
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 February 2025
Sec. Renal Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1484320
This article is part of the Research TopicCell Death in Kidney Diseases: Novel Biomarkers, Mechanisms, and Therapeutic StrategiesView all 15 articles
 Maierhaba Maitiyaer1†
Maierhaba Maitiyaer1† Peiyi Li1†
Peiyi Li1† Huangdong Li2†
Huangdong Li2† Dingwen Jiang3
Dingwen Jiang3 Jingyu Zhang2
Jingyu Zhang2 Chuwei Yang1
Chuwei Yang1 Xiaobin Yang4
Xiaobin Yang4 Chengmei Chen1
Chengmei Chen1 Wenhui Huang1
Wenhui Huang1 Zhiping Liu2*
Zhiping Liu2* Shuilian Yu1*
Shuilian Yu1*Background: Lupus nephritis (LN) frequently manifests as a significant complication in systemic lupus erythematosus (SLE) patients, with emerging research indicating a plausible correlation between subclinical retinal involvement and renal manifestations. This study aims to investigate the relationship between the neutrophil-to-C3 ratio (NC3R) and both LN as well as retinal microvasculature damage among SLE patients.
Methods: In this cross-sectional study, a cohort of 220 participants (78 LN and 142 non-LN) was recruited. We assessed clinical indicators and organ involvement and conducted correlation analyses between NC3R and markers of lupus activity. Additionally, we analyzed the diagnostic performance of NC3R in diagnosing LN and constructed ROC curves. Variables such as clinical and laboratory data were screened by univariate and least absolute shrinkage and selection operator (LASSO) logistic regression modeling. After 10-fold cross-validation, the predictive model was built by multivariate logistic regression. We further examined the influence of NC3R on retinal vasculature density based on its cutoff value and conducted correlation analyses accordingly.
Results: The LN group exhibited a significant increase in NC3R compared to the group without LN (5.9 vs. 4.5, p = 0.007). NC3R demonstrated positive correlations with 24-h proteinuria (R = 0.329, p < 0.001) and systemic lupus erythematosus disease activity index (SLEDAI) score (R = 0.268, p < 0.001). Multiple regression analysis revealed NC3R as an independent risk factor for LN (OR: 2.03, p = 0.025). NC3R was proven valuable in distinguishing LN patients (AUC: 0.613), with an optimal cutoff value of 6.40 (sensitivity: 48.1%, specificity: 72.0%). Our results indicated that the lower NC3R group (NC3R < 6.40) exhibited reduced vascular density, particularly within the macular region. Furthermore, we observed a positive correlation between NC3R levels and vascular density.
Conclusion: NC3R demonstrated promising potential as a reliable indicator for predicting both LN and retinal microvasculature involvement. Consequently, the pre-treatment evaluation of NC3R had the potential to assist clinicians in identifying potential organ involvement among SLE patients.
Systemic lupus erythematosus (SLE) is an autoimmune disease that triggers inflammation in multiple organs due to the loss of self-tolerance and the formation of autoantigens and immune complexes (Kiriakidou and Ching, 2020). Among the complications of SLE, lupus nephritis (LN) exhibits rapid progression, an unfavorable prognosis, and contributes significantly to morbidity and mortality (Hahn et al., 2012). Ocular involvement, including retinopathy, is prevalent in approximately one-third of SLE patients and plays a crucial role in assessing disease activity and prognosis, primarily due to active SLE retinal vasculopathy and reduced complement levels (Silpa-archa et al., 2016; Luboń et al., 2022).
Neutrophil immune dysregulation is implicated in SLE pathogenesis by activating type Ⅰ interferon, leading to the production of effector leukocytes, inflammatory mediators, and autoantibodies, which contribute to tissue damage affecting various organs, including the kidneys and eyes (Mistry et al., 2019). Complement system activation contributes to neutrophil recruitment and activation, with increased neutrophils in the kidneys associated with nephritis activity (Mohan and Putterman, 2015). Recent studies have identified a critical role for neutrophils in SLE disease progression, particularly in patients with lower complement levels and increased inflammatory cells such as neutrophils (Yu et al., 2019). The association between the neutrophil-to-C3 ratio (NC3R) and SLE disease activity has been shown, but its diagnostic value for identifying LN remains uncertain.
Identifying reliable biological markers for predicting renal and retinal vasculopathy in SLE patients is of utmost importance for effective monitoring and treatment. This study aims to investigate the predictive efficacy of NC3R in LN patients and its correlation with retinal microvascular damage, thereby assisting clinicians in early screening and prevention of organ damage.
