- 1Clinical Laboratory, Qingdao Traditional Chinese Medicine Hospital, Qingdao Hiser Hospital Affiliated of Qingdao University, Qingdao Key Laboratory of Immunodiagnosis, Qingdao, China
- 2Shandong Provincial Key Laboratory of Pathogenesis and Prevention of Neurological Disorders, Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders, Institute of Brain Science and Disease, Qingdao University, Qingdao, China
Neurodegenerative diseases are primarily characterized by the selective loss of neurons in the brain, leading to a significant and widespread global public health burden. Although numerous mechanisms underlying neurodegenerative diseases have been elucidated, effective therapeutic strategies are still being explored. Several drugs have been proposed to halt disease progression; however, they often come with severe side effects. Recently, polysaccharides have garnered considerable attention due to their antioxidant, anti-neuroinflammatory, anticholinesterase, and anti-amyloidogenic properties. The ocean contains a large number of animals, plants, algae and fungal species. Its rich sources and wide availability make the research on marine drugs become a hot topic. Recently, polysaccharides dominated by fucoidan and chitosan have been reported to inhibit the progression of neurodegenerative diseases in a variety of ways. In this review article, we provide a comprehensive summary of reported polysaccharides that intervene in neurodegenerative diseases with the aim of exploring their potential as therapeutic agents.
1 Introduction
Neurodegenerative diseases (NDDs) arise from the progressive loss of neurons or their myelin sheathing and involve a fundamental role of neuroinflammation in the pathophysiology, resulting in functional impairment over time (Heemels, 2016; Agnello and Ciaccio, 2022). The prevalence of NDDs is extensive and encompasses Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI)and age-related macular degeneration (AMD). These disorders may have genetic, environmental, or multifactorial etiologies (Höglund and Salter, 2013; Temple, 2023). The alterations in markers of inflammation could initiate or exacerbate neuroinflammation and perpetuate the neurodegenerative process (Furman et al., 2019; Tansey et al., 2022). ND affects individuals across all age groups and imposes substantial economic burdens on patients and their families (Nichols et al., 2022; Nandi et al., 2022). To date, no effective cure has been identified for any neurodegenerative disease (Heemels, 2016; Agnello and Ciaccio, 2022). Currently, drug therapy dominates the treatment landscape for neurodegenerative diseases. However, challenges such as drug toxicity, high costs, limited availability of drugs, and the emergence of drug resistance persist. Therefore, there is a pressing need to develop novel therapeutic agents.
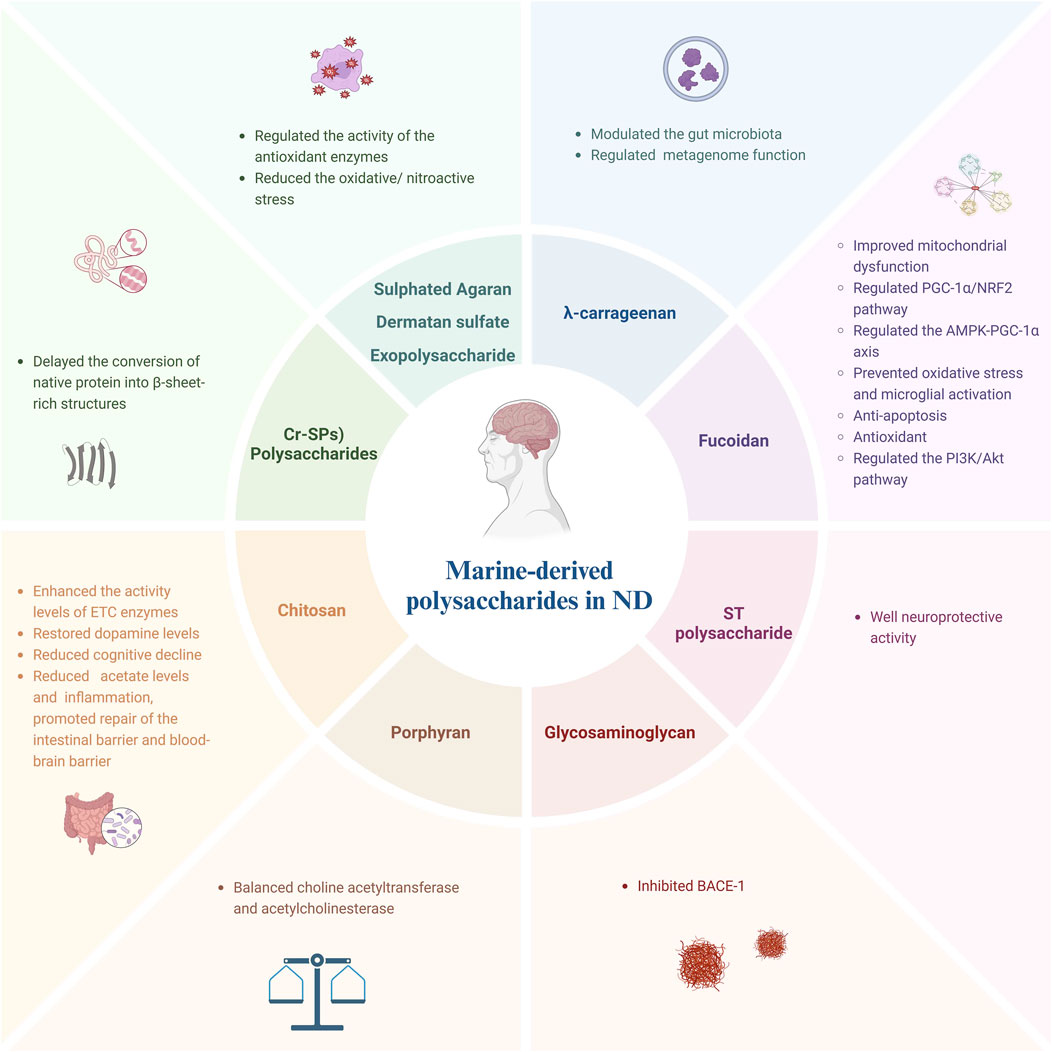
Marine species exhibit remarkable diversity. China has been at the forefront of utilizing marine resources for medicinal purposes (Fu et al., 2016). In recent years, significant emphasis has been placed on developing new drugs and products derived from marine sources within pharmaceutical research (Hu et al., 2011). Marine drugs encompass various categories including polysaccharides, peptides, terpenoids, steroids, tannins, flavonoids and other t compounds (Hu et al., 2011). Polysaccharides are widely distributed in nature. Marine polysaccharides are a kind of polysaccharides isolated and purified from Marine and lake organisms (Wang et al., 2012). Based on their sources, marine polysaccharides can be categorized into three types: animal polysaccharides, plant polysaccharides, and microbial polysaccharides (Wang et al., 2012). Polysaccharides exhibit various effects such as anti-aging properties, antioxidant activity, ulcer prevention abilities, virus inhibition capabilities,cancer-fighting potential, hypoglycemic effects,and regulation of body immunity (Wang et al., 2012; Yu et al., 2018; Zeng et al., 2019; Jiang et al., 2021; Zhou et al., 2022). This review provides a comprehensive summary of recent advancements in utilizing marine polysaccharides for the treatment of neurodegenerative diseases as shown in Figure 1.
