
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 28 February 2025
Sec. Experimental Pharmacology and Drug Discovery
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1516038
This article is part of the Research Topic Pharmacological Mechanisms of Drugs Affecting Bone Formation and Bone Resorption Volume II View all 16 articles
 Qing Lin
1,2
†
Qing Lin
1,2
†
 Biyi Zhao
1
†
Biyi Zhao
1
† Jiajia Huang3
†
Jiajia Huang3
† Rumeng Chen4
Rumeng Chen4 Weipeng Sun1
Weipeng Sun1
 Qianyun Ye
2,3
Qianyun Ye
2,3
 Li Yang
2,4
Li Yang
2,4
 Xiaofeng Zhu
1,2,3*
Xiaofeng Zhu
1,2,3*
 Xiaoyun Li
2,4*
Xiaoyun Li
2,4*
 Ronghua Zhang
2,4*
Ronghua Zhang
2,4*Osteoporosis (OP) is a complex bone metabolism disorder disease that affects the skeleton, nervous system, muscles, and multiple tissues. Neuropeptides, which are endogenous substances derived from both bone and brain, play a critical role in maintaining the balance of bone metabolism. This review summarizes research conducted from 1986 to 2024 on the pathological mechanisms of neuropeptides and their receptors in the context of OP. Specifically, the roles of Neuropeptide Y, Vasoactive Intestinal Peptide, Calcitonin Gene-Related Peptide, and Substance P and their receptors in key processes of OP were examined, including their function of bone formation and resorption, osteoblast differentiation, and osteoclast differentiation. Our study showed that these neuropeptides could promote bone formation and inhibit bone resorption, while their receptors in osteocytes exhibit distinct functions, indicating complex regulatory mechanisms that require further investigation. Additionally, we summarize the progress of Traditional Chinese Medicine (TCM) formulae, single TCM herbs, and bioactive compounds derived from TCM in exerting anti-OP effects through neuropeptide modulation. These studies highlight the multi-targeted and multi-mechanistic pharmacological actions of TCM in treating OP. By integrating these findings, we aim to enhance the understanding of neuropeptides’ roles in bone metabolism and to explore the development of neuropeptide-targeted TCM therapies for OP management. This comprehensive perspective highlights the potential of neuropeptides as therapeutic targets, paving the way for innovative approaches to treating OP.
Osteoporosis (OP) is a systemic bone metabolic disorder characterized by reduced bone strength and an increased risk of fractures, primarily resulting from decreased bone mass and the deterioration of bone microstructure. Globally, OP is estimated to affect approximately 18.3% of the population, with a notably higher prevalence among women at 23.1% compared to at 11.7% among men. In the aging demographic, this figure soars to 35.3% (Salari et al., 2021), representing a significant burden on healthcare systems, economies, and societies at large. The root cause of OP lies in the imbalance between bone formation and resorption, where insufficient bone formation fails to counteract excessive bone resorption (Li et al., 2021a). Bone formation is commonly associated with bone mesenchymal stem cells and osteoblasts, while osteoclasts are responsible for bone resorption. Consequently, the dynamic interplay between these cell types determines both the quality and quantity of bone. Studies have highlighted that signaling pathways, methylation modifications, and non-coding RNAs mediate these processes, along with the presence of endogenous active substances in the microenvironment.
Neuropeptides, the largest and most diverse class of signaling molecules in the brain, play multifaceted roles beyond neurotransmission. They can function as neurotransmitters, modulate ongoing neurotransmission by other transmitters, act as autocrine or paracrine regulators within localized cellular environments, and serve as hormones over the long term (Wilkinson and Brown, 2015). Neuropeptides exhibit high activity and a broad spectrum of effects, involving in the modulation of social valence, sleep, appetite, anxiety, stress response, pain perception (Smith et al., 2020; Wang et al., 2018). Research has indicated that neuropeptides regulate bone turnover and endogenous levels in the skeleton, influencing the occurrence and progression of OP (Chen et al., 2023; Wang et al., 2021). Specifically, Neuropeptide Y (NPY), Vasoactive Intestinal Peptide (VIP), Calcitonin Gene-Related Peptide (CGRP), and Substance P (SP), along with their respective receptors, have been identified as being expressed in both brain and bone tissue, as key contributors to bone growth and development (Liu et al., 2018a). Their absence can lead to bone metabolism imbalance and bone mass loss. These findings highlight the significant regulatory role of neuropeptides in bone metabolism.
In Traditional Chinese Medicine (TCM), the primary pathogenesis of OP is attributed to kidney deficiency, blood stasis, and spleen deficiency. Treatment focuses on nourishing the kidney and spleen, promoting blood circulation, and resolving blood stasis (Cao et al., 2024). TCM is widely used in the management of OP, recognized for its safety and efficacy. Clinical trials have shown that TCM can improve bone mineral density (BMD), alleviate pain, and cause minimal side effects in OP patients (Jia et al., 2022; Li et al., 2022a). For example, a randomized controlled trial involving 200 patients demonstrated that Zuogui and Yougui pills significantly improved lumbar spine and femoral BMD, reduced pain, and enhanced quality of life (Li et al., 2018). Similarly, our previous study revealed that Yigu capsules increased lumbar and hip BMD, relieved ostealgia, and extended motion time without causing new fractures or adverse reactions (Zhang et al., 2004). Unlike conventional treatments, TCM takes a holistic approach, addressing systemic imbalances and targeting multiple pathways. This approach facilitates personalized treatments with fewer adverse effects, aiming to optimize bone metabolism and overall health (Cao et al., 2024). Recent research highlights TCM’s multifaceted mechanisms in preserving and enhancing bone health. Specifically, TCM stimulates osteoblast activity (essential for bone formation) and inhibits osteoclast function (reducing bone resorption), maintaining the balance between bone formation and resorption. These effects are partly mediated by neuropeptides, suggesting that TCM may act as a neuropeptide modulator (Cao et al., 2024; Lei et al., 2021; Li et al., 2023; Peng et al., 2022).
To date, research publications on neuropeptides have exceeded 2,600, with sources from the Chinese National Knowledge Infrastructure (http://www.cnki.net/), the National Science and Technology Library (http://www.nstl.gov.cn/), and approximately 1,253 from the PubMed (www.pubmed.gov) database. This review aims to enhance the understanding of the role of neuropeptides in bone metabolism and explore potential anti-OP strategies using TCM based on neuropeptide targets. More than 70 references were consulted from various databases, spanning the period from their inception to October 2024. These findings highlight the increasing number of therapeutic approaches.
Until now, synapses have not been identified within bone; however, various neuropeptides have been found in bone tissue, including NPY, VIP, SP and CGRP. Neuropeptides commonly released into the extracellular space through non-synaptic vesicular fusion within axon varicosities. After their release into the extracellular fluid, these signaling molecules are transported to receptors on the targeted bone tissues via energy gradients, subsequently stimulating the activities of related cells (Figure 1; Table 1).
NPY, a 36-amino acid polypeptide, belongs to the pancreatic polypeptide family and is widely distributed throughout both the central and peripheral nervous systems. In the central nervous system, NPY is particularly abundant in several key regions, including the hypothalamus, cerebral cortex, brainstem, striatum, and limbic system, with especially high concentrations found in the arcuate nucleus of the hypothalamus. In the peripheral nervous system, NPY plays a crucial role in the sympathetic nervous system, where it is co-stored with norepinephrine in sympathetic neurons and released alongside norepinephrine upon neural stimulation (Baldock et al., 2009; Shende and Desai, 2020). The role of NPY and its receptors, specifically the Neuropeptide Y1 receptor (NPY1R) and the Neuropeptide Y2 receptor (NPY2R), is closely linked to the pathogenesis of OP, suggesting that NPY signaling may significantly influence bone metabolism (Khor and Baldock, 2012).
