- Department of Anesthesiology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
Background: Patients with breast cancer experience varying degrees of pain, depression, and anxiety after surgery, which affect their postoperative recovery. Although ketamine/esketamine exhibit potential for opioid-sparing and controlling postoperative pain and depression, their effects on postoperative pain and depression remain unclear. This meta-analysis aimed to evaluate whether perioperative administration of ketamine/esketamine could reduce postoperative pain and depression, improve postoperative recovery, and reduce the incidence of adverse events in patients after breast cancer surgery.
Material and methods: PubMed, Embase, Web of Science, Cochrane Library, and Clinical Trials were searched from inception until June 2, 2024 for randomized controlled trials in English language on the effect of perioperative ketamine/esketamine on postoperative pain in patients undergoing breast cancer surgery. The primary outcome was the postoperative pain score, and the secondary outcomes were the postoperative depression score, quality of postoperative recovery, incidence of adverse events, and extubation time. The standardized mean difference and 95% confidence interval (CI) were calculated for continuous outcomes, and the risk ratio and 95% CI were calculated for binary variables.
Results: Seven studies involving 748 patients were included in this meta-analysis. No significant differences were found in postoperative pain scores at 2 h, 4 h, 1 day, 3 days, 7 days, and 3 months after surgery. Postoperative depression scores at 3 and 7 days after surgery were lower in the ketamine/esketamine group. The incidence of dizziness was lower in ketamine/esketamine group. No statistically significant differences were observed in postoperative depression scores at 30 days after surgery, quality of postoperative recovery at 1 and 3 days after surgery, extubation time, or the incidence of nausea, vomiting, and nightmares.
Conclusion: Perioperative ketamine/esketamine administration did not significantly reduce postoperative pain in patients undergoing breast cancer surgery; however, it may reduce depression within a short period after the surgery.
Clinical Trial Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42024572414, identifier CRD42024572414.
Introduction
In 2020, breast cancer overtook lung cancer as the most common cancer in females worldwide. (Sung et al., 2021). A meta-analysis further revealed that approximately half of all women who undergo breast cancer surgery experience persistent postoperative pain, with approximately a quarter experiencing moderately to severely persistent postoperative pain. (Wang et al., 2020). Acute pain can become persistent through Sp4-dependent overexpression of transient receptor potential (TRP) channels and sustained production of inflammatory mediators (Schumacher, 2024). Studies have shown that approximately 50% of patients with acute post-operative pain will develop chronic pain. (Schumacher, 2024). Long term chronic pain and undergoing radical breast cancer surgery greatly increase the risk of postoperative depression in breast cancer patients. (Kim et al., 2017; Gohari et al., 2022). Notably, postoperative pain and depression affect patient wellbeing and are associated with a decreased quality of life, increased risk of unemployment, and increased healthcare costs. (Wang et al., 2020).
Ketamine, a racemic mixture of (S)-ketamine and (R)-ketamine, (Adams et al., 1978), has been used clinically as an anesthetic since 1970. (Dundee et al., 1970). In addition to its primary dissociative anesthetic properties, (Domino, 2010), ketamine exerts its analgesic effect by binding to the N-methyl-D-aspartate (NMDA) receptor and blocking the inward flow of calcium ions, inhibiting central sensitisation and pain signalling. (Wang K. et al., 2024; Zanos and Gould, 2018; Laskowski et al., 2011). Furthermore, ketamine may also exert antidepressant effects by affecting the Mechanistic Target of Rapamycin and Brain Derived Neurotrophic Factor (mTOR-BDNF) signalling pathway, modulating synaptic plasticity and neurotransmitter release (Zanos and Gould, 2018; Chen et al., 2024). However, the potential side effects of ketamine, including dissociative, psychotomimetic effects and cognitive impairment, limit its clinical application. (Laskowski et al., 2011; Cohen et al., 2018; Avidan et al., 2017; Shaffer et al., 2014; Zanos et al., 2018; Shinohara et al., 2021). In contrast, esketamine, the S-isomer of ketamine, exhibits a stronger affinity for NMDA receptors, requires a smaller dose for the onset of action, and has fewer side effects than ketamine. (Mion and Himmelseher, 2024). Clinical trials have demonstrated the advantages of esketamine in perioperative settings. For instance, a randomized controlled trial reported that the perioperative use of low-dose esketamine significantly reduced postoperative pain scores through anti-inflammation in elderly patients undergoing lumbar spine surgery. (Hou et al., 2025). Meanwhile, esketamine is also able to reduce the use of opioids, which is beneficial for maintaining intraoperative haemodynamic stability in patients and reducing the incidence of postoperative respiratory depression. (Hou et al., 2025). Additionally, esketamine has a faster onset of action than ketamine in the antidepressant setting. It has been shown that esketamine improves depression by inhibiting TREK-1 (TWIK-related K+ channel 1) channels and modulating neurotransmitters in postoperative breast cancer patients. (Xu et al., 2025). Notably, ketamine/esketamine have received considerable research attention in recent years because of their potential rapid antidepressant and analgesic effects in perioperative pain management and antidepressant applications. (Miziara et al., 2016; Su et al., 2022; Jiang et al., 2016). However, the effects of perioperative ketamine/esketamine administration on postoperative pain and depression in patients undergoing breast cancer surgery remain controversial. Moreover, the widespread perioperative use of ketamine/esketamine is limited by the uncertainty of their long-term effects and safety. (Avidan et al., 2017; Wang et al., 2019; Zhu et al., 2022). Therefore, this meta-analysis was aimed to explore the effects of ketamine and esketamine on postoperative pain and depression in patients after breast cancer surgery to guide their perioperative application.
Material and methods
This systematic review and meta-analysis was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021) and Assessing the methodological quality of systematic reviews (AMSTAR) Guidelines (Shea et al., 2017) and registered in PROSPERO.
Search strategy and eligibility criteria
PubMed, Embase, Web of Science, Cochrane Library, and Clinical Trials were systematically searched from inception until June 2, 2024 using MeSH and free-text terms. The PubMed search was performed using the following keywords: “((Esketamine [Title/Abstract]) OR (ketamine [Title/Abstract])) AND (((Breast cancer [Title/Abstract]) OR (Breast tumor [Title/Abstract])) OR (breast surgery [Title/Abstract])).” The language was restricted to English. The inclusion criteria were defined according to the PICOS framework: 1) Population: adult patients (≥18 years) undergoing breast cancer surgery; 2) Intervention: Perioperative (pre-, intra- or postoperative) single or continuous infusion of ketamine/esketamine; 3) Comparison: placebo (normal saline); 4) Outcomes: primary outcome as postoperative pain scores, secondary outcomes including depression scores, quality of recovery, adverse events, and extubation time; 5) Study design: only randomized controlled trials (RCTs).
Exclusion criteria
Non-RCTs, case reports, conference abstracts, comments, systematic reviews, and studies involving animal experiments, non-intubation general anesthesia, pediatric surgery, ketamine/esketamine as an adjuvant to regional anesthesia, and a combination of ketamine/esketamine and bupivacaine, lidocaine, or dexmedetomidine, as well as studies that did not report postoperative pain scores, were excluded.
