- 1Department of Clinical Pharmacy, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan
- 2Department of Emergency and Disaster Medical Pharmacy, Faculty of Pharmaceutical Sciences, Fukuoka University, Fukuoka, Japan
- 3Department of Pharmacotherapy, School of Pharmacy, Shujitsu University, Okayama, Japan
Introduction: In this study, we aimed to examine the effects of chotosan, a traditional Japanese botanical drug, and its active component, Uncaria hook, on anxiety-like behaviors induced by systemic inflammation in mice.
Methods: To induce systemic inflammation, the mice were treated with lipopolysaccharide (LPS), a bacterial endotoxin. Prior to LPS treatment, the mice were administered chotosan or Uncaria hook orally each day for 14 days. Anxiety-like behavior of the mice was evaluated using the light–dark test 24 h after LPS treatment.
Results: Repeated administration of chotosan prevented anxiety-like behavior in both normal and LPS-treated mice. Similarly, administration of Uncaria hook suppressed LPS-induced anxiety-like behavior in mice. Furthermore, treatment with tandospirone, a 5-HT1A receptor agonist, alleviated anxiety-like behavior in mice, whereas treatment with DOI, a 5-HT2A receptor agonist, enhanced anxiety-like behavior in mice. LPS treatment significantly increased serotonin (5-HT)2A receptor mRNA expression in the frontal cortex, whereas 5-HT1A receptor mRNA expression remained unchanged in the hippocampus. Notably, chotosan significantly suppressed the mRNA expression of 5-HT2A receptor.
Discussion: These findings indicate that chotosan exerts anxiolytic-like effects in the context of inflammation-induced anxiety, potentially mediated by the inhibition of 5-HT2A receptor hyperfunction in LPS-treated mice. Consequently, we postulate that chotosan may be effective in managing inflammation-induced anxiety-like behaviors.
1 Introduction
Inflammation significantly contributes to psychiatric disorders, including anxiety, bipolar disorder, and depression (Leonard and Maes, 2012; Yang et al., 2015). Elevated levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6), have been observed in patients with anxiety and major depressive disorders (Choi et al., 2022; Dowlati et al., 2010). Previous studies by our group and others have demonstrated that systemic administration of lipopolysaccharide (LPS), a bacterial endotoxin, elicits anxiety-like behavior in mice (Matsumoto et al., 2021; Sabedra Sousa et al., 2019; Ushio et al., 2022).
Anxiety disorders are characterized by excessive fear or worry, often accompanied by physiological symptoms such as increased heart rate, sweating, and trembling. Their known causes include genetic predisposition, environmental stressors, and neurochemical imbalances (Bandelow and Michaelis, 2015). Therapeutic strategies for anxiety include pharmacological treatments such as benzodiazepines and selective serotonin reuptake inhibitors, as well as non-pharmacological approaches such as cognitive-behavioral therapy (CBT) (Bandelow and Michaelis, 2015; Bandelow et al., 2017; Hofmann et al., 2012; Savignac et al., 2016).
Several subtypes of serotonin (5-HT) receptors, especially the 5-HT2A receptor, are hypothesized to play a major role in the development of emotional memory as well as in mediating anxiety and defensive responses (Homberg, 2012; Murnane, 2019). It has been reported that LPS treatment markedly increases the expression of 5-HT2A receptor proteins in the cortex of mice (Savignac et al., 2016). Upregulated expression of cortical 5-HT2A receptors has been implicated in the central pathophysiological mechanisms of inflammation (Savignac et al., 2016). This pharmacological evidence supports the hypothesis that 5-HT2A receptors modulate anxiety. Furthermore, numerous studies have suggested that 5-HT1A receptors are involved in anxiety, depression, and their treatment (Kitamura et al., 2003; Kitamura and Nagatani, 1996). Tandospirone, an agonist of the 5-HT1A receptor, is currently clinically used as an anxiolytic agent. However, the anxiolytic effects of 5-HT1A receptor agonists in inflammatory conditions remain poorly understood.
Phytotherapy, the use of plant-based remedies, has been employed in traditional medicine systems worldwide. Notably, in Indian traditional medicine (ayurveda), Withania somnifera (ashwagandha) extracts have been used for their anxiolytic and anti-inflammatory properties. Studies have shown that ashwagandha can effectively reduce stress and anxiety levels in humans and animal models (Costa et al., 2023; Dar et al., 2015; Gupta and Kaur, 2018; Kaur et al., 2017; Maccioni et al., 2022; Pratte et al., 2014). Similarly, Kampo medicine, a traditional Japanese medicine, includes various phytotherapeutic remedies for anxiety and other conditions.
Chotosan, also known as Diago Teng San in Chinese and Jopungsan in Korean, is a Kampo formula composed of 10 botanical drugs and gypsum fibrosum. It is extensively used in clinical practice to treat brain and heart diseases (Satoh, 2013). Chotosan is typically prescribed for individuals with moderate physical strength who experience chronic conditions, such as headaches, dizziness, stiff shoulders, neurosis, and a predisposition to hypertension (Ministry of Health, Labour, and Welfare MHLW, 2021). In a previous study, chotosan ameliorated cognitive impairment and hippocampal neuronal loss in a common carotid artery occlusion model of vascular dementia (Jiang et al., 2019). In addition, chotosan has demonstrated antidepressant-like effects in mice (Sasaki-Hamada et al., 2017) and alleviated anxiety-like behavior in a mouse model of type 2 diabetes (Zhao et al., 2012). Thus, chotosan has demonstrated potential benefits for the treatment of psychiatric disorders. Moreover, Kampo medicines, which include crude drugs, such as Uncaria hook found in chotosan, are frequently prescribed for a broad spectrum of conditions ranging from mental disorders to physical weakness. Uncaria hook refers to the dried stem and hook of the Uncaria plant, which belongs to the family Rubiaceae (Ndagijimana et al., 2013; Liang et al., 2020). Although many botanical origins have been identified for Uncaria hook (Zhang et al., 2015), the Japanese Pharmacopoeia recognizes three primary sources: Uncaria rhynchophylla Miquel, Uncaria sinensis Haviland, and Uncaria macrophylla Wallich. Geissoschizine methyl ether, an indole alkaloid metabolite of Uncaria hook, reportedly alleviates aggressive behavior in socially isolated mice through a partial agonist effect on the 5-HT1A receptor (Nishi et al., 2012).
In this study, we aimed to investigate the anxiolytic effects of chotosan and Uncaria hook on LPS-induced anxiety-like behavior in mice using a light–dark test. Additionally, we assessed the effects of chotosan on the mRNA expression of 5-HT1A and 5-HT2A receptors in LPS-treated mice.
2 Materials and methods
2.1 Animals
This study was conducted in compliance with the recommendations outlined in the Guide for Animal Experiments of the Advanced Science Research Center of Okayama University and Shujitsu University. The animal study protocol was approved by the Animal Care and Use Committee of the Advanced Science Research Center of Okayama University (OKU-2023527) and Shujitsu University (054–001). In total, 430 six-week-old male ICR mice were used in this study. The mice were purchased from Jackson Laboratory, Japan (Yokohama, Japan). The mice were housed in a climate-controlled room with a temperature of approximately 23°C ± 1°C and a humidity level of approximately 60%. They were housed in groups of five per cage under a regular light–dark cycle (lights on from 08:00 to 20:00 h). The mice were provided standard laboratory food and tap water.
