- 1Metabolic Syndrome Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Department of Medical Physiology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 3Department of Clinical Biochemistry, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 4Department of Medical Sciences, Faculty of Nursing, Warith Al-Anbiyaa University, Kerbala, Iraq
- 5Department of Medical Sciences, Faculty of Dentistry, University of Kerbala, Kerbala, Iraq
- 6Department of Pharmacology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 7Medical School, Mashhad University of Medical Sciences, Mashhad, Iran
- 8Department of Physiology and Pharmacology, Afzalipour Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran
- 9Department of Obstetrics and Gynecology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 10Department of Human Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 11School of Medicine, Saint Louis University, Saint Louis, MO, United States
Introduction: The therapeutic efficacy of oral administration of Ziziphus jujube in the context of uterine adhesion (UA) and its impact on pregnancy outcomes was investigated.
Methods: In a rat UA model, Z. jujube was evaluated for its ability to mitigate injury-induced uterine adhesion bands, uterine shortening, and enhance endometrial regeneration. The assessment included analysis of gland numbers, uterine endometrial thickness, and regulation of inflammatory cytokines. The antioxidant properties of Z. jujube were also studied through antioxidant enzyme activity in uterine tissue homogenates. Fibrotic changes were examined through histological Trichrome staining and analysis of pro-fibrotic factors.
Results: Treatment with Z. jujube resulted in a significant reduction in uterine tissue fibrosis, as evidenced by histological evaluation and reduced expression of fibrotic markers. The intervention demonstrated positive outcomes in embryonic development, pregnancy rates, and pregnancy outcomes. Z. jujube effectively inhibited the formation of extra-uterine adhesion bands to internal organs. No toxicity-related morphological changes were observed in vital organs of the Z. Jujube-treated group.
Discussion: The results collectively indicate that Z. jujube is a safe and potent natural product with anti-inflammatory and anti-fibrotic properties, highlighting its potential as a novel candidate for clinical studies targeting UA in patients.
Introduction
Asherman’s syndrome is a worldwide disease affecting women of reproductive age (Deans and Abbott, 2010). Typically, when the basal layer of the endometrium undergoes mechanical or infectious damage, it sets off an inflammatory response that triggers the formation of fibrous adhesion bands within the uterine cavity. This disruption of the endometrial regeneration process results in an alteration of the normal biological activity of the endometrium (Evans-Hoeker and Young, 2014; Chen et al., 2021). IUA is a leading cause of secondary infertility in women, affecting approximately 25%–30% of individuals struggling with infertility (Rein et al., 2011; Zhang et al., 2019). Moreover, menstrual irregularities, notably amenorrhea, and decreased pregnancy rates with poor outcomes are significant complications associated with IUA (Deans and Abbott, 2010; Al-Inany, 2001). Today, various therapeutic approaches are employed worldwide to inhibit the fibrosis process and the subsequent formation of adhesion bands. These approaches include hysteroscopic adhesiolysis, a surgical procedure for removing visible adhesion bands, the use of intrauterine devices (IUDs), intrauterine gel injections, and estrogen therapy aimed at preventing adhesion recurrence and stimulating endometrial regeneration (Zhang et al., 2019; Myers and Hurst, 2012; Tsui et al., 2014). Despite demonstrating effectiveness in treating IUA, these methods are somewhat invasive, outcomes can vary among individuals, and they are not universally accessible. Therefore, the development and proposal of new methods with greater effectiveness in endometrial regeneration and lower invasiveness are warranted (Yu et al., 2008; Dreisler and Kjer, 2019).
Inflammation and its successive fibrosis are the principal players in the adhesion formation process (Wang et al., 2017; Leung et al., 2021; Xue et al., 2015). Following an injury to the endometrium, inflammatory signaling pathways become activated, leading to an imbalance in the oxidant/antioxidant ratio and increased secretion of inflammatory mediators, including IL-6, IL-1β, IFN-γ, TNF-α, and the key regulator of endometrial fibrosis, TGF-β (Wang et al., 2017; Wyble et al., 1997; Tak and Firestein, 2001; Freudlsperger et al., 2013; Jones et al., 2006). Once TGF-β is activated, it stimulates collagen deposition, increases fibrosis percentage, and enhances endometrial stiffness, ultimately resulting in the formation of uterine adhesion bands (Leung et al., 2021; Liu and Wang, 2022).
The fruit of Ziziphus jujube (Z. jujube) is a traditional medicinal herb (Plastina et al., 2012; Chen and Tsim, 2020; Huang et al., 2007) with known anti-inflammatory and fibrinolytic properties, containing high concentrations of vitamins C and B, as well as various minerals such as magnesium, phosphorus, potassium, sodium, and zinc (Li et al., 2007). The major active components in Z. jujube responsible for its bioactivities include triterpenic acids, flavonoids, polysaccharides, and saponins. These bioactive compounds exhibit various pharmacological properties such as antioxidant, anti-inflammatory, and immunomodulatory effects. In terms of absorption, studies suggest that these components can be effectively absorbed due to their optimal molecular size and the presence of transporters in the body that facilitate their uptake. Research on the bioavailability and pharmacokinetics of these active components in Z. jujube is ongoing to further understand their absorption mechanisms and potential health benefits (Hong et al., 2020; Gao et al., 2013). Numerous studies have demonstrated the potent ability of either the fruit or extract of Z. jujube to attenuate inflammation (Zhan et al., 2018; Mesaik et al., 2018; Shen et al., 2022; Ruan et al., 2021; Chen et al., 2014; Liu et al., 2020; Farhadnejad et al., 2022), oxidative stress (Khajavi Rad et al., 2021; Wang et al., 2012), and fibrosis (Wang et al., 2012) in cellular, animal, and clinical studies.
In our previous studies, we explored the therapeutic potential of various components against adhesion band formation in different organs, including tendons (Askarnia-Faal et al., 2023), the abdomen (Arjmand et al., 2020; Soleimani et al., 2020), among others. This study uniquely investigates the therapeutic potential of Z. jujube, a natural product with anti-inflammatory and anti-fibrotic properties, in addressing uterine adhesions, which has not been extensively explored in prior studies. By highlighting the innovative approach of utilizing Z. jujube to improve fertility outcomes and endometrial regeneration, this research contributes valuable insights that may pave the way for new, effective treatment strategies for women suffering from Asherman’s syndrome, thereby filling a critical gap in the current therapeutic landscape.
Materials and methods
Materials
Jujube was purchased locally and powdered using a blender. Rat TNF-α, IL-6 and TGF-β ELISA kits were obtained from Zellbio (Lonsee, Germany). Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Catalase (CAT) assay reagents were acquired from Kushan Zist Azma Co. (Tehran, Iran). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, United States).
Study design
Female albino Wistar rats aged 8 weeks were purchased from the laboratory animal center of Mashhad University of Medical Sciences, Mashhad, Iran. The present study was conducted in compliance with the ARRIVE guidelines and guidelines of the Research Ethics Committee of Mashhad University of Medical Sciences with the ID number IR. MUMS.AEC.1401.084.
The model induction involved mechanically injuring the uterus during the estrus cycle. Vaginal smearing was performed daily at 8 a.m. to assess the estrus cycle of rats. The induction procedure included anesthesia via intra-peritoneal injection of a ketamine (80 mg/kg)/xylazine (8 mg/kg) mixture. Subsequently, midline incisions were made to access the uterus, and the endometrium layer of both uteri was gently scratched using a 7-gauge needle (Liang et al., 2022). The incision area was then sutured. A total of 72 female rats were divided into three groups (n = 24 for each group): Sham Group (midline excision without uterine damage), IUA Positive Control Group (uterine damage with no treatment), and Z. jujube-Treated Group (uterine damage followed by 10 days of 400 mg/kg/day Z. jujube oral treatment). In our previous study (Khalili-Tanha SEN et al., 2024), we employed 400 mg/kg/day Z. jujube and observed notable anti-inflammatory and anti-fibrotic effects, along with a reduction in adhesive bands in the abdomen. The positive outcomes obtained from our earlier research further solidified our decision to utilize the same dosage in the current study. However, considering the evidence from the existing literature (Resim et al., 2020; Wang et al., 2012) and our research, we believe that the 400 mg/kg/day Z. jujube was the most appropriate choice for our study.