Study selection and data collection
Two authors independently selected eligible studies and extracted data based on the predefined study selection criteria and clinical endpoints. Disagreements between the two authors were resolved through discussion with another senior researcher. The data, including first author/year, ASA grade, sample size, age, ketamine/esketamine administration details (dosage and timing), and country of origin, were extracted from the selected studies. The primary outcome of the study was the postoperative visual analog scale score for pain, whereas the secondary outcomes were the postoperative depression scores, quality of postoperative recovery, risk of adverse events (such as nausea, vomiting, dizziness, and nightmares), and extubation time.
Assessment of risk of bias
The revised Cochrane Risk of Bias 2 (RoB 2) tool was used to assess the quality of the included RCTs in five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selective reporting by two authors independently, and the risk of overall bias was graded as high, unclear, or low (Higgins et al., 2011; Nejadghaderi et al., 2024). Disagreements were resolved through discussion with a third author.
Statistical analysis
Statistical analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and STATA 16.0. The chi-square and I2 tests were employed for all meta-analyses to evaluate statistical heterogeneity, which was classified as low (I2 < 50%), moderate (I2 = 50–75%), and high (I2 > 75%). (Melsen et al., 2014). The choice between fixed-effect and random-effects models was based on both statistical and clinical heterogeneity (Melsen et al., 2014; Borenstein et al., 2010). A random-effects model was applied if significant heterogeneity was detected (I2 > 50% or p < 0.05), accounting for variability across studies in surgical techniques, dosing regimens, and outcome assessment (Melsen et al., 2014; Borenstein et al., 2010). Otherwise, a fixed effects model was used. This approach aligns with recommendations for meta-analyses with heterogeneous populations or interventions. Standardized mean difference (SMD) and 95% confidence interval (CI) were calculated for continuous outcomes, whereas risk ratio (RR) with 95% CI were used to compare binary variables. The median and interquartile range (IQR) or the median and 95% CI of continuous data were converted to mean and standard deviation (SD) based on the method described by Wan et al. (Wan et al., 2014) Statistical significance was set at p < 0.05. A sensitivity analysis was performed to evaluate the stability of the primary outcomes.
Assessment of publication bias and quality of evidence
If the number of included studies is greater than 10, we planned to use funnel plots to assess the potential for publication bias. (Sterne et al., 2011). We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework to assess the quality and strength of the evidence base. (Guyatt et al., 2008; Granholm et al., 2019). All assessments were performed independently by two investigators, followed by discussions to reach a consensus.
Results
Search results
Initially, 479 potentially eligible studies were identified. After removing 175 duplicate records, 304 studies were screened based on their titles and abstracts, and 33 full-text articles were evaluated for their eligibility. After excluding 11 non-RCT studies, 6 studies that included local anesthetic nerve blocks, and 9 studies without primary endpoints, 7 studies were finally included. (Zhu et al., 2022; Mahran and Hassan, 2015; Ranran et al., 2017; Kang et al., 2020; Liu et al., 2021; Zhao et al., 2021; Wang H. et al., 2024). A flowchart of our study selection process is presented in Figure 1.
Study characteristics
Overall, the included studies involved 748 patients, of which 390 and 358 received ketamine/esketamine and NS as a control, respectively. The characteristics of the included studies are summarized in Supplementary Table 1. Of the seven RCT studies included, five (Zhu et al., 2022; Ranran et al., 2017; Kang et al., 2020; Zhao et al., 2021; Wang H. et al., 2024) were modified radical mastectomies and the other two (Mahran and Hassan, 2015; Liu et al., 2021) did not describe the specific surgical procedure for breast cancer. Most studies enrolled patients with an ASA classification of I–II, (Zhu et al., 2022; Mahran and Hassan, 2015; Ranran et al., 2017; Kang et al., 2020; Zhao et al., 2021; Wang H. et al., 2024), and only one study included patients with an ASA physical status of III. (Liu et al., 2021). Three (Zhu et al., 2022; Liu et al., 2021; Wang H. et al., 2024) and four (Mahran and Hassan, 2015; Ranran et al., 2017; Kang et al., 2020; Zhao et al., 2021) studies used esketamine and ketamine, respectively. Five studies (Zhu et al., 2022; Ranran et al., 2017; Liu et al., 2021; Zhao et al., 2021; Wang H. et al., 2024) involved intraoperative administration of ketamine/esketamine, and two studies (Mahran and Hassan, 2015; Kang et al., 2020) involved preoperative and intraoperative administration. In addition, the dosing regimen differed in each study, with loading doses ranging from 0.125 to 0.5 mg/kg and infusion rates from 0.002 to 0.25 mg/kg/h. Four studies used the postoperative VAS score, (Mahran and Hassan, 2015; Ranran et al., 2017; Liu et al., 2021; Wang H. et al., 2024), and three studies used the postoperative numeric rating scale (NRS) pain score. (Zhu et al., 2022; Kang et al., 2020; Zhao et al., 2021). Only three studies involved postoperative depression scoring using the Hamilton Depression Scale (Ranran et al., 2017; Liu et al., 2021) and the Hospital Anxiety and Depression Scale. (Zhao et al., 2021). Three studies assessed the quality of postoperative recovery using three different scores: 40-Item Quality of Recovery scale, (Zhao et al., 2021), quality of recovery-15 scores, (Zhu et al., 2022), and the Patient Health Questionnaire-9 scores. (Wang H. et al., 2024).
Risk of bias in included studies
Figure 2 displays the quality assessment results of the included studies, conducted according to the revised Cochrane RoB 2 tool. In total, there are 5 studies with a low overall risk of bias, which indicates reliable methodologies and findings (Wang et al., 2020; Zhu et al., 2022; Mahran and Hassan, 2015; Kang et al., 2020; Liu et al., 2021). One study raised some concerns due to missing outcome data (Zhao et al., 2021), while one study was rated as unclear one study’s risk of bias was rated unclear for deviations from the intended interventions (Ranran et al., 2017).
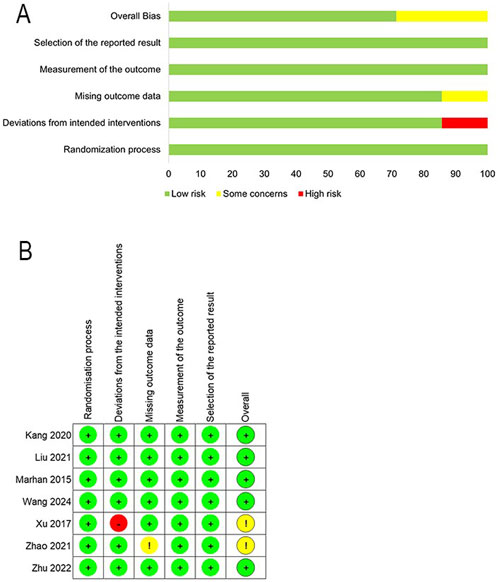
Figure 2. Risk of bias assessment. (A) Risk-of-bias summary. (B) Risk of bias in individual studies. Risk of bias methods: (+), a low risk of bias; (?), unclear risk of bias; and (−), high risk of bias.
