
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 March 2025
Sec. Pharmacoepidemiology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1521358
Background: Faricimab is the first and only bispecific antibody approved by the U.S. Food and Drug Administration (FDA) for intravitreal injection. Given its increasingly widespread use in retinal vascular diseases, understanding its adverse events (AEs) in real-world settings is crucial. This study employed the FDA Adverse Event Reporting System (FAERS) database to investigate potential safety concerns, with the aim of providing new insights for clinical practice.
Methods: This study conducted a disproportionality analysis of adverse event data from the FAERS database, in which faricimab was identified as the primary suspect, covering the period from the first quarter of 2022 to the second quarter of 2024. To ensure the accuracy and reliability of the study, we employed four types of disproportionality analyses: the reporting odds ratio (ROR), proportional reporting ratio (PRR), multi-item gamma Poisson shrinker (MGPS), and Bayesian confidence propagation neural network (BCPNN). Additionally, the Weibull distribution was utilized to model the risk of adverse events over time.
Results: A total of 2,735 adverse reaction reports, in which faricimab was identified as the primary suspect, were retrieved from the FAERS database. The analysis showed that faricimab-induced AEs occurred across 25 system organ classes (SOCs), with eye disorders meeting the positive threshold for all four algorithms. Significant AEs were mapped to preferred terms (PT), identifying the adverse reactions listed on the drug label: endophthalmitis, elevated intraocular pressure, cataract, retinal pigment epithelial tear, vitreous floaters, retinal vasculitis, retinal artery occlusion, and retinal vein occlusion. In addition to the AEs listed on the drug label, several previously unreported AEs were identified, including blindness, cerebral infarction, retinal hemorrhage, retinal occlusive vasculitis, glaucoma, dry eye, metamorphopsia, and unilateral blindness.
Conclusion: This study provided valuable evidence on the real-world safety of faricimab, suggesting that clinicians should place greater emphasis on monitoring its adverse effects during use.
Age-related macular degeneration (AMD), diabetic macular edema (DME), and retinal vein occlusion-related macular oedema (RVO-MO) are among the leading global causes of blindness and visual impairment (Sharma et al., 2024). AMD is a major cause of visual impairment among the elderly and occurs in two forms: dry (non-neovascular) and wet (neovascular, nAMD). Approximately 90% of severe vision loss associated with AMD is attributable to the wet form (Yang et al., 2022). DMO and RVO-MO are vascular complications linked to systemic diseases such as diabetes and hypertension, DME is marked by fluid accumulation in the macula due to vascular damage, whereas RVO-MO results from retinal vein occlusion, which causes retinal hemorrhages and macular oedema (Panos et al., 2023). Intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) drugs is the current mainstream treatment approach. Faricimab is the first and currently the only bispecific antibody approved by the U.S. Food and Drug Administration (FDA), administered through intravitreal injection (Liberski et al., 2022). Faricimab binds with high affinity to angiopoietin-2 (Ang-2) and neutralizes VEGF-A, targeting two key pathways involved in the pathology of nAMD and DME, this dual inhibition addresses distinct mechanisms contributing to the progression of these diseases (Zhang et al., 2024). Multiple clinical studies had demonstrated that faricimab is highly effective, leading to improvements in best corrected visual acuity (BCVA), reductions in retinal thickness, and significant anatomical enhancements (Zarbin et al., 2024; Wykoff et al., 2022; Wong et al., 2024). A meta-analysis conducted by Watkins et al. (2023) demonstrated that faricimab achieves greater improvements in BCVA compared to Ranibizumab, while also requiring fewer injections. A phase II clinical trial indicated that the duration of faricimab’s effect may exceed that of existing intravitreal anti-VEGF treatments like ranibizumab, allowing for less frequent dosing regimens (Khanani et al., 2020).
Faricimab exhibits a unique mechanism of action and shows promising potential for expanded clinical applications. Although several studies (Wijesingha et al., 2024; Li et al., 2023) had assessed its safety, real-world safety data remains limited. The FDA Adverse Event Reporting System (FAERS) is one of the largest post-market safety monitoring databases. It collects standardized real-world data to support the FDA’s safety surveillance program for drugs and therapeutic biologics through spontaneous reports submitted by consumers, healthcare professionals, pharmaceutical manufacturers, and other non-medical individuals (Zhao and Tao, 2024). This study performed a retrospective pharmacovigilance analysis using the FAERS database to assess adverse event reports related to faricimab. Signal detection methods were employed to identify potential drug safety signals and offer critical insights into faricimab’s safety profile, providing new evidence for clinical ophthalmologists.
Data for this study were sourced from the FAERS database, a comprehensive pharmacovigilance resource supporting the FDA’s post-marketing surveillance program for approved drugs and therapeutic biologics. Compared to other pharmacovigilance databases, such as the EV database managed by the European Medicines Agency (EMA), which primarily collects, manages, and analyzes individual case safety reports (ICSRs) related to authorized drugs or vaccines in clinical trials within the European Economic Area (EEA) and makes these data publicly available through the EMA website (www.adrreports.eu) (Gozzo et al., 2023; Brancati et al., 2021), or the Italian spontaneous ADR reporting database (Rete Nazionale di Farmacovigilanza, RNF) (Gozzo et al., 2021), the FAERS database exhibits the following characteristics. The FAERS dataset is structured into seven sections: DEMO (patient demographic and administrative information), DRUG (drug-specific details), REAC (coded adverse events), OUTC (patient outcomes), RPSR (report sources), THER (therapy initiation and cessation dates), and INDI (indications for drug use). Reported drugs in FAERS are categorized into four groups: PS (Primary Suspect), SS (Secondary Suspect), C (Concomitant), and I (Interacting). Both the brand name and the generic name are employed to identify records related to faricimab. The search terms include “VABYSMO,” “FARICIMAB,” “Vascular Endothelial Growth Factor Inhibitors Faricimab,” and “Blinded Faricimab.” In this study, we focused exclusively on data that designated faricimab as a PS. Adverse events (AEs) and medication errors are coded using terminology from the Medical Dictionary for Regulatory Activities (MedDRA), a comprehensive and detailed standard developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). To address duplicate reports, we adopted the methodology recommended by the FDA. From the DEMO table, we extracted the PRIMARYID, CASEID, and FDA_DT fields, selecting entries with the maximum FDA_DT value according to FDA guidelines to ensure that we retained the most recent report for each CASEID. In cases where CASEID and FDA_DT are identical, the report with the highest PRIMARYID was retained. The detailed flowchart of the research design can be found in Figure 1.
