
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 28 February 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1525313
Zanthoxylum bungeanum Maxim (Z. bungeanum) is a medicinal and edible plant commonly used to improve the flavor of Chinese cuisine due to its unique numbing taste. It is recognized for its medicinal properties, including bodywarming, relieving cold, promoting blood circulation, and alleviating pain. Additionally, Z. bungeanum has been extensively studied for its antitumor properties. In this study, various scientific databases and network pharmacology were used to search for information about Z. bungeanum and its components for the treatment of tumors. Numerous active components of Z. bungeanum have been identified, demonstrating antitumor properties. We discovered that Z. bungeanum can modulate multiple signaling pathways across various targets using network pharmacological predictions, highlighting its strong antitumor potential. The components of Z. bungeanum and the traditional Chinese medicine compound containing Z. bungeanum can promote apoptosis, arrest the cell cycle, inhibit cell invasion and metastasis, promote autophagy, and increase the sensitivity of chemotherapeutic drugs through P53, PI3K/AKT, Wnt/β-catenin and other signaling pathways, which are effective against various cancers, including hepatocellular cancer, gastric cancer, and breast cancer. Z. bungeanum and its extracts have demonstrated promising effects against various tumors, indicating their potential use in future cancer therapies and offering new strategies for tumor treatment. However, clinical studies evaluating the antitumor efficacy and toxicity of Z. bungeanum in humans are scarce. Therefore, well-designed clinical trials should be prioritized in the future to establish a solid foundation for its use in cancer treatment.
Cancer is one of the leading causes of human mortality, and its prevention and treatment remain among the most challenging clinical problems (Siegel et al., 2023). With the aging population and poor lifestyle, the incidence of cancer is increasing every year. Currently, the common methods of cancer treatment include surgical intervention, radiotherapy, chemotherapy, targeted therapy, and immunotherapy (Mun et al., 2018). However, the mortality and recurrence rates of cancer remain high, and the side effects of treatment also cause significant pain to patients. This is attributed to the fact that most antitumor drugs kill cancer cells while severely damaging normal cells, making it crucial to find highly effective and low-toxicity drugs to treat and prevent tumor development.
There is a growing interest in medicinal and edible plants that have both culinary and therapeutic properties. Medicinal and edible plants are rich in polysaccharides, proteins, fats, and vitamins (Ma et al., 2022). The concept of replacing pharmacies with kitchens and medicines with food has gained widespread acceptance. Medicinal and edible plants have demonstrated remarkable therapeutic potential for the treatment of various diseases, often exhibiting low toxicity (Qu et al., 2023). Medicinal and edible plants hold significant promise for managing dyslipidemia, offering superior efficacy, acceptability, and commercial value compared to lipid-lowering medications that frequently cause adverse effects (Hu et al., 2022). Various components of edible and medicinal plants exhibit numerous physiological effects, including anti-inflammatory, antiviral, and antioxidant properties (Lu et al., 2022; Wang et al., 2022; Yang et al., 2022; Xiao et al., 2023). Similarly, these active components have demonstrated significant antitumor activity. Angelica sinensis, a medicinal and edible plant, can inhibit liver cancer growth (Wang et al., 2017). Hawthorn is a promising candidate for the management of melanoma (Mustapha et al., 2016). The anticancer properties of ginseng have been demonstrated in various types of cancers of the stomach, lungs, liver, colon, and skin (Wargovich, 2001; Cai et al., 2013; Sharma and Goyal, 2015; Yoo et al., 2017; Chen et al., 2021). Consequently, medicinal and edible plants have significant potential in the development of therapeutic agents for treating tumors.
Z. bungeanum is also known as Chinese prickly ash or Huajiao in Mandarin. Currently, it is extensively available in China, Korea, Japan, India, and other Asian nations. In 2002, it was officially recognized by the Chinese Ministry of Health as a plant suitable for both medicinal and food applications. It is primarily used as a seasoning in cuisine due to its unique pungency and numbing sensation and its appetite-enhancing effect. Z. bungeanum is particularly popular in Sichuan cuisine (Luo et al., 2022). In addition to its culinary applications, Z. bungeanum possesses significant medicinal properties. It exhibits detoxifying, hemostatic, analgesic, anti-inflammatory, and antiplasmodial properties (Goodman et al., 2019; Alam et al., 2020; Qi et al., 2024; Wang et al., 2024). Additionally, Z. bungeanum benefits the digestive system and is frequently used as a herbal remedy for treating stomach discomfort and relieving physical ailments. Recent research has demonstrated that Z. bungeanum and its active components exhibit significant antitumor properties. Z. bungeanum extracts have demonstrated efficacy against various cancers, including skin, gastric, and liver cancers. Therefore, the antitumor effects of Z. bungeanum and the mechanisms underlying these effects were investigated in this study. We anticipate that developing medicinally active ingredients and related products derived from Z. bungeanum for oncological applications may become a significant research hotspot in the future.
First, the composition and blood-entry components of Z. bungeanum were investigated using network pharmacological analysis to identify potential components for tumor treatment. Combined with the literature and KEGG analysis, it was discovered that Z. bungeanum can fight against various tumors and that Z. bungeanum and its constituents inhibit the growth of tumors through multiple target sites and signaling pathways. Additionally, the herbal compounds containing Z. bungeanum demonstrated significant antitumor activity, while its hepatoprotective and gastrointestinal protective effects indicate a potential role in preventing tumor development. These findings support the antitumor properties of Z. bungeanum and provide new insights into its potential for cancer prevention and treatment (Figure 1).
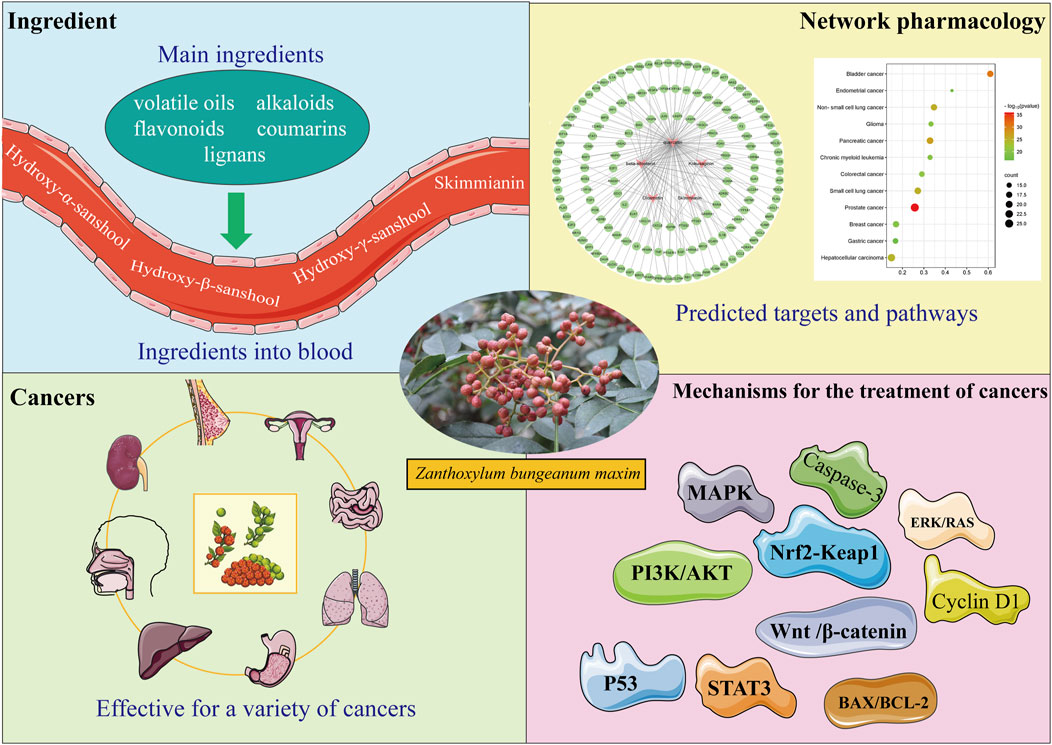
Figure 1. Through literature retrieval and network pharmacology analysis of the components by which Zanthoxylum bungeanum Maxim (Z. bungeanum) performs its functions, it was discovered that Z. bungeanum has curative effects on multiple tumors. Further studies on the antitumor mechanism of Z. bungeanum must be conducted to determine the components and mechanisms of its antitumor effects.
One of the active constituents of Z. bungeanum is a volatile oil that contains terpenes, alcohols, and esters. Various early investigations examining the chemical makeup of plants revealed that linalool and limonene were the primary constituents of volatile oil (Yang, 2008; Sun J. et al., 2020).
In Ehrlich ascites tumor model mice, volatile oil demonstrated significant immunomodulatory effects and anticancer efficacy (da Silva et al., 2007). Among the volatile oil components, linalool and limonene have demonstrated promising advantages in the treatment of tumors. Research indicates that linalool exhibits a significant antitumor proliferative effect and lowers the expression of PCNA and Ki-67 in prostate cancer cells 22RV1. These findings demonstrate that linalool can be used as a drug for prostate cancer treatment (Zhao et al., 2020). Colorectal cancer cells undergo apoptosis when exposed to linalool, probably due to cancer-specific hydroxyl radical formation. The linalool group of mice exhibited a 55% lower average tumor weight than the control group (Iwasaki et al., 2016). Additionally, linalool induces cell cycle arrest and stimulates apoptosis by generating oxidative stress and activating MAPK and AKT pathways in hepatocellular carcinoma cells (Rodenak-Kladniew et al., 2018).
D-limonene-induced apoptosis in lung cancer cells was inhibited using the autophagy inhibitor chloroquine and ATG5 knockdown, confirming that D-limonene inhibits tumor growth via the autophagy pathway (Yu et al., 2018). In gastric cancer, D-limonene exhibited anti-angiogenic and pro-apoptotic effects, thereby inhibiting its growth and metastasis (Lu et al., 2004). Besides, D-limonene functions well against numerous cancers, including breast cancer (Mandal et al., 2023), neuroblastoma (Berliocchi et al., 2018), and melanoma (Alipanah et al., 2021).
Alkaloids are the primary active compounds in Z. bungeanum, with over 80 different alkaloids being extracted from this plant (Fu et al., 2020; Ji et al., 2022). Among these, quinoline and isoquinoline alkaloids, including skimmianine, leucine, goitrogenine, and chelerythrine, were the most prevalent. Several studies have reported that alkaloids from the roots of Z. bungeanum exhibit cytotoxic and antiproliferative effects against various tumor cell lines, including those from liver, lung, cervical, and stomach cancers (Mbaveng et al., 2019; Fu et al., 2020; Qin et al., 2022). Moreover, the total alkaloids extracted from Z. bungeanum root inhibited tumor growth in mice by regulating the blood levels of TNF-α and interleukin-2, as well as by promoting apoptosis. The underlying mechanism of action is likely associated with immune system modulation and induction of tumor cell death (Long et al., 2022).