Pooled results of included studies
Primary outcome
Seven studies reported postoperative pain scores, (Zhu et al., 2022; Mahran and Hassan, 2015; Ranran et al., 2017; Kang et al., 2020; Liu et al., 2021; Zhao et al., 2021; Wang H. et al., 2024), and three of them reported NRS scores as medians (IQRs), (Zhu et al., 2022; Kang et al., 2020; Zhao et al., 2021), which were converted into means ± SDs. The results revealed that ketamine/esketamine did not reduce pain scores in patients with breast cancer at 2 h (SMD: −0.70, 95% CI: −1.50 to 0.11, p = 0.09, I2 = 82%), 4 h (SMD: −0.04, 95% CI: −0.36 to 0.27, p = 0.79, I2 = 0%), 1 day (SMD: −0.44, 95% CI: −0.98 to 0.11, p = 0.12, I2 = 89%), 3 days (SMD: −0.52, 95% CI: −1.65 to 0.61, p = 0.37, I2 = 95%), 7 days (SMD: 0.07, 95% CI: −0.47 to 0.61, p = 0.80, I2 = 78%) and 3 months (SMD: 0.00, 95% CI: −0.26 to 0.26, p = 1.00, I2 = 0%) after surgery (Figure 3). We also analyzed the postoperative pain scores according to the drug type and found no statistically significant difference between ketamine and esketamine for reducing postoperative pain scores at 1 day after surgery (SMD: −0.44, 95% CI: −1.00 to 0.12, p = 0.52, I2 = 0%) (Figure 4).
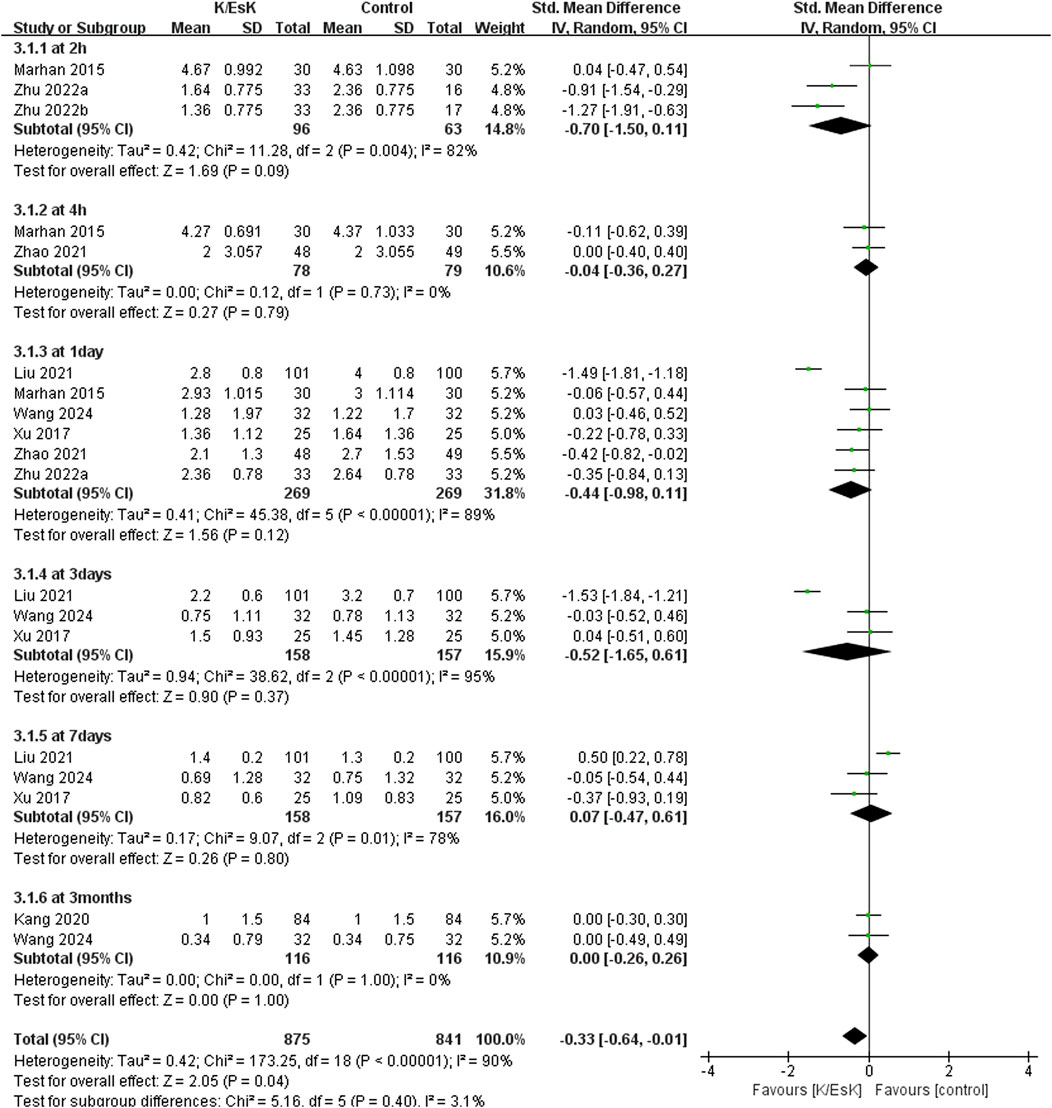
Figure 3. Forest plot of the effect of perioperative administration of ketamine/esketamine (k/esk) on postoperative pain scores within 3 months of surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
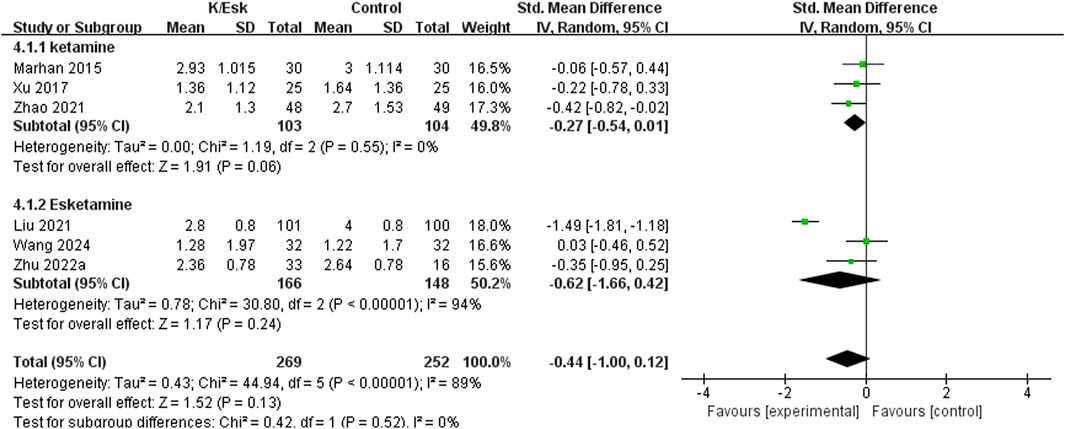
Figure 4. Forest plot of the subgroup analysis of the effect of perioperative administration of ketamine/esketamine (k/esk) on postoperative pain scores at 1 day after surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
Secondary outcomes
