- 1Institute of Digestive Diseases, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate school, China Academy of Chinese Medical Sciences, Beijing, China
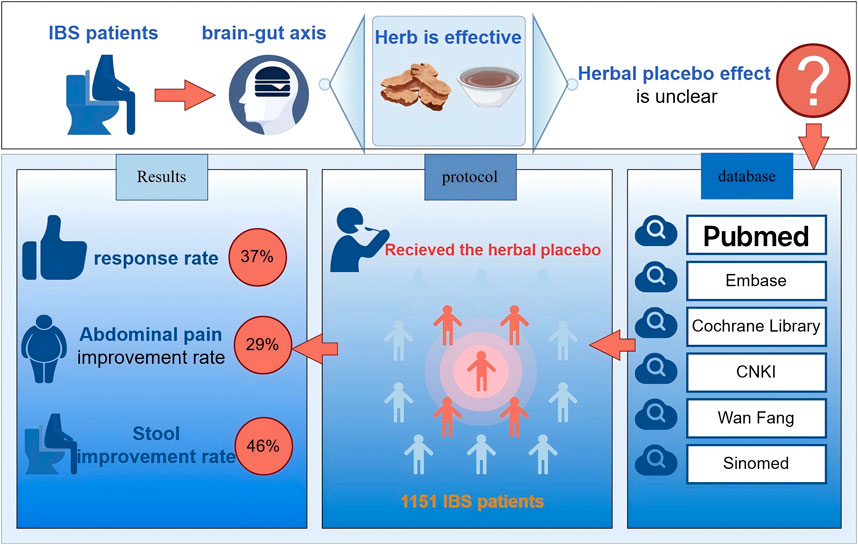
Aim of the study: To systematically evaluate the herbal placebo response in randomized controlled trials (RCTs) of herbal medicine on irritable bowel syndrome (IBS).
Materials and methods: We searched for RCTs with herbal placebo groups for IBS in PubMed, EMBASE, the Cochrane Library, the China National Knowledge Infrastructure (CNKI), the Wan Fang database and Sinomed database from 31 January 1994 to November 2023, and the quality of the literature was evaluated by the Cochrane risk of bias assessment criteria. The primary outcome indicators were response rate, abdominal pain and stool improvement rate, which were analyzed by single-group rate meta-analysis. Secondary outcomes were analyzed in subgroups based on diagnostic criteria, duration of treatment, subtype, research locations, placebo form, and presence of herbal ingredients to look for factors affecting respond rate.
Results: The study included 24 papers, involving a total of 2,596 patients. Of these, 1151 IBS patients were treated with the herbal placebo. The placebo response rate in IBS patients in the herbal placebo group was 37% (P < 0.01,I2 = 75%). A total of 287 patients in five studies were given the herbal placebo, and the improvement rate of abdominal pain was 29% (P = 0.83, I2 = 0%). Four studies enrolled a total of 212 patients with IBS who received herbal placebo, and the stool improvement rate was 46% (P = 0.02 < 0.05, I2 = 71%). The research locations and treatment duration were sources of heterogeneity (P < 0.05).
Conclusion: There is a significant herbal placebo response in patients with IBS. Different research locations and treatment durations are major sources of heterogeneity that may affect IBS patient response rates. The addition of a low dose of herbal ingredients when simulating an herbal placebo does not exaggerate the therapeutic effect of the placebo. There is a lack of uniformity and standardization in the preparation and evaluation of herbal placebos.
1 Introduction
In the past, irritable bowel syndrome (IBS) was thought to be a functional bowel disease that could not be explained by organic, structural abnormalities, but it is now widely recognized that IBS is mediated by brain-gut interaction disorders in functional gastrointestinal diseases (FGIDs) (Huang et al., 2023). Although the pathogenesis of IBS is not fully understood, it is now thought to be associated with genetics (e.g., SCN5A mutation), intestinal infections, microbial disorders, low-grade mucosal inflammation, immune activation, and bile acid metabolism disorders, and there is strong evidence for a brain-related connection (Holtmann et al., 2016). The prevalence of IBS varies significantly between locations, with the prevalence of IBS ranging from 1.1% (France and Iran) to 35.5% (Mexico), with large differences in prevalence between Europe and Asia (Black and Ford, 2020; Huang et al., 2023; Rahman et al., 2017; Sperber et al., 2017). Drugs recommended in the pharmacological treatment of IBS-D (diarrhea) include rifaximin to regulate intestinal flora, the mixed μ- and κ-opioid agonist/delta-opioid antagonist eludoline, and the selective 5-hydroxytryptamine 5-HT3 antagonist alosetron for the treatment of diarrhea and abdominal pain, but there are many limitations, such as unsatisfactory efficacy, strict indications, and adverse effects (Brenner and Sayuk, 2020; Nee et al., 2015). Due to the disappointment of conventional treatments, more and more people are looking for complementary and alternative medicine (CAM) treatments (Wu, 2010). Therefore, finding effective and safe CAM therapies is a critical issue that requires immediate attention nowadays. Herbal medicine, a multi-targeted therapy for the treatment of IBS, is a potential treatment for IBS by improving gastrointestinal motility, decreasing visceral hypersensitivity, and modulating intestinal flora (Chen et al., 2023; Shapiro and Deutsch, 2021). Herbs improved abdominal pain and global symptoms in patients with IBS compared to placebo (RR = 1.57, 95% CI: 1.31–1.88, I2 = 77%), but the quality of the current evidence is low and the lack of high-quality and rigorous RCTs of herbal medicines remains a challenge (Billings et al., 2021).
A high placebo response has been demonstrated in FGIDs with brain-gut axis interactions (Enck and Klosterhalfen, 2020), and IBS is one of the most common FGIDs associated with psychological effects, stress, and strain. One study showed that 27.3% of patients with IBS felt an overall improvement in symptoms after receiving placebo (Bosman et al., 2021). Therefore, in clinical RCTs, the IBS placebo response is important to control for the influence of non-treatment factors on the outcome; however, the herbal placebo response rate in IBS trials has not received attention. High-quality RCTs should account for the placebo effect. Therefore, we conducted a meta-analysis to clarify the herbal placebo response rate in patients with IBS in clinical trials to look for factors that may influence the placebo response rate and heterogeneity.
2 Materials and methods
This study was conducted in accordance with the updated 2020 Statement on Systematic Evaluation and Meta-Analysis. The protocol for this study is registered under PROSPERO registration number CRD42024509587, which can be found at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=509587.
2.1 Search strategy
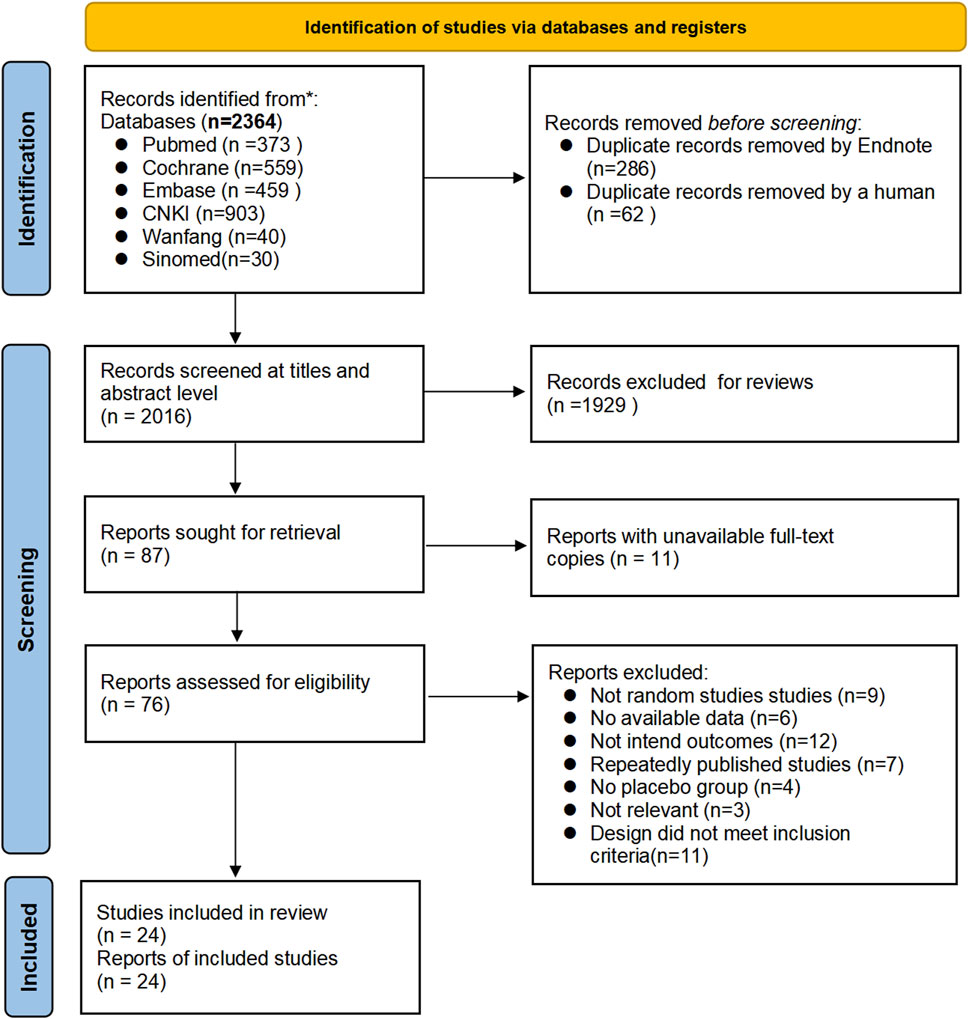
The English databases of PubMed, Embase, and Cochrane Library and the Chinese databases of CNKI, WanFang, and Sinomed were searched by two investigators combining the subject terms and free text words from January 1994 to November 2023. The terms of IBS: Irritable Bowel Syndrome and functional disease, colon [both as medical subject heading (MeSH) and free text terms] and Irritable Bowel Syndromes, Syndrome, Irritable Bowel, Syndromes, Irritable Bowel, Colon, Irritable, Irritable Colon, Colitis, Mucous, Colitides, Mucous, Mucous Colitides, Mucous Colitis, IBS, spastic colon (as free text terms). We combine these using the set operator AND with term identifiers of herbal and randomized controlled trials: Traditional Chinese Medicine, Drugs, Chinese Herba, Phytotherapy (both as MeSH and free text terms), and chinese medic*, chinese herb*, chinese drug*, chinese formul*, chinese plant, chinese prescri*, complementary therap*, alternativ* treatment*, alternativ* therap*, alternativ* medicin*, complementary therap* (as free text terms). And randomized controlled trial (publication type), randomized, placebo (as free text terms). And detailed search strategies for other databases are shown in Supplement. With the help of Endnote X9 to screen for duplicate literature, two researchers (H.K.Y. and Z.T.) independently read and evaluated the abstracts for literature screening, and when disagreements were encountered, L.L. participated in discussion and problem solving.
