- Department of Clinical Pharmacy, Weifang People’s Hospital, Shandong Second Medical University, Weifang, China
Background: Recombinant human granulocyte-colony stimulating factors (G-CSF)-induced aortitis is a rare but particularly serious adverse event, commonly seen in cancer patients undergoing chemotherapy. The aim of this article is to clarify the clinical characteristics of G-CSF- induced aortitis and provide effective references for clinical diagnosis and intervention.
Methods: Case reports of adverse reactions of aortitis induced by G-CSF were collected from the relevant databases. The patients’ basic information and adverse reaction process were recorded and subjected to descriptive analysis.
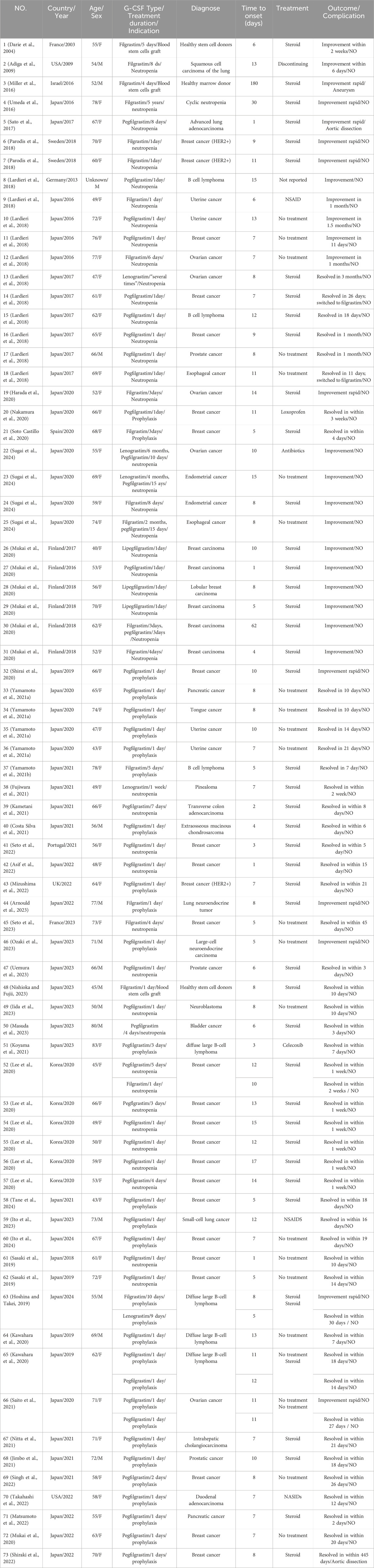
Results: A total of 72 patients were enrolled, including 14 males and 58 females, with a mean age of 61.83 ± 10.30 years. The G-CSF type with the highest frequency of occurrence of aortitis is pegfilgrastim. Apart from three healthy stem cell donors, G-CSF-induced aortitis was primarily found in patients with underlying malignancies, especially in patients with breast cancer. The most common anticancer drugs used at onset were docetaxel, cyclophosphamide, and doxorubicin. CT scan showed that aortitis most commonly occured in the aortic arch and its branches. Most patients had a good prognosis, but 3 cases developed complications. Importantly, G-CSF-induced aortitis was also found in 4 asymptomatic patients.
Conclusion: This article found that G-CSF-induced aortitis not only occured in cancer patients undergoing chemotherapy as previously reported in literature, but also in healthy stem cell donors. Especially, asymptomatic patients with G-CSF-induced aortitis faced a greater risk of being missed by the attending physician.
1 Introduction
Recombinant human granulocyte-colony stimulating factors (G-CSF) is a member of the hematopoietic growth factor family that mobilizes and increases peripheral blood hematopoietic stem cells in both blood donors and cancer patients. G-CSF is primarily used to promote an increase in neutrophil count during bone marrow transplantation and prevent chemotherapy-induced febrile neutropenia (Link, 2022). The common adverse reactions of G-CSF include fever, back pain, headache, bone pain, and myalgia. Severe adverse events include acute lung injury, acute coronary syndrome, and acute aortitis (D'Souza et al., 2008). According to Japanese Adverse Drug Event Report database, aortitis occurring after G-CSF administration is considered an adverse reaction of G-CSF (Oshima et al., 2019). Aortitis induced-by G-CSF is extremely rare, with an incidence rate of 0.3%–0.74% in patients with malignant neoplasms (Sasaki et al., 2021; Takamatsu et al., 2022; Lee et al., 2020).
Aortitis associated with G-CSF administration primarily presents as a systemic symptoms, manifesting as fever and increased levels of c-reactive protein (CRP) (Lee et al., 2020). Due to the similarity of symptoms, it is highly likely to be misdiagnosed as an infection, thereby delaying the timely treatment of aortitis. CT is one of the crucial diagnostic tools for definitive diagnosis.
Based on the cases reported to date, there are four types of G-CSF associated with aortitis, including filgrastim, lenograstim, pegfilgrastim and lipegfilgrastim. Filgrastim and lenograstim, are short-acting G-CSF, which have a similar structure and biological activity to endogenous human G-CSF (Lee et al., 2020). Pegfilgrastim and lipegfilgrastim are long-acting agent of G-CSF (Lee et al., 2020). Most notably, pegfilgrastim and filgrastim account for the majority of reported cases of aortitis. Although adverse events of arteritis caused by G-CSF have been reported in previous studies (Ito et al., 2023; Ito et al., 2024; Hoshina and Takei, 2019), adverse events of arteritis have not been described in large-scale cohort studies.
In the present study, we attempted to explore distribution, occurrence and combination therapy characteristics of G-CSF-induced aortitis. To achieve this aim, we analyzed data from case reports of aortitis induced by G-CSF published in databases.