Furthermore, skimmianine, a major alkaloid in Z. bungeanum, induces apoptosis in non-small cell lung cancer (NSCLC) cells, significantly inhibiting their proliferation. The effects on apoptosis and growth inhibition were concentration-dependent and mediated through caspase activation (Zuo et al., 2019). In addition, some scholars have investigated the antitumor activity of chelerythrine from Z. bungeanum and discovered that it could reduce p-FAK expression, thereby altering the cytoskeletal structure and inhibiting hepatocellular carcinoma by downregulating MMP-2/9 expression through the PI3K/AKT/mTOR signaling pathway (Zhu et al., 2018).
Z. bungeanum contains a high concentration of flavonoids, predominantly in the form of flavonoid glycosides. The major flavonoid components of Z. bungeanum include quercetin, chrysin, rutin, and other active ingredients with anticancer properties (Zhang et al., 2014).
Quercetin is a natural flavonoid component of Z. bungeanum that exhibits various activities, including cardiovascular protection, anti-inflammatory, and antitumor activities (Li et al., 2016; Patel et al., 2018; Mirazimi et al., 2022). In the context of antitumor activity, quercetin increases lysosomal activation and ferritin degradation mediated by the transcription factor EB, leading to iron death and P53-independent cell death (Wang et al., 2021). Quercetin inhibits proliferation and metastasis and promotes apoptosis in lung, cervical, and liver cancers (Bishayee et al., 2013; Chang et al., 2017; Ren et al., 2017). Hyperin, extracted from the leaves of Z. bungeanum, inhibited the growth of SW620 colon cancer cells through the P53 signaling pathway and caspase-dependent apoptosis (Zhang et al., 2017). Furthermore, hyperin may suppress the progression of gastric cancer by modulating the Wnt/β-catenin signaling pathway (Ping, 2020). Moreover, hyperin glycoside demonstrated good efficacy in the treatment of skin, lung, and ovarian cancers (Zhu et al., 2017; Li et al., 2018; Kong et al., 2020). Rutin, recognized as a safe anticancer agent, exerts its anticancer effects by modulating multiple signaling pathways (Imani et al., 2021).
Lignans and coumarins from Z. bungeanum have demonstrated cytotoxic effects in lung and pancreatic cancer cell lines (Mukhija et al., 2014; Chai and Qiang, 2022). Steroid A, a C34 pentacyclic steroid analog extracted from Capsicum annuum, exhibited antiproliferative effects against HeLa, MCF-7, and HepG2 cell lines, exhibiting promising antitumor activity (Meng et al., 2020).
Rong et al. discovered that when Z. bungeanum extract was injected subcutaneously, the absolute bioavailability of the amide constituents, hydroxy-α-sanshool, hydroxy-β-sanshool, and hydroxy-γ-sanshool, was 100.2%, 76.2%, and 90.3%, respectively (Rong et al., 2016). Lin et al. used UPLC-Q-TOF-MS to determine the alkaloidal constituents in the plasma of rats after oral administration of Z. bungeanum extract and detected 18 alkaloids, with skimmianine having the highest maximum plasma drug concentration (377.90 ± 52.65 ng/mL) (Lin et al., 2020). These results indicated that hydroxy-α-sanshool, hydroxy-β-sanshool, hydroxy-γ-sanshool, and skimmianine can be used for medicinal purposes.
Hydroxy-γ-sanshool significantly reduced the mRNA and protein expression levels of Cyclin D1, CDK4, PCNA, P53, P21, Fas, and Caspase 8 in HCT-116 colon cancer cells. Furthermore, Caspase 8 and P53 protein inhibitors significantly reduced the apoptosis and cell cycle arrest induced by hydroxy-γ-sanshool. These findings confirmed that hydroxy-γ-sanshool inhibits tumor growth through P53 and Caspase 8 pathways (Zhaojun et al., 2022).
Skimmianine significantly reduced the growth of xenograft tumors in nude mice, inhibited the proliferation of esophageal squamous cell carcinoma by preventing the activation of ERK1/2, and modulated epithelial-mesenchymal transition (EMT) to limit tumor cell migration and invasion (Liu et al., 2022). Skimmianine induced apoptosis in lung cancer cells and significantly suppressed the growth of four NSCLC cell lines (Zuo et al., 2019). Skimmianine proved to be an active ingredient against tumors.
Z. bungeanum was searched in the Traditional Chinese Medicine Database and Analysis Platform database (TCMSP, https://tcmsp-e.com/), according to the oral bioavailability value ≥30% and drug-likeness value ≥0.18. Five active ingredients were selected: kokusaginin, skimmianine, diosmetin, beta-sitosterol, and quercetin. Cytoscape (version 3.8.2) was used to construct the protein-protein interaction network (Figure 2A). The number of targets corresponding to the five components (Figure 2B). KEGG enrichment analysis, conducted using Metascape software (https://metascape.org/), revealed that the targets of Z. bungeanum were mainly enriched in cancer pathways (Figure 2C). We further analyzed the cancer pathways and discovered that the targets of Z. bungeanum may exhibit a certain effect on bladder, lung, liver, and gastric cancers, among other tumors (Figure 2D).
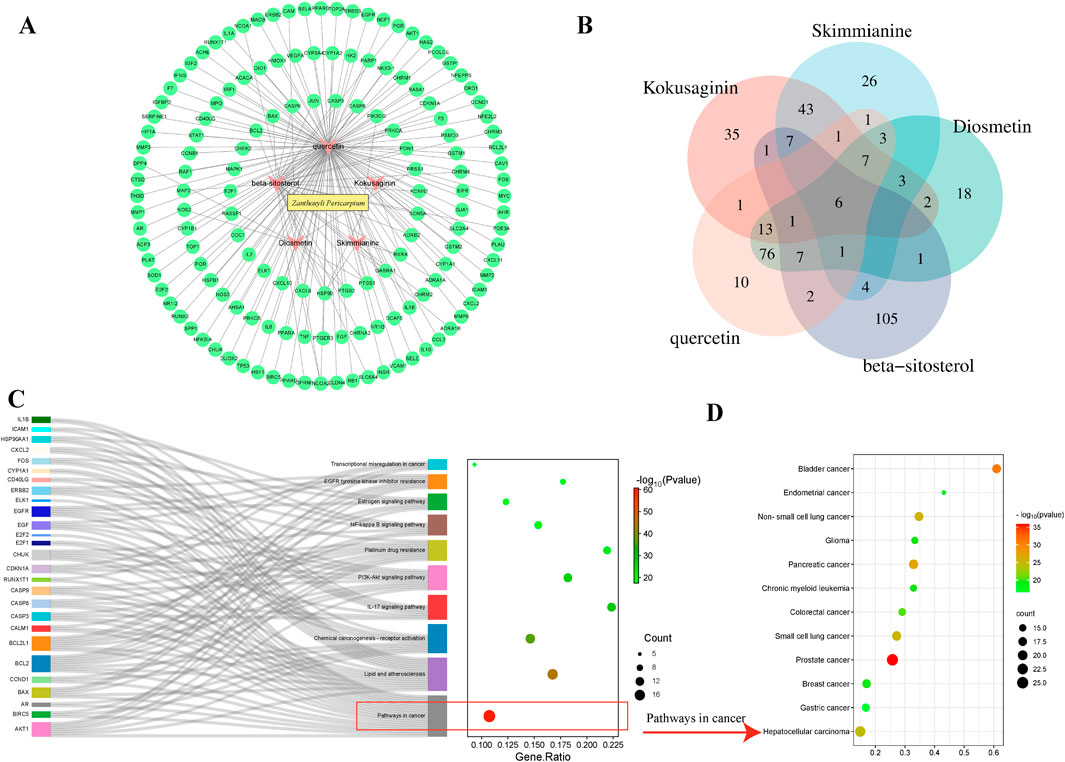
Figure 2. Network pharmacology analysis. (A) Components and targets through which Z. bungeanum exerts its effects. (B) Venn diagram of the targets corresponding to the five components of Z. bungeanum. (C) Related pathways enriched by the targets of Z. bungeanum, among which most targets were enriched in cancer pathways. (D) Pathways involved in cancer development.
Among the five active ingredients predicted by network pharmacology, skimmianine, an alkaloid component of Z. bungeanum, has been identified as the main component due to its multiple therapeutic effects, including antitumor properties. This validates the network pharmacology predictions and supports the feasibility of further investigating other predicted components for their potential therapeutic benefits. Quercetin, one of the major alkaloidal components of Z. bungeanum, has been discussed for its antitumor properties, implying that it can fight cancer.
Kokusaginin significantly increased apoptosis in breast cancer-resistant cells by decreasing P-gp protein levels and inhibiting P-gp function. Kokusaginin has been proposed as an anti-multidrug-resistant drug for the treatment of breast cancer (Chen et al., 2018).
Diosmetin inhibits melanoma tumor metastasis by inducing apoptosis and inhibiting tumor angiogenesis (Choi et al., 2019). Inhibition of RPA2 and RAD51 recruitment at the onset of DNA double-strand breaks during radiotherapy inhibits homologous recombination in endometrial cancer, thereby improving the sensitivity to radiotherapy (Hu et al., 2020). Diosmetin significantly inhibited the proliferation of hepatocellular carcinoma cells and promoted cell cycle arrest in the G2/M phase (Ma and Zhang, 2020). In addition, Diosmetin inhibits the proliferation and metastasis of lung, ovarian, gastric, and colorectal cancers (Koosha et al., 2019; Zhao F. et al., 2021; Song et al., 2022; Zhang and Luo, 2023).
Beta-sitosterol has been demonstrated to reduce the size and scope of tumor metastasis in vivo, as well as the proliferation of numerous tumor cell types. Beta-sitosterol promotes apoptosis in breast cancer cells by activating the caspase-8 and Fas receptor pathway proteins (Awad et al., 2007). Additionally, beta-sitosterol is effective against colon cancer, prostate cancer, intracranial aneurysms, ovarian cancer, and fibrosarcoma (von Holtz et al., 1998; Moon et al., 2007; Baskar et al., 2010; Yang et al., 2019; Bae et al., 2021), indicating its potential as an effective compound for preventing and treating tumors.
