). Although a direct causal link has not been established, these data suggest that low levels of miR-133 and miR-590 could contribute to atrial fibrosis. In an experimental study in rats with AF (Yao et al., 2020), suppression of MIAT, an inhibitor of miR133a-3p expression, reduced atrial fibrosis, inhibited the expression of genes that promote collagen deposition, and led to a decrease in CTGF and TGF-β1 levels. These changes were reversed by miR-133a-3p inhibition. However, implementation of such a strategy requires additional safety studies, given that miR-133 also intervenes in other diseases (Hua et al., 2021).
In an experimental model of AF in dogs, miR-30 levels were significantly lower 3 weeks after initiation of AF by rapid stimulation of the pulmonary veins (Li et al., 2012). Although atrial fibrosis was not evaluated in that study, previous studies have shown that miR-30 regulates CTGF, a key profibrotic protein, and controls structural changes in the extracellular matrix of the ventricular myocardium (Li et al., 2012). In a model of atrial fibrosis induced by angiotensin II, increased expression of the Snail-1 gene was associated with a decrease in miR-30a expression (Duisters et al., 2009). Meanwhile, an increase in miR-30a levels led to Snail-1 inhibition and decreased periostin expression, suggesting a direct involvement of miR-30a in atrial fibrosis (Yuan et al., 2015).
In patients undergoing coronary bypass surgery, preoperative levels of miR-483-5p were significantly higher in patients who developed postoperative AF compared to those who did not, suggesting that miR-483-5p levels may reflect the existence of an arrhythmogenic substrate (Harling et al., 2017). In studies with experimental pulmonary hypertension, miR-483 had important profibrotic effects (Zhang et al., 2020). The use of miR-483-5p as a biomarker for postoperative AF has also been proposed (Yuan et al., 2015). However, the presence of altered miR-483 expression in other pathologies and the ability of miR-483 to self-regulate its expression affects its specificity and sensitivity as an AF biomarker (Zhang et al., 2020; Matson et al., 2023).
Increased miR-155-5p and miR-24-3p levels were observed in pigs with AF, while ablation reduced the expression of these miRNAs in both pigs and patients with AF (Wang W. et al., 2019). These post-ablation changes were associated with nitric oxide (NO) reduction, suggesting the involvement of these miRNAs in endothelial NOS signaling pathway regulation (Wang M. et al., 2019). Unfortunately, neither miR-155 nor miR-24 are specific for AF, as their expression has been reported to be increased or decreased in many other pathologies (Wang W. et al., 2019).
Studies have also associated increased miR-328 levels with atrial dilation and with increased left atrial volume index and voltage zone index (Soeki et al., 2016). Reduced miR-499-5p and increased SOX6 levels were also reported in rats with AF, while miR-499-5p overexpression attenuated atrial fibrosis in that model, probably via SOX6 reduction (Yao et al., 2020).
The role of miRNAs in autonomic cardiac remodeling has been less studied. However, studies have associated various miRNAs with changes in the autonomic nervous system of the heart. miR-133a has been shown to regulate β1-adrenergic receptor transduction cascade, whereas miR-1/133a clusters regulate adrenergic control of cardiac repolarization (Ishizaki et al., 2011; Besser et al., 2014). It remains to be established whether these miRNAs also influence atrial repolarization.
miR-30 overexpression led to KCNJ3/Kir3.1 downregulation and reduced IK-Ach activity (Morishima et al., 2016). Since IK-Ach is involved in the regulation of AERP, miR-30 could affect the occurrence of AF (Morishima et al., 2016). In a canine model, atrial tachypacing increased AF inducibility and mean nerve density in the superior left fat pads. Overexpression of miR-206 additionally increased nerve density, while anti-miR-206 exerted opposite effects (Zhang et al., 2015). The mechanisms that explain this hyperinnervation are related to an increase of reactive oxygen species, coupled with a decrease in superoxide dismutase 1 induced by miR-206 (Zhang et al., 2015).
Recently, bioinformatics analysis of miRNA-predicted target genes suggested that miR-155-5p and miR-302a-3p could regulate nerve growth factor (NGF) signaling (Tran et al., 2019). The same study demonstrated that the two miRNAs are also associated with left atrial epicardial adipose tissue in AF patients. Given that the epicardial adipose tissue secretes cytokines and growth factors, including NGF, and that NGF can increase the activity of ganglionated plexi, located in the epicardial fat, NGF could represent a link between miR-155-5p and miR-302a-3p and proarrhythmic autonomic remodeling (Tran et al., 2019).
The onset and progression of AF has been associated with numerous risk factors and with altered levels of several biomarkers. However, none of these risk factors and biomarkers have proven convincing reliability for AF diagnosis or prediction, especially since they are also involved in other cardiac and non-cardiac conditions (Kornej et al., 2020). Significant efforts have been made to develop risk scores to identify patients prone to AF, but their clinical role is not convincing (Olivia et al., 2019). The multifactorial etiology and the lack of risk factors for AF in certain patients are likely to contribute to the low reliability of such risk scores (Kornej et al., 2020; Olivia et al., 2019; Fan et al., 2019).
