- 1Faculty of Medicine, Tehran University of Medical Sciences (TUMS), Tehran, Iran
- 2College of Nursing, Michigan State University, East Lansing, MI, United States
- 3Department of Traditional Medicine, School of Persian Medicine, Shahed University, Tehran, Iran
- 4Neuroscience Institute, Tehran University of Medical Sciences (TUMS), Tehran, Iran
- 5Research Center for Antibiotic Stewardship and Antimicrobial Resistance, Department of Infectious Diseases, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Introduction: Klebsiella poses a significant global threat due to its high antibiotic resistance rate. In recent years, researchers have been seeking alternative antimicrobial agents, leading to the introduction of natural compounds such as monoterpenes, specifically thymol and carvacrol. This review aims to illustrate the potential antimicrobial, anti-biofilm, and synergistic traits of thymol and carvacrol in combat against Klebsiella.
Methods: Searching PubMed, Scopus, and Web of Science, we reviewed available evidence on the antibacterial effects of thymol, carvacrol, or combined with other compounds against Klebsiella until May 2024. Reference checking was performed after the inclusion of studies. Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), fractional inhibitory concentration (FIC), and anti-biofilm activity were gathered, and the MBC/MIC ratio was calculated to assess the bactericidal efficacy.
Results: We retrieved 38 articles out of 2,652 studies screened. The gathered data assessed the anti-microbial activity of thymol, carvacrol, and both compounds in 17, 10, and 11 studies, respectively. The mean (± standard deviation) non-weighted MIC was 475.46 μg/mL (±509.95) out of 60 MIC for thymol and 279.26 μg/mL (±434.38) out of 68 MIC for carvacrol. Thymol and carvacrol showed anti-biofilm activities in the forms of disruption, inhibition, and mass reduction of biofilms. The MBC/MIC ratio was lower than 4 in 45 out of 47 cases, showing high bactericidal efficacy. FIC values were gathered for 68 combinations of thymol and carvacrol with other compounds, and they were mostly synergistic or additive.
Conclusion: Thymol and carvacrol alone or in combination with other compounds, specifically known antibiotics, show great antimicrobial activity.
1 Introduction
Klebsiella pneumoniae (K. pneumoniae), a member of the Enterobacteriaceae family, is a part of the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter), known primarily for their antibiotic resistance and association with hospital-acquired infections (Nanayakkara et al., 2021; Ma et al., 2020). Over the years, the ESKAPE pathogens have transformed into multi-drug resistance (MDR) microorganisms. They are now prioritized as a global health threat by the World Health Organization (WHO) due to the mortality, morbidity, and economic burden they cause (Jesudason, 2024). In a systematic review by Ayobami et al., the antibiotic resistance rate of the ESKAPE pathogens in lower and middle-income countries was estimated to be as high as 85.5% for critical antibiotics. They found that the most commonly reported antibiotic resistance was against third-generation-cephalosporins and was particularly among Escherichia coli (E. coli), K. pneumoniae, and Enterobacter spp. (Ayobami et al., 2022). According to reports, K. pneumoniae resistance to carbapenem rates exceeded 50% in two WHO regions (Zhen et al., 2019). K. pneumoniae is isolated from patients with pyogenic liver abscess (Zhang et al., 2019), community-acquired, ventilator-associated, and intensive care unit (ICU)-associated pneumonia (Sharma et al., 2023; Bodmann, 2005), wound infection (Chang et al., 2021), and meningitis (Pu et al., 2023).
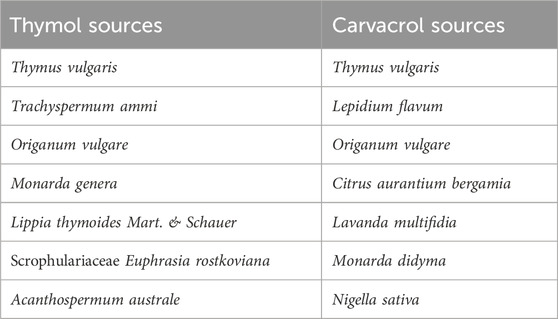
The growing emergence of antimicrobial-resistant pathogens has shifted attention to alternative antibacterial agents, including medicinal plants, which have been used since the beginning of humanity (Idris and Nadzir, 2023). According to the WHO, in 2019, antimicrobial resistance (AMR) directly caused 1.27 million deaths, contributed to 4.95 million deaths, and in total, was responsible for 6.22 million deaths globally (Antimicrobial Resistance Collaborators, 2022). Essential oils (EOs), such as lavender, tea tree, and peppermint, are secretions of herbal plants obtained through fermentation, expression, extraction, or enfleurage. They are used in various industries, including culinary, cosmetics, perfumes, insecticides, and pharmaceuticals (Hoffmann, 2020). These natural products have been meticulously studied for antimicrobial purposes over the years, leading to the identification of several components. One of these components is monoterpenes, which are secondary metabolites found in the EOs of aromatic plants, such as Thymus, Lamiaceae, Origanum, and Lippa peppercorn family (Dehsheikh et al., 2020; Jurevičiūtė et al., 2019; Zhao et al., 2022; Sukmawan et al., 2021). Monoterpenes can be classified into alkaloids, terpenes, flavonoids, phenolic compounds, resins, polypeptides, coumarins, and glucosinolates (Peter et al., 2024). They exhibit antimicrobial, anticancer, antioxidant, and anti-inflammatory activities, making them an interesting field of research (Durugbo, 2013; Sahoo et al., 2021). Thymol and carvacrol are phenolic monoterpenes, approved by the Federal Drug Administration as safe for human consumption (US Food & Drug Administration, 2024). They are considered potent bioactive compounds due to their chemical structure, specifically the presence of the hydroxyl group, which enhances the antibacterial potential of these compounds, and their mechanism of action (Ultee et al., 2002). Furthermore, the antibiofilm activities of thymol and carvacrol have attracted attention to these phenolic monoterpenes in recent years (Campana and Baffone, 2018; Liu et al., 2021). A brief list of plants containing thymol and carvacrol is presented in Table 1. Studies show that they can demonstrate antibacterial properties through biofilm reduction, inhibition of motility, inhibition of membrane-bound adenosine triphosphatases (ATPases) and efflux pumps, and cell wall membrane disruption (Kachur and Suntres, 2020). Their antibacterial role has been commonly studied against S. aureus, Salmonella, Shigella, and E. coli (Ngome et al., 2018; Abdelhamid and Yousef, 2021; Heckler et al., 2021; Cid-Pérez et al., 2024). However, given the significant burden Klebsiella infections impose on the healthcare system in terms of mortality and morbidity, there was a pressing need for a systematic review study. Our study, therefore, aimed to systematically review the antibacterial activities of thymol and carvacrol against Klebsiella, including their bacteriostatic, bactericidal, anti-biofilm, and synergistic effects, offering a potential solution to the growing concern of multi-drug resistance pathogens.
2 Methods
We used the PICO strategy for formulating research questions. The strategy was based on population (P): Klebsiella, Intervention (I): thymol or carvacrol, Control (C): not applicable, and outcome (O): antibacterial effect. This study followed Systematic Review and Meta-Analysis (PRISMA) guidelines (Page et al., 2021).
2.1 Search strategy
A comprehensive and systematic search was conducted on databases, including PubMed, Scopus, and Web of Science to identify the relevant articles published until May 2024.
“Thymol,” “carvacrol,” “antibacterial,” “Klebsiella pneumoniae,” “Klebsiella infections,” “Klebsiella oxytoca,” and related keywords were used. Backward and forward citations were tracked by examining the references of the included studies. No restriction on the year of publication was applied.
