- 1Wenzhou Key Laboratory for the Diagnosis and Prevention of Diabetic Complications, The Third Affiliated Hospital of Wenzhou Medical University (Ruian People’s Hospital), Ruian, China
- 2College of Pharmacy, Yanbian University, Yanji, Jilin, China
- 3Liji Medical Research Academy, The Third Affiliated Hospital of Wenzhou Medical University (Ruian People’s Hospital), Ruian, China
Background: Fibrosis is key in the development and progression of diabetic kidney disease (DKD). Baicalin (BA), wogonin (WGN), and wogonoside (WGS) have renoprotective effects. The mechanism of alleviation of DKD progression, by improving renal fibrosis, is unclear. This study aimed to investigate the mechanisms and effects of a Scutellaria baicalensis Georgi. (Lamiaceae) mixture (MIX, WGN:BA:WGS = 4:2:1) on DKD in a spontaneous DKD model.
Methods: Male db/m mice were controls, and db/db mice were diabetes models. Both groups received daily oral gavage of normal saline. Treatment groups received daily oral gavage of BA or MIX (20 mg/kg) for 10 weeks. Biochemical indicators and kidney lesions were assessed. Fibrosis-related proteins were detected by immunoblotting, immunohistochemistry, and real-time fluorescence quantitative PCR.
Results: MIX significantly reduced body weight (40.97 ± 1.43 vs. 42.26 ± 1.60), improved insulin sensitivity (63.70 ± 8.98 vs. 109.48 ± 0.69), lowered the renal hypertrophy index (19.81 ± 2.86 vs. 28.94 ± 0.256), and decreased blood urea nitrogen levels (7.57 ± 0.79 vs. 9.57 ± 0.38) and the urine protein/creatinine ratio (0.50 ± 0.06 vs. 0.80 ± 0.18). MIX also enhanced lipid profiles and renal function by improving renal tubular dilation, restoring renal structures, and reducing glomerulosclerosis, basal membrane thickening, and glycogen deposition. These effects were achieved by reducing the protein and gene expression of collagen II (Col-II), connective tissue growth factor, and collagen I (Col-I).
Conclusion: MIX inhibits the transforming growth factor-β/Smads signaling pathway, thus alleviating renal fibrosis, and can be used to develop a treatment for DKD.
Highlights
• MIX outperformed BA in reducing renal fibrosis markers.
• MIX significantly lowered the renal hypertrophy index in DKD mice.
• BA and MIX improved insulin sensitivity and reduced blood urea nitrogen.
• MIX inhibited TGF-β/Smad signaling, alleviating renal fibrosis.
• MIX shows potential as a natural treatment for diabetic nephropathy.
Introduction
Diabetic kidney disease (DKD) is the main cause of end-stage lesions in diabetic patients (Aschner et al., 2021; Du et al., 2017). It is one of the most serious complications, affecting 10%–40% of patients (Adeshara et al., 2016). In China, the incidence of DKD is expected to increase in the coming decades as the prevalence of diabetes rises (Guo et al., 2016; Tang et al., 2017).
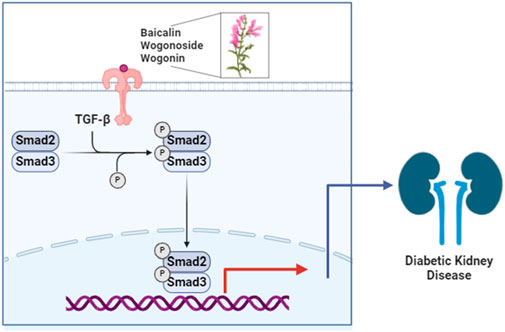
The pathogenesis of DKD involves many factors including metabolic disorders, immune-inflammatory mechanisms, oxidative stress, and genetic factors (Li et al., 2017; Lavoz et al., 2020; Meshkani and Vakili, 2016). Renal fibrosis is considered a complex and irreversible process occurring in the DKD lesions at later stages, further exacerbating disease progression. Renal fibrosis is a multifactorial dynamic process, with studies indicating the involvement of numerous factors in DKD fibrosis development including transforming growth factor-ß (TGF-ß), interleukin (IL)-1, IL-6, tumor necrosis factor-α, and oxidative stress. Research has found that NAD(P)H quinone oxidoreductase 1 mitigates diabetes-induced renal inflammation and fibrosis by modulating the Toll-like receptor 4 (TLR4)/NF-κB and TGF-ß/Smads signaling pathways (Qiu et al., 2023). Under the influence of multiple factors leading to inflammation and damage, excessive extracellular matrix deposition and the epithelial-mesenchymal transition occur, resulting in a loss of differentiated epithelial cells and their capillaries, and the accumulation of myofibroblasts and inflammatory cells, ultimately leading to structural and functional damage of the kidney (Hu et al., 2021; Yamashita and Kramann, 2024).
Scutellaria baicalensis Georgi. (Lamiaceae), a plant with a medicinal history spanning at least 2000 years, was first recorded around 200 AD in Shennong’s Classic of Materia Medica (Liao et al., 2021; Zhao et al., 2019). The roots of S. baicalensis are known to promote wound healing, remove heat and dampness, clear fires, detoxify and reconcile toxins, and regulate immunity. It is used for the prevention and treatment of diabetes, have the effects of improving the renal function, insulin resistance and retinopathy of type 2 diabetic patients (Lai and Li, 2024). And exhibits various pharmacological properties, including antitumor, free-radical-scavenging, antioxidant, anti-inflammatory, and antiviral activity (Shang et al., 2023). Baicalin (BA), wogonin (WGN), and wogonoside (WGS) are bioactive flavonoids found in S. baicalensis (Wu et al., 2014). BA has numerous health benefits, including anti-inflammatory, antibacterial, antitumor, and antioxidant activity (Liao et al., 2021; Shang et al., 2023). BA has been proposed as a potential drug for the treatment of DKD (Hu et al., 2024), alleviate podocyte damage (Ou et al., 2021), diminish renal fibrosis [ (Zhang et al., 2020; Wang H. et al., 2022)], enhance renal functionality (Yang et al., 2019), oxidative stress and inflammation (Ma et al., 2021) and provide relief in DKD scenarios. WGN mitigates glomerulopathy and podocyte injury by regulating Bcl-2-mediated crosstalk between autophagy and apoptosis (Liu et al., 2022). WGS and WGN can reduce the expression levels of TLR4 mRNA and NF-κBp65 mRNA in renal tissue. Consequently, the proteins TLR4 and NF-κBp65 are decreased, inhibiting the TLR/NF-κB signaling pathway and playing a protective role in renal tissue (Wang YR. et al., 2022).
Scutellaria baicalensis Georgi. (Lamiaceae) mixture (MIX, WGN:BA:WGS = 4:2:1), that using multi-chamber electrophoresis screening technology, three components that bind to the target protein were selected from a multi-component mixture of S. baicalensis. These components, qualitatively identified as WGN, BA, and WGS through LC-MS/MS in the multiple reaction monitoring mode, were subsequently quantified. The molar ratio of the components was 4:2:1 (v/v). Using a high glucose-induced human renal tubular epithelial cell (HK-2) model, we demonstrated that these components suppress inflammatory responses and mitigate apoptosis in high glucose-induced HK-2 cells (Suo, 2022). There is insufficient in vivo evidence validating the therapeutic efficacy and safety of MIX in animal models of DKD and inadequate understanding of the molecular pathways modulated by these combined components, specifically through the TGF-β/Smads signaling pathway.