This study was a retrospective, single-center observational study and 220 patients with SLE were recruited from the Second Affiliated Hospital of Guangzhou Medical University between September 2019 and April 2024. All SLE patients were diagnosed according to the 2019 EULAR/ACR classification criteria for SLE (Aringer et al., 2019). The inclusion criteria for patients in the LN group were: (1) persistent proteinuria >0.5 g/day or >3+ by dipstick, and/or the presence of cellular casts (red cell, hemoglobin, granular, tubular, or mixed); or (2) renal biopsy demonstrating immune complex-mediated glomerulonephritis consistent with LN (Hahn et al., 2012). SLE disease activity was evaluated utilizing the systemic lupus erythematosus disease activity index (SLEDAI)-2K score (Gladman et al., 2002). The ocular inclusion criteria were defined as follows: (1) best-corrected visual acuity better than 0.1 log of the Minimum Angle of Resolution (LogMAR); (2) intraocular pressure <21 mmHg; (3) spherical equivalent < +2.5 D or >−6.0 D. Patients with infections, malignancies, and other inflammatory diseases were excluded for this study. The ocular exclusion criteria were defined as follows: (1) spherical equivalent > +6.0 D or <-6.0 D; (2) axial length ≥26 mm; (3) any ocular pathological changes detected on slit lamp, fundus color photography, or optical coherence tomography imaging; (4) history of previous ocular diseases such as glaucoma, cataract or ocular surgery, including refractive surgeries. Informed consent was obtained from all participants, and the study received approval from the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University.
Demographic data, clinical manifestations, and laboratory data were retrieved from patients’ medical records. Patient characteristics encompassed age, gender, disease duration, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complement 3(C3), C4, neutrophil-to-lymphocyte ratio (NLR), NC3R. Kidney function was evaluated through the following biochemical assays: serum creatinine, glomerular filtration rate (GFR), and 24-h proteinuria. Additionally, we collected information regarding organ involvement in patients, including gastrointestinal involvement, neurological damage, arthritis, skin involvement, hematological abnormalities, and serositis. The NLR and NC3R are calculated by dividing the absolute neutrophil count by the absolute lymphocyte count or serum C3 value.
According to the ETDRS (1991), a standard LogMAR chart was used to assess the best-corrected visual acuity (BCVA) in each eye, thereby evaluating central visual acuity. In the analysis of ocular vasculature, the region of interest (ROI) in the macular region was characterized by circular areas centered on the macula with diameters of 1 mm and 2.5 mm, respectively. The optic disc region was a circular zone centered on the optic disc with a diameter of 1.5 mm, 2.5 mm, 3.5 mm, and 5 mm, respectively. The parafoveal region referred to macular area 0.5–1.5 mm from the foveal center and the peripapillary region encompassed the optic nerve head. The ratio of flow pixels to total pixels was used to calculate macular vessel density (VD). The macular vessel length density (VLD) was calculated using the ratio of vessel length to total area. The foveal avascular zone (FAZ) area and perimeter were calculated by manually delineating the central avascular zone of the macula. The whole image referred to the entire 3 × 3 mm2 area centered on the macula/the entire 6 × 6 mm2 area centered on the optic disc. This circular region was subdivided further into superior, inferior, temporal, and nasal regions (Li et al., 2024) (Figures 1A–C).
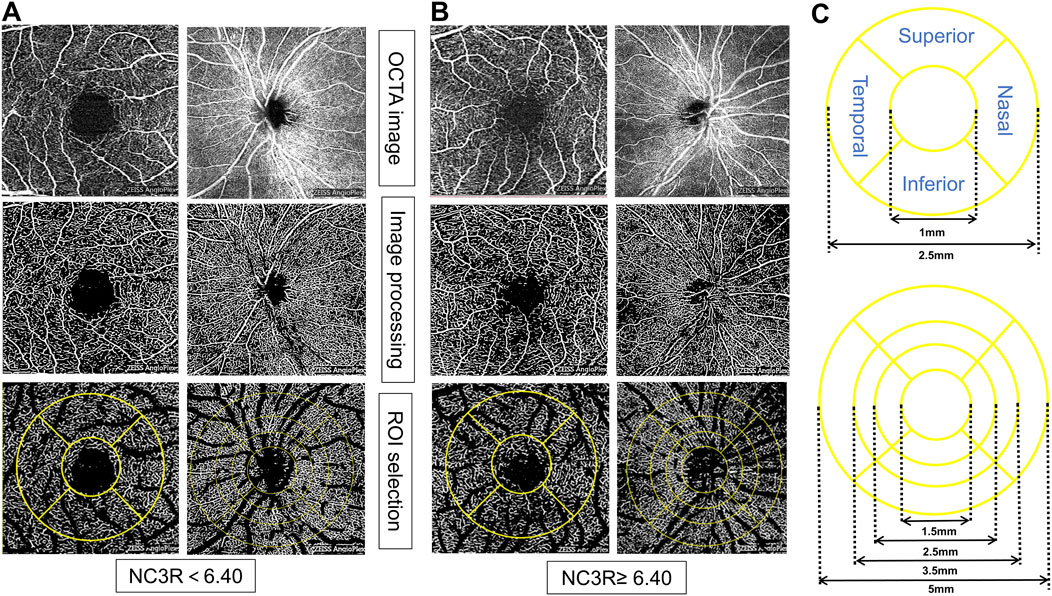
Figure 1. Representative OCTA images for measuring retinal microvasculature parameters in different NC3R groups. In (A), the patient from the NC3R < 6.40 group exhibited a decreased vessel density, while in (B), the patient from the NC3R ≥ 6.4 group displayed an increased vessel density. (C) showed the different circles and sides of macular and optic disc vascular density measured by OCTA. NC3R, neutrophil -to-C3 ratio; OCTA, Optical Coherence Tomography Angiography; ROI: region of interest.