2.2 Drugs
The following drugs were used in this study: LPS (from Escherichia coli O127:B8; Sigma-Aldrich, St. Louis, MO, United States), WAY100635 (Sigma-Aldrich), tandospirone (Sediel® Tablets; Sumitomo Pharma, Osaka, Japan), and (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; Sigma-Aldrich). The drugs were dissolved in saline solution and administered to the mice via intraperitoneal (i.p.) injection at a volume of 10 mL/kg. Chotosan (Lot No. 2190047010) and chotokou (Uncaria hook) (Lot. no.: 2201089010) were provided by Tsumura & Co., (Tokyo, Japan). Chotosan comprises 10 dried botanical drugs and gypsum fibrosum. Chotosan was used in the form of an extract powder made of the following raw materials: 3.0 parts Japanese Pharmacopoeia (JP) Uncaria Hook [U. rhynchophylla Miquel, U. sinensis Haviland, or U. macrophylla Wallich, hook], 3.0 parts JP Citrus unshiu peel [C. unshiu Marcowicz, or Citrus reticulata Blanco (Rutaceae), pericarpium], 2.0 parts JP Chrysanthemum flower [Chrysanthemum indicum Linné, or Chrysanthemum morifolium Ramatulle, capitulum], 2.0 parts JP Saposhnikovia root and rhizome [Saposhnikovia divaricata Schischkin, root, and rhizome], 3.0 parts JP Pinellia tuber [Pinellia ternata Breitenbach, tuber], 3.0 parts JP Ophiopogon root [ Opiopogon japonicus Ker-Gawlerm, root], 3.0 parts JP Poria sclerotium [Wolfiporia cocos Ryvarden et Gilbertson, sclerotium], 2.0 parts JP Ginseng [Panax ginseng C. A. Meyer (Panax schinseng Nees) (Araliaceae), radix], 1.0 part JP Ginger [Zingiber officinale Roscoe (Zingiberaceae), rhizoma], 1.0 part JP Glycyrrhiza [Glycyrrhiza uralensis Fischer, or Glycyrrhiza glabra Linné (Leguminosae), radix], and 5.0 parts JP gypsum fibrosum [CaSO4 2H2O]. The plants were identified based on their morphology and marker components, as per JP and company standards. The extract quality was standardized based on good manufacturing practices, as defined by the Ministry of Health, Labour, and Welfare of Japan. The concentrate was spray-dried to obtain the chotosan powder. A three-dimensional high-performance liquid chromatogram of chotosan, provided by Tsumura & Co., is shown in Supplementary Figure 1. The chotosan and Uncaria hook powders were dissolved in distilled water and administered by peroral (p.o.) injection.
2.3 Light–dark test
The light–dark test serves as an anxiogenic challenge, as it assesses the conflict between the desire to explore new environments and aversion to brightly lit zones (Ushio et al., 2022). We evaluated the preventative effects of repeated administration of chotosan and Uncaria hook solutions and tandospirone on LPS-induced anxiety-like behavior in mice using the light–dark test. The light–dark box consisted of a light zone (20 cm × 20 cm × 25 cm) and a dark zone (20 cm × 20 cm × 25 cm) with black walls and a black floor. The two zones were separated by a partition with a single opening (5 cm × 8 cm) to allow the mice to move between them. The light zone was illuminated at an intensity of 500 × l, whereas the dark zone was covered with a lid to prevent illumination. Individual mice were placed in the box for a total duration of 10 min. At the beginning of the test, the mice were placed in the center of the light zone, and the total time spent in the light zone was recorded. The floor of the light–dark box was covered with breeding wooden chips. These chips were replaced for each experiment. In addition, the behavior of the mice was recorded on video, and one researcher measured the time spent by mice in the light zone.
2.4 Locomotor activity
Locomotor activity was monitored for 10 min using automated activity monitoring chambers (DAS-8; Neuroscience, Inc., Tokyo, Japan). The plastic chambers measured 28 cm (width) × 20 cm (length) × 13 cm (height). Locomotor activity of mice was recorded for 10 min. The assay was conducted the day after the final administration of chotosan and Uncaria hook. Different mice were used for the locomotor activity test and the light–dark box test.
2.5 In vivo experimental schedule
Mice were injected with LPS (500 μg/kg, i. p.) 1 day prior to the light–dark test. The injection schedule and doses of LPS (500 μg/kg, i. p.) selected were based on our previous studies (Matsumoto et al., 2021; Ushio et al., 2022). Chotosan (100–1,000 mg/kg) or Uncaria hook (10–100 mg/kg) were administered orally (p.o.) once a day for 14 days until the day before the experiments. The doses and duration of the injection of chotosan and Uncaria hook were based on a previous report (Nishi et al., 2012). Following the administration of the final dose of chotosan or Uncaria hook, the animals were injected with LPS after a duration of 1 h. Single doses of tandospirone (0.1–1 mg/kg, i. p.) and DOI (1 mg/kg, i. p.) were administered intraperitoneally 30 min before the light–dark test. The doses of tandospirone and DOI were based on our previous study (Nakamura et al., 2018). Previous studies, including our own, have shown that tandospirone exhibits significant anxiolytic effects following a single administration in animal models (Nakamura et al., 2018; Shimizu et al., 1992). In the antagonism experiment using WAY100635, we performed the light–dark test at 1 h after the final administration of chotosan or Uncaria hook. WAY100635, a 5-HT1A receptor antagonist, was administered 15 min before the light–dark test. The dose of WAY100635 was based on a previous study (Egashira et al., 2008). Mice used for the light–dark test and those used for the locomotor activity assay were different, because we considered that anxiety induced by the light–dark test could influence the locomotor activity.
2.6 Measurement of the frontal cortex 5-HT2A receptor mRNA and hippocampal 5-HT1A receptor mRNA expression using real-time quantitative polymerase chain reaction
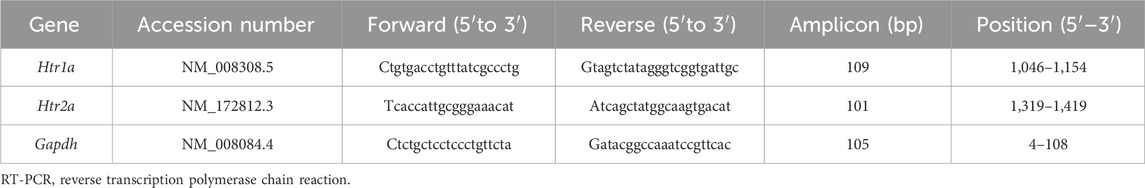
At 24 h after LPS treatment, the mice were decapitated without anesthesia. The hippocampus and frontal cortex were collected, quickly frozen, and stored at −80°C until homogenization. Total RNA was isolated and purified from individual hippocampus and frontal cortex tissues using the Maxwell® RSC RNA tissue kit (Promega, Madison, WI, United States), according to the manufacturer’s instructions. Thereafter, 1 µg of total RNA was reverse-transcribed to cDNA using ReverTra Ace® quantitative reverse transcription polymerase chain reaction (RT-qPCR) master mix (TOYOBO, Osaka, Japan). Quantitative real-time PCR was performed using a StepOnePlus Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, United States) and THUNDERBIRD® SYBR® qPCR mix (TOYOBO) under the following amplification conditions: 95°C for 20 s for early denaturation, followed by 40 cycles for 1 s at 95°C for heat denaturation and 20 s at 60°C for annealing. To quantify the total expression of Htr1a and Htr2a, forward and reverse primers and probes were designed (Integrated DNA Technologies, Coralville, IO, United States). Gapdh was used as an internal control. The relative fold changes in the expression level of each target gene were calculated using the comparative CT method and the 2−ΔΔCT equation. The sequences of the primers used, target genes, and other related information are presented in Table 1.