2 Materials and methods
2.1 Patients selection
PubMed, MEDLINE, Embase, Web of Science, Science Direct, China National Knowledge Internet, Wan fang, and Wei Pu databases were searched until 23 March 2024. The keywords “arteritis,” “aortitis,” “large vessel vasculitis,” “adverse events,” and “granulocyte colony-stimulating factor” were used as search terms to collect case reports concerning adverse events of aortitis induced by G-CSF. The inclusion criteria were (Link, 2022) reports published in open journals and (D'Souza et al., 2008) studies including the patients’ basic information, symptoms, and treatment of adverse aortitis events. The exclusion criteria were (Link, 2022) cases repeated in the databases, and (D'Souza et al., 2008) articles with incomplete patient information, therapeutic process and prognosis of adverse events of aortitis.
2.2 Methods
A literature review research design included 44 papers, was accepted to collect well-documented information (Table 1). A total of 73 cases with aortitis induced by G-CSF were collected, with one case excluded due to insufficient information. For all 72 cases, age, sex, nationality, primary disease, types of G-CSF, combined chemotherapy regimen, onset time of aortitis adverse events, symptoms, aortitis extension, clinical intervention, and outcome were recorded. The relevant data was analysed statistically.
2.3 Statistical analysis
Baseline characteristics were described as numbers and percentages for all patients treated with G-CSF. Continuous variables were presented as mean ± standard deviation (M±SD). Categorical variables were presented as percentages. All statistical analysis was performed using Graphpad Prism version 8.0.2.
3 Results
3.1 Frequency and clinical information related to G-CSF-induced aortitis
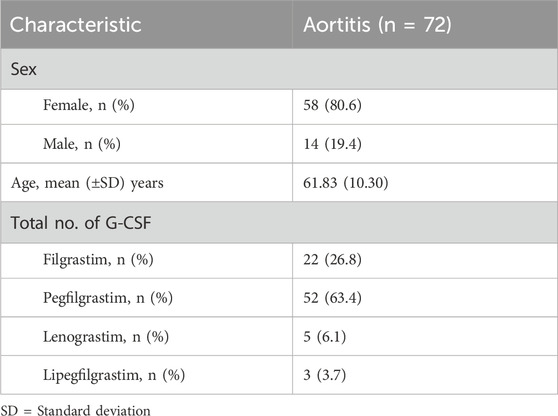
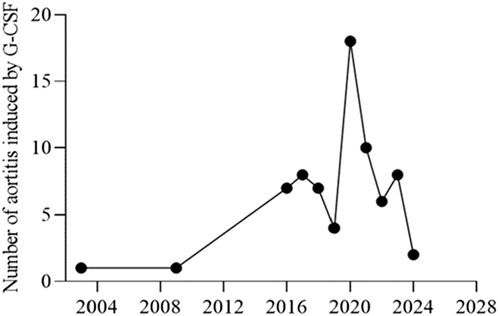
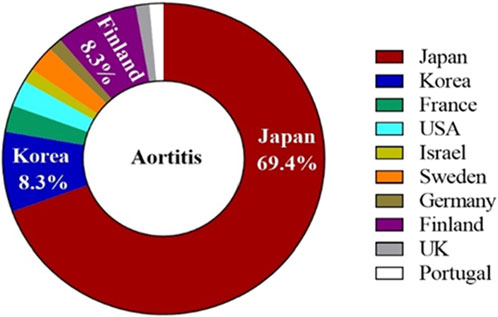
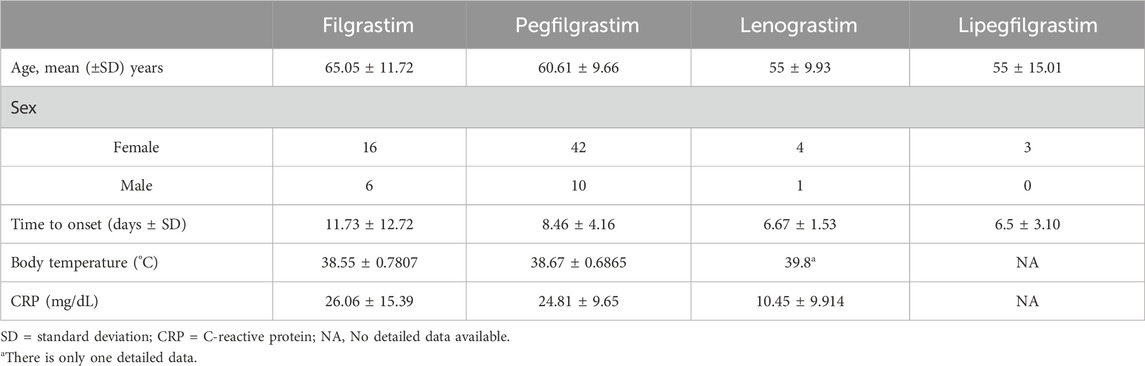
The characteristics of the patients were presented in Table 2. There were 58 women and 14 men, with a mean age of 61.83 years ±10.30 (SD) (age range, 40–80 years) at the occurrence of adverse events related to aortitis. Patients with G-CSF-induced aortitis were predominantly female (male, 19.4%; female, 80.6%). The annual trends in the incidence of G-CSF-induced aortitis are shown in Figure 1, showing that the incidence of G-CSF-induced aortitis increased each year until 2020. Figure 2 shows the relationship between the incidence of G-CSF-induced aortitis and global distribution. The countries with the highest incidence of G-CSF-induced aortitis are Japan (50 cases, 69.4%), South Korea (6 cases, 8.3%) and Finland (6 cases, 8.3%). A total of 72 patients had a medical or prophylactic aim for filgrastim (n = 22), pegfilgrastim (n = 52), lenograstim (n = 5) or lipegfilgrastim (n = 3) during the identification period (Table 2). Of all, pegfilgrastim was characterized by the highest frequency (63.4%) of arteritis. Patients with G-CSF-induced aortitis of these four types were remarkably similar in terms of sex, age and time to onset. Patients who use filgrassim (CRP 26.06 ± 15.39 mg/dL), pegfililgrassim (CRP 24.81 ± 9.65 mg/dL), and lenograstim (CRP 10.45 ± 9.914 mg/dL) all presented varying degrees of elevated serum CRP levels (Table 3). Unfortunately, no CRP related data were provided for the four cases using lipegfilgrastim. In addition, Patients with G-CSF-induced aortitis of these four types were similar in clinical manifestations, such as fever, chest pain, abdominal pain, neck pain, back pain, earache, sore throat, headache and myalgia, etc. Meanwhile, the four types of G-CSF-induced aortitis also exhibited similarities in their common occurrence sites, all of which were prone to occur in the aortic arch, abdominal aorta, and thoracic aorta, etc.