SPSS software (version 25.0) and GraphPad Prism (version 10; GraphPad software) and Hiplot tools were used for analysis. The normality of distributions was tested by the Kolmogorov-Smirnov test. Data that followed a normal distribution were expressed as mean ± standard deviation and analyzed using Student’s t-test, while non-normally distributed data were presented as median and interquartile range (IQR) and evaluated with the Mann-Whitney U-test. The association between NC3R and relevant clinical indicators in SLE patients was assessed using Spearman’s correlation coefficient. The univariate logistic regression and multivariate logistic regression models examined if NLR and NC3R were associated with increased or decreased risk of LN in diagnosed patients. LASSO was applied to manage multiple predictors and reduce overfitting by selecting the most significant variables. A 10-fold cross-validation process was used to identify the optimal lambda value, based on the minimum binomial deviance. ROC and calibration curves evaluated the efficacy of the model. The maximum Youden index, calculated as sensitivity + specificity-1, from the ROC curve analysis, was used to determine the optimal cutoff values for NC3R and NLR. p < 0.05 constituted statistically significant results.
A total of 220 Chinese SLE patients were recruited, including 206 women, and 14 men. They were divided into two groups based on the diagnosis of LN, 142 patients without LN and 78 patients with LN. Demographic and clinical characteristics were summarized in Table 1. The mean disease duration was no significant difference between the two groups. Participants with LN showed significantly higher levels of SLEDAI score (12.5 vs. 10.0, p = 0.017). Notably, LN patients showed a higher NC3R level compared to those without LN (5.9 vs. 4.5, p = 0.007) (Figure 2A). However, there were no significant differences observed in NLR between the two groups (Figure 2B). Serologically, LN patients had a significantly lower GFR, creatinine, and albumin level, and a higher 24-h proteinuria level (all p < 0.01). Gastrointestinal involvement was significantly higher in LN patients compared to non-LN patients (11.5% vs. 2.1%, p = 0.005). However, there were no significant differences in other organ involvements, including neurological damage, APS, arthritis, skin involvement, hematological damage, and serositis (Table 1). A total of 47 patients with LN underwent renal biopsy, including 2 with class II, 4 with class III, 2 with class III + V, 13 with class IV, 21 with class IV + V, and 5 with class V (Table 1). We further investigated the immunotherapy treatment in both groups. The results showed that the proportions of patients using prednisolone, hydroxychloroquine, and cyclosporine were similar between the two groups. However, a higher proportion of patients in the LN group received tacrolimus (7.7% vs. 0.7%, p = 0.009) and mycophenolate mofetil treatment (65.4% vs. 12.7%, p < 0.001). In contrast, a greater percentage of patients without LN were treated with methotrexate (11.3% vs. 2.6%, p = 0.024).
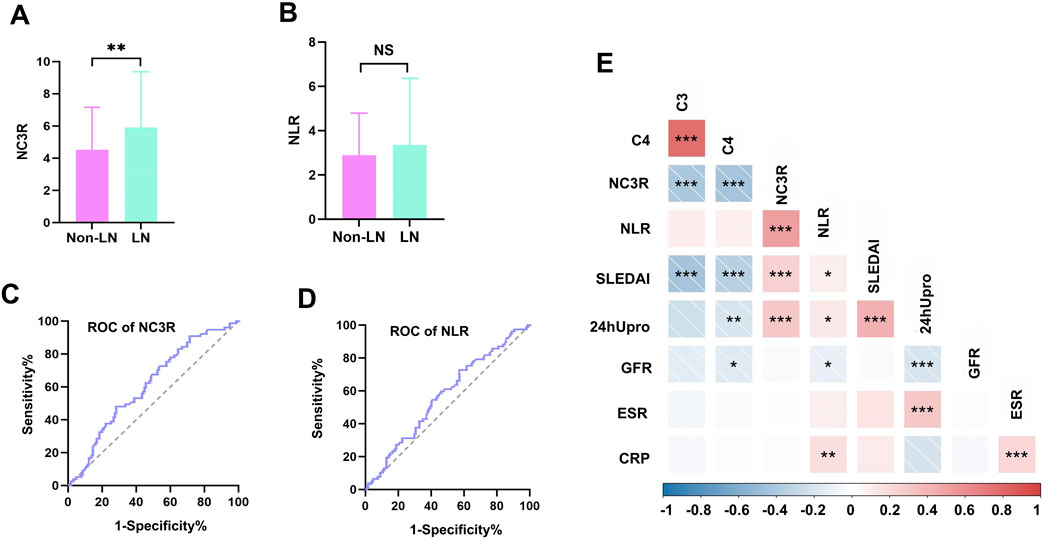
Figure 2. Comparison of NC3R and NLR in different groups, receiver operating characteristic (ROC) curve for predicting LN and correlations with clinical parameters. (A, B) The LN group showed a higher NC3R level compared to those without LN (5.9 vs. 4.5, P = 0.007), and no significant differences were observed in NLR between the two groups. (C, D) Area under curve (AUC) for NC3R and NLR are 0.613 (95% CI: 0.535-0.690), and 0.570 (95% CI: 0.491-0.649), respectively. (E) Correlations of NC3R, and NLR with clinical parameters in SLE patients. LN, lupus nephritis; NLR, neutrophil-to-lymphocyte ratio; NC3R, neutrophil -to-C3 ratio; 24 h Upro, 24-h proteinuria; CRP, C-reactive protein; ESR,erythrocyte sedimentation rate; C3, complement 3; C4, complement 4; GFR, glomerular filtration rate. *means P < 0.05; ** means P < 0.01; *** means P < 0.001.