NPY functions as a regulator of bone homeostasis, with its effects on bone mass closely associated with fluctuations in hypothalamic NPY levels and energy intake. Research has demonstrated that in ovariectomized (OVX) rats, a decrease in bone density coincides with a significant increase in NPY expression in both the hypothalamus and femur (Li et al., 2022b). In NPY knockout (KO) mouse, both trabecular and cortical bone volumes are elevated. Furthermore, reducing NPY expression in the hypothalamus has been shown to enhance bone mass in rats with adequate energy intake. Conversely, NPY expression in the hypothalamus decreases during fasting, which is accompanied by a reduction in bone mass, likely linked to decreased energy consumption (Baldock et al., 2009).
Additionally, NPY is expressed in osteoblasts, bone marrow mesenchymal stem cells (BMSCs), and osteoclasts, and it is directly involved in the differentiation and proliferation of these cells. Research has shown that NPY can inhibit the expression of markers associated with osteoblast differentiation and suppress the differentiation of osteoblasts (Baldock et al., 2009). The specific knockout of NPY derived from BMSCs has been found to enhance cortical bone in KO rats, promoting the expression of bone sialoprotein and osteocalcin in BMSCs, thereby facilitating the osteogenic differentiation of these cells (Wee et al., 2019; Wee et al., 2020). Other studies suggest that NPY may also be involved in regulating bone resorption. NPY can inhibit the cAMP/PKA pathway activated by isoproterenol, which promotes the secretion of receptor activator of nuclear factor-κB ligand (RANKL) by osteoblasts, consequently increasing the bone resorption activity of osteoclasts (Amano et al., 2007). In summary, with adequate energy intake, the inhibition of NPY expression of NPY in bone tissue leads to an increase in bone mass and enhances the processes of bone healing. In conclusion, the mechanisms by which NPY influences OP may involve promoting osteogenic differentiation in BMSCs and inhibiting bone resorption in osteoclasts.
NPY1R is expressed in both central and peripheral tissues, demonstrating an inverse correlation in its expression levels between the central nervous system and bone tissues in OP model animals. This makes NPY1R an important target for regulating bone metabolism. Studies indicate that NPY1R expression is elevated in the bone tissue of OVX rats, while its expression decreases in the central nervous system. The administration of NPY1R could mitigate bone tissue damage caused by the OVX operation (Xie et al., 2020a). Baldock et al. found that NPY1R KO rats exhibit increased bone mass. Further investigations revealed that the specific knockout of NPY1R in the hypothalamus does not affect bone mass, whereas the specific knockout of NPY1R in osteoblasts enhances the rates of bone mineral deposition and bone formation, thereby increasing bone mass in mouse (Baldock et al., 2007; Lee et al., 2011). NPY1R also regulates the healing process of fractures. Zou et al. found that during the initial stage of fracture healing, the expression level of NPY1R is relatively low. However, it increases significantly during the intermediate stage and then declines back to a lower level in the later stage. This pattern indicates that NPY1R is involved in the mid-to-late stages of fracture healing and aids in the formation and remodeling of callus tissue (Zou et al., 2020). Recent studies have shown that NPY1R deficiency can enhance bone strength and reduce fracture risk by improving the ultrastructure of the extracellular matrix and increasing matrix maturity (Sousa et al., 2020).
Research indicates that the knockout of NPY1R gene not only enhances the activity of osteoblasts in mouse, increasing bone turnover and bone mass, but also promotes the proliferation and osteogenic differentiation of BMSCs (Baldock et al., 2009; Liu et al., 2016b). Further exploration has revealed that activating NPY1R expression can inhibit the osteogenic differentiation of BMSCs via the cAMP/PKA/CREB signaling pathway (Xie et al., 2020b), whereas inhibiting NPY1R expression enhances the osteogenic differentiation capabilities of BMSCs (Liu et al., 2018a). Similarly, inhibiting the expression of NPY1R on pre-osteoblastic MC3T3-E1 can also enhance osteogenic differentiation, with the underlying mechanism linked to the regulation of the extracellular signal-regulated kinase signaling pathway (Yu et al., 2016). Thus, NPY1R is expressed in osteoblasts on both cortical and trabecular bone surfaces, and its inhibition of osteoblast and BMSC may be related to the regulation of NPY. Additionally, Dong et al. suggested that regulating the NPY/NPY1R signaling pathway promotes osteogenic differentiation of BMSCs and fracture healing (Dong et al., 2018).
NPY2R expression increases in the tibia and dorsal root ganglia of OVX rats. The administration of NPY2R antagonists in OVX rats can enhance bone density and mitigate bone loss (Seldeen et al., 2018). In contrast to NPY1R, both global and conditional knockout of NPY2R in the hypothalamus result in an increased number of trabecular bones in the femurs of rats (Baldock et al., 2002). However, the conditional knockout of peripheral NPY2R has no effect on the skeletal system (Shi et al., 2011). Therefore, hypothalamic NPY2R might play a more critical role in regulating bone metabolism compared to peripheral NPY2R. Furthermore, unlike other receptors, NPY2R functions as an auto-receptor, providing negative feedback regulation of NPY expression. The conditional knockout of NPY2R on NPY neurons in the hypothalamus of rats leads to an increase in NPY levels within the hypothalamus, which diminishes the stimulatory effect on bone formation and may even inhibit it (Hohmann et al., 1986). Thus, apart from NPY2R on NPY neurons, NPY2R on other neurons in the hypothalamus may play a significant role in regulating bone metabolism.
Moreover, promoting bone formation following NPY2R knockout may be associated with an increased osteogenic differentiation of osteoblasts and the proliferation of BMSCs (Baldock et al., 2002). Lundberg et al. found that the number of BMSCs in rats with systemic NPY2R deficiency increased nearly doubled in global NPY2R knockout mice, and NPY2R knockout led to a decrease in NPY1R expression on osteoblasts (Lundberg et al., 2007). Therefore, in addition to the role of NPY2R expressed on NPY neurons, NPY2R on other hypothalamic neurons may also significantly contribute to the regulation of bone metabolism.
VIP, composed of 28 amino acids, is a small neuropeptide that belongs to the glucagon-like polypeptide family. It is primarily released by intestinal neurons, as well as by endocrine and immune cells. The main receptors for VIP are vasoactive intestinal peptide receptor 1 (VIPR1) and vasoactive intestinal peptide receptor 2 (VIPR2). In bone tissue, VIP is predominantly found in the parasympathetic neurons of the skeleton, with smaller amounts present in postganglionic sympathetic neurons and primary sensory neurons. VIP plays a crucial role in regulating bone metabolic balance (Hohmann et al., 1986).
VIP plays a dual role in inhibiting bone resorption and promoting bone formation. Clinical studies have demonstrated that serum levels of VIP are inversely correlated with the severity of postmenopausal OP (Wang et al., 2019), indicating a significant relationship between VIP and bone resorption. Research suggests that inhibiting sympathetic VIP secretion can lead to an increase in the number of osteoclasts and elevated levels of cortical bone resorption on the surface of the mandible in rats (Hill et al., 1991; Joo et al., 2004). Furthermore, VIP can promote osteoclast activity without affecting the number of osteoclast precursors (Qu et al., 2021). VIP not only acts directly on osteoclasts but also indirectly regulates bone resorption by influencing osteoblasts. In vitro studies by Mukohyama et al. have shown that VIP can enhance the secretion of bone protective proteins from BMSCs or osteoblasts, inhibit the expression of nuclear factor kappa B receptor activator (RANK) and RANKL (receptor activator of RANK), and thereby indirectly inhibit osteoclasts differentiation and bone resorption (Mukohyama et al., 2000). Additionally, VIP accelerates the formation of mineralized nodules in osteoblasts, promoting osteogenic differentiation (Lundberg et al., 1999; Ma et al., 2013). Shi et al. has found that VIP can activate the Wnt/β-catenin signaling pathway to enhance BMSCs and stimulate the expression of vascular endothelial growth factor to promote angiogenesis (Shi et al., 2020).
