- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Introduction: Brodifacoum is a highly potent superwarfarin rodenticide that leads to coagulopathy. Although the effect of warfarin during pregnancy is well understood, reports on superwarfarin poisoning, like brodifacoum, during pregnancy are scarce.
Case Presentation: We report a case involving a woman with a singleton pregnancy who experienced sudden nasal hemorrhage accompanied by hematuria at 34 weeks and 3 days of gestation, with no apparent etiology. It was ultimately diagnosed as acquired coagulopathy resulting from brodifacoum poisoning. Fetal ultrasonography revealed significant intracranial hemorrhage, leading to intrauterine fetal demise and subsequent pregnancy termination. Following the correction of the patient’s coagulation profile through intravenous and oral administration of vitamin K1, tailored to the serum levels of brodifacoum, diligent monitoring confirmed that the patient was in stable condition.
Conclusion: The primary hazard associated with the ingestion of brodifacoum is hemorrhage, with clinical presentations varying from asymptomatic cases to overt bleeding, which may present as hematuria, epistaxis, menometrorrhagia, and intracranial hemorrhage. Therefore, the diagnosis and management of this type of poisoning pose significant challenges. Prompt recognition and ongoing care for pregnant individuals affected by rodenticide toxicity are crucial for optimizing maternal health outcomes.
Introduction
Superwarfarins, long-acting anticoagulant rodenticides (LAARs) derived from warfarin, are characterized by extended half-lives ranging from 24 to 120 days and enhanced potency in inducing coagulopathy compared to warfarin (Yaqoob et al., 2019). LAARs, like brodifacoum (BDF) and diphacinone-sodium, are common household hazards that can be ingested accidently or due to psychiatric disorders. In contrast to warfarin, LAARs demonstrate a high affinity for vitamin K epoxide reductase (VKOR), which is essential for the reactivation of vitamin K in the liver (King and Tran, 2015). The ingestion of LAARs results in the body’s inability to produce vitamin K-dependent coagulation factors, including factors II, VII, IX, and X, leading to an extension of prothrombin time (PT) and activated partial thromboplastin time (APTT).
The main risk of LAAR compound ingestion is bleeding, with symptoms ranging from asymptomatic to severe, including hematuria, epistaxis, menometrorrhagia, contusions, hemarthrosis, anemia, hemoptysis, and both retroperitoneal and intracranial hemorrhages. However, mucocutaneous bleeding sites are the most commonly reported bleeding sites, with hematuria being the most prevalent symptom (King and Tran, 2015). Studies shown that prothrombin time (PT) or international normalized ratio (INR) may be the most reliable screening test when conducted 48–72 h after exposure (Smolinske et al., 1989).
The diagnosis of LAAR poisoning depends on the careful selection and interpretation of laboratory tests. The levels of APTT, PT, and INR serve as initial indicators in the condition. If the results are disordered, the next step is to evaluate the activity levels of each individual factor, choosing between vitamin K-independent factors (typically FV) and vitamin K-dependent coagulation factors (FII, FVII, FIX, and FX). While these tests suggest vitamin K antagonism or deficiency, they do not confirm the presence of a specific agent. Only high-performance liquid chromatography (HPLC) can detect particular anticoagulant drugs. Blood or liver samples are mixed with a solvent and analyzed via HPLC (Vudathala et al., 2010).
While the impact of warfarin in pregnancy is well-documented, instances of superwarfarin toxicity, particularly from BDF, during gestation are limited. Warfarin, a common anticoagulant, is contraindicated in pregnancy due to its placental transfer and associated embryopathy risks, such as midface hypoplasia and limb deformities. Later use can lead to fetal intracranial hemorrhage and schizencephaly. In contrast, unfractionated heparin and low-molecular-weight heparin do not cross the placenta, making them the preferred anticoagulants during pregnancy (Bulletins, 2018; Kolettis and Craigo, 2018).
