- 1Division of Chinese Internal and Pediatric Medicine, Center for Traditional Chinese Medicine, Chang Gung Memorial Hospital, Taipei, Taiwan
- 2Division of Chinese Acupuncture and Traumatology, Center of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 3School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 4Department of Pediatrics and Neonatology, Chang Gung Memorial Hospital, Linkou, Taiwan
- 5Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
Introduction: Chinese herbal medicine (CHM) taken orally is frequently utilized to enhance functional ability and independence in cerebral palsy (CP); nonetheless, there is a lack of current evidence regarding the efficacy of oral CHM in treating CP. Additionally, the general complexities of CHM prescriptions often obscure the underlying mechanisms. Our study aims to assess the efficacy of oral CHM in treating CP, a meta-analysis will be conducted on randomized clinical trials (RCTs).
Materials and methods: We searched Cochrane Library, PubMed, Embase, Scopus, PubMed Central, ClinicalTrials.gov, and China National Knowledge Infrastructure (CNKI), from 1990 to 2022. The primary outcome was the improvement in Effectiveness rate (ER). The secondary outcome was the improvement of motor function (GMFM). Subgroup analysis and trial sequential analysis (TSA) were conducted to confirm results consistency. Core CHMs were investigated through system pharmacology analysis.
Results: Seventeen RCTs were analyzed, in which CHMs with Standard treatment (ST) were compared to ST alone. All participants were aged <11 years. More participants in the CHM group achieved prominent improvement in ER (RR: 1.21, 95% CI: 1.13–1.30, p-value < 0.001, I2 = 32%) and higher GMFM improvement (SMD: 1.49; 95% CI: 1.33–1.65, p-value < 0.001, I2 = 92%). TSA also showed similar results with proper statistical power. Core CHMs, such as Glycyrrhiza uralensis Fisch. Ex DC., Poria cocos (Schw.) Wolf, Paeonia lactiflora Pall., processed Rehmannia glutinosa (Gaertn.) DC., Astragalus mongholicus Bunge, and Angelica sinensis (Oliv.) Diels, exerted effects on immune modulation and metabolism systems. The subgroup analysis showed participants using core CHMs or longer CHM treatment duration, and studies enrolling CP with spastic or mixed type, or mild-to-moderate severity had better outcomes in CHM groups with less heterogeneity.
Conclusion: CHMs may have a positive impact on managing pediatric CP; however, the potential bias in study design should be improved.
Systematic Review Registration: Identifier CRD42023424754.
1 Introduction
Cerebral palsy (CP) is the most common cause of disability in childhood, with an estimated global prevalence between 0.16% and 0.37% (McIntyre et al., 2022). The term refers to a group of neurological disorders that affect movement and posture along the lifespan, caused by damage to the developing brain. It results in motor disability, and some patients may also develop epilepsy or disturbance of cognition, behavior, communication, sensation, and perception (Rosenbaum et al., 2007; Novak et al., 2012). In terms of socioeconomic aspects, individuals with CP face the impact of multiple disabilities; consequently, they require long-term medication, rehabilitation, and care. Their necessary expenses are significantly higher compared with those of their healthy age-matched counterparts, thus imposing substantial burdens on caregiving families (Vadivelan et al., 2020).
The treatment of CP focuses on improving movement and reducing the disruptions caused to daily activities (Colver et al., 2014). Therefore, physical rehabilitation is currently the standard first-line therapy for CP (Demont et al., 2022). Other therapies include medication, speech rehabilitation, occupational rehabilitation, and surgical intervention (Vitrikas, Dalton, and Breish, 2020). However, recent research indicates that the improvement in gross motor skills through rehabilitation remains limited (Liang et al., 2021). Recent advances in treatment strategies, such as robot-assisted devices and virtual reality, have been used for motor learning and cortical reorganization; nevertheless, the efficacy of these approaches remains uncertain (Bekteshi et al., 2023; Paul et al., 2022).
Consequently, there is a growing interest in exploring alternative medical therapies for improving functional ability and independence of patients with CP. Traditional Chinese medicine (TCM) has been commonly used for centuries as adjunctive therapy in Asia. Studies have found that combining TCM with Standard treatment (ST) can improve motor function and activities of daily living in patients with CP (Zhang et al., 2010; Liao et al., 2017). Moreover, a recent systematic review demonstrated that the combination of TCM and modern rehabilitation therapies may resulted in effective improvements in gross motor function, muscle tone, and functional independence in children with CP (Chen et al., 2023). Thereby, TCM seems to enhance the independence of patients’ daily activity and may reduce the burden on caregivers and the healthcare system. However, previous review articles on TCM interventions often encompassed oral Chinese herbal medicine (CHM), acupuncture, massage, or low-level laser therapy, whereas studies focusing exclusively on CHM remain relatively scarce.
As to CHM efficacy on CP, a recent study utilizing network pharmacology and bioinformatics has elucidated the therapeutic potential of Liuwei Dihuang pills, a traditional CHM, in the treatment and management of CP. The key bioactive constituents of Liuwei Dihuang pills, including quercetin, stigmasterol, and kaempferol, exert their effects of modulating immunological and inflammatory responses through the regulation of several critical signaling pathways, including the PI3K-Akt, IL-17, Jak-STAT, and NF-κB pathways, which are integral to the pathophysiology of CP (Wang et al., 2024). Additionally, in animal study, tanshinone IIA, ingredient of Salvia miltiorrhiza Bunge, showed neuroprotective effect and weakened spasticity through inflammation, p38MAPK and VEGF pathway (Zhang et al., 2018). Moreover, a review article reported improved daily activity outcomes when Oriental herbal medicine was integrated into rehabilitation programs (Lee et al., 2018). However, there is still a lack of extensive and up-to-date literature, robust bias assessment, and statistical analysis regarding the efficacy and safety of oral CHMs as well as the core CHMs for CP.
The aim of this study was to compile evidence from recent Randomized clinical trials (RCT) on the use of oral CHM for pediatric CP and assess its potential effectiveness. Additionally, network pharmacology analysis was also undertaken to identify core CHMs utilized in the examined trials and elucidate potential pharmacological pathways involved.
2 Materials and methods
This study protocol was prospectively registered in PROSPERO (No. CRD42023424754).
2.1 Eligibility criteria
The inclusion criteria were as follows:
1) RCT studies.
2) CP diagnosis was based on diagnostic criteria evaluated by a physician.
3) Age < 18 years.
4) Interventions involved the oral administration of single or mixed traditional CHMs.
5) No limitations based on ethnicity, age, or language.
The exclusion criteria were as follows:
1) Non-RCT studies.
2) Use of folk medicine or traditional medicine other than CHM (i.e., acupuncture, or massage).
3) Studies evaluating the effectiveness of CHMs administered topically (i.e., moxibustion, herbal bath, fumigation therapy).
4) Lack of a control group.
5) The control group did not receive ST.
6) The intervention group did not receive CHM combined with ST.
7) Outcome assessment other than Effectiveness rate (ER), Gross Motor Function Measure score (GMFM), Activities of Daily Living for CP recover evaluation (ADL) (Shuchun, 2000; Yingyuan, 2009), and Modified Ashworth Scale (MAS) score.
8) Studies not published in peer-reviewed journals.
9) Lack of search strategy and information sources.
We conducted thorough searches in various electronic databases from 1 January 1990 to December 2022. The databases included Cochrane Library, PubMed, Embase, Scopus, PubMed Central, ClinicalTrials.gov, and China National Knowledge Infrastructure (CNKI). The specific search approaches are provided in Supplementary Appendix S1. The search terms were used as follow: “Cerebral Palsy” (MeSH Terms) for patient group, and [“Medicine, Chinese Traditional” (Mesh) or “Herbal Medicine” (Mesh) or “Medicine, Korean Traditional” (Mesh) or “Medicine, Kampo” (Mesh)] for intervention.
2.2 Data extraction
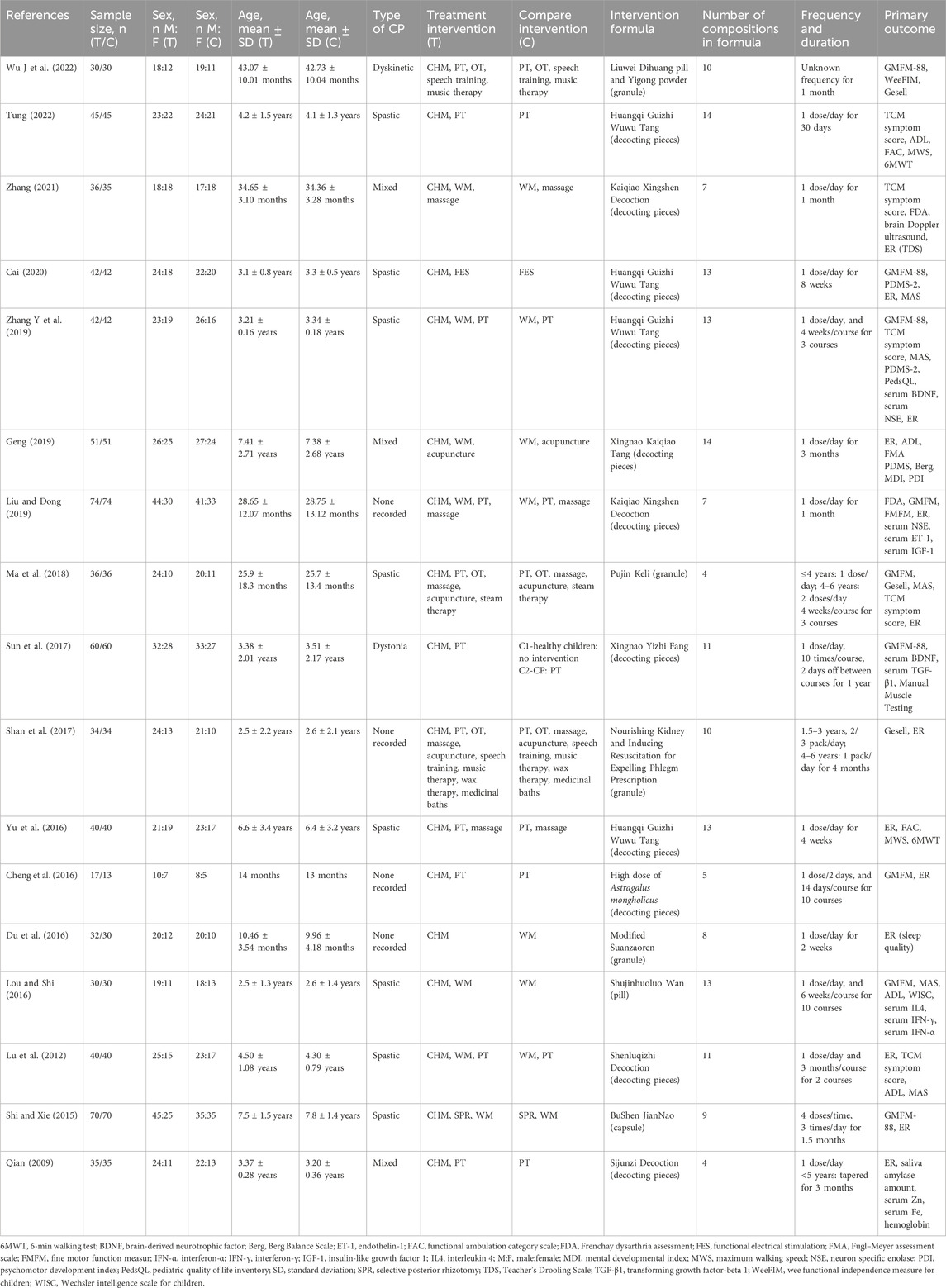
Huang independently extracted data using a predefined format, as outlined in Table 1, which includes details on the study authors, publication year, sample size, sex, age, intervention, and primary outcomes. Any discrepancies were resolved via deliberations with Cheng, Yang, and Chen. The extracted information included the publication year, study country, study design, CHM content and duration, type of standard management, diagnostic criteria, sample size, participant age and sex, and outcome assessments. Additionally, information regarding interventions, including composition, dosage, and frequency of usage for both control and intervention groups, was recorded. If necessary, and at the discretion of the reviewing author, the corresponding authors of the clinical studies were contacted to obtain any missing data.