Based on existing studies and web-based pharmacological analyses, we summarized the constituents and blood-entry components of Z. bungeanum that may exhibit antitumor properties in Figure 3. The monomers of traditional Chinese medicines are the active ingredients that form the material basis of the mechanism of action. The therapeutic effects of the active ingredients of traditional Chinese medicines on cancer have been widely researched in the medical profession (Li et al., 2020). Identifying the components of Z. bungeanum involved in tumor treatment provides a comprehensive understanding of its antitumor mechanisms and a robust foundation for its use as an antitumor agent.
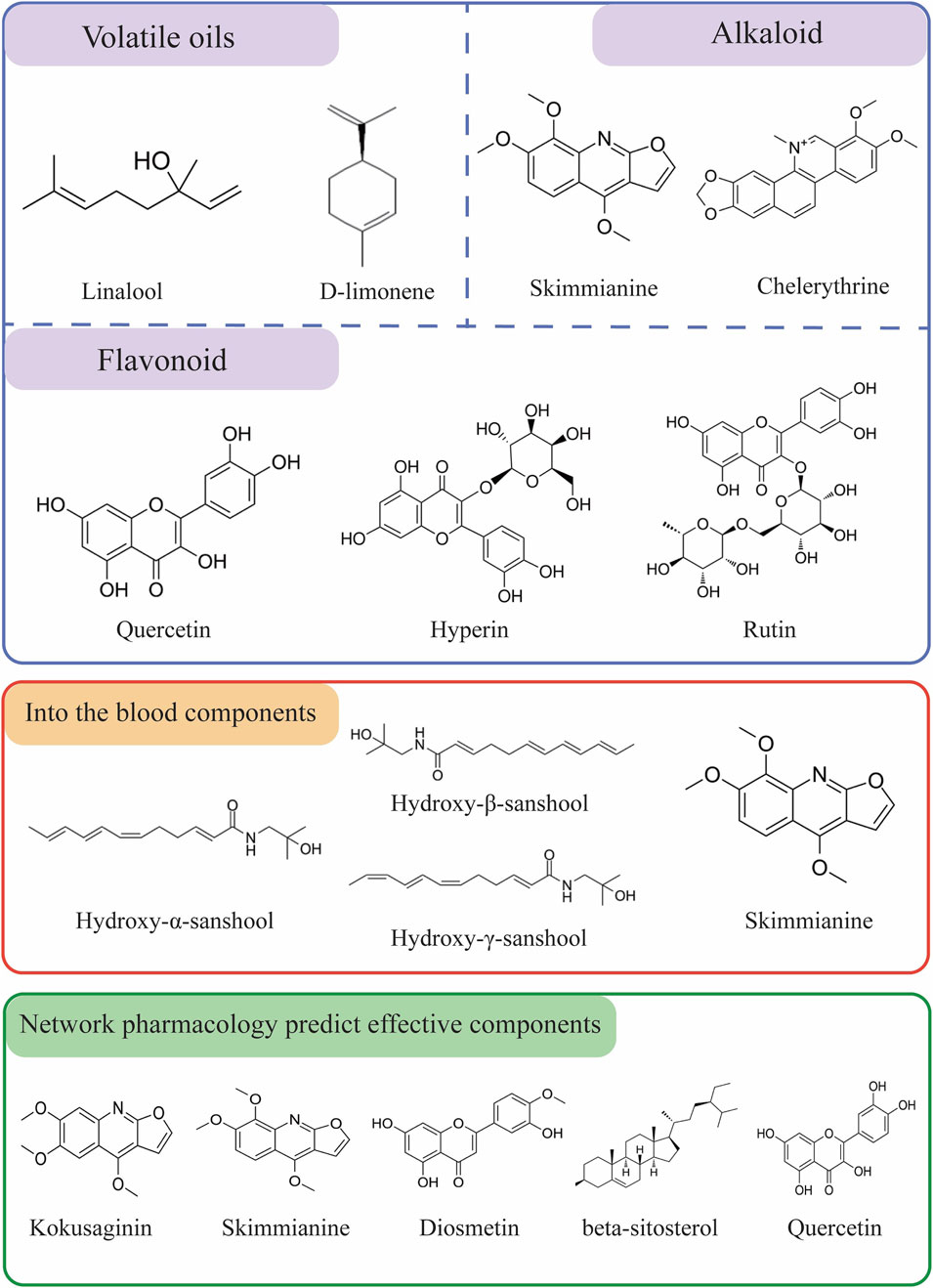
Figure 3. Chemical structures of Z. bungeanum components with potential medicinal functions and those entering the bloodstream.
The infinite proliferation of tumor cells is the uncontrolled rapid reproduction and growth of cells, producing tumors that are difficult to cure. Inhibition of cell proliferation is an effective strategy to treat tumors, and numerous components of Z. bungeanum extract can inhibit the proliferation of tumor cells, thereby achieving the purpose of tumor treatment.
Z. bungeanum bark extract inhibited the cellular activity of B16-F10 mouse melanoma cells without exhibiting toxicity to human dermal fibroblasts (Santhanam et al., 2016). Zantholic acid, an active ingredient extracted from Z. bungeanum, exhibited cytotoxicity against breast cancer cells, effectively inhibiting cell proliferation (Vyry Wouatsa et al., 2013). Moreover, Z. bungeanum leaf extracts exhibited cytotoxicity against promyelocytic and myelomonocytic leukemia cells, further supporting the antiproliferative effects of this medicinal herb (Chou et al., 2011).
Tumors acquire special abilities during the transition of normal cells into tumor cells, including the ability of tumor cells to proliferate indefinitely due to severe cell cycle dysregulation. The rate of cell proliferation is determined by rhythmic regulation of the cell cycle, which is tightly controlled by various cell cycle-related factors. Z. bungeanum extracts may disrupt this regulation by stabilizing specific proteins, thereby impeding cell cycle progression (Hydbring et al., 2016). In hepatocellular carcinoma, Z. bungeanum extract significantly inhibited HA22T cell viability in the G2/M phase, which was further confirmed in a nude mouse model, where Z. bungeanum extract inhibited tumor growth and activated PP2A proteins to downregulate cell cycle regulatory proteins (Dung et al., 2012). In melanoma, Z. bungeanum seed oil inhibited the CDC25A/CyclinB1/CDK1 signaling pathway to block the G0/G1 phase of human malignant melanoma A-375 cells and regulated the MAPK signaling pathway to inhibit cell proliferation, implying that Z. bungeanum seed oil exhibits anticancer activity but does not produce toxicity in mice (Pang et al., 2019; Wang et al., 2023). Z. bungeanum extract Z. bungeanum toxin-triazole derivative induced S/G2 phase arrest in gastric cancer AGS cells by inhibiting cell growth, exhibiting better therapeutic activity and specificity for gastric cancer (Shen et al., 2017). Z. bungeanum extract inhibited androgen receptor (AR) signaling and downregulated nuclear levels of AR by inhibiting AKT and Cyclin D1 levels in prostate cancer cells (Yang et al., 2006). The specific mechanism of cell cycle arrest by Z. bungeanum and its components is depicted in Figure 4.

Figure 4. Z. bungeanum and its components inhibit cell proliferation by blocking the cell cycle through multiple pathways.
Apoptosis is a tightly controlled mode of cell death characterized by nuclear consolidation, cellular crumpling, cell membrane vesiculation, and DNA fragmentation. Caspases are cysteine proteases that are crucial for controlling apoptosis (Kopeina et al., 2018). Bax and Bcl-2 are two proteins that play key roles in apoptosis regulation. Their interaction and regulation are essential for the balance between cell survival and death (Hafezi and Rahmani, 2021).
Z. bungeanum leaf extract inhibited the activation of the PI3K/AKT pathway and enhanced reactive oxygen species (ROS) production, thereby inducing apoptosis in bladder cancer cells in a dose-dependent manner (Park et al., 2022). Z. bungeanum seed oil promoted apoptosis in the laryngeal cancer cell line by inducing autophagy and inhibiting the expression and phosphorylation of PI3K/AKT/mTOR proteins (Bai et al., 2021). Z. bungeanum fruit, bark, and leaf extracts, as well as saponins, may exert cytotoxic effects on breast cancer cells through a mechanism involving apoptosis (Vyry Wouatsa et al., 2013; Alam et al., 2017). The decreased expression of IL1β, TGFβ1, and VEGFR1 promotes apoptosis in breast cancer cells (Simanullang et al., 2022).
An alkaloid from Z. bungeanum, nitidine chloride, promoted apoptosis in renal cancer cells by inhibiting their proliferation in an effective, time- and dose-dependent manner, thereby inhibiting the growth of renal cancer cells. Moreover, it decreased phosphorylation of AKT and ERK, upregulated BAX, P53, cleavage caspase-3, and cleavage PARP while downregulating the expression of Bcl-2, Caspase-3, and PARP (Fang et al., 2014).
In Huh7 hepatocellular cancer cells, ailanthoidol, which was extracted from the bark of Z. bungeanum, increased Bax expression and decreased Bcl-xL/Bcl-2 expression. Furthermore, it reduced the expression of mutant P53 protein, thereby preventing STAT3 activation and promoting apoptosis (Tseng et al., 2022). The specific mechanisms by which Z. bungeanum and its components promote apoptosis are depicted in Figure 5.
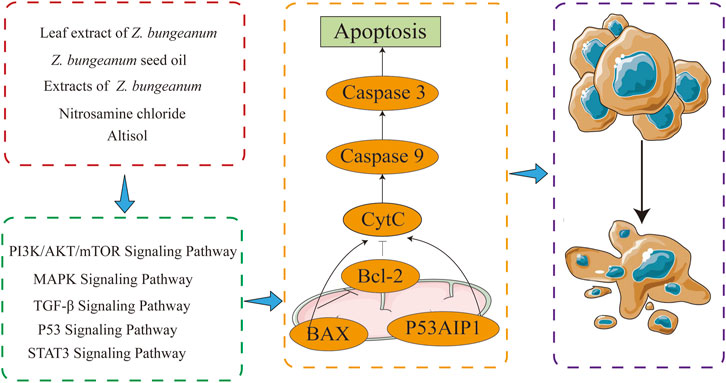
Figure 5. Z. bungeanum and its components inhibit cell proliferation by facilitating cell apoptosis through multiple pathways.
Cancer cells invade local tissues and spread to distant sites through invasion and migration, which is the main cause of tumor recurrence. The matrix metalloproteinase family, including MMP1, MMP2, and MMP9, causes cancer cell invasion and migration during cancer progression (Zucker et al., 1994). EMT is a key step in the infiltration and metastasis of tumor cells and is an important marker of malignant tumor progression. Tumor invasion and metastasis are crucial for EMT (Lo and Zhang, 2018).