Three studies reported postoperative depression scores (Ranran et al., 2017; Liu et al., 2021; Zhao et al., 2021), with a total sample size of 348 patients (174 in the ketamine/eketamine group and 174 in the control group), one of which was reported at as medians (IQRs), and these data were converted into means ± SDs. (Zhao et al., 2021). We also performed an analysis based on different postoperative times for the postoperative depression scores. Notably, the postoperative depression scores of patients in the ketamine/esketamine group were lower than those of the control group at 3 days (SMD: −1.84, 95% CI: −2.93 to −0.76, p < 0.001, I2 = 89%) and 7 days (SMD: −0.57, 95% CI: −0.82 to −0.31, p < 0.001, I2 = 0%) after surgery. However, no statistically significant difference was observed in postoperative depression scores between the two groups at 30 days after surgery (SMD: −0.12, 95% CI: −0.55 to 0.32, p = 0.60, I2 = 69%) (Figure 5). One study presented the results for the quality of postoperative recovery as medians (IQRs), which were converted into means ± SDs. (Zhu et al., 2022). No statistically significant difference was observed in the quality of recovery at 1 day (SMD: 0.81, 95% CI: −0.25 to 1.88, p = 0.13, I2 = 93%) and 3 days (SMD: 0.55, 95% CI: −0.30 to 1.41, p = 0.20, I2 = 85%) after surgery (Figure 6). Three studies reported data for extubation time, (Ranran et al., 2017; Kang et al., 2020; Wang H. et al., 2024), and the results revealed no significant difference (SMD: 0.17, 95% CI: −0.42 to 0.76, p = 0.58, I2 = 81%) between the groups. (Figure 7). The pooled results revealed a lower incidence of dizziness in ketamine/esketamine group compared to the NS group (RR: 1.96, 95% CI: 1.28 to 3.01, p = 0.002, I2 = 77%) (Figure 8). No statistical differences were found between the groups regarding the incidence of nausea (RR: 1.06, 95% CI: 0.86 to 1.32, p = 0.58, I2 = 0%), vomiting (RR: 0.98, 95% CI: 0.61, 1.59, p = 0.94, I2 = 0%), and nightmares (RR: 1.34, 95% CI: 0.38 to 4.71, p = 0.65, I2 = 0%) (Figure 8).