2.3 Quality assessment
Huang and Cheng evaluated the methodological quality using the Risk-of-bias (RoB) assessment tool established by the Cochrane Collaboration (Higgins et al., 2011). Any discrepancies in the assessment were resolved through consultations with Yang and Chen.
2.4 Outcome measurements
The primary outcome was the percentage of participants in whom the treatment showed prominent effectiveness. ER was selected since it was commonly used in most studies and provided a composite outcome for participants. It was commonly presented by classifying the clinical response at the end of the study into three grades, such as prominent effectiveness, effectiveness, and ineffectiveness. Prominent improvement, including prominent effectiveness and effectiveness, was confirmed according to the following criteria varied according to different RCTs: 1) GMFM total score improved by ≥1% (Cheng et al., 2016); 2) ≥1/3 symptoms improved (Geng, 2019; Yu, 2016; Lu et al., 2012); 3) TCM syndrome score improved by ≥20% (Zhang L. Q. et al., 2019; Ma et al., 2018); 4) efficacy index improved by ≥1% (Liu and Dong, 2019); 5) drooling improved by ≥1 level (Zhang, 2021); 6) MAS score decreased by ≥1 grade (Shi and Xie, 2015); 7) Peabody developmental motor scale-2 (PDMS-2) improved by ≥1% (Cai, 2020); 8) sleep quality significantly improved (Du et al., 2016); and 9) 10 sports function score improved ≥10 (Qian, 2009). The percentage of prominent improvement was compared between the CHM + ST and ST groups, and this information was extracted as the primary outcome. The secondary outcome included improvement of solely evaluated clinical score systems, such as the Gesell Developmental Scale (Gesell), GMFM indicating motor function, ADL, and MAS presenting the severity of limb spasticity.
2.5 Statistical analysis
The analysis of all data was conducted utilizing Cochrane Review Manager 5.4.1. (The Cochrane Collaboration, 2014). The proportion of participants with prominent improvement in ER was analyzed using the Risk ratio (RR) and a 95% Confidence interval (CI). Numerical outcomes were analyzed using the Standardized mean difference (SMD) and/or Mean difference (MD). For data synthesis, a random-effects model with the Mantel–Haenszel test was used to summarize inverse variance and dichotomous data for continuous data. Heterogeneity between the studies was assessed using the I2-statistic. A funnel plot was used to detect publication bias. If bias was present, the trim and fill method (Peters et al., 2007) would be applied for correction. Additionally, Trial sequential analysis (TSA) was performed to confirm the efficacy of CHM. TSA is a novel method for evaluating treatment efficacy through interventional meta-analysis study in a more robust manner to mimic large-scale clinical trials (Wetterslev, Jakobsen, and Gluud, 2017; De Cassai et al., 2021; Kang, 2021). In this study, we adopted 5% type I error with 90% power of statistical examination in TSA to evaluate the consistency of results and the adequacy of the number of cases. TSA was carried out using proprietary software (Lan and DeMets, 1983). A system pharmacology analysis was conducted on the prescriptions from the included studies. Detailed methodologies are outlined in Supplementary Appendix S2. In summary, the Chinese herbal medicine network (CMN) was employed to identify the core CHMs, illustrating graphically the commonly used CHMs for CP. The pharmacological pathways of these core CHMs were clarified by referencing online databases for pharmacology pathways. Utilizing this well-established approach, we previously compared the effectiveness of CHM versus Western medicine (WM) in managing Coronavirus disease 2019 (COVID-19), allergic diseases, and diabetic nephropathy (Lu et al., 2022; Chen et al., 2015; Wu et al., 2021; Wang et al., 2023).
We conducted four subgroup analyses. Firstly, based on the type of CP, we divided the participants into spastic and mixed types. Secondly, dividing different initial symptom severity into three groups due to CP baseline severity was a main influencing factor to prognosticate long-term functional outcome. We used the ADL, Gesell, and MAS scores for categorization (mild, ADL: ≥91, Gesell: ≥55, MAS: <2; moderate, ADL: 61–90, Gesell: 40–54, MAS: 2; and severe, ADL: ≤60, Gesell: ≤39, MAS: >2) (Yuan et al., 2021; Huifang, 2012; Winstein et al., 2016). We divided the subgroup into mild-to-moderate and severe. Thirdly, we used the duration of the treatment course. We divided the duration into three subgroups, namely, 0–1 month, 1–3 months, and 3–6 months. We selected 1 month as the first cut point due to the shortest period for observing the efficacy of CHM (Yoo et al., 2016). Three months was the fastest time for neural recovery (Boecker et al., 2022; Schalow, 2002), and 6 months represented chronic phase of recovery (Gao et al., 2022). Finally, we extracted the studies that utilized core CHMs. For all analyses, excluding TSA, p-values < 0.05 denoted statistical significance.
3 Results
3.1 Literature search
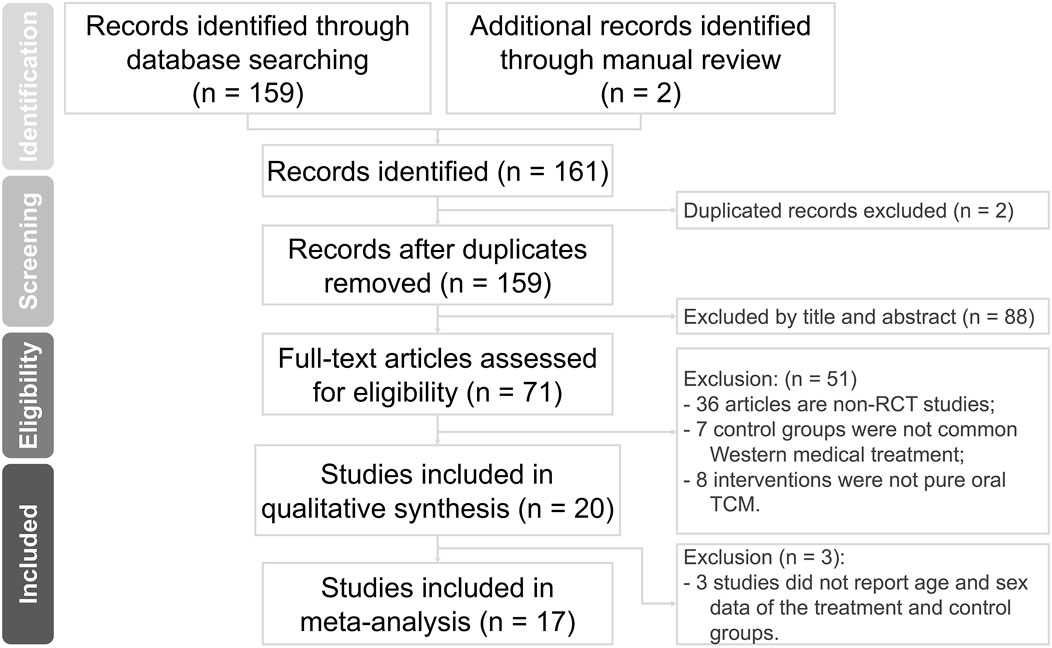
Our electronic and manual searches yielded a total of 161 references. After removing two duplicate records, 159 studies remained. A detailed examination of titles and abstracts led to the exclusion of 88 studies.
After this initial screening, we proceeded to retrieve and carefully evaluate the complete texts of 71 references. Based on the inclusion and exclusion criteria, 51 studies were removed. Furthermore, three studies were excluded due to the lack of detailed data on the CHM and ST groups. Finally, our comprehensive assessment led to the inclusion of 17 studies, involving a total of 1,421 participants. These data are illustrated in Figure 1.
3.2 Description of included studies
3.2.1 Characteristics of studies
Table 1 shows the detailed information of the analyzed studies, which were all RCTs. Sixteen studies adopted a two-arm parallel design, and only one used a three-arm design, in which only data from CHM and the control arm were extracted. All selected studies were sourced from China.
3.2.2 Characteristics of participants
In the analyzed studies, the age of the participants ranged from 0 to 11 years old. In terms of diagnostic criteria and classification, 11 studies followed the Chinese national clinical diagnostic criteria and classification as their standard, whereas six studies referred to the Rehabilitation Guideline for CP in China. Regarding the type of CP, eight studies enrolled only patients with the spastic type, while the remaining enrolled participants with all types of CP.
