- 1The Affiliated Kangning Hospital of Wenzhou Medical University, Zhejiang Provincial Clinical Research Center for Mental Health, Wenzhou, Zhejiang, China
- 2Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Introduction: Anxiety diagnoses have surged recently during and after the COVID-19 pandemic. Lorazepam is widely recognized for its efficacy in treatment of anxiety, as well as insomnia, etc. However, the long-term safety profile of lorazepam in extensive patient populations has not been thoroughly established.
Methods: This study aims to evaluate the potential lorazepam-associated adverse events (AEs) using data mining of the Food and Drug Administration Adverse Event Reporting System (FAERS) of the United States, seeking to provide a guidance for the future therapeutic practices.
Results: Our study revealed drug abuse, suicide attempt, sopor, delirium, and psychotic disorder were among the most prevalent AEs linked to lorazepam. In addition to common AEs, we also found that patients using lorazepam may have the risk of abnormal fat metabolism, cardiac impairment, and immunosuppression-related disorders.
Discussion: In general, our research has unveiled novel AE signals and expanded our understanding of the safety profile of lorazepam in clinical practices, providing guidance for its rational use.
1 Introduction
Anxiety is a mental condition featured by unease, worry, or fear that can be triggered by a variety of factors, such as stressful life situations, uncertainties regarding the future, or personal vulnerabilities, which may cause serious harm to individual psychological wellbeing, and the overall harmony of society. Anxiety-related symptoms are one of major mental health disorders, with a weighted prevalence of about 20% in China (Tang et al., 2024). With the intensification of social competition, anxiety seems to have become a common aspect of daily life for many individuals (Nekar et al., 2023; Rabby et al., 2023). Especially during and after the COVID-19 pandemic, the prevalence of anxiety increased in children, adolescents, and young adults (Scott et al., 2023; Fischer-Grote et al., 2024).
The etiologies of anxiety are complex and multifaceted, encompassing genetic predispositions, and psychological factors and environmental influences. Consequently, the treatment strategies of anxiety are also diversified, usually involving in a combination of psychological therapies and pharmacological interventions (Bandelow et al., 2023; Goldstein, 2024). Lorazepam, a benzodiazepine (BDZ) medication, exerts sedative and anti-anxiety effects by enhancing inhibitory circuit in the hippocampus and inhibiting excitability in the central nervous system (CNS). Lorazepam is a prominent and effective psychotropic drug approved by the Food and Drug Administration (FDA) in the treatment of anxiety in clinical practices, as well as in the treatment of other conditions such as insomnia (Ameer and Greenblatt, 1981). As lorazepam becomes more widely used, a range of adverse effects have been identified, predominantly impacting the central nervous system (such as drowsiness, dizziness), mental health (such as emotional fluctuations, fantasies), and causing withdrawal symptoms (Neale and Smith, 2007; Cosci and Chouinard, 2020; Pham Nguyen et al., 2022).
The FDA Adverse Event Reporting System (FAERS) is a database designed for monitoring the post-market drugs and therapeutic bioproducts, which covers tens of millions of case reports of AEs submitted by physicians, pharmacists, manufacturers, healthcare professionals, and others. This database includes all AEs information and medication error information collected by the FDA and serves as a critical approach for evaluating drug safety (Yu et al., 2024; Zhou et al., 2024).
In this study, we aimed to delve into the data concerning lorazepam from the FAERS database over the last 20 years, to retrospectively summarize the AEs of lorazepam, dig out potential unreported AEs and provide guidance for its rational use.
2 Methods
2.1 Data source
This study collected the American Standard Code for Information Interchange (ASCII) report files from the FAERS database for the period from the first quarter of 2004 to the fourth quarter of 2023 (2004Q1 - 2023Q4). The FAERS data file includes patient demographic and administrative information (DEMO), drug information (DRUG), reaction (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start dates and end dates for reported drugs (THER), and indications for drug administration (INDI). The data was imported into MySQL 15.0 and processed using Navicat Premium 15 software.
2.2 Signal filtering and categorization
Drug names were standardized using Medex_UIMA_1.8.3 system. All the AEs documented in the FAERS database were coded by Medical Dictionary for Regulatory Activities 24.0 (MedDRA). During the initial screening phase, we selected preferred terms (PTs) with a reported frequency ≥3 for further analysis. These PTs, along with their corresponding System Organ Class (SOCs) in MedDRA were employed to systematically categorize and analyze the signals.
2.3 Data extraction and analysis
Reports suggesting that lorazepam as the primary drug associated with adverse events (AEs) were extracted. Various signal quantification techniques including the reporting odds ratios (ROR), proportional reporting ratios (PRR), Bayesian confidence propagation neural network (BCPNN), and empirical Bayesian geometric mean (EBGM) from the disproportionality methods were employed to evaluate the data from different perspectives to offer a more comprehensive and reliable outcome and rapidly detect rare and unpredictable adverse drug reactions with strong drug-attributable component (Shu et al., 2022). The ROR is a measure of association that compares the odds of an AE occurring for users of a specific drug to the odds of it occurring for non-users, which is calculated by comparing the number of reports for the drug and AE to the number of reports for other drugs and the adverse event. The PRR is a frequency-based method that compares the reporting rate of an AE for a specific drug to the reporting rate for all drugs, which measures how often an AE is reported for a drug relative to all other drugs. BCPNN is used for signal detection in pharmacovigilance, analyzing AE reports to identify potential safety signals associated with medications, and EBGM is a Bayesian method used to assess the strength of the association between a specific drug and an adverse event (AE), which adjusts for the overall reporting rate of the AE across all drugs and provides a measure that is less sensitive to random variation.
The detailed formula for the above methods and prerequisites (Table 1) were listed in below.
