
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 27 November 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1497072
This article is part of the Research Topic Prevention and Treatment of Infectious Diseases by Herbal Medicine View all 10 articles
Background: Dachaihu decoction (Dachaihu tang) plays a crucial role in treating acute illnesses. Recently, a significant number of clinical studies on Dachaihu decoction for acute cholecystitis (AC) have been published. This study was conducted to assess the efficacy and safety of Dachaihu decoction in patients with this condition.
Methods: To identify relevant randomized controlled trials (RCTs), eight databases and three clinical trial registries were searched from inception to 30 June 2024. Two researchers independently screened and extracted data from eligible studies using EndNote X9 and Microsoft Office Excel 2019. RoB 2.0 was used to assess the risk of bias in the included studies. Stata 17.0 was used for data analysis. Publication bias and its impact on result stability were evaluated using a funnel plot and the “trim-and-fill” method. The quality of evidence was graded using the GRADE assessment system.
Results: Thirty-three RCTs involving 2,851 participants were included. The treatment group demonstrated improved clinical efficacy (RR = 1.18; 95% CI = 1.13 to 1.24), significantly reduced length of hospital stay (MD = −1.78 days; 95% CI = –2.02 to −1.53), and the incidence of adverse events (RR = 0.31; 95% CI = 0.20 to 0.48). Additionally, there appeared to be reductions in the time for abdominal pain to resolve (MD = −1.92 days; 95% CI = –2.33 to −1.51), fever to disappear (MD = −1.52 days; 95% CI = –1.90 to −1.14), white blood cell count to return to normal (MD = −2.89 days; 95% CI = –3.32 to −2.46), alanine aminotransferase (ALT) levels (MD = −11.88 U/L; 95% CI = –15.29 to −8.47), aspartate aminotransferase (AST) levels (MD = −8.74 U/L; 95% CI = –9.76 to −7.72), neutrophil percentage (MD = −9.68; 95% CI = –11.33 to −8.03), TNF-α levels (SMD = −2.10 pg/L; 95% CI = –2.43 to −2.78), and certainty of evidence (moderate-to-low certainty).
Conclusion: Dachaihu decoction may be an effective botanical formula for managing AC and a lower incidence of adverse events. However, due to the substantial risk of bias and heterogeneity across the included studies, these findings should be interpreted with caution and require further validation through well-designed, high-quality trials.
Systematic Review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=573332.
Acute cholecystitis (AC) is an acute infectious gallbladder disease caused by cystic duct obstruction, chemical stimulation, and bacterial infection. It represents 10% of all acute abdominal cases in clinical practice (Abdulrahman et al., 2022; Kimura et al., 2007; China Association Of Integrative Medicine Emergency Medicine Committee et al., 2019). The clinical manifestations include paroxysmal colic in the right upper abdomen, often accompanied by nausea, vomiting, fever, and jaundice. The incidence of AC increases among individuals aged 50 and above, with an overall mortality rate of approximately 3%, increasing significantly in patients with comorbidities. In cases without gallstones, mortality can escalate to 15%–40% (Bedirli et al., 2001; González-Castillo et al., 2021). This higher risk is not only due to AC being secondary to critical illnesses—such as trauma, surgery, shock, burns, sepsis, total parenteral nutrition, and mechanical ventilation—but also because it is more likely to lead to gangrene, perforation, and pyothorax, or empyema compared to calculous cholecystitis (Kimura et al., 2007; Gluhovschi et al., 2023).
AC arises from a confluence of factors, including systemic inflammatory responses, multiorgan dysfunction, and gallbladder damage caused by gallstones and infection (Markaki et al., 2021; Nitzan et al., 2017; Regimbeau et al., 2014; Halpin, 2014). Treatment typically involves medication (such as anti-infection agents, antispasmodics, and analgesics) and surgical interventions. However, in elderly patients with comorbidities, a conservative approach is favored as it effectively relieves symptoms and controls infection during the acute phase (Mencarini et al., 2024). Despite targeting the initial pathological event, there are no specific agents for AC treatment. The risk of postoperative infectious complications in grade 1 and 2 AC is approximately 17% (Regimbeau et al., 2014). The role of antibiotics and surgical interventions, whether administered early in the disease or during the perioperative period, remains a topic of debate (Singh et al., 2023; Dietrich et al., 2024). Therefore, on the basis of modern medical treatments, exploring alternative treatments for AC is crucial.
A large number of traditional Chinese medicine (TCM) formulas are utilized in Chinese clinical practice to treat diseases with complex mechanisms. Recently, the focus has shifted toward combination therapies involving multi-target and multi-component drugs, which has become fundamental in exploring the molecular mechanisms of TCM prescription components for disease treatment (Li et al., 2023). TCM formulas have demonstrated significant efficacy and safety over thousands of years of clinical practice (Sheehan et al., 1992; Deng and Xu, 2017). Dachaihu decoction (Dachaihu Tang), a classic TCM formula, was documented in the Treatise on Febrile Diseases by Zhang Zhongjing, a prominent traditional Chinese physician from 150 to 219 AD. Comprising eight botanical drugs, namely, Bupleurum chinense DC. [Apiaceae; Bupleuri radix], Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae radix], Citrus aurantium L. [Rutaceae; Citri aurantii fructus immaturus], Pinellia ternata (Thunb.) Makino [Araceae; Pinelliae rhizoma], Rheum officinale Baill. [Polygonaceae; Rhei radix et rhizoma], Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae radix alba], Zingiber officinale Roscoe [Zingiberaceae; Zingiberis rhizoma], and Ziziphus jujuba Mill. [Rhamnaceae; Ziziphi jujubae fructus], it is noted for its ability to relieve abdominal pain, reduce fever, alleviate gastrointestinal symptoms, and lower inflammation (Bi et al., 2023).
In 1994, Japanese Kampo medicine scholars first demonstrated its capability to ameliorate liver injury in a mechanistic study of Dachaihu decoction (Ji et al., 2024). The main bioactive components include saikosaponin B2, baicalin, baicalein, epiberberine, naringin, hesperidin, neohesperidin, and nobiletin. These efficacy markers may influence various signaling pathways such as MAPK, NF-κB, and Toll-like receptors by targeting molecules like JUN, TNF, EGFR, MAPK3, RELA, TNF, HIF1A, AKT1, IGF1R, CREB1, and SIRT1, HIF-1, PI3K-AKT, and AMPK, thus regulating the ERK1/ERK2 cascade, NF-κB transcription factor activity, inflammatory responses, glucose metabolism, angiogenesis, and adipocyte differentiation (Mao et al., 2017; Ohta et al., 1995). Dachaihu decoction protects the liver, promotes bile flow, and exerts anti-inflammatory effects, making it a commonly prescribed botanical drug treatment for AC among clinicians (Bi et al., 2023; Zhou, 2020). It can reduce inflammatory responses and alleviate gastrointestinal symptoms in patients with AC by regulating CRP levels and inflammatory factors (TNF-α, IL-6, and IL-8), without apparent side effects (Liu et al., 2021). Given the global prevalence of AC, the high rate of perioperative infections, and the limitations of Western medications such as anti-infective drugs and analgesics in managing this condition, along with extensive clinical study data from clinical studies, there is a pressing need for a comprehensive review of existing research. This study aims to review the relevant literature to summarize previous findings on the efficacy of Dachaihu decoction alone or in combination with conventional treatment for managing AC, thereby providing a deeper understanding of its overall efficacy and safety in AC treatment.
This study has been registered on the PROSPERO platform (Registration No.: CRD42024573332; https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=573332).
(1) Participants: Individuals aged 18 years or older who met the diagnostic criteria for AC, as outlined in the Tokyo Guidelines (2018) (Kimura et al., 2007), including ① obvious right upper-abdominal pain with tenderness or a positive Murphy sign; ② fever, elevated C-reactive protein, or elevated white blood cell count; and ③ imaging examination suggested acute cholecystitis. The diagnosis of acute cholecystitis could be confirmed if any one of the criteria in ①, ②, and ③ was met. Exclusion criteria encompassed patients with severe cardiovascular, hepatic, or renal insufficiency; malignant tumors or significant organic lesions; a history of severe neurological dysfunction or mental illness; and pregnant or lactating women, or those allergic to Dachaihu decoction.
(2) Interventions: The treatment group received Dachaihu decoction alone/or in combination with the conventional treatment (CT)/laparoscopic cholecystectomy (LC). The composition of Dachaihu decoction includes B. chinense DC. [Apiaceae; B. radix], S. baicalensis Georgi [Lamiaceae; S. radix], C. aurantium L. [Rutaceae; C. aurantii fructus immaturus], P. ternata (Thunb.) Makino [Araceae; P. rhizoma], R. officinale Baill. [Polygonaceae; R. radix et rhizoma], P. lactiflora Pall. [Paeoniaceae; P. radix alba], Z. officinale Roscoe [Zingiberaceae; Z. rhizoma], and Z. jujuba Mill. [Rhamnaceae; Z. jujubae fructus]. All botanical drugs were selected and reported according to the guidelines of the “Consortium for Phytochemical Characterization of Medicinal Plants (ConPhyMP)” (Heinrich et al., 2022; Heinrich et al., 2020). Moreover, in accordance with the modification principles of TCM, there were no restrictions on the dosage (grams), slight adjustments, formulation, or the route of administration for this decoction. Information on the botanical drugs, standard dosage (grams), and decoction preparation method of Dachaihu decoction is given in Table 1 and Supplementary Material S1.
(3) Comparisons: The control group was treated with CT, including antibiotics (β-lactams, 4-quinolones, and nitroimidazoles), along with antispasmodic, hepatoprotective, and choleretic drugs. Surgical intervention (LC), specifically laparoscopic cholecystectomy, was carried out when indicated.