Three phases of the study
Phase 1: Ten days post-surgery, 24 rats (8 per group) were sacrificed to investigate the stimulatory effect of Z. jujube on endometrial layer regeneration. Molecular and histological examinations of uterine tissues were conducted to evaluate inflammatory and fibrotic markers as well as regeneration indicators. Extra-uterine adhesions to other abdominal sites and adjacent organs were assessed using an adhesion scoring system (Güney et al., 2017).
Phase 2: On the 10th day post-injury, 24 rats (8 per group) were housed with male rats for mating. After 15 days post-mating, pregnant rats were sacrificed, and parameters such as the number, weight, implantation of embryos, and placental weight were compared between groups. Non-pregnant rats were subsequently housed with male rats to induce further pregnancies.
Phase 3: This phase evaluated the phenotypic and neonatal outcomes of the pregnancy. Twenty-four rats (8 per group) were housed with male rats for mating. Similar to Phase 2, animals were maintained under normal housing conditions until birth. Outcomes included the number and weight of babies, the number of live births, conception time, and a 2-month evaluation of the growth rate of live babies. A schematic representation of the study design is provided in Figure 1. After the experiment, euthanasia was conducted via carbon dioxide (CO2) inhalation.
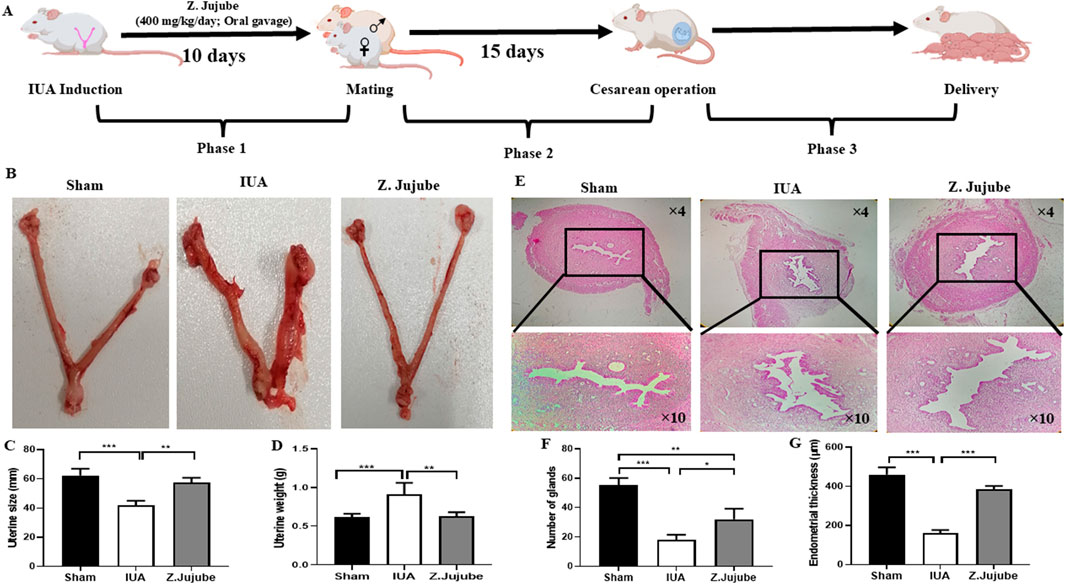
Figure 1. Ziziphus jujube decreased adhesion bands and enhanced endometrial regeneration. (A) The schematic presentation of the study. The therapeutic effects of Ziziphus jujube on (B, C) uterine size and (D) weight were investigated in different groups. (E) H&E-stained sections of the rats’ uterine. (F) The number of glands and (G) endometrial thickness were also measured in the H&E-stained sections of the uterine. *P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
Histological evaluation
The histological assessment involved Hematoxylin and Eosin (H&E) staining to examine endometrial alterations and morphology as described (Feldman and Wolfe, 2014). Measurements of endometrial thickness and the number of glands were performed. Trichrome staining was utilized to evaluate the extent of fibrosis in uterine samples as described (Sridharan et al., 2022) and fibrotic areas were quantified using ImageJ software (NIH, Maryland, United States).
Quantitative RT-PCR
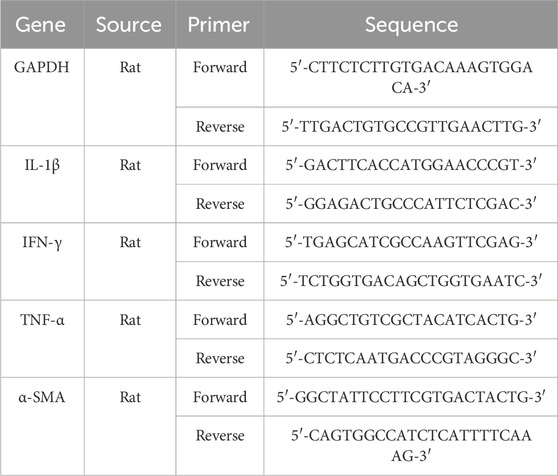
Real-time quantitative PCR (qRT-PCR) was performed as previously described (Hassanian et al., 2014). Total RNA was extracted from liquid nitrogen-frozen tissues, followed by cDNA synthesis via kit (Yekta Tajhiz, Tehran, Iran). qRT-PCR amplification was carried out using Ampliqon SYBR Green PCR Master Mix, with GAPDH serving as the control housekeeping gene. Primer sequences are presented in Table 1.
ELISA assay
The tissue concentrations of TNF-α, IL-6, and TGF-β were measured using Zellbio ELISA kits according to the manufacturer’s protocol.
Oxidative stress evaluation
Oxidative stress markers, including MDA as an oxidative marker, and the enzyme activities of SOD and CAT as antioxidant markers, were measured in uterine tissue homogenates as previously described (Hashemzehi et al., 2020).
Scoring of extra-uterine adhesions
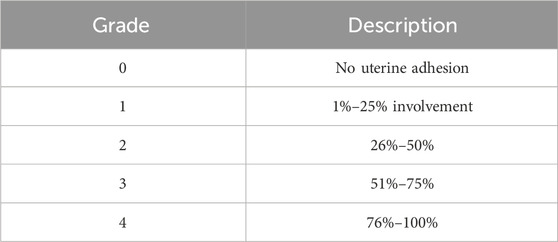
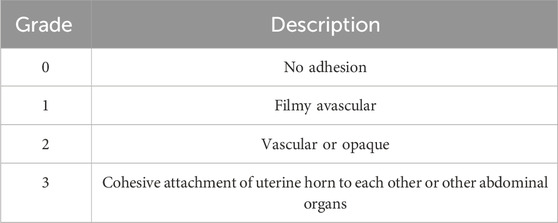
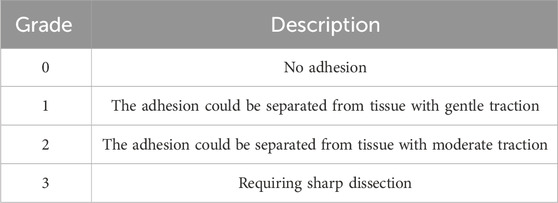
At the end of Phase 1, two independent observers, blinded to the groups, scored pelvic adhesions from sacrificed animals according to the adhesion scoring system developed by Mazuji et al. (1964) (Tables 2–4).