2.2 Study selection and eligibility criteria
Two independent researchers screened the studies by reading titles and abstracts and then full texts using Rayyan, a web-based tool for systematic reviews, and selected relevant studies. Any discrepancies were resolved through consensus between reviewers, and if necessary, a third reviewer made a decision.
2.3 Inclusion and exclusion criteria
Original in vivo and in vitro studies in the English language that reported effects of thymol and carvacrol, simultaneously or independently, in conjunction with other antibacterial agents, were included.
Review articles, editorials/letters, protocols, abstracts, conference articles, meta-analyses, and comments were excluded. Studies without full texts or those involving a mixture of compounds (e.g., herbal essential oils) without the pure forms of thymol and carvacrol were not eligible (Figure 1).
2.4 Data extraction
The following data were extracted from the included studies: first author, publication year, country of study, methodology, analyzed compound (thymol or carvacrol or both), Klebsiella species, resistance against carbapenems, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), synergistic effects (fractional inhibitory concentration or FIC), and anti-biofilm effects.
Data on antimicrobial resistance to carbapenems were gathered from the studies. If this information was not available, http://ATCC.org was searched using the strain code provided in the study.
2.5 Quality assessment
Quality appraisal was conducted by two authors using an adapted version of the Quality Assessment Tool For In Vitro Studies (QUIN Tool) (Sheth et al., 2024).
2.6 Antibacterial strength
We reported bacteriostatic activity and bactericidal activity of thymol and carvacrol against Klebsiella in the form of MIC and MBC, respectively. To compare the antibacterial strength of phenolic compounds, we used the criteria by Taguri et al. (2006) and considered the activity of thymol and carvacrol against Klebsiella as strong for MIC <400 μg/mL, moderate for 400 μg/mL < MIC <800 μg/mL, and weak for MIC >800 μg/mL.
Bactericidal efficacy was then calculated using the MBC/MIC ratio, with values less than four considered as good bactericidal efficiency (Bury-Moné, 2014). The methodology and results of studies on anti-biofilm effects were also gathered and presented.
2.7 Synergistic activity
We gathered data on the combination of thymol and carvacrol with other compounds and antimicrobials and reported their combination effect using FIC and changes in MIC. The combination effect was considered as synergistic for FIC < 0.5, additive for 0.5 < FIC < 1.0, non-interactive for 1.0 < FIC < 4.0, and antagonistic for FIC > 4.0 (van Vuuren and Viljoen, 2011).
3 Results
3.1 Search results
Of 2,652 studies screened, 38 (Abdel-halim et al., 2022; Addo et al., 2022; Al-Ani et al., 2015; Alavi and Karimi, 2019; Ndezo et al., 2022; Cordeiro et al., 2020; de Souza et al., 2021; de Souza et al., 2024; Drobac et al., 2017; Gan et al., 2023; Hamoud et al., 2014; Höferl et al., 2009; Huang et al., 2023; Ilić et al., 2017; Iten et al., 2009; Köse, 2022; Kwiatkowski et al., 2022; Liu et al., 2022; Marinelli et al., 2019; Mbese et al., 2022; Mbese et al., 2023; Moghtaderi et al., 2023; Mohammed and Al-Bayati, 2009; Muftah et al., 2020; Bisso et al., 2021; Raei et al., 2017; Rani et al., 2022a; Rani et al., 2022b; Sabour et al., 2019; Salaria et al., 2022; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Tashakor et al., 2024; Yao et al., 2022; Yehia et al., 2024; Zhang et al., 2011; Pormohammad et al., 2022; Choi et al., 2009) studies from 19 different countries were included (Figure 1). All studies showed scores above 70% in quality appraisal using QUIN, indicating a low risk of bias (Supplementary Material S1). A summary of characteristics is available in Table 2.
3.2 Anti-microbial and anti-biofilm effects
Data on the anti-microbial activity of thymol (Abdel-halim et al., 2022; Addo et al., 2022; Alavi and Karimi, 2019; Ndezo et al., 2022; Drobac et al., 2017; Gan et al., 2023; Hamoud et al., 2014; Huang et al., 2023; Ilić et al., 2017; Moghtaderi et al., 2023; Mohammed and Al-Bayati, 2009; Muftah et al., 2020; Bisso et al., 2021; Sabour et al., 2019; Salaria et al., 2022; Tashakor et al., 2024; Yao et al., 2022), carvacrol (Al-Ani et al., 2015; Cordeiro et al., 2020; de Souza et al., 2021; de Souza et al., 2024; Köse, 2022; Marinelli et al., 2019; Mbese et al., 2022; Mbese et al., 2023; Yehia et al., 2024; Choi et al., 2009) and both compounds (Höferl et al., 2009; Iten et al., 2009; Kwiatkowski et al., 2022; Liu et al., 2022; Raei et al., 2017; Rani et al., 2022a; Rani et al., 2022b; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Zhang et al., 2011; Pormohammad et al., 2022) were obtained from 17, 10, and 11 studies, respectively. All studies used purchased pure forms of thymol and carvacrol, except the study by Mohammed and Al-Bayati (2009), which isolated thymol from essential oils.
All studies assessed anti-bacterial activity against K. pneumoniae, except four against K. oxytoca (Mbese et al., 2022; Yehia et al., 2024; Zhang et al., 2011; Choi et al., 2009). Two studies compared K. pneumoniae and K. oxytoca (Al-Ani et al., 2015; Mbese et al., 2023), and one compared K. aerogenes and K. pneumonia (Gan et al., 2023).
Regarding the sources of isolates, 14 studies used clinical isolates (Abdel-halim et al., 2022; Alavi and Karimi, 2019; Ndezo et al., 2022; Cordeiro et al., 2020; de Souza et al., 2021; de Souza et al., 2024; Höferl et al., 2009; Huang et al., 2023; Köse, 2022; Kwiatkowski et al., 2022; Bisso et al., 2021; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Yao et al., 2022), 2 used isolates derived from chicken broilers (Liu et al., 2022; Yehia et al., 2024), 1 from animal feed (Zhang et al., 2011), and others purchased reference strains.
Regarding the availability of MIC data, 37 MIC were available for reference strains, 84 for clinical stains, 5 for strains derived from chicken broiler and 2 for strains from animal feed. The values of non-weighted MIC mean (median) were calculated as follows: for reference strains: 464.65 (250) µg/mL for thymol and 257 (200) µg/mL for carvacrol, for clinical strains: 505.17 (256) µg/mL for thymol and 288.83 (128) µg/mL for carvacrol, for chicken broiler strains: 198.40 (198.40) µg/mL for thymol and 212.93 (241.40) µg/mL for carvacrol, and for animal feed strains: 187 (187) µg/mL for thymol and 375 (375) µg/mL for carvacrol.
Regarding the methods used for MIC assessment, all studies used broth dilution except 1 (Addo et al., 2022), which used well diffusion, 3 (Alavi and Karimi, 2019; Mbese et al., 2023; Choi et al., 2009), which used disc diffusion, and 3 (Höferl et al., 2009; Raei et al., 2017; Yehia et al., 2024), which used agar dilution.