Disproportionality analysis was employed to characterize the features of adverse event reports associated with faricimab. This analysis typically comprises two components: frequentist statistics and Bayesian statistics. Frequentist Statistics includes the reporting odds ratio (ROR) and proportional reporting ratio (PRR) (Rothman et al., 2004; Evans et al., 2001), while Bayesian Statistics encompasses the multi-item gamma Poisson shrinker (MGPS) and Bayesian confidence propagation neural network (BCPNN) (Bate et al., 1998; Szarfman et al., 2004). In this study, AEs that met the positive thresholds for all four methods were classified as adverse reactions. Combining the ROR, PRR, MGPS, and BCPNN algorithms leveraged the strengths of multiple approaches and mitigated potential bias associated with reliance on a single algorithm. The interval between the occurrence of AEs recorded in the DEMO file and the initiation of faricimab treatment documented in the THER file was defined as the onset time of faricimab-related AEs. Temporal changes in the incidence of AEs were modeled using the Weibull distribution. All analyses were conducted using R software version 4.3.0. The 2 × 2 contingency table used in the descriptive analysis is provided in Table 1, and the specific algorithms and positive threshold criteria for the four methods are detailed in Table 2.
Between the first quarter of 2022 and the second quarter of 2024, the FAERS database received a total of 4,304,335 reports. Following data deduplication and screening, 5,691 adverse reaction reports involving 2,735 patients were identified, with faricimab designated as the PS drug. Table 3 demonstrated the basic population characteristics with faricimab-related AEs. The proportion of female patients (39.6%) exceeded that of male patients (35.5%), with the gender of the remaining patients unspecified. Regarding age distribution, patients aged 65–85 represented the largest group (32.1%), followed by those aged 18–65 (17%). The United States accounted for more than half (55.6%) of reported cases, with Japan, Canada, the United Kingdom, and India comprising the remainder of the top five reporting countries. In terms of reporting sources, clinicians contributed the largest proportion of reports (61.3%), followed by consumers, health professionals, and pharmacists. Since its launch in 2022, the number of reported AEs has shown a steady annual increase, peaking in 2024 with 67.7% of the total reports.
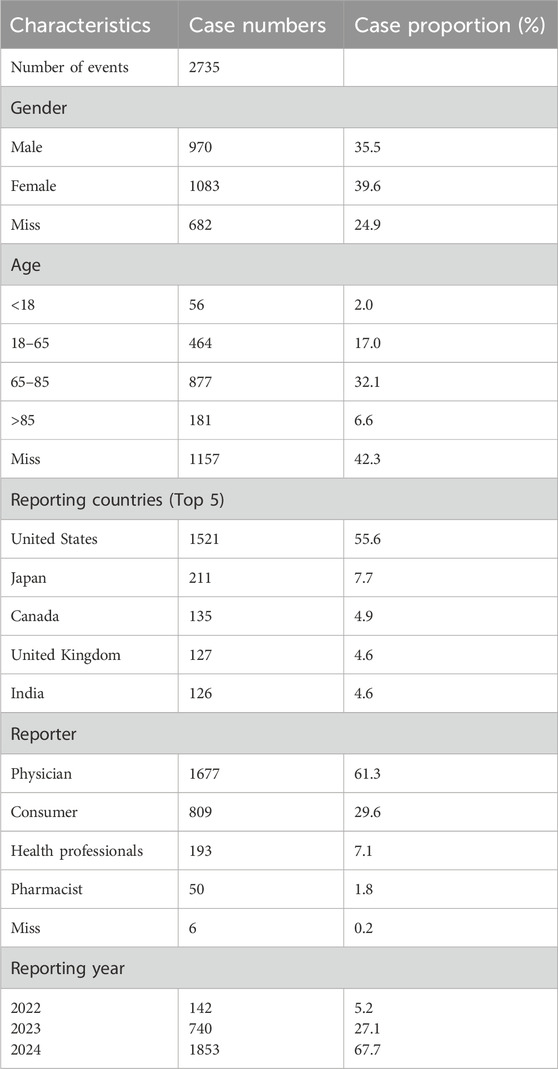
Table 3. Clinical characteristics of faricimab adverse event reports from the FAERS database (Q1 2022 – Q2 2024).
The results of faricimab’s AE reports at the SOC level are presented in Table 4. The proportion of SOC reported in faricimab-related AEs can be found in Figure 2. The final analysis revealed that adverse reactions associated with faricimab spanned 25 SOCs. Ranked by the number of reported cases, the top three SOCs were Eye Disorders (n = 2109, 37.1%), General Disorders and Administration Site Conditions (n = 1354, 23.8%), and Injury, Poisoning, and Procedural Complications (n = 1148, 20.1%). Eye Disorders (ROR = 29.56, PRR = 18.97, EBGM = 18.81, IC = 4.23) demonstrated a strong positive signal across all four algorithms, aligning with descriptions in the faricimab drug label, which suggests high data reliability. Additionally, General Disorders and Administration Site Conditions (ROR = 1.43) met the positive threshold only in the ROR algorithm, while displaying negative signals in the other algorithms.
The final results showed that 123 PTs met the positive criteria across all four algorithms. Table 5 presents the top 50 AEs associated with faricimab at the PT level. The Venn diagram in Figure 3 visually illustrated the AEs that met the positive threshold of all four algorithms at the PT level. Among the top 50 most common AEs, several events were identified that aligned with those listed on the drug label, including endophthalmitis (Including AEs classified as part of endophthalmitis), elevated intraocular pressure, cataract, retinal pigment epithelial tear, vitreous floaters, retinal vasculitis, retinal artery occlusion, and retinal vein occlusion. Additionally, several noteworthy AEs not included on the drug label were identified, such as blindness, cerebral infarction, retinal hemorrhage, retinal occlusive vasculitis, glaucoma, dry eye, metamorphopsia, and unilateral blindness.
A total of 450 AEs were associated with onset times, predominantly occurring within the first month. The distribution of onset times for these AEs is shown in Figure 4. Analysis using the Weibull distribution revealed an early failure mode, with detailed parameters presented in Table 6. Additionally, the cumulative incidence curve of AEs is depicted in Figure 5.