Statistical analysis
The data obtain from groups were statistically compared using one-way ANOVA followed by LSD post hoc test. All the quantified numeral values are presented as mean ± SEM. P values < 0.05 indicated a significant difference between the given groups. *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Z. jujube decreased adhesion band formation and enhanced endometrial regeneration in rat IUA model
This study is comprised of three phases (Figure 1A). At the end of the first phase, we evaluated the macroscopic and microscopic effects of Z. jujube treatment on the formation of adhesion bands and endometrial regeneration. Our results showed that oral administration of Z. jujube significantly decreased uterine shortening (Figures 1B, C) and decreased uterine weight (Figure 1D) compared to the untreated IUA group. Uterine shortening was associated with fibrosis, while uterine weight was associated with inflammation (Kim et al., 2020). Additionally, H&E-stained sections of the uterine revealed enhanced endometrial regeneration, visualized by a significant increase in the number of glands (Figure 1E) and endometrial thickness (Figure 1F) in Z. jujube-treated rats.
Z. jujube attenuated uterine inflammation in rat IUA model
To investigate the protective mechanism of Z. jujube, we compared the expression levels of pro-inflammatory molecules including IL-1β, IL-6, IFN-γ, and TNF-α in the uterine tissue samples. Our results showed that Z. jujube potently downregulated mRNA expression of TNF-α (Figure 2A), IL-1β (Figure 2B), and IFN-γ (Figure 2C) compared to the un-treated IUA group. Furthermore, protein concentrations of IL-6 (Figure 2D) and TNF-α (Figure 2E) were significantly decreased in the Z. jujube-treated group. To further determine the anti-inflammatory properties of Z. jujube, concentrations and the enzymatic activities of oxidant/antioxidant markers were evaluated in uterine tissue homogenates. Z. jujube reduced MDA concentrations (Figure 2F), an oxidant marker, while increasing the enzymatic activities of CAT (Figure 2G) and SOD (Figure 2H), two antioxidant markers, in uterine tissue.

Figure 2. Z. jujube attenuated uterine inflammation in rat IUA model. The transcriptional mRNA level of (A) TNF-α, (B) IL-1β, and (C) IFN-γ were measured in the uterine tissue samples of the animals. Protein expression level of (D) IL-6 and (E) TNF-α was also evaluated in the tissue homogenates of the uterine samples. (F) Z. jujube attenuated MDA concentration, whereas (G) increased enzymatic activities of CAT, and (H) SOD, in uterine tissue samples. *P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
Z. jujube elicited fibrinolytic effects in the uterine tissues
Fibrosis is a key factor in the pathogenesis of adhesion band formation in the uterus. The anti-fibrotic properties of Z. jujube were investigated in uterine tissue samples of animals. Results showed that Z. Jujube potently decreased collagen deposition in the uterine tissue sections as visualized by trichrome staining (Figure 3A). Similarly, the percentage of fibrosis was decreased in the uterine histological sections of rats orally treated with Z. jujube compared with the untreated IUA group (Figure 3B). Transforming Growth Factor-β (TGF-β) is a pivotal player in tissue fibrosis (Verrecchia and Mauviel, 2007). Our results showed that Z. Jujube downregulated the expression of TGF-β (Figure 3C) and pro-fibrotic genes such as α-SMA (Figure 3D) in uterine tissue samples.
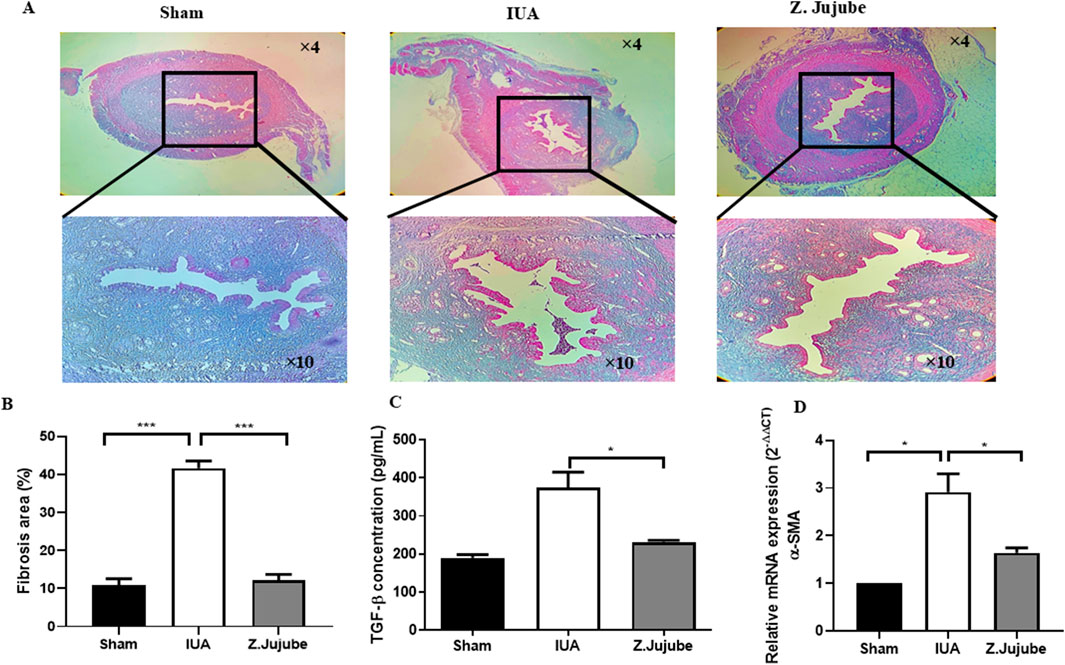
Figure 3. Z. jujube elicited anti-fibrotic effects in the uterine tissues. (A) Z. jujube decreased collagen deposition in the uterine tissue sections as visualized by Trichrome staining. (B) Fibrosis percentage was compared and quantified in the uterine histological sections of rats. Z. jujube decreased (C) protein concentrations of TGF-β and (D) mRNA level of α-SMA in the uterine tissue samples. *P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
Effect of Z. jujube on the embryonic development in the rat IUA model
In the second phase of the study, the effects of Z. jujube on factors related to embryonic development were assessed in the uterine of pregnant rats. Our results showed that injury to the un-treated uterine IUA group decreased the total number of embryos (Figure 4A), while oral administration of Z. jujube post-injury increased the number (Figure 4B), percent of live embryos (Figure 4C), size (Figure 4D), and weight of embryos (Figure 4E). Moreover, injury-induced decrease in fetus implantation factors, including placenta size and weight in the untreated group, were significantly improved in the presence of Z. jujube treatment (Figures 4F, G), demonstrating potent protective effects of gavage administration of Z. jujube on embryonic development in the rat uterus post-injury.