2 Polysaccharides from animals
2.1 Chitosan
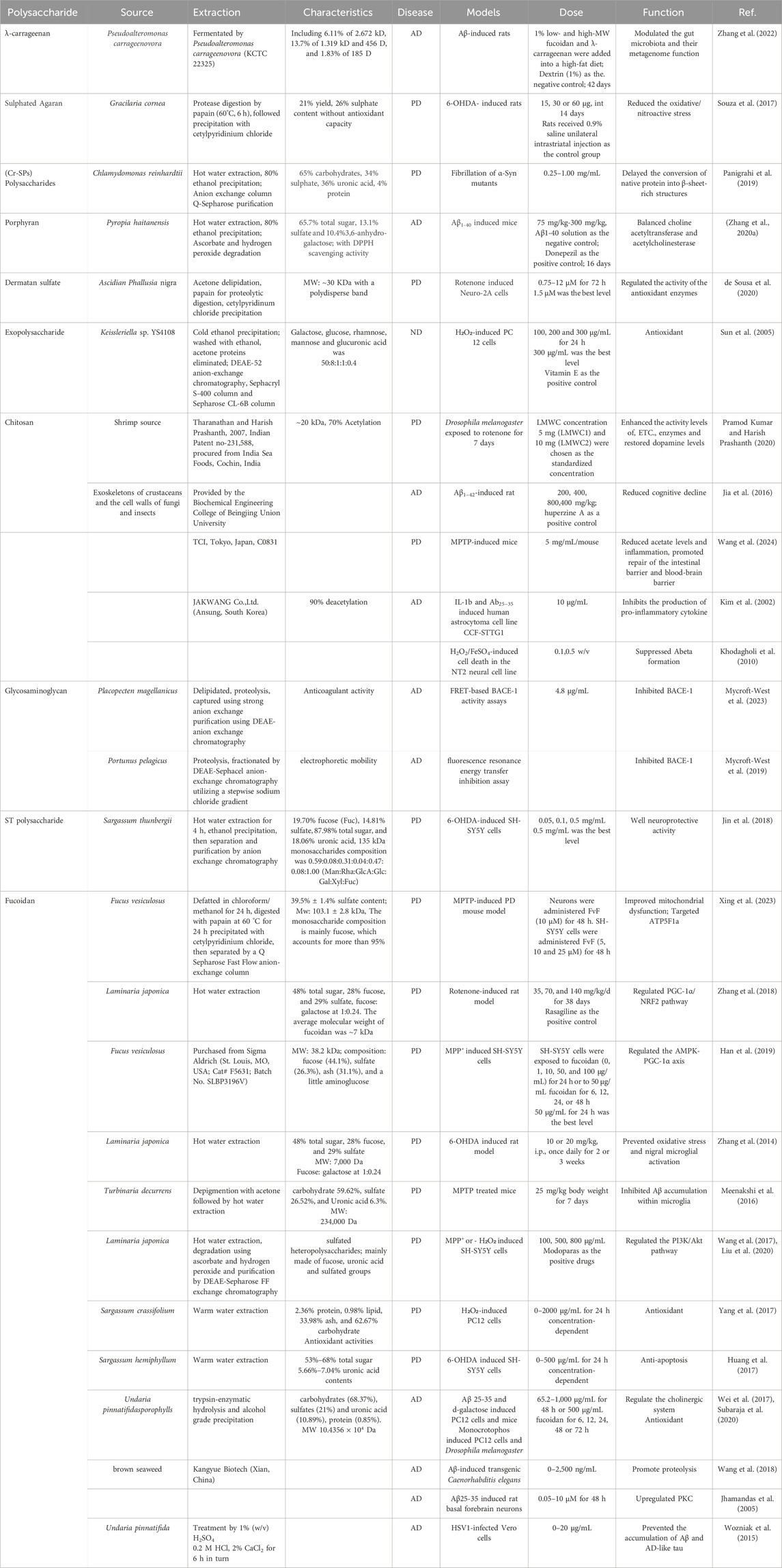
Chitosan, a biopolymer derived from chitin found in the exoskeleton of crustaceans such as shrimp and crabs (Ouyang et al., 2017; González-Chavarría et al., 2023), has gained significant attention across various fields due to its biocompatibility, biodegradability, and non-toxic nature (Ouyang et al., 2017; Thirupathi et al., 2022; Budai et al., 2023). Chitosan has been investigated for its potential role in the management and treatment of neurodegenerative diseases by scavenging free radicals and reducing oxidative stress (Ahlawat et al., 2020; Pramod Kumar and Harish Prashanth, 2020; Etxebeste-Mitxeltorena et al., 2024). Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation (Huang et al., 2015), Chitosan, contributed to the attenuation of oxidative damage and could be used as a nutritional agent for AD treatment. Additionally, both chitosan (CHT) and its quarternary derivative N-trimethyl chitosan chloride exhibited a concentration-dependent inhibiting activity on Aβ 40 fibrillogenesis mainly depending on the attractive electrostatic interactions between the positively charged moieties in chitosan and the negatively charged residues in Aβ40 (Liu et al., 2015). Several animal studies have suggested that chitosan has the potential to enhance cognitive function and memory, which could be beneficial for conditions such as AD (Amini and Abdolmaleki, 2022; Saleem et al., 2022; Yang et al., 2023b). Following a 10-day induction with rotenone (500 μM) in male adult flies, the administration of low molecular weight chitosan demonstrated improvements in drosophila dyskinesia, exploratory defects, and increased survival up to 16 days (Pramod Kumar and Harish Prashanth, 2020). Chronic inflammation is recognized as a contributing factor in NDDs (Yang et al., 2022). Chitosan has demonstrated anti-inflammatory effects that may help alleviate neuroinflammatory processes associated with these conditions (Yang et al., 2018; Mohebichamkhorami et al., 2023; Rajkumar et al., 2024). It could modulate inflammatory mediators in lipopolysaccharides (LPS)-stimulated BV2 microglia via the MAPK signaling pathway, which was negative to its molecular weight, with lower molecular weight showed higher activity (Pangestuti et al., 2011). Wang et al. administered chitosan to MPTP-induced Parkinson’s disease mouse models which resulted in reduced inflammation along with effective protection against dopamine neuron damage while improving motor symptoms (Wang et al., 2024).
The blood-brain barrier (BBB) is the block of the delivery of drugs. As a non-toxic, safe, and potential agent, chitosan has attracted attention for use in drug delivery systems. Efficient delivery of dopamine (DA) is crucial for Parkinson’s disease treatment but pure dopamine cannot penetrate BBB due to its hydrophilicity (De Virgilio et al., 2016). Ren et al. (2017) highlighted chitosan hydrogel as a promising carrier system for drug delivery since their synthesized injectable quaternized chitosan hydrogel exhibited no cytotoxicity while effectively enabling local release of DA along with anti-inflammatory drugs. Hassan et al. (2024) confirmed significant anti-Parkinson’s and antidepressant effects achieved through chitosan-coated-Tanshinone IIA-nanostructured lipid carriers compared to uncoated counterparts in rotenone-induced PD rat models. Yang et al. discovered that Res-loaded CS/TPP nanoparticles can effectively penetrate the blood-brain barrier and inhibit microglia activation, thereby regulating brain glucose homeostasis, oxidative stress, and neuroinflammation (Yang et al., 2023a). Insulin exhibits a potent therapeutic effect in the treatment of Alzheimer’s disease (AD), and intranasal delivery of insulin represents an effective treatment strategy. Notably, chitosan Transfersulin nanovesicles prepared through thin film hydration have been demonstrated to enhance the neuroprotective efficacy on the hippocampus of diabetic model rats by facilitating continuous intracellular drug uptake and mediating insulin transport to the brain. This study highlights their potential as a promising approach for AD therapy (Bedse et al., 2015; Nojoki et al., 2022). The neuroprotective potential of chitosan-based FTY720 nanoformulation for preventing Parkinson’s disease (PD) through pP2A-mediated epigenetic regulation was investigated by Sardoiwala et al. (2021). It facilitates the transport of therapeutic agents across the BBB, potentially enhancing the efficacy of drugs used in treating NDDs (Ren et al., 2017; Zhang L. et al., 2020; Omidian et al., 2024). While there is promising research on chitosan’s potential in addressing neurodegenerative diseases, much of the work is still in the preclinical or early clinical stages. More extensive human trials are required to fully understand its benefits, optimal dosages, and mechanisms of action. The application of this native polysaccharide is limited by its high molecular weight and highly viscous nature resulting in its low solubility in acid-free aqueous media.