2.7 Statistical analysis
Data are presented as mean ± standard error of the mean. Data analysis was conducted using the Shapiro–Wilk test to assess the normality of data distribution before selecting the appropriate statistical tests. For normally distributed data, the student’s t-test or one-way or two-way analysis of variance (ANOVA) was used, followed by Tukey’s test (Excel-Toukei ver 7.0; ESUMI Co., Ltd., Tokyo, Japan) to determine the significance of differences among the groups. Statistical significance was set at p < 0.05. In cases where the number of animals was reduced, this occurred solely due to administration errors that resulted in the death of the animals, as detailed in the Methods section. No data were excluded based on statistical outliers or significant deviations from other data points.
3 Results
3.1 Effect of chotosan and Uncaria hook on LPS-induced anxiety-like behavior during the light–dark test in mice
Treatment with both chotosan (300–1,000 mg/kg) and Uncaria hook (30–100 mg/kg) significantly increased the amount of time the mice spent in the light zone [chotosan: F (3,19) = 3.60, p < 0.05; Uncaria hook: F (3,19) = 8.65, p < 0.01] (Figures 1A, B). In contrast, LPS treatment significantly reduced the amount of time the mice spent in the light zone. In the LPS-treated mice, treatment with effective dose of both chotosan (300 mg/kg) and Uncaria hook (30 mg/kg) significantly increased the amount of time the mice spent in the light zone [chotosan: LPS: F (1, 11) = 10.48, p < 0.01; chotosan: F (1, 11) = 12.99, p < 0.01; LPS × chotosan F (1, 11) = 1.10, p = 0.32] (Figure 2A) [Uncaria hook: LPS: F (1, 11) = 17.63, p < 0.01; Uncaria hook: F (1, 11) = 38.96, p < 0.01; LPS × Uncaria hook: F (1, 11) = 0.48, p = 0.51] (Figure 2B). In the course of the experiment, we observed the death of two mice due to administration errors. Specifically, in Figure 1A, 1 mouse in the 300 mg/kg chotosan group died on day 3, and in Figure 1B, 1 mouse in the 10 mg/kg Uncaria hook group died on day 10. We believe that the cause of death was likely due to the administered substance inadvertently entering the trachea instead of the esophagus, leading to respiratory failure, as the deaths occurred immediately after oral administration.
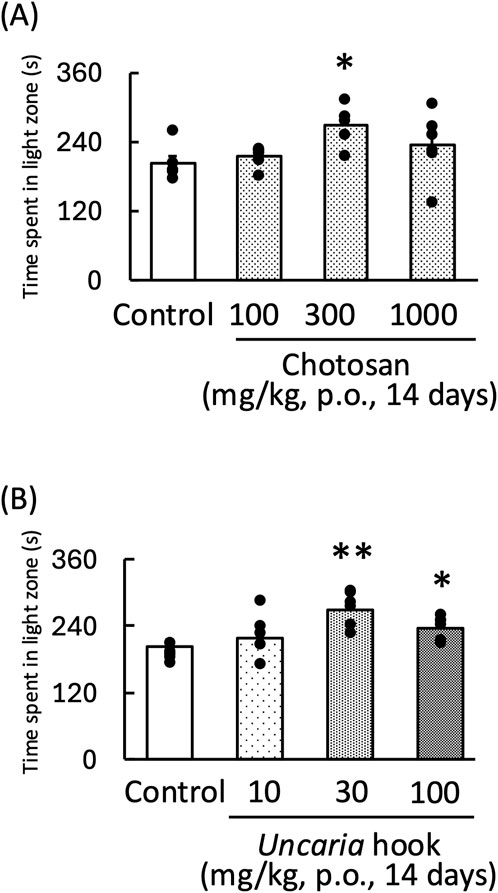
Figure 1. Effects of chotosan (A) and Uncaria hook (B) on the amount of time mice spent in the light zone during the light–dark test. Chotosan (100, 300, and 1,000 mg/kg, p. o.) or Uncaria hook (10, 30, and 100 mg/kg, p. o.) was administered for 14 days prior to the light–dark test. Data are shown as mean ± standard error of the mean; n = 5–6 per group. Data were analyzed using one-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. control), **p < 0.01 (vs. control).
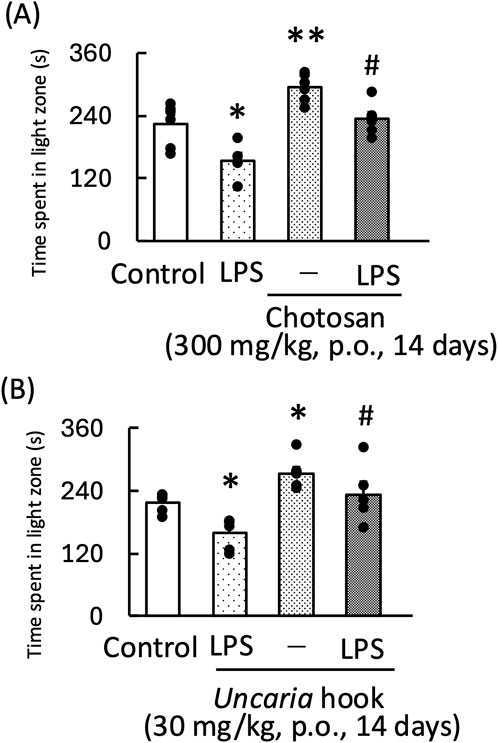
Figure 2. Effects of chotosan (A) and Uncaria hook (B) on the amount of time LPS-treated mice spent in the light zone during the light–dark test. Chotosan (300 mg/kg, p. o.) or Uncaria hook (30 mg/kg, p. o.) was administered for 14 days prior to the light–dark test. LPS (500 μg/kg, i.p.) was administered 1 day prior to the light–dark test. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. control), **p < 0.01 (vs. control), #p < 0.05 (vs. LPS) i.p., intraperitoneal; LPS, lipopolysaccharide.
3.2 Effect of chotosan and Uncaria hook on the locomotor activity in mice
We evaluated the effect of chotosan (300 mg/kg) and Uncaria hook (30 mg/kg) on the locomotor activity of mice. Chotosan and Uncaria hook did not change the locomotor activity (chotosan: p = 0.95; Uncaria hook: p = 0.58) (Figure 3).
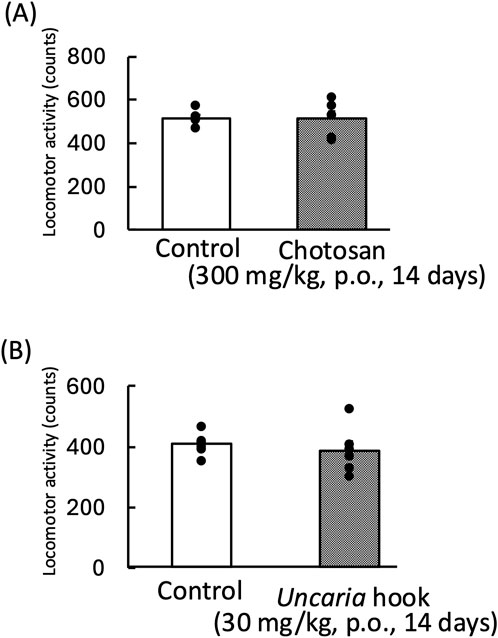
Figure 3. Effects of chotosan (A) and Uncaria hook (B) on locomotor activity in mice. Chotosan (300 mg/kg) or Uncaria hook (30 mg/kg) was administered orally once a day for 14 days until the day prior to locomotor activity measurement. The locomotor activity was monitored for 10 min. Data are shown as mean ± standard errors of the mean; n = 6 per group. Data were analyzed using the student’s t-test.