2.3.1 ROR formula
The threshold of positive AE signals: reported cases ≥3 and 95% CI (lower limit) > 1, suggesting that use of the drug may be associated with an increased risk of the AE;
2.3.2 PRR formula
The threshold of positive AE signals: PRR ≥2,
2.3.3 BCPNN formula
The threshold of positive AE signals: IC025 (the lower limit of 95% CI) > 0, suggesting a possible association between the drug and the adverse event;
2.3.4 EBGM formula
The threshold of positive AE signals: EBGM05 (the lower limit of 95% CI) > 2, suggesting a possible association between the drug and the adverse event.
3 Results
3.1 Descriptive results
From January 2004 to September 2023, a total of 20,750,364 reports were obtained from the FAERS database. Following the FDA’s guidelines for identifying duplicates using CASEID and FDA_DT, we removed the redundant entries. Consequently, after the exclusion of 3,367,326 duplicate reports, a final dataset comprising 17, 383, 038 reports was obtained (Figure 1). There were 174,145 reports of lorazepam as the primarily suspected (PS) drug, and 931,661 AEs preferred terms (PTs) induced by lorazepam (as the primarily suspected drug) were identified. The detailed information of lorazepam-associated adverse events reports was described in Figure 2. Since 2004, the number of AE reports of lorazepam has gradually increased, with the highest volume recorded in 2019 (15,079 reports). The majority of these AE reports came from United States (59.79%), followed by Canada (10.55%), Italy (5.38%), Germany (3.73%), and United Kingdom (3.26%). The AE reports were mainly submitted by consumers (30.07%), physician (27.82%), pharmacist (18.19%) and other health-professional (15.75%), which were consistent with indications approved by FDA. Among all reports, females (60.25%) accounted for a larger proportion than males (33.77%). Patients were mainly aged >20 years old in the reports recording age (95.03%). Hospitalization (38.45%) was the most frequently reported serious outcome. Additionally, death or life-threatening events were reported in 23,818 cases (12.40%) and 11,195 cases (5.83%), respectively. The administration of lorazepam was predominantly oral, with 87.48% of the reports indicating this route of administration. Notably, a significant percentage of AEs occurred within the first 80 days of treatment, representing 91.57% of the cases.
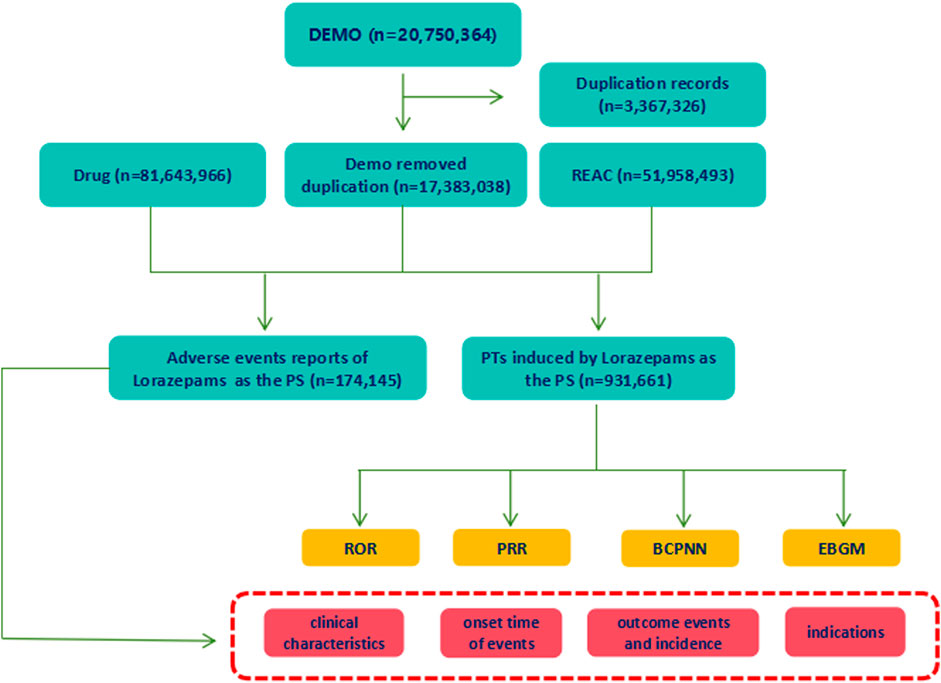
Figure 1. The process of selecting lorazepam-associated adverse events (AEs) from Food and Drug Administration adverse event reporting database (FAERS). Duplicate reports were removed according to the CASEID and FDA_DT. Out of total reports of 17, 383, 038, 174,145 AEs reports that lorazepam was believed as the primarily suspected (PS) drug that causes AEs, and 931,661 preferred terms (PTs) induced by lorazepam (as the primarily suspected drug) were extracted. The basic information of lorazepam-associated reports was collected. And the signal strengths of AEs at PTs levels were detected using ROR, PRR, BCPNN, EBGM methods.