2.2 Glycosaminoglycan
Glycosaminoglycans play a pivotal role in the regeneration of the mammalian CNS. Glycosaminoglycan, especially those coating the surface of endothelial cells and leukocytes, regulate some of the major events during the inflammation process by which exogenous sulfated structures downregulating inflammation processes (Morla, 2019). As the rate-limiting step in Aβ production, the β-site amyloid precursor protein cleaving enzyme 1 (BACE-1) has emerged as a key drug target of AD (Prati et al., 2018; Monteiro et al., 2023). Previous studies have reported that heparin, a glycosaminoglycan, exhibits significant inhibitory effects on BACE-1 activity. However, the high anticoagulant potential limits heparin’s suitability as a therapeutic biomolecule. Therefore, alternative sources with reduced anticoagulant properties have garnered more attention (Engelberg, 2004; Alavi Naini and Soussi-Yanicostas, 2018; Yang et al., 2021). Notably, chondroitin sulfate extracted from Placopecten magellanicus containing a high content of 6-sulphated N-acetyl glucosamine demonstrates potent inhibition against BACE-1 (IC50 = 4.8 μg/mL), suggesting a more favorable therapeutic profile (Mycroft-West et al., 2023). Additionally, a marine-derived glycosaminoglycan containing heparan sulphate from Portunus pelagicus, was identified to inhibit human BACE1. Importantly, the interactions between P. pelagicus glycosaminoglycan and BACE1 differ significantly from those observed with heparin (Mycroft-West et al., 2019). Further investigations are warranted to elucidate the underlying mechanisms. Besides, de Sousa et al., obtained a type of Glycosaminoglycans, dermatan sulfate from the ascidian Phallusia nigra and demonstrated its neuroprotective and antioxidant properties in rotenone induced Neuro-2A cells. The underlying mechanisms probably resulted from regulating the activity of the antioxidant enzymes superoxide dismutase and catalase, leading to the decreased levels of lipid peroxidation to protected cells from damage (de Sousa et al., 2020). Recently, Phallusia nigra dermatan sulfate (PnD2,6S) was also reported with neuritogenic and neuroprotective role. It had a better neuritogenic effect than chondroitin sulfate and dermatan sulfate at a lower concentration (0.05 μg/mL) in pesticide rotenone induced a neuro 2A murine neuroblastoma cell line, suggesting the sulfation pattern was important for neuritogenic activity (Medeiros et al., 2023).
3 Polysaccharides from algae
Generally, the chemical composition of polysaccharides from microalgae and macroalgae is very complex and in high variance depending on the algal source, harvesting time, cultivation method. Due to the huge biodiversity and wide application in foods and folk medicine, seaweeds are considered as an attractive source of bioactive compounds. Many bioactive compounds, partially purified polysaccharides have been tested for various therapeutic activities against various human diseases (Pradhan et al., 2020).
3.1 Fucoidan
Fucoidans, abundant in the brown seaweeds and other marine species, are a group of sulfated polysaccharides composed of fucose, other monosaccharides (mannose, galactose, glucose, xylose, etc.) and uronic acids, even acetyl groups and proteins (Luthuli et al., 2019). Despite its complex structure, repeated (1→3)-l-fucopyranose, or alternating and repeated (1→3)- and (1→4)-l-fucopyranose, are recognized as the structural backbone. C-2 and C-4 on fucosyl residues could be substituted with sulfate and/or acetate groups (Zahan et al., 2022). Fucoidans derived from different sources possess variations in structural characteristics, leading to a wide spectrum of biological effects. Wozniak et al. obtained five sulfated fucans from brown algae (Scytothamnus australis, Marginariella boryana, Papenfussiella lutea, Splachnidium rugosum and Undaria pinnatifida), four of which could prevent the accumulation of beta-amyloid and AD-like tau in Herpes simplex virus type 1 (HSV1)-infected Vero cells. HSV1 was reported to induces the formation of beta-amyloid, and abnormally phosphorylated tau (P-tau), inducing AD (Wozniak et al., 2015). heparan sulfate (HS) in cell surface mediated Tau spreading in Alzheimer’s disease. fucoidans were expected to compete with HS to bind tau, inhibiting tau spreading. Jin et al. determined the binding abilities of sixty fucoidans/glycans with tau using SPR and AlphaLISA. They found that sulfated galactofucan (SJ-I) and sulfated heteropolysaccharide (SJ-GX-3) inhibited tau-cell interaction and uptake in wild type mouse lung endothelial cell lines, displying strong binding abilities than heparin, suggesting that fucoidans might be potential for inhibiting tau spreading (Jin et al., 2023). Xing et al. displayed that fucoidan from Fucus vesiculosus (FvF) more significantly improved mitochondrial dysfunction, reduced dopaminergic neuron loss, and improved motor deficits than other isolated and purified three different fucoidan species with different chemical structures in an 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model. Furthermore, the ATP5F1a protein was identified as the key target responsible for alleviating mitochondrial dysfunction (Xing et al., 2023). Yang et al. extracted fucoidans from Sargassum crassifolium by warm water, which all showed antioxidant activity dose-dependently in H₂O₂-induced cytotoxicity in rat pheochromocytoma PC-12 cells. Despite the diverse structure, they possessed high and similar neuronal protective properties (Yang et al., 2017). Huang et al. improved the technology of extracting fucoidan from Sargassum hemiphyllum via a compressional-puffing process. All extracts showed antioxidant activities and protected SH-SY5Y cells from 6-hydroxydopamine (6-OHDA)-induced apoptosis, among which, phosphorylation of Akt play a vital role against 6-OHDA-induced neurotoxicity (Huang et al., 2017).
Fucoidan could inhibit the release of cytochrome c from the mitochondria to cytosol and caspase dependent apoptosis in beta-amyloid 25-35 (Aβ25-35) and d-galactose induced PC12 cells. Notablely, Fucoidan could regulate the cholinergic system by improving activity of acetylcholine and choline acetyl transferase and attenuating activity of acetylcholine esterase in AD model mice induced by infusion of d-Gal. Furthermore, fucoidan improved antioxidant activity to reduce oxidative stress (Wei et al., 2017). In monocrotophos induced PC12 cells, fucoidan also showed inhibitory activity against cholinergic and monoamine-metabolized enzymes. In addition, fucoidan prevented changes in neurochemicals and latency time of locomotor, learning and memory induced by monocrotophos in Drosophila melanogaster (Subaraja et al., 2020). Adella Putri et al., verified that fucoidan possessed good binding affinity with acetylcholinesterase (AChE) with the binding free energy values at −7.4 kcal/mol via a molecular docking approach (Adella Putri et al., 2024). In a transgenic Caenorhabditis elegans (C. elegans) AD model, fucoidan alleviated Abeta induced paralyzed phenotype through promoting proteolysis. Leading to the reduce the production of reactive oxygen species (ROS) (Wang et al., 2018). Jhamandas et al. used whole-cell patch clamp recording fucoidan on A beta-induced whole-cell currents in acutely dissociated rat basal forebrain neurons and in primary neuronal cultures. The results showed that Fucoidan not only blocks the A beta (25–35) reduction of whole-cell currents in a dose-dependent manner, but also attenuated the downregulation of phosphorylated protein kinase C (Jhamandas et al., 2005). These results demonstrated the potential neuroprotective effects of fucoidan.
