- 1Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Department of Virology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 4School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 5Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
- 6Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Background: Cisplatin-based chemotherapy as a common therapeutic regimen for cervical cancer patients, is becoming more and more ineffective due to high resistance. This urges the need for introducing novel metabolics such as botanical drugs with the capacity to increase the cisplatin effectiveness. In that regard, here we investigated the anticancer effects of the Cisplatin-Vitex pseudo-Negundo combination in cervical cancer cell lines.
Method and Material: V. pseudo-Negundo fruits were dried and extracted methanolic fraction. The MTT assay was performed to evaluate cytotoxicity of both drugs in CaSki and HeLa cells. Then, apoptosis, ROS production, and cell cycling were assessed by flow cytometry assay in cells treated with V. pseudo-Negundo and Cisplatin and their combination. Also, the rate of cell migration and colony formation were measured, using wound healing and colony formation assay, respectively. Also, the expression level of related genes (CD133, BAX, BCL2, Casp-3/8/9, MMP-3) was evaluated using the RT-PCR method.
Results: The obtained results established that the V. pseudo-Negundo plant has medicinal properties to induce apoptotic and antioxidant signals. The combination treatment of methanol extraction and Cisplatin had a cytotoxic effect on cervical cancer cell lines (HeLa and CaSki) compared to monotherapy. Also, combination therapy resulted in an increased apoptosis rate and diminished ROS production in both CaSki and HeLa cell lines. Furthermore, V. pseudo-Negundo and Cisplatin combination therapy leads to cell cycle arrest in the G2-M and G0-G1 phase in HeLa and CaSki cell lines, respectively. Moreover, combination therapy decreased the colony formation and cell motility in both cell lines and upregulated caspases gene expression.
Conclusion: The combination of V. pseudo-Negundo with Cisplatin therapy results in a significant anti-cancer and antioxidant effect compared to cisplatin, representing a promising candidate for future clinical investigations.
1 Introduction
Accounting for more than a quarter of a million annual fatalities, cervical cancer (CC) is a vital health concern worldwide (Small et al., 2017). The financial and medical burden of CC requires global efforts to achieve advancements in treatment methods. The International Federation of Gynecology and Obstetrics staging system provides the foundation for cervical cancer treatment (Tsikouras et al., 2016). Patients can get standard therapies such as surgery, chemotherapy, and radiation depending on the stage of their malignancy; however, women with metastatic cervical cancer are not yet eligible for any mainstream therapies (Li et al., 2016). Chemotherapy and preventative immunization are now the main treatment interventions for cervical cancer (Shiri Aghbash et al., 2023). Similar to other treatments, chemotherapy is faced with several limitations, including the requirement for high dosages, toxicity, and activation of pro-survival pathways after prolonged exposure, all of which result in diminished effectiveness and chemo-resistance (Florea and Büsselberg, 2011; Rastogi et al., 2015; Liu et al., 2019a). Moreover, one of the side effects of chemotherapy medicines such as Cisplatin is nephrotoxicity the related mechanism of which is not known during the use of Cisplatin. Also, currently, there is no effective methods or strategies to protect the kidneys during chemotherapy (Ahmad et al., 2019). In this regard, research indicates that the side effects caused by the use of Cisplatin, such as oxidative damage, are reduced or improved following the use of antioxidants (Almutairi et al., 2017; Darwish et al., 2018; Hazman et al., 2018). In other words, human cancer antitumor therapy frequently involves the administration of antioxidant and apoptosis-inducing medicines that prevent growth, invasion, and metastasis (Talib et al., 2022). Advances in the field of cancer treatment have become a high-profile and multi-billion-dollar industry; hence, targeted therapy has led to the reduction of limitations associated with chemotherapy (Dasari et al., 2022). Human cancer antitumor therapy frequently involves the administration of antioxidant and apoptosis-inducing medicines that prevent growth, invasion, and metastasis. Also, for decades herbal medicines have been utilized to treat various disorders and malignancies (Graham et al., 2000). Some species of Vitex, such as Vitex agnus-castus, Vitex trifolia, Vitex rotundifolia, and Vitex negundo can be used as herbal medicines (Jing et al., 2019). In this way, in the study conducted by Puglia et al., in 2023, it was shown that Vitex-agnus-castus can lead to the regulation of prolactin in individuals with mild prolactinemia (Puglia et al., 2023). Therefore, developing novel and potent anticancer substances derived from natural sources are useful for cancer treatment. This encourages further investigations to develop promising cancer treatments. In this regard, with the ultimate objective of identifying and creating new anti-CC drugs, medicinal plants have been thoroughly considered. It is also suggested that botanical drugs and natural medicines be considered in order to investigate the effectiveness of chemotherapy and reduce drug resistance (Nimlamool et al., 2022). Vitex Pseudo-Negundo (Hausskn.) is a plant from the Lamiaceae family and grows naturally near the seasonal rivers of Iran (Salehpour et al., 2018). This botanical drug is a traditional medicine that is used for the treatment of various diseases and disorders that affect women, including amenorrhea, dysmenorrhea, corpus luteum failure, hyperprolactinemia, infertility, acne, and menopause (Tandon and Gupta, 2005). Preclinical investigations have confirmed pharmacological roles for several of these substances. The existence of estrogenic, dopaminergic, opioids, antineoplastic, immunomodulatory, and antioxidant activities has therefore been established by several researchers (Adamov et al., 2022). Moreover, studies on phytochemistry have revealed that this genus’s members include substances such as flavonoids, terpenoids, ecdysteroids, and iridoid glycosides (Kirtikar and Basu, 1918). The V. pseudo-Negundo fruit extraction includes 20 flavonoids, 11 phenolic acids, 10 iridoids, 12 terpenes, and 3 diterpene alkaloids. Therefore, it can be used as an anti-cancer and antioxidant medicine in malignancies. Moreover, while many novel medicines are currently being developed in cancer treatment, combination therapies are highly anticipated. Most regions of the world have historically used natural botanical drugs for cancer treatment (Liu et al., 2019b). Natural botanical drugs have showed the capacity to elevate the efficiency of conventional chemo- and radio-therapy and promote cell death in cancer cells, prevent metastasis, as well as trigger the anticancer immunity (Liu et al., 2019b). In this regard, in 2022, Dasari et al., reported that the combined treatment of cisplatin and natural products as new strategic approaches to cancer treatment, are effective (Dasari et al., 2022). In addition, due to the existence of some bioactive metabolites in medicinal plants, they can be considered as promising sources for the treatment of various human malignancies (Dasari et al., 2022). During clinical studies, it has been established that the metabolites in medicinal plants can increase the therapeutic activity of Cisplatin as well as reduce the toxicity caused by chemotherapy. Also, metabolites found in medicinal plants modulate multiple gene transcription factors and induce cell death via apoptosis or necrosis, as a result lead to protection against organ toxicity caused by Cisplatin (Dasari et al., 2022).
Considering the need for overcoming the cisplatin-based chemo-resistance and the effectiveness of botanical drug’s metabolites for increasing the efficacy of this therapeutic regimens, here we investigated the combined effect of Vitex botanical medicine and cisplatin chemotherapy drug on synergistic toxicity, improving the effectiveness of chemotherapy drugs and inhibiting the drug resistance of CC cells.
2 Methods
2.1 Methanolic extraction of Vitex pseudo-Negundo fruit
This plant with the local name Five Fingers belongs to the Lamiaceae family. The Vitex pseudo-Negundo is a shrub with a height of about 3 m and is used as a medicinal species and grows naturally around the seasonal rivers and streams of Iran. After collection of the V. pseudo-Negundo in winter, the V. pseudo-Negundo fruits were dried and ground into a powder before being subjected to a soaking extraction method. In this process, an alcoholic solvent was utilized, with 100 mL employed for each extraction to yield the methanolic extract. To purify the final extract, 1 gr of the plant material underwent freeze-drying. The final V. pseudo-Negunda extract (3 mg of 1 gr) was dissolved in 30 μL of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, United States) as a stock. Using a sensitive scale, 3 mg of the desired extract was weighed and placed in a microtube. Then about 30 μL of DMSO and 1,000 μL of distilled water were added to the extract to reduce the cytotoxicity of DMSO. The final volume of our stock reached 3 mg/mL. To reduce the toxicity effect of DMSO on the cells in the per wells, the concentration of this substance in each well was less than 1% (Aldaghi et al., 2016; Garbi et al., 2015).The obtained solution was stored on covered glass plates at −20°C, at dark, for cell culture experiments (Aldaghi et al., 2016; Gong et al., 2021). Also, according to the Heinrich et al., we speculated that our botanical drug is in the type C group (Heinrich et al., 2022).
2.2 Cell culture and treatment
Human cervical cancer cell lines, CaSki and HeLa, were sourced from the Iranian Biological Resource Centre (IBRC). They were cultured in a monolayer form using Roswell Park Memorial Institute medium (RPMI 1640; Gibco, United States), supplemented with 10% fetal bovine serum (FBS; Gibco, United States), along with 100 units/mL of penicillin and streptomycin in T25 flasks. The cells were maintained at 37°C in a humidified atmosphere with 5% CO2 until they reached logarithmic growth. When the cells achieved about 70% confluency, they were detached using a 0.25% Trypsin-EDTA solution (Gibco, United States) for subsequent experiments.
2.3 MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the viability and proliferation of CaSki and HeLa cells, determining the IC50 values for Cisplatin in both monotherapy and conjunction with varying concentrations of V. pseudo-Negundo extract (1, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200 μg/mL). Among other treatments. Normal human foreskin fibroblast (HFF2) cells were included for comparison. Approximately 1.5 × 104 CaSki and 1 × 104 HeLa and HFF2 cells were seeded in a 96-well plate. The cell lines were divided into four treatment groups: control, Cisplatin only, V. pseudo-Negundo extract only, and a combination of both. After a 24-h incubation, various concentrations of the agents replaced the medium. Subsequent incubation for another 24 h was followed by adding 150 µL of MTT solution (2 mg/mL) and incubating for another 4 h at 37°C with 5% CO2. The solution was then removed, and to dissolve the resultant formazan crystals, 150 μL of DMSO was added. Absorbance at 570 nm (reference at 620 nm) was measured using a microplate reader (Tecan, Switzerland). Each assay was conducted in triplicate. Also, we examined 50% cell viability at different concentrations of both agents in both cell lines, using a [0.5:10] ratio of cisplatin to V. pseudo-Negundo, respectively.
