- 1Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Clinical Pharmacy Center, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 4Department of Pharmacy, Peking Union Medical College Hospital, Beijing, China
- 5State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Beijing, China
- 6Department of Pediatrics, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 7Department of Pharmacy, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 8Department of Hematology, Shenzhen Second People’s Hospital, Shenzhen, China
- 9Department of Pharmacy, Guangzhou Women and Children’s Medical Center, Guangzhou, China
- 10Department of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou, China
- 11Department of Pharmacy, Panyu Central Hospital, Guangzhou, China
- 12Department of Hematology, Panyu Central Hospital, Guangzhou, China
- 13Department of Pharmacy, Guangdong Provincial People’s Hospital, Southern Medical University, Guangzhou, China
- 14Department of Hematology, Huizhou Central People’s Hospital, Huizhou, China
- 15Department of Clinical Pharmacy, General Hospital of Southern Theatre Command of PLA, Guangzhou, China
- 16Department of Pharmacy, Shenzhen Second People’s Hospital, Shenzhen, China
- 17Department of Pharmacy, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 18Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, China
- 19Department of Pharmacy, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 20Pharmacy Department, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 21Department of Pharmacy, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
- 22Department of Pharmacy, First Affiliated Hospital of Kunming Medical University, Kunming, China
- 23Department of Pharmacy, The Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 24Guangdong Pharmaceutical Association, Guangzhou, China
Drug package inserts are a crucial foundation for clinical medication practices and serve as the legal basis for guiding rational drug use and ensuring patient safety and efficacy. As rare disease treatments evolve, current package inserts often need to meet the clinical requirements for treating such conditions, frequently resulting in off-label drug use. This consensus is derived from discussions between Guangdong Pharmaceutical Association Hematologic Rare Diseases Group experts. The consensus aims to provide a framework and reference for the clinical application of off-label drug use in treating rare hematologic diseases.
1 Introduction
Package inserts are crucial for the use of clinical medications and serve as the legal standard that guides clinicians and pharmacists toward rational drug use. Despite rapid advances in diagnosing and treating rare diseases, updates to package inserts lag, leading to widespread off-label drug use in clinical settings. This is often unavoidable due to the unique characteristics of the rare disease patient population. Article 29, Clause 2 of the Chinese “Law on Doctors” states “in the absence of effective or superior treatment methods and under special circumstances, physicians may use drugs not explicitly indicated in the drug instructions but supported by evidence-based medical evidence, with the patient’s informed consent.” (Physician Law on Doctors of the People’s Republic of China, 2021) However, standardized guidelines or expert consensus on off-label use for rare disease patients remain absent. Although the current progress in rare diseases has seen significant advancements, particularly in the areas of gene therapy, immunotherapy, antibody-drug conjugate agents and innovative drugs for rare hematological diseases, there are still obstacles in the accessibility of these drugs or treatments in China. The new use of old drugs still dominates the treatment of rare hematological diseases, however, off-label drug use is common because no updated of the instructions and less clinical trials for rare diseases. To address this, the Guangdong Pharmaceutical Association Rare Disease Expert Committee of the has compiled the “Expert Consensus on Off-Label Use of Drugs for Rare Hematological Diseases (2024 Edition)” (hereafter referred to as the “Consensus”). This document seeks to provide evidence-based guidance for the off-label use of commonly used drugs in the diagnosis and treatment of rare hematologic diseases, standardize related drug use, and improve pharmaceutical supervision and management in the individualized treatment of special populations. The “Consensus” is intended for use by physicians when prescribing and pharmacists when reviewing prescriptions at medical institutions. However, the management of off-label drug use in clinical practice should still adhere to the relevant regulations. Clinicians are urged to assess the benefits and risks of off-label drug use for patients and to avoid such practices whenever satisfactory clinical efficacy can be achieved with standard drug instructions.
2 Materials and methods
2.1 The consensus scope, target professionals, and target patient population
This consensus applies to healthcare institutions at all levels for treating rare hematologic diseases. The target patient population includes individuals with rare hematologic diseases listed in the first Chinese list of rare diseases. The target healthcare professionals include physicians, pharmacists, nurses, other healthcare workers, and policymakers involved in managing rare diseases.
2.2 The methodology of the consensus development
A nominal group technique was used to discuss a specific topic (off-label drug use in treating rare hematologic diseases) in an online conference format organized by an experienced facilitator and attended by 26 relevant experts. The consensus development process and reporting adhered to the World Health Organization Handbook for Guideline Development (2nd edition) (Word Health Organization, 2014), the Statement of Reporting Items for Practice Guidelines in Healthcare (RIGHT) (Chen Y et al., 2017), and the specification of evidence-based pharmaceutical evaluation methods for off-label drug use (T/GDPA 1-2021, 2021, Guangdong Pharmaceutical Association) (The specification of evidence-base pharmaceutical evaluation method for off-label drug use, 2021). Conflicts of interest and disclosures were managed according to the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals of the International Committee of Medical Journal Editors.
2.3 Consensus panels
The group members comprised experts from the following disciplines: hematologic medicine, clinical pharmacy, and pharmaceutical administration. The composition and positions of the panel members are shown in Supplementary Table S1.
2.4 Evidence retrieval and data extraction
The “Consensus” includes drugs commonly used off-label in treating rare hematologic diseases, presented in a tabular format for clarity and organized by therapeutic properties and application fields. The inclusion criteria for these drugs are based on the “2023 Guangdong Pharmaceutical Association Off-Label Drug Use Directory” (Guangdong Pharmaceutical Association, 2023), with adjustments for the unique aspects of rare disease medications: (1) included in package inserts from the United States, Europe, or Japan; (2) listed in the “Chinese Pharmacopoeia Clinical Medication Instructions” or “Clinical Diagnosis and Treatment Guidelines” (published by the Chinese Medical Association and People’s Medical Publishing House); (3) included in leading international and Chinese guidelines or consensus documents; (4) rated by Micromedex® with an efficacy rating and recommendation level IIb or evidence level C or above; and (5) supported by published randomized controlled trial studies in first-quartile (Q1) SCI journals of the relevant field.
When Micromedex® lacks evaluations for certain off-label drugs commonly used in rare diseases, the consensus adopts the Thomson Micromedex® classification system standards to assess the efficacy, recommendation, and evidence levels of the included drugs, as described by the expert drafting group. Details of this classification system are provided in Supplementary Table S1.
2.5 Comprehensive analysis of evidence and compilation of evidence report
After analyzing and summarizing the retrieved data, expert opinions were gathered through meetings and online consultations to supplement the evidence. Finally, the writing team prepared an evidence report, which was reviewed by the consensus conference.
2.6 The process of formulating recommendations
The writing team developed recommendations based on the best available evidence. When direct supportive evidence was insufficient, expert clinical experience was collected online to supplement recommendations. After discussions of expert evidence, the team formulated an initial draft of the expert consensus. The guideline members provided feedback on recommendations and the expert consensus statement, ultimately establishing linguistic consensus.
The “Consensus” is structured in a tabular format to enhance clarity and conciseness. Each entry in the consensus table includes the following elements: “Generic Name,” specifying the drug’s official generic designation; “Dosage Form,” detailing the dosage form such as tablet or injection form; “Off-Label Type,” describing how the drug is used beyond its approved indications; “Off-Label Content,” providing specific details on the conditions or symptoms treated off-label; “Specific Usage,” outlining the dosage and administration details for off-label use; “Evidence and References,” including citations that support off-label use; and “Evidence Level,” indicating the grade or level of evidence supporting the drug’s efficacy and safety for off-label use.
3 Result
3.1 Off-label drug use for hemophilia treatment
Hemophilia, a rare X-linked recessive hereditary bleeding disorder, is categorized into Hemophilia A and Hemophilia B. Hemophilia A results from a deficiency of clotting factor VIII. At the same time, Hemophilia B is due to a deficiency of clotting factor IX, each due to mutations in their respective genes. The prevalence of hemophilia in China is approximately 2.73–3.09 per 100,000 (Xue and Yang, 2022). Primary treatment approaches include plasma-derived or recombinant clotting factor replacement, non-factor replacement, and gene therapy. However, there are no approved indications in the drug package inserts for immune tolerance therapy for Factor VIII inhibitors or the hemostatic treatment of low-titer Factor VIII inhibitors (Xue F, et al., 2023). Supplementary Table S2 presents the expert consensus on off-label drug use in the treatment of hemophilia.