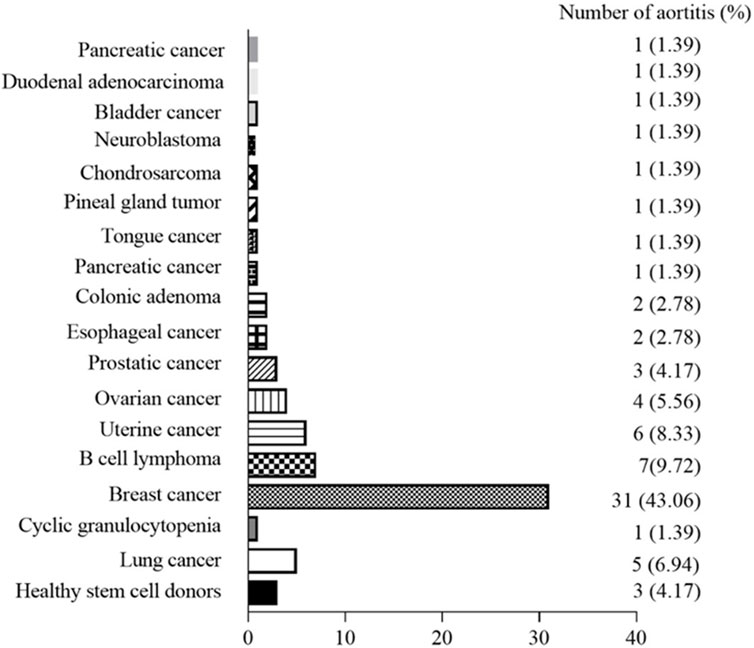
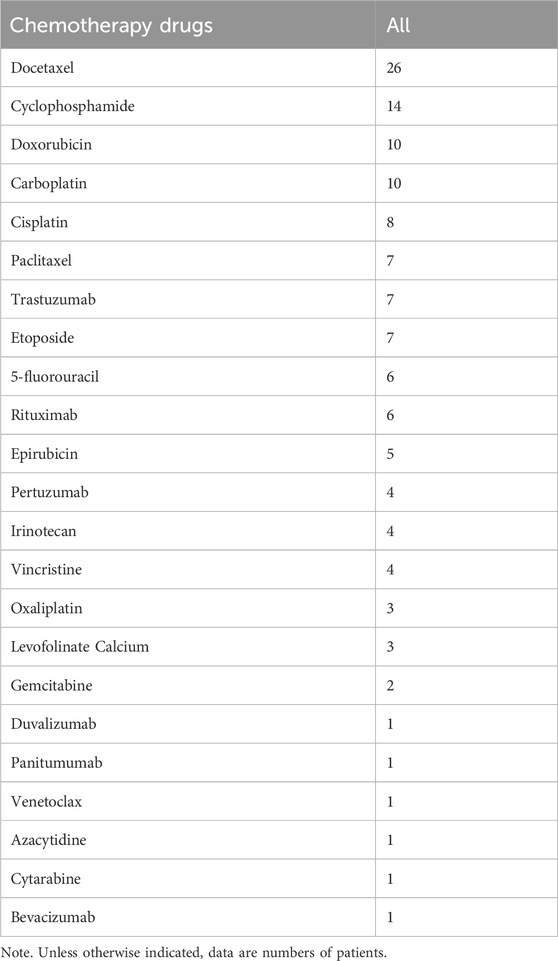
The relationship between the occurrence of G-CSF-induced aortitis and background diseases were shown in Figure 3. Apart from healthy stem cell donors (3 cases, 4.17%), G-CSF-induced aortitis was primarily found in patients with underlying malignancies (69 cases, 95.83%). Among them, the most common malignancies were breast cancer (31 cases, 43.06%), diffuse large B-cell lymphoma (7 cases, 9.72%) and uterine cancer (6 cases, 8.33%). The background chemotherapeutic agents for G-CSF-induced aortitis were shown in Table 4 and Figure 4. Common agents included docetaxel (26 cases), cyclophosphamide (14 cases), doxorubicin (10 cases) and carboplatin (10 cases), followed by cisplatin (7 cases), paclitaxel (7 cases), etoposide (7 cases) and trastuzumab (7 cases).