To further investigate the differences in NC3R levels among different types of LN, we compared NC3R levels across the various LN subtypes. Our results indicate that there were no statistically significant differences in NC3R levels between the groups (Supplementary Table S1).
ROC curve analyses were conducted to compare the diagnostic performance of NC3R, and NLR. The AUC for NC3R was 0.613 (95% CI: 0.535-0.690), which was higher than the AUC for NLR 0.570 (95% CI: 0.491-0.649) (Figures 2C, D). The cutoff value of NC3R in identifying LN was 6.40, with a sensitivity of 48.1% and specificity of 72.0%. The cutoff value of NLR was 2.55, with a sensitivity of 72.7% and a specificity of 42.9% (Supplementary Table S2).
We further investigated the relationship between NC3R, NLR, and various clinical parameters including 24-h proteinuria, GFR, C3, C4, ESR, CRP, and SLEDAI scores. NC3R exhibited a positive correlation with 24-h proteinuria (R = 0.329, p < 0.001) and SLEDAI score (R = 0.268, p < 0.001), but a negative association with C3 (R = −0.422, p < 0.001) and C4 (R = −0.408, p < 0.001). Similarly, we found that NLR exhibited a positive correlation with 24-h proteinuria (R = 0.148, p = 0.048), SLEDAI score (R = 0.152, p = 0.028), and CRP (R = 0.271, p = 0.002), but exhibited a negative correlation with GFR (R = −0.177, p = 0.012) as shown in Table 2; Figure 2E.
Univariate logistic analysis revealed possible variables correlated with LN. This was performed on the LN patients and non-LN patients. Results are presented in Table 3. NC3R and NLR were stratified based on a predefined cutoff value. Of the clinical features, NC3R ≥ 6.40 (OR = 2.37, p = 0.004), NLR ≥ 2.55 (OR = 2.01, p = 0.024), GFR (OR = 0.99, p = 0.005) and 24-h proteinuria (OR = 1, p < 0.001) were statistically significantly associated with LN. To avoid multicollinearity and overfitting, LASSO regression was applied following univariate logistic regression screening. A 10-fold cross-validation process identified the optimal lambda value (Figure 3A), with the minimum binomial deviance ensuring the best model performance (Figure 3B). Variables with non-zero coefficients, including NC3R (≥6.40) and NLR (≥2.55), were included in the final logistic regression model (Supplementary Table S3). Our findings revealed that NC3R (≥6.40) independently contributed to the risk of developing LN, with an odds ratio of 2.03 (95% CI: 1.09–3.78, p = 0.025).
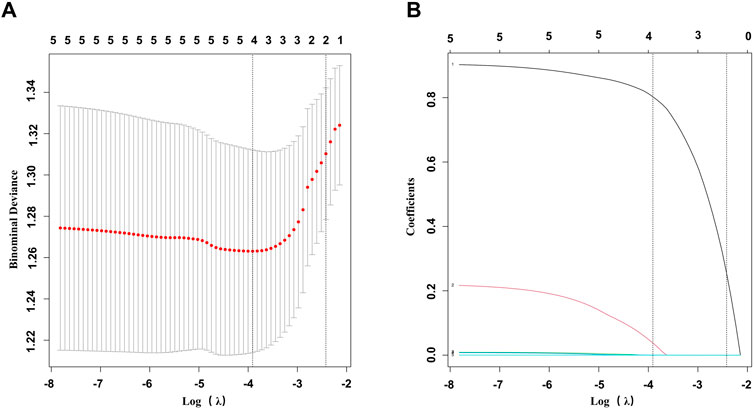
Figure 3. Feature selection was performed using LASSO binary logistic regression. (A) Filtering the best lambda by 10-fold cross-validation in the LASSO model. (B) LASSO model coefficient curves for candidate variables. LASSO, least absolute shrinkage and selection operator.
We conducted ophthalmological assessments on 52 patients and categorized them into two groups based on the NC3R cutoff value: 17 patients in the higher NC3R group (NC3R ≥ 6.40) and 35 patients in the lower NC3R group (NC3R < 6.40). No significant differences were observed in spherical equivalent (SEQ), BCVA (LogMAR), and intraocular pressure (IOP) between both groups of patients (p > 0.05, Table 4).
