
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 29 January 2025
Sec. Drugs Outcomes Research and Policies
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1534479
This article is part of the Research Topic Clinical Pharmacist Service Promotes the Improvement of Medical Quality Volume II View all 51 articles
Background: Coronary artery disease (CAD) is the leading cause of death worldwide, and inflammation is a significant factor in its development. While the hemoglobin-to-red blood cell distribution width ratio (HRR), an indicator of inflammation, has been linked to various diseases, its association with CAD is not well established.
Methods: We conducted an analysis using data from the National Health and Nutrition Examination Survey (NHANES) spanning from 2011 to 2018. After excluding participants due to age, missing data, and potential confounding factors, 6,881 individuals were included in our study. CAD was identified through self-reported questionnaires, and HRR was determined from laboratory measurements. We controlled for factors such as hypertension, waist circumference, systolic blood pressure, fasting plasma glucose, and others in our logistic regression analysis to explore the relationship between HRR and CAD.
Results: We found that higher HRR levels were associated with a lower risk of CAD. In our fully adjusted model, the odds ratios for CAD for the second, third, and fourth quartiles of HRR were 0.38, 0.42, and 0.51, respectively, compared to the first quartile (P < 0.001). An increase in HRR by one unit was associated with a 49% decrease in the likelihood of CAD. Furthermore, linear regression models indicated a 74% reduction in CAD risk for each one-unit increase in HRR (P = 0.0002). There was a notable threshold at HRR 1.02; beyond this point, each unit increase in HRR was associated with a 91% decrease in CAD odds. This suggests that for individuals with an HRR above 1.02, strategies to increase body water content and reduce blood viscosity could potentially lower their risk of developing CAD.
Conclusion: Our study revealed an inverse linear relationship between HRR and CAD risk, indicating that HRR may serve as a protective factor against CAD.
Coronary artery disease (CAD) is one of the common diseases in cardiovascular medicine, including but not limited to coronary atherosclerosis, angina, myocardial ischemia, myocardial infarction, and coronary artery spasm (Crook et al., 2023; Chen et al., 2024; Kalyani et al., 2014). According to data from the World Health Organization, 7.4 million people worldwide die from CAD (Szilveszter et al., 2022; Manolis et al., 2024). The incidence of CAD is increasing year by year, and it is also a significant factor in global disability and mortality rates, having a major impact on individual health and socio-economics (Tada et al., 2024; Tsukihashi et al., 2021; Omori et al., 2019). The causes of CAD are diverse, including hypertension (Honigberg et al., 2019; Wang et al., 2023), diabetes (Mäenpää et al., 2023; Lin et al., 2024; Luo et al., 2019), hyperlipidemia (Li et al., 2023; Andersen, 1992), obesity (Yang et al., 2023; Jahangir et al., 2014; Pedersen et al., 2019), inflammation (Attiq et al., 2024; Zhao Z. et al., 2024; Kinoshita et al., 2023), and lifestyle factors (Cho et al., 2023; Fu et al., 2023; Said et al., 2019). Among these, inflammation is an important etiology, and in-depth research on it can help improve outcomes for CAD patients.
The hemoglobin-to-red blood cell distribution width ratio (HRR) is recognized as an inflammatory marker associated with the incidence and adverse events of various diseases (Chen et al., 2023; Zhao W. X. et al., 2024; Del Rosso et al., 2022), such as diabetes (Liu et al., 2016), atrial fibrillation (Ralevic et al., 2016; Cortigiani et al., 2020), and even non-small cell lung cancer (Marzio et al., 2022; Meng et al., 2022). HRR is primarily composed of two hematological indicators: hemoglobin (HB) and red blood cell distribution width (RDW) (Su et al., 2021). HB mainly reflects the degree of anemia, while RDW indicates the heterogeneity in red blood cell volume and is indirectly used for diagnosing anemia (Jang et al., 2023). Previous studies have shown that HRR undergoes dynamic changes in response to disease stimuli, and its level variations can serve as a sensitive measure of inflammation (Yılmaz et al., 2021; Eyiol and Ertekin, 2024). Although HRR, as an inflammatory marker, is related to numerous diseases, the clinical relationship between HRR and CAD remains unclear. The hemoglobin-to-red blood cell distribution width ratio (HRR) is calculated using routine blood parameters, and it has been shown to correlate with systemic inflammation. In the context of CAD, inflammation plays a critical role in plaque formation and destabilization, which can lead to acute cardiovascular events. By examining HRR in relation to CAD, we aim to explore its potential as an accessible and cost-effective biomarker for risk stratification in CAD patients. This study attempts to explore the association between HRR and CAD from the perspective of inflammation.