In this study, we investigated the impact of multiple components of S. baicalensis on the TGF-β/Smads signaling pathway in the renal tissues of DKD db/db mice. We aimed to explore the mechanisms by which BA and MIX ameliorate DKD and provide an experimental foundation for the potential clinical applications of BA and MIX.
Methods
Ethics statement
All animal experiments were conducted in accordance with internationally recognized animal welfare guidelines and were approved by the Medical Ethics Committee of Experimental Animal Ethics at Ruian People’s Hospital (SYSQ-2023-008, 30 April 2023).
Drugs
BA (>98.0% purity, catalog no. MB6698), WGN (>98.0% purity; catalog no. MB6662), and WGS (>98.0% purity; catalog no. MB6663) were purchased from Meilunbio (Dalian, China). A MIX of S. baicalensis was prepared using WGN, BA, and WGS in a molar ratio of 4:2:1. Isoflurane was obtained from RWD Life Science Co. Ltd. (Shenzhen, China).
Experimental animals and treatment
db/db mice (males, 7 weeks, weighing 36 ± 2 g, n = 18), and db/m mice (males, 7 weeks, weighing 22 ± 2 g, n = 6) which were non-diabetic mice born in the same litter as the db/db mice, were obtained from Jiangsu Changzhou Kavins Laboratory Animal Co., Ltd. (license number: SCXK (Su) 2021-0013). They were housed in separate individually ventilated cages (temperature: 23°C ± 3°C, humidity 40%–70%, light/dark cycle 12 h). The mice had ad libitum access to a standard diet and water.
The db/db mice were divided randomly into three groups based on fasting blood glucose levels and body weight (n = 6): the db/db group (model of diabetes), the db/db mice treated with MIX group, and the BA group. The db/m mice (n = 6) served as the normal control group of non-diabetic mice (Jin et al., 2023). Mice in the db/m and db/db groups received normal saline, while those in the BA group received a daily dose of 20 mg/kg BA, and those in the MIX group received 20 mg/kg MIX. The three components were calculated according to a WGN: BA: WGS ratio of 4:2:1. All treatments were administered by oral gavage once daily at 10:00 a.m. for 10 weeks.
Metabolic and biochemical parameters
The fasting blood glucose levels of the mice were assessed using a blood glucose meter and test strips once a week throughout the treatment period, with blood samples obtained from the tail tip. At 18 weeks old, the mice were weighed and placed in metabolic cages for 24 h for urine collection. The mice were anesthetized using an anesthesia machine with 1.5%–2% isoflurane. After anesthesia, blood samples were collected from the orbital venous plexus, and both kidneys were carefully removed, washed with phosphate-buffered saline, and weighed. The following parameters were measured using an automated biochemical analyzer (IDEXX Laboratories, Shanghai, China): urine protein, urine creatinine, blood urea nitrogen (BUN), cholesterol, and triglycerides.
Intraperitoneal glucose tolerance
The mice were fasted for 12 h. An intraperitoneal injection of a glucose solution (2 g/kg, 50%) was administered. Blood samples were collected from the tail vein 0, 30, 60, and 120 min after the injection, and the corresponding blood glucose values were recorded. The area under the curve (AUC) for the glucose tolerance test was calculated (Huang et al., 2024).
Kidney histology
The kidney tissues were fixed in 4% paraformaldehyde buffer (P1110, Beijing Solaibao Technology Co., Ltd.), embedded in paraffin, and sectioned. These sections were further processed for histological staining using hematoxylin and eosin (HE) (G1120, Beijing Solaibao Technology Co., Ltd.), Masson’s modified trichrome (G1340, Beijing Solaibao Technology Co., Ltd.), or periodic acid-Schiff (PAS) (C0142S, Beijing Solaibao Technology Co., Ltd.) and viewed under a 400× power lens.
Immunohistochemistry staining
Following the standard deparaffinization and hydration protocol. Antigen repair was performed by microwaving 10 mM sodium citrate (pH 6.0) buffer solution for 10 min. To block endogenous peroxidase activity, the slides were incubated with 3% hydrogen peroxide (H2O2) at room temperature for 30 min. The slides were blocked with 20% normal goat serum for 1 h. The sections were incubated overnight with the appropriate primary antibody TGF-β (25 kDa, 1:500, #ab92486, Abcam, Cambridge, United Kingdom) and Smad2/3 (60 kDa, 1:500, #3700, CST, Danvers, MA, United States) were applied to the slides and left overnight at 4°C. (Negative control: no primary antibody was added during the staining process). DAB color development (DAB chromogenic reagent for histochemical kit; G1212; Wuhan servicebio technology CO.,LTD.) and counterstained with hematoxylin (G1004; G1039; G1040; Wuhan servicebio technology CO.,LTD.). Images were captured at a magnification of ×400 and analyzed Immunohistochemistry positive area using ImagePro Plus 6.0 quantitative software (NIH, Bethesda, MD, United States).
RT-qPCR
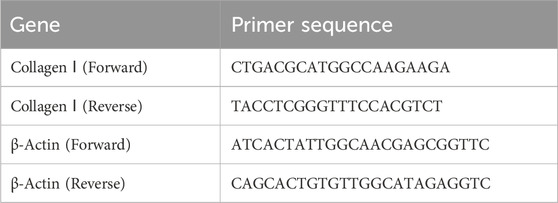
Total RNA was extracted from mouse kidney tissues using the RNAiso Plus reagent following the standard protocol (MF846, Mei5 Biotechnology, Beijing, China). The cDNA was reverse-transcribed (MF012, Mei5 Biotechnology, Beijing, China). RT-qPCR (MF049, Mei5 Biotechnology, Beijing, China) was performed on the QuantStudio™ 3 system (Thermo Fisher Scientific, Waltham, MA, United States). The mRNA levels of the target genes were normalized and analyzed using the 2−ΔΔCT method. The primer sequences are listed in Table 1.
Western blot assays
Renal tissues (approximately 100 mg) were collected and lysed in radioimmunoprecipitation assay buffer (P0013B, Beyotime Biotechnology Co., Ltd.). The lysates were centrifuged at 4°C and 12,000 g for 15 min, and the resulting supernatants were collected. Total protein was extracted from the supernatant, and the protein concentration was ascertained using a bicinchoninic acid assay (BCA, P0010 Beyotime Biotechnology Co., Ltd.). Equivalent amounts of protein (35 μg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, P0010, Beyotime Biotechnology Co., Ltd.) and transferred to polyvinylidene difluoride membranes by electroblotting.