This case report details a woman with a singleton pregnancy who presented with sudden onset nasal hemorrhage and hematuria at 34 weeks and 3 days of gestation, which was linked to acquired coagulopathy resulting from BDF toxicity. Fetal ultrasound examination revealed significant intracranial hemorrhage, ultimately resulting in intrauterine fetal demise and the necessity for pregnancy termination. After the patient’s coagulation parameters were normalized through intravenous and oral administration of vitamin K1, guided by serum BDF concentrations, diligent monitoring confirmed the patient’s stable condition.
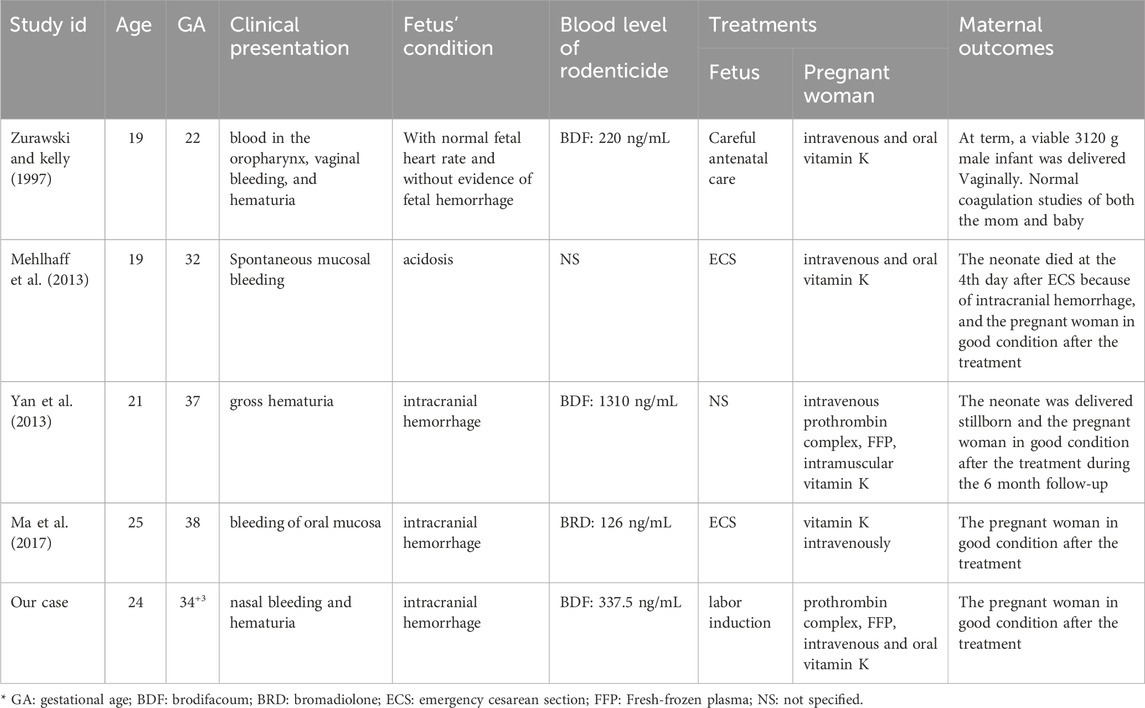
Additionally, a comprehensive literature review was conducted utilizing keywords such as BDF and pregnancy in both English and Chinese contexts (as shown in Table 1) (Zurawski and kelly, 1997; Mehlhaff et al., 2013; Yan et al., 2013; Ma et al., 2017). Currently, there have been a total of four reported cases of long-acting anticoagulant rodenticide poisoning during pregnancy that were similar to our case. Most of the affected pregnant women presented with hematuria, epistaxis, or mucocutaneous bleeding, and their fetuses succumbed to intracranial hemorrhage. Each of the pregnant women received the treatment of vitamin K, and subsequent follow-ups indicated a restoration of normal coagulation function. This study received approval from the Ethics Committee of West China Second University Hospital.
Case presentation
A 24 year-old female patient (gravida 1, para 0) with a singleton pregnancy conceived naturally was recently admitted to our hospital after experiencing sudden nasal hemorrhage and hematuria at 34 weeks and 3 days of gestation, with no identifiable causes. Laboratory results indicated a PT of 73.55 s, APTT of 42.61 s, and an INR of 6.13. Her social and family histories were unremarkable; however, her medical history noted an episode of unexpected nasal bleeding at 33 weeks and 4 days of gestation. The bleeding was resolved following the nasal application of bovine basic fibroblast growth factor gel.