3.2.3 Design of the control group
ST, including Physical therapy (PT) and Occupational therapy (OT), was found in the control group of 11 trials. Five trials only used WM, and three trials used rehabilitation plus WM in the control group. The WM prescribed in trials included baclofen, dantrolene sodium, midazolam, phenobarbital, cerebrolysin, ligustrazine hydrochloride, or other medicines for nourishing neurons. With regard to ST, five trials added massage, and some added complementary therapy, such as speech training, dry needle therapy, steam therapy, music therapy, wax therapy, and medicinal baths. Notably, one study used Functional electrical stimulation (FES) in the control group, while another used Selective posterior rhizotomy (SPR) plus WM. The disparities among the experimental herbal formulas combined with PT, PT alone, and no treatment (i.e., healthy children) were discussed in a three-arm parallel trial.
3.2.4 Design of the intervention group
All included trials involved a combination of CHM with ST, and all prescriptions were mixed CHMs. The number of CHMs used in trials ranged from 4 to 14 (mean: 9; SD: 3). The frequency of CHMs combination usage was shown in Supplementary Appendix S3. Among all CHMs, Glycyrrhiza uralensis Fisch. ex DC. (GU) and Poria cocos (Schw.) Wolf. (PC) are the most frequently used combination of medications (47.059%). The duration of treatment ranged from 2 weeks to 15 months.
3.3 Quality of trials
Quality assessment was performed using Cochrane RoB (Figures 2A, B). Within the RoB assessment, most studies displayed unclear statuses of allocation bias, performance bias, and detection bias. Evaluation of the selection bias indicated that nine of the RCTs included in this analysis were at a low RoB, while the status of others remained unclear. Similarly, the risk of attrition bias was low across the majority of RCTs, except for two studies that were linked to high risk. Notably, all studies were rated as having a low risk of reporting bias.
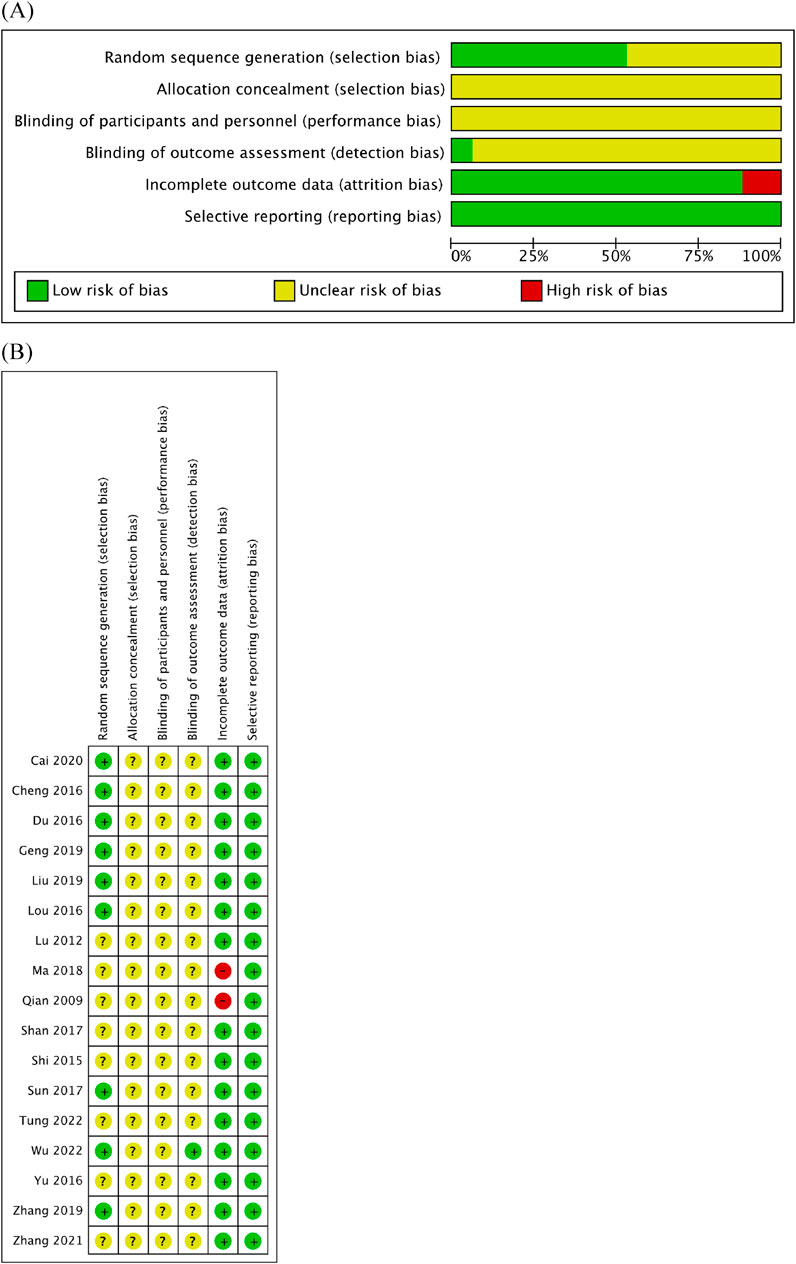
Figure 2. Quality assessment of 17 included studies using the RoB assessment tool established by the Cochrane Collaboration. (A) RoB graph. (B) RoB summary.
3.4 Meta-analysis of included studies
3.4.1 Primary outcome: the RR of achieving prominent improvement in ER
Generally, the CHM + ST group had better outcomes than the ST group. In 13 RCTs analyzed, the CHM + ST group had a superior proportion of participants with prominent improvement (495/549, 90.16%) compared with the ST group (398/542, 73.43%). The CHM + ST group demonstrated a 21% higher proportion of prominent improvement compared with the ST groups (RR: 1.21, 95% CI: 1.13–1.30, p-value < 0.001, I2 = 32%) (Figure 3). Moreover, the TSA confirmed this result, and the total pooled case number (n = 1,091) achieved the threshold of 90% statistical examination power (n = 310) (Supplementary Appendix S4).
3.4.2 Secondary outcome: improvement of Gesell, GMFM, ADL, and MAS scores
More than half of the studies used GMFM (n = 9) to measure motor function disorder. The mean of improvement of GMFM scores of the intervention and control groups ranged from 13.56 (minimum)–135.63 (maximum) and 5.5 (minimum)–77.89 (maximum), respectively. The pooled analysis revealed a significantly better improvement in the GMFM score in the CHM + ST group versus the ST group (SMD: 1.49; 95% CI: 1.33–1.65, p-value < 0.001, I2 = 92%) (Figure 4). Three RCTs were included in the Gesell analysis. The CHM + ST group exhibited a more significant change in scores compared to the ST group (MD: 10.91; 95% CI: 8.95–12.87, p-value < 0.001, I2 = 0%) (Figure 5). Four studies reported the improvement of daily living function using the ADL score. Greater ADL improvement was noted in the CHM + ST group compared with the ST group (MD: 7.33; 95% CI: 6.08–8.58, p-value < 0.001, I2 = 70%) (Figure 6). Five studies were included in the MAS analysis. Greater MAS improvement was recorded in the CHM + ST group versus the ST group (MD: 0.46; 95% CI: 0.40–0.51, p-value < 0.001, I2 = 90%) (Figure 7).
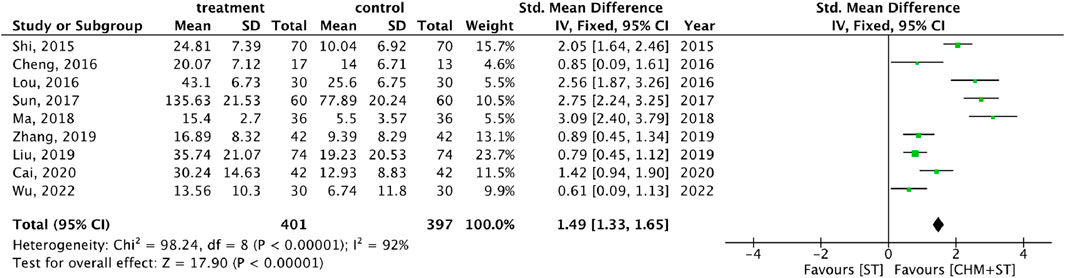
Figure 4. Forest plot of meta-analysis comparing CHM + ST with ST in terms of improvement of GMFM score change from the baseline.
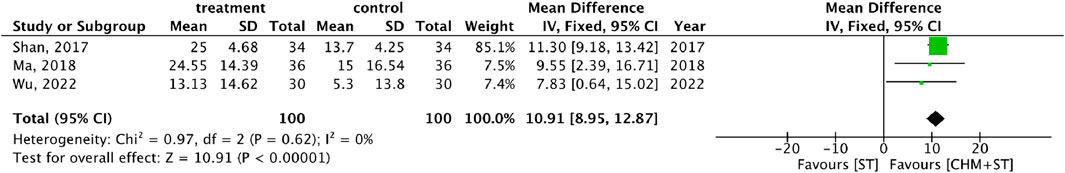
Figure 5. Meta-analysis comparing CHM + ST with ST in terms of Gesell Developmental Schedule improvement from baseline.
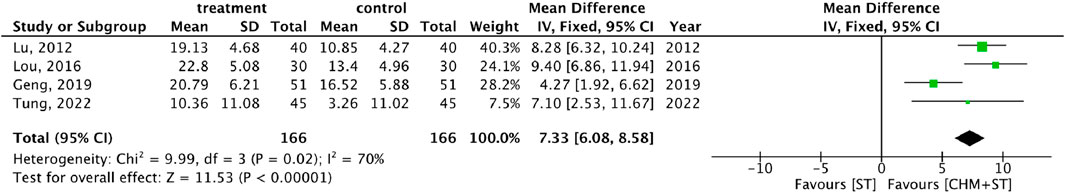
Figure 6. Meta-analysis comparing CHM + ST with ST in terms of improvement of ADL score from baseline.