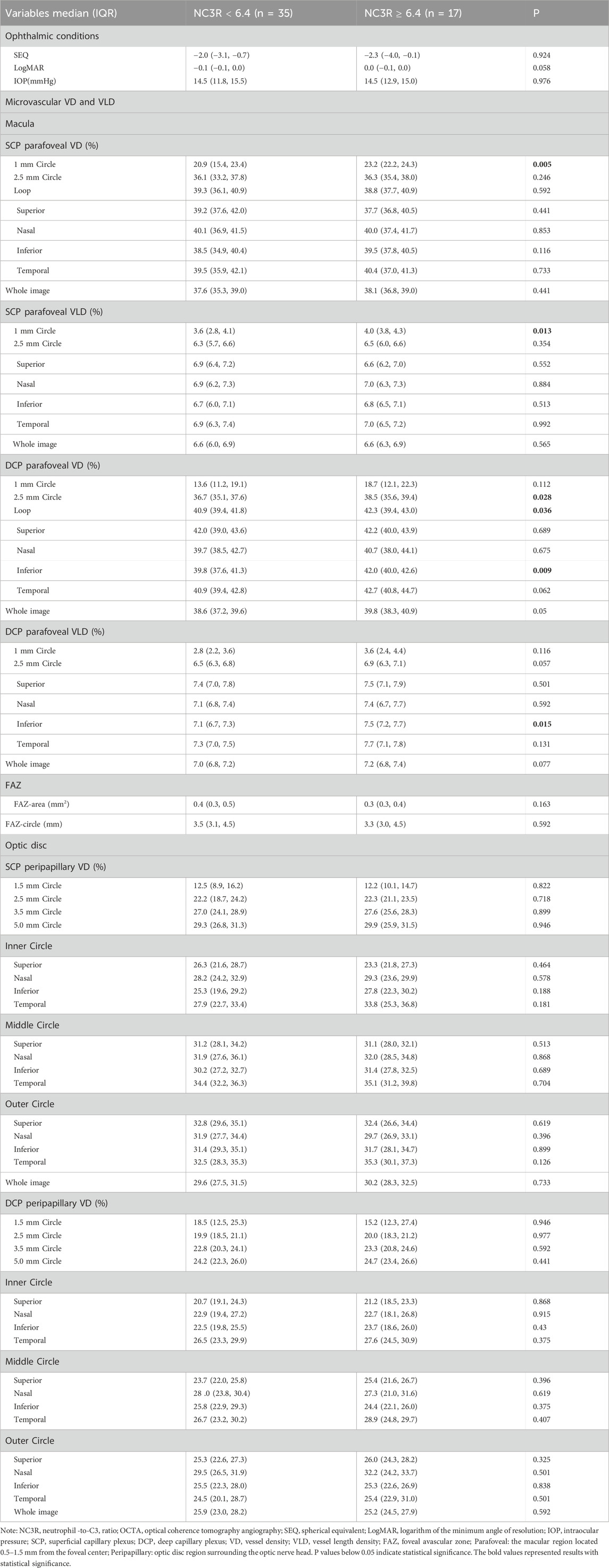
Table 4. Primary ocular examination parameters and comparison of macular and optic disc vascular density and vessel length density between different NC3R groups of patient.
The patients in the NC3R ≥ 6.40 group exhibited increased superficial capillary plexus vessel density (SCP-VD) and superficial capillary plexus vessel length density (SCP-VLD) in the 1 mm circle area (all p < 0.05). Additionally, an increase in deep capillary plexus vessel density (DCP-VD) in the 2.5 mm circle area, loop area, inferior area, and an increased deep capillary plexus vessel length density (DCP-VLD) in the inferior area were observed (all p < 0.05) (Table 4; Figures 1A, B).
A comparative analysis of FAZ parameters was undertaken between two groups of patients. Nevertheless, no statistically significant differences were identified in either FAZ area or FAZ circle measurements between them (all p > 0.05, Table 4).
There were no significant differences observed in optic disc SCP-VD and DCP-VD in both groups (all p > 0.05, Table 4).
We conducted a correlation analysis between NC3R and retinal vascular density. Our findings indicated a positive correlation between NC3R and SCP-VD in the 1 mm circle area, 2.5 mm circle area, and SCP-VLD in the 1 mm circle area. In DCP-VD, there was a positive correlation with the 2.5 mm circle, loop area, inferior side, and temporal side, as well as with DCP-VLD in the 2.5 mm circle, inferior side, and temporal side. Notably, there is a negative correlation between NC3R and the FAZ area. (all p < 0.05) (Supplementary Table S4).
Early diagnosis of LN is crucial due to its severe impact, with only 50%–70% of SLE patients achieving remission and 10%–20% progressing to end-stage renal disease within 5 years, highlighting its role in disease progression and adverse prognosis (Hahn et al., 2012). Lupus retinopathy primarily manifests as immune complex-mediated microangiopathy, indicating severe disease activity and its correlation with the central nervous system and renal involvement, making it a prognostic marker for poor survival outcomes (Palejwala et al., 2012; Gallagher et al., 2015). Conigliaro et al. found that SLE patients, especially those with kidney involvement, exhibited decreased retinal microvascular density compared to normal subjects (Conigliaro et al., 2018; Conigliaro et al., 2019). Additionally, Ushiyama et al. observed higher levels of serum creatinine in SLE patients with retinopathy compared to those without retinopathy (Ushiyama et al., 2000).