2.2 Inclusion criteria
We included the literature of clinical studies with herbal placebo control groups for the treatment of IBS, applying the following specific inclusion criteria: 1) Participants in the study must be over 18 years old, diagnosed with IBS based on Rome Criteria I-IV, and undergo a gastroscopy examination to rule out other organic diseases before recruitment. 2) Type of study design: RCTs meeting the criteria for double-blind, RCTs with a placebo group; 3) Intervention group setup: administration of herbal placebo soup, granules, proprietary Chinese medicines, poultices, and other herbal placebos as a test group, not receiving other basic treatments for IBS, and herbal medicines as a test group, with a treatment duration of ≥4 weeks; 4) Outcome indicators: the results of the study must report the response rate of the placebo group: response rate = (significant + improvement)/total patient cases × 100%; the number of extracted placebo-responsive subjects; and the information can be obtained directly from the articles or by calculation; 5) Language: English and Chinese.
2.3 Exclusion criteria
1) Duplicate papers with the most complete information were included, and all other duplicates were excluded; 2) Dissertations in Chinese databases; 3) Conference papers in Chinese databases; 4) Patients with significant symptom overlap in the study population. Dissertations and conference papers from Chinese databases were excluded because they were not peer-reviewed and there may be hidden risks to the rigor of the data (Supplementary Table S2). Furthermore, we excluded overlapping functional dyspepsia and gastroesophageal reflux disease due to their potential of biasing the results.
2.4 Data extraction
The basic information of the articles, including the first author, year of publication, title of the paper, country, language, diagnostic criteria, subtype, sample size, form of placebo, age, duration of administration, etc., was extracted by H.K.Y. and L.M., respectively, and recorded in the Excel 2021 form. If the efficiency is given directly, it is extracted directly; if it is given separately as relieve, significantly effective, improve, or ineffective, the data of the first two are taken (significant efficiency); the patients who fall off after enrollment in the placebo group are counted as invalid. Since it belongs to the analysis of the rate of a single group, when there is a full analysis set (FAS) and a per protocol set (PPS), the choice of the FAS is the result. When disagreements arose with the data, two individuals reexamined the entire text and reached a consensus through discussion.
2.5 Quality and risk of bias evaluation
The risk of bias evaluation of the included RCTs was evaluated using the evaluation entries of the RCT risk of bias evaluation tool recommended by the Cochrane Collaboration (Harbord et al., 2006; Higgins et al., 2011) and was evaluated by two reviewers (Z.T. and H.K.Y.) according to the entries, respectively. If differences were encountered, both of them reread the full text and resolved the differences by reaching a consensus through discussion. Also, the methods of Egger’s and Begg’s test (Sterne et al., 2011) were used to determine whether there was publication bias based on the P-value (the calibration level was α < 0.05).
2.6 Statistical analysis
R 4.2.3 software and the metaprop package were used to analyze the single group rate of response rate, abdominal pain improvement rate, and stool improvement rate, and the test of heterogeneity of the results of the included studies was analyzed using the X2 test, which was combined with the I2 quantification to determine the magnitude of the heterogeneity, and the inverted variance was used in the weighting method. The effect target data were transformed using the arcsine square root transformation, which was independent of the P-value without the need for continuous calibration. Setting the significance threshold as P< 0.05. We performed subgroup analyses based on various diagnostic criteria, treatment duration, subtype, study site, placebo form, and presence of herbal components. We used I2 and the p-value of Q tests to assess heterogeneity, which was defined as significant if I2 ≥ 50% and the p-value of Q tests <0.1 (Higgins et al., 2003). We analyzed placebo response rates using a random-effects model if there was significant statistical heterogeneity between studies.
3 Results
3.1 Search results
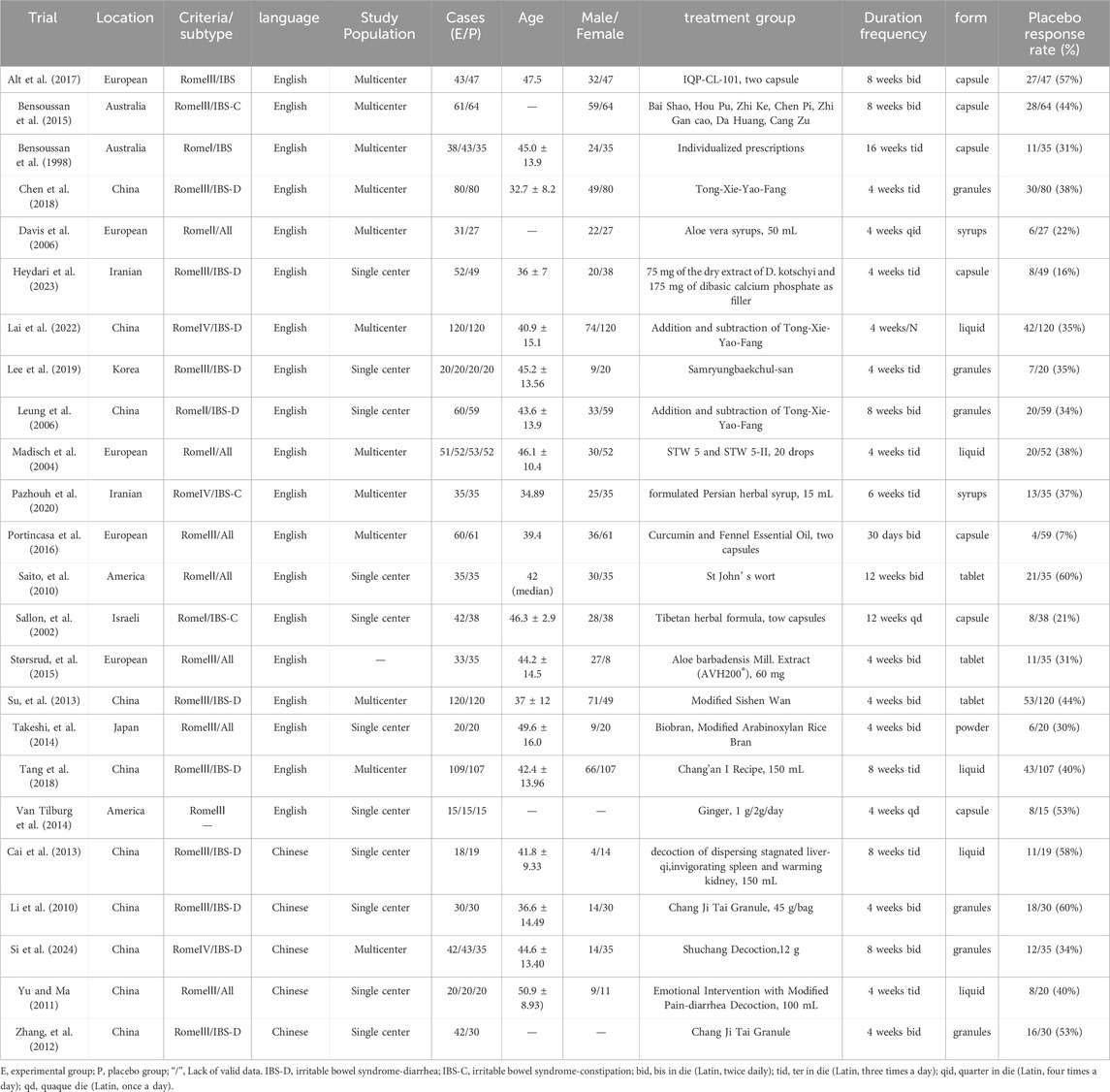
Twenty-four articles (Alt et al., 2017; Bensoussan et al., 2015; Bensoussan et al., 1998; Cai et al., 2013; Chen et al., 2018; Davis et al., 2006; Heydari et al., 2023; Lai et al., 2022; Lee et al., 2019; Leung et al., 2006; Li et al., 2010; Madisch et al., 2004; Pazhouh et al., 2020; Portincasa et al., 2016; Saito et al., 2010; Sallon et al., 2002; Si et al., 2024; Størsrud et al., 2015; Su et al., 2013; Takeshi et al., 2014; Tang et al., 2018; Van Tilburg et al., 2014; Yu and Ma, 2011; Zhang et al., 2012) were finally screened to meet the inclusion criteria, including 19 English articles (Alt et al., 2017; Bensoussan et al., 2015; Bensoussan et al., 1998; Chen et al., 2018; Davis et al., 2006; Heydari et al., 2023; Lai et al., 2022; Lee et al., 2019; Leung et al., 2006; Madisch et al., 2004; Pazhouh et al., 2020; Portincasa et al., 2016; Saito et al., 2010; Sallon et al., 2002; Størsrud et al., 2015; Su et al., 2013; Takeshi et al., 2014; Tang et al., 2018; Van Tilburg et al., 2014) (79.17%) and 5 Chinese articles (Cai et al., 2013; Li et al., 2010; Si et al., 2024; Yu and Ma, 2011; Zhang et al., 2012) (20.83%), in which 1,151 patients with IBS were treated with herbal placebo, and the process of literature search is shown in Figure 1.