(4) Outcomes: ① Clinical efficacy was assessed using criteria adapted from the Guiding Principles for Clinical Research of New Chinese Medicines (Ministry of Health of the PRC, 2002): cured—significant improvement in symptoms and signs, evidence point reduction ≥95%; obvious efficacy—notable improvement in symptoms and signs, evidence point reduction ≥70% but <95%; efficacy—some improvement in symptoms and signs, evidence point reduction ≥30% but <70%; inefficacy—no significant improvement in symptoms and signs, evidence point reduction <30%; calculated using the nimodipine method—efficacy index (%) = [(pre-treatment points - post-treatment points)/pre-treatment points × 100%]. ② Time for resolution of abdominal pain. ③ Time for resolution of fever. ④ Time for white blood cell counts to normalize. ⑤ Length of hospital stay. ⑥ Levels of alanine aminotransferase (ALT). ⑦ Levels of aspartate aminotransferase (AST). ⑧ Neutrophil percentage (NEUT%). ⑨ Levels of tumor necrosis factor-α (TNF-α). ⑩ Incidence of adverse events. Primary outcomes included ⑤and⑩, and secondary outcomes included ①, ②, ③, ④, ⑥, ⑦, ⑧, and ⑨.
(5) Study design: All randomized controlled trials (RCTs).
Two researchers (Xin-xin Liu and You-zhu Su) systematically searched eight databases, i.e., four English databases (Web of Science, Cochrane Database of Systematic Reviews, PubMed, and EMBASE) and four Chinese databases (CNKI, Wanfang, VIP, and SinoMed). Additionally, three clinical trial registration platforms (International Clinical Trials Registry Platform, ClinicalTrials.gov, and Chinese Clinical Trial Registry) were searched from their inception to 30 June 2024. The search terms included “acute cholecystitis” and “Dachaihu decoction.” There were no language restrictions. The complete search strategy is given in Supplementary Material S2.
Two researchers (Xin-xin Liu and Ying-qi Ma) independently reviewed the literature, extracted data, and cross-checked the findings. Any disagreements were resolved by consulting a third reviewer (Jian-ping Liu). The initial screening involved reviewing titles and abstracts to filter out irrelevant studies, followed by a full-text review to decide on final inclusion. The extracted data covered the first author, year of publication, sample size, gender and age of participants, symptom onset, intervention types, treatment duration, and outcomes. In this study, species classification and validation of the phytomedicines in Dachaihu decoction were performed using the ConPhyMP tool to meet drug classification standards.
Two researchers (Xin-xin Liu and Ling-yao Kong) independently evaluated the risk of bias in the included studies using the Cochrane’s Risk of Bias 2.0 tools, and the results were cross-checked. Any disagreements were resolved through consultation with a third reviewer (Jian-ping Liu). The assessment criteria included random sequence generation, allocation concealment, blinding (of implementers, participants, and outcome evaluators), incomplete outcome data, selective reporting, and other potential bias sources. Risks were categorized as “low risk,” “high risk,” or “some concerns” based on this assessment.
A meta-analysis was conducted using Stata 17.0 software, with the results reported as 95% confidence intervals (95% CIs) and statistical significance defined as p < 0.05. (1) Heterogeneity across studies was assessed using the I2 test. A fixed-effect model was used when heterogeneity was low (p > 0.1; I2 < 50%), while a random-effects model was applied for high heterogeneity (p < 0.1; I2 > 50%). (2) For a dichotomous variable, the effect size was calculated using relative risk (RR), and for continuous variables, mean difference (MD) was used. When studies used different units of measurement, standardized mean difference (SMD) was applied.
Subgroup analyses were carried out according to the type of intervention to evaluate the consistency and reliability of the results. Sensitivity analyses were used to assess whether the results and heterogeneity changed when a single study was excluded.
Publication bias was assessed if the funnel plot showed asymmetry or if the Egger test indicated bias (p < 0.05). In such cases, the “trim-and-fill” method was used to estimate the adjusted combined effect size.
The certainty of evidence for an outcome measure was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) assessment system. Two researchers (Xin-xin Liu and Ling-yao Kong) independently evaluated GRADE. Any disagreements were resolved by consulting a third reviewer (Jian-ping Liu). This evaluation considers five factors: risk of bias, consistency of results, indirectness of evidence, imprecision, and potential publication bias. Based on these criteria, the evidence was classified as “high,” “moderate,” “low,” or “very low” (Grade Working Group, 2017).
A comprehensive computerized search retrieved 549 studies (121 studies in CNKI, 163 in VIP, 153 in Wanfang, 97 in SinoMed, 1 in PubMed, 1 in Embase, 0 in Web of Science, 13 in Cochrane Library, 0 in International Clinical Trials Registry Platform, 0 in ClinicalTrials.gov, and 0 in Chinese Clinical Trial Registry). A total of 296 duplicate records were removed, and full texts of the remaining 253 studies were reviewed. Studies that did not meet the inclusion criteria were excluded. Ultimately, 33 RCTs (Zhou, 2020; Liu et al., 2021; Cai, 2022; Chen et al., 2023; Deng, 2018; Deng et al., 2018; Gu, 2021; Guo, 2012; Guo, 2023; Hong and Gao, 2018; Li, 2016; Liang and Deng, 2013; Liang et al., 2013; Liao, 2014; Liu, 2021; Liu, 2015; Su, 2016; Sun, 2005; Wang et al., 2020; Wang and Li, 2014; Wang, 2010; Wei, 2016; Xia, 2020; Xiong and Yi, 2021; Xu, 2017; Yang, 2016; Yu, 2009; Zhang et al., 2003; Zhang, 2018; Zhou, 2019; Zhu, 2017; Zhuang, 2021; Zuo, 2021) were included. The flow diagram of the selection process is shown in Figure 1.
The selected studies, published between 2003 and 2023, accounted for 81.81% (27/33) of the studies conducted in the past decade. The 33 RCTs included 2,851 Chinese patients diagnosed with AC, comprising 1,404 male and 1,447 female individuals. Sample sizes ranged from 40 to 206 cases. Nineteen studies did not specify the duration of the onset of illness, and the intervention was Dachaihu decoction or a combination of conventional treatment/LC, with the treatment duration ranging from 5 to 30 days. Outcomes included time to the disappearance of clinical symptoms, inflammation levels, reports of adverse events, clinical efficacy, length of hospital stay, and liver function. Study characteristics are given in Table 2.
Among the 33 RCTs, 16 (Liu et al., 2021; Deng et al., 2018; Gu, 2021; Hong and Gao, 2018; Liao, 2014; Liu, 2021; Liu, 2015; Wang et al., 2020; Wang and Li, 2014; Xia, 2020; Xiong and Yi, 2021; Xu, 2017; Yang, 2016; Zhang, 2018; Zhu, 2017; Zhuang, 2021) used random number tables, 1 (Chen et al., 2023) utilized lotteries, and 1 (Zhou, 2020) adopted two-color ballots, while the remaining 15 merely mentioned “random.” All studies were comparable at baseline; however, none provided details on the implementation of allocation concealment, making it unclear whether it was properly executed. As such, this aspect was rated as having “some concerns” regarding randomization. Furthermore, none of the studies reported blinding of participants or outcome assessors, leading to a rating of “some concerns” for potential deviation from the intended intervention. Despite these limitations, all included studies presented complete data, thus receiving a “low-risk” rating for data completeness. In terms of outcome assessment, 26 studies were rated as “low risk,” whereas 7 (Liu et al., 2021; Li, 2016; Liang and Deng, 2013; Liu, 2015; Wang et al., 2020; Wang and Li, 2014; Wei, 2016) were considered “high risk” due to inappropriate measurement methods. Regarding selective reporting, only one study was rated as “low risk,” while the others lacked registration program documentation, resulting in a “some concerns” rating due to potential selective reporting bias (Figures 2, 3).
Among the 33 included studies, 14 (Zhou, 2020; Liu et al., 2021; Chen et al., 2023; Deng et al., 2018; Li, 2016; Liang and Deng, 2013; Liu, 2015; Sun, 2005; Wang et al., 2020; Wang and Li, 2014; Wei, 2016; Yu, 2009; Zhang, 2018; Zhou, 2019) (n = 1,219) assessed clinical efficacy, revealing a statistically significant difference between the groups (RR = 1.18; 95% CI = 1.13 to 1.24; I2 = 0.00%; Q (13) = 6.39; p = 0.00) (Figure 4A).
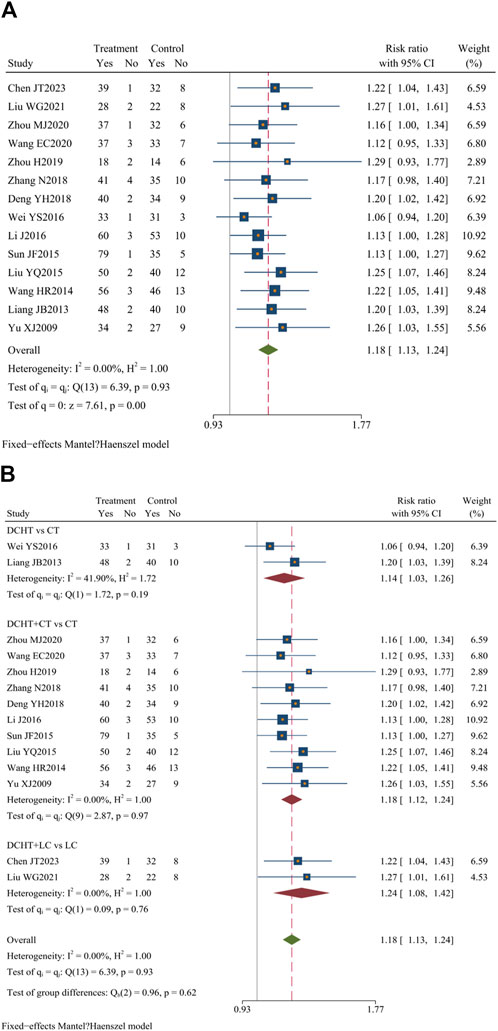
Figure 4. (A) Clinical efficacy, (B) Subgroup analysis of clinical efficacy. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
Subgroup analysis revealed that, compared with the CT group, the Dachaihu decoction group showed an improvement in clinical efficacy (RR = 1.14; 95% CI = 1.03 to 1.26; I2 = 41.90%). The combination of Dachaihu decoction and CT also showed notable improvements (RR = 1.18; 95% CI = 1.12 to 1.24; I2 = 0.00%). Similarly, combining Dachaihu decoction with LC significantly enhanced clinical efficacy (RR = 1.24; 95% CI = 1.08 to 1.42; I2 = 0.00%) Figure 4B.
