
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 25 September 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1464654
This article is part of the Research Topic Plant and Fungal Extracts and Metabolites in Neurotherapy: Exploring Their Pharmacology and Potential Clinical Uses View all 11 articles
Sleep disorders are becoming more and more common, leading to many health problems. However, most of current available medications to treat sleep disorders are addictive and even impair cognitive abilities. Therefore, it is important to find a natural and safe alternative to treat sleep disorders. In this study, twenty-four 8-week-old male ICR mice (25 ± 2 g) were equally divided into three groups: the control group (gavage of 0.9% saline), the eucalyptus essential oil (EEO) group (10 mg/kg B.W.), and the diazepam group (1 mg/kg B.W.). Firstly, open field test and sleep induction test were used to determine the sedative-hypnotic effect of EEO. Secondly, the effect of EEO on neurotransmitters in the mice brain was determined. Finally, based on the gut microbiota-brain axis (GMBA), the effect of EEO on the intestinal flora of mice was explored. It was found that EEO significantly reduce the activity and prolong the sleep duration of mice, exhibiting a good sedative-hypnotic effect. In the brain, EEO could increase the levels of sleep-promoting neurotransmitters, such as glutamine, Gamma-aminobutyric acid (GABA), glycine, tryptophan, N-acetylserotonin, and 5-hydroxyindoleacetic acid (5-HIAA). In the intestine, EEO was found to increase the diversity of gut microbes, the abundance of short chain fatty acid (SCFA) producing flora, and the abundance of functional flora synthesizing GABA and glycine neurotransmitters. These studies suggested that EEO exerted a sedative-hypnotic effect by acting on gut microbes and neurotransmitters in the brain. EEO has the potential to become a natural and safe alternative to traditional hypnotic sedative drugs.
Insomnia can seriously influence people quality of life, and recent studies have revealed its association with obesity, diabetes, heart disease, coronary artery disease, depression and other serious problems (Walia and Mehra, 2016; Broussard et al., 2016). It is estimated that 50–70 million American adults suffer from one or more sleep disorders (Harding and Feldman, 2008), so does 31% of Western Europeans and 23% of Japanese (Dasdelen et al., 2022). In recent decades, a large number of medications have appeared on the market for the treatment of various types of sleep disorders such as chloral hydrate, barbiturates, benzodiazepine, benzodiazepine agonists, modafinil, and antidepressants. However, these medications can cause dependence even addiction, and some may even gradually impair cognitive abilities (Matheson and Hainer, 2017). Therefore, it is important to find safe and effective substances to replace existing commercially available medications to treat sleep disorders.
Eucalyptus essential oil (EEO) is extracted from eucalyptus branches and leaves by steam distillation and has a wide range of applications. The main components of EEO are 1,8-eudesmusin and terpineol (Ieri et al., 2019). Many of the components of EEO (Bampidis et al., 2023) and EEO (Regulation (EC) No 1831/2003) are authorized as a food additive and animal feed additive. EEO has been reported to have antibacterial, anti-inflammatory, insecticidal, antioxidant, analgesic, and other excellent biological activities (Caceres et al., 2017; Aziz et al., 2019; Barbosa et al., 2021; Sathantriphop et al., 2014). It has been found that 1,8-eudesmusin can act on the nervous system, affecting the synthesis of neurotransmitters and then exerting analgesic effects (Takaishi et al., 2012). A study has shown that 1,8-eudesmusin can also inhibit locomotion in mice, showing a good sedative effect (Santos and Rao, 2000). However, the sedative-hypnotic effect of EEO has not been studied thoroughly and systematically.
Neurotransmitters in the central nervous system play an important role in the regulation of the sleep-wake cycle as key mediators of neuronal signal (Ngo and Vo, 2019). In recent years, the gut microbiota-brain axis (GMBA) has been a hot topic in biomedical researches and suggested as a potential therapeutic target for central nervous system disorders (Pesarico et al., 2023). Gut microbes not only affect the digestive, metabolic, and immune functions of the host, but also regulate the sleep state of the host through the GMBA (Poroyko et al., 2016; Golubeva et al., 2017; Niu et al., 2022). High-quality sleep has been found to be closely related to the composition of gut microbes, and in addition, gut microbes dysbiosis can exacerbate the cognitive dysfunction in sleep-deprived mice (Torres-Fuentes et al., 2017; Li N. et al., 2023). Gut microbes have been shown to be involved in the production of a variety of neurotransmitters and cytokines (Galland, 2014). Besides, gut microbes and their metabolites may also influence the expression of genes associated with the biological clock in the central nervous system and liver, then affecting sleep quality (Leone et al., 2015). It is necessary to study how EEO works on neurotransmitters and gut microbes for deep understanding the observed improvement in sleep quality. Therefore, the study was conducted to investigate the effects of EEO on brain neurotransmitters and gut microbes in mice through GMBA to find the possible targets of EEO for the treatment of sleep disorders.