3.2 Basic characteristics
The basic characteristics of the 24 studies are shown in Table 1, including 12 (Alt et al., 2017; Bensoussan et al., 2015; Bensoussan et al., 1998; Chen et al., 2018; Davis et al., 2006; Lai et al., 2022; Madisch et al., 2004; Pazhouh et al., 2020; Portincasa et al., 2016; Si et al., 2024; Su et al., 2013; Tang et al., 2018) multicenter studies and 11 (Cai et al., 2013; Heydari et al., 2023; Lee et al., 2019; Leung et al., 2006; Li et al., 2010; Saito et al., 2010; Sallon et al., 2002; Takeshi et al., 2014; Van Tilburg et al., 2014; Yu and Ma, 2011; Zhang et al., 2012) single-center studies, 10 of which were conducted in China (Cai et al., 2013; Chen et al., 2018; Lai et al., 2022; Leung et al., 2006; Li et al., 2010; Si et al., 2024; Su et al., 2013; Tang et al., 2018; Yu and Ma, 2011; Zhang et al., 2012) and the rest in Australia, Europe, the Middle East, etc. (see Table 1 for details).
3.3 Risk of bias assessment
Twenty-four studies were evaluated using Cochrane’s RCT risk of bias, as described in the Supplement (Figures 2, 3; Supplementary Table S1). Three studies (Van Tilburg et al., 2014; Yu and Ma, 2011; Zhang et al., 2012) did not state the specific method of randomization. Nine studies (Alt et al., 2017; Heydari et al., 2023; Portincasa et al., 2016; Sallon et al., 2002; Si et al., 2024; Su et al., 2013; Takeshi et al., 2014; Van Tilburg et al., 2014; Yu and Ma, 2011; Zhang et al., 2012) did not state the method of allocating concealment. Seven studies (Heydari et al., 2023; Portincasa et al., 2016; Su et al., 2013; Takeshi et al., 2014; Van Tilburg et al., 2014; Yu and Ma, 2011; Zhang et al., 2012) did not state the specific recipients of the blinding method or the method of implementation, and one study (Lee et al., 2019) blinded patients only. The majority of studies had a fallout of the placebo group, and only two studies (Madisch et al., 2004; Takeshi et al., 2014) reported no placebo group fallout. The risk of bias was detected using R4.2.3 software, Begg’s test (P = 0.4534) and Egger’s test (P = 0.5157), and the P values were both > 0.05, indicating that there was no significant publication bias.
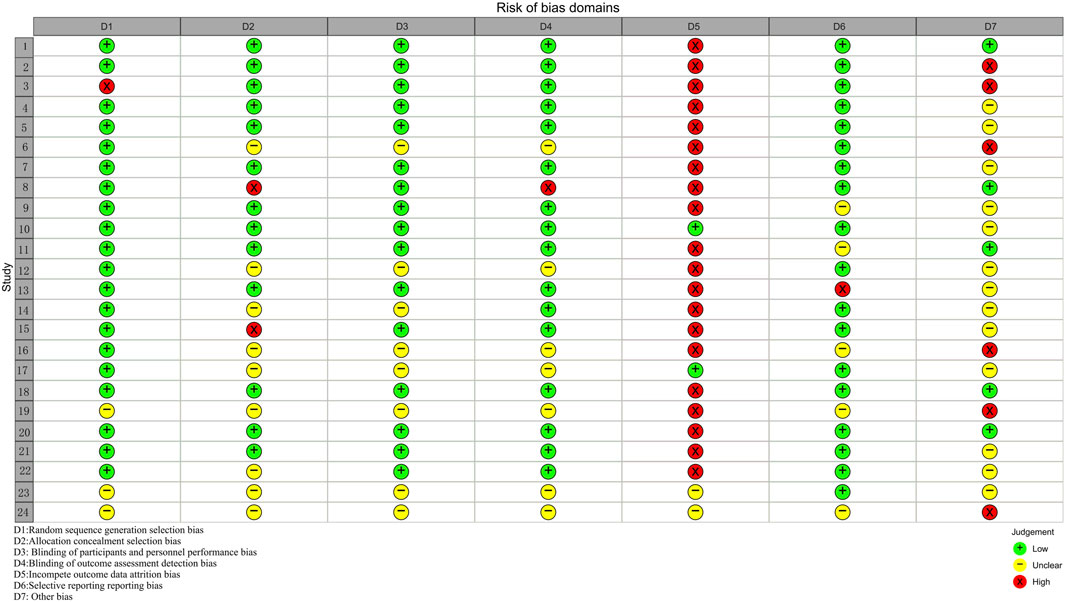
Figure 3. Risk of bias domains (Study 1–24 correspond in turn to references (Alt et al., 2017; Bensoussan et al., 2015; Bensoussan et al., 1998; Chen et al., 2018; Davis et al., 2006; Heydari et al., 2023; Lai et al., 2022; Lee et al., 2019; Leung et al., 2006; Madisch et al., 2004; Pazhouh et al., 2020; Portincasa et al., 2016; Saito et al., 2010; Sallon et al., 2002; Størsrud et al., 2015; Su et al., 2013; Takeshi et al., 2014; Tang et al., 2018; Van Tilburg et al., 2014; Cai et al., 2013; Li et al., 2010; Si et al., 2024; Yu and Ma, 2011; Zhang et al., 2012).
3.4 Placebo response rate
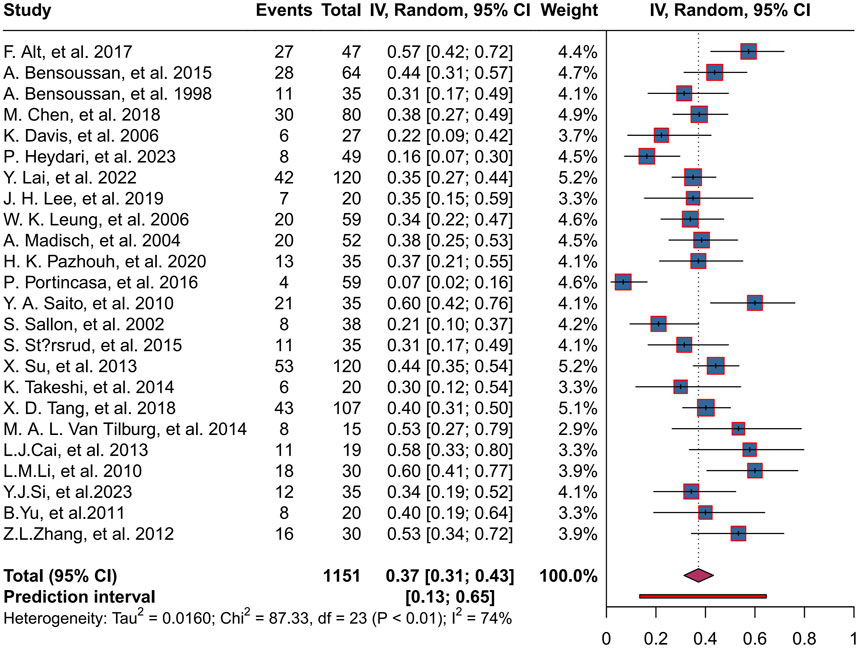
A total of 1,151 patients were enrolled in the placebo group in 24 trials, with a combined herbal placebo response rate of 37% (95% CI, 0.31 to 0.43; P < 0.01). There was significant heterogeneity between trials (I2 = 74%), using a random-effects model. The range of placebo response rates in the trials was 7%–60% (Figure 4).