2.4 Wound healing assay
To evaluate cell migration, an assay was performed using CaSki (3 × 105 cells) and HeLa (2 × 105 cells) lines after treatment with Cisplatin and V. pseudo-Negundo extract. Cells were plated as a singular layer in 24-well plates and incubated for 24 h at 37°C with 5% CO2. A linear wound was created with a 100 μL pipette, and the respective IC50 concentrations of the treatments were administered. Migration was tracked at three-time intervals—0, 24, and 48 hours—utilizing an inverted microscope (Optika, XDS-3, Italy).
2.5 Real-time PCR (RT-PCR)
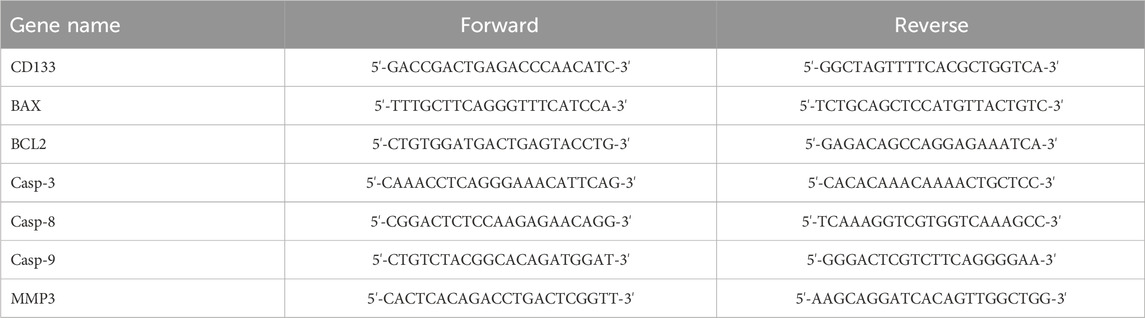
For this experiment, 3 × 105 CaSki cells and 2 × 105 HeLa cells were cultured in 6-well plates, and divided into four treatment groups as specified earlier. Following a 24-h incubation at 37°C in a 5% CO2 atmosphere, cells were treated for an additional 24 h with their respective IC50 doses of Cisplatin and V. pseudo-Negundo. RNA extraction was executed using TRIzol reagent (RiboEX, South Korea), and RNA concentrations were quantified with a Nano-Drop spectrophotometer (Thermo Scientific, United States) at various wavelengths (230, 260, and 280 nm). Complementary DNA (cDNA) synthesis followed using the 2X RT-PCR Pre-Mix (BioFACT™, South Korea) to assess relative gene expression levels. Genes of interest included CD133, BAX, BCL2, Casp-3, Casp-8, Casp-9, and MMP-3, evaluated using the 2X Real-Time PCR Master Mix (BioFACT™, South Korea) on a StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, United States). GAPDH served as a normalization control, and analysis was performed with the 2−ΔΔCT method (Shiri Aghbash et al., 2023). All primer sequences were confirmed with NCBI’s Primer-BLAST before the experiment; their sequences are presented in Table 1.
2.6 Apoptosis assay
In the apoptosis evaluation, 3 × 105 CaSki cells and 2 × 105 HeLa cells were cultured in separate 6-well plates (Shiri Aghbash et al., 2023). After 24 h, the groups were treated with their respective IC50 concentrations of Cisplatin and V. pseudo-Negundo. The Annexin V/PI staining kit (Exbio-Czech) was employed to prepare samples for flow cytometry. Cells were washed with 1X PBS and labeled with FITC-conjugated Annexin V and propidium iodide (PI) per the manufacturer’s protocol. Apoptotic cells were then quantified via flow cytometry.
2.7 Cell cycle assay
Following the seeding of 3 × 105 CaSki cells and 2 × 105 HeLa cells in 6-well plates, the cells were treated with their respective IC50 concentrations. After washing with PBS, cells were fixed in 70% ethanol and stored at −20°C overnight. They were then re-suspended in PBS with RNase A (200 μg/mL) and incubated at 37°C for 30 min before being stained with DAPI (50 μg/mL) for analysis. Flow cytometry (Miltenyi Biotec MACSQuant 10) was used to assess cell cycle distributions, and data were analyzed using FlowJo FACS software.
2.8 Assessment of cellular ROS generation
Intracellular ROS levels were evaluated using the dichloro-dihydro-fluorescein diacetate (DCFH-DA; Meilunbio, China) assay. CaSki and HeLa cells (3 × 105 and 2 × 105 respectively) were grown in 6-well plates. Following treatment with their respective IC50 concentrations, cells were washed twice with PBS in a dark setting and stained with 20 μM DCFH-DA for 30 min at 37°C.
2.9 Colony formation assay
For the colony formation assessment, 104 CaSki cells and 5 × 103 HeLa cells were placed in 6-well plates. After an incubation period of 24 h at 37°C with 5% CO2, cells were treated with their designated drug doses. The medium was refreshed every 2–3 days, with colony counts performed after 10–12 days. Colonies were stained with crystal violet for 30 min, and the number of colonies was quantified using ImageJ software.
2.10 Statistical analysis
Statistical analyses were carried out using SPSS version 26 (SPSS Inc., Chicago, IL, United States), Prism 8.0 (GraphPad, San Diego, CA, United States), and CompuSyn software. The distribution of quantitative variables was assessed through the Kolmogorov-Smirnov test. Data were presented as mean ± standard deviation (SD), with a significance level established at p < 0.05. The Wilcoxon signed-rank test or paired t-test tested paired measurements, while one-way ANOVA compared group differences.
3 Results
3.1 Combination of cisplatin and Vitex pseudo-negundo suppresses cervical cancer cells viability
The treatment with Cisplatin alone resulted in approximately 50% inhibition of cell viability and proliferation in CaSki and HeLa cells at concentrations of 10.3 μg/mL and 6.6 μg/mL, respectively, compared to controls. In contrast, the extraction of V. pseudo-Negundo displayed a notable growth-inhibiting effect on cervical cancer cells, particularly at 81.47 μg/mL for CaSki and 77.49 μg/mL for HeLa. The application of various V. pseudo-Negundo extract concentrations demonstrated a significant reduction in cell viability for both cancer cell lines. Specifically, the concentrations at which cell viability in CaSki cells dropped were between 125 μg/mL and 150 μg/mL, while for HeLa cells, it ranged from 150 μg/mL to 200 μg/mL. For comparison, HFF2 cells showed an IC50 value of 270.5 μg/mL for V. pseudo-Negundo (Figure 1).
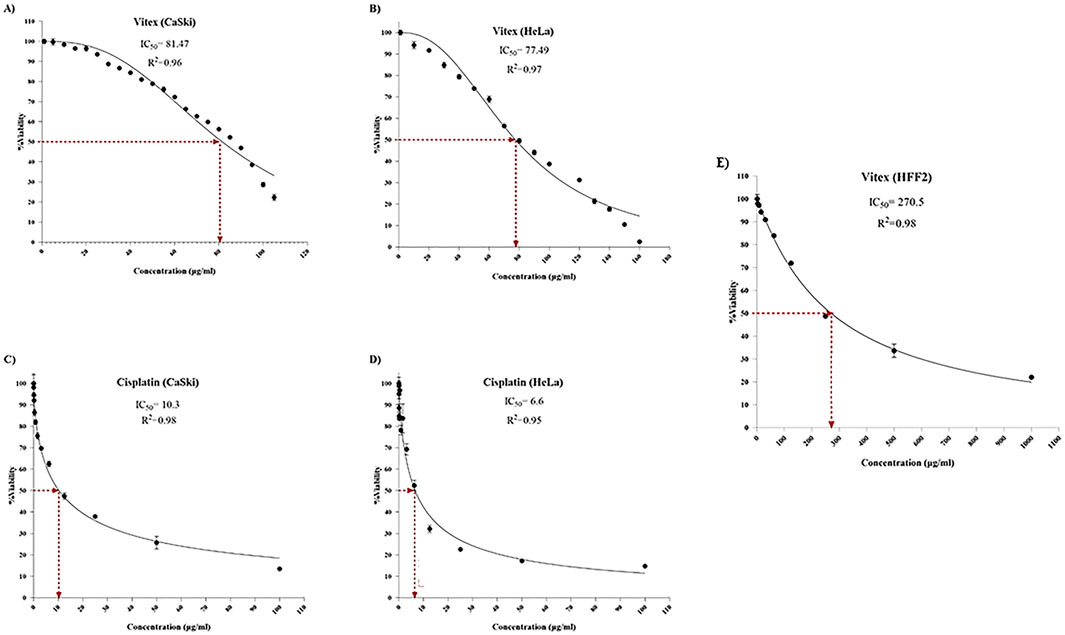
Figure 1. The impact of Vitex extraction and Cisplatin monotherapy on the viability of (A) Vitex (CaSki); (B) Vitex (HeLa); (C) Cisplatin (CaSki); (D) Cisplatin (HeLa); (E) Vitex (HFF2).
Importantly, V. pseudo-Negundo extraction was found to lower the IC50 threshold for Cisplatin, effectively reducing the required concentration of Cisplatin to achieve less than 50% viability. The synergy between V. pseudo-Negundo and Cisplatin was evident, with a 10:0.5 ratio leading to IC50 values of 32.07 μg/mL and 42.3 μg/mL for HeLa and CaSki cells, respectively (Figure 2).
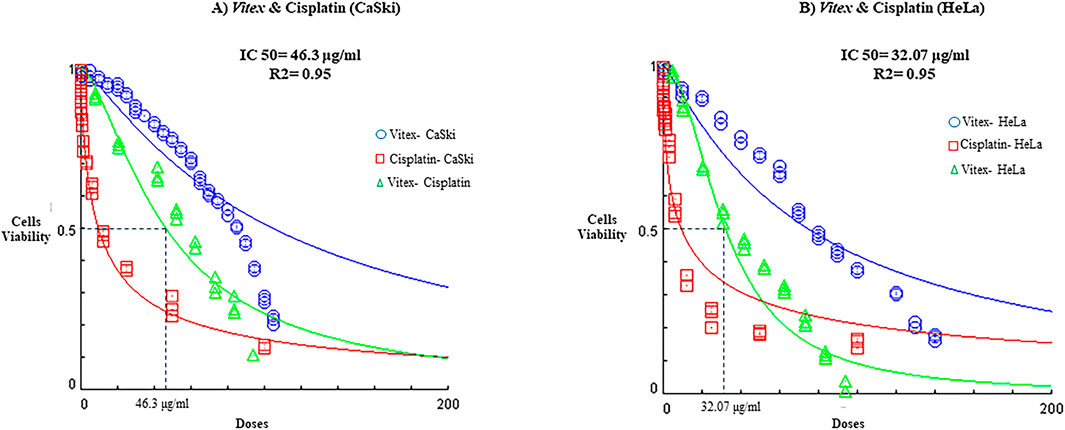
Figure 2. The impact of Vitex extraction and cisplatin combination on the viability of (A) CaSki cell line; (B) HeLa cell line.