3.2 Off-label drug use for Castleman’s disease treatment
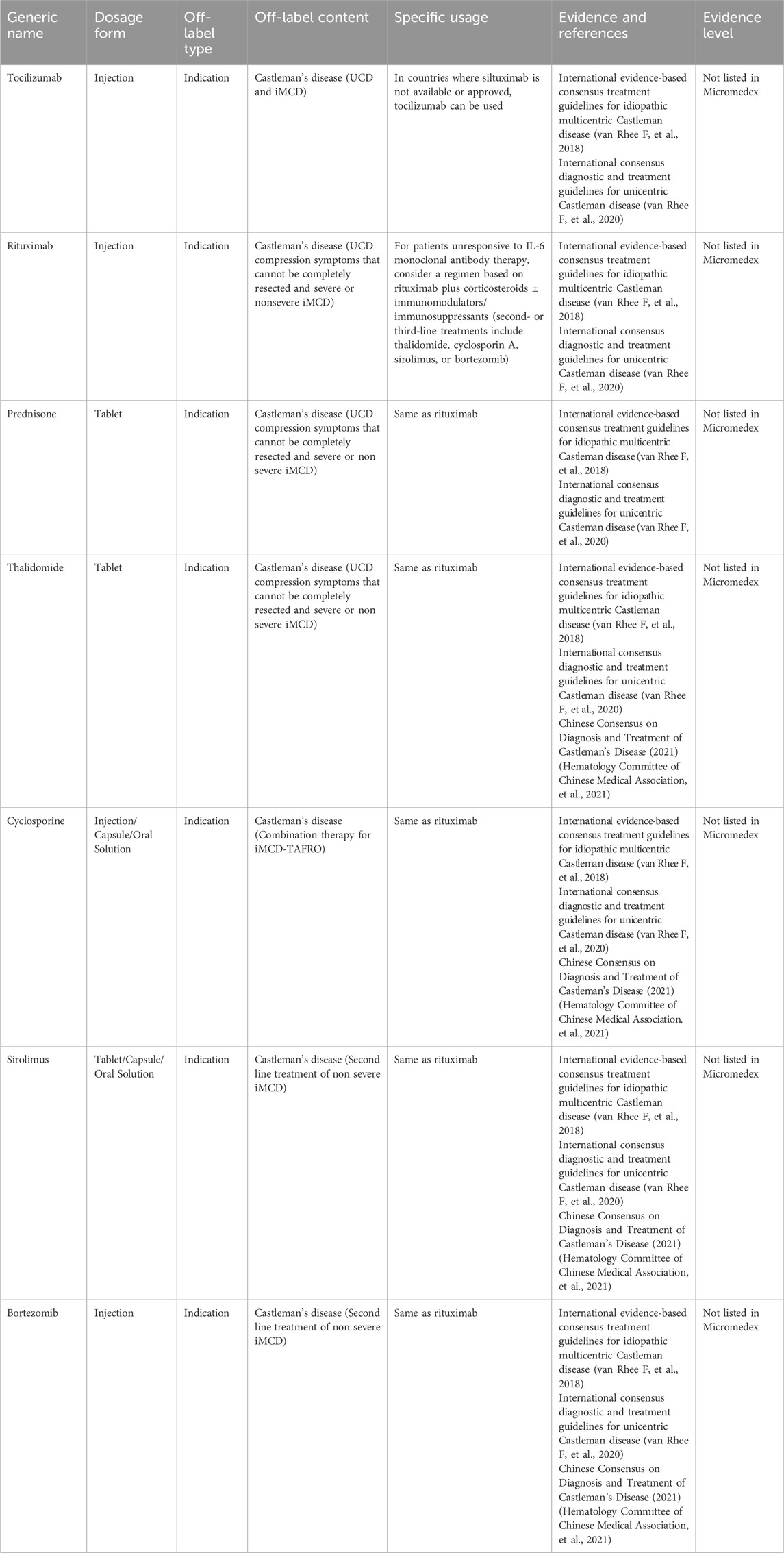
Castleman’s disease (CD), or giant lymph node hyperplasia or angiofollicular lymph node hyperplasia, is a relatively rare lymphoproliferative disorder. The generally accepted pathogenic mechanisms of CD mainly involve cytokine interleukin-6 (IL-6), human herpesvirus 8 (HHV-8), and human immunodeficiency virus (HIV) infection. The incidence rate is approximately 0.2 per 10,000, although no data is available on the incidence of CD in China (Zhang L, et al. 2023). According to current guidelines and consensus, targeting IL-6 is the preferred first-line treatment for newly diagnosed idiopathic multicentric Castleman’s disease (iMCD). However, IL-6 targeted therapy is not universally effective, achieving an efficacy rate of only 34% in randomized controlled trials (RCTs). Siltuximab, the only IL-6-targeted drug approved in China for iMCD, is costly and increases the economic burden on patients. Additionally, drug-targeting pathways beyond IL-6 have yet to receive approval in China. Table 1 presents the expert consensus on off-label drug use to treat Castleman’s disease.
3.3 Off-label drug use for Erdheim-Chester disease treatment
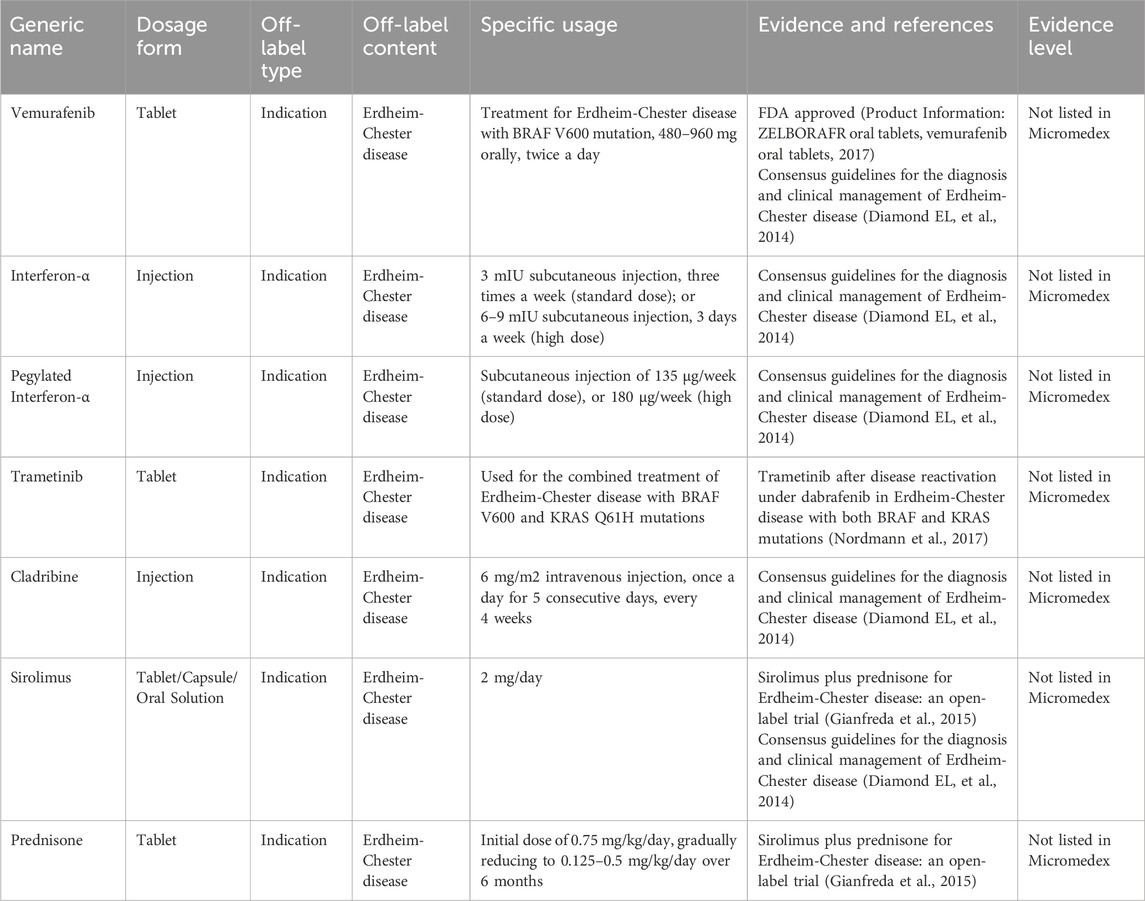
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans cell histiocytosis known as lipid granulomatosis. This disease predominantly affects middle-aged and older individuals, with no marked gender differences in incidence rates. ECD can involve the skeletal system and multiple organs, most frequently affecting the diaphyseal and metaphyseal regions of the long bones, particularly in the lower extremities. Approximately 1,000 cases of ECD have been reported globally, yet comprehensive epidemiological data is lacking in China (Merai H, et al., 2020). There are no RCTs investigating treatments for ECD. Glucocorticoids and immunosuppressants have shown effectiveness in some cases. Additionally, BRAF inhibitors are applicable to patients with BRAF mutations, and other kinase inhibitors have also demonstrated efficacy in patients without such mutations. Interferon-alpha is commonly used to treat this condition, and recent studies highlight the potential of TNF-alpha antagonists, IL-6 antagonists, and IL-1 antagonists (Haroche J, et al., 2020). However, these therapeutic agents have not received approval for these specific indications. Table 2 presents the expert consensus on the off-label use of these drugs to treat ECD.