Accumulating studies have identified numerous miRNAs that are differentially expressed in the cardiac tissue of patients with AF compared to those in sinus rhythm, suggesting that they could be used as diagnostic markers for AF (Table 1). Although promising, this strategy involves sampling of the atrial tissue, which is not feasible in most AF patients. Subsequent research has thus focused on identifying miRNAs for which the circulating levels reflect their atrial expression. Due to their ability to associate with various microstructures such as exosomes, microvesicles, and apoptotic bodies, miRNAs exhibit remarkable stability in plasma (Takahashi et al., 2017). Also, they are often bound to proteins and high-density lipoproteins, which gives them protection against RNase enzymes (Takahashi et al., 2017). Studies demonstrated modified expression levels of several miRNAs, both in the left atrium and in the peripheral blood of patients with AF compared to those in sinus rhythm (Wei et al., 2015; Ishizaki et al., 2011). Plasma levels of miR-29b, miR-21, miR-328, and miR-150 seem to be modified in patients with AF compared to those without AF (Soeki et al., 2016; Dawson et al., 2013; Nishi et al., 2013). In patients with AF, miR-126 serum levels were found to be lower than in the control group (Wei et al., 2015). However, not all circulating miRNAs reflect their atrial expression. In a study performed in patients with postoperative AF (POAF), although in the atrium there was a difference between miR-208a expression in patients who developed POAF and those who did not, serum levels of miR-208a were not significantly different between the two groups (Harling et al., 2017). When analyzing the whole blood, among the 385 miRNAs evaluated in patients with AF, only miR-328 remained significantly associated with AF after adjusting for risk factors and RNA concentration and quality (McManus et al., 2014).
Studies, including from our team, demonstrated that atrial remodeling precedes the occurrence of AF (Șerban et al., 2019). Considering that several miRNAs have been associated with atrial remodeling, evaluating these miRNAs at an early stage could help identify patients predisposed to AF. In patients undergoing coronary bypass grafting, miR-483-5p atrial expression level was higher in patients who developed POAF than in those who did not. More importantly, the peripheral level of miR-483-5p was also significantly higher in POAF compared to control patients even before surgery (Harling et al., 2017). In a rat model of AF, we have also shown that changes in miR-328 expression preceded the occurrence of AF (Scridon et al., 2023). These results suggest that dynamic changes in peripheral miR-483-5p and/or miR-328 levels could emerge as biomarkers for AF prediction. Unfortunately, none of these miRNAs are specific for AF (Wang et al., 2023; Matson et al., 2023).
The potential role of miRNAs as biomarkers of AF recurrence after cardioversion or catheter ablation has also been investigated. miR-21 serum levels negatively correlated with ablation success at 1-year follow-up, suggesting that miR-21 levels could stratify patients according to the risk of recurrence and help plan more extensive ablative treatment in patients at high risk of recurrence (Zhou et al., 2018). miR-155 levels measured post-cardioversion also proved useful for AF recurrence risk evaluation (Zhang et al., 2019).
Also, miR-22-3p and miR-107 levels were significantly higher and miR-146a-5p levels were significantly lower in patients with AF who had an increased risk of major adverse cardiovascular events (Rivera-Caravaca et al., 2020). Adding these miRNAs to the 2MACE score increased its predictive ability in AF patients (Rivera-Caravaca et al., 2020). Although these hypotheses need further confirmation from larger-scale studies, they can be a starting point for establishing miRNAs as biomarkers for major cardiovascular events in patients with AF. Many other miRNAs have or are currently being studied in the context of FA, indicating a rapidly expanding field that holds significant promise for the discovery of new biomarkers.
Studies have demonstrated that changes in miRNA expression can induce a substrate that promotes AF onset and/or maintenance, while restoring normal miRNA expression can reduce AF risk (Table 2).
Decreased expression of miRNAs associated with AF can be addressed using synthetic miRNA duplex approaches (miRNA mimics) or virus-mediated miRNA transfer (Ishida and Selaru, 2013). miRNA mimics have been used in experimental studies to evaluate the impact of miR-9, miR-23a, and miR-34a overexpression on the mechanisms involved in AF, but the vast majority have not been yet tested in vivo (Wiedmann et al., 2022). Importantly, in vivo studies that have used this strategy for other pathologies have not reported significant adverse reactions (Lhamyani et al., 2021). Lentivirus and adenovirus transfection containing precursors of miR-1 and miR-328 were used to increase the expression of these miRNAs, while an adeno-associated virus containing miR-26a mimic was used to increase the expression of miR-26a (Table 2) (Jia et al., 2013; Lu et al., 2010; Luo et al., 2013). Although the use of cardiotropic adeno-associated virus-mediated transfer seems safe in terms of extracardiac effects, unwanted immune or inflammatory responses and relatively low load capacity remain the main disadvantages of this method (Dawson et al., 2013; Ishida and Selaru, 2013).