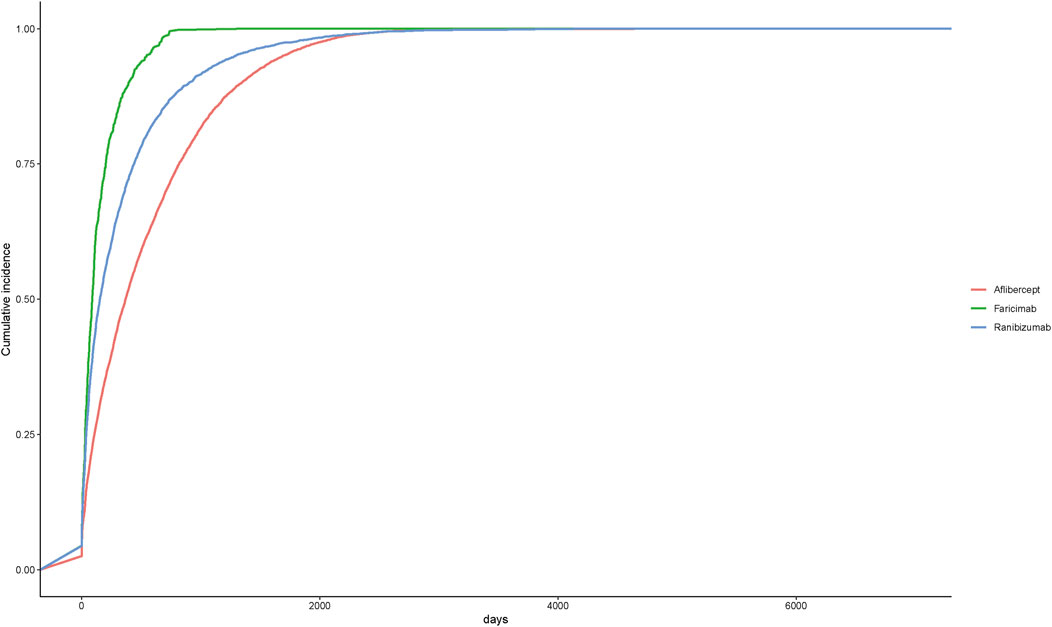
Figure 5. Cumulative incidence of adverse events related to three different anti-VEGF drugs over time.
In order to further explore the safety differences between faricimab and other anti-VEGF drugs, this study also utilized the FAERS database to analyze the adverse reactions of ranibizumab and aflibercept and directly compared them with faricimab. The results revealed that all three anti-VEGF drugs exhibited several similar AEs, including endophthalmitis, retinal vein occlusion, retinal artery occlusion, glaucoma, dry eye syndrome, and additional AEs not mentioned in the drug’s prescribing information. Additionally, among the ocular-related AEs, retinal occlusive vasculitis exhibited positive signals exclusively in aflibercept and faricimab, macular ischemia showed a positive signal only in ranibizumab, and eye edema, retinal depigmentation, and ulcerative keratitis demonstrated positive signals exclusively in ranibizumab and aflibercept. Among the non-ocular AEs, cerebral infarction was a common event across all three drugs, whereas myocardial infarction, transient ischemic attack, and deafness exhibited positive signals solely in ranibizumab. The detailed adverse reactions can be found in Tables 7, 8.
This study is the first to apply the FAERS database to assess the safety of faricimab since its approval in 2022. We employed four disproportionality algorithms-ROR, PRR, MGPS, and BCPNN to evaluate AEs with faricimab as the PS drug. The ROR method is known for its sensitivity and ease of calculation, but it can generate false-positive signals when report volumes are low. PRR is a simple disproportionality measure designed to detect potential signals of adverse drug reactions. In contrast, BCPNN and MGPS offer higher specificity in identifying true signals (Wang et al., 2024b). BCPNN uses Bayesian methods to estimate the likelihood of a causal relationship between a drug and an adverse event, while MGPS adjusts effect sizes-such as PRR or ROR-to reduce the occurrence of false-positive signals (Wang et al., 2024a). Each of these algorithms has distinct advantages and limitations. Therefore, this study focused on discussing AEs that met the criteria of all four algorithms.
The global prevalence of w-AMD is projected to increase from approximately 196 million patients today to 288 million by 2040, similarly, the rising incidence of diabetes has led to a 64% increase in DR-related visual loss and a 27% increase in blindness (Keenan et al., 2021; Lin et al., 2021). Anti-VEGF therapies remain the mainstay for treating exudative retinal diseases, including n-AMD and DME. Faricimab, a bispecific antibody that binds with high affinity to both VEGF-A and Ang-2, was recently approved by the FDA (Sharma et al., 2020). The angiopoietin (Ang) yrosine kinase endothelial receptors (Tie) pathway plays a crucial role in the regulation of vascular homeostasis, vascular permeability, angiogenesis, and pro-inflammatory processes, notably, Tie-2 is a transmembrane receptor localized specifically on vascular endothelial cells, where it functions as a binding site for Ang-1 and Ang-2 (Ferro Desideri et al., 2023). Ang-1 serves as a complete agonist for Tie-2, promoting its phosphorylation and activating downstream signaling, which enhances vascular stability while suppressing vascular permeability and leakage. Conversely, Ang-2 functions as a partial agonist or antagonist of Tie-2. Consequently, Ang-2 binding to the Tie-2 receptor inhibits pathway activation, resulting in increased vascular leakage (Ferro Desideri et al., 2023). Under normal physiological conditions, Ang-1 is expressed at higher constitutive levels than Ang-2, however, under pathological conditions, Ang-2 expression is upregulated, amplifying the effects of VEGF through inhibition of the endothelial-specific Tie-2 receptor (Wolfrum et al., 2024). Faricimab’s dual mechanism of action demonstrated favorable outcomes in the YOSEMITE and RHINE trials, with improvements in baseline visual acuity, retinal structure, and extended treatment intervals maintained for up to 2 years (Wong et al., 2024). A meta-analysis by Watkins et al. (2023) found that Faricimab offers superior visual outcomes compared to Ranibizumab, with a safety profile similar to Aflibercept. The efficacy of faricimab is comparable to established first-line therapies, and the incidence of most adverse events is similar to that of existing alternative treatments (Zhang et al., 2024). However, Faricimab presents some potential adverse effects that warrant consideration. Based on our analysis, we highlight signals categorized as strong in adverse event reports for Faricimab, providing new insights into its safety in clinical applications.