3.5 Abdominal pain improvement rate
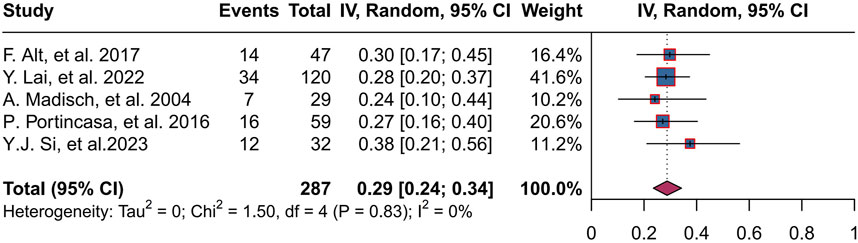
Five studies (Alt et al., 2017; Lai et al., 2022; Madisch et al., 2004; Portincasa et al., 2016; Si et al., 2024) reported an improvement rate in abdominal pain, totaling 287 patients, with a combined abdominal pain improvement rate of 29% (95% CI, 0.24 to 0.34; P = 0.83, I2 = 0%), and a range of abdominal pain improvement rates of 24%–38% across trials (Figure 5).
3.6 Stool improvement rate
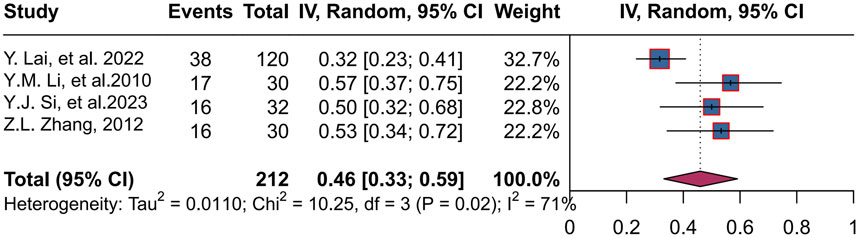
Four studies (Lai et al., 2022; Li et al., 2010; Si et al., 2024; Zhang et al., 2012) enrolled 212 patients, with a combined rate of 46% (95% CI, 0.33 to 0.59; P = 0.02 < 0.05, I2 = 71%), using a random-effects mode. The range of stool improvement rates across trials was 32%–57% (Figure 6).
3.7 Subgroup analysis
3.7.1 Effect of diagnostic criteria on response rate
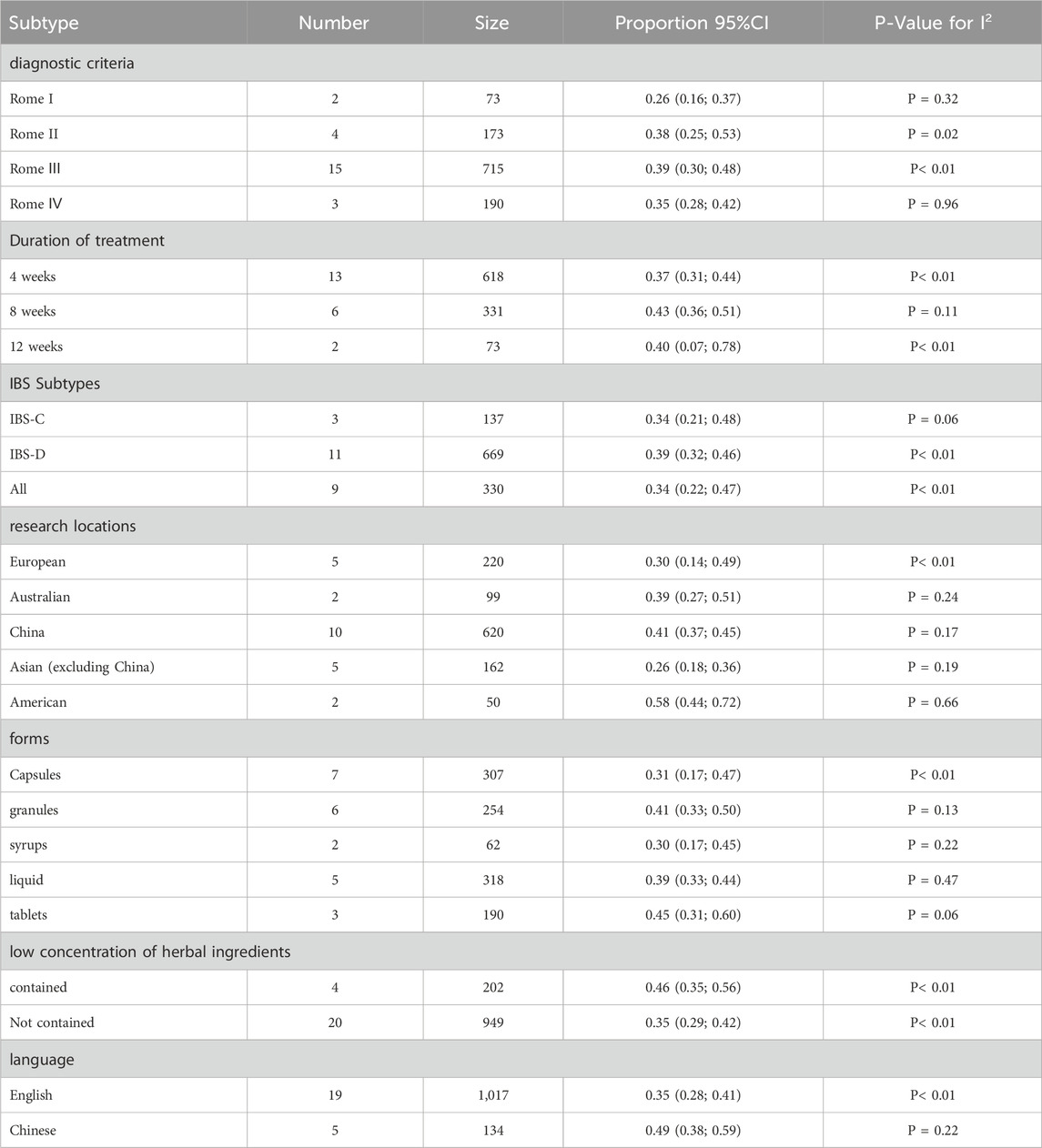
Subgroup analysis was conducted according to the different diagnostic criteria of Rome I-IV, among which 2 studies used Rome I diagnostic criteria, 4 studies used Rome II diagnostic criteria, 15 studies used Rome III diagnostic criteria, and 3 studies used Rome IV diagnostic criteria. The placebo response rates of Chinese medicine were 26%, 38%, 39%, and 35%, respectively (I2 = 0%, I2 = 70%, I2 = 80%, and I2 = 0%), and the difference between groups with different diagnostic criteria was not statistically significant (P = 0.28). For more information, please see Supplementary Figure S1.
3.7.2 Effect of treatment duration on response rate
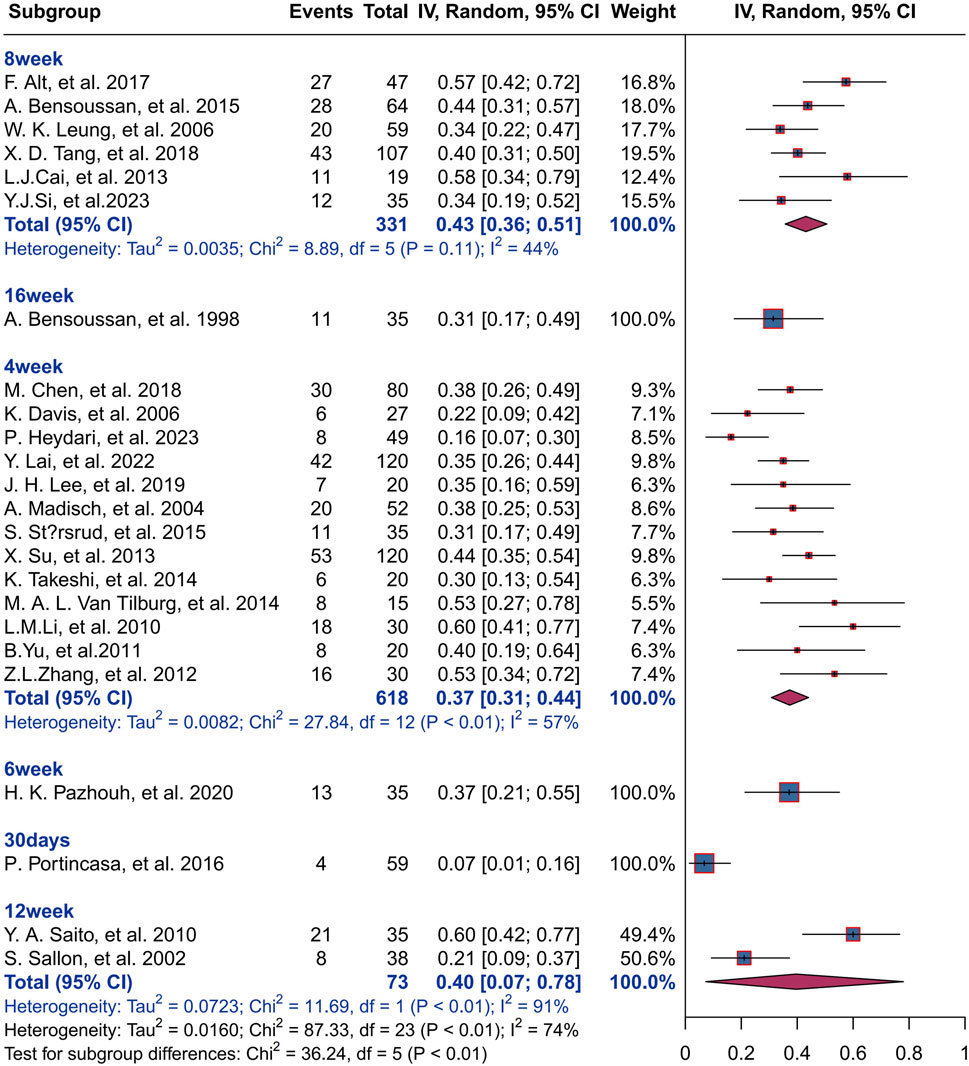
The result of subgroup analysis based on treatment duration shows that 618 IBS patients in 13 studies were treated with herbal placebo for 4 weeks with a response rate of 37% (I2 = 57%, P< 0.01), and 331 IBS patients were treated for 8 weeks with a response rate of 43% (I2 = 44%, P< 0.01).73 IBS patients were treated for 12 weeks with a response rate of 40% (I2 = 91%, P< 0.01)There was statistically significant difference between groups for different treatment durations (P< 0.01). For more information, please see Figure 7.
