
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol., 10 March 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1489696
This article is part of the Research TopicThe Molecular Mechanism in Anti-tumor Therapy ResistanceView all 14 articles
Background: The MET proto-oncogene (MET) plays a crucial role as an oncogenic driver gene in non-small cell lung cancer (NSCLC). At present, numerous types of MET exon 14 (METex14) skipping mutation have been identified, but different splice variants often exhibit varying treatment responses. There is currently no standardized treatment approach for rare METex14 mutation after resistance to epidermal growth factor receptor tyrosine kinases inhibitor (EGFR-TKI). Herein, we present for the first time a case of advanced lung adenocarcinoma with a novel METex14 skipping mutation following resistance to EGFR-TKI and subsequent sensitivity to savolitinib. In addition, the patient developed a novel METex14 skipping mutation after EGFR-TKI resistance, which we suspect may be a potential new mechanism of EGFR-TKI resistance that has not been reported.
Materials and methods: We conducted surgical specimen pathology diagnosis and next-generation sequencing (NGS) of peripheral blood to ascertain the patient’s pathological and molecular characteristics.
Results: NGS testing identified a novel METex14 (c.2888-23_2888-8del) skipping mutation in the patient with advanced lung adenocarcinoma who developed resistance to EGFR-TKI, suggesting its potential involvement as one of the mechanisms underlying the resistance to EGFR-TKI. Following administration of savolitinib with a daily dose of 400 mg, the patient exhibited a partial response and achieved progression-free survival (PFS) exceeding 8 months.
Conclusion: The case presents a novel METex14 skipping mutation that emerges subsequent to the progression of advanced lung adenocarcinoma following EGFR-TKI treatment. Importantly, this mutation may serve as one of the mechanisms contributing to resistance against EGFR-TKI and exhibit sensitivity towards savolitinib treatment, providing reference for future similar cases in terms of treatment options.
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the first-line treatment for patients with locally advanced or metastatic EGFR mutation non-small cell lung cancer (NSCLC) (Hanna et al., 2017; Planchard et al., 2018). However, the development of resistance is inevitable in patients receiving EGFR-TKIs. The current mechanism of acquired EGFR-TKIs resistance mainly include the emergence of bypass pathways or histologic alterations, and the Mesenchymal–epithelial transition proto-oncogene (MET) exon 14 (METex14) skipping mutation does not appear to include.
Generally speaking, in approximately 3%–4% of NSCLC patients, the presence of METex14 skipping mutation is observed, which rarely coexists with other known driver mutations in NSCLC, except for MET amplification (Cancer Genome Atlas Research Network, 2014; Baldacci et al., 2018; Frampton et al., 2015; Tong et al., 2016). The METex14 skipping mutation acts as an independent oncogenic driver in NSCLC, and patients with this mutation experience unfavorable prognosis and lower survival rates (Wolf et al., 2020; Yang et al., 2020). Savolitinib is a highly potent and selective MET-TKI that has shown remarkable efficacy and safety in advanced NSCLC patients with METex14 skipping mutation (Lu et al., 2021; Wang et al., 2022). Furthermore, it can be effectively combined with EGFR-TKIs (such as gefitinib, Osimertinib, etc.) to overcome acquired resistance caused by MET alterations (MET amplification or c-MET overexpression), extending the benefits to patients who have previously undergone EGFR-TKIs treatment and experienced disease progression (Sequist et al., 2020; Markham, 2021; Oxnard et al., 2020).
At present, the locations that may lead to METex14 skipping mutation are diverse, warranting further investigation into potential heterogeneity in function and treatment among these distinct MET exon splice variants. It is still unclear whether these newly discovered rare METex14 skipping mutation are sensitive to MET-TKIs. Here, we present a case of advanced lung adenocarcinoma, harboring a novel and rare METex14 skipping mutation, which potentially represents a new mechanism of resistance to EGFR-TKIs.
