- 1Laboratory of Neuroinflammation, Institute of Biological Sciences, Federal University of Pará, Belém, Brazil
- 2Laboratory of Morphophysiology Applied to Health, Department of Morphology and Physiological Sciences, State University of Pará, Belém, Brazil
- 3Laboratory of Chemical Analysis, Coordination of Earth Sciences and Ecology, Emílio Goeldi Museum, Belém, Brazil
- 4Laboratory of Central Extraction, Institute of Exact and Natural Sciences, Federal University of Pará, Belém, Brazil
- 5Laboratory of Experimental Neuropharmacology, Institute of Biological Sciences, Federal University of Pará, Belém, Brazil
- 6Laboratory of Developmental Biology, Department of Morphology, Federal University of São Paulo, São Paulo, Brazil
Background: Montrichardia linifera (Arruda) Schott is popularly known as “aninga,” “aningaçu,” “aningaíba,” and “aninga-do-igapó.” Compresses and plasters made from the leaves of this medicinal plant are used to treat abscesses, tumors, and pain caused by stingray stings.
Aim of the study: This study aimed to chemically characterize the methanolic extract of M. linifera leaves (MEMLL), as well as to verify their acute oral toxicity and antinociceptive potential.
Materials and methods: The leaves were collected during the rainy season, and the methanolic extract was obtained after gradient extraction using different solvents. MEMLL was analyzed using high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR). Acute oral toxicity testing followed the Organization for Economic Co-operation and Development (OECD) guideline 423. Subsequently, acetic acid, hot plate, and formalin tests were used to evaluate the analgesic effects.
Results: In the chemical characterization of MEMLL by HPLC, three flavonoids were identified: rutin, quercetin, and epicatechin. In addition, when NMR spectroscopy was performed, rutin and quercetin were again identified, as well as the chemical compounds luteolin and chrysoeriol. In the acute oral toxicity test, MEMLL showed no physiological or behavioral changes. In the nociceptive study, MEMLL showed an effect at doses of 50 and 100 mg/kg in the 0.6% acetic acid test, i.e., 51.46% and 75.08%, respectively. In the hot plate test, the MEMLL group at a dose of 50 mg/kg was effective at times of 30 and 60 min, i.e., 164.43% and 122.95%, respectively. Similarly, the MEMLL group at a dose of 100 mg/kg was also effective in increasing latency at times of 30 and 60 min, i.e., 162.62% and 136.68%, respectively. In the formalin test, MEMLL showed an antinociceptive effect on neurogenic pain at doses of 50 and 100 mg/kg when compared to the control group, 35.25% and 52.30%, respectively. In the inflammatory phase, inhibition was observed in the MEMLL at doses of 50 and 100 mg/kg, i.e., 66.39% and 72.15%, respectively.
Conclusion: MEMLL has analgesic properties and is non-toxic, validating the Brazilian ethnopharmacological use of this plant for pain treatment. The leaves of the species M. linifera showed central and peripheral antinociceptive effects.
1 Introduction
Pain affects not only wellbeing but also the health and productivity of individuals, a growing public health concern that has become a global problem (Del Gaudio et al., 2023). In many diseases, pain serves as a warning system amongst noxious stimuli, a defense mechanism when something in the body is not working properly (Vargas-Ruiz et al., 2023). It is the main reason people seek medical treatment (Cohen et al., 2021). Pain is currently defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage,” according to the International Association for the Study of Pain (IASP) (Raja et al., 2020).
Pharmacological intervention is the most widely used treatment for pain relief among several treatments. It includes the use of non-steroidal anti-inflammatory drugs (NSAIDs) and opioids for peripheral and central analgesic action, respectively (Marinho et al., 2023). These are potentially effective in the treatment of pain, but the administration of these drugs often causes adverse effects (Nascimento et al., 2024). The use of NSAIDs can cause gastrointestinal bleeding and renal dysfunction, and opioids are associated with potentially fatal respiratory depression, dependence, and constipation (Jabbari et al., 2024). Thus, there is a current need to find new analgesic agents (Del Gaudio et al., 2023).
Natural products serve as a source of several therapeutic agents that constitute modern medical practice (Ema et al., 2023). They have gained prominence for their promising effects in treating diseases, resulting from numerous chemical compounds, the most successful source of potential medicines (Marinho et al., 2023; Pires et al., 2024). The World Health Organization (WHO) estimates that traditional medicine, plant extracts, or their active ingredients are used to meet primary health needs and improve the quality of life in 80% of the world’s population (Del Gaudio et al., 2023). So, medicinal plants have emerged as an alternative for treating diseases in folk medicine since ancient civilizations (Santana et al., 2023), playing an important role in scientific research to reduce side effects and regulate pain (Nascimento et al., 2024).
In this context, Brazil has a great capacity to develop studies with medicinal plants, being the country with the most incredible plant diversity, with around 20% of the world’s flora (Ferreira et al., 2022). Montrichardia linifera (Arruda) Schott is a species of aquatic macrophyte native and non-endemic to Brazil (Miranda et al., 2015), belonging to the genus Montrichardia Crueg of the Araceae family (Lopes et al., 2016). It is popularly known as “aninga,” “aningaçu,” “aningaíba,” and “aninga-do-igapó” (Costa et al., 2009).
Its sap is traditionally used in Brazilian ethnopharmacology as a healing, antirheumatic, antidiuretic, and expectorant medicine. However, its excessive use can be considered toxic due to its ability to cause burns, skin eruptions, spots, and, in the case of eye contact, blindness (Lima et al., 2021). The powder from the root of this plant is used as an antifungal medicine and anesthetic against stingray stings (Batista et al., 2019). The compresses and plasters made from the leaves are used to treat abscesses, tumors, and also against stingray stings (Amarante et al., 2011). In a previous phytochemical study, Faria et al. (2021) proved the methanolic extract of the leaves to be most promising for biological studies as the presence of groups of secondary metabolites such as coumarins, flavonic heterosides, tannins, polyphenols, and saponins was observed. Despite this, not many studies in the scientific literature demonstrate its biological potential.
Based on its traditional use, the current study aimed to investigate the analgesic effects of the methanolic extract of M. linifera leaves (MEMLL). For this purpose, chemical characterization, acute oral toxicity test, and nociceptive tests were performed on MEMLL.
2 Materials and methods
2.1 Collection and extraction of the plant material
The M. linifera (Arruda) Schott leaves were collected during the rainy season from January to April 2015 in the city of Belém, Pará, Brazil (coordinates: 1°27′53.0″S, 48°30′24.3″W). The specimen was registered in the National Management System of Genetic Heritage and Associated Traditional Knowledge (SisGen, Brazil) under the registration number A91B68B, and the exsiccata was deposited in the João Murça Pires Herbarium of the Emílio Goeldi Museum under the registration number MG 216695.
The collected leaves were washed with running water and deionized water, cut into small pieces, and dried in a refrigerated environment at 16°C with 35% humidity. Afterward, drying was completed in an oven at 45°C, and the samples were ground in an industrial blender to obtain leaf powder (1,000 g) and subjected to gradient extraction (5 L for each solvent) in an increasing order of polarity (hexane, ethyl acetate, and methanol). The extracts were filtered and concentrated in a rotary evaporator model Q344M2 (QUIMIS®; São Paulo, Brazil) using an ultra-thermostatic bath model Q214M2 (QUIMIS®; São Paulo, Brazil) and then dried in an oven at 45°C.
