- College of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
The seed of Herpetospermum pedunculosum (Ser.) C.B. Clarke, known in Chinese as Bo-Leng-Gua-Zi and in Tibetan as Sejimedo, are here abbreviated as H. pedunculosum seeds. Herpetospermum pedunculosum seeds is a traditional Chinese medicine for protecting the liver, clearing heat, and detoxifying. A total of 125 chemical metabolites of H. pedunculosum seeds are found, including lignans, fatty acids, terpenes, coumarins, and others. The pharmacological activities of H. pedunculosum seeds are mainly in hepatoprotective, antioxidant, anti-cancer cells, and anticholestatic effects. In clinical application, it is mainly used in combination with other traditional Chinese medicines to play a key role in treating the liver disease. This paper gives a systematic review of above research aspects, proposes the potential limitations and put forward plausible solutions. Relevant literatures were searched in PubMed, Web of Science and Chinese National Knowledge Infrastructure with Herpetospermum as the key word. A number of studies have shown that H. pedunculosum seeds exert excellent hepatoprotective effects by acting on NF-κB, TGF-β, and Keap1-Nrf2 signaling pathways, which provide a solid base for its clinic application. However, more research is needed to explore the standard cultivation and quality evaluation of H. pedunculosum seeds and systematical structure-activity relationship of its active metabolites.
1 Introduction
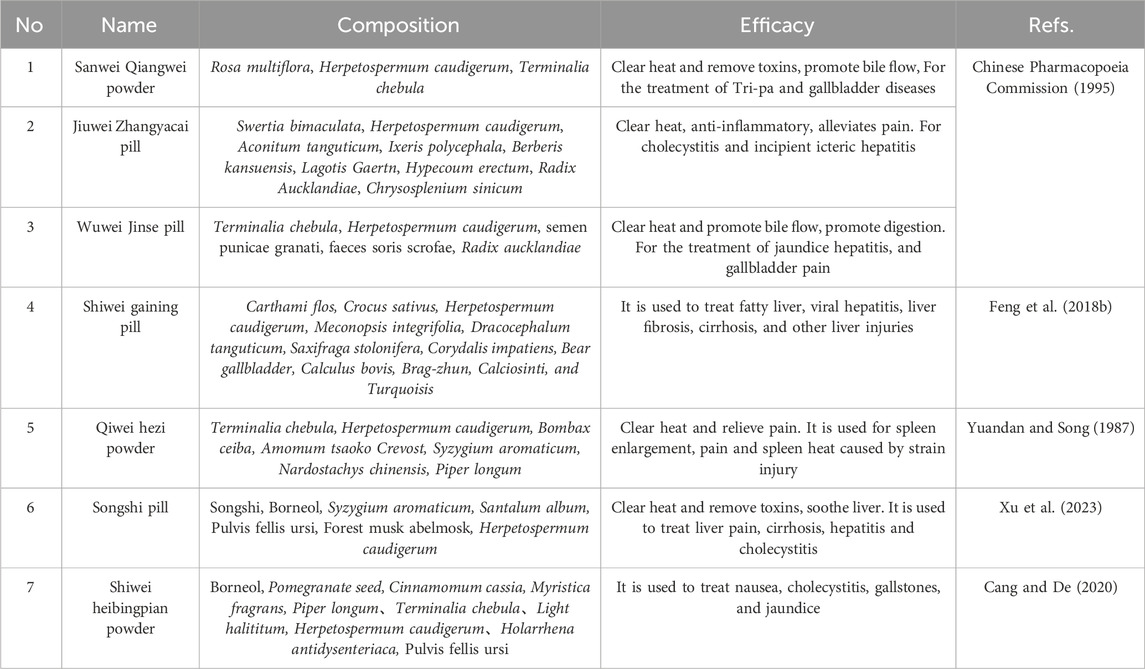
With the continuous development of various drugs, phytomedicines with fewer side effects and significant effects are gradually attracting people. Especially for some effective ethnodrugs, further development of their hidden medicinal value through in-depth research is increasingly popular and desirable. Herpetospermum pedunculosum (Ser.) C.B. Clarke (H. pedunculosum) is mainly distributed in several high-altitude areas such as Tibet, Yunnan, India and Nepal. As the main medicinal part of H. pedunculosum (Ser.) C.B. Clarke, H. pedunculosum seeds are traditional Tibetan drug, which have traditional effects of clearing heat and softening liver. At the same time, H. pedunculosum seeds are also the core ingredient of clinical traditional Chinese prescriptions, such as Jiuwei Zhangya Pill (for treating cholecystitis), Wuwei Jinse Pill (for treating jaundice hepatitis), and Songshi pill (for treating hepatitis and liver fibrosis). Modern studies have shown that H. pedunculosum seeds contain a variety of chemical metabolites, including lignans, coumarins, terpenes, etc. (Xu, 2012), and have shown a variety of pharmacological activities, including liver protection, antioxidant, anti-tumor, and anti-cholestasis effects (Gong, 2012; Shen et al., 2015).
The excellent pharmacological effects and particular characteristics of H. pedunculosum seeds undoubtedly deserve systematical induction and summary, which is hardly reported to the best of our knowledge. Therefore, we investigated relevant literatures in Web of Science, PubMed and CNKI with Herpetospermum as the key word, and focused on the literatures of the H. pedunculosum seeds with excluding that on other parts, such as stem, leaf and flesh. Based on these literatures, this paper comprehensively reviews the traditional use, botany, chemical metabolites, pharmacological effects, pharmaceutical analysis, processing and application in Chinese herbal prescriptions of H. pedunculosum seeds, which can provide scientific basis for further research and promote the potential for development.
2 Traditional uses
As a classic Tibetan medicine, H. pedunculosum seeds often used in the treatment of Tri-pa (a disease be traditionally characterized by diffusion of bile, disorders of the blood-heat, and yellow color in the muscles and eyes), which was first recorded in Yue Wang Yao Zhen (《月王药诊》) in the early 8th century. At the same time, in the middle of the 8th century, Tara Materia Medica (《度母本草》, Shivatso) recorded that H. pedunculosum seeds can treat heat disease, and bacon disease (diseases caused by the combination of food accumulation and cold). Beside these, Si Bu Yi Dian (《四部医典》, Yutog Yontan Gonpo), written and revised during the late 8th to 12th century, further proposed that H. pedunculosum seeds can remove the heat of the lower organs. In addition, it supplemented the bitter taste of H. pedunculosum seeds. Jingzhu Materia Medica (《晶珠本草》, Dema Tenpe Nyima), written in 1840, proposed that H. pedunculosum seeds could treat Tri-pa in the viscera. Diqing Tibetan medicine (《迪庆藏药》, Yang and Chu cheng) and Chinese Tibetan medicine (《中华藏本草》Luo, 1997) supplement recorded its effects of treating liver and gallbladder heat and indigestion, which was also supported by the record of Chinese Materia Medica (《中华本草》, National Administration of Traditional Chinese Medicine). In 2015, the “Interpretation of Tibetan Medicine Jinsui Materia Medica” (《藏药金穗本草诠释》, Gama Qupei) concluded that H. pedunculosum seeds could treat the liver and gallbladder diseases of the Tri-pa type. In summary, H. pedunculosum seeds have been used as its prototype medicine for over 13 centuries, and its effects on protecting the liver and treating indigestion have gained tremendous application as recorded in traditional medical books.
3 Botany
Herpetospermum pedunculosum (Ser.) C. B. Clarke, is usually harvested at around October, and adapts to grow on warm, humid subtropical roadsides, hillsides, shrubland, and forest edges at the altitude of 2,300–3,500 m (Flora Reipublicae Popularis Sinicae Commission, 1983) and its botanical organs including the flower, leaf, fruit and seed were shown in Figures 1A–D. As displayed in Figure 1D, H. pedunculosum seeds is slightly oblong with uneven carving and the surface from brown to black brown. One end of H. pedunculosum seeds has triangular protrusions, and the other end is tapered, slightly wedge-shaped and slightly concave in the center (Chinese Materia Medica Commission, 1998). The further investigation of H. pedunculosum seeds characters and sources can enhance the standardization of commercial H. pedunculosum seeds and is of great significance in cultivating it.
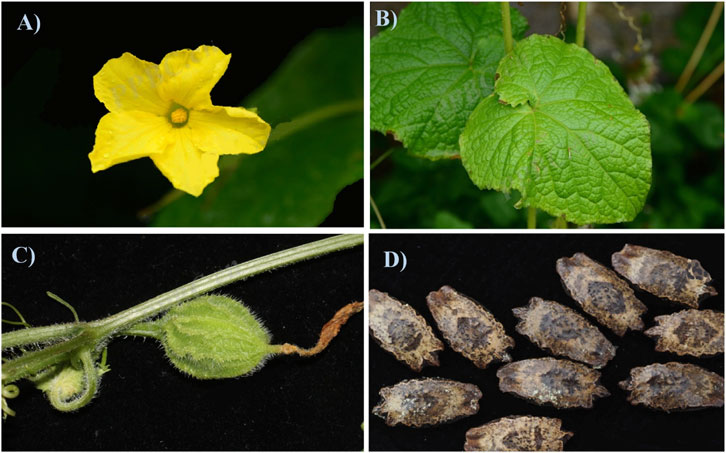
Figure 1. Flower (A), leaf (B), fruit (C) and seed (D) of Herpetospermum pedunculosum (Ser.) C.B. Clarke.