All animal testing procedures were conducted in accordance with the guidelines of the Animal Ethics Committee of Zhejiang University (ethical programme code ZJU 20160403). A total of twenty-four 8-week-old male ICR mice (25 ± 2 g) were purchased from Shanghai Lingchang for this study. The 24 mice were equally divided into three groups: the control group (gavage of 0.9% saline), the EEO group (10 mg/kg B.W.), and the diazepam group (1 mg/kg B.W.). Daily gavage was administered at 9:00 a.m. for 14 days. The test environment was controlled at 20°C–25°C and 40%–70% relative humidity. Acclimatisation in the animal house had been carried out for 1 week till the test. EEO was provided by Shandong Longchang Animal Health Products Co. Diazepam was purchased from Shanghai Xudong Haipu Pharmaceutical Co., Ltd., Batch No. H13021864. Pentobarbital sodium salt (CAS no: 57-33-0) was purchased from Sigma-Aldrich, United States.
The method of preparing EEO refers to CN 110558324B. Australian eucalyptus leaves were cleaned, dried at 180°C and then crushed, and the eucalyptus oil was obtained by using water vapour distillation, and finally the eucalyptus oil was added to a vacuum distillation column for distillation to remove impurities, resulting in EEO. Measure out 100 mg of EEO and mix it with 1 mL of Tween 80, then dissolve the mixture in 49 mL of 0.9% saline solution. Shake the mixture thoroughly using a shaker, and store the EEO in a sealed, light-proof container in a refrigerator at 4°C for future experimental use. At this point, the concentration of EEO was 2 mg/mL. The EEO of 2 mg/mL is used for subsequent mice gavage experiment.
Thirty min following the final drug administration to the mice in each group, the mice were placed into the open field with a space of 50 cm3 × 50 cm3 × 40 cm3, made of acrylic sheet and aluminium alloy frame, where the photographic equipment (model 550D, Canon digital camera) was suspended at 2 m directly above. After 5 min of acclimatisation, the activities of the mice during the 5 min were recorded. Trajectory analysis was performed by the video analysis system (SANS, SA215).
The sleep induction test was referred to the Medeiros study (Medeiros et al., 2018). After the last treatment, all mice were injected intraperitoneally with sodium pentobarbital solution salt according to 50 mg/kg B.W. standard. Sleep onset was determined by the disappearance of reflex for 1 min after administration of pentobarbital sodium salt solution. Sleep latency was the time from the injection of the drug to the disappearance of the reflex, and sleep duration was the time from the disappearance of the reflex to its appearance. The sleep latency and sleep duration were recorded for each group.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) was used to determine the content of neurotransmitters in mouse brain (Horvath et al., 2022). The mouse brain was homogenised and extracted with 20% acetonitrile in methanol for 3 min, then centrifuged (12,000 × g, 4°C, 10 min), and the supernatant was refrigerated at −20°C for 30 min, then centrifuged again, the supernatant was taken as the test solution. The chromatographic column used was a Waters ACQUITY UPLCC HSS T3 C18 column (1.8 μm, 100 mm × 2.1 mm i.d.), and the column temperature was controlled at 40°C. Mobile phases A and B were ultrapure water (containing 0.1% formic acid) and acetonitrile (containing 0.1% formic acid), respectively, and the control flow rate was 0.35 mL/min. The mass spectrometry conditions were based on the use of an electrospray ionization source, with the mass spectral voltage (MSV) set to 5,500 V in the positive ion mode and to −4,500 V in the negative ion mode.