A 79-year-old non-smoking man visited the hospital because of a 5-month history of cough with sputum. The patient was previously in good health, without hypertension and diabetes. A computed tomography (CT) scan revealed a nodule in the right upper lung (the diameter is approximately 1.2 cm), without bone, liver, kidney, or brain metastases. On 9 January 2018, the patient underwent right upper lobectomy plus systematic lymph node dissection and was diagnosed with right upper lung adenocarcinoma (stage IA, pT1N0M0) as the immunohistochemical (IHC) staining indicated the tumor was positive for Napsin A and thyroid transcription factor-1 (TTF-1) (Figure 1). The gene testing of the patient’s pathological tissue revealed the presence of epidermal growth factor receptor (EGFR) exon 21 L858R point mutation. The patient did not receive postoperative adjuvant therapy and underwent regular follow-up. In November 2019, a chest CT scan revealed local recurrence of the residual right upper lobe of the lung. Subsequently, the patient initiated gefitinib treatment at a daily dose of 250 mg. Four months later, the patient stopped taking gefitinib due to progressive lesion growth and then the patient received chest radiotherapy, followed by continuous treatment with a combination of Icotinib and Bevacizumab in November 2020. Due to a significant decline in renal function (serum creatinine level:123umol/L; estimated glomerular filtration rate (eGFR):48.1 mL/min), Icotinib and Bevacizumab were discontinued in November 2022, and the patient was subsequently switched to Osimertinib. In April 2023, the patient’s chest CT revealed interstitial changes in the lungs, which led to the consideration of possible intolerance to Osimertinib, and the treatment was adjusted to Almonertinib. In June 2023, a CT scan showed bilateral lung metastases and a growth of the tumor in the lower segment of the right posterior lobe of the liver, indicating disease progression (Figure 2), then the peripheral blood next-generation sequencing (NGS) testing was conducted, and the NGS testing revealed a novel METex14 skipping mutation (c.2888–23_2888-8delTCTTTCTTTCTCTCTG IVS13, 1.0%), accompanied by a TP53 mutation (p.Y163C, 0.1%) (Figure 3). Combination therapy with Almonertinib and savolitinib were recommended, but the patient chose to receive savolitinib alone at a daily dose of 400 mg after considering the cost. Fortunately, after a 2-month period of treatment, the patient had a partial response in lung lesions and complete response in liver metastasis (Figure 2). As of the time of writing this manuscript, the patient is still taking savolitinib with a progression-free survival (PFS) over 8 months and no significant adverse events have been observed so far. The patient’s treatment process is shown in Figure 4.
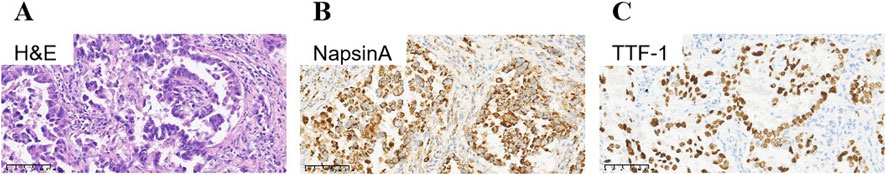
Figure 1. Immunohistochemical staining of primary tumor: Lung adenocarcinoma. (A) Hematoxylin-eosin staining of lung adenocarcinoma. Tumor cells were positive for NapsinA (B), and TTF-1 (C). TTF-1, thyroid transcription factor-1.
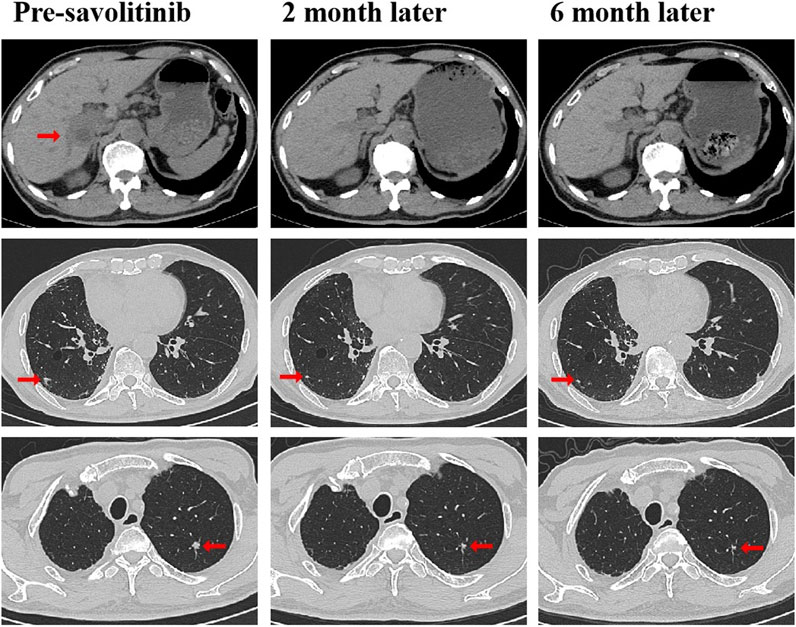
Figure 2. CT scans before and after savolitinib treatment. CT scans at pre-savolitinib showed the presence of liver metastasis and bilateral lung metastases. CT scans after treatment with savolitinib showed partial response in bilateral lung lesions and complete response in liver metastasis. CT, computed tomography.