Enormous evidence suggest that mitochondria hold a central position in ageing-related neurodegenerative diseases, which regulates cell death, a key feature of neurodegeneration. Mitochondrial DNA mutations and oxidative stress both contribute to ageing. Moreover, an impressive number of disease-specific proteins interact with mitochondria. Thus, therapies targeting energy metabolism or free-radical generation hold great promise (Klemmensen et al., 2024; Zong et al., 2024). Fucoidan was reported to reserve mitochondrial function by regulating PGC-1α/NRF2 pathway in a rotenone-induced rat model (Zhang et al., 2018), and via regulating the AMPK-PGC-1α axis in MPP+ induced SH-SY5Y cells (Han et al., 2019). In a 6-hydroxydopamine (6-OHDA) rat model of PD, fucoidan also showed neuroprotective effects. It prevented NADPH oxidases-1 (Nox1)-sensitive oxidative stress and cell damage in both tyrosine hydroxylase (TH)-positive neurons and non-TH-positive neurons. Fucoidan also effectively inhibited nigral microglial activation (Zhang et al., 2014).
In addition, Fucoidan improved the TH protein levels in MPP+-induced MN9D cells and in substantia nigra and corpus striatum of MPTP treated mice. Notably, the increase is greater than the level of dopamine and DOPAC, verifying that the dopaminergic terminals are more sensitive than the dopaminergic cell bodies to MPTP toxicity (Luo et al., 2009; Meenakshi et al., 2016). Fucoidan can be purified into several fractions, various fractions of FPS differing in uronic acid and sulfate content showed variable activities. fucoidan fraction sulfated heterosaccharide possessed protective effect against MPP + or - H₂O₂ induced SH-SY5Y cells apoptosis by affecting the PI3K/Akt pathway (Wang et al., 2017; Liu et al., 2020). The high sulfate content of fucoidan may have contributed to its bioactivity. Fucoidan could intervene in many inflammation-related metabolic syndrome, mainly act on several inflammatory process, including modulating inflammation-related gene expression, blocking lymphocyte adhesion and invasion, inhibiting multiple enzymes, and inducing apoptosis (Apostolova et al., 2020; Jayawardena et al., 2022). Fucoidan suppressed tumor necrosis factor-alpha (TNF-alpha)- and interferon-gamma (IFN-gamma)-induced NO production and iNOS expression in C6 glioma cells. Additionally, the activation AP-1, IRF-1, JAK/STAT and p38 mitogen-activated protein kinase (MAPK) was inhibited and the level of scavenger receptor B1 (SR-B1) was improved, suggesting its potential roles for treating inflammatory-related neuronal injury in neurodegenerative disease (Do et al., 2010). Additionally, fucoidan downregulate intracellular ROS and subsequent proinflammatory cytokine release in LPS-activated microglia, NF-κB/MAPK/Akt pathway played an important role (Park et al., 2011; Cui et al., 2012). However, in another study by Murgas et al., fucoidan accelerated the NO production by microglia induced by β amyloid (Aβ) and activated JNK/NF-κB signal pathway (Murgas et al., 2012). The details can be found in the Table 1. Fucoidan is a seaweed polysaccharide with a promising application in the prevention and treatment of NDDs. However, due to its wide source and diverse extraction methods, its molecular weight, sulfate content and structure change, which will affect its pharmacological activity.
3.2 λ-carrageenan
Carrageenan, a sulphated linear polysaccharide extracted from red seaweeds, was composed of D-galactose residues linked in β-1,4 and α-1,3 galactose-galactose bond (Borsani et al., 2021). Carrageenans have been extensively investigated for various bioactivities such as immunomodulatory activity, antiviral, anticoagulant, antioxidant, and cholesterol-lowering effects (Pangestuti and Kim, 2014). Based on the special negative charge and gelling, it has been used as a viscosity enhancing agent for controlled drug release and prolonged retention and tissue regeneration (Li et al., 2014; Borsani et al., 2021). Low-molecular-weight λ-carrageenan showed improvement on the memory function of rats with an infusion of toxic amyloid-β(Aβ) mainly by increasing the BDNF content and the insulin signaling through enhancing the pSTAT3→pAkt→pGSK-3β pathway. Additionally, regardless of the MW, λ-carrageenan intake could improve gut microbiota to reduce secondary bile acid biosynthesis degradation of toxic compounds, which effects were similar to low-molecular-weight fucoidan, as showed in the increased the abundance of probiotic bacteria Lactobacillus and Akkermentia. Furthermore, low-molecular-weight fucoidan potentiated hippocampal insulin signaling, increased the expression of ciliary neurotrophic factor and improved glucose tolerance the most (Zhang et al., 2022). Amyloids, with their β-sheet-rich structure, contribute to diabetes and AD. Liposomal nanoformulated iota carrageenan was formulated to effectively disrupt insulin amyloids, which shared structural resemblances with amyloids. The well biocompatibility facilitates its application in prevention and cure of AD (Udayakumar et al., 2024). Limited by the solubility of λ-carrageenan, more studies have been conducted on its oligosaccharides.
3.3 Sargassum polysaccharides
Jin et al. extracted several heteropolysaccharides from Sargassum integerrimum, Sargassum maclurei, Sargassum naozhouense, Spiraea thunbergii, S. hemiphyllum and Sargassum fusiforme. The result showed that neuroprotective activities varied according to the structure of the polysaccharides. Although the fragments represented the principal difference between the active and non-active compounds and structures with superoxide-radical scavenging effect were identified, these structures were not associated with neuroprotective effects. Thus, the effect of neuroprotective activities is determined by multiple factors (Jin et al., 2014). Sargassum thunbergii, a common intertidal seaweed species, is commonly used as bait and a component of artificial Sargassum beds due to its wide ecological amplitude and high economic and ecological value. Subsequently, Jin et al., prepared two polysaccharides (ST-1 and ST-2) from S. thunbergii using anion exchange chromatography. And ST showed well neuroprotective activity. The structure of ST was analyzed by ESI-MS with collision-induced dissociation tandem mass spectrometry (ESI-CID-MS/MS), suggesting that glucuronomannan contained alternating 2-linked Man and 4-linked GlcA, while fucoglucuronan contained 4-linked glucuronan with branched Fuc at C-3. The structure-function relationship need to be further study (Jin et al., 2018).
3.4 Polysaccharide from Chlamydomonas reinhardtii
α-Synuclein (α-Syn) is an intrinsically disordered presynaptic protein, participates in the progress of PD in an aggregation manner. The sulfated polysaccharides from Chlamydomonas reinhardtii (Cr-SPs) were isolated and verified to effectively inhibit α-Syn fibrillation by binding with α-Syn and delaying the conversion of α-helical intermediate into β-sheet rich structures. Cr-SPs are also effective even if onset of α-Syn fibrillation has already started and they also have the ability to dissolve pre-formed fibrils (Choudhary et al., 2018). Fibrillation/aggregation of α-Syn mutants affect specific tertiary interactions essential for stability of the native state. Panigrahi et al., demonstrated Cr-SPs inhibiting effects on fibrillation of α-Syn mutants through efficiently delaying the conversion of native protein into β-sheet-rich structures (Panigrahi et al., 2019). Thus, the finds have substantial therapeutic implications towards PD treatment.
3.5 Porphyran
The sulphated polysaccharides drived from marine algae are heterogenic molecules with different biological activities. Polysaccharides from red algae Pyropia haitanensis, named porphyrin, has been reported as a good antioxidant to stimulate immune response in aging mice. Considering its high viscosity, Zhang et al., degraded the polysaccharide and obtained several samples with similar sulfate content to that of natural porphyrin. These composition ameliorated the learning and memory impairment induced by Aβ1-40 through increasing choline acetyltransferase (CHAT) activity and decreased acetylcholinesterase (AChE) activity in the cortical and hippocampal tissue, implying that porphyrin might be the potential agents to target aging-related neurodegenerative disease (Zhang Z. et al., 2020). In addition, Wang et al. reported that acetylated porphyran and phosphorylated porphyran, rather than porphyran, significantly antagonized 25 μmol/L 6-OHDA-induced cytotoxicity, but without effect on 75 μmol/L 6-OHDA induced cytotoxicity. However, none could improve mitochondrial transmembrane potential, suggesting their minor neuroprotective effects independent of mitochondria restoration (Wang et al., 2015). An antioxidant degraded porphyran, was regarded as a protective agent against neurotoxicity-induced amyloid β peptide (Aβ) of AD mice. Porphyran significantly ameliorated the learning and memory impairment, balanced ChAT activity and AChE activity in the cortical and hippocampal tissue induced by Aβ1-40 (Zhang Z. et al., 2020).