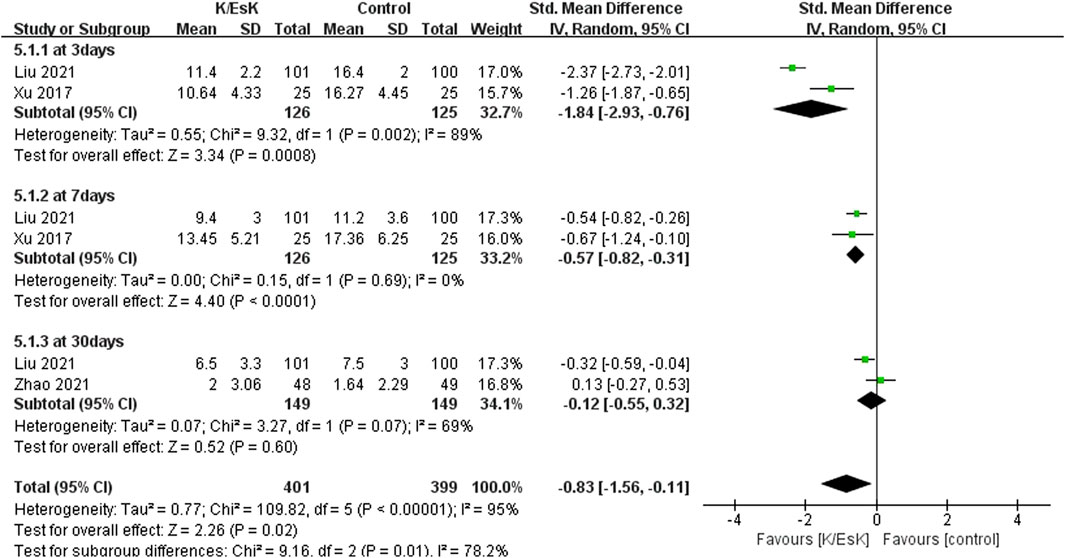
Figure 5. Forest plot of the effect of perioperative administration of ketamine/esketamine (k/esk) on postoperative depression scores within 30 days of surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
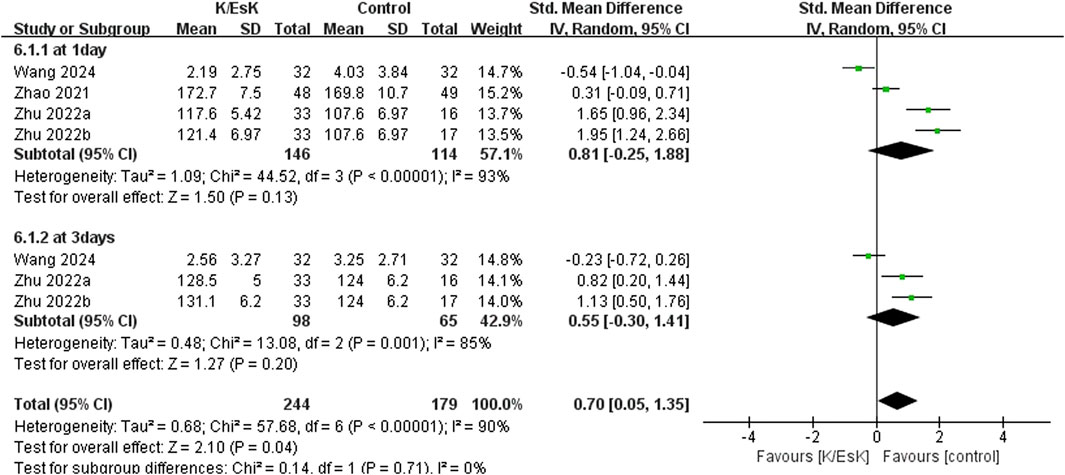
Figure 6. Forest plot of the effect of perioperative administration of ketamine/esketamine (k/esk) application on the quality of postoperative recovery within 3 days after surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
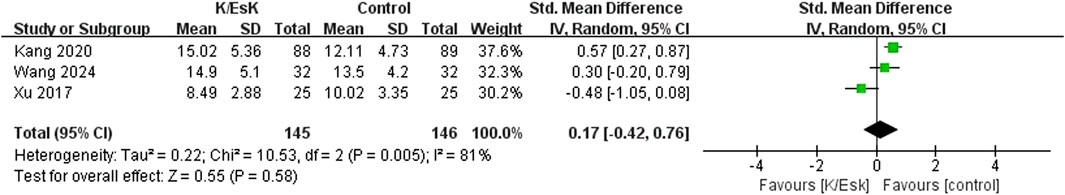
Figure 7. Forest plot of the effect of perioperative administration of ketamine/esketamine (k/esk) on extubation time after surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
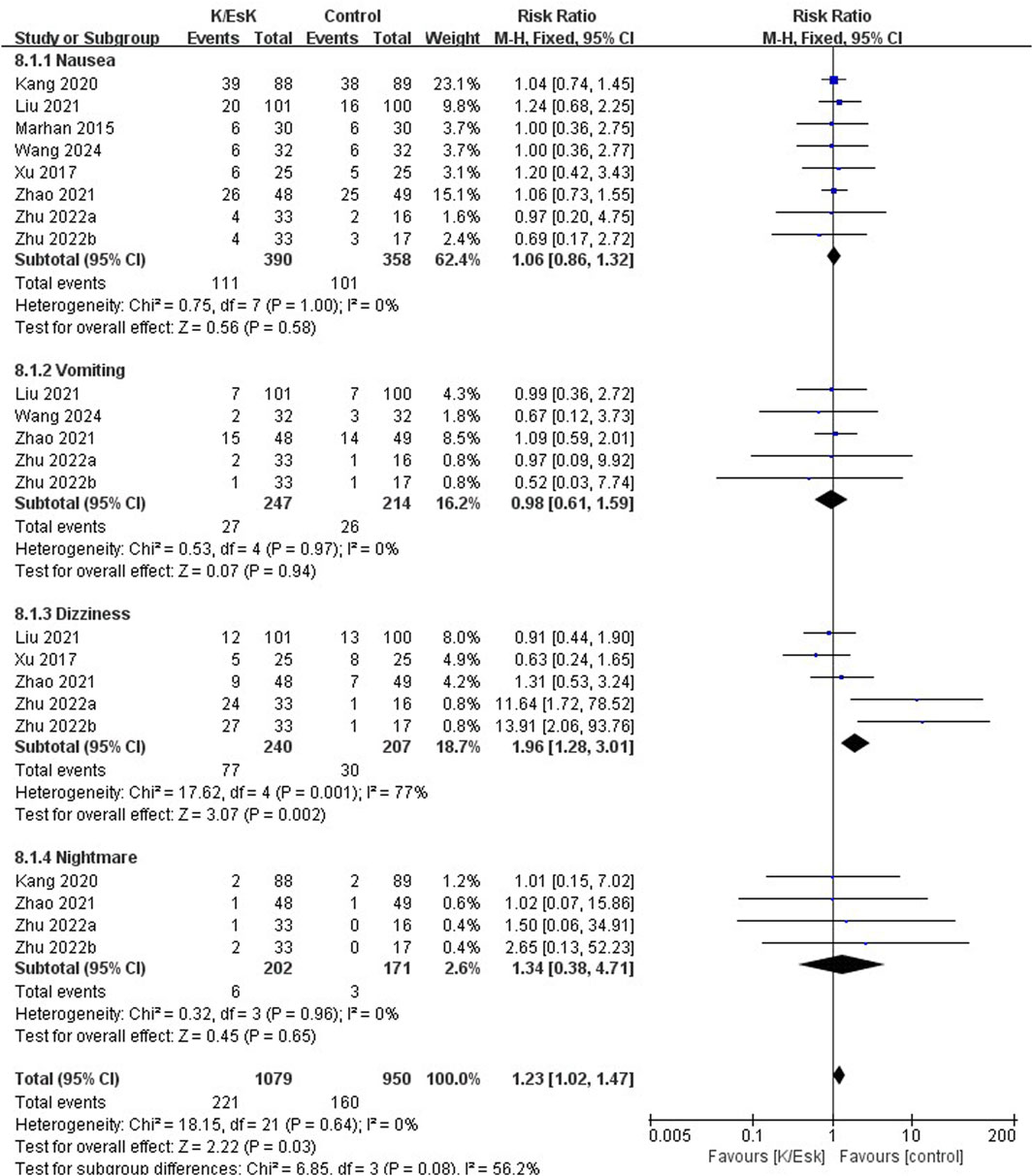
Figure 8. Forest plot of the incidence of postoperative adverse effects following perioperative ketamine/esketamine (k/esk) administration. CI, confidence interval; df, degrees of freedom.
Sensitivity analysis
Sensitivity analyses performed to evaluate the stability of the primary outcomes. The results revealed that one study had a significant impact on the stability of ketamine/esketamine on pain scores at 2 h after surgery (Mahran and Hassan, 2015) (Figure 9A). Furthermore, the results of 4 h and 3 months postoperative pain scores were more stable (Figures 9B,C). Additionally, a greater effect of ketamine/esketamine on postoperative pain scores at 1 day, 3 days, and 7 days after surgery was observed in the study of Liu (Liu et al., 2021) (Figures 9D–F).
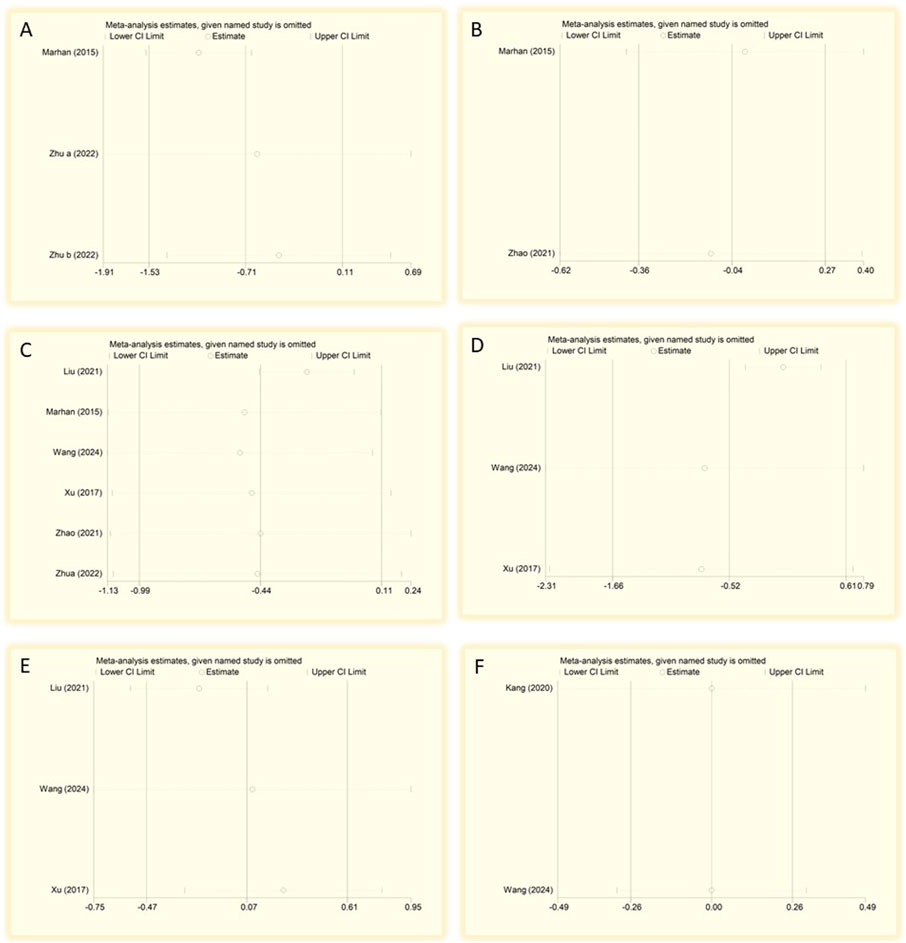
Figure 9. Sensitivity analysis of postoperative pain scores at different time points after surgery. (A) 2 h, (B) 4 h, (C) 3 months, (D) 1 day, (E) 3 days, and (F) 7 days after surgery. CI, confidence interval; df, degrees of freedom; Std, standardized.