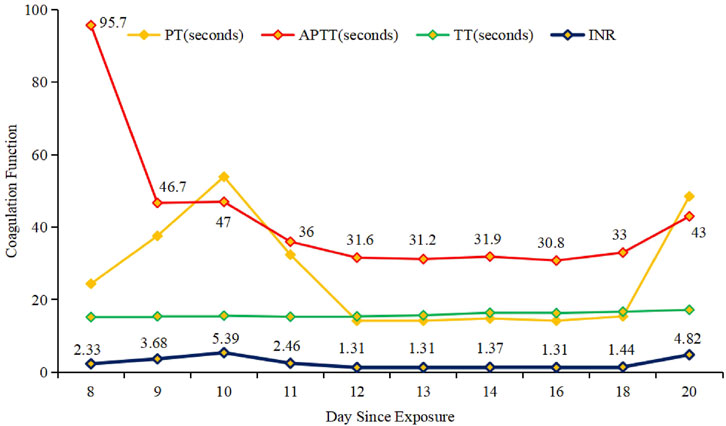
The recurrent hemorrhage was significant, exhibiting a greater volume than previous one, and nasal packing proved ineffective at this time. However, there were no discernible bleeding sites or petechiae in the subcutaneous tissue throughout the body, nor were there any indications of abdominal pain or vaginal bleeding. Laboratory analyses indicated reductions in the activities of coagulation factors II (3.5%), VII (8.7%), IX (1.4%), and X (1.2%), alongside a prolonged APTT of 95.7 s, while factors V, VIII, and XI were found to be within the normal range. Imaging studies, including urinary ultrasound, echocardiogram, chest ultrasound, and liver ultrasound, showed no apparent abnormalities in the patient. Additionally, fetal heart monitoring demonstrated a stable baseline fetal heart rate with minimal variability and deceleration, while fetal ultrasonography revealed evidence of intracranial hemorrhage. The patient’s unexplained bleeding posed a diagnostic dilemma, necessitating the implementation of blood component therapy, including fresh-frozen plasma (FFP), prothrombin complex concentrate (PCC), hemostatic agents, and vitamin K. Unluckily, the patient and her family opted to forgo further intervention for the fetus, citing the mother’s critical health status and financial constraints as primary factors in their decision.
Due to the patient’s critical condition, a series of coagulation tests were conducted by a multidisciplinary team (MDT) including obstetricians, hematologists, and pharmacists. The examinations revealed normal levels of fibrinogen, D-dimer, fibrin degradation products (FDP), von Willebrand factor, lupus anticoagulant, and antithrombin. Serological tests for antinuclear antibodies, anti-dsDNA antibodies, and anticardiolipin antibodies were negative, as was the antiglobulin (Coombs') test. The introduction of normal plasma in a 1:1 ratio corrected the coagulation abnormalities (APTT test), suggesting a coagulation factor deficiency. We hypothesized a potentially life-threatening coagulation disorder linked to deficiencies in vitamin K–dependent proteins. The differential diagnosis at this stage includes: (1) vitamin K deficiency; (2) hereditary deficiency of vitamin K–dependent carboxylase or vitamin K epoxide reductase; or (3) accidental or covert ingestion of a vitamin K antagonist, such as warfarin, phenprocoumon, or a potent rodenticide (“superwarfarin”).