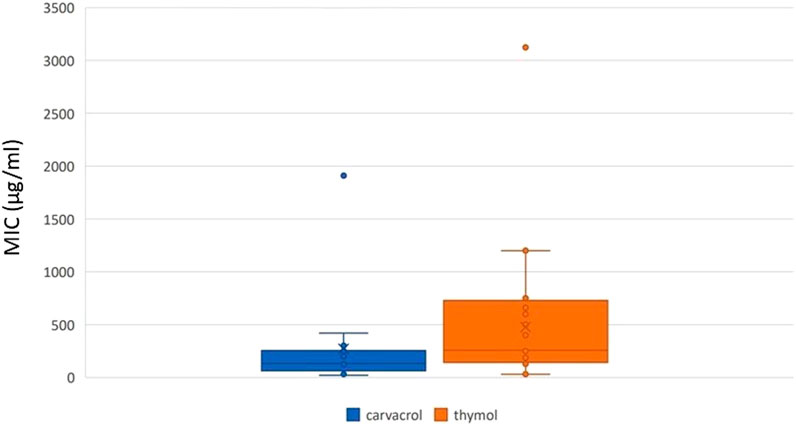
Regarding the assessment of antibacterial activity, two studies provided inhibition zone diameter (Addo et al., 2022; Mbese et al., 2023), one provided sessile MIC calculated for anti-biofilm activity (Scandorieiro et al., 2023), two studies did not provide MIC in µg/mL (Salaria et al., 2022; Zhang et al., 2011), and one provided MIC 50% and 90% (Marinelli et al., 2019). A total of 128 MIC in µg/mL were gathered, with 60 MIC reported for thymol, ranging from 30 μg/mL to 3,123 μg/mL, and 68 MIC reported for carvacrol, ranging from 32 μg/mL to 1910 μg/mL. Additionally, 99 MIC values were lower than 400 μg/mL and considered strong, while 16 were moderate and 13 were weak. The mean (± standard deviation, median) non-weighted MIC was 475.46 μg/mL (±509.95, 256 μg/mL) for thymol and 279.26 μg/mL (±434.38, 130 μg/mL) for carvacrol (Figure 2), with carvacrol MIC being significantly lower than thymol MIC (P = 0.022).
Carbapenem-resistant Klebsiella was reported in 11 studies (Abdel-halim et al., 2022; Addo et al., 2022; de Souza et al., 2021; de Souza et al., 2024; Huang et al., 2023; Köse, 2022; Kwiatkowski et al., 2022; Raei et al., 2017; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Yao et al., 2022), with 68 MIC ranging from 32 μg/mL to 1910 μg/mL. The mean (± standard deviation, median) non-weighted MIC for carbapenem-resistant Klebsiella was 681.04 μg/mL (±216.61, 600 μg/mL) for thymol and 247.76 μg/mL (±68.44, 128 μg/mL) for carvacrol.
The anti-biofilm effect against Klebsiella was reported in 11 studies, with 6 studies assessing thymol (Alavi and Karimi, 2019; Ndezo et al., 2022; Huang et al., 2023; Moghtaderi et al., 2023; Bisso et al., 2021; Yao et al., 2022), 1 assessing carvacrol (de Souza et al., 2024), and 4 examining both (Kwiatkowski et al., 2022; Raei et al., 2017; Scandorieiro et al., 2023; Pormohammad et al., 2022). The studies showed that thymol and carvacrol can have multiple anti-biofilm mechanisms against Klebsiella, including changing the cell morphology, inhibition of biofilm formation, disruption of preformed biofilm, reduction of bacterial mass, and synergistic activity with antibiotics.
3.3 Bactericidal effects
To evaluate the bactericidal efficacy of thymol and carvacrol, we calculated the MBC/MIC ratio in studies reporting MBC. Of the 14 studies providing MBC values (Al-Ani et al., 2015; Alavi and Karimi, 2019; Ndezo et al., 2022; de Souza et al., 2021; Gan et al., 2023; Hamoud et al., 2014; Ilić et al., 2017; Kwiatkowski et al., 2022; Bisso et al., 2021; Rani et al., 2022a; Rani et al., 2022b; Sabour et al., 2019; Scandorieiro et al., 2022; Pormohammad et al., 2022), a total of 47 MBC/MIC ratios were calculated. Of these, 45 ratios were four or less, and only two ratios from two studies (Ndezo et al., 2022; Bisso et al., 2021) were higher, indicating substantial bactericidal activities for thymol and carvacrol. These two ratios, both equal to eight, were against clinical isolates.
3.4 Combination effects
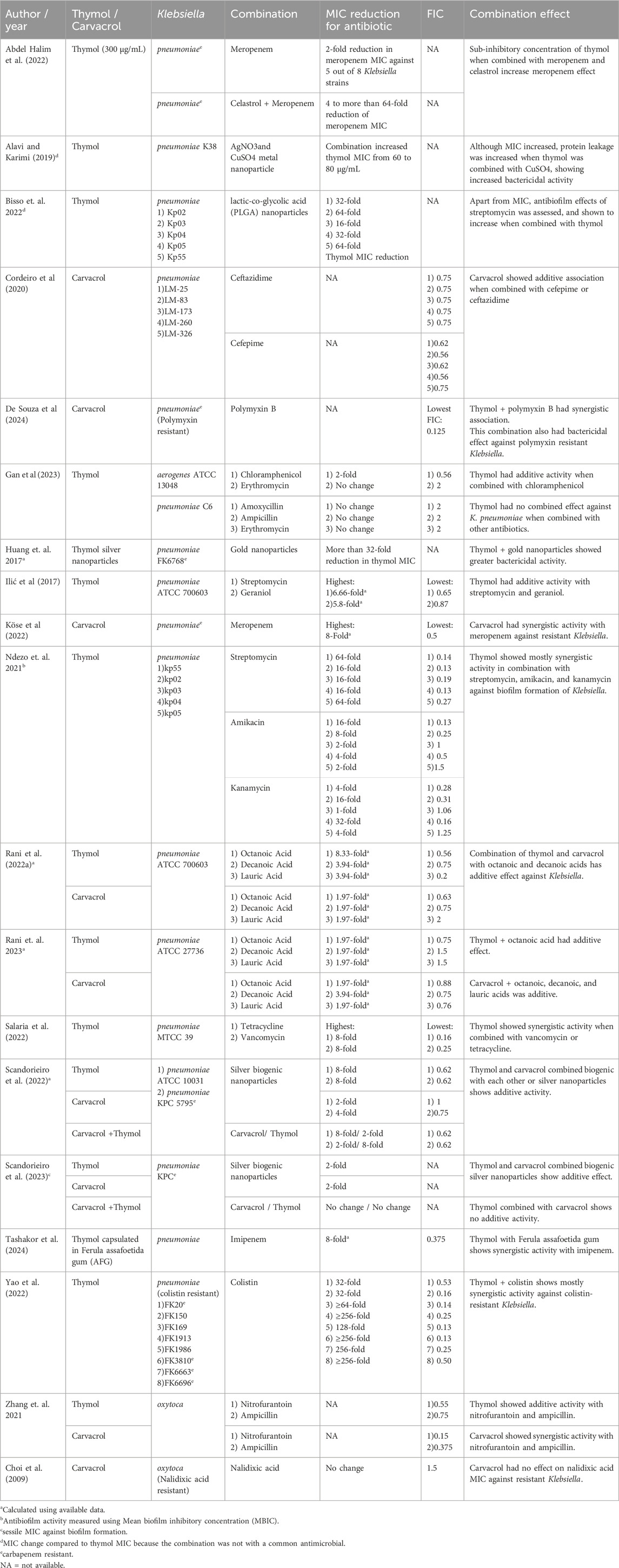
The anti-bacterial combination effects of thymol and carvacrol with other compounds (Table 3), were assessed in 19 studies (Abdel-halim et al., 2022; Alavi and Karimi, 2019; Ndezo et al., 2022; Cordeiro et al., 2020; de Souza et al., 2024; Gan et al., 2023; Huang et al., 2023; Ilić et al., 2017; Köse, 2022; Bisso et al., 2021; Rani et al., 2022a; Rani et al., 2022b; Salaria et al., 2022; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Tashakor et al., 2024; Yao et al., 2022; Zhang et al., 2011; Choi et al., 2009), with 10 studies assessing thymol (Abdel-halim et al., 2022; Alavi and Karimi, 2019; Ndezo et al., 2022; Gan et al., 2023; Huang et al., 2023; Ilić et al., 2017; Salaria et al., 2022; Tashakor et al., 2024; Yao et al., 2022), four assessing carvacrol (Cordeiro et al., 2020; de Souza et al., 2024; Köse, 2022; Choi et al., 2009), and 5 assessing both (Rani et al., 2022a; Rani et al., 2022b; Scandorieiro et al., 2022; Scandorieiro et al., 2023; Zhang et al., 2011).