2.2 Chemical characterization of MEMLL
MEMLL was characterized by adapting the methods described by Williams et al. (2015), Singh and Dhepe (2018), Tomazi et al. (2021), Pinheiro et al. (2022), and Kuhn and Nuzillard (2023). The organic compounds were identified using the following analytical techniques: high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy.
2.2.1 Chromatographic profile by HPLC
A clean-up was carried out on the investigated MEMLL to remove hydrophobic pigments, such as chlorophylls. For this purpose, these interferents were removed by solid-phase extraction (SPE). Initially, C18 SPE cartridges (1 g/6 mL; Strata, Phenomenex) were cleaned and activated with water and acetonitrile. Subsequently, 180 mg of MEMLL solubilized in water/acetonitrile (30:70, v/v) was injected and extracted with two volumes in the same ratio. After obtaining the extracting solutions, they were dried in an oven with air circulation.
The chromatographic analysis by HPLC of the MEMLL was performed in a liquid chromatograph Prominence LC-20AT model (Shimadzu, Tokyo, Japan) with a diode array detector (DAD) and Gemini C18 column (250 mm × 4.6 mm, 5 μm) (Phenomenex; Torrance, CA, United States). LCSolution 1.20 software was used for data processing. Then, 20 µL/injection of standardized MEMLL was applied at 1,000 ppm and eluted in a water/acetonitrile gradient, with an organic modifier ranging linearly from 5% to 100%, in 60 min and a flow rate of 1 mL/min, according to the method described by Pinheiro et al. (2022). MEMLL was monitored from the compound’s absorbance under ultraviolet radiation, from 200 to 400 nm.
To obtain fingerprinting, the retention times (tr), ultraviolet spectra, and NMR data on the flavonoid standards epicatechin, rutin, quercetin, myricetin, and kaempferol were compared with those observed for the MEMLL.
2.2.2 Spectroscopy profile by NMR
The NMR spectra of 1H, 13C, and the correlation maps homonuclear correlation spectroscopy (HOMO-COSY), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple-bond coherence (HMBC) were obtained on a Bruker spectrometer, Ascend™ (Rheinstetten, Germany) model, operating at 400 and 100 MHz. Preparation involved dissolving 50 mg of MEMLL in 600 µL of deuterated dimethyl sulfoxide (DMSO-d6), with subsequent data control and processing done using TopSpin software (version 3.6.0). The free induction decays (FIDs) were transformed using Fourier transform, applying a line broadening (LB) of 0.3 Hz to enhance the resolution. The manual adjustments were made for baseline correction and calibration using the residual solvent peak as an internal reference, DMSO at 2.49 (1H) and 39.5 ppm (13C), according to the adaptation of the methods proposed by Williams et al. (2015), Singh and Dhepe (2018), Tomazi et al. (2021), and Kuhn and Nuzillard (2023).
The abundance of functional groups present in the classes of metabolites of interest was analyzed in the processing of FIDs, grouped, and normalized into specific regions (δ = 0.5–1.5/1.5–3.0/3.0–04.5/4.5–6.0/6.0–9.0/9.0–10.0) (Pinheiro et al., 2022). To indicate the selected peaks, chemical shift data (δ) and coupling constant (J) of one-dimensional spectra (1D) 1H e 13C were used, in addition to two-dimensional correlation maps 2D 1H 1H (HOMO-COSY), 1H13C (HSQC), and 1H13C (HMBC). The experimental data were compared with the respective literature signals for flavonoid structures.
2.3 Experimental animals and ethical statement
All animals in this research were treated according to the ethical guidelines for the care and use of laboratory animals. Eighty-eight adult male mice (Mus musculus; 30–40 g, 8–10 weeks old, Swiss) were obtained from the Laboratory Animal Breeding and Production Section of the Evandro Chagas Institute and retained in the “Luiz Carlos de Lima Silveira” animal facility of the State University of Pará. Before testing, the animals were acclimatized for 1 week, maintained under a 12-h light/dark cycle, controlled temperature of 22°C ± 2°C, and with access to food Nuvilab CR1 (NUVITAL Nutrientes Ltda., Curitiba, Brazil) and water available ad libitum. This research was approved by the State University of Pará Animal Use Ethics Committee under protocol 09/2017.
2.4 Acute oral toxicity test
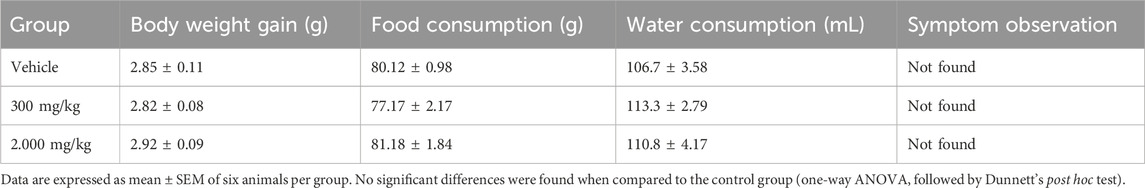
The acute oral toxicity test was performed according to the Organization for Economic Co-operation and Development (OECD) guideline 423 (OECD, 2002). The mice were divided into three groups of six animals: the control group received a vehicle (1% Tween 80 diluted in saline solution, v/v, p.o.; n = 6) and two treatment groups (MEMLL at doses of 300 and 2.000 mg/kg, p.o.; n = 6 per group). A single oral dose was administered individually to each animal. Subsequently, they were observed for 4 h and then every 24 h for 14 days to evaluate possible behavioral and physiological changes such as aggression, alertness, apathy, ataxia, convulsions, diarrhea, lack of appetite, locomotion, nasal secretion, piloerection, response to touch, spontaneous motor activity, stereotypy, sweating, and urination.
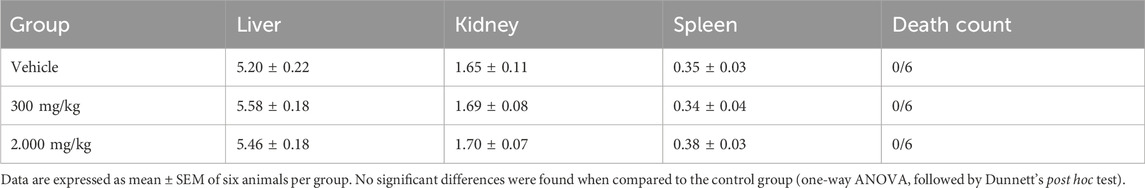
During this period, body weight gain and food and water consumption were recorded. On day 14, the mice were subjected to induced euthanasia (300 mg/kg ketamine and 30 mg/kg xylazine) for the macroscopic evaluation of internal organs (the liver, kidney, and spleen) and masses obtained using an analytical balance.
2.5 Evaluation of antinociceptive activity
2.5.1 Acetic acid-induced writhing test
The experiment in mice was performed according to the method reported by Koster et al. (1959). Abdominal writhing was induced in animals by intraperitoneal administration of 0.6% acetic acid in a volume of 10 mL/kg. The mice were divided into six groups of five animals: the control group received the vehicle (1% Tween 80 diluted in saline solution, v/v, p.o.; n = 5), four treatment groups (MEMLL at doses of 10, 25, 50, and 100 mg/kg, p.o.; n = 5), and a positive control group (5 mg/kg indomethacin, i.p.; n = 5). After 60 min of treatment, the animals received an intraperitoneal administration of 0.6% acetic acid, and then, the abdominal contortions and hind paw extensions were recorded for 30 min.
The doses that showed the best results in this experiment were chosen for the other nociception tests.