4 Phytochemistry
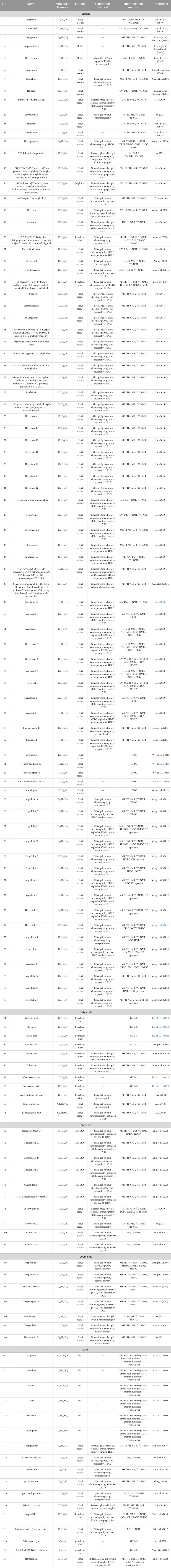
The chemical metabolites of H. pedunculosum seeds are reported to include lignans, fatty acids, terpenoids, and coumarins. The other metabolites such as amino acids, alkaloids, and flavones were also discussed. Details can be found in Figures 2–6 and Table 1.
4.1 Lignans
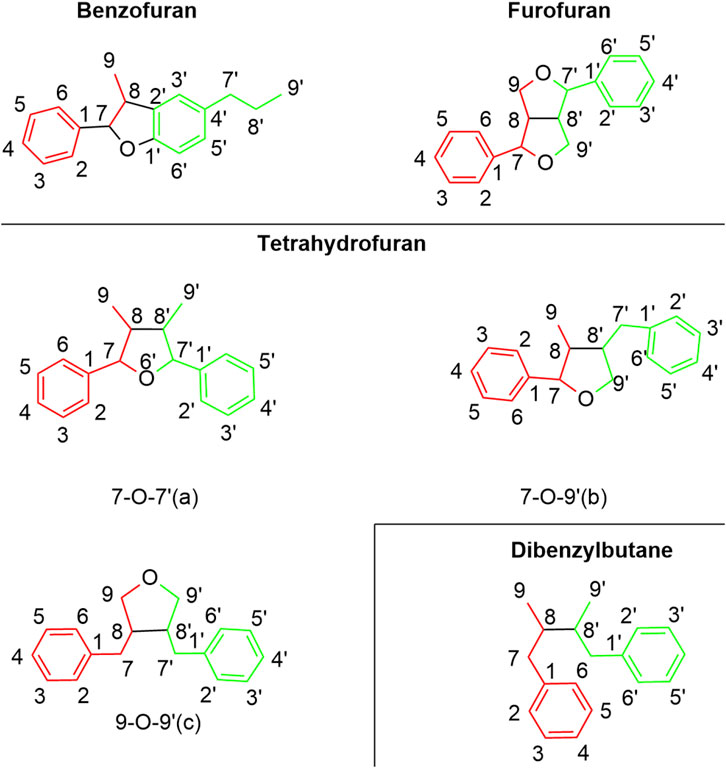
As collected in Figure 2 and summarized in Figure 3, lignans in H. pedunculosum seeds can be mainly divided into benzofurans, tetrahydrofurans and furofuran. In benzofuran lignan such as dehydrodiconiferyl alcohol (9) and herpetotriol (12), the benzene ring is linked to the side chain to form the furan oxygen ring (Figure 2; Table 1). In furfuran lignan, bimolecular phenylpropanin side chains are connected to form a bis-tetrahydrofuran ring, such as herpetetradione (4), herpetetrone (5) and herpetrione (6). Tetrahydrofurans lignans can be further divided into three types with 7-O-7' (a), 7-O-9' (b), and 9-O-9' (c) structures (Figure 2). The tetrahydrofuran of 7-O-9′ is predominant in H. pedunculosum seeds, represented by herpetriol (1) and herpetetrol (2). Beside above three main lignan types, H. pedunculosum seeds also contains dibenzylbutane (chemicals of 29 and 30) as shown in Figure 2.
4.2 Fatty acids
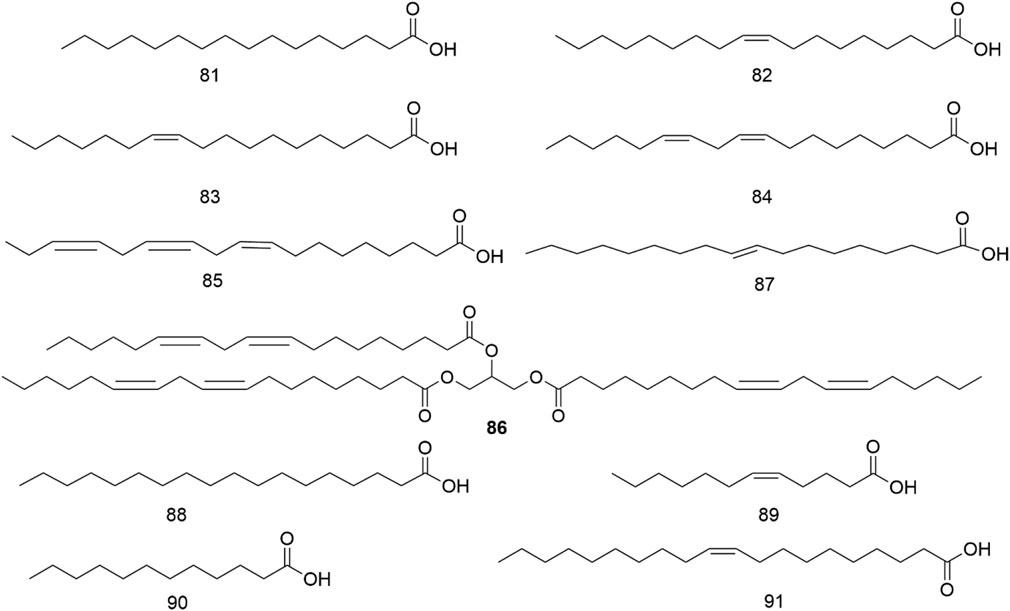
It has been found that H. pedunculosum seeds contain various fatty acids (81-91, Figure 4; Table 1), with comparatively greater concentrations of linoleic (84) and linolenic acid (85) (Zhao et al., 2009). Oleic (82), palmitic (81), and linoleic acids (84) are reported to be physiologically active in decreasing blood cholesterol levels and alleviating the formation of cholesterol in the vascular wall (Dobrzyńska and Przysławski, 2020). Therefore, it is essential to study the fatty acids in H. pedunculosum seeds.
4.3 Terpenoids
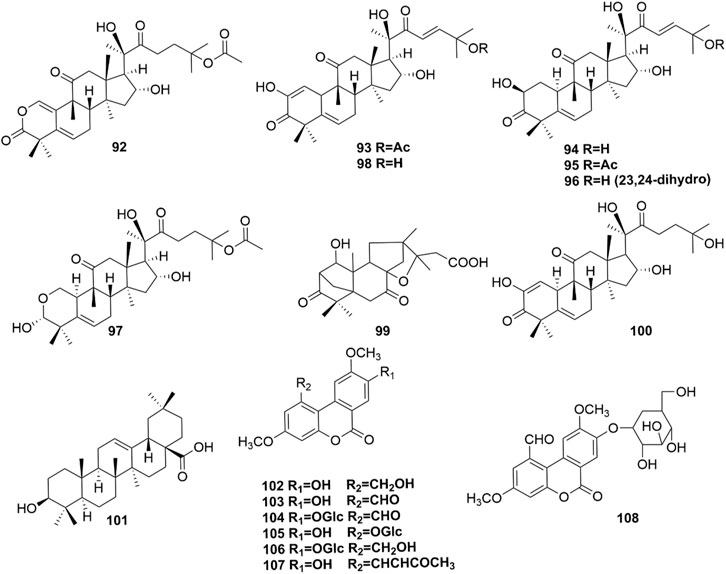
Ten terpenoids (92-101, Figure 5) were identified in H. pedunculosum seeds, and triterpenoid was the dominant type among them. Triterpenoids have the activities of anti-inflammatory, antibacterial, and antiviral properties (Xiao et al., 2018). For example, cucurbitacin B (95) was reported to show anti-inflammatory, antioxidant, and neuroprotective effects (Dai et al., 2023). These bioactive triterpenoids in H. pedunculosum seeds doubtlessly contribute to its favorable pharmacologic actions.
4.4 Coumarins
Coumarin is widely acknowledged to have extensive biological activities including anti-tumor, anti-oxidation, anti-inflammation, and anti-coagulation (Kirsch et al., 2016; Wu et al., 2020). And there are 7 coumarins (102-108 in Figure 5) found in H. pedunculosum seeds up to now. For example, Huang et al. (2021) found that herpetolide H (107) from H. pedunculosum seeds had the effects of anti-inflammatory in vitro.