3.7.3 Effects of IBS subtypes on herbal placebo response rates
The funding of the subgroup analysis based on the IBS subtype revealed that in 11 studies including IBS-D subjects, the herbal placebo response rate was 39% in 669 IBS-D patients (I2 = 62%, P < 0.01) and a herbal placebo response rate of 34% in 137 IBS-C patients in 3 studies (I2 = 64%, P = 0.06). Nine studies did not differentiate between the subtypes of the study subjects. The response rate of IBS patients was 34% (I2 = 84%, P < 0.01). The difference between groups of different sybtypes was not statistically significant (P = 0.69). For more information, please see Supplementary Figure S2.
3.7.4 Effects of research locations on herbal placebo response rates
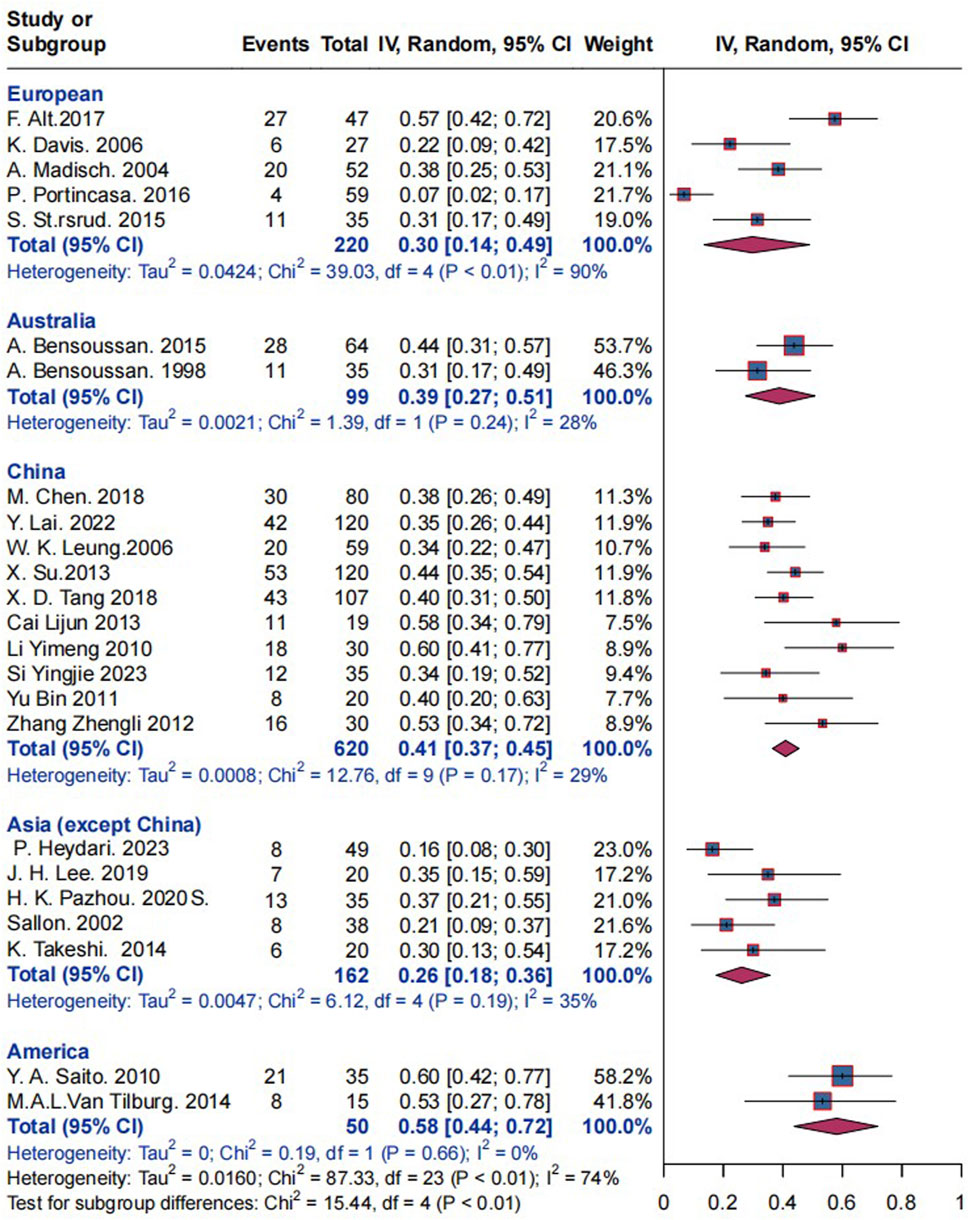
Subgroup analysis based on research locations revealed that the placebo response rate in 220 European patients with IBS studies in five trials was 30% (I2 = 90%, P< 0.01), and the response rate in 99 Australian patients with IBS was 39% (I2 = 28%, P = 0.24). In 620 Chinese patients with IBS, the response rate was 41% (I2 = 29%, P = 0.17). The response for 162 Asian (excluding China) individuals with IBS was 26% (I2 = 35%, P = 0.19), while for 50 American IBS patients it was 58% (I2 = 0%, P = 0.17). Statistically significant differences were observed between the groups of the various research locations (P < 0.01); refer to Figure 8 for further information. China was analyzed separately and Asia was not included in the analysis because after many analyses, we found that there were significant differences in response rates while China was included in Asia (Supplementary Figure S6), and China was the country where herbal medicine was most commonly used, so we analyzed it independently.
3.7.5 Effect of placebo forms on response rate
Seven studies used capsules with a placebo response rate of 31% (I2 = 88%, P< 0.01), five studies used granules with a response rate of 41% (I2 = 41%, P = 0.13), two studies used syrups with a response rate of 30% (I2 = 34%, P = 0.22), five studies used liquid with a response rate of 39% (I2 = 0%, P = 0.47), and three studies used tablets with a response rate of 45% (I2 = 65%, P = 0.06). There was no statistically significant difference between the groups (P = 0.5). Refer to the Supplementary Figure S3 for further information.
3.7.6 Effect of low concentrations of herbal ingredients on response rate
According to the report in the results, four studies mentioned that the placebo ingredients contained low doses of the test group herbs, and 20 studies had placebos that did not contain the test group herbs. Subgroup analyses were performed based on this data, which showed that the response rate in the group containing the herbs was 46% (I2 = 50%, P = 0.11) and in the group not containing the herbs was 35% (I2 = 75%, P< 0.01), and the difference between the groups was not statistically significant (P = 0.12). See Supplementary Figure S4 for more details.
3.7.7 Further analysis
Based on the results of subgroup analyses (Table 2), which revealed that the location of the study was a source of heterogeneity, we used it as a breakthrough point to further analyze the results. ① The research’s location of the study in and out of China: no difference between the two groups (P = 0.14). ② The language of the published article (Chinese and English), the difference between groups was statistically significant (P = 0.03 < 0.05), with 19 studies included in SCI journals published in English, with a response rate of 35% (I2 = 75%, P < 0.01). Five studies published in Chinese, with a response rate of 49% (I2 = 31%, P = 0.22). Overall, geography is a factor influencing herbal placebo response rates, especially in China, which is closely related to the historical development of herbal medicine in China. Returning to the study itself, it is reasonable that Chinese patients trust herbal medicines more, leading to a relatively higher response rate. See Supplementary Figure S6 for more details.