3.6 Other polysaccharides
Souza et al. isolated and obtained a sulphated polysaccharide (SA-Gc) from red marine alga Gracilaria cornea presenting a structural 3,6-anhydro-α-l-galactose component. SA-Gc was identified as a polysaccharide of the type agaran, displayed neuroprotective effects through reducing the oxidative/nitroactive stress and modulating the transcription of neuroprotective and inflammatory genes in rat model PD induced by 6-hydroxydopamine (6-OHDA) (Souza et al., 2017). Polysaccharides from Chlorella pyrenoidosa (CPS) showed neuroprotective effect in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of pD. The polysaccharides improved motor activity and dopamine expressions, inhibited peripheral immunomodulatory responses in serum and gut. The neuroprotective mechanism might be related to its immunomodulatory action (Chen et al., 2014). Additionally, polysaccharide obtained from Spirulina platensis also played a neuroprotective role in MPTP-induced mice, which was likely related to their antioxidative properties (Zhang et al., 2015). Marine microorganisms are considered as efficient producers of biologically active compounds. Sun et al., isolated and authenticated an exopolysaccharide from a marine filamentous fungus-Keissleriella sp. YS4108. The polysaccharide was composed of galactose, glucose, rhamnose, mannose and glucuronic acid, with a mean molecular weight of 1.3 × 105 Da. It showed that the polysaccharide possesses pronounced protective effects against H2O2-induced cell toxicity through elevating the cell survival and antioxidant activity in a dose-dependent manner (Sun et al., 2005).
4 Conclusion and perspective
Polysaccharides, especially marine polysaccharides showed related to regulating the biological processes with anti-apoptosis, anti-inflammatory, antioxidant, anticancer and many other effects. The study of fucoidan and chitosan applied in the treatment of neurodegenerative disorders via inhibiting cell death, inflammation, oxidation and acetylcholinesterase enzymatic activity drew more attention of marine polysaccharides. Now, the reported polysaccharides are mainly from animal and algal sources. A large number of fungi derived polysaccharides need to be further investigated. In addition, Polysaccharides, a heterogeneous class of macromolecules, possess distinct properties depending on their sources and extraction, which determinates their monosaccharide composition and functional groups. The development of polysaccharides as drugs requires a more rigorous standard for extraction and identification, and the polysaccharides containing sulfuric acid groups seem to have better opening value. Furthermore, drug delivery systems, such as chitosan, further expand the application of polysaccharides.
Author contributions
LZ: Writing–original draft. YR: Writing–original draft, Supervision. SZ: Writing–original draft, Data curation. YG: Writing–review and editing. JZ: Data curation, Writing–original draft. YL: Writing–review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (32200802) and Qingdao West Coast New District Science and Technology Project (2020-3-2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adella Putri, A. D., Sembiring, M. H., and Tuba, S. (2024). Phytochemical constituents analysis in laminaria digitata for Alzheimer's disease: molecular docking and in-silico toxicity approach. Commun. Integr. Biol. 17 (1), 2357346. doi:10.1080/19420889.2024.2357346
Agnello, L., and Ciaccio, M. (2022). Neurodegenerative Diseases: from molecular basis to therapy. Int. J. Mol. Sci. 23 (21), 12854. doi:10.3390/ijms232112854
Ahlawat, J., Neupane, R., Deemer, E., Sreenivasan, S. T., and Narayan, M. (2020). Chitosan-ellagic acid nanohybrid for mitigating rotenone-induced oxidative stress. ACS Appl. Mater Interfaces 12 (16), 18964–18977. doi:10.1021/acsami.9b21215
Alavi Naini, S. M., and Soussi-Yanicostas, N. (2018). Heparan sulfate as a therapeutic target in Tauopathies: insights from zebrafish. Front. Cell Dev. Biol. 6, 163. doi:10.3389/fcell.2018.00163
Amini, M., and Abdolmaleki, Z. (2022). The effect of cannabidiol coated by nano-chitosan on learning and memory, hippocampal CB1 and CB2 levels, and amyloid plaques in an Alzheimer's disease rat model. Neuropsychobiology 81 (3), 171–183. doi:10.1159/000519534
Apostolova, E., Lukova, P., Baldzhieva, A., Katsarov, P., Nikolova, M., Iliev, I., et al. (2020). Immunomodulatory and anti-inflammatory effects of fucoidan: a review. Polym. (Basel) 12 (10), 2338. doi:10.3390/polym12102338
Bedse, G., Di Domenico, F., Serviddio, G., and Cassano, T. (2015). Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front. Neurosci. 9, 204. doi:10.3389/fnins.2015.00204
Borsani, B., De Santis, R., Perico, V., Penagini, F., Pendezza, E., Dilillo, D., et al. (2021). The role of carrageenan in inflammatory bowel diseases and allergic reactions: where do we stand? Nutrients 13 (10), 3402. doi:10.3390/nu13103402
Budai, L., Budai, M., Fülöpné Pápay, Z. E., Vilimi, Z., and Antal, I. (2023). Rheological considerations of pharmaceutical formulations: focus on viscoelasticity. Gels 9 (6), 469. doi:10.3390/gels9060469
Chen, P. B., Wang, H.-C., Liu, Y.-W., Lin, S.-H., Chou, H.-N., and Sheen, L.-Y. (2014). Immunomodulatory activities of polysaccharides from Chlorella pyrenoidosa in a mouse model of Parkinson's disease. J. Funct. Foods 11, 103–113. doi:10.1016/j.jff.2014.08.019
Choudhary, S., Save, S. N., and Vavilala, S. L. (2018). Unravelling the inhibitory activity of Chlamydomonas reinhardtii sulfated polysaccharides against α-Synuclein fibrillation. Sci. Rep. 8 (1), 5692. doi:10.1038/s41598-018-24079-7
Cui, Y. Q., Jia, Y. J., Zhang, T., Zhang, Q. B., and Wang, X. M. (2012). Fucoidan protects against lipopolysaccharide-induced rat neuronal damage and inhibits the production of proinflammatory mediators in primary microglia. CNS Neurosci. Ther. 18 (10), 827–833. doi:10.1111/j.1755-5949.2012.00372.x
de Sousa, G. F., Palmero, C. Y., de Souza-Menezes, J., Araujo, A. K., Guimarães, A. G., and de Barros, C. M. (2020). Dermatan sulfate obtained from the Phallusia nigra marine organism is responsible for antioxidant activity and neuroprotection in the neuroblastoma-2A cell lineage. Int. J. Biol. Macromol. 164, 1099–1111. doi:10.1016/j.ijbiomac.2020.06.285
De Virgilio, A., Greco, A., Fabbrini, G., Inghilleri, M., Rizzo, M. I., Gallo, A., et al. (2016). Parkinson's disease: autoimmunity and neuroinflammation. Autoimmun. Rev. 15 (10), 1005–1011. doi:10.1016/j.autrev.2016.07.022
Do, H., Pyo, S., and Sohn, E. H. (2010). Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 21 (8), 671–679. doi:10.1016/j.jnutbio.2009.03.013