Nineteen studies (Zhou, 2020; Liu et al., 2021; Cai, 2022; Chen et al., 2023; Deng, 2018; Deng et al., 2018; Gu, 2021; Guo, 2023; Liang and Deng, 2013; Liang et al., 2013; Liao, 2014; Liu, 2015; Wang and Li, 2014; Wang, 2010; Wei, 2016; Xia, 2020; Xiong and Yi, 2021; Yang, 2016; Yu, 2009) (n = 1,648) evaluated the time required for abdominal pain to disappear. The results showed that the treatment group significantly shortened the time for abdominal pain to subside compared to the control group (MD = −1.92 days; 95% CI = –2.33 to −1.51; I2 = 97.98%; Q (18) = 893.00; p = 0.00) (Figure 5A).
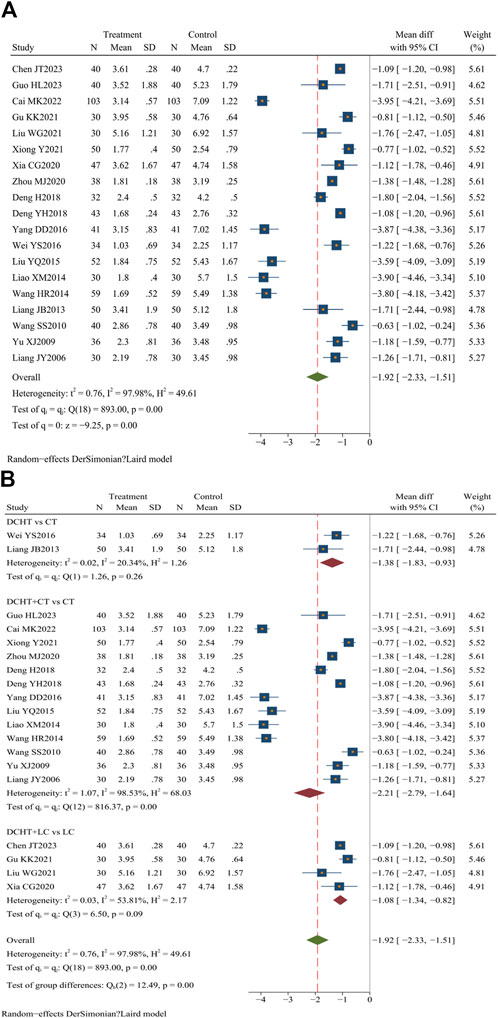
Figure 5. (A) Time for resolution of abdominal pain, (B) Subgroup analysis of time for resolution of abdominal pain. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
Subgroup analysis demonstrated that the Dachaihu decoction group significantly reduced the time for abdominal pain relief compared to the CT group (MD = −1.38 days; 95% CI = –1.83 to −0.93; I2 = 20.34%). The Dachaihu decoction combined with CT also significantly reduced abdominal pain duration (MD = −2.21 days; 95% CI –2.79 to −1.64; I2 = 98.53%). When combined with LC, the decoction further reduced the time for abdominal pain to disappear (MD = −1.08 days; 95% CI = −1.34 to −0.82; I2 = 53.81%) (Figure 5B).
In the Dachaihu decoction combined with CT compared to the CT group, we further analyzed the reason for heterogeneity. Two studies (Zhou, 2020; Liao, 2014) were excluded because the time of treatment was >28 days, one (Deng, 2018) was excluded as a result of patients being ≥80 years old, two (Cai, 2022; Liu, 2015) were excluded because the onset time was >3 days, and two studies (Liu et al., 2021; Xu, 2017) were excluded because they did not mention the type and dosage of anti-infective drugs. The heterogeneity was reduced when the above studies were excluded (I2 = 62%). There were still statistical differences (p < 0.05) (Supplementary Material S3).
Twenty studies (Zhou, 2020; Liu et al., 2021; Cai, 2022; Chen et al., 2023; Deng, 2018; Deng et al., 2018; Gu, 2021; Guo, 2023; Liang and Deng, 2013; Liang et al., 2013; Liao, 2014; Liu, 2015; Wang and Li, 2014; Wang, 2010; Wei, 2016; Xia, 2020; Xiong and Yi, 2021; Xu, 2017; Yang, 2016; Yu, 2009) (n = 1717) assessed the time for fever resolution. Compared with the control group, the Dachaihu decoction group appeared to significantly shorten the duration of fever in patients (MD = −1.52 days; 95% CI = –1.90 to −1.14; I2 = 95.86%; Q (19) = 459.11; p = 0.00) (Figure 6A).
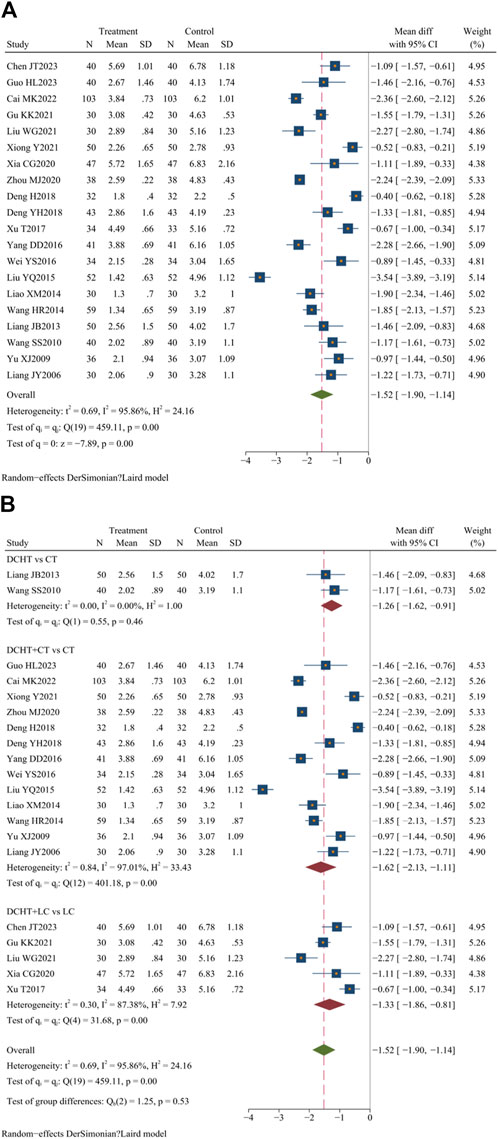
Figure 6. (A) Time for resolution of fever, (B) Subgroup analysis of time for resolution of fever. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
The subgroup analysis showed that compared with the CT group, the Dachaihu decoction group significantly reduced fever duration (MD = −1.26 days; 95% CI = –1.62 to −0.91; I2 = 0.00%). The combination of Dachaihu decoction and CT also significantly reduced fever duration (MD = −1.62 days; 95% CI = –2.13 to −1.11; I2 = 97.01%). When combined with LC, the duration of fever was significantly reduced (MD = −1.33 days; 95% CI = –1.86 to −0.81; I2 = 87.38%) (Figure 6B).
Further analysis of heterogeneity was conducted. In the Dachaihu decoction combined with the CT group versus the CT group, two studies (Zhou, 2020; Guo, 2012) were excluded due to treatment durations exceeding 28 days, one study (Deng, 2018) was excluded due to the inclusion of patients aged ≥80 years, and three studies (Cai, 2022; Liu, 2015; Xiong and Yi, 2021) were excluded because the onset time exceeded 3 days, resulting in reduced heterogeneity (I2 = 79.00%). In the Dachaihu decoction combined with the LC group versus the LC group, two studies (Liu et al., 2021; Xu, 2017) were excluded because they did not mention the type and dosage of anti-infective drugs, significantly reducing heterogeneity (I2 = 40%). Statistical differences remained (p < 0.05) (Supplementary Material S3).
Of the 33 included studies, 7 (Cai, 2022; Chen et al., 2023; Guo, 2023; Liu, 2015; Xia, 2020; Yang, 2016; Yu, 2009) (n = 704) assessed the time required for white blood cell counts to normalize. The results showed that the treatment group significantly reduced the time compared to the control group (MD = −2.89 days; 95% CI = –3.32 to −2.46; I2 = 83.26%; Q (6) = 35.84; p = 0.00) (Figure 7A).