2.5.2 Hot plate test
The central analgesic activity was evaluated using a model established by Macdonald et al. (1946). The animals were placed on a hot plate at a fixed temperature (55°C ± 0.5°C), and the latency time to respond to the temperature change, such as licking one of the hind paws or jumping, was recorded. The mice were divided into four groups of five animals: the control group received the vehicle (1% Tween 80 diluted in saline solution, v/v, p.o.; n = 5), two treatment groups (MEMLL at doses of 50 and 100 mg/kg, p.o.; n = 5), and a positive control group (10 mg/kg morphine, s.c.; n = 5). Subsequently, the animals were individually placed on the hot plate, and the response was evaluated at times 0, 30, 60, 90, and 120 min. The maximum latency time of 40 s was respected to avoid tissue damage.
2.5.3 Formalin-induced licking response
To confirm the antinociceptive effect of the MEMLL, the formalin nociception test was performed according to the methodology described by Hunskaar et al. (1985). The mice were divided into four groups of five animals: the control group received the vehicle (1% Tween 80 diluted in saline solution, v/v, p.o.; n = 5), two treatment groups (MEMLL at doses of 50 and 100 mg/kg, p.o.; n = 5), and a positive control group (4 mg/kg morphine, s.c.; n = 5). After 60 min of treatment, the test involved an intraplantar injection of 20 µL of 1% formalin solution into the right hind paw of each animal. The time during which the animal licked its paw was recorded in the first phase from 0 to 5 min (neurogenic pain) and in the second phase from 15 to 30 min (inflammatory pain).
2.6 Statistical analysis
GraphPad Prism® version 10.2.3 (GraphPad Software Inc., San Diego, CA, United States) was used to perform statistical tests. First, the data normality test (Shapiro–Wilk) was used to determine the type of statistical tests. Then, one-way analysis of variance (ANOVA) was performed on the results of the acute oral toxicity test, the writhing test, and the formalin-induced licking response test, and two-way ANOVA was performed on the hot plate test. All tests were followed by Dunnett’s multiple comparison post hoc test. The data are expressed as the mean ± standard error of the mean (SEM). Statistical differences were considered significant when p < 0.05.
3 Results
3.1 Chemical characterization of MEMLL
3.1.1 Chromatographic profile by HPLC
To obtain the initial information about the compounds present in MEMLL, the chromatographic profile was obtained by HPLC-DAD, as well as from the comparison of tr. The extract was fingerprinted against the flavonoid standards (Figure 1). In the chromatogram analysis of the extract at wavelength 270 nm, chromatographic bands of chromophoric substances were observed between 3.5 and 23 min, attributed to more polar and hydrophilic substances, and an isolated chromatographic band was also observed at 39.7 min. In the individual analysis of the ultraviolet spectra of each peak observed in the chromatogram, all bands showed the same spectral pattern, with absorption maxima at 220 and 270 nm or 220, 270, and 339 nm, typical of the absorption of the A rings (270 nm), B (220), and C (339 nm–observed when the C ring contains unsaturation between carbons C2 and C3) of flavonoids. The presence of flavonoids rutin (tr 16.6 min/λmax = 255 and 354 nm) in Figure 1C, quercetin (tr 23.8 min/λmax = 255 and 371 nm) in Figure 1D, and epicatechin (tr 39.7 min/λmax = 220 and 270 nm) in Figure 1B was determined in the MEMLL (Figure 1A) in the fingerprint of the chromatograms of the standards used. Myricetin (Figure 1E) and kaempferol (Figure 1F) were absent in MEMLL.
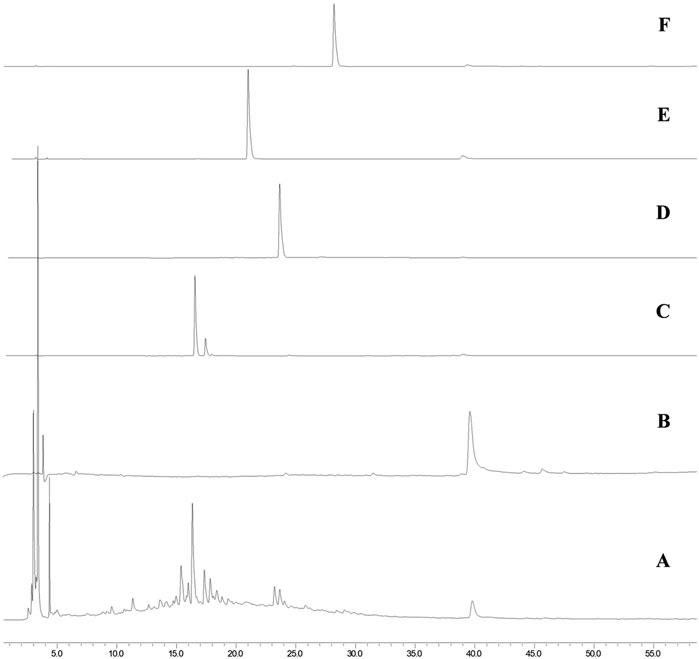
Figure 1. HPLC analysis. (A) MEMLL; (B) epicatechin; (C) rutin; (D) quercetin; (E) myricetin; (F) and kaempferol.
3.1.2 Spectroscopy profile by NMR
The spectroscopic profile of the MEMLL was obtained by 1H and 13C NMR (Supplementary Figures S1, S2), which allowed the evaluation of the chemical composition by identifying the sub-structural fractions (functional groups), as well as evaluating their abundance by normalizing the integrals by spectral regions. Thus, δH was observed to be 0.5–1.5, which comprises the hydrogen signals of methyl (-CH3), methylene (-CH2), and methine (-CH) groups, typical of alkyl groups, with approximately 15.79%. The region of the spectrum from δH 1.5 to 3.0, typical of -CH3/-CH2/-CH hydrogens, bonded to unsaturated carbon (CH3-C=C/CH2-C=C/CH-C=C), the ketone carbonyl or aldehyde (CH3-COR/CH2-COR/CH-COR), the carboxyl (CH3-COOR/CH2-COOR/CH-COOR), the nitrogen atoms (CH3-N/CH2-N/CH-N), or directly linked to the aromatic ring (CH3-Ph/CH2-Ph/CH-Ph), a percentage area of 10.63%. The region of δH 3.0 to 4.5, corresponding to the signals of hydrogens bonded to oxidized carbons (CH2-OH/CH-OH) or alkoxy, phenoxy, and acylate groups (PhO-CH3/PhO-CH2/RCOO-CH3/RCOO-CH2/RCOO-CH), presented the highest percentage area of the studied extract, 62.37%, thus indicating a high sugar composition. Furthermore, in the hydrogen region of olefinic groups, δH 4.5 to 6.0 (CH2 = CH-R/R-CH = CH-R) presented 6.08% of spectrum integration. Another notable region was that of hydrogens linked to aromatic carbons (Ph-H) or olefinic carbons vicinal to the aromatic ring (Ph-CH = CH-R), which showed a higher content of aromatic hydrogens for the 5.13% extract (Supplementary Table S1).
The 13C NMR spectrum of the MEMLL was obtained to corroborate the information evidenced in the 1H NMR spectrum, having confirmed the presence of signals from aliphatic carbons (-CH3, -CH2-, -CH -, CH3-CO-, and -CH2-NH2) in the spectral region between δC 10.0 and 50.0. The region δC 50.0–90.0, typical of oxidized aliphatic carbons of ether and alcohol (-C-O-C- or -C-OH), showed very intense signals, which highlights the hypothesis of the abundance of sugars, as observed in NMR 1H (δH 3 .0–4.5). For the region of aromatic and olefinic carbons, δC 100.0–140.0 and between 140.0 and 160.0 indicate oxidized aromatic carbons, highlighting the content of phenolic compounds.