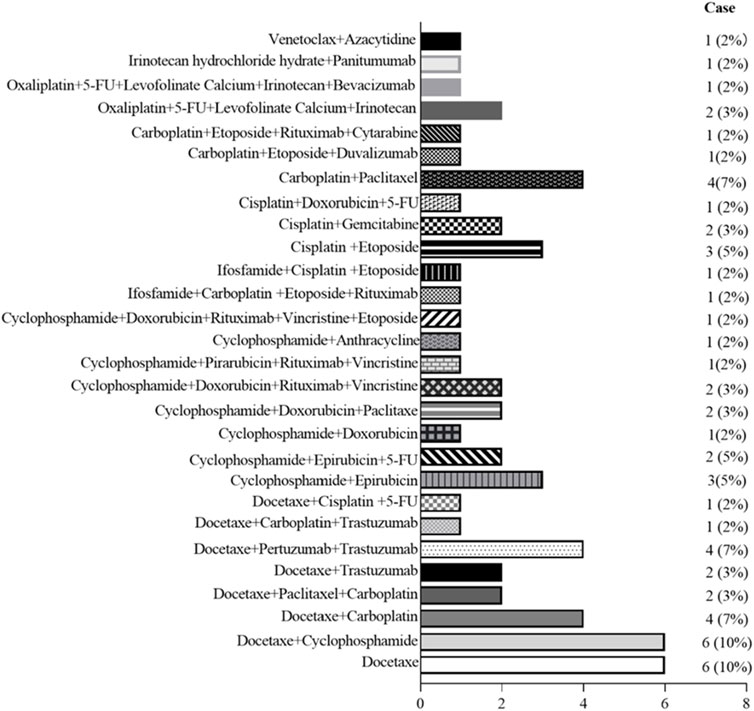
Figure 4. Graph shows the frequency of primary aortitis in patients with different chemotherapy regimen.
3.2 Clinical presentation and Radiologic findings of G-CSF-induced aortitis
The frequency of G-CSF-induced aortitis in patients with different chemotherapy regimens was shown in Figure 4. The chemotherapy regimen that was most frequently associated with the occurrence of arteritis was docetaxel and the combination of docetaxel and cyclophosphamide. The onset of aortitis may be presented as symptomatic or be asymptomatic. Symptomatic patients mainly presented with fever, chest pain, back pain, abdominal pain, neck pain, and sore throat, etc (Table 3). The blood test results revealed an elevated CRP (Table 3). The lesions were primarily located at the aortic arch in 26 cases (36.11%), abdominal aorta in 19 cases (26.39%) and thoracic aorta in 16 cases (22.22%) (Figure 5). The blood culture tests of all patients were negative. None of these patients met diagnostic criteria for conditions such as macro-arteritis, granuloma with polyangiitis, or giant cell arteritis. None of the patients had a history of IgG4-related diseases, and there were no other organ involvements attributed to IgG4-related diseases post the arteritis event.
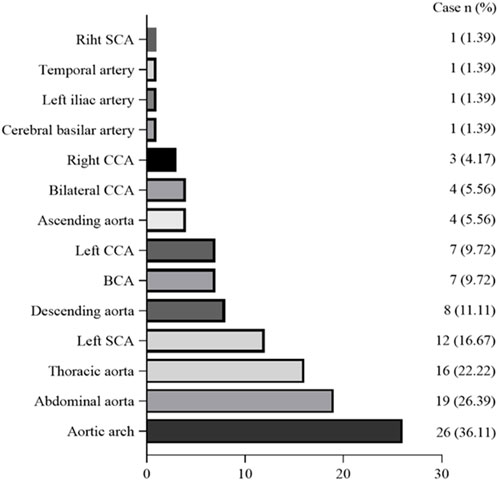
Figure 5. Graph shows distribution of G-CSF-induced aortitis at CT. Vertical axis indicates location of the aorta and its branches, and abscissa indicates the number of patients with G-CSF-induced aortitis. BCA = brachiocephalic artery; CCA = common carotid artery; SCA = subclavian artery.
Among the 72 patients with G-CSF-induced aortitis, 68 cases (96%) were symptomatic, while the remaining 4 (6%) were asymptomatic. Symptomatic patients with steroids or nonsteroidal anti-inflammatory drugs (NSAIDs) treatment exhibited an improvement in symptoms within an average of 15.26 ± 15.48 days, while untreated patients needed an average of 19.25 ± 10.23 days to achieve remission. Forty-seven patients accepted corticosteroids therapy, five were treated with NSAIDs, and the remaining patients did not receive any medication treatment, merely undergoing G-CSF discontinuation and conservative observation. For the 4 asymptomatic patients, after observation, the inflammatory changes in the aortitis also recovered. The specific recovery time of three asymptomatic patients with arteritis was unknown, and one asymptomatic patient recovered in 27 days. Five patients developed recurrent aortitis due to re-using the same type of G-CSF. Despite switching to another type of G-CSF, one patient still developed aortitis. CT findings indicated recovery in most cases, but one case led to aortic aneurysm, and two case led aortic dissection.
4 Discussion
Among all the published case reports, G-CSF-induced aortitis tended to affect elderly women over the age of 60. In addition, the cases of male G-CSF-induced aortitis observed in this review may reflect a higher likelihood of occurrence of prostatic cancer in men. The reason why G-CSF-induced aortitis is more likely to occur in females compared to males is not yet clear, so more samples would be needed to verify the gender differences in G-CSF-induced aortitis. Moreover, G-CSF-induced aortitis was frequently observed in patients with breast cancer as the primary tumor and in patients who received the anticancer drugs docetaxel and cyclophosphamide. The two drugs were used as combination therapy for patients with esophageal cancer (docetaxel and cyclophosphamide). Although, several reports have discussed the relationship between G-CSF-induced aortitis and anticancer drugs, especially docetaxel (Hoshina and Takei, 2021; Taimen et al., 2020). Our finding was that more patients with G-CSF induced aortitis have been using docetaxel, which is consistent with previous reports. However, the causal relationship between docetaxel and G-CSF-induced aortitis remains unclear. In addition, the drug instructions indicate that cyclophosphamide may lead to adverse reactions such as vasculitis. Therefore, when G-CSF is necessary, close attention should be paid to the clinical symptoms of patients when using cyclophosphamide in combination.