3.4 Off-label drug use for fanconi anemia treatment
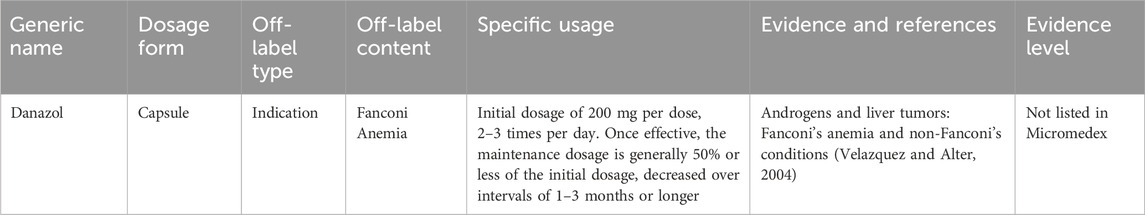
Fanconi anemia is a rare genetic blood disorder that predominantly manifests in childhood, with an incidence rate of approximately 1 in 136,000 (Che R, et al., 2018). Androgens have been found to improve blood cell counts in approximately 50% of affected patients. However, androgen treatments currently available in China lack approval for this specific therapeutic indication. Table 3 presents the expert consensus on the off-label use of androgen medications for Fanconi anemia.
3.5 Off-label drug use for langerhans cell histiocytosis treatment
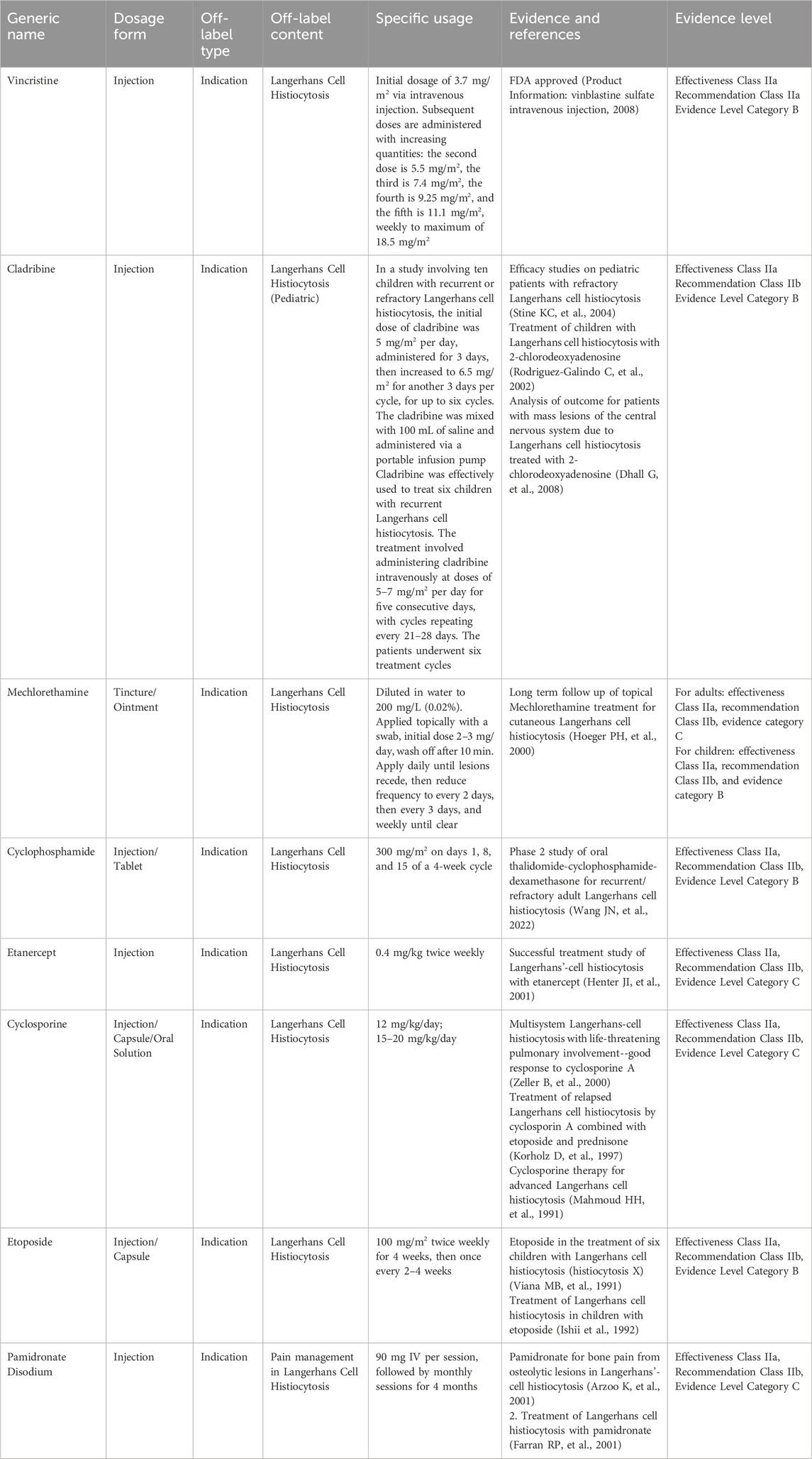
Langerhans cell histiocytosis (LCH) is a group of histiocyte proliferative disorders characterized by an unknown etiology. It is traditionally categorized into three clinical types: Letterer-Siwe disease (L-S disease), Hand-Schüller-Christian disease (H-S-C disease), and eosinophilic granuloma (EGB), each marked by the pathological proliferation of Langerhans cells. The incidence rate among children is approximately 3–5 per million, whereas in adults, it is less than 1 to 2 cases per million (Baumgartner I, et al., 1997). There are no widely accepted treatment recommendations for adult patients. Recent advances in chemotherapy have significantly improved the prognosis of this disease. However, these chemotherapeutic agents have not received approval for the indication of LCH in China (Dai and Cao, 2023). Table 4 presents the expert consensus on the off-label use of drugs for LCH.