Publication bias test
We did not perform a publication bias test, as we included <10 studies.
Quality of the evidence
According to the GRADE, for the primary outcome, the quality of evidence for pain scores at 4 h and 3 months after surgery was considered “moderate,” the quality of evidence for pain scores at 2 h, and 1 day after surgery was considered “low,” and the quality of evidence for pain scores at other times was considered “very low.” In addition, for secondary outcomes, the quality of evidence for vomiting was considered “high”, and the quality of evidence for nausea and nightmare was considered “moderate”. The evidence of depression scores at 3 days after surgery and the extubation time was considered “very low”. The quality of evidence for the remaining secondary outcomes was considered “low”. (Supplementary Table 1).
Discussion
This meta-analysis revealed that perioperative administration of ketamine/esketamine prevents depressive symptoms in the early postoperative period to a certain extent; however, their effectiveness in reducing postoperative pain, promoting the quality of recovery, and reducing adverse effects was limited. This phenomenon may be related to the complexity of the mechanism of action of these drugs, individual patient differences, and their effects on the body. Our findings align with previous studies demonstrating the antidepressant effects of ketamine/esketamine in perioperative settings. However, unlike prior research, which primarily focused on analgesic efficacy, our study highlights the potential of these drugs in preventing early-onset depressive symptoms. This distinction is clinically significant, as postoperative depression is often underdiagnosed and undertreated. While ketamine/esketamine is widely used, our findings provide additional evidence supporting their role in managing postoperative mental health, particularly in high risk populations.
Breast cancer is one of the most common malignant tumors among females, which affects the physical and mental health of patients. (Sung et al., 2021). Although modified radical mastectomy is considered the most effective treatment for breast cancer, most patients experience different degrees of postoperative pain as well as emotional disturbances such as anxiety, depression, and fear because of surgical resection, nerve damage, and inflammatory stimulation. This in turn reduces patient satisfaction and leads to poor wound healing, thus affecting postoperative recovery and the quality of life of the patients. (Zhu et al., 2022; Wang H. et al., 2024; Werner and Kongsgaard, 2014).
Ketamine, as an NMDA receptor antagonist, has been used in clinical anesthesia for many years because of its powerful sedative and analgesic effects. Esketamine, the S-(+) enantiomer of ketamine with all substituents on the same side and a stereochemically chiral center, exhibits approximately three to four times greater affinity for the NMDA receptors than that of R-ketamine, thus resulting in a higher bioactivity and fewer adverse effects, particularly as an analgesic and antidepressant. (Wang et al., 2019; Li et al., 2022). Ketamine and esketamine act as noncompetitive antagonists of NMDA receptors, and their pharmacological properties mainly involve the modulation of the central nervous system. Their mechanism of action may also be related to neuroplasticity and altered mood states in addition to modulating pain perception. (Wang et al., 2019; Zhu et al., 2022; Autry et al., 2011; Li et al., 2010).
Sustained injurious stimuli can lead to pain sensitization by activating NMDA receptors. The mechanism of ketamine-induced antinociceptive sensitization primarily involves noncompetitive antagonism of NMDA receptors. Previous studies have reported the perioperative use of esketamine in relieving postoperative pain and reducing opioid consumption. (Miziara et al., 2016; Su et al., 2022). A meta-analysis reported that the perioperative use of ketamine/eketamine was associated with improvements in early subjective quality of recovery, pain severity, and psychological symptoms without increasing the likelihood of adverse events. (Hung et al., 2024). However, Brinck et al. found that the intraoperative administration of esketamine did not reduce postoperative pain or oxycodone consumption during lumbar fusion surgery, which is consistent with the findings of our meta-analysis. (Brinck et al., 2021). This may be attributed to several factors, including, but not limited to, type of surgery, drug dose, route of administration, age, and individual differences in pain thresholds. (Laskowski et al., 2011; Avidan et al., 2017; Zhao et al., 2021). Notably, intraoperative ketamine application improved postoperative depression scores and elevated serum BDNF levels in patients undergoing elective orthopaedic surgery. (Jiang et al., 2016). Ketamine/esketamine can rapidly increase presynaptic glutamate release and BDNF synthesis by antagonizing NMDA receptors, (Autry et al., 2011; Li et al., 2010), which in turn promotes structural synaptic connectivity, resulting in a prolonged antidepressant effect. (Liu et al., 2012; Li et al., 2011). Tu et al. reported that eketamine administration during the induction of anesthesia reduced the perioperative inflammatory response and promoted the recovery of postoperative cognitive function in older patients after surgery. (Tu et al., 2021). However, the analgesic and antidepressant effects of ketamine and esketamine are not exclusively dependent on NMDA receptor antagonists and may involve multiple metabolites and mechanisms. (Zanos and Gould, 2018; Zanos et al., 2016).
Although ketamine and esketamine may potentially improve postoperative pain and early depression, the conclusion remain inconsistent, and their potential adverse effects and long-term safety issues limit their widespread perioperative use. (Shaffer et al., 2014). Perioperative esketamine administration significantly reduced pain intensity at 24 h postoperatively but increased Bispectral Index values and the incidence of drowsiness. (Zhu et al., 2022). In addition, a multicenter study found that the perioperative ketamine administration did not improve postoperative delirium in older adults after major surgery and increased the incidence of postoperative hallucinations and nightmares, thus inducing negative experiences. (Avidan et al., 2017). Therefore, clinicians should thoroughly assess the risks and benefits of ketamine and esketamine in perioperative management and develop individualized perioperative regimens to ensure patient safety.
This meta-analysis provides evidence supporting the potential of ketamine and esketamine to improve early postoperative depression in patients with breast cancer. Nevertheless, this study also has some limitations. First, only seven studies with relatively small sample sizes were included in our meta-analysis, which may have affected the statistical validity. Future larger trials and longer follow-up times are needed to further validate the findings of this meta-analysis. Second, the baseline characteristics of most studies were well-balanced, (Zhu et al., 2022; Mahran and Hassan, 2015; Ranran et al., 2017; Kang et al., 2020; Liu et al., 2021; Wang H. et al., 2024), and one study exhibited comparable baseline characteristics, which might have affected the accuracy of our results. (Zhao et al., 2021). Third, the measurement method of postoperative pain scores differed among the studies, with four (Mahran and Hassan, 2015; Ranran et al., 2017; Liu et al., 2021; Wang H. et al., 2024) and three (Zhu et al., 2022; Kang et al., 2020; Zhao et al., 2021) studies using the VAS and NRS scores, respectively, which may have affected the accuracy of our results. Fourth, four of the seven studies did not include postoperative depression scores as the primary outcome (Zhu et al., 2022; Mahran and Hassan, 2015; Kang et al., 2020; Wang H. et al., 2024); therefore, the data we extracted might be the occasional findings of these studies. Fifth, there may be heterogeneity in the type of surgery included in the study (modified radical versus breast-conserving surgery), and the severity of postoperative pain and depression may vary depending on the invasiveness of the surgery. Future studies need to be further stratified to analyse the effect of type of surgery on outcomes. Finally, we could not explore the mechanisms for improving postoperative depression and pain, as only one study assessed the perioperative serum BDNF and 5-hydroxytryptamine levels. (Liu et al., 2021).