4.5 Others
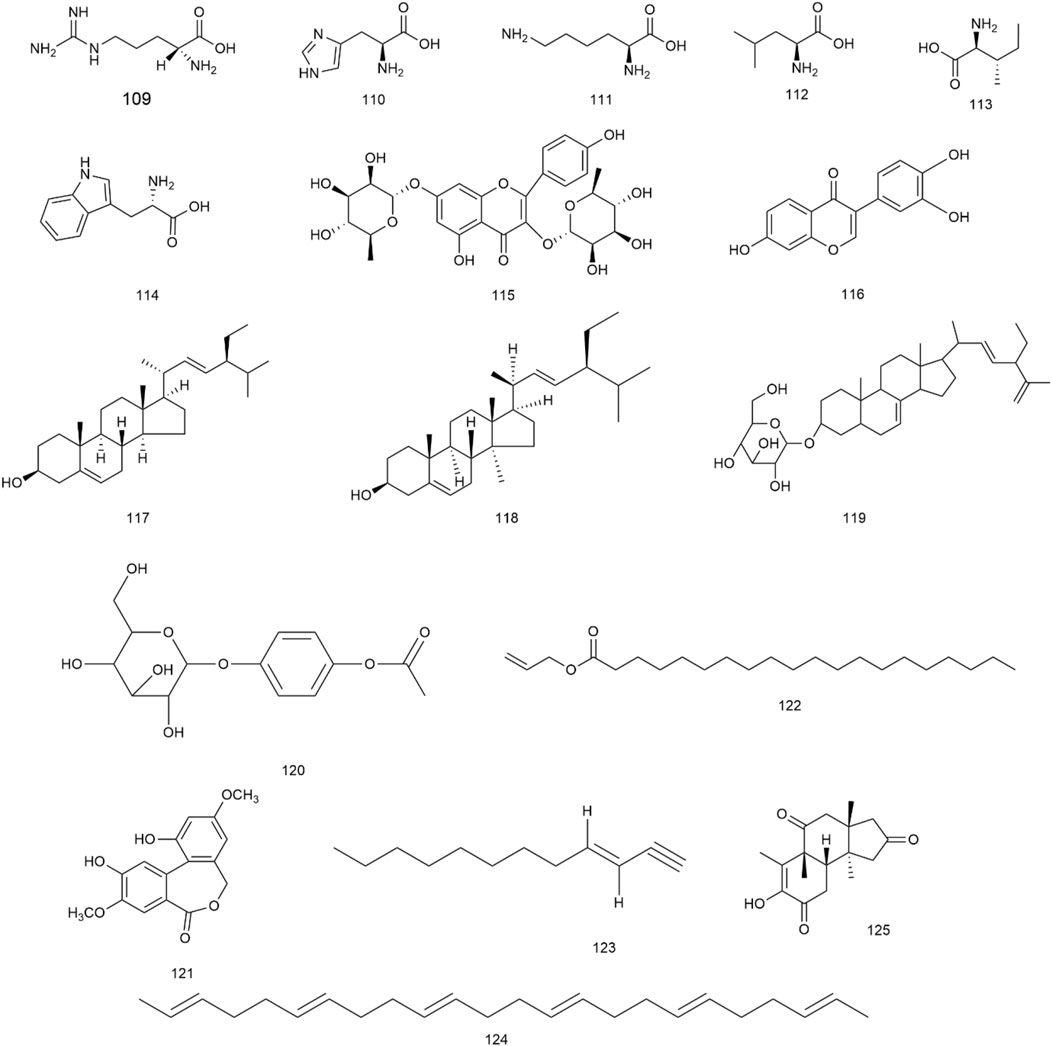
In addition to the aforementioned metabolites, H. pedunculosum seeds also contain amino acids (109–114), flavonoids (115, 116), sterols (117–119), glucosides (120), esters (121, 122), olefin (123–124), and ketones (125) as illustrated in Figure 6. It is reported that leucine (112) and isoleucine (113) can prevent the fat accumulation from in hepatocyte (Zhang et al., 2022). Kaempferitrin (115) has anti-inflammatory and anti-oxidation effects (Patel D. K., 2021). The biological activity of stigmasterol (117) is found to include anti-inflammatory, antioxidant, and anti-cancer properties (Bakrim et al., 2022). Therefore, the role of these metabolites in the application of H. pedunculosum seeds deserves further research.
5 Pharmacology
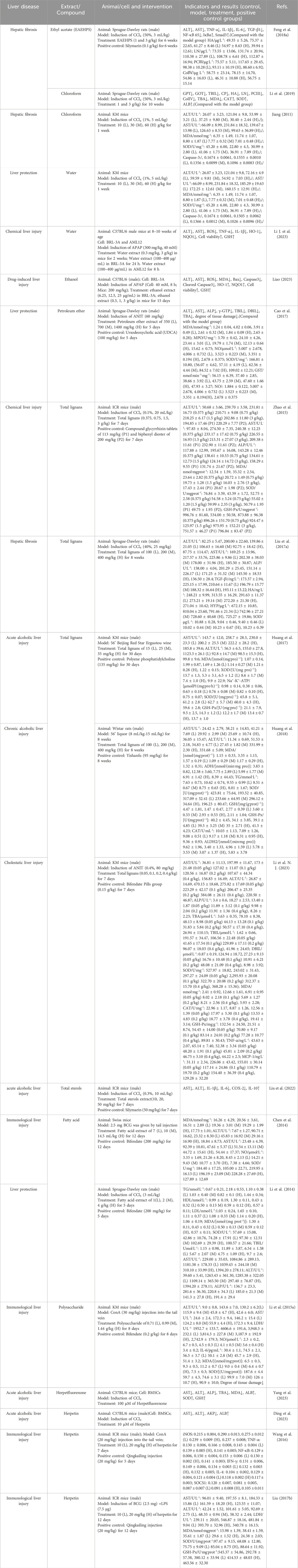
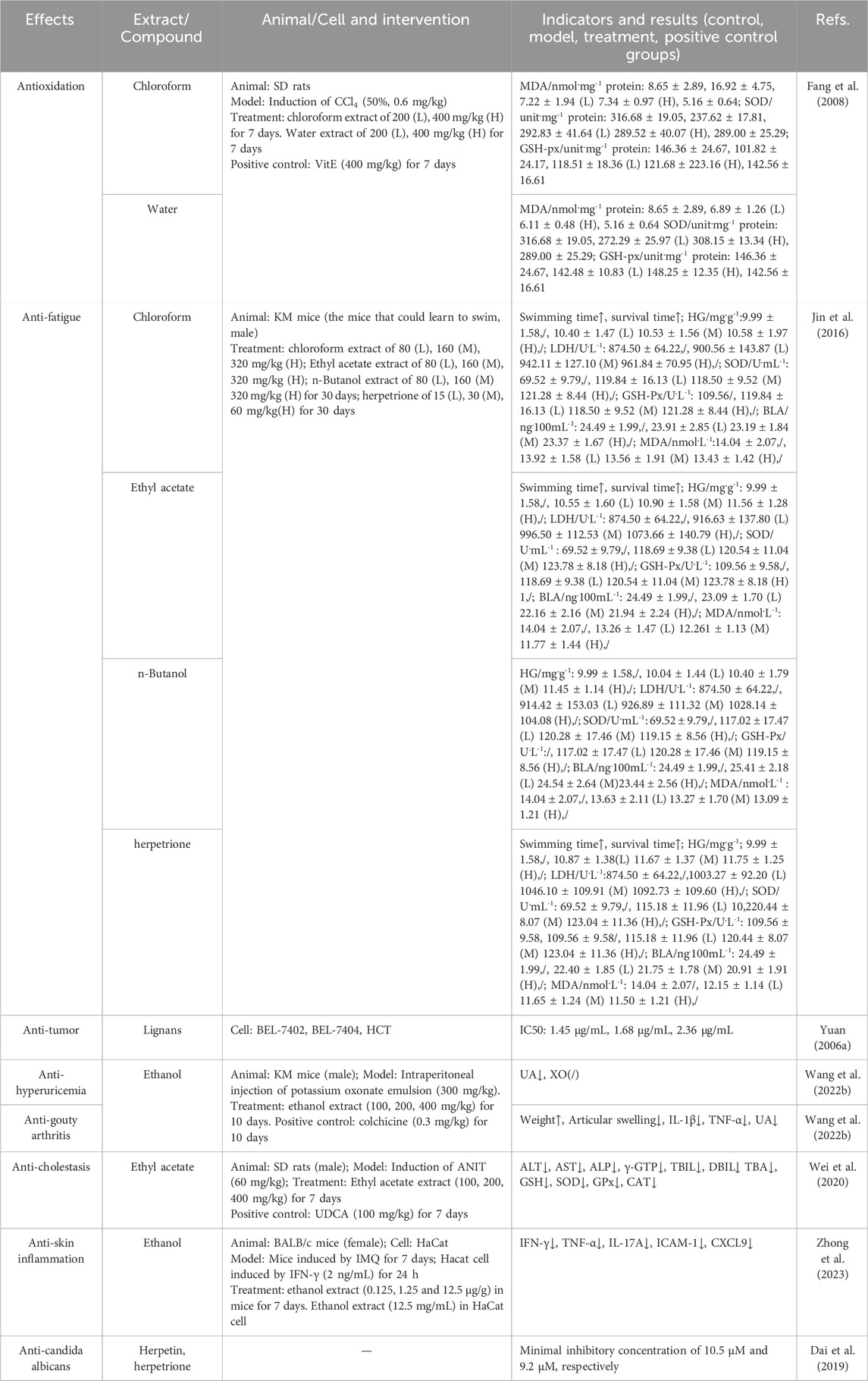
Diverse studies have demonstrated the hepatoprotective, antioxidant, and anti-cholestasis effects of H. pedunculosum seeds and aforementioned metabolites. Especially, the action mechanism on liver protection effect of H. pedunculosum seeds was systematically generalized. The specific hepatoprotective action and other pharmacological effects were summarized in Table 2 and Table 3, respectively.