Analysis of two-dimensional NMR spectra (COSY, HSQC, and HMBC) carried out with the MEMLL allowed the evaluation of phytochemical diversity through the observation of correlations 1H/1H (2,3JH, H), 1H/13C (1JC, H), and 1H/13C (2,3JC, H) of the typical signals of bonds and sub-structural fractions present in flavonoids, which allowed the identification of four secondary metabolites: rutin, quercetin, luteolin, and chrysoeriol (Supplementary Figures S3–S5; Supplementary Table S2).
In the homonuclear correlation map COSY 1H/1H (2,3JH, H), correlation patterns common to the annotated compounds were observed, such as the related meta-doublets, in δH 6.20 (d 1.9)/6.41 (d 1.9); 6.21 (d 1.9)/6.45 (d 1.9); and 6.19 (d 2.7)/6.45 (d 2.7), which indicate the presence of 1,2,3,5-tetra-substituted aromatic rings. Another correlation pattern observed indicates the presence of 1,3,4-tri-substituted aromatic rings as follows: δH 7.53 (d 2.4)/6.91 (d 7.8)/7.54 (dd 2.4; 7.8); 7.55 (d 2.3)/6.91 (d 8.4)/7.52 (dd 2.3; 8.4); and 7.35 (d 2.0)/6.82 (d 8.3)/7.39 (dd 2.0; 8.3). These spectral patterns, in association with the 13C NMR spectrum in which carbonyl carbon signals were observed (δC 177.8; 181.1; 182.9; and 175.7), allow us to relate the sub-structural fractions of flavonoids (rings A and B) (Pinheiro et al., 2022).
In the HSQC spectrum, the correlations between the signals of the aromatic ring sub-structural fraction stand out at δH/δC 6.20 (d 1.9)/99.2; 6.41 (d 1.9)/94.1; 7.53 (d 2.4)/116.2; 6.91 (d 7.8)/116.1; and 7.54 (dd 2.4; 7.8)/122.0, two correlated pairs that indicate anomeric H/C at 5.45 (d 7.2)/102.3 and 4.4 (d 1.6)/100.5; in addition to these, the typical signals of the rhamnose methyl group, at 4.42 (d 1.6)/18.4, suggest the presence of a glycosylated flavonoid. After confirming the correlations of the COSY 1H/1H (2,3JH, H) and HMBC 1H/13C (2,3JC, H) spectra, comparison with literature data (Moura et al., 2011) allowed us to note the structure of the glycosylated flavonoid rutin.
Another set of correlations visualized in the HSQC, COSY, and HMBC spectra, similar to those of the rutin glycoside but with lower intensity, were δH/δC 6.18 (d 2.0)/98.9; 6.40 (d 2.0)/94.3; 7.63 (d 2.3)/116.3; 6.86 (d 8.2)/115.4; and 7.54 (dd 8.2; 2.3)/119.5, which allow the presence of the flavonoid quercetin (Cuong et al., 2019).
In the region of hydrogens and aromatic carbons of the HSQC spectrum, correlations are observed at δH/δC 6.80 (s)/102.5; 6.19 (d 2.7)/99.2; 6.45 (d 2.7)/93.9; 7.35 (d 2.0)/113.3; 6.82 (d 8.3)/115.9; and 7.39 (dd 2.0; 8.3)/119.1. After correlations in the HMBC spectrum and comparison with the literature (Cuong et al., 2019), spectral data were recorded for the compound luteolin. With a spectroscopic profile similar to luteolin, the compound chrysoeriol was noted with spectral data in δH/δC 7.00 (s)/106.6; 6.21 (d 1.9)/99.3; 6.45 (d 1.9)/94.2; 7.55 (d 2.3)/109.7; 6.91 (d 8.4)/116.7; and 7.52 (dd 2.3; 8.4)/119.4 and the typical sign of a methoxy substituent at 3.59 (s)/51.7. In the HMBC spectrum, a correlation between the signal of methoxyl hydrogen at δH 3.59 and the aromatic carbons at δC 109.7 (C-2′) was observed, 147.9 (C-4′) and 149.3 (C-3′), thus confirming the position of the methoxyl group on the C-3′ carbon of ring B (Park et al., 2007).
3.2 Acute oral toxicity test
Oral administration of the MEMLL at doses of 300 and 2.000 mg/kg did not promote signs of changes in the animals’ body weight gain or food and water consumption (Table 1). There were neither behavioral or physiological changes in the first 4 h of observation nor was any mortality observed in the first 24 h or during the following 14 days. Furthermore, no significant differences in the relative organ masses were observed between the MEMLL groups and the control group (Table 2).
3.3 Evaluation of antinociceptive activity
3.3.1 Acetic acid-induced writhing test
The results were obtained with the oral gavage treatment of the MEMLL, as shown in Figure 2. A significant reduction in the percentage of abdominal contortions was observed for the MEMLL at doses of 50 and 100 mg/kg when compared to the control group, 51.46% (p < 0.05) and 75.08% (p < 0.001), respectively. No reduction in abdominal contortions was observed for MEMLL at doses of 10 and 25 mg/kg. The positive control group with 5 mg/kg indomethacin showed inhibition of 78.64% (p < 0.001).
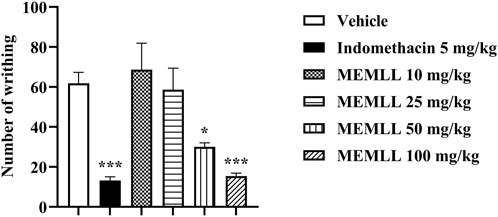
Figure 2. Peripheral analgesic effect of the methanolic extract of MEMLL at 10, 25, 50, and 100 mg/kg in the acetic acid writhing test. Data are expressed as the mean ± SEM of five animals per group. * = p < 0.05 and *** = p < 0.001 were considered statistically significant when compared to the control group (one-way ANOVA, followed by Dunnett’s post hoc test).
3.3.2 Hot plate test
The graph of the hot plate test demonstrates the result obtained when the animals were treated with the MEMLL (Figure 3). The control group maintained low latency at all analysis times. The MEMLL group at a dose of 50 mg/kg was effective when compared to the control group at times of 30 and 60 min, 164.43% (p < 0.01) and 122.95% (p < 0.05), respectively. Similarly, the MEMLL group at a dose of 100 mg/kg was also effective in increasing latency at times of 30 and 60 min, 162.62% (p < 0.01) and 136.68% (p < 0.05), respectively. The positive control group with 10 mg/kg morphine showed a longer latency time at 0, 30, 60, and 90 min than the control group, 167.74% (p < 0.05), 357.92% (p < 0.0001), 277.88% (p < 0.0001), and 231.49% (p < 0.001), respectively.
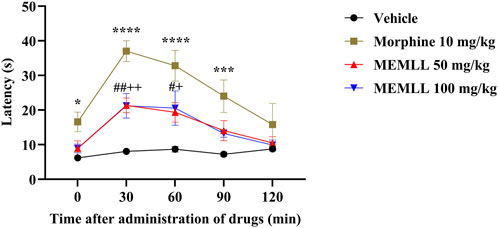
Figure 3. Central analgesic effect of the methanolic extract of MEMLL at 50 and 100 mg/kg in the hot plate test. Data are expressed as the mean ± SEM of five animals per group. * or + or # = p < 0.05, ++ or ## = p < 0.01, *** = p < 0.001, **** = p < 0.0001 were considered statistically significant when compared to the control group (two-way ANOVA, followed by Dunnett’s post hoc test).