The lowest FIC value for each Klebsiella strain and compound, in combination with thymol or carvacrol, was gathered, resulting in 68 FIC, as shown in Table 3. We found that 25 combinations were synergistic, 32 were additive, and 11 were non-interactive. The change in antibiotic MIC is also available, ranging from no change for erythromycin, amoxicillin, and ampicillin when combined with thymol (Gan et al., 2023), and for nalidixic acid when combined with carvacrol (Choi et al., 2009) to more than 256-fold antibiotic MIC reduction for colistin when combined with thymol against colistin-resistant Klebsiella (Yao et al., 2022). This substantial reduction in colistin MIC was possibly due to the increased permeability of the Klebsiella outer membrane in the presence of thymol (Yao et al., 2022). Overall, thymol was assessed in more combinations and showed more synergistic activities with other compounds than carvacrol.
When combined with known antimicrobial agents (i.e., meropenem, ceftazidime, cefepime, polymyxin B, chloramphenicol, erythromycin, amoxicillin, ampicillin, streptomycin, amikacin, kanamycin, tetracycline, vancomycin, imipenem, colistin, nitrofurantoin, and nalidixic acid), pure thymol showed FIC<1 or at least a 2-fold reduction in the antimicrobial agent MIC for 35 out of 42 combinations (83.3%), while pure carvacrol showed FIC<1 or at least a 2-fold reduction in the antimicrobial agent MIC for 14 out of 15 combinations (93.3%).
4 Discussion
In this systematic review, we aimed to provide new insights into the activities of two terpenoids, carvacrol and its isomer thymol, against an ESKAPE pathogen, Klebsiella. We gathered data regarding MIC, MBC, MBC/MIC ratio, anti-biofilm, and the combination effect with antibiotics in order to appraise antimicrobial activities of these two compounds.
The MIC values, used as a measure of antimicrobial inhibition, were collected and found to vary widely. In a systematic review by Truong et al. investigating the antibacterial effects of Lavender EOs against methicillin-resistant S. aureus, inconsistent results were noticed due to variability in materials, bacterial strains, and methodology (Truong and Mudgil, 2023). Similarly, we observed variability in Klebsiella strain, type of Klebsiella sampling, antimicrobial resistance pattern, and methodology of MIC measurement. Nevertheless, results indicated strong bacteriostatic activity (104 out of 132 MIC, 78.8%) for both thymol (44 out of 65 strong, 67.7%) and carvacrol (60 out of 67 strong, 89.5%).
Additionally, we observed variability in MBC values. To deal with this variability in results, we calculated the MBC/MIC ratios, and found that 45 out of 47 ratios were lower than four, showing the homogeneity in bactericidal effect and high bactericidal efficacy of both thymol and carvacrol. The bactericidal activity of thymol and carvacrol was previously demonstrated against S. aureus (Zhou et al., 2019; Rúa et al., 2011), Shigella flexnri (Ngome et al., 2018), Actinobacillus pleuropneumoniae (Wang et al., 2017), A. baumannii (Hassannejad et al., 2019), Staphylococcus pseudintermedius, Proteus mirabilis, and P. aeruginosa (Sim et al., 2019).
The antibacterial activities of EOs against Klebsiella were previously demonstrated for Monarda didyma (Chen et al., 2023), Satureja nabateorum (Al-Maharik and Jaradat, 2021), and Althaea officinalis (Arab et al., 2023), which constituted mostly of thymol (69.75%, 46.07%, 58.91%, respectively) and for Lavandula coronopifolia (Ait Said et al., 2015), Thymus capitatus (Ben Selma et al., 2024), and Satureja spicigera (Eftekhar et al., 2009), which constituted mostly of carvacrol (48.9%, 69.28%, 53.74%, respectively). The antibacterial activities of these Eos against Klebsiella can therefore be attributed partly to thymol and carvacrol.
The anti-biofilm activity of antimicrobials is crucial in combating K. pneumoniae, especially considering the increased risk of infection when medical devices are present (Vuotto et al., 2017). Our collected data showed the anti-biofilm activity of thymol and carvacrol against biofilm formation and pre-formed biofilms. The anti-biofilm activity of thymol and carvacrol was previously demonstrated against S. aureus and P. aeruginosa (Walczak et al., 2021). It was also reported against carbapenem-resistant Gram-negative bacilli, such as Klebsiella, Pseudomonas, and Acinetobacter by Raei et al. (2017).
Our study demonstrated antibacterial activity against carbapenem-resistant Klebsiella, with strong activity observed in 53 out of 78 available MIC. This activity was not restricted to Klebsiella; it also extended to other resistant bacteria, such as Pseudomonas and Acinetobacter (Raei et al., 2017). Furthermore, the activity was not limited to resistance to carbapenems; it also included resistance to polymyxin B (de Souza et al., 2021; de Souza et al., 2024), nalidixic acid (Choi et al., 2009), colistin (Yao et al., 2022), and ESBL (Al-Ani et al., 2015; Hamoud et al., 2014; Ilić et al., 2017; Marinelli et al., 2019; Rani et al., 2022a; Tashakor et al., 2024). Additionally, in an in vivo study using a pneumonic mouse model, Hassannejad et al. illustrated the antibacterial activities of thymol, carvacrol, and Zataria multiflora boiss extract, the major constituents of which are thymol and carvacrol, against colistin-resistant A. baumannii (Hassannejad et al., 2019).
Interestingly, these two compounds not only demonstrated significant antibacterial activity alone but also when combined with a range of antibiotics, showed additive to synergistic activities. This property can be substantially beneficial, especially against K. pneumoniae resistant to carbapenems, polymyxin B, and colistin, where the choice of treatment becomes complicated (Ardebili et al., 2023). In our study, we demonstrated not only the synergistic activities of thymol and carvacrol with meropenem (FIC = 0.5) but also a reduction in meropenem MIC when combined with these two compounds against carbapenem-resistant K. pneumoniae (Abdel-halim et al., 2022; Köse, 2022). The same results were also available for colistin against colistin-resistant K. pneumoniae (Yao et al., 2022) and for polymyxin B against polymyxin B-resistant K. pneumoniae (de Souza et al., 2024). This synergistic activity of antibiotics with thymol and carvacrol could be due to their ability to increase bacterial cell wall permeability and cause disruption (Xu et al., 2008). This activity is maintained by permeability to hydrogen and potassium ions through lipid layer destabilization, decrease in elasticity, and increase in fluidity, and by interaction with bacterial proteins (Kowalczyk et al., 2020). These factors may allow the combined antibacterial compound to affect the resistant bacteria.