We discovered that over three-fourths of all cases occured in Asian populations, particularly in Japan and Korea. Human leucocyte antigen (HLA) as the genetic system with the richest polymorphism in humans, plays a crucial role in intercellular recognition, antigen recognition, and antigen presentation, resulting in different susceptibility of HLA genes to diseases. Evidence shows that HLA-DRB1*09:01 is one of the most common HLA-DRB1 alleles in Asians but is rare in European and American populations (Tsuchiya, 2013). Meanwhile, HLA-DRB1*09:01 has been shown to be associated with antineutrophil cytoplasmic antibody-associated vasculitis in Japan (Tsuchiya, 2013). In addition, previous literature has shown that the HLA-DRB1*04 and DRB1*07 alleles were strongly associated with aortitis in a Chinese Han population (Dang et al., 2002). The reason why G-CSF-induced aortitis was more common in Asian regions such as Japan and South Korea may be related to susceptibility genes in the Asian population that predispose to the development of aortitis. However, there is no evidence to support the correlation between HLA genes and G-CSF-induced aortitis. Therefore, it is necessary that further research will be conducted on the correlation between aortitis and HLA genes in populations prone to G-CSF- induced aortitis.
Our research findings also provided a deeper understanding of asymptomatic G-CSF-induced aortitis, which occurred in 4 out of 72 cases. These 4 asymptomatic patients with aortitis were found to have occurred between 1 and 2 weeks after using G-CSF through CT examination. Due to early detection, only G-CSF was discontinued for these asymptomatic patients and they did not receive treatment with glucocorticoids or NSAIDs. If G-CSF failed to discontinue medication timely in this type of patient, it was not clear whether it would lead to severe symptoms in the later stage. Therefore, considering the rarity of asymptomatic vasculitis and the issue of health economics, whether it is necessary to use ultrasound or CT for screening is not yet conclusive.
Currently, two types of G-CSF are clinically available, one being short-acting G-CSF including filgrastim and lenograstim, and another type being long-acting G-CSF including pegfilgrastim and lipegfilgrastim. We found that the proportion of pegfilgrastim was highest in G-CSF-induced aortitis cases, followed by filgrastim. In terms of pharmacokinetics, the half-life of filgrastim is 3.5 h, and that of pegfilgrastim is 33.2 h (Zamboni, 2003). Compared with filgrastim, pegfilgrastim was more likely to induce aortic inflammation, potentially due to its longer pharmacological effect. G-CSF-induced aortitis lesions were reported to be common in the aortic arch and proximal bifurcation. The distribution patterns of the aortitis are consistent with the findings of a single-center analysis by Takamatsu et al. (Lee et al., 2020). Owing to the unique anatomical structure of the aortic arch, hemodynamic instability heightens the risk of aortic dissection occurring at this location (Williams et al., 2022). Therefore, for patients with arteritis in the aortic arch and its branches, the treating physicians should be vigilant and actively treat to prevent the occurrence of aortic aneurysm and aortic dissection.
According to etiology, arteritis can be broadly classified as infectious and non-infectious (Benhuri et al., 2020). However, the underlying mechanism of aortitis-induced by G-CSF is still unclear. Functionally, G-CSF is a growth factor that regulates many aspects of neutrophil biology, including proliferation, differentiation, release, trafficking, and survival of granulocytes (Martin et al., 2021). However, in autoimmune and inflammatory diseases, the neutrophil response must be strictly regulated, as excessive recruitment and activation, or prolonged neutrophil survival time, can lead to chronic inflammation and sometimes irreversible organ damage (Martin et al., 2021). In this study, pegfilgrastim had the highest proportion of arterial inflammation, which may be related to its longer biological half-life.
G-CSF-induced aortitis exhibited good responsiveness to corticosteroids or NSAIDs and had a favorable prognosis. However, among all the collected cases, 2 cases had aortic dissection (Shiraki et al., 2022; Sato et al., 2017) and 1 case had aortic aneurysm (Miller et al., 2016). One case was a 58-year-old healthy male who was injected with filgrastim to donate bone marrow stem cells. Six months later, an iliac artery aneurysm was discovered (Miller et al., 2016). In the other two cases, after corticosteroids treatment, the symptoms of G-CSF-induced aortitis improved, but CT scans revealed aortic dissection (Shiraki et al., 2022; Sato et al., 2017). These cases suggested that even after the symptoms improve during corticosteroids therapy, there was still a risk of developing aortic dissection. Therefore, before corticosteroids reduction, it should be recommended to recheck CT to clarify the recovery status of arteritis.
There is controversy concerning the acceptability of re-administration or change dosage form of G-CSF in patients with a history of G-CSF-induced aortitis. Among all collected cases, three underwent pegfilgrastim re-administration later; three of the patients exhibited recurrence of pegfilgrastim-induced aortitis. One case who had clinically diagnosed pegfilgrastim-induced aortitis, after switching to filgrastim, still developed G-CSF-induced aortitis. One case who had clinically diagnosed filgrastim-induced aortitis, after switching to lenograstim, also reappeared with G-CSF-induced aortitis. This indicated that patients with a history of G-CSF-induced aortitis, whether through repeated use or changes in dosage form, cannot rule out the possibility of recurrent G-CSF-induced aortitis. However, considering the reporting bias, further validation of clinical data is needed for this possibility.
In conclusion, regardless of the dosage form of G-CSF, there is a risk of leading to arteritis. Due to the increased use of prophylactic treatment for chemotherapy-related neutropenia, the frequency of G-CSF-induced aortitis had also increased. In addition, G-CSF-induced aortitis also occured in healthy stem cell donors. Especially, asymptomatic patients with G-CSF-induced aortitis faced a greater risk of being missed by the attending physician. Given the regional characteristics of G-CSF-induced aortitis, it is recommended that physicians should pay close attention to the Asian population, especially elderly women after using G-CSF, to prevent the occurrence of complications of vasculitis.