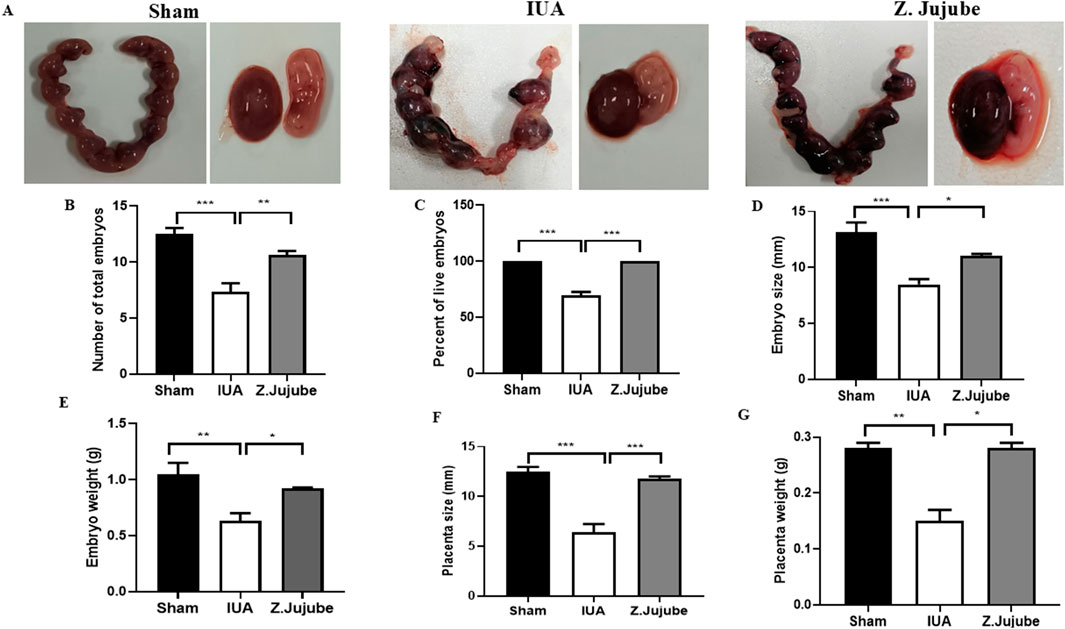
Figure 4. Z. jujube enhanced embryonic development in the rat model of IUA. (A) Macroscopic comparison of uterine, embryo and placenta in different groups of the study. Gavage administration of Z. jujube post-injury enhanced embryonic development as indicated by an increase in (B) number of total embryos, (C) the percent of live embryos, (D) embryo size, (E) embryo weight, (F) placenta size and (G) placenta weight. *P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
Z. jujube enhanced the pregnancy outcomes in a rat model of IUA
In the third phase of the study, we compared the pregnancy outcomes between groups. Injury-induced adhesion formation in the IUA group resulted in a significant reduction in the pregnancy rate in rats. However, this reduction was abrogated by Z. jujube treatment, as all the animals in the Z. jujube-treated group had successful pregnancies by the second mating attempt, in contrast to the IUA group, where about 40% of the rats got pregnant at the fourth mating attempt (Figure 5A). The number of babies (Figures 5B, C), and the percentage of live babies (Figure 5D) were significantly increased by Z. jujube treatment and the babies were also heavier than in the IUA group (Figure 5E). Furthermore, Z. jujube-treated rats conceived significantly faster, in fewer days, compared to the IUA control rats (Figure 5F).
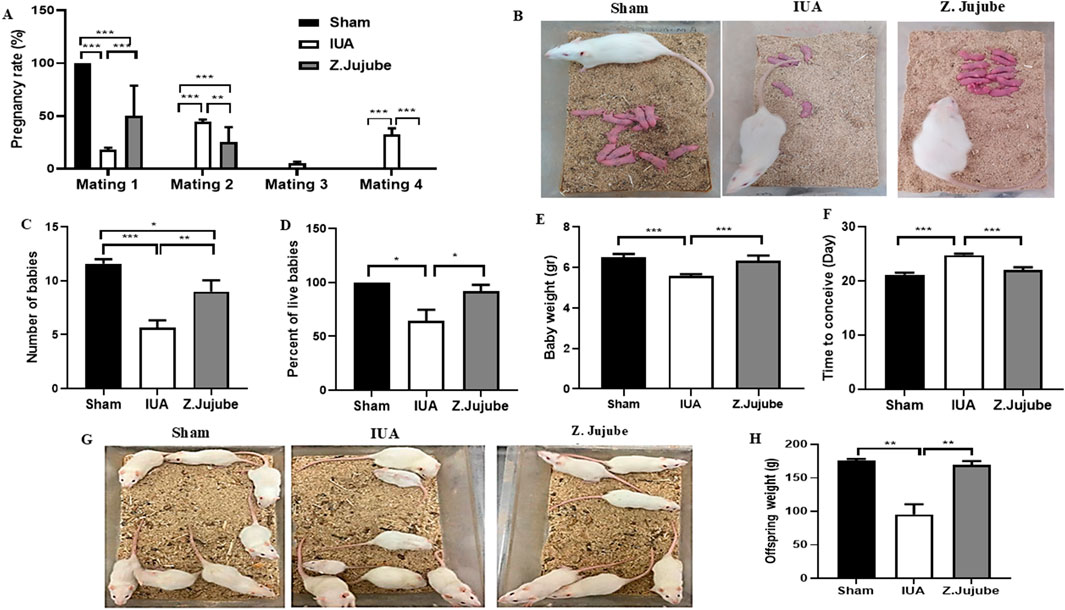
Figure 5. Pregnancy outcomes were enhanced by Z. jujube treatment. (A) The rate of pregnancy is compared between groups. Z. jujube treatment improved the pregnancy outcomes as (B–C) the number of babies, (D) the percent of live babies, and (E) the weight of babies increased in the jujube-treated rats. (F) Mean conception time of each given group. (G) Babies of Z. jujube-treated rats showed normal growth patterns into adulthood and (H) were significantly heavier than the ones in the IUA group.*P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
To further evaluate the effect of IUA on the normal growth of the animals, we maintained the babies in the standard condition for 2 months. Interestingly, we observed impaired growth patterns in some of the babies in the IUA control group, as indicated by a wide variation in their sizes (Figure 5G). On the other hand, the babies of the Z. jujube-treated rats showed a more balanced and normal growth pattern. Consistently, the weight of the babies in the Z. jujube-treated group was the same as the Sham group and significantly higher than that of the IUA rats (Figure 5H).
Z. jujube suppressed extra-uterine adhesion band formation to the internal organs
Surgical procedures on the uterus, including cesarean sections, induce the formation of adhesion bands between the uterus and internal organs (Awonuga et al., 2011). Induction of IUA requires the midline excisions of the uterus, which indirectly causes extra-uterine adhesion as well. Therefore, we evaluated the therapeutic potency of Z. jujube in decreasing extra-uterine adhesion to internal organs using Mazuji et al. (1964) scoring system. As presented in Figure 6A, oral administration of Z. jujube decreased the formation of extra-uterine adhesion to internal organs. Furthermore, using Mazuji et al. scoring system, the adhesion extent score (Figure 6B) (Table 2), severity score (Figure 6C) (Table 3), degree score (Figure 6D) (Table 4), and total adhesion score (Figure 6E) were all attenuated in the Z. jujube-treated rats, compared with the untreated IUA group.
