- 1Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
- 3Department of Urological Organ Transplantation, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 4International Research Center for Precision Medicine, Transformative Technology and Software Services, Changsha, Hunan, China
Background: The concurrent administration of tacrolimus and voriconazole in kidney transplant recipients can lead to drug interactions, potentially resulting in severe adverse reactions. This study aimed to establish a robust population pharmacokinetic model to explore the interaction between tacrolimus and voriconazole in greater depth.
Methods: Tacrolimus blood samples and laboratory data were prospectively collected from eligible patients enrolled between April 2023 and April 2024, following predefined inclusion and exclusion criteria. Using Phoenix (version 8.1), a pharmacokinetic prediction model was developed. Model performance was assessed using model fitting plots, bootstrap analysis, and visual predictive checks (VPC).
Results: This study ultimately included 51 eligible patients, with a total of 281 blood samples collected. Analysis revealed a significant negative correlation between voriconazole concentration (Cvrc) and tacrolimus volume of clearance rate (CL), a significant positive correlation between platelets (PLT) and tacrolimus clearance (CL), and a significant negative correlation between blood cells (RBC) and tacrolimus clearance (CL).
Conclusion: This study successfully established a population pharmacokinetic model for renal transplant patients concurrently receiving tacrolimus and voriconazole. The model demonstrated good predictive performance and offers valuable insights to clinicians for optimizing tacrolimus dosing in this patient population.
1 Introduction
Kidney transplantation significantly improves survival rates for patients with kidney disease (Augustine, 2018; Voora and Adey, 2019). However, effective management is crucial, particularly in immunotherapy and infection control. Voriconazole is commonly used in combination with tacrolimus to treat invasive fungal infections, but this can lead to pharmacokinetic (PK) drug interactions and adverse effects (Gong et al., 2023; Maertens et al., 2016). Because Tacrolimus is mainly metabolized by CYP3A4 and CYP3A5 (Coto et al., 2011; Choi et al., 2017; Staatz et al., 2010), while voriconazole is mainly metabolized by CYP3A4 and CYP2C19. Voriconazole is also a strong inhibitor of CYP3A4/5 (Jeong et al., 2009; Kluwe et al., 2023). The Vfend instruction book recommended a decreased initial dose of tacrolimus during voriconazole co-therapy. The results varied widely and were not suitable for clinical use. Lloberas et al. (2023) found that PPK-based Tac dosing had significant advantages over classic labeled dosing based on body weight when initiating Tac prescription, However, most studies only analyze voriconazole as an influencing factor, which can only prove that the use of voriconazole is a key influencing factor, which has great limitations (Vanhove et al., 2017; Ota et al., 2019; Chen et al., 2022). In another study, Burrows et al. (2023), reviewed 8 transplant recipients (5 lung, 2 redo lung, 1 heart) treated concurrently with flucloxacillin, voriconazole, and tacrolimus. A significant three-way interaction was found between flucloxacillin, voriconazole, and tacrolimus, but no further insight was gained regarding the effects of voriconazole. In addition, A previous retrospective study suggested that voriconazole concentrations could be used to optimize tacrolimus dosing in lung transplant recipients, offering an important perspective (Chen et al., 2022). However, this study was limited by its exclusive reliance on trough concentration measurements and the inherent constraints of retrospective data analysis. Notably, there remains a lack of research focusing on renal transplant patients. To address this gap, our study employed a prospective data collection approach combined with a sparse sampling design, allowing us to randomly capture blood concentration data that thoroughly represented both absorption and elimination phases. This methodology enabled a robust and high-resolution pharmacokinetic analysis, providing a more reliable foundation for optimizing voriconazole therapy in renal transplant patients.
2 Methods
2.1 Study subjects
Study participants consisted of renal transplant patients admitted to the Second Xiangya Hospital of Central South University between April 2023 and September 2024. This research received approval from the hospital ethics committee [LYEC2024-K0106]. This is a non-interventional clinical study, all patients signed informed consent forms, and patient information is strictly confidential.
2.2 Inclusion and exclusion criteria
The study employed the following inclusion criteria: (a) Patients undergoing kidney transplantation at the Renal Transplantation Department of Xiangya Second Hospital; (b)Patients aged 18 years or older; (c) Patients administered a triple immunosuppressive regimen comprising tacrolimus, mycophenolate mofetil, and glucocorticoids, alongside oral voriconazole; (d) At least five tacrolimus concentration values were collected for each patient, etc. (e) Patients at least one-year post-kidney transplantation. The exclusion criteria were as follows: (a) Patients concurrently prescribed cyclosporine, rapamycin, or other immunosuppressants; (b) Patients receiving rifampicin, isoniazid, phenytoin, or other potent CYP450 inducers or inhibitors; (c) Patients with incomplete or missing pertinent experimental data, etc.