5.1 Hepatoprotective effect
Liver is a vital metabolic organ implementing multiple functions such as toxicant detoxification, protein synthesis, and special compound production, thus the increasing prevalence of liver illnesses including fatty liver, liver damage, fibrosis, cirrhosis, and cancer aroused great attention nowadays (Asrani et al., 2019). As collected in Table 2, plentiful researches showed the remarkable hepatoprotective effect of H. pedunculosum seeds through the adjustment of some enzymes in animal models with the induction of CCl4, paracetamol (APAP), concanavalin A (ConA), α-naphthyl isothiocyanate (ANIT), liquor, bacillus calmette-guérin (BCG) and lipopolysaccharides (LPS). For instance, the ethyl acetate extract of H. pedunculosum seeds (EAEHPS) showed hepatoprotective activity against CCl4-induced hepatic fibrosis in rats via the inflammatory pathway with obviously inhibiting the expression of NF-κB (IκBα), Samd3, and TGF-β1 proteins (Feng et al., 2018a). The water extract of H. pedunculosum seeds could alleviate APAP-induced liver injury by inhibiting oxidative stress and ferroptosis through activating the Nrf2 signal pathway (Li N. Z. et al., 2023). In addition, some proteins, such as NLRP3, TLR-2, TLR-4, and JNK, will have their expression reduced by the total lignan of H. pedunculosum seeds (TLHPS), so as to protect mice against ANIT-induced liver damage (Li J. et al., 2023). Some metabolites such as herpetfluorenone (HPF, 23) and herpetin (18) from H. pedunculosum seeds were further found to have a positive pharmaceutical effect on acute liver injury by promoting the differentiation of bone marrow mesenchymal stem cells into hepatocellular-like cells and controlling autoimmune oxidation (Yang et al., 2023; Ding et al., 2023).
Based on above discussions and previous literatures, the hepatoprotective mechanism of H. pedunculosum seeds can be summarized into three pathways as illustrated in Figure 7. The first one is the inhibition of NF-κB signaling pathway to alleviate the inflammation during liver diseases (Figure 7A). Herpetospermum pedunculosum seeds can inhibit the phosphorylation of IκB through the inhibition of IKK, which in turn has anti-inflammatory and hepatoprotective effects (Li N. Z. et al., 2023). The second mechanism is inhibiting the TGF-β signaling pathway (Figure 7B). EAEHPS an inhibit the phosphorylation of Smad3, which in turn inhibits the expression of relevant genes after the complex enters the nucleus, thus playing a role in inhibiting liver fibrosis (Feng et al., 2018a). The third one is the promotion of Keap1-Nrf2 signaling pathway (Figure 7C). Nrf2 plays a crucial role in cellular defense against oxidative stress. When activated by H. pedunculosum seeds, the stability of Nrf2 increases, leading to reduced degradation and subsequent activation of genes driven by the antioxidant response element (ARE), thereby exerting a protective effect against liver damage (Li N. Z. et al., 2023; Liao, 2023).
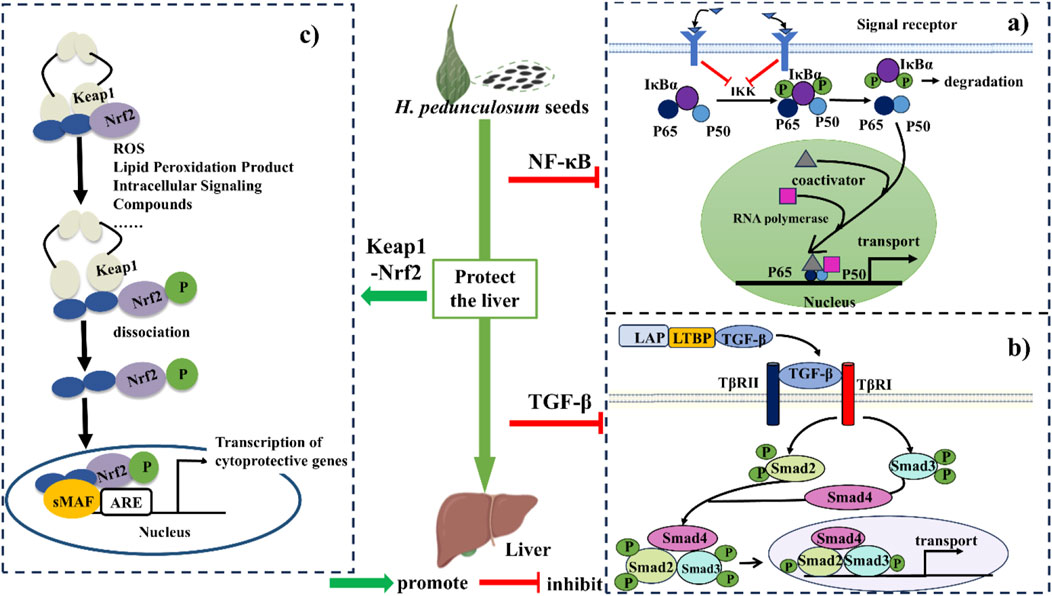
Figure 7. The mechanism of protective effect of Herpetospermum pedunculosum seeds on liver. (A) NF-κB signaling pathway; (B) TGF-β signaling pathway; (C) Keap1-Nrf2 signaling pathway.
5.2 Antioxidation
Fang et al. (2007), Fang et al. (2008) demonstrated the antioxidant activities of CHCl3, water and ethanol extracts of H. pedunculosum seeds to prevent lipid peroxidation brought on by CCl4 in vivo experiments. Jiang et al. (2020) tested the significant inhibitory activity of neocucurbitacin D (92) (IC50 = 15.27 ± 0.29 μM) and 23, 24-dihydrocucurbitacin B (97) (IC50 = 24.18 ± 0.26 μM) on XOD. Gong, (2013) showed that herpetone (7) has good DPPH free radical scavenging ability and antioxidant activity. Although many studies have shown that H. pedunculosum seeds has antioxidant effects, there are still some problems, such as the simplistic evaluation index and the unclear relationship between dose and activity.
5.3 Anti-cancer cells
The lignan of H. pedunculosum seeds demonstrated considerable in vitro inhibitory action against several cancer cells. The IC50 of lignans in H. pedunculosum seeds were 1.45 μg/mL, 1.68 μg/mL, and 2.36 μg/mL for human hepatocellular carcinoma cells (BEL-7402, BEL-7404), and HCT, respectively (Yuan, 2006a). Zhang et al. (2007) demonstrated the inhibitory effects of herpetolide A (102) and herpetolide B (116) on the growth of human promyelocytic leukemia cells (HL-60). The metabolites of H. pedunculosum seeds including including herpetosiol A (42), herpetosiol C (44), 7′, 8′-didehydlroherpepetotriol (14), herpetetrol (2), herpepropenal (13), herpetrione (6) showed significant cytotoxicity on human gastric adenocarcinoma cells (SGC7901), human lung cancer cells (A549), human breast cancer cells (MDA-MB-231), and human hepatocellular carcinoma cells (HepG2) (Ma, 2020; Kong et al., 2023). However, these studies only perform a simple detection of IC 50 and cytotoxicity, and lack other powerful indicators to reflect the efficacy of the drug. In addition, it is worth noting that the anti-tumor effects are mainly tested at the cellular level, lacking in animal and mechanism investigations, which are noteworthy in further research.
5.4 Anticholestasis effects
EAEHPS exerted an anti-cholestatic effect with increasing bile flow in a dose-dependent manner, which promoted bile acid transport by activating the farnesoid X receptor (FXR) signaling pathway (Wei, 2020). Meanwhile, the EAEHPS activated the Keap1-Nrf2 pathway to alleviate oxidative stress and inhibit of NF-κB/Are signaling pathway to inhibit inflammatory response, which could prevent and treat ANIT-induced cholestasis in rats (Wei et al., 2020).
5.5 Other effects
Wang S. W. et al. (2022) found that EAEHPS also had anti-hyperuricemia and anti-gouty arthritis activities, through reducing serum uric acid (UA) levels, suppressing the production and releasing pertinent inflammatory components, and lessening inflammatory damage and pathological tissue necrosis. Jin et al. (2016) demonstrated the anti-fatigue effects of ethanol extract of H. pedunculosum seeds with longer swimming time and hypoxia tolerance of experimental mice than that of the control group. The ethanol extract further showed a therapeutic effect on skin inflammation caused by imiquimod (Zhong et al., 2023). Moreover, Dai et al. (2019) showed that herpetin (18) and herpetrione (6) had favorable anti-candida albicans effects with minimal inhibitory connection of 10.5 μM and 9.2 μM, respectively.