Engelberg, H. (2004). Pathogenic factors in vascular dementia and Alzheimer's disease. Multiple actions of heparin that probably are beneficial. Dement. Geriatr. Cogn. Disord. 18 (3-4), 278–298. doi:10.1159/000080034
Etxebeste-Mitxeltorena, M., Niza, E., Fajardo, C. M., Gil, C., Gómez-Gómez, L., Martinez, A., et al. (2024). Neuroprotective properties of exosomes and chitosan nanoparticles of Tomafran, a bioengineered tomato enriched in crocins. Nat. Prod. Bioprospect 14 (1), 9. doi:10.1007/s13659-023-00425-9
Fu, X. M., Zhang, M. Q., Shao, C. L., Li, G. Q., Bai, H., Dai, G. L., et al. (2016). Chinese marine materia medica resources: status and potential. Mar. Drugs 14 (3), 46. doi:10.3390/md14030046
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25 (12), 1822–1832. doi:10.1038/s41591-019-0675-0
González-Chavarría, I., Roa, F. J., Sandoval, F., Muñoz-Flores, C., Kappes, T., Acosta, J., et al. (2023). Chitosan microparticles enhance the intestinal release and immune response of an immune stimulant peptide in Oncorhynchus mykiss. Int. J. Mol. Sci. 24 (19), 14685. doi:10.3390/ijms241914685
Han, Y. S., Lee, J. H., and Lee, S. H. (2019). Fucoidan suppresses mitochondrial dysfunction and cell death against 1-methyl-4-phenylpyridinum-induced neuronal cytotoxicity via regulation of PGC-1α expression. Mar. Drugs 17 (9), 518. doi:10.3390/md17090518
Hassan, D. M., El-Kamel, A. H., Allam, E. A., Bakr, B. A., and Ashour, A. A. (2024). Chitosan-coated nanostructured lipid carriers for effective brain delivery of Tanshinone IIA in Parkinson's disease: interplay between nuclear factor-kappa β and cathepsin B. Drug Deliv. Transl. Res. 14 (2), 400–417. doi:10.1007/s13346-023-01407-7
Höglund, K., and Salter, H. (2013). Molecular biomarkers of neurodegeneration. Expert Rev. Mol. Diagn 13 (8), 845–861. doi:10.1586/14737159.2013.850033
Hu, G. P., Yuan, J., Sun, L., She, Z. G., Wu, J. H., Lan, X. J., et al. (2011). Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 9 (4), 514–525. doi:10.3390/md9040514
Huang, C. Y., Kuo, C. H., and Chen, P. W. (2017). Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the Brown seaweed Sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 23 (1), 78. doi:10.3390/molecules23010078
Huang, H. C., Hong, L., Chang, P., Zhang, J., Lu, S. Y., Zheng, B. W., et al. (2015). Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox. Res. 27 (4), 411–420. doi:10.1007/s12640-014-9512-x
Jayawardena, T. U., Nagahawatta, D. P., Fernando, I. P. S., Kim, Y. T., Kim, J. S., Kim, W. S., et al. (2022). A review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar. Drugs 20 (12), 755. doi:10.3390/md20120755
Jhamandas, J. H., Wie, M. B., Harris, K., MacTavish, D., and Kar, S. (2005). Fucoidan inhibits cellular and neurotoxic effects of beta-amyloid (A beta) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 21 (10), 2649–2659. doi:10.1111/j.1460-9568.2005.04111.x
Jia, S., Lu, Z., Gao, Z., An, J., Wu, X., Li, X., et al. (2016). Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-β1-42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 83, 416–425. doi:10.1016/j.ijbiomac.2015.11.011
Jiang, Y., Zhou, W., Zhang, X., Wang, Y., Yang, D., and Li, S. (2021). Protective effect of blood cora polysaccharides on H9c2 rat heart cells injury induced by oxidative stress by activating Nrf2/HO-1 signal pathway. Front. Nutr. 8, 632161. doi:10.3389/fnut.2021.632161
Jin, W., Liu, B., Li, S., Chen, J., Tang, H., Jiang, D., et al. (2018). The structural features of the sulfated heteropolysaccharide (ST-1) from Sargassum thunbergii and its neuroprotective activities. Int. J. Biol. Macromol. 108, 307–313. doi:10.1016/j.ijbiomac.2017.12.009
Jin, W., Lu, C., Zhu, Y., Zhao, J., Zhang, W., Wang, L., et al. (2023). Fucoidans inhibited tau interaction and cellular uptake. Carbohydr. Polym. 299, 120176. doi:10.1016/j.carbpol.2022.120176
Jin, W., Zhang, W., Wang, J., Yao, J., Xie, E., Liu, D., et al. (2014). A study of neuroprotective and antioxidant activities of heteropolysaccharides from six Sargassum species. Int. J. Biol. Macromol. 67, 336–342. doi:10.1016/j.ijbiomac.2014.03.031
Khodagholi, F., Eftekharzadeh, B., Maghsoudi, N., and Rezaei, P. F. (2010). Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol. Cell. Biochem. 337 (1–2), 39–51. doi:10.1007/s11010-009-0284-1
Kim, M. S., Sung, M. J., Seo, S. B., Yoo, S. J., Lim, W. K., and Kim, H. M. (2002). Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 321 (1–2), 105–109. doi:10.1016/s0304-3940(02)00066-6
Klemmensen, M. M., Borrowman, S. H., Pearce, C., Pyles, B., and Chandra, B. (2024). Mitochondrial dysfunction in neurodegenerative disorders. Neurotherapeutics 21 (1), e00292. doi:10.1016/j.neurot.2023.10.002
Li, L., Ni, R., Shao, Y., and Mao, S. (2014). Carrageenan and its applications in drug delivery. Carbohydr. Polym. 103, 1–11. doi:10.1016/j.carbpol.2013.12.008
Liu, H., Ojha, B., Morris, C., Jiang, M., Wojcikiewicz, E. P., Rao, P. P., et al. (2015). Positively charged chitosan and N-trimethyl chitosan inhibit Aβ40 fibrillogenesis. Biomacromolecules 16 (8), 2363–2373. doi:10.1021/acs.biomac.5b00603
Liu, H., Wang, J., Zhang, Q., Geng, L., Yang, Y., and Wu, N. (2020). Protective effect of fucoidan against MPP(+)-induced SH-SY5Y cells apoptosis by affecting the PI3K/Akt pathway. Mar. Drugs 18 (6), 333. doi:10.3390/md18060333
Luo, D., Zhang, Q., Wang, H., Cui, Y., Sun, Z., Yang, J., et al. (2009). Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 617 (1-3), 33–40. doi:10.1016/j.ejphar.2009.06.015
Luthuli, S., Wu, S., Cheng, Y., Zheng, X., Wu, M., and Tong, H. (2019). Therapeutic effects of fucoidan: a review on recent studies. Mar. Drugs 17 (9), 487. doi:10.3390/md17090487
Medeiros, T. B., Cosendey, P., Gerin, D. R., de Sousa, G. F., Portal, T. M., and Monteiro-de-Barros, C. (2023). The effect of the sulfation patterns of dermatan and chondroitin sulfate from vertebrates and ascidians on their neuritogenic and neuroprotective properties. Int. J. Biol. Macromol. 247, 125830. doi:10.1016/j.ijbiomac.2023.125830
Meenakshi, S., Umayaparvathi, S., Saravanan, R., Manivasagam, T., and Balasubramanian, T. (2016). Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 86, 425–433. doi:10.1016/j.ijbiomac.2015.12.025
Mohebichamkhorami, F., Faizi, M., Mahmoudifard, M., Hajikarim-Hamedani, A., Mohseni, S. S., Heidari, A., et al. (2023). Microfluidic synthesis of ultrasmall chitosan/graphene quantum dots particles for intranasal delivery in Alzheimer's disease treatment. Small 19 (40), e2207626. doi:10.1002/smll.202207626