VIP1R and VIP2R are the primary receptors for VIP. VIP1R is closely associated with the osteogenic differentiation of osteoblasts, whereas VIP2R is involved in both bone resorption and formation. Studies have demonstrated that osteoblasts derived from the periosteum and osteosarcoma express VIP1R but not VIP2R (Togari et al., 1997). The expression of VIP1R increases during the later stages of osteoblast differentiation, indicating a strong correlation between VIP1R and bone formation (Lundberg et al., 2001). In contrast, its activation can elevate the RANKL/OPG ratio and increase the expression of interleukin-6 by activating various signaling pathways, including cAMP-ERK, p44/p42 MAPK, and cAMP/PKA/CREB (Natsume et al., 2010; Persson and Lerner, 2011; Persson et al., 2005), thereby promoting osteoclast-mediated bone resorption. Furthermore, the activation of VIP2R can enhance the levels of cyclic adenosine monophosphate (cAMP) in osteoblasts, stimulating the expression of alkaline phosphatase and osteocalcin, which in turn promotes osteogenic differentiation (Lundberg et al., 1999; Ma et al., 2013).
CGRP, a neuropeptide consisting of 37 amino acids, is released by sensory nerve terminals and is widely distributed throughout both the central and peripheral nervous systems. Research has shown that CGRP is primarily localized in the periosteum and bone marrow. It can bind to the calcitonin receptor-like protein (CALCRL) on bone-related cells, thereby influencing their activity and playing a crucial regulatory role in bone growth and repair (Irie et al., 2002).
CGRP has significant benefits for bone growth and repair. In OVX rats, CGRP levels are upregulated in the spinal cord but downregulated in the serum and femoral tissue (Zhang et al., 2021). In a rat model of femoral fracture, α-CGRP deficiency leads to severe impairments in bone regeneration, characterized by a significant reduction in osteoblast numbers, incomplete healing of the callus, and high rates of nonunion. These impairments are closely associated with the differential expression of specific genes related to ossification, bone remodeling, and adipogenesis. Among these, CGRP-induced peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling plays a pivotal role in fracture healing (Appelt et al., 2020). CGRP may enhance the proliferation and migration of BMSCs extracted from OP rats, via the Wnt/β-catenin signaling pathway (Liang et al., 2015). CGRP stimulates the homing and differentiating into osteoblasts. Subsequently, these osteoblasts produce increased levels of insulin-like growth factor 1 (IGF-1) while inhibiting the generation of tumor necrosis factor-α (TNF-α), thereby further regulating osteoblast function (Valentijn et al., 1997). Additional studies have demonstrated that CGRP enhances bone formation mediated by osteoblasts by stimulating Wnt signaling, promoting the expression of bone morphogenetic protein 2 (BMP-2) and Runt-related transcription factor 2 (Runx2), inhibiting the expression of inflammatory factors, and preventing apoptosis (Cai et al., 2016; Mrak et al., 2010; Tuzmen and Campbell, 2018). Consequently, the effects of CGRP on bone metabolism are primarily related to the regulation of BMSCs and osteoblasts.
CALCRL is a known receptor for CGRP. Its expression is decreased in brains of OVX rats, demonstrating a negative correlation with bone formation (Liu et al., 2018b). Overexpression of the CALCRL gene enhances bone mass in the femora of rats, whereas silencing the CALCRL gene exhibits the opposite effect (Zhang et al., 2016). Research has also indicated that CALCRL is expressed in bone-related cells, including induced pluripotent stem cells, hematopoietic precursor cells, and BMSCs, which are closely linked to their differentiation processes. CALCRL and receptor activity-modifying protein one form a functional heterodimer complex that can be activated by CGRP, leading to the expression of the Creb1 gene and the osteoblast-specific gene Sp7, thereby facilitating the osteogenic differentiation of periosteal-derived stem cells (Zhang et al., 2016). CALCRL expression increases during the osteogenic differentiation of induced pluripotent stem cells and periosteal-derived osteoblasts, participating in the local regulation of human bone metabolism alongside norepinephrine (Nagao et al., 2014; Wang et al., 2011; Zhang B. et al., 2022). Furthermore, CGRP can promote the osteogenic differentiation of osteosarcoma cells, which is associated with an increase in intracellular free calcium ion concentration (Drissi et al., 1999). Additionally, the CALCRL gene has an inhibitory effect on bone resorption. Studies have shown that monocyte-macrophage cells with CALCRL gene defects cannot mature into mature osteoclasts following after RANKL stimulation (Cho et al., 2022).
SP is a neuropeptide located in primary sensory neurons and is released from the axons of these neurons following neural stimulation. It belongs to the tachykinin family and is primarily synthesized by neurons in the dorsal root ganglion. SP binds to the neurokinin-1 receptor to exert various physiological effects. Research has demonstrated that SP is present in bones, bone marrow, epiphyseal plates, ligaments, and muscles, where it is distributed in active sites of bone formation during bone metabolism. It serves as a crucial local regulator of bone-related cellular functions (Liu et al., 2007).
SP can delay the onset and progression of OP while promoting bone repair. In OVX rat models, the expression of SP in bone tissue is significantly reduced (Liu X. et al., 2018). Similarly, in the OVX fracture rat model, the fracture healing rate in OVX rats is notably decreased, which is accompanied by a reduction in SP content at the fracture site (Ding et al., 2010). Conversely, SP intervention can protect against the loss of H-type blood vessels in early estrogen-deficient bone tissue by regulating oxidative stress, thereby preventing the development of OP (Kim et al., 2023). Furthermore, research indicates that SP can mitigate chronic inflammation by promoting the polarization of regulatory T cell and inhibiting the development of T helper 17 cells, which are associated with osteoclast generation and activation. This process enhances the proliferation and osteoclastogenesis of BMSCs from OVX rats, thereby accelerating bone formation (Piao et al., 2020). Inhibition of SP signaling not only reduces bone mass in normal rats but also accelerates bone loss in OVX rats (Zheng et al., 2016). Furthermore, SP promotes the proliferation and osteogenic differentiation of BMSCs or osteoblasts, with its effects dependent on the concentration of SP. In vitro cultures of BMSCs, both the number and size of bone marrow cell colonies are positively correlated with SP concentration, indicating that it stimulates osteogenic differentiation (Shih and Bernard, 1997). Additionally, SP enhances the proliferation of osteoblasts in rat models of spinal cord injury through the RANKL/OPG signaling axis; however, it inhibits their osteogenic differentiation and mineralization (Liu et al., 2016a).
Other studies suggest that low concentrations of SP can enhance the proliferation of BMSCs, while high concentrations of SP have the opposite effect (Wang et al., 2009). Furthermore, SP induction can accelerate bone resorption by osteoclasts, with the underlying mechanisms linked to inflammatory responses. SP can induce the osteoclastic differentiation of RAW 264.7 cells through the upregulation of NF-κB and TNF-α, thereby promoting bone resorption (Lam et al., 2000; Wang et al., 2009). A deficiency of SP may reduce the number of osteoclasts and subsequently diminish the capacity for bone resorption (Niedermair et al., 2018). In summary, SP promotes the bone resorption activity of osteoclasts and exhibits beneficial effects on bone repair in OP animal models. The underlying mechanism may involve an acceleration of the bone remodeling rate by SP, with its effects on bone formation outweighing its effects on bone resorption.
TACR1 is a well-known receptor for SP, and it is involved in the mediation of phosphatidylinositol metabolism of SP. In OVX rats, the expression of TACR1 was also significantly reduced in the brain, but increased in the bone (Liu et al., 2018a). Further studies have demonstrated that activating TACR1 can promote the osteogenic differentiation of late-stage osteoblasts, while blocking TACR1 produces the opposite effect (Goto et al., 2007). Therefore, TACR1 expressed on osteoblasts presents a promising therapeutic target for the treatment of OP.