6 Structure-activity relationship of lignan
Considering the key role of lignans in H. pedunculosum seeds, their structure-activity relationship was summarized according to previous literatures. For benzofuran lignans (Figure 2), H-5 can improve its anti-inflammatory capacity when it remains unchanged (Wang L. X. et al., 2022). The electron-withdrawing or electron-donor groups on the benzene ring of benzofuran lignans can decrease their anti-tuberculosis activity (Xu Z. et al., 2019). The anti-tumor activity of tetrahydrofuran lignans with the 7-O-9′ structure (Figure 2B.) can be increased by fixing the following sites: C-7′ is carbonyl group, H-5/5′is not substituted, and C-4/4′is methoxy (Wang L. X. et al., 2022). The antioxidant capacity of furofuran lignans is reported to decrease with the number of substituted methoxy groups on their benzene ring (Wang L. X. et al., 2022). Meanwhile, the presence of methoxy benzene in furofuran lignans enhances its toxicity to tumor cells (Xu W. H. et al., 2019), which could provide a structure-activity basis for the anti-tumor effect of herpetrione (6, Figure 3) (Yuan, 2006a; Yuan et al., 2006b; Yuan et al., 2011). For dibenzylbutane lignans (Figure 2), stronger antiviral activity can be achieved when the hydrogens at C-4 and C-5 are substituted by hydroxyl and methoxy groups respectively, and that at C-3'/4'/5′are substituted by methoxy or hydroxyl groups (Xu et al., 2022). Therefore, the separation and structural modification of lignan compounds from H. pedunculosum seeds show great potential for the development of drug leads.
7 Pharmaceutical analysis
Herpetospermum pedunculosum seeds are only stipulated qualitatively in the Chinese Pharmacopoeia, and their quantitative provisions are still lacking. The existing regulations are not enough to accurately evaluate the quality of H. pedunculosum seeds. Therefore, this section briefly introduces the latest research on modern analytical methods to provide guidance for quality evaluation for H. pedunculosum seeds.
Lignans such as herpetrione (6), herpetotriol (12), herpetin (18), and herpetfluorenone (23) are considered to be typical metabolites of the genus Herpetospermum and also the main active metabolites of H. pedunculosum seeds, which undoubtedly have a direct effect on the quality research of H. pedunculosum seeds and are indispensable to be detected. Wang, (2014) used herpetotriol (12) as the chemical reference materials in TLC to compare H. pedunculosum seeds from different areas. Cong et al. (2008) detected seven lignans from different areas by reversed-phase HPLC method. The results showed that herpetrione (6) was the most abundant among the seven metabolites, followed by herpetotriol (12). Qian et al. (2011) further accurately analyzed the average content of herpetrione (6) in 10 batches of H. pedunculosum seeds from different areas by UPLC, which was found to be 3.7223 mg g-1. Except lignan, other metabolites such as fatty acids and polysaccharides also contribute to the bioactivity of H. pedunculosum seeds, and their analysis are meaningful for the quality evaluation of H. pedunculosum seeds. Ling et al. (2018) detected four fatty acids in H. pedunculosum seeds by GC, the result showed that the content of oleic acid (82) was highest, followed by palmitic acid (81). Liu M. L., (2017a) combined UV-Vis and phenol-sulfuric acid methods to detect the polysaccharides in 10 batches of H. pedunculosum seeds, indicating higher polysaccharide content (2.16%) of H. pedunculosum seeds produced in Yunnan province. However, it is difficult to accurately evaluate the quality of H. pedunculosum seeds based on single or several metabolite analyses owing to its plentiful active metabolites, and establishing their fingerprint for similarity evaluation and principal component analysis could be a feasible choice in this aspect. Wang, (2014) found that there were 18 common peaks in the HPLC fingerprint, and the content of herpetolide A (102) was relatively high in all the samples to be analyzed. Subsequently, the HPLC fingerprint of H. pedunculosum seeds from Nyingchi region of Tibet was also studied, and 17 common peaks were identified, among which herpetrione (6) was the highest (Chen et al., 2020b).
In brief, among the active metabolites suitable for quantitative analysis, herpetrione (6) and herpetolide (102) exhibit various pharmacological activities with relatively high content, which have the potential to serve as markers for evaluating the quality of H. pedunculosum seeds. The current analysis methods for H. pedunculosum seeds are still far from perfect to establishing their quality evaluation system. It is urgent to elucidate the key indicative metabolites of H. pedunculosum seeds and develop standard determination methods capable of evaluating its quality comprehensively.
8 Processing
Processing methods are able to change the effect of H. pedunculosum seeds. For example, the stir-frying with grit can effectively alleviate the side effects of diarrhea caused by the shell of H. pedunculosum seeds. Meanwhile, the content of herpetrione (6) significantly decreased by 40.9% during this process, which can affect the clinical efficacy (Ling et al., 2018). Research further revealed that H. pedunculosum seeds processed by stir-frying with vinegar has better effects on protecting the liver and reducing enzymes, compared with sand owing to the lower herpetrione loss (12%) than that stir-frying with sand (41.4%). (Chen et al., 2016). Additionally, preparing the lignans of H. pedunculosum seeds into nanosuspension can improve their bioavailability and stability (Li et al., 2018; Shen et al., 2016). Therefore, the processing optimization could be a feasible approach to enhance the efficacy of H. pedunculosum seeds, which deserves more detailed research.
9 Application
The commercial herbal formulae including H. pedunculosum seeds and related details were collected in Table 4. For example, H. pedunculosum seeds is often combined with Swertia bimaculata, Terminalia schedule, and Carthami flos (1, 2, 3 in Table 4) to soothe the liver, promote bile flow, clear heat, and detoxify. When it is paired with Rosa multiflora, T. schedule, Phylanthus emblica (1, 2, 3, 4 in Table 4), formed compound medicines have the effects of strengthening the spleen, as well as promoting digestion. These summarizations and analyses supported the clinical practice of H. pedunculosum seeds and provided reference value for the development of other H. pedunculosum seed-derived prescriptions.
10 Conclusions and prospects
Herpetospermum pedunculosum seeds is a traditional Tibetan medicine with long history, rich chemical metabolites and high medicinal value. The research of H. pedunculosum seeds has achieved fruitful results and provided a scientific basis for the clinical medication. However, there are some shortcomings that need to be addressed in follow-up studies.
Although H. pedunculosum seeds is present in many Chinese patent medicines for the treatment of liver diseases, the interaction between the chemical metabolites in the prescriptions remains unclear and needs further investigation. Secondly, the supply of H. pedunculosum seeds is restricted due to the particular growth environment and limited wild resources. The large-scale cultivation of H. pedunculosum seeds could be of high research and economy value. Herpetospermum pedunculosum seeds are reported to contain 125 chemical metabolites, and lignan, terpenoids and coumarin are the main metabolites. Among them, lignan has been widely studied, which is usually recognized as the main pharmacological metabolite of H. pedunculosum seeds to exert hepatoprotective effect. However, the research on many other potentially active components such as polysaccharide is still in shortage. More advanced technologies can be used to extract, enrich, separate and purify the metabolites with low content and attention for better understanding of the medicinal material base of H. pedunculosum seeds. Moreover, there is a lack of structure-activity relationship studies of other active mmetabolites except lignans in H. pedunculosum seeds. The systematic structure-activity relationship studies can accelerate the synthesis of active metabolites and the development of related drugs derived from H. pedunculosum seeds.
The pharmacological effects of H. pedunculosum seeds, especially its effects on liver diseases, have been extensively researched. However, there are few in-depth studies on other pharmacological effects, and the current pharmacological research only remains at the cell and animal levels without comprehensive clinical research. Future research should take this as the direction to accelerate the clinical translation of drugs. Moreover, the quality standard of H. pedunculosum seeds still lacks the indicative components and standard detection method, which can be disadvantageous for standard pharmacology research and clinic practice. At present, some analytical methods have been used to detect the main bioactive ingredients with relatively high content such as herpetrione (6) and herpetolide (102), which may be a promising direction for better quality evaluation.
Although the current medical use of H. pedunculosum seeds is without processing, some studies have shown that H. pedunculosum seeds stir-fried with sand and vinegar can reduce their side effect of diarrhea and also the content of active ingredients. Therefore, the effect of processing method needs to be systematically determinated and optimized in combination with pharmacology and clinical research. In summary, this paper has comprehensively reviewed and analyzed the botany, phytochemistry, pharmacology, analytical methods and quality evaluation, processing and application of H. pedunculosum seeds, which can provide more insights for further research and development of traditional Tibetan medicine.