3.8 Meta-regression analysis
We conducted a meta-regression analysis of the subgroups using R 4.2.3 to identify the source of heterogeneity. We used criteria, duration of treatment, IBS subtype, research locations, placebo forms, and low concentration of herbal ingredients as covariates. We used a random effects model to determine whether p > 0.05 indicates that the factor is not a significant influence on heterogeneity, and p < 0.05 indicates that it is a source of heterogeneity. The results indicate that the location (p = 0.0438) and duration (p = 0.0365) may both be significant influencing factors for heterogeneity (see Supplementary Table S3 for an in-depth analysis).
3.9 Trial sequential analysis
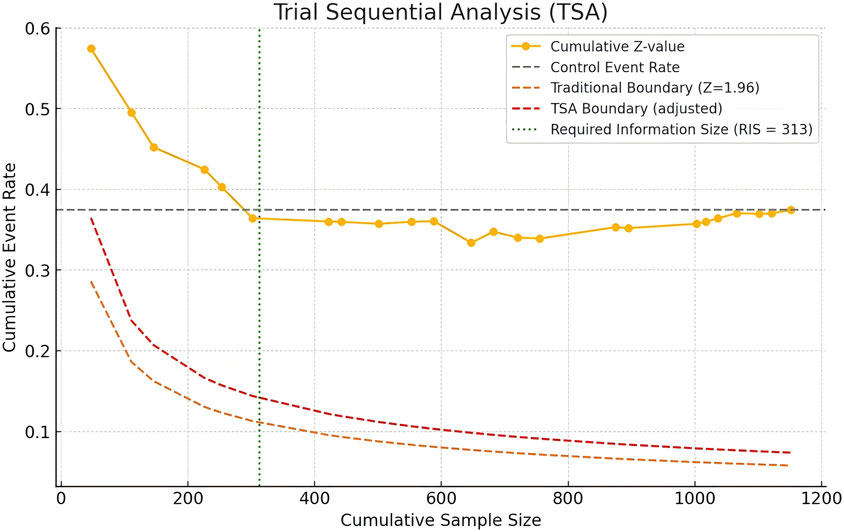
We used Python software to analyze the trial sequential analysis (TSA), allowing for a Type I error rate of 0.05, statistical efficacy of 80%. Z-curves did not cross the traditional boundaries, but whose cumulative information reaches the required information size (RIS), and the Z-curves has flattened out, indicating that there is no significant difference between the herbal placebo treatments and that this conclusion is also stable (Figure 9).
4 Discussion
Placebo is a tool that is frequently used in RCTs, and numerous studies in recent years have demonstrated significant placebo response rates for many diseases (Enck and Klosterhalfen, 2019; Jakovljevic, 2014); therefore, the placebo effect in patients should not be ignored in RCTs. Our study was the first to examine herbal placebos for IBS, and the results revealed a high herbal placebo response rate (37% in patients with IBS), with 29% reporting an improvement in abdominal pain and 49% reporting an improvement in bowel movements. Another study by Glissen Brown and Lembo (2022), showed that the combined composite response rate, abdominal pain rate, and fecal response rate for patients in the IBS-C placebo group, assessed at week 6, were 18.9%, 34.6%, and 30.1%, respectively. As per FDA standards, in the IBS-D placebo group, the combined placebo response rate for the composite endpoint was 16.2%, the abdominal pain improvement rate was 40.2%, and the fecal improvement rate was 16.2%. Bosman et al. (2021) included 73 RCTs of IBS in 2021, which showed an overall response rate of 34.4% and an improvement in abdominal pain of 17.9% in the placebo group for IBS. Comparing previous relevant studies, the placebo response rate in IBS patients varied considerably, and our findings are similar to the study by Bosman et al. Furthermore, our findings indicated that research locations and treatment durations were sources of heterogeneity in the placebo response rate and also affected the results of the herbal placebo response rate. The study by Bosman, M. et al. also found that the location of the study affected the placebo response rates in people with IBS. The pooled placebo response rate was significantly higher in trials conducted in Europe than in the United States (38.9% vs. 25.7%, p = 0.0032), but there was no significant difference in the pooled placebo response rate when compared to trials conducted in Asia (30.3%, p = 0.068). Our results also indicated that location plays a significant role in the effectiveness of herbal placebos in IBS patients, revealing a significantly higher herbal placebo response rate in China compared to other regions.
Because our study was focused on herbal medicines and China is a country where herbal medicines are more frequently used, we further analyzed the studies conducted in China and found that there was no difference in the herbal placebo response rate between China and countries outside of China (P = 0.14), but the difference in the herbal placebo response rate between the groups of studies where the language was English and Chinese was statistically significant (P = 0.03), so we believe that the design and rigor of the study is one of the reasons for the effect.
Based on the results of our study and the factors affecting the placebo effect, we will analyze it based on location, the patients themselves, and the placebo drug itself. In addition to this, placebo response rates are also related to the doctor-patient relationship, the recruitment process, and other external factors (Pardo-Cabello et al., 2022). To begin with, epidemiologic research on IBS has shown that the prevalence of IBS varies from country to country (Huang et al., 2023), and studies conducted globally by the Rome Foundation have shown comparable prevalence rates in Europe and the United States and slightly lower prevalence rates in Asia and Australia (Sperber, 2021). While there is no direct effect of prevalence and herbal placebo response rates, high prevalence and disease progression can affect efficacy as well as placebo response rates. The combination of IBS epidemiology and China is one of the most widely used countries for herbal medicine; the result is reliable and reasonable. Surprisingly, the IBS herbal placebo response rate in China was not the highest, and the herbal placebo response rate in the US was the highest in the two studies. As only two studies were conducted in the US, with only 50 participants, we suspect potential bias. In 10 studies in China, the herbal placebo response rate was as high as 41% (I2 = 29%) in 620 patients with IBS. We suspect potential bias. Given that China is the birthplace of Chinese herbal medicine and one of the countries with the widest use of herbal medicine, it is important to consider this high herbal placebo response rate when designing trials.
The second pertains to the placebo itself, including its visual, color, smell, dosage for, and pharmacological characteristics. The salience of the placebo plays a crucial role in determining the rates of placebo response (Buckalew and Coffield, 1982; Meissner and Linde, 2018). Currently herbal placebos lack uniform standards for production and evaluation, and there are challenges with odor and taste simulation, which can lead to low placebo response rates or even outright shedding if the placebo is easily recognized. So for the herbal placebo, we conducted subgroup analyses for the dosage form of the placebo and whether or not it contained an active ingredient, which also yielded reasonable and interesting results. Although there were no differences between the groups, the results showed that the highest response rates were found in the tablet and granule groups, and that granules are the most commonly used herbal placebo dosage form and the one that most closely resembles the original drug, which reduces patient recognition and reduces the error associated with placebo medications. The fact that tablets are less difficult to simulate in appearance is one of the reasons why they are less likely to be recognized. In addition to this, what is more interesting is that we found that the response rate of the placebo group made with added herbs was 35%, and the response rate of the placebo group made without herbs was 46%, which could also confirm that the addition of a small amount of herbs when making herbal placebos does not affect the outcome of the trial, and there are other relevant literature to prove this as well (Huang et al., 2024). Although there are fewer studies on the addition of herbs to make placebos, the results also proved that the addition of a small amount of the test group’s drug to the placebo does not cause the bias of placebo group’s response rate, and that the addition of a certain concentration of the original drug can make the placebo simulate the color, taste, and mouthfeel of the study drug better, which is a feasible option.
Both subgroup analyses and meta-regression analyses show that treatment duration does affect placebo response rates. We noticed that placebo response rates peaked at 8 weeks, with a positive correlation between medication duration and placebo response rates before 8 weeks and a negative correlation between medication duration and placebo response rates after 8 weeks. Expectation and learning are two of the most characterized primary mechanisms mediating the placebo response. At the beginning of the trial, patients may think that the treatment will alleviate their condition. This could lead to higher placebo response rates both at the beginning and as the trial goes on. However, as soon as the expectation is insufficient to mask the placebo’s inadequacy, the placebo response weakens.
Regarding the risk of bias, as the subject of this study was herbal placebos, and all studies set up a placebo group, so most of the trials achieved strict randomization, blinding, and allocation concealment. However, more than 80% (20/24) of the studies were found to have dropouts in the placebo group, and if the patients who dropped out were excluded from the study, the total number of participants in the study would have been reduced, and therefore would have inflated the placebo response rate. However, we believe that there is an inevitable link between patients who fall off and poor placebo efficacy, so we believe that all patients who fall off are due to poor efficacy, so our study categorizes patients who fall off as ineffective, so although most of the articles suffered from the bias of incomplete reporting, we tried to minimize the bias by not overstating the efficacy rate of the herbal placebo after treating the data in a uniform manner.