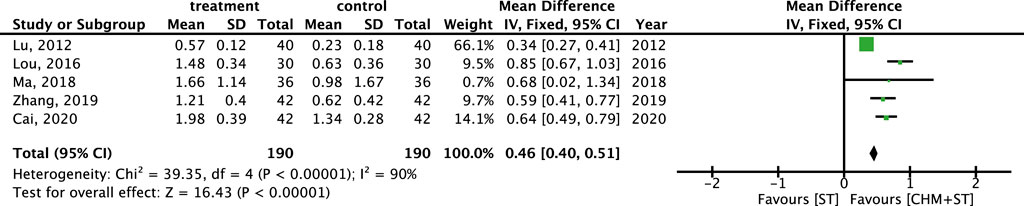
Figure 7. Meta-analysis comparing CHM + ST with ST in terms of improvement of MAS level from baseline.
3.5 Subgroup meta-analysis
Within different subtypes of CP, durations, and degrees of severity, the subgroup analysis showed lower heterogeneity with consistent results. For both spastic (n = 540) and mixed types (n = 243) of CP, the CHM + ST group had a significantly higher proportion of prominent improvement in ER compared with the ST group (RR: 1.20, 95% CI: 1.11–1.29, p-value < 0.001, I2 = 0% vs. RR: 1.25, 95% CI: 1.10–1.43, p-value < 0.001, I2 = 19%) (Figure 8).
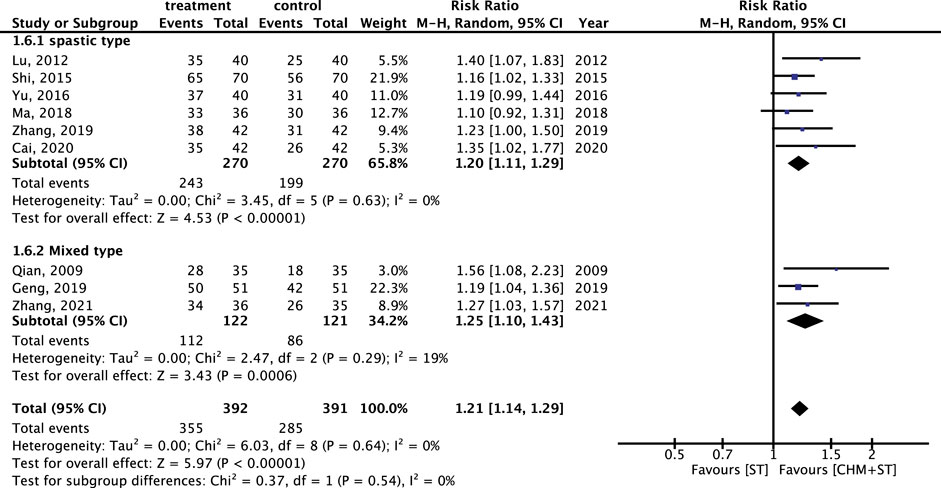
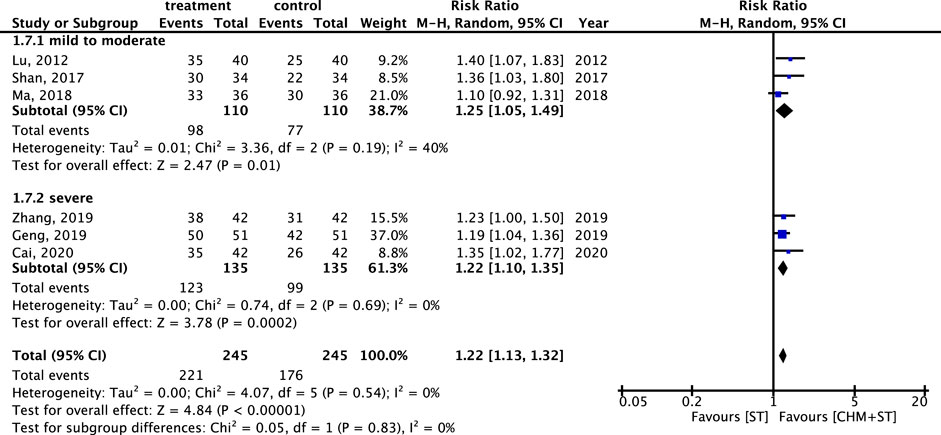
Figure 8. Subgroup analysis of RCTs. Comparison of spastic type with mixed type CP based on improvement in the ER.
The RR of ER within different degrees of CP severity was also analyzed. Six studies were analyzed after screening. In both categories, there was a notable enhancement in ER in the CHM + ST group versus the ST group. In addition, the mild-to-moderate subgroup (n = 220, RR: 1.25, 95% CI: 1.05–1.49, p-value = 0.001, I2 = 40%) exhibited better improvement than the severe subgroup (n = 270, RR: 1.22, 95% CI: 1.10–1.35, p-value < 0.001, I2 = 0%) (Figure 9).
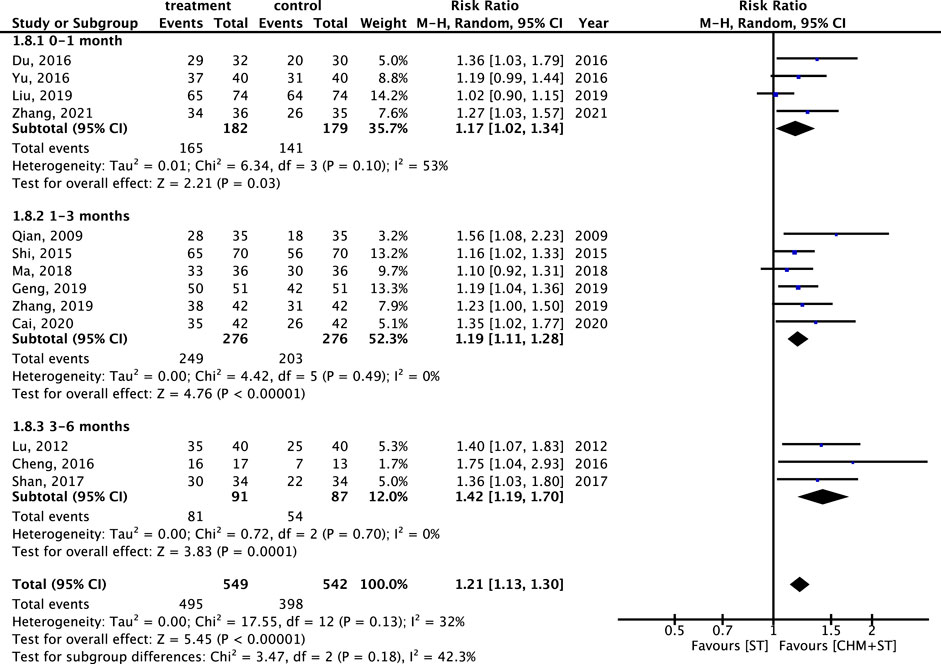
Furthermore, regarding the duration of the treatment course, the CHM + ST group also showed better results compared with the ST group. Patients receiving treatment for 3–6 months (n = 178, RR: 1.42, 95% CI: 1.19–1.70, p-value < 0.001, I2 = 0%) showed the greatest improvement, followed by those treated for 1–3 months (n = 552, RR: 1.19, 95% CI: 1.11–1.28, p-value < 0.001, I2 = 0%), and 0–1 month (n = 361, RR: 1.17, 95% CI: 1.02–1.34, p-value = 0.03, I2 = 53%) (Figure 10).
3.6 CMN for CP obtained from the included trials
The components of CHMs in each included study were itemized in Supplementary Appendix S5. The prevalence of CHM applied was presented in order in Supplementary Appendix S6. CMN could be constructed based on these CHM connections and present as Figure 11. Among these, three sets of core CHMs were found, i.e., core CHM1: GU, PC, and Paeonia lactiflora Pall. (PL); core CHM2: Rehmannia glutinosa (Gaertn.) DC. (RG) (present in 29% of all studies); and core CHM3: Angelica sinensis (Oliv.) (AS) and Astragalus mongholicus Bunge (AM). Compared with CHM + WM that did not include core medicines, CHM + WM including the three aforementioned sets of core CHMs exhibited better effectiveness (core CHM1, n = 512, RR: 1.25, 95% CI: 1.15–1.36, p-value < 0.001; core CHM2, n = 679, RR: 1.21, 95% CI: 1.10–1.33, p-value < 0.001; core CHM3, n = 310, RR: 1.19, 95% CI: 1.09–1.31, p-value < 0.001; without core medicine, n = 72, RR: 1.10, 95% CI: 0.92–1.31, p-value = 0.29) (Figure 12).
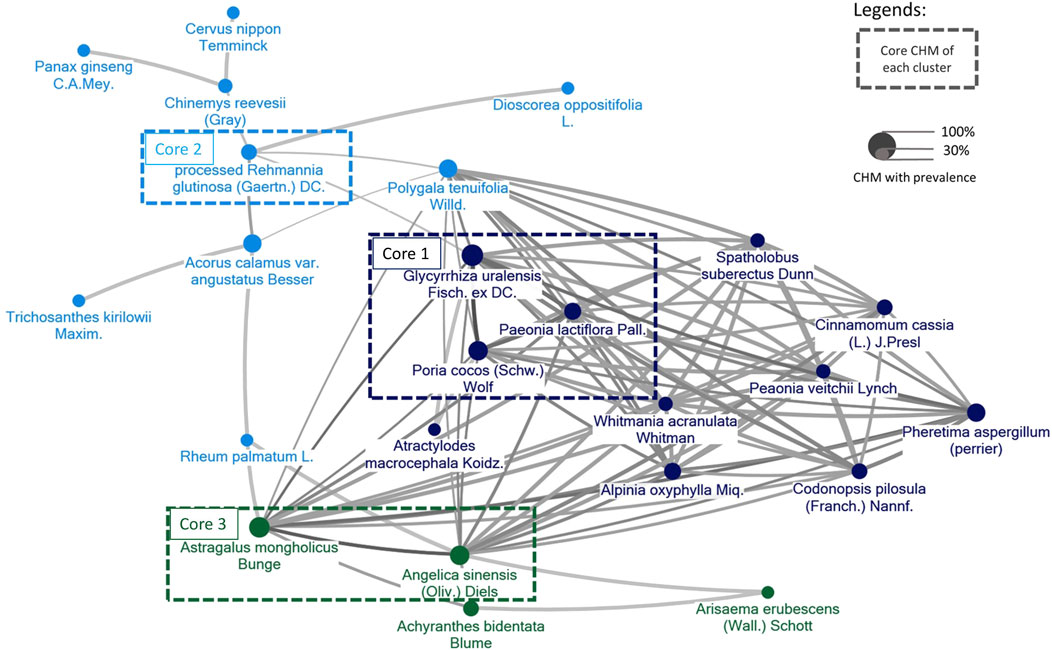
Figure 11. The CMN for CP was derived from the trials included in the study. Colors assigned to each node (agent) represent distinct clusters of CHM, with higher prevalence represented by larger node sizes. Darker colors and thicker connecting lines signify a more pronounced and frequent mixture of two CHMs.