The retina, along with the ciliary body, choroid, and iris, forms the posterior segment of the eye. Due to the low ocular bioavailability (less than 5%–10%) of topical medications, conventional treatments for posterior segment diseases often require peribulbar, retrobulbar, or subconjunctival injections, these procedures are invasive and may result in severe ocular complications (Puglia et al., 2021). Post-injection endophthalmitis is a rare yet severe complication of intravitreal anti-VEGF injections, potentially leading to significant vision loss (McCannel, 2011). In this study, diseases and symptoms associated with endophthalmitis showed strong signals across all four algorithms, consistent with the information provided in the drug label. The most common symptoms and signs of endophthalmitis include visual impairment, eye irritation, elevated intraocular pressure, vitreous floaters, eye pain, ocular hyperaemia, eyelid swelling, hypopyon, conjunctival congestion, corneal edema, and keratic precipitates (Haddock et al., 2014), each presenting strong signals as adverse reactions. Ben Ghezala et al. (2024). reported a case series of 6 patients with severe endophthalmitis, including 5 cases of severe anterior uveitis and intermediate uveitis resembling endophthalmitis. Several other clinical trials have also reported severe endophthalmitis associated with faricimab, suggesting a higher incidence of severe endophthalmitis with faricimab compared to first-generation anti-VEGF drugs (Thangamathesvaran et al., 2024; Chen et al., 2024; Palmieri et al., 2024). Endophthalmitis associated with faricimab administration may be attributed to several factors. Primarily, faricimab is supplied in a single-dose vial with a filter needle rather than in a prefilled syringe, which may increase the risk of contamination during handling (Alkhawaldeh and Abu Serhan, 2024). Secondly, thermal stability may contribute to these phenomena. The melting temperatures at which the heavy and light chains of faricimab unfold are approximately 64°C and 71.5°C, respectively, necessitating refrigeration (Akiba et al., 2019; Liberski et al., 2022). Additionally, faricimab’s anti-ANG-2 activity may also play a role, faricimab may possess pro-inflammatory properties, though this hypothesis is not yet fully supported by data (Ben Ghezala et al., 2024). Furthermore, cataract, retinal pigment epithelial tear, retinal vasculitis, retinal artery occlusion, and retinal vein occlusion all reached the positive thresholds in the four algorithms, aligning with the data presented in the drug label. Siddiqui et al. (2024) documented a case of faricimab-associated retinal vasculitis in a patient presenting with painless vision loss. Fluorescein angiography revealed delayed vascular filling with extensive leakage, characteristic of non-occlusive vasculitis, the patient’s symptoms improved following intravenous steroid treatment. Clemens et al. (2023) reported a case in which a patient developed a retinal pigment epithelial tear 4 weeks after transitioning from aflibercept to faricimab for intravitreal injections. In the phase three TENAYA and LUCERNE trials for nAMD, the incidence of retinal pigment epithelial tears was 2.7% and 3.0% in the faricimab group, compared to 1.8% and 0.9% in the aflibercept group (Heier et al., 2022). One possible explanation is that anti-VEGF therapy may induce fibrotic contraction of neovascular tissue beneath the retinal pigment epithelium, leading to a tear in the overlying epithelium and contributing to this observed complication (Ma et al., 2022). A single-center, prospective cohort study by Cancian et al. (2024). found that one patient with a history of ischemic heart disease died from acute myocardial infarction after 37 weeks of follow-up. Additionally, one patient developed retinal pigment epithelium changes following two injections, and another patient experienced two episodes of uveitis. A study by Mukai et al. (2024), conducted over the course of 1 year, found two cases of retinal pigment epithelium tear and one patient developed iritis after six intraocular injections of faricimab. The study by Mori et al. (2023) reported only allergic conjunctivitis associated with faricimab.
In addition to the AEs explicitly stated in the drug label, this study identified several unreported signals, including blindness, cerebral infarction, retinal hemorrhage, retinal occlusive vasculitis, glaucoma, dry eye, metamorphopsia, and unilateral blindness. While retinal occlusive vasculitis is rare, it remains a potential complication of faricimab treatment. Reichel et al. (2024) documented a case involving a 52-year-old male who developed sudden vision loss, new retinal hemorrhage, marked retinal vascular occlusion, and ischemia after receiving monthly faricimab injections for diabetic macular edema. In a separate case, a 72-year-old male receiving treatment for polypoidal choroidal vasculopathy developed occlusive vasculitis 2 weeks following his second faricimab injection (Chen et al., 2024). Faricimab and Brolucizumab may share similar mechanisms, with retinal occlusive vasculitis associated with both agents typically occurring between 1 week and 12 months after the first injection, rather than immediately post-injection (Monés et al., 2021). Anti-drug antibodies (ADAs) against a non-native form of Brolucizumab, formed upon prolonged incubation at body temperature in the vitreous, lead to immune complex formation, which mediates hypersensitivity reactions and triggers inflammation and platelet aggregation, this may be similar to faricimab (Reichel et al., 2024). Although the drug label for faricimab warns about the risk of thromboembolic events following its use, it does not explicitly mention any associated diseases. This study found that cerebral infarction is an adverse event that met the high-signal criteria across all four algorithms. Changes in blood flow, a hypercoagulable state, and vessel wall damage are three critical factors in the pathogenesis of thrombosis (Zhang et al., 2022). Intravitreal injection of anti-VEGF therapy may enter systemic circulation (Fogli et al., 2018). Following intravitreal administration, faricimab plasma concentrations increase in proportion to the dose, ranging from 0.5 to 3 mg, and reach peak plasma levels within 2 days. Faricimab is believed to undergo lysosomal degradation, resulting in peptides and amino acids similar to endogenous IgG (Panos et al., 2023). However, further research is needed to thoroughly understand the exact clearance rate, systemic absorption, and comprehensive pharmacokinetic profile of faricimab in humans. This study also found that glaucoma may be a potential adverse reaction to faricimab. A major risk factor for the development and progression of glaucoma is elevated intraocular pressure (IOP) (Zimmermann et al., 2021). Past experience suggested that repeated intravitreal injections of anti-VEGF drugs may reduce the function of the aqueous outflow system and be associated with the development of glaucoma (Wingard et al., 2019). A study by Wen et al. (2017) found that the aqueous outflow facility in eyes with AMD receiving 20 or more anti-VEGF injections decreased by 12% compared to the untreated fellow eye. Nitric oxide (NO) is a crucial signaling molecule. Anti-VEGF drugs disrupt the NO signaling pathway, potentially lowering NO levels below physiological baseline. This reduction in NO may contribute to glaucoma pathogenesis through mechanisms that lead to increased intraocular pressure (IOP), retinal vascular dysfunction, and retinal nerve fiber layer (RNFL) thinning (Daka et al., 2023). Endogenous VEGF expression in the trabecular meshwork functions as a paracrine regulator of conventional outflow pathways, Anti-VEGF drugs may disrupt this