3.3 Effect of tandospirone on LPS-induced anxiety-like behavior during the light–dark test and locomotor activity in mice
Tandospirone, a 5-HT1A receptor agonist, significantly increased the amount of time the mice spent in the light zone [F (3,20) = 3.35, p < 0.05] (Figure 4A). The anxiolytic effect of tandospirone was blocked by WAY100635, a 5-HT1A receptor antagonist [WAY100635: F (1, 11) = 4.09, p = 0.07; tandospirone: F (1, 11) = 4.46, p = 0.06; WAY100635 × tandospirone: F (1, 11) = 4.70, p = 0.05] (Figure 4B). Additionally, tandospirone significantly increased the amount of time the LPS-treated mice spent in the light zone [LPS: F (1, 11) = 63.60, p < 0.01; tandospirone: F (1, 11) = 61.94, p < 0.01; LPS × tandospirone: F (1, 11) = 0.07, p = 0.80] (Figure 4C). However, the measurement of locomotor activity in this treatment group showed no significant changes [tandospirone: F (1, 11) = 7.73, p < 0.05; WAY100635: F (1, 11) = 0.84, p = 0.38; tandospirone × WAY100635: F (1, 11) = 0.002, p = 0.96] (Figure 5A); [LPS: F (1, 11) = 0.27, p = 0.62; tandospirone: F (1, 11) = 2.24, p = 0.16; LPS × tandospirone: F (1, 11) = 0.17, p = 0.68] (Figure 5B).
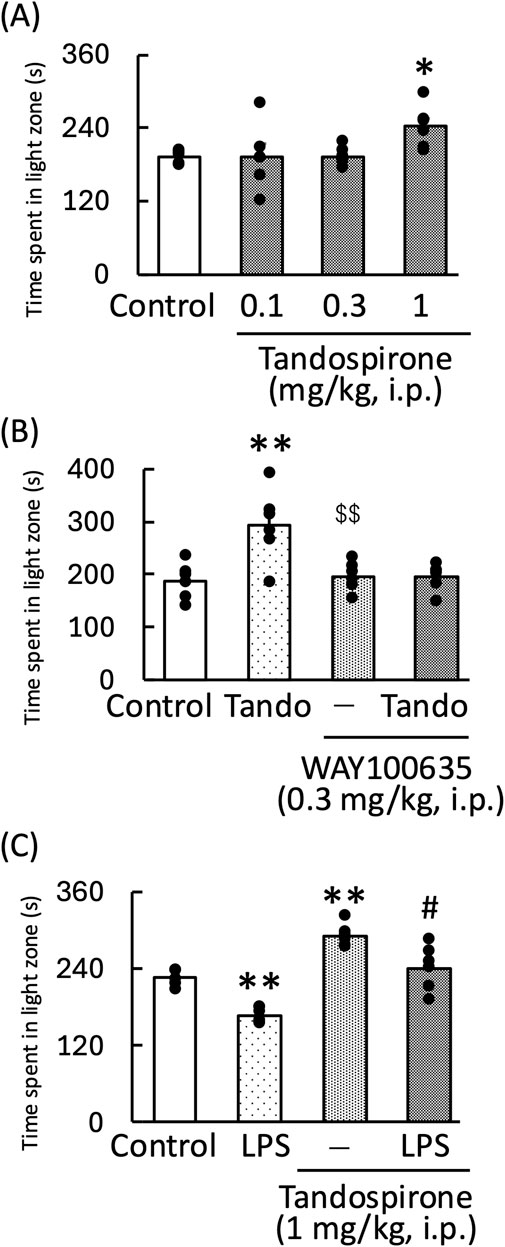
Figure 4. Effects of tandospirone on the amount of time spent by the normal (A,B) and LPS-treated (C) mice in the light zone during the light–dark test. Tandospirone (0.1, 0.3, and 1 mg/kg, i. p.) (A) and tandospirone (1 mg/kg, i. p.) (B,C) were administered 30 min before the light–dark test. WAY100635 (0.3 mg/kg, i. p.) was administered to normal mice 15 min before the light–dark test. LPS (500 μg/kg, i. p.) was administered 1 day prior to the light–dark test. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using one-way ANOVA; group means were compared using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. control), **p < 0.01 (vs. control), $$p < 0.01 (vs. tandospirone), #p < 0.05 (vs. LPS). i.p., intraperitoneal; LPS, lipopolysaccharide; Tando, tandospirone.
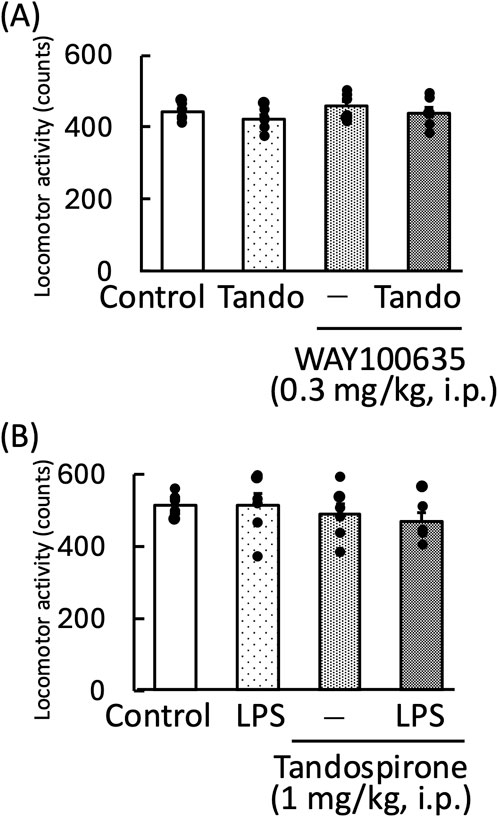
Figure 5. Effects of tandospirone on locomotor activity in WAY100635-treated (A) and LPS-treated (B) mice. Tandospirone (1 mg/kg, i. p.) was administered 30 min before the locomotor activity measurement. WAY100635 (0.3 mg/kg, i. p.) was administered 15 min before the light–dark test. LPS (500 μg/kg, i. p.) was administered 1 day prior to the locomotor activity measurement. The locomotor activity was monitored for 10 min. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using one-way ANOVA, group means were compared using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. i.p., intraperitoneal; LPS, lipopolysaccharide; Tando, tandospirone.
3.4 Effect of WAY100635 on the anxiolytic effects of chotosan and Uncaria hook on mice during the light–dark test
In the light–dark test, the anxiolytic effects of chotosan and Uncaria hook on mice were blocked by WAY100635, a 5-HT1A receptor antagonist [WAY100635: F (1, 11) = 6.14, p < 0.05; chotosan: F (1, 11) = 4.36, p = 0.06; WAY100635 × chotosan: F (1, 11) = 4.38, p = 0.06] (Figure 6A) [WAY100635: F (1, 11) = 2.24, p = 0.16; Uncaria hook: F (1, 11) = 0.87, p = 0.37; WAY100635 × Uncaria hook: F (1, 11) = 16.22, p < 0.05] (Figure 6B).