3.2 Vitex pseudo-negundo can sensitize cervical cancer cells to cisplatin-induced apoptosis
Data suggest that the combination therapy led to an increased rate of apoptosis in cervical cancer cells when compared to control and monotherapy conditions. In HeLa cells, the rates of apoptosis following individual treatments of V. pseudo-Negundo and Cisplatin were recorded at 14.2% and 18.1%, respectively, while the combination treatment achieved a rate of 20.8%. There were no significant differences between the monotherapy and combination groups; however, each was significantly different from the controls (p< 0.0001) (Figures 3C–F). In CaSki cells, the monotherapy groups showed marked differences (V. pseudo-Negundo: 5.20%, Cisplatin: 7.11%) compared to the combination group (10.7%), with all treatments yielding significant differences from the control (p < 0.0001). Additionally, a remarkable increase in the expression of apoptosis-related genes such as Casp-3, -8, -9, and Bax was observed after combination therapy, contrasting with a notable decrease in the expression of the anti-apoptotic gene Bcl2 (Figures 4C–F).
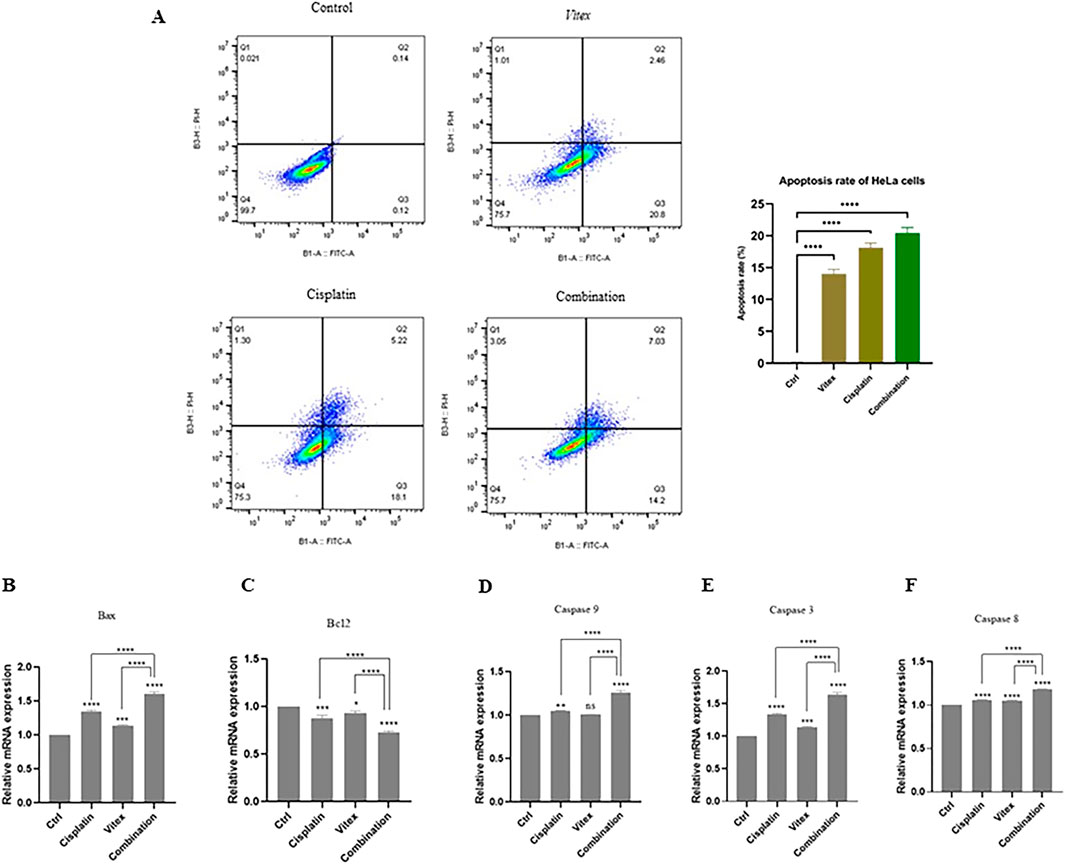
Figure 3. The effect of the Vitex extract combined with Cisplatin on apoptosis in HeLa cells. (A) The combination notably enhances HeLa cell apoptosis; (B, C) Bax levels increasing and Bcl2 levels decreasing compared to controls. (D, E, F) Caspase gene expression significantly rose following combination therapy versus monotherapy with either Vitex or Cisplatin (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
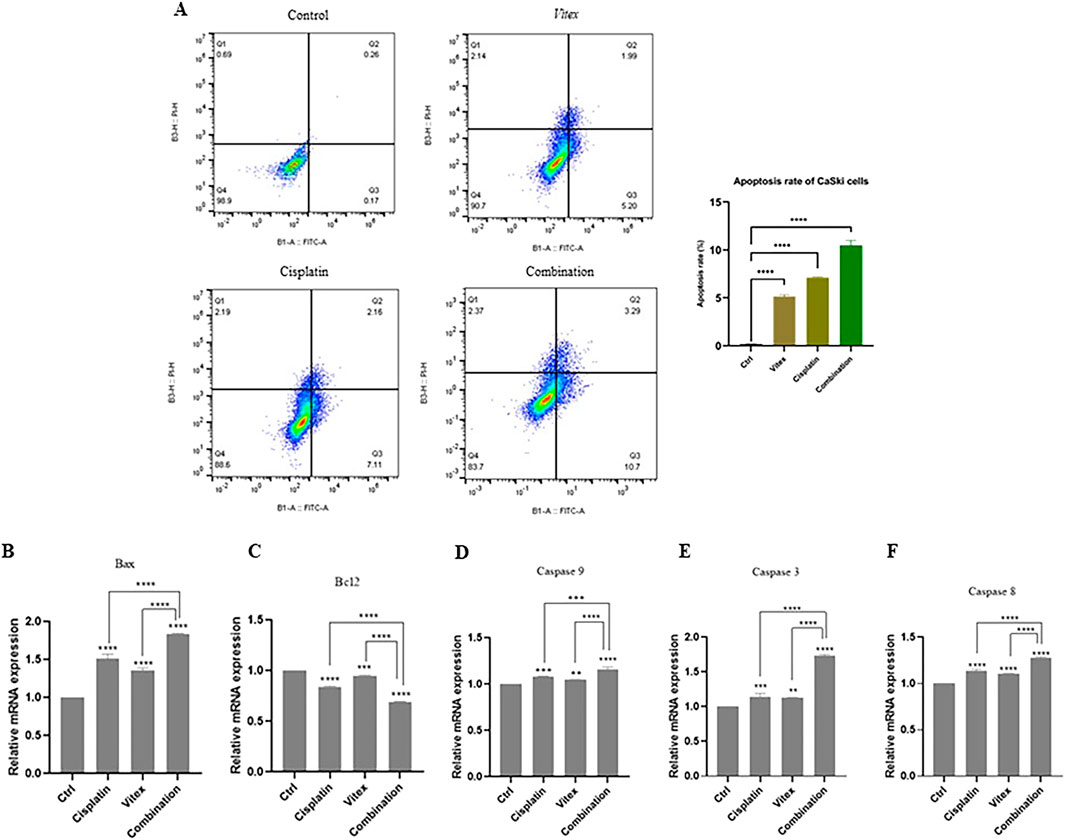
Figure 4. The impact of the Vitex extract and Cisplatin combination on apoptosis in CaSki cells. (A) the combination promotes increased apoptosis in CaSki cells, (B, C) expression level of Bax and Bcl2 changed, (D, E, F) significant rise in Caspase gene expression compared to monotherapy (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.3 Vitex pseudo-negundo extraction in combination with cisplatin arrested CaSki and HeLa cells on G0-G1 and G2-M stage
To better understand the impact of the combined treatment on cell division, the distribution of cell cycles in both cell lines was examined. The findings revealed that CaSki cells underwent an arrest in the G0-G1 phase, while HeLa cells showed arrest predominantly in the G2-M phase after treatment (Figures 5, 6). Following combined treatment with V. pseudo-Negundo and Cisplatin (p < 0.0001), a significant increase in cell populations was noted within the G2-M phase, moving from 3.60% to 14.7% for CaSki cells and from 11.1% to 20.1% for HeLa cells, relative to controls. This indicates that the combination therapy enhanced the presence of HeLa cells in the G2-M phase, thereby effectively promoting cell cycle arrest at this juncture (Figure 5). In addition, CaSki cells were also observed to be arrested in the G0-G1 or sub-G1 phase (Figure 6).
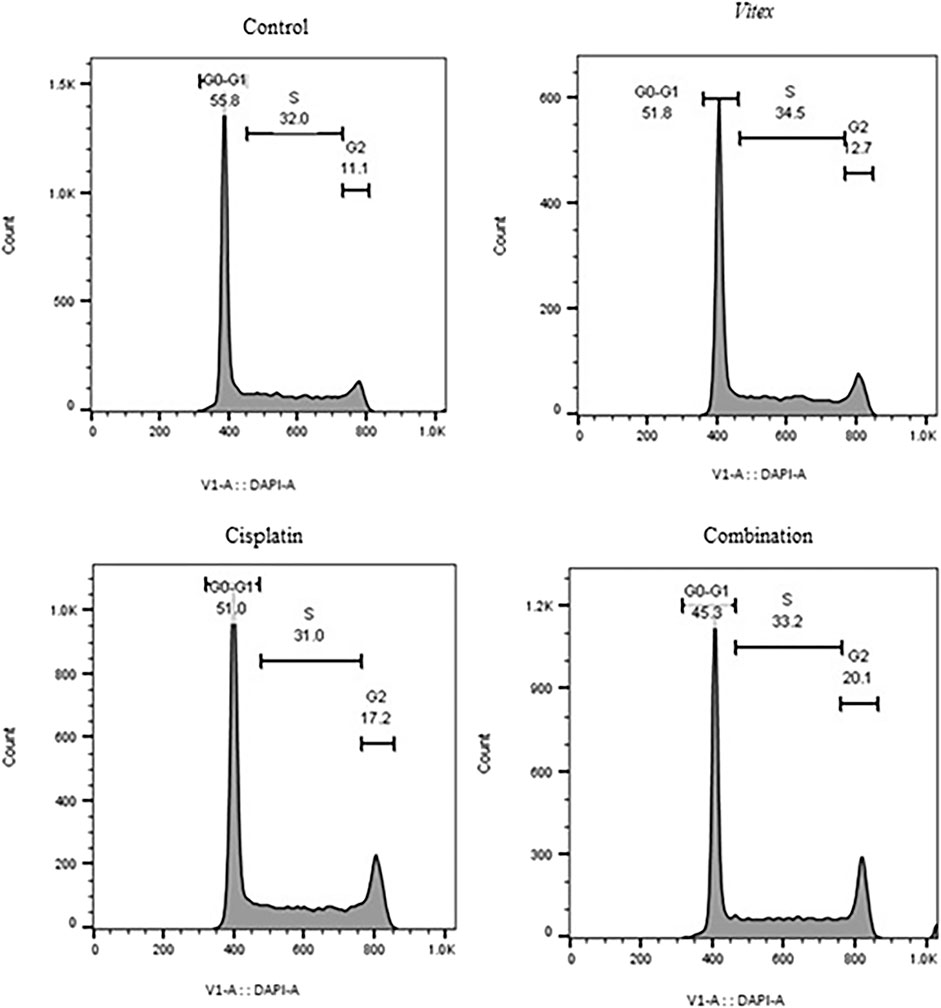
Figure 5. The effect of the Vitex extract combined with Cisplatin on the cell cycle in HeLa cells, resulting in cell cycle arrest at the G2-M phase.