3.3.3 Formalin-induced licking response
The formalin test graph shows the results obtained when the animals were treated with MEMLL in Figure 4. The groups treated with the MEMLL showed promising results, suppressing paw licking activity in both the first and second phases. The antinociceptive effect in the neurogenic phase occurs with inhibition in the paw licking time of mice treated with the MEMLL at doses of 50 and 100 mg/kg compared to the control group, 35.25% (p < 0.05) and 52.30% (p < 0.01), respectively. In the inflammatory phase, inhibition was also observed in the MEMLL at doses of 50 and 100 mg/kg, 66.39% (p < 0.0001) and 72.15% (p < 0.0001), respectively. The positive control with 4 mg/kg morphine was effective in both the neurogenic phase (88.48%, p < 0.0001) and the inflammatory phase (94.37%, p < 0.0001).
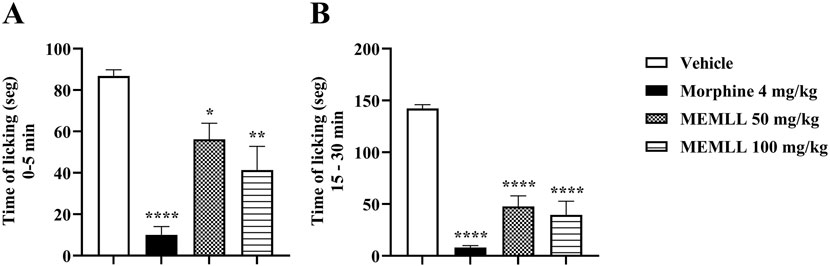
Figure 4. Central and peripheral analgesic effect of the methanolic extract of MEMLL at doses of 50 and 100 mg/kg in the formalin test. (A) First phase assesses neurogenic pain. (B) Second phase assesses inflammatory pain. Data are expressed as the mean ± SEM of five animals per group. * = p < 0.05, ** = p < 0.01, and **** = p < 0.0001 were considered statistically significant when compared to the control group (one-way ANOVA, followed by Dunnett’s post hoc test).
4 Discussion
Natural products are widely accepted and recognized as effective therapeutics for various diseases and drug development (Umeh et al., 2024). Historically, natural products have provided a wide range of chemical molecules and remain one of the main sources of new and effective modern drugs (Nahar and Sarker, 2020). In this work, we used the methanolic extract after performing a bioassay-guided fractionation analysis (data not shown). So, the current investigation aimed to evaluate the ethnopharmacological use of M. linifera regarding its antinociceptive potential. For this purpose, chemical characterization, acute oral toxicity test, and nociceptive tests on MEMLL were carried out.
The HPLC fingerprint analysis identified three phenolic compounds classified as flavonoids: rutin, quercetin, and epicatechin. Similarly, to corroborate the study, the analysis of NMR spectra allowed the identification of rutin and quercetin again, as well as luteolin and chrysoeriol. Plant secondary metabolism produces flavonoids with several pharmacological effects (Liu et al., 2024). In short, rutin is known for biological activities such as its anticarcinogenic, antioxidant, anti-inflammatory, antinociceptive, cardioprotective, and neuroprotective effects (Ganeshpurkar and Saluja, 2017). Quercetin has antioxidant, anti-inflammatory, and antinociceptive effects (Azevedo et al., 2013). Epicatechin is reported to have antidiabetic, antioxidant, anti-inflammatory, antinociceptive, and neuroprotective activities (Qu et al., 2021). Luteolin has anticarcinogenic, antioxidant, anti-inflammatory, and antinociceptive effects (Hara et al., 2014). Chrysoeriol has antiarthritic, anticancer, antidiabetic, antioxidant, anti-inflammatory, and antinociceptive properties (Law et al., 2024). The chemical compounds found may contribute to the antinociceptive activities of the MEMLL.
In the present study, we report acute oral toxicity in the species M. linifera for the first time. The use of medicinal plants has become an increasingly common practice due to the low cost and high consumption of natural products by society. However, there is still a belief that medicinal plants do not have toxic effects, and therefore, it is necessary to conduct research to obtain data on their safety (Araldi et al., 2021). The acute oral toxicity test is an experiment that precedes the execution of pharmacological tests. It is important to obtain preliminary data on the toxic effects of a substance and, therefore, avoid harm when administered.
The results demonstrate that no effects are considered toxic; the MEMLL lethal dose (LD50) is greater than 2.000 mg/kg. According to OECD guideline 423, drugs that have LD50 between 2.000 and 5.000 mg/kg are classified as category 5, presenting low acute oral toxicity (OECD, 2002). Pereira et al. (2022) performed cytotoxicity tests on tumor cell lines, with the MEMLL collected in different locations on the Píaui Coast, and it was observed that the methanolic extract of the leaves showed high antiproliferative effects on the B16-F10 cell line (murine metastatic melanoma). Therefore, further studies are needed to understand the anticarcinogenic potential of M. linifera leaves as our study sought to evaluate acute oral toxicity in healthy animals.
Furthermore, we evaluated the peripheral and central antinociceptive effects of MEMLL in the acetic acid, hot plate, and formalin tests. The acetic acid writhing test is used to assess the inflammatory-based analgesic activity of a potential drug (de Jesus et al., 2023). In this nociception model, the intensity of the painful stimulus is proportional to the number of abdominal writhings (da Fonseca et al., 2021).The physical writhing response involves the conversion of arachidonic acid to eicosanoids by lipoxygenases (LOX) and cyclooxygenases (COX), which cause hyperalgesia by sensitizing peripheral pain neurons (Alqudah et al., 2023; Oqal et al., 2023). Eicosanoids are responsible for cellular signaling and inflammatory mediators by releasing prostaglandins, mainly prostaglandin E2 (PGE2), which are related to pathological circumstances (Silva et al., 2023).
The antinociceptive activities of the acetic acid test are closely linked to the MEMLL compounds since all the flavonoids have anti-inflammatory activities and may have inhibited nociception by suppressing peripheral LOX and COX levels, reducing the production of inflammatory mediators such as PGE2. This is the first report of a dose-related inhibition curve of an extract from M. linifera. In addition, the result of the effect with MEMLL corroborates a previous initial study found in the literature, in which a dose of 400 mg/kg of the ethanolic extract of M. linifera leaves was used and also obtained a reduction in peripheral pain (Silva et al., 2013).
The hot plate test is a widely used model to evaluate antinociceptive effects on the central nervous system, such as opioids, through thermal stimulation (Asiri et al., 2024). Animals are placed on a hot plate at a fixed temperature, where the response time of licking one of the paws or jumping is observed, which requires coordination of the central nervous system (Xie et al., 2023). In this test, opioid drugs increase the nociception threshold of rodents in relation to heat via spinal and supraspinal receptors (Manouze et al., 2017). Therefore, the MEMLL showed central antinociceptive effects linked to the flavonoids’ properties and their probable action on opioid receptors. This is the first report of the species M. linifera with the hot plate test.