Neuropeptides are highly susceptible to degradation, presenting significant challenges for their application and stability in both in vivo and in vitro studies. This instability often limits their therapeutic potential and necessitates alternative strategies to harness their benefits. TCM has demonstrated a significant role in combating OP by promoting the secretion of neuropeptides in both bone and brain tissues. These neuropeptides, in turn, modulate the activity of bone-related cells, such as osteoblasts and osteoclasts, which are critical for maintaining bone homeostasis. By enhancing neuropeptide production, TCM indirectly supports bone formation while inhibiting bone resorption, contributing to the preservation of bone density and structural integrity (Figure 2; Table 2). This unique mechanism suggests that TCM may serve as a valuable adjunct in OP treatment, addressing challenges associated with neuropeptide degradation while offering a holistic and multifaceted approach to bone health.
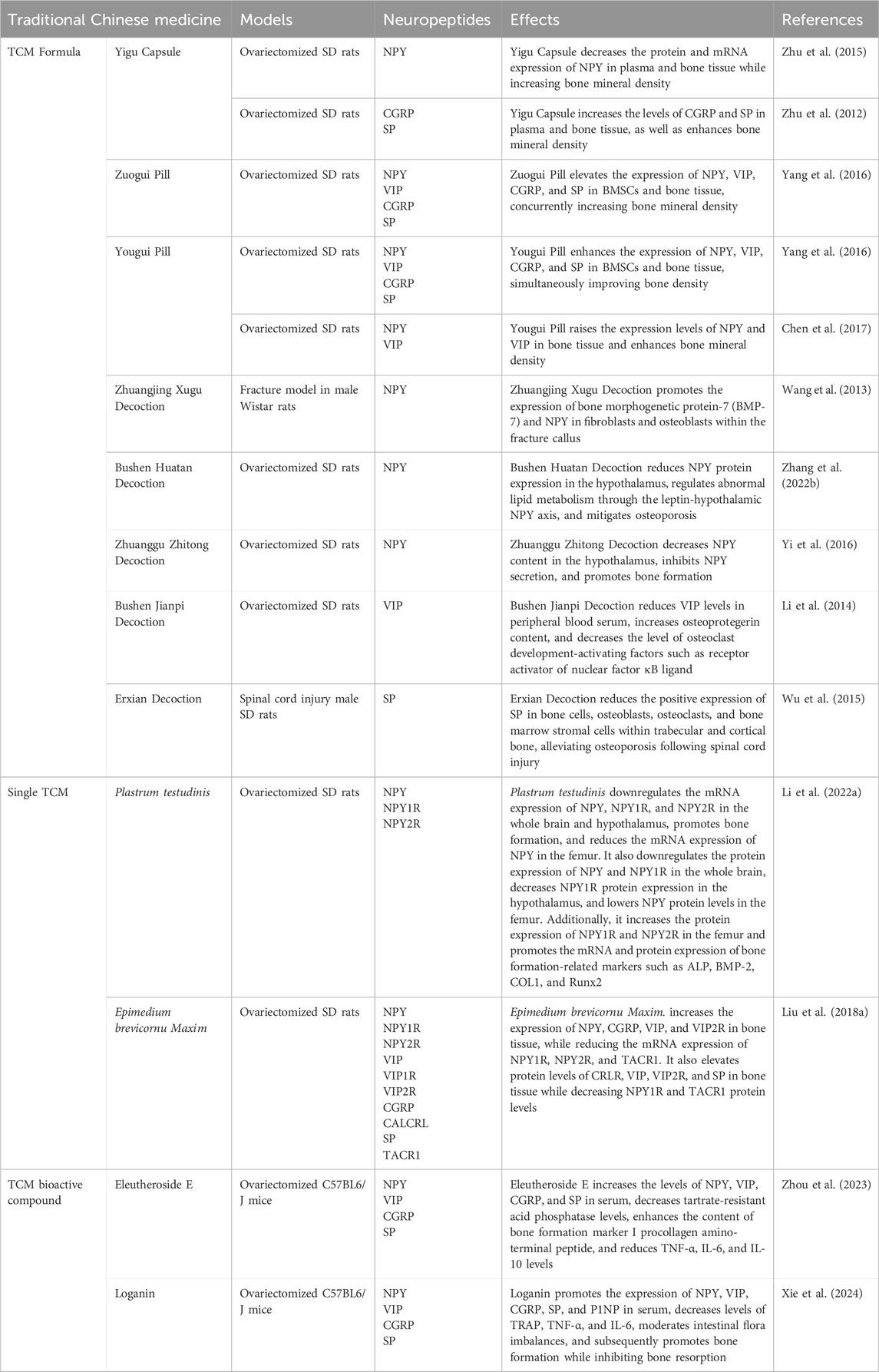
Table 2. The role of traditional Chinese medicine in modulating bone metabolism through neuropeptide regulation.
The role of TCM in enhancing bone health and treating OP has been extensively studied. Among these, the Yigu Capsule has demonstrated the ability to increase bone density in OVX rats, accompanied by elevated NPY levels in both plasma and bone tissue (Zhu et al., 2015). Similarly, the Yougui Pill has been found to significantly enhance NPY levels in the bone tissue, contributing to improvements in bone mineral density in OVX rats (Chen et al., 2017). The Zhuangjing Xugu Decoction, on the other hand, facilitates fracture healing by upregulating NPY expression in callus tissue fibroblasts (Wang et al., 2013). Furthermore, the Bushen Huatan Decoction has been reported to modulate the dysregulated adipose-bone metabolism in OVX rats via the hypothalamic leptin-NPY axis (Zhang Y. et al., 2022). Meanwhile, the Zhuanggu Zhitong Decoction has demonstrated the ability to reduce hypothalamic NPY levels while concurrently increasing bone density in OP rats (Yi et al., 2016).
In addition to NPY, other neuropeptides have been implicated in bone health. The Zuogui Pill and Yougui Pill enhance bone repair by increasing the levels of VIP in BMSCs and bone tissue of OVX rats (Yang et al., 2016). Similarly, the Bushen Jianpi Decoction elevates bone mineral density and VIP levels in the peripheral blood serum of OP rats (Li et al., 2014).
The CGRP is another key factor influenced by traditional medicines. The Yigu Capsule improves bone mineral density in OVX rats by elevating CGRP levels in bone tissue and plasma, thus offering a potent therapeutic approach for OP (Zhu et al., 2012). In a similar vein, the Zuogui Pill and Yougui Pill foster bone repair by increasing CGRP levels in BMSCs and the bone tissue of OVX rats (Yang et al., 2016).
Moreover, SP has been identified as a critical mediator of bone repair. Both the Zuogui Pill and Yougui Pill promote bone repair and stimulate BMSC proliferation in OVX rats by increasing SP content in bone tissue (Yang et al., 2016). The Yigu Capsule also raises SP levels in bone tissue and plasma, leading to increased bone density (Zhu et al., 2012). Finally, the Erxian Decoction enhances bone density through the upregulation of SP expression in the distal femur of OVX rats (Wu et al., 2015).
We conducted an analysis of these formulas and identified Lycium chinense Miller., Epimedium brevicornu Maxim., and Angelica sinensis (Oliv.) Diels. as the most abundant components. Notably, the whole extract of Sida cordifolia L. and its most potent aqueous fraction were shown to significantly upregulate neuropeptides, including CGRP and SP, in nerve-injured rats exhibiting pain-like behavior (Tiwari and Hemalatha, 2024). Additionally, the combination of ferulic acid was found to reduce neurological deficits, decrease infarct volume, and inhibit the expression of IL-1β and NPY in a transient middle cerebral artery occlusion rat model (Ge et al., 2015). Furthermore, Epimedium brevicornu Maxim. and its active component, icariin, were observed to downregulate the expression of key proteins such as NPY, NPY1R, SP R, and 5-HT1B R, while significantly reducing VIP levels in a KOA rat model (Li et al., 2021b). Collectively, these findings provide strong evidence that these formulas play a role in the regulation of neuropeptides.