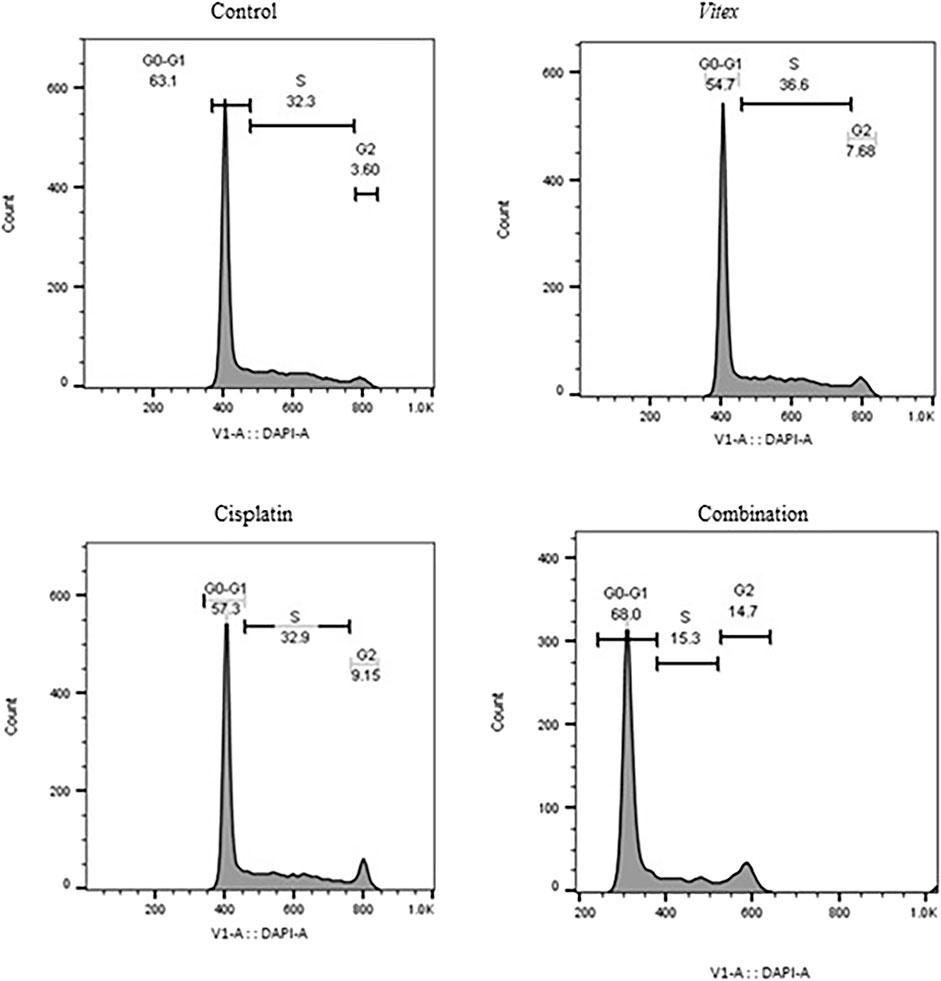
Figure 6. The effect of the Vitex extract combined with Cisplatin on the cell cycle in CaSki cells, leading to cell cycle arrest in the sub-G1 phase.
3.4 ROS production was inhibited by Vitex pseudo-negundo in combination with cisplatin
To investigate whether the combined treatment with V. pseudo-Negundo and Cisplatin affected intracellular ROS levels in cervical cancer cells, measurements were taken after 24 h of either combination therapy or monotherapy. The results demonstrated a significant increase in intracellular ROS levels (DCF) in the combination therapy group compared to controls (p < 0.0001). Notably, in the HeLa cells, ROS levels were found to rise from 0.74% in controls to 89.9% following combination treatment, whereas in CaSki cells, levels increased from 0.68% to 62.6% (Figures 7, 8). Overall, there were no statistically meaningful differences in ROS production among the monotherapy groups (Cisplatin: 89.7%, V. pseudo-Negundo: 83.7%) compared to the combination group (89.9%); however, each exhibited significant variations when contrasted with the control (p< 0.0001) (Figure 7). Additionally, the highest ROS production was seen in the CaSki cells treated with both agents, peaking at 62.6%, followed by monotherapy with Cisplatin (35.4%) and V. pseudo-Negundo (33%) (Figure 8).
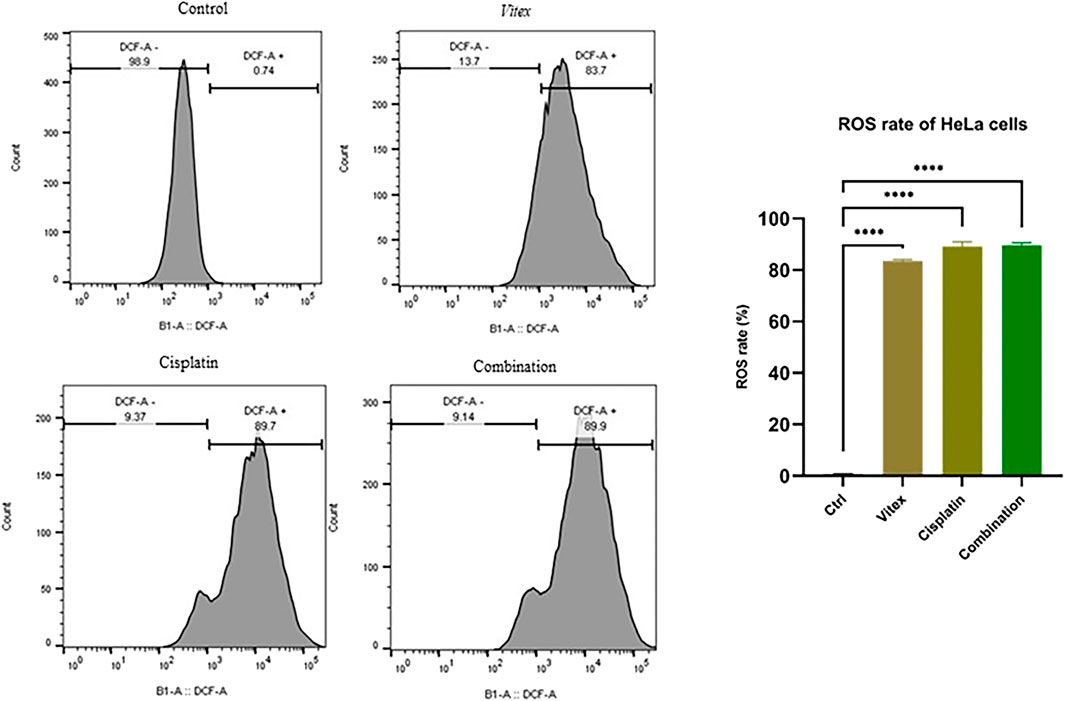
Figure 7. The influence of the Vitex and Cisplatin combination on ROS levels in HeLa cells. Cells in exponential growth were treated with the specified concentrations, and ROS levels were measured via flow cytometry, showing ROS (DCF) levels (%) in relation to the control (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
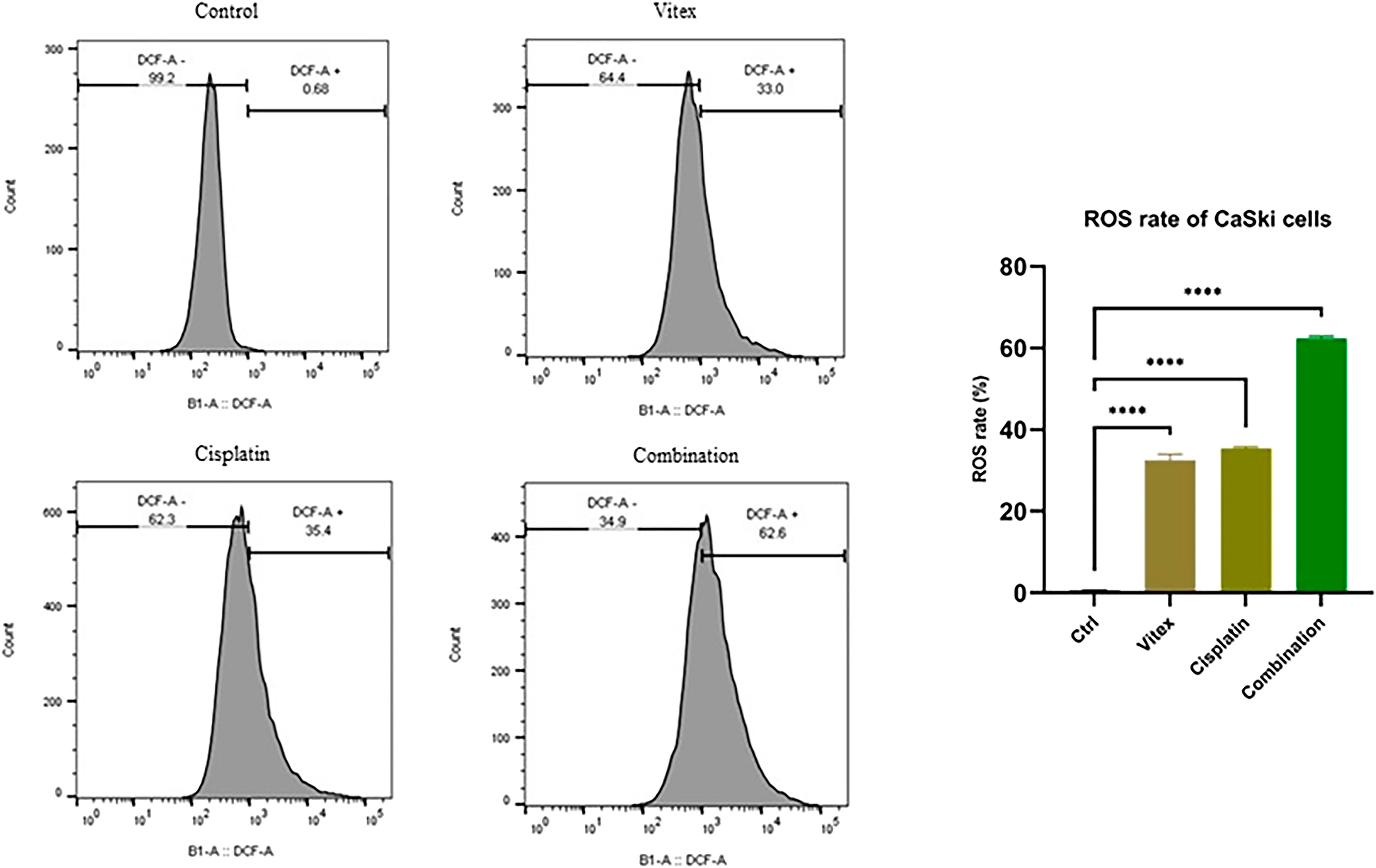
Figure 8. The influence of the Vitex and Cisplatin combination on ROS levels in CaSki cells. Cells in exponential growth were treated with the specified concentrations, and ROS levels were measured via flow cytometry, showing ROS (DCF) levels (%) in relation to the control (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.5 Stemness features in cervical cancer cells were decreased by Vitex pseudo-negundo fraction in combination with cisplatin
To assess the influence of V. pseudo-Negundo extract combined with Cisplatin on the stemness traits of cervical cancer cells, a colony formation assay was conducted. Treatments involving both CaSki and HeLa cell lines with the dual agents resulted in a marked reduction in colony formation, particularly notable in the combination group when compared to controls (Figures 9A, B). Treatments with either V. pseudo-Negundo or Cisplatin alone also exhibited declines in colony numbers compared to controls, but the combination showed a more substantial impact. To support these findings, RT-PCR analysis was utilized to investigate the expression of stemness-related genes like CD133. Results revealed a significant disparity in CD133 expression levels between the combination treatment group and controls (p < 0.0001) for both cell lines. While reductions in CD133 were noted across all treatment groups, no significant differences were observed among those groups.