The membranes were blocked in 5% (w/v) nonfat milk at room temperature for 1 h. They were then incubated overnight at 4°C with specific primary antibodies against TGF-β (25kDa, 1:1,000 dilution; #ab92486, Abcam, Cambridge, United Kingdom), Smad2/3 (60 kDa, 1:1,000 dilution; #12470, CST, Danvers, MA, United States), collagen I (Col-Ⅰ) (220 kDa1:1,000 dilution, #72026 CST, Danvers, MA, United States), collagen II (Col-Ⅱ) (200 kDa, 1:1,000 dilution, #43306S, CST, Danvers, MA, United States), and connective tissue growth factor (CTGF) (35 kDa, 1:1,000 dilution, #86641S, CST, Danvers, MA, United States). The internal control β-actin/GAPDH calibration was used to correct the errors in the quantification and loading of protein samples to ensure the accuracy of the experimental results. After washing with tris-buffered saline/0.1% Tween 20 (TBST), the membranes were incubated with the appropriate secondary antibody at room temperature for 1 h. Following washing with TBST, the membranes were stained with an enhanced chemiluminescence reagent and visualized using a gel imaging system. ImagePro Plus 6.0 was used to analyze the gray scale of the obtained strips.
Statistical analyses
Data analysis was conducted using SPSS version 22.0. Results are presented as mean ± standard deviation. Differences among multiple sample groups were assessed using one-way analysis of variance. Pairwise comparisons between groups with homogeneous variances were performed using the Bonferroni method, while Tamhane’s T2 test was employed for groups with heterogeneous variances. p < 0.05 was considered statistically significant.
Results
Effect of baicalin and MIX on weight, blood sugar, and glucose tolerance of db/db mice
During the experiment, both the db/m and db/db groups exhibited significant increases in average body weight. However, after 4 weeks of treatment, the db/db MIX treatment group showed a marked decrease in body weight, surpassing the decrease observed in the db/db BA group. The db/db group demonstrated a notable elevation in blood glucose levels compared to the db/m group. Neither BA nor MIX administration in the db/db group reduced the blood glucose level. This indicates that both BA and MIX were ineffective in mitigating the onset and progression of DKD by lowering blood glucose levels.
In the db/m group, blood glucose levels gradually decreased after intraperitoneal insulin injections and reached their lowest value 30 min after injection. In contrast, blood glucose levels in the db/db group did not change significantly after insulin injection, indicating a marked decrease in insulin sensitivity and the presence of insulin resistance. The AUC of the insulin tolerance test also increased significantly in the db/db group compared to the control group. In the BA and MIX treatment groups, blood glucose levels decreased after intraperitoneal insulin injection, reaching their lowest values at 60 min. This indicates that BA and MIX restored insulin sensitivity in mice. Among the treatment groups, the MIX group demonstrated the greatest improvement in glucose and insulin tolerance, as indicated by the insulin tolerance test AUC values shown in Figure 1.
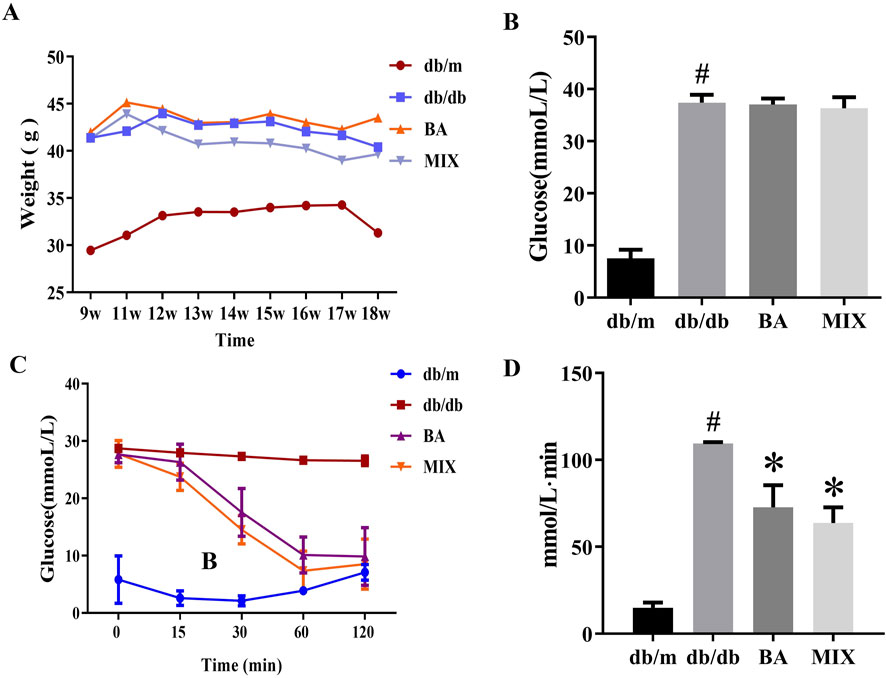
Figure 1. Changes in body weight, blood glucose, and glucose tolerance in each group (A) The weight change (g) of each group over the study period. (B) The blood glucose levels (mmol/L) measured at the end of the study. (C) The time trend of blood glucose levels (mmol/L) after insulin injection. (D) The area under the curve of the insulin tolerance test for each group (n = 6, compared to the db/m group, #p < 0.05; compared to the db/db group, *p < 0.05). Abbreviations: db/m: normal control group of non-diabetic mice, db/db: model of diabetes, BA: baicalin, MIX: multi-component mixture.
Effect of baicalin and MIX on renal function and serum biochemical indicators in db/db mice
The ratio of kidney weight to tibial length in mice serves as an indicator of renal hypertrophy. Compared to the db/m group, the db/db group exhibited a significant increase in the renal hypertrophy index. However, the BA and MIX treatment groups significantly reduced the renal hypertrophy index compared with the db/db group. In particular, the MIX treatment group showed a more substantial decrease in the renal hypertrophy index than the BA treatment group did, as shown in Figure 2A.