Xanthotoxol from Z. bungeanum arrests the cell cycle and induces apoptosis and EMT-related genes in NSCLC cells by downregulating PI3K-AKT signaling to inhibit migration and invasion (Lin et al., 2022). Z. bungeanum extract enhances GSK-3β and attenuates β-catenin via PP2A, inhibiting the metastasis of HA22T cells and hepatocytes in vivo (Dung et al., 2012; Wu et al., 2017). Nitidine chloride, a constituent of Z. bungeanum, inhibited EMT and reduced the invasiveness of osteosarcoma cells through the AKT/GSK-3 β/Snail signaling pathway (Cheng et al., 2016). MMP-9 and GLUT-1 enzymes are involved in tumor cell invasion, metastasis, and angiogenesis. Z. bungeanum extract can inhibit the metastasis of cervical cancer by reducing the expression of MMP-9 and GLUT-1 in serum and tissues while elevating the expression of Myc and reducing the expression of Wee1 (Ilyas et al., 2022). In addition, quercetin, a component of Z. bungeanum, can inhibit the metastasis of pancreatic ductal adenocarcinoma through TGF-β1/Smad2/3 signaling and promote EMT (Guo et al., 2021). The mechanism of inhibition of tumor metastasis by Z. bungeanum and its components is depicted in Figure 6.
One of the causes of tumor recurrence is the development of resistance in tumor cells. Similar to antibacterial drugs, chemotherapeutic drugs selectively kill non-resistant cancer cells, while the resistant cancer cells frequently reappear, leading to tumor recurrence (Vasan et al., 2019). Consequently, improving the sensitivity of the tumors to drugs is crucial.
In cervical cancer, Z. bungeanum leaf extract increased the susceptibility of HeLa cells to cisplatin and other chemotherapeutic drugs by activating the MAPK signaling pathway, which can be used in conjunction with chemotherapeutic drugs to treat cervical cancer (Singh et al., 2015). Nitdumpeptins A and B, cyclic hexapeptides isolated from Z. bungeanum, exhibited synergistic antiproliferative effects when combined with gefitinib in gefitinib-resistant NSCLC cells. This combination increases cellular sensitivity to drugs, potentially by suppressing YAP expression in drug-resistant cells (Qin et al., 2021). Z. bungeanum contains hyperin, which enhanced the sensitivity of colon cancer cell line HCT8/VCR to vincristine by downregulating P-glycoprotein and inhibiting TLR4 signaling. Besides, hyperin increased the susceptibility of breast cancer cells to paclitaxel (Wang et al., 2018; Sun T. et al., 2020). In conclusion, these results demonstrate that Z. bungeanum and its active components could enhance the sensitivity to chemotherapeutic drugs (Figure 7).
In cancer cell lines, including HepG2, DLD-1, and Caco-2, Z. bungeanum fruit extract increased LC3-II expression, leading to significant autophagy-like cytosolic vesiculation, inhibition of cell division, and, ultimately, induction of cell death (Nozaki et al., 2016). Angoline, extracted from Z. bungeanum, was identified as a novel inhibitor of the STAT3 pathway, an important pathway for cancer therapy. Angoline inhibited STAT3 phosphorylation and target gene expression. The identification of small molecules targeting the STAT3 signaling pathway is crucial for the development of novel anticancer therapies (Liu et al., 2014). D-limonene, a key volatile oil constituent of Z. bungeanum, can modulate inflammation, oxidative stress, and MAPK pathways, thereby inhibiting skin tumorigenesis in mice (Chaudhary et al., 2012).
Therefore, we concluded that the antitumor mechanisms of Z. bungeanum are complex and diverse; the specific mechanisms are depicted in Figure 8.
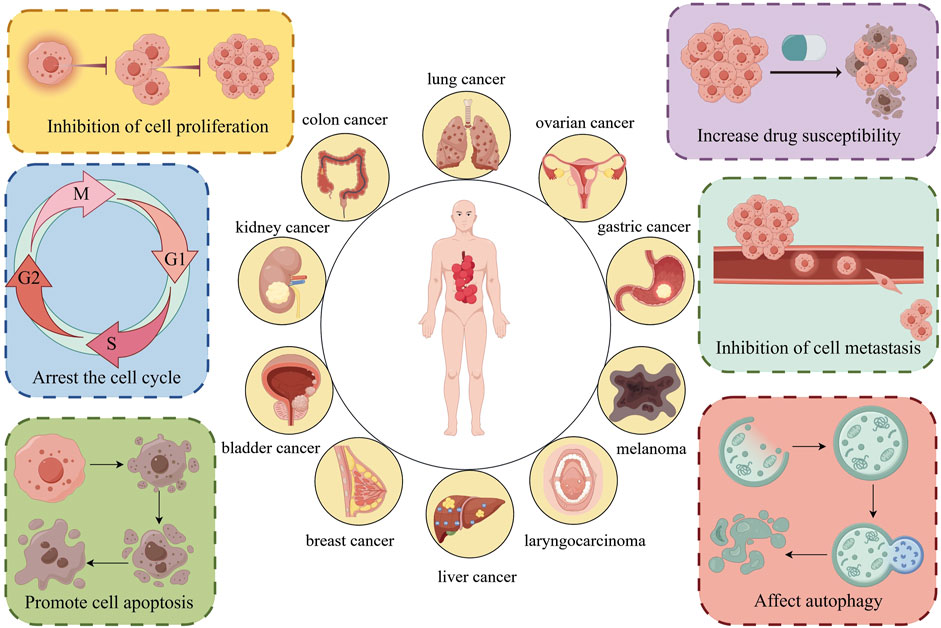
Figure 8. Z. bungeanum and its components treat various tumors through multiple mechanisms, providing a basis for their use in tumor therapy. The figure was drawn using Figdraw, http://www.figdraw.com/.
We discovered that the antitumor function of Z. bungeanum may be achieved through multiple targets and signaling pathways, thereby affecting the mechanisms of tumor cell proliferation, cycle, apoptosis, and metastasis. Studies have demonstrated that the antitumor function of Z. bungeanum may be achieved through PI3K/AKT, P53, WNT, STAT3, MAPK, and other signaling pathways. These signaling pathways are closely associated with tumor growth. Table 1 illustrates the precise mechanisms by which Z. bungeanum functions as an antitumor agent.
Z. bungeanum has been part of the Chinese Pharmacopoeia since 1977. It is included in over 30 types of prescription medications used to treat numerous illnesses, including dermatitis, dyspepsia, vomiting, diarrhea, and abdominal discomfort. Dajianzhong decoction and Wumei pill are the most widely used. In modern research, numerous Chinese herbal compound prescriptions containing Z. bungeanum have been demonstrated to be effective in treating tumors.
Dajianzhong decoction can inhibit gastric cancer proliferation and metastasis by regulating MMP-9 expression by modulating the ERK1/2 signaling pathway (He et al., 2017). Wumei pill suppressed lung cancer progression by inhibiting the HGF/C-Met signaling pathway (Yu et al., 2022). Moreover, it significantly inhibited the proliferation, migration, and invasion of pancreatic cancer and induced apoptosis by inhibiting the PI3K/AKT signaling pathway (Wang and Zhang, 2022). Furthermore, Wumei pill inhibited the development of gastric cancer and precancerous lesions and significantly downregulated the expression of c-myc and surviving genes (Li et al., 2010). Xingma Biejia decoction can improve the survival status and survival time of mice with acute myeloid leukemia, as well as the histopathological damage of the liver, spleen, and bone marrow. This could be due to controlling the rate at which mitochondria divide and triggering cellular autophagy (Si et al., 2023).
In conclusion, it has been proven that the compound formula containing Z. bungeanum can treat certain tumors, in which Z. bungeanum plays an indispensable role. Plasma and urine analyses of people who had taken Dajianzhong decoction revealed that hydroxy-α-sanshool and hydroxy-β-sanshool were present in plasma, with maximum values of these two compounds appearing at 0.5 h after drug administration. Additionally, glucuronic acid conjugates of these two chemical compounds were detected in the urine, confirming the role of Z. bungeanum in antitumor therapy (Iwabu et al., 2010; Jiang et al., 2023).
Tumor development is a long process, and timely intervention before cancer onset can significantly reduce tumor occurrence. Early intervention can effectively lower the cancer risk, facilitate preventive measures, and promote overall health maintenance. Such strategies play a crucial role in reducing the incidence of tumors and mortality rates (Vineis and Wild, 2014).
In terms of liver protection, Z. bungeanum alkaloids improved liver and kidney function markers in olive oil-induced liver cancer (Acheampong et al., 2021). Glycoproteins isolated from Z. bungeanum fruits can act as potent hepatoprotective agents via the antioxidant pathway (Lee and Lim, 2008). Z. bungeanum and its components improve non-alcoholic fatty liver disease by regulating fatty acid and cholesterol metabolism, intestinal flora, and activating the AMPK/Nrf2 signaling pathway (Huang et al., 2023; Peng et al., 2024). These studies indicate that Z. bungeanum might be effective in preventing liver cancer.
The gastroprotective function of Z. bungeanum has been confirmed by several studies. Unsaturated fatty acid amides isolated from Z. bungeanum pericarp can relax the circular muscles of isolated guinea pig stomachs and contract the longitudinal muscles of the ileum and distal colon, thereby regulating gastrointestinal motility (Hashimoto et al., 2001). Z. bungeanum stem bark extract has also demonstrated significant gastroprotective effects (Freitas et al., 2011). Z. bungeanum pericarp extract increased the body weight and decreased gastric lesions in rats. Z. bungeanum and its components play a role in tumor prevention by protecting the liver and stomach (Figure 9).
The uncontrolled proliferation and metastasis of tumors make their treatment difficult, while resistance to chemotherapeutic drugs and the off-target effects on normal cells hinder their complete eradication. This study highlighted the substantial role of medicinal and edible plants in cancer therapy by increasing the sensitivity of tumor cells to chemotherapy with minimal toxicity to normal cells. Therefore, medicinal and edible plants can be used as treatments for cancer or as adjuvant therapies to conventional treatments, helping alleviate the burden on cancer patients.