Multicenter studies with larger sample sizes should be conducted in the future to improve the reliability and general applicability of the results regarding the use of ketamine/esketamine in postoperative management. In addition, exploring more precise strategies for the use of ketamine/esketamine in patients undergoing breast cancer surgery, such as optimal dosage, timing of administration, and patient screening criteria, would help further optimize their clinical use. Moreover, long-term follow-up studies may help assess the long-term effects of these drugs on postoperative pain and depression, as well as their combined effects on the quality of life of the patients.
Conclusion
Perioperative ketamine/esketamine administration did not significantly reduce postoperative pain in patients after breast cancer surgery; however, ketamine/esketamine may reduce depression in patients within a short period after the surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XS: Formal Analysis, Investigation, Writing–original draft. CwL: Investigation, Writing–original draft. LX: Data curation, Software, Writing–review and editing. XL: Data curation, Writing–review and editing, Formal Analysis. ZZ: Data curation, Writing–review and editing, Investigation, Methodology. ClL: Methodology, Writing–review and editing. JL: Funding acquisition, Supervision, Writing–review and editing. PW: Funding acquisition, Investigation, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support for this study was provided from the China Postdoctoral Science Foundation (2024M761822), the Natural Science Foundation of Shandong Province (ZR2020QH291 and ZR2020MH126), the Qingdao Key Health Discipline Development Fund (2025), and the Qingdao Outstanding Health Professional Development Fund (2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1532524/full#supplementary-material
References
Autry, A. E., Adachi, M., Nosyreva, E., Na, E. S., Los, M. F., Cheng, P. f., et al. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475 (7354), 91–95. doi:10.1038/nature10130
Avidan, M. S., Maybrier, H. R., Abdallah, A. B., Jacobsohn, E., Vlisides, P. E., Pryor, K. O., et al. (2017). Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 390 (10091), 267–275. doi:10.1016/S0140-6736(17)31467-8
Borenstein, M., Hedges, L. V., Higgins, J. P., and Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1 (2), 97–111. doi:10.1002/jrsm.12
Brinck, E. C. V., Maisniemi, K., Kankare, J., Tielinen, L., Tarkkila, P., and Kontinen, V. K. (2021). Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth. Analg. 132 (1), 69–79. doi:10.1213/ANE.0000000000004729
Chen, M., Ma, S., Liu, H., Dong, Y., Tang, J., Ni, Z., et al. (2024). Brain region-specific action of ketamine as a rapid antidepressant. Science 385 (6709), eado7010. doi:10.1126/science.ado7010
Cohen, S. P., Bhatia, A., Buvanendran, A., Schwenk, E. S., Wasan, A. D., Hurley, R. W., et al. (2018). Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. Reg. Anesth. Pain Med. 43 (5), 521–546. doi:10.1097/AAP.0000000000000808
Domino, E. F. (2010). Taming the ketamine tiger. 1965. Anesthesiology 113 (3), 678–684. doi:10.1097/ALN.0b013e3181ed09a2
Dundee, J. W., Knox, J. W., Black, G. W., Moore, J., Pandit, S. K., Bovill, J., et al. (1970). Ketamine as an induction agent in anaesthetics. Lancet 1 (7661), 1370–1371. doi:10.1016/s0140-6736(70)91273-0
Gohari, J., Grosman-Rimon, L., Arazi, M., Caspi-Avissar, N., Granot, D., Gleitman, S., et al. (2022). Clinical factors and pre-surgical depression scores predict pain intensity in cardiac surgery patients. BMC Anesthesiol. 22 (1), 204. doi:10.1186/s12871-022-01740-3
Granholm, A., Alhazzani, W., and Møller, M. H. (2019). Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 123 (5), 554–559. doi:10.1016/j.bja.2019.08.015
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj, 343d5928. doi:10.1136/bmj.d5928
Hou, N. N., Zhang, M. Y., Zhang, Y. W., Wu, H. J., Luo, H., and Yang, H. (2025). Safety and efficacy of low-dose esketamine weakly opioidized anesthesia in elderly patients with lumbar spinal stenosis undergoing surgery: a prospective, double-blind randomized controlled trial. BMC Anesthesiol. 25 (1), 57. doi:10.1186/s12871-025-02908-3
Hung, K. C., Kao, C. L., Ho, C. N., Hsing, C. H., Chang, Y. J., Wang, L. K., et al. (2024). The impact of perioperative ketamine or esketamine on the subjective quality of recovery after surgery: a meta-analysis of randomised controlled trials. Br. J. Anaesth. 132 (6), 1293–1303. doi:10.1016/j.bja.2024.03.012
Jiang, M., Wang, M. H., Wang, X. B., Liu, L., Wu, J. L., Yang, X. L., et al. (2016). Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. J. Anesth. 30 (2), 232–237. doi:10.1007/s00540-015-2096-7
Kang, C., Cho, A. R., Kim, K. H., Lee, E. A., Lee, H. J., Kwon, J. Y., et al. (2020). Effects of intraoperative low-dose ketamine on persistent postsurgical pain after breast cancer surgery: a prospective, randomized, controlled, double-blind study. Pain Physician 23 (1), 37–47.