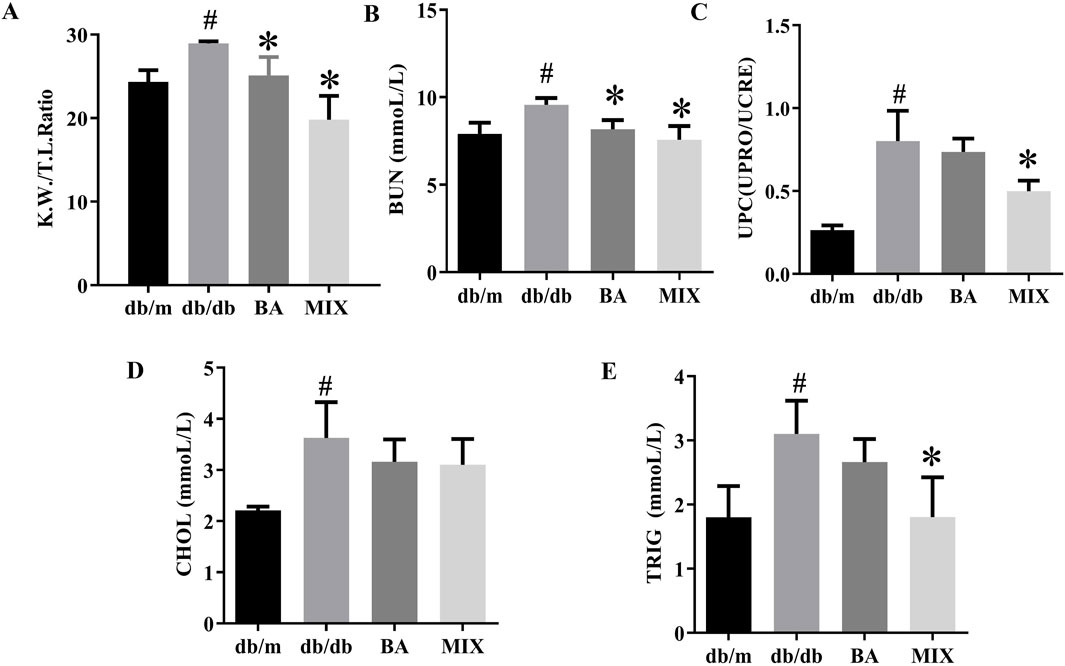
Figure 2. Effect on renal function, and serum biochemical indicators, in each group of type 2 diabetic mice (A) The ratio of renal weight to tibial length. (B) Urea nitrogen. (C) Ratio of urinary protein to urinary creatinine. (D) Cholesterol level. (E) Triglyceride level (n = 6, compared to the db/m group, #p < 0.05; compared to the db/db group, *p < 0.05). Abbreviations: K.W: renal weight, T. I: tibial length, BUN: blood urea nitrogen, UPRO: urinary protein, UCRE: urinary creatinine, CHOL: cholesterol, TRIG: triglyceride, db/m: normal control group of non-diabetic mice, db/db: model of diabetes, BA: baicalin, MIX: multi-component mixture, UPC: ratio of urinary protein to urinary creatinine.
The db/db group showed a substantial increase in BUN levels compared with the db/m group. In contrast, the BA and MIX treatment groups exhibited varying degrees of reduction in BUN levels compared with the db/db group, suggesting improved renal function. Among the treatment groups, the MIX treatment group showed a significant decrease in BUN levels compared with the BA treatment group (Figure 2B).
The urinary protein-to-creatinine ratio was significantly higher in the db/db group than in the db/m group. However, the treatment groups demonstrated varying degrees of reduction in this ratio, indicative of improved renal function. The MIX treatment group exhibited the most pronounced reduction in the urinary protein-to-creatinine ratio, especially when compared to the BA treatment group (Figure 2C). These findings suggest that MIX enhanced renal filtration and alleviated the progression of DKD.
The db/db group demonstrated a significant increase in cholesterol (CHOL) and triglyceride (TRIG) levels compared with the control group. However, the treatment groups showed varying degrees of reduction in TRIG and CHOL levels compared with the db/db group, indicating a beneficial effect. In particular, the MIX treatment group exhibited a significant decrease in TRIG levels compared with the db/db group. The results are shown in Figure 2E.
Effect of baicalin and MIX on renal pathological morphology of db/db mice
HE and PAS staining revealed a well-preserved renal tissue structure in the db/m group, with normal glomerular size and morphology and healthy interstitial space without mesangial proliferation. The nuclear membrane boundaries were clear and showed no abnormalities and the renal tubules maintained their integrity and compact arrangement, with no anomalies detected in the interstitium. In contrast, the db/db group exhibited substantial abnormalities, including extensive strip-shaped blank areas, significant reduction in nuclei, loss of renal tubular shape, noticeable tubular dilation, epithelial cell apoptosis and necrosis, cell loss, and abnormal changes in the renal interstitium. Following the administration of various doses of BA and MIX, pathological improvements were observed to varying degrees, with a reduction in interstitial fibrosis. The BA treatment group showed more pronounced improvement than the MIX treatment group did, indicating greater efficacy in mitigating these pathological changes.
In the db/m group, the boundaries of the kidney cells remained distinct. The glomeruli showed no significant signs of sclerosis, with no collagen production or minimal filamentous deposition. In contrast, in the db/db group, the glomeruli exhibited marked fibrosis, intercellular congestion, substantial accumulation of collagen fibers around the renal tubules, widening of the renal interstitium, a significant increase in collagen fibers, the presence of protein casts, and diffuse distribution throughout the renal interstitium. The areas of collagen deposition and fibrosis were significantly reduced in each treatment group compared to the model group. Only a small number of filamentous fibers were observed in the BA and MIX treatment groups, with the most substantial improvement seen in these groups, as shown in Figure 3.
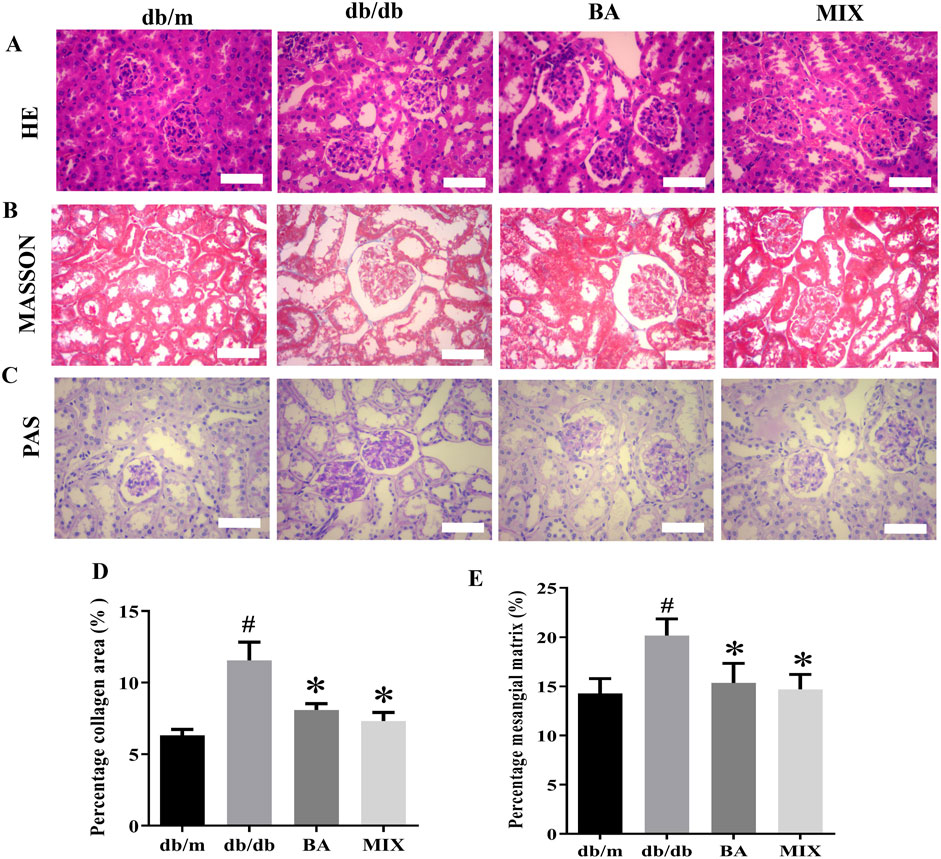
Figure 3. Photomicrographs of kidney sections from each group of mice, stained with hematoxylin and eosin (A) Masson’s trichome (B) and periodic acid-Schiff (C) observed under a light microscope (×400). (D) Changes in the glomerular area are seen with Masson’s trichome staining. (E) The percentage of the mesangial matrix observed with PAS staining (n = 6, compared to the db/m group, #p < 0.05; compared to the db/db group, *p < 0.05). Abbreviations: HE: hematoxylin and eosin, PAS: periodic acid-Schiff, db/m: normal control group of non-diabetic mice, db/db: model of diabetes, BA: baicalin, MIX: multi-component mixture.