The rind of Z. bungeanum possesses a strong hemp flavor and serves as an important seasoning, primarily due to its high volatile oil content, making it a valuable raw material for spices and flavoring agents. Additionally, Z. bungeanum leaves can be used in stir-fries, cold dishes, and tea preparations. Recently, Z. bungeanum has been increasingly used in healthcare products, including foot bath packs, foot patches, and teas, where it has demonstrated antibacterial, insecticidal, analgesic, and cold-repelling properties. Additionally, Z. bungeanum is commonly used in pest control for the storage of archives, food, clothing, and Chinese medicinal tablets. The versatility of this plant in both medicinal and culinary applications highlights its significance across multiple domains.
This review provides a systematic account of the antitumor properties of Z. bungeanum, providing solid theoretical support for researchers investigating its anticancer potential. The antitumor properties of Z. bungeanum were thoroughly explored and analyzed using network pharmacology. Previous studies have confirmed that Z. bungeanum extract exhibits therapeutic effects on various tumors, including hepatocellular, gastric, and lung carcinomas. These effects are mediated through the modulation of multiple signaling pathways, including P53, WNT, and PI3K/AKT, leading to the inhibition of cell proliferation, suppression of cell migration and invasion, and enhancement of the sensitivity to chemotherapeutic agents, among other multifaceted mechanisms.
Avicularin, a flavonoid component of Z. bungeanum peel, can inhibit ferroptosis and improve cognitive impairment in Alzheimer’s disease by regulating the NOX4/Nrf2 axis (Li et al., 2024). WGX50 from Z. bungeanum alleviated doxorubicin-induced cardiotoxicity by inhibiting mitochondrial ROS and ferroptosis (Tai et al., 2023), demonstrating that the components of Z. bungeanum play a regulatory role in ferroptosis. Moreover, ethyl acetate extract of Z. bungeanum can improve cognitive dysfunction in aged mice by inhibiting NLRP3 inflammasome activation and pyroptosis (Zhao M. et al., 2021). Compounds isolated from Z. bungeanum inhibited LPS-induced nitric oxide production in RAW264.7 cells (Liu et al., 2023), indicating that Z. bungeanum affects immune function. Z. bungeanum polysaccharides also exhibit antioxidant activity (Liu et al., 2024). However, there are no relevant reports on the effect of Z. bungeanum on tumor growth by promoting ferroptosis and pyroptosis. More research is required to elucidate the role of Z. bungeanum in tumor treatment. The antitumor properties of Z. bungeanum have not been comprehensively investigated, and there is still a partial lack of understanding of the mechanism, thereby requiring in-depth research to investigate the antitumor potential of Z. bungeanum thoroughly.
The anticancer potential of Z. bungeanum has yet to be conclusively established through extensive rigorously designed clinical trials. While network pharmacology, as well as in vivo and ex vivo studies, have demonstrated its potential as an adjuvant therapy in cancer treatment, further validation through clinical trial data is necessary due to the complexity of integrating traditional Chinese medicine into cancer therapy. We anticipate that Z. bungeanum and its active ingredients can be applied in clinical practice to treat tumors, offering new therapeutic options for cancer patients. Our research aimed to take advantage of the low toxicity and high efficacy of Z. bungeanum to address the current challenges in cancer therapy and alleviate treatment-related side effects in patients.
YD: Writing–original draft, Investigation, Writing–review and editing. SD: Investigation, Writing–review and editing. YY: Data curation, Writing–review and editing. JT: Writing–review and editing, Data curation. SH: Investigation, Writing–review and editing. YN: Formal Analysis, Writing–review and editing. ZZ: Validation, Writing–review and editing. LY: Conceptualization, Funding acquisition, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Ningxia Key Research and Development Program (No. 2023BEG02015); Ningxia Natural Science Foundation (No. 2022AAC02039); National Natural Science Foundation of China (No. 82374261); Talent Development Projects of Young Qihuang of National Administration of Traditional Chinese Medicine (2020, 2022).
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acheampong, D. O., Baffour, I. K., Atsu Barku, V. Y., Addo, J. K., Essuman, M. A., and Boye, A. (2021). Zanthoxylum zanthoxyloides alkaloidal extract improves CCl(4)-induced hepatocellular carcinoma-like phenotypes in rats. Evid. Based Complement. Altern. Med. 2021, 3804379. doi:10.1155/2021/3804379
Alam, F., Din, K. M., Rasheed, R., Sadiq, A., Jan, M. S., Minhas, A. M., et al. (2020). Phytochemical investigation, anti-inflammatory, antipyretic and antinociceptive activities of Zanthoxylum armatum DC extracts-in vivo and in vitro experiments. Heliyon 6 (11), e05571. doi:10.1016/j.heliyon.2020.e05571
Alam, F., Najum Us Saqib, Q., and Waheed, A. (2017). Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 17 (1), 368. doi:10.1186/s12906-017-1882-1
Alipanah, H., Farjam, M., Zarenezhad, E., Roozitalab, G., and Osanloo, M. (2021). Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. Ther. 21 (1), 186. doi:10.1186/s12906-021-03362-7
Awad, A. B., Chinnam, M., Fink, C. S., and Bradford, P. G. (2007). beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 14 (11), 747–754. doi:10.1016/j.phymed.2007.01.003
Bae, H., Park, S., Ham, J., Song, J., Hong, T., Choi, J. H., et al. (2021). ER-mitochondria calcium flux by β-sitosterol promotes cell death in ovarian cancer. Antioxidants (Basel) 10 (10), 1583. doi:10.3390/antiox10101583
Bai, Y., Hou, J., Zhang, X. T., Gao, J. P., and Zhou, J. T. (2021). Zanthoxylum bungeanum seed oil elicits autophagy and apoptosis in human laryngeal tumor cells via PI3K/AKT/mTOR signaling pathway. Anticancer Agents Med. Chem. 21 (18), 2610–2619. doi:10.2174/1871520621666210401103820
Baskar, A. A., Ignacimuthu, S., Paulraj, G. M., and Al Numair, K. S. (2010). Chemopreventive potential of beta-sitosterol in experimental colon cancer model--an in vitro and in vivo study. BMC Complement. Altern. Med. 10, 24. doi:10.1186/1472-6882-10-24
Berliocchi, L., Chiappini, C., Adornetto, A., Gentile, D., Cerri, S., Russo, R., et al. (2018). Early LC3 lipidation induced by d-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 40, 98–105. doi:10.1016/j.phymed.2018.01.005
Bishayee, K., Ghosh, S., Mukherjee, A., Sadhukhan, R., Mondal, J., and Khuda-Bukhsh, A. R. (2013). Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: signal cascade and drug-DNA interaction. Cell Prolif. 46 (2), 153–163. doi:10.1111/cpr.12017
Cai, J. P., Wu, Y. J., Li, C., Feng, M. Y., Shi, Q. T., Li, R., et al. (2013). Panax ginseng polysaccharide suppresses metastasis via modulating Twist expression in gastric cancer. Int. J. Biol. Macromol. 57, 22–25. doi:10.1016/j.ijbiomac.2013.03.010
Chai, T., and Qiang, Y. (2022). Two new coumarins from branches of Zanthoxylum schinifolium. J. Asian Nat. Prod. Res. 24 (9), 820–826. doi:10.1080/10286020.2021.1992391
Chang, J. H., Lai, S. L., Chen, W. S., Hung, W. Y., Chow, J. M., Hsiao, M., et al. (2017). Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. Biochim. Biophys. Acta Mol. Cell Res. 1864 (10), 1746–1758. doi:10.1016/j.bbamcr.2017.06.017
Chaudhary, S. C., Siddiqui, M. S., Athar, M., and Alam, M. S. (2012). D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 31 (8), 798–811. doi:10.1177/0960327111434948
Chen, C., Wang, Y. S., Zhang, E. T., Li, G. A., Liu, W. Y., Li, Y., et al. (2021). (20S) ginsenoside Rh2 exerts its anti-tumor effect by disrupting the HSP90a-cdc37 system in human liver cancer cells. Int. J. Mol. Sci. 22 (23), 13170. doi:10.3390/ijms222313170
Chen, H., Li, S., Wang, S., Li, W., Bao, N., and Ai, W. (2018). The inhibitory effect of kokusaginine on the growth of human breast cancer cells and MDR-resistant cells is mediated by the inhibition of tubulin assembly. Bioorg Med. Chem. Lett. 28 (14), 2490–2492. doi:10.1016/j.bmcl.2018.05.059
Cheng, Z., Guo, Y., Yang, Y., Kan, J., Dai, S., Helian, M., et al. (2016). Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3β/Snail signaling pathway. Oncol. Rep. 36 (2), 1023–1029. doi:10.3892/or.2016.4846
Choi, J., Lee, D. H., Park, S. Y., and Seol, J. W. (2019). Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed. Pharmacother. 117, 109091. doi:10.1016/j.biopha.2019.109091
Chou, S. T., Chan, H. H., Peng, H. Y., Liou, M. J., and Wu, T. S. (2011). Isolation of substances with antiproliferative and apoptosis-inducing activities against leukemia cells from the leaves of Zanthoxylum ailanthoides Sieb. Zucc. Phytomedicine 18 (5), 344–348. doi:10.1016/j.phymed.2010.08.018
da Silva, S. L., Figueiredo, P. M., and Yano, T. (2007). Chemotherapeutic potential of the volatile oils from Zanthoxylum rhoifolium Lam leaves. Eur. J. Pharmacol. 576 (1-3), 180–188. doi:10.1016/j.ejphar.2007.07.065
Dung, T. D., Chang, H. C., Binh, T. V., Lee, M. R., Tsai, C. H., Tsai, F. J., et al. (2012). Zanthoxylum avicennae extracts inhibit cell proliferation through protein phosphatase 2A activation in HA22T human hepatocellular carcinoma cells in vitro and in vivo. Int. J. Mol. Med. 29 (6), 1045–1052. doi:10.3892/ijmm.2012.938
Fang, Z., Tang, Y., Jiao, W., Xing, Z., Guo, Z., Wang, W., et al. (2014). Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem. Toxicol. 66, 210–216. doi:10.1016/j.fct.2014.01.049
Freitas, F. F., Fernandes, H. B., Piauilino, C. A., Pereira, S. S., Carvalho, K. I., Chaves, M. H., et al. (2011). Gastroprotective activity of Zanthoxylum rhoifolium Lam. in animal models. J. Ethnopharmacol. 137 (1), 700–708. doi:10.1016/j.jep.2011.06.026
Fu, Y. H., Guo, J. M., Xie, Y. T., Hua, J., Dai, Y. Y., Zhang, W., et al. (2020). Structural characterization, antiproliferative and anti-inflammatory activities of alkaloids from the roots of Zanthoxylum austrosinense. Bioorg Chem. 102, 104101. doi:10.1016/j.bioorg.2020.104101
Goodman, C. D., Hoang, A. T., Diallo, D., Malterud, K. E., McFadden, G. I., and Wangensteen, H. (2019). Anti-plasmodial effects of Zanthoxylum zanthoxyloides. Planta Med. 85 (13), 1073–1079. doi:10.1055/a-0973-0067
Guo, Y., Tong, Y., Zhu, H., Xiao, Y., Guo, H., Shang, L., et al. (2021). Quercetin suppresses pancreatic ductal adenocarcinoma progression via inhibition of SHH and TGF-β/Smad signaling pathways. Cell Biol. Toxicol. 37 (3), 479–496. doi:10.1007/s10565-020-09562-0
Hafezi, S., and Rahmani, M. (2021). Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers (Basel) 13 (6), 1292. doi:10.3390/cancers13061292
Hashimoto, K., Satoh, K., Kase, Y., Ishige, A., Kubo, M., Sasaki, H., et al. (2001). Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med. 67 (2), 179–181. doi:10.1055/s-2001-11513
He, H., Wang, J., Yang, Y., Kang, J., and Gong, X. (2017). Djianzhong decoction regulates the expression of MMP-9 in gastric cancer animal model with spleen-yang deficiency through ERK1/2 signaling pathway. Chin. Natl. Folk Med. 26 (22), 24–27.