The research on TCM and its effects on neuropeptides remains quite limited. Plastrum testudinis has been found to promote bone formation in OVX rats by reducing the expression of NPY and its receptor NPY1R in both brain and hypothalamic tissues, along with lowering NPY protein levels in femoral tissue (Li et al., 2022b). Additionally, Epimedium brevicornu Maxim. has been found to increase the expression of NPY, CGRP, VIP, and VIP2R in bone tissue, while reducing the mRNA expression of NPY1R, NPY2R, and TACR1. It also elevates protein levels of CRLR, VIP, VIP2R, and SP in bone tissue while decreasing NPY1R and TACR1 protein levels (Liu et al., 2018a).
Recent studies highlight the potential of bioactive compounds in modulating neuropeptide levels to regulate bone metabolism and improve bone health in OVX rats. Loganin, a bioactive compound derived from Strychnos nux-vomica L., has been shown to significantly elevate serum NPY levels in OVX rats, thereby contributing to the regulation of bone metabolism and promoting a balance in bone remodeling (Xie et al., 2024). Similarly, Eleutheroside E, extracted from Acanthopanax senticosus, exhibits a dual role in enhancing bone health. It significantly increases serum concentrations of both NPY and procollagen type 1 N-terminal propeptide in OVX rats, indirectly influencing bone metabolism and resulting in improved bone mass (Zhou et al., 2023). Additionally, Eleutheroside E has also been found to elevate serum VIP levels in OVX rats, further regulating bone metabolism and promoting bone mass accrual (Zhou et al., 2023).
This review explores the roles of the neuropeptides NPY, VIP, CGRP, and SP in bone metabolism, as well as the TCM that can regulate these neuropeptides. However, current research is still limited. For one thing, the interactions among different neuropeptides and their specific regulatory mechanisms in bone metabolism have not been fully elucidated. For another, despite the potential demonstrated by TCM in regulating neuropeptides, its specific mechanisms and active ingredients still need thorough exploration. We thought that future research should focus on the following strategies. Firstly, the application of single-cell spatial transcriptomics is recommended to comprehensively map the heterogeneity of neuropeptide receptors across various bone cell subtypes. This approach will facilitate a detailed understanding of the molecular mechanisms underlying bone-nerve interactions at the cellular level. Secondly, the development of biomaterial-assisted delivery systems, such as mesoporous silica nanoparticles, should be pursued to achieve site-specific modulation of neuropeptides. This strategy has the potential to effectively overcome off-target effects, thereby enhancing the precision and efficacy of therapeutic interventions. Thirdly, the establishment of TCM component libraries paired with neuropeptide receptor CRISPR screening platforms is proposed to identify synergistic phytochemical combinations. This integrative approach will leverage the rich pharmacological diversity of TCM to identify novel therapeutic agents that can modulate bone-nerve crosstalk.
Our findings advocate for a paradigm shift from the traditional single-target inhibition approach to the restoration of multi-neuropeptide equilibrium. This shift positions TCM-derived formulations as precision modulators of bone-nerve interactions, offering a novel and potentially more effective therapeutic strategy. By bridging the current gap between mechanistic understanding and clinical application, this approach holds significant promise for improving the management of bone-related disorders, particularly in elderly patients with comorbid metabolic and neurological conditions.
QL: Writing–original draft. BZ: Writing–original draft. JH: Investigation, Writing–original draft. RC: Writing–original draft. WS: Investigation, Writing–original draft. QY: Investigation, Writing–original draft. LY: Writing–review and editing. XZ: Writing–review and editing. XL: Writing–review and editing. RZ: Conceptualization, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Joint Funds of the National Natural Science Foundation of China (U24A6013), the National Natural Science Foundation of China (82074287, 82274232, 82274376 and 82405121), National Key R&D Program of China (2018YFC2002500), Science and Technology Program Project of Guangdong Province - Guangdong Provincial Key laboratory of Traditional Chinese Informatization (2021B1212040007), Fundamental Research Funds for the Central Universities (21622329, 21623115, 21624315), Basic and Applied Basic Research Fund of Guangdong Province (2022B1515120022), and Construction project of Guangdong Famous Traditional Chinese Medicine Inheritance Studio of RZ (Guangdong Traditional Chinese Medicine Letter (2023) No. 108). We would like to express gratitude to the MCMIA and Vincent and Lily Woo Foundation for the Vincent and Lily Woo Fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amano, S., Arai, M., Goto, S., and Togari, A. (2007). Inhibitory effect of NPY on isoprenaline-induced osteoclastogenesis in mouse bone marrow cells. Biochim. Biophys. Acta 1770, 966–973. doi:10.1016/j.bbagen.2007.02.009
Appelt, J., Baranowsky, A., Jahn, D., Yorgan, T., Köhli, P., Otto, E., et al. (2020). The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine 59, 102970. doi:10.1016/j.ebiom.2020.102970
Baldock, P. A., Allison, S. J., Lundberg, P., Lee, N. J., Slack, K., Lin, E. J., et al. (2007). Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J. Biol. Chem. 282, 19092–19102. doi:10.1074/jbc.M700644200
Baldock, P. A., Lee, N. J., Driessler, F., Lin, S., Allison, S., Stehrer, B., et al. (2009). Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One 4, e8415. doi:10.1371/journal.pone.0008415
Baldock, P. A., Sainsbury, A., Couzens, M., Enriquez, R. F., Thomas, G. P., Gardiner, E. M., et al. (2002). Hypothalamic Y2 receptors regulate bone formation. J. Clin. Invest. 109, 915–921. doi:10.1172/JCI14588
Cai, J., Lv, J., Li, S. T., Gao, Q. G., and Zhang, G. (2016). Calcitonin gene-related peptide promotes the differentiation of mouse osteoblasts by inhibiting the expression of NOD-like receptor protein 3. West China J. Stomatology 34, 12–16. doi:10.7518/hxkq.2016.01.003
Cao, G., Hu, S., Ning, Y., Dou, X., Ding, C., Wang, L., et al. (2024). Traditional Chinese medicine in osteoporosis: from pathogenesis to potential activity. Front. Pharmacol. 15, 1370900. doi:10.3389/fphar.2024.1370900
Chen, J., Xie, L., Tao, Y., Xu, G., Ma, Y. M., and Dai, M. (2017). Effects of Yougui pill on bone density, neuropeptide Y, and vasoactive intestinal peptide in ovariectomy induced osteoporosis rats. Chin. J. Exp. Surg. doi:10.3760/cma.j.issn.1001-9030.2017.05.059
Chen, Z., Lv, M., Liang, J., Yang, K., Li, F., Zhou, Z., et al. (2023). Neuropeptide Y-mediated gut microbiota alterations aggravate postmenopausal osteoporosis. Adv. Sci. (Weinh) 10, e2303015. doi:10.1002/advs.202303015