Monteiro, K. L. C., Dos Santos Alcântara, M. G., Freire, N. M. L., Brandão, E. M., do Nascimento, V. L., Dos Santos Viana, L. M., et al. (2023). BACE-1 inhibitors targeting Alzheimer's disease. Curr. Alzheimer Res. 20 (3), 131–148. doi:10.2174/1567205020666230612155953
Morla, S. (2019). Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int. J. Mol. Sci. 20 (8), 1963. doi:10.3390/ijms20081963
Murgas, P., Godoy, B., and von Bernhardi, R. (2012). Aβ potentiates inflammatory activation of glial cells induced by scavenger receptor ligands and inflammatory mediators in culture. Neurotox. Res. 22 (1), 69–78. doi:10.1007/s12640-011-9306-3
Mycroft-West, C. J., Cooper, L. C., Devlin, A. J., Procter, P., Guimond, S. E., Guerrini, M., et al. (2019). A glycosaminoglycan extract from Portunus pelagicus inhibits BACE1, the β secretase implicated in Alzheimer's disease. Mar. Drugs 17 (5), 293. doi:10.3390/md17050293
Mycroft-West, C. J., Devlin, A. J., Cooper, L. C., Guimond, S. E., Procter, P., Miller, G. J., et al. (2023). A sulphated glycosaminoglycan extract from Placopecten magellanicus inhibits the Alzheimer's disease β-site amyloid precursor protein cleaving enzyme 1 (BACE-1). Carbohydr. Res. 525, 108747. doi:10.1016/j.carres.2023.108747
Nandi, A., Counts, N., Chen, S., Seligman, B., Tortorice, D., Vigo, D., et al. (2022). Global and regional projections of the economic burden of Alzheimer's disease and related dementias from 2019 to 2050: a value of statistical life approach. EClinicalMedicine 51, 101580. doi:10.1016/j.eclinm.2022.101580
Nichols, E., Steinmetz, J. D., Vollset, S. E., Fukutaki, K., Chalek, J., Allah, F.-A., et al. (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7 (2), e105–e125. doi:10.1016/s2468-2667(21)00249-8
Nojoki, F., Ebrahimi-Hosseinzadeh, B., Hatamian-Zarmi, A., Khodagholi, F., and Khezri, K. (2022). Design and development of chitosan-insulin-transfersomes (Transfersulin) as effective intranasal nanovesicles for the treatment of Alzheimer's disease: in vitro, in vivo, and ex vivo evaluations. Biomed. Pharmacother. 153, 113450. doi:10.1016/j.biopha.2022.113450
Omidian, H., Gill, E. J., Dey Chowdhury, S., and Cubeddu, L. X. (2024). Chitosan nanoparticles for intranasal drug delivery. Pharmaceutics 16 (6), 746. doi:10.3390/pharmaceutics16060746
Ouyang, Q. Q., Zhao, S., Li, S. D., and Song, C. (2017). Application of chitosan, chitooligosaccharide, and their derivatives in the treatment of Alzheimer's Disease. Mar. Drugs 15 (11), 322. doi:10.3390/md15110322
Pangestuti, R., Bak, S. S., and Kim, S. K. (2011). Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 49 (4), 599–606. doi:10.1016/j.ijbiomac.2011.06.014
Pangestuti, R., and Kim, S. K. (2014). Biological activities of carrageenan. Adv. Food Nutr. Res. 72, 113–124. doi:10.1016/b978-0-12-800269-8.00007-5
Panigrahi, G. P., Rane, A. R., Vavilala, S. L., and Choudhary, S. (2019). Deciphering the anti-Parkinson's activity of sulphated polysaccharides from Chlamydomonas reinhardtii on the α-Synuclein mutants A30P, A53T, E46K, E57K and E35K. J. Biochem. 166 (6), 463–474. doi:10.1093/jb/mvz064
Park, H. Y., Han, M. H., Park, C., Jin, C. Y., Kim, G. Y., Choi, I. W., et al. (2011). Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 49 (8), 1745–1752. doi:10.1016/j.fct.2011.04.020
Pradhan, B., Patra, S., Nayak, R., Behera, C., Dash, S. R., Nayak, S., et al. (2020). Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: a journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 164, 4263–4278. doi:10.1016/j.ijbiomac.2020.09.019
Pramod Kumar, P., and Harish Prashanth, K. V. (2020). Diet with low molecular weight chitosan exerts neuromodulation in rotenone induced Drosophila model of Parkinson's disease. Food Chem. Toxicol. 146, 111860. doi:10.1016/j.fct.2020.111860
Prati, F., Bottegoni, G., Bolognesi, M. L., and Cavalli, A. (2018). BACE-1 inhibitors: from recent single-target molecules to multitarget compounds for Alzheimer's disease. J. Med. Chem. 61 (3), 619–637. doi:10.1021/acs.jmedchem.7b00393
Rajkumar, M., Govindaraj, P., Vimala, K., Thangaraj, R., and Kannan, S. (2024). Chitosan/PLA-loaded Magnesium oxide nanocomposite to attenuate oxidative stress, neuroinflammation and neurotoxicity in rat models of Alzheimer’s disease. Metab. Brain Dis. 39 (4), 487–508. doi:10.1007/s11011-023-01336-x
Ren, Y., Zhao, X., Liang, X., Ma, P. X., and Guo, B. (2017). Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson's disease. Int. J. Biol. Macromol. 105 (Pt 1), 1079–1087. doi:10.1016/j.ijbiomac.2017.07.130
Saleem, S., Banerjee, R., and Rajesh Kannan, R. (2022). Chrysin-loaded chitosan nanoparticle-mediated neuroprotection in Aβ(1-42)-induced neurodegenerative conditions in zebrafish. ACS Chem. Neurosci. 13 (13), 2017–2034. doi:10.1021/acschemneuro.2c00240
Sardoiwala, M. N., Karmakar, S., and Choudhury, S. R. (2021). Chitosan nanocarrier for FTY720 enhanced delivery retards Parkinson's disease via PP2A-EzH2 signaling in vitro and ex vivo. Carbohydr. Polym. 254, 117435. doi:10.1016/j.carbpol.2020.117435
Souza, R. B., Frota, A. F., Sousa, R. S., Cezario, N. A., Santos, T. B., Souza, L. M., et al. (2017). Neuroprotective effects of sulphated agaran from marine alga Gracilaria cornea in rat 6-hydroxydopamine Parkinson's disease model: Behavioural, neurochemical and transcriptional alterations. Basic Clin. Pharmacol. Toxicol. 120 (2), 159–170. doi:10.1111/bcpt.12669
Subaraja, M., Anantha Krishnan, D., Edwin Hillary, V., William Raja, T. R., Mathew, P., Ravikumar, S., et al. (2020). Fucoidan serves a neuroprotective effect in an Alzheimer's disease model. Front. Biosci. Elite Ed. 12 (1), 1–34. doi:10.2741/e855
Sun, C., Shan, C. Y., Gao, X. D., and Tan, R. X. (2005). Protection of PC12 cells from hydrogen peroxide-induced injury by EPS2, an exopolysaccharide from a marine filamentous fungus Keissleriella sp. YS4108. J. Biotechnol. 115 (2), 137–144. doi:10.1016/j.jbiotec.2004.08.011
Tansey, M. G., Wallings, R. L., Houser, M. C., Herrick, M. K., Keating, C. E., and Joers, V. (2022). Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22 (11), 657–673. doi:10.1038/s41577-022-00684-6
Temple, S. (2023). Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 30 (5), 512–529. doi:10.1016/j.stem.2023.03.017
Thirupathi, K., Raorane, C. J., Ramkumar, V., Ulagesan, S., Santhamoorthy, M., Raj, V., et al. (2022). Update on chitosan-based hydrogels: preparation, characterization, and its antimicrobial and antibiofilm applications. Gels 9 (1), 35. doi:10.3390/gels9010035
Udayakumar, S., Metkar, S. K., Girigoswami, A., Deepika, B., Janani, G., Kanakaraj, L., et al. (2024). Exploring the amyloid degradation potential of nanoformulated carrageenan-bridging in vitro and in vivo perspectives. Int. J. Biol. Macromol. 279 (Pt 1), 134814. doi:10.1016/j.ijbiomac.2024.134814