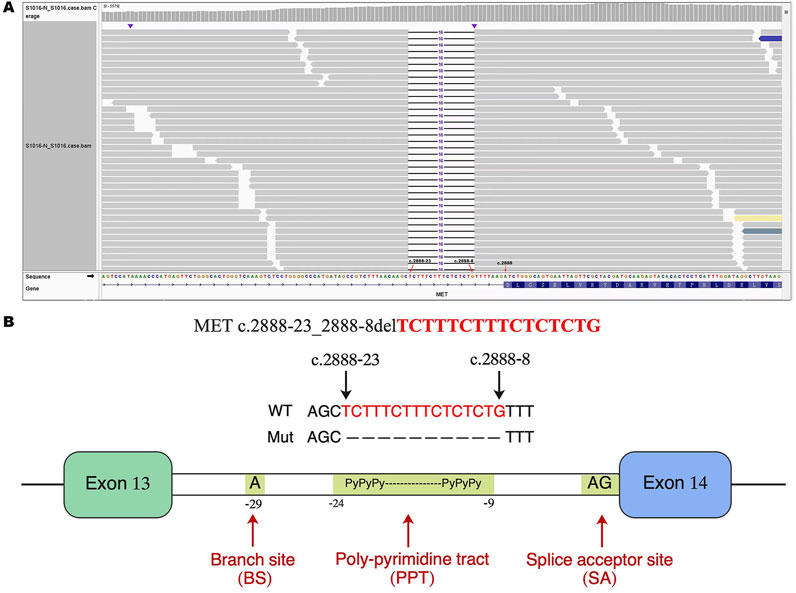
Figure 3. Identification of a novel METex14 skipping mutation by NGS. (A) The Integrative Genomics Viewer (IVG) shows the novel METex14 skipping mutation detected by NGS. (B) Illustration of METex 14 skipping mutation (the new variant is located near the PPT). METex14, Mesenchymal–epithelial transition (MET) exon 14; NGS, Next-generation sequencing; PPT, poly-pyrimidine tract.
The novel METex14 skipping mutation (c.2888-23_2888-8del) reported here is located near the poly-pyrimidine tract (PPT), which may lead to the skipping mutation in exon 14. To our knowledge, this study presents the first report of a novel METex14 skipping mutation observed in NSCLC patients following resistance to EGFR-TKIs treatment. We propose that this mutation may serve as a potential mechanism of resistance to EGFR-TKIs, which has not been previously reported. In addition, this study provides the first clinical evidence demonstrating that savolitinib effectively reverses acquired resistance to EGFR-TKIs driven by novel METex14 skipping mutation in patients with advanced lung adenocarcinoma harboring EGFR mutations, thereby yielding long-term PFS benefits.
The bypassing of activation mediated by the MET signaling pathway is a crucial mechanism contributing to resistance against EGFR-TKI, which can manifest as gene-level amplification or protein-level overexpression (Reis et al., 2018; Wood et al., 2021). The effective approach for managing acquired resistance to EGFR-TKIs driven by MET is combination therapy involving EGFR-TKIs and MET-TKIs (Oxnard et al., 2020; York et al., 2017; Gainor et al., 2016; Wu et al., 2018). Currently, savolitinib is primarily employed as a monotherapy for advanced NSCLC with METex14 skipping mutation, as well as in combination with EGFR-TKIs to overcome acquired resistance driven by MET to EGFR-TKIs (Sequist et al., 2020; Markham, 2021; Oxnard et al., 2020; Yu et al., 2024; Lee et al., 2023). However, the resistance mechanisms acquired to EGFR-TKIs driven by MET do not appear to involve METex14 skipping mutation. Our case report showed that the patient developed a new METex14 skipping mutation after acquiring resistance to EGFR-TKIs. We suggested combining savolitinib with the current EGFR-TKI treatment, but the patient chose to only use savolitinib alone and still experienced a significant improvement in PFS. Therefore, in such cases, it may be advisable to assess the viability of MET-TKI monotherapy, considering the patient’s physical tolerance, family financial circumstances, and personal willingness.