2.3 Date collection and analysis
2.3.1 Blood sample collection and monitoring for tacrolimus and voriconazole
In this study, both drugs were administered orally. Tacrolimus was provided in an immediate-release (IR) formulation, typically dosed twice daily. The doses of both tacrolimus and voriconazole were adjusted by clinicians based on therapeutic drug monitoring (TDM), established clinical guidelines, and patient-specific factors.
A total of 51 patients were enrolled in the study and randomly assigned to three groups using a sparse sampling design. Each group followed a predefined sampling schedule: Group 1 collected samples at 0, 0.5, and 1 h; Group 2 collected samples at 2, 4, and 6 h; and Group 3 collected samples at 8 h and 30 min before the next dose.
Tacrolimus blood concentration was measured using a chemiluminescent particle immunoassay with the ARCHITECT Tacrolimus Kit IL77-35. Detailed information on standard operating procedures, assay methodology, and stability data is provided in the Prograf assay kit instructions (IL77-G08363R10-B1L77C) (Laboratories, 2009).
Voriconazole plasma concentration was determined through a fully automated two-dimensional liquid chromatography system (2D-HPLC, Changsha Demeter Instrument Co., Ltd.). Chromatographic conditions included: Column A (FRO C18, 5 μm, 100 mm × 3.0 mm, ANAX) with a mobile phase of 20 mmol/L ammonium acetate-acetonitrile (48:52, V/V) at a flow rate of 1.0 mL/min, and Column B (ASTON HD C18, 150 mm × 4.6 mm, 5 μm, ANAX) with a mobile phase of 40 mmol/L ammonium acetate-acetonitrile (85:15, V/V) at a flow rate of 1.2 mL/min. The detection wavelength was set at 273 nm, the column temperature maintained at 45°C, and the injection volume set to 200 μL. The method exhibited a linear range of 0.35–11.26 μg/mL. All laboratories underwent annual quality assessments conducted by the National Health Commission Clinical Testing Center to ensure compliance with quality standards.
2.3.2 Pharmacokinetic data analysis and model evaluation
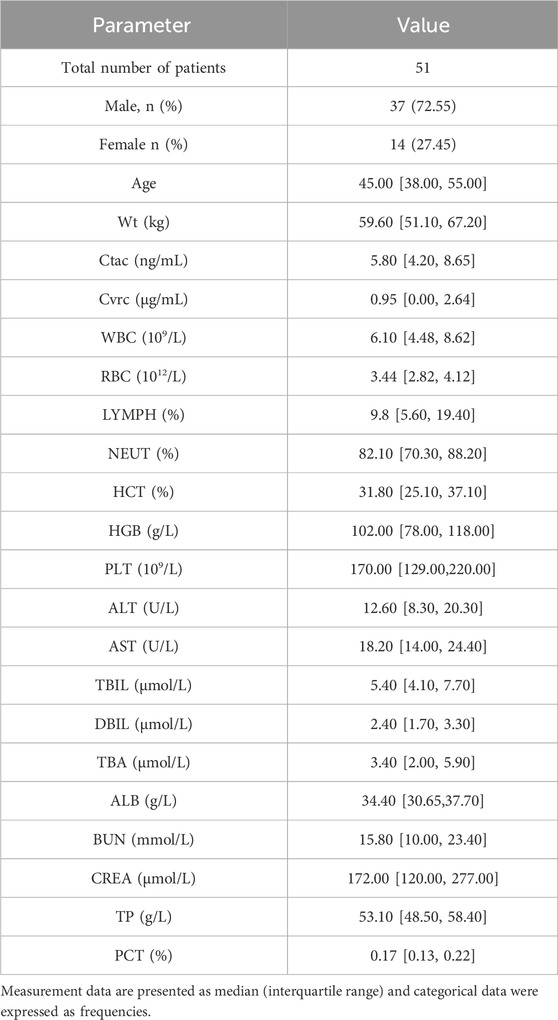
Laboratory data including sex, weight, age, albumin, hematocrit, creatinine, aspartate aminotransferase, C-reactive protein, and total bilirubin were collected in this study.Data analysis was performed using Phoenix NLME pharmacokinetic software (version 8.1). For the description of baseline characteristics, mean and standard deviation were used to describe normally distributed continuous variables, median and interquartile range were used to describe non-normally distributed continuous variables, and frequency and percentage were used for categorical variables.