Author contributions
ZJ: Writing–original draft. CZ: Writing–original draft. XY: Writing–original draft. KW: Writing–original draft. ZS: Writing–original draft. WG: Writing–original draft. QaZ: Writing–original draft. XM: Formal analysis, Funding acquisition, Writing–review and editing. LQ: Writing–review and editing. QmZ: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support from Zhejiang Provincial Natural Science Foundation of China (LY24H290005), National Natural Science Foundation of China (82004208), and Research Project of Zhejiang Chinese Medical University (2023GJYY16; GQD23SH23, BZXCG-2022-37).
Acknowledgments
It is also appreciated for the assistance from the Public Platform of Pharmaceutical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University and Shiyanjia Lab (www.shiyanjia.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70 (1), 151–171. doi:10.1016/j.jhep.2018.09.014
Bakrim, S., Benkhaira, N., Bourais, I., Benali, T., Lee, L. H., El Omari, N., et al. (2022). Health benefits and pharmacological properties of stigmasterol. Antioxidants (Basel.) 11 (10), 1912. doi:10.3390/antiox11101912
Cang, J., and De, J. (2020). Clinical observation on the treatment of jaundice hepatitis with Tibetan medicine Shiwei heibing tables. J. Med. Pharm. Chin. Minor. 26, 3–5. doi:10.16041/j.cnki.cn15-1175.2020.05.002
Cao, W. R., Ge, J. Q., Xie, X., Fan, M. L., Fan, X. D., Wang, H., et al. (2017). Protective effects of petroleum ether extracts of Herpetospermum caudigerum against α-naphthylisothiocyanate-induced acute cholestasis of rats. J. Ethnopharmacol. 198, 139–147. doi:10.1016/j.jep.2017.01.003
Chen, H. G., Shen, B. D., Shen, C. Y., Li, Z. M., and Yuan, H. L. (2016). Effects of different processing technology on active ingredients diarrhea and hepatoprotection of Herpetospermum caudigerum. Pharm. J. Chin. People's Lib. Army. 32, 289–292+298.
Chen, J. F. (2020). Study on potential toxicity, antibacterial activity, and chemical constituents of the shell of Herpetospermum pedunculosum. Master. Jiangsu: Nanjing University of Science and Technolog.
Chen, L., Zhang, M., Yao, H. E., Li, C. Q., and He, C. Y. (2014). Protective effect of Herpetospermum seed oil on immunologic liver injury in the mice. West China J. Pharm. Sci. 29, 143–145.
Chen, X., Lu, X. G., Feng, X., and Zhong, G. J. (2020b). HPLC fingerprint of Tibetan medicine hertospermi semen from Linzhi Tibet. Cent. South Pharm. 18, 664–667.
Chinese Materia Medica Commission (1998). Chinese Materia Medica. Shanghai: Shanghai Science and Technology Press.
Chinese Pharmacopoeia Commission (1995). Pharmacopoeia of the people’s Republic of China, Beijing: China. Med. Sci. Press.
Cong, L. B., Yuan, H. L., Wang, Q., Li, X. Y., Gong, Q. F., and Xiao, X. H. (2008). Simultaneous determination of seven bioactive lignans in Herpetospermum caudigerum by RP-HPLC method. Biomed. Chromatogr. BMC 22 (10), 1084–1090. doi:10.1002/bmc.1028
Dai, S., Wang, C., Zhao, X. T., Ma, C., Fu, K., Liu, Y. F., et al. (2023). Cucurbitacin B: a review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 187, 106587. doi:10.1016/j.phrs.2022.106587
Dai, Y. X., Hu, S., Jiang, H. Z., and Tan, R. (2019). Study on phenolic constituents from Herpetospermum caudigerum. Nat. Prod. R&D. 31 (02), 280–283. doi:10.16333/j.1001-6880.2019.2.016
Dai, Y. X., Wu, W. J., and Tan, R. (2017). Study on the chemical components of the ethyl acetate extract from Herpetospermum caudigerum. North Am. J. Med. Sci. 10 (04), 136–138.
Ding, Y., Tan, R., Gu, J., and Gong, P. Y. (2023). Herpetin promotes bone marrow mesenchymal stem cells to alleviate carbon tetrachloride-induced acute liver injury in mice. Molecules 28 (9), 3842. doi:10.3390/molecules28093842
Dobrzyńska, M. A., and Przysławski, J. (2020). The effect of camelina oil (α-linolenic acid) and canola oil (oleic acid) on lipid profile, blood pressure, and anthropometric parameters in postmenopausal women. Arch. Med. Sci. 17 (6), 1566–1574. doi:10.5114/aoms.2020.94033
Dong, Z. Y., Wang, H., Ma, Y. X., Lan, X. Z., and Chen, M. (2019). Chemical constituents from Herpetospermum caudigerum. Chin. Tradit. Pat. Med. 41, 341–344.
Fan, X. D., Wang, G. W., Wang, H., Ma, J. X., Lan, X. Z., Liao, Z. H., et al. (2016). Herpetolide C: one new 7H-dibenzo[c,e]oxepin-5-one from Herpetospermum caudigerum. Acta Pharm. Sin. 51 (5), 770–774. doi:10.16438/j.0513-4870.2015-0938
Fang, Q. M., Zhang, H., and Cao, Y. (2008). Anti-oxidant activities of the seed extracts of Herpetospermum pedunculosum against liver injury in rats. West China J. Pharm. Sci. 02, 147–149. doi:10.13375/j.cnki.wcjps.2008.02.007
Fang, Q. M., Zhang, H., Cao, Y., and Wang, C. (2007). Anti-inflammatory and free radical scavenging activities of ethanol extracts of three seeds used as “Bolengguazi”. J. Ethnopharmacol. 114 (1), 61–65. doi:10.1016/j.jep.2007.07.024
Feng, X., Li, M. H., Xia, J., Deng Ba, D. J., Ruan, L. Y., Xing, Y. X., et al. (2018b). Tibetan medical formula Shi-Wei-Gan-Ning-Pill protects against carbon tetrachloride-induced liver fibrosis-an NMR-based metabolic profiling. Front. Pharmacol. 9, 965. doi:10.3389/fphar.2018.00965
Feng, X., Zhong, G. J., Deng Ba, D. J., Yang, B., Chen, L. Y., and Du, Y. (2018a). Hepatoprotective effect of Herpetospermum caudigerum Wall. on carbon tetrachloride-induced hepatic fibrosis in rats. J. Cell. Mol. Med. 22 (07), 3691–3697. doi:10.1111/jcmm.13568
Flora Reipublicae Popularis Sinicae Commission (1983). Flora Reipublicae Popularis Sinicae. Beijing: Science Publishing House.
Gong, F. Q. (2013). Studies on chemical constituents and pharmacological activity of the seeds of Herpetospermum caudigerum Wall. Master. Shandong: Shandong University of Traditional Chinese Medicine.
Gong, P. Y., Yuan, Z. X., Gu, J., Tan, R., Li, J. C., Ren, Y., et al. (2016). Anti-HBV activities of three compounds extracted and purified from Herpetospermum seeds. Molecules 22 (1), 14. doi:10.3390/molecules22010014
Hu, S. (2016). Study on the chemical constitutions of the ethyl acetate extract from Herpetospermum caudigerum. Master. Shandong: Southwest Jiaotong University.
Huang, D., Ma, Y. X., Wei, L., Sun, Y., Zeng, Q. H., Lan, X. Z., et al. (2021). One new coumarin from seeds of Herpetospermum pedunculosum. China. J. Chin. Mater. Med. 46 (10), 2514–2518. doi:10.19540/j.cnki.cjcmm.20210125.601
Huang, S. Y., Gu, J., Tan, R., Shi, Y. C., Liu, W., and Shi, L. L. (2018). The protective effect and mechanism of total lignans from Tibetan medicinal Herpetospermum pedunculosum seeds on chronic alcohol-induced hepatic injury in rats. J. Chin. Med. Mater. 41 (02), 432–436. doi:10.13863/j.issn1001-4454.2018.02.041
Huang, S. Y., Sun, N. Y., Sun, W., Fu, X. H., and Gu, J. (2017). Protective effect and mechanism of total lignans from Tibetan medicinal Herpetospermum seeds on alcohol-inducing acute hepatic injury in mice. China J. Chin. Mater. Med. 33, 66–70. doi:10.13412/j.cnki.zyyl.2017.05.018
Jiang, H. Z., Hu, S., Tan, R. X., Tan, R., and Jiao, R. H. (2020). Neocucurbitacin D, a novel lactone-type norcucurbitacin as xanthine oxidase inhibitor from Herpetospermum pedunculosum. Nat. Prod. Res. 34 (8), 1728–1734. doi:10.1080/14786419.2018.1528592
Jiang, H. Z., Tan, R., Jiao, R. H., Deng, X. Z., and Tan, R. X. (2016). Herpecaudin from Herpetospermum caudigerum, a xanthine oxidase inhibitor with a novel isoprenoid scaffold. Planta. Med. 82 (11-12), 1122–1127. doi:10.1055/s-0042-108210
Jiang, X. (2011). Protective effect of extracting solution of the Tibetan medicine Herpetospermum caudigerum seeds on acute liver injury mice produced by carbon tetrachloride. Master. Hubei: Huazhong Agricultural University.