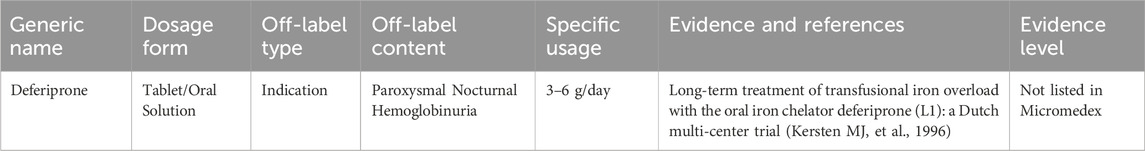
3.6 Off-label drug use for paroxysmal nocturnal hemoglobinuria treatment
Paroxysmal nocturnal hemoglobinuria (PNH) is a disorder resulting from acquired mutations in the PIG-A gene of hematopoietic stem cells, which leads to increased sensitivity of blood cells to complement. This increased sensitivity causes intravascular hemolysis, thrombosis, and bone marrow failure. The annual global incidence rate of PNH is approximately 1–10 per million (Brodsky RA, 2014). The primary treatment for symptomatic management currently includes complement C5 inhibitors, such as eculizumab. However, there is still off-label use of other medications for the symptomatic treatment of PNH. Table 5 shows the expert consensus on the off-label drug use for PNH.
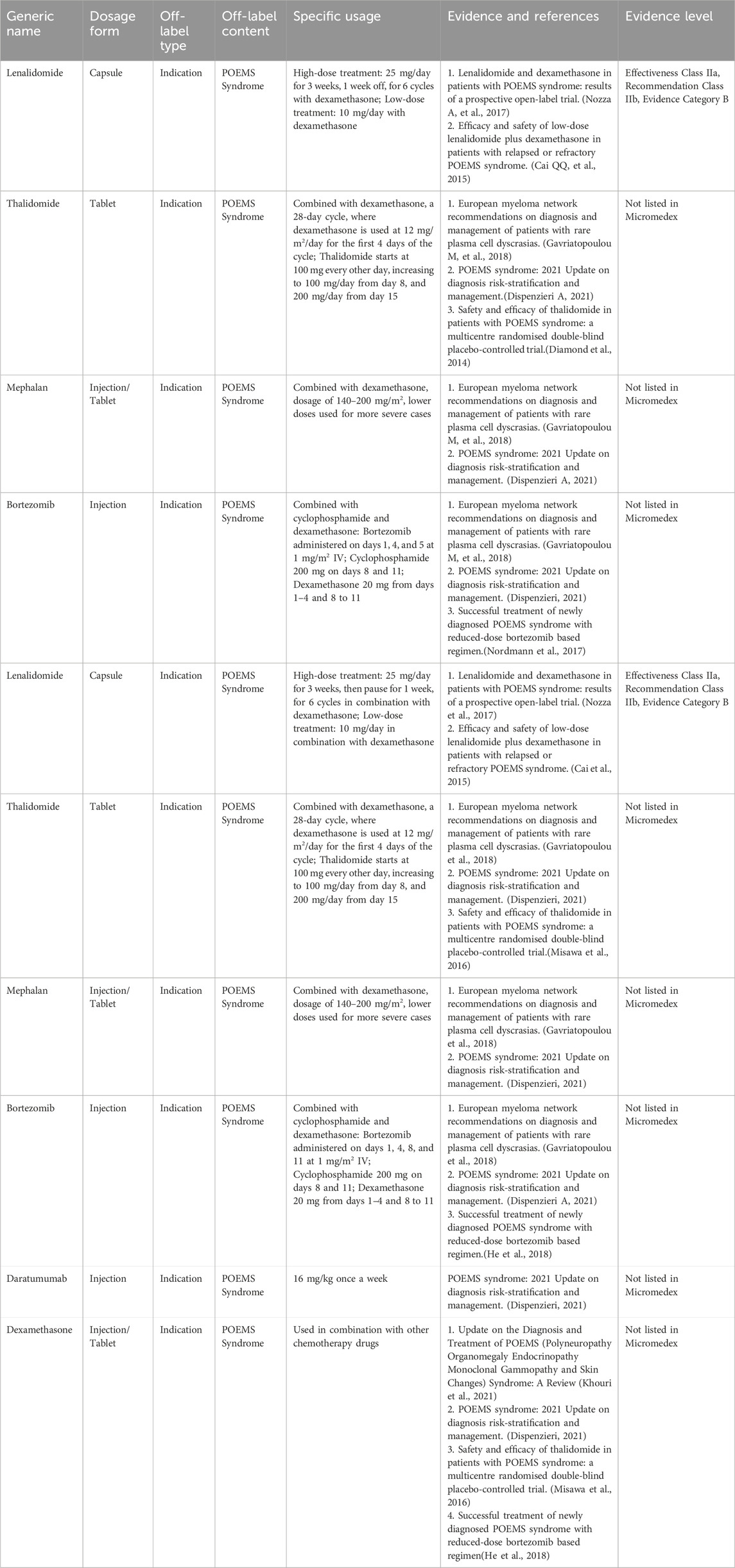
3.7 Off-label drug use for POEMS syndrome
POEMS syndrome is a multisystem disorder associated with plasma cell disease, characterized clinically by polyneuropathy, organomegaly, endocrinopathy, monoclonal proteinemia, and skin changes. The prevalence rate is estimated to be approximately 0.3 per 100,000 (Soubrier MJ, et al., 1994; Li J, et al., 2011; Kulkarni et al., 2011), and as a group of clinical disorders attributable to plasma cell malignancies. To date, no RCTs have been reported for the treatment of POEMS syndrome, with treatment recommendations derived primarily from case reports. Table 6 presents the expert consensus on the off-label use of drugs to treat POEMS syndrome.
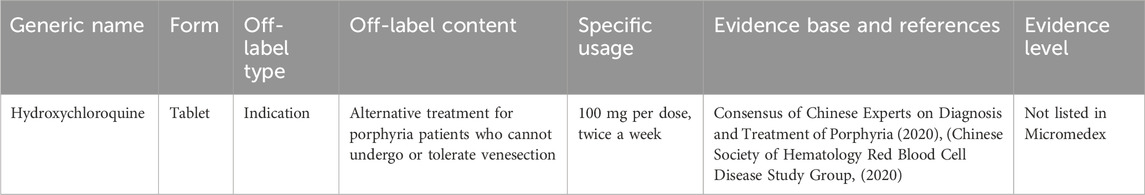
3.8 Off-label drug use for treating porphyria
Porphyria includes a group of metabolic disorders resulting from abnormalities in the production and excretion of porphyrins, often influenced by genetic factors. In adults, the most common types are porphyria cutanea tarda, acute intermittent porphyria, and erythropoietic protoporphyria, with incidence rates varying between the different types. There is no epidemiological data available on the prevalence of these disorders in China (Chinese Society of HematologyRed Blood Cell Disease Study Group, 2020). Table 7 presents the expert consensus on the off-label use of drugs to treat porphyria.