According to our results, carvacrol exhibited a lower MIC and better synergistic activity. Additionally, previous clinical trials showed the use of carvacrol in patients with asthma (Ghorani et al., 2021a) and veterans exposed to sulfur mustard (Khazdair and Boskabady, 2019). Moreover, a phase I clinical study assessed carvacrol in healthy patients and showed safety and tolerability when carvacrol was used in 1 and 2 mg/kg/day doses (Ghorani et al., 2021b). Therefore, carvacrol seems to be a better candidate for use as an antibacterial agent. The mechanisms of action of carvacrol and thymol are speculated to involve disrupting membrane integrity by integrating into its lipid fragments, depleting the cell of its ATPs and intracellular materials, and thus causing cellular death (Trombetta et al., 2005).
Notably, using thymol and carvacrol as antibacterial agents has some limitations due to their high vaporization and volatility (Escobar et al., 2020). In addition, the low oxidation rate of thymol requires the use of a catalyst to enhance oxidation, which is a common degradation method (Gabrič et al., 2022; Günay et al., 2016). Moreover, carvacrol exhibits low stability, low water solubility, and high sensitivity to the acidity of the digestive system (Günay et al., 2016; Mączka et al., 2023).
Although one of the objectives of this study was to assess the effects of thymol and carvacrol on antimicrobial-resistant Klebsiella, many of the included studies did not provide the resistance pattern of the Klebsiella strains studied. Also, MIC values were not reported with ranges or standard deviations, preventing us from conducting a meta-analysis. For further research, we recommend reporting all MIC values with standard deviations and providing the resistance pattern of all bacterial strains.
5 Conclusion
The results of this systematic review show that thymol and carvacrol have strong bacteriostatic activity and high bactericidal efficacy. They also exhibit anti-biofilm activities and additive to synergistic combination effects with other compounds against Klebsiella. Therefore, thymol and, especially, carvacrol possess great potential for future studies on antimicrobial resistance. However, their inherent limitations must be considered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KF: Conceptualization, Data curation, Methodology, Project administration, Writing–original draft. ER: Data curation, Investigation, Writing–original draft. HV: Formal Analysis, Validation, Visualization, Writing–original draft. MI: Conceptualization, Methodology, Validation, Writing–original draft. SK: Methodology, Validation, Writing–review and editing. MS: Conceptualization, Project administration, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Mrs. Fariba Zamani, MSc, ELS, for language editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1487083/full#supplementary-material
References
Abdel-halim, M., Saad, M., Askoura, M., Mansour, B., Metwally, G., and El-Ganiny, A. (2022). In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates.
Abdelhamid, A. G., and Yousef, A. E. (2021). Carvacrol and thymol combat desiccation resistance mechanisms in Salmonella enterica serovar tennessee. Microorganisms 10 (1), 44. doi:10.3390/microorganisms10010044
Addo, J. K., Owusu-Ansah, E., Dayie, N., Cheseto, X., and Torto, B. (2022). Synthesis of 1,2,3-triazole-thymol derivatives as potential antimicrobial agents. HELIYON 8 (10), e10836. doi:10.1016/j.heliyon.2022.e10836
Ait Said, L., Zahlane, K., Ghalbane, I., El Messoussi, S., Romane, A., Cavaleiro, C., et al. (2015). Chemical composition and antibacterial activity of Lavandula coronopifolia essential oil against antibiotic-resistant bacteria. Nat. Prod. Res. 29 (6), 582–585. doi:10.1080/14786419.2014.954246
Al-Ani, I., Zimmermann, S., Reichling, J., and Wink, M. (2015). Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 22 (2), 245–255. doi:10.1016/j.phymed.2014.11.019
Alavi, M., and Karimi, N. (2019). Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int. J. Biol. Macromol. 128, 893–901. doi:10.1016/j.ijbiomac.2019.01.177
Al-Maharik, N., and Jaradat, N. (2021). Phytochemical profile, antimicrobial, cytotoxic, and antioxidant activities of fresh and air-dried Satureja nabateorum essential oils. Molecules 27 (1), 125. doi:10.3390/molecules27010125
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399 (10325), 629–655. doi:10.1016/S0140-6736(21)02724-0
Arab, M., Arab, N., Kahrizi, D., and Ebadi, A. G. (2023). Evaluating antibacterial effects of alcoholic extracts and essential oil of Althaea officinalis against two types of gram-positive and gram-negative bacteria (Bacillus cereus and Klebsiella pneumonia). J. Med. plants By-Products 12 (1), 107–115. doi:10.22092/jmpb.2021.355329.1385
Ardebili, A., Izanloo, A., and Rastegar, M. (2023). Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: is it superior to polymyxin monotherapy? Expert Rev. Anti Infect. Ther. 21 (4), 387–429. doi:10.1080/14787210.2023.2184346
Ayobami, O., Brinkwirth, S., Eckmanns, T., and Markwart, R. (2022). Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: a systematic review and meta-analysis. Emerg. Microbes Infect. 11 (1), 443–451. doi:10.1080/22221751.2022.2030196
Ben Selma, W., Alibi, S., Ferjeni, M., Ghezal, S., Gallala, N., Belghouthi, A., et al. (2024). Synergistic activity of Thymus capitatus essential oil and cefotaxime against ESBL-producing Klebsiella pneumoniae. Int. J. Environ. Health Res. 34 (8), 2936–2946. doi:10.1080/09603123.2023.2280149
Bisso, N. B., Tokam Kuaté, C. R., and Dzoyem, J. P. (2021). Synergistic antibiofilm efficacy of thymol and piperine in combination with three aminoglycoside antibiotics against Klebsiella pneumoniae biofilms. Can. J. Infect. Dis. Med. Microbiol. 2021, 7029944. doi:10.1155/2021/7029944
Bodmann, K. F. (2005). Current guidelines for the treatment of severe pneumonia and sepsis. Chemotherapy 51 (5), 227–233. doi:10.1159/000087452
Bury-Moné, S. (2014) “Antibacterial therapeutic agents: antibiotics and bacteriophages,” in Reference module in biomedical sciences. Elsevier.