Given that CAD may be associated with inflammation, there might be a unique link between HRR and CAD. However, the relationship between HRR and CAD is not clear in current research. Therefore, this study investigates whether HRR, an emerging inflammatory marker, is independently associated with CAD prevalence, addressing a significant gap in existing literature.
NHANES is a cross-sectional, nationally representative survey conducted in the United States. It encompasses data collection through basic information, anthropometric measurements, blood samples, and self-reported questionnaires. Additionally, informed consent is obtained from each participant at the outset of NHANES, thereby eliminating the need for further ethical approval for this study. Figure 1 provides a detailed depiction of the participant selection process for this study (Figure 1). Initially, we extracted demographic, laboratory, physical examination, and questionnaire data from a total of 39,156 participants in NHANES from 2011 to 2018. Subsequently, individuals less than 18 years old or over 80 years old were excluded, totaling 15,331 individuals. Following this, 1,359 participants lacking information related to coronary artery disease (CAD) were excluded. Next, we removed 13,342 participants due to missing data required for the calculation of the HRR. To further enhance the credibility of the study, we continued to screen for potential confounding factors, leading to the exclusion of an additional 2,243 participants who had missing information regarding these factors. Specifically, six individuals did not provide information on smoking, 219 lacked measured systolic blood pressure, 273 were missing data related to hypertension, 2 lacked glycated hemoglobin data, 4 had unknown levels of education, 106 did not have LDL data, 895 were missing Poverty Income Ratio (PIR) income level data, 2 lacked diagnosed diabetes-related information, and another 732 did not provide information on alcohol consumption. Ultimately, a total of 6,881 participants were included in the study.
The diagnosis of CAD was derived from the questionnaire data in the NHANES database. It was primarily determined by three questions in the Medical Conditions Section: 1) Have you ever been told you had coronary heart disease? 2) Have you ever been told you had angina or angina pectoris? 3) Have you ever been told you had a heart attack? This is also called a myocardial infarction. An affirmative response to any of the above three questions was used to define CAD, and the absence of such a response was considered negative for CAD.
The Hemoglobin-to-Red Blood Cell Distribution Width Ratio (HRR) is calculated by dividing the Hemoglobin (HB) levels by the Red Blood Cell Distribution Width (RDW), utilizing laboratory data from NHANES. In subsequent analyses, HRR is stratified into quartiles.
Building on previous relevant studies, this study included hypertension, waist circumference (WC), systolic blood pressure (SBP), fasting plasma glucose (FPG), triglycerides (TG), low-density lipoprotein (LDL), marital status, body mass index (BMI), total cholesterol (TC), age, poverty-income ratio (PIR), glycated hemoglobin (HbA1C), diabetes, race, education level, high-density lipoprotein (HDL), and gender as covariates.
BMI is calculated from anthropometric data, specifically height (HT in meters, m) and weight (WT in kilograms, kg), using the formula BMI = WT/(HT)^2. Marital status is categorized into three main groups: 1) Married/cohabiting, 2) Widow/widower/separated, and 3) Unmarried. Race is classified into five categories based on NHANES survey results: Mexican, Hispanic, White, Black, and Other Race. Education level is primarily divided into three categories: Less than high school, High school, and More than high school.
Hypertension is defined by three main criteria: 1) Systolic blood pressure of 140 mmHg or higher in anthropometric data, 2) Diastolic blood pressure of 90 mmHg or higher in anthropometric data, and 3) Participants who have been told by a doctor that they have hypertension, as reported in the blood pressure questionnaire. Meeting any one of these criteria is sufficient to be defined as having hypertension.
Diabetes is defined by four criteria: 1) FPG of 7 mmol/L or higher, 2) HbA1C of 6.5% or higher, 3) Participants who have been told by a doctor that they have diabetes, as reported in the questionnaire, and 4) Participants who have taken hypoglycemic medications, as reported in the questionnaire. Meeting any one of these criteria is sufficient to be defined as having diabetes.
Gender and age are well-known demographic factors that influence the prevalence and outcomes of coronary artery disease (CAD). The Poverty Income Ratio (PIR) and level of education reflect socioeconomic status, which is associated with health outcomes, including cardiovascular diseases. Race is a significant factor affecting health disparities and the prevalence of CAD. Alcohol use and smoking status indicate that both drinking and smoking are risk factors for CAD. Body Mass Index (BMI) shows that obesity is a known risk factor for CAD. Lipids such as high-density lipoprotein, triglycerides, and low-density lipoprotein are directly related to cardiovascular health. Systolic blood pressure (SBP) indicates that hypertension is a major risk factor for CAD. HbA1c and fasting plasma glucose (FPG) suggest that glycemic control is crucial in CAD, especially among diabetic patients. Waist circumference (WC), as a measure of central obesity, is associated with an increased risk of CAD. Marital status has been shown to affect health behaviors and outcomes, including CAD. Lastly, hypertension and diabetes, as well-recognized comorbidities, significantly increase the risk of CAD.