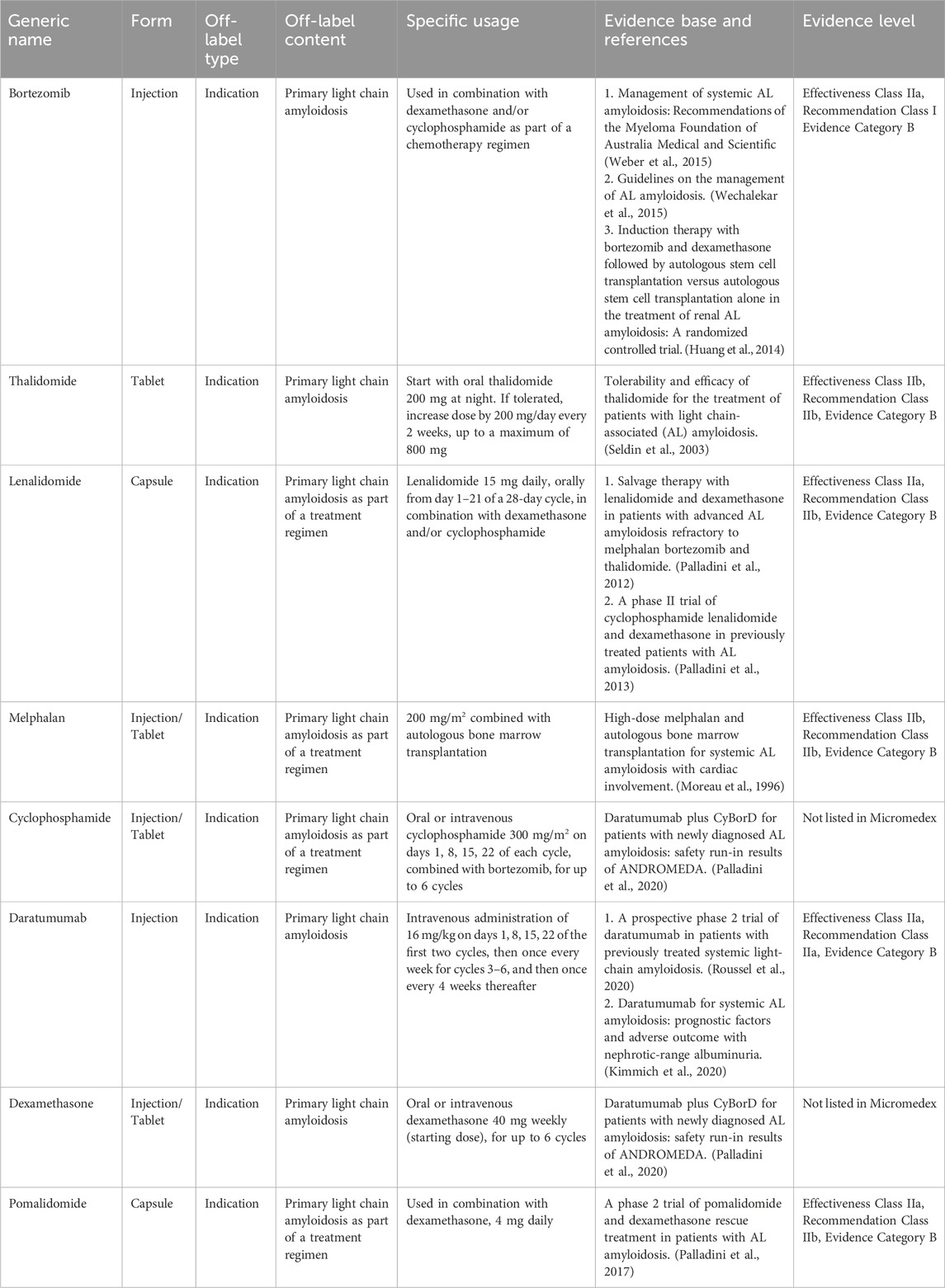
3.9 Off-label drug use for treating primary light Chain Amyloidosis
Primary light chain amyloidosis is a systemic disease characterized by the deposition of monoclonal immunoglobulin light chains, which possess an antiparallel β-sheet structure in organ tissues, leading to organ dysfunction. The annual incidence rate is estimated to be between 3 and 5 per 1,000,000 people (Kyle RA, et al., 1992), with a higher prevalence observed in males than in females. The clinical manifestations are varied and may include foamy urine, shortness of breath following activity, edema, and discomfort in the liver area. Treatment options include hematopoietic stem cell transplantation, chemotherapy, and supportive care. Chemotherapy regimens commonly use agents such as bortezomib, melphalan, and immunomodulatory drugs (Hematology Oncology Committee of China Anti-Cancer Association, Leukemia & Lymphoma Group Society of Hematology at Chinese Medical Association, 2016). However, these therapeutic drugs have not yet received approval for these specific indications in China. Table 8 presents the expert consensus on the off-label use of drugs for primary light-chain amyloidosis.
3.10 Off-label drug use for treating sickle cell anemia
Sickle cell anemia is a hereditary hemoglobinopathy characterized by substituting valine for glutamic acid at the sixth position of the β-globin chain, leading to the formation of sickle hemoglobin, which replaces normal hemoglobin. Clinically, it is associated with chronic hemolytic anemia, increased susceptibility to infections, and recurrent pain crises that cause chronic local ischemia and subsequent organ and tissue damage (Payne et al., 2020; Rees DC, et al., 2010). In China, the incidence of sickle cell anemia is relatively low, and specific prevalence data are not yet available. Treatment primarily involves blood transfusions and symptomatic drug therapy. The use of hydroxyurea and L-glutamine is currently off-label. Table 9 presents the expert consensus on the off-label use of drugs for sickle cell anemia.
3.11 Off-label drug use for treating eczema, thrombocytopenia, and immunodeficiency syndrome (Wiskott-Aldrich syndrome)
Wiskott-Aldrich syndrome (WAS) is a rare X-linked recessive genetic disorder characterized by eczema, thrombocytopenia, and immune deficiency, accompanied by an increased risk of autoimmune diseases and malignant tumors. Clinically, WAS is relatively rare, with an estimated annual incidence rate ranging from 1 to 10 in 1,000,000 males and rarer in females (Massaad MJ, et al., 2013; Blundell MP, et al., 2010). Allogeneic hematopoietic stem cell transplantation is the only recognized effective treatment for WAS. Other symptomatic treatments used to manage the condition lack approved indications specifically for WAS. Table 10 presents the expert consensus on the off-label use of drugs for AWS.
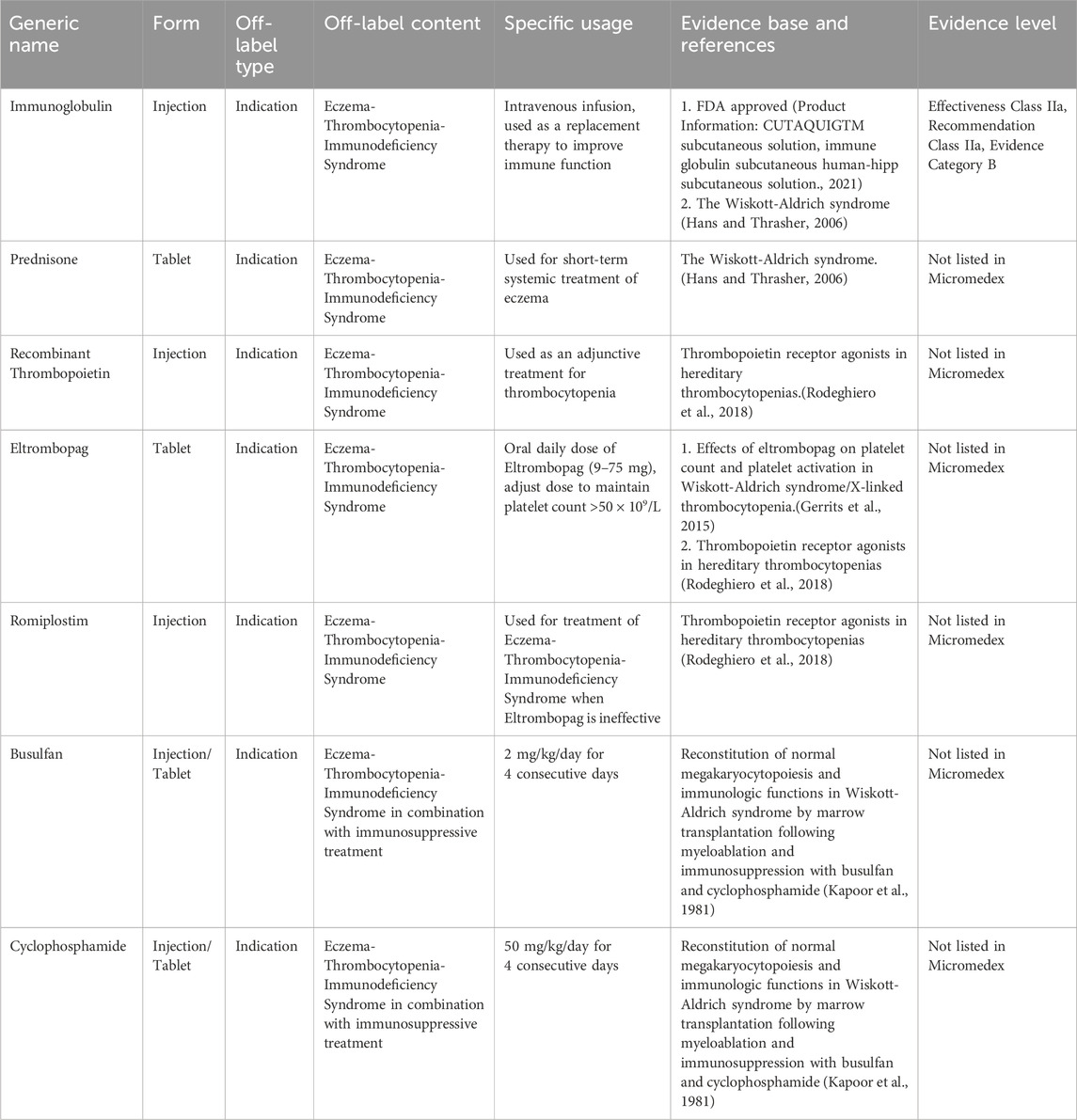
Table 10. Off-label drug usage catalog for treating eczema, thrombocytopenia, and immunodeficiency syndrome.