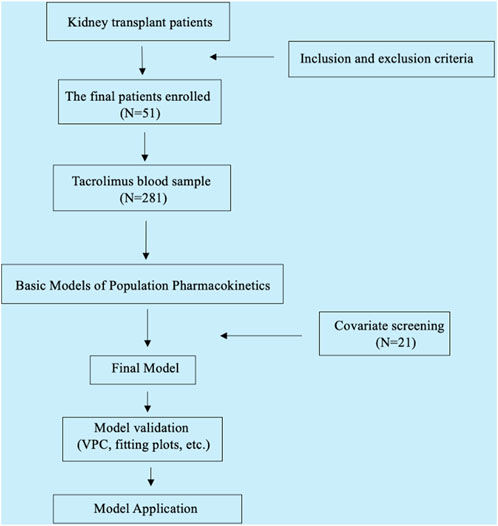
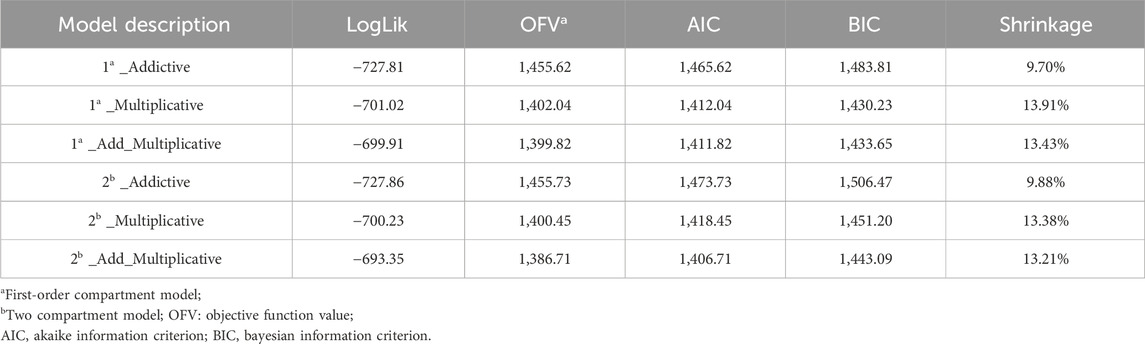
In the process of establishing the structural model, both one-compartment and two-compartment models were evaluated. Key parameters such as LogLik, AIC, OFV, and Shrinkage were compared between the models, alongside the analysis of the fitting diagram (Supplementary Figure SA). Based on these comparisons, the one-compartment Add-Multiplicative model for oral absorption and elimination was selected as the most suitable. The final covariate model was selected by a stepwise method based on the least squares principle, including forward inclusion (p < 0.05) and backward elimination (p < 0.01). The change in the model objective function value (ΔOFV) after the inclusion of covariates was evaluated. The final model was evaluated using methods such as goodness-of-fit plots, bootstrap analysis, and visual predictive tests (VPC). The research flow chart is shown in Figure 1.
3 Results
3.1 Patient demographics and characteristics
A total of 51 patients were ultimately enrolled in this study, comprising 38 males. The study gathered a total of 281 tacrolimus concentration points, with a median concentration of 5.8 ng/mL. Detailed demographic data can be found in Table 1.
3.2 Establishment of population pharmacokinetic model
After thorough consideration of various models encompassing one-compartment/two-compartment additive, multiplicative, and mixture of oral absorption and elimination, the Add-Multiplicative model emerged as the chosen basic model, with AIC = 1,411.82, BIC = 1,433.65 (Table 2). For comparison of model fitting plots, please refer to Supplementary Figure SA.