Compared to the db/m group, the glomeruli in the db/db group showed noticeable swelling, evident glycogen deposition, prominent extracellular matrix deposition by glomerular mesangial cells, and thickening of the basement membrane. Various degrees of improvement were observed in each treatment group. The BA and MIX treatment groups exhibited reduced glomerular volume, a significant reduction in extracellular matrix deposition by glomerular mesangial cells, decreased thickness of the glomerular mesangial basement membrane, and decreased glycogen deposition. However, these changes were not significant, as shown in Figure 3.
Effect of baicalin and MIX on fibrosis in db/db mice
The Western blot results and data analysis are presented in Figure 4. These results demonstrate that, compared to the db/m group, the model group exhibited a significant increase in the protein expression levels of CTGF, Col-Ⅰ, and Col-ⅠI. In the treatment groups, both BA and MIX demonstrated varying degrees of improvement in CTGF, Col-Ⅰ, and Col-ⅠI levels. Specifically, the BA treatment group showed significantly reduced expression of Col-I protein compared to that of the db/db group. Simultaneously, the MIX treatment group also exhibited a reduction in the expression of Col-I protein, although the difference was not statistically significant. The BA and MIX treatment groups showed significantly decreased expression of Col-I protein compared with that of the model group. Regarding CTGF protein expression, the BA group showed significantly reduced levels compared to those in the db/db group, and the MIX treatment group also showed a reduction, although this was not statistically significant. These results indicated that BA and MIX could effectively ameliorate renal fibrosis in diabetic mice, with BA demonstrating a slightly more pronounced effect.
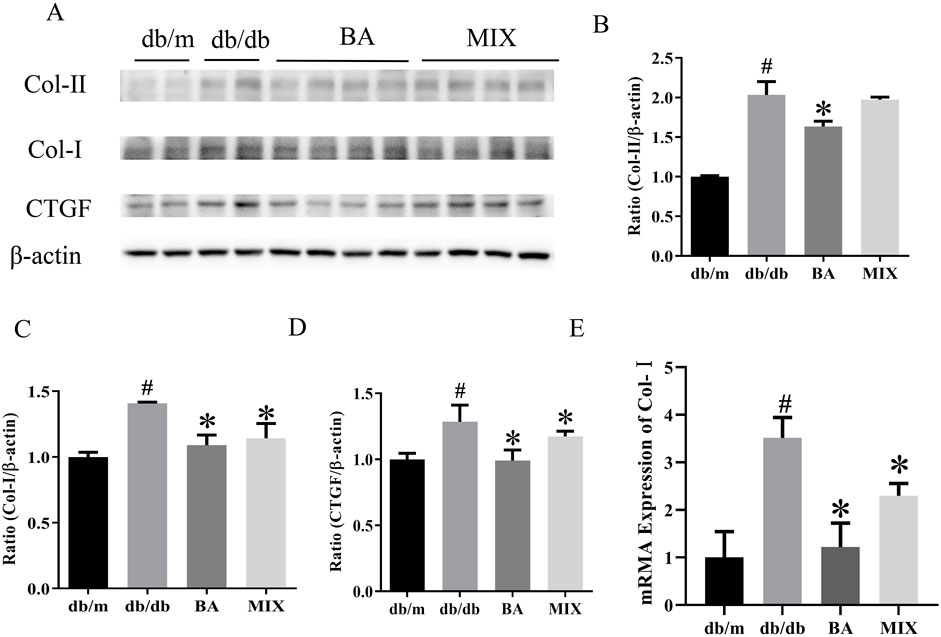
Figure 4. Effects on the expression of fibrotic protein in type 2 diabetic mice (A) Immunoblotting bands depicting collagen-II, collagen-I, and CTGF protein levels in mice in each group. (B) Expression of collagen-II protein in the mice of each group. (C) Expression of collagen-I protein in the mice of each group. (D) Expression of the CTGF protein in the mice of each group. (E) Relative expression of collagen-I mRNA in each group (n = 6, compared to the db/m group, #p < 0.05; compared to the db/db group, *p < 0.05). Abbreviations: HE: hematoxylin and eosin, PAS: periodic acid-Schiff, db/m: normal control group of non-diabetic mice, db/db: model of diabetes, BA: baicalin, MIX: multi-component mixture, Col- (I) collagen I, Col-II: collagen II, CTGF: connective tissue growth factor.
RT-PCR revealed that, compared to the db/m group, Col-Ⅰ mRNA expression levels were markedly elevated in the db/db group, indicating severe fibrosis in the db/db group. In contrast, the BA and MIX treatment groups showed varying degrees of improvement in Col-I gene levels. The BA treatment group exhibited the most pronounced reduction in gene expression. Simultaneously, the MIX treatment group demonstrated a decrease in fibrosis, although not as extensively as that in the BA treatment group.
The findings from the transcriptional to translational levels of fibrotic factors clearly demonstrate a substantial increase in these factors in diabetic organisms. BA emerged as an effective agent for improving diabetes-induced renal fibrosis, thereby slowing the progression of diabetic kidney sclerosis.
Baicalin and MIX inhibited the TGF-β/Smads signaling pathway in the renal tissues of db/db mice
Our study evaluated the expression of TGF-β, Smad2/3, and p-Smad2/3 by Western blots and immunohistochemistry staining (Figure 5). Western blot results and data analysis are presented in Figures 5A–D. These results demonstrate that, compared to the db/m group, the db/db group exhibited a significant increase in protein expression levels of TGF-β, Smad2/3, and p-Smad2/3. In the administration groups, BA and MIX demonstrated varying degrees of improvement in TGF-β, Smad2/3 and p-Smad2/3 protein levels. Specifically, the MIX treatment group significantly reduced the expression of TGF-β, Smad2/3, and p-Smad2/3 proteins compared to that of the db/db group. Concurrently, the BA treatment group also showed a reduction in the expression of TGF-β, Smad2/3, and p-Smad2/3 proteins, although it was not statistically significant. These results indicate that BA and MIX can inhibit the TGF-β/Smads signaling pathway in the renal tissues of db/db mice, with MIX demonstrating a slightly more pronounced effect.