Complement system activation, complement fragment production, and deposition, as well as subsequent inflammation, are integral to the pathogenesis of SLE. Moreover, the release of compounds and antimicrobial peptides from neutrophils in SLE patients contributes to tissue and organ damage and inflammation (Kaplan, 2011). Previous reports have indicated lower complement levels and higher inflammatory cell counts in SLE patients compared to healthy individuals (Charlesworth et al., 1989; Bosch, 2011; Fresneda Alarcon et al., 2021). Recent research has shed light on the fact that many components involved in NETosis function as autoantigens in autoimmune processes (Knight et al., 2012). Studies have also indicated that remnants of neutrophil activation, such as NET structures, can be deposited in glomeruli even in the absence of intact neutrophils within the kidneys (Frangou et al., 2019). In patients with LN, significantly elevated levels of autoantibodies are found in their serum, exceeding concentrations in the kidneys by a factor of 103. Moreover, target antigens have been detected in the NETosis matrix, providing support for the underlying concept that anti-podocyte and anti-implanted antigen antibodies form in circulation and subsequently accumulate within the glomeruli (Bonanni et al., 2015). Therefore, this study aimed to explore the association between NC3R, and LN.
Previous studies have established NLR as a valuable marker for assessing disease activity in various conditions such as rheumatoid arthritis, Bechet disease, psoriatic arthritis, and adult-onset Still’s disease (Kim et al., 2016; Avci et al., 2017; Seo et al., 2017; Erre et al., 2019). Similarly, Liu et al., Tang et al., and Firizal et al. have reported NLR potential utility in monitoring disease activity and kidney damage in SLE patients (Firizal et al., 2020; Liu et al., 2020; Tang et al., 2022). Given the pivotal role of C3 in immune complex deposition and organ damage in SLE, particularly in LN, we chose NC3R for its ability to offer more specific insights into the disease mechanisms and provide a comprehensive understanding of both immune dysregulation and inflammation. Notably, our analysis revealed that NC3R had superior predictive capabilities in differentiating LN patients compared to NLR. Consistent with the findings of Yu et al. (2019), who reported its utility as an inflammatory marker for evaluating disease activity in SLE, we observed significantly higher levels of NC3R in LN patients. Furthermore, our study demonstrated a positive association between NC3R and 24-h proteinuria, a crucial indicator for diagnosing LN. Through multivariate logistic regression analysis, we determined that NC3R remained an independent risk factor for poor renal outcomes, even after adjusting for other variables (OR: 2.03, P = 0.035). In addition, our ROC analysis indicated that NC3R, not NLR, could serve as an additional diagnostic tool for identifying LN, displaying higher specificity compared to NLR (72.0% vs 42.9%). These findings suggested that NC3R may serve as an indicator of renal involvement in SLE patients.
Lupus retinopathy is characterized by microangiopathy, severe vascular occlusion, and vasculitis (Silpa-archa et al., 2016; Luboń et al., 2022). The vasculitis involves the deposition of immune complexes on endothelial cells, initiating complement activation and heightened phagocytosis, subsequently leading to the release of additional inflammatory mediators (Giorgi et al., 1999). Notably, our results indicated that the lower NC3R group (NC3R < 6.40) showed reduced vascular density, particularly pronounced in the macular retina. Lower NC3R levels may reflect increased complement activation, particularly C3, suggesting ongoing inflammation in small vessels (Sun et al., 2013), including those in the retina. Furthermore, we found a positive correlation between vascular density and NC3R levels, suggesting that NC3R may serve as a potential marker for vascular inflammation and complement activation in SLE retinopathy. Consequently, we hypothesize that the reduced vascular density in SLE patients is attributable to complement-mediated vasculitis, and NC3R may indicate potential retinal damage.
This study has several limitations. First, the cross-sectional design restricts our ability to fully explore the role of NC3R in LN. Second, further longitudinal studies are necessary to validate these findings. Third, the absence of a normal control group, along with potential influences from therapeutic medications, SLE itself, and coexisting conditions, may have affected NC3R levels. Despite these limitations, we observed significant correlations between NC3R, LN, and ocular involvement in SLE patients.