Cho, E., Cheon, S., Ding, M., Lim, K., Park, S. W., Park, C., et al. (2022). Identification of novel genes for cell fusion during osteoclast formation. Int. J. Mol. Sci. 23, 6421. doi:10.3390/ijms23126421
Ding, W. G., Zhang, Z. M., Zhang, Y. H., Jiang, S. D., Jiang, L. S., and Dai, L. Y. (2010). Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 159, 28–34. doi:10.1016/j.regpep.2009.11.004
Dong, P., Gu, X., Zhu, G., Li, M., Ma, B., and Zi, Y. (2018). Melatonin induces osteoblastic differentiation of mesenchymal stem cells and promotes fracture healing in a rat model of femoral fracture via neuropeptide Y/neuropeptide Y receptor Y1 signaling. Pharmacology 102, 272–280. doi:10.1159/000492576
Drissi, H., Lieberherr, M., Hott, M., Marie, P. J., and Lasmoles, F. (1999). Calcitonin gene-related peptide (CGRP) increases intracellular free Ca2+ concentrations but not cyclic AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4). Cytokine 11, 200–207. doi:10.1006/cyto.1998.0415
Ge, L. J., Fan, S. Y., Yang, J. H., Wei, Y., Zhu, Z. H., Lou, Y. J., et al. (2015). Pharmacokinetic and pharmacodynamic analysis of ferulic acid-puerarin-astragaloside in combination with neuroprotective in cerebral ischemia/reperfusion injury in rats. Asian Pac J. Trop. Med. 8, 299–304. doi:10.1016/S1995-7645(14)60334-5
Goto, T., Nakao, K., Gunjigake, K. K., Kido, M. A., Kobayashi, S., and Tanaka, T. (2007). Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 41, 25–31. doi:10.1016/j.npep.2006.11.002
Hill, E. L., Turner, R., and Elde, R. (1991). Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience 44, 747–755. doi:10.1016/0306-4522(91)90094-5
Hohmann, E. L., Elde, R. P., Rysavy, J. A., Einzig, S., and Gebhard, R. L. (1986). Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232, 868–871. doi:10.1126/science.3518059
Irie, K., Hara-Irie, F., Ozawa, H., and Yajima, T. (2002). Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc. Res. Tech. 58, 85–90. doi:10.1002/jemt.10122
Jia, Y., Sun, J., Zhao, Y., Tang, K., Zhu, R., Zhao, W., et al. (2022). Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered 13, 5581–5597. doi:10.1080/21655979.2022.2038941
Joo, K. M., Chung, Y. H., Kim, M. K., Nam, R. H., Lee, B. L., Lee, K. H., et al. (2004). Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J. Comp. Neurol. 476, 388–413. doi:10.1002/cne.20231
Khor, E. C., and Baldock, P. (2012). The NPY system and its neural and neuroendocrine regulation of bone. Curr. Osteoporos. Rep. 10, 160–168. doi:10.1007/s11914-012-0102-7
Kim, D., Piao, J., Park, J. S., Lee, D., Hwang, D. Y., and Hong, H. S. (2023). Substance P-mediated vascular protection ameliorates bone loss. Oxid. Med. Cell Longev. 2023, 9903336. doi:10.1155/2023/9903336
Lam, J., Takeshita, S., Barker, J. E., Kanagawa, O., Ross, F. P., and Teitelbaum, S. L. (2000). TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488. doi:10.1172/JCI11176
Lee, N. J., Nguyen, A. D., Enriquez, R. F., Doyle, K. L., Sainsbury, A., Baldock, P. A., et al. (2011). Osteoblast specific Y1 receptor deletion enhances bone mass. Bone 48, 461–467. doi:10.1016/j.bone.2010.10.174
Lei, S. S., Su, J., Zhang, Y., Huang, X. W., Wang, X. P., Huang, M. C., et al. (2021). Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: a review. Int. J. Biol. Macromol. 193, 1996–2005. doi:10.1016/j.ijbiomac.2021.11.030
Li, H. H., Yu, Z., Zhao, H. Y., Yi, X. L., Pan, J. H., Liu, H., et al. (2014). Exploration of the mechanism of “spleen kidney correlation” and the effect of kidney tonifying and spleen strengthening formula on the levels of OPG, RANKL, VIP, MTL, and GAS in peripheral blood of rats with spleen kidney deficiency type osteoporosis. China J. Basic Med. inTraditional Chin. Med. 20, 1628–1631. doi:10.19945/j.cnki.issn.1006-3250.2014.12.015
Li, J., Fu, S. F., Yang, Y., An, R., Liu, H. Y., and Mao, H. P. (2022). Clinical practice of traditional Chinese medicine for the treatment of postmenopausal osteoporosis: a literature review. Climacteric 25, 562–569. doi:10.1080/13697137.2022.2102894
Li, K., Jiang, Y., Wang, N., Lai, L., Xu, S., Xia, T., et al. (2023). Traditional Chinese medicine in osteoporosis intervention and the related regulatory mechanism of gut microbiome. Am. J. Chin. Med. 51, 1957–1981. doi:10.1142/S0192415X23500866
Li, Q., Cheng, J. C., Jiang, Q., and Lee, W. Y. (2021). Role of sirtuins in bone biology: potential implications for novel therapeutic strategies for osteoporosis. Aging Cell 20, e13301. doi:10.1111/acel.13301
Li, W., Liu, Z., Liu, L., Yang, F., Li, W., Zhang, K., et al. (2018). Effect of Zuogui pill and Yougui pill on osteoporosis: a randomized controlled trial. J. Tradit. Chin. Med. 38, 33–42. doi:10.1016/j.jtcm.2018.01.005
Li, X., Xu, Y., Li, H., Jia, L., Wang, J., Liang, S., et al. (2021). Verification of pain-related neuromodulation mechanisms of icariin in knee osteoarthritis. Biomed. Pharmacother. 144, 112259. doi:10.1016/j.biopha.2021.112259
Li, X. Y., Lin, Q., Chen, R. M., Wang, H. Y., Cui, Y., and Zhang, R. H. (2022). Mechanism of Plastrum testudinis extracts regulating bone formation in ovariectomized rats through neuropeptides. China J. Traditional Chin. Med. Pharm. 37, 7076–7081.
Liang, W., Zhuo, X., Tang, Z., Wei, X., and Li, B. (2015). Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol. Cell Biochem. 402, 101–110. doi:10.1007/s11010-014-2318-6
Liu, D., Jiang, L. S., and Dai, L. Y. (2007). Substance P and its receptors in bone metabolism. Neuropeptides 41, 271–283. doi:10.1016/j.npep.2007.05.003
Liu, H., Xiong, Y., Wang, H., Yang, L., Wang, C., Liu, X., et al. (2018). Effects of water extract from epimedium on neuropeptide signaling in an ovariectomized osteoporosis rat model. J. Ethnopharmacol. 221, 126–136. doi:10.1016/j.jep.2018.04.035
Liu, H. J., Yan, H., Yan, J., Li, H., Chen, L., Han, L. R., et al. (2016). Substance P promotes the proliferation, but inhibits differentiation and mineralization of osteoblasts from rats with spinal cord injury via RANKL/OPG system. PLoS One 11, e0165063. doi:10.1371/journal.pone.0165063
Liu, S., Jin, D., Wu, J. Q., Xu, Z. Y., Fu, S., Mei, G., et al. (2016). Neuropeptide Y stimulates osteoblastic differentiation and VEGF expression of bone marrow mesenchymal stem cells related to canonical Wnt signaling activating in vitro. Neuropeptides 56, 105–113. doi:10.1016/j.npep.2015.12.008
Liu, S., Wu, J. Q., Hu, J. J., Wang, L., Wang, Z., Meng, H., et al. (2018). Neuropeptide Y Y1 receptor antagonist PD160170 promotes osteogenic differentiation of rat bone marrow mesenchymal stem cells in vitro and femoral defect repair in rats. J. South. Med. Univ. 38, 669–676. doi:10.3969/j.issn.1673-4254.2018.06.05
Liu, X., Liu, H., Xiong, Y., Yang, L., Wang, C., Zhang, R., et al. (2018c). Postmenopausal osteoporosis is associated with the regulation of SP, CGRP, VIP, and NPY. Biomed. Pharmacother. 104, 742–750. doi:10.1016/j.biopha.2018.04.044
Lundberg, P., Allison, S. J., Lee, N. J., Baldock, P. A., Brouard, N., Rost, S., et al. (2007). Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J. Biol. Chem. 282, 19082–19091. doi:10.1074/jbc.M609629200