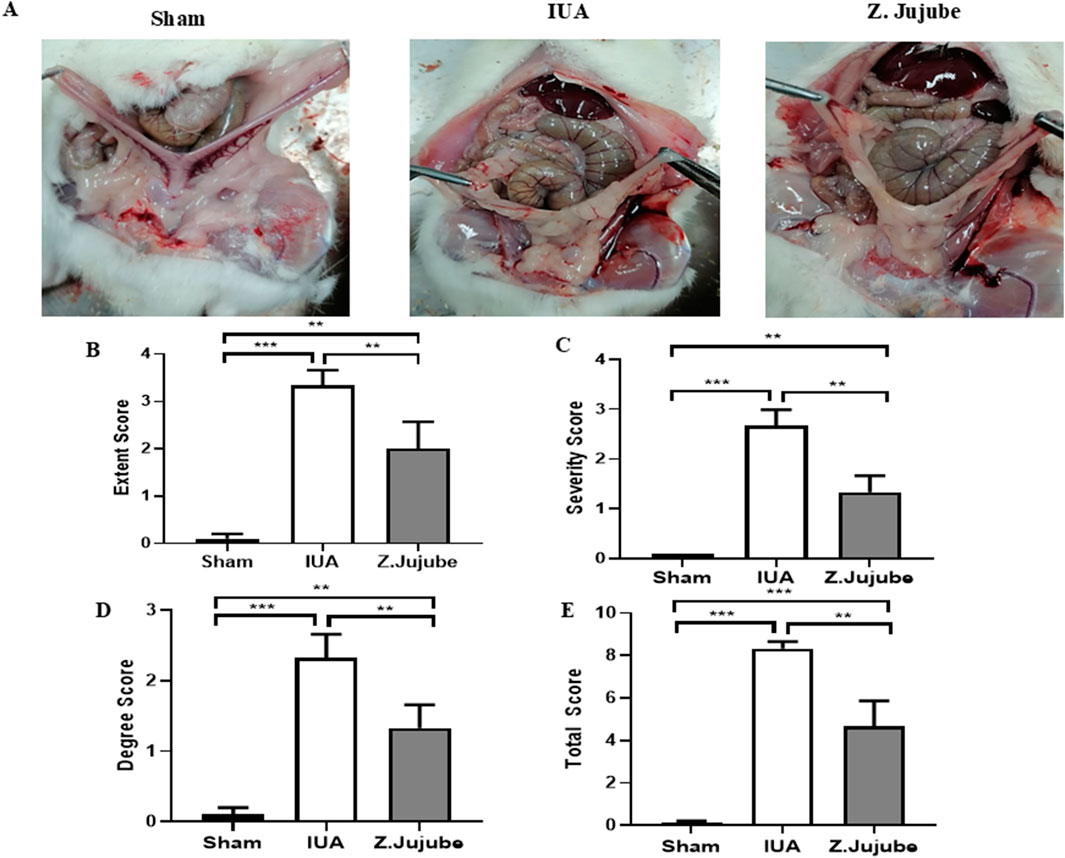
Figure 6. Z. jujube decreased extra-uterine adhesions. (A) Compared to the IUA group, adhesion of uterine to internal organs were decreased in the Z. jujube-treated group. (B) The extent, (C) severity, (D) degree, and (E) Total score of extra-uterine adhesions were compared between different groups. *P < 0.05, **P < 0.01, ***P < 0.001. Data were presented as Mean ± SEM.
Z. jujube did not exhibit any histopathological changes in the organs of rats
To investigate the potential side effects of Z. jujube on treated rats, the heart, kidney, and liver tissues were collected and evaluated by H&E staining. No toxicity-associated morphological changes, no infiltration of inflammatory cells to the liver or kidney, nor re-arrangement of myofibers in the heart were observed in Z. jujube-treated groups (Figure 7), supporting the safety of the oral administration of Z. jujube on the treated group.
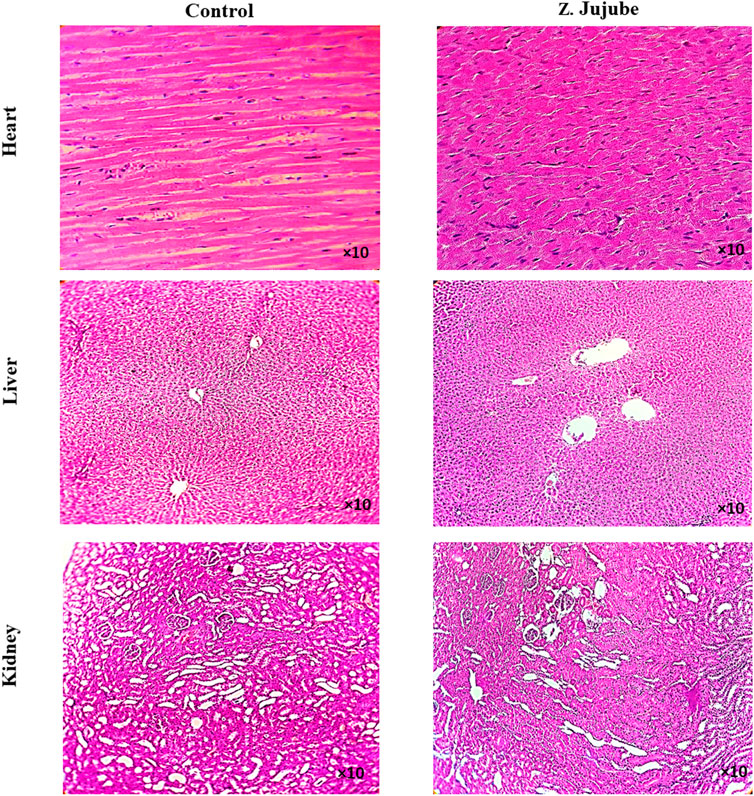
Figure 7. Oral administrations of Z. jujube is safe to internal organs. The H&E staining of heart, liver, and kidney tissues showed no histo-pathological changes, infiltration of inflammatory cells to liver or kidney, nor re-arrangement of myofibers in heart in Z. jujube-treated groups.
Discussion
This is the first study investigating the therapeutic potential of Z. jujube in a rat model of intra- and extra-uterine adhesion, shedding light on its promising effects. Our results demonstrate that oral administration of Z. jujube exerts a significant mitigating influence on the formation and severity of adhesion bands. This effect is demonstrated by the suppression of inflammatory and fibrotic factors, a conclusion drawn from both molecular and histological assessments. Notably, the administration of Z. jujube also leads to improvements in pregnancy outcomes, encompassing increased pregnancy and implantation rates, reduced time to conception, and enhanced parameters related to pregnancy, such as the number and weight of offspring in the rat model of IUA.
Intrauterine adhesion (IUA) frequently results from mechanical or infectious injury to the endometrium layer of the uterus, initiating a pro-inflammatory response marked by the expression and release of pro-inflammatory mediators, including TNF-α, IL-6, and IFN-γ (Chen et al., 2018). The anti-inflammatory effects of Z. jujube have been investigated in several cellular and animal studies. In line with this, Shen et al. (2022) showed that the immunomodulatory properties of Z. jujube polyphenols reduced LPS-induced inflammation by inhibition of NF-κB and MAPK signaling and the reduced production of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β. Z. jujube triterpenes were also reported to exert anti-inflammatory effects by ameliorating the production of IL-6 and TNF-α in LPS-stimulated RAW264.7 cells (Ruan et al., 2021). Jujube polysaccharides also ameliorated colitis-related clinical symptoms and histological alterations in C57BL/6 mice by inhibiting NF-κB and MAPK inflammatory signaling pathways (Liu et al., 2020). The Z. jujube extract was found to decrease the expression of IL-6 and IL-1β in murine macrophages induced by LPS (Chen et al., 2014). In line with these studies, we showed that oral Z. jujube inhibited inflammatory response in the uterine tissue of animals as indicated by downregulated tissue expression of key inflammatory markers including TNF-α, IL-β, and IL-6. Moreover, the tissue concentration of IL-6 and TNF-α -proteins was reduced in Z. jujube-treated animals.
The major inflammatory cells in uterine adhesions are typically macrophages and neutrophils. Macrophages play a crucial role in the inflammatory response and tissue repair processes, while neutrophils are involved in the initial stages of inflammation (Zhu et al., 2023).
Regarding the effect of jujube on these immune cells, studies have shown that certain bioactive compounds present in Ziziphus jujube, such as flavonoids and triterpenoids, have anti-inflammatory properties. These compounds can modulate the activity of inflammatory cells and cytokines, thereby reducing the inflammatory response in various tissues, including the uterus. While specific research on the direct effect of jujube on inflammatory cells in uterine adhesions is limited, the anti-inflammatory properties of jujube suggest potential benefits in mitigating inflammation and immune cell activation in this context (Batovska et al., 2024; Ruan et al., 2023).