Campana, R., and Baffone, W. (2018). Carvacrol efficacy in reducing microbial biofilms on stainless steel and in limiting re-growth of injured cells. Food control. 90, 10–17. doi:10.1016/j.foodcont.2018.02.029
Chang, D., Sharma, L., Dela Cruz, C. S., and Zhang, D. (2021). Clinical epidemiology, risk factors, and control strategies of Klebsiella pneumoniae infection. Front. Microbiol. 12, 750662. doi:10.3389/fmicb.2021.750662
Chen, Y., Zhao, J., Liu, C., Wu, D., and Wang, X. (2023). In-vitro antibacterial activity and mechanism of Monarda didyma essential oils against Carbapenem-resistant Klebsiella pneumoniae. BMC Microbiol. 23 (1), 263. doi:10.1186/s12866-023-03015-4
Choi, J. G., Kang, O. H., Lee, Y. S., Oh, Y. C., Chae, H. S., Jang, H. J., et al. (2009). Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 14 (5), 1773–1780. doi:10.3390/molecules14051773
Cid-Pérez, T. S., Munguía-Pérez, R., Nevárez-Moorillón, G. V., Ochoa-Velasco, C. E., Navarro-Cruz, A. R., and Avila-Sosa, R. (2024). Carvacrol and thymol effect in vapor phase on Escherichia coli and Salmonella serovar Typhimurium growth inoculated in a fresh salad. Heliyon 10 (9), e29638. doi:10.1016/j.heliyon.2024.e29638
Cordeiro, L. V., Figueiredo, P. T.Rd, Sousa, A.Pd, Andrade Júnior, F.Pd, Souza, H. D. S., Araújo, D. L., et al. (2020). Association of carvacrol with ceftazidime and cefepime against Klebsiella pneumoniae. Soc. Dev. 9 (7), e264974089. doi:10.33448/rsd-v9i7.4089
Dehsheikh, A. B., Sourestani, M. M., Dehsheikh, P. B., Mottaghipisheh, J., Vitalini, S., and Iriti, M. (2020). Monoterpenes: essential oil components with valuable features. Mini Rev. Med. Chem. 20 (11), 958–974. doi:10.2174/1389557520666200122144703
de Souza, G. H., Vaz, M. S., Dos Santos Radai, J. A., Fraga, T. L., Rossato, L., and Simionatto, S. (2024). Synergistic interaction of polymyxin B with carvacrol: antimicrobial strategy against polymyxin-resistant Klebsiella pneumoniae. Future Microbiol. 19, 181–193. doi:10.2217/fmb-2023-0070
de Souza, G. H. A., dos Santos Radai, J. A., Mattos Vaz, M. S., Esther da Silva, K., Fraga, T. L., Barbosa, L. S., et al. (2021). In vitro and in vivo antibacterial activity assays of carvacrol: a candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae. PLOS ONE 16 (2), e0246003. doi:10.1371/journal.pone.0246003
Drobac, M., Petrović, S., Milenković, M., Couladis, M., Kukić-Marković, J., and Niketić, M. (2017). Composition and antimicrobial properties of essential oils of laser trilobum rhizomes and fruits. Nat. Product. Commun. 12 (3), 445–448. doi:10.1177/1934578x1701200335
Durugbo, E. (2013). Medico-ethnobotanical inventory of ogii, okigwe imo state, south eastern Nigeria – I. Glob. Adv. Res. J. Med. Plants (GARJMP) 2 (2), 30–44.
Eftekhar, F., Raei, F., Yousefzadi, M., Ebrahimi, S. N., and Hadian, J. (2009). Antibacterial activity and essential oil composition of Satureja spicigera from Iran. Z Naturforsch C J. Biosci. 64 (1-2), 20–24. doi:10.1515/znc-2009-1-204
Escobar, A., Pérez, M., Romanelli, G., and Blustein, G. (2020). Thymol bioactivity: a review focusing on practical applications. Arabian J. Chem. 13 (12), 9243–9269. doi:10.1016/j.arabjc.2020.11.009
Gabrič, A., Hodnik, Ž., and Pajk, S. (2022). Oxidation of drugs during drug product development: problems and solutions. Pharmaceutics 14 (2), 325. doi:10.3390/pharmaceutics14020325
Gan, C., Langa, E., Valenzuela, A., Ballestero, D., and Pino-Otín, M. R. (2023). Synergistic activity of thymol with commercial antibiotics against critical and high WHO priority pathogenic bacteria. Plants 12 (9), 1868. doi:10.3390/plants12091868
Ghorani, V., Alavinezhad, A., Rajabi, O., and Boskabady, M. H. (2021a). Carvacrol improves pulmonary function tests, oxidant/antioxidant parameters and cytokine levels in asthmatic patients: a randomized, double-blind, clinical trial. Phytomedicine 85, 153539. doi:10.1016/j.phymed.2021.153539
Ghorani, V., Alavinezhad, A., Rajabi, O., Mohammadpour, A. H., and Boskabady, M. H. (2021b). Safety and tolerability of carvacrol in healthy subjects: a phase I clinical study. Drug Chem. Toxicol. 44 (2), 177–189. doi:10.1080/01480545.2018.1538233
Günay, T., Çimen, Y., Karabacak, R. B., and Türk, H. (2016). Oxidation of thymol and carvacrol to thymoquinone with KHSO5 catalyzed by iron phthalocyanine tetrasulfonate in a methanol–water mixture. Catal. Lett. 146 (11), 2306–2312. doi:10.1007/s10562-016-1850-2
Hamoud, R., Zimmermann, S., Reichling, J., and Wink, M. (2014). Synergistic interactions in two-drug and three-drug combinations (thymol, EDTA and vancomycin) against multi drug resistant bacteria including E. coli. Phytomedicine 21 (4), 443–447. doi:10.1016/j.phymed.2013.10.016
Hassannejad, N., Bahador, A., Rudbari, N. H., Modarressi, M. H., and Parivar, K. (2019). In vivo antibacterial activity of Zataria multiflora Boiss extract and its components, carvacrol, and thymol, against colistin-resistant Acinetobacter baumannii in a pneumonic BALB/c mouse model. J. Cell Biochem. 120 (11), 18640–18649. doi:10.1002/jcb.28908
Heckler, C., Sant'anna, V., Brandelli, A., and Malheiros, P. S. (2021). Combined effect of carvacrol, thymol and nisin against Staphylococcus aureus and Salmonella Enteritidis. An Acad Bras Cienc 93 (Suppl. 4), e20210550. doi:10.1590/0001-3765202120210550
Höferl, M., Buchbauer, G., Jirovetz, L., Schmidt, E., Stoyanova, A., Denkova, Z., et al. (2009). Correlation of antimicrobial activities of various essential oils and their main aromatic volatile constituents. J. Essent. Oil Res. 21 (5), 459–463. doi:10.1080/10412905.2009.9700218
Hoffmann, K. H. (2020). Essential oils. Z Naturforsch C J. Biosci. 75 (7-8), 177. doi:10.1515/znc-2020-0124
Huang, Z., Zhang, X., Yao, Z., Han, Y., Ye, J., Zhang, Y., et al. (2023). Thymol-decorated gold nanoparticles for curing clinical infections caused by bacteria resistant to last-resort antibiotics. mSphere 8 (3), e0054922. doi:10.1128/msphere.00549-22
Idris, F. N., and Nadzir, M. M. (2023). Multi-drug resistant ESKAPE pathogens and the uses of plants as their antimicrobial agents. Archives Microbiol. 205 (4), 115. doi:10.1007/s00203-023-03455-6
Ilić, B. S., Miladinović, D. L., Kocić, B. D., Spalović, B. R., Marković, M. S., Čolović, H., et al. (2017). Chemoinformatic investigation of antibiotic antagonism: the interference of thymus glabrescens essential oil components with the action of streptomycin. Nat. Product. Commun. 12 (10), 1934578X1701201–8. doi:10.1177/1934578x1701201033
Iten, F., Saller, R., Abel, G., and Reichling, J. (2009). Additive antimicrobial [corrected] effects of the active components of the essential oil of Thymus vulgaris--chemotype carvacrol. Planta Medica 75 (11), 1231–1236. doi:10.1055/s-0029-1185541