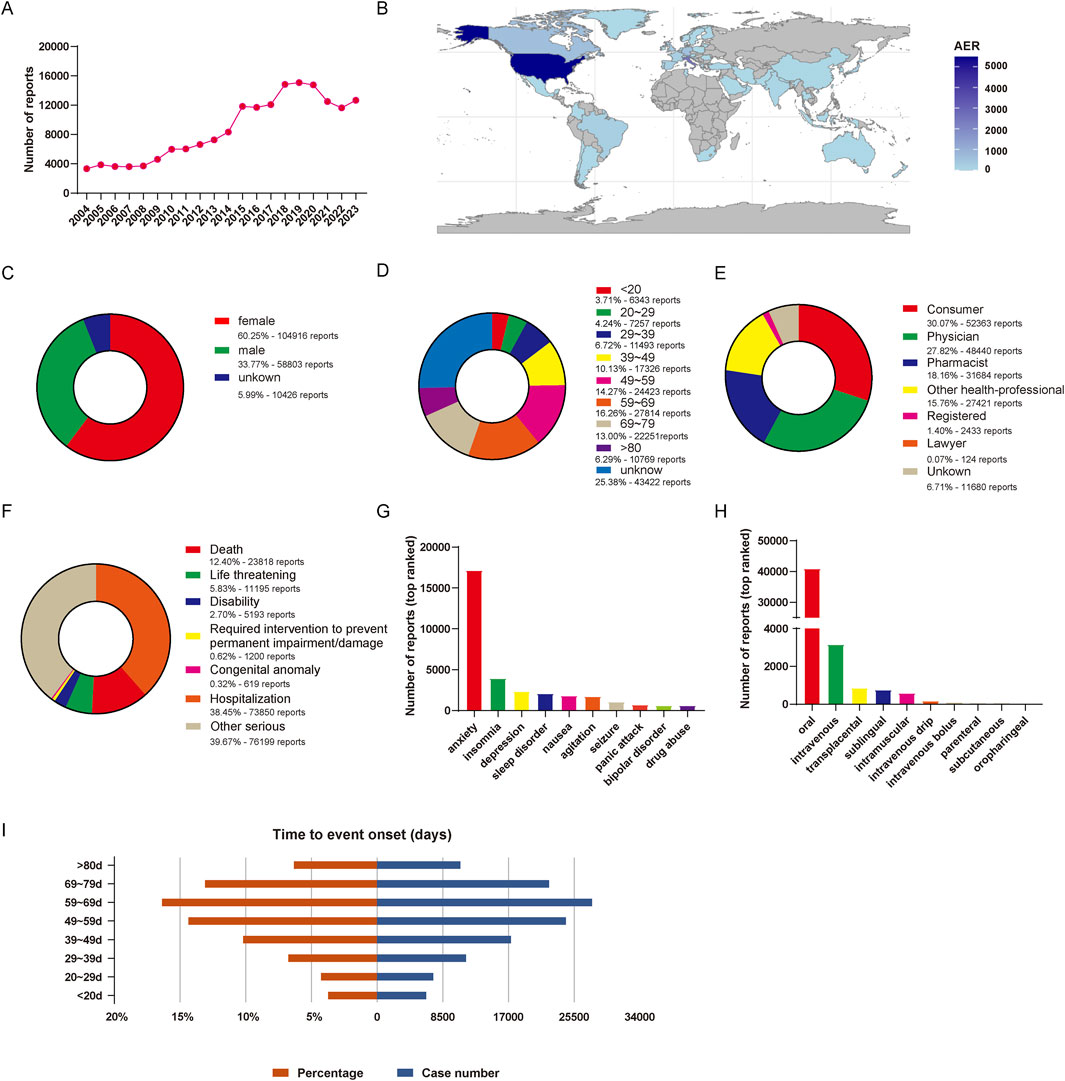
Figure 2. Basic information of lorazepam-associated AE reports in the FAERS from 2004 Q1 to 2023 Q4. (A) The annual number of AE reports. (B) The world map of countries that report lorazepam-associated AEs. (C) The gender distribution of patients. (D) The age distribution of patients. (E) The occupational distribution of the reporter. (F) The outcome distribution of AE reports in patients. (G) Top 10 ranked indications distribution of lorazepam. (H) Top 10 ranked routes distribution of lorazepam. (I) Distribution of the time of AEs occurrence.
3.2 AE signal mining results
We firstly investigated the signal strength of lorazepam-associated AEs at the System Organ Class (SOC) level (Table 2). Statistically, we found that lorazepam-induced AEs occurrence targeted 24 SOCs. The top three AEs occurred and ranked by case numbers were general disorders (symptoms like fatigue, fever, chills, edema (swelling), and other general systemic effects) and administration site conditions (local adverse effects, such as inflammation, pain, or infection, that manifest at the location where a drug is applied or injected) (126,277 cases, ROR (95% CI) = 0.74 (0.74, 0.74), PRR (95% CI) = 0.78 (0.78, 0.78), IC (IC025) = −0.36 (−0.37), EBGM (EBGM05) = 0.78 (0.78)), nervous system disorders (97,378 cases, ROR (95% CI) = 1.25 (1.25, 1.26), PRR (95% CI) = 1.23 (1.23, 1.23), IC (IC025) = 0.29 (0.28), EBGM (EBGM05) = 1.22 (1.22)), psychiatric disorders (97,378 cases, ROR (95% CI) = 1.89 (1.88, 1.9), PRR (95% CI) = 1.79 (1.79, 1.79), IC (IC025) = 0.82 (0.81), EBGM (EBGM05) = 1.77 (1.76)), which is consistent with the common AEs of lorazepam. The significant SOCs that at least one of the four algorithm meets the criteria were psychiatric disorders (IC (IC025) = 0.82 (0.81)), metabolism and nutrition disorders (IC (IC025) = 0.46 (0.45)), nervous system disorders, investigations (IC (IC025) = 0.29 (0.28)), investigations (AE that related to clinical laboratory tests and other diagnostic procedures) (IC (IC025) = 0.25 (0.24)), blood and lymphatic system disorders (IC (IC025) = 0.23 (0.21)), endocrine disorders (IC (IC025) = 0.21 (0.15)), renal and urinary disorders (IC (IC025) = 0.18 (0.16)), cardiac disorders (IC (IC025) = 0.18 (0.16)), respiratory, thoracic and mediastinal disorders (IC (IC025) = 0.13 (0.12)), gastrointestinal disorders (IC (IC025) = 0.09 (0.08)), vascular disorders (IC (IC025) = 0.04 (0.02)), musculoskeletal and connective tissue disorders (IC (IC025) = 0.02 (0.01)).