4 Conclusion
This consensus standardizes the management of off-label drug use for rare hematologic diseases, helping medical institutions develop lists of off-label drugs for rare diseases, promoting rational drug use, and addressing the diagnostic and treatment needs of patients with rare diseases. It also contributes to exploring and establishing an evaluation and management system for off-label drug use in rare diseases.
Author contributions
BxZ: Writing–review and editing, Writing–original draft. XZ: Writing–original draft, Investigation. PZ: Writing–review and editing, Methodology, Investigation. BZ: Writing–review and editing. XF: Writing–review and editing. JC: Writing–review and editing. LC: Writing–review and editing. YC: Writing–review and editing. LH: Writing–review and editing. JaS: Writing–review and editing. SC: Writing–review and editing. YZ: Writing–review and editing. GL: Writing–review and editing. BJ: Writing–review and editing. JW: Writing–review and editing. WF: Writing–review and editing. ML: Writing–review and editing. YJ: Writing–review and editing. TL: Writing–review and editing. XM: Writing–review and editing. JW: Writing–review and editing. HW: Writing–review and editing. HZ: Writing–review and editing. ZcZ: Writing–review and editing. ZhZ: Writing–review and editing, Project administration, Methodology. JnS: Writing–review and editing, Project administration. YL: Writing–review and editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1477550/full#supplementary-material
References
Arzoo, K., Sadeghi, S., and Pullarkat, V. (2001). Pamidronate for bone pain from osteolytic lesions in Langerhans'-cell histiocytosis. N. Engl. J. Med. 345 (3), 225. doi:10.1056/NEJM200107193450318
Baumgartner, I., Hochstetter, A., Baumert, B., Luetolf, U., and Follath, F. (1997). Langerhans'-cell histiocytosis in adults. Med. Pediatr. Oncol. 28 (1), 9–14. doi:10.1002/(sici)1096-911x(199701)28:1<9::aid-mpo3>3.0.co;2-p
Blundell, M. P., Worth, A., Bouma, G., and Thrasher, A. J. (2010). The Wiskott-Aldrich syndrome: the actin cytoskeleton and immune cell function. Dis. Markers 29 (3-4), 157–175. doi:10.3233/DMA-2010-0735
Brodsky, R. A. (2014). Paroxysmal nocturnal hemoglobinuria. Blood 124 (18), 2804–2811. doi:10.1182/blood-2014-02-522128
Cai, Q. Q., Wang, C., Cao, X. X., Cai, H., Zhou, D. B., and Li, J. (2015). Efficacy and safety of low-dose lenalidomide plus dexamethasone in patients with relapsed or refractory POEMS syndrome. Eur. J. Haematol. 95 (4), 325–330. doi:10.1111/ejh.12492
Che, R., Zhang, J., Nepal, M., Han, B., and Fei, P. (2018). Multifaceted Fanconi anemia signaling. Trends Genet. 34 (3), 171–183. doi:10.1016/j.tig.2017.11.006
Chen, Y., Yang, K., Marušic, A., Qaseem, A., Meerpohl, J. J., Flottorp, S., et al. (2017). A reporting tool for practice guidelines in Health care: the RIGHT statement. Ann. Intern. Med. 166, 128–132. doi:10.7326/M16-1565
Chinese Society of Hematology, Red Blood Cell Disease (Anemia) Study Group (2020). Expert consensus on the diagnosis and treatment of porphyria in China. Chin. Med. J. 100 (14), 1051–1056. doi:10.3760/cma.j.cn112137-20200219-00349
Dai, J., and Cao, X. (2023). Research progress in the treatment of adult Langerhans cell histiocytosis. Chin. J. Intern. Med. 62 (1), 97–102. doi:10.3760/cma.j.cn112138-20220106-00014
Dhall, G., Finlay, J. L., Dunkel, I. J., Ettinger, L. J., Kellie, S. J., Allen, J. C., et al. (2008). Analysis of outcome for patients with mass lesions of the central nervous system due to Langerhans cell histiocytosis treated with 2-chlorodeoxyadenosine. Pediatr. Blood Cancer 50 (1), 72–79. doi:10.1002/pbc.21225
Diamond, E. L., Dagna, L., Hyman, D. M., Cavalli, G., Janku, F., Estrada-Veras, J., et al. (2014). Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 124 (4), 483–492. doi:10.1182/blood-2014-03-561381
Dispenzieri, A. (2021). POEMS syndrome: 2021 Update on diagnosis, risk-stratification, and management. Am. J. Hematol. 96 (7), 872–888. doi:10.1002/ajh.26240
Farran, R. P., Zaretski, E., and Egeler, R. M. (2001). Treatment of Langerhans cell histiocytosis with pamidronate. J. Pediatr. Hematol. Oncol. 23 (1), 54–56. doi:10.1097/00043426-200101000-00013
Gavriatopoulou, M., Musto, P., Caers, J., Merlini, G., Kastritis, E., van de Donk, N., et al. (2018). European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia 32 (9), 1883–1898. doi:10.1038/s41375-018-0209-7
Gerrits, A. J., Leven, E. A., Frelinger, A. L. 3rd, Brigstocke, S. L., Berny-Lang, M. A., Mitchell, W. B., et al. (2015). Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood 126 (11), 1367–1378. doi:10.1182/blood-2014-09-602573
Gianfreda, D., Nicastro, M., Galetti, M., Alberici, F., Corradi, D., Becchi, G., et al. (2015). Sirolimus plus prednisone for Erdheim-Chester disease: an open-label trial. Blood 126 (10), 1163–1171. doi:10.1182/blood-2015-01-620377
Guangdong Pharmaceutical Association (2023). Notice on the release of the Off-Label drug use catalog. Available at: http://www.sinopharmacy.com.cn/notification/2797.html (Guangdong Pharmaceutical Association [2023] No. 72). Guangzhou: Guangdong Pharmaceutical Association, 2023-07-04 [accessed on 2023-07-04].