Jesudason, T. (2024). WHO publishes updated list of bacterial priority pathogens. Lancet Microbe 5 (9), 100940. doi:10.1016/j.lanmic.2024.07.003
Jurevičiūtė, R., Ložienė, K., Bruno, M., Maggio, A., and Rosselli, S. (2019). Composition of essential oil of lemon thyme (Thymus × citriodorus) at different hydrodistillation times. Nat. Prod. Res. 33 (1), 80–88. doi:10.1080/14786419.2018.1434642
Kachur, K., and Suntres, Z. (2020). The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 60 (18), 3042–3053. doi:10.1080/10408398.2019.1675585
Khazdair, M. R., and Boskabady, M. H. (2019). The effect of carvacrol on inflammatory mediators and respiratory symptoms in veterans exposed to sulfur mustard, a randomized, placebo-controlled trial. Respir. Med. 150, 21–29. doi:10.1016/j.rmed.2019.01.020
Köse, E. O. (2022). In vitro activity of carvacrol in combination with meropenem against carbapenem-resistant Klebsiella pneumoniae. Folia Microbiol. 67 (1), 143–156. doi:10.1007/s12223-021-00908-7
Kowalczyk, A., Przychodna, M., Sopata, S., Bodalska, A., and Fecka, I. (2020). Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules 25 (18), 4125. doi:10.3390/molecules25184125
Kwiatkowski, P., Sienkiewicz, M., Pruss, A., Łopusiewicz, Ł., Arszyńska, N., Wojciechowska-Koszko, I., et al. (2022). Antibacterial and anti-biofilm activities of essential oil compounds against new DelhiMetallo-β-lactamase-1-producing uropathogenic Klebsiella pneumoniae strains. Antibiotics 11 (2), 147. doi:10.3390/antibiotics11020147
Liu, F., Jin, P., Sun, Z., Du, L., Wang, D., Zhao, T., et al. (2021). Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food control. 119, 107473. doi:10.1016/j.foodcont.2020.107473
Liu, X., Liu, R., Zhao, R., Wang, J., Cheng, Y., Liu, Q., et al. (2022). Synergistic interaction between paired combinations of natural antimicrobials against poultry-borne pathogens. Front. Microbiol. 13, 811784. doi:10.3389/fmicb.2022.811784
Ma, Y. X., Wang, C. Y., Li, Y. Y., Li, J., Wan, Q. Q., Chen, J. H., et al. (2020). Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. (Weinh) 7 (1), 1901872. doi:10.1002/advs.201901872
Mączka, W., Twardawska, M., Grabarczyk, M., and Wińska, K. (2023). Carvacrol-A natural phenolic compound with antimicrobial properties. Antibiot. (Basel) 12 (5), 824. doi:10.3390/antibiotics12050824
Marinelli, L., Fornasari, E., Eusepi, P., Ciulla, M., Genovese, S., Epifano, F., et al. (2019). Carvacrol prodrugs as novel antimicrobial agents. Eur. J. Med. Chem. 178, 515–529. doi:10.1016/j.ejmech.2019.05.093
Mbese, Z., Nell, M., Fonkui, Y. T., Ndinteh, D. T., Steenkamp, V., and Aderibigbe, B. A. (2022). Hybrid compounds containing carvacrol scaffold: in vitro antibacterial and cytotoxicity evaluation. Recent Adv. Anti-Infective Drug Discov. 17 (1), 54–68. doi:10.2174/1574891X16666220124122445
Mbese, Z., Peteni, S., Fotsing, M. C., Fonkui, T. Y., Ndinteh, D. T., Ray, S. S., et al. (2023). Antibacterial study of carbopol-mastic gum/silver nanoparticle-based topical gels with carvacrol/neem bark extract in vitro. J. Wound Care 32. CLXXXI–CLXXXIX. doi:10.12968/jowc.2023.32.sup9a.clxxxi
Moghtaderi, M., Bazzazan, S., Sorourian, G., Sorourian, M., Akhavanzanjani, Y., Noorbazargan, H., et al. (2023). Encapsulation of thymol in gelatin methacryloyl (GelMa)-Based nanoniosome enables enhanced antibiofilm activity and wound healing. Pharmaceutics 15 (6), 1699. doi:10.3390/pharmaceutics15061699
Mohammed, M. J., and Al-Bayati, F. A. (2009). Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine 16 (6), 632–637. doi:10.1016/j.phymed.2008.12.026
Muftah, H., Ozçelik, B., Oyardı, O., Kutluk, İ., and Orhan, N. (2020). A comparative evaluation of Juniperus species with antimicrobial magistrals. Pak. J. Pharm. Sci. 33 (4), 1443–1449. doi:10.36721/pjps.2020.33.4.reg.1443-1449
Nanayakkara, A. K., Boucher, H. W., Fowler, V. G., Jezek, A., Outterson, K., and Greenberg, D. E. (2021). Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA Cancer J. Clin. 71 (6), 488–504. doi:10.3322/caac.21697
Ndezo, B. B., Tokam Kuaté, C. R., Boulens, N., Allémann, E., Delie, F., and Dzoyem, J. P. (2022). Antibiofilm synergistic activity of streptomycin in combination with thymol-loaded poly (Lactic-co-glycolic acid) nanoparticles against Klebsiella pneumoniae isolates. Evidence-based Complementary Altern. Med. 2022, 1936165. doi:10.1155/2022/1936165
Ngome, M. T., Alves, J., de Oliveira, A. C. F., da Silva Machado, P., Mondragón-Bernal, O. L., and Piccoli, R. H. (2018). Linalool, citral, eugenol and thymol: control of planktonic and sessile cells of Shigella flexneri. Amb. Express 8 (1), 105. doi:10.1186/s13568-018-0634-z
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Peter, S., Sotondoshe, N., and Aderibigbe, B. A. (2024). Carvacrol and thymol hybrids: potential anticancer and antibacterial therapeutics. Molecules 29 (10), 2277. doi:10.3390/molecules29102277
Pormohammad, A., Hansen, D., and Turner, R. J. (2022). Antibacterial, antibiofilm, and antioxidant activity of 15 different plant-based natural compounds in comparison with ciprofloxacin and gentamicin. Antibiot. (Basel) 11 (8), 1099. doi:10.3390/antibiotics11081099
Pu, D., Zhao, J., Chang, K., Zhuo, X., and Cao, B. (2023). Superbugs with hypervirulence and carbapenem resistance in Klebsiella pneumoniae: the rise of such emerging nosocomial pathogens in China. Sci. Bull. (Beijing) 68 (21), 2658–2670. doi:10.1016/j.scib.2023.09.040
Raei, P., Pourlak, T., Memar, M. Y., Alizadeh, N., Aghamali, M., Zeinalzadeh, E., et al. (2017). Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cell Mol. Biol. (Noisy-le-grand). 63 (5), 108–112. doi:10.14715/cmb/2017.63.5.20
Rani, S., Singh, H., and Ram, C. (2022a). Efficacy and mechanism of carvacrol with octanoic acid against mastitis causing multi-drug-resistant pathogens. Braz. J. Microbiol. 53 (1), 385–399. doi:10.1007/s42770-021-00639-4
Rani, S., Verma, S., Singh, H., and Ram, C. (2022b). Antibacterial activity and mechanism of essential oils in combination with medium-chain fatty acids against predominant bovine mastitis pathogens. Lett. Appl. Microbiol. 74 (6), 959–969. doi:10.1111/lam.13675
Rúa, J., Fernández-Álvarez, L., de Castro, C., Del Valle, P., de Arriaga, D., and García-Armesto, M. R. (2011). Antibacterial activity against foodborne Staphylococcus aureus and antioxidant capacity of various pure phenolic compounds. Foodborne Pathog. Dis. 8 (1), 149–157. doi:10.1089/fpd.2010.0659
Sabour, A., El Asbahani, A., Bentahar, S., Ait Taleb, M., Lacherai, A., and Jilale, A. (2019). Synthesis of some thymol derivatives for enhanced antibacterial activity. Moroc. J. Chem. 7 (4), 748–757. doi:10.48317/IMIST.PRSM/morjchem-v7i4.17494