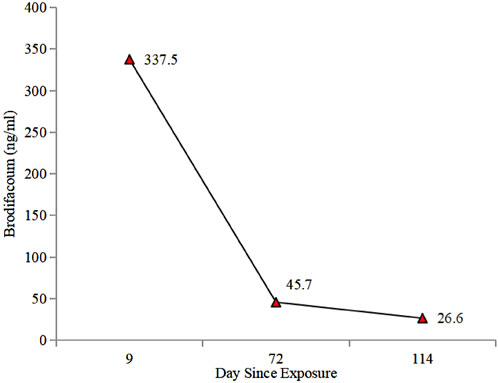
Ultimately, the patient’s blood tested positive for BDF at 337.5 ng/mL. A detailed history revealed unintentional rodenticide exposure 2 days prior to symptom onset. If BDF poisoning is suspected, prompt coagulopathy correction with FFP and vitamin K is crucial for effective maternal resuscitation (Gunja et al., 2011). Thus, FFP and vitamin K1 were administered to reverse the coagulopathy status (Figure 1). However, it is important to highlight that currently, there are no established protocols regarding the administration and dosage of vitamin K1 in cases of poisoning. In this context, the decision to modify treatment approaches and the specific dosing schedule were informed by the clinical experience of hematologist, as well as the blood levels of BDF and the patient’s coagulation status.
At 34 weeks and 5 days of gestation (the 10th day of BDF exposure), the fetal ultrasound indicated significant intracranial hemorrhage, ultimately leading to intrauterine fetal death. Following the correction of the active coagulation function, the patient exhibited no apparent bleeding tendency by the 13th day of BDF exposure. After consultations with obstetricians and hematologists, labor induction was initiated. On the 14th day of BDF exposure, the patient delivered a stillborn fetus. We continuously monitored the patient’s coagulation function in relation to the duration of BDF exposure (Figure 1). Additionally, we constructed a timeline detailing the patient’s treatments, the concentration of BDF in the maternal bloodstream, and her health status during the follow-up (Figures 2, 3).
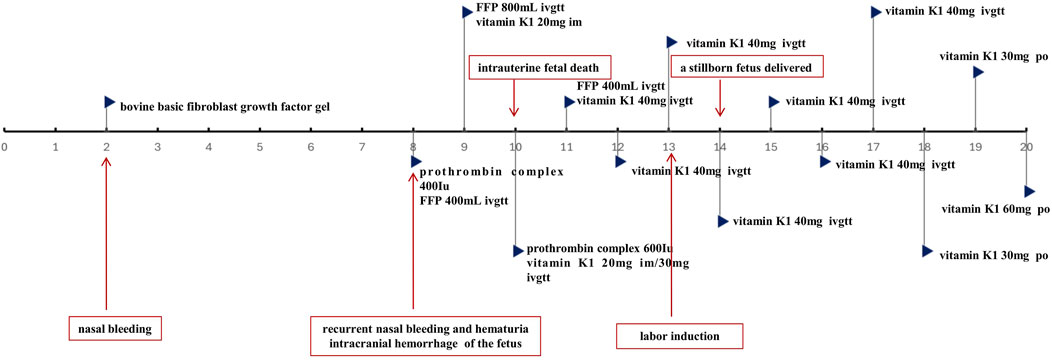
Figure 2. Timeline of the treatments and key events. *FFP:fresh-frozen plasma; im: intramuscular injection; ivgtt: intravenous drip; po: per os.
Discussion
BDF, a long-acting vitamin K antagonist rodenticide, is associated with intentional ingestions and contamination in synthetic cannabinoids, leading to prolonged PT and APTT (Moritz et al., 2018). The evidence of extended PT, APTT, and INR, along with significantly reduced vitamin K-dependent coagulation factors, complicates differential diagnosis between hemophilia and acquired hemophilia (AH). Hemophilia A and B are congenital disorders due to deficiencies in clotting factors VIII and IX, respectively, with female cases being rare due to the recessive X-linked inheritance. Women are often heterozygous carriers, exhibiting reduced clotting factor levels and mild symptoms. A family history of spontaneous bleeding aids in diagnosing hemophilia (Berntorp et al., 2021). AH, a rare condition caused by autoantibodies against endogenous factor VIII, is typically identified postpartum. IgG antibodies can cross the placenta, heightening the neonate’s hemorrhage risk. Corticosteroids are the first-line treatment for pregnancy-related AH (Berntorp et al., 2021). However, the patient in our case denied any familial or genetic history.