Table 2. Signal strength of lorazepam-associated AEs at the System Organ Class (SOC) level in the FAERS database.
We next investigated the most frequent lorazepam-associated AEs at preferred terms (PT) level. A total of 552 signals of lorazepam-induced AEs were detected after conforming to the four algorithms simultaneously. And the number of case reporting AEs >300 was presented in Table 3, including 33 PTs and 13 corresponding SOCs. Notably, drug abuse (4,135 cases, ROR (95% CI) = 3.29 (3.19, 3.4), PRR (95% CI) = 3.28 (3.15, 3.41), IC (IC025) = 1.66 (1.61), EBGM (EBGM05) = 3.15 (3.07)), suicide attempt (2,866 cases, ROR (95% CI) = 3.13 (3.01, 3.25), PRR (95% CI) = 3.12 (3, 3.24), IC (IC025) = 1.59 (1.53), EBGM (EBGM05) = 1.59 (1.53)), sopor (2,407 cases, ROR (95% CI) = 13.1 (12.53, 13.69), PRR (95% CI) = 13.06 (12.56, 13.58), IC (IC025) = 3.42 (3.36), EBGM (EBGM05) = 10.7 (10.31)), delirium (1896 cases, ROR (95% CI) = 3.84 (3.67, 4.03), PRR (95% CI) = 3.84 (3.69, 3.99), IC (IC025) = 1.87 (1.8), EBGM (EBGM05) = 3.65 (3.51)), psychotic disorder (1,441 cases, ROR (95% CI) = 3.21 (3.05, 3.39), PRR (95% CI) = 3.21 (3.03, 3.4), IC (IC025) = 1.63 (1.55), EBGM (EBGM05) = 3.09 (2.95)) had high signal frequencies, aligning with the drug’s label information of lorazepam. Of note, a lot of unexpected significant AEs that uncovered in the label were found in our data mining, such as PTs of pneumonia aspiration (1,178 cases, ROR (95% CI) = 3.19 (3.01, 3.39), PRR (95% CI) = 3.19 (3.01, 3.38), IC (IC025) = 1.62 (1.53), EBGM (EBGM05) = 3.07 (2.92)), obesity (709 cases, ROR (95% CI) = 3.07 (2.92), PRR (95% CI) = 3.18 (2.94, 3.44), IC (IC025) = 1.61 (1.5), EBGM (EBGM05) = 3.06 (2.87)), sinus tachycardia (676 cases, ROR (95% CI) = 3.11 (2.88, 3.36), PRR (95% CI) = 3.11 (2.88, 3.36), IC (IC025) = 1.58 (1.47), EBGM (EBGM05) = 2.99 (2.8)), pericarditis (629 cases, ROR (95% CI) = 3.09 (2.85, 3.35), PRR (95% CI) = 3.09 (2.85, 3.35), IC (IC025) = 1.57 (1.46), EBGM (EBGM05) = 2.97 (2.78)), duodenal ulcer perforation (507 cases, ROR (95% CI) = 7.99 (7.28, 8.77), PRR (95% CI) = 7.99 (7.24, 8.81), IC (IC025) = 2.82 (2.69), EBGM (EBGM05) = 7.08 (6.55)).
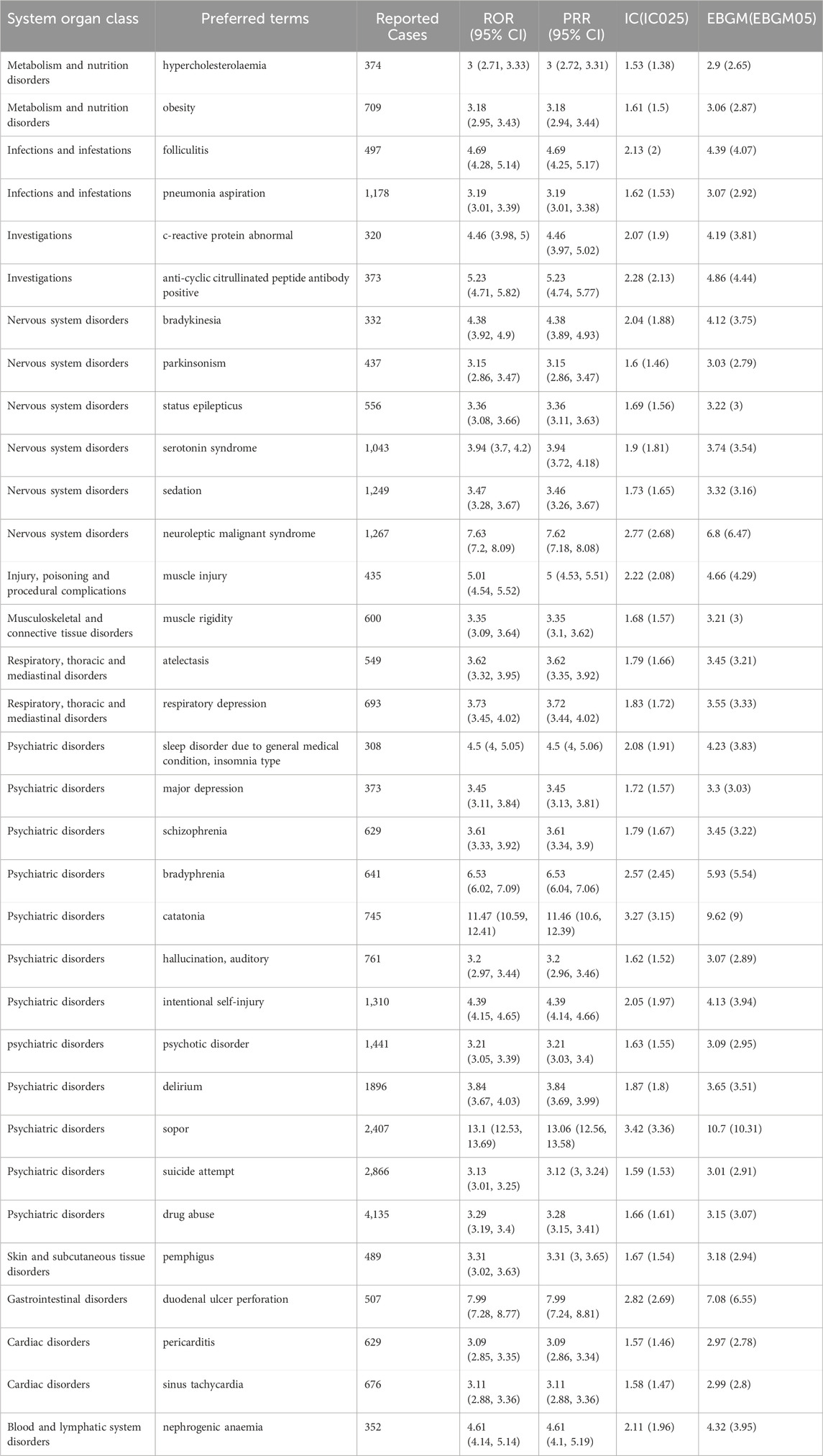
Table 3. The top 30 signal strength of lorazepam-associated AEs ranked by AE numbers at the PTs level in FAERS database.