In this study, we conducted thorough processing and quality control of the data. Given the non-normal distribution of the data, continuous variables are expressed as medians with interquartile ranges (Q1, Q3), while categorical variables are presented as counts and percentages. The Kruskal-Wallis test was used to compare continuous variables between the CAD and non-CAD groups, and the chi-squared test was used for categorical variables. A p-value less than 0.05 was considered to indicate a statistically significant difference.
Using logistic regression analysis appropriate for the survey design, we explored the relationship between HRR and CAD. The robustness of the model was incrementally enhanced by adjusting for different covariates. Specifically, Model 1 is the most basic model, with no adjustments for any variables. Model 2 is adjusted for race, age, and gender. Finally, Model 3 includes adjustments for potential confounding factors such as BMI, HDL, gender, HbA1C, WC, age, PIR, TG, race, LDL, education level, TC, FPG, SBP, hypertension, and diabetes to further strengthen the robustness of the model.
The study selected 6,881 adult Americans from the NHANES database spanning from 2011 to 2018, with a higher number of males, totaling 3,449 individuals, accounting for approximately 50.12% of the study population. Within this cohort, 499 individuals were diagnosed with CAD, representing 7.25% of the sample size. The majority of participants were identified as non-Hispanic white people, with 2,807 individuals making up 40.79% of the total study cohort. Table 1 summarizes the demographic and baseline characteristics of the study subjects, stratified by HRR quartiles (Table 1). Individuals with higher HRR tended to be younger (P < 0.001), have a smaller waist circumference (P < 0.001), higher income (P < 0.001), lower BMI (P < 0.001), drink less alcohol (P < 0.001), have a higher level of education (P = 0.007), and were free from hypertension (P < 0.001). Additionally, individuals with higher HRR levels may have lower HDL levels (P < 0.001), higher LDL levels (P < 0.001), and higher TG levels (P < 0.01) in their blood.
As shown in Table 2, in the univariate analysis, WC (OR = 1.02, 95%CI: 1.02, 1.03, P < 0.0001), TG (OR = 1.28, 95%CI: 1.15, 1.43, P < 0.0001), SBP (OR = 1.02, 95%CI: 1.02, 1.03, P < 0.0001), FPG (OR = 1.16, 95%CI: 1.13, 1.20, P < 0.0001), BMI (OR = 1.02, 95%CI: 1.02, 1.03, P = 0.0004), RDW (OR = 1.27, 95%CI: 1.20, 1.33, P < 0.0001), HRR (OR = 1.13, 95%CI: 1.09, 1.16, P < 0.0001) were positively associated with CAD. In contrast, LDL (OR = 0.54, 95%CI: 0.49, 0.61, P < 0.0001) and HB (OR = 0.92, 95%CI: 0.86, 0.97, P = 0.0033) were negatively associated with CAD (Table 2).

Table 2. Weighted univariate logistic analyses between Variables and CAD (odds ratios, 95% confidence intervals).
Referencing Table 3, elevated HRR quartiles are associated with a decreased risk of CAD (Table 3). The unadjusted model reveals an inverse relationship between HRR quartiles and the prevalence of CAD (Model 1). This correlation remains significant after partial adjustments in Model 2. With comprehensive consideration of all potential confounding factors, the OR for CAD in relation to the second (Q2), third (Q3), and fourth (Q4) quartiles compared to the reference quartile (Q1) are approximately 0.38 (95% CI: 0.29–0.51), 0.42 (95% CI: 0.30–0.58), and 0.51 (95% CI: 0.38–0.70), respectively, for Model 3 (P < 0.001). Additionally, under strict control for potential confounding variables, at the HRR Q4 level, each unit increase in HRR corresponds to a 49% reduction in the probability of CAD.
Using smooth curve fitting and threshold effect analysis, we assessed the potential linear correlation between HRR and the risk of CAD. As shown in Figure 2, a significant linear relationship was found between these variables (Figure 2). Table 4 details the outcomes from a standard linear regression model, revealing a 74% reduction in CAD risk for each one-unit increment in HRR (P = 0.0002). Two-piecewise linear regression models identified a critical point at an HRR value of 1.02. For HRR values exceeding 1.02, each unit increase in HRR is associated with a 91% decrease in CAD odds (OR = 0.09, 95%CI = 0.02–0.43, P = 0.0026), while for HRR values at or below 1.02, each unit increase in HRR is associated with a 53% decrease in CAD odds (OR = 0.47, 95%CI = 0.17–1.35, P = 0.1626). The log-likelihood ratio test yielded a P value of 0.131. Consequently, when synthesizing these findings, a definite inverse linear relationship is observed between HRR and CAD risk (Table 5). This implies that for individuals with an HRR value exceeding 1.02, increasing body water content and reducing blood viscosity to a certain extent may potentially lower the risk of CAD. However, such measures may not be effective in individuals with an HRR equal to or below 1.02.