Consistent with the anti-inflammatory properties of Z. jujube, its inhibitory effects on oxidative stress have been investigated in previous studies. For instance, Resim et al. showed that Z. jujube induced the activity of SOD and CAT, reducing MDA levels of cavernous nerve injury (CNI)-induced erectile dysfunction (ED) in a rat model (Wang et al., 2012). Similarly, Z. jujube was reported to exert anti-oxidant properties in a rat model of adriamycin-induced organ toxicity (Khajavi Rad et al., 2021). In another study conducted by Hong et al., Z. jujube effectively decreased the oxidative stress in a model of alcohol-induced liver damage, which was indicated by a significant reduction in MDA level (Hong et al., 2020). Using an animal model of diabetes, Hemmati et al. (2015) showed an increase in total antioxidant capacity in the sera of animals accompanied by a reduction in MDA level following Z. jujube treatment. Our results showed that Z. jujube decreased oxidative stress and regulated the oxidant/antioxidant balance by stimulating the antioxidant markers including SOD and CAT enzymes and inhibiting the oxidative stress marker, MDA.
During the inflammatory phase, immune cells rapidly release pro-inflammatory cytokines to the uterine cavity leading to initiation of fibrotic responses (Leung et al., 2021). Inflammation-induced TGF-β activation stimulates the release of collagens, smooth muscle actin, and other extracellular matrix (ECM) components. Excessive release of ECM contents forms adhesion bands within the uterine cavity (Chen et al., 2021; Leung et al., 2021). Z. jujube has been previously reported to suppress the expression of fibrosis-associated proteins including COL1, COL3, and smooth muscle actins (Wang et al., 2012). We also found that oral Z. jujube decreased the tissue concentration of TGF-β and the subsequent formation of adhesion bands. The TGF-β-induced mRNA upregulation of α-SMA was also significantly diminished by Z. jujube treatment.
In the clinical context, the formation of adhesion bands in the uterus presents numerous complications, impacting both maternal health and pregnancy outcomes. Adhesion bands prevent normal endothelial regeneration in the menstrual cycles resulting in infertility (Evans-Hoeker and Young, 2014). Adhesion bands interfere with the blastocyte implantation process and restrain the blood supply of the uterus resulting in a low pregnancy rate and/or unsuccessful pregnancy among individuals affected by IUA (March, 2011). The results obtained in this study demonstrated the protective effect of Z. jujube on IUA complications. The implantation success rate and pregnancy rate were significantly enhanced by Z. jujube treatment. In addition, the pregnancy outcomes were significantly enhanced by Z. jujube as the number of total and live babies increased in pregnant rats and babies had higher weights. Another noteworthy observation was the normal and balanced growth pattern in the babies of Z. jujube-treated rats compared to the IUA control group.
Briefly, Z. jujube is a plant with potential therapeutic properties that could facilitate endometrial regeneration and alleviate adhesion formation. Several key attributes contribute to its efficacy in this context: First, Z. jujube possesses anti-inflammatory properties, which may help reduce inflammation and granuloma tissue formation (Zhu et al., 2024). Inflammation is a critical factor in various diseases and conditions, including endometrial dysfunction. By mitigating inflammation, Z. jujube may create a more favorable environment for endometrial regeneration. Additionally, Z. jujube has been shown to inhibit the activity of NF-κB, a transcription factor that regulates the production of pro-inflammatory cytokines (Miao et al., 2020). This modulation of NF-κB activity could play a crucial role in reducing inflammation and promoting tissue regeneration (Miao et al., 2020). It has also demonstrated the ability to inhibit the formation of bacterial biofilms, leading to a reduction in the thickness and adhesion of these biofilms. The inhibitory effects on biofilm formation could be beneficial in preventing infection and promoting a healthy environment for endometrial regeneration (Miao et al., 2020). This plant contains various active compounds, such as triterpenic acids, ursolic acid, and oleanolic acid, which are believed to possess anti-inflammatory properties (Yu et al., 2012). These compounds may work synergistically to promote endometrial regeneration and reduce adhesion formation (Kang et al., 2015). Furthermore, Z. jujube contains alkaloids, which are known for their antioxidant and antiviral properties (Zare-Zardini et al., 2013). These alkaloids may further contribute to the overall therapeutic effects of Z. jujube in the context of endometrial health.
The endometrium is a highly regenerative tissue that undergoes cyclical processes of growth, differentiation, and shedding, regulated by hormones such as estrogen and progesterone (Gargett et al., 2012). The potential therapeutic properties of Z. jujube, as discussed above, may provide additional support to the endometrium during these cyclical changes, promoting optimal tissue regeneration and reducing adhesion formation. However, further research is necessary to fully understand the effects of Z. jujube on endometrial health and its potential clinical applications.
In conclusion, our findings suggest that Z. jujube holds promise as a safe therapeutic candidate for mitigating intra- and extra-uterine adhesion bands. The protective effects of Z. jujube in decreasing the formation of adhesion bands may be attributed to its inhibitory effects on inflammation, oxidative stress, and fibrosis. However, further research is warranted to elucidate the exact mechanisms underpinning Z. jujube’s protective effects and to assess its efficacy in other animal models and clinical trials.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Mashhad University of Medical Sciences Mashhad, Iran. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FA: Conceptualization, Formal Analysis, Methodology, Project administration, Validation, Visualization, Writing–review and editing. MA: Investigation, Methodology, Visualization, Writing–original draft. MK: Formal Analysis, Supervision, Writing–review and editing. AA-A: Resources, Writing–original draft. SM: Investigation, Methodology, Writing–original draft. HN: Methodology, Visualization, Writing–original draft. ME: Visualization, Writing–original draft. AK: Conceptualization, Methodology, Writing–original draft. SN: Data curation, Formal Analysis, Validation, Writing–original draft. AkA: Investigation, Software, Writing–original draft. MF: Data curation, Validation, Visualization, Writing–original draft. EE: Methodology, Visualization, Writing–original draft. AmA: Data curation, Formal Analysis, Writing–original draft. MR: Writing–review and editing. MH: Writing–review and editing. SH: Funding acquisition, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants awarded by the Mashhad University of Medical Sciences (4002161) to SH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAT, catalase; H&E, Hematoxylin and Eosin; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IFN-γ, Interferon gamma; MDA, malonyl dialdehyde; SOD, superoxide dismutase; TGF-β, transforming growth factor β; TNF-α, necrosis factor-alpha; α-SMA, α-smooth muscle actin; IUA, Intrauterine adhesion; Z. jujube, Ziziphus jujube.