Hu, Y., Chen, X., Hu, M., Zhang, D., Yuan, S., Li, P., et al. (2022). Medicinal and edible plants in the treatment of dyslipidemia: advances and prospects. Chin. Med. 17 (1), 113. doi:10.1186/s13020-022-00666-9
Hu, Z., Cai, B., Wang, M., Wen, X., Geng, A., Hu, X., et al. (2020). Diosmetin enhances the sensitivity of radiotherapy by suppressing homologous recombination in endometrial cancer. Cell Cycle 19 (22), 3115–3126. doi:10.1080/15384101.2020.1831257
Huang, X., Yuan, Z., Liu, X., Wang, Z., Lu, J., Wu, L., et al. (2023). Integrative multi-omics unravels the amelioration effects of Zanthoxylum bungeanum Maxim. on non-alcoholic fatty liver disease. Phytomedicine 109, 154576. doi:10.1016/j.phymed.2022.154576
Hydbring, P., Malumbres, M., and Sicinski, P. (2016). Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 17 (5), 280–292. doi:10.1038/nrm.2016.27
Ilyas, S., Simanullang, R. H., Hutahaean, S., Rosidah, R., and Situmorang, P. C. (2022). Correlation of myc expression with Wee1 expression by Zanthoxylum acanthopodium in cervical carcinoma histology. Pak J. Biol. Sci. 25 (11), 1014–1020. doi:10.3923/pjbs.2022.1014.1020
Imani, A., Maleki, N., Bohlouli, S., Kouhsoltani, M., Sharifi, S., and Maleki Dizaj, S. (2021). Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 35 (5), 2500–2513. doi:10.1002/ptr.6977
Iwabu, J., Watanabe, J., Hirakura, K., Ozaki, Y., and Hanazaki, K. (2010). Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab. Dispos. 38 (11), 2040–2048. doi:10.1124/dmd.110.033589
Iwasaki, K., Zheng, Y. W., Murata, S., Ito, H., Nakayama, K., Kurokawa, T., et al. (2016). Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 22 (44), 9765–9774. doi:10.3748/wjg.v22.i44.9765
Ji, K. L., Liu, W., Yin, W. H., Li, J. Y., and Yue, J. M. (2022). Quinoline alkaloids with anti-inflammatory activity from Zanthoxylum avicennae. Org. Biomol. Chem. 20 (20), 4176–4182. doi:10.1039/d2ob00711h
Jiang, M., Peng, W., Wu, C., and Wang, J. (2023). Pharmacochemistry of Dajianzhong decoction based on UPLC-Q-TOF/MS technology. Chin. Herb. Med. 54 (16), 5154–5164.
Kong, Y., Sun, W., and Wu, P. (2020). Hyperoside exerts potent anticancer activity in skin cancer. Front. Biosci. Landmark Ed. 25 (3), 463–479. doi:10.2741/4814
Koosha, S., Mohamed, Z., Sinniah, A., and Alshawsh, M. A. (2019). Investigation into the molecular mechanisms underlying the anti-proliferative and anti-tumorigenesis activities of diosmetin against HCT-116 human colorectal cancer. Sci. Rep. 9 (1), 5148. doi:10.1038/s41598-019-41685-1
Kopeina, G. S., Prokhorova, E. A., Lavrik, I. N., and Zhivotovsky, B. (2018). Alterations in the nucleocytoplasmic transport in apoptosis: caspases lead the way. Cell Prolif. 51 (5), e12467. doi:10.1111/cpr.12467
Lee, S. J., and Lim, K. T. (2008). Glycoprotein of Zanthoxylum piperitum DC has a hepatoprotective effect via anti-oxidative character in vivo and in vitro. Toxicol Vitro 22 (2), 376–385. doi:10.1016/j.tiv.2007.10.002
Li, J. P., Liao, X. H., Xiang, Y., Yao, A., Song, R. H., Zhang, Z. J., et al. (2018). Hyperoside and let-7a-5p synergistically inhibits lung cancer cell proliferation via inducing G1/S phase arrest. Gene 679, 232–240. doi:10.1016/j.gene.2018.09.011
Li, R., Li, Q., and Ji, Q. (2020). Molecular targeted study in tumors: from western medicine to active ingredients of traditional Chinese medicine. Biomed. Pharmacother. 121, 109624. doi:10.1016/j.biopha.2019.109624
Li, Y., Huang, L., Yang, X., and Ye, Z. (2010). Effects of Wumei pill on the expression of c-myc and survivin in gastric cancer and precancerous lesions. Sci. Technol. Chin. Med. 17 (05), 385–386+429+376.
Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M. T., Wang, S., et al. (2016). Quercetin, inflammation and immunity. Nutrients 8 (3), 167. doi:10.3390/nu8030167
Li, Z., Lu, Y., Zhen, Y., Jin, W., Ma, X., Yuan, Z., et al. (2024). Avicularin inhibits ferroptosis and improves cognitive impairments in Alzheimer's disease by modulating the NOX4/Nrf2 axis. Phytomedicine 135, 156209. doi:10.1016/j.phymed.2024.156209
Lin, Q., Pu, H., Guan, H., Ma, C., Zhang, Y., Ding, W., et al. (2020). Rapid identification and pharmacokinetic studies of multiple active alkaloids in rat plasma through UPLC-Q-TOF-MS and UPLC-MS/MS after the oral administration of Zanthoxylum nitidum extract. J. Pharm. Biomed. Anal. 186, 113232. doi:10.1016/j.jpba.2020.113232
Lin, X., Liu, J., Zou, Y., Tao, C., and Chen, J. (2022). Xanthotoxol suppresses non-small cell lung cancer progression and might improve patients' prognosis. Phytomedicine 105, 154364. doi:10.1016/j.phymed.2022.154364
Liu, J., Zhang, Q., Ye, Y., Li, W., Qiu, J., Liu, J., et al. (2014). Angoline: a selective IL-6/STAT3 signaling pathway inhibitor isolated from Zanthoxylum nitidum. Phytomedicine 21 (8-9), 1088–1091. doi:10.1016/j.phymed.2014.04.001
Liu, J. Y., Wang, Y. F., Dai, Y. Q., Huang, S., Xie, J., Chen, L., et al. (2023). Compounds isolated from the pericarp of Zanthoxylum bungeanum and inhibitory activity against LPS-induced NO production in RAW264.7. J. Asian Nat. Prod. Res. 25 (10), 1012–1020. doi:10.1080/10286020.2023.2188203
Liu, Y., Kang, L., Shi, S. M., Li, B. J., Zhang, Y., Zhang, X. Z., et al. (2022). Skimmianine as a novel therapeutic agent suppresses proliferation and migration of human esophageal squamous cell carcinoma via blocking the activation of ERK1/2. Neoplasma 69 (3), 571–582. doi:10.4149/neo_2022_211118N1640
Liu, Z., Ye, J., Zhang, R., Li, Y., Guan, F., Zhang, T., et al. (2024). Fractionation and antioxidation activities of polysaccharides from Zanthoxylum bungeanum Maxim. Food Chem. 439, 138050. doi:10.1016/j.foodchem.2023.138050
Lo, H. C., and Zhang, X. H. (2018). EMT in metastasis: finding the right balance. Dev. Cell 45 (6), 663–665. doi:10.1016/j.devcel.2018.05.033
Long, Y., Meng, Y., and Jiang, X. (2022). Antitumor activity of total alkaloids from rhizome of Zanthoxylum bungeanum. Chin. J. Pharm. 31 (04), 63–66.