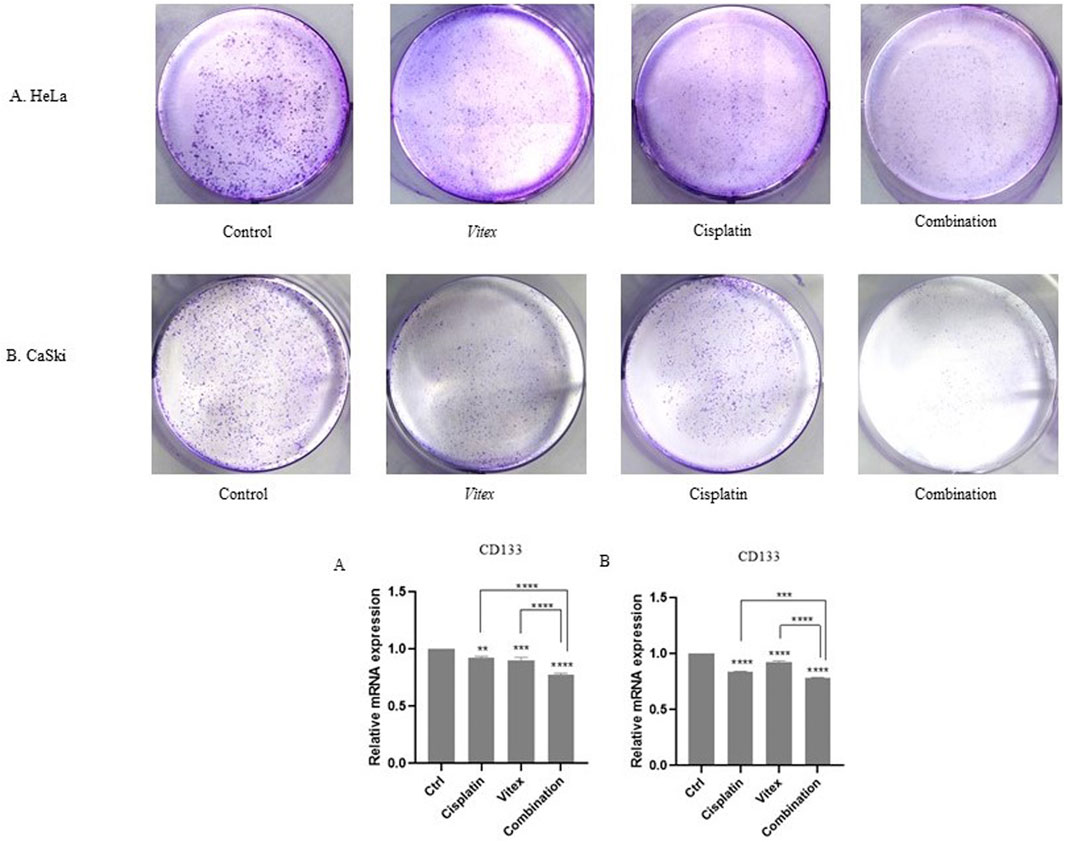
Figure 9. The effect of the Vitex and Cisplatin combination on stemness characteristics. (A) In HeLa cells, the number of colonies greatly decreases in the combination group when compared to the control. (B) A similar decrease in colony numbers is observed in the CaSki cell line with combination treatment. Changes in the stem cell marker CD133 are noted in the combination group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
3.6 Cervical cancerous cells’ motility and related genes have been inhibited by Vitex pseudo-negundo -cisplatin combination
The wound healing assay results indicated that individual treatments with Cisplatin or V. pseudo-Negundo had minimal effects on cervical cancer cell migration. In contrast, the synergistic application of both agents resulted in a significant reduction in the migration of CaSki and HeLa cells following 48 h of treatment compared to controls (Figures 10, 11). Further analysis into the effects of combination treatment on cellular motility employed RT-PCR techniques to evaluate MMP-3 gene expression. This analysis confirmed that the combination of V. pseudo-Negundo and Cisplatin effectively reduced MMP-3 levels in both cell lines (p < 0.0001) (Figures 10B, 11B). Although each monotherapy presented reductions in migration and MMP-3 expression, the combination treatment yielded far greater reductions than Cisplatin alone (p < 0.0001), demonstrating significant efficacy even at lower Cisplatin doses.
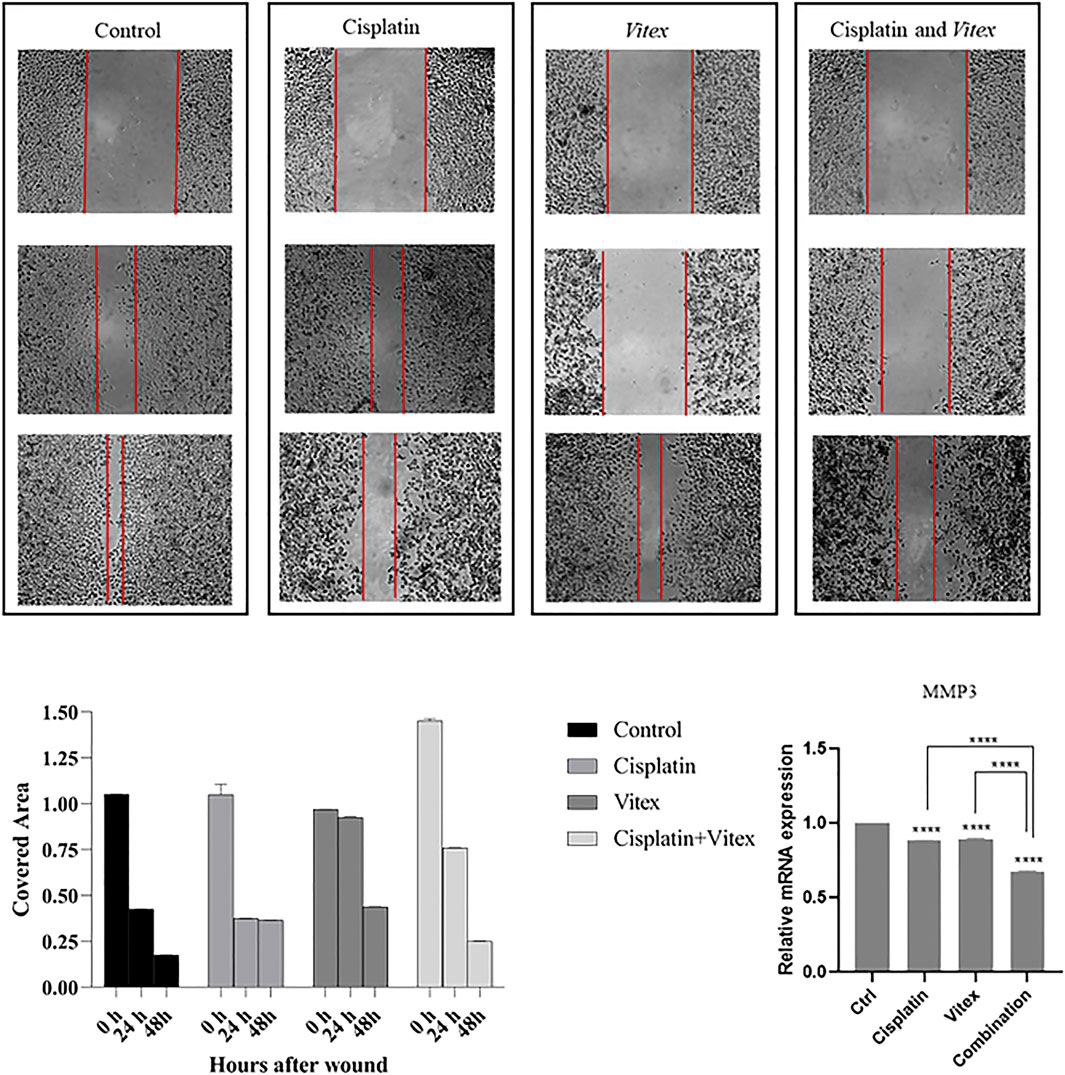
Figure 10. The effect of the Vitex and Cisplatin combination on migration and motility in HeLa Cell. After 48 h, this combination results in a significant reduction of motility characteristics compared to control groups. (A) Shows covered area by cells after 48 h treatment in which groups. (B) Shows alteration of MMP3 expression and compression of it in treatment groups and control group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
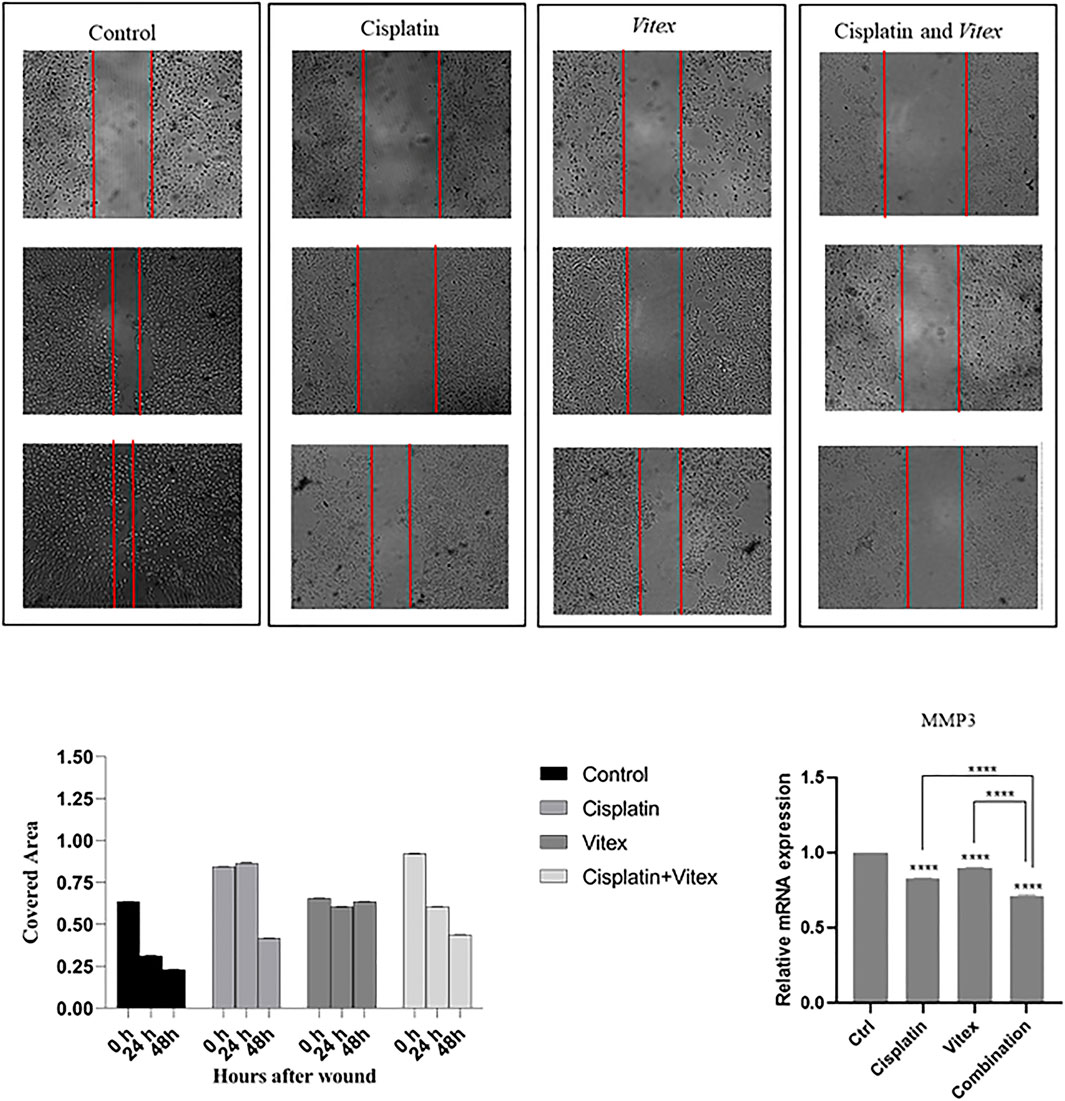
Figure 11. The effect of the Vitex and Cisplatin combination on migration and motility in CaSki Cell. After 48 h, this combination results in a significant reduction of motility characteristics compared to control groups. (A) Shows covered area by cells after 48 h treatment in which groups. (B) Shows alteration of MMP3 expression and compression of it in treatment groups and control group (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
4 Discussion
In this study, we explored the effects of combining V. pseudo-Negundo extraction with Cisplatin for cervical cancer (CC) therapy. Our findings indicate that V. pseudo-Negundo extract significantly inhibits the growth and proliferation of tumor cells through its growth-inhibitory properties. Specifically, the methanolic extract of V. pseudo-Negundo demonstrated a growth inhibitory impact on HeLa and CaSki cell lines at concentrations of 150 μg/mL and 200 μg/mL, with IC50 values of 77.49 μg/mL and 81.47 μg/mL, respectively. Notably, the IC50 values were higher for CaSki cells than HeLa cells, likely due to the former’s resistance to treatment. Additionally, we observed that the Cisplatin combination with botanical drug remedies enhances the sensitivity of HeLa cells to lower doses of Cisplatin (Madhulika Singh et al., 2013). This suggests that certain plant extracts can improve the response of cancer cells that are resistant to Cisplatin, thereby enhancing the overall efficacy of the treatment (Dasari et al., 2022; Liu et al., 2020). For instance, Erdogan et al. demonstrated that flavonoids could increase the sensitivity of CD44+ prostate cancer cells to Cisplatin (Erdogan et al., 2017). In contrast, Li et al. noted similar effects in ovarian cancer cells treated with platinum-based therapies (Li et al., 2014).
Cisplatin is a widely utilized chemotherapeutic agent for various cancers, functioning by disrupting DNA repair mechanisms, causing DNA damage, and promoting apoptosis in cancer cells. However, its effectiveness is often hampered by drug resistance, as well as a range of side effects. Consequently, combination therapies involving Cisplatin and other agents have emerged as a strategy to overcome resistance and mitigate toxicity (Dasari and Bernard Tchounwou, 2014). Herbal medicines in combination therapies have resulted in long-lasting and more potent therapeutic results. Furthermore, they might shield the healthy tissues and trigger sensitivity to chemotherapy and radiotherapy, as well as suppress tumor metastasis and recurrence. Considering the unique characteristics, low side effects, availability, and ease of access, botanical drugs from