Author contributions
TZ: Writing–original draft, Writing–review and editing. HX: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from Weifang City Science and Technology Development Plan (2022YX084).
Acknowledgments
We are grateful for the support and assistance provided by all colleagues in the Clinical Pharmacy Department of Weifang People’s Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adiga, G. U., Elkadi, D., Malik, S. K., Fitzpatrick, J. D., and Madajewicz, S. (2009). Abdominal aortitis after use of granulocyte colony-stimulating factor. Clin. Drug Investig. 29 (12), 821–825. doi:10.2165/11530790-000000000-00000
Arnould, B., Miranda, S., Mignon, F., and Camus, V. (2023). G-CSF-induced TIPIC syndrome and large vessel vasculitis: a case report. Clin. Case Rep. 11 (9), e7918. doi:10.1002/ccr3.7918
Asif, R., Edwards, G., Borley, A., and Jones, S. (2022). Granulocyte colony stimulating factor (G-CSF)-induced aortitis in a patient undergoing adjuvant chemotherapy for breast cancer. BMJ Case Rep. 15 (1), e247237. doi:10.1136/bcr-2021-247237
Benhuri, B., Eljack, A., Kahaleh, B., and Chakravarti, R. (2020). Mechanism and biomarkers in aortitis--a review. J. Mol. Med. Berl. 98 (1), 11–23. doi:10.1007/s00109-019-01838-1
Costa Silva, R., Monteiro, M., Dias, R. P., Silva, I., Rodrigues Dos Santos, J., Vassalo, T., et al. (2021). Large-vessel vasculitis induced by pegfilgrastim. Acta Reumatol. Port. 46 (4), 355–359.
Dang, A., Wang, B., Zhang, Y., Zhang, P., Huang, J., Liu, G., et al. (2002). Association of the HLA-DRB1 gene with susceptibility to aortoarteritis in a Chinese Han population. Hypertens. Res. 25 (4), 631–634. doi:10.1291/hypres.25.631
Darie, C., Boutalba, S., Fichter, P., Huret, J. F., Jaillot, P., Deplus, F., et al. (2004). Aortitis after G-CSF injections. Rev. Med. Interne 25 (3), 225–229. doi:10.1016/j.revmed.2003.10.015
D'Souza, A., Jaiyesimi, I., Trainor, L., and Venuturumili, P. (2008). Granulocyte colony-stimulating factor administration: adverse events. Transfus. Med. Rev. 22 (4), 280–290. doi:10.1016/j.tmrv.2008.05.005
Fujiwara, Y., Yamaguchi, T., and Nakane, M. (2021). Granulocyte colony-stimulating factor-associated aortitis: treatment suggestion for this complication. JCO Oncol. Pract. 17 (1), 57–58. doi:10.1200/OP.20.00121
Harada, M., Motoki, H., Sakai, T., and Kuwahara, K. (2020). Granulocyte colony stimulating factor-associated aortitis evaluated via multiple imaging modalities including vascular echography: a case report. Eur. Heart J. Case Rep. 5 (2), ytaa503. doi:10.1093/ehjcr/ytaa503
Hoshina, H., and Takei, H. (2019). Granulocyte-colony stimulating factor-associated aortitis in a woman with advanced breast cancer: a case report and review of the literature. BMC Cancer 19 (1), 1217. doi:10.1186/s12885-019-6403-9
Hoshina, H., and Takei, H. (2021). Granulocyte-colony stimulating factor-associated aortitis in cancer: a systematic literature review. Cancer Treat. Res. Commun. 29, 100454. doi:10.1016/j.ctarc.2021.100454
Iida, K., Honda, Y., and Homma, Y. (2023). Granulocyte colony-stimulating factor-induced aortitis with temporal arteritis and monoarthritis. BMJ Case Rep. 16 (2), e251216. doi:10.1136/bcr-2022-251216
Ito, M., Amari, M., Sato, A., and Hikichi, M. (2024). Granulocyte-colony stimulating factor (G-CSF)-Induced aortitis: a case report. Cureus 16 (2), e54845. doi:10.7759/cureus.54845
Ito, T., Kanai, O., Saito, Z., Imakita, T., Oi, I., Fujita, K., et al. (2023). Pegfilgrastim-induced aortitis in a patient with small-cell lung cancer who received immunotherapy combined with chemotherapy. Case Rep. Oncol. 16 (1), 1466–1474. doi:10.1159/000534931
Jimbo, H., Horimoto, Y., Okazaki, M., Ishizuka, Y., Kido, H., and Saito, M. (2021). Drug-induced aortitis of the subclavian artery caused by pegfilgrastim: a case report. Surg. Case Rep. 7 (1), 197. doi:10.1186/s40792-021-01282-9
Kametani, T., Otani, Y., Ohigashi, T., Kubo, T., Sakuda, T., Furuta, D., et al. (2021). Granulocyte colony-stimulating factor-induced aortitis with lung injury, splenomegaly, and a rash during treatment for recurrent extraosseous mucinous chondrosarcoma. Intern Med. 60 (8), 1311–1315. doi:10.2169/internalmedicine.5913-20
Kawahara, H., Endo, A., Yoshitomi, H., and Tanabe, K. (2020). Recurrent granulocyte colony-stimulating factor-induced aortitis after pegfilgrastim administration. Circ. Rep. 2 (12), 764–765. doi:10.1253/circrep.CR-20-0092
Koyama, Y., Adachi, K., Yagi, M., Go, Y., Orimoto, K., Kawai, S., et al. (2021). Successful treatment of G-CSF-related aortitis with prednisolone during preoperative chemotherapy for breast cancer: a case report. Surg. Case Rep. 7 (1), 23. doi:10.1186/s40792-021-01111-z