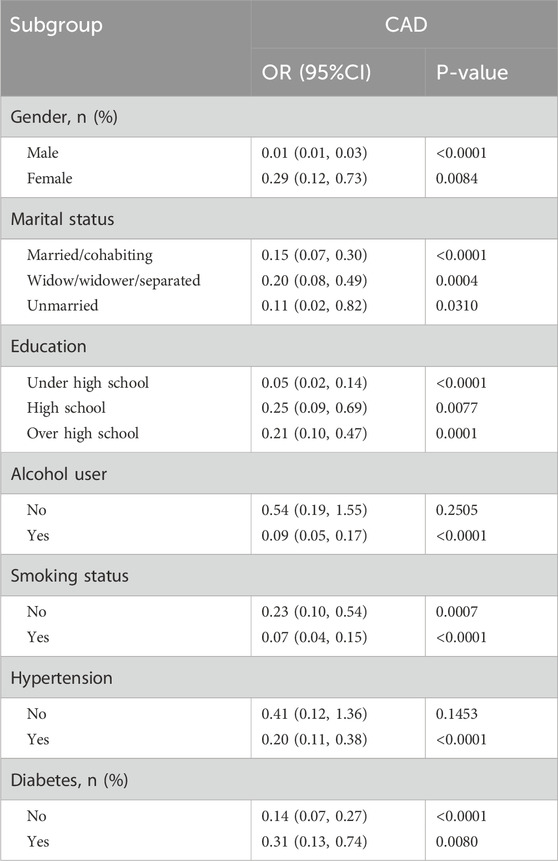
Table 5. Stratified analysis of the correlation between HRR and CAD in adults in the NHANES 2013–2018.
CAD is a prevalent condition in cardiovascular medicine, encompassing a spectrum of pathologies from coronary atherosclerosis to angina and myocardial infarction (Liu et al., 2023; Larsen et al., 2015). According to the World Health Organization, CAD accounts for 7.4 million deaths globally, highlighting its significant impact on individual health and socio-economics (Dromain et al., 2013). Among the multifaceted etiologies of CAD, inflammation is a pivotal factor (Kryczka et al., 2021; Sun et al., 2024). Our study, leveraging data from NHANES between 2011 and 2018, reveals a significant negative correlation between the HRR and the likelihood of CAD, indicating an approximate linear association.
Inflammation is a cornerstone in the pathophysiology of CAD, a condition inherently linked with inflammatory processes (Gadidala et al., 2023). Both HRR and RDW, recognized as novel inflammatory biomarkers, have been implicated in inflammation and are associated with the incidence and adverse outcomes of various diseases, including CAD (Ackland et al., 2018). Previous research has demonstrated the utility of RDW in predicting mortality rates among CAD patients. A study examining 1,039 outpatients from 1997 to 2007, with follow-up through 2009, revealed that patients in the highest RDW quartile had a 66% higher risk of death compared to those in the lowest quartile, with a 1% increase in RDW associated with a 10% increase in all-cause mortality risk (Qiu et al., 2017). RDW emerged as an independent prognostic indicator for CAD patients (Fava et al., 2019) A study on non-small cell lung cancer (NSCLC) included 245 NSCLC patients, 97 patients with benign pulmonary nodules, and 94 healthy volunteers, analyzing the relationship between various hematological indicators such as red blood cell distribution width (RDW) and tumor progression. It was found that RDW could be used to distinguish NSCLC patients from healthy controls (p < 0.05). RDW was positively correlated with NSCLC staging, while the hemoglobin-to-red blood cell distribution width ratio (HRR) was negatively correlated with NSCLC staging. The study concluded that RDW could serve as a simple and effective biomarker for diagnosing and assessing the progression of NSCLC (Chen et al., 2020). A study has investigated the association between dietary patterns of patients with coronary artery disease and their sociodemographic and health-related characteristics. The study included 250 coronary artery disease patients who were aged 40 and above. It was found that dietary patterns are related to sociodemographic and health-related factors (Esmaili et al., 2015). Another study conveniently recruited 124 full-time male and female firefighters from the Fire and Rescue Services in Cape Town, South Africa, between September and November 2020. It was found that alcohol consumption was significantly associated with gender, race, total minutes of low-intensity physical activity, diastolic blood pressure, and hypertension (p = 0.005). Gender (p = 0.021) and race (p = 0.042) were significantly related to the type of alcohol consumed. Alcohol intake was a significant predictor of total low-intensity physical activity as well as systolic blood pressure (p = 0.048) and diastolic blood pressure (p = 0.036). This suggests that targeting physical activity may potentially improve Coronary Artery Disease (CAD) (Dąbek et al., 2020).