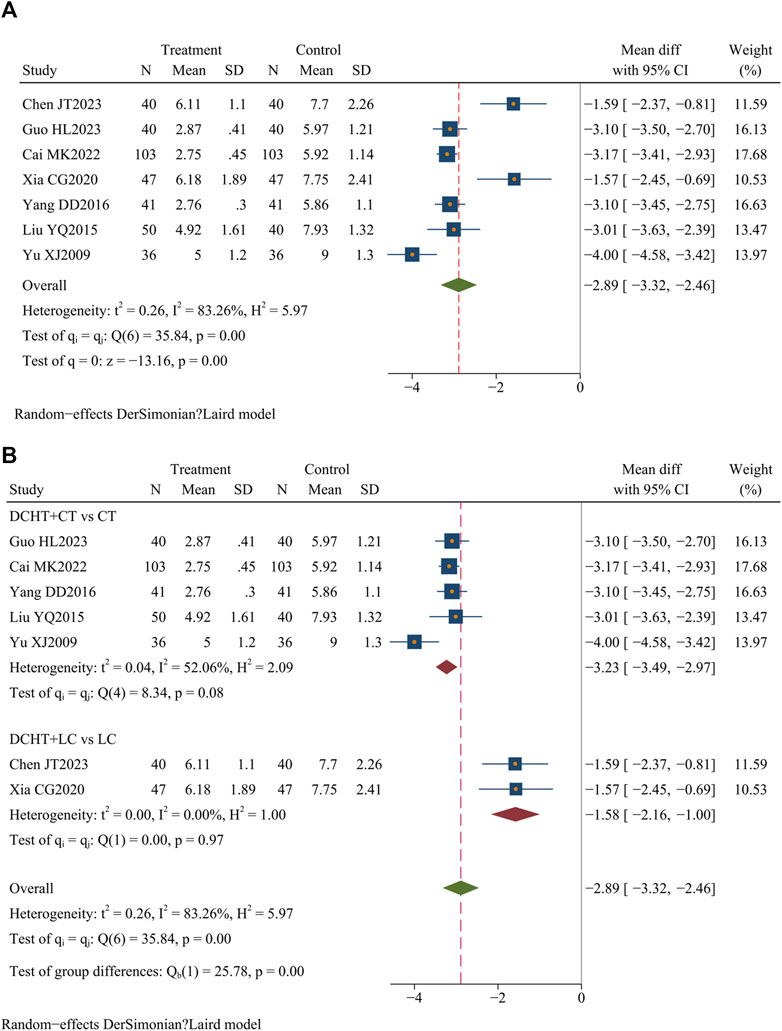
Figure 7. (A) Time for white blood cell counts to normalize, (B) Subgroup analysis of time for white blood cell counts to normalize. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
The subgroup analysis revealed that compared with the CT group, the Dachaihu decoction combined with CT significantly reduced the time required for white blood cell counts to normalize (MD = −3.23 days; 95% CI = –3.49 to −2.97; I2 = 52.06%). Compared with the LC group, the combination of Dachaihu decoction and LC also significantly shortened this time (MD = −1.58 days; 95% CI = –2.16 to −1.00; I2 = 0.00%) (Figure 7B).
In the Dachaihu decoction combined with the CT group versus the CT group, one study (Yu, 2009) was excluded due to the treatment duration exceeding 14 days and publication before 2010, significantly reducing heterogeneity (I2 = 0.00%). The results remained statistically significant (p < 0.05) (Supplementary Material S3).
Five studies (Chen et al., 2023; Gu, 2021; Guo, 2012; Xia, 2020; Xu, 2017) (n = 371) assessed the length of stay. The results demonstrated that the Dachaihu decoction significantly shortened the length of hospital stay compared to the control group (MD = −1.78 days; 95% CI = –2.02 to −1.53; I2 = 3.56%; Q (4) = 4.15; p = 0.00) (Figure 8A).
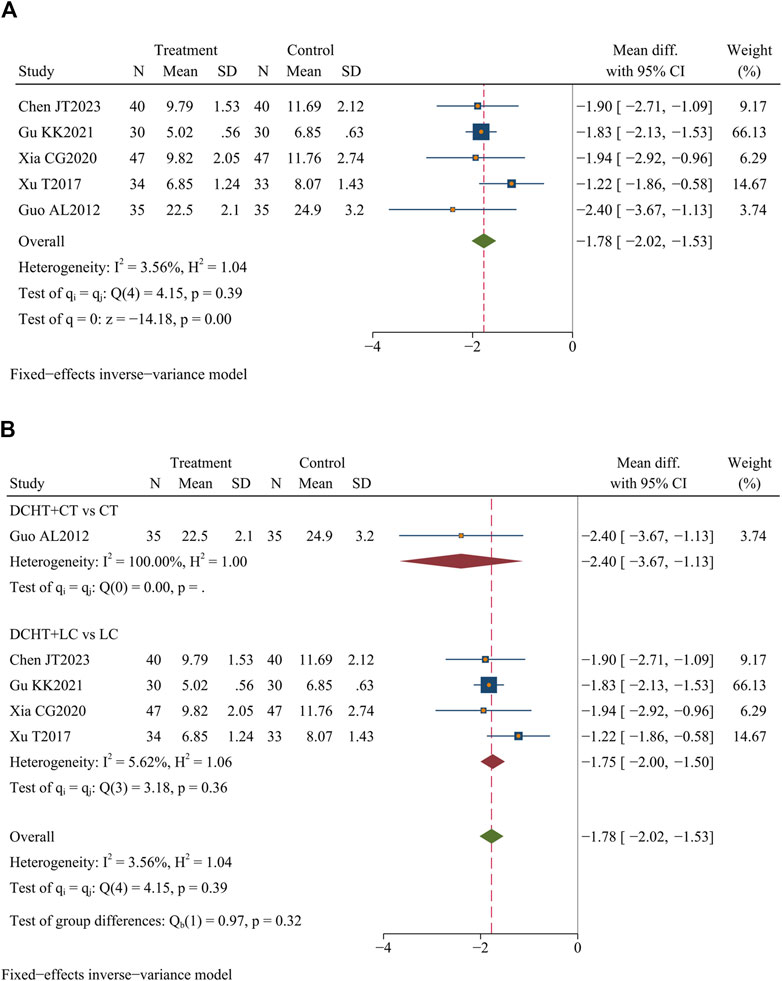
Figure 8. (A) Length of hospital stay, (B) Subgroup analysis of length of hospital stay. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
Subgroup analysis revealed that compared with the LC, the combination of Dachaihu decoction and LC significantly shortened the length of hospital stay (MD = −1.75 days; 95% CI = –2.00 to −1.50; I2 = 5.62%) (Figure 8B).
Eight studies (Chen et al., 2023; Gu, 2021; Wang et al., 2020; Xia, 2020; Zhang et al., 2003; Zhang, 2018; Zhou, 2019; Zuo, 2021) (n = 638) assessed ALT levels. The results indicated that Dachaihu decoction significantly lowered ALT levels compared to the control group (MD = −11.88 U/L; 95% CI = –15.29 to −8.47; I2 = 89.25%; Q (7) = 65.13; p = 0.00) (Figure 9A).
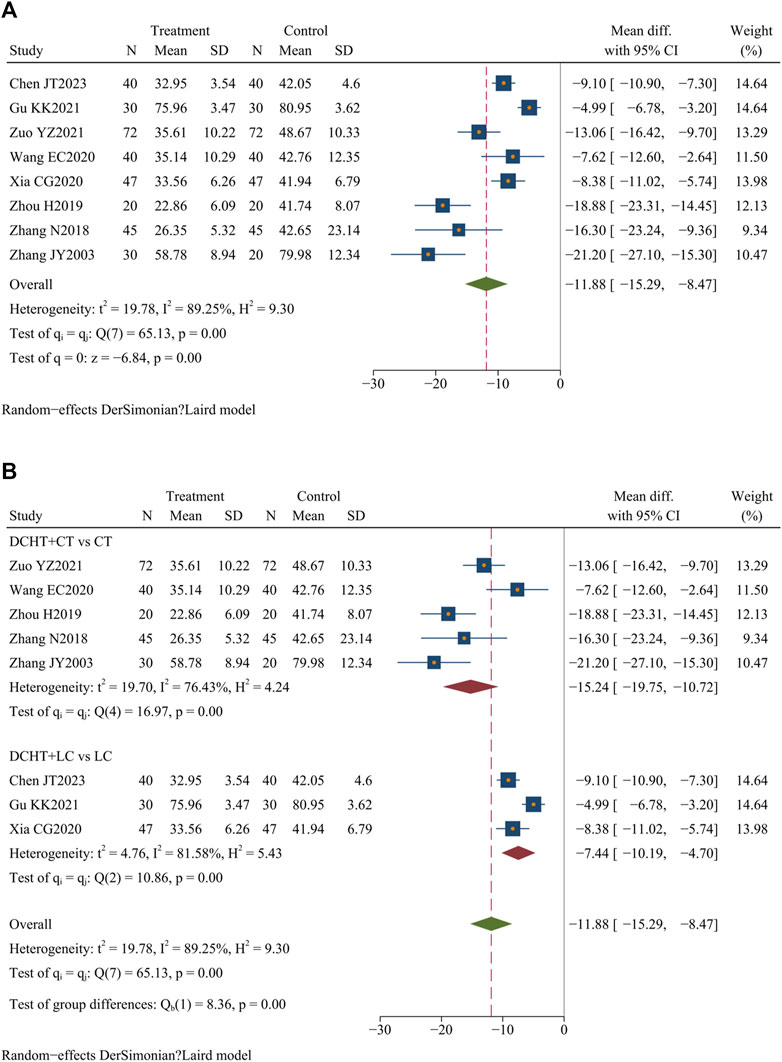
Figure 9. (A) ALT levels, (B) Subgroup analysis of ALT levels. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
The subgroup analysis indicated that the Dachaihu decoction combined with the CT group significantly reduced ALT levels compared with the CT group alone (MD = −15.24 U/L; 95% CI = –19.75 to −10.72; I2 = 76.43%). Dachaihu decoction combined with LC also significantly lowered ALT levels (MD = −7.44 U/L; 95% CI = −10.19 to −4.70; I2 = 81.58%) (Figure 9B).
In the Dachaihu decoction combined with the CT group versus the CT group, heterogeneity was significantly reduced after excluding studies for specific reasons: one study (Wang et al., 2020) due to the mean age of included patients being <40 years, one (Zuo, 2021) for differing onset times, and one (Gu, 2021) because the included patients were elderly. Heterogeneity was significantly reduced (I2 = 0.00%), and the results remained statistically significant (p < 0.05) (Supplementary Material S3).
Two studies (Chen et al., 2023; Xia, 2020) (n = 174) evaluated the AST levels in patients with AC, and the findings showed that Dachaihu decoction significantly decreased AST levels compared to the control group (MD = −8.74 U/L; 95% CI = –9.76 to −7.72; I2 = 0.00%; Q (1) = 0.14; p = 0.00) (Figure 10).