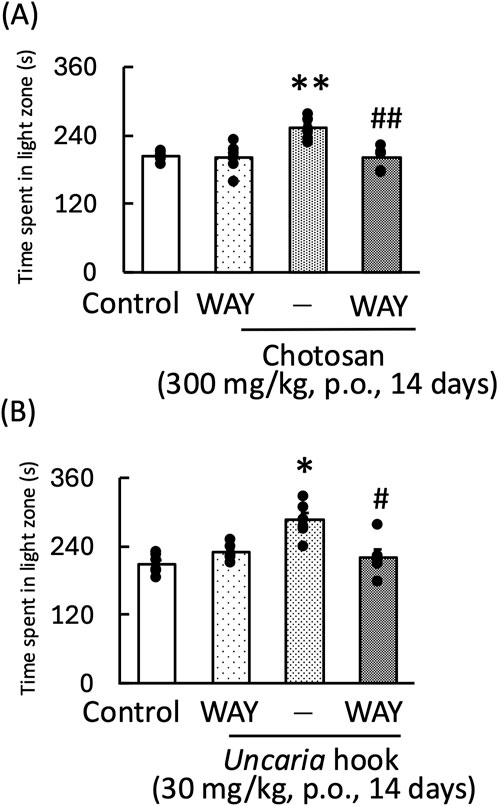
Figure 6. Effects of WAY100635 on chotosan-induced (A) and Uncaria hook-induced (B) increase in the amount of time spent by mice in the light zone during the light–dark test. Chotosan (300 mg/kg, p. o.) or Uncaria hook (30 mg/kg, p. o.) was administered for 14 days prior to the light–dark test. The light–dark test performed 1 h after the final administration of chotosan and Uncaria hook. WAY100635 (0.3 mg/kg, i. p.) was administered 15 min before the light–dark test. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. control), **p < 0.01 (vs. control), #p < 0.05 (vs. chotosan), ##p < 0.01 (vs. Uncaria hook) i.p., intraperitoneal; p.o., peroral.
3.5 Effect of DOI on LPS-induced anxiety-like behavior during the light–dark test and locomotor activity in mice
DOI, a 5-HT2A receptor agonist, significantly decreased the amount of time spent in the light zone by both normal and LPS-treated mice [LPS: F (1, 11) = 33.96, p < 0.01; DOI: F (1, 11) = 32.70, p < 0.01; LPS × DOI: F (1, 11) = 0.73, p = 0.73] (Figure 7A). However, the measurement of locomotor activity in this treatment group showed no significant changes [LPS: F (1, 11) = 3.32, p = 0.10; DOI: F (1, 11) = 11.59, p < 0.01; LPS × DOI: F (1, 11) = 0.87, p = 0.37] (Figure 7B).
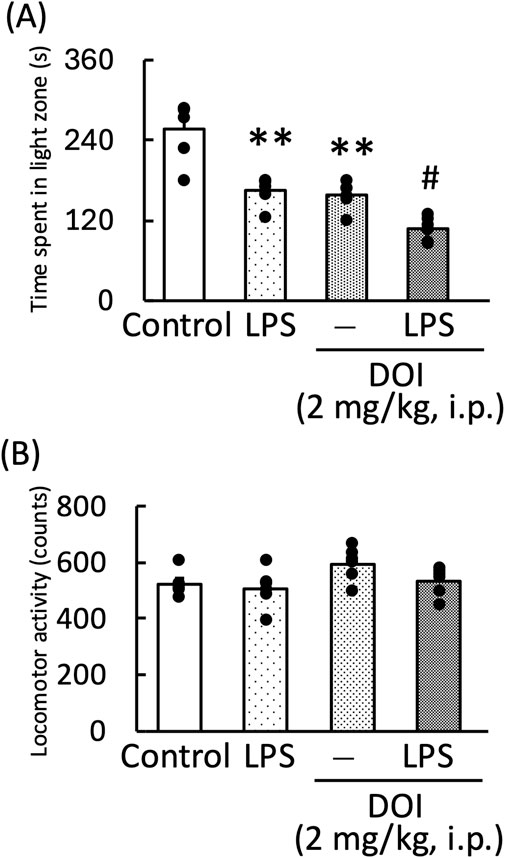
Figure 7. Effects of DOI on the amount of time mice spent in the light zone during the light–dark test (A) and locomotor activity of mice (B). DOI (1 mg/kg, i. p.) was administered 30 min before the light–dark test and locomotor activity measurement. LPS (500 μg/kg, i. p.) was administered 1 day prior to the light–dark test and locomotor activity measurement. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. **p < 0.01 (vs. Control), #p < 0.05 (vs. DOI) i.p., intraperitoneal.
3.6 Effects of chotosan and Uncaria hook on the mRNA expression of frontal cortex 5-HT2A receptor and hippocampal 5-HT1A receptor in LPS-treated mice
LPS treatment significantly increased the frontal cortex 5-HT2A receptor mRNA expression in mice. Chotosan decreased the frontal cortex 5-HT2A receptor mRNA expression in LPS-treated mice [LPS: F (1, 11) = 10.51, p < 0.01; chotosan: F (1, 11) = 0.35, p = 0.56; LPS × chotosan: F (1, 11) = 0.94, p = 0.35] (Figure 8A). In contrast, the hippocampal mRNA expression of the 5-HT1A receptor was not affected by LPS treatment. Chotosan did not alter the hippocampal 5-HT1A receptor mRNA expression in normal or LPS-treated mice [LPS: F (1, 11) = 2.92, p = 0.11; chotosan: F (1, 11) = 4.39, p = 0.06; LPS × chotosan: F (1, 11) = 0.68, p = 0.43] (Figure 8B). Uncaria hook significantly decreased the frontal cortex 5-HT2A receptor mRNA expression in LPS-treated mice [LPS: F (1, 11) = 1.80, p = 0.21; Uncaria hooks: F (1, 11) = 4.29, p = 0.06; LPS × Uncaria hook: F (1, 11) = 5.11, p < 0.05] (Figure 9A). In contrast, the hippocampal mRNA expression of the 5-HT1A receptor was not affected by treatment with LPS or Uncaria hook [LPS: F (1, 11) = 0.05, p = 0.83; Uncaria hook: F (1, 11) = 0.38, p = 0.55; LPS × Uncaria hook: F (1, 11) = 3.80, p = 0.08] (Figure 9B).
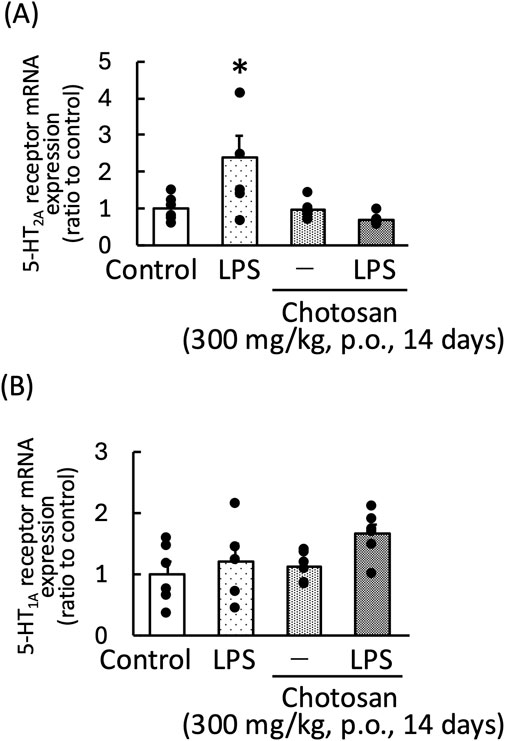
Figure 8. Effects of chotosan on the frontal cortex 5-HT2A receptor mRNA (A) and hippocampal 5-HT1A receptor mRNA expression (B) in mice. Chotosan (300 mg/kg) was administered orally for 14 days. 5-HT2A receptor and 5-HT1A receptor mRNA levels measured 1 day after LPS (500 μg/kg, i. p.) administration. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. Control). i.p., intraperitoneal.