The role of HRR in CAD, however, has been less clear. Our study, being the first to explore the potential link between HRR and CAD using a large dataset from NHANES, found that high levels of HRR are protective against CAD, reducing the probability of CAD by 56% for each unit increase in HRR. This contrasts with findings in other disease contexts, such as nasopharyngeal carcinoma, where HRR was associated with poorer outcomes. The discrepancy may be attributed to the different physiological responses to disease stimuli in oncological versus cardiovascular settings, suggesting that the role of HRR may vary significantly across disease types.
Under stringent inclusion criteria, our study meticulously selected indicators and populations from the reputable NHANES database, ensuring a certain level of persuasiveness in our results. We endeavored to adjust for a range of covariates to ensure the reliability of our findings. However, it is important to acknowledge the limitations inherent in our study. The cross-sectional nature of the study precludes the establishment of causal relationships between HRR and CAD, limiting our exploration to potential associations. HRR is primarily determined by two factors, HB and RDW, with HB being a relatively easily modifiable indicator. In the elderly, whose blood is relatively viscous, moderately diluting blood viscosity and increasing body water content within the normal range of HB could potentially reduce HRR to some extent, thereby reducing the risk of CAD. Of course, these hypotheses require further validation through multicenter clinical trials. Additionally, while we controlled for multiple covariates, there may be other confounding factors that could influence the relationship between HRR and CAD, which were not accounted for in this study. Despite these limitations, our study is novel in identifying HRR as a potential protective factor against CAD, providing a foundation and new insights for future research.
In conclusion, this study demonstrates a significant inverse linear relationship between the HRR and the risk of CAD, suggesting that higher HRR values are associated with a reduced likelihood of CAD. These findings provide valuable insights into the role of inflammation in CAD and highlight HRR as a potential inflammatory marker that could aid in CAD risk stratification.
Publicly available datasets were analyzed in this study. This data can be found here: The data analyzed in this study are publicly available from the National Health and Nutrition Examination Survey (NHANES), conducted by the Centers for Disease Control and Prevention (CDC). You can access the datasets through the NHANES website: Repository: NHANES Data and Documentation Access Link: https://wwwn.cdc.gov/nchs/nhanes/.
X-DW: Investigation, Methodology, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing. CL: Writing–original draft, Writing–review and editing, Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Visualization. JH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Visualization, Writing–original draft, Writing–review and editing. FC: Conceptualization, Investigation, Project administration, Writing–original draft. LZ: Investigation, Methodology, Resources, Writing–review and editing. YZ: Conceptualization, Formal Analysis, Investigation, Project administration, Validation, Writing–original draft, Writing–review and editing. ZW: Conceptualization, Methodology, Resources, Software, Supervision, Validation, Writing–original draft, Writing–review and editing. JL: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ackland, G. L., Minto, G., Clark, M., Whittle, J., Stephens, R. C. M., Owen, T., et al. (2018). Autonomic regulation of systemic inflammation in humans: a multi-center, blinded observational cohort study. Brain Behav. Immun. 67, 47–53. doi:10.1016/j.bbi.2017.08.010
Andersen, P. (1992). Hypercoagulability and reduced fibrinolysis in hyperlipidemia: relationship to the metabolic cardiovascular syndrome. J. Cardiovasc Pharmacol. 20 (Suppl. 8), S29–S31. doi:10.1097/00005344-199200208-00007