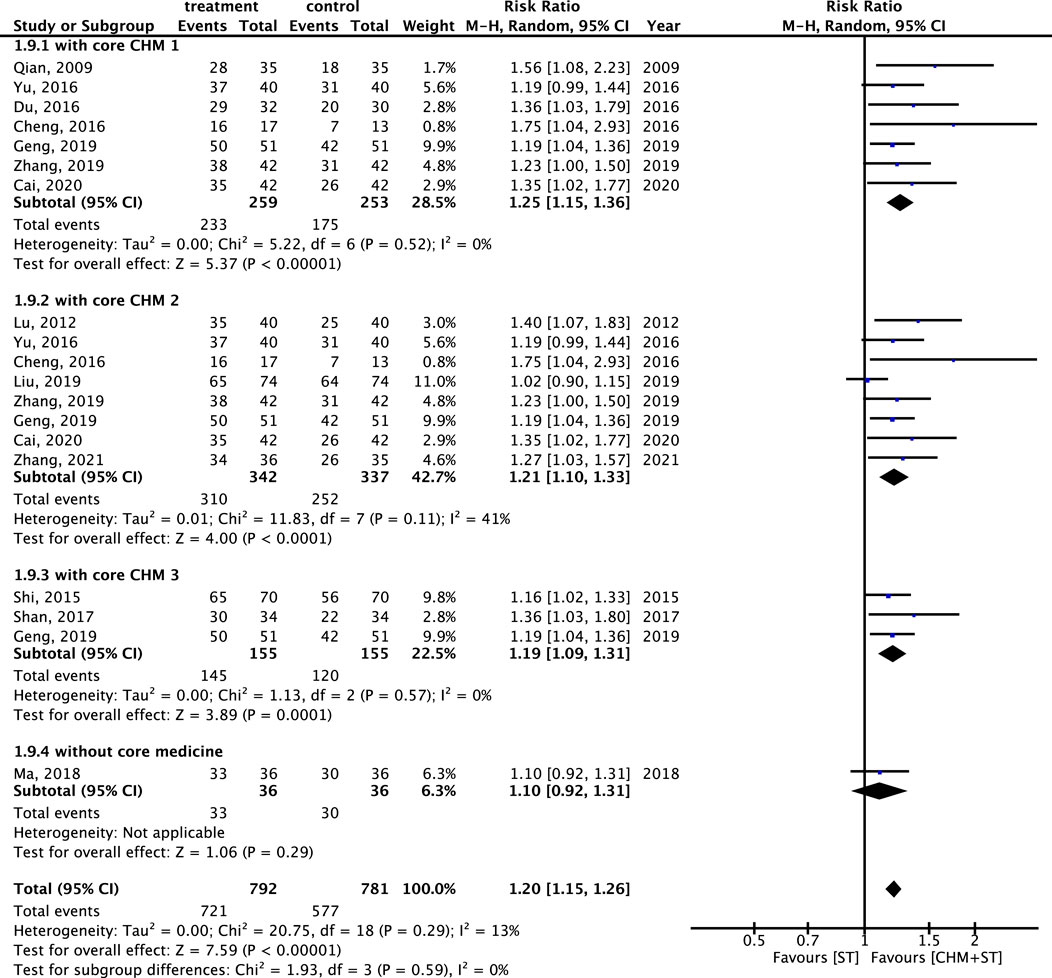
Figure 12. Subgroup analysis of RCTs. Comparison of the effects of three clusters of core medicines on the improvement of ER.
Moreover, noticeable disparities were observed in the proposed pharmacological pathways between the core CHMs (Figure 13). In terms of the immune system, core CHM1 (PC, GU, and PL) acted on Interleukin 4 (IL4) and Interleukin 13 (IL13) signaling. Additionally, core CHM3 (AS and AM) played a crucial role in modulating the activation of the γ-aminobutyric acid (GABA) receptor. Moreover, with regard to metabolism pathways, cores CHM1 and CHM3 demonstrated multiple advantages, particularly in aspects of arachidonic acid metabolism.

Figure 13. Pharmacologic pathways of CHM. Core 1: PC, GU, with PL; Core 2: RG; Core 3: AM with AS. (PC, Poria cocos (Schw.) Wolf; GU, Glycyrrhiza uralensis Fisch. ex DC.; PL, Paeonia lactiflora Pall.; RG, processed Rehmannia glutinosa (Gaertn.) DC.; AM, Astragalus mongholicus Bunge; AS, Angelica sinensis (Oliv.) Diels.
3.7 Publication bias
The funnel plots exhibited a low risk of publication bias (Supplementary Appendix S7). Moreover, the corrected results using Trim and Fill approach remained significant (Effective size 1.170, 95% CI: 1.084–1.256) (Supplementary Appendix S8).
3.8 Adverse drug events (ADEs) of CHM
Of the 17 included studies, only five reported side effects. In one study, side effects were only observed in the control group. The remaining four studies described that the patients treated with CHM + ST experienced side effects, such as gastrointestinal discomfort (i.e., nausea, vomiting, or diarrhea), although of no significance in comparison to the ST group. Moreover, there were no significant changes in liver and renal function in the CHM groups.
4 Discussion
To the best of our knowledge, this is the first meta-analysis for pediatric CP involving core CHM exploration and TSA. In all studies, we found the use of oral CHM in combine with ST led to a significantly higher proportion of patients achieving prominent improvement in ER compared with control. Improvements in motor skills, developmental status, self-care abilities, and muscle rigidity were consistently observed. In addition, the ER was higher for CHM + ST versus ST regardless of the type or severity of CP. Additionally, our meta-analysis showed that a longer duration of treatment is associated with better results. Regarding prescriptions, various types of CHM were used in the studies as other meta-analyses of CHMs (Wieland et al., 2013; Chung et al., 2015). Through the CMN, it was possible to efficiently identify the potential core CHMs for pediatric CP.
Recent advances in the treatment of CP include numerous methods, such as hyperbaric oxygen (Laureau et al., 2022), stem cell therapy (Novak et al., 2023), virtual reality rehabilitation (Han and Park, 2023), and robot-assisted devices (Vezér et al., 2024; Conner et al., 2022), which are currently under investigation. However, pharmacological options for CP remain limited. For children with CP, early intervention is more beneficial, as it minimizes the potential impact of muscle tension and poor posture on motor skills, thereby preventing hindrances in daily activities (Bobath, 1967). Therefore, CHM could be used as a complementary therapy with a good safety profile. Additionally, according to our results, incorporating the use of CHM for 1 month can lead to noticeable improvements in CP syndromes. Moreover, continuing combined therapy for 3–6 months seems appropriate, as supported by the subgroup analysis conducted in this study. CHMs might not only have the effect of muscle relax like WM, but also the effect of motor function and developmental status improvement.
CP is caused by disturbance or injury to the developing brain, often as a consequence of hypoxia, infection, stroke, or hypotension; the subsequent inflammatory cascade follows the original insult (Wimalasundera and Stevenson, 2016). Recent studies found higher levels of inflammatory markers in infants and children with CP, which might have a relationship between inflammation and neural damage at the perinatal period and during development of children (Paton et al., 2022; Magalhães et al., 2019; Malaeb and Dammann, 2009; Bashiri et al., 2006). Increased of cytokine IL-4 and IL-13 in CP patient were mentioned in some studies and were thought to have relationship with neural injury (Than et al., 2023; Djukic et al., 2009; Kaukola et al., 2004). And arachidonic acid, which might activate neuroinflammatory response and overproduction of proinflammatory cytokine, might lead to the serious of white matter damage and CP development as a consequence (Kapitanović Vidak et al., 2017; Chun et al., 2015; Strickland, 2014). Therefore, there is a growing interest in neuroprotective effect in CP through anti-inflammatory agent (Salomon, 2024; Mallah et al., 2020). Based on the current hypothesis and our findings on the anti-inflammatory effects of CHM, core CHM1 (PC, PL, GU) can modulate IL4 and IL13, while cores CHM1 and CHM3 (PC, PL, GU, AS, AM) are related to the arachidonic acid pathway. Previous studies also revealed that all these agents possess anti-inflammatory properties (Li et al., 2022; Wu J et al., 2022; He and Dai, 2011; Li et al., 2020; Gong et al., 2022), which may have benefit in reducing nerve damage caused by inflammation.
As to core CHM2 (RG), catalpol is one of the active ingredients in RG; it exerts a neuroprotective effect against hypoxic/ischemic injury by inhibiting apoptosis and regulating Aquaporin-4 (AQP4) expression (Jiang et al., 2015; Zhang Y et al., 2019). Besides, catalpol can promote angiogenesis via enhancing vascular endothelial growth factor-phosphatidylinositol 3 kinase/protein kinase B (VEGF-PI3K/AKT) and VEGF- Mitogen Activated Protein Kinase Kinase 1/2/extracellular signal-regulated kinase 1/2 (VEGF-MEK1/2/ERK1/2) signaling (Wang et al., 2020; Wang et al., 2022). Moreover, rehmannioside A, which is derived from RG, has neuroprotection effects and improves cognitive impairment by inhibiting ferroptosis and activating the PI3K/AKT/Nuclear factor erythroid 2–related factor 2 (Nrf2) and solute carrier family 7 member 11/glutathione peroxidase 4 (SLC7A11/GPX4) signaling pathway (Fu et al., 2022). Additionally, catalpol and mannitol, which are two components of RG, have anticonvulsant effects via GABAA receptor regulation (Kim et al., 2020).