References
Al-Inany, H. (2001). Intrauterine adhesions. An update. Acta Obstet. Gynecol. Scand. 80, 986–993. doi:10.1080/obs.80.11.986.993
Arjmand, M.-H., Zahedi-Avval, F., Barneh, F., Mousavi, S. H., Asgharzadeh, F., Hashemzehi, M., et al. (2020). Intraperitoneal administration of telmisartan prevents postsurgical adhesion band formation. J. Surg. Res. 248, 171–181. doi:10.1016/j.jss.2019.10.029
Askarnia-Faal, M.-M., Sayyed-Hosseinian, S.-H., Nazari, S. E., Asgharzadeh, F., Vahedi, E., Eskandari, M., et al. (2023). Exploring new therapeutic potentials of curcumin against post-surgical adhesion bands. BMC Complement. Med. Ther. 23, 27. doi:10.1186/s12906-022-03808-6
Awonuga, A. O., Fletcher, N. M., Saed, G. M., and Diamond, M. P. (2011). Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod. Sci. 18, 1166–1185. doi:10.1177/1933719111414206
Batovska, D., Gerasimova, A., and Nikolova, K. (2024). Exploring the therapeutic potential of jujube (Ziziphus jujuba mill.) extracts in cosmetics: a review of bioactive properties for skin and hair wellness. Cosmetics 11, 181. doi:10.3390/cosmetics11050181
Chen, J., Du, C. Y. Q., Lam, K. Y. C., Zhang, W. L., Lam, C. T. W., Yan, A. L., et al. (2014). The standardized extract of Ziziphus jujuba fruit (jujube) regulates pro-inflammatory cytokine expression in cultured murine macrophages: suppression of lipopolysaccharide-stimulated NF-κB activity. Phytother. Res. 28, 1527–1532. doi:10.1002/ptr.5160
Chen, J., and Tsim, K. W. K. (2020). A review of edible jujube, the Ziziphus jujuba fruit: a heath food supplement for anemia prevalence. Front. Pharmacol. 11, 593655. doi:10.3389/fphar.2020.593655
Chen, J.-M., Huang, Q.-Y., Zhao, Y.-X., Chen, W.-H., Lin, S., and Shi, Q.-Y. (2021). The latest developments in immunomodulation of mesenchymal stem cells in the treatment of intrauterine adhesions, both allogeneic and autologous. Front. Immunol. 12, 785717. doi:10.3389/fimmu.2021.785717
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., et al. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218. doi:10.18632/oncotarget.23208
Deans, R., and Abbott, J. (2010). Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 17, 555–569. doi:10.1016/j.jmig.2010.04.016
Dreisler, E., and Kjer, J. J. (2019). Asherman's syndrome: current perspectives on diagnosis and management. Int. J. Womens Health 11, 191–198. doi:10.2147/IJWH.S165474
Evans-Hoeker, E. A., and Young, S. L. (2014). Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 32, 392–401. doi:10.1055/s-0034-1376358
Farhadnejad, H., Asghari, G., Hedayati, M., Sahranavard, S., Teymoori, F., Mirmiran, P., et al. (2022). Effect of Ziziphus jujube on cardiometabolic factors and systemic inflammation in type 2 diabetic patients: a randomized controlled trial. Clin. Nutr. ESPEN 49, 53–60. doi:10.1016/j.clnesp.2022.03.043
Feldman, A. T., and Wolfe, D. (2014). Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 1180, 31–43. doi:10.1007/978-1-4939-1050-2_3
Freudlsperger, C., Bian, Y., Contag Wise, S., Burnett, J., Coupar, J., Yang, X., et al. (2013). TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32, 1549–1559. doi:10.1038/onc.2012.171
Gao, Q.-H., Wu, C.-S., and Wang, M. (2013). The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 61, 3351–3363. doi:10.1021/jf4007032
Gargett, C. E., Nguyen, H. P., and Ye, L. (2012). Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. and metabolic Disord. 13, 235–251. doi:10.1007/s11154-012-9221-9
Güney, G., Kaya, C., Oto, G., Yıldırım, S., Özdemir, H., and Tokmak, A. (2017). Effects of quercetin and surgicel for preventing adhesions after gynecological surgery: a rat uterine horn model. J. Obstet. Gynaecol. Res. 43, 179–184. doi:10.1111/jog.13185
Hashemzehi, M., Naghibzadeh, N., Asgharzadeh, F., Mostafapour, A., Hassanian, S. M., Ferns, G. A., et al. (2020). The therapeutic potential of losartan in lung metastasis of colorectal cancer. EXCLI J. 19, 927–935. doi:10.17179/excli2020-2093
Hassanian, S. M., Dinarvand, P., and Rezaie, A. R. (2014). Adenosine regulates the proinflammatory signaling function of thrombin in endothelial cells. J. Cell Physiol. 229, 1292–1300. doi:10.1002/jcp.24568
Hemmati, M., Zohoori, E., Mehrpour, O., Karamian, M., Asghari, S., Zarban, A., et al. (2015). Anti-atherogenic potential of jujube, saffron and barberry: anti-diabetic and antioxidant actions. EXCLI J. 14, 908–915. doi:10.17179/excli2015-232
Hong, S., Kim, Y., Sung, J., Lee, H., Heo, H., Jeong, H. S., et al. (2020). Jujube (Ziziphus jujuba mill.) protects hepatocytes against alcohol-induced damage through Nrf2 activation. Evid. Based Complement. Altern. Med. 2020, 6684331. doi:10.1155/2020/6684331
Huang, X., Kojima-Yuasa, A., Norikura, T., Kennedy, D. O., Hasuma, T., and Matsui-Yuasa, I. (2007). Mechanism of the anti-cancer activity of Zizyphus jujuba in HepG2 cells. Am. J. Chin. Med. 35, 517–532. doi:10.1142/S0192415X0700503X
Jones, R. L., Stoikos, C., Findlay, J. K., and Salamonsen, L. A. (2006). TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 132, 217–232. doi:10.1530/rep.1.01076
Kang, K. B., Ming, G., Kim, G. J., Choi, H., Oh, W. K., Sung, S. H., et al. (2015). Jubanines F–J, cyclopeptide alkaloids from the roots of Ziziphus jujuba. Phytochemistry 119, 90–95. doi:10.1016/j.phytochem.2015.09.001
Khajavi Rad, A., Entezari Heravi, N., Kamkar-Del, Y., Abbasnezhad, A., Jalili-Nik, M., Shafei, M. N., et al. (2021). A standardized extract of Ziziphus jujuba Mill protects against adriamycin-induced liver, heart, and brain toxicity: an oxidative stress and biochemical approach. J. Food Biochem. 45, e13698. doi:10.1111/jfbc.13698
Khalili-Tanha Sen, G., Asgharzadeh, F., Mobasheri, L., Babaei, M., Naimi, H., Maftooh, M., et al. (2024). The therapeutic potential of ganoderma lucidum karst and Ziziphus jujuba mill for postsurgical adhesion band formation. Lett. Drug Des. and Discov. 21. doi:10.2174/0115701808298079240818170758
Kim, S. W., Kim, Y. Y., Kim, H., and Ku, S.-Y. (2020). Animal models closer to intrauterine adhesive pathology. Ann. Transl. Med. 8, 1125. doi:10.21037/atm-20-3598
Leung, R. K.-K., Lin, Y., and Liu, Y. (2021). Recent advances in understandings towards pathogenesis and treatment for intrauterine adhesion and disruptive insights from single-cell Analysis. Reprod. Sci. 28, 1812–1826. doi:10.1007/s43032-020-00343-y
Li, J.-W., Fan, L.-P., Ding, S.-D., and Ding, X.-L. (2007) Nutritional composition of five cultivars of Chinese jujube. Food Chem. 103: 454–460. doi:10.1016/j.foodchem.2006.08.016
Liang, S., Huang, Y., Xia, Y., Liang, S., Wu, Q., and Zhi, Z. (2022). Animal models in intrauterine adhesion research. J. Obstet. Gynaecol. 42, 3409–3415. doi:10.1080/01443615.2022.2124854
Liu, H.-D., and Wang, S.-W. (2022). Role of noncoding RNA in the pathophysiology and treatment of intrauterine adhesion. Front. Genet. 13, 948628. doi:10.3389/fgene.2022.948628