Wang, J., Liu, H., Zhang, X., Li, X., Geng, L., Zhang, H., et al. (2017). Sulfated hetero-polysaccharides protect SH-SY5Y cells from H₂O₂-induced apoptosis by affecting the PI3K/Akt signaling pathway. Mar. Drugs 15 (4), 110. doi:10.3390/md15040110
Wang, W., Song, N., Jia, F., Xie, J., Zhang, Q., and Jiang, H. (2015). Neuroprotective effects of porphyran derivatives against 6-hydroxydopamine-induced cytotoxicity is independent on mitochondria restoration. Ann. Transl. Med. 3 (3), 39. doi:10.3978/j.issn.2305-5839.2015.01.33
Wang, W., Wang, S. X., and Guan, H. S. (2012). The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs 10 (12), 2795–2816. doi:10.3390/md10122795
Wang, X., Yi, K., and Zhao, Y. (2018). Fucoidan inhibits amyloid-β-induced toxicity in transgenic Caenorhabditis elegans by reducing the accumulation of amyloid-β and decreasing the production of reactive oxygen species. Food Funct. 9 (1), 552–560. doi:10.1039/c7fo00662d
Wang, Y., Chen, R., Shi, G., Huang, X., Li, K., Wang, R., et al. (2024). Chitosan alleviates symptoms of Parkinson's disease by reducing acetate levels, which decreases inflammation and promotes repair of the intestinal barrier and blood-brain barrier. Neural Regen. Res. doi:10.4103/nrr.nrr-d-23-01511
Wei, H., Gao, Z., Zheng, L., Zhang, C., Liu, Z., Yang, Y., et al. (2017). Protective effects of fucoidan on aβ25-35 and d-gal-induced neurotoxicity in PC12 cells and d-gal-induced cognitive dysfunction in mice. Mar. Drugs 15 (3), 77. doi:10.3390/md15030077
Wozniak, M., Bell, T., Dénes, Á., Falshaw, R., and Itzhaki, R. (2015). Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer's disease. Int. J. Biol. Macromol. 74, 530–540. doi:10.1016/j.ijbiomac.2015.01.003
Xing, M., Li, G., Liu, Y., Yang, L., Zhang, Y., Zhang, Y., et al. (2023). Fucoidan from Fucus vesiculosus prevents the loss of dopaminergic neurons by alleviating mitochondrial dysfunction through targeting ATP5F1a. Carbohydr. Polym. 303, 120470. doi:10.1016/j.carbpol.2022.120470
Yang, D., Wang, X., Zhang, L., Fang, Y., Zheng, Q., Liu, X., et al. (2022). Lipid metabolism and storage in neuroglia: role in brain development and neurodegenerative diseases. Cell Biosci. 12 (1), 106. doi:10.1186/s13578-022-00828-0
Yang, L., Wang, Y., Li, Z., Wu, X., Mei, J., and Zheng, G. (2023a). Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery: impact on insulin resistance and gut microbiota in obesity-related Alzheimer's disease. Carbohydr. Polym. 310, 120714. doi:10.1016/j.carbpol.2023.120714
Yang, L., Wang, Y., Zheng, G., Li, Z., and Mei, J. (2023b). Resveratrol-loaded selenium/chitosan nano-flowers alleviate glucolipid metabolism disorder-associated cognitive impairment in Alzheimer's disease. Int. J. Biol. Macromol. 239, 124316. doi:10.1016/j.ijbiomac.2023.124316
Yang, R., Chen, M., Zheng, J., Li, X., and Zhang, X. (2021). The role of heparin and glycocalyx in blood-brain barrier dysfunction. Front. Immunol. 12, 754141. doi:10.3389/fimmu.2021.754141
Yang, R., Zheng, Y., Wang, Q., and Zhao, L. (2018). Curcumin-loaded chitosan-bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer's disease. Nanoscale Res. Lett. 13 (1), 330. doi:10.1186/s11671-018-2759-z
Yang, W. N., Chen, P. W., and Huang, C. Y. (2017). Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of Crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 15 (6), 183. doi:10.3390/md15060183
Yu, Y., Shen, M., Song, Q., and Xie, J. (2018). Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr. Polym. 183, 91–101. doi:10.1016/j.carbpol.2017.12.009
Zahan, M. S., Hasan, A., Rahman, M. H., Meem, K. N., Moni, A., Hannan, M. A., et al. (2022). Protective effects of fucoidan against kidney diseases: pharmacological insights and future perspectives. Int. J. Biol. Macromol. 209 (Pt B), 2119–2129. doi:10.1016/j.ijbiomac.2022.04.192
Zeng, P., Li, J., Chen, Y., and Zhang, L. (2019). The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 163, 423–444. doi:10.1016/bs.pmbts.2019.03.003
Zhang, F., Lu, J., Zhang, J. G., and Xie, J. X. (2015). Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson's disease model in C57BL/6J mice. Neural Regen. Res. 10 (2), 308–313. doi:10.4103/1673-5374.152387
Zhang, F. L., He, Y., Zheng, Y., Zhang, W. J., Wang, Q., Jia, Y. J., et al. (2014). Therapeutic effects of fucoidan in 6-hydroxydopamine-lesioned rat model of Parkinson's disease: role of NADPH oxidase-1. CNS Neurosci. Ther. 20 (12), 1036–1044. doi:10.1111/cns.12340
Zhang, L., Hao, J., Zheng, Y., Su, R., Liao, Y., Gong, X., et al. (2018). Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of Parkinson's disease. Aging Dis. 9 (4), 590–604. doi:10.14336/ad.2017.0831
Zhang, L., Yang, S., Wong, L. R., Xie, H., and Ho, P. C. (2020a). In vitro and in vivo comparison of curcumin-encapsulated chitosan-coated poly(lactic-co-glycolic acid) nanoparticles and curcumin/hydroxypropyl-β-cyclodextrin inclusion complexes administered intranasally as therapeutic strategies for Alzheimer's disease. Mol. Pharm. 17 (11), 4256–4269. doi:10.1021/acs.molpharmaceut.0c00675
Zhang, T., Wu, X., Yuan, H., Huang, S., and Park, S. (2022). Mitigation of memory impairment with fermented fucoidan and λ-carrageenan supplementation through modulating the gut microbiota and their Metagenome function in hippocampal amyloid-β infused rats. Cells 11 (15), 2301. doi:10.3390/cells11152301
Zhang, Z., Wang, X., Pan, Y., Wang, G., and Mao, G. (2020b). The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 146, 725–729. doi:10.1016/j.ijbiomac.2019.09.243
Zhou, H., Dai, C., Cui, X., Zhang, T., Che, Y., Duan, K., et al. (2022). Immunomodulatory and antioxidant effects of Glycyrrhiza uralensis polysaccharide in Lohmann Brown chickens. Front. Vet. Sci. 9, 959449. doi:10.3389/fvets.2022.959449
Keywords: neurodegenerative diseases, polysaccharides, marine, fucoidan, chitosan
Citation: Zhu L, Ren Y, Zhang S, Guo Y, Zong J and Liu Y (2024) Marine-derived polysaccharides: the potential agents against neurodegenerative diseases. Front. Pharmacol. 15:1506789. doi: 10.3389/fphar.2024.1506789
Received: 06 October 2024; Accepted: 04 December 2024;
Published: 18 December 2024.
Edited by:
Peipei Wang, Shanghai Ocean University, ChinaReviewed by:
Mariia I. Shanaida, Ternopil State Medical University, UkraineCopyright © 2024 Zhu, Ren, Zhang, Guo, Zong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjuan Liu, bGl1eWluZ2p1YW44MjlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Lin Zhu1†
Lin Zhu1† Yunliang Guo
Yunliang Guo Yingjuan Liu
Yingjuan Liu