Lu, Q., Li, R., Yang, Y., Zhang, Y., Zhao, Q., and Li, J. (2022). Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chem. 368, 130610. doi:10.1016/j.foodchem.2021.130610
Lu, X. G., Zhan, L. B., Feng, B. A., Qu, M. Y., Yu, L. H., and Xie, J. H. (2004). Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J. Gastroenterol. 10 (14), 2140–2144. doi:10.3748/wjg.v10.i14.2140
Luo, J., Ke, J., Hou, X., Li, S., Luo, Q., Wu, H., et al. (2022). Composition, structure and flavor mechanism of numbing substances in Chinese prickly ash in the genus Zanthoxylum: a review. Food Chem. 373 (Pt B), 131454. doi:10.1016/j.foodchem.2021.131454
Ma, A., and Zhang, R. (2020). Diosmetin inhibits cell proliferation, induces cell apoptosis and cell cycle arrest in liver cancer. Cancer Manag. Res. 12, 3537–3546. doi:10.2147/cmar.S240064
Ma, A., Zou, F., Zhang, R., and Zhao, X. (2022). The effects and underlying mechanisms of medicine and food homologous flowers on the prevention and treatment of related diseases. J. Food Biochem. 46 (12), e14430. doi:10.1111/jfbc.14430
Mandal, D., Sahu, B. R., and Parija, T. (2023). Combination of tamoxifen and D-limonene enhances therapeutic efficacy in breast cancer cells. Med. Oncol. 40 (8), 216. doi:10.1007/s12032-023-02081-y
Mbaveng, A. T., Damen, F., Çelik, İ., Tane, P., Kuete, V., and Efferth, T. (2019). Cytotoxicity of the crude extract and constituents of the bark of Fagara tessmannii towards multi-factorial drug resistant cancer cells. J. Ethnopharmacol. 235, 28–37. doi:10.1016/j.jep.2019.01.031
Meng, X. H., Chai, T., Shi, Y. P., and Yang, J. L. (2020). Bungsteroid A: one unusual C(34) pentacyclic steroid analogue from Zanthoxylum bungeanum Maxim. J. Org. Chem. 85 (16), 10806–10812. doi:10.1021/acs.joc.0c01312
Mirazimi, S. M. A., Dashti, F., Tobeiha, M., Shahini, A., Jafari, R., Khoddami, M., et al. (2022). Application of quercetin in the treatment of gastrointestinal cancers. Front. Pharmacol. 13, 860209. doi:10.3389/fphar.2022.860209
Moon, D. O., Lee, K. J., Choi, Y. H., and Kim, G. Y. (2007). Beta-sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells. Int. Immunopharmacol. 7 (8), 1044–1053. doi:10.1016/j.intimp.2007.03.010
Mukhija, M., Lal Dhar, K., and Nath Kalia, A. (2014). Bioactive Lignans from Zanthoxylum alatum Roxb. stem bark with cytotoxic potential. J. Ethnopharmacol. 152 (1), 106–112. doi:10.1016/j.jep.2013.12.039
Mun, E. J., Babiker, H. M., Weinberg, U., Kirson, E. D., and Von Hoff, D. D. (2018). Tumor-treating fields: a fourth modality in cancer treatment. Clin. Cancer Res. 24 (2), 266–275. doi:10.1158/1078-0432.Ccr-17-1117
Mustapha, N., Mokdad-Bzéouich, I., Maatouk, M., Ghedira, K., Hennebelle, T., and Chekir-Ghedira, L. (2016). Antitumoral, antioxidant, and antimelanogenesis potencies of Hawthorn, a potential natural agent in the treatment of melanoma. Melanoma Res. 26 (3), 211–222. doi:10.1097/cmr.0000000000000240
Nozaki, R., Kono, T., Bochimoto, H., Watanabe, T., Oketani, K., Sakamaki, Y., et al. (2016). Zanthoxylum fruit extract from Japanese pepper promotes autophagic cell death in cancer cells. Oncotarget 7 (43), 70437–70446. doi:10.18632/oncotarget.11926
Pang, W., Liu, S., He, F., Li, X., Saira, B., Zheng, T., et al. (2019). Anticancer activities of Zanthoxylum bungeanum seed oil on malignant melanoma. J. Ethnopharmacol. 229, 180–189. doi:10.1016/j.jep.2018.10.012
Park, C., Choi, E. O., Hwangbo, H., Lee, H., Jeong, J. W., Han, M. H., et al. (2022). Induction of apoptotic cell death in human bladder cancer cells by ethanol extract of Zanthoxylum schinifolium leaf, through ROS-dependent inactivation of the PI3K/Akt signaling pathway. Nutr. Res. Pract. 16 (3), 330–343. doi:10.4162/nrp.2022.16.3.330
Patel, R. V., Mistry, B. M., Shinde, S. K., Syed, R., Singh, V., and Shin, H. S. (2018). Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 155, 889–904. doi:10.1016/j.ejmech.2018.06.053
Peng, W., He, C. X., Li, R. L., Qian, D., Wang, L. Y., Chen, W. W., et al. (2024). Zanthoxylum bungeanum amides ameliorates nonalcoholic fatty liver via regulating gut microbiota and activating AMPK/Nrf2 signaling. J. Ethnopharmacol. 318 (Pt A), 116848. doi:10.1016/j.jep.2023.116848
Ping, M. H. (2020). Hyperin controls the development and therapy of gastric cancer via regulating wnt/β-catenin signaling. Cancer Manag. Res. 12, 11773–11782. doi:10.2147/cmar.S270544
Qi, J., Pan, Z., Wang, X., Zhang, N., He, G., and Jiang, X. (2024). Research advances of Zanthoxylum bungeanum Maxim. polyphenols in inflammatory diseases. Front. Immunol. 15, 1305886. doi:10.3389/fimmu.2024.1305886
Qin, F., Li, M. S., Li, J. J., Wang, Y., Wang, K., Zhou, J. Y., et al. (2022). Four new sesquiterpenoids from Zanthoxylum nitidum. Chem. Biodivers. 19 (7), e202200449. doi:10.1002/cbdv.202200449
Qin, F., Wang, C. Y., Kim, D., Wang, H. S., Zhu, Y. K., Lee, S. K., et al. (2021). Nitidumpeptins A and B, cyclohexapeptides isolated from Zanthoxylum nitidum var. tomentosum: structural elucidation, total synthesis, and antiproliferative activity in cancer cells. J. Org. Chem. 86 (2), 1462–1470. doi:10.1021/acs.joc.0c02057
Qu, S., Yu, S., Ma, X., and Wang, R. (2023). Medicine food homology plants promote periodontal health: antimicrobial, anti-inflammatory, and inhibition of bone resorption. Front. Nutr. 10, 1193289. doi:10.3389/fnut.2023.1193289
Ren, K. W., Li, Y. H., Wu, G., Ren, J. Z., Lu, H. B., Li, Z. M., et al. (2017). Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 50 (4), 1299–1311. doi:10.3892/ijo.2017.3886
Rodenak-Kladniew, B., Castro, A., Stärkel, P., De Saeger, C., García de Bravo, M., and Crespo, R. (2018). Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 199, 48–59. doi:10.1016/j.lfs.2018.03.006
Rong, R., Cui, M. Y., Zhang, Q. L., Zhang, M. Y., Yu, Y. M., Zhou, X. Y., et al. (2016). Anesthetic constituents of Zanthoxylum bungeanum Maxim.: a pharmacokinetic study. J. Sep. Sci. 39 (14), 2728–2735. doi:10.1002/jssc.201600295
Sahu, R., Kar, R. K., Sunita, P., Bose, P., Kumari, P., Bharti, S., et al. (2021). LC-MS characterized methanolic extract of zanthoxylum armatum possess anti-breast cancer activity through Nrf2-Keap1 pathway: an in-silico, in-vitro and in-vivo evaluation. J. Ethnopharmacol. 269, 113758. doi:10.1016/j.jep.2020.113758
Santhanam, R. K., Ahmad, S., Abas, F., Safinar Ismail, I., Rukayadi, Y., Tayyab Akhtar, M., et al. (2016). Bioactive constituents of Zanthoxylum rhetsa bark and its cytotoxic potential against B16-F10 melanoma cancer and normal human dermal fibroblast (HDF) cell lines. Molecules 21 (6), 652. doi:10.3390/molecules21060652
Sharma, J., and Goyal, P. K. (2015). Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J. Ginseng Res. 39 (3), 265–273. doi:10.1016/j.jgr.2015.01.005
Shen, Q. K., Liu, C. F., Zhang, H. J., Tian, Y. S., and Quan, Z. S. (2017). Design and synthesis of new triazoles linked to xanthotoxin for potent and highly selective anti-gastric cancer agents. Bioorg Med. Chem. Lett. 27 (21), 4871–4875. doi:10.1016/j.bmcl.2017.09.040
Si, Y., Cui, S., Wei, W., Yin, X., Li, Z., Ma, G., et al. (2023). To investigate the effects of Xingma Biejia decoction on mitochondrial fission and autophagy-related protein expression in bone marrow of mice with acute myeloid leukemia. J. Traditional Chin. Med. 64 (14), 1475–1482. doi:10.13288/j.11-2166/r.2023.14.012
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. doi:10.3322/caac.21763
Simanullang, R. H., Situmorang, P. C., Herlina, M., Silalahi, B., and Manurung, S. S. (2022). Histological changes of cervical tumours following Zanthoxylum acanthopodium DC treatment, and its impact on cytokine expression. Saudi J. Biol. Sci. 29 (4), 2706–2718. doi:10.1016/j.sjbs.2021.12.065
Singh, T. D., Meitei, H. T., Sharma, A. L., Robinson, A., Singh, L. S., and Singh, T. R. (2015). Anticancer properties and enhancement of therapeutic potential of cisplatin by leaf extract of Zanthoxylum armatum DC. Biol. Res. 48 (1), 46. doi:10.1186/s40659-015-0037-4
Song, C., Deng, S., Hu, H., Zheng, Z., Shen, B., Wu, X., et al. (2022). Diosmetin affects gene expression on human lung adenocarcinoma cells. J. Oncol. 2022, 5482148. doi:10.1155/2022/5482148
Sun, J., Sun, B., Ren, F., Chen, H., Zhang, N., and Zhang, Y. (2020a). Characterization of key odorants in hanyuan and hancheng fried pepper (Zanthoxylum bungeanum) oil. J. Agric. Food Chem. 68 (23), 6403–6411. doi:10.1021/acs.jafc.0c02026
Sun, T., Liu, Y., Li, M., Yu, H., and Piao, H. (2020b). Administration with hyperoside sensitizes breast cancer cells to paclitaxel by blocking the TLR4 signaling. Mol. Cell Probes 53, 101602. doi:10.1016/j.mcp.2020.101602
Tai, P., Chen, X., Jia, G., Chen, G., Gong, L., Cheng, Y., et al. (2023). WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 21 (1), 823. doi:10.1186/s12967-023-04715-1
Tseng, T. H., Wang, C. J., Lee, Y. J., Shao, Y. C., Shen, C. H., Lee, K. C., et al. (2022). Suppression of the proliferation of Huh7 hepatoma cells involving the downregulation of mutant p53 protein and inactivation of the STAT 3 pathway with ailanthoidol. Int. J. Mol. Sci. 23 (9), 5102. doi:10.3390/ijms23095102
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575 (7782), 299–309. doi:10.1038/s41586-019-1730-1