different regions around the world have been explored, in particular for cancer research (Zulfiker et al., 2017). For instance, the consumption of flavonoids as a part of the V. pseudo-Negundo plant leads to cancer prevention and increased sensitivity in Cisplatin-resistant cases, and as a result, promotes Cisplatin treatment. In combined treatments with Cisplatin and flavonoid-rich plants, flavonoids have been associated with increased effectiveness in disrupting the cellular oxidative system, inducing mitochondrial dysfunction, and ultimately leading to apoptosis (Kachadourian et al., 2007). Consistent with our findings, we observed that combining V. pseudo-Negundo with Cisplatin resulted in higher levels of reactive oxygen species (ROS) and more pronounced apoptotic responses via a mitochondrial-dependent pathway. Moreover, since Cisplatin is known to induce nephrotoxicity, flavonoids have shown protective effects against this side effect (Casanova et al., 2021). Specifically, they can prevent kidney cell damage by inhibiting apoptosis through the inactivation of p53, mitogen-activated protein kinase (MAPK), and protein kinase B (AKT) signaling pathways (Ju et al., 2015; Wang et al., 2018).
A study conducted by Lui, N et al., in 2018, on V. negundo L found that its metabolic, VB1 (vitexin metabolic 1), can induce apoptosis by increasing the expression of pro-apoptotic Bax and decreasing the anti-apoptotic Bcl-2, thereby leading to cell cycle arrest at the G2/M phase in melanoma cells (Liu et al., 2018).
Our data also confirmed that the V. pseudo-Negundo can promote cell cycle arrest at the G2-M phase in HeLa cell lines, whereas Cisplatin causes the cell cycle arrest in the G0-G1 phase. Additionally, following treatment with Cisplatin and V. pseudo-Negundo fraction, cell cycle arrest in CaSki cell line is comparable to that in the HeLa cell line; however, the rate of cell cycle arrest in the G0-G1 phase remains greater than that in the G2-M phase in the CaSki cell lines. These suggest a synergistic effect for the V. pseudo-Negundo and Cisplatin combination. In this regard, Meng et al. reported that Cyclin B1 and CDK1 proteins are significantly reduced after treatment of prostate cancer cells, which results in cell cycle arrest in the G2/M phase (Meng et al., 2012).
Moreover, we observed that monotherapy with V. pseudo-Negundo extraction significantly induced apoptosis at high concentrations while inhibiting apoptosis at low concentrations in both cell lines. With that context, we observed that combination therapy enables more effectiveness at lower dosages of V. pseudo-Negundo than monotherapy in both cell lines.
Furthermore, our results indicated that the methanolic extraction of V. pseudo-Negundo promotes apoptosis through downregulating Bcl-2-related genes and upregulating Bax. Furthermore, the high apoptotic levels were associated with a significant upregulation in the expression level of caspase-3/8/9 in both cell lines, suggesting that apoptosis induction occurs through the caspase-dependent mitochondrial pathway following the combination therapy of V. pseudo-Negundo extraction and Cisplatin. Lui et al. also highlighted that changes in gene expression of p53 significantly alter apoptosis and cell cycle pathways (Liu et al., 2018). Thus, we hypothesize that the observed apoptosis rates in our study may hinge on p53 induction, subsequently promoting caspase gene expression and apoptosis through the mitochondrial pathway. Moreover, Khan et al., noted that increased levels of ROS could lead to oxidative damage and DNA damage, ultimately modulating Bcl-2 and reducing mitochondrial membrane integrity, which triggers mitochondrial-dependent apoptosis (Khan et al., 2012).
Furthermore, ROS generation following the use of specific chemotherapeutic agents, can effectively destroy tumor cells through promoting apoptosis. Consequently, it is useful to consider effective ways to achieve significant synergy by combining agents with similar ability to alter redox conditions. Cisplatin is capable of producing ROS that leads to oxidative stress (Poveda et al., 2007; Serkies and Jassem, 2004). While increased ROS can facilitate cancer progression, excessive accumulation leads to apoptosis induction (Gorrini et al., 2013). As a result, to promote the induction of cell death as an anticancer strategy, it is suggested to increase the level of ROS (Raj et al., 2011; Wang et al., 2015; Hang et al., 2018). It has been reported that medicinal metabolites extracted from plants can lead to the induction of intracellular ROS and subsequently DNA damage and increased apoptosis (Hang et al., 2018; Yang et al., 2017).