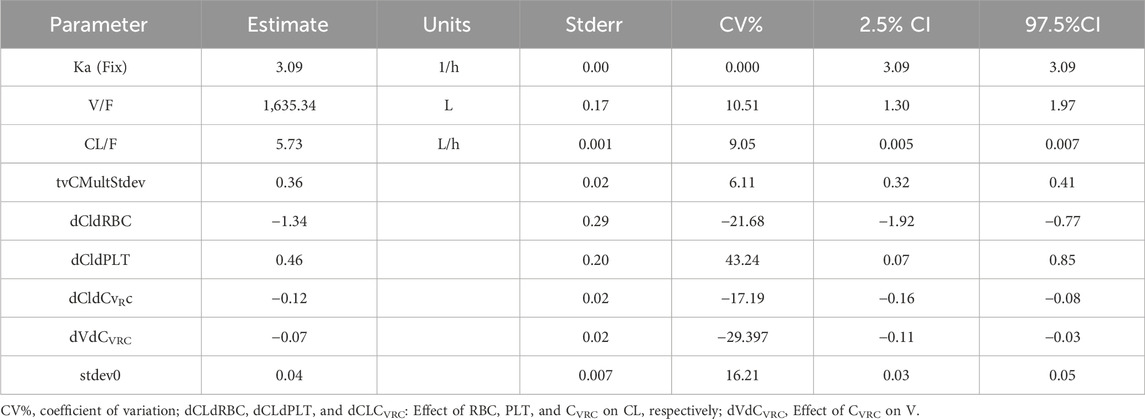
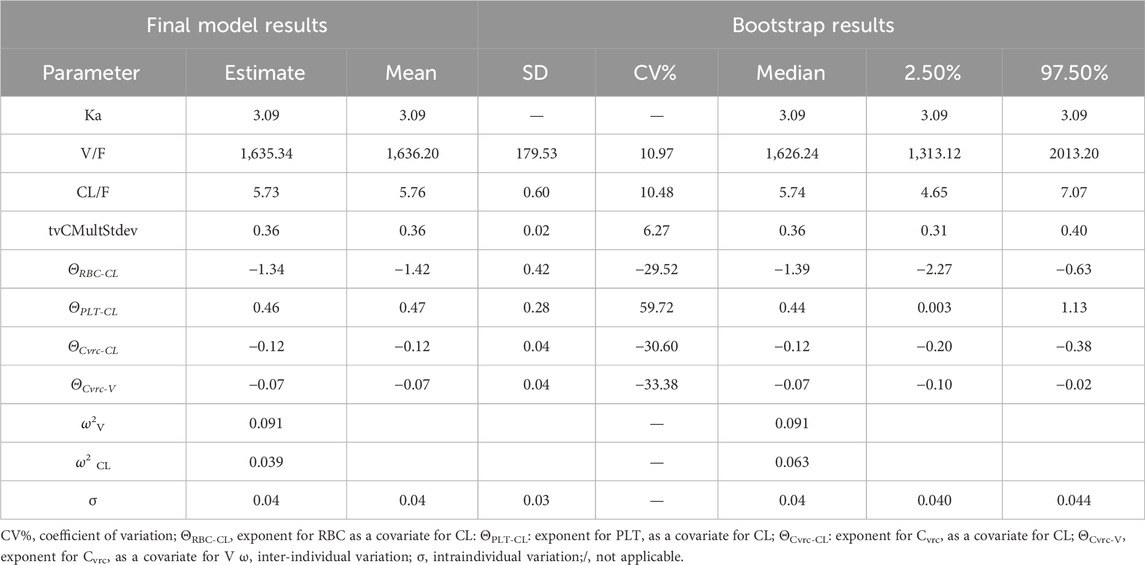
After screening all 21 covariates, it was found that Cvrc was significantly negatively correlated with CL; PLT was significantly positively correlated with CL, and RBC was significantly negatively correlated with CL (Supplementary Table SA). The final equation obtained by incorporating relevant variables was Ka = 3.09; V/F = 1,635.34, CL/F = 5.73 (Table 3).
The formulas for V and CL were as follows:
3.3 Validation of the population pharmacokinetic model
3.3.1 Bootstrap validation and model fit plots
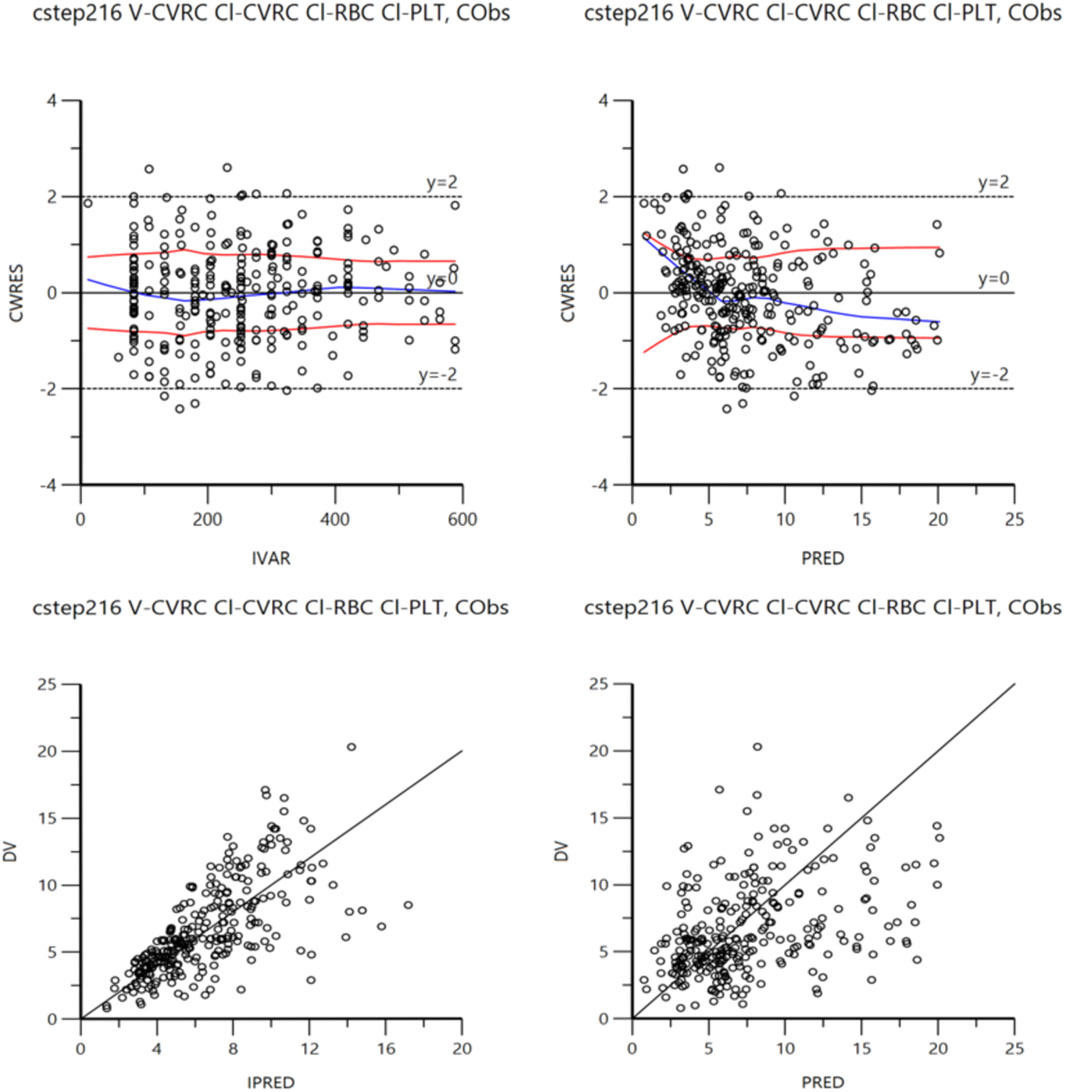