In our analysis, we have not only focused on AEs with a high volume of reports but have also identified the top 30 AEs with significant signal strength ranked by EBGM, which are listed in Table 4. The top five AEs with strongest signal were natural killer cell count (4 cases, ROR (95% CI) = 214.65 (23.99, 1920.5), PRR (95% CI) = 214.65 (23.9, 1927.94), IC (IC025) = 5.45 (3.74), EBGM (EBGM05) = 43.73 (6.99)), affective ambivalence (4 cases, ROR (95% CI) = 214.65 (23.99, 1920.5), PRR (95% CI) = 214.65 (23.9, 1927.94), IC (IC025) = 5.45 (3.74), EBGM (EBGM05) = 43.73 (6.99)), morbihan disease (4 cases, ROR (95% CI) = 214.65 (23.99, 1920.5), PRR (95% CI) = 214.65 (23.9, 1927.94), IC (IC025) = 5.45 (3.74), EBGM (EBGM05) = 43.73 (6.99)), computerised tomogram thorax (36 cases, ROR (95% CI) = 175.63 (89.4, 345.02), PRR (95% CI) = 175.62 (90.19, 341.97), IC (IC025) = 5.39 (4.77), EBGM (EBGM05) = 41.87 (23.8)), and parachute mitral valve (9 cases, ROR (95% CI) = 160.99 (43.58, 594.66), PRR (95% CI) = 160.98 (43.3, 598.53), IC (IC025) = 5.36 (4.17), EBGM (EBGM05) = 41 (13.74)). Additionally, we have also observed that AEs with strong signals that have been reported with relatively higher frequency, such as deep vein thrombosis postoperative (275 cases, ROR (95% CI) = 42.42 (36.21, 49.69, PRR (95% CI) = 42.4 (36.25, 49.6), IC (IC025) = 4.59 (4.39), EBGM (EBGM05) = 24.13 (21.14)), lupus vulgaris (99 cases, ROR (95% CI) = 33 (25.69, 42.39), PRR (95% CI) = 33 (25.58, 42.58), IC (IC025) = 4.38 (4.05), EBGM (EBGM05) = 20.81 (16.88)). The combination of a strong signal with a higher frequency of reported cases for these AEs suggests a potential increased risk associated with lorazepam use and warrants close clinical attention and further research to understand their implications fully.
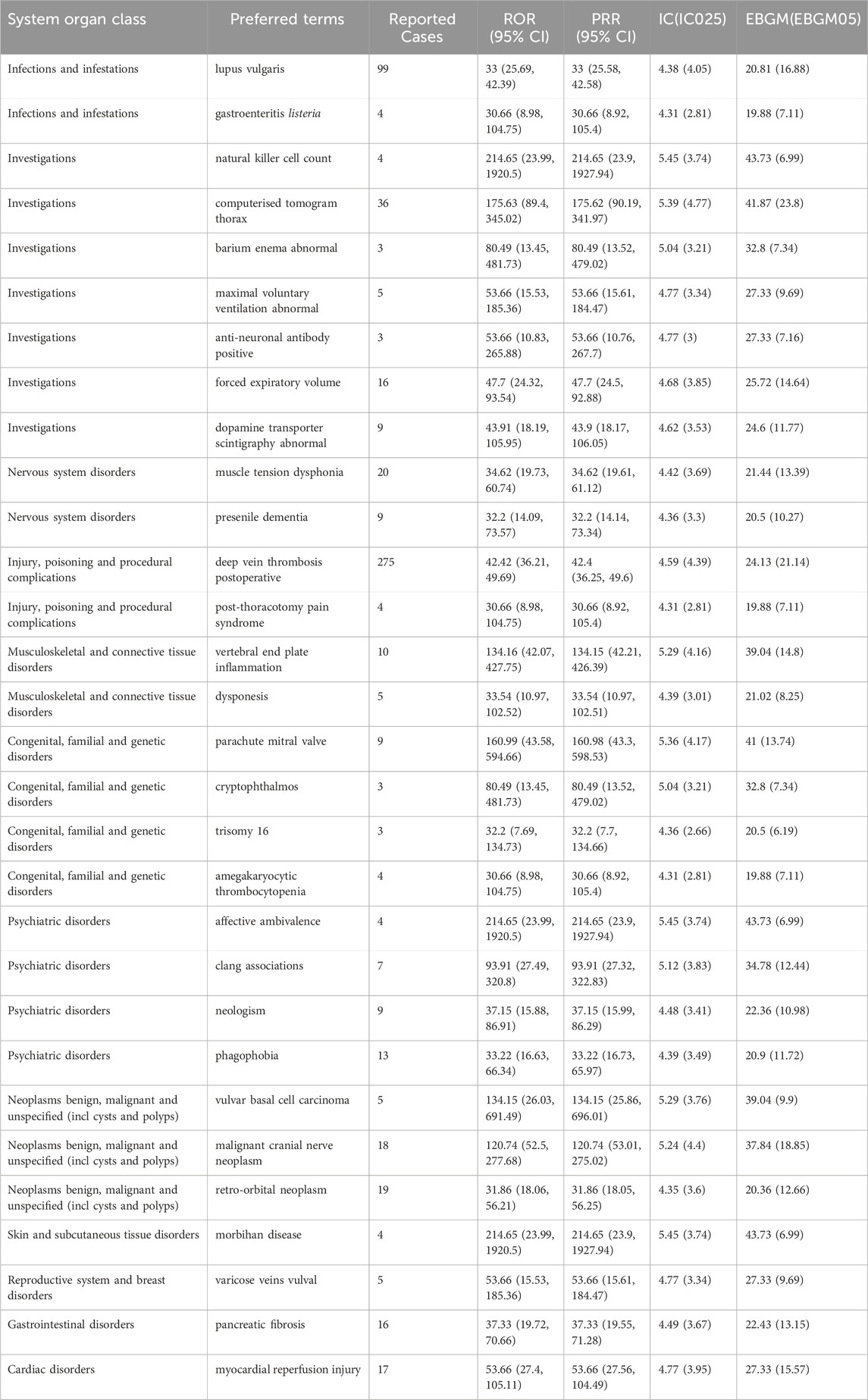
Table 4. The top 30 signal strength of lorazepam-associated AEs ranked by EBGM at the PTs level in FAERS database.
4 Discussion
In this study, we carried out a comprehensive and systematic pharmacovigilance data mining on lorazepam-associated AEs reports based on the FAERS database. We strictly collected and analyzed lorazepam-induced AEs over the past 20 years. The reports of lorazepam AEs since 2004 have continued to increase and reach to its peak in 2019 with 15,079 cases, due to the excellent therapeutic effect of the medication, as well as increasing number of patients. In this study, lorazepam showed a high proportion of AEs in older patients with the highest percentage observed in the 59–69 years age group, accounting for 16.26% of the total AEs This demographic trend may be linked to the natural decline in physical functioning and the cumulative effects of social stress experienced by this age group. The proportion of lorazepam utilization in females is nearly twice as high as that in males, which is also related to the fact that females are physically and psychologically more prone to anxiety (Warner et al., 2023). In addition, the outcomes of lorazepam treatment can be less than favorable, with a notable percentage of cases resulting in death (12.4%), life-threatening conditions (5.83%), and other serious consequences (39.67%), there is a pressing need to closely monitor and understand the AEs associated with its use.