expression. Furthermore, several mechanisms, including inflammation, particle obstruction from injected solutions, or secondary angle-closure, could lead to increased IOP (Wen et al., 2017; Wen et al., 2016). A key characteristic of glaucoma is the death of retinal ganglion cells (RGCs). Multiple factors contribute to RGC damage, including oxidative stress, mitochondrial dysfunction, axonal transport blockade, synaptic impairment, glutamate-induced excitotoxicity, and alterations in pro-inflammatory cytokines, increasing evidence supports the role of TNF-α as a mediator of RGC death in glaucoma, acting through its binding to TNF receptor-1 (TNF-R1) (Romano et al., 2023). Prior to RGC death, there is typically a reversible phase of functional impairment and structural remodeling, non-IOP-dependent neuroprotection, or neuroprotection as an adjunct to IOP-lowering therapies for glaucoma, remains a significant challenge (Chou et al., 2018). RGC degeneration is commonly associated with ischemia resulting from central retinal artery occlusion and ischemic optic neuropathy. Gliosis, a pivotal event in the pathogenesis of glaucoma, serves as a hallmark of retinal degeneration. Reactive glial cells in the retina exhibit elevated immunoreactivity for glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (Iba1), injury-induced gliosis in both the optic nerve head and retina accelerates retinal ganglion cell (RGC) death through the excessive release of pro-inflammatory mediators (Conti et al., 2021b; Conti et al., 2021a). A study by Amato R et al. found that diabetes can serve as an intraocular pressure (IOP)-independent risk factor for the early progression of glaucoma, contributing to oxidative stress and inflammation-induced RGC dysfunction, gliosis, and cell death (Amato et al., 2021). A significant proportion of patients treated with faricimab have diabetes, which itself increases the risk of developing glaucoma. In this study, retinal damage and changes associated with intravitreal injection of faricimab were observed, and glaucoma was also identified as a potential adverse reaction. Additionally, focusing on the protection of the optic nerve after treatment is crucial. Therefore, it is essential to monitor both the long-term and short-term IOP of patients after intravitreal injection of faricimab. In addition, clinicians should also be aware of the intraocular pressure increase and glaucoma induced by other intravitreal anti-VEGF drugs. Dry eye disease is a chronic inflammatory condition of the ocular surface (Kwaku Akowuah et al., 2024). Topical antibiotics are commonly used before and after intravitreal injection of anti-VEGF drugs, which may have toxic side effects on ocular surface cells. Additionally, most of these drugs contain preservatives (Laude et al., 2017).
Intravitreal injection of anti-VEGF drugs is widely used to mitigate disease progression and enhance visual outcomes in affected patients. Furthermore, it is important to compare the safety profiles of different anti-VEGF agents (Ventrice et al., 2013). Xiong et al. (2024) investigated ocular and systemic AEs following the market approval of brolucizumab through the FAERS database. They identified ocular events, including keratic precipitates (KPs), retinal perivascular sheathing, vitreal cells, dry eye, and glaucoma, as well as systemic events, such as arterial thromboembolic events, cerebral infarction, and rhinorrhea, which were not included in the drug’s prescribing information. Ma et al. (2022) analyzed three anti-VEGF drugs (ranibizumab, aflibercept, brolucizumab) using the FAERS database and identified several positive signals that were not mentioned in the drug’s prescribing information. These included macular ischemia and retinal pigment epithelial tear associated with ranibizumab, increased intraocular pressure and endophthalmitis associated with aflibercept, and retinal vasculitis and/or retinal vascular occlusion and dry eye associated with brolucizumab. Sakai et al. (2022) conducted a focused analysis of the relationship between intravitreal anti-VEGF injections and miscarriage using the JAPIC AERS and FAERS databases. They identified 19 miscarriage cases associated with ranibizumab, 6 with bevacizumab, and 4 with aflibercept. No cases of miscarriage associated with faricimab were observed in this study. However, considering that faricimab has been available for a shorter period and the sample size is smaller compared to other anti-VEGF drugs, this event should still be closely monitored. This study also compared the safety profiles of the three anti-VEGF drugs, identifying common adverse reactions. Furthermore, macular ischemia was found to be a potentially unique adverse reaction associated with ranibizumab, a finding consistent with the research of Ma et al. (2022) and others. Anti-VEGF drugs inhibit the normal physiological functions of VEGF. Consequently, VEGF blockade-induced vasoconstriction may exacerbate hypoxic damage in the already compromised macular capillary bed, potentially leading to detrimental effects on macular function and visual outcomes (Sun et al., 2021). Ranibizumab, which blocks all VEGF isoforms and has a Fab fragment that penetrates all retinal layers more effectively, exhibits a stronger effect (Ba et al., 2015). Additionally, while some ocular AEs were observed only in ranibizumab and aflibercept, the relatively short market availability of faricimab and its small sample size must be taken into account. Therefore, the results for this section should be interpreted with caution. Among the non-ocular AEs, myocardial infarction, transient ischemic attack, and deafness showed positive signals only in ranibizumab, cerebral infarction is a common side effect shared by the three anti-VEGF drugs. A recent study by Yang et al. (2025) found that the incidence of cardiovascular-related adverse events with Ranibizumab is higher than that with Aflibercept. This may be due to the drug’s impact on vascular tone (e.g., hypertension), rheological properties (e.g., promoting thrombosis), or cardiac electrophysiology, all of which increase the risk of cardiovascular events (Zakaria et al., 2022). Therefore, enhanced monitoring of the patient’s cardiovascular and cerebrovascular functions is recommended when administering anti-VEGF drugs via intravitreal injection.
This study has several limitations. First, the FAERS database presents a potential bias risk, as all reported information is voluntarily submitted by pharmaceutical companies, healthcare providers, and consumers. Although the majority of data in this study originates from physicians, a substantial portion is also provided by consumers, which may compromise data completeness and reliability. Furthermore, the FAERS database is the primary system in the United States for monitoring post-marketing adverse drug reactions. Approximately 55.6% of the data originates from the United States, the limited contributions from other regions undermine the external validity for other populations, ethnic group differences warrant consideration. Thus, cross-validation with other databases is recommended.
This study assessed the safety of intravitreal faricimab injections using the FAERS database, with data analyzed through four algorithms. Alongside confirming adverse reactions listed in the drug label, some previously unreported potential adverse reactions were also identified. Nevertheless, given the limitations of the FAERS database, these findings should be interpreted with caution. Future validation through rigorous prospective clinical trials or epidemiological studies is recommended.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The data were extracted from previously published studies, so ethical approval and patient consent were unnecessary.