Hans, D. O., and Thrasher, A. J. (2006). The Wiskott-Aldrich syndrome. J. Allergy Clin. Immunol. 117 (4), 725–738. quiz 739. doi:10.1016/j.jaci.2006.02.005
Haroche, J., Cohen-Aubart, F., and Amoura, Z. (2020). Erdheim-Chester disease. Blood 135 (16), 1311–1318. doi:10.1182/blood.2019002766
He, H., Fu, W., Du, J., Jiang, H., and Hou, J. (2018). Successful treatment of newly diagnosed POEMS syndrome with reduced-dose bortezomib based regimen. Br. J. Haematol. 181 (1), 126–128. doi:10.1111/bjh.14497
Hematology Committee of Chinese Medical Association, Hematological Oncology Committee of China Anti-Cancer Association, China Castleman Disease Network (CCDN) (2021). The consensus of the diagnosis and treatment of Castleman disease in China (2021). Chin. J. Hematol. 42 (7), 529–534. doi:10.3760/cma.j.issn.0253-2727.2021.07.001
Hematology Oncology Committee of China Anti-Cancer Association, Leukemia & Lymphoma Group Society of Hematology at Chinese Medical Association (2016). The consensus of the diagnosis and treatment of primary light chain amyloidosis in China (2016 version). Chin. J. Hematol. 37 (9), 742–746. doi:10.3760/cma.j.issn.0253-2727.2016.09.003
Henter, J. I., Karlén, J., Calming, U., Bernstrand, C., Andersson, U., and Fadeel, B. (2001). Successful treatment of Langerhans'-cell histiocytosis with etanercept. N. Engl. J. Med. 345 (21), 1577–1578. doi:10.1056/NEJM200111223452118
Hoeger, P. H., Nanduri, V. R., Harper, J. I., Atherton, D. A., and Pritchard, J. (2000). Long term follow up of topical mustine treatment for cutaneous Langerhans cell histiocytosis. Arch. Dis. Child. 82, 483–487. doi:10.1136/adc.82.6.483
Huang, X., Wang, Q., Chen, W., Zeng, C., Chen, Z., Gong, D., et al. (2014). Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med. 12 (2), 2. doi:10.1186/1741-7015-12-2
Ishii, E., Matsuzaki, A., Okamura, J., Inoue, T., Kajiwara, M., Uozumi, T., et al. (1992). Treatment of Langerhans cell histiocytosis in children with etoposide. Am. J. Clin. Oncol. 15, 515–517. doi:10.1097/00000421-199212000-00011
Kapoor, N., Kirkpatrick, D., Blaese, R. M., Oleske, J., Hilgartner, M. H., Chaganti, R. S., et al. (1981). Reconstitution of normal megakaryocytopoiesis and immunologic functions in Wiskott-Aldrich syndrome by marrow transplantation following myeloablation and immunosuppression with busulfan and cyclophosphamide. Blood 57 (4), 692–696. doi:10.1182/blood.v57.4.692.692
Kersten, M. J., Lange, R., Smeets, M. E., Vreugdenhil, G., Roozendaal, K. J., Lameijer, W., et al. (1996). Long-term treatment of transfusional iron overload with the oral iron chelator deferiprone (L1): a Dutch multi-center trial. Ann. Hematol. 73, 247–252. doi:10.1007/s002770050236
Khouri, J., Nakashima, M., and Wong, S. (2021). Update on the diagnosis and treatment of POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome: a review. JAMA Oncol. 7 (9), 1383–1391. doi:10.1001/jamaoncol.2021.0586
Kimmich, C. R., Terzer, T., Benner, A., Dittrich, T., Veelken, K., Carpinteiro, A., et al. (2020). Daratumumab for systemic AL amyloidosis: prognostic factors and adverse outcome with nephrotic-range albuminuria. Blood 135 (18), 1517–1530. doi:10.1182/blood.2019003633
Korholz, D., Janben, G., and Gobel, U. (1997). Treatment of relapsed Langerhans cell histiocytosis by cyclosporin A combined with etoposide and prednisone. Pediatr. Hematol. Oncol. 14, 443–449. doi:10.3109/08880019709028774
Kulkarni, G. B., Mahadevan, A., Taly, A. B., Yasha, T. C., Seshagiri, K. S., Nalini, A., et al. (2011). Clinicopathological profile of polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes (POEMS) syndrome. J. Clin. Neurosci. 18 (3), 356–360. doi:10.1016/j.jocn.2010.07.124
Kyle, R. A., Linos, A., Beard, C. M., Linke, R. P., Gertz, M. A., O'Fallon, W. M., et al. (1992). Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989 [see comments]. Blood 79 (7), 1817–1822. doi:10.1182/blood.v79.7.1817.1817
Li, J., Zhou, D. B., Huang, Z., Jiao, L., Duan, M. H., Zhang, W., et al. (2011). Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann. Hematol. 90 (7), 819–826. doi:10.1007/s00277-010-1149-0
Mahmoud, H. H., Wang, W. C., and Murphy, S. B. (1991). Cyclosporine therapy for advanced Langerhans cell histiocytosis [see comments]. Blood 77, 721–725. doi:10.1182/blood.v77.4.721.bloodjournal774721
Massaad, M. J., Ramesh, N., and Geha, R. S. (2013). Wiskott-Aldrich syndrome: a comprehensive review. Ann. N. Y. Acad. Sci. 1285, 26–43. doi:10.1111/nyas.12049
Merai, H., Collas, D., Bhagat, A., and Mandalia, U. (2020). Erdheim-Chester disease: a case report and review of the literature. J. Clin. Imaging Sci. 10, 37. doi:10.25259/JCIS_68_2020
Misawa, S., Sato, Y., Katayama, K., Nagashima, K., Aoyagi, R., Sekiguchi, Y., et al. (2016). Safety and efficacy of thalidomide in patients with POEMS syndrome: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 15 (11), 1129–1137. doi:10.1016/S1474-4422(16)30157-0
Moreau, P., Milpied, N., de Faucal, P., Petit, T., Herbouiller, P., Bataille, R., et al. (1996). High-dose melphalan and autologous bone marrow transplantation for systemic AL amyloidosis with cardiac involvement [letter]. Blood 87, 3063–3064. doi:10.1182/blood.v87.7.3063.bloodjournal8773063
Nordmann, T. M., Juengling, F. D., Recher, M., Berger, C. T., Kalbermatten, D., Wicki, A., et al. (2017). Trametinib after disease reactivation under dabrafenib in Erdheim-Chester disease with both BRAF and KRAS mutations. Blood 129 (7), 879–882. doi:10.1182/blood-2016-09-740217
Nozza, A., Terenghi, F., Gallia, F., Adami, F., Briani, C., Merlini, G., et al. (2017). Lenalidomide and dexamethasone in patients with POEMS syndrome: results of a prospective, open-label trial. Br. J. Haematol. 179 (5), 748–755. doi:10.1111/bjh.14966
Palladini, G., Kastritis, E., Maurer, M. S., Zonder, J., Minnema, M. C., Wechalekar, A. D., et al. (2020). Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood 136 (1), 71–80. doi:10.1182/blood.2019004460
Palladini, G., Milani, P., Foli, A., Basset, M., Russo, F., Perlini, S., et al. (2017). A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood 129 (15), 2120–2123. doi:10.1182/blood-2016-12-756528
Palladini, G., Russo, P., Foli, A., Milani, P., Lavatelli, F., Obici, L., et al. (2012). Salvage therapy with lenalidomide and dexamethasone in patients with advanced AL amyloidosis refractory to melphalan, bortezomib, and thalidomide. Ann. Hematol. 91 (1), 89–92. doi:10.1007/s00277-011-1244-x
Palladini, G., Russo, P., Milani, P., Foli, A., Lavatelli, F., Nuvolone, M., et al. (2013). A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica 98 (3), 433–436. doi:10.3324/haematol.2012.073593
Payne, A. B., Mehal, J. M., Chapman, C., Haberling, D. L., Richardson, L. C., Bean, C. J., et al. (2020). Trends in sickle cell disease-related mortality in the United States, 1979 to 2017. Ann. Emerg. Med. 76 (3S), S28-S36–S36. doi:10.1016/j.annemergmed.2020.08.009
Physician Law on Doctors of the People’s Republic of China (2021). The national People’s congress of the People’s Republic of China. Available at: http://www.npc.gov.cn/npc/c2/c30834/202108/t20210820_313104.html.
Product Information: HEMLIBRA(R) subcutaneous injection, emicizumab-kxwh subcutaneous injection (2021). South San Francisco, CA: Genentech Inc per FDA.
Product Information: CUTAQUIG(TM) subcutaneous solution (2021). Immune globulin subcutaneous (human)-hipp subcutaneous solution. Pfizer Labs (per FDA). N. Y. Available at: https://www.fda.gov/media/119234/download.
Product Information: CYKLOKAPRON(R) intravenous injection (2020). Tranexamic acid intravenous injection. New York, NY: Pharmacia & Upjohn Company per FDA.