For miRNAs with increased levels involved in AF, studies used three strategies to decrease miRNA levels: antimiRs, miRNA sponges, and miRNA erasers (Ishida and Selaru, 2013). Numerous studies have demonstrated the therapeutic potential of antimiRs in AF (Table 2), without major adverse reactions (Jia et al., 2013; Lu et al., 2010; Cardin et al., 2012). However, considering the systemic delivery, antimiRs could have extracardiac adverse effects (Ishida and Selaru, 2013). miRNA sponges and miRNA erasers seem to be safer from this point of view. In AF, decreasing the expression of miR-29b and miR-378 using a sponge-based strategy had no adverse reactions (Dawson et al., 2013; Wu et al., 2023). However, controlling the administered dose for successfully inhibiting the miRNA of interest is challenging (Ebert and Sharp, 2010). A much more specific approach involves the use of single-stranded masking oligonucleotides (miR-masks) (Ishida and Selaru, 2013). The advantage is that a specific mRNA target is blocked, while the other miRNA targets are not affected. miR-masks were used to study the effect of miR-26 on KCNJ2/KIR2.1 expression in AF, without adverse effects (Luo et al., 2013).
Experimental studies have shown a potential therapeutic role for miRNA-based approaches in AF. However, their long-term efficacy, safety, and pharmacokinetics in humans remain unknown. Future studies will have to elucidate if the results observed in experimental studies are also applicable in humans and if these approaches are sufficiently safe for clinical use.
Identification of reliable AF diagnostic and predictive biomarkers is an area of ongoing research. The diagnosis of AF is established based on the identification of a typical arrhythmic episode on an ECG tracing. However, AF is often intermittent and asymptomatic. Hence, it often escapes detection, exposing patients to increased risk of complications. Readily available diagnostic AF biomarkers could identify patients in whom AF (ECG-based) diagnostic strategies should be intensified. This would be of particular interest in patients at risk of stroke, in whom AF diagnosis will be followed by prophylactic anticoagulation. In parallel, a reliable AF predictive biomarker could guide AF screening strategies and intensify substrate-modifying approaches to reduce AF. Such biomarkers could also guide antiarrhythmic therapies and improve selection for catheter ablation, reducing the rates of complications and costs. miRNAs are seen as highly promising in these regards, and several miRNAs have been associated with the presence and recurrence of AF. However, to date, with the exception of miR-31, no miRNA has been identified as being specific for AF, and the predictive ability of miRNAs identified so far is rather modest. More will have to be done to identify miRNAs specific for AF and validate them as AF biomarkers. Maybe the solution will come from a combination of multiple miRNAs with or without protein biomarkers on the same panel. Given the complexity of AF, such a tool would provide a more comprehensive picture of AF and could improve diagnostic accuracy.
Currently available antiarrhythmic drugs have limited efficacy and high side-effect profiles. Although promising in the preclinical studies, most upstream therapies have failed to show similar success in the clinical trials. Given their contribution to AF pathogenesis, miRNAs could represent the key to a novel therapeutic approach. Experimental studies are again very promising in this regard, although no clinical data is available at this point. Given the complexity and the multitude of mechanisms in which miRNAs are involved, miRNA manipulation could influence several pathophysiological pathways involved in AF, which places them as potential multifunctional therapeutic strategies in AF. However, the same features of miRNAs raise concerns regarding the potential off-target effects of miRNA manipulation, which could affect cardiac regions other than the atria or even organs other than the heart. For instance, miR-328 levels were significantly higher in animals with AF than in those without (Scridon et al., 2023), suggesting that miR-328 could represent a possible target for AF therapy. miR-328 is, however, a known tumor suppressor, and decreased miR-328 levels have been reported in several types of cancers, precluding its inhibition as a potential therapeutic approach for AF. Similar features are also seen for other miRNAs associated with AF. Strategies designed to specifically target atrial miRNA expression will probably provide the solution, but technical challenges remain to be overcome.
With the advent of miRNAs, we may have discovered the Holy Grail. However, extensive investigations and additional research are still needed before miRNAs become therapeutic targets or biomarkers in AF. Collaboration between multidisciplinary teams that include academic researchers, clinicians, biotechnologists and pharmaceutical companies is essential for the discovery of the specific miRNA or a panel of miRNAs with potential clinical utility. Performing more robust preclinical studies that use sensitive and specific miRNA detection methods are imperative. Many studies have used microarray techniques, which is a semi-quantitative method with high rates of false negative and positive results. The use of quantitative methods (e.g., qRT-PCR) could improve our knowledge about miRNA involvement in AF. The limitations of the clinical studies performed so far cannot be overlooked. The small number of patients and the high inter-individual variability in terms of sex, age, drug therapies, and concomitant conditions contribute to the inconsistencies of the results. Large multi-center datasets and registries will be needed to identify AF-specific miRNAs that could be used for AF prediction, diagnosis, and therapy, if such miRNAs exist. Last but not least, the great efforts of consortia are crucial for the standardization of experimental protocols. They ensure the experimental protocols reproducibility and the miRNAs validation in extended cohorts of patients, thus accelerating the use of miRNAs in clinical practice. The demonstrated success of other miRNAs that have proven effective in the treatment of other diseases such as cancer offer hope for similar achievements in AF, emphasizing the importance of comprehensive preclinical studies followed by rigorous clinical trials.