Attiq, A., Afzal, S., Ahmad, W., and Kandeel, M. (2024). Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 966, 176338. doi:10.1016/j.ejphar.2024.176338
Chen, J. L., Wu, J. N., Lv, X. D., Yang, Q. C., and Zhang, D. M. (2020). The value of red blood cell distribution width, neutrophil-to-lymphocyte ratio, and hemoglobin-to-red blood cell distribution width ratio in the progression of non-small cell lung cancer. PLoS One 15 (8), e0237947. doi:10.1371/journal.pone.0237947
Chen, K., Li, S., Xie, Z., Liu, Y., Li, Y., Mai, J., et al. (2024). Association between oxidative balance score, systemic inflammatory response index, and cardiovascular disease risk: a cross-sectional analysis based on NHANES 2007-2018 data. Front. Nutr. 11, 1374992. doi:10.3389/fnut.2024.1374992
Chen, X., Wang, S., Yang, J., Wang, X., Yang, L., and Zhou, J. (2023). The predictive value of hematological inflammatory markers for acute kidney injury and mortality in adults with hemophagocytic Lymphohistiocytosis: a retrospective analysis of 585 patients. Int. Immunopharmacol. 122, 110564. doi:10.1016/j.intimp.2023.110564
Cho, S. M. J., Koyama, S., Honigberg, M. C., Surakka, I., Haidermota, S., Ganesh, S., et al. (2023). Genetic, sociodemographic, lifestyle, and clinical risk factors of recurrent coronary artery disease events: a population-based cohort study. Eur. Heart J. 44 (36), 3456–3465. doi:10.1093/eurheartj/ehad380
Cortigiani, L., Carpeggiani, C., Landi, P., Raciti, M., Bovenzi, F., and Picano, E. (2020). Prognostic value of heart rate reserve in patients with permanent atrial fibrillation during dipyridamole stress echocardiography. Am. J. Cardiol. 125 (11), 1661–1665. doi:10.1016/j.amjcard.2020.02.046
Crook, J. M., Yoon, S. J. L., Grundmann, O., Horgas, A., and Johnson-Mallard, V. (2023). Subclinical vitamin C plasma levels associated with increased risk of CAD diagnosis via inflammation: results from the NHANES 2003-2006 surveys. Nutrients 15 (3), 584. doi:10.3390/nu15030584
Dąbek, J., Knapik, A., Gallert-Kopyto, W., Brzęk, A. M., Piotrkowicz, J., and Gąsior, Z. (2020). Fear of movement (kinesiophobia) - an underestimated problem in Polish patients at various stages of coronary artery disease. Ann. Agric. Environ. Med. 27 (1), 56–60. doi:10.26444/aaem/106143
Del Rosso, S., Baraquet, L., Bergero, G., Muñoz, F., Mazzocco, Y. L., Aoki, M. P., et al. (2022). Associations between objectively measured physical activity, sedentary time, and cardiorespiratory fitness with inflammatory and oxidative stress markers and heart rate variability. J. Public Health Res. 11 (2), 22799036221106580. doi:10.1177/22799036221106580
Dromain, C., Boyer, B., Ferré, R., Canale, S., Delaloge, S., and Balleyguier, C. (2013). Computed-aided diagnosis (CAD) in the detection of breast cancer. Eur. J. Radiol. 82 (3), 417–423. doi:10.1016/j.ejrad.2012.03.005
Esmaili, H., Mohd Yusof, R., Abu Saad, H., Ghaemian, A., and Darani Zad, N. (2015). Association of dietary patterns with sociodemographic and health-related factors among coronary artery disease (CAD) patients. Ecol. Food Nutr. 54 (1), 4–19. doi:10.1080/03670244.2014.930031
Eyiol, A., and Ertekin, B. (2024). The relationship between hemoglobin-to-red cell distribution width (RDW) ratio (HRR) and mortality in stroke patients. Eur. Rev. Med. Pharmacol. Sci. 28 (4), 1504–1512. doi:10.26355/eurrev_202402_35480
Fava, C., Cattazzo, F., Hu, Z. D., Lippi, G., and Montagnana, M. (2019). The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: useful or hype? Ann. Transl. Med. 7 (20), 581. doi:10.21037/atm.2019.09.58
Fu, Z., Liu, Q., Liang, J., Weng, Z., Li, W., Xu, J., et al. (2023). Association between NMR metabolomic signatures of healthy lifestyle and incident coronary artery disease. Eur. J. Prev. Cardiol. 30 (3), 243–253. doi:10.1093/eurjpc/zwac252
Gadidala, S. K., Johny, E., Thomas, C., Nadella, M., Undela, K., and Adela, R. (2023). Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: a systematic review and meta-analysis. Phytother. Res. 37 (6), 2242–2254. doi:10.1002/ptr.7729
Honigberg, M. C., Zekavat, S. M., Aragam, K., Klarin, D., Bhatt, D. L., Scott, N. S., et al. (2019). Long-term cardiovascular risk in women with hypertension during pregnancy. J. Am. Coll. Cardiol. 74 (22), 2743–2754. doi:10.1016/j.jacc.2019.09.052