Pregnant women with spontaneous bleeding should be assessed for coagulopathy through history and laboratory tests, including complete blood count, PT, APTT, fibrinogen, and metabolic panel. Differential diagnoses include obstetric coagulopathies like disseminated intravascular coagulation (DIC) with abruption and fetal demise, fulminant liver failure, hereditary deficiencies, and drug effects. Abnormalities in both intrinsic and extrinsic pathways indicate a common pathway deficiency or inhibition of vitamin K-dependent factors. Therefore, in cases of severe painless hematuria, after excluding other conditions, impaired coagulation possibly due to poisoning should be considered.
In our case, the woman’s bleeding symptoms ranged from nasal bleeding to hematuria, resembling pregnancy complications due to DIC. However, her hematuria persisted for several days, which DIC could not explain. Additionally, routine blood tests, liver function tests, fibrinogen levels, fibrinogen degradation product (FDP), and D-dimer were normal, consistent with previous LAAR poisoning cases (Yan et al., 2013). Clinically significant LAAR poisoning is often challenging to identify due to: 1) its prevalence in adults who intentionally or covertly expose themselves; 2) delayed symptom onset post-exposure; and 3) variability in symptoms among individuals.
Previous studies indicate that the primary manifestation of BDF poisoning is spontaneous bleeding, including nosebleeds, hematuria, and mucosal bleeding (Mehlhaff et al., 2013). A review of 174 LAAR exposure cases identified hematuria, gingival bleeding, epistaxis, and gastrointestinal bleeding as the top four hemorrhagic features. Notably, intracranial hemorrhage was the leading cause of death, with BDF being the most common agent in LAAR exposures (King and Tran, 2015).
According to earlier reports, maternal-fetal outcomes from BDF poisoning vary from healthy fetuses to intracranial hemorrhaging or abortion, depending on pregnancy stage (Lipton and Klass, 1984; Zurawski and Kelly, 1997; Tosounidou and Gordon, 2019; Rubinstein et al., 2018). There is no specific information on whether BDF crosses the placenta or is present in breast milk. We have summarized the clinical manifestations and maternal outcomes of reported cases, including our case of LAARs poisoning during pregnancy (Table 1). The first case (Zurawski and Kelly, 1997) in 1997 involved a 19 year-old woman at 22 weeks gestation, experienced oral mucosa bleeding, vaginal bleeding, and hematuria, yet delivered vaginally at term with normal coagulation studies for both mother and baby. The absence of fetal hemorrhage led investigators to hypothesize that BDF might not cross the placenta. However, subsequent cases showed all fetuses had intracranial hemorrhage and died, contradicting the initial hypothesis. Possible explanations for the lack of fetal hemorrhage in the first case include: 1) BDF crosses the placenta but its effects are mitigated by maternal vitamin K treatment; 2) BDF may have teratogenic effects that are influenced by individual fetal factors.
LAARs tend to accumulate in the liver, exhibiting prolonged half-lives due to increased lipid solubility and slow metabolism in specific areas. Accordingly, vitamin K1 maintenance treatment is often necessary post-LAAR poisoning, as premature cessation can result in abnormal coagulation and bleeding, even with normal coagulation tests. In our case, we reduced the vitamin K1 dosage on the 18th day of exposure, leading to evident coagulation abnormalities in the patient.
Previous clinical experiences indicate that BDF poisoning is primarily managed with vitamin K1, though treatment duration varies based on blood concentration and coagulation status, complicating management. Vitamin K1 acts as a specific antidote for LAAR poisoning, rapidly addressing intrinsic and extrinsic coagulation pathway abnormalities, with normalization of coagulation parameters (PT, APTT, INR) typically occurring within 2, 3 days post-treatment (Tsutaoka et al., 2003).
However, vitamin K1 may also induce thrombotic complications, such as a popliteal deep vein thrombosis (Franco et al., 2013) and intracranial venous thrombosis (Papin et al., 2007), likely due to early depletion of anticoagulant proteins C and S (Lipton and Klass, 1984). Other rare adverse events include angioedema with airway compromise, diffuse alveolar hemorrhage, hemarthrosis, miscarriage, and bowel obstruction due to bowel wall hematoma (Barnett et al., 1992).