MicroRNAs play an essential role in regulating structural, electrical, and autonomic remodeling in AF. Experimental studies have shown that miRNA manipulation could prevent or even reverse pathological remodeling in AF. Clinical and preclinical studies suggest that changes in miRNA levels in blood or atrial tissue could emerge as novel biomarkers for the diagnosis and/or prediction of AF. However, it remains to be determined if and which of the miRNAs associated with AF have sufficient specificity and sensitivity to be used as AF biomarkers in clinical practice. Further research is also needed to establish if therapeutic strategies based on miRNA manipulation could emerge as effective and safe approaches for patients with AF.
AB: Writing–original draft. AS: Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the University of Medicine, Pharmacy, Science and Technology “George Emil Palade” Târgu Mureș [grant number 171/5/09.01.2024].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adam, O., Löhfelm, B., Thum, T., Gupta, S. K., Puhl, S. L., Schäfers, H. J., et al. (2012). Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol. 107, 278. doi:10.1007/s00395-012-0278-0
Bai, X., and Bian, Z. (2022). MicroRNA-21 is a versatile regulator and potential treatment target in central nervous system disorders. Front. Mol. Neurosci. 15, 842288–842313. doi:10.3389/fnmol.2022.842288
Banach, E., Dmitrzak-Weglarz, M., Pawlak, J., Kapelski, P., Szczepankiewicz, A., Rajewska-Rager, A., et al. (2017). Dysregulation of miR-499, miR-708 and miR-1908 during a depression episode in bipolar disorders. Neurosci. Lett. 654, 117–119. doi:10.1016/j.neulet.2017.06.019
Barana, A., Matamoros, M., Dolz-Gaitón, P., Pérez-Hernández, M., Amorós, I., Núñez, M., et al. (2014). Chronic atrial fibrillation increases microRNA-21 in human atrial myocytes decreasing L-type calcium current. Arrhythmia Electrophysiol. 7, 861–868. doi:10.1161/CIRCEP.114.001709
Bautista-Sánchez, D., Arriaga-Canon, C., Pedroza-Torres, A., De La Rosa-Velázquez, I. A., González-Barrios, R., Contreras-Espinosa, L., et al. (2020). The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol. Ther. Nucleic Acids 20, 409–420. doi:10.1016/j.omtn.2020.03.003
Benito, B., García-Elías, A., Ois, Á., Tajes, M., Vallès, E., Ble, M., et al. (2022). Plasma levels of miRNA-1-3p are associated with subclinical atrial fibrillation in patients with cryptogenic stroke. Rev. Esp. Cardiol. Engl. Ed. 75, 717–726. doi:10.1016/j.rec.2021.12.001
Besser, J., Malan, D., Wystub, K., Bachmann, A., Wietelmann, A., Sasse, P., et al. (2014). MiRNA-1/133a clusters regulate adrenergic control of cardiac repolarization. PLoS One 9, e113449. doi:10.1371/journal.pone.0113449
Cañón, S., Caballero, R., Herraiz-Martínez, A., Pérez-Hernández, M., López, B., Atienza, F., et al. (2016). miR-208b upregulation interferes with calcium handling in HL-1 atrial myocytes: implications in human chronic atrial fibrillation. J. Mol. Cell. Cardiol. 99, 162–173. doi:10.1016/j.yjmcc.2016.08.012
Cardin, S., Guasch, E., Luo, X., Naud, P., Quang, K.Le, Shi, Y. F., et al. (2012). Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ. Arrhythmia Electrophysiol. 5, 1027–1035. doi:10.1161/CIRCEP.112.973214
Chen, H., Zhang, F., Zhang, Y. L., and Yang, X. C. (2021). Relationship between circulating miRNA-21, atrial fibrosis, and atrial fibrillation in patients with atrial enlargement. Ann. Palliat. Med. 10, 12742–12749. doi:10.21037/apm-21-3518
Chen, X., Zhang, Y., Meng, H., Chen, G., Ma, Y., Li, J., et al. (2024). Identification of miR-1 and miR-499 in chronic atrial fibrillation by bioinformatics analysis and experimental validation. Front. Cardiovasc. Med. 11, 1400643. doi:10.3389/fcvm.2024.1400643
Cushing, L., Kuang, P. P., Qian, J., Shao, F., Wu, J., Little, F., et al. (2011). miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 45, 287–294. doi:10.1165/rcmb.2010-0323OC