Five studies (Deng et al., 2018; Hong and Gao, 2018; Xiong and Yi, 2021; Zhou, 2020; Zhuang, 2021) (n = 419) assessed neutrophil levels. The results demonstrated that Dachaihu decoction significantly reduced neutrophil levels compared with the control group (MD = −9.68; 95% CI = –11.33 to −8.03; I2 = 56.04%; Q (4) = 9.10; p = 0.00) (Figure 11).
Three studies (Liu et al., 2021; Guo, 2023; Zhang, 2018) (n = 230) assessed the TNF-α levels. The results showed that Dachaihu decoction significantly lowered TNF-α levels compared to the control group (SMD = −2.10 pg/L; 95% CI = –2.43 to −1.78; I2 = 36.52%; Q (2) = 3.15; p = 0.00) (Figure 12).
Fifteen studies (Liu et al., 2021; Cai, 2022; Chen et al., 2023; Guo, 2012; Hong and Gao, 2018; Liao, 2014; Liu, 2021; Su, 2016; Wang and Li, 2014; Wei, 2016; Xiong and Yi, 2021; Xu, 2017; Yang, 2016; Zhu, 2017; Zhuang, 2021) (n = 1,333) evaluated the incidence of adverse events. Cai (2022) and Guo (2012) indicated that the combination of Dachaihu decoction with CT was more effective in minimizing mild adverse reactions, such as diarrhea, nausea, insomnia, and palpitations, compared to using conventional treatment alone. Additionally, four studies (Liu et al., 2021; Chen et al., 2023; Su, 2016; Xu, 2017) observed that Dachaihu decoction combined with LC significantly reduced the occurrence of incision infection, biliary fistula, and bile duct injury. Furthermore, two studies (Wei, 2016; Zhu, 2017) found a lower incidence of mild adverse events, such as gastrointestinal discomfort, dizziness, and skin itching, in the Dachaihu decoction group than in routine treatment. In summary, Dachaihu decoction demonstrated better safety and tolerability in the treatment of acute cholecystitis, with potential benefits in reducing postoperative complications and alleviating gastrointestinal discomfort (RR = 0.31; 95% CI = 0.20 to 0.48; I2 = 0.00%; Q (14) = 4.05; p = 0.00) (Figure 13A; Table 3).
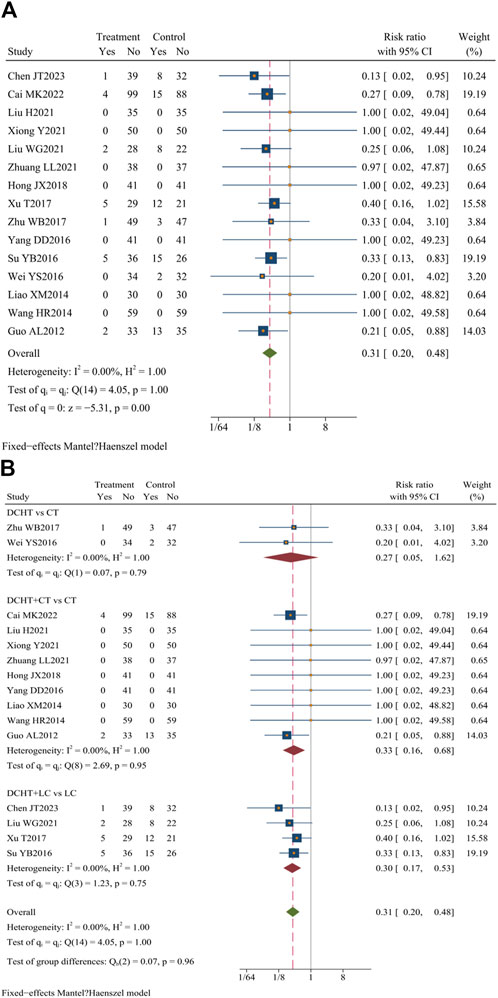
Figure 13. (A) Incidence of adverse events, (B) Subgroup analysis of incidence of adverse events. DCHT, Dachaihu tang; CT, Conventional treatment; LC, Laparoscopic cholecystectomy.
The subgroup analysis revealed that the Dachaihu decoction group significantly lowered the incidence of adverse events (RR = 0.27; 95% CI = 0.05 to 1.62; I2 = 0.00%). Compared with the CT group, the Dachaihu decoction combined with CT markedly decreased the incidence of adverse events (RR = 0.33; 95% CI = 0.16 to 0.68; I2 = 0.00%), including gastrointestinal symptoms, insomnia, and palpitations. Compared with the LC group, the Dachaihu decoction combined with LC significantly reduced the incidence of adverse events (surgical incision bleeding/infection, biliary fistula, bile duct injury, abdominal abscess, and fall) (RR = 0.30; 95% CI = 0.17 to 0.53; I2 = 0.00%). Seven studies (Hong and Gao, 2018; Liao, 2014; Liu, 2021; Wang and Li, 2014; Xiong and Yi, 2021; Yang, 2016; Zhuang, 2021) reported no adverse events (Figure 13B).
As funnel plot analysis requires at least 10 original studies, this method was used to evaluate clinical efficacy, time required for the resolution of abdominal pain and fever, and the incidence of adverse events. Subsequent Egger’s test results indicated a significant difference in clinical efficacy (p < 0.05), suggesting publication bias. To address this, the “trim-and-fill” method was used to estimate combined effect sizes adjusted for publication bias. The adjusted pooled effect size remained significant (p < 0.01), suggesting that publication bias did not influence the assessment of clinical efficacy (Figures 14A–E).
Certainty of evidence and the reasons for the upgrade and downgrade are presented in Table 4. The evidence for all outcomes was assessed as moderate to low certainty due to the risk of bias, imprecision, inconsistency, or indirectness.
A total of 33 eligible RCTs involving 2,851 participants evaluated the use of Dachaihu decoction alone or in combination with conventional treatment/LC for treating AC. Dachaihu decoction demonstrated potential benefits in improving clinical symptoms, shortening hospital stays, protecting liver function, reducing inflammatory responses, and decreasing adverse events (adverse drug reactions and postoperative complications). Subgroup analyses revealed that Dachaihu decoction alone may alleviate abdominal pain and fever, with no significant adverse events reported. When combined with CT or LC, it may further improve liver function, reduce inflammation, and enhance the symptoms of fever and abdominal pain in AC. Additionally, it may shorten the length of hospital stay, increase clinical efficacy, and decrease adverse event rates, indicating potential for clinical use. The sensitivity analysis showed that excluding different studies did not significantly alter the primary outcome’s direction or significance, suggesting consistent findings. Despite variations in study quality, the results remained applicable and interpretable. Due to an insufficient description of the randomization process and blinding design, these studies demonstrated a high risk of bias. No publication bias was observed, except in clinical efficacy. The evidence was assessed as having moderate-to-low certainty.
Classical TCM formulas (Dachaihu decoction) have distinct components and therapeutic properties. According to evidence-based TCM principles, clinicians can slightly adjust the grammage and composition of botanical drugs of Dachaihu decoction to meet individual patient needs. Multiple studies have shown that modifying Dachaihu decoction can improve symptom management through synergistic and complementary effects (Cheang et al., 2024; Dai et al., 2016; Li et al., 2014). The inclusion of multiple RCTs showed significant improvements in efficacy by customizing Dachaihu decoction’s grammage of certain botanical drugs to target the primary symptoms of diverse patients, underscoring its potential as an extension of the therapeutic system.
In the United States, approximately 20 million people experience AC annually, leading to healthcare costs exceeding $6.3 billion and imposing a substantial economic burden (Anderloni and Fugazza, 2022). A study reported that perioperative AC requires prolonged antibacterial treatment, which significantly increases hospitalization medical expenses and contributes to antibiotic resistance (Murata et al., 2013). The association of surgical infection is put forward for the quiet line in low-risk patients with laparoscopic cholecystectomy, not recommended for the routine use of perioperative antibiotics (Colling et al., 2022). Another study found that the complication rate associated with antibiotic therapy in elderly patients with AC was approximately 33% (Kivivuori et al., 2023). Our study found that the treatment of this disease with Dachaihu decoction might shorten the average length of hospital stay by nearly 2 days. This potential shortened the duration of symptoms but also reduced the nation’s health expenditure and the patients’ individual economic burden.
AC, a common inflammatory condition of the gallbladder, is characterized by inflammation and dyskinesia. Research has demonstrated that neutrophils contribute to both inflammation and gallbladder dysmotility in AC, potentially leading to gallbladder injury through the inhibition of SCF and c-kit expression (Zhou, 2020; Huang et al., 2016; Lin et al., 2019). TNF-α, a polymorphic hormone, is essential for regulating the body’s inflammatory and immune responses while promoting monocyte activity. It is involved in various local and systemic inflammatory reactions (Kalliolias and Ivashkiv, 2016). During AC episodes, a significant release of TNF-α occurs, resulting in the local inflammation of the gallbladder, fever, increased exudation, and right upper quadrant abdominal pain (Chen and Chen, 2023; Psaltis et al., 2024). This study suggested a potential reduction in the duration of abdominal pain and fever, neutrophil percentages, TNF-α levels, and white blood cell recovery times, following Dachaihu decoction treatment. These effects may be attributed to various active ingredients such as saikosaponin a, paeoniflorin, and aloe emodin, which inhibit IL-6, TNF-α, and CCK, thus modulating inflammation, enhancing immunity, repairing gallbladder damage, and promoting bile excretion and stone expulsion (Lin et al., 2019).