Jahangir, E., De Schutter, A., and Lavie, C. J. (2014). The relationship between obesity and coronary artery disease. Transl. Res. 164 (4), 336–344. doi:10.1016/j.trsl.2014.03.010
Jang, T. K., Kim, H., Eo, W., Kim, K. H., Lee, C. M., and Kim, M. (2023). Clinical significance of the combination of serum HE4 levels, hemoglobin-to-red cell distribution width ratio, and CT imaging for the pretreatment assessment of adnexal masses. J. Cancer 14 (4), 600–610. doi:10.7150/jca.81174
Kalyani, R. R., Lazo, M., Ouyang, P., Turkbey, E., Chevalier, K., Brancati, F., et al. (2014). Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 37 (3), 830–838. doi:10.2337/dc13-1755
Kinoshita, D., Suzuki, K., Yuki, H., Niida, T., Fujimoto, D., Minami, Y., et al. (2023). Coronary artery disease reporting and data system (CAD-RADS), vascular inflammation and plaque vulnerability. J. Cardiovasc Comput. Tomogr. 17 (6), 445–452. doi:10.1016/j.jcct.2023.09.008
Kryczka, K. E., Kruk, M., Demkow, M., and Lubiszewska, B. (2021). Fibrinogen and a triad of thrombosis, inflammation, and the renin-angiotensin system in premature coronary artery disease in women: a new insight into sex-related differences in the pathogenesis of the disease. Biomolecules 11 (7), 1036. doi:10.3390/biom11071036
Larsen, S. B., Grove, E. L., Würtz, M., Neergaard-Petersen, S., Hvas, A. M., and Kristensen, S. D. (2015). The influence of low-grade inflammation on platelets in patients with stable coronary artery disease. Thromb. Haemost. 114 (3), 519–529. doi:10.1160/TH14-12-1007
Li, J., Dong, Z., Wu, H., Liu, Y., Chen, Y., Li, S., et al. (2023). The triglyceride-glucose index is associated with atherosclerosis in patients with symptomatic coronary artery disease, regardless of diabetes mellitus and hyperlipidaemia. Cardiovasc Diabetol. 22 (1), 224. doi:10.1186/s12933-023-01919-z
Lin, Z., He, J., Yuan, S., Song, C., Bian, X., Yang, M., et al. (2024). Glycemic control and cardiovascular outcomes in patients with diabetes and coronary artery disease according to triglyceride-glucose index: a large-scale cohort study. Cardiovasc Diabetol. 23 (1), 11. doi:10.1186/s12933-023-02112-y
Liu, S., Li, Y., and Wu, C. (2023). Paeoniflorin suppresses the apoptosis and inflammation of human coronary artery endothelial cells induced by oxidized low-density lipoprotein by regulating the Wnt/β-catenin pathway. Pharm. Biol. 61 (1), 1454–1461. doi:10.1080/13880209.2023.2220360
Liu, Y., Liu, S. X., Zheng, F., Cai, Y., Xie, K. L., and Zhang, W. L. (2016). Cardiovascular autonomic neuropathy in patients with type 2 diabetes. J. Diabetes Investig. 7 (4), 615–621. doi:10.1111/jdi.12438
Luo, F., Das, A., Chen, J., Wu, P., Li, X., and Fang, Z. (2019). Metformin in patients with and without diabetes: a paradigm shift in cardiovascular disease management. Cardiovasc Diabetol. 18 (1), 54. doi:10.1186/s12933-019-0860-y
Mäenpää, M., Kujala, I., Harjulahti, E., Stenström, I., Nammas, W., Knuuti, J., et al. (2023). The impact of diabetes on the relationship of coronary artery disease and outcome: a study using multimodality imaging. Cardiovasc Diabetol. 22 (1), 129. doi:10.1186/s12933-023-01850-3
Manolis, A. A., Manolis, T. A., and Manolis, A. S. (2024). Early-onset or premature coronary artery disease. Curr. Med. Chem. 31. doi:10.2174/0109298673303891240528114755
Marzio, A., Kurz, E., Sahni, J. M., Di Feo, G., Puccini, J., Jiang, S., et al. (2022). EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell 185 (1), 169–183.e19. doi:10.1016/j.cell.2021.12.005
Meng, C., Yang, Y., Ren, P., Ju, Q., Jin, X., Long, Q., et al. (2022). FIGNL1 is a potential biomarker of cisplatin resistance in non-small cell lung cancer. Int. J. Biol. Markers 37 (3), 260–269. doi:10.1177/03936155221110249
Omori, K., Katakami, N., Yamamoto, Y., Ninomiya, H., Takahara, M., Matsuoka, T. A., et al. (2019). Identification of metabolites associated with onset of CAD in diabetic patients using CE-MS analysis: a pilot study. J. Atheroscler. Thromb. 26 (3), 233–245. doi:10.5551/jat.42945