Lardieri, A., McCulley, L., Christopher Jones, S., and Woronow, D. (2018). Granulocyte colony-stimulating factors and aortitis: a rare adverse event. Am. J. Hematol. 93 (10), E333–E336. doi:10.1002/ajh.25220
Lee, S. Y., Kim, E. K., Kim, J. Y., Park, T. K., Choi, S. H., Im, Y. H., et al. (2020). The incidence and clinical features of PEGylated filgrastim-induced acute aortitis in patients with breast cancer. Sci. Rep. 10 (1), 18647. doi:10.1038/s41598-020-75620-6
Link, H. (2022). Current state and future opportunities in granulocyte colony-stimulating factor (G-CSF). Support Care Cancer 30 (9), 7067–7077. doi:10.1007/s00520-022-07103-5
Martin, K. R., Wong, H. L., Witko-Sarsat, V., and Wicks, I. P. (2021). G-CSF - a double edge sword in neutrophil mediated immunity. Semin. Immunol. 54, 101516. doi:10.1016/j.smim.2021.101516
Masuda, Y., Oyama, T., Nakazaki, K., Nakai, Y., Sasaki, K., Matsuda, K., et al. (2023). Aortitis associated with prophylactic short-acting granulocyte colony-stimulating factor administration: a case report and review of the literature. Intern Med. 62 (11), 1647–1652. doi:10.2169/internalmedicine.0599-22
Matsumoto, N., Kondo, N., Wanifuchi-Endo, Y., Asano, T., Hisada, T., Uemoto, Y., et al. (2022). Granulocyte colony-stimulating factor-associated aortitis in a woman with breast cancer: a case report. Surg. Case Rep. 8 (1), 157. doi:10.1186/s40792-022-01514-6
Miller, E. B., Grosu, R., and Landau, Z. (2016). Isolated abdominal aortitis following administration of granulocyte colony stimulating factor (G-CSF). Clin. Rheumatol. 35 (6), 1655–1657. doi:10.1007/s10067-016-3253-6
Mizushima, R., Kikuchi, R., Takoi, H., Shioiri, N., Toriyama, K., and Abe, S. (2022). Double-ring sign in granulocyte colony-stimulating factor-induced vasculitis. Respirol. Case Rep. 10 (6), e0976. doi:10.1002/rcr2.976
Mukai, T., Kubo, S., Morita, Y., Yamamoto, M., and Ikeda, M. (2020). Aortitis which developed after the administration of granulocyte-colony stimulating factor. Mod. Rheumatol. Case Rep. 4 (1), 74–78. doi:10.1080/24725625.2019.1629570
Nakamura, J., Nishi, T. M., Yamashita, S., Nakamura, H., Sato, K., Oda, Y., et al. (2020). Pegfilgrastim-associated large-vessel vasculitis developed during adjuvant chemotherapy for breast cancer: a case report and review of the literature. J. Oncol. Pharm. Pract. 26 (7), 1785–1790. doi:10.1177/1078155220910800
Nishioka, H., and Fujii, M. (2023). Granulocyte colony-stimulating factor-associated aortitis. Intern Med. 62 (21), 3263. doi:10.2169/internalmedicine.1357-22
Nitta, S., Tanaka, T., Yanagihashi, R., Nonaka, H., Suzuki, S., Kimura, T., et al. (2021). Granulocyte colony-stimulating factor associated arteritis in a patient with castration-resistant prostate cancer. IJU Case Rep. 5 (1), 29–31. doi:10.1002/iju5.12376
Oshima, Y., Takahashi, S., Tani, K., and Tojo, A. (2019). Granulocyte colony-stimulating factor-associated aortitis in the Japanese adverse drug event report database. Cytokine 119, 47–51. doi:10.1016/j.cyto.2019.02.013
Ozaki, H., Takemura, K., Kizawa, R., Yamaguchi, T., Komiyama, C., Tachi, M., et al. (2023). Granulocyte colony-stimulating factor-associated aortitis on gallium scintigraphy. Intern Med. 62 (21), 3163–3166. doi:10.2169/internalmedicine.1453-22
Parodis, I., Dani, L., Notarnicola, A., Martenhed, G., Fernström, P., Matikas, A., et al. (2018). G-CSF-induced aortitis: two cases and review of the literature. Autoimmun. Rev. 18 (6), 615–620. doi:10.1016/j.autrev.2018.12.011
Saito, H., Suda, T., Oishi, N., and Matsushita, E. (2021). Pegfilgrastim-induced large vessel vasculitis. BMJ Case Rep. 14 (6), e243757. doi:10.1136/bcr-2021-243757
Sasaki, K., Matsuda, K., Miyauchi, M., Honda, A., Shimura, A., Masamoto, Y., et al. (2021). A retrospective analysis on arteritis after administration of granulocyte colony-stimulating factor. Ann. Hematol. 100 (5), 1341–1343. doi:10.1007/s00277-021-04453-8
Sasaki, K., Miyauchi, M., Ogura, M., Shimura-Nukina, A., Toyama, K., Nakazaki, K., et al. (2019). Arteritis after administration of granulocyte colony-stimulating factor: a case series. Int. J. Hematol. 110 (3), 370–374. doi:10.1007/s12185-019-02662-6
Sato, Y., Kaji, S., Ueda, H., and Tomii, K. (2017). Thoracic aortitis and aortic dissection following pegfilgrastim administration. Eur. J. Cardiothorac. Surg. 52 (5), 993–994. doi:10.1093/ejcts/ezx165
Seto, Y., Kittaka, N., Taniguchi, A., Kanaoka, H., Nakajima, S., Oyama, Y., et al. (2022). Pegfilgrastim-induced vasculitis of the subclavian and basilar artery complicated by subarachnoid hemorrhage in a breast cancer patient: a case report and review of the literature. Surg. Case Rep. 8 (1), 155. doi:10.1186/s40792-022-01499-2