The accuracy of the final model was examined by bootstrap validation (simulation 1,500 times), and it was found that key data such as PK parameters were within a reasonable range (Table 4). In addition, the final model fitting graph had good convergence (Figure 2).
3.3.2 Visual forecast checking
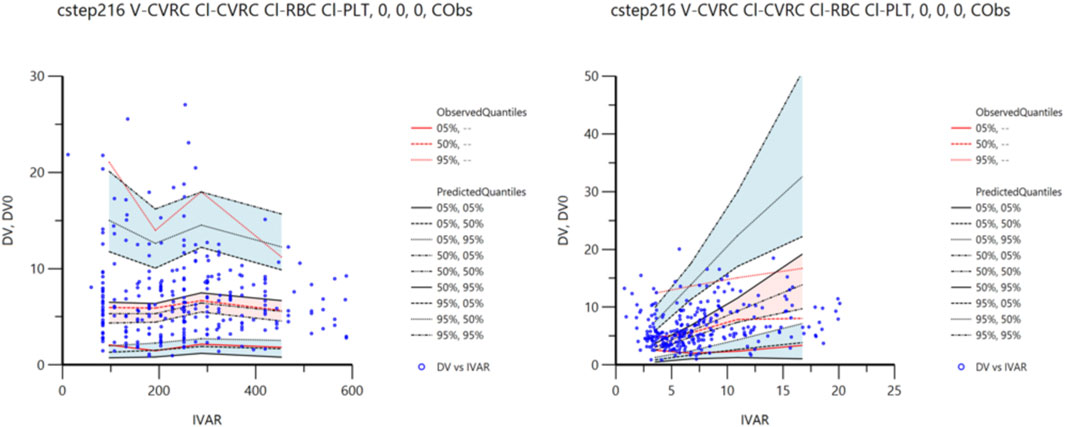
The VPC method was used for 1,500 simulation simulations to verify the final model. The VPC diagnosis figures are shown in Figure 3. As can be seen from the figure, the 5th, 50th, and 95th quantiles of all the observed values fell within the 90%CI of the corresponding predicted values, indicating that the predicted values were in high agreement with the observed values, indicating that the prediction performance of the model was good.