In non-pregnant cases, while vitamin K1 can reverse LAAR-induced coagulopathy, this process requires at least 12 h (Haesloop et al., 2016). Patients experiencing active bleeding may benefit from PCC (II, VII, IX, and X) infusion; a case study reported normalization of INR within 30 min following 2000 U of PCC in BDF toxicity patients (Doyle et al., 2018). Another study noted rapid and sustained coagulopathy reversal in a LAAR poisoning patient after receiving 45 IU/kg PCC.
The timing for discontinuing vitamin K1 therapy in LAAR poisoning remains contentious, Experts recommend using HPLC to track the serum levels to determine the course of treatment. Furthermore, the elimination kinetic can be plotted and a treatment course determined using the BDF concentration in the peripheral blood. However, in clinical practice, treatment has often been discontinued arbitrarily, with subsequent monitoring of the coagulation profile. A retrospective study of 21 LAAR poisoning patients analyzed serum rodenticide levels before and after vitamin K1 treatment, revealing that those with concentrations >10 ng/mL were at higher risk for re-bleeding (Rubinstein et al., 2018). For BDF poisoning, it is suggested that vitamin K1 can be safely discontinued at serum concentrations <10 ng/mL (Bruno et al., 2000). Whereas, low circulating BDF levels (<10 ng/mL) can still be detected in cases, the harmful effects of these concentrations on fetal development remain unclear. Future multicenter, randomized controlled studies with larger sample sizes are needed to further validate our findings regarding treatment duration, dosing, and withdrawal timing.
A report revealed the development of a straightforward fluorescence micro-plate immunoassay utilizing an in situ fluorogenic reaction for detecting bromadiolone (BRD) and BDF in food and biological matrices, demonstrating significant potential for food safety monitoring and BRD/BRF poisoning diagnostics (Li et al., 2024).
Blood perfusion theoretically could eliminate LAAR due to its large molecular weight and high fat solubility; however, further research is needed to assess its applications and therapeutic benefits. As a cytochrome P450 inducer, phenobarbital enhances liver microsome metabolism and the detoxification capacity of liver enzymes, yet its effect on BDF metabolism or clearance remains unclear (Rubinstein et al., 2019).
Acute hemorrhagic symptoms typically necessitate intravenous vitamin K1 doses exceeding 50–100 mg, while chronic maintenance often involves 100 mg of oral vitamin K1 daily to manage coagulopathy, with treatment durations averaging 168 days. Adjunctive hemostatic therapies, including recombinant factor VIIa and PCC, have been documented, and phenobarbital has been employed to accelerate LAAR metabolism (King and Tran, 2015).
It is clear that appropriate blood products (FFP/red blood cells) and intravenous or oral vitamin K1 are essential treatments for BDF-induced coagulopathy and bleeding. Furthermore, the administration and dosage of vitamin K1 must be tailored to the drug concentration and coagulation status. Post-discharge, we meticulously monitored BDF levels in peripheral blood (Figure 2) and adjusted the treatment plan according to the hematologist’s guidance.
In summary, two primary challenges in managing long-acting anticoagulant rodenticides are diagnosis difficulty and coagulopathy correction, particularly in pregnant women. Treatment for these cases may involve: differential diagnosis, bleeding control (coagulation correction), vitamin K antagonist therapy, long-term follow-up, and psychosocial evaluation. Severe bleeding in previously healthy patients or laboratory signs indicating high bleeding risk poses diagnostic challenges. Rapid assessment of prolonged PT and PTT, along with specific vitamin K-dependent coagulation deficiencies, significantly narrows the differential diagnosis. Given the life-threatening nature of this condition and its resolution with proper treatment, swift and precise diagnosis is essential.
It is important to recognize that our study is constrained by the limited availability of BDF concentrations in the placenta, amniotic fluid, and the fetus, which could not be collected following fetal death attributed to intracranial hemorrhage. Thus, it remains uncertain whether the fetal death resulting from intracranial hemorrhage is linked to acquired coagulation dysfunction induced by BDF. This further substantiates the notion that BDF may influence fetal coagulation function via the placenta.