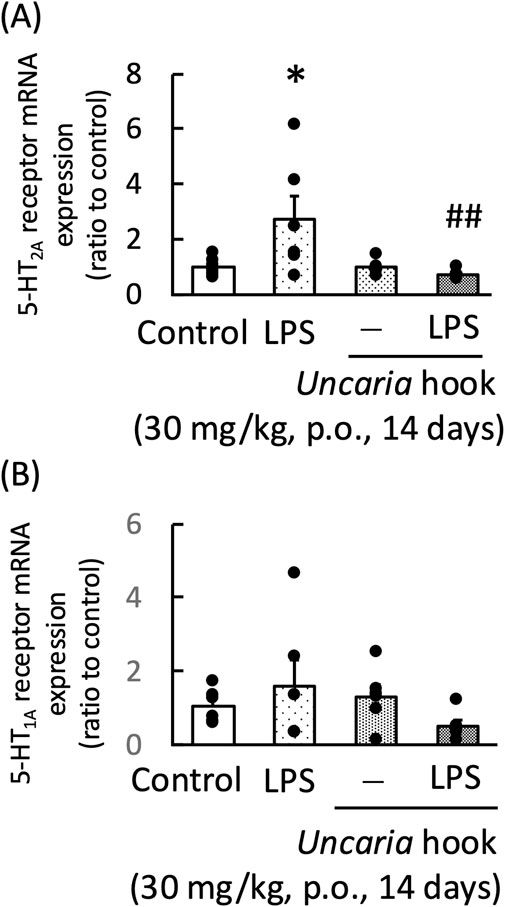
Figure 9. Effects of Uncaria hook on the frontal cortex 5-HT2A receptor mRNA (A) and hippocampal 5-HT1A receptor mRNA expression (B) in mice at 24 h after LPS treatment. Uncaria hook (30 mg/kg) was administered orally for 14 days. 5-HT2A receptor and 5-HT1A receptor mRNA levels measured 1 day after LPS (500 μg/kg, i. p.) administration. Data are shown as mean ± standard error of the mean; n = 6 per group. Data were analyzed using two-way ANOVA, and Tukey’s test was used to compare the means of multiple groups. *p < 0.05 (vs. Control), ##p < 0.01 (vs. LPS). i.p., intraperitoneal; LPS, lipopolysaccharide.
4 Discussion
The light–dark test employed in this study has been extensively utilized to evaluate states of anxiety and anxiolysis. Notably, we previously reported that inflammatory conditions induced by LPS administration elicited anxiety-like behaviors during the light–dark test in mice (Ushio et al., 2022). In the present study, we used the light–dark test to demonstrate that chotosan exerted anxiolytic effects in both normal and LPS-treated mice. Zhao et al. (2012) explored the effect of chotosan on emotional deficits in type 2 diabetic db/db mice, revealing these mice displayed heightened anxiety levels as assessed using the elevated plus maze. Chotosan was found to alleviate emotional deficits in these mice. Collectively, these findings suggest that chotosan possesses anxiolytic-like properties and can mitigate anxiety-like behaviors under inflammatory conditions. Regarding the active component of chotosan responsible for these effects, Uncaria hook, a key component of chotosan, has been previously identified (Yuzurihara et al., 2002). Our findings indicate that treatment with Uncaria hook significantly prolonged the duration spent by mice in light areas in the light–dark test compared with that by both vehicle- and LPS-treated mice. This observation implies that Uncaria hook may be the component of chotosan that contributes to its anxiolytic-like effects, particularly under inflammatory conditions. Furthermore, we acknowledge the small number of animals used in each group as a limitation of this study. As this was a screening study, the small sample size may limit the statistical power of our findings. Future studies with larger samples are necessary to confirm these results.
The 5-HT nerve system plays a pivotal role in modulating anxiety and anxiolytic effects. Among the various subtypes of 5-HT receptors, 5-HT1A receptors are thought to be critically involved in the pathogenesis of mood-related disorders (Sharp and Barnes, 2020). Tandospirone, a partial agonist of the 5-HT1A receptor, has anxiolytic properties (Nishitsuji et al., 2004). In this study, tandospirone induced anxiolytic-like behavior in both normal and LPS-treated mice. Notably, the anxiolytic effect of tandospirone was inhibited by WAY100635, a 5-HT1A receptor antagonist. These observations suggest that agonists of the 5-HT1A receptor may play a therapeutic role in alleviating anxiety-like behaviors induced by inflammation in mice. Furthermore, mice treated with chotosan spent a significantly longer time in the light zone than those treated with vehicle, and the anxiolytic effect of chotosan was attenuated by pretreatment with WAY100635. This finding implies that chotosan may induce anxiolytic-like behavior by enhancing the activity of the 5-HT1A receptor. Additionally, Uncaria hook demonstrated anxiolytic-like effects in both normal and LPS-treated mice. This effect was also mitigated by WAY100635 treatment. A previous study reported that Uncaria hook exhibits partial agonistic activity toward the 5-HT1A receptor (Terawaki et al., 2010). These observations collectively suggest that chotosan functions as a 5-HT1A receptor agonist, potentially through its active component Uncaria hook, thereby exerting anxiolytic effects, especially under conditions of systemic inflammation.
It is well known that 5-HT2A receptor hyperfunction is associated with neuropsychiatric disorders such as stress responses, anxiety, and depression (Shelton et al., 2009; Weisstaub et al., 2006). However, the specific role of the 5-HT2A receptors in states of inflammation remains poorly understood. In this study, we investigated the mechanisms underlying LPS-induced anxiety-like behaviors, with particular emphasis on the role of 5-HT2A receptor hyperactivity. Therefore, we aimed to elucidate the involvement of 5-HT2A receptors in LPS-induced anxiety-like behaviors in mice. Our findings demonstrated a notable upregulation in frontal cortex 5-HT2A receptor mRNA expression following LPS treatment, corroborating with the results from previous research (Couch et al., 2015). Furthermore, mice administered DOI, a 5-HT2A receptor agonist, spent a shorter time in the light compartment than those subjected to normal and LPS treatments. Thus, the LPS-induced anxiety-like behaviors observed in the light–dark test might be attributed to hyperfunction of the 5-HT2A receptor. Notably, we found that chotosan mitigated the elevation in 5-HT2A receptor mRNA levels in LPS-treated mice, and Uncaria hook markedly reduced 5-HT2A receptor mRNA expression in the frontal cortex of LPS-treated mice. Previous studies have documented that repeated administration of Yokukansan, which includes Uncaria hook, significantly reduces 5-HT2A receptor protein levels in the prefrontal cortex and alleviates behaviors mediated by the 5-HT2A receptor in mice (Egashira et al., 2008). Consequently, the anxiolytic-like effects observed with chotosan and Uncaria hooks under inflammatory conditions may be attributed to the suppression of 5-HT2A receptor hyperfunction. Numerous studies have demonstrated a possible interaction between 5-HT2A and 5-HT1A receptors in rats and mice (Darmani et al., 1990; Dursun and Handley, 1993; Kitamura et al., 2007). Our research and previous studies have indicated that 8-OH-DPAT, a 5-HT1A receptor full agonist, inhibits DOI-induced wet-dog shake behavior in rats (Darmani et al., 1990; Kitamura et al., 2007). These results support the hypothesis that 5-HT1A receptors may exert an inhibitory effect on the activation of 5-HT2A receptors. Furthermore, in this study, LPS treatment did not significantly modify the hippocampal 5-HT1A receptor mRNA expression in mice. Additionally, Uncaria hook exhibits partial agonistic effects on 5-HT1A receptors (Terawaki et al., 2010). These findings suggest that the anxiolytic effects of Uncaria hook may involve suppressive modulation of 5-HT2A receptor function via the activation of 5-HT1A receptors. The model of inflammatory conditions used in this study is particularly intriguing from the perspective of understanding the interplay between 5-HT2A and 5-HT1A receptor functions or the action of 5-HT1A receptor agonists. These results contribute to a growing body of evidence suggesting that 5-HT1A receptor agonists play an important role in mitigating inflammation-induced anxiety-like behaviors in mice.