Dai, M., Jiang, T., Luo, C., dong, D. W., Wang, M., yan, Q., et al. (2022). Radiofrequency ablation reduces expression of SELF by upregulating the expression of microRNA-26a/b in the treatment of atrial fibrillation. J. Interv. Card. Electrophysiol. 65, 663–673. doi:10.1007/s10840-022-01305-x
Dawson, K., Wakili, R., Ördög, B., Clauss, S., Chen, Y., Iwasaki, Y., et al. (2013). MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation 127, 1466–1475. doi:10.1161/CIRCULATIONAHA.112.001207
De Gonzalo-Calvo, D., Van Der Meer, R. W., Rijzewijk, L. J., Smit, J. W. A., Revuelta-Lopez, E., Nasarre, L., et al. (2017). Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci. Rep. 7, 1–14. doi:10.1038/s41598-017-00070-6
Duisters, R. F., Tijsen, A. J., Schroen, B., Leenders, J. J., Lentink, V., Van Der Made, I., et al. (2009). MiR-133 and miR-30 Regulate connective tissue growth factor: implications for a role of micrornas in myocardial matrix remodeling. Circ. Res. 104, 170–178. doi:10.1161/CIRCRESAHA.108.182535
Ebert, M. S., and Sharp, P. A. (2010). MicroRNA sponges: progress and possibilities. Rna 16, 2043–2050. doi:10.1261/rna.2414110
Fan, S. M., Fann, A., Nah, G., Pletcher, M. J., Olgin, J. E., and Marcus, G. M. (2019). Characteristics of atrial fibrillation patients with a family history of atrial fibrillation. J. Atr. Fibrillation 12, 2198–2207. doi:10.4022/jafib.2198
Gao, J., and Liu, Q. G. (2011). The role of miR-26 in tumors and normal tissues (Review). Oncol. Lett. 2, 1019–1023. doi:10.3892/ol.2011.413
Girmatsion, Z., Biliczki, P., Bonauer, A., Wimmer-Greinecker, G., Scherer, M., Moritz, A., et al. (2009). Changes in microRNA-1 expression and Ik1 up-regulation in human atrial fibrillation. Hear. Rhythm. 6, 1802–1809. doi:10.1016/j.hrthm.2009.08.035
Harling, L., Lambert, J., Ashrafian, H., Darzi, A., Gooderham, N. J., and Athanasiou, T. (2017). Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardio-thoracic Surg. 51, 73–78. doi:10.1093/ejcts/ezw245
Horita, M., Farquharson, C., and Stephen, L. A. (2021). The role of miR-29 family in disease. J. Cell. Biochem. 122, 696–715. doi:10.1002/jcb.29896
Hua, Y. T., Xu, W. X., Li, H., and Xia, M. (2021). Emerging roles of MiR-133a in human cancers. J. Cancer 12, 198–206. doi:10.7150/JCA.48769
Huang, H., Chen, H., Liang, X., Chen, X., Chen, X., and Chen, C. (2022). Upregulated miR-328-3p and its high risk in atrial fibrillation: a systematic review and meta-analysis with meta-regression. Med. Baltim. 101, e28980. doi:10.1097/md.0000000000028980
Ishida, M., and Selaru, F. M. (2013). miRNA-based therapeutic strategies. Curr. Pathobiol. Rep. 1, 63–70. doi:10.1007/s40139-012-0004-5
Ishizaki, T., Tamiya, T., Taniguchi, K., Morita, R., Kato, R., Okamoto, F., et al. (2011). miR126 positively regulates mast cell proliferation and cytokine production through suppressing Spred1. Genes. Cells 16, 803–814. doi:10.1111/j.1365-2443.2011.01529.x
Jia, H., Qu, M., Fan, G., Wu, H., and Wang, L. (2020). miR-499-5p suppresses C-reactive protein and provides neuroprotection in hypoxic-ischemic encephalopathy in neonatal rat. Neurosci. Res. 161, 44–50. doi:10.1016/j.neures.2019.12.002
Jia, X., Zheng, S., Xie, X., Zhang, Y., Wang, W., Wang, Z., et al. (2013). MicroRNA-1 accelerates the shortening of atrial effective refractory period by regulating KCNE1 and KCNB2 expression: an atrial tachypacing rabbit model. PLoS One 8, 856399–e85711. doi:10.1371/journal.pone.0085639
Kornej, J., Börschel, C. S., Börschel, C. S., Benjamin, E. J., Benjamin, E. J., Schnabel, R. B., et al. (2020). Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ. Res. 127, 4–20. doi:10.1161/CIRCRESAHA.120.316340
Lhamyani, S., Gentile, A. M., Giráldez-Pérez, R. M., Feijóo-Cuaresma, M., Romero-Zerbo, S. Y., Clemente-Postigo, M., et al. (2021). miR-21 mimic blocks obesity in mice: a novel therapeutic option. Mol. Ther. - Nucleic Acids. 26, 401–416. doi:10.1016/j.omtn.2021.06.019
Li, H., Li, S., Yu, B., and Liu, S. (2012). Expression of miR-133 and miR-30 in chronic atrial fibrillation in canines. Mol. Med. Rep. 5, 1457–1460. doi:10.3892/mmr.2012.831