4 Discussion
In this study, we developed a single-compartment model of oral first-level elimination and described a PPK model of nvasive fungal infections (IFIs) in renal transplant recipients treated with tacrolimus plus voriconazole. Specifically, a median tacrolimus concentration of 5.80 [4.20, 8.65] ng/mL was observed, which is consistent with the European Consensus Conference recommendations (Wallemacq et al., 2009). These guidelines recommend maintaining tacrolimus (FK506) whole blood concentrations between 5 and 10 ng/mL during the first 12 months after transplantation. Furthermore, the median voriconazole concentration was 0.95 [0.00, 2.64] ng/mL, which was notably higher than the median concentration of 0.00 [0.00, 0.50] ng/mL reported in a retrospective study of renal transplant recipients 15 days post-surgery (Zhao et al., 2024). This difference may be attributed to variations in dosing strategies between long-term transplant recipients and those in the early. This study found that voriconazole concentration was negatively correlated with tacrolimus clearance, RBC level was negatively correlated with clearance, and PLT level was positively correlated with clearance. Because voriconazole significantly reduced the metabolic rate of tacrolimus by inhibiting the CYP3A4 enzyme system, resulting in a decrease in its clearance. At the same time, the effect of voriconazole on liver and kidney function can indirectly affect the distribution of tacrolimus (Theuretzbacher et al., 2006; Neofytos et al., 2012). Studies have found that Cvrc is significantly negatively correlated with V; Similarly, Staats et al. The interaction between voriconazole and tacrolimus at the level of drug transporters such as P-glycoprotein (P-gp) can further influence the distribution of tacrolimus (Fu et al., 2019). (Yuan et al., 2020) Additionally, changes in protein binding due to coadministration of voriconazole may lead to changes in tacrolimus distribution (Yuan et al., 2020).
Low red blood cell levels may reflect potential liver dysfunction or other systemic problems, thereby reducing the metabolism of tacrolimus (Venkataramanan et al., 1995). Instead, higher platelet levels may reflect certain body states (such as inflammation or other stimuli) that may promote the metabolism of tacrolimus through complex physiological mechanisms (such as increased blood flow to the liver), thereby increasing its clearance. These findings are consistent with previous studies.
The high temperature and humidity during the plum rain season create an ideal environment for the growth and dissemination of pathogenic fungi, particularly Aspergillus species (Valencia-Quintana et al., 2020), leading to a significant increase in the incidence of invasive fungal infections (IFIs) among kidney transplant patients. This study specifically focuses on kidney transplant recipients suffering from invasive fungal infections. As a typical plum rain region, Changsha, Hunan, further elevating the infection risk for immunosuppressed patients. Therefore, kidney transplant recipients should adopt preventive measures such as improved air quality management, early screening, and prophylactic antifungal therapy to reduce infection rates and mortality. This phenomenon demonstrates strong regional and seasonal characteristics, warranting clinical attention. In addition, we previously conducted a retrospective study on the combined use of medications in renal transplant patients 15 days post-surgery, which provided valuable insights for subsequent research (Zhao et al., 2024). Compared with the previous study, the present study adopts a prospective design, focusing on patients 1 year or more after transplantation, with an emphasis on the long-term effects in this population. The sample size has also increased significantly, from 19 participants in the earlier study to 51 in this one, with a similarly high proportion of male participants (78.9% and 72.5%, respectively). Furthermore, the methods of blood sampling differed between the two studies. The current study utilized random sampling, encompassing the entire phase of drug absorption and elimination, which allowed for real-time measurement of tacrolimus and voriconazole blood concentrations. This approach minimizes the risk of data lag and enhances the accuracy of drug concentration assessments. The findings revealed significant differences in drug concentrations at the two time points. Specifically, the median tacrolimus concentration in patients one-year post-surgery was lower than in those within 15 days post-surgery (5.80 ng/mL vs. 7.90 ng/mL, respectively), whereas voriconazole concentrations were higher. Moreover, in both studies, a significant effect of voriconazole concentration on the volume of distribution and clearance of tacrolimus was observed. These results provide critical insights into the pharmacokinetics of these drugs in long-term renal transplant recipients and underscore the importance of individualized treatment strategies for this population.
At the same time, the study unexpectedly found that for patients taking tacrolimus for a long time, clinicians often gave smaller doses. This may be due to a decrease in the target concentration range of tacrolimus as time after transplantation increases.