In conclusion, LN patients exhibited higher levels of NC3R compared to those without LN, with a positive correlation with 24-h proteinuria. NC3R emerged as a risk factor for LN and displayed significant diagnostic utility in identifying LN. Remarkably, patients in the lower NC3R group displayed a decreased retinal vessel density. These findings underscore the potential of NC3R as a predictive marker for LN and for evaluating retinal microvasculature vessel density in SLE patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MM: Writing–review and editing, Conceptualization, Writing–original draft, Data curation, Formal Analysis, Investigation, Methodology, Software. PL: Conceptualization, Writing–original draft, Data curation, Formal Analysis, Investigation, Methodology, Writing–review and editing. HL: Conceptualization, Writing–original draft, Data curation, Formal Analysis, Methodology, Software. DJ: Conceptualization, Writing–original draft, Formal Analysis, Investigation, Methodology, Supervision. JZ: Writing–original draft, Data curation, Software. CY: Writing–original draft, Formal Analysis, Methodology. XY: Writing–original draft, Formal Analysis, Methodology, Supervision. CC: Writing–original draft, Methodology, Supervision. WH: Writing–original draft, Conceptualization, Funding acquisition, Supervision. ZL: Supervision, Writing–review and editing, Conceptualization, Funding acquisition. SY: Supervision, Writing–review and editing, Funding acquisition, Conceptualization, Investigation, Resources.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangdong Basic and Applied Basic Research Foundation of Guangdong Province, China (Grant No. 2019A1515011094); the Guangdong Basic and Applied Basic Research Foundation of Guangdong Province, China (Grant No. 2022A1515010471); the Guangzhou Science and Technology Planning Project of Guangdong Province, China (Grant No. 202102010139), Guangzhou Science and Technology Project (No. 2024A03J0207); Multi-center clinical research Project Fund of the Second Affiliated Hospital of Guangzhou Medical University of Guangdong Province, China (Grant No. 2022-LCYJ-YYDZX-05).
We thank all the participants of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1484320/full#supplementary-material
Aringer, M., Costenbader, K., Daikh, D., Brinks, R., Mosca, M., Ramsey-Goldman, R., et al. (2019). 2019 European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheumatic Dis. 78 (9), 1151–1159. doi:10.1136/annrheumdis-2018-214819
Avci, A., Avci, D., Erden, F., Ragip, E., Cetinkaya, A., Ozyurt, K., et al. (2017). Can we use the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume values for the diagnosis of anterior uveitis in patients with Behcet's disease? Ther. Clin. Risk Manag. 13, 881–886. doi:10.2147/TCRM.S135260
Bonanni, A., Vaglio, A., Bruschi, M., Sinico, R. A., Cavagna, L., Moroni, G., et al. (2015). Multi-antibody composition in lupus nephritis: isotype and antigen specificity make the difference. Autoimmun. Rev. 14 (8), 692–702. doi:10.1016/j.autrev.2015.04.004
Bosch, X. (2011). Systemic lupus erythematosus and the neutrophil. N. Engl. J. Med. 365 (8), 758–760. doi:10.1056/NEJMcibr1107085
Charlesworth, J. A., Peake, P. W., Golding, J., Mackie, J. D., Pussell, B. A., Timmermans, V., et al. (1989). Hypercatabolism of C3 and C4 in active and inactive systemic lupus erythematosus. Ann. Rheumatic Dis. 48 (2), 153–159. doi:10.1136/ard.48.2.153
Conigliaro, P., Cesareo, M., Chimenti, M. S., Triggianese, P., Canofari, C., Aloe, G., et al. (2019). Evaluation of retinal microvascular density in patients affected by systemic lupus erythematosus: an optical coherence tomography angiography study. Ann. Rheumatic Dis. 78 (2), 287–289. doi:10.1136/annrheumdis-2018-214235
Conigliaro, P., Triggianese, P., Draghessi, G., Canofari, C., Aloe, G., Chimenti, M. S., et al. (2018). Evidence for the detection of subclinical retinal involvement in systemic lupus erythematosus and sjögren syndrome: a potential association with therapies. Int. Archives Allergy Immunol. 177 (1), 45–56. doi:10.1159/000488950
Erre, G. L., Paliogiannis, P., Castagna, F., Mangoni, A. A., Carru, C., Passiu, G., et al. (2019). Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur. J. Clin. Investigation 49 (1), e13037. doi:10.1111/eci.13037
ETDRS (1991). Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 98 (5 Suppl. l), 741–756. doi:10.1016/s0161-6420(13)38009-9
Firizal, A. S., Sugianli, A. K., and Hamijoyo, L. (2020). Cut off point of neutrophil-to-lymphocyte ratio as a marker of active disease in systemic lupus erythematosus. Lupus 29 (12), 1566–1570. doi:10.1177/0961203320950822
Frangou, E., Chrysanthopoulou, A., Mitsios, A., Kambas, K., Arelaki, S., Angelidou, I., et al. (2019). REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann. Rheum. Dis. 78 (2), 238–248. doi:10.1136/annrheumdis-2018-213181
Fresneda Alarcon, M., McLaren, Z., and Wright, H. L. (2021). Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Front. Immunol. 12, 649693. doi:10.3389/fimmu.2021.649693
Gallagher, K., Viswanathan, A., and Okhravi, N. (2015). Association of systemic lupus erythematosus with uveitis. JAMA Ophthalmol. 133 (10), 1190–1193. doi:10.1001/jamaophthalmol.2015.2249
Giorgi, D., Pace, F., Giorgi, A., Bonomo, L., and Gabrieli, C. B. (1999). Retinopathy in systemic lupus erythematosus: pathogenesis and approach to therapy. Hum. Immunol. 60 (8), 688–696. doi:10.1016/s0198-8859(99)00035-x
Gladman, D. D., Ibañez, D., and Urowitz, M. B. (2002). Systemic lupus erythematosus disease activity index 2000. J. Rheumatology 29 (2), 288–291.