Jin, S. Y., Li, R. S., Shen, B. D., Bai, J. X., Xu, P. H., Dai, L., et al. (2016). Lignans-rich extract from Herpetospermum caudigerum alleviate physical fatigue in mice. Chin. J. Integr. Med. 22 (11), 840–845. doi:10.1007/s11655-016-2254-2
Kaouadji, M., and Favre-Bonvin, J. (1984). Herpetol, a new dimeric lignoid. from Herpetospermum caudigerum Wall. Z. Naturforsch., C. J. Biosci. 39, 307–308. doi:10.1515/znc-1984-3-419
Kaouadji, M., and Jean, F. B. (1983). Herpetrione, lignoide trimere isoled’Herpetospermum caudigerum. Tetrahedron Lett. 24, 5881–5884. doi:10.1016/S0040-4039(00)94226-6
Kaouadji, M., Jean, F. B., and Mariotte, A. M. (1978). Herpetal, benzofuranne isolede Herpetospermum caudigerum. Phytochemistry 17, 2134–2135. doi:10.1016/s0031-9422(00)89299-7
Kaouadji, M., Jean, F. B., and Mariotte, A. M. (1979). Herpetriol and Herpetetrol, new lignoids isolated from Herpetospermum caudigerum Wall. Z. Naturforsch., C. J. Biosci. 34, 1129–1132. doi:10.1515/znc-1979-1208
Kaouadji, M., Jean, F. B., Sarrazin, F., and Davoust, D. (1987). Herpetetrone, another tetrameric lignoid from Herpetospermum caudigerum seeds. J. Nat. Prod. 50, 1089–1094. doi:10.1021/np50054a013
Kaouadji, M., and Pieraccini, E. (1984a). Herpetetradione, nouveau lignoide tetramere isoled’Herpetospermum caudigerum wall. Tetrahedron Lett. 25, 5135–5136. doi:10.1016/s0040-4039(01)81544-6
Kirsch, G., Abdelwahab, A. B., and Chaimbault, P. (2016). Natural and synthetic coumarins with effects on inflammation. Molecules 21, 1322. doi:10.3390/molecules21101322
Kong, F. M., Wang, C. R., Zhao, L. L., Liao, D. Y., Wang, X. Q., Sun, B. X., et al. (2023). Traditional Chinese medicines for non-small cell lung cancer: therapies and mechanisms. Chin. Herb. Med. 15 (4), 509–515. doi:10.1016/j.chmed.2023.05.004
Li, C. Q., Wang, Z. Y., Tang, H. J., Wang, C. D., and Zhang, M. (2015a). Protective effects of Herpetospermum polysaccharide on immunological liver injury induced by concanavalin A in mice. Pharmacol. Clin. Chin. Mater. Med. 31 (04), 94–97. doi:10.13412/j.cnki.zyyl.2015.04.029
Li, G., Wang, X. Y., Suo, Y. R., and Wang, H. L. (2014). Protective effect of seed oil of Herpetospermum pedunculosum against carbon tetrachloride-induced liver injury in rats. Saudi Med. J. 35 (9), 981–987.
Li, J., Lu, Q., Peng, M., Liao, J., Zhang, B., Yang, D., et al. (2023). Water extract from Herpetospermum pedunculosum attenuates oxidative stress and ferroptosis induced by acetaminophen via regulating Nrf2 and NF-κB pathways. J. Ethnopharmacol. 305, 116069. doi:10.1016/j.jep.2022.116069
Li, J. J., Cheng, L., Shen, G., Qiu, L., Shen, C. Y., Zheng, J., et al. (2018). Improved stability and oral bioavailability of Ganneng dropping pills following transforming lignans of Herpetospermum caudigerum into nanosuspensions. Chin. J. Nat. Med. 16 (1), 70–80. doi:10.1016/S1875-5364(18)30031-1
Li, L. Y., Shu, S., Wei, Y. F., Zhong, G. Y., and Chen, J. H. (2005). Morphological and histological studies of Herpetospermum pedunculosum seeds and other substitutes. China J. Chin. Mater. Med. 14, 1073–1076.
Li, M. H., Feng, X., Deng Ba, D. J., Chen, C., Ruan, L. Y., Xing, Y. X., et al. (2019). Hepatoprotection of Herpetospermum caudigerum Wall. against CCl4-induced liver fibrosis on rats. J. Ethnopharmacol. 229, 1–14. doi:10.1016/j.jep.2018.09.033
Li, N. Z., Shi, Y. W., Guo, M. X., Zhu, J. X., Liang, J., and Feng, Y. L. (2023). Study on protective effect and mechanism of total lignans from Tibetan medicine Herpetospermun caudigerum Wall against α-Naphthylisothiocyanate-induced liver injury in mice. Tradit. Chin. Drug Res. Clin. Pharmacol. 34 (01), 49–56. doi:10.19378/j.issn.1003-9783.2023.01.007
Li, Y. L., Zhou, F. C., Wang, H. L., Ding, C. X., and Suo, Y. R. (2005). Analysis of amino acids in Tibetan medicine. Herpetospermum penduculosum Biot. Resour. 04, 11–12.
Liao, J. Q. (2023). Molecular mechanism research of ethanol extract of Herpetospermum pedunculosum seeds alleviating acetaminophen-induced liver injury. Master. Sichuan: Chengdu University.
Ling, H. L., Liu, M. L., and Zhang, M. (2018). Simultaneous determination of four fatty acids in the fatty oil from Bolenggua seeds by GC-MS. Asia-Pacific Tradit. Med. 14, 37–40.
Liu, F., Zhu, M. Q., and Liu, W. Y. (2022). Hepatoprotective effects of total sterols from Herpetospermum caudigerum Wall. shell on CCl4-induced acute liver injury in mice and quantification of major ingredients by RP-HPLC. World Sci. Technology-Modernization Traditional Chin. Med. 24, 2319–2330.
Liu, J., Chen, X., Zhang, Y., and Zhang, M. (2010). Chemical constituents of the ethyl acetate extract from Semen herpetospermi. Pharmacol. Clin. Chin. Mater. Med. 1 (03), 15–18.
Liu, M. L. (2017a). Primary studies on the methods for quality control and liver injury of active fractions of Herpetospermum caudigerum. Master. Sichuan: Chengdu University of Traditional Chinese Medicine.
Liu, S. Y. (2017b). Study on the protective effect and mechanism of herpetin, an effective component of Tibetan medicine Herpetospermum caudigerum Wall, on immune liver injury. Master. Sichuan: Southwest Minzu University.
Liu, W., Fu, X. H., Huang, S. Y., Shi, Y. C., Shi, L. L., and Gu, J. (2017a). Protective effect and mechanism of total lignans from Herpetospermum seeds on carbon tetrachloride-induced liver fibrosis in rats. China J. Chin. Mater. Med. 42 (3), 567–571. doi:10.19540/j.cnki.cjcmm.20161222.063
Liu, X. L., Han, Y. P., Xu, X. S., and Song, X. W. (2005). Analysis of fat oil from Bolenggua seeds by GC-MS. J. Southwest Univ. Nat. Sci. 05, 710–711.
Ma, Y. X. (2020). Study on the chemical constitutions of the ethyl acetate extract from Herpetospermum pedunculosum. Master. Sichuan: Southwest University.
Meng, F. C., Ma, Y. X., Zhan, H. H., Zong, W., Linghu, L., Wang, Z., et al. (2022). Lignans from the seeds of Herpetospermum pedunculosum and their farnesoid X receptor-activating effect. Phytochemistry 193, 113010. doi:10.1016/j.phytochem.2021.113010
Patel, D. K. (2021). Pharmacological activities and therapeutic potential of kaempferitrin in medicine for the treatment of human disorders: a review of medicinal importance and health benefits. Cardiovasc Hematol. Disord. Drug Targets 21 (2), 104–114. doi:10.2174/1871529X21666210812111931
Qian, Y. Z., Han, J., Liu, L. P., Zhang, Y. Y., Guo, J. J., and Yuan, H. L. (2011). Determination of Herpetrione in Herpetospermum caudigerum by ultrahigh performance liquid chromatography. Pharm. J. Chin. People's Lib. Army. 27, 527–528+531.
Shen, B., Chen, H. G., Shen, C. Y., Xu, P. H., Li, J. J., Shen, G., et al. (2015). Hepatoprotective effects of lignans extract from Herpetospermum caudigerum against CCl4-induced acute liver injury in mice. J. Ethnopharmacol. 164, 46–52. doi:10.1016/j.jep.2015.01.044
Shen, G., Chen, L., Wang, L. Q., Zhong, L. H., Shen, B. D., Liao, W. B., et al. (2016). Formulation of dried lignans nanosuspension with high redispersibility to enhance stability, dissolution, and oral bioavailability. Chin. J. Nat. Med. 14 (10), 757–768. doi:10.1016/S1875-5364(16)30090-5
Wang, H. (2005). Studies on the chemical components in bioactive part and HPLC fingerprint of the Tibetan medicinal substance Herpetospermum seed. Master. Sichuan: Chengdu University of Traditional Chinese Medicine.
Wang, L., Liu, S. Y., Sun, N. Y., Li, J. C., and Gu, J. (2016). Protection of herpetin from Tibetan medicine Herpetospermum seeds on ConA-induced immunologic liver injury in mice. J. Southwest Univ. Nat. Sci. 42, 492–495.