Liu, Y., Zhao, X., Lin, T., Wang, Q., Zhang, Y., and Xie, J. (2020). Molecular mechanisms of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds regulating the bioavailability of spinosin and preventing colitis. Int. J. Biol. Macromol. 163, 1393–1402. doi:10.1016/j.ijbiomac.2020.07.229
March, C. M. (2011). Management of Asherman's syndrome. Reprod. Biomed. Online 23, 63–76. doi:10.1016/j.rbmo.2010.11.018
Mazuji, M. K., Kalambaheti, K., and Pawar, B. (1964). Prevention of adhesions with polyvinylpyrrolidone. preliminary report. Arch. Surg. 89, 1011–1015. doi:10.1001/archsurg.1964.01320060079015
Mesaik, A. M., Poh, H. W., Bin, O. Y., Elawad, I., and Alsayed, B. (2018). In vivo anti-inflammatory, anti-bacterial and anti-diarrhoeal activity of Ziziphus jujuba fruit extract. Open Access Maced. J. Med. Sci. 6, 757–766. doi:10.3889/oamjms.2018.168
Miao, W., Sheng, L., Yang, T., Wu, G., Zhang, M., Sun, J., et al. (2020). The impact of flavonoids-rich Ziziphus jujuba Mill. Extract on Staphylococcus aureus biofilm formation. BMC complementary Med. Ther. 20, 187. doi:10.1186/s12906-020-2833-9
Myers, E. M., and Hurst, B. S. (2012). Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil. Steril. 97, 160–164. doi:10.1016/j.fertnstert.2011.10.036
Plastina, P., Bonofiglio, D., Vizza, D., Fazio, A., Rovito, D., Giordano, C., et al. (2012). Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol. 140, 325–332. doi:10.1016/j.jep.2012.01.022
Rein, D. T., Schmidt, T., Hess, A. P., Volkmer, A., Schöndorf, T., and Breidenbach, M. (2011). Hysteroscopic management of residual trophoblastic tissue is superior to ultrasound-guided curettage. J. Minim. Invasive Gynecol. 18, 774–778. doi:10.1016/j.jmig.2011.08.003
Resim, S., Koluş, E., Barut, O., Kucukdurmaz, F., Bahar, A. Y., and Dagli, H. (2020). Ziziphus jujube ameliorated cavernosal oxidative stress and fibrotic processes in cavernous nerve injury-induced erectile dysfunction in a rat model. Andrologia 52, e13632. doi:10.1111/and.13632
Ruan, J., Li, H., Lu, M., Hao, M., Sun, F., Yu, H., et al. (2023). Bioactive triterpenes of jujube in the prevention of colorectal cancer and their molecular mechanism research. Phytomedicine 110: 154639. doi:10.1016/j.phymed.2022.154639
Ruan, J., Sun, F., Hao, M., Han, L., Yu, H., Lin, F., et al. (2021). Structurally diverse triterpenes obtained from the fruits of Ziziphus jujuba Mill. as inflammation inhibitors by NF-κB signaling pathway. Food Funct. 12, 4496–4503. doi:10.1039/d1fo00117e
Shen, D., Wu, C., Fan, G., Li, T., Dou, J., Zhu, J., et al. (2022). Jujube peel polyphenols synergistically inhibit lipopolysaccharide-induced inflammation through multiple signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 164, 113062. doi:10.1016/j.fct.2022.113062
Soleimani, A., Asgharzadeh, F., Rahmani, F., Avan, A., Mehraban, S., Fakhraei, M., et al. (2020). Novel oral transforming growth factor-β signaling inhibitor potently inhibits postsurgical adhesion band formation. J. Cell Physiol. 235, 1349–1357. doi:10.1002/jcp.29053
Sridharan, D., Pracha, N., Dougherty, J. A., Akhtar, A., Alvi, S. B., and Khan, M. (2022). A one-stop protocol to assess myocardial fibrosis in frozen and paraffin sections. Methods Protoc. 5, 13. doi:10.3390/mps5010013
Tak, P. P., and Firestein, G. S. (2001). NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 107, 7–11. doi:10.1172/JCI11830
Tsui, K.-H., Lin, L.-T., Cheng, J.-T., Teng, S.-W., and Wang, P.-H. (2014). Comprehensive treatment for infertile women with severe Asherman syndrome. Taiwan J. Obstet. Gynecol. 53, 372–375. doi:10.1016/j.tjog.2014.04.022
Verrecchia, F., and Mauviel, A. (2007). Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 13, 3056–3062. doi:10.3748/wjg.v13.i22.3056
Wang, D., Zhao, Y., Jiao, Y., Yu, L., Yang, S., and Yang, X. (2012). Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 88: 1453–1459. doi:10.1016/j.carbpol.2012.02.046
Wang, X., Ma, N., Sun, Q., Huang, C., Liu, Y., and Luo, X. (2017). Elevated NF-κB signaling in Asherman syndrome patients and animal models. Oncotarget 8, 15399–15406. doi:10.18632/oncotarget.14853
Wyble, C. W., Hynes, K. L., Kuchibhotla, J., Marcus, B. C., Hallahan, D., and Gewertz, B. L. (1997). TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. J. Surg. Res. 73, 107–112. doi:10.1006/jsre.1997.5207
Xue, X., Chen, Q., Zhao, G., Zhao, J.-Y., Duan, Z., and Zheng, P.-S. (2015). The overexpression of TGF-β and CCN2 in intrauterine adhesions involves the NF-κB signaling pathway. PLoS One 10, e0146159. doi:10.1371/journal.pone.0146159
Yu, D., Li, T.-C., Xia, E., Huang, X., Liu, Y., and Peng, X. (2008). Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil. Steril. 89, 715–722. doi:10.1016/j.fertnstert.2007.03.070
Yu, L., Jiang, B. P., Luo, D., Shen, X. C., Guo, S., Duan, J. A., et al. (2012). Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine 19, 239–244. doi:10.1016/j.phymed.2011.09.071
Zare-Zardini, H., Tolueinia, B., Hashemi, A., Ebrahimi, L., and Fesahat, F. (2013). Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer's Dis. other dementias 28, 702–709. doi:10.1177/1533317513500839
Zhan, R., Xia, L., Shao, J., Wang, C., and Chen, D. (2018). Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. J. Funct. Foods 40: 461–470. doi:10.1016/j.jff.2017.11.026
Zhang, S., Li, P., Yuan, Z., and Tan, J. (2019). Platelet-rich plasma improves therapeutic effects of menstrual blood-derived stromal cells in rat model of intrauterine adhesion. Stem Cell Res. Ther. 10, 61. doi:10.1186/s13287-019-1155-7
Zhu, D., Jiang, N., Wang, N., Zhao, Y., and Liu, X. (2024). A literature review of the pharmacological effects of jujube. Foods 13, 193. doi:10.3390/foods13020193
Keywords: Ziziphus jujube, uterine adhesion, pregnancy outcomes, endometrial regeneration, Asherman’s syndrome, embryonic development
Citation: Asgharzadeh F, Attarian M, Khazaei M, Al-Asady AM, Mansoori S, Naimi H, Eskandari M, Khorrami A, Nazari SE, Aminian A, Farazastanian M, Eshtad E, Avan A, Ryzhikov M, Hasanzadeh M and Hassanian SM (2025) Ziziphus jujube promotes fertility and pregnancy outcomes in Rat model of uterine adhesions. Front. Pharmacol. 15:1496136. doi: 10.3389/fphar.2024.1496136
Received: 13 September 2024; Accepted: 30 December 2024;
Published: 27 January 2025.
Edited by:
Chris A. Bashur, Florida Institute of Technology, United StatesReviewed by:
James David Adams, Independent Researcher, Benicia, CA, United StatesLuoyang Wang, Qingdao University, China
Tian Xia, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Copyright © 2025 Asgharzadeh, Attarian, Khazaei, Al-Asady, Mansoori, Naimi, Eskandari, Khorrami, Nazari, Aminian, Farazastanian, Eshtad, Avan, Ryzhikov, Hasanzadeh and Hassanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed Mahdi Hassanian, aGFzYW56YWRlbW9mcmFkbUBtdW1zLmFjLmly; Malihe Hasanzadeh, RmFyYXplc3Rhbmlhbk1AbXVtcy5hYy5pcg==
†These authors have contributed equally to this work
 Fereshteh Asgharzadeh
Fereshteh Asgharzadeh Mahsa Attarian3†
Mahsa Attarian3† Majid Khazaei
Majid Khazaei Malihe Hasanzadeh
Malihe Hasanzadeh