The presence of TP53 gene mutations is frequently observed in NSCLC with METex14 skipping mutation (Frampton et al., 2015). While the coexistence of TP53 mutations has been associated with reduced efficacy of EGFR-TKIs in NSCLC, there is no evidence to suggest that alterations in TP53 impact the effectiveness of MET-TKIs (Canale et al., 2017; Fujino et al., 2021). The optimal diagnostic method for METex14 skipping mutation is based on NGS of DNA or RNA (Kim et al., 2019). Based on the NGS analysis, our case report demonstrated the concurrent presence of METex14 skipping mutation and TP53 mutation in the patient, with a PFS exceeding 8 months. Further investigation is necessary to understand the impact of TP53 coexistence on the effectiveness of MET-TKIs.
Currently, the indications for administering MET-TKIs to rare MET exon splice variants are still a subject of controversy due to the heterogeneous response observed among them. Hence, it is essential to utilize NGS for a thorough identification of MET exon splicing variants, aiming to optimize treatment strategies and improve patient survival with MET-TKIs. The novel METex14 skipping mutation, detected by NGS in this case, showed a positive response to savolitinib, resulting in a PFS of over 8 months. Therefore, we propose that this mutation is sensitive to MET-TKIs. Until the submission of the manuscript, the patient is still undergoing savolitinib treatment, and we will continue to monitor the therapeutic efficacy.
In summary, our case report presented a lung adenocarcinoma patient who developed a novel METex14 skipping mutation (c.2888-23_2888-8del) after acquiring resistance to EGFR-TKIs treatment and exhibited clinical benefits from savolitinib therapy. Moreover, we proposed that this newly identified METex14 skipping mutation may represent an emerging mechanism of acquired resistance to EGFR-TKI treatment, warranting further investigation for validation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the West China Hospital, Sichuan University ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YX: Conceptualization, Methodology, Writing–original draft. WL: Conceptualization, Methodology, Writing–original draft. PL: Data curation, Investigation, Writing–review and editing. KH: Data curation, Investigation, Writing–review and editing. QZ: Supervision, Writing–review and editing. QW: Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Youth Project of Sichuan Natural Science Foundation (No. 2023NSFSC1892; No. 2024NSFSC1918) and Postdoctoral Research Project of West China Hospital, Sichuan University (No. 2020HXBH132).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baldacci, S., Kherrouche, Z., Descarpentries, C., Wislez, M., Dansin, E., Furlan, A., et al. (2018). MET exon 14 splicing sites mutations: a new therapeutic opportunity in lung cancer. Rev. Mal. Respir. 35 (8), 796–812. doi:10.1016/j.rmr.2018.01.011
Canale, M., Petracci, E., Delmonte, A., Chiadini, E., Dazzi, C., Papi, M., et al. (2017). Impact of TP53 mutations on outcome inEGFR-mutated patients treated with first-line tyrosine kinase inhibitors. Clin. Cancer Res. 23 (9), 2195–2202. doi:10.1158/1078-0432.CCR-16-0966
Cancer Genome Atlas Research Network (2014). Comprehensive molecular profiling of lung adenocarcinoma. Nature 511 (7511), 543–550. doi:10.1038/nature13385
Frampton, G. M., Ali, S. M., Rosenzweig, M., Chmielecki, J., Lu, X., Bauer, T. M., et al. (2015). Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 5 (8), 850–859. doi:10.1158/2159-8290.CD-15-0285
Fujino, T., Suda, K., and Mitsudomi, T. (2021). Lung cancer with MET exon 14 skipping mutation: genetic feature, current treatments, and future challenges. Lung Cancer (Auckl) 12, 35–50. doi:10.2147/LCTT.S269307
Gainor, J. F., Niederst, M. J., Lennerz, J. K., Dagogo-Jack, I., Stevens, S., Shaw, A. T., et al. (2016). Dramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-mutant lung cancer harboring de novo MET amplification. J. Thorac. Oncol. 11 (7), e83–e85. doi:10.1016/j.jtho.2016.02.021
Hanna, N., Johnson, D., Temin, S., Baker, S. Jr, Brahmer, J., Ellis, P. M., et al. (2017). Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 35 (30), 3484–3515. doi:10.1200/JCO.2017.74.6065
Kim, E. K., Kim, K. A., Lee, C. Y., Kim, S., Chang, S., Cho, B. C., et al. (2019). Molecular diagnostic assays and clinicopathologic implications of MET exon 14 skipping mutation in non-small-cell lung cancer. Clin. Lung Cancer 20 (1), e123–e132. doi:10.1016/j.cllc.2018.10.004