C-ZH: Data curation, Formal Analysis, Methodology, Software, Writing–original draft, Writing–review and editing. QQ: Formal Analysis, Methodology, Software, Writing–original draft. S-JL: Conceptualization, Writing–original draft. F-LX: Investigation, Visualization, Writing–original draft. J-QL: Investigation, Visualization, Writing–original draft. YH: Conceptualization, Methodology, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The contributions of each author in the process of literature collection, data extraction, and quality assessment are gratefully appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akiba, H., Satoh, R., Nagata, S., and Tsumoto, K. (2019). Effect of allotypic variation of human IgG1 on the thermal stability of disulfide-linked knobs-into-holes mutants of the Fc for stable bispecific antibody design. Antib. Ther. 2, 65–69. doi:10.1093/abt/tbz008
Alkhawaldeh, I. M., and Abu Serhan, H. (2024). Intraocular inflammation with faricimab: insights from manufacturer and user facility device experience (MAUDE) database. Eye (Lond) 38, 2494–2496. doi:10.1038/s41433-024-03079-0
Amato, R., Lazzara, F., Chou, T. H., Romano, G. L., Cammalleri, M., Dal Monte, M., et al. (2021). Diabetes exacerbates the intraocular pressure-independent retinal ganglion cells degeneration in the DBA/2J model of glaucoma. Invest Ophthalmol. Vis. Sci. 62, 9. doi:10.1167/iovs.62.9.9
Ba, J., Peng, R. S., Xu, D., Li, Y. H., Shi, H., Wang, Q., et al. (2015). Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des. Devel Ther. 9, 5397–5405. doi:10.2147/DDDT.S86269
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. doi:10.1007/s002280050466
Ben Ghezala, I., Gabrielle, P. H., Sibert, M., Steinberg, L. A., Dautriche, A., Arnould, L., et al. (2024). Severe intraocular inflammation after intravitreal injection of faricimab: a single-site case series of six patients. Am. J. Ophthalmol. 269, 11–19. doi:10.1016/j.ajo.2024.08.008
Brancati, S., Gozzo, L., Romano, G. L., Vetro, C., Dulcamare, I., Maugeri, C., et al. (2021). Venetoclax in relapsed/refractory acute myeloid leukemia: are supporting evidences enough? Cancers (Basel) 14, 22. doi:10.3390/cancers14010022
Cancian, G., Paris, A., Agliati, L., Rizzato, A., Clerici, M., Volpe, G., et al. (2024). One-year real-world outcomes of intravitreal faricimab for previously treated neovascular age-related macular degeneration. Ophthalmol. Ther. 13, 2985–2997. doi:10.1007/s40123-024-01036-4
Chen, X., Wang, X., and Li, X. (2024). Intra-ocular inflammation and occlusive retinal vasculitis following intravitreal injections of faricimab: a case report. Ocul. Immunol. Inflamm. 32, 2544–2547. doi:10.1080/09273948.2024.2361834
Chou, T. H., Musada, G. R., Romano, G. L., Bolton, E., and Porciatti, V. (2018). Anesthetic preconditioning as endogenous neuroprotection in glaucoma. Int. J. Mol. Sci. 19, 237. doi:10.3390/ijms19010237
Clemens, C. R., Alten, F., Zimmermann, J. A., and Eter, N. (2023). Old problem in a new guise: retinal pigment epithelium tear after intravitreal faricimab (Vabysmo(®)) injection. Case Rep. Ophthalmol. 14, 241–244. doi:10.1159/000529930
Conti, F., Lazzara, F., Romano, G. L., Platania, C. B. M., Drago, F., and Bucolo, C. (2021a). Caffeine protects against retinal inflammation. Front. Pharmacol. 12, 824885. doi:10.3389/fphar.2021.824885
Conti, F., Romano, G. L., Eandi, C. M., Toro, M. D., Rejdak, R., DI Benedetto, G., et al. (2021b). Brimonidine is neuroprotective in animal paradigm of retinal ganglion cell damage. Front. Pharmacol. 12, 705405. doi:10.3389/fphar.2021.705405
Daka, Q., Špegel, N., Atanasovska Velkovska, M., Steblovnik, T., Kolko, M., Neziri, B., et al. (2023). Exploring the relationship between anti-VEGF therapy and glaucoma: implications for management strategies. J. Clin. Med. 12, 4674. doi:10.3390/jcm12144674
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. doi:10.1002/pds.677
Ferro Desideri, L., Traverso, C. E., Nicolò, M., and Munk, M. R. (2023). Faricimab for the treatment of diabetic macular edema and neovascular age-related macular degeneration. Pharmaceutics 15, 1413. doi:10.3390/pharmaceutics15051413
Fogli, S., Del Re, M., Rofi, E., Posarelli, C., Figus, M., and Danesi, R. (2018). Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond) 32, 1010–1020. doi:10.1038/s41433-018-0021-7
Gozzo, L., Nardo, A., Brancati, S., Judica, A., Duminuco, A., Maugeri, C., et al. (2023). Severe gastrointestinal toxicity following the use of gilteritinib: a case series and analysis of postmarketing surveillance data. Healthc. (Basel) 11, 1479. doi:10.3390/healthcare11101479
Gozzo, L., Vetro, C., Brancati, S., Longo, L., Vitale, D. C., Romano, G. L., et al. (2021). Off-label use of venetoclax in patients with acute myeloid leukemia: single center experience and data from pharmacovigilance database. Front. Pharmacol. 12, 748766. doi:10.3389/fphar.2021.748766
Haddock, L. J., Ramsey, D. J., and Young, L. H. (2014). Complications of subspecialty ophthalmic care: endophthalmitis after intravitreal injections of anti-vascular endothelial growth factor medications. Semin. Ophthalmol. 29, 257–262. doi:10.3109/08820538.2014.959616
Heier, J. S., Khanani, A. M., Quezada Ruiz, C., Basu, K., Ferrone, P. J., Brittain, C., et al. (2022). Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 399, 729–740. doi:10.1016/S0140-6736(22)00010-1
Keenan, T. D. L., Cukras, C. A., and Chew, E. Y. (2021). Age-related macular degeneration: epidemiology and clinical aspects. Adv. Exp. Med. Biol. 1256, 1–31. doi:10.1007/978-3-030-66014-7_1