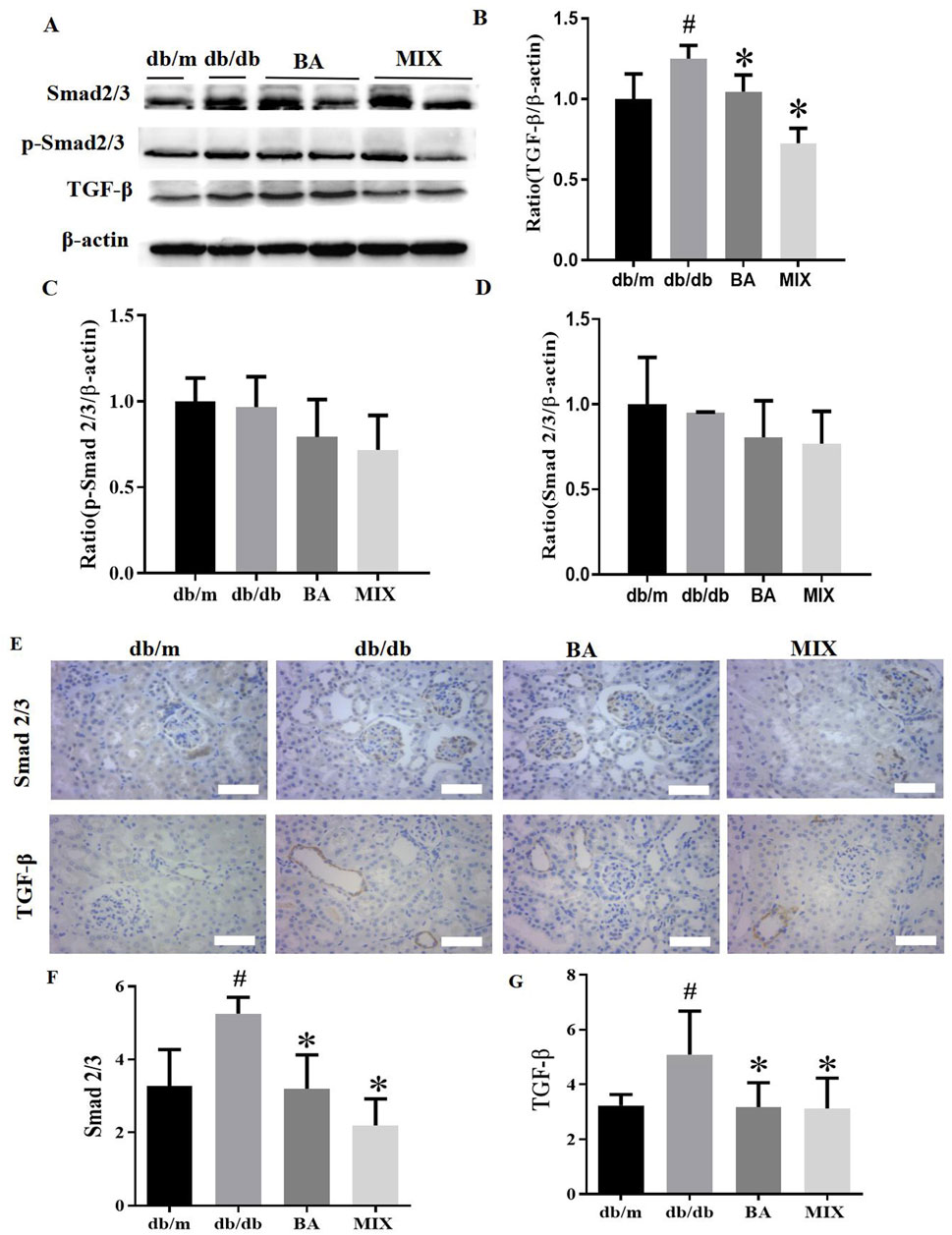
Figure 5. Effects indicating that baicalin and Scutellaria baicalensis mixture can inhibit the TGF-β/Smads signaling pathway in the renal tissues of HG mice (A) Immunoblotting bands depicting TGF-β, Smad2/3, and p- Smad2/3 protein levels in the mice from each group. (B) Expression levels of TGF-β protein in mice from each group. (C) Expression levels of p-Smad2/3 protein in mice from each group. (D) Expression levels of Smad2/3 protein in the mice from each group. (E) Photomicrographs of Smad2/3 and TGF-β staining of the kidney sections from each group, observed under a light microscope (×400). (F) Quantitative statistical plot of Smad2/3 in kidney sections. (G) Quantitative statistical plot of TGF-β in kidney sections (n = 6, compared to the db/m group, #p < 0.05; compared to the db/db group, *p < 0.05). Abbreviations: HE: hematoxylin and eosin, PAS: periodic acid-Schiff, db/m: normal control group of non-diabetic mice, db/db: model of diabetes, BA: baicalin, MIX: multi-component mixture, Col- (I) collagen, I Col-II: collagen II, TGF-β: transforming growth factor-β.
TGF-ß and Smad2/3 were weakly expressed in the kidneys of db/m mice but showed strong expression in the kidneys of db/db mice, with brown staining detected predominantly in glomerular epithelial cells and renal tubules. The levels of TGF-ß and Smad2/3 were significantly reduced in both treatment groups compared to the db/db group, although they remained higher than those in the db/m group (Figures 5E–G). Meanwhile, no significant differences were observed between the BA and MIX treatment groups, indicating that both BA and MIX reduced the abundance of TGF-β and Smad2/3 proteins in the renal tissues of db/db mice.
Discussion
DKD is a chronic microvascular disease caused by sustained hyperglycemia. The incidence of DKD in patients with diabetes exceeds 40% and is a key factor in the development of end-stage renal disease (Chen et al., 2022). Without timely treatment, it can lead to kidney failure, significantly increasing patient disability and mortality rates. With the improvement of living standards in recent years, the incidence and mortality of DKD have been on the rise. Currently, the treatment of DKD mainly involves comprehensive therapies, such as glycemic control, lipid regulation, anti-inflammatory drugs, and calcium channel blockers. However, the treatment outcomes are not entirely satisfactory. Therefore, actively exploring new therapeutic drugs for DKD holds significant importance. DKD in traditional Chinese medicine belongs to diabetes syndrome, the main pathogenesis of which is Qi-yin deficiency, stasis, and turbidity-poison stopping. Scutellaria baicalensis is known for its high content of active components. These components have been shown to reduce mouse body weight, regulate blood lipid metabolism, and significantly improve insulin sensitivity (Miao et al., 2023).
Renal tissue fibrosis is a pathological, morphological, and structural change that occurs in the early stage of DKD and progressively aggravates, which is an important reason for the continuous deterioration of renal function. In studies of the pathogenesis of DKD, researchers have found alterations in cytokine levels in such patients, which can exacerbate renal function loss. Examples include the activation of signaling pathways such as TGF-β, Smad, and p38MAPK.
To investigate the effects of BA and MIX (WGN, BA, and WGS), we used a spontaneous mouse model of type 2 diabetes (db/db) to assess how these interventions impact diabetic kidney disease (DKD) in mice. These components improve glomerular filtration function, reduce the kidney enlargement index, and improve kidney function. They alleviate interstitial fibrosis, reduce glomerular volume, and inhibit the formation of the glomerular mesangial matrix (Hu et al., 2024). Reduces the synthesis of Col-I at both the gene and protein levels. Decreases the production of CTGF, an early marker of fibrosis. TGF-β1 is a universal cytokine in mammalian cells; it can induce the transcription expression and deposition of extracellular matrix genes and accelerate tissue fibrosis. Col-I and Col-II are essential components of the extracellular matrix (Hu et al., 2018; Chen et al., 2021). Multiple signaling pathways regulate the transformation of renal tubular epithelial cells into fibroblasts, with the TGF-β1/Smads pathway playing an important role (Gewin, 2020). The expression of Smads is regulated by TGF-β1, with Smad2 and Smad3 being most closely related to renal fibrosis. TGF-β1 can induce their polymerization (Smad2/3), which shifts to the nucleus and induces excessive expression of the extracellular matrix to promote tissue fibrosis (Budi et al., 2021; Walton et al., 2017).