Seto, Y., Yamada, T., Egami, M., Sugimoto, T., Sato, I., Tanaka, S., et al. (2023). Recurrence of large-vessel vasculitis induced by multiple types of granulocyte colony-stimulating factor preparation in patient with large-cell neuroendocrine lung carcinoma: a case report. Case Rep. Oncol. 16 (1), 771–778. doi:10.1159/000533375
Shirai, T., Komatsu, H., Sato, H., Fujii, H., Ishii, T., and Harigae, H. (2020). Migratory aortitis associated with granulocyte-colony-stimulating factor. Intern Med. 59 (12), 1559–1563. doi:10.2169/internalmedicine.4331-19
Shiraki, E., Hamada-Nishimoto, M., Kang, Y., and Tsuyuki, S. (2022). Aortitis and aortic dissection after administration of pegfilgrastim during adjuvant chemotherapy for early breast cancer. Int. Cancer Conf. J. 11 (2), 138–141. doi:10.1007/s13691-022-00540-3
Singh, H., Rosenthal, M. H., and Wolpin, B. M. (2022). G-CSF-induced carotid inflammation. Lancet Oncol. 23 (5), e235. doi:10.1016/S1470-2045(22)00074-2
Soto Castillo, J. J., Loarce-Martos, J., and Blanc-Molina, J. M. (2020). Large vessel vasculitis secondary to granulocyte-colony stimulating factor. Vasa 49 (6), 509–513. doi:10.1024/0301-1526/a000872
Sugai, Y., Toyoguchi, Y., Kanoto, M., Kirii, K., Hiraka, T., Konno, Y., et al. (2024). Clinical and image features: large-vessel vasculitis after granulocyte colony stimulating factor administration. Acta Radiol. 65 (4), 284185120931685–391. doi:10.1177/0284185120931685
Taimen, K., Heino, S., Kohonen, I., Relas, H., Huovinen, R., Hänninen, A., et al. (2020). Granulocyte colony-stimulating factor- and chemotherapy-induced large-vessel vasculitis: six patient cases and a systematic literature review. Rheumatol. Adv. Pract. 4 (1), rkaa004. doi:10.1093/rap/rkaa004
Takahashi, T., Yamamoto, K., Yamaguchi, T., Miura, Y., and Kizawa, R. (2022). Granulocyte colony-stimulating factor-associated aortitis. Lancet Oncol. 23 (3), e155. doi:10.1016/S1470-2045(22)00020-1
Takamatsu, A., Yoshida, K., Toshima, F., Kozaka, K., Yamamoto, N., Sai, Y., et al. (2022). Single-center analysis of pegfilgrastim-induced aortitis using a drug prescription database and CT findings. Radiology 305 (3), 729–740. doi:10.1148/radiol.220357
Tane, M., Kosako, H., Hosoi, H., Furuya, Y., Hori, Y., Yamashita, Y., et al. (2024). Aortitis after switching short-acting granulocyte colony-stimulating factors in a lymphoma patient with HLA-B52. Int. J. Hematol. 119 (5), 608–612. doi:10.1007/s12185-024-03744-w
Tsuchiya, N. (2013). Genetics of ANCA-associated vasculitis in Japan: a role for HLA-DRB1*09:01 haplotype. Clin. Exp. Nephrol. 17 (5), 628–630. doi:10.1007/s10157-012-0691-6
Uemura, Y., Oshima, K., Fuseya, A., Hosokai, A., Ohashi, A., Kanno, M., et al. (2023). Aortitis after administration of pegfilgrastim to a healthy donor for peripheral blood stem cell collection. Int. J. Hematol. 118 (6), 772–775. doi:10.1007/s12185-023-03649-0
Umeda, M., Ikenaga, J., Koga, T., Michitsuji, T., Shimizu, T., Fukui, S., et al. (2016). Giant cell arteritis which developed after the administration of granulocyte-colony stimulating factor for cyclic neutropenia. Intern Med. 55 (16), 2291–4. doi:10.2169/internalmedicine.55.6704
Williams, J. G., Marlevi, D., Bruse, J. L., Nezami, F. R., Moradi, H., Fortunato, R. N., et al. (2022). Aortic dissection is determined by specific shape and hemodynamic interactions. Ann. Biomed. Eng. 50 (12), 1771–1786. doi:10.1007/s10439-022-02979-0
Yamamoto, K., Tamura, N., Oka, K., Hasegawa, K., Hagiya, H., Hokama, M., et al. (2021b). Large-vessel vasculitis induced by granulocyte colony-stimulating factor administration after chemotherapy. Mod. Rheumatol. Case Rep. 5 (2), 322–326. doi:10.1080/24725625.2020.1857022
Yamamoto, S., Waki, D., and Maeda, T. (2021a). Granulocyte-colony stimulating factor-induced vasculitis successfully treated with short-term corticosteroid therapy: a case report. Cureus 13 (12), e20563. doi:10.7759/cureus.20563
Keywords: recombinant human granulocyte-colony stimulating factor, aortitis, adverse event, cancer, chemotherapy
Citation: Zhao T and Xu H (2024) Literature review analysis of aortitis induced by granulocyte-colony stimulating factor. Front. Pharmacol. 15:1487501. doi: 10.3389/fphar.2024.1487501
Received: 28 August 2024; Accepted: 04 December 2024;
Published: 18 December 2024.
Edited by:
Masataka Umeda, Nagasaki University, JapanReviewed by:
Tsuyoshi Shirai, Tohoku University, JapanRyo Hisada, Hokkaido University Hospital, Japan
Copyright © 2024 Zhao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanhuan Xu, cm15eXh1aHVhbmh1YW5Ac2RzbXUuZWR1LmNu
 Ting Zhao
Ting Zhao Huanhuan Xu
Huanhuan Xu