Li, J., Dong, X., Wang, Z., and Wu, J. (2014). MicroRNA-1 in cardiac diseases and cancers. Korean J. Physiol. Pharmacol. 18, 359–363. doi:10.4196/kjpp.2014.18.5.359
Li, Y. D., Hong, Y. F., Yusufuaji, Y., Tang, B. P., Zhou, X. H., Xu, G. J., et al. (2015). Altered expression of hyperpolarization-activated cyclic nucleotide-gated channels and microRNA-1 and -133 in patients with age-associated atrial fibrillation. Mol. Med. Rep. 12, 3243–3248. doi:10.3892/mmr.2015.3831
Ling, T. Y., Wang, X. L., Chai, Q., Lu, T., Stulak, J. M., Joyce, L. D., et al. (2017). Regulation of cardiac CACNB2 by microRNA-499: potential role in atrial fibrillation. BBA Clin. 7, 78–84. doi:10.1016/j.bbacli.2017.02.002
Liu, G., Friggeri, A., Yang, Y., Milosevic, J., Ding, Q., Thannickal, V. J., et al. (2010). miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207, 1589–1597. doi:10.1084/jem.20100035
Lu, Y., Zhang, Y., Wang, N., Pan, Z., Gao, X., Zhang, F., et al. (2010). MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation 122, 2378–2387. doi:10.1161/CIRCULATIONAHA.110.958967
Luo, X., Pan, Z., Shan, H., Xiao, J., Sun, X., Wang, N., et al. (2013). MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Invest. 123, 1939–1951. doi:10.1172/JCI62185
Lv, X., Lu, P., Hu, Y., and Xu, T. (2021). Overexpression of MiR-29b-3p inhibits atrial remodeling in rats by targeting PDGF-B signaling pathway. Oxid. Med. Cell. Longev. 2021, 3763529. doi:10.1155/2021/3763529
Ma, Q., Ma, Y., Wang, X., Li, S., Yu, T., Duan, W., et al. (2020). Circulating miR-1 as a potential predictor of left ventricular remodeling following acute ST-segment myocardial infarction using cardiac magnetic resonance. Quant. Imaging Med. Surg. 10, 1490–1503. doi:10.21037/QIMS-19-829
Ma, R., Wang, J., Wu, X., Chen, Z., Zhang, X., Yang, X., et al. (2017). MiR-499 is a diagnostic biomarker of paroxysmal atrial fibrillation involved in the development of atrial fibrillation. Int. J. Clin. Exp. Pathology 10, 4221–4231.
Malpeli, G., Barbi, S., Tosadori, G., Greco, C., Zupo, S., Pedron, S., et al. (2018). MYC-related microRNAs signatures in non-Hodgkin B-cell lymphomas and their relationships with core cellular pathways. Oncotarget 9, 29753–29771. doi:10.18632/oncotarget.25707
Matson, K., Macleod, A., Mehta, N., Sempek, E., and Tang, X. (2023). Impacts of MicroRNA-483 on human diseases. Non-coding RNA 9, 37. doi:10.3390/ncrna9040037
McManus, D. D., Lin, H., Tanriverdi, K., Quercio, M., Yin, X., Larson, M. G., et al. (2014). Relations between circulating microRNAs and atrial fibrillation: data from the framingham offspring study. Hear. Rhythm 11, 663–669. doi:10.1016/j.hrthm.2014.01.018
Morishima, M., Iwata, E., Nakada, C., Tsukamoto, Y., Takanari, H., Miyamoto, S., et al. (2016). Atrial fibrillation-mediated upregulation of miR-30d regulates myocardial electrical remodeling of the G-protein-gated K(+) channel, Ik.ACh. IK.ACh. Circ. J. 80, 1346–1355. doi:10.1253/circj.CJ-15-1276
Nishi, H., Sakaguchi, T., Miyagawa, S., Yoshikawa, Y., Fukushima, S., Saito, S., et al. (2013). Impact of microRNA expression in human atrial tissue in patients with atrial fibrillation undergoing cardiac surgery. PLoS One 8, e73397–e73398. doi:10.1371/journal.pone.0073397
Olivia, L. H., Khurshid, S., Weng, L. C., Anderson, C. D., Wang, E. Y., Ashburner, J. M., et al. (2019). Development and validation of a prediction model for atrial fibrillation using electronic health records. JACC Clin. Electrophysiol. 5, 1331–1341. doi:10.1016/j.jacep.2019.07.016
Paul, A., Pai, P. G., Ariyannur, P. S., and Joy, R. A. (2021). Diagnostic accuracy of MicroRNA 208b level with respect to different types of atrial fibrillation. Indian Heart J. 73, 506–510. doi:10.1016/j.ihj.2021.06.018
Reilly, S. N., Liu, X., Carnicer, R., Recalde, A., Muszkiewicz, A., Jayaram, R., et al. (2016). Up-regulation of MIR-31 in human atrial fibrillation begets the arrhythmia by depleting dystrophin and neuronal nitric oxide synthase. Sci. Transl. Med. 8, 340ra74. doi:10.1126/scitranslmed.aac4296