The formalin test was performed to support the central and peripheral analgesic effects of the MEMLL. This model is widely used in rodents, causing painful stimuli in the intraplantar region of the animal’s hind paw. The licking is perceived after injection of the chemical substance that corresponds to the painful response (Hirota et al., 2023). The first phase, which evaluates neurogenic pain, occurs through direct formalin stimulation in primary afferent nociceptors, releasing bradykinin and substance P (Santos et al., 2024). The second phase of inflammatory pain occurs through the release of inflammatory mediators at the site of the injury, such as prostaglandins, serotonin, and bradykinin (Todorov et al., 2024). The central and peripheral analgesic activity of the MEMLL was observed in the formalin test, corroborating the antinociceptive effects observed in the hot plate test and acetic acid test, respectively. This is the first report on the species M. linifera with the formalin nociception test.
Finally, the data support the use of M. linifera in folk medicine for the treatment of pain. The current study is a significant step toward the sustainable development of family farming as it highlights the potential of the species as a source of income due to its traditional use. Thus, we propose that a more detailed investigation of the species M. linifera will provide information to determine the specific pathways and its use as a new therapeutic agent for pain management in the future.
5 Conclusion
The flavonoids identified in the MEMLL showed analgesic activities in nociceptive tests, validating the Brazilian ethnopharmacological use of this plant for pain treatment. The MEMLL did not demonstrate toxic effects or mortality, highlighting its safety profile. In this important work, we present the first report on these compounds identified in the chemical characterization of the leaves of the species M. linifera. This is a significant contribution to the ethnopharmacology field. Furthermore, we are the first to evaluate the acute oral toxicity, dose-related inhibition in the acetic acid test, and antinociceptive effects in the hot plate and formalin tests in the leaves of the species M. linifera, which are of great interest to the Amazon community.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Ethics Committee on Animal Use from the State University of Pará (ethics approval number: 09/2017). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WC: data curation, conceptualization, writing–original draft, visualization, methodology, investigation, and formal analysis. LC: writing–original draft, investigation, formal analysis, and data curation. AT: formal analysis, writing–original draft, investigation, and data curation. RM: writing–original draft, investigation, formal analysis, and data curation. JA: writing–original draft and investigation. KL: writing–original draft and investigation. AB: writing–original draft and investigation. RB: writing–review and editing, methodology, and investigation. JF: writing–review and editing, methodology, and investigation. WP: methodology, investigation, writing–original draft, formal analysis, and data curation. FO: formal analysis, data curation, writing–original draft, resources, and conceptualization. KO: resources, writing–review and editing, investigation, and conceptualization. AL: methodology, formal analysis, data curation, writing–original draft, investigation, and conceptualization. CA: validation, supervision, resources, writing–review and editing, investigation, and conceptualization. GB: writing–review and editing, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Pró-Reitoria de Pesquisa e Pós-Graduação / Programa de Apoio à Publicação Qualificada (PROPESP / PAPQ) of Federal University of Pará and the Government of Pará.
Acknowledgments
The authors acknowledge the Laboratory Chemical Analysis of the Emílio Goeldi Museum for supplying the extract and the partnerships between the Laboratory of Central Extraction and Laboratory of Experimental Neuropharmacology of the Federal University of Pará and the Laboratory Morphophysiology Applied to Health of the State University of Pará.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1475157/full#supplementary-material
References
Alqudah, A., Qnais, E. Y., Wedyan, M. A., AlKhateeb, H., Abdalla, S. S., Gammoh, O., et al. (2023). Lysionotin exerts antinociceptive effects in various models of nociception induction. Heliyon 9, e15619. doi:10.1016/j.heliyon.2023.e15619
Amarante, C. B. do, Silva, J. C. F. da, Müller, R. C. S., and Müller, A. H. (2011). Avaliação da composição mineral do chá da folha senescente de Montrichardia linifera (arruda) schott (araceae) por espectrometria de absorção atômica com chama (FAAS). Quim Nova 34, 419–423. doi:10.1590/S0100-40422011000300010
Araldi, I. C. da C., Souza, T. P. de, Vencato, M. de S., Fortes, T. de A., Mello, C. B. E., Oliveira, J. S. de, et al. (2021). Preclinical safety assessment of the crude extract from Sida rhombifolia L. aerial parts in experimental models of acute and repeated-dose 28 days toxicity in rats. Regul. Toxicol. Pharmacol. 124, 104974. doi:10.1016/j.yrtph.2021.104974
Asiri, S. A., Shabnam, M., Zafar, R., Alshehri, O. M., Alshehri, M. A., Sadiq, A., et al. (2024). Evaluation of Habenaria aitchisonii Reichb. for antioxidant, anti-inflammatory, and antinociceptive effects with in vivo and in silico approaches. Front. Chem. 12, 1351827. doi:10.3389/fchem.2024.1351827
Azevedo, M. I., Pereira, A. F., Nogueira, R. B., Rolim, F. E., Brito, G. A., Wong, D. V. T., et al. (2013). The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 9, 53. doi:10.1186/1744-8069-9-53
Batista, R. J. D. R., Do Amarante, C. B., Botelho, A. D. S., Andrade, E. H. D. A., and Do Nascimento, L. D. (2019). Constituintes voláteis da raiz e do rizoma de Montrichardia linifera (Arruda) Schott (Araceae). Bol. do Mus. Para. Emílio Goeldi - Ciências Nat. 14, 197–207. doi:10.46357/bcnaturais.v14i2.174
Cohen, S. P., Vase, L., and Hooten, W. M. (2021). Chronic pain: an update on burden, best practices, and new advances. Lancet 397, 2082–2097. doi:10.1016/S0140-6736(21)00393-7
Costa, E. S. S., Dolabela, M. F., Póvoa, M. M., Oliveira, D. J., and Müller, A. H. (2009). Estudos farmacognósticos, fitoquímicos, atividade antiplasmódica e toxicidade em Artemia salina de extrato etanólico de folhas de Montrichardia linifera (Arruda) Schott, Araceae. Rev. Bras. Farmacogn. 19, 834–838. doi:10.1590/S0102-695X2009000600006