Sahoo, C. R., Paidesetty, S. K., and Padhy, R. N. (2021). The recent development of thymol derivative as a promising pharmacological scaffold. Drug Dev. Res. 82 (8), 1079–1095. doi:10.1002/ddr.21848
Salaria, D., Rolta, R., Patel, C. N., Dev, K., Sourirajan, A., and Kumar, V. (2022). In vitro and in silico analysis of Thymus serpyllum essential oil as bioactivity enhancer of antibacterial and antifungal agents. J. Biomol. Struct. Dyn. 40 (20), 10383–10402. doi:10.1080/07391102.2021.1943530
Scandorieiro, S., Rodrigues, B. C. D., Nishio, E. K., Panagio, L. A., de Oliveira, A. G., Durán, N., et al. (2022). Biogenic silver nanoparticles strategically combined with Origanum vulgare derivatives: antibacterial mechanism of action and effect on multidrug-resistant strains. Front. Microbiol. 13, 842600. doi:10.3389/fmicb.2022.842600
Scandorieiro, S., Teixeira, F., Nogueira, M. C. L., Panagio, L. A., de Oliveira, A. G., Durán, N., et al. (2023). Antibiofilm effect of biogenic silver nanoparticles combined with oregano derivatives against carbapenem-resistant Klebsiella pneumoniae. ANTIBIOTICS-BASEL 12 (4), 756. doi:10.3390/antibiotics12040756
Sharma, A., Thakur, A., Thakur, N., Kumar, V., Chauhan, A., and Bhardwaj, N. (2023). Changing trend in the antibiotic resistance pattern of Klebsiella pneumonia isolated from endotracheal aspirate samples of ICU patients of a tertiary care hospital in north India. Cureus 15 (3), e36317. doi:10.7759/cureus.36317
Sheth, V. H., Shah, N. P., Jain, R., Bhanushali, N., and Bhatnagar, V. (2024). Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: the QUIN. J. Prosthet. Dent. 131 (6), 1038–1042. doi:10.1016/j.prosdent.2022.05.019
Sim, J. X. F., Khazandi, M., Chan, W. Y., Trott, D. J., and Deo, P. (2019). Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol 30 (6), 524–e159. doi:10.1111/vde.12794
Sukmawan, Y. P., Anggadiredja, K., and Adnyana, I. K. (2021). Anti-neuropathic pain activity of ageratum conyzoides L due to the essential oil components. CNS Neurol. Disord. Drug Targets 20 (2), 181–189. doi:10.2174/1871527319666201120144228
Taguri, T., Tanaka, T., and Kouno, I. (2006). Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 29 (11), 2226–2235. doi:10.1248/bpb.29.2226
Tashakor, A. H., Rezaei, A., Fouladseresht, H., and Mansury, D. (2024). Characterization and investigation of cytotoxicity and antimicrobial properties of coencapsulated limonene and thymol into the Ferula assafoetida gum microparticles. Int. J. Biol. Macromol. 263, 130338. doi:10.1016/j.ijbiomac.2024.130338
Trombetta, D., Castelli, F., Sarpietro, M. G., Venuti, V., Cristani, M., Daniele, C., et al. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49 (6), 2474–2478. doi:10.1128/AAC.49.6.2474-2478.2005
Truong, S., and Mudgil, P. (2023). The antibacterial effectiveness of lavender essential oil against methicillin-resistant Staphylococcus aureus: a systematic review. Front. Pharmacol. 14, 1306003. doi:10.3389/fphar.2023.1306003
Ultee, A., Bennik, M. H. J., and Moezelaar, R. (2002). The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68 (4), 1561–1568. doi:10.1128/aem.68.4.1561-1568.2002
van Vuuren, S., and Viljoen, A. (2011). Plant-based antimicrobial studies – methods and approaches to study the interaction between natural products. Planta Med. 77 (11), 1168–1182. doi:10.1055/s-0030-1250736
Vuotto, C., Longo, F., Pascolini, C., Donelli, G., Balice, M. P., Libori, M. F., et al. (2017). Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 123 (4), 1003–1018. doi:10.1111/jam.13533
Walczak, M., Michalska-Sionkowska, M., Olkiewicz, D., Tarnawska, P., and Warżyńska, O. (2021). Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules 26 (9), 2723. doi:10.3390/molecules26092723
Wang, L., Zhao, X., Zhu, C., Xia, X., Qin, W., Li, M., et al. (2017). Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of Actinobacillus pleuropneumoniae strain L20. Vet. Microbiol. 203, 202–210. doi:10.1016/j.vetmic.2017.02.021
Xu, J., Zhou, F., Ji, B. P., Pei, R. S., and Xu, N. (2008). The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 47 (3), 174–179. doi:10.1111/j.1472-765X.2008.02407.x
Yao, Z., Feng, L., Zhao, Y., Zhang, X., Chen, L., Wang, L., et al. (2022). Thymol increases sensitivity of clinical col-R gram-negative bacteria to colistin. Microbiol. Spectr. 10 (4). doi:10.1128/spectrum.00184-22
Yehia, M., Gamal, A., El-Ela, F. I. A., Abdel-Baki, A. A. S., Ibrahium, S. M., Shokier, K. A., et al. (2024). Carvacrol-loaded invasomes biocidal effect against multidrug resistant isolates of Enterobacteriaceae and housefly. Austral J. Veterinary Sci. 56 (1), 25–33. doi:10.4206/ajvs.561.04
Zhang, D., Hu, H., Rao, Q., and Zhao, Z. (2011). Synergistic effects and physiological responses of selected bacterial isolates from animal feed to four natural antimicrobials and two antibiotics. Foodborne Pathogens Dis. 8 (10), 1055–1062. doi:10.1089/fpd.2010.0817
Zhang, S., Zhang, X., Wu, Q., Zheng, X., Dong, G., Fang, R., et al. (2019). Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrob. Resist Infect. Control 8, 166. doi:10.1186/s13756-019-0615-2
Zhao, H., Ren, S., Yang, H., Tang, S., Guo, C., Liu, M., et al. (2022). Peppermint essential oil: its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 154, 113559. doi:10.1016/j.biopha.2022.113559
Zhen, X., Lundborg, C. S., Sun, X., Hu, X., and Dong, H. (2019). Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob. Resist Infect. Control 8, 137. doi:10.1186/s13756-019-0590-7
Keywords: Klebsiella, K. pneumoniae, antimicrobial resistance, thymol, carvacrol, synergistic, biofilm
Citation: Farhadi K, Rajabi E, Varpaei HA, Iranzadasl M, Khodaparast S and Salehi M (2024) Thymol and carvacrol against Klebsiella: anti-bacterial, anti-biofilm, and synergistic activities—a systematic review. Front. Pharmacol. 15:1487083. doi: 10.3389/fphar.2024.1487083
Received: 27 August 2024; Accepted: 04 October 2024;
Published: 24 October 2024.
Edited by:
Tushar Dhanani, Florida Agricultural and Mechanical University, United StatesReviewed by:
Azazahemad A. Kureshi, Pharmanza Herbal Private Limited, IndiaArka Banerjee, NewYork-Presbyterian, United States
Copyright © 2024 Farhadi, Rajabi, Varpaei, Iranzadasl, Khodaparast and Salehi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammadreza Salehi, c2FsZWhpLm1vaGFtYWQzQGdtYWlsLmNvbQ==
 Kousha Farhadi
Kousha Farhadi Erta Rajabi
Erta Rajabi Hesam Aldin Varpaei
Hesam Aldin Varpaei Maryam Iranzadasl
Maryam Iranzadasl Sepideh Khodaparast4
Sepideh Khodaparast4 Mohammadreza Salehi
Mohammadreza Salehi