Vineis, P., and Wild, C. P. (2014). Global cancer patterns: causes and prevention. Lancet 383 (9916), 549–557. doi:10.1016/s0140-6736(13)62224-2
von Holtz, R. L., Fink, C. S., and Awad, A. B. (1998). beta-Sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 32 (1), 8–12. doi:10.1080/01635589809514709
Vyry Wouatsa, N. A., Misra, L. N., Venkatesh Kumar, R., Darokar, M. P., and Tchoumbougnang, F. (2013). Zantholic acid, a new monoterpenoid from Zanthoxylum zanthoxyloides. Nat. Prod. Res. 27 (21), 1994–1998. doi:10.1080/14786419.2013.811662
Wang, D., Bu, T., Li, Y., He, Y., Yang, F., and Zou, L. (2022). Pharmacological activity, pharmacokinetics, and clinical research progress of puerarin. Antioxidants (Basel) 11 (11), 2121. doi:10.3390/antiox11112121
Wang, F., Li, J., Li, R., Pan, G., Bai, M., and Huang, Q. (2017). Angelicin inhibits liver cancer growth in vitro and in vivo. Mol. Med. Rep. 16 (4), 5441–5449. doi:10.3892/mmr.2017.7219
Wang, L., Hao, H., Meng, X., Zhang, W., Zhang, Y., Chai, T., et al. (2024). A novel isoquinoline alkaloid HJ-69 isolated from Zanthoxylum bungeanum attenuates inflammatory pain by inhibiting voltage-gated sodium and potassium channels. J. Ethnopharmacol. 330, 118218. doi:10.1016/j.jep.2024.118218
Wang, L. M., Zhang, M. Y., Zhu, Q. S., Lu, C. F., and Bai, X. (2018). Hyperin enhances the sensitivity of HCT8/VCR colon cancer cell line to vincristine by down-regulating P-glycoprotein. Clin. Lab. 64 (3), 269–275. doi:10.7754/Clin.Lab.2017.170923
Wang, W., Pang, W., Yan, S., Zheng, X., Han, Q., Yao, Y., et al. (2023). Zanthoxylum bungeanum seed oil inhibits tumorigenesis of human melanoma A375 by regulating CDC25A/CyclinB1/CDK1 signaling pathways in vitro and in vivo. Front. Pharmacol. 14, 1165584. doi:10.3389/fphar.2023.1165584
Wang, X., and Zhang, Y. (2022). To investigate the effects of Wumei pill containing serum on the proliferation, invasion, migration and apoptosis of pancreatic cancer cells based on PI3K/Akt signaling pathway. Chin. J. Exp. Med. 28(06), 34–42. doi:10.13422/j.cnki.syfjx.20220529
Wang, Z. X., Ma, J., Li, X. Y., Wu, Y., Shi, H., Chen, Y., et al. (2021). Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 178 (5), 1133–1148. doi:10.1111/bph.15350
Wargovich, M. J. (2001). Colon cancer chemoprevention with ginseng and other botanicals. J. Korean Med. Sci. 16, S81–S86. doi:10.3346/jkms.2001.16.S.S81
Wu, H. C., Lay, I. S., Shibu, M. A., Ho, T. J., Cheng, S. M., Lin, C. H., et al. (2017). Zanthoxylum avicennae extract enhances GSK-3β to attenuate β-catenin via phosphatase 2A to block metastatic effects of HA22T cells and hepatocellular carcinoma xenografted nude mice. Environ. Toxicol. 32 (9), 2133–2143. doi:10.1002/tox.22426
Xiao, J., Liu, P., Hu, Y., Liu, T., Guo, Y., Sun, P., et al. (2023). Antiviral activities of Artemisia vulgaris L. extract against herpes simplex virus. Chin. Med. 18 (1), 21. doi:10.1186/s13020-023-00711-1
Yang, Q., Yu, D., and Zhang, Y. (2019). β-Sitosterol attenuates the intracranial aneurysm growth by suppressing TNF-α-mediated mechanism. Pharmacology 104 (5-6), 303–311. doi:10.1159/000502221
Yang, R., Pei, T., Huang, R., Xiao, Y., Yan, J., Zhu, J., et al. (2022). Platycodon grandiflorum triggers antitumor immunity by restricting PD-1 expression of CD8(+) T cells in local tumor microenvironment. Front. Pharmacol. 13, 774440. doi:10.3389/fphar.2022.774440
Yang, X. (2008). Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 56 (5), 1689–1696. doi:10.1021/jf0728101
Yang, Y., Ikezoe, T., Takeuchi, T., Adachi, Y., Ohtsuki, Y., Koeffler, H. P., et al. (2006). Zanthoxyli Fructus induces growth arrest and apoptosis of LNCaP human prostate cancer cells in vitro and in vivo in association with blockade of the AKT and AR signal pathways. Oncol. Rep. 15 (6), 1581–1590. doi:10.3892/or.15.6.1581
Yoo, H. S., Kim, J. M., Jo, E., Cho, C. K., Lee, S. Y., Kang, H. S., et al. (2017). Modified Panax ginseng extract regulates autophagy by AMPK signaling in A549 human lung cancer cells. Oncol. Rep. 37 (6), 3287–3296. doi:10.3892/or.2017.5590
Yu, X., Lin, H., Wang, Y., Lv, W., Zhang, S., Qian, Y., et al. (2018). d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 11, 1833–1847. doi:10.2147/ott.S155716
Yu, Y., Shangguan, X., and Li, X. (2022). To explore the anti-tumor effect and mechanism of Wumei pill on Lewis lung cancer mice with cold and heat syndrome based on HGF/C-Met signaling pathway. Chin. J. Exp. formulas Chin. Med. 28 (21), 32–41. doi:10.13422/j.cnki.syfjx.202201722
Zhang, F., and Luo, H. (2023). Diosmetin inhibits the growth and invasion of gastric cancer by interfering with M2 phenotype macrophage polarization. J. Biochem. Mol. Toxicol. 37 (10), e23431. doi:10.1002/jbt.23431
Zhang, Y., Dong, H., Zhang, J., and Zhang, L. (2017). Inhibitory effect of hyperoside isolated from Zanthoxylum bungeanum leaves on SW620 human colorectal cancer cells via induction of the p53 signaling pathway and apoptosis. Mol. Med. Rep. 16 (2), 1125–1132. doi:10.3892/mmr.2017.6710
Zhang, Y., Wang, D., Yang, L., Zhou, D., and Zhang, J. (2014). Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS One 9 (8), e105725. doi:10.1371/journal.pone.0105725
Zhao, F., Hong, X., Li, D., Wei, Z., Ci, X., and Zhang, S. (2021a). Diosmetin induces apoptosis in ovarian cancer cells by activating reactive oxygen species and inhibiting the Nrf2 pathway. Med. Oncol. 38 (5), 54. doi:10.1007/s12032-021-01501-1
Zhao, M., Dai, Y., Li, P., Wang, J., Ma, T., and Xu, S. (2021b). Inhibition of NLRP3 inflammasome activation and pyroptosis with the ethyl acetate fraction of Bungeanum ameliorated cognitive dysfunction in aged mice. Food Funct. 12 (21), 10443–10458. doi:10.1039/d1fo00876e
Zhao, Y., Cheng, X., Wang, G., Liao, Y., and Qing, C. (2020). Linalool inhibits 22Rv1 prostate cancer cell proliferation and induces apoptosis. Oncol. Lett. 20 (6), 289. doi:10.3892/ol.2020.12152
Zhaojun, C., Lulin, T., Xin, F., Abdel-Nasser, S., Zunguo, L., and Xiong, L. (2022). Hydroxy-γ-sanshool from Zanthoxylum bungeanum (prickly ash) induces apoptosis of human colorectal cancer cell by activating P53 and Caspase 8. Front. Nutr. 9, 914638. doi:10.3389/fnut.2022.914638
Zhu, X., Ji, M., Han, Y., Guo, Y., Zhu, W., Gao, F., et al. (2017). PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int. J. Oncol. 50 (3), 835–846. doi:10.3892/ijo.2017.3873
Zhu, Y., Pan, Y., Zhang, G., Wu, Y., Zhong, W., Chu, C., et al. (2018). Chelerythrine inhibits human hepatocellular carcinoma metastasis in vitro. Biol. Pharm. Bull. 41 (1), 36–46. doi:10.1248/bpb.b17-00451
Zucker, S., Lysik, R. M., Zarrabi, H. M., Moll, U., Tickle, S. P., Stetler-Stevenson, W., et al. (1994). Plasma assay of matrix metalloproteinases (MMPs) and MMP-inhibitor complexes in cancer. Potential use in predicting metastasis and monitoring treatment. Ann. N. Y. Acad. Sci. 732, 248–262. doi:10.1111/j.1749-6632.1994.tb24740.x
Zuo, Y., Pu, J., Chen, G., Shen, W., and Wang, B. (2019). Study on the activity and mechanism of skimmianine against human non-small cell lung cancer. Nat. Prod. Res. 33 (5), 759–762. doi:10.1080/14786419.2017.1408096
Z. bungeanum Zanthoxylum bungeanum Maxim
P53 Cellular tumor antigen p53
PI3K Phosphoinositide 3-kinase
AKT Protein kinase B
Wnt Wingless/integrated
UPLC Ultra Performance Liquid Chromatography
MS Mass spectrum
KEGG Kyoto Encyclopedia of Genes and Genomes
OB Oral bioavailability
DL Drug-like properties
PCNA Proliferating Cell Nuclear Antigen
Ki-67 Protein phosphatase 1
MAPK Mitogen-activated protein kinase
ATG5 Autophagy-related Gene 5
TNF-α Tumor necrosis factor-α
IL-2 Interleukin-2
NSCLC Non-small cell lung cancer
FAK Focal adhesion kinase
mTOR Mammalian target of rapamycin
MMP2/9 Matrix metalloproteinase 2/9
CDK4 Cyclin-dependent kinase 4
HGF Hepatocyte growth factor
ERK1/2 Extracellular signal-regulated kinase
EMT Epithelial-mesenchymal transition
ROS Reactive oxygen species
P-gp P-glycoprotein
BAX BCL-2-associated X protein
BCL-2 B cell lymphoma-2
GLUT-1 Facilitative glucose transporter-1
PARP Poly ADP-ribose polymerase
STAT3 Signal transducer and activator of transcription 3
LC3-II Microtubule-associated protein 1 light chain 3 II
MYC Myc proto-oncogene protein
CDC25A M-phase inducer phosphatase 1
Nrf2 Nuclear factor erythroid2-related factor 2
GSK-3β Glycogen Synthase Kinase 3β
TLR4 Toll-like receptor 4
YAP Yes-associated protein
TGFβ1 Transforming growth factor beta 1
Keywords: Zanthoxylum bungeanum Maxim, medicine food homology plant, cancer, anticancer mechanism, anticancer active ingredients
Citation: Du Y, Duan S, Yang Y, Tibenda JJ, Huang S, Nan Y, Zhang Z and Yuan L (2025) Antitumor components and mechanisms of Zanthoxylum bungeanum Maxim with medicine and food homology. Front. Pharmacol. 16:1525313. doi: 10.3389/fphar.2025.1525313
Received: 09 November 2024; Accepted: 07 February 2025;
Published: 28 February 2025.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Sinem Aslan Erdem, Ankara University, TürkiyeCopyright © 2025 Du, Duan, Yang, Tibenda, Huang, Nan, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Zhang, emhhbmd6aGVAenJ5aHl5LmNvbS5jbg==; Ling Yuan, MjAwODAwMTdAbnhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.