Cuong, D. T. D., Dat, H. T., Duan, N. T., Thuong, P. D., Phat, N. T., Tri, M. D., et al. (2019). Isolation and characterization of six flavonoids from the leaves of Sterculia foetida Linn. Vietnam J. Chem. 57, 438–442. doi:10.1002/vjch.201900084
Da Fonseca, A., Fernandes Ribeiro Dantas, L., Rodrigues, J., Alencar Filho, M. P., De Melo Rêgo, M., Da Rocha Pitta, M., et al. (2021). PA-Int5: an isatin-thiosemicarbazone derivative that exhibits anti-nociceptive and anti-inflammatory effects in Swiss mice. Biomed. Rep. 15, 61. doi:10.3892/br.2021.1437
de Jesus, E. N. S., Tavares, M. S., Barros, P. A. C., Miller, D. C., da Silva, P. I. C., Freitas, J. J. S., et al. (2023). Chemical composition, antinociceptive and anti-inflammatory activities of the curzerene type essential oil of Eugenia uniflora from Brazil. J. Ethnopharmacol. 317, 116859. doi:10.1016/j.jep.2023.116859
Del Gaudio, M. P., Kraus, S. I., Melzer, T. M., Bustos, P. S., and Ortega, M. G. (2023). Antinociceptive effect and identification of berberine alkaloid in Berberis ruscifolia extracts. J. Ethnopharmacol. 305, 116066. doi:10.1016/j.jep.2022.116066
Ema, R. S., Kabir Zihad, S. M. N., Islam, M. N., Sifat, N., Rouf, R., Shilpi, J. A., et al. (2023). Analgesic, anti-inflammatory activity and metabolite profiling of the methanolic extract of Callicarpa arborea Roxb. leaves. J. Ethnopharmacol. 300, 115757. doi:10.1016/j.jep.2022.115757
Faria, L. V., Brígido, H. P. C., Bentaberry-Rosa, A. A., Correa-Barbosa, J., Silva-Silva, J. V., Bastos, M. L. C., et al. (2021). Anti-leishmania activity of extract and fractions from the stem and leaf of Montrichardia linifera (Arruda) schott (Araceae) against Leishmania amazonensis. Res. Soc. Dev. 10, e9310212312. doi:10.33448/rsd-v10i2.12312
Ferreira, O. O., Cruz, J. N., de Moraes, Â. A. B., de Jesus Pereira Franco, C., Lima, R. R., Anjos, T. O. dos, et al. (2022). Essential oil of the plants growing in the Brazilian Amazon: chemical composition, antioxidants, and biological applications. Molecules 27, 4373. doi:10.3390/molecules27144373
Ganeshpurkar, A., and Saluja, A. K. (2017). The pharmacological potential of rutin. Saudi Pharm. J. 25, 149–164. doi:10.1016/j.jsps.2016.04.025
Hara, K., Haranishi, Y., Terada, T., Takahashi, Y., Nakamura, M., and Sata, T. (2014). Effects of intrathecal and intracerebroventricular administration of luteolin in a rat neuropathic pain model. Pharmacol. Biochem. Behav. 125, 78–84. doi:10.1016/j.pbb.2014.08.011
Hirota, I., Koyama, Y., and Shimada, S. (2023). Histochemical analysis of the biphasic properties of formalin pain-induced behavior. Biochem. Biophys. Rep. 34, 101467. doi:10.1016/j.bbrep.2023.101467
Hunskaar, S., Fasmer, O. B., and Hole, K. (1985). Formalin test in mice, a useful technique for evaluating mild analgesics. J. Neurosci. Methods 14, 69–76. doi:10.1016/0165-0270(85)90116-5
Jabbari, S., Zakaria, Z. A., Ahmadimoghaddam, D., and Mohammadi, S. (2024). The oral administration of Lotus corniculatus L. attenuates acute and chronic pain models in male rats. J. Ethnopharmacol. 319, 117181. doi:10.1016/j.jep.2023.117181
Koster, R., Anderson, M., and De Beer, E. J. (1959). “Acetic acid for analgesic screening,” Federation Proceedings, 412–417.
Kuhn, S., and Nuzillard, J. (2023). Easy structural dereplication of natural products by means of predicted carbon-13 nuclear magnetic resonance spectroscopy data. Chemistry–Methods 3. doi:10.1002/cmtd.202200054
Law, S. K., Wu, X. X., Jiang, Z., Tong, C. W. S., Chow, W. Y. L., and Au, D. C. T. (2024). Pharmacological activities of lonicerae japonicae flos and its Derivative—“Chrysoeriol” in skin diseases. Molecules 29, 1972. doi:10.3390/molecules29091972
Lima, C., Andrade, D., Moreira, G., Sousa, Â., Leal, A., Figuerêdo, J., et al. (2021). Antibacterial, antibiofilm, and antischistosomal activity of Montrichardia linifera (arruda) schott (araceae) leaf extracts. Sci. Pharm. 89, 31. doi:10.3390/scipharm89030031
Liu, Y., Luo, J., Peng, L., Zhang, Q., Rong, X., Luo, Y., et al. (2024). Flavonoids: potential therapeutic agents for cardiovascular disease. Heliyon 10, e32563. doi:10.1016/j.heliyon.2024.e32563
Lopes, A., Parolin, P., and Piedade, M. T. F. (2016). Morphological and physiological traits of aquatic macrophytes respond to water chemistry in the Amazon Basin: an example of the genus Montrichardia Crueg (Araceae). Hydrobiologia 766, 1–15. doi:10.1007/s10750-015-2431-x
Macdonald, A. D., Woolfe, G., Bergel, F., Morrison, A. L., and Rinderknecht, H. (1946). Analgesic action of pethidine derivatives and related compounds. Br. J. Pharmacol. Chemother. 1, 4–14. doi:10.1111/j.1476-5381.1946.tb00022.x
Manouze, H., Bouchatta, O., Gadhi, A. C., Bennis, M., Sokar, Z., and Ba-M’hamed, S. (2017). Anti-inflammatory, antinociceptive, and antioxidant activities of methanol and aqueous extracts of anacyclus pyrethrum roots. Front. Pharmacol. 8, 598. doi:10.3389/fphar.2017.00598
Marinho, A. de O., Brito, J. de S., da Costa, J. A., da Silva, A. R., da Silva, S. P., de Amorim, L. C., et al. (2023). Schinus terebinthifolia leaf lectin has central and peripheral antinociceptive action mediated by its carbohydrate-recognition domain and delta-opioid receptors. J. Ethnopharmacol. 301, 115817. doi:10.1016/j.jep.2022.115817
Miranda, J. A. L., Rocha, J. A., Araújo, K. M., Quelemes, P. V., Mayo, S. J., and Andrade, I. M. (2015). Atividade antibacteriana de extratos de folhas de Montrichardia linifera (Arruda) Schott (Araceae). Rev. Bras. plantas Med. 17, 1142–1149. doi:10.1590/1983-084x/14_169
Moura, A. C. da S., Vilega, W., and Santos, L. C. dos (2011). Identificação de alguns constituintes químicos de Indigofera hirsuta Linn. (Fabaceae) por CLAE-IES-EM (TOF) e avaliação da atividade antirradicalar. Quim Nova 34, 1136–1140. doi:10.1590/S0100-40422011000700006
Nahar, L., and Sarker, S. D. (2020). Medicinal natural products—an introduction. Annu. Rep. Med. Chem., 1–44. doi:10.1016/bs.armc.2020.02.008
Nascimento, M. F. do, Costa, W. K., Aguiar, J. C. R. de O. F. de, Navarro, D. M. do A. F., Silva, M. V. da, Paiva, P. M. G., et al. (2024). Essential oil from leaves of Croton blanchetianus Baill does not present acute oral toxicity, has antigenotoxic action and reduces neurogenic and inflammatory nociception in mice. J. Ethnopharmacol. 318, 116908. doi:10.1016/j.jep.2023.116908
OECD (2002). Test No. 423: acute oral toxicity - acute toxic class method. OECD. doi:10.1787/9789264071001-en
Oqal, M., Qnais, E., Alqudah, A., and Gammoh, O. (2023). Analgesic effect of the flavonoid herbacetin in nociception animal models. Eur. Rev. Med. Pharmacol. Sci. 27, 11236–11248. doi:10.26355/eurrev_202312_34563