Dachaihu decoction reduces liver injury and enhances liver function through multiple mechanisms such as scavenging oxygen free radicals, reducing lipid peroxidation, promoting liver cell regeneration, and enhancing liver blood flow (Yang et al., 2019). AST, ALT, and serum total bilirubin (TBIL) are recognized as sensitive markers of hepatocyte injury. Extensive clinical and experimental research has demonstrated that Dachaihu decoction may significantly reduce serum AST and ALT levels, potentially aiding in liver function recovery and minimizing liver cell damage (Law et al., 2014; Han et al., 2016; Mou et al., 2024), which was consistent with our findings. This effect is likely mediated by the activation of the PI3K/AKT/STAT3 and PPARα signaling pathways, which regulate the expression of E-cadherin, N-cadherin, p53, Bax, Bcl-2, PI3K, p-AKT, AKT, STAT3, CYP7a1, and Cyp8b1, thereby reducing liver cell damage (Xu et al., 2022; Duan et al., 2024).
Antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used to treat infections and relieve biliary colic. However, their use may increase the risk of severe and potentially life-threatening side effects, including gastrointestinal bleeding, kidney damage, and cardiovascular complications (Fraquelli et al., 2016). The risk of adverse events in perioperative patients ranges from 16% to 25% (Ishii et al., 2023), and significant controversy persists regarding the risk associated with different surgical protocols (Do et al., 2023; Gurusamy and Samraj, 2006; Cucchetti et al., 2022). Dachaihu decoction has been well-documented in the TCM literature, indicating a long-standing history of safe use. Several studies have shown that the eight botanicals in its formula usually have a better safety profile under conventional dosage (grams) (Yang et al., 2017; Song et al., 2020; Ali et al., 2008; Bai et al., 2022). Clinical reports showed that adverse reactions were typically mild gastrointestinal symptoms, which usually resolved on their own without the need for special intervention. Furthermore, Dachaihu decoction exhibits hepatoprotective effects in alpha-naphthyl isothiocyanate (ANIT)-induced liver injury models (Chen et al., 2016). Recent studies further demonstrate that Dachaihu decoction has protective effects on liver and kidney function, indicating a relatively safe profile (Huang et al., 2023; Qiao et al., 2022). However, while most clinical trials on drug interactions have highlighted its effects in reducing toxicity and enhancing efficacy, experimental research remains relatively limited, underscoring the need for careful monitoring during clinical use. Although some studies suggested that Dachaihu decoction did not increase adverse reactions and might have reduced complications when used preoperatively, a comprehensive assessment is still necessary. Current toxicity data are mostly derived from small-scale studies, lacking large-scale randomized controlled trials. More research is needed to further evaluate its safety and inform clinical applications.
This study performed a systematic search across eight databases and three clinical trial registries to ensure broad literature coverage. A language-unrestricted search strategy was used to minimize selection bias. A total of 33 RCTs involving 2,851 patients were included, providing sufficient evidence to evaluate the efficacy and safety of Dachaihu decoction in treating acute cholecystitis. In terms of outcome measures, this study assessed a variety of indicators, such as clinical efficacy, time to resolution of abdominal pain and fever, time to white blood cell normalization, length of hospital stay, liver function, and inflammatory markers. These diverse measures allowed for a comprehensive evaluation of Dachaihu decoction’s effects, showcasing its potential multi-target and multi-mechanism benefits in managing acute cholecystitis. In addition, Dachaihu decoction is widely used in clinical practice and generally has a higher safety profile. Subgroup and sensitivity analyses were also conducted to identify the sources of heterogeneity and verify the stability of the results, thereby enhancing the findings’ reliability. The GRADE assessment system was utilized to evaluate the quality of evidence, offering a structured framework for interpreting results and guiding clinical decision-making and future research.
Nonetheless, our study has several limitations. First, the included studies were all conducted in China and involved only Chinese patients, which may introduce regional publication bias; therefore, additional international clinical trials are recommended. Second, the risk-of-bias assessment showed that most studies reported random sequence generation, but most lacked proper blinding or did not report it. It is essential to recognize that this methodological issue presents a frequent obstacle in many investigations of botanical drug tonics. Given that these tonics are mainly used in practical clinical environments, achieving full implementation of double-blind methods and allocation concealment is often difficult. Furthermore, the complex nature of botanical drug formulations, combined with the distinctive smells or colors of certain botanical drugs, adds to the challenge of blinding. To determine the potential influence of bias on study outcomes, a sensitivity analysis was performed, showing that the results maintained a reasonable degree of credibility. To improve the validity and consistency of future research on botanical drugs of tonic effectiveness, researchers are encouraged to apply stricter randomization and blinding techniques. Additionally, the control groups in these studies were limited to traditional modern medicine, with no placebo controls, and most studies did not include a follow-up period. Moreover, the GRADE assessment system found that most studies were of low quality, indicating a need for better quality and improved methods in current randomized controlled trials. The small sample size in this study may limit the generalizability and reliability of the results. Therefore, larger-scale studies are needed to confirm these findings. We also suggest that future researchers focus on implementing blinding and registering clinical trials to enhance study quality and credibility.
Dachaihu decoction may provide benefits in treating acute cholecystitis, such as reducing the length of hospital stay and improving symptoms, with a lower incidence of adverse events. However, due to the varying quality of the included studies and potential bias and heterogeneity, the accuracy of these findings may be limited. Caution is needed when interpreting the results. Future high-quality, large-scale, multi-center studies are necessary to further confirm the efficacy and safety of Dachaihu decoction for acute cholecystitis.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
X-xL: conceptualization, data curation, formal analysis, methodology, software, writing–original draft, and writing–review and editing. Y-qM: data curation, validation, and writing–original draft. L-yK: formal analysis, methodology, software, and writing–review and editing. Y-zS: data curation, software, and writing–review and editing. NR: funding acquisition, supervision, and writing–review and editing. J-pL: conceptualization, funding acquisition, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the High-Level Talent Research Start-up Fund (90011451310039) and project “Research and Development of Evidence-Based Chinese Medicine” (B23041). Funders did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1497072/full#supplementary-material
Abdulrahman, R., Hashem, J., and Walsh, T. N. (2022). A review of acute cholecystitis. JAMA 328 (1), 76–77. doi:10.1001/jama.2022.7768
Ali, B. H., Blunden, G., Tanira, M. O., and Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 46 (2), 409–420. doi:10.1016/j.fct.2007.09.085
Anderloni, A., and Fugazza, A. (2022). Acute cholecystitis: which is the best therapeutic option? Gastrointest. Endosc. 95 (3), 407–409. doi:10.1016/j.gie.2021.11.010
Bai, J., Qi, J., Yang, L., Wang, Z., Wang, R., and Shi, Y. (2022). A comprehensive review on ethnopharmacological, phytochemical, pharmacological and toxicological evaluation, and quality control of Pinellia ternata (Thunb.) Breit. J. Ethnopharmacol. 298, 115650. doi:10.1016/j.jep.2022.115650
Bedirli, A., Sakrak, O., Sözüer, E. M., Kerek, M., and Güler, I. (2001). Factors effecting the complications in the natural history of acute cholecystitis. Hepatogastroenterology 48 (41), 1275–1278.
Bi, S., Liu, Y., Lv, T., Ren, Y., Liu, K., Liu, C., et al. (2023). Preliminary exploration of method for screening efficacy markers compatibility in TCM prescriptions based on Q-markers: anti-inflammatory activity of Dachaihu decoction as an example. J. Ethnopharmacol. 312, 116539. doi:10.1016/j.jep.2023.116539
Cai, M. K. (2022). The therapeutic effect of Dachaihu decoction on acute cholecystitis and cholelithiasis and its impact on psychological indicators. Med. HEALTH (09), 150–153.
Cheang, I., Yao, W., Zhou, Y., Zhu, X., Ni, G., Lu, X., et al. (2024). The traditional Chinese medicine Qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial. Nat. Med. 30 (8), 2295–2302. doi:10.1038/s41591-024-03169-2
Chen, J. T., Li, X., Liu, L., Hou, X. X., and Wang, J. (2023). Clinical study on the promotion of postoperative rehabilitation of acute cholecystitis and cholecystectomy with modified Dachaihu decoction. Med. Health (11), 170–173.
Chen, L., and Chen, X. (2023). Th1/Th2 cytokine profile in patients with acute and chronic calculus cholecystitis. Eur. Cytokine Netw. 34 (3), 22–28. doi:10.1684/ecn.2023.0488
Chen, Z., Zhu, Y., Zhao, Y., Ma, X., Niu, M., Wang, J., et al. (2016). Serum metabolomic profiling in a rat model reveals protective function of paeoniflorin against ANIT induced cholestasis. Phytother. Res. 30 (4), 654–662. doi:10.1002/ptr.5575
China Association Of Integrative Medicine Emergency Medicine Committee, Editorial Committee Of Chinese Journal Of Integrated Traditional And Western Medicine Li, Z., Wang, D., and Li, Y. (2019). Expert consensus on diagnosis and treatment of septic shock with integrated traditional Chinese and Western medicine. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 31 (11), 1317–1323. doi:10.3760/cma.j.issn.2095-4352.2019.11.002
Colling, K. P., Besshoff, K. E., Forrester, J. D., Kendrick, D., Mercier, P., and Huston, J. M. (2022). Surgical infection society guidelines for antibiotic use in patients undergoing cholecystectomy for gallbladder disease. Surg. Infect. (Larchmt) 23 (4), 339–350. doi:10.1089/sur.2021.207
Cucchetti, A., Binda, C., Dajti, E., Sbrancia, M., Ercolani, G., and Fabbri, C. (2022). Trial sequential analysis of EUS-guided gallbladder drainage versus percutaneous cholecystostomy in patients with acute cholecystitis. Gastrointest. Endosc. 95 (3), 399–406. doi:10.1016/j.gie.2021.09.028
Dai, M., Yang, Y. W., Guo, W. H., Wang, F. L., Xiao, G. M., Li, Y. M., et al. (2016). Addition and subtraction theory of TCM using xiao-chaihu-decoction and naturopathy in predicting survival outcomes of primary liver cancer patients: a prospective cohort study. Evid. Based Complement. Altern. Med. 2016, 4723530. doi:10.1155/2016/4723530
Deng, H., Chen, J. F., She, L., and Li, Z. X. (2018). Randomized controlled study on the treatment of elderly acute cholecystitis with modified Dachaihu decoction. Diet Sci. (6X), 47.