Placebo response in clinical trials is the result of a combination of psychological and neurobiological mechanisms, as well as natural history disease processes (Jones and Holtmann, 2023). Therefore, the herbal placebo response is influenced by numerous factors, especially the patient’s psychological feelings, and is a complex outcome. As countries with frequent use of herbal medicines, including China, Japan, Korea and so on. should pay more attention to the herbal placebo response when designing clinical trials involving herbal placebos. However, the search process could discover that many clinical trials of Chinese herbal medicines were not set up with standard blinding, even ignoring the herbal placebo effect. Therefore, setting up the placebo group is the key to improving the rigor and quality of clinical research on herbal medicines. In addition, our study found that adding a low level of herbal ingredients to the herbal placebo does not exaggerate the effect of the placebo and has no therapeutic effect on the placebo group. Herbal placebos are easily recognized by patients because of the difficulty in simulating the appearance, taste, and smell of herbal placebos. Adding a low level of the herbal ingredients can better simulate the herb placebo, which can solve the contradiction of the difficulty in simulating the placebo without setting a placebo group.
5 Limitations
1) Heterogeneity is high due to the different trial group prescriptions in each RCT and the uncontrollable baseline of patients taking herbal placebos. 2) Although most studies implemented blinding and allocation concealment, herbal placebos are difficult to simulate and so are easily recognized by patients, so the resulting outcomes are inevitably at risk of bias. In addition, the placebo group also shed a large number of patients, inevitably resulting in follow-up bias. 3) The criteria for evaluating the outcomes of the herbal RCTs are also not standardized, which would result in a risk of bias. 4) Herbal placebo response is related to the psychology of the patient, which varies between studies and can be deviated by the psychological status of the patient.
6 Conclusion
Primarily, there is a high herbal placebo response in IBS disease, where patient adherence and trust in the herbal medicine affects the outcome, and the placebo response should be emphasized in both trials and clinics. At the same time, we found that most Chinese RCTs of herbal medicines lacked placebo groups, and fewer studies met the inclusion criteria than English-language journals. Moreover, there was also a significant difference in the placebo response rates for the herbs summarized in the Chinese and English articles, indicating that the quality of the studies also affected the placebo response rates for the herbs. Finally, in light of the non-negligible placebo response rate for herbal medicines, we suggest that when conducting herbal medicine-related RCTs, if the disease or drug involved is ethical, there should be a placebo control group to exclude errors and to prove the validity and safety of the positive control drug, so that the trial design and results will be more rigorous and scientific. We should quantify and scientifically develop the production of herbal placebos and the standardized evaluation of appearance, taste, and odor to establish a standard and scientific evaluation system. This will reduce patient or researcher blindness, exclude the placebo response and confounding factors, and ensure the experimental group’s results are relatively reliable, rigorous, and scientific.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KH: Writing–original draft, Visualization, Software, Methodology, Formal Analysis, Data curation, Conceptualization. ML: Writing–original draft, Software, Data curation. TZ: Writing–original draft, Methodology, Formal Analysis, Data curation. FW: Writing–review and editing. XT: Writing–review and editing, Supervision, Project administration. LL: Writing–review and editing, Visualization, Project administration, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the National Natural Science Foundation of China, No. 81873297; The Fundamental Research Funds for the Central Public Welfare Research Institutes, China, No. ZZ13-YQ-006; Innovation Fund of Chinese Academy of Chinese Medical Sciences, China, No. CI 2021A01003; Hospital capability enhancement project of Xiyuan Hospital, CACMS(No. XYZX0303-07).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1475366/full#supplementary-material
Abbreviations
RTCs, randomized controlled trial; IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome-diarrhea; IBS-C, irritable bowel syndrome-constipation; CNKI, China National Knowledge Infrastructure; WanFang, Wan Fang data knowledge service platform; SinoMed, China biomedicine literature database; FGIDs, functional gastrointestinal diseases; FAS, full analysis set; PPS, per protocol set.
References
Alt, F., Chong, P. W., Teng, E., and Uebelhack, R. (2017). Evaluation of benefit and tolerability of IQP-CL-101 (xanthofen) in the symptomatic improvement of irritable bowel syndrome: a double-blinded, randomised, placebo-controlled clinical trial. Phytotherapy Res. PTR 31 (7), 1056–1062. doi:10.1002/ptr.5826
Bensoussan, A., Kellow, J. E., Bourchier, S. J., Fahey, P., Shim, L., Malcolm, A., et al. (2015). Efficacy of a Chinese herbal medicine in providing adequate relief of constipation-predominant irritable bowel syndrome: a randomized controlled trial. Clin. gastroenterology hepatology 13 (11), 1946–1954. doi:10.1016/j.cgh.2015.06.022
Bensoussan, A., Talley, N. J., Hing, M., Menzies, R., Guo, A., and Ngu, M. (1998). Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. Jama 280 (18), 1585–1589. doi:10.1001/jama.280.18.1585
Billings, W., Mathur, K., Craven, H. J., Xu, H., and Shin, A. (2021). Potential benefit with complementary and alternative medicine in irritable bowel syndrome: a systematic review and meta-analysis. Clin. gastroenterology hepatology 19 (8), 1538–1553.e14. doi:10.1016/j.cgh.2020.09.035
Black, C. J., and Ford, A. C. (2020). Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterology and hepatology 17 (8), 473–486. doi:10.1038/s41575-020-0286-8
Bosman, M., Elsenbruch, S., Corsetti, M., Tack, J., Simrén, M., Winkens, B., et al. (2021). The placebo response rate in pharmacological trials in patients with irritable bowel syndrome: a systematic review and meta-analysis. lancet. Gastroenterology and hepatology 6 (6), 459–473. doi:10.1016/s2468-1253(21)00023-6
Brenner, D. M., and Sayuk, G. S. (2020). Current US food and drug administration-approved pharmacologic therapies for the treatment of irritable bowel syndrome with diarrhea. Adv. Ther. 37 (1), 83–96. doi:10.1007/s12325-019-01116-z
Buckalew, L. W., and Coffield, K. E. (1982). An investigation of drug expectancy as a function of capsule color and size and preparation form. J. Clin. Psychopharmacol. 2 (4), 245–248. PMID:7119132. doi:10.1097/00004714-198208000-00003
Cai, L. J., Lv, B., Meng, L. N., Ma, L. j., and Fan, Y. H. (2013). Efficacy of patients with diarrhea- predominant irritable bowel syndrome treated with treatment of dispersing stagnated liver- qi invigorating spleen and warming kidney. Chin. J. Traditional Chin. Med. 31 (05), 1097–1099. (In Chinese).
Chen, G. R., Xie, X. F., and Peng, C. (2023). Treatment of irritable bowel syndrome by Chinese medicine: a review. Chin. J. Integr. Med. 29 (4), 377–384. doi:10.1007/s11655-021-3521-4
Chen, M., Tang, T. C., Wang, Y., Shui, J., Xiao, X. H., Lan, X., et al. (2018). Randomised clinical trial: tong-Xie-Yao-Fang granules versus placebo for patients with diarrhoea-predominant irritable bowel syndrome. Alimentary Pharmacol. and Ther. 48 (2), 160–168. doi:10.1111/apt.14817
Davis, K., Philpott, S., Kumar, D., and Mendall, M. (2006). Randomised double-blind placebo-controlled trial of aloe vera for irritable bowel syndrome. Int. J. Clin. Pract. 60 (9), 1080–1086. doi:10.1111/j.1742-1241.2006.00980.x
Enck, P., and Klosterhalfen, S. (2019). Placebos and the placebo effect in drug trials. Handb. Exp. Pharmacol. 260, 399–431. doi:10.1007/164_2019_269
Enck, P., and Klosterhalfen, S. (2020). Placebo responses and placebo effects in functional gastrointestinal disorders. Front. psychiatry 11, 797. doi:10.3389/fpsyt.2020.00797
Glissen Brown, J. R., and Lembo, A. J. (2022). Therapeutics, placebo, and the importance of hard outcomes in irritable bowel syndrome research. Clin. gastroenterology hepatology 20 (5), e921–e922. doi:10.1016/j.cgh.2021.10.041
Harbord, R. M., Egger, M., and Sterne, J. A. (2006). A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics Med. 25 (20), 3443–3457. doi:10.1002/sim.2380
Heydari, P., Ghanadian, M., Asghari, G., Azimi, M., Babaeian, M., and Adibi, P. (2023). A double-blind randomized clinical trial of Dracocephalum kotschyi Boiss. in the patients with diarrhea-predominant irritable bowel syndrome. Res. Pharm. Sci. 18 (1), 89–99. doi:10.4103/1735-5362.363599
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. ed.) 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ (Clin. Res. ed.) 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Holtmann, G. J., Ford, A. C., and Talley, N. J. (2016). Pathophysiology of irritable bowel syndrome. lancet. Gastroenterology and hepatology 1 (2), 133–146. doi:10.1016/s2468-1253(16)30023-1
Huang, K., Huang, J., Wang, F., Ma, X., Tang, X., and Lv, L. (2024). Meta-analysis of the placebo effect of Chinese herbal medicine in clinical trials of traditional Chinese. J. Traditional Chin. Med. 65 (05), 479–488. (In Chinese). doi:10.13288/j.11-2166/r.2024.05.008
Huang, K. Y., Wang, F. Y., Lv, M., Ma, X. X., Tang, X. D., and Lv, L. (2023). Irritable bowel syndrome: epidemiology, overlap disorders, pathophysiology and treatment. World J. Gastroenterol. 29 (26), 4120–4135. doi:10.3748/wjg.v29.i26.4120
Jakovljevic, M. (2014). The placebo-nocebo response: controversies and challenges from clinical and research perspective. Eur. Neuropsychopharmacol. 24 (3), 333–341. doi:10.1016/j.euroneuro.2013.11.014
Jones, M. P., and Holtmann, G. (2023). Placebo effects in functional dyspepsia: causes and implications for clinical trials. Neurogastroenterol. Motil. 35 (2), e14527. doi:10.1111/nmo.14527
Lai, Y., Liang, X., Fan, H., Liu, Y., Zheng, L., Lu, W., et al. (2022). Assessing the post-treatment therapeutic effect of tongxie in irritable bowel syndrome: a randomized controlled trial. Complementary Ther. Med. 68, 102839. doi:10.1016/j.ctim.2022.102839
Lee, J. H., Kim, J. I., Baeg, M. K., Sunwoo, Y. Y., Do, K., Lee, J. H., et al. (2019). Effect of samryungbaekchul-san combined with otilonium bromide on diarrhea-predominant irritable bowel syndrome: a pilot randomized controlled trial. J. Clin. Med. 8 (10), 1558. doi:10.3390/jcm8101558
Leung, W. K., Wu, J. C. Y., Liang, S. M., Chan, L. S., Chan, F. K. L., Xie, H., et al. (2006). Treatment of diarrhea-predominant irritable bowel syndrome with traditional Chinese herbal medicine: a randomized placebo-controlled trial. Am. J. Gastroenterology 101 (7), 1574–1580. doi:10.1111/j.1572-0241.2006.00576.x
Li, Y. M., Zhang, Y. L., Cai, G., and Lin, J. (2010). A randomized double-blinded and placebo-controlled trial of chang ji tai Granule in treating diarrhea-predominant diarrhea. Shanghai J. Traditional Chin. Med. 44 (12), 33–36, 45. (In Chinese).