The mice were dissected at the end of the test and the contents of their cecum were collected in sterile conical tubes. DNA was extracted from the cecal content using the CTAB method according to manufacturer’s instructions. The V3 to V4 regions of the microbiota 16S rDNA gene were amplified using 338F/806R primers, then the amplicon was purified using the Qiagen gel extraction kit (Qiagen, Hilden, Germany) and sequenced by the Illumina HiSeq 2500 PE250 platform (Novogene Bioinformatics Technology Co., Ltd., Tianjin, China). Analysis and visualization of high-throughput sequencing data were collected via the online Novomagic Cloud Platform.
Data were tested for significance using one-way ANOVA in SPSS version 22.0 software (IBM, United States), and data were expressed as mean ± standard deviation (M ± SD), with *P < 0.05 being significant and **P < 0.01 being highly significant. Other statistical methods were detailed in the relevant figures.
The effects of EEO on the total distance, movement speed and rest time of mice were shown in Figures 1A, E, F. Compared with the control group, the total distance travelled and the speed of movement of mice in the EEO group and the diazepam group were highly significantly reduced (P < 0.01), with a reduction in the total distance travelled of 32.5% and 52.5%, and the speed of movement was reduced to 25.13 ± 3.01 and 21.88 ± 6.55 mm/s. Figures 1B–D showed the activity trajectories of mice in the control group, EEO group, and diazepam group, respectively, from which it can also be observed that both EEO and diazepam reduced the activity of mice. In terms of resting time, EEO had no significant effect on the resting time of mice (P > 0.05), however, diazepam significantly increased the resting time of mice (P < 0.01).
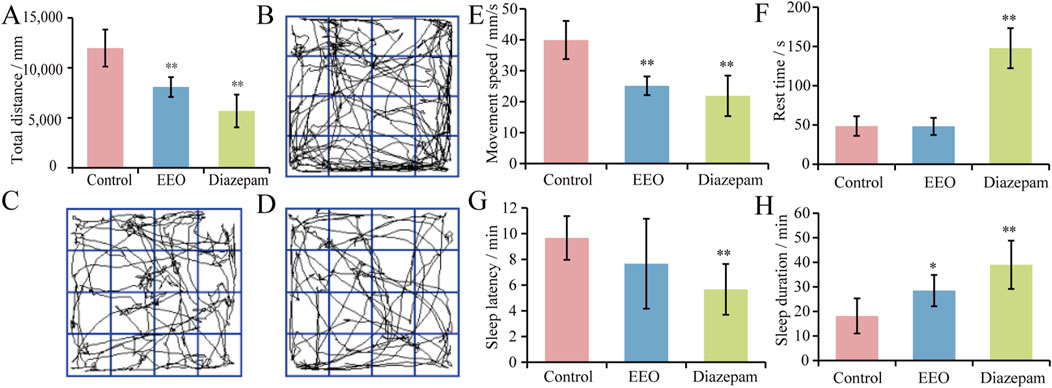
Figure 1. Effects of EEO on the behaviors of mice. (A) Total distance. (B–D) The behavioural trajectories of mice in the control group, EEO group, and diazepam group, respectively. (E) Movement speed. (F) Rest time. (G) Sleep latency. (H) Sleep duration. *p < 0.05, **p < 0.01; n = 7–8.
The effects of EEO on sleep latency and sleep duration in mice were shown in Figures 1G, H. Gavage of EEO resulted in a shortening of sleep latency time, but there was no significant difference, and diazepam reduced the sleep latency of mice with a highly significantly (P < 0.01). The sleep duration of mice in control, EEO and diazepam groups were 18.17 ± 7.15, 28.50 ± 6.37 and 39.00 ± 9.85 min, respectively. Compared with the control group, EEO significantly increased the sleep duration of mice (P < 0.05), diazepam highly significantly increased the sleep duration of mice (P < 0.01). EEO and diazepam prolonged the sleep duration of mice by 56.9% (P < 0.05) and 114.7% (P < 0.01), respectively.
We analysed the effects of EEO on neurotransmitters in the mouse brain. As shown in the PCoA and OPLS-DA score plots, compared with the control group, EEO or diazepam treatment significantly altered neurotransmitters in the mouse brain. The neurotransmitter profile in the brain of mice in the EEO group was similar to that of the diazepam group, with a greater difference from the control group (Figures 2A, B). In addition, volcano plot revealed neurotransmitters that differed significantly between groups (Figure 2C). EEO and diazepam treatments significantly elevated the levels of 7 and 3 neurotransmitters, respectively, compared to the control group. There was no significant difference in neurotransmitter levels between the EEO and diazepam groups.