Deng, H., and Xu, J. (2017). Wendan decoction (Traditional Chinese medicine) for schizophrenia. Cochrane Database Syst. Rev. 6 (6), CD012217. doi:10.1002/14651858.CD012217.pub2
Deng, Y. H., Chen, J. F., She, L., et al. (2018). Clinical study on the treatment of acute cholecystitis (gallbladder heat syndrome) with modified Dachaihu decoction. J. Emerg. Traditional Chin. Med. 27 (03), 462–464. doi:10.3969/j.issn.1004-745X.2018.03.025
Dietrich, C. F., Arcidiacono, P. G., Bhutani, M. S., Braden, B., Burmester, E., Fusaroli, P., et al. (2024). Controversies in endoscopic ultrasound-guided biliary drainage. Cancers (Basel) 16 (9), 1616. doi:10.3390/cancers16091616
Do, Y. A., Yoon, C. J., Lee, J. H., Choi, W. S., and Lee, C. H. (2023). Percutaneous cholecystostomy as a definitive treatment for acute acalculous cholecystitis: clinical outcomes and risk factors for recurrent cholecystitis. Br. J. Radiol. 96 (1147), 20220943. doi:10.1259/bjr.20220943
Duan, Z. W., Liu, Y., Zhang, P. P., Hu, J. Y., Mo, Z. X., Liu, W. Q., et al. (2024). Da-Chai-Hu-Tang Formula inhibits the progression and metastasis in HepG2 cells through modulation of the PI3K/AKT/STAT3-induced cell cycle arrest and apoptosis. J. Ethnopharmacol. 331, 118293. doi:10.1016/j.jep.2024.118293
Fraquelli, M., Casazza, G., Conte, D., and Colli, A. (2016). Non-steroid anti-inflammatory drugs for biliary colic. Cochrane Database Syst. Rev. 9 (9), CD006390. doi:10.1002/14651858.CD006390.pub2
Gluhovschi, C., Gadalean, F., Velciov, S., Petrica, L., Duta, C., Botoca, M., et al. (2023). Acute acalculous cholecystitis associated with abscesses-an unknown dual pathology. Biomedicines 11 (2), 632. doi:10.3390/biomedicines11020632
González-Castillo, A. M., Sancho-Insenser, J., De Miguel-Palacio, M., Morera-Casaponsa, J. R., Membrilla-Fernández, E., Pons-Fragero, M. J., et al. (2021). Mortality risk estimation in acute calculous cholecystitis: beyond the Tokyo Guidelines. World journal of emergency surgery. WJES 16 (1), 24. doi:10.1186/s13017-021-00368-x
Gu, K. K. (2021). Effect of laparoscopic cholecystectomy combined with da chaihu decoction on calculous cholecystitis. Reflexology Rehabilitation Med. 2 (10), 70–72.
Guo, A. L. (2012). Clinical analysis of integrated traditional Chinese and Western medicine in the treatment of acute cholecystitis. China Med. Pharm. 2 (01), 118+147.
Guo, H. L. (2023). Exploration of the clinical efficacy of modified Dachaihu decoction in the treatment of acute cholecystitis and gallstones. Med. (12), 181–184.
Gurusamy, K. S., and Samraj, K. (2006). Early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Cochrane Database Syst. Rev. (4), CD005440. doi:10.1002/14651858.CD005440.pub2
Han, L., Wang, X. W., Shan, H. Y., and Zhang, X. (2016). World Chinese Medicine. 11 (06), 1063–1065+1069. doi:10.3969/j.issn.1673.7202.2016.06.034
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research–Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Hong, J. X., and Gao, Y. H. (2018). Modified Dachaihu decoction combined with drug therapy treatment of acute cholecystitis (stagnation of gallbladder heat) randomized parallel control study. J. Pract. Traditional Chin. Intern. Med. 32 (06), 40–43. doi:10.13729/j.issn.1671-7813.z20170547
Huang, N., Wei, Y., Liu, M., Yang, Z., Yuan, K., Chen, J., et al. (2023). Dachaihu decoction ameliorates septic intestinal injury via modulating the gut microbiota and glutathione metabolism as revealed by multi-omics. J. Ethnopharmacol. 312, 116505. doi:10.1016/j.jep.2023.116505
Huang, Z. P., Qiu, H., Yang, Y., and Yu, B. P. (2016). Effect of neutrophils on gallbladder interstitial Cajal like cells in Guinea pig model of acute cholecystitis. Cell. Physiology Biochem. 39, 2033–2043. doi:10.1159/000447899
Ishii, K., Fujita, Y., Suzuki, E., Koyama, Y., Tsujino, S., Nagao, A., et al. (2023). The efficacy and safety of EUS-guided gallbladder drainage as a bridge to surgery for patients with acute cholecystitis. J. Clin. Med. 12 (8), 2778. doi:10.3390/jcm12082778
Ji, X. L., Zhou, Y., Li, Z. H., Qiang, F. J., Bing, Y. F., Zhao, J. Y., et al. (2024). Progress on historical evolution, clinical application, and pharmacological effects of Dachaihu decoction and predictive analysis of its quality markers. Zhongguo Zhong Yao Za Zhi 49 (8), 2064–2075. doi:10.19540/j.cnki.cjcmm.20231209.201
Kalliolias, G. D., and Ivashkiv, L. B. (2016). TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12 (1), 49–62. doi:10.1038/nrrheum.2015.169
Kimura, Y., Takada, T., Kawarada, Y., Nimura, Y., Hirata, K., Sekimoto, M., et al. (2007). Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat.Surg. 14, 15–26. doi:10.1007/s00534-006-1152-y
Kivivuori, A., Salminen, P., Ukkonen, M., Ilves, I., Vihervaara, H., Zalevskaja, K., et al. (2023). Laparoscopic cholecystectomy versus antibiotic therapy for acute cholecystitis in patients over 75 years: randomized clinical trial and retrospective cohort study. Scand. J. Surg. 112 (4), 219–226. doi:10.1177/14574969231178650
Law, B. Y., Mo, J. F., and Wong, V. K. (2014). Autophagic effects of chaihu (dried roots of Bupleurum chinense DC or Bupleurum scorzoneraefolium WILD). Chin. Med. 9, 21. doi:10.1186/1749-8546-9-21
Li, B., Tao, W., Zheng, C., Shar, P. A., Huang, C., Fu, Y., et al. (2014). Systems pharmacology-based approach for dissecting the addition and subtraction theory of traditional Chinese medicine: an example using Xiao-Chaihu-Decoction and Da-Chaihu-Decoction. Comput. Biol. Med. 53, 19–29. doi:10.1016/j.compbiomed.2014.05.007
Li, J. (2016). Clinical observation of Dachaihu decoction in the treatment of acute cholecystitis. China Snaturopathy. 24 (07), 61–62. doi:10.19621/j.cnki.11-3555/r.2016.07.053
Li, X., Liu, Z., Liao, J., Chen, Q., Lu, X., and Fan, X. (2023). Network pharmacology approaches for research of Traditional Chinese Medicines. Chin. J. Nat. Med. 21 (5), 323–332. doi:10.1016/S1875-5364(23)60429-7
Liang, J. B., and Deng, H. J. (2013). The clinical efficacy decoction Dachaihu for acute cholecystitis in 50 patients. Int. Med. Health Guid. News. 19 (15), 2304–2307. doi:10.3760/cma.j.issn.1007-1245.2013.15.018
Liang, J. Y., Liu, X. Y., and Zhing, F. P. (2013). 30 cases of acute cholecystitis treated with integrated traditional Chinese and Western medicine. 19 (15), 2304–2307. doi:10.13457/j.cnki.jncm.2006.04.048
Liao, X. M. (2014). Clinical efficacy of integrated traditional and western in the treatment of acute cholecystitis. Mod. Diagnosis Treat. 25 (12), 2684–2686.
Lin, M. J., Chen, L., Huang, Z. P., Qiu, H., and Yu, B. P. (2019). Neutrophils injure gallbladder interstitial Cajal-like cells in a Guinea pig model of acute cholecystitis. J. Cell Physiol. 234 (4), 4291–4301. doi:10.1002/jcp.27197
Liu, H. (2021). Effects of Dachaihu decoction combined with western medicine non-surgical conventional treatment of acute cholecystitis. Med. J. Chin. People’s Health 33 (15), 68–70. doi:10.3969/j.issn.1672-0369.2021.15.027
Liu, W. G., Ding, X. J., and Yu, C. (2021). Clinical observation on the therapeutic effect of modified Dachaihu decoction as an adjuvant therapy for acute cholecystitis with gallstones (damp heat syndrome of liver and gallbladder). J. Emerg. Traditional Chin. Med. 30 (12), 2192–2194. doi:10.3969/j.issn.1004-745X.2021.12.033
Liu, Y. Q. (2015). Clinical observation of integrated traditional Chinese and western medicine in the treatment of acute cholecystitis. J. Emerg. Traditional Chin. Med. 24 (10), 1834–1835. doi:10.3969/j.issn.1004-745X.2015.10.051
Mao, C., Zhou, Y., Ji, D., Tan, X., Tao, Y., Zang, W., et al. (2017). Chemical fingerprint of Dachaihu granule and its chemical correlation between raw herbs. J. Chromatogr. Sci. 55 (4), 405–410. doi:10.1093/chromsci/bmw194
Markaki, I., Konsoula, A., Markaki, L., Spernovasilis, N., and Papadakis, M. (2021). Acute acalculous cholecystitis due to infectious causes. World J. Clin. Cases 9 (23), 6674–6685. doi:10.12998/wjcc.v9.i23.6674
Mencarini, L., Vestito, A., Zagari, R. M., and Montagnani, M. (2024). The diagnosis and treatment of acute cholecystitis: a comprehensive narrative review for a practical approach. J. Clin. Med. 13 (9), 2695. doi:10.3390/jcm13092695
Ministry of Health of the PRC (2002). Reference to the guiding principles for clinical research of New Chinese medicines. Beijing: China Medical Science and Technology Press.