Lee, T. S., Kim, J. Y., Lee, M. H., Cho, I. R., Paik, W. H., Ryu, J. K., et al. (2023). Savolitinib: a promising targeting agent for cancer. Cancers (Basel) 15 (19), 4708. doi:10.3390/cancers15194708
Lu, S., Fang, J., Li, X., Cao, L., Zhou, J., Guo, Q., et al. (2021). Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir. Med. 9 (10), 1154–1164. doi:10.1016/S2213-2600(21)00084-9
Markham, A. (2021). Savolitinib: first approval. Drugs 81 (14), 1665–1670. doi:10.1007/s40265-021-01584-0
Oxnard, G. R., Yang, J. C., Yu, H., Kim, S. W., Saka, H., Horn, L., et al. (2020). TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 31 (4), 507–516. doi:10.1016/j.annonc.2020.01.013
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C., et al. (2018). Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29 (Suppl. 4), iv192–iv237. doi:10.1093/annonc/mdy275
Reis, H., Metzenmacher, M., Goetz, M., Savvidou, N., Darwiche, K., Aigner, C., et al. (2018). MET expression in advanced non-small-cell lung cancer: effect on clinical outcomes of chemotherapy, targeted therapy, and immunotherapy. Clin. Lung Cancer 19 (4), e441–e463. doi:10.1016/j.cllc.2018.03.010
Sequist, L. V., Han, J. Y., Ahn, M. J., Cho, B. C., Yu, H., Kim, S. W., et al. (2020). Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 21 (3), 373–386. doi:10.1016/S1470-2045(19)30785-5
Tong, J. H., Yeung, S. F., Chan, A. W., Chung, L. Y., Chau, S. L., Lung, R. W., et al. (2016). MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin. Cancer Res. 22 (12), 3048–3056. doi:10.1158/1078-0432.CCR-15-2061
Wang, Y., Liu, T., Chen, G., Gong, J., Bai, Y., Zhang, T., et al. (2022). Phase ia/ib study of the selective MET inhibitor, savolitinib, in patients with advanced solid tumors: safety, efficacy, and biomarkers. Oncologist 27 (5), 342–e383. doi:10.1093/oncolo/oyab066
Wolf, J., Seto, T., Han, J. Y., Reguart, N., Garon, E. B., Groen, H. J. M., et al. (2020). Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N. Engl. J. Med. 383, 944–957. doi:10.1056/NEJMoa2002787
Wood, G. E., Hockings, H., Hilton, D. M., and Kermorgant, S. (2021). The role of MET in chemotherapy resistance. Oncogene 40 (11), 1927–1941. doi:10.1038/s41388-020-01577-5
Wu, Y. L., Zhang, L., Kim, D. W., Liu, X., Lee, D. H., Yang, J. C., et al. (2018). Phase ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. Met. Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 36 (31), 3101–3109. doi:10.1200/JCO.2018.77.7326
Yang, H., Zhou, Z., Lin, L., Yang, M., Li, C., Li, Z., et al. (2020). Characterization of MET exon 14 alteration and association with clinical outcomes of crizotinib in Chinese lung cancers. Lung Cancer 148, 113–121. doi:10.1016/j.lungcan.2020.08.009lungcan.2020.08.009
York, E. R., Varella-Garcia, M., Bang, T. J., Aisner, D. L., and Camidge, D. R. (2017). Tolerable and effective combination of full-dose crizotinib and osimertinib targeting MET amplification sequentially emerging after T790M positivity in EGFR-mutant non-small cell lung cancer. J. Thorac. Oncol. 12 (7), e85–e88. doi:10.1016/j.jtho.2017.02.020
Yu, Y., Guo, Q., Zhang, Y., Fang, J., Zhong, D., Liu, B., et al. (2024). Savolitinib in patients in China with locally advanced or metastatic treatment-naive non-small-cell lung cancer harbouring MET exon 14 skipping mutations: results from a single-arm, multicohort, multicentre, open-label, phase 3b confirmatory study. Lancet Respir. Med. 12 (12), 958–966. doi:10.1016/S2213-2600(24)00211-X
Keywords: novel MET exon 14 skipping mutation, EGFR-TKI resistance, advanced lung adenocarcinoma, savolitinib, sustained clinical response
Citation: Xue Y, Li W, Li P, Huang K, Zhou Q and Wu Q (2025) Case Report: A novel MET exon 14 skipping mutation after EGFR-TKI resistance in advanced lung adenocarcinoma and sustained clinical response to savolitinib. Front. Pharmacol. 16:1489696. doi: 10.3389/fphar.2025.1489696
Received: 01 September 2024; Accepted: 24 February 2025;
Published: 10 March 2025.
Edited by:
Chunbo He, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Akshita Bhatt, American Association For Cancer Research, United StatesCopyright © 2025 Xue, Li, Li, Huang, Zhou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wu, d3VxaWFuZzgxOEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.