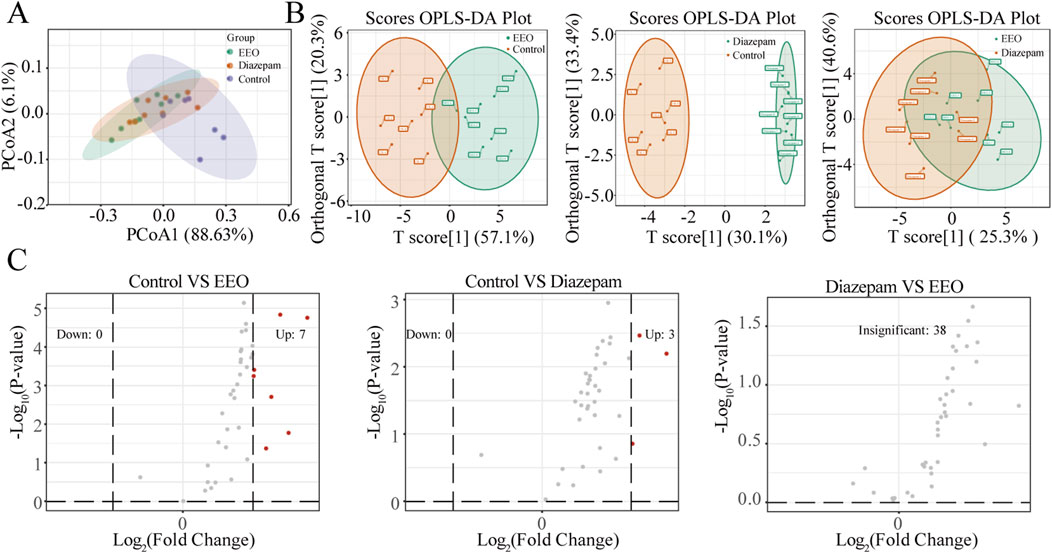
Figure 2. Effects of EEO gavage on neurotransmitters diversities of mice. (A) Principal coordinate analysis (PCoA) of weighted UniFrac distance. PCoA was performed by statistics function ggplot2 within R (http://www.r-project.org). (B) Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA). After pre-processing the original data in OPLS-DA by
The changes of neurotransmitters were observed after EEO treatment through a clustering heat map (Figure 3A). Brain levels of Gamma-aminobutyric acid (GABA), glutamine, glycine, tryptophan, 5-hydroxyindoleacetic acid (5-HIAA) and N-acetylserotonin were significantly higher in the EEO-treated and diazepam-treated groups of mice than in the control group (Figure 3B).
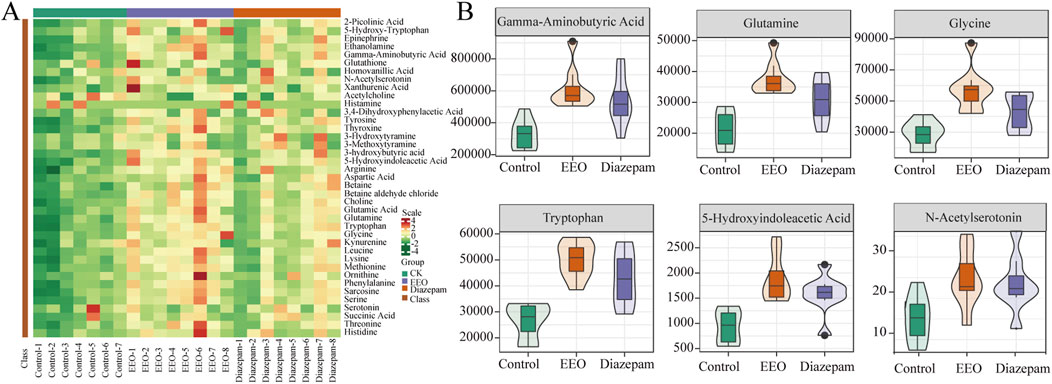
Figure 3. Effects of EEO supplementation on the levels of various neurotransmitters in the mice. (A) Heat map of neurotransmitter in different groups. The original levels of differential neurotransmitters are normalized using Unit Variance Scaling and heat maps are generated using R software. (B) Neurotransmitters with group differences. Using Variable Importance in Projection (VIP) based on the OPLS-DA model for differential testing, and neurotransmitters considered to be significant if VIP > 0.8. n = 8.