Wang, L. X., Wang, H. L., Huang, J., Chu, T. Z., Peng, C., Zhang, H., et al. (2022a). Review of lignans from 2019 to 2021: newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry 202, 113326. doi:10.1016/j.phytochem.2022.113326
Wang, P. L. (2014). Research on quality control of the seeds of Herpetospermum pedunculosum (Sex.). Master. Chongqing: Southwest University.
Wang, S. W., Li, Y. X., Du, C. Y., Fan, H. B., Wu, X. Q., Chen, X., et al. (2022b). Chemical constituents, anti-hyperuricemic and anti-gouty arthritis activities of extract of Herpetospermum caudigerum. Pharmacol. Res. 3, 100102. doi:10.1016/j.prmcm.2022.100102
Wei, X. D. (2020). Study on FXR-mediated anti-cholestasis effect and mechanism of the seeds of Herpetospermum pedunculosum. Master. Chongqing: Southwest University.
Wei, X. D., Fan, X. D., Feng, Z. Y., Ma, Y. X., Lan, X. Z., and Chen, M. (2020). Ethyl acetate extract of Herpetospermum pedunculosum alleviates α-naphthylisothiocyanate-induced cholestasis by activating the farnesoid x receptor and suppressing oxidative stress and inflammation in rats. Phytomedicine 76, 153257. doi:10.1016/j.phymed.2020.153257
Wu, Y., Xu, J., Liu, Y. T., Zeng, Y. Y., and Wu, G. J. (2020). A review on anti-tumor mechanisms of coumarins. Front. Oncol. 10, 592853. doi:10.3389/fonc.2020.592853
Xiao, S. L., Tian, Z. Y., Wang, Y. F., Si, L. L., Zhang, L. Z., and Zhou, D. M. (2018). Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 38 (3), 951–976. doi:10.1002/med.21484
Xu, B. (2012). Studies on the chemical constituents of Herpetospermum caudigerum Wall. Master. Chongqing: Southwest University.
Xu, B., Liu, S., Fan, X. D., Deng, L. Q., Ma, W. H., and Chen, M. (2015). Two new coumarin glycosides from Herpetospermum caudigerum. J. Asian Nat. Prod. Res. 17 (7), 738–743. doi:10.1080/10286020.2014.996137
Xu, W. H., Zhao, P., Wang, M., and Liang, Q. (2019b). Naturally occurring furofuran lignans: structural diversity and biological activities. Nat. Prod. Res. 33 (9), 1357–1373. doi:10.1080/14786419.2018.1474467
Xu, X., Lu, H. G., Xie, X., Liu, Y. T., Zhao, H. Q., Lei, C., et al. (2023). Ershiwuwei Songshi Pills improve CCL4-induced liver fibrosis in rats by regulating macrophage phenotype. Pharmacol. Clin. Chin. Mater. Med. 39, 17–23. doi:10.13412/j.cnki.zyyl.20230525.002
Xu, X. Y., Wang, D. Y., Li, Y. P., Deyrup, S. T., and Zhang, H. J. (2022). Plant-derived lignans as potential antiviral agents: a systematic review. Phytol. Clin. Chin.Mater.Med. 21 (1), 239–289. doi:10.1007/s11101-021-09758-0
Xu, Z., Zhao, S., Lv, Z., Feng, L., Wang, Y., Zhang, F., et al. (2019a). Benzofuran derivatives and their anti-tubercular, anti-bacterial activities. Eur.J. Med. Chem. 162, 266–276. doi:10.1016/j.ejmech.2018.11.025
Yang, B., Luo, Q. L., Wang, N., Hu, Y. T., Zheng, W. X., Li, H., et al. (2023). HPF modulates the differentiation of BMSCs into HLCs and promotes the recovery of acute liver injury in mice. Int. J. Mol. Sci. 24 (6), 5686. doi:10.3390/ijms24065686
Yang, F., Zhang, H. J., Zhang, Y. Y., Chen, W. S., Yuan, H. L., and Lin, H. W. (2010). A hepatitis B virus inhibitory neolignan from Herpetospermum caudigerum. Chem. Pharm. Bull. 58 (3), 402–404. doi:10.1248/cpb.58.402
Yu, J. Q., Hang, W., Duan, W. J., Wang, X., Wang, D. J., and Qin, X. M. (2014). Two new anti-HBV lignans from Herpetospermum caudigerum. Phytochem. Lett. 10, 230–234. doi:10.1016/j.phytol.2014.10.001
Yuan, H. L. (2006a). Extracts from Herpetospermum caudigerum Wall, their Dropping Pills, preparation methods, and applications. Patent. No: CN1857367A.
Yuan, H. L., Guo, J. J., Li, X. Y., Han, J., Lv, J. L., and Fu, S. S. (2011). Preparation and application of herperione and its capsule. Patent. No: CN102140101A.
Yuan, H. L., Liu, Y., Zhao, Y. L., and Xiao, X. H. (2005). Herpetin, a new bioactive lignan isolated from Herpetospermum caudigerum. J. Chin. Pharm. Sci. 03, 140–143.
Yuan, H. L., Yang, M., Li, X. Y., You, R. H., Liu, Y., Zhu, J., et al. (2006b). Hepatitis B virus inhibiting constituents from Herpetospermum caudigerum. Chem. Pharm. Bull. 54 (11), 1592–1594. doi:10.1248/cpb.54.1592
Zhang, M., Deng, Y., Zhang, H. B., and Chen, H. L. (2007). Herpetolide, preparation method and application. Patent. No: CN101311173.
Zhang, M., Deng, Y., Zhang, H. B., Su, X. L., Chen, H. L., Yu, T., et al. (2008). Two new coumarins from Herpetospermum caudigerum. Chem. Pharm. Bull. 56, 192–193. doi:10.1248/cpb.56.192
Zhang, M., Dong, X. P., Deng, Y., Wang, H., Li, X. N., and Song, Q. (2006). A new sesqui-norlignan from Herpetospermum pedunculosum. Acta Pharmacol. Sin. 41 (07), 659–661. doi:10.16438/j.0513-4870.2006.07.015
Zhang, M., Dong, X. P., Wang, H., Li, X. N., and Song, Q. (2004). GC-MS analysis on the fatty oils of the Tibetan medicinal substance Herpetospermum. J. Chengdu Univ. Tradit. Chin. Med. 04, 49–52.
Zhang, Y., Lin, S., Peng, J., Liang, X., Yang, Q., Bai, X., et al. (2022). Amelioration of hepatic steatosis by dietary essential amino acid-induced ubiquitination. Mol. cell 82 (8), 1528–1542.e10. doi:10.1016/j.molcel.2022.01.021
Zhao, X. E., Liu, R. J., Wang, H. L., and Su, Y. R. (2009). HPLC and GC-MS analysis of fatty acids Tibetan medicine Herpetospermum seed oil. Nat. Prod. R&D. 21 (01), 76–83. doi:10.16333/j.1001-6880.2009.01.022
Zhao, X. R., Zhang, L., Li, J. C., and Gu, J. (2015). Protective effect of total lignans from Tibetan medicinal Herpetospermum seeds on experimental liver injury by CCl4. J. Southwest Univ. Nat. Sci. 41 (02), 181–185.
Zhong, Y., Zhang, B. W., Li, J. T., Zeng, X., Pei, J. X., Zhang, Y. M., et al. (2023). Ethanol extract of Herpetospermum caudigerum Wall ameliorates psoriasis-like skin inflammation and promotes degradation of keratinocyte-derived ICAM-1 and CXCL9. J. Integr. Med. 21 (6), 584–592. doi:10.1016/j.joim.2023.11.004
Keywords: Herpetospermum pedunculosum (Ser.) C.B. Clarke, phytochemistry, pharmacological activity, liver protection, lignan
Citation: Jiang Z, Zhang C, Yu X, Wang K, Sang Z, Gong W, Zhang Q, Meng X, Qin L and Zhao Q (2024) Traditional utilization, botany, phytochemistry, pharmacology, pharmaceutical analysis, processing and application of the seeds of Herpetospermum pedunculosum (Ser.) C.B. Clarke: a comprehensive review. Front. Pharmacol. 15:1498768. doi: 10.3389/fphar.2024.1498768
Received: 19 September 2024; Accepted: 26 November 2024;
Published: 19 December 2024.
Edited by:
Hongbo Li, Shaanxi University of Science and Technology, ChinaReviewed by:
Claudio Frezza, Università Link Campus, ItalyChangfu Wang, Guangdong Pharmaceutical University, China
Copyright © 2024 Jiang, Zhang, Yu, Wang, Sang, Gong, Zhang, Meng, Qin and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiongyu Meng, bWVuZ3hpb25neXVAemNtdS5lZHUuY24=; Lupin Qin, bHBxaW5AemNtdS5lZHUuY24=; Qiming Zhao, cW16aGFvQHpjbXUuZWR1LmNu
†These authors have contributed equally to this work
 Zhixia Jiang†
Zhixia Jiang† Qiaoyan Zhang
Qiaoyan Zhang Lupin Qin
Lupin Qin Qiming Zhao
Qiming Zhao