Product Information: DROXIA(R) oral capsules (2015). Hydroxyurea oral capsules. Princeton, NJ: Bristol-Myers Squibb Company per FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-hydroxyurea-treatment-pediatric-patients-sickle-cell-anemia.
Product Information: ENDARI(TM) oral powder (2017). L-glutamine oral powder. Torrance, CA: Emmaus Medical, Inc per FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approved-l-glutamine-powder-treatment-sickle-cell-disease.
Product Information: vinblastine sulfate intravenous injection, vinblastine sulfate intravenous injection (2008). APP pharmaceuticals. Schaumburg, IL: LLC.
Product Information: ZELBORAF(R) oral tablets, vemurafenib oral tablets (2017). South San Francisco, CA: Genentech USA, Inc per FDA. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202429-s012lbl.pdf.
Rees, D. C., Williams, T. N., and Gladwin, M. T. (2010). Sickle-cell disease. Lancet 376 (9757), 2018–2031. doi:10.1016/S0140-6736(10)61029-X
Rodeghiero, F., Pecci, A., and Balduini, C. L. (2018). Thrombopoietin receptor agonists in hereditary thrombocytopenias. J. Thromb. Haemost. 16 (9), 1700–1710. doi:10.1111/jth.14217
Rodriguez-Galindo, C., Kelly, P., Jeng, M., Presbury, G. G., Rieman, M., and Wang, W. (2002). Treatment of children with Langerhans cell histiocytosis with 2-chlorodeoxyadenosine. Am. J. Hematol. 69, 179–184. doi:10.1002/ajh.10053
Roussel, M., Merlini, G., Chevret, S., Arnulf, B., Stoppa, A. M., Perrot, A., et al. (2020). A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood 135 (18), 1531–1540. doi:10.1182/blood.2019004369
Seldin, D. C., Choufani, E. B., Dember, L. M., Wiesman, J. F., Berk, J. L., Falk, R. H., et al. (2003). Tolerability and efficacy of thalidomide for the treatment of patients with light chain-associated (AL) amyloidosis. Clin. Lymphoma 3 (4), 241–246. doi:10.3816/clm.2003.n.005
Soubrier, M. J., Dubost, J. J., and Sauvezie, B. J. (1994). POEMS syndrome: a study of 25 cases and a review of the literature. French Study Group on POEMS syndrome. Am. J. Med. 97 (6), 543–553. doi:10.1016/0002-9343(94)90350-6
Stine, K. C., Saylors, R. L., Saccente, S., McClain, K. L., and Becton, D. L. (2004). Efficacy of continuous infusion 2-CDA (cladribine) in pediatric patients with Langerhans cell histiocytosis. Pediatr. Blood Cancer 43 (1), 81–84. doi:10.1002/pbc.20053
T/GDPA 1-2021, GuangDong Pharmaceutical Association. (2021) The specification of evidence-base pharmaceutical evaluation method for off-label drug use.
van Rhee, F., Oksenhendler, E., Srkalovic, G., Voorhees, P., Lim, M., Dispenzieri, A., et al. (2020). International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. 4 (23), 6039–6050. doi:10.1182/bloodadvances.2020003334
van Rhee, F., Voorhees, P., Dispenzieri, A., Fosså, A., Srkalovic, G., Ide, M., et al. (2018). International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood 32 (20), 2115–2124. doi:10.1182/blood-2018-07-862334
Velazquez, I., and Alter, B. P. (2004). Androgens and liver tumors: fanconi's anemia and non-Fanconi's conditions. Am. J. Hematol. 77 (3), 257–267. doi:10.1002/ajh.20183
Viana, M. B., Oliveira, B. M., Silva, C. M., and Rios Leite, V. H. (1991). Etoposide in the treatment of six children with Langerhans cell histiocytosis (histiocytosis X). Med. Ped Oncol. 19, 289–294. doi:10.1002/mpo.2950190414
Wang, J. N., Liu, T., Zhao, A. L., Pan, B. J., Sun, J., Li, J., et al. (2022). Phase 2 study of oral thalidomide-cyclophosphamide-dexamethasone for recurrent/refractory adult Langerhans cell histiocytosis. Leukemia 36 (6), 1619–1624. doi:10.1038/s41375-022-01555-8
Weber, N., Mollee, P., Augustson, B., Brown, R., Catley, L., Gibson, J., et al. (2015). Management of systemic AL amyloidosis: recommendations of the myeloma foundation of Australia medical and scientific advisory group. Intern Med. J. 45 (4), 371–382. doi:10.1111/imj.12566
Wechalekar, A. D., Gillmore, J. D., Bird, J., Cavenagh, J., Hawkins, S., Kazmi, M., et al. (2015). Guidelines on the management of AL amyloidosis. Br. J. Haematol. 168 (2), 186–206. doi:10.1111/bjh.13155
Word Health Organization (2014). WHO Handbook for guideline development. 2nd ed. Geneva, Switzerland: WHO.
Xue, F., Dai, J., Chen, L. X., Liu, W., Zhang, H. Q., Wu, R. H., et al. (2023). Report on diagnosis and treatment of hemophilia in China 2023. J. Diagn. Concepts Pract. 22 (02), 89–115. doi:10.16150/j.1671-2870.2023.02.001
Xue, F., and Yang, R. C. (2022). Establishment and evolution of China national hemophilia registry. J. Rare Dis. 1 (4), 370–374. doi:10.12376/j.issn.2097-0501.2022.04.002
Zeller, B., Storm-Mathisen, I., Smevik, B., and Lie, S. O. (2000). Multisystem Langerhans-cell histiocytosis with life-threatening pulmonary involvement--good response to cyclosporine A. Med. Pediatr. Oncol. 35, 438–442. doi:10.1002/1096-911x(20001001)35:4<438::aid-mpo12>3.0.co;2-4
Keywords: rare diseases, hematological disease, off-label drug use, expert consensus, evidence based pharmacy
Citation: Zhao B, Zhou X, Zheng P, Zhang B, Feng X, Chen J, Cai L, Chen Y, He L, Su J, Cheng S, Zeng Y, Li G, Ji B, Wu J, Feng W, Liu M, Jin Y, Liu T, Mo X, Wu J, Wu H, Zhang H, Zheng Z, Zheng Z, Sun J, Li Y and Guangdong Pharmaceutical Association, Hematology Group of Rare Disease Expert Committee of Guangdong Pharmaceutical Association (2024) Expert consensus on the off-label use in China of drugs for rare hematologic diseases (2024 edition). Front. Pharmacol. 15:1477550. doi: 10.3389/fphar.2024.1477550
Received: 08 August 2024; Accepted: 17 September 2024;
Published: 22 November 2024.
Edited by:
Shusen Sun, Western New England University, United StatesReviewed by:
Hongtao Xiao, University of Electronic Science and Technology of China, ChinaQuanjun Yang, Shanghai Jiao Tong University, China
Copyright © 2024 Zhao, Zhou, Zheng, Zhang, Feng, Chen, Cai, Chen, He, Su, Cheng, Zeng, Li, Ji, Wu, Feng, Liu, Jin, Liu, Mo, Wu, Wu, Zhang, Zheng, Zheng, Sun, Li and Guangdong Pharmaceutical Association, Hematology Group of Rare Disease Expert Committee of Guangdong Pharmaceutical Association. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihua Zheng, c25vd25vdHJhY2VAMTI2LmNvbQ==; Jing Sun, anN1bl9jbkBob3RtYWlsLmNvbQ==; Yilei Li, bGl5aWxlaTE5NzVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Boxin Zhao
Boxin Zhao Xuan Zhou3†
Xuan Zhou3† Xiaoqin Feng
Xiaoqin Feng Jie Chen
Jie Chen Yilu Chen
Yilu Chen Bo Ji
Bo Ji Jianlong Wu
Jianlong Wu Maobai Liu
Maobai Liu Yiran Jin
Yiran Jin Taotao Liu
Taotao Liu Xiaolan Mo
Xiaolan Mo Hongliang Zhang
Hongliang Zhang Zhihua Zheng
Zhihua Zheng Yilei Li
Yilei Li