Khanani, A. M., Patel, S. S., Ferrone, P. J., Osborne, A., Sahni, J., Grzeschik, S., et al. (2020). Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 138, 964–972. doi:10.1001/jamaophthalmol.2020.2699
Kwaku Akowuah, P., Junior Obinwanne, C., Owusu, E., Kyeremeh, S., Bonsu, K., Karikari, L. A. A., et al. (2024). Platelet-rich plasma for treating dry eye disease - a systematic review and meta-analysis. Cont. Lens Anterior Eye 47, 102091. doi:10.1016/j.clae.2023.102091
Laude, A., Lim, J. W., Srinagesh, V., and Tong, L. (2017). The effect of intravitreal injections on dry eye, and proposed management strategies. Clin. Ophthalmol. 11, 1491–1497. doi:10.2147/OPTH.S136500
Liberski, S., Wichrowska, M., and Kocięcki, J. (2022). Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: a review. Int. J. Mol. Sci. 23, 9424. doi:10.3390/ijms23169424
Li, G., Zhu, N., and Ji, A. (2023). Comparative efficacy and safety of Faricimab and other anti-VEGF therapy for age-related macular degeneration and diabetic macular edema: a systematic review and meta-analysis of randomized clinical trials. Med. Baltim. 102, e36370. doi:10.1097/MD.0000000000036370
Lin, K. Y., Hsih, W. H., Lin, Y. B., Wen, C. Y., and Chang, T. J. (2021). Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 12, 1322–1325. doi:10.1111/jdi.13480
Ma, P., Pan, X., Liu, R., Qu, Y., Xie, L., Xie, J., et al. (2022). Ocular adverse events associated with anti-VEGF therapy: a pharmacovigilance study of the FDA adverse event reporting system (FAERS). Front. Pharmacol. 13, 1017889. doi:10.3389/fphar.2022.1017889
Mccannel, C. A. (2011). Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 31, 654–661. doi:10.1097/IAE.0b013e31820a67e4
MonéS, J., Srivastava, S. K., Jaffe, G. J., Tadayoni, R., Albini, T. A., Kaiser, P. K., et al. (2021). Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology 128, 1050–1059. doi:10.1016/j.ophtha.2020.11.011
Mori, R., Honda, S., Gomi, F., Tsujikawa, A., Koizumi, H., Ochi, H., et al. (2023). Efficacy, durability, and safety of faricimab up to every 16 weeks in patients with neovascular age-related macular degeneration: 1-year results from the Japan subgroup of the phase 3 TENAYA trial. Jpn. J. Ophthalmol. 67, 301–310. doi:10.1007/s10384-023-00985-w
Mukai, R., Kataoka, K., Tanaka, K., Miyara, Y., Maruko, I., Nakayama, M., et al. (2024). One-year outcomes and safety assessment of faricimab in treatment-naïve patients with neovascular age-related macular degeneration in Japan. Sci. Rep. 14, 11681. doi:10.1038/s41598-024-62559-1
Palmieri, F., Younis, S., Bedan Hamoud, A., and Fabozzi, L. (2024). Uveitis following intravitreal injections of faricimab: a case report. Ocul. Immunol. Inflamm. 32, 1873–1877. doi:10.1080/09273948.2023.2293925
Panos, G. D., Lakshmanan, A., Dadoukis, P., Ripa, M., Motta, L., and Amoaku, W. M. (2023). Faricimab: transforming the future of macular diseases treatment - a comprehensive review of clinical studies. Drug Des. Devel Ther. 17, 2861–2873. doi:10.2147/DDDT.S427416
Puglia, C., Santonocito, D., Romeo, G., Intagliata, S., Romano, G. L., Strettoi, E., et al. (2021). Lipid nanoparticles traverse non-corneal path to reach the posterior eye segment: in vivo evidence. Molecules 26, 4673. doi:10.3390/molecules26154673
Reichel, F. F., Kiraly, P., Vemala, R., Hornby, S., de Silva, S. R., and Fischer, M. D. (2024). Occlusive retinal vasculitis associated with intravitreal Faricimab injections. J. Ophthalmic Inflamm. Infect. 14, 45. doi:10.1186/s12348-024-00429-7
Romano, G. L., Gozzo, L., Maurel, O. M., DI Martino, S., Riolo, V., Micale, V., et al. (2023). Fluoxetine protects retinal ischemic damage in mice. Pharmaceutics 15, 1370. doi:10.3390/pharmaceutics15051370
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13, 519–523. doi:10.1002/pds.1001
Sakai, T., Mori, C., and Ohtsu, F. (2022). Potential safety signal of pregnancy loss with vascular endothelial growth factor inhibitor intraocular injection: a disproportionality analysis using the Food and Drug Administration Adverse Event Reporting System. Front. Pharmacol. 13, 1063625. doi:10.3389/fphar.2022.1063625
Sharma, A., Kumar, N., Kuppermann, B. D., Bandello, F., and Loewenstein, A. (2020). Faricimab: expanding horizon beyond VEGF. Eye (Lond) 34, 802–804. doi:10.1038/s41433-019-0670-1
Sharma, A., Kumar, N., Parachuri, N., Loewenstein, A., Bandello, F., and Kuppermann, B. D. (2024). Global experience of faricimab in clinical settings - a review. Expert Opin. Biol. Ther. 24, 263–268. doi:10.1080/14712598.2024.2336087
Siddiqui, M. Z., Durrani, A., and Smith, B. T. (2024). Faricimab-associated retinal vasculitis. J. Vitr. Dis. 8, 627–630. doi:10.1177/24741264241253899
Sun, T., Wei, Q., Gao, P., Zhang, Y., and Peng, Q. (2021). Cytokine and chemokine profile changes in patients with neovascular age-related macular degeneration after intravitreal ranibizumab injection for choroidal neovascularization. Drug Des. Devel Ther. 15, 2457–2467. doi:10.2147/DDDT.S307657
Szarfman, A., Tonning, J. M., and Doraiswamy, P. M. (2004). Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy 24, 1099–1104. doi:10.1592/phco.24.13.1099.38090
Thangamathesvaran, L., Kong, J., Bressler, S. B., Singh, M., Wenick, A. S., Scott, A. W., et al. (2024). Severe intraocular inflammation following intravitreal faricimab. JAMA Ophthalmol. 142, 365–370. doi:10.1001/jamaophthalmol.2024.0530
Ventrice, P., Leporini, C., Aloe, J. F., Greco, E., Leuzzi, G., Marrazzo, G., et al. (2013). Anti-vascular endothelial growth factor drugs safety and efficacy in ophthalmic diseases. J. Pharmacol. Pharmacother. 4, S38–S42. doi:10.4103/0976-500X.120947