Lundberg, P., Boström, I., Mukohyama, H., Bjurholm, A., Smans, K., and Lerner, U. H. (1999). Neuro-hormonal control of bone metabolism: vasoactive intestinal peptide stimulates alkaline phosphatase activity and mRNA expression in mouse calvarial osteoblasts as well as calcium accumulation mineralized bone nodules. Regul. Pept. 85, 47–58. doi:10.1016/s0167-0115(99)00069-5
Lundberg, P., Lundgren, I., Mukohyama, H., Lehenkari, P. P., Horton, M. A., and Lerner, U. H. (2001). Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor subtypes in mouse calvarial osteoblasts: presence of VIP-2 receptors and differentiation-induced expression of VIP-1 receptors. Endocrinology 142, 339–347. doi:10.1210/endo.142.1.7912
Ma, W., Zhang, X., Shi, S., and Zhang, Y. (2013). Neuropeptides stimulate human osteoblast activity and promote gap junctional intercellular communication. Neuropeptides 47, 179–186. doi:10.1016/j.npep.2012.12.002
Mrak, E., Guidobono, F., Moro, G., Fraschini, G., Rubinacci, A., and Villa, I. (2010). Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. J. Cell Physiol. 225, 701–708. doi:10.1002/jcp.22266
Mukohyama, H., Ransjö, M., Taniguchi, H., Ohyama, T., and Lerner, U. H. (2000). The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem. Biophys. Res. Commun. 271, 158–163. doi:10.1006/bbrc.2000.2599
Nagao, S., Goto, T., Kataoka, S., Toyono, T., Joujima, T., Egusa, H., et al. (2014). Expression of neuropeptide receptor mRNA during osteoblastic differentiation of mouse iPS cells. Neuropeptides 48, 399–406. doi:10.1016/j.npep.2014.10.004
Natsume, H., Tokuda, H., Mizutani, J., Adachi, S., Matsushima-Nishiwaki, R., Minamitani, C., et al. (2010). Synergistic effect of vasoactive intestinal peptides on TNF-alpha-induced IL-6 synthesis in osteoblasts: amplification of p44/p42 MAP kinase activation. Int. J. Mol. Med. 25, 813–817. doi:10.3892/ijmm_00000409
Niedermair, T., Schirner, S., Seebröker, R., Straub, R. H., and Grässel, S. (2018). Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci. Rep. 8, 9199. doi:10.1038/s41598-018-27432-y
Peng, Z., Xu, R., and You, Q. (2022). Role of traditional Chinese medicine in bone regeneration and osteoporosis. Front. Bioeng. Biotechnol. 10, 911326. doi:10.3389/fbioe.2022.911326
Persson, E., and Lerner, U. H. (2011). The neuropeptide VIP regulates the expression of osteoclastogenic factors in osteoblasts. J. Cell Biochem. 112, 3732–3741. doi:10.1002/jcb.23304
Persson, E., Voznesensky, O. S., Huang, Y. F., and Lerner, U. H. (2005). Increased expression of interleukin-6 by vasoactive intestinal peptide is associated with regulation of CREB, AP-1 and C/EBP, but not NF-kappaB, in mouse calvarial osteoblasts. Bone 37, 513–529. doi:10.1016/j.bone.2005.04.043
Piao, J., Park, J. S., Hwang, D. Y., Son, Y., and Hong, H. S. (2020). Substance P blocks ovariectomy-induced bone loss by modulating inflammation and potentiating stem cell function. Aging (Albany NY) 12, 20753–20777. doi:10.18632/aging.104008
Qu, H., Zhuang, Y., Zhu, L., Zhao, Z., and Wang, K. (2021). The effects of vasoactive intestinal peptide on RANKL-induced osteoclast formation. Ann. Transl. Med. 9, 127. doi:10.21037/atm-20-7607
Salari, N., Darvishi, N., Bartina, Y., Larti, M., Kiaei, A., Hemmati, M., et al. (2021). Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 669. doi:10.1186/s13018-021-02821-8
Seldeen, K. L., Halley, P. G., Volmar, C. H., Rodríguez, M. A., Hernandez, M., Pang, M., et al. (2018). Neuropeptide Y Y2 antagonist treated ovariectomized mice exhibit greater bone mineral density. Neuropeptides 67, 45–55. doi:10.1016/j.npep.2017.11.005
Shende, P., and Desai, D. (2020). Physiological and therapeutic roles of neuropeptide Y on biological functions. Adv. Exp. Med. Biol. 1237, 37–47. doi:10.1007/5584_2019_427
Shi, L., Feng, L., Zhu, M. L., Yang, Z. M., Wu, T. Y., Xu, J., et al. (2020). Vasoactive intestinal peptide stimulates bone marrow-mesenchymal stem cells osteogenesis differentiation by activating wnt/β-catenin signaling pathway and promotes rat skull defect repair. Stem Cells Dev. 29, 655–666. doi:10.1089/scd.2019.0148
Shi, Y. C., Lin, S., Castillo, L., Aljanova, A., Enriquez, R. F., Nguyen, A. D., et al. (2011). Peripheral-specific y2 receptor knockdown protects mice from high-fat diet-induced obesity. Obes. (Silver Spring) 19, 2137–2148. doi:10.1038/oby.2011.99
Shih, C., and Bernard, G. W. (1997). Calcitonin gene related peptide enhances bone colony development in vitro. Clin. Orthop. Relat. Res. 334, 335–344. doi:10.1097/00003086-199701000-00043
Smith, S. J., Hawrylycz, M., Rossier, J., and Sümbül, U. (2020). New light on cortical neuropeptides and synaptic network plasticity. Curr. Opin. Neurobiol. 63, 176–188. doi:10.1016/j.conb.2020.04.002
Sousa, D. M., Martins, P. S., Leitão, L., Alves, C. J., Gomez-Lazaro, M., Neto, E., et al. (2020). The lack of neuropeptide Y-Y(1) receptor signaling modulates the chemical and mechanical properties of bone matrix. Faseb J. 34, 4163–4177. doi:10.1096/fj.201902796R
Tiwari, V., and Hemalatha, S. (2024). Sida cordifolia L. attenuates behavioral hypersensitivity by interfering with KIF17-NR2B signaling in rat model of neuropathic pain. J. Ethnopharmacol. 319, 117085. doi:10.1016/j.jep.2023.117085
Togari, A., Arai, M., Mizutani, S., Mizutani, S., Koshihara, Y., and Nagatsu, T. (1997). Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 233, 125–128. doi:10.1016/s0304-3940(97)00649-6
Tuzmen, C., and Campbell, P. G. (2018). Crosstalk between neuropeptides SP and CGRP in regulation of BMP2-induced bone differentiation. Connect. Tissue Res. 59, 81–90. doi:10.1080/03008207.2017.1408604
Valentijn, K., Gutow, A. P., Troiano, N., Gundberg, C., Gilligan, J. P., and Vignery, A. (1997). Effects of calcitonin gene-related peptide on bone turnover in ovariectomized rats. Bone 21, 269–274. doi:10.1016/s8756-3282(97)00142-7
Wang, L., Zhao, R., Shi, X., Wei, T., Halloran, B. P., Clark, D. J., et al. (2009). Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone 45, 309–320. doi:10.1016/j.bone.2009.04.203
Wang, S. L., Han, Y., Xie, W. X., Li, Z. C., and Xiao, J. (2018). Experimental study on differential expression of neuropeptides in bone tissue of ovariectomized rats. Chin. J. Osteoporos. 24, 437–440+457. doi:10.3969/j.issn.1006-7108.2018.04.004
Wang, W., Wang, Z. P., Huang, C. Y., Chen, Y. D., Yao, W. F., and Shi, B. M. (2019). The neuropeptide vasoactive intestinal peptide levels in serum are inversely related to disease severity of postmenopausal osteoporosis: a cross-sectional study. Genet. Test. Mol. Biomarkers. 23, 480–486. doi:10.1089/gtmb.2019.0041
Wang, X., Xu, J., and Kang, Q. (2021). Neuromodulation of bone: role of different peptides and their interactions (Review). Mol. Med. Rep. 23, 32. doi:10.3892/mmr.2020.11670
Wang, X. J., Pan, Y. X., Du, Z. X., Zhou, C., Liu, M., and Guo, Y. L. (2013). Effect of Zhuangjin Xugu Tang on the expression of bone morphogenetic protein-7 and neuropeptide Y in rat fracture callus. China J. Traditional Chin. Med. Pharm. 28, 2420–2422.