We demonstrated that chotosan alleviated anxiogenic-like behavior in mice under LPS-induced inflammatory conditions, as assessed using the light–dark test. Moreover, we found that Uncaria hook, the key component of botanical drug chotosan, effectively suppresses anxiety-like behavior induced by LPS. The anxiety-like behavior elicited by LPS treatment appears to be associated with the hyperactivity of the 5-HT2A receptor. Additionally, our findings indicate that chotosan alleviates the behavioral consequences of LPS-induced 5-HT2A receptor hyperfunction. This effect is potentially mediated by its agonistic activity on the 5-HT1A receptors. These results indicate the therapeutic potential of chotosan and its component (Uncaria hook) in ameliorating anxiety-like behavior, particularly under inflammatory conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Animal Experiments of the Advanced Science Research Center of Okayama University and Shujitsu University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YO: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. SU: Data curation, Formal Analysis, Funding acquisition, Writing–review and editing, Writing–original draft. YI: Formal Analysis, Writing–review and editing. YK: Data curation, Formal Analysis, Investigation, Supervision, Writing–review and editing. YZ: Supervision, Writing–review and editing. TS: Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS KAKENHI (Grant Number 22K15339).
Acknowledgments
The authors are grateful to Tsumura & Co., (Tokyo, Japan) for providing chotosan and Uncaria hook.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1471602/full#supplementary-material
Abbreviations
LPS, lipopolysaccharide; 5-HT, serotonin; JP, Japanese Pharmacopoeia; N.D., not detected.
References
Bandelow, B., and Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues. Clin. Neurosci. 17, 327–335. doi:10.31887/DCNS.2015.17.3/bbandelow
Bandelow, B., Michaelis, S., and Wedekind, D. (2017). Treatment of anxiety disorders. Dialogues. Clin. Neurosci. 19, 93–107. doi:10.31887/DCNS.2017.19.2/bbandelow
Choi, W., Kang, H. J., Kim, J. W., Kim, H. K., Kang, H. C., Lee, J. Y., et al. (2022). Interaction effect of the serum interleukin-6 level and anxiety on the 12-week pharmacotherapeutic responses of patients with depressive disorders. J. Affect. Disord. 308, 166–171. doi:10.1016/j.jad.2022.04.048
Costa, G., Serra, M., Maccioni, R., Casu, M. A., Kasture, S. B., Acquas, E., et al. (2023). Withania somnifera influences MDMA-induced hyperthermic, cognitive, neurotoxic and neuroinflammatory effects in mice. Biomed. Pharmacother. 161, 114475. doi:10.1016/j.biopha.2023.114475
Couch, Y., Xie, Q., Lundberg, L., Sharp, T., and Anthony, D. C. (2015). A model of post-infection fatigue Is associated with increased TNF and 5-HT2A receptor expression in mice. PLOS ONE 10, e0130643. doi:10.1371/journal.pone.0130643
Dar, N. J., Hamid, A., and Ahmad, M. (2015). Pharmacologic overview of Withania somnifera, the Indian ginseng. Cell. Mol. Life. Sci. 72, 4445–4460. doi:10.1007/s00018-015-2012-1
Darmani, N. A., Martin, B. R., Pandey, U., and Glennon, R. A. (1990). Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol. Biochem. Behav. 36, 901–906. doi:10.1016/0091-3057(90)90098-3
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry. 67, 446–457. doi:10.1016/j.biopsych.2009.09.033
Dursun, S. M., and Handley, S. L. (1993). The effects of alpha 2-adrenoceptors antagonists on the inhibition of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced head shakes by 5-HT1A receptor agonists in the mouse. Br. J. Pharmacol. 109, 1046–1052. doi:10.1111/j.1476-5381.1993.tb13727.x
Egashira, N., Iwasaki, K., Ishibashi, A., Hayakawa, K., Okuno, R., Abe, M., et al. (2008). Repeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT)2A receptors in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1516–1520. doi:10.1016/j.pnpbp.2008.05.010
Gupta, M., and Kaur, G. (2018). Withania somnifera as a potential anxiolytic and anti-inflammatory candidate against systemic lipopolysaccharide-induced neuroinflammation. Neuromolecular. Med. 20, 343–362. doi:10.1007/s12017-018-8497-7
Hofmann, S. G., Asnaani, A., Vonk, I. J., Sawyer, A. T., and Fang, A. (2012). The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cogn. Ther. Res. 36, 427–440. doi:10.1007/s10608-012-9476-1
Homberg, J. R. (2012). Serotonergic modulation of conditioned fear. Sci. (Cairo) 2012, 821549. doi:10.6064/2012/821549
Jiang, P., Chen, L., Sun, J., Li, J., Xu, J., Liu, W., et al. (2019). Chotosan ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia via activating the Nrf2-mediated antioxidant pathway. J. Pharmacol. Sci. 139, 105–111. doi:10.1016/j.jphs.2018.12.003
Kaur, T., Singh, H., Mishra, R., Manchanda, S., Gupta, M., Saini, V., et al. (2017). Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol. Cell. Biochem. 427, 91–101. doi:10.1007/s11010-016-2900-1
Kitamura, Y., Araki, H., Shibata, K., Gomita, Y., and Tanizaki, Y. (2003). 5-HT (1A) receptor full agonist, 8-OH-DPAT, exerts antidepressant-like effects in the forced swim test in ACTH-treated rats. Eur. J. Pharmacol. 481, 75–77. doi:10.1016/j.ejphar.2003.09.008
Kitamura, Y., Kitagawa, K., Fujitani, Y., Shibata, K., Araki, H., Sendou, T., et al. (2007). The 5-HT1A receptor full agonist, 8-OH-DPAT inhibits ACTH-induced 5-HT2A receptor hyperfunction in rats: involvement of 5-HT1A receptors in the DOI-induced wet-dog shakes in ACTH-treated rats. Biol. Pharm. Bull. 30, 117–120. doi:10.1248/bpb.30.117
Kitamura, Y., and Nagatani, T. (1996). Buspirone enhances immobility in the forced swim test in mice. Pharmacol. Biochem. Behav. 55, 445–451. doi:10.1016/s0091-3057(96)00116-5
Leonard, B., and Maes, M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav Rev. 36, 764–785. doi:10.1016/j.neubiorev.2011.12.005
Liang, J.-H., Wang, C., Huo, X.-K., Tian, X.-G., Zhao, W.-Y., Wang, X., et al. (2020). The genus Uncaria: a review on phytochemical metabolites and biological aspects. Fitoterapia 147, 104772. doi:10.1016/j.fitote.2020.104772
Maccioni, R., Serra, M., Marongiu, J., Cottiglia, F., Maccioni, E., Bassareo, V., et al. (2022). Effects of docosane ferulate, a constituent of Withania somnifera, on ethanol- and morphine-elicited conditioned place preference and ERK phosphorylation in the accumbens shell of CD1 mice. Psychopharmacol. Berl. 239, 795–806. doi:10.1007/s00213-022-06069-w
Matsumoto, D., Ushio, S., Wada, Y., Noda, Y., Esumi, S., Izushi, Y., et al. (2021). Bumetanide prevents diazepam-modified anxiety-like behavior in lipopolysaccharide-treated mice. Eur. J. Pharmacol. 904, 174195. doi:10.1016/j.ejphar.2021.174195
Ministry of Health, Labour, and Welfare MHLW (2021). “Japanese Pharmacopoeia,” in 18th edition: the MHLW ministerial notification No. 220. Available at: https://www.mhlw.go.jp/content/11120000/000912390.pdf (Accessed August 15, 2024).