Park, Y., Moon, B., Lee, E., Lee, Y., Yoon, Y., Ahn, J., et al. (2007). 1 H and 13 C-NMR data of hydroxyflavone derivatives. Magnetic Reson. Chem. 45, 674–679. doi:10.1002/mrc.2010
Pereira, J. I. A., Pereira, F. I. A., Araújo, G. S., Rodrigues, J. M. A., Sá, R. E. de, Souza, J. M. T., et al. (2022). Phytochemical investigation and biological activities of extracts obtained from Montrichardia linifera (Arruda) Schott leaves collected in different localities from Piauí coast. Res. Soc. Dev. 11, e23011427231. doi:10.33448/rsd-v11i4.27231
Pinheiro, W. B. S., Pinheiro Neto, J. R., Botelho, A. S., Dos Santos, K. I. P., Da Silva, G. A., Muribeca, A. J. B., et al. (2022). The use of Bagassa guianensis aubl. forestry waste as an alternative for obtaining bioproducts and bioactive compounds. Arabian J. Chem. 15, 103813. doi:10.1016/j.arabjc.2022.103813
Pires, P. G. da S., Sarrazin, S. L. F., Souza, D. J., dos, A. de, Mourão, R. H. V., Massing, L. T., et al. (2024). Antiedema and antinociceptive potential of the essential oil of Pectis elongata Kunt (Asteraceae) from the Brazilian Amazon. J. Ethnopharmacol. 322, 117643. doi:10.1016/j.jep.2023.117643
Qu, Z., Liu, A., Li, P., Liu, C., Xiao, W., Huang, J., et al. (2021). Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 61, 211–233. doi:10.1080/10408398.2020.1723057
Raja, S. N., Carr, D. B., Cohen, M., Finnerup, N. B., Flor, H., Gibson, S., et al. (2020). The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982. doi:10.1097/j.pain.0000000000001939
Santana, F. R., de Santana Souza, M. T., Camargo, E. A., and Silva, J. A. da (2023). Anti-inflammatory and antinociceptive effects of a pectinolide-enriched fraction from Mesosphaerum pectinatum (L.) Kuntze. J. Ethnopharmacol. 302, 115916. doi:10.1016/j.jep.2022.115916
Santos, P. V. L., Jerônimo, L. B., Ribeiro, W. S. C., Lopes, G. M., Leão Neto, J. H. de C., da Silva, H. B. O., et al. (2024). Exploring the impact of seasonal variations on the chemical composition, antinociceptive, and anti-inflammatory properties of Pogostemon heyneanus Benth. essential oil. Front. Pharmacol. 15, 1336878. doi:10.3389/fphar.2024.1336878
Silva, J. V. da S., Rosário, D. M. do, Veiga, A. do S. S. da, Vasconcelos, F. de, Percário, S., and Dolabela, M. F. (2013). A bibliographic review on the family Araceae with foccus on the genera Pistia, Philodendron and Montrichardia: botanical, phytochemical and biological activity aspects. Rev. Fitos 8. doi:10.5935/1808-9569.20130006
Silva, V. B. G., Fonsêca, B. M. B. da, Aguiar, J. C. R. de O. F. de, Navarro, D. M. do A. F., Oliveira, A. M. de, Napoleão, T. H., et al. (2023). Chemical composition, antinociceptive and anti-inflammatory effects in mice of the essential oil of Psidium cattleyanum Sabine leaves. J. Ethnopharmacol. 312, 116443. doi:10.1016/j.jep.2023.116443
Singh, S. K., and Dhepe, P. L. (2018). Experimental evidences for existence of varying moieties and functional groups in assorted crop waste derived organosolv lignins. Ind. Crops Prod. 119, 144–151. doi:10.1016/j.indcrop.2018.04.002
Todorov, P., Georgieva, S., Peneva, P., Nikolov, S., Rangelov, M., Todorova, N., et al. (2024). Synthesis, molecular docking, electrochemical and fluorimetric analysis of new caffeic and cinnamic acid-conjugated hemorphin derivatives designed as potential anticonvulsant and antinociceptive agents. Bioorg Chem. 143, 107063. doi:10.1016/j.bioorg.2023.107063
Tomazi, R., Figueira, Â. C., Ferreira, A. M., Ferreira, D. Q., de Souza, G. C., de Souza Pinheiro, W. B., et al. (2021). Hypoglycemic activity of aqueous extract of latex from hancornia speciosa gomes: a study in zebrafish and in silico. Pharmaceuticals 14, 856. doi:10.3390/ph14090856
Umeh, N. E., Onuorah, R. T., Ekweogu, C. N., Ijioma, S. N., Egeduzu, O. G., Nwaru, E. C., et al. (2024). Chemical profiling, toxicity assessment, anti-diarrhoeal, anti-inflammatory and antinociceptive activities of Canarium schweinfurthii Engl. (Burseraceae) bark in rats. J. Ethnopharmacol. 333, 118460. doi:10.1016/j.jep.2024.118460
Vargas-Ruiz, R., Montiel-Ruiz, R. M., Zamilpa, A., Gonzalez-Cortazar, M., Herrera-Ruiz, M. L., Molina-Cabrera, J., et al. (2023). Bio-guided study of the antinociceptive, anti-inflammatory, and free-radical scavenging capacity of the leaves of Rhus virens Lindh. ex A. Gray and its possible mechanism of antinociception. J. Ethnopharmacol. 300, 115756. doi:10.1016/j.jep.2022.115756
Williams, R. B., O’Neil-Johnson, M., Williams, A. J., Wheeler, P., Pol, R., and Moser, A. (2015). Dereplication of natural products using minimal NMR data inputs. Org. Biomol. Chem. 13, 9957–9962. doi:10.1039/C5OB01713K
Keywords: acute oral toxicity, antinociceptive, flavonoid, medicinal plant, Montrichardia linifera
Citation: Costa WJTN, Coelho LPdF, Tembra AL, Monteiro RFM, Almeida JRG, Lima KT, Botelho AdS, Batista RJdR, Freitas JJdS, Pinheiro WBdS, Oliveira FRT, Oliveira KRHM, Lima ABd, Amarante CBd and Bastos GdNT (2024) Chemical characterization, assessment of acute oral toxicity, and antinociceptive potential of the methanolic extract of Montrichardia linifera (Arruda) Schott leaves from Brazil. Front. Pharmacol. 15:1475157. doi: 10.3389/fphar.2024.1475157
Received: 02 August 2024; Accepted: 21 October 2024;
Published: 20 November 2024.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Yugal Kishore Mohanta, University of Science and Technology, IndiaNoemi Waksman, Autonomous University of Nuevo León, Mexico
Copyright © 2024 Costa, Coelho, Tembra, Monteiro, Almeida, Lima, Botelho, Batista, Freitas, Pinheiro, Oliveira, Oliveira, Lima, Amarante and Bastos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilmara de Nazareth Tavares Bastos, YmFzdG9zZ250QGdtYWlsLmNvbQ==
 Wellington Junior Taisho Nagahama Costa
Wellington Junior Taisho Nagahama Costa Leticia Prazeres de Farias Coelho
Leticia Prazeres de Farias Coelho Alan Luz Tembra2
Alan Luz Tembra2 Rayan Fidel Martins Monteiro
Rayan Fidel Martins Monteiro Jose Ramon Gama Almeida
Jose Ramon Gama Almeida Klinsmann Thiago Lima
Klinsmann Thiago Lima Anderson de Santana Botelho
Anderson de Santana Botelho Raimundo Junior da Rocha Batista
Raimundo Junior da Rocha Batista Jofre Jacob da Silva Freitas
Jofre Jacob da Silva Freitas Wandson Braamcamp de Souza Pinheiro
Wandson Braamcamp de Souza Pinheiro Karen Renata Herculano Matos Oliveira
Karen Renata Herculano Matos Oliveira Anderson Bentes de Lima
Anderson Bentes de Lima Cristine Bastos do Amarante
Cristine Bastos do Amarante Gilmara de Nazareth Tavares Bastos
Gilmara de Nazareth Tavares Bastos