In our study, we found that core CHM3 (AS and AM) played a crucial role in modulating GABA receptor activation and arachidonic acid metabolism. Previous studies showed that AM and AS upregulated VEGF expression to modulate the function of capillaries (Song et al., 2009). Gelispirolide and riligustilide, which are two phthalide dimmers isolated from AS, exert a GABAergic effect to relax spastic muscle (Deng et al., 2006). Besides, previous studies have demonstrated the anti-inflammatory effects of AS and AM (Li et al., 2020; Gong et al., 2022). Collectively, the available evidence indicates that the combination of CHM with conventional therapy may bring more advantages for patients with CP.
The safety of treatment using CHM was assessed by analyzing reported adverse reactions. Only mild side effects, such as gastrointestinal discomfort (nausea, vomiting, or diarrhea), were reported, without significant differences compared with the ST group. Furthermore, commonly used WM, such as baclofen, may cause central nervous system adverse reactions (e.g., confusion, dizziness, drowsiness, sedation, and asthenia) (Alstermark et al., 2008). Diazepam and clonazepam may be associated with side effects including sedation, cognitive impairment, amnesia, and ataxia (Vinkers and Olivier, 2012). Botulinum toxin injections lead to muscle atrophy and muscle weakness (Kaya Keles and Ates, 2022). Unlike WM, CHM does not cause drowsiness or muscle weakness; furthermore, it does not affect the daily life and rehabilitation schedule of children with CP.
5 Limitations
There are some limitations in this study. Firstly, there was a high heterogeneity observed in the results for the GMFM, ADL, and MAS scores. This heterogeneity may be due to the various types of CHM used. Therefore, we presented the core medicine network for CP to simplify the intervention and eliminate the diversity for future clinical trials. Secondly, although selection, attrition, and reporting biases were low, the allocation and performance biases were mostly unclear. Common sources of biases included uncertain concealment, lack of a specific blinding process, and randomization. Consequently, there is a need for high-quality clinical trials with improved designs. Thirdly, the generalizability of the present findings is poor. All studies included in this analysis were conducted in China; hence, the ethnic diversity is limited. These studies did not include participants from Caucasian, African, or Hispanic populations. Fourthly, the sample size in the included trials was comparatively modest (Sakpal, 2010). Therefore, we used TSA to confirm the results in this meta-analysis, which achieved the threshold of 90% statistical examination power. Fifthly, the hypothesis that CHM could improve CP through anti-inflammatory effects remains speculative. There was evidence supporting that CHM had anti-inflammatory properties and that inflammation can be mitigated in CP, but direct evidence is lacking. Therefore, larger CHM related RCTs with inflammatory biomarker analysis was needed to detail the mechanistic aspects and the relationship with CP.
6 Conclusion
CHM has the potential to treat pediatric CP in terms of improving motor function, developmental status, daily living function, and spasticity, as well as avoiding the occurrence of serious ADEs. We also identified core medications for treating CP and possible drug action pathways for reference in future clinical use. Subgroup analysis revealed that the combination of CHM with conventional treatment demonstrated better efficacy when core CHMs were included, the treatment duration was extended, or when patients had mild-to-moderate baseline severity. However, the included studies exhibited considerable biases over allocation and performance, high level of heterogeneity, poor generalizability, small sample size in the analysis. Therefore, further rigorous, multicenter, larger, and high-quality research is warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Y-YH: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft. Y-YC: Conceptualization, Data curation, Writing–original draft. H-YC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Writing–review and editing. R-HF: Resources, Supervision, Validation, Writing–review and editing. Y-JC: Resources, Supervision, Validation, Writing–review and editing. T-HY: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Ministry of Science and Technology in Taiwan (MOST111-2320-B-182-035-MY3), and the Ministry of Health and Welfare (MOHW112-CMAP-M-113-000006-D).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1500095/full#supplementary-material
References
Alstermark, C., Amin, K., Dinn, S. R., Elebring, T., Fjellström, O., Fitzpatrick, K., et al. (2008). Synthesis and pharmacological evaluation of novel gamma-aminobutyric acid type B (GABAB) receptor agonists as gastroesophageal reflux inhibitors. J. Med. Chem. 51, 4315–4320. doi:10.1021/jm701425k
Bashiri, A., Burstein, E., and Mazor, M. (2006). Cerebral palsy and fetal inflammatory response syndrome: a review. J. Perinat. Med. 34, 5–12. doi:10.1515/JPM.2006.001
Bekteshi, S., Monbaliu, E., McIntyre, S., Saloojee, G., Hilberink, S. R., Tatishvili, N., et al. (2023). Towards functional improvement of motor disorders associated with cerebral palsy. Lancet Neurol. 22, 229–243. doi:10.1016/S1474-4422(23)00004-2
Bobath, B. (1967). The very early treatment of cerebral palsy. Dev. Med. Child. Neurol. 9, 373–390. doi:10.1111/j.1469-8749.1967.tb02290.x
Boecker, A. H., Lukhaup, L., Aman, M., Bergmeister, K., Schwarz, D., Bendszus, M., et al. (2022). Evaluation of MR-neurography in diagnosis and treatment in peripheral nerve surgery of the upper extremity: a matched cohort study. Microsurgery 42, 160–169. doi:10.1002/micr.30846
Cai, M. (2020). Clinical observation on 42 cases of children with spastic cerebral palsy treated by Huangqi Guizhi Wuwu Tang combined with functional electrical stimulation. J. Pediatr. Traditional Chin. Med. 16, 73–76. doi:10.16840/j.issn1673-4297.2020.02.22
Chen, H. Y., Lin, Y. H., Huang, J. W., and Chen, Y. C. (2015). Chinese herbal medicine network and core treatments for allergic skin diseases: implications from a nationwide database. J. Ethnopharmacol. 168, 260–267. doi:10.1016/j.jep.2015.04.002
Chen, Z., Huang, Z., Li, X., Deng, W., Gao, M., Jin, M., et al. (2023). Effects of traditional Chinese medicine combined with modern rehabilitation therapies on motor function in children with cerebral palsy: a systematic review and meta-analysis. Front. Neurosci. 17, 1097477. doi:10.3389/fnins.2023.1097477
Cheng, W., Zhang, Z., Feng, K., Cai, Z., Li, A., and Lu, Y. (2016). Effect observation of child cerebral palsy gross movement disorders treated with high dose of Astragalus mongholicus. Lishizhen Med. Materia Medica Res. 27, 2192–2194. doi:10.3969/j.issn.1008-0805.2016.09.050
Chun, P. T., McPherson, R. J., Marney, L. C., Zangeneh, S. Z., Parsons, B. A., Shojaie, A., et al. (2015). Serial plasma metabolites following hypoxic-ischemic encephalopathy in a nonhuman primate model. Dev. Neurosci. 37, 161–171. doi:10.1159/000370147
Chung, V. C., Ho, R. S., Wu, X., Fung, D. H., Lai, X., Wu, J. C., et al. (2015). Are meta-analyses of Chinese herbal medicine trials trustworthy and clinically applicable? a cross-sectional study. J. Ethnopharmacol. 162, 47–54. doi:10.1016/j.jep.2014.12.028
Colver, A., Fairhurst, C., and Pharoah, P. O. (2014). Cerebral palsy. Lancet 383, 1240–1249. doi:10.1016/S0140-6736(13)61835-8
Conner, B. C., Remec, N. M., and Lerner, Z. F. (2022). Is robotic gait training effective for individuals with cerebral palsy? A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 36, 873–882. doi:10.1177/02692155221087084
De Cassai, A., Tassone, M., Geraldini, F., Sergi, M., Sella, N., Boscolo, A., et al. (2021). Explanation of trial sequential analysis: using a post-hoc analysis of meta-analyses published in Korean Journal of Anesthesiology. Korean J. Anesthesiol. 74, 383–393. doi:10.4097/kja.21218
Demont, A., Gedda, M., Lager, C., de Lattre, C., Gary, Y., Keroulle, E., et al. (2022). Evidence-Based, implementable motor rehabilitation guidelines for individuals with cerebral palsy. Neurology 99, 283–297. doi:10.1212/WNL.0000000000200936
Deng, S., Chen, S. N., Lu, J., Wang, Z. J., Nikolic, D., van Breemen, R. B., et al. (2006). GABAergic phthalide dimers from Angelica sinensis (Oliv.) Diels. Phytochem. Anal. 17, 398–405. doi:10.1002/pca.937
Djukic, M., Gibson, C. S., Maclennan, A. H., Goldwater, P. N., Haan, E. A., McMichael, G., et al. (2009). Genetic susceptibility to viral exposure may increase the risk of cerebral palsy. Aust. N. Z. J. Obstet. Gynaecol. 49, 247–253. doi:10.1111/j.1479-828X.2009.00999.x
Du, X., Zheng, G., Feng, B., and Ma, B. (2016). Clinical research of modified suanzaoren granule in treating sleep disorders of cerebral palsy. Guangming J. Chin. Med. 31, 1576–1577. doi:10.3969/j.issn.1003-8914.2016.11.026
Fu, C., Wu, Y., Liu, S., Luo, C., Lu, Y., Liu, M., et al. (2022). Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 289, 115021. doi:10.1016/j.jep.2022.115021
Gao, Z., Pang, Z., Chen, Y., Lei, G., Zhu, S., Li, G., et al. (2022). Restoring after central nervous system injuries: neural mechanisms and translational applications of motor recovery. Neurosci. Bull. 38, 1569–1587. doi:10.1007/s12264-022-00959-x
Geng, L. (2019). The efficacy of Xingnao Kaiqiao decoction combined with acupuncture in the treatment of cerebral palsy and its impact on children’s movement and daily living abilities. Shaanxi J. Traditional Chin. Med. 40, 664–666. doi:10.3969/j.issn.1000-7369.2019.05.035
Gong, F., Qu, R., Li, Y., Lv, Y., and Dai, J. (2022). Astragalus Mongholicus: a review of its anti-fibrosis properties. Front. Pharmacol. 13, 976561. doi:10.3389/fphar.2022.976561
Han, Y., and Park, S. (2023). Effectiveness of virtual reality on activities of daily living in children with cerebral palsy: a systematic review and meta-analysis. PeerJ 11, e15964. doi:10.7717/peerj.15964
He, D. Y., and Dai, S. M. (2011). Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall, a traditional Chinese herbal medicine. Front. Pharmacol. 2, 10. doi:10.3389/fphar.2011.00010
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huifang, Y. (2012). A study on the correlation between work-related stress and perceived health status among home care providers. Chin. J. Occup. Med. 19, 135–145.