Murnane, K. S. (2019). Serotonin 2A receptors are a stress response system: implications for post-traumatic stress disorder. Behav. Pharmacol. 30, 151–162. doi:10.1097/FBP.0000000000000459
Nakamura, Y., Kitamura, Y., Sumiyoshi, Y., Naito, N., Kan, S., Ushio, S., et al. (2018). Involvement of 5-HT (2A) receptor hyperfunction in the anxiety-like behavior induced by doxorubicin and cyclophosphamide combination treatment in rats. J. Pharmacol. Sci. 138, 192–197. doi:10.1016/j.jphs.2018.10.001
Ndagijimana, A., Wang, X., Pan, G., Zhang, F., Feng, H., and Olaleye, O. (2013). A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 86, 35–47. doi:10.1016/j.fitote.2013.01.018
Nishi, A., Yamaguchi, T., Sekiguchi, K., Imamura, S., Tabuchi, M., Kanno, H., et al. (2012). Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin ₁A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience 207, 124–136. doi:10.1016/j.neuroscience.2012.01.037
Nishitsuji, K., To, H., Murakami, Y., Kodama, K., Kobayashi, D., Yamada, T., et al. (2004). Tandospirone in the treatment of generalised anxiety disorder and mixed anxiety-depression: results of a comparatively high dosage trial. Clin. Drug. Investig. 24, 121–126. doi:10.2165/00044011-200424020-00007
Pratte, M. A., Nanavati, K. B., Young, V., and Morley, C. P. (2014). An alternative treatment for anxiety: a systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J. Altern. Complement. Med. 20, 901–908. doi:10.1089/acm.2014.0177
Sabedra Sousa, F. S., Birmann, P. T., Bampi, S. R., Fronza, M. G., Balaguez, R., Alves, D., et al. (2019). Lipopolysaccharide-induced depressive-like, anxiogenic-like and hyperalgesic behavior is attenuated by acute administration of α-(phenylselanyl) acetophenone in mice. Neuropharmacology 146, 128–137. doi:10.1016/j.neuropharm.2018.11.028
Sasaki-Hamada, S., Suzuki, A., Ueda, Y., Matsumoto, K., and Oka, J. I. (2017). Serotonergic and dopaminergic systems are implicated in antidepressant-like effects of chotosan, a Kampo formula, in mice. J. Pharmacol. Sci. 133, 110–113. doi:10.1016/j.jphs.2017.01.002
Satoh, H. (2013). Pharmacological characteristics of Kampo medicine as a mixture of constituents and ingredients. J. Integr. Med. 11, 11–16. doi:10.3736/jintegrmed2013003
Savignac, H. M., Couch, Y., Stratford, M., Bannerman, D. M., Tzortzis, G., Anthony, D. C., et al. (2016). Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 52, 120–131. doi:10.1016/j.bbi.2015.10.007
Sharp, T., and Barnes, N. M. (2020). Central 5-HT receptors and their function; present and future. Neuropharmacology 177, 108155. doi:10.1016/j.neuropharm.2020.108155
Shelton, R. C., Sanders-Bush, E., Manier, D. H., and Lewis, D. A. (2009). Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience 158, 1406–1415. doi:10.1016/j.neuroscience.2008.11.036
Shimizu, H., Tatsuno, T., Tanaka, H., Hirose, A., Araki, Y., and Nakamura, M. (1992). Serotonergic mechanisms in anxiolytic effect of tandospirone in the Vogel conflict test. Jpn. J. Pharmacol. 59, 105–112. doi:10.1254/jjp.59.105
Terawaki, K., Ikarashi, Y., Sekiguchi, K., Nakai, Y., and Kase, Y. (2010). Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J. Ethnopharmacol. 127, 306–312. doi:10.1016/j.jep.2009.11.003
Ushio, S., Wada, Y., Nakamura, M., Matsumoto, D., Hoshika, K., Shiromizu, S., et al. (2022). Anxiolytic-like effects of hochuekkito in lipopolysaccharide-treated mice involve interleukin-6 inhibition. Front. Pharmacol. 13, 890048. doi:10.3389/fphar.2022.890048
Weisstaub, N. V., Zhou, M., Lira, A., Lambe, E., Gonzalez-Maeso, J., Hornung, J. P., et al. (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313, 536–540. doi:10.1126/science.1123432
Yang, C., Shirayama, Y., Zhang, J. C., Ren, Q., and Hashimoto, K. (2015). Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 27, 312–316. doi:10.1017/neu.2015.36
Yuzurihara, M., Ikarashi, Y., Goto, K., Sakakibara, I., Hayakawa, T., and Sasaki, H. (2002). Geissoschizine methyl ether, an indole alkaloid extracted from Uncariae Ramulus et Uncus, is a potent vasorelaxant of isolated rat aorta. Eur. J. Pharmacol. 444, 183–189. doi:10.1016/s0014-2999(02)01623-0
Zhang, Q., Zhao, J. J., Xu, J., Feng, F., and Qu, W. (2015). Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. J. Ethnopharmacol. 173, 48–80. doi:10.1016/j.jep.2015.06.011
Zhao, Q., Niu, Y., Matsumoto, K., Tsuneyama, K., Tanaka, K., Miyata, T., et al. (2012). Chotosan ameliorates cognitive and emotional deficits in an animal model of type 2 diabetes: possible involvement of cholinergic and VEGF/PDGF mechanisms in the brain. BMC Complement. Altern. Med. 12, 188. doi:10.1186/1472-6882-12-188
Keywords: anxiolytic, chotosan, inflammation, serotonin receptor, Uncaria hook
Citation: Okawa Y, Ushio S, Izushi Y, Kitamura Y, Zamami Y and Sendo T (2024) Ameliorating effect of chotosan and its active component, Uncaria hook, on lipopolysaccharide-induced anxiety-like behavior in mice. Front. Pharmacol. 15:1471602. doi: 10.3389/fphar.2024.1471602
Received: 28 July 2024; Accepted: 23 August 2024;
Published: 04 September 2024.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Riccardo Maccioni, The Scripps Research Institute, United StatesMagdalena Kotańska, Jagiellonian University Medical College, Poland
Copyright © 2024 Okawa, Ushio, Izushi, Kitamura, Zamami and Sendo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soichiro Ushio, cy11c2hpb0BmdWt1b2thLXUuYWMuanA=
 Yasumasa Okawa1
Yasumasa Okawa1 Soichiro Ushio
Soichiro Ushio Yasuhisa Izushi
Yasuhisa Izushi Yoshihisa Kitamura
Yoshihisa Kitamura Toshiaki Sendo
Toshiaki Sendo