This study showed that MIX enhanced lipid profiles and renal function by improving renal tubular dilation, restoring the tight arrangement of renal structures, and reducing glomerulosclerosis, basal membrane thickening, and glycogen deposition. These effects were achieved by reducing the protein and gene expressions of Col-II, Col-I, and CTGF. This indicates that inhibiting the TGF-β1/Smad2/3 pathway may be an important molecular mechanism by which MIX inhibits renal fibrosis in db/db mice. The results suggest that BA and MIX play protective roles against DKD. The therapeutic effect of MIX was superior to that of BA. MIX significantly reduced body weight, improved insulin sensitivity, lowered the renal hypertrophy index, and reduced BUN levels and the urine protein/creatinine ratio. MIX inhibited the TGF-β/Smads signaling pathway, thus alleviating renal fibrosis.
In the study, we focused on investigating the effects of BA and MIX, specifically examining the potential of MIX in alleviating fibrosis associated with DKD. BA is one of the primary active flavonoid compounds in S. baicalensis and has demonstrated beneficial effects in reducing fibrosis, inflammation, and oxidative stress in DKD, making it a promising candidate for further development and application. WGN and WGS were a kind of flavonoids, which have many pharmacological effects, such as anti-oxidation, antiinflammatory and anti-fibrosis. WGN treats DKD by in mitigating tubulointerstitial fibrosis and renal tubular cell injury via regulating PI3K/Akt/NF-κB signaling pathway-mediated autophagy and inflammation (Lei et al., 2021), glomerulopathy and podocyte injury by regulating Bcl-2-mediated crosstalk between autophagy and apoptosis (Liu et al., 2022). WGS can inhibit inflammation, oxidative stress regulates renal endothelial injury (Wang YR. et al., 2022). Although the results of the study suggest that WGN and WGS have a certain application in the treatment of DKD. However, the main purpose of this study was to evaluate the effect of MIX combination therapy. And focused on investigating the effects of BA and MIX, specifically examining the potential of MIX in alleviating fibrosis associated with DKD with the aim of advancing the development of MIX.
The value of comparing the individual effects of WGN and WGS with MIX is also recognized (Liu et al., 2022; Wang YR. et al., 2022; Lei et al., 2021), as this would provide insights into potential synergistic effects. Future studies will explore the interactions among BA, WGN, and WGS in more detail. To this end, additional experiments will be conducted to compare WGN and WGS with MIX, in order to better understand each component’s contribution and the overall effectiveness of MIX. It remains unclear how MIX regulates downstream genes and proteins in the TGF-β1/Smad2/3 pathway. Additionally, the impact of MIX on fibrosis in DKD mice involves multiple pathways, requiring further investigation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Medical Ethics Committee of Experimental Animal Ethics at Ruian People’s Hospital. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JL: Writing–review and editing, Methodology, Validation. YZ: Formal Analysis, Methodology, Validation, Writing–original draft. GF: Formal Analysis, Visualization, Writing–original draft. SW: Data curation, Methodology, Validation, Writing–original draft. EY: Formal Analysis, Methodology, Validation, Writing–original draft. JG: Writing–review and editing. CZ: Funding acquisition, Writing–review and editing. SJ: Funding acquisition, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant from the Wenzhou Bureau of Science and Technology (grant number: Y2023936), the Ruian Bureau of Science and Technology (MS2022017), and the National Science Foundation of China (82370368; 82073843).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeshara, K. A., Diwan, A. G., and Tupe, R. S. (2016). Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr. Drug Targets 17, 1309–1328. doi:10.2174/1389450117666151209124007
Aschner, P., Karuranga, S., James, S., Simmons, D., Basit, A., Shaw, J. E., et al. (2021). The International Diabetes Federation's guide for diabetes epidemiological studies. Diabetes Res. Clin. Pract. 172, 108630. doi:10.1016/j.diabres.2020.108630
Budi, E. H., Schaub, J. R., Decaris, M., Turner, S., and Derynck, R. (2021). TGF-β as a driver of fibrosis: physiological roles and therapeutic opportunities. J. Pathol. 254, 358–373. doi:10.1002/path.5680
Chen, X., Sun, L., Li, D., Lai, X., Wen, S., Chen, R., et al. (2022). Green tea peptides ameliorate diabetic nephropathy by inhibiting the TGF-β/Smad signaling pathway in mice. Food Funct. 13, 3258–3270. doi:10.1039/d1fo03615g
Chen, Y., Lin, X., Zheng, Y., Yu, W., Lin, F., and Zhang, J. (2021). Dendrobium mixture ameliorates diabetic nephropathy in db/db mice by regulating the TGF-β1/Smads signaling pathway. Evid. Based Complement. Altern. Med. 2021, 9931983. doi:10.1155/2021/9931983
Du, N., Liu, S., Cui, C., Zhang, M., Jia, J., and Cao, X. (2017). DMP-1 attenuates oxidative stress and inhibits TGF-β activation in rats with diabetic kidney disease. Ren. Fail 39, 229–235. doi:10.1080/0886022X.2016.1256319
Gewin, L. S. (2020). TGF-Β and diabetic nephropathy: lessons learned over the past 20 years. Am. J. Med. Sci. 359, 70–72. doi:10.1016/j.amjms.2019.11.010
Guo, K., Zhang, L., Zhao, F., Lu, J., Pan, P., Yu, H., et al. (2016). Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: cross-sectional study. J. Diabetes Complicat. 30, 803–810. doi:10.1016/j.jdiacomp.2016.03.020
Hu, H., Li, W., Hao, Y., Peng, Z., Zou, Z., and Liang, W. (2024). Baicalin ameliorates renal fibrosis by upregulating CPT1α-mediated fatty acid oxidation in diabetic kidney disease. Phytomedicine 122, 155162. doi:10.1016/j.phymed.2023.155162
Hu, H. H., Chen, D. Q., Wang, Y. N., Feng, Y. L., Cao, G., Vaziri, N. D., et al. (2018). New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 292, 76–83. doi:10.1016/j.cbi.2018.07.008
Hu, L., Ding, M., and He, W. (2021). Emerging therapeutic strategies for attenuating tubular EMT and kidney fibrosis by targeting wnt/β-catenin signaling. Front. Pharmacol. 12:830340. doi:10.3389/fphar.2021.830340