Rivera-Caravaca, J. M., Teruel-Montoya, R., Roldán, V., Cifuentes-Riquelme, R., Crespo-Matas, J. A., de los Reyes-García, A. M., et al. (2020). Pilot study on the role of circulating mirnas for the improvement of the predictive ability of the 2mace score in patients with atrial fibrillation. J. Clin. Med. 9, 3645–3711. doi:10.3390/jcm9113645
Scridon, A., Balan, A. I., Halatiu, V. B., Cozac, D. A., Bobarnac, R. L., Moldovan, V., et al. (2023). miR-328 - a novel diagnostic and predictive atrial fibrillation biomarker in spontaneously hypertensive rats. Europace 25, 2023. doi:10.1093/europace/euad122.576
Scridon, A., Şerban, R. C., and Chevalier, P. (2018). Atrial fibrillation: neurogenic or myogenic? Arch. Cardiovasc. Dis. 111, 59–69. doi:10.1016/j.acvd.2017.11.001
Șerban, R. C., Balan, A. I., Perian, M., Pintilie, I., Somkereki, C., Huțanu, A., et al. (2019). Atrial electrical remodeling induced by chronic ischemia and inflammation in patients with stable coronary artery disease. Chin. J. Physiol. 62, 11–16. doi:10.4103/CJP.CJP_2_19
Shan, H., Zhang, Y., Lu, Y., Zhang, Y., Pan, Z., Cai, B., et al. (2009). Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc. Res. 83, 465–472. doi:10.1093/cvr/cvp130
Soeki, T., Matsuura, T., Bando, S., Tobiume, T., Uematsu, E., Ise, T., et al. (2016). Relationship between local production of microRNA-328 and atrial substrate remodeling in atrial fibrillation. J. Cardiol. 68, 472–477. doi:10.1016/j.jjcc.2015.12.007
Sugiyama, Y., Yoshimi, R., Takeno, M., Kunishita, Y., Kishimoto, D., Kamiyama, R., et al. (2020). miR-1 is a novel biomarker for polymyositis/dermatomyositis-associated interstitial lung disease. Mod. Rheumatol. 30, 878–883. doi:10.1080/14397595.2019.1661584
Takahashi, R. U., Prieto-Vila, M., Hironaka, A., and Ochiya, T. (2017). The role of extracellular vesicle microRNAs in cancer biology. Clin. Chem. Lab. Med. 55, 648–656. doi:10.1515/cclm-2016-0708
Terentyev, D., Belevych, A. E., Terentyeva, R. M. M. M., Malana, G. E., Kuhn, D. E., Abdellatif, M., et al. (2009). miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ. Res. 104, 514–521. doi:10.1161/CIRCRESAHA.108.181651
Thum, T., Gross, C., Fiedler, J., Fischer, T., Kissler, S., Bussen, M., et al. (2008). MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984. doi:10.1038/nature07511
Tran, K. V., Majka, J., Sanghai, S., Sardana, M., Lessard, D., Milstone, Z., et al. (2019). Micro-RNAs are related to epicardial adipose tissue in participants with atrial fibrillation: data from the MiRhythm study. Front. Cardiovasc. Med. 6, 115–118. doi:10.3389/fcvm.2019.00115
Tsoporis, J. N., Fazio, A., Rizos, I. K., Izhar, S., Proteau, G., Salpeas, V., et al. (2018). Increased right atrial appendage apoptosis is associated with differential regulation of candidate MicroRNAs 1 and 133A in patients who developed atrial fibrillation after cardiac surgery. J. Mol. Cell. Cardiol. 121, 25–32. doi:10.1016/j.yjmcc.2018.06.005
Van Rooij, E., Sutherland, L. B., Thatcher, J. E., DiMaio, J. M., Naseem, R. H., Marshall, W. S., et al. (2008). Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U. S. A. 105, 13027–13032. doi:10.1073/pnas.0805038105
Wang, J., Ye, Q., Bai, S., Chen, P., Zhao, Y., Ma, X., et al. (2021). Inhibiting microRNA-155 attenuates atrial fibrillation by targeting CACNA1C. J. Mol. Cell. Cardiol. 155, 58–65. doi:10.1016/j.yjmcc.2021.02.008
Wang, M., Sun, L., Ding, W., Cai, S., and Zhao, Q. (2019a). Ablation alleviates atrial fibrillation by regulating the signaling pathways of endothelial nitric oxide synthase/nitric oxide via miR-155-5p and miR-24-3p. J. Cell. Biochem. 120, 4451–4462. doi:10.1002/jcb.27733
Wang, W., Li, T., Gao, L., Li, Y., Sun, Y., and Yao, H. C. (2019b). Plasma miR-208b and miR-499: potential biomarkers for severity of coronary artery disease. Dis. Markers 2019, 9842427. doi:10.1155/2019/9842427
Wang, Z., Xie, W., and Guan, H. (2023). The diagnostic, prognostic role and molecular mechanism of miR-328 in human cancer. Biomed. Pharmacother. 157, 114031. doi:10.1016/j.biopha.2022.114031