Madisch, A., Holtmann, G., Plein, K., and Hotz, J. (2004). Treatment of irritable bowel syndrome with herbal preparations: results of a double-blind, randomized, placebo-controlled, multi-centre trial. Alimentary Pharmacol. and Ther. 19 (3), 271–279. doi:10.1111/j.1365-2036.2004.01859.x
Meissner, K., and Linde, K. (2018). Are blue pills better than green? How treatment features modulate placebo effects. Int. Rev. Neurobiol. 139, 357–378. doi:10.1016/bs.irn.2018.07.014
Nee, J., Zakari, M., and Lembo, A. J. (2015). Current and emerging drug options in the treatment of diarrhea predominant irritable bowel syndrome. Expert Opin. Pharmacother. 16 (18), 2781–2792. doi:10.1517/14656566.2015.1101449
Pardo-Cabello, A. J., Manzano-Gamero, V., and Puche-Cañas, E. (2022). Placebo: a brief updated review. Naunyn Schmiedeb. Arch. Pharmacol. 395 (11), 1343–1356. doi:10.1007/s00210-022-02280-w
Pazhouh, H. K., Hosseini, S. M. A., Taghipour, A., Hamedi, S., and Noras, M. (2020). Anti-irritable bowel syndrome syrup improves constipation-predominant irritable bowel syndrome: a randomized, placebo-controlled trial. Chin. J. Integr. Med. 26 (10), 729–735. doi:10.1007/s11655-020-3267-4
Portincasa, P., Bonfrate, L., Scribano, M. L., Kohn, A., Caporaso, N., Festi, D., et al. (2016). Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointest. liver Dis. JGLD. 25 (2), 151–157. doi:10.15403/jgld.2014.1121.252.ccm
Rahman, M. M., Mahadeva, S., and Ghoshal, U. C. (2017). Epidemiological and clinical perspectives on irritable bowel syndrome in India, Bangladesh and Malaysia: a review. World J. gastroenterology 23 (37), 6788–6801. doi:10.3748/wjg.v23.i37.6788
Saito, Y. A., Rey, E., Almazar-Elder, A. E., Harmsen, W. S., Zinsmeister, A. R., Locke, G. R., et al. (2010). A randomized, double-blind, placebo-controlled trial of St John's wort for treating irritable bowel syndrome. Am. J. gastroenterology 105 (1), 170–177. doi:10.1038/ajg.2009.577
Sallon, S., Ben-Arye, E., Davidson, R., Shapiro, H., Ginsberg, G., and Ligumsky, M. (2002). A novel treatment for constipation-predominant irritable bowel syndrome using Padma Lax, a Tibetan herbal formula. Digestion 65 (3), 161–171. doi:10.1159/000064936
Shapiro, J. M., and Deutsch, J. K. (2021). Complementary and alternative medicine therapies for irritable bowel syndrome. Gastroenterology Clin. N. Am. 50 (3), 671–688. doi:10.1016/j.gtc.2021.03.009
Si, Y. J., Wen, H. Z., Bian, H., Lu, L., Huo, L., Fei, X. Y., et al. (2024). Shuchang decoction for treatment of diarrhea predominant irritable bowel syndrome with Gan depression and pi deficiency: a multicenter, randomized, double-blind, placebo controlled trial. Chin. J. Integr. Med. 43 (11), 1–10. (In Chinese).
Sperber, A. D. (2021). Epidemiology and burden of irritable bowel syndrome: an international perspective. Gastroenterology Clin. N. Am. 50 (3), 489–503. doi:10.1016/j.gtc.2021.04.001
Sperber, A. D., Dumitrascu, D., Fukudo, S., Gerson, C., Ghoshal, U. C., Gwee, K. A., et al. (2017). The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut 66 (6), 1075–1082. doi:10.1136/gutjnl-2015-311240
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clin. Res. ed.) 343, d4002. doi:10.1136/bmj.d4002
Størsrud, S., Pontén, I., and Simrén, M. (2015). A pilot study of the effect of aloe barbadensis mill. Extract (AVH200®) in patients with irritable bowel syndrome: a randomized, double-blind, placebo-controlled study. J. Gastrointest. liver Dis. JGLD 24 (3), 275–280. doi:10.15403/jgld.2014.1121.243.sst
Su, X., Tang, Y., Zhang, J., Dong, Y., Wei, W., Bai, Y., et al. (2013). Curative effect of warming kidney and fortifying spleen recipe on diarrhea-predominant irritable bowel syndrome. J. traditional Chin. Med. = Chung i tsa chih ying wen pan 33 (5), 615–619. doi:10.1016/s0254-6272(14)60030-3
Takeshi, K., Michiko, S., Mamoru, T., Keiji, O., Masahide, E., Takahito, K., et al. (2014). Therapeutic effects of biobran, modified arabinoxylan rice bran, in improving symptoms of diarrhea predominant or mixed type irritable bowel syndrome: a pilot, randomized controlled study. Evidence-based complementary and Altern. Med. (ecam) 2014, 1–6. doi:10.1155/2014/828137
Tang, X. D., Lv, B., Li, Z. H., Wei, W., Meng, L. N., Li, B. S., et al. (2018). Therapeutic effect of chang'an I recipe (I) on irritable bowel syndrome with diarrhea: a multicenter randomized double-blind placebo-controlled clinical trial. Chin. J. Integr. Med. 24 (9), 645–652. doi:10.1007/s11655-016-2596-9
Van Tilburg, M. A. L., Palsson, O. S., Ringel, Y., and Whitehead, W. E. (2014). Is ginger effective for the treatment of irritable bowel syndrome? A double blind randomized controlled pilot trial. Complementary Ther. Med. 22 (1), 17–20. doi:10.1016/j.ctim.2013.12.015
Wu, J. C. (2010). Complementary and alternative medicine modalities for the treatment of irritable bowel syndrome: facts or myths? Gastroenterology and hepatology 6 (11), 705–711. PMID:21437019.
Yu, B., and Ma, F. (2011). Summary of emotional intervention with modified pain-diarrhea decoctionon 20 patients with irritable bowel syndrome. Hunan J. Traditional Chin. Med. 27 (3), 6–8. (In Chinese).
Keywords: herbs, complementary treatment, placebo, irritable bowel syndrome, meta-analysis
Citation: Huang K, Lv M, Zheng T, Wang F, Tang X and Lv L (2024) Herbal placebo response in clinical trials on irritable bowel syndrome: a systematic review and meta-analysis. Front. Pharmacol. 15:1475366. doi: 10.3389/fphar.2024.1475366
Received: 03 August 2024; Accepted: 11 November 2024;
Published: 28 November 2024.
Edited by:
Yuanyuan Wu, Nanjing University of Chinese Medicine, ChinaReviewed by:
Renjun Gu, Nanjing University of Chinese Medicine, ChinaLinshuoshuo Lyu, Vanderbilt University Medical Center, United States
Copyright © 2024 Huang, Lv, Zheng, Wang, Tang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Lv, bHVzaGFuZ3NoaXRvdUBxcS5jb20=
 Kaiyue Huang
Kaiyue Huang Mi Lv1,2
Mi Lv1,2