There are some limitations. This study utilized the immediate-release (IR) formulation of tacrolimus, which is typically dosed twice daily. Due to the differences in pharmacokinetics, the findings of this study may not be directly applicable to other formulations of tacrolimus, such as prolonged-release or extended-release formulations. The limitation of sample size may affect the generalizability of the study conclusions. Although this study revealed a significant effect of voriconazole on the pharmacokinetics of tacrolimus, the small sample size may limit the generalizability of the study results in different populations. Therefore, a larger multicenter study should be conducted in the future to expand the sample size, further verify the conclusions of this study, and improve the statistical power and the wide applicability of the conclusions. At present, we have begun to prepare for a multicenter study. The study of group behavior and drug interaction in this study mainly focused on clinical observation data, and the exploration of its potential mechanisms was insufficient. Group behavior is inherently dynamic and complex and is affected by multiple factors. Drug interactions often produce different reactions with changes in physiological state. Future studies should combine longer longitudinal tracking data to explore drug metabolic pathways under different environments and conditions and reveal the multidimensional mechanisms of drug interactions. In summary, the limitations of this study provide guidance for future multicenter, large sample, and interdisciplinary in-depth research, aiming to optimize medication strategies and improve clinical treatment effects through more comprehensive data and theoretical support.
5 Conclusion
This study successfully constructed a population pharmacokinetic model for renal transplant patients taking tacrolimus and voriconazole simultaneously. The model had good predictive ability and provided valuable insights to clinicians to help optimizing tacrolimus dosing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Z-HS: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. Y-CZ: Software, Writing–review and editing. J-KL: Investigation, Writing–review and editing. FP: Project administration, Writing–review and editing. FY: Methodology, Writing–review and editing. B-KZ: Project administration, Visualization, Writing–review and editing. MY: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This research was funded by Hunan Medical Association with the founding number of [HMA202001002]; This research was funded by the Interna-tional Research Center for Precision Medicine, Transformative Technology, and Software Services, Hunan, China; This research was funded by Research Project established by Chinese Pharmaceutical Association Hospital Phamacy department [NO.CPA-Z05-ZC-2024002]; This research was funded by Hunan Provincial Health High-Level Talent Scientific Research [R2023061].
Acknowledgments
Thanks to all participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1502097/full#supplementary-material
References
Augustine, J. (2018). Kidney transplant: new opportunities and challenges. Cleve Clin. J. Med. 85 (2), 138–144. doi:10.3949/ccjm.85gr.18001
Burrows, F. S., Carlos, L. M., Stojanova, J., and Marriott, D. J. E. (2023). It cuts both ways: a single-center retrospective review describing a three-way interaction between flucloxacillin, voriconazole and tacrolimus. Int. J. Antimicrob. Agents 62 (3), 106908. doi:10.1016/j.ijantimicag.2023.106908
Chen, W., Wang, X., Li, B., Qin, W., Li, S., Wang, X., et al. (2022). Effects of voriconazole exposure on the pharmacokinetics of tacrolimus in lung transplantation patients, based on therapeutic drug monitoring data. J. Clin. Pharmacol. 62 (10), 1310–1320. doi:10.1002/jcph.2066
Choi, Y., Jiang, F., An, H., Park, H. J., Choi, J. H., and Lee, H. (2017). A pharmacogenomic study on the pharmacokinetics of tacrolimus in healthy subjects using the DMETTM Plus platform. Pharmacogenomics J. 17 (1), 105–106. doi:10.1038/tpj.2016.85
Coto, E., Tavira, B., Suárez-Álvarez, B., López-Larrea, C., Díaz-Corte, C., Ortega, F., et al. (2011). Pharmacogenetics of tacrolimus: ready for clinical translation? Kidney Int. Suppl. 1 (2), 58–62. doi:10.1038/kisup.2011.14
Fu, R., Tajima, S., Suetsugu, K., Watanabe, H., Egashira, N., and Masuda, S. (2019). Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharmacol. Sin. 40 (2), 151–159. doi:10.1038/s41401-018-0070-2