Here, we demonstrated that V. pseudo-Negundo can increase the toxic effect of cisplatin through promoting ROS generation, thereby promoting apoptosis in CC cells. It has been shown that V. pseudo-Negundo can enhance the cytotoxic effects of Cisplatin by promoting ROS generation, resulting in increased apoptosis in cervical cancer cells. ROS facilitates the migration of immune cells to damaged tissues and plays a role in infection control during the inflammatory response (Gulumian et al., 2018). Furthermore, ROS promotes the proliferation of keratinocytes, endothelial cells, and fibroblasts, potentially leading to apoptosis and tissue damage (Dunnill et al., 2017). As suggested in this study, elevated ROS levels post-combination treatment was associated with enhanced apoptosis through a mitochondria-dependent pathway in Cisplatin-resistant cells. Liu et al. similarly found that VB1 enhances intracellular ROS accumulation, resulting in DNA damage and increased apoptosis alongside G2/M phase cell cycle arrest in vemurafenib-resistant melanoma cells (Liu et al., 2018).
In addition to apoptosis, which is a critical aspect of cancer progression, we assessed other hallmarks of cancer progression through this experiment. Metastasis is one the most pivotal processes in cancer progression, and MMPs appear to a play critical role in both metastasis and apoptosis processes (Mannello et al., 2005). It has been demonstrated that Cisplatin can suppress cell migration through targeting MMPs (Urso et al., 2010; Montiel et al., 2009). With that context, we established that the Cisplatin- V. pseudo-Negundo combination therapy might decrease the expression level of MMPs such as MMP-3 in CC cells.
In addition, significant morphological changes have appeared in CaSki and HeLa cells following V. pseudo-Negundo treatment. The combination treatment persuaded a notable number of CC cells to adopt a floating and round-shaped morphology. In this regard, it is speculated that V. pseudo-Negundo extract leads to interference in the normal physiological conditions of HeLa and CaSki cells. Moreover, the effect of Cisplatin on morphological alterations has been previously demonstrated in different cells (Gonçalves et al., 2006). Also, the results of the colony formation test indicated that the number of colonies decreased significantly after 14 days compared to the initial days following combination therapy in both cell lines. In other words, the number of colonies in combination groups compared to monotherapy and control groups decreased dramatically, while the colony numbers are not significant between treatment groups (p < 0.0001).
In addition to the prominent anticancer activity of Cisplatin through induction of mitochondrial apoptosis, the high side effects and frequent chemoresistance results in therapeutic failure (Galluzzi et al., 2012). Here, we introduced the ethanolic extraction of the V. pseudo-Negundo plant as a promising candidate for combination therapy with Cisplatin in CC treatment. We demonstrated that V. pseudo-Negundo has a lethal and antioxidant effect on CaSki and HeLa cancerous cells. Our data also suggested that this natural metabolic exhibited synergistic effects with Cisplatin and can increase cervical cancerous cell features such as apoptosis, ROS production, colony formation, and cell motility suggesting a novel approach for CC treatment.
5 Conclusion
In recent decades, preclinical studies have indicated the interaction between chemotherapy drugs and medicinal plant extracts as a therapeutic strategy for many malignancies. Improving the effectiveness of treatment while reducing side effects is one of the aims of combination therapy. Despite the advantages of combined treatments, they may also have disadvantages (Déciga-Campos et al., 2022). So far, the combination therapy of Cisplatin and Paclitaxel is considered a standard chemotherapeutic regimen for the treatment of recurrent or metastatic CC. However, the overall response rate and the median overall survival of patients receiving this regimen are unsatisfactory. In addition, either intrinsic or acquired resistance to Cisplatin can seriously compromise the efficacy of this widely used drug. This underscores the urgent need to introduce novel metabolites that can overcome this resistance, and increase efficacy while exerting minimal cytotoxicity. In this regard, natural metabolites appear to be promising for the treatment of malignancies including CC. Here, we established that the ethanolic extraction of V. pseudo-Negundo in combination with Cisplatin can increase its efficacy for the treatment of cervical cancer, suggesting a novel potential for further investigations and offering promise for future clinical therapeutic methods. According to the conducted studies, it has been reported that natural products can prevent the toxicity caused by Cisplatin in different parts of the body, such as the liver, kidney, and nervous system. As a result, the combination therapy of Cisplatin with natural products leads to a reduction in the side effects caused by Cisplatin. Therefore, it is suggested that formulations based on the natural metabolites of Cisplatin be considered and developed as a novel treatment method to prevent the progression of malignancy.
6 Limitations
Unfortunately, the number of studies and reports published in our area of study is very limited. Reported studies varied significantly according to the type of cell line, which may have affected the results. In this study, we focused on the main extract; however, it would be preferable to examine the individual active metabolites in the methanolic extract of the V. pseudo-Negundo plant in future clinical studies. Also, the lack of access to latest techniques and technologies such as HPLC, NMR, and lack of time for researchers had been influenced. Furthermore, future studies should investigate other genes involved in cellular mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
PSA: Writing–review and editing, Writing–original draft, Methodology, Investigation, Formal Analysis. JSN: Writing–review and editing, Project administration, Formal Analysis. ORF: Writing–review and editing, Formal Analysis. SMRH: Writing–review and editing, Formal Analysis. MB: Writing–review and editing. TEM: Writing–review and editing. HBB: Writing–review and editing, Supervision, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by a grant from Tabriz University of Medical Sciences, (Grant No: IR.TBZMED.VCR.REC.1400.162).
Acknowledgments
This project was supported by the Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamov, G. V., Rendyuk, T. D., Saybel, O. L., Dargaeva, T. D., Tsitsilin, A. N., and Bokov, D. O. (2022). Vitex agnus-castus: botanical features and area, chemical composition of fruit, pharmacological properties, and medicinal uses. J. Appl. Pharm. Sci. 12 (3), 034–044. doi:10.7324/JAPS.2022.120304
Ahmad, S. N. S., Rashtchizadeh, N., Argani, H., Roshangar, L., Ghorbanihaghjo, A., Sanajou, D., et al. (2019). Tangeretin protects renal tubular epithelial cells against experimental cisplatin toxicity. Iran. J. basic Med. Sci. 22 (2), 179–186. doi:10.22038/ijbms.2018.32010.7691
Aldaghi, L. S., Rezaee Seresht, H., and Cheshomi, H. (2016). The cytotoxic effect of Vitex pseudo negundo fruit in breast cancer (MCF7) cell line. J. Sabzevar Univ. Med. Sci. 23 (2), 353–359.
Almutairi, M. M., Alanazi, W. A., Alshammari, M. A., Alotaibi, M. R., Alhoshani, A. R., Al-Rejaie, S. S., et al. (2017). Neuro-protective effect of rutin against Cisplatin-induced neurotoxic rat model. BMC complementary Altern. Med. 17, 472–479. doi:10.1186/s12906-017-1976-9
Casanova, A. G., Prieto, M., Colino, C. I., Gutiérrez-Millán, C., Ruszkowska-Ciastek, B., de Paz, E., et al. (2021). A micellar formulation of quercetin prevents cisplatin nephrotoxicity. Int. J. Mol. Sci. 22 (2), 729. doi:10.3390/ijms22020729
Darwish, M. A., Abo-Youssef, A. M., Khalaf, M. M., Abo-Saif, A. A., Saleh, I. G., and Abdelghany, T. M. (2018). Resveratrol influences platinum pharmacokinetics: a novel mechanism in protection against cisplatin-induced nephrotoxicity. Toxicol. Lett. 290, 73–82. doi:10.1016/j.toxlet.2018.03.023
Dasari, S., and Bernard Tchounwou, P. (2014). Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378. doi:10.1016/j.ejphar.2014.07.025
Dasari, S., Njiki, S., Mbemi, A., Yedjou, C. G., and Tchounwou, P. B. (2022). Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 23 (3), 1532. doi:10.3390/ijms23031532
Déciga-Campos, M., Ventura-Martínez, R., González-Trujano, M. E., and Silveira, D. (2022). Editorial: Pharmacological interaction between drugs and medicinal plants. Front. Pharmacol. 13, 1081090. doi:10.3389/fphar.2022.1081090
Dunnill, C., Patton, T., Brennan, J., Barrett, J., Dryden, M., Cooke, J., et al. (2017). Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. wound J. 14 (1), 89–96. doi:10.1111/iwj.12557
Erdogan, S., Turkekul, K., Serttas, R., and Erdogan, Z. (2017). The natural flavonoid apigenin sensitizes human CD44+ prostate cancer stem cells to cisplatin therapy. Biomed. and Pharmacother. 88, 210–217. doi:10.1016/j.biopha.2017.01.056
Florea, A.-M., and Büsselberg, D. (2011). Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 3 (1), 1351–1371. doi:10.3390/cancers3011351
Galluzzi, L., Senovilla, L., Vitale, I., Michels, J., Martins, I., Kepp, O., et al. (2012). Molecular mechanisms of cisplatin resistance. Oncogene 31 (15), 1869–1883. doi:10.1038/onc.2011.384
Garbi, M. I., Osman, E. E., Kabbashi, A. S., Saleh, M. S., Yuosof, Y. S., Mahmoud, S. A., et al. (2015). Cytotoxicity of Vitex trifolia leaf extracts on MCF-7 and Vero cell lines. J. Sci. Innov. Res. 4 (2), 89–93. doi:10.31254/jsir.2015.4208
Gonçalves, E. M., Ventura, C. Â., Yano, T., Rodrigues Macedo, M. L., and Genari, S. C. (2006). Morphological and growth alterations in Vero cells transformed by cisplatin. Cell Biol. Int. 30 (6), 485–494. doi:10.1016/j.cellbi.2005.12.007
Gong, G., Shen, Y.-L., Lan, H.-Y., Jin, J.-M., An, P., Zhang, L.-J., et al. (2021). The Cyr61 is a potential target for rotundifuran, a natural labdane-type diterpene from Vitex trifolia L., to trigger apoptosis of cervical cancer cells. Oxidative Med. Cell. Longev. 2021 (1), 6677687. doi:10.1155/2021/6677687
Gorrini, C., Harris, I. S., and Mak, T. W. (2013). Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12 (12), 931–947. doi:10.1038/nrd4002
Graham, J. G., Quinn, M. L., Fabricant, D. S., and Farnsworth, N. R. (2000). Plants used against cancer–an extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 73 (3), 347–377. doi:10.1016/s0378-8741(00)00341-x
Gulumian, M., Yahaya, E. S., and Steenkamp, V. (2018). African herbal remedies with antioxidant activity: a potential resource base for wound treatment. Evidence-Based Complementary Altern. Med. 2018 (1), 4089541. doi:10.1155/2018/4089541
Hang, W., Yin, Z.-X., Liu, G., Zeng, Q., Shen, X.-F., Sun, Q.-H., et al. (2018). Piperlongumine and p53-reactivator APR-246 selectively induce cell death in HNSCC by targeting GSTP1. Oncogene 37 (25), 3384–3398. doi:10.1038/s41388-017-0110-2
Hazman, Ö., Bozkurt, M. F., Fidan, A. F., Uysal, F. E., and Çelik, S. (2018). The effect of boric acid and borax on oxidative stress, inflammation, ER stress and apoptosis in cisplatin toxication and nephrotoxicity developing as a result of toxication. Inflammation 41, 1032–1048. doi:10.1007/s10753-018-0756-0
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Jing, R., Ban, Y., Xu, W., Nian, H., Guo, Y., Geng, Y., et al. (2019). Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine 58, 152825. doi:10.1016/j.phymed.2019.152825
Ju, S. M., Kang, J. G., Bae, J. S., Pae, H. O., Lyu, Y. S., and Jeon, B. H. (2015). The flavonoid apigenin ameliorates cisplatin-induced nephrotoxicity through reduction of p53 activation and promotion of PI3K/akt pathway in human renal proximal tubular epithelial cells. Evidence-Based Complementary Altern. Med. 2015 (1), 186436. doi:10.1155/2015/186436
Kachadourian, R., Leitner, H. M., and Day, B. J. (2007). Selected flavonoids potentiate the toxicity of cisplatin in human lung adenocarcinoma cells: a role for glutathione depletion. Int. J. Oncol. 31 (1), 161–168. doi:10.3892/ijo.31.1.161
Khan, M., Zheng, B., Yi, F., Rasul, A., Gu, Z., Li, T., et al. (2012). Pseudolaric Acid B induces caspase-dependent and caspase-independent apoptosis in u87 glioblastoma cells. Evidence-based Complementary Altern. Med. 2012 (1), 957568. doi:10.1155/2012/957568
Kirtikar, K. R., and Basu, B. D. (1918). Indian medicinal plants: publisher not identified Basu. Bhuwaneśwari Âśrama.