Mou, Z., Gong, T., Wu, Y., Liu, J., Yu, J., and Mao, L. (2024). The efficacy and safety of Dachaihu decoction in the treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Front. Med. (Lausanne) 11, 1397900. doi:10.3389/fmed.2024.1397900
Murata, A., Okamoto, K., Matsuda, S., Kuwabara, K., Ichimiya, Y., Matsuda, Y., et al. (2013). Multivariate analysis of factors influencing length of hospitalization and medical costs of cholecystectomy for acute cholecystitis in Japan: a national database analysis. Keio J. Med. 62 (3), 83–94. doi:10.2302/kjm.2012-0015-oa
Nitzan, O., Brodsky, Y., Edelstein, H., Hershko, D., Saliba, W., Keness, Y., et al. (2017). Microbiologic data in acute cholecystitis: ten years' experience from bile cultures obtained during percutaneous cholecystostomy. Surg. Infect. (Larchmt) 18 (3), 345–349. doi:10.1089/sur.2016.232
Ohta, Y., Sasaki, E., Nishida, K., Kobayashi, T., Nagata, M., and Ishiguro, I. (1995). Preventive effect of dai-saiko-to (da-chai-hu-tang) extract on disrupted hepatic active oxygen metabolism in rats with carbon tetrachloride-induced liver injury. Am. J. Chin. Med. 23 (1), 53–64. doi:10.1142/S0192415X95000080
Psaltis, E., Zaitoun, A. M., Neal, K. R., and Lobo, D. N. (2024). Immunohistochemical inflammation in histologically normal gallbladders containing gallstones. World J. Surg. 48 (7), 1662–1673. doi:10.1002/wjs.12219
Qiao, X., Xu, S. H., Wang, Y. W., Peng Pk, , and Shen, K. K. (2022). Da chaihutang inhibits hepatocellular carcinoma by regulating p38 MAPK/IL-6/STAT3 signaling pathway. Chin. J. Exp. Tradit. Med. Form. 28 (16), 19–31. doi:10.13422/j.cnki.syfjx.20221026
Regimbeau, J. M., Fuks, D., Pautrat, K., Mauvais, F., Haccart, V., Msika, S., et al. (2014). Effect of postoperative antibiotic administration on postoperative infection following cholecystectomy for acute calculous cholecystitis: a randomized clinical trial. JAMA 312 (2), 145–154. doi:10.1001/jama.2014.7586
Sheehan, M. P., Rustin, M. H., Atherton, D. J., Buckley, C., Harris, D. W., Brostoff, J., et al. (1992). Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis. Lancet 340 (8810), 13–17. doi:10.1016/0140-6736(92)92424-e
Singh, A., Kaur, M., Swaminathan, C., Subramanian, A., Singh, K. K., and Sajid, M. S. (2023). Preoperative antibiotic prophylaxis in acute cholecystectomy: a systematic review and meta-analysis of randomised controlled trials. Transl. Gastroenterol. Hepatol. 8, 37. doi:10.21037/tgh-23-48
Song, J., Kim, Y. S., Lee, D., and Kim, H. (2020). Safety evaluation of root extract of Pueraria lobata and Scutellaria baicalensis in rats. BMC Complement. Med. Ther. 20 (1), 226. doi:10.1186/s12906-020-02998-1
Su, Y. B. (2016). Clinical observation of the effect of laparoscopic cholecystectomy and Dachaihu decoction in the treatment of acute calculous cholecystitis. Contemp. Med. Symp. 14 (21), 64–65.
Sun, J. F. (2005). Clinical observation on the therapeutic effect of modified Dachaihu decoction on acute cholecystitis. J. Sichuan Traditional Chin. Med. (11), 55–56.
Wang, E. C., Yao, W., Li, J., Wang, X. D., Li, B., and Mi, S. P. (2020). Clinical observation on oral administration of Dachaihu decoction combined with external application of shaoyao gancao in the treatment of acute cholecystitis. Chin. Med. Mod. Distance Educ. China. 18 (03), 69–70. doi:10.3969/j.issn.1672-2779.2020.03.028
Wang, H. R., and Li, L. (2014). Clinical observation of Dachaihu decoction combined with western medicine in the treatment of acute cholecystitis. J. Emerg. Traditional Chin. Med. 23 (11), 2070–2071. doi:10.3969/j.issn.1004-745X.2014.11.043
Wang, S. S. (2010). Clinical observation on the diagnosis and treatment of 80 cases of acute cholecystitis with modified Dachaihu decoction. Chin. Med. Mod. Distance Educ. Chin, 8 (20), 21. doi:10.3969/j.issn.1672-2779.2010.20.017
Wei, Y. S. (2016). Clinical analysis of traditional Chinese medicine treatment for acute cholecystitis. Asia-Pacific Tradit. Med. 12 (16), 136–137. doi:10.11954/ytctyy.201616059
Xia, C. G. (2020). Clinical study on Dachaihu tang combined with routine therapy in promoting postoperative rehabilitation for acute cholecystitis after cholecystectomy. J. New Chin. Med. 52 (5), 26–29. doi:10.13457/j.cnki.jncm.2020.05.007
Xiong, Y., and Yi, X. L. (2021). Clinical research of Macro-bupleuri Decoction on acute cholecystitis of syndrome of stagnation of heat in gallbladder. China Med. Pharm. 11 (7), 106–109. doi:10.3969/j.issn.2095-0616.2021.07.028
Xu, S., Qiao, X., Peng, P., Zhu, Z., Li, Y., Yu, M., et al. (2022). Da-Chai-Hu-tang protects from acute intrahepatic cholestasis by inhibiting hepatic inflammation and bile accumulation via activation of PPARα. Front. Pharmacol. 13, 847483. doi:10.3389/fphar.2022.847483
Xu, T. (2017). Clinical observation of the therapeutic effect of Da Chai Hu Tang combined with surgery on 34 cases of acute cholecystitis caused by Gallstones. Yunnan J. Traditional Chin. Med. Materia Medica, 38 (6), 58–59. doi:10.16254/j.cnki.53-1120/r.2017.06.029
Yang, D. D. (2016). Clinical observation on the clinical efficacy of modified Dachaihu decoction in the treatment of acute cholecystitis and gallstones. For All Health. 10 (5), 77–78.
Yang, F., Dong, X., Yin, X., Wang, W., You, L., and Ni, J. (2017). Radix bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed. Res. Int. 2017, 7597596. doi:10.1155/2017/7597596
Yang, J. M., Sun, Y., Wang, M., Zhang, X. L., Zhang, S. J., Gao, Y. S., et al. (2019). Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J. Gastroenterol. 25 (34), 5105–5119. doi:10.3748/wjg.v25.i34.5105
Yu, X. J. (2009). 36 cases of acute cholecystitis treated with integrated traditional Chinese and Western medicine. Chin. J. Ethnomedicine Ethnopharmacy. 18 (20), 100–101. doi:10.3969/j.issn.1007-8517.2009.20.083
Zhang, J. Y., Guo, Y. Z., and Yu, A. S. (2003). Treating 30 cases of acute cholecystitis and cholecystolithiasis with Dachaihu decoction. Chin. J. Ethnomedicine Ethnopharmacy, (2), 96–97. doi:10.3969/j.issn.1007-8517.2003.02.015
Zhang, N. (2018). The therapeutic effect of modified Dachaihu decoction on elderly patients with acute cholecystitis of liver gallbladder damp heat type and its impact on inflammatory factors and liver function indicators. Yunnan J. Traditional Chin. Med. Materia Medica, 39 (11), 42–43. doi:10.3969/j.issn.1007-2349.2018.11.018
Zhou, H. (2019). Clinical observation on Dachaihu decoction in the treatment of senile acute cholecystitis with liver gallbladder dampness-heat. Guangming J. Chin. Med. 34 (9), 1349–1351. doi:10.3969/j.issn.1003-8914.2019.09.018
Zhou, M. J. (2020). Clinical efficacy of jiawei Dachaihu decoction in the treatment of acute cholecystitis (syndrome of stagnation and heat of gallbladder and viscera). World J. Complex Med. 6 (02), 144–146. doi:10.11966/j.issn.2095-994X.2020.06.02.47
Zhu, W. B. (2017). Observation of effect of modified Dachaihu decoction on elderly acute cholecystitis. Shanxi J. Traditional Chin. Med. 33 (07), 7–8.
Zhuang, L. L. (2021). “Study on the clinical effect of modified Dachaihu decoctionon acute cholecystitis complicated with gallstone (Syndrome of heat stagnation in biliary organs),”. Sichuan, China: Chengdu University of Traditional Chinese Medicine. [Master Dissertation]. doi:10.26988/d.cnki.gcdzu.2021.000180
Keywords: Dachaihu decoction, acute cholecystitis, clinical efficacy, systematic review, meta-analysis
Citation: Liu X-x, Ma Y-q, Kong L-y, Su Y-z, Robinson N and Liu J-p (2024) Unveiling the therapeutic role of Dachaihu decoction in acute cholecystitis: a comprehensive systematic review and meta-analysis of its efficacy and safety. Front. Pharmacol. 15:1497072. doi: 10.3389/fphar.2024.1497072
Received: 16 September 2024; Accepted: 05 November 2024;
Published: 27 November 2024.
Edited by:
Qingquan Liu, Capital Medical University, ChinaReviewed by:
Wentai Pang, Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2024 Liu, Ma, Kong, Su, Robinson and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-ping Liu, bGl1anBAYnVjbS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.