As a medication primarily used in the treatment of psychiatric conditions, the most reported and significant SOCs of lorazepam are psychiatric disorders, followed by a range of other SOCs. It is noteworthy that metabolism and nutrition disorders, endocrine disorders, thoracic and mediastinal disorders are not mentioned in the drug’s leaflet. This oversight raises concerns and highlights the need for further research and attention to these potential risks associated with lorazepam use. Among the SOC of psychiatric disorders, most frequently reported in association with lorazepam use include drug abuse, suicide attempt, sopor, delirium, psychotic disorder, intentional self-injury, which have been wildly recognized and documented by numerous studies, highlighting their significance in the safety profile of lorazepam. Additionally, we also observed other common PTs, such as neuroleptic malignant syndrome, sedation (Babakhanian et al., 2012; Friedrich et al., 2020; Gibbons et al., 2024; Midkiff et al., 2024). Notably, we found some unexpected PTs, such as pneumonia aspiration, obesity, sinus tachycardia, pericarditis, duodenal ulcer perforation, pemphigus, hypercholesterolaemia. Pneumonia aspiration, a type of pulmonary infection, is caused by inhalation of oral secretions, gastric contents, or both. This situation may occur in patients with reduced consciousness or swallowing dysfunction stem from various reasons, such as unconscious due to anesthesia, sedatives, or medical conditions (Kong et al., 2022). Lorazepam has the effects of sedation and hypnosis, which may have the potential to cause pneumonia aspiration. Drug induced obesity may occur after taking various medications, such as antipsychotics (clozapine, olanzapine) (Larsen et al., 2017; Miyakoshi et al., 2023), antidepressants (selective serotonin reuptake inhibitors SSRIs) (Suchacki et al., 2023), corticosteroids (glucocorticoids) (Zhang et al., 2024). It was noteworthy that patients used lorazepam may exhibit metabolism and nutrition disorders (obesity and hypercholesterolaemia), suggesting that the underlying influence of lorazepam in fat metabolism. Meanwhile, numerous AE signal of sinus tachycardia and pericarditis were also found, indicating the cardiac impairment potential of lorazepam. The long-term use of non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin, may damage gastrointestinal mucosa and increase the risk of duodenal ulcer perforation (Niv et al., 2005). At present, there is no report that lorazepam can increase the risk of duodenal ulcer perforation, which may be caused by indirectly affecting gastrointestinal function through affecting the central nervous system. Pemphigus is a group of serious autoimmune bullous diseases characterized by the formation of loose blisters on the skin and mucosa (Timoteo et al., 2024). The occurrence of pemphigus indicated the inhibitory effect of lorazepam in immune system.