Li, H., Wu, X., and Cheng, X. (2016). Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 27 (4), e43. doi:10.3802/jgo.2016.27.e43
Li, J., Wang, Y., Lei, J.-C., Hao, Y., Yang, Y., Yang, C.-X., et al. (2014). Sensitisation of ovarian cancer cells to cisplatin by flavonoids from Scutellaria barbata. Nat. Prod. Res. 28 (10), 683–689. doi:10.1080/14786419.2013.871547
Liu, N., Wang, K. S., Qi, M., Zhou, Y. J., Zeng, G. Y., Tao, J., et al. (2018). Vitexin compound 1, a novel extraction from a Chinese herb, suppresses melanoma cell growth through DNA damage by increasing ROS levels. J. Exp. and Clin. Cancer Res. 37, 269–316. doi:10.1186/s13046-018-0897-x
Liu, W., Yang, B., Yang, L., Kaur, J., Jessop, C., Fadhil, R., et al. (2019a). Therapeutic effects of ten commonly used Chinese herbs and their bioactive compounds on cancers. Evidence-Based Complementary Altern. Med. 2019 (1), 6057837. doi:10.1155/2019/6057837
Liu, W., Yang, B., Yang, L., Kaur, J., Jessop, C., Fadhil, R., et al. (2019b). Therapeutic effects of ten commonly used Chinese herbs and their bioactive compounds on cancers. Evidence-Based Complementary Altern. Med. 2019, 6057837. doi:10.1155/2019/6057837
Liu, Y., Wang, X., Li, W., Xu, Y., Zhuo, Y., Li, M., et al. (2020). Oroxylin A reverses hypoxia-induced cisplatin resistance through inhibiting HIF-1α mediated XPC transcription. Oncogene 39 (45), 6893–6905. doi:10.1038/s41388-020-01474-x
Madhulika Singh, M. S., Kulpreet Bhui, K. B., Richa Singh, R. S., and Yogeshwer Shukla, Y. S. Tea polyphenols enhance cisplatin chemosensitivity in cervical cancer cells via induction of apoptosis. 2013.
Mannello, F., Luchetti, F., Falcieri, E., and Papa, S. (2005). Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis 10 (1), 19–24. doi:10.1007/s10495-005-6058-7
Meng, F.-M., Yang, J.-B., Yang, C.-H., Jiang, Y., Zhou, Y.-F., Yu, B., et al. (2012). Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest. Asian Pac. J. Cancer Prev. 13 (12), 6369–6374. doi:10.7314/apjcp.2012.13.12.6369
Montiel, M., Urso, L., de la Blanca, E. P., Marsigliante, S., and Jiménez, E. (2009). Cisplatin reduces endothelial cell migration via regulation of type 2-matrix metalloproteinase activity. Cell. Physiology Biochem. 23 (4-6), 441–448. doi:10.1159/000218191
Nimlamool, W., Chansakaow, S., Potikanond, S., Wikan, N., Hankittichai, P., Ruttanapattanakul, J., et al. (2022). The leaf extract of Mitrephora chulabhorniana suppresses migration and invasion and induces human cervical cancer cell apoptosis through caspase-dependent pathway. BioMed Res. Int. 2022, 2028082. doi:10.1155/2022/2028082
Poveda, A., Salazar, R., Del Campo, J. M., Mendiola, C., Cassinello, J., Ojeda, B., et al. (2007). Update in the management of ovarian and cervical carcinoma. Clin. Transl. Oncol. 9, 443–451. doi:10.1007/s12094-007-0083-7
Puglia, L. T., Lowry, J., and Tamagno, G. (2023). Vitex agnus castus effects on hyperprolactinaemia. Front. Endocrinol. 14, 1269781. doi:10.3389/fendo.2023.1269781
Raj, L., Ide, T., Gurkar, A. U., Foley, M., Schenone, M., Li, X., et al. (2011). Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475 (7355), 231–234. doi:10.1038/nature10167
Rastogi, N., Duggal, S., Singh, S. K., Porwal, K., Srivastava, V. K., Maurya, R., et al. (2015). Proteasome inhibition mediates p53 reactivation and anti-cancer activity of 6-gingerol in cervical cancer cells. Oncotarget 6 (41), 43310–43325. doi:10.18632/oncotarget.6383
Salehpour, Z., Jahantab, E., Morshedloo, M. R., Iraji, A., and Mohamadi, J. (2018). Essential oil composition of aerial parts of Vitex pseudo-negundo populations collected from Southwest of Iran. J. Essent. Oil Bear. Plants 21 (2), 570–576. doi:10.1080/0972060x.2018.1438928
Serkies, K., and Jassem, J. (2004). Concurrent weekly cisplatin and radiotherapy in routine management of cervical cancer: a report on patient compliance and acute toxicity. Int. J. Radiat. Oncology* Biology* Phys. 60 (3), 814–821. doi:10.1016/j.ijrobp.2004.04.042
Shiri Aghbash, P., Hemmat, N., Baradaran, B., and Bannazadeh Baghi, H. (2023). siRNA-E6 sensitizes HPV-16-related cervical cancer through Oxaliplatin: an in vitro study on anti-cancer combination therapy. Eur. J. Med. Res. 28 (1), 42. doi:10.1186/s40001-023-01014-9
Small, Jr W., Bacon, M. A., Bajaj, A., Chuang, L. T., Fisher, B. J., Harkenrider, M. M., et al. (2017). Cervical cancer: a global health crisis. Cancer 123 (13), 2404–2412. doi:10.1002/cncr.30667
Talib, W. H., Awajan, D., Hamed, R. A., Azzam, A. O., Mahmod, A. I., and Al-Yasari, I. H. (2022). Combination anticancer therapies using selected phytochemicals. Mol. Basel, Switz. 27 (17), 5452. doi:10.3390/molecules27175452
Tandon, V. R., and Gupta, R. K. (2005). An experimental evaluation of anticonvulsant activity of Vitex-negundo. Indian J. physiology Pharmacol. 49 (2), 199–205.
Tsikouras, P., Zervoudis, S., Manav, B., Tomara, E., Iatrakis, G., Romanidis, C., et al. (2016). Cervical cancer: screening, diagnosis and staging. J. buon 21 (2), 320–325.
Urso, L., Muscella, A., Calabriso, N., Vetrugno, C., Jiménez, E., Montiel, M., et al. (2010). Effects of cisplatin on matrix metalloproteinase-2 in transformed thyroid cells. Biochem. Pharmacol. 79 (6), 810–816. doi:10.1016/j.bcp.2009.10.013
Wang, L., Yu, Y., Chow, D.-C., Yan, F., Hsu, C.-C., Stossi, F., et al. (2015). Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer cell 28 (2), 240–252. doi:10.1016/j.ccell.2015.07.005
Wang, S.-w., Xu, Y. I., Weng, Y.-y., Fan, X.-y., Bai, Y.-f., Zheng, X.-y., et al. (2018). Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem. Toxicol. 114, 227–236. doi:10.1016/j.fct.2018.02.041
Yang, Y., Zhang, Y., Wang, L., and Lee, S. (2017). Levistolide A induces apoptosis via ROS-mediated ER stress pathway in colon cancer cells. Cell. Physiology Biochem. 42 (3), 929–938. doi:10.1159/000478647
Keywords: Vitex pseudo-negundo, cisplatin, combination therapy, human papillomavirus, cervical cancer, botanical drug
Citation: Shiri Aghbash P, Sadri Nahand J, Rahbar Farzam O, Hosseini SMR, Bayat M, Entezari Maleki T and Bannazadeh Baghi H (2024) Combination of Vitex pseudo-negundo methanolic-extract with cisplatin can induce antioxidant activity and apoptosis in HeLa and Caski cells. Front. Pharmacol. 15:1476152. doi: 10.3389/fphar.2024.1476152
Received: 05 August 2024; Accepted: 18 November 2024;
Published: 04 December 2024.
Edited by:
Qingquan Liu, Capital Medical University, ChinaReviewed by:
Zhixing Wu, Texas A and M University, United StatesIbrahim Ahmed Shaikh, Najran University, Saudi Arabia
Copyright © 2024 Shiri Aghbash, Sadri Nahand, Rahbar Farzam, Hosseini, Bayat, Entezari Maleki and Bannazadeh Baghi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossein Bannazadeh Baghi, aGJhbm5hemFkZWhAdGJ6bWVkLmFjLmly, aGIuemFkZWhAZ21haWwuY29t
†ORCID: Hossein Bannazadeh Baghi, orcid.org/0000-0002-2513-5361
 Parisa Shiri Aghbash
Parisa Shiri Aghbash Javid Sadri Nahand
Javid Sadri Nahand Omid Rahbar Farzam3
Omid Rahbar Farzam3 Hossein Bannazadeh Baghi
Hossein Bannazadeh Baghi