Gong, F., Hu, H., Ouyang, Y., Liao, Z. Z., Kong, Y., Hu, J. F., et al. (2023). Physiologically-based pharmacokinetic modeling-guided rational combination of tacrolimus and voriconazole in patients with different CYP3A5 and CYP2C19 alleles. Toxicol. Appl. Pharmacol. 466, 116475. doi:10.1016/j.taap.2023.116475
Jeong, S., Nguyen, P. D., and Desta, Z. (2009). Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: major effect on CYPs 2B6, 2C9, 2C19, and 3A. Antimicrob. Agents Chemother. 53 (2), 541–551. doi:10.1128/aac.01123-08
Kluwe, F., Michelet, R., Huisinga, W., Zeitlinger, M., Mikus, G., and Kloft, C. (2023). Towards model-informed precision dosing of voriconazole: challenging published voriconazole nonlinear mixed-effects models with real-world clinical data. Clin. Pharmacokinet. 62 (10), 1461–1477. doi:10.1007/s40262-023-01274-y
Lloberas, N., Grinyó, J. M., Colom, H., Vidal-Alabró, A., Fontova, P., Rigo-Bonnin, R., et al. (2023). A prospective controlled, randomized clinical trial of kidney transplant recipients developed personalized tacrolimus dosing using model-based Bayesian Prediction. Kidney Int. 104 (4), 840–850. doi:10.1016/j.kint.2023.06.021
Maertens, J. A., Raad, I. I., Marr, K. A., Patterson, T. F., Kontoyiannis, D. P., Cornely, O. A., et al. (2016). Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387 (10020), 760–769. doi:10.1016/s0140-6736(15)01159-9
Neofytos, D., Lombardi, L. R., Shields, R. K., Ostrander, D., Warren, L., Nguyen, M. H., et al. (2012). Administration of voriconazole in patients with renal dysfunction. Clin. Infect. Dis. 54 (7), 913–921. doi:10.1093/cid/cir969
Ota, R., Hirata, A., Noto, K., Yokoyama, S., Hosomi, K., Takada, M., et al. (2019). Relationship between the blood concentrations of tacrolimus and voriconazole in hematopoietic stem cell transplant recipients. Int. J. Clin. Pharmacol. Ther. 57 (11), 561–566. doi:10.5414/cp203539
Staatz, C. E., Goodman, L. K., and Tett, S. E. (2010). Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin. Pharmacokinet. 49 (3), 141–175. doi:10.2165/11317350-000000000-00000
Theuretzbacher, U., Ihle, F., and Derendorf, H. (2006). Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45 (7), 649–663. doi:10.2165/00003088-200645070-00002
Valencia-Quintana, R., Milić, M., Jakšić, D., Šegvić Klarić, M., Tenorio-Arvide, M. G., Pérez-Flores, G. A., et al. (2020). Environment changes, aflatoxins, and Health issues, a review. Int. J. Environ. Res. Public Health 17 (21), 7850. doi:10.3390/ijerph17217850
Vanhove, T., Bouwsma, H., Hilbrands, L., Swen, J. J., Spriet, I., Annaert, P., et al. (2017). Determinants of the magnitude of interaction between tacrolimus and voriconazole/posaconazole in solid organ recipients. Am. J. Transpl. 17 (9), 2372–2380. doi:10.1111/ajt.14232
Venkataramanan, R., Swaminathan, A., Prasad, T., Jain, A., Zuckerman, S., Warty, V., et al. (1995). Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 29 (6), 404–430. doi:10.2165/00003088-199529060-00003
Voora, S., and Adey, D. B. (2019). Management of kidney transplant recipients by general nephrologists: core curriculum 2019. Am. J. Kidney Dis. 73 (6), 866–879. doi:10.1053/j.ajkd.2019.01.031
Wallemacq, P., Armstrong, V. W., Brunet, M., Haufroid, V., Holt, D. W., Johnston, A., et al. (2009). Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther. Drug Monit. 31 (2), 139–152. doi:10.1097/FTD.0b013e318198d092
Yuan, Z. Q., Qiao, C., Yang, Z. C., Yu, L., Sun, L. N., Qian, Y., et al. (2020). The impact of plasma protein binding characteristics and unbound concentration of voriconazole on its adverse drug reactions. Front. Pharmacol. 11, 505. doi:10.3389/fphar.2020.00505
Keywords: tacrolimus, population pharmacokinetics, voriconazole, renal transplantation, predictive model
Citation: Sun Z-H, Zhao Y-C, Li J-K, Peng F, Yu F, Zhang B-K and Yan M (2025) Population dynamics analysis of the interaction between tacrolimus and voriconazole in renal transplant recipients. Front. Pharmacol. 15:1502097. doi: 10.3389/fphar.2024.1502097
Received: 26 September 2024; Accepted: 30 December 2024;
Published: 29 January 2025.
Edited by:
Youssef Daali, University of Geneva, SwitzerlandReviewed by:
Guo Ma, Fudan University, ChinaBin Lin, Changxing People’s Hospital, China
Pengmei Li, China-Japan Friendship Hospital, China
Copyright © 2025 Sun, Zhao, Li, Peng, Yu, Zhang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bi-Kui Zhang, NTA1OTk1QGNzdS5lZHUuY24=; Miao Yan, eWFubWlhb0Bjc3UuZWR1LmNu
 Zhi-Hua Sun1,2
Zhi-Hua Sun1,2 Yi-Chang Zhao
Yi-Chang Zhao Fenghua Peng
Fenghua Peng Bi-Kui Zhang
Bi-Kui Zhang Miao Yan
Miao Yan