As shown in Figure 4A, the OTUs of mice intestinal flora in the control, EEO and diazepam groups were 2,342, 3,659 and 3,609, EEO and diazepam treatments increased the OTUs of mice intestinal flora, EEO and diazepam groups had more concurrent OTUs (1,114) than the control group duplicated with EEO (812) and diazepam (868) groups, respectively. The α diversity index including shannon, ace and chao1 results were shown in Figures 4B–D. Both EEO and diazepam significantly increased the shannon, ace and chao1 indices of the intestinal microbes in mice compared to the control group (P < 0.05). The β diversity index results showed that EEO and diazepam highly significantly increased the intestinal flora of the mouse β diversity (Figure 4E, P < 0.01). Based on the weighted UniFrac distance metric, the community composition of each sample was analysed for similarity using principal co-ordinates analysis (PCoA). The microbial communities in the EEO and diazepam groups were altered compared with the control group, and the microbial community species composition was structurally similar in the EEO and diazepam groups (Figure 4F). The similarity between individuals was analysed using the Unweighted UniFrac clustering tree based on the UPGMA method (Figure 4G), and in agreement with the results of the PCoA analysis, the gut microbial community composition in the EEO and diazepam groups was dramatically different compared to the control group.
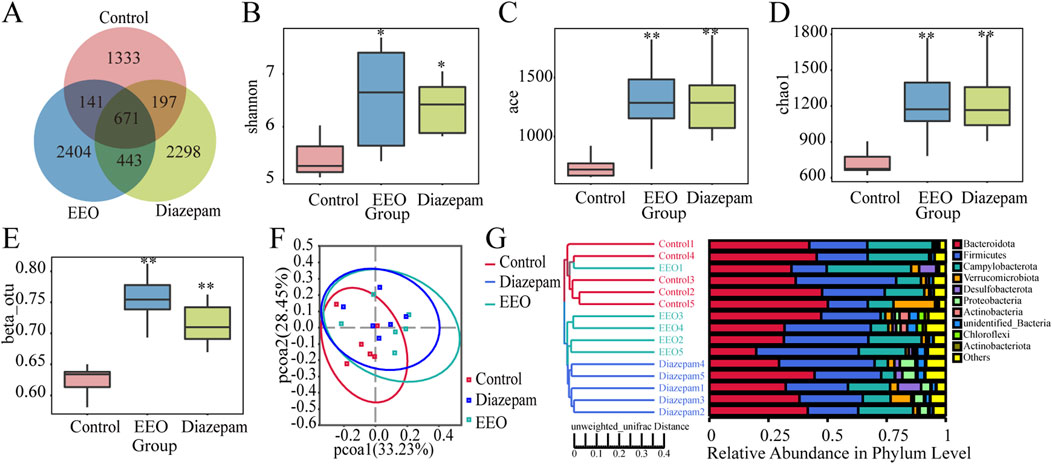
Figure 4. Effects of EEO on the diversity of microbial communities in the cecum of mice. (A) Wayne plots at ou level. (B–D) Box plots of α-diversity indices: Shannon (B), ACE (C) and Chao1 (D). (E) β-Diversity indices based on otu level. Species diversity difference is analyzed using Tukey and Kruskal–Wallis rank sum tests. *p < 0.05, **p < 0.01. (F) Scatter plots obtained from PCoA based on the weighted UniFrac distance metric. (G) UPGMA clustering tree and community structure map based on unweighted UniFrac distance. n = 5–6.
Differences in dominant species among the three groups of samples at the phylum level are shown in Figure 5A, the study found that the relative abundance of Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidota/Firmicutes differed among the three groups. EEO significantly increased the relative abundance of relative abundance of Firmicutes, Proteobacteria and Actinobacteria (P < 0.01) and decreased the abundance ratio of Bacteroidota/Firmicutes (Figure 5B). At the genus level, EEO and diazepam treatment altered the relative abundance of Bacteroides, Dubosiella, Prevotella_9, and Lachnospiraceae_NK4A136_groups in the gut microbial community of mice (Figure 5C). EEO and diazepam significantly increased the elevated relative abundance of Lachnospiraceae_NK4A136_group, Bacteroides, Dubosiella, Prevotella_9 (Figure 5D).