Conclusion
Bleeding is the major risk following ingestion of BDF, and the clinical manifestations range from being asymptomatic to active bleeding manifested as hematuria, epistaxis, menometrorrhagia, and intracranial hemorrhage. Therefore, diagnosis and treatment of this intoxication remain a challenge. Early identification and long-term management of pregnant women with rodenticide poisoning play a vital role in the maternal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YJ: Data curation, Writing–original draft, Investigation. YZ: Data curation, Supervision, Writing–review and editing. GH: Data curation, Supervision, Writing–review and editing. CD: Data curation, Supervision, Writing–review and editing. HY: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the doctors and staff who were involved in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barnett, V. T., Bergmann, F., Humphrey, H., and Chediak, J. (1992). Diffuse alveolar hemorrhage secondary to superwarfarin ingestion. Chest 102 (4), 1301–1302. doi:10.1378/chest.102.4.1301
Berntorp, E., Fischer, K., Hart, D. P., Mancuso, M. E., Stephensen, D., Shapiro, A. D., et al. (2021). Haemophilia. Nat. Rev. Dis. Primer 7 (1), 45. doi:10.1038/s41572-021-00278-x
Bruno, G. R., Howland, M. A., McMeeking, A., and Hoffman, R. S. (2000). Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann. Emerg. Med. 36, 262–267. doi:10.1067/mem.2000.108317
Bulletins—Obstetrics ACoOaGCoP (2018). ACOG practice bulletin No. 196: thromboembolism in pregnancy. Obstet. Gynecol. 132 (1), e1–e17. doi:10.1097/AOG.0000000000002706
Doyle, R. J., Elfessi, Z., and Kolman, K. (2018). Fixed dose 4-factor prothrombin complex concentrate for bleeding caused by long acting anticoagulant rodenticides. Am. J. Emerg. Med. 36 (10), 1922.e3–1922. doi:10.1016/j.ajem.2018.06.040
Franco, D., Everett, G., and Manoucheri, M. (2013). I smell a rat: a case report and literature review of paradoxical thrombosis and hemorrhage in a patient with brodifacoum toxicity. Blood Coagulation and Fibrinolysis 24 (2), 202–204. doi:10.1097/MBC.0b013e328358e959
Gunja, N., Coggins, A., and Bidny, S. (2011). Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin. Toxicol. (Phila) 49 (5), 385–390. doi:10.3109/15563650.2011.587126
Haesloop, O., Tillick, A., Nichol, G., and Strote, J. (2016). Superwarfarin ingestion treated successfully with prothrombin complex concentrate. Am. J. Emerg. Med. 234 (1), 116.e1–e2. doi:10.1016/j.ajem.2015.05.033
King, N., and Tran, M. H. (2015). Long-acting anticoagulant rodenticide (superwarfarin) poisoning: a review of its historical development, epidemiology, and clinical management. Transfus. Med. Rev. 29 (4), 250–258. doi:10.1016/j.tmrv.2015.06.002
Kolettis, D., and Craigo, S. (2018). Thromboprophylaxis in pregnancy. Obstet. Gynecol. Clin. North Am. 45 (2), 389–402. doi:10.1016/j.ogc.2018.01.007
Li, H., Chen, J., Xu, W., Huang, B., Peng, C., Cai, H., et al. (2024). A facile fluorescence microplate immunoassay based on an in situ fluorogenic reaction for the detection of two highly toxic anticoagulant rodenticides in food and biological matrix. Food Chem. 437 (Pt 1), 137792. doi:10.1016/j.foodchem.2023.137792
Lipton, R. A., and Klass, E. M. (1984). Human ingestion of a “superwarfarin” rodenticide resulting in a prolonged anticoagulant effect. JAMA 252 (21), 3004–3005. doi:10.1001/jama.1984.03350210052030
Ma, M., Zhang, M., Tang, X., and Li, Z. (2017). Massive neonatal intracranial hemorrhage caused by bromadiolone: a case report. Med. Baltim. 96 (45), e8506. doi:10.1097/MD.0000000000008506