Jiang, B., Shen, R. F., Bi, J., Tian, X. S., Hinchliffe, T., and Xia, Y. (2015). Catalpol: a potential therapeutic for neurodegenerative diseases. Curr. Med. Chem. 22, 1278–1291. doi:10.2174/0929867322666150114151720
Kang, H. (2021). Trial sequential analysis: novel approach for meta-analysis. Anesth. Pain Med. Seoul. 16, 138–150. doi:10.17085/apm.21038
Kapitanović Vidak, H., Catela Ivković, T., Vidak, Z., and Kapitanović, S. (2017). COX-1 and COX-2 polymorphisms in susceptibility to cerebral palsy in very preterm infants. Mol. Neurobiol. 54, 930–938. doi:10.1007/s12035-016-9713-9
Kaukola, T., Satyaraj, E., Patel, D. D., Tchernev, V. T., Grimwade, B. G., Kingsmore, S. F., et al. (2004). Cerebral palsy is characterized by protein mediators in cord serum. Ann. Neurol. 55, 186–194. doi:10.1002/ana.10809
Kaya Keles, C. S., and Ates, F. (2022). Botulinum toxin intervention in cerebral palsy-induced spasticity management: projected and contradictory effects on skeletal muscles. Toxins (Basel) 14, 772. doi:10.3390/toxins14110772
Kim, M., Acharya, S., Botanas, C. J., Custodio, R. J., Lee, H. J., Sayson, L. V., et al. (2020). Catalpol and mannitol, two components of Rehmannia glutinosa, exhibit anticonvulsant effects probably via GABA(A) receptor regulation. Biomol. Ther. Seoul. 28, 137–144. doi:10.4062/biomolther.2019.130
Lan, G., and DeMets, D. L. (1983). Discrete sequential boundaries for clinical trials. Biometrika 70, 659–663. doi:10.2307/2336502
Laureau, J., Pons, C., Letellier, G., and Gross, R. (2022). Hyperbaric oxygen in children with cerebral palsy: a systematic review of effectiveness and safety. PLoS One 17, e0276126. doi:10.1371/journal.pone.0276126
Lee, B., Kwon, C. Y., and Chang, G. T. (2018). Oriental herbal medicine for neurological disorders in children: an overview of systematic reviews. Am. J. Chin. Med. 46, 1701–1726. doi:10.1142/S0192415X18500866
Li, M. M., Zhang, Y., Wu, J., and Wang, K. P. (2020). Polysaccharide from Angelica sinensis suppresses inflammation and reverses anemia in complete Freund’s adjuvant-induced rats. Curr. Med. Sci. 40, 265–274. doi:10.1007/s11596-020-2183-3
Li, M. T., Xie, L., Jiang, H. M., Huang, Q., Tong, R. S., Li, X., et al. (2022). Role of licochalcone A in potential pharmacological therapy: a review. Front. Pharmacol. 13, 878776. doi:10.3389/fphar.2022.878776
Liang, X., Tan, Z., Yun, G., Cao, J., Wang, J., Liu, Q., et al. (2021). Effectiveness of exercise interventions for children with cerebral palsy: a systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 53, jrm00176. doi:10.2340/16501977-2772
Liao, H. H., Yen, H. R., Muo, C. H., Lee, Y. C., Wu, M. Y., Chou, L. W., et al. (2017). Complementary traditional Chinese medicine use in Children with cerebral palsy: a nationwide retrospective cohort study in Taiwan. BMC Complementary Altern. Med. 17, 155. doi:10.1186/s12906-017-1668-5
Liu, Y., and Dong, H. (2019). Clinical study of massaging acupuncture points in head combined with kaiqiao xingshen decoction in treatment of motor and language dysfunction in children with cerebral palsy. Liaoning J. Traditional Chin. Med. 46, 611–614. doi:10.13192/j.issn.1000-1719.2019.03.050
Lou, Y., and Shi, H. (2016). Observing the clinical efficacy of pastic cerebral palsy with Shujinhuoluo Wan. Pharmacol. Clin. Chin. Materia Medica 32, 185–188. doi:10.13412/j.cnki.zyyl.2016.03.054
Lu, C., Zhou, S., and Guo, G. (2012). Clinical effect of Shenluqizhi decoction on patients with spastic cerebral palsy. Hebei J. Tradit. Chin. Med. 34, 21–23.
Lu, Y. C., Tseng, L. W., Huang, Y. C., Yang, C. W., Chen, Y.-C., and Chen, H.-Y. (2022). The potential complementary role of using Chinese herbal medicine with western medicine in treating COVID-19 patients: pharmacology network analysis. Pharmaceuticals 15, 794. doi:10.3390/ph15070794
Ma, B., Zhang, J. F., Chen, T., and Zheng, H. (2018). Clinical observation on the effect of Pujin Keli in adjunctive treating of 34 cases of children with spastic cerebral palsy caused by phlegm and blood retention in head. J. Pediatr. Tradit. Chin. Med. 14, 26–30. doi:10.16840/j.issn1673-4297.2018.03.08
Magalhães, R. C., Moreira, J. M., Lauar, A. O., da Silva, A. A. S., Teixeira, A. L., and Silva, E. (2019). Inflammatory biomarkers in children with cerebral palsy: a systematic review. Res. Dev. Disabil. 95, 103508. doi:10.1016/j.ridd.2019.103508
Malaeb, S., and Dammann, O. (2009). Fetal inflammatory response and brain injury in the preterm newborn. J. Child. Neurol. 24, 1119–1126. doi:10.1177/0883073809338066
Mallah, K., Couch, C., Borucki, D. M., Toutonji, A., Alshareef, M., and Tomlinson, S. (2020). Anti-inflammatory and neuroprotective agents in clinical trials for CNS disease and injury: where do we go from here? Front. Immunol. 11, 2021. doi:10.3389/fimmu.2020.02021
McIntyre, S., Goldsmith, S., Webb, A., Ehlinger, V., Hollung, S. J., McConnell, K., et al. (2022). Global prevalence of cerebral palsy: a systematic analysis. Dev. Med. Child. Neurol. 64, 1494–1506. doi:10.1111/dmcn.15346
Novak, I., Hines, M., Goldsmith, S., and Barclay, R. (2012). Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics 130, e1285–e1312. doi:10.1542/peds.2012-0924
Novak, I., Paton, M. C., Griffin, A. R., Jackman, M., Blatch-Williams, R. K., and Finch-Edmondson, M. (2023). The potential of cell therapies for cerebral palsy: where are we today? Expert Rev. Neurother. 23, 673–675. doi:10.1080/14737175.2023.2234642
Paton, M. C. B., Finch-Edmondson, M., Dale, R. C., Fahey, M. C., Nold-Petry, C. A., Nold, M. F., et al. (2022). Persistent inflammation in cerebral palsy: pathogenic mediator or comorbidity? a scoping review. J. Clin. Med. 11, 7368. doi:10.3390/jcm11247368
Paul, S., Nahar, A., Bhagawati, M., and Kunwar, A. J. (2022). A review on recent advances of cerebral palsy. Oxid. Med. Cell. Longev. 2022, 2622310. doi:10.1155/2022/2622310
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2007). Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat. Med. 26, 4544–4562. doi:10.1002/sim.2889
Qian, S. (2009) The role of Sijunzi Decoction in the treatment of patients with cerebral palsy in replenishing qi and strengthening the spleen. J. North China Univ. Sci. Technol. North China 11 310–311.
Rosenbaum, P., Paneth, N., Leviton, A., Goldstein, M., Bax, M., Damiano, D., et al. (2007). A report: the definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. Suppl. 109, 8–14.
Sakpal, T. V. (2010). Sample size estimation in clinical trial. Perspect. Clin. Res. 1, 67–69. doi:10.4103/2229-3485.71856
Salomon, I. (2024). Neurobiological insights into cerebral palsy: a review of the mechanisms and therapeutic strategies. Brain Behav. 14, e70065. doi:10.1002/brb3.70065
Schalow, G. (2002). Recovery from spinal cord injury achieved by 3 months of coordination dynamic therapy. Electromyogr. Clin. Neurophysiol. 42, 367–376.
Shan, H., Zhang, Y., Zhang, P., Cao, C., Lou, Y., and Hou, Y. (2017). Observation on the therapeutic effect of nourishing kidney and inducing resuscitation for expelling phlegm prescription in the treatment of cerebral palsy with mental retardation. Chin. Med. Mod. Distance Educ. 15, 78–80. doi:10.3969/j.issn.1672-2779.2017.01.034
Shi, J., and Xie, Q. (2015). Integrated medicine therapy in treating 70 children with cerebral palsy of convulsion pattern. West. J. Tradit. Chin. Med. 28, 104–106.
Song, J., Meng, L., Li, S., Qu, L., and Li, X. (2009). A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, improved renal microvascular insufficiency in 5/6 nephrectomized rats. Vasc. Pharmacol. 50, 185–193. doi:10.1016/j.vph.2009.01.005
Strickland, A. D. (2014). Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med. Hypotheses 82, 522–528. doi:10.1016/j.mehy.2014.02.003
Sun, J., Wang, J., Lin, X., Xie, B., and Wu, F. (2017). Effects of xingnao yizhi fang combined with rehabilitation training on children with cerebral palsy due to dystonia and its effect on transforming growth factor beta 1 and nerve remodeling in children. World Chin. Med. 12, 2975–2978. doi:10.3969/j.issn.1673-7202.2017.12.028
Than, U. T. T., Nguyen, L. T., Nguyen, P. H., Nguyen, X. H., Trinh, D. P., Hoang, D. H., et al. (2023). Inflammatory mediators drive neuroinflammation in autism spectrum disorder and cerebral palsy. Sci. Rep. 13, 22587. doi:10.1038/s41598-023-49902-8
Tung, S. (2022). Clinical observation on rehabilitation training combined with huangqi guizhi wuwu decoction in the treatment of spastic cerebral palsy in children. J. Pract. Tradit. Chin. Med. 38, 364–366.