Wei, X. J., Han, M., Yang, F. Y., Wei, G. C., Liang, Z. G., Yao, H., et al. (2015). Biological significance of miR-126 expression in atrial fibrillation and heart failure. Braz. J. Med. Biol. Res. 48, 983–989. doi:10.1590/1414-431X20154590
Wiedmann, F., Kraft, M., Kallenberger, S., Büscher, A., Paasche, A., Blochberger, P. L., et al. (2022). MicroRNAs regulate TASK-1 and are linked to myocardial dilatation in atrial fibrillation. J. Am. Heart Assoc. 11, e023472. doi:10.1161/JAHA.121.023472
Wu, L., Gao, B., Shen, M., Wei, L., Li, Z., and Zhuang, W. (2023). lncRNA LENGA sponges miR-378 to promote myocardial fibrosis in atrial fibrillation. Open Med. 18, 20230831. doi:10.1515/med-2023-0831
Xinyuan, H., Wang, S., Yong, Z., Xueting Zhang, X. W., and Wang, X. (2022). miR-29b ameliorates atrial fibrosis in rats with atrial fibrillation by targeting TGFβRΙ and inhibiting the activation of Smad-2/3 pathway. J. Bioenerg. Biomembr. 54, 81–91. doi:10.1007/s10863-022-09934-7
Xu, L., Xu, Y., Jing, Z., Wang, X., Zha, X., Zeng, C., et al. (2014). Altered expression pattern of miR-29a, miR-29b and the target genes in myeloid leukemia. Exp. Hematol. Oncol. 3, 17–7. doi:10.1186/2162-3619-3-17
Yan, H., Zhang, X., and Xu, Y. (2020). Aberrant expression of miR-21 in patients with inflammatory bowel disease: a protocol for systematic review and meta analysis. Med. Baltim. 99, e19693. doi:10.1097/MD.0000000000019693
Yang, B., Lin, H., Xiao, J., Lu, Y., Luo, X., Li, B., et al. (2007). The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 13, 486–491. doi:10.1038/nm1569
Yao, L., Zhou, B., You, L., Hu, H., and Xie, R. (2020). LncRNA MIAT/miR-133a-3p axis regulates atrial fibrillation and atrial fibrillation-induced myocardial fibrosis. Mol. Biol. Rep. 47, 2605–2617. doi:10.1007/s11033-020-05347-0
Yuan, C. T., Li, X. X., Cheng, Q. J., Wang, Y. H., Wang, J. H., and Liu, C. L. (2015). MiR-30a regulates the atrial fibrillation-induced myocardial fibrosis by targeting snail 1. Int. J. Clin. Exp. Pathol. 8, 15527–15536.
Zhang, J., He, Y., Yan, X., Chen, S., He, M., Lei, Y., et al. (2020). Micro RNA-483 amelioration of experimental pulmonary hypertension. EMBO Mol. Med. 12, e11303–e11313. doi:10.15252/emmm.201911303
Zhang, K., Zhao, L., Ma, Z., Wang, W., Li, X., Zhang, Y., et al. (2018). Doxycycline attenuates atrial remodeling by interfering with microRNA-21 and downstream phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K) signaling pathway. Med. Sci. Monit. 24, 5580–5587. doi:10.12659/MSM.909800
Zhang, X., Xiao-Ping, X., Xu-Ai, R., and Cui, T. (2019). Plasma miRNA-155 levels predict atrial fibrillation recurrence after cardioversion. Heart Surg. Forum 22, E140–E148. doi:10.1532/hsf.2281
Zhang, Y., Zheng, S., Geng, Y., Xue, J., Wang, Z., Xie, X., et al. (2015). MicroRNA profiling of atrial fibrillation in canines: MiR-206 modulates intrinsic cardiac autonomic nerve remodeling by regulating SOD1. PLoS One 10, 01226744–e122716. doi:10.1371/journal.pone.0122674
Zhou, Q., Maleck, C., Von Ungern-Sternberg, S. N. I., Neupane, B., Heinzmann, D., Marquardt, J., et al. (2018). Circulating MicroRNA-21 correlates with left atrial low-voltage areas and is associated with procedure outcome in patients undergoing atrial fibrillation ablation. Circ. Arrhythmia Electrophysiol. 11, 1–9. doi:10.1161/CIRCEP.118.006242
Keywords: antiarrhythmic therapy, atrial fibrillation, electrical remodeling, microRNA, structural remodeling
Citation: Balan AI and Scridon A (2025) MicroRNAs in atrial fibrillation – have we discovered the Holy Grail or opened a Pandora’s box?. Front. Pharmacol. 16:1535621. doi: 10.3389/fphar.2025.1535621
Received: 27 November 2024; Accepted: 24 January 2025;
Published: 12 February 2025.
Edited by:
Felix Wiedmann, Heidelberg University Hospital, GermanyReviewed by:
Manuel Kraft, Heidelberg University Hospital, GermanyCopyright © 2025 Balan and Scridon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alina Scridon, YWxpbmFzY3JpZG9uQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.