Hahn, B. H., McMahon, M. A., Wilkinson, A., Wallace, W. D., Daikh, D. I., Fitzgerald, J. D., et al. (2012). American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. Hob. 64 (6), 797–808. doi:10.1002/acr.21664
Kaplan, M. J. (2011). Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 7 (12), 691–699. doi:10.1038/nrrheum.2011.132
Kim, D. S., Shin, D., Lee, M. S., Kim, H. J., Kim, D. Y., Kim, S. M., et al. (2016). Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J. Dermatology 43 (3), 305–310. doi:10.1111/1346-8138.13061
Kiriakidou, M., and Ching, C. L. (2020). Systemic lupus erythematosus. Ann. Intern. Med. 172 (11), ITC81–ITC96. doi:10.7326/AITC202006020
Knight, J. S., Carmona-Rivera, C., and Kaplan, M. J. (2012). Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front. Immunol. 3, 380. doi:10.3389/fimmu.2012.00380
Li, H., Yu, S., Li, P., Zhang, J., Maitiyaer, M., Huang, A. M., et al. (2024). Microvasculature alterations of peripapillary retina and macula in systemic lupus erythematosus patients without ocular involvement by optical coherence tomography angiography. Retina Phila. Pa. 44 (3), 515–526. doi:10.1097/IAE.0000000000003989
Liu, P., Li, P., Peng, Z., Xiang, Y., Xia, C., Wu, J., et al. (2020). Predictive value of the neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-neutrophil ratio, and neutrophil-to-monocyte ratio in lupus nephritis. Lupus 29 (9), 1031–1039. doi:10.1177/0961203320929753
Luboń, W., Luboń, M., Kotyla, P., and Mrukwa-Kominek, E. (2022). Understanding ocular findings and manifestations of systemic lupus erythematosus: update review of the literature. Int. J. Mol. Sci. 23 (20), 12264. doi:10.3390/ijms232012264
Mistry, P., Nakabo, S., O'Neil, L., Goel, R. R., Jiang, K., Carmona-Rivera, C., et al. (2019). Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 116 (50), 25222–25228. doi:10.1073/pnas.1908576116
Mohan, C., and Putterman, C. (2015). Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11 (6), 329–341. doi:10.1038/nrneph.2015.33
Palejwala, N. V., Walia, H. S., and Yeh, S. (2012). Ocular manifestations of systemic lupus erythematosus: a review of the literature. Autoimmune Dis. 2012, 290898. doi:10.1155/2012/290898
Seo, J.-Y., Suh, C.-H., Jung, J.-Y., Kim, A.-R., Yang, J. W., and Kim, H.-A. (2017). The neutrophil-to-lymphocyte ratio could be a good diagnostic marker and predictor of relapse in patients with adult-onset Still's disease: a STROBE-compliant retrospective observational analysis. Medicine 96 (29), e7546. doi:10.1097/MD.0000000000007546
Silpa-archa, S., Lee, J. J., and Foster, C. S. (2016). Ocular manifestations in systemic lupus erythematosus. Br. J. Ophthalmol. 100 (1), 135–141. doi:10.1136/bjophthalmol-2015-306629
Sun, W., Jiao, Y., Cui, B., Gao, X., Xia, Y., and Zhao, Y. (2013). Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Laboratory Investigation; a J. Tech. Methods Pathology 93 (6), 626–638. doi:10.1038/labinvest.2013.61
Tang, D., Tang, Q., Zhang, L., and Wang, H. (2022). High neutrophil-lymphocyte ratio predicts serious renal insufficiency in patients with lupus nephritis. Iran. J. Immunol. IJI 19 (1), 5. doi:10.22034/IJI.2022.92554.2154
Ushiyama, O., Ushiyama, K., Koarada, S., Tada, Y., Suzuki, N., Ohta, A., et al. (2000). Retinal disease in patients with systemic lupus erythematosus. Ann. Rheumatic Dis. 59 (9), 705–708. doi:10.1136/ard.59.9.705
Keywords: systemic lupus erythematosus, neutrophil-to-C3 ratio, lupus nephritis, retinal microvasculature, biomarker
Citation: Maitiyaer M, Li P, Li H, Jiang D, Zhang J, Yang C, Yang X, Chen C, Huang W, Liu Z and Yu S (2025) The neutrophil-to-C3 ratio: unveiling diagnostic efficacy for lupus nephritis and association with reduced retinal vascular density in systemic lupus erythematosus. Front. Pharmacol. 16:1484320. doi: 10.3389/fphar.2025.1484320
Received: 21 August 2024; Accepted: 20 January 2025;
Published: 19 February 2025.
Edited by:
José Luis Maravillas-Montero, National Autonomous University of Mexico, MexicoReviewed by:
Zhiming Lin, Third Affiliated Hospital of Sun Yat-Sen University, ChinaCopyright © 2025 Maitiyaer, Li, Li, Jiang, Zhang, Yang, Yang, Chen, Huang, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuilian Yu, c2h1aWxpYW4yMDA4QGdtYWlsLmNvbQ==; Zhiping Liu, enhsaXUyMDEzQGd6aG11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.