Pedersen, L. R., Olsen, R. H., Anholm, C., Astrup, A., Eugen-Olsen, J., Fenger, M., et al. (2019). Effects of 1 year of exercise training versus combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: a randomized trial. Cardiovasc Diabetol. 18 (1), 127. doi:10.1186/s12933-019-0934-x
Qiu, S., Cai, X., Sun, Z., Li, L., Zuegel, M., Steinacker, J. M., et al. (2017). Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 6 (5), e005505. doi:10.1161/JAHA.117.005505
Ralevic, S., Perunicic, J., Lasica, R., Marinkovic, J., Blagojevic, T., Simanic, I., et al. (2016). Prognostic significance of atrial fibrillation in lower limb amputee patients. Eur. J. Vasc. Endovasc. Surg. 52 (6), 823–829. doi:10.1016/j.ejvs.2016.09.009
Said, M. A., van de Vegte, Y. J., Zafar, M. M., van der Ende, M. Y., Raja, G. K., Verweij, N., et al. (2019). Contributions of interactions between lifestyle and genetics on coronary artery disease risk. Curr. Cardiol. Rep. 21 (9), 89. doi:10.1007/s11886-019-1177-x
Su, Y. C., Wen, S. C., Li, C. C., Su, H. C., Ke, H. L., Li, W. M., et al. (2021). Low hemoglobin-to-red cell distribution width ratio is associated with disease progression and poor prognosis in upper tract urothelial carcinoma. Biomedicines 9 (6), 672. doi:10.3390/biomedicines9060672
Sun, M., Zhu, S., Wang, Y., Zhao, Y., Yan, K., Li, X., et al. (2024). Effect of inflammation on association between cancer and coronary artery disease. BMC Cardiovasc Disord. 24 (1), 72. doi:10.1186/s12872-023-03613-0
Szilveszter, B., Vattay, B., Bossoussou, M., Vecsey-Nagy, M., Simon, J., Merkely, B., et al. (2022). CAD-RADS may underestimate coronary plaque progression as detected by serial CT angiography. Eur. Heart J. Cardiovasc Imaging 23 (11), 1530–1539. doi:10.1093/ehjci/jeab215
Tada, H., Yamagami, K., Sakata, K., Usui, S., Kawashiri, M. A., and Takamura, M. (2024). Healthy lifestyle, lipoprotein (a) levels and the risk of coronary artery disease. Eur. J. Clin. Invest 54 (1), e14093. doi:10.1111/eci.14093
Tsukihashi, Y., Shiga, Y., Suematsu, Y., Idemoto, Y., Tashiro, K., Yano, Y., et al. (2021). Presence and severity of coronary artery disease in patients who achieved intensive blood pressure reduction at the time of coronary computed tomography angiography. Hypertens. Res. 44 (2), 206–214. doi:10.1038/s41440-020-00545-6
Wang, J., Campos, A. I., Rentería, M. E., and Xu, L. (2023). Causal associations of sleep apnea, snoring with cardiovascular diseases, and the role of body mass index: a two-sample Mendelian randomization study. Eur. J. Prev. Cardiol. 30 (7), 552–560. doi:10.1093/eurjpc/zwad005
Yang, F., Xu, F., Zhang, H., Gill, D., Larsson, S. C., Li, X., et al. (2023). Proteomic insights into the associations between obesity, lifestyle factors, and coronary artery disease. BMC Med. 21 (1), 485. doi:10.1186/s12916-023-03197-8
Yılmaz, H., Yılmaz, A., and Demirağ, G. (2021). Prognostic significance of hemoglobin-to-red cell distribution width ratio in patients with metastatic renal cancer. Future Oncol. 17 (29), 3853–3864. doi:10.2217/fon-2021-0040
Zhao, W. X., Wu, Z. Y., Zhao, N., Diao, Y. P., Lan, Y., and Li, Y. J. (2024b). Novel systemic inflammatory markers predict all-cause mortality in patients undergoing endovascular abdominal aortic aneurysm repair. Rev. Cardiovasc Med. 25 (6), 202. doi:10.31083/j.rcm2506202
Keywords: coronary artery disease, hemoglobin-to-red blood cell distribution width ratio, inflammation, NHANES, cross-sectional study
Citation: Wang X-D, Li C, Hu J, Cao F, Zhu L, Zhu Y, Wen Z and Liu J (2025) Hemoglobin-to-red blood cell distribution width ratio as a protective factor against coronary artery disease: a cross-sectional analysis of NHANES (2011-2018). Front. Pharmacol. 16:1534479. doi: 10.3389/fphar.2025.1534479
Received: 26 November 2024; Accepted: 07 January 2025;
Published: 29 January 2025.
Edited by:
Zhijie Xu, Central South University, ChinaReviewed by:
He Huang, The Third Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2025 Wang, Li, Hu, Cao, Zhu, Zhu, Wen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongzhi Zhu, MzU4MTk2MzZAcXEuY29t; Zhongzheng Wen, ZHJ3enpoZW5nQDE2My5jb20=; Jun Liu, bGl1a2VqdW4xOUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.