Other than common AEs, we also listed some AEs with relatively less occurrence but higher significance (ranked by EBMG). It is obvious that the use of lorazepam is closely related to the occurrence of deep vein thrombosis postoperative. As we mentioned above, lorazepam has good sedative and hypnotic effects, so it is also widely used to relieve postoperative anxiety and pain and promote sleep (Morgan and Malison, 2008). Long-term bed rest after surgery may reduce and slow the blood flow reflux of patients, which leads to deep vein thrombosis, which may explain the occurrence of deep vein thrombosis postoperative in patients used lorazepam. Meanwhile, we also observed the occurrence of lupus vulgaris, a disease caused by the infection of mycobacterium tuberculosis (Couppoussamy et al., 2024), which is also an immune related disease, further suggesting the potential of immune inhibition of lorazepam.
It must be emphasized that the above discussion on AEs and their potential mechanism with lorazepam is only preliminary conjecture. Therefore, we must combine clinical and basic research to reach a positive conclusion, when interpreting AEs. At the same time, medical professionals should continue to monitor the occurrence of serious AEs in clinical practice and take intervention measures as soon as possible. Although this study provides a reliable scientific basis for the safety evaluation of lorazepam from multiple perspectives, there are still some limitations. Firstly, the database collects AE reports through a voluntary reporting system, which lacks rigorous oversight of patient confidentiality and actual medication usage. This could result in underreporting, incorrect reporting, or the exclusion of certain data. Meanwhile, our statistical findings merely suggest a link between lorazepam and certain AEs, not a definitive causal relationship. Additionally, there are many confounders that can bias statistical analysis outcomes such as individual health status, gender differences, patients’ existing diseases and unknown concomitant medication use. To obtain a more comprehensive and accurate research perspective, future studies must consider using more rigorous prospective studies combined with clinical trials and epidemiological studies.
5 Conclusion
Our pharmacovigilance study explored reports of lorazepam-associated AEs using FAERS database. 174,145 reports of lorazepam as the PS and 931,661 AEs induced by lorazepam were identified over the last 20 years. Common AEs in SOC levels, such as drug abuse, suicide attempt, sopor, delirium, psychotic disorder should be overly concerned. Unexpected and novel significant AEs such as pneumonia aspiration, sinus tachycardia, pericarditis, obesity, hypercholesterolaemia, duodenal ulcer perforation, lupus vulgaris and deep vein thrombosis postoperative and others might also occur. In addition to the common adverse reactions, we found that use of lorazepam may have the potential to lead to abnormal fat metabolism, cardiac impairment, and immunosuppression-related disorders, but this needs further clinical and basic research evidence to prove. In general, our research has identified novel AE signals and expanded our understanding of the safety of lorazepam, providing guidance for its rational use.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committees of The Affiliated Kangning Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZS: Writing–original draft, Data curation, Conceptualization. ZH: Writing–original draft, Data curation. XC: Writing–original draft, Data curation. XL: Writing–review and editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The data of this study were provided by the FDA Adverse Event Reporting System. We thank Dr. Baolong Liu for the computer program processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ameer, B., and Greenblatt, D. J. (1981). Lorazepam: a review of its clinical pharmacological properties and therapeutic uses. Drugs 21, 162–200. doi:10.2165/00003495-198121030-00001
Babakhanian, M., Sadeghi, M., Mansoori, N., Alam Mehrjerdi, Z., and Tabatabai, M. (2012). Nonmedical abuse of benzodiazepines in opiate-dependent patients in tehran, Iran. Iran. J. Psychiatry Behav. Sci. 6, 62–67.
Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D., Denys, D., Dilbaz, N., et al. (2023). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders - version 3. Part I: anxiety disorders. World J. Biol. Psychiatry 24, 79–117. doi:10.1080/15622975.2022.2086295
Cosci, F., and Chouinard, G. (2020). Acute and persistent withdrawal syndromes following discontinuation of psychotropic medications. Psychother. Psychosom. 89, 283–306. doi:10.1159/000506868
Couppoussamy, K. I., Shanmugam, S., Devanda, R., and Murugan, R. (2024). Lupus vulgaris: a narrative review. Int. J. Dermatol 63, 431–437. doi:10.1111/ijd.16987
Fischer-Grote, L., Fossing, V., Aigner, M., Fehrmann, E., and Boeckle, M. (2024). Effectiveness of online and remote interventions for mental health in children, adolescents, and young adults after the onset of the COVID-19 pandemic: systematic review and meta-analysis. JMIR Ment. Health 11, e46637. doi:10.2196/46637
Friedrich, J. M., Sun, C., Geng, X., Calello, D. P., Gillam, M., Medeiros, K. L., et al. (2020). Child and adolescent benzodiazepine exposure and overdose in the United States: 16 years of poison center data. Clin. Toxicol. (Phila) 58, 725–731. doi:10.1080/15563650.2019.1674321
Gibbons, R. D., Hur, K., Lavigne, J. E., and Mann, J. J. (2024). Risk of suicide attempts and intentional self-harm on alprazolam. Psychiatry Res. 335, 115857. doi:10.1016/j.psychres.2024.115857
Goldstein, B. I. (2024). Editorial: the challenge of psychopharmacological treatment of anxiety among youth offspring of parents with bipolar disorder. J. Am. Acad. Child. Adolesc. Psychiatry 63, 396–398. doi:10.1016/j.jaac.2023.11.002
Kong, X., Wu, Y., Wen, B., Meng, D., Wei, L., and Yu, P. (2022). Effect of stress ulcers prophylaxis, sedative and statin on ventilator-associated pneumonia: a retrospective analysis based on mimic database. Front. Pharmacol. 13, 921422. doi:10.3389/fphar.2022.921422
Larsen, J. R., Vedtofte, L., Jakobsen, M. S. L., Jespersen, H. R., Jakobsen, M. I., Svensson, C. K., et al. (2017). Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 74, 719–728. doi:10.1001/jamapsychiatry.2017.1220
Midkiff, J. P., Gaba, A., and Munjal, S. (2024). When lorazepam worsens delirium in your catatonic patient. Prim. Care Companion CNS Disord. 26, 23cr03616. doi:10.4088/PCC.23cr03616
Miyakoshi, T., Ishikawa, S., Okubo, R., Hashimoto, N., Sato, N., Kusumi, I., et al. (2023). Risk factors for abnormal glucose metabolism during antipsychotic treatment: a prospective cohort study. J. Psychiatr. Res. 168, 149–156. doi:10.1016/j.jpsychires.2023.10.055
Morgan, P. T., and Malison, R. T. (2008). Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. Am. J. Drug Alcohol Abuse 34, 692–702. doi:10.1080/00952990802308221
Neale, G., and Smith, A. J. (2007). Self-harm and suicide associated with benzodiazepine usage. Br. J. Gen. Pract. 57, 407–408.