Kim, M. S., Kim, S. Y., Kim, J. H., Park, B., and Choi, H. G. (2017). Depression in breast cancer patients who have undergone mastectomy: a national cohort study. PLoS One 12 (4), e0175395. doi:10.1371/journal.pone.0175395
Laskowski, K., Stirling, A., McKay, W. P., and Lim, H. J. (2011). A systematic review of intravenous ketamine for postoperative analgesia. Can. J. Anaesth. 58 (10), 911–923. doi:10.1007/s12630-011-9560-0
Li, J., Wang, Z., Wang, A., and Wang, Z. (2022). Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: a randomized controlled trial. J. Clin. Pharm. Ther. 47 (6), 759–766. doi:10.1111/jcpt.13604
Li, N., Lee, B., Liu, R. J., Banasr, M., Dwyer, J. M., Iwata, M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 (5994), 959–964. doi:10.1126/science.1190287
Li, N., Liu, R. J., Dwyer, J. M., Banasr, M., Lee, B., Son, H., et al. (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 69 (8), 754–761. doi:10.1016/j.biopsych.2010.12.015
Liu, P., Li, P., Li, Q., Yan, H., Shi, X., Liu, C., et al. (2021). Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J. Invest. Surg. 34 (8), 883–888. doi:10.1080/08941939.2019.1710626
Liu, R. J., Lee, F. S., Li, X. Y., Bambico, F., Duman, R. S., and Aghajanian, G. K. (2012). Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry 71 (11), 996–1005. doi:10.1016/j.biopsych.2011.09.030
Mahran, E., and Hassan, M. E. (2015). Comparison of pregabalin versus ketamine in postoperative pain management in breast cancer surgery. Saudi J. Anaesth. 9 (3), 253–257. doi:10.4103/1658-354X.154697
Melsen, W. G., Bootsma, M. C., Rovers, M. M., and Bonten, M. J. (2014). The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 20 (2), 123–129. doi:10.1111/1469-0691.12494
Mion, G., and Himmelseher, S. (2024). Esketamine: less drowsiness, more analgesia. Anesth. Analg. 139 (1), 78–91. doi:10.1213/ANE.0000000000006851
Miziara, L. E., Simoni, R. F., Esteves, L. O., Cangiani, L. H., Grillo-Filho, G. F. R., and Paula, A. G. L. E. (2016). Efficacy of continuous S(+)-Ketamine infusion for postoperative pain control: a randomized placebo-controlled trial. Anesthesiol. Res. Pract. 2016, 20166918327. doi:10.1155/2016/6918327
Nejadghaderi, S. A., Balibegloo, M., and Rezaei, N. (2024). The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: a perspective on the pros and cons. Health Sci. Rep. 7 (6), e2165. doi:10.1002/hsr2.2165
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj, 372n71. doi:10.1136/bmj.n71
Ranran, Xu Y. Z., and Chen, S. (2017). Effect of intraoperative single administration of sub-anesthesia ketamine on breast cancer patients with depression. Biomed. Res. Special Issue: S552-S556(Special Issue):S552-S6.
Schumacher, M. A. (2024). Peripheral neuroinflammation and pain: how acute pain becomes chronic. Curr. Neuropharmacol. 22 (1), 6–14. doi:10.2174/1570159X21666230808111908
Shaffer, C. L., Osgood, S. M., Smith, D. L., Liu, J., and Trapa, P. E. (2014). Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology 86, 86174–86180. doi:10.1016/j.neuropharm.2014.07.008
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj, 358j4008. doi:10.1136/bmj.j4008
Shinohara, R., Aghajanian, G. K., and Abdallah, C. G. (2021). Neurobiology of the rapid-acting antidepressant effects of ketamine: impact and opportunities. Biol. Psychiatry 90 (2), 85–95. doi:10.1016/j.biopsych.2020.12.006
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 343, 343d4002. doi:10.1136/bmj.d4002
Su, Y., Zhang, J., Wang, H., Gu, Y., Ouyang, H., and Huang, W. (2022). The use of Esketamine in CT-guided percutaneous liver tumor ablation reduces the consumption of remifentanil: a randomized, controlled, double-blind trial. Ann. Transl. Med. 10 (12), 704. doi:10.21037/atm-22-2756
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tu, W., Yuan, H., Zhang, S., Lu, F., Yin, L., Chen, C., et al. (2021). Influence of anesthetic induction of propofol combined with esketamine on perioperative stress and inflammatory responses and postoperative cognition of elderly surgical patients. Am. J. Transl. Res. 13 (3), 1701–1709.
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 14135. doi:10.1186/1471-2288-14-135
Wang, H., Te, R., Zhang, J., Su, Y., Zhou, H., Guo, N., et al. (2024b). Effects of a single subanesthetic dose of esketamine on postoperative subthreshold depressive symptoms in patients undergoing unilateral modified radical mastectomy: a randomised, controlled, double-blind trial. BMC Psychiatry 24 (1), 315. doi:10.1186/s12888-024-05753-9
Wang, J., Huang, J., Yang, S., Cui, C., Ye, L., Wang, S. Y., et al. (2019). Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in Comparison with ketamine: a randomized, open-label clinical study. Drug Des. Devel Ther. 13, 134135–134144. doi:10.2147/DDDT.S224553
Wang, K., Tan, X., Ding, K. M., Feng, X. Z., Zhao, Y. Y., Zhu, W. L., et al. (2024a). Dynamic regulation of phosphorylation of NMDA receptor GluN2B subunit tyrosine residues mediates ketamine rapid antidepressant effects. Pharmacol. Res. 205, 205107236. doi:10.1016/j.phrs.2024.107236
Wang, L., Cohen, J. C., Devasenapathy, N., Hong, B. Y., Kheyson, S., Lu, D., et al. (2020). Prevalence and intensity of persistent post-surgical pain following breast cancer surgery: a systematic review and meta-analysis of observational studies. Br. J. Anaesth. 125 (3), 346–357. doi:10.1016/j.bja.2020.04.088
Werner, M. U., and Kongsgaard, U. E. I. (2014). Defining persistent post-surgical pain: is an update required? Br. J. Anaesth. 113 (1), 1–4. doi:10.1093/bja/aeu012
Xu, J., Li, M., Hu, Y., Yang, Q., Long, Q., and Zhou, H. (2025). Esketamine reduces postoperative depression in breast cancer through TREK-1 channel inhibition and neurotransmitter modulation. Cancer Cell Int. 25 (1), 51. doi:10.1186/s12935-025-03664-7
Yang, C., Shirayama, Y., Zhang, J. C., Ren, Q., Yao, W., Ma, M., et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry, (2025), 5 (9), e632. doi:10.1038/tp.2015.136
Zanos, P., and Gould, T. D. (2018). Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23 (4), 801–811. doi:10.1038/mp.2017.255
Zanos, P., Moaddel, R., Morris, P. J., Georgiou, P., Fischell, J., Elmer, G. I., et al. (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533 (7604), 481–486. doi:10.1038/nature17998
Zanos, P., Moaddel, R., Morris, P. J., Riggs, L. M., Highland, J. N., Georgiou, P., et al. (2018). Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol. Rev. 70 (3), 621–660. doi:10.1124/pr.117.015198
Zhao, Z., Xu, Q., Chen, Y., Liu, C., Zhang, F., Han, Y., et al. (2021). The effect of low-dose ketamine on postoperative quality of recovery in patients undergoing breast cancer surgery: a randomised, placebo-controlled trial. Int. J. Clin. Pract. 75 (12), e15010. doi:10.1111/ijcp.15010
Keywords: ketamine, esketamine, breast cancer surgery, postoperative pain, postoperative depression, meta-analysis
Citation: Sun X, Li C, Xu L, Lin X, Zhang Z, Lin C, Li J and Wei P (2025) Effect and safety of perioperative ketamine/esketamine administration on postoperative pain and depression after breast cancer surgery: a systematic review and meta-analysis. Front. Pharmacol. 16:1532524. doi: 10.3389/fphar.2025.1532524
Received: 25 November 2024; Accepted: 14 March 2025;
Published: 28 March 2025.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Shaochun Liu, Second Hospital of Anhui Medical University, ChinaJie Hao, Southeast Colorado Hospital, United States
Copyright © 2025 Sun, Li, Xu, Lin, Zhang, Lin, Li and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Li, bGpqOTU3M0AxNjMuY29t; Penghui Wei, d2VpcGVuZ2h1aWh1aUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Xinyi Sun†
Xinyi Sun† Zheng Zhang
Zheng Zhang Jianjun Li
Jianjun Li Penghui Wei
Penghui Wei