Wang, Z., Jin, D., Tuo, Y. H., Guo, X. L., Wen, J., Zhou, J., et al. (2011). Calcitonin gene-related peptide promoting migration of rat bone marrow mesenchymal stem cells and stimulating expression of vascular endothelial growth factor. Chin. J. reparative Reconstr. Surg. 25, 1371–1376.
Wee, N. K. Y., Sinder, B. P., Novak, S., Wang, X., Stoddard, C., Matthews, B. G., et al. (2019). Skeletal phenotype of the neuropeptide Y knockout mouse. Neuropeptides 73, 78–88. doi:10.1016/j.npep.2018.11.009
Wee, N. K. Y., Vrhovac Madunic, I., Ivanisevic, T., Sinder, B. P., and Kalajzic, I. (2020). Divergent effects of peripheral and global neuropeptide Y deletion. J. Musculoskelet. Neuronal Interact. 20, 579–590.
Wilkinson, M., and Brown, R. (2015). “Neuropeptides I: classification, synthesis, and co-localization with classical neurotransmitters,” in An introduction to neuroendocrinology (Cambridge University Press), 257–285.
Wu, J. Z., Wang, W. Q., Zhou, X. M., Wu, M. Y., Zeng, Y. G., and Shi, X. G. (2015). The effect of Erxian Tang on neuropeptide substance P in osteoporotic rats after spinal cord injury. Chin. Med. Mater. 38, 1254–1257. doi:10.13863/j.issn1001-4454.2015.06.036
Xie, W., Han, Y., Li, F., Gu, X., Su, D., Yu, W., et al. (2020a). Neuropeptide Y1 receptor antagonist alters gut microbiota and alleviates the ovariectomy-induced osteoporosis in rats. Calcif. Tissue Int. 106, 444–454. doi:10.1007/s00223-019-00647-5
Xie, W., Li, F., Han, Y., Qin, Y., Wang, Y., Chi, X., et al. (2020b). Neuropeptide Y1 receptor antagonist promotes osteoporosis and microdamage repair and enhances osteogenic differentiation of bone marrow stem cells via cAMP/PKA/CREB pathway. Aging (Albany NY) 12, 8120–8136. doi:10.18632/aging.103129
Xie, Y. H., Zhou, T. Y., Wang, J. Z., Wang, J. Y., Zhou, Y. L., Tang, L., et al. (2024). Mechanism of Ma qian zi glycoside inhibiting bone loss in OVX mice through gut microbiota and neuropeptides. Chin. J. Osteoporos. 30, 538–545. doi:10.3969/j.issn.1006-7108.2024.04.012
Yang, F., Yang, L. X., Li, X. Q., and Li, Y. M. (2016). The effects of zuo gui wan and you gui wan on neuropeptides CGRP, SP, VIP, and NPY in ovariectomized osteoporotic rats. Chin. J. Osteoporos. 22, 761–765. doi:10.3969/j.issn.1006-7108.2016.06.022
Yi, Z. Y., Gan, G. X., Guo, X. L., Liu, Y., Rao, J. H., and Li, B. F. (2016). The effect of Zhuanggu Zhitong Fang on hypothalamic neuropeptide Y and alpha melanocyte stimulating hormone in ovariectomized osteoporotic rats. China Med. Her. 13, 20–23.
Yu, W., Zhu, C., Xu, W., Jiang, L., and Jiang, S. (2016). Neuropeptide Y1 receptor regulates glucocorticoid-induced inhibition of osteoblast differentiation in murine mc3t3-E1 cells via ERK signaling. Int. J. Mol. Sci. 17, 2150. doi:10.3390/ijms17122150
Zhang, B., Zhao, J., Yan, H., Zhao, Y., Tian, H., Wang, C., et al. (2022a). A novel nano delivery system targeting different stages of osteoclasts. Biomater. Sci. 10, 1821–1830. doi:10.1039/d2bm00076h
Zhang, R. H., Chen, K. J., and Lu, D. X. (2004). Clinical study on treatment of postmenopausal osteoporosis by Yigu capsule. Zhongguo Zhong Xi Yi Jie He Za Zhi 24, 680–684.
Zhang, R. H., Zhang, X. B., Lu, Y. B., Hu, Y. C., Chen, X. Y., Yu, D. C., et al. (2021). Calcitonin gene-related peptide and brain-derived serotonin are related to bone loss in ovariectomized rats. Brain Res. Bull. 176, 85–92. doi:10.1016/j.brainresbull.2021.08.007
Zhang, Y., Xiang, N., Zhou, G. W., Li, Z. Q., Tan, Z. K., Huang, S. Y., et al. (2022b). Study on the effect of tonifying kidney and resolving phlegm formula on lipid bone metabolism and central regulation mechanism in osteoporosis rats after castration. Chin. Archives Traditional Chin. Med. 40, 21–27+259-262. doi:10.13193/j.issn.1673-7717.2022.05.006
Zhang, Y., Xu, J., Ruan, Y. C., Yu, M. K., O'Laughlin, M., Wise, H., et al. (2016). Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 22, 1160–1169. doi:10.1038/nm.4162
Zheng, X. F., Zhao, E. D., He, J. Y., Zhang, Y. H., Jiang, S. D., and Jiang, L. S. (2016). Inhibition of substance P signaling aggravates the bone loss in ovariectomy-induced osteoporosis. Prog. Biophys. Mol. Biol. 122, 112–121. doi:10.1016/j.pbiomolbio.2016.05.011
Zhou, T. Y., Xie, Y. H., Wang, J. Y., Tang, L., Dong, Q. W., and Sun, P. (2023). The effect of Ciwujia glycoside E on bone mass and neuropeptides in ovariectomized mice. Chin. J. Osteoporos. 29, 655–659. doi:10.3969/j.issn.1006-7108.2023.05.007
Zhu, X. F., Wang, Y. C., Yang, L., Wang, P. P., and Zhang, R. H. (2015). The effect of kidney tonifying and blood activating traditional Chinese medicine formula on bone density and neuropeptide Y in bone tissue of ovariectomized rats. Chin. J. Gerontology 35, 2884–2886. doi:10.3969/j.issn.1005-9202.2015.11.002
Zhu, X. F., Wang, Y. C., Zhang, R. H., Wang, P. P., Han, L., and Yang, L. (2012). Effect of Yigu capsule on bone density, plasma and bone tissue CGRP and SP content in ovariectomy rats. Shizhen Med. Materia Medica Res. 23, 1054–1056. doi:10.3969/j.issn.1008-0805.2012.05.002
Keywords: neuropeptide, bone formation, bone resorption, osteoporosis, traditional Chinese medicine
Citation: Lin Q, Zhao B, Huang J, Chen R, Sun W, Ye Q, Yang L, Zhu X, Li X and Zhang R (2025) Neuropeptides as regulators of bone metabolism: from molecular mechanisms to traditional Chinese medicine intervention strategies. Front. Pharmacol. 16:1516038. doi: 10.3389/fphar.2025.1516038
Received: 23 October 2024; Accepted: 03 February 2025;
Published: 28 February 2025.
Edited by:
Raghuram Kandimalla, James Graham Brown Cancer Center, United StatesReviewed by:
Sanjeeb Kalita, DBT-APSCS&T CoE for Bioresources and Sustainable Development, IndiaCopyright © 2025 Lin, Zhao, Huang, Chen, Sun, Ye, Yang, Zhu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronghua Zhang, dHpyaEBqbnUuZWR1LmNu; Xiaoyun Li, bGl4eTIxQGpudS5lZHUuY24=; Xiaofeng Zhu, enhpYW9mQGpudS5lZHUuY24=
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.