Nekar, D. M., Kang, H. Y., Lee, J. W., Oh, S. Y., and Yu, J. H. (2023). Effects of cooperative, competitive, and solitary exergames on cognition and anxiety levels in children with developmental disabilities. Games Health J. 12, 405–413. doi:10.1089/g4h.2023.0050
Niv, Y., Battler, A., Abuksis, G., Gal, E., Sapoznikov, B., and Vilkin, A. (2005). Endoscopy in asymptomatic minidose aspirin consumers. Dig. Dis. Sci. 50, 78–80. doi:10.1007/s10620-005-1281-1
Pham Nguyen, T. P., Soprano, S. E., Hennessy, S., Brensinger, C. M., Bilker, W. B., Miano, T. A., et al. (2022). Population-based signals of benzodiazepine drug interactions associated with unintentional traumatic injury. J. Psychiatr. Res. 151, 299–303. doi:10.1016/j.jpsychires.2022.04.033
Rabby, M. R., Islam, M. S., Orthy, M. T., Jami, A. T., and Hasan, M. T. (2023). Depression symptoms, anxiety, and stress among undergraduate entrance admission seeking students in Bangladesh: a cross-sectional study. Front. Public Health 11, 1136557. doi:10.3389/fpubh.2023.1136557
Scott, H. R., Stevelink, S. a.M., Gafoor, R., Lamb, D., Carr, E., Bakolis, I., et al. (2023). Prevalence of post-traumatic stress disorder and common mental disorders in health-care workers in England during the COVID-19 pandemic: a two-phase cross-sectional study. Lancet Psychiatry 10, 40–49. doi:10.1016/S2215-0366(22)00375-3
Shu, Y., Ding, Y., Liu, Y., Wu, P., He, X., and Zhang, Q. (2022). Post-Marketing safety concerns with secukinumab: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 13, 862508. doi:10.3389/fphar.2022.862508
Suchacki, K. J., Ramage, L. E., Kwok, T. C., Kelman, A., Mcneill, B. T., Rodney, S., et al. (2023). The serotonin transporter sustains human brown adipose tissue thermogenesis. Nat. Metab. 5, 1319–1336. doi:10.1038/s42255-023-00839-2
Tang, Z., Yang, X., Tan, W., Ke, Y., Kou, C., Zhang, M., et al. (2024). Patterns of unhealthy lifestyle and their associations with depressive and anxiety symptoms among Chinese young adults: a latent class analysis. J. Affect Disord. 352, 267–277. doi:10.1016/j.jad.2024.02.055
Timoteo, R. P., Pessoa-Goncalves, Y. M., Do Carmo Neto, J. R., Rodrigues, W. F., Da Silva, M. V., and Oliveira, C. J. F. (2024). A global view of pemphigus: geographical variations. Clin. Rev. Allergy Immunol. 66, 14–29. doi:10.1007/s12016-024-08980-w
Warner, E. N., Ammerman, R. T., Glauser, T. A., Pestian, J. P., Agasthya, G., and Strawn, J. R. (2023). Developmental epidemiology of pediatric anxiety disorders. Child. Adolesc. Psychiatr. Clin. N. Am. 32, 511–530. doi:10.1016/j.chc.2023.02.001
Yu, Z., Luo, J., and Wei, H. (2024). Novel insights into post-marketing adverse events associated with lenvatinib: a comprehensive analysis utilizing the FAERS database. Heliyon 10, e28132. doi:10.1016/j.heliyon.2024.e28132
Zhang, Y., Du, C., Wang, W., Qiao, W., Li, Y., Zhang, Y., et al. (2024). Glucocorticoids increase adiposity by stimulating Kruppel-like factor 9 expression in macrophages. Nat. Commun. 15, 1190. doi:10.1038/s41467-024-45477-8
Zhou, J., Zheng, Y., Xu, B., Long, S., Zhu, L. E., Liu, Y., et al. (2024). Exploration of the potential association between GLP-1 receptor agonists and suicidal or self-injurious behaviors: a pharmacovigilance study based on the FDA Adverse Event Reporting System database. BMC Med. 22, 65. doi:10.1186/s12916-024-03274-6
Keywords: anxiety, lorazepam, FAERS, adverse events, data mining
Citation: Su Z, Huang Z, Chen X and Li X (2024) Adverse event profile of lorazepam: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Front. Pharmacol. 15:1465245. doi: 10.3389/fphar.2024.1465245
Received: 16 July 2024; Accepted: 23 October 2024;
Published: 22 November 2024.
Edited by:
Tommaso Cassano, University of Foggia, ItalyReviewed by:
David Tattersall, GlaxoSmithKline, United StatesDimy Fluyau, Emory University, United States
Copyright © 2024 Su, Huang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Li, eGlsaV9paGJAMTI2LmNvbQ==
 Zhengkang Su
Zhengkang Su Zhengwei Huang
Zhengwei Huang Xiaoyu Chen
Xiaoyu Chen Xi Li
Xi Li