Wang, K., Wang, M., Li, W., and Wang, X. (2024a). A real-world disproportionality analysis of Tivozanib data mining of the public version of FDA adverse event reporting system. Front. Pharmacol. 15, 1408135. doi:10.3389/fphar.2024.1408135
Wang, X., Chen, H., Han, S., Li, L., Chen, H., and Yang, B. (2024b). The real-world analysis of adverse events with teduglutide: a pharmacovigilance study based on the FAERS database. Front. Pharmacol. 15, 1404658. doi:10.3389/fphar.2024.1404658
Watkins, C., Paulo, T., BüHRER, C., Holekamp, N. M., and Bagijn, M. (2023). Comparative efficacy, durability and safety of faricimab in the treatment of diabetic macular edema: a systematic literature review and network meta-analysis. Adv. Ther. 40, 5204–5221. doi:10.1007/s12325-023-02675-y
Wen, J. C., Cousins, S. W., Schuman, S. G., and Allingham, R. R. (2016). Dynamic changes of the another chamber angle produced by intravitreal anti-vascular growth factor injections. Retina 36, 1874–1881. doi:10.1097/IAE.0000000000001018
Wen, J. C., Reina-Torres, E., Sherwood, J. M., Challa, P., Liu, K. C., Li, G., et al. (2017). Intravitreal anti-VEGF injections reduce aqueous outflow facility in patients with neovascular age-related macular degeneration. Invest Ophthalmol. Vis. Sci. 58, 1893–1898. doi:10.1167/iovs.16-20786
Wijesingha, N., Kotecha, A., Margaron, P., and Sivaprasad, S. (2024). Infographic: 2-year efficacy, durability and safety of intravitreal faricimab with treat-and-extend dosing up to 16 weeks in neovascular age-related macular degeneration (pooled results from TENAYA and LUCERNE). Eye (Lond). 39, 186–187. doi:10.1038/s41433-024-03209-8
Wingard, J. B., Delzell, D. A., Houlihan, N. V., Lin, J., and Gieser, J. P. (2019). Incidence of glaucoma or ocular hypertension after repeated anti-vascular endothelial growth factor injections for macular degeneration. Clin. Ophthalmol. 13, 2563–2572. doi:10.2147/OPTH.S232548
Wolfrum, P., BöHM, E. W., Lorenz, K., Stoffelns, B., Pfeiffer, N., and Korb, C. A. (2024). Short-term clinical outcomes of patients with diabetic macular edema following a therapy switch to faricimab. J. Clin. Med. 13, 4508. doi:10.3390/jcm13154508
Wong, T. Y., Haskova, Z., Asik, K., Baumal, C. R., Csaky, K. G., Eter, N., et al. (2024). Faricimab treat-and-extend for diabetic macular edema: two-year results from the randomized phase 3 YOSEMITE and RHINE trials. Ophthalmology 131, 708–723. doi:10.1016/j.ophtha.2023.12.026
Wykoff, C. C., Abreu, F., Adamis, A. P., Basu, K., Eichenbaum, D. A., Haskova, Z., et al. (2022). Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 399, 741–755. doi:10.1016/S0140-6736(22)00018-6
Xiong, X., Zhang, X., Li, X., and Huang, T. (2024). Adverse events associated with brolucizumab: a disproportionality analysis of the FDA adverse event reporting system (FAERS). Expert Opin. Drug Saf. 23, 1447–1452. doi:10.1080/14740338.2024.2322712
Yang, J. M., Jung, S. Y., Kim, M. S., Lee, S. W., Yon, D. K., Shin, J. I., et al. (2025). Cardiovascular and cerebrovascular adverse events associated with intravitreal anti-VEGF monoclonal antibodies: a world health organization pharmacovigilance study. Ophthalmology 132, 62–78. doi:10.1016/j.ophtha.2024.07.008
Yang, S., Li, T., Jia, H., Gao, M., Li, Y., Wan, X., et al. (2022). Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci. Transl. Med. 14, eabj2177. doi:10.1126/scitranslmed.abj2177
Zakaria, N., Guerard, N., Emanuelli, A., Dugel, P., Watts, J., Liew, M., et al. (2022). Evaluation of cardiac parameters and other safety outcomes of brolucizumab treatment in patients with neovascular age-related macular degeneration. Pharmacol. Res. Perspect. 10, e00897. doi:10.1002/prp2.897
Zarbin, M., Tabano, D., Ahmed, A., Amador, M., Ding, A., Holekamp, N., et al. (2024). Efficacy of faricimab versus aflibercept in diabetic macular edema in the 20/50 or worse vision subgroup in phase III YOSEMITE and RHINE trials. Ophthalmology 131, 1258–1270. doi:10.1016/j.ophtha.2024.05.025
Zhang, G., Wen, X., Li, Y., Sun, J., Jia, H., and Sun, X. (2024). Comprehensive assessment of the impact of intravitreal faricimab on retinal diseases: a systematic review, meta-analysis, and trial sequential analysis. Pharmacol. Res. 208, 107335. doi:10.1016/j.phrs.2024.107335
Zhang, Q., Zhang, X., Zhang, J., Wang, B., Tian, Q., Meng, X., et al. (2022). Vascular endothelial growth factor and the risk of venous thromboembolism: a genetic correlation and two-sample Mendelian randomization study. Thromb. J. 20, 67. doi:10.1186/s12959-022-00427-6
Zhao, J., and Tao, Y. (2024). Adverse event reporting of the IGF-1R monoclonal antibody teprotumumab: a real-world study based on the US food and drug administration adverse event reporting system. Front. Pharmacol. 15, 1393940. doi:10.3389/fphar.2024.1393940
Keywords: faricimab, adverse events, FAERS database, disproportionality, pharmacovigilance
Citation: He C-Z, Qiu Q, Lu S-J, Xue F-L, Liu J-Q and He Y (2025) Adverse event reporting of faricimab: a disproportionality analysis of FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 16:1521358. doi: 10.3389/fphar.2025.1521358
Received: 01 November 2024; Accepted: 26 February 2025;
Published: 12 March 2025.
Edited by:
Giovanni Luca Romano, Kore University of Enna, ItalyReviewed by:
Lucia Gozzo, University of Catania, ItalyCopyright © 2025 He, Qiu, Lu, Xue, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu He, MzA2MjQ0ODQzQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.