Mehlhaff, K. M., Baxter, C. C., Rudinsky, K., and McKenna, D. S. (2013). Lethal neonatal coagulopathy after maternal ingestion of a superwarfarin. Obstet. Gynecol. 122 (Pt 2), 500–502. doi:10.1097/AOG.0b013e31829267c4
Moritz, E., Austin, C., Wahl, M., DesLauriers, C., Navon, L., Walblay, K., et al. (2018). Notes from the field: outbreak of severe illness linked to the vitamin K antagonist brodifacoum and use of synthetic cannabinoids-Illinois, March–April 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 607–608. doi:10.15585/mmwr.mm6721a4
Papin, F., Clarot, F., Vicomte, C., Gaulier, J. M., Daubin, C., Chapon, F., et al. (2007). Lethal paradoxical cerebral vein thrombosis due to suspicious anticoagulant rodenticide intoxication with chlorophacinone. Forensic Sci. Int. 166 (2-3), 85–90. doi:10.1016/j.forsciint.2006.04.003
Rubinstein, I., van Breemen, R., Nosal, D. G., Weinberg, G., Hershow, R. C., and Feinstein, D. L. (2019). Should cytochrome P450 inducers be used to accelerate clearance of brodifacoum from poisoned patients? Drugs R. D. 19 (1), 67–71. doi:10.1007/s40268-019-0261-4
Rubinstein, I., Weinberg, G., van Breemen, R., Hershow, R. C., and Feinstein, D. L. (2018). Treatment for long acting anticoagulant rodenticide poisoning-beyond INR monitoring? Toxicol. Commun. 2 (1), 59–61. doi:10.1080/24734306.2018.1500152
Smolinske, S. C., Scherger, D. L., Kearns, P. S., Wruk, K. M., Kulig, K. W., and Rumack, B. H. (1989). Superwarfarin poisoning in children: a prospective study. Pediatrics 84 (3), 490–494. doi:10.1542/peds.84.3.490
Tosounidou, S., and Gordon, C. (2019). Medications in pregnancy and breastfeeding. Best. Pract. Res. Clin. Obstet. Gynaecol. 64, 68–76. doi:10.1016/j.bpobgyn.2019.10.007
Tsutaoka, B. T., Miller, M., Fung, S. M., Patel, M. M., and Olson, K. R. (2003). Superwarfarin and glass ingestion with prolonged coagulopathy requiring high-dose vitamin K1 therapy. Pharmacotherapy 23, 1186–1189. doi:10.1592/phco.23.10.1186.32755
Vudathala, D., Cummings, M., and Murphy, L. (2010). Analysis of multiple anticoagulant rodenticides in animal blood and liver tissue using principles of QuEChERS method. J. Anal. Toxicol. 34 (5), 273–279. doi:10.1093/jat/34.5.273
Yan, J., Shi, Y., Sun, C., and Yang, H. (2013). Vitamin K treatment of brodifacoum poisoning in a pregnant woman. Int. J. Gynaecol. Obstet. 122 (2), 162–163. doi:10.1016/j.ijgo.2013.03.008
Yaqoob, M., Feinstein, D. L., and Rubinstein, I. (2019). Pregnancy in women with life-threatening poisoning with long-acting anticoagulant rodenticides. Mayo Clin. Proc. 94 (8), 1646–1647. doi:10.1016/j.mayocp.2019.05.007
Keywords: brodifacoum, long-acting anticoagulant rodenticides, vitamin K, pregnancy, coagulation dysfunction, case report
Citation: Jia Y, Zhan Y, Huang G, Deng C and Yu H (2024) Acute long-acting anticoagulant rodenticide poisoning in pregnancy: a case report . Front. Pharmacol. 15:1502596. doi: 10.3389/fphar.2024.1502596
Received: 27 September 2024; Accepted: 12 November 2024;
Published: 27 November 2024.
Edited by:
Margherita Neri, University of Ferrara, ItalyReviewed by:
Catherine M. T. Sherwin, University of Western Australia, AustraliaTayyab Ali, University of Agriculture, Pakistan
Copyright © 2024 Jia, Zhan, Huang, Deng and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Yu, ZmFuank0MjJAMTYzLmNvbQ==
 Yanxi Jia
Yanxi Jia Yongchi Zhan
Yongchi Zhan Guiqiong Huang1,2
Guiqiong Huang1,2 Haiyan Yu
Haiyan Yu