Huang, W., Huang, G. P., Zhang, L. X., Yu, E., Yang, W. K., Ye, M., et al. (2024). Lignan-rich extract from Cinnamomum camphora leaf attenuates metabolic syndrome by modulating glycolipid metabolism and gut microbiota in T2DM mice. Phytomedicine 135, 156118. doi:10.1016/j.phymed.2024.156118
Jin, X., Zhu, L., Lu, S., Li, C., Bai, M., Xu, E., et al. (2023). Baicalin ameliorates CUMS-induced depression-like behaviors through activating AMPK/PGC-1α pathway and enhancing NIX-mediated mitophagy in mice. Eur. J. Pharmacol. 938, 175435. doi:10.1016/j.ejphar.2022.175435
Lai, J., and Li, C. X. (2024). Review on the pharmacological effects and pharmacokinetics of scutellarein. Arch. Pharm. Weinh. 357 (9), e2400053. doi:10.1002/ardp.202400053
Lavoz, C., Rodrigues-Diez, R. R., Plaza, A., Carpio, D., Egido, J., Ruiz-Ortega, M., et al. (2020). VEGFR2 blockade improves renal damage in an experimental model of type 2 diabetic nephropathy. J. Clin. Med. 9, 302. doi:10.3390/jcm9020302
Lei, L., Zhao, J., Liu, X. Q., Chen, J., Qi, X. M., Xia, L. L., et al. (2021). Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by Inhibiting PI3K/Akt/NF-κB signaling pathways. Drug Des. Devel Ther. 15, 3131–3150. doi:10.2147/DDDT.S310882
Li, L., Zhang, X., Li, Z., Zhang, R., Guo, R., Yin, Q., et al. (2017). Renal pathological implications in type 2 diabetes mellitus patients with renal involvement. J. Diabetes Complicat. 31, 114–121. doi:10.1016/j.jdiacomp.2016.10.024
Liao, H., Ye, J., Gao, L., and Liu, Y. (2021). The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: a comprehensive review. for alleviation of inflammatory cytokines: a comprehensive review. Biomed. Pharmacother. 133, 110917. doi:10.1016/j.biopha.2020.110917
Liu, X. Q., Jiang, L., Li, Y. Y., Huang, Y. B., Hu, X. R., Zhu, W., et al. (2022). Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol. Sin. 43, 96–110. doi:10.1038/s41401-021-00721-5
Ma, L., Wu, F., Shao, Q., Chen, G., Xu, L., and Lu, F. (2021). Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des. Devel Ther. 15, 3207–3221. doi:10.2147/DDDT.S319260
Meshkani, R., and Vakili, S. (2016). Tissue resident macrophages: key players in the pathogenesisof type 2 diabetes and its complications. Clin. Chim. Acta 462, 77–89. doi:10.1016/j.cca.2016.08.015
Miao, L., Liu, C., Cheong, M. S., Zhong, R., Tan, Y., Rengasamy, K. R. R., et al. (2023). Exploration of natural flavones’ bioactivity and bioavailability in chronic inflammation induced-type-2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 63, 11640–11667. doi:10.1080/10408398.2022.2095349
Ou, Y., Zhang, W., Chen, S., and Deng, H. (2021). Baicalin improves podocyte injury in rats with diabetic nephropathy by inhibiting PI3K/Akt/mTOR signaling pathway. Open Med. (Wars) 16, 1286–1298. doi:10.1515/med-2021-0335
Qiu, D., Song, S., Chen, N., Bian, Y. W., Zhang, W., Wei, Z., et al. (2023). NQO1 alleviates renal fibrosis by inhibiting the TLR4/NF-κB and TGF-β/Smad signaling pathways in diabetic nephropathy. Cell Signal 108, 110712. doi:10.1016/j.cellsig.2023.110712
Shang, L. Y., Zhou, M. H., Cao, S. Y., Zhang, M., Wang, P. J., Zhang, S., et al. (2023). Effect of polyethylene glycol 400 on the pharmacokinetics and tissue distribution of baicalin by intravenous injection based on the enzyme activity of UGT1A8/1A9. Eur. J. Pharm. Sci. 180, 106328. doi:10.1016/j.ejps.2022.106328
Suo, J. Y. (2022). Screening of anti-inflammatory active ingredients combined with myd88 in qian-jin-wen-Wu decoction and its synergistic effect. Master’s Thesis. Yanbian University.
Tang, F., Hao, Y., Zhang, X., and Qin, J. (2017). Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des. Devel Ther. 11, 2813–2826. doi:10.2147/DDDT.S143805
Walton, K. L., Johnson, K. E., and Harrison, C. A. (2017). Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 8, 461. doi:10.3389/fphar.2017.00461
Wang, H., Jiang, Q., and Zhang, L. (2022a). Baicalin protects against renal interstitial fibrosis in mice by inhibiting the TGF-β/Smad signalling pathway. Pharm. Biol. 60, 1407–1416. doi:10.1080/13880209.2022.2097700
Wang, Y. R., Liu, Z., Liu, G. Y., and Wang, H. J. (2022b). Research progress of active ingredients of Scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 148, 112690. doi:10.1016/j.biopha.2022.112690
Wu, H., Long, X., Yuan, F., Chen, L., Pan, S., Liu, Y., et al. (2014). Combined use of phospholipid complexes and self-emulsifying microemulsions for improving the oral absorption of a BCS class IV compound, baicalin. Acta Pharm. Sin. B 4, 217–226. doi:10.1016/j.apsb.2014.03.002
Yamashita, N., and Kramann, R. (2024). Mechanisms of kidney fibrosis and routes towards therapy. Trends Endocrinol. Metab. 35 (1), 31–48. doi:10.1016/j.tem.2023.09.001
Yang, M., Kan, L., Wu, L., Zhu, Y., and Wang, Q. (2019). Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp. Ther. Med. 3, 2071–2076. doi:10.3892/etm.2019.7181
Zhang, S., Xu, L., Liang, R., Yang, C., and Wang, P. (2020). Baicalin suppresses renal fibrosis through microRNA-124/TLR4/NF-κB axis in streptozotocin-induced diabetic nephropathy mice and high glucose-treated human proximal tubule epithelial cells. J. Physiol. Biochem. 3, 407–416. doi:10.1007/s13105-020-00747-z
Keywords: flavonoids, traditional Chinese medicine, diabetic nephropathy, baicalin, renal fibrosis, insulin sensitivity
Citation: Li J, Zhuang Y, Fan G, Wang S, Yan E, Guo J, Zhang C and Jiang S (2024) Impact of baicalin and components of Scutellaria baicalensis on renal fibrosis of diabetic kidney disease. Front. Pharmacol. 15:1480626. doi: 10.3389/fphar.2024.1480626
Received: 14 August 2024; Accepted: 25 November 2024;
Published: 06 December 2024.
Edited by:
Ochuko Lucky Erukainure, University of the Free State, South AfricaReviewed by:
Gloria O. Izu, Central University of Technology, South AfricaAkingbolabo Daniel Ogunlakin, Bowen University, Nigeria
Copyright © 2024 Li, Zhuang, Fan, Wang, Yan, Guo, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shicui Jiang, c2hpY3VpamlhbmdAd211LmVkdS5jbg==; Chi Zhang, emhhbmdjaGk1MTVAd211LmVkdS5jbg==
†These authors have contributed equally to this work
 Jiarui Li1,2†
Jiarui Li1,2† Shicui Jiang
Shicui Jiang