Vadivelan, K., Sekar, P., Sruthi, S. S., and Gopichandran, V. (2020). Burden of caregivers of children with cerebral palsy: an intersectional analysis of gender, poverty, stigma, and public policy. BMC Public Health 20, 645. doi:10.1186/s12889-020-08808-0
Vezér, M., Gresits, O., Engh, M. A., Szabó, L., Molnar, Z., Hegyi, P., et al. (2024). Evidence for gait improvement with robotic-assisted gait training of children with cerebral palsy remains uncertain. Gait Posture 107, 8–16. doi:10.1016/j.gaitpost.2023.08.016
Vinkers, C. H., and Olivier, B. (2012). Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABA(A) receptor modulators? Adv. Pharmacol. Sci. 2012, 416864. doi:10.1155/2012/416864
Vitrikas, K., Dalton, H., and Breish, D. (2020). Cerebral palsy: an overview. Am. Fam. Physician 101, 213–220.
Wang, H., Xu, X., Yin, Y., Yu, S., Ren, H., Xue, Q., et al. (2020). Catalpol protects vascular structure and promotes angiogenesis in cerebral ischemic rats by targeting HIF-1α/VEGF. Phytomedicine 78, 153300. doi:10.1016/j.phymed.2020.153300
Wang, H. J., Ran, H. F., Yin, Y., Xu, X. G., Jiang, B. X., Yu, S. Q., et al. (2022). Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol. Sin. 43, 1670–1685. doi:10.1038/s41401-021-00803-4
Wang, L., Chen, B., Xie, D., and Wang, Y. (2024). Bioinformatics and network pharmacology discover the molecular mechanism of Liuwei Dihuang pills in treating cerebral palsy. Med. Baltim. 103, e40166. doi:10.1097/MD.0000000000040166
Wang, M. C., Chou, Y. T., Kao, M. C., Lin, Q. Y., Chang, S. Y., and Chen, H. Y. (2023). Topical Chinese herbal medicine in treating atopic dermatitis (eczema): a systematic review and meta-analysis with core herbs exploration. J. Ethnopharmacol. 317, 116790. doi:10.1016/j.jep.2023.116790
Wetterslev, J., Jakobsen, J. C., and Gluud, C. (2017). Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 17, 39. doi:10.1186/s12874-017-0315-7
Wieland, L. S., Manheimer, E., Sampson, M., Barnabas, J. P., Bouter, L. M., Cho, K., et al. (2013). Bibliometric and content analysis of the Cochrane Complementary Medicine Field specialized register of controlled trials. Syst. Rev. 2, 51. doi:10.1186/2046-4053-2-51
Wimalasundera, N., and Stevenson, V. L. (2016). Cerebral palsy. Pract. Neurol. 16, 184–194. doi:10.1136/practneurol-2015-001184
Winstein, C. J., Stein, J., Arena, R., Bates, B., Cherney, L. R., Cramer, S. C., et al. (2016). Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 47, e98–e169. doi:10.1161/STR.0000000000000098
Wu, C. W., Chen, H. Y., Yang, C. W., and Chen, Y. C. (2021). Deciphering the efficacy and mechanisms of Chinese herbal medicine for diabetic kidney disease by integrating web-based biochemical databases and real-world clinical data: retrospective cohort study. JMIR Med. Inf. 9, e27614. doi:10.2196/27614
Wu, J., Zong, H., Wang, Y., and Xu, D. M. (2022). Clinical observation on Liuwei Dihuang pill and yigong power in the treatment of dyskinetic cerebral palsy. Guangming J. Chin. Med. 37, 3514–3517. doi:10.3969/j.issn.1003-8914.2022.19.018
Wu, Y., Li, D., Wang, H., and Wan, X. (2022). Protective effect of Poria cocos polysaccharides on fecal peritonitis-induced sepsis in mice through inhibition of oxidative stress, inflammation, apoptosis, and reduction of treg cells. Front. Microbiol. 13, 887949. doi:10.3389/fmicb.2022.887949
Yoo, J. E., Yun, Y. J., Shin, Y. B., Kim, N. K., Kim, S. Y., Shin, M. J., et al. (2016). Protocol for a prospective observational study of conventional treatment and traditional Korean medicine combination treatment for children with cerebral palsy. BMC Complement. Altern. Med. 16, 172. doi:10.1186/s12906-016-1161-6
Yu, M. (2016). The influence of clinical effects and walking function on 40 cases of children with spastic cerebral palsy treated by the combination of Chinese herbs and rehabilitation training. J. Pediatr. Tradit. Chin. Med. 12, 78–81. doi:10.16840/j.issn1673-4297.2016.05.25
Yuan, J. J., Wu, D., Wang, W. W., Duan, J., Xu, X. Y., and Tang, J. L. (2021). A prospective randomized controlled study on mouse nerve growth factor in the treatment of global developmental delay in children. Zhongguo Dang Dai Er Ke Za Zhi 23, 786–790. doi:10.7499/j.issn.1008-8830.2106042
Yingyuan, Hu (2009). “Evaluation standards for rehabilitation efficacy and prediction of rehabilitation outcomes for children with cerebral palsy,” in The 6th national pediatric cerebral palsy academic exchange and international exchange conference, 8–9.
Zhang, C. (2021). Kaiqiao Xingshen Decoction combined with head acupoint massage in the treatment of cerebral palsy in children. J. Pract. Tradit. Chin. Med. 37, 745–746.
Zhang, L. Q., Chen, K. X., and Li, Y. M. (2019). Bioactivities of natural catalpol derivatives. Curr. Med. Chem. 26, 6149–6173. doi:10.2174/0929867326666190620103813
Zhang, W. L., Cao, Y. A., Xia, J., Tian, L., Yang, L., and Peng, C. S. (2018). Neuroprotective effect of tanshinone IIA weakens spastic cerebral palsy through inflammation, p38MAPK and VEGF in neonatal rats. Mol. Med. Rep. 17, 2012–2018. doi:10.3892/mmr.2017.8069
Zhang, Y., Liu, J., Wang, J., and He, Q. (2010). Traditional Chinese medicine for treatment of cerebral palsy in children: a systematic review of randomized clinical trials. J. Altern. Complement. Med. 16, 375–395. doi:10.1089/acm.2009.0609
Zhang, Y., Xiang, S., and Wang, M. C. (2019). Influences of huangqi guizhi wuwu decoction combined with aerobic rehabilitation exercise on therapeutic effect, serum BDNF, NSE levels, and motor function in the treatment of children with spastic cerebral palsy. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 17, 2744–2747. doi:10.12102/j.issn.1672-1349.2019.18.006
Glossary
6MWT 6-minute walking test
ADEs Adverse drug events
ADL Activities of Daily Living for CP recover evaluation
AKT Protein kinase B
AM Astragalus mongholicus Bunge
AQP4 Aquaporin-4
AS Angelica sinensis (Oliv.)
BDNF Brain-derived neurotrophic factor
Berg Berg Balance Scale
CHM Chinese herbal medicine
CI Confidence interval
CMN Chinese herbal medicine network
CNKI China National Knowledge Infrastructure
COVID-19 Coronavirus disease 2019
CP Cerebral palsy
ER Effectiveness rate
ERK1/2 Extracellular signal-regulated kinase 1/2
ET-1 Endothelin-1
FAC Functional ambulation category scale
FDA Frenchay Dysarthria Assessment
FES Functional electrical stimulation
FMA Fugl–Meyer assessment scale
FMFM Fine motor function measure
GABA γ-aminobutyric acid
Gesell Gesell Developmental Scale
GMFM Gross Motor Function Measure score
GPX4 Glutathione peroxidase 4
GU Glycyrrhiza uralensis Fisch. ex DC.
IFN-α Interferon-α
IGF-1 Insulin-like growth factor 1
IL13 Interleukin 13
IL4 Interleukin 4
M:F Male:Female
MAS Modified Ashworth Scale
MD Mean difference
MDI Mental Developmental Index
MEK1/2 Mitogen Activated Protein Kinase Kinase 1/2
MWS Maximum walking speed
Nrf2 Nuclear factor erythroid 2–related factor 2
NSE Neuron specific enolase
OT Occupational therapy
PC Poria cocos (Schw.) Wolf.
PDI Psychomotor Development Index
PDMS-2 Peabody developmental motor scale-2
PedsQL Pediatric Quality of Life Inventory
PI3K Phosphatidylinositol 3 kinase
PL Paeonia lactiflora Pall.
PT Physical therapy
RCT Randomized clinical trial
RG Rehmannia glutinosa (Gaertn.) DC.
RoB Risk-of-bias
RR Risk ratio
SD Standard deviation
SLC7A11 Solute carrier family 7 member 11
SMD Standardized mean difference
SPR Selective posterior rhizotomy
ST Standard treatment
TCM Traditional Chinese medicine
TDS Teacher’s Drooling Scale
TGF-β1 Transforming growth factor-beta 1
TSA Trial sequential analysis
VEGF Vascular endothelial growth factor
WeeFIM Wee Functional Independence Measure for Children
WISC Wechsler Intelligence Scale for Children
WM Western medicine
Keywords: meta-analysis, cerebral palsy, Chinese herbal medicine, system pharmacology, traditional Chinese medicine
Citation: Huang Y-Y, Cheng Y-Y, Chen H-Y, Fu R-H, Chang Y-J and Yang T-H (2025) Chinese herbal medicine for the treatment of children with cerebral palsy: a meta-analysis of randomized controlled trials with core herbs exploration. Front. Pharmacol. 16:1500095. doi: 10.3389/fphar.2025.1500095
Received: 22 September 2024; Accepted: 02 January 2025;
Published: 26 February 2025.
Edited by:
Karim Hosni, Institut National de Recherche et d'Analyse Physico-Chimique (INRAP), TunisiaReviewed by:
Kylie Crompton, Royal Children’s Hospital, AustraliaAfef Amri, Institut National des Sciences et Technologies de la Mer, Tunisia
Copyright © 2025 Huang, Cheng, Chen, Fu, Chang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsung-Hsien Yang, ODkwNTAwMUBjZ21oLm9yZy50dw==
 Ying-Yu Huang
Ying-Yu Huang Ya-Yun Cheng2
Ya-Yun Cheng2 Hsing-Yu Chen
Hsing-Yu Chen Ren-Huei Fu
Ren-Huei Fu Yi-Jung Chang
Yi-Jung Chang Tsung-Hsien Yang
Tsung-Hsien Yang