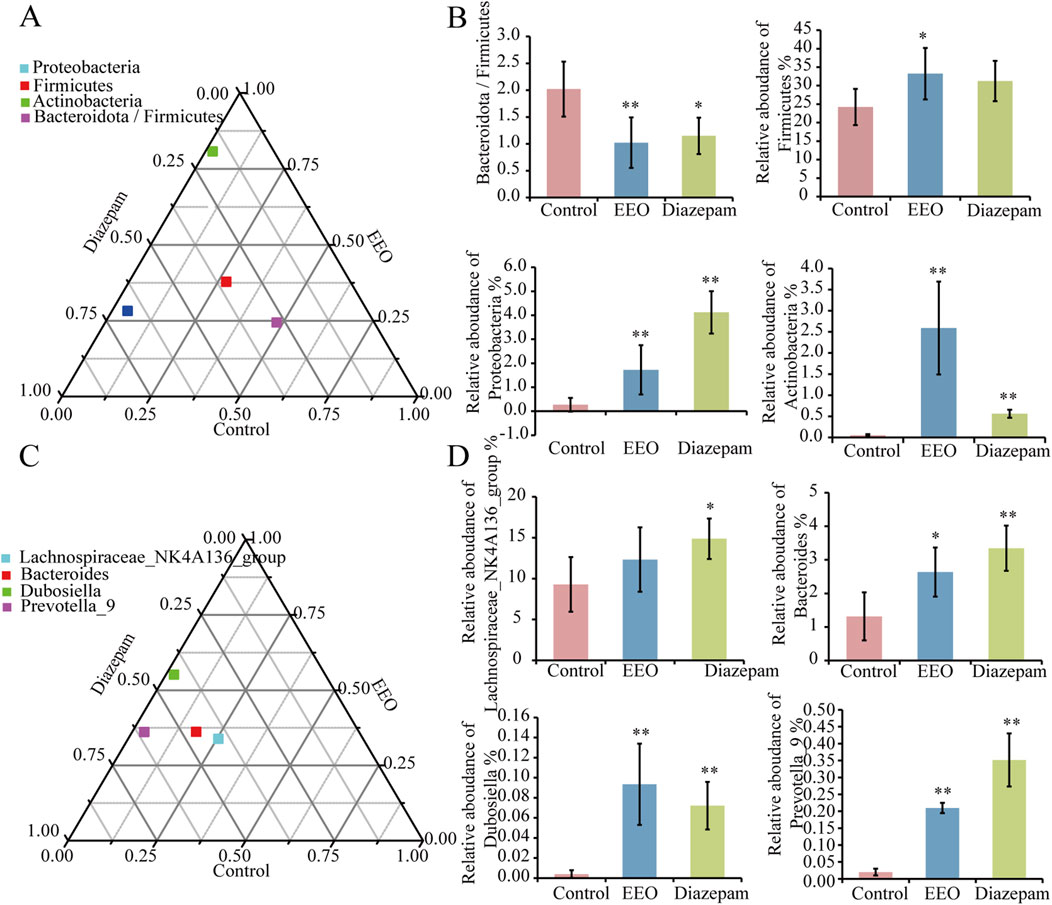
Figure 5. Differences in the abundance of intestinal flora in each group at the phylum and genus levels. Ternary phase diagrams of the differences in intestinal flora at the phylum (A) and genus (C) levels. (B) Histograms of the abundance of intestinal flora at the phylum level in each group after treatment with EEO. (D) Histograms of the abundance of intestinal flora at the genus level in each group after eucalyptus essential oils treatment. Find significantly different microbes through t-test analysis. *p < 0.05, **p < 0.01; n = 6.
Linear discriminant analysis of LDA effect size (LEfSe) was used to identify taxa most likely to explain differences in microbiota composition between the control and EEO groups. A comparison of the individuals from both groups revealed that Actinobacteria at phylum level, as well as Acidothermus, Rothia, Conexibacter and Bacillus at the genus level exhibited significant influences in the EEO group, while in the control, Bacteroidota in the phylum level made contributions (Figure 6A). A taxonomic cladogram derived from the LEfSe analysis (with the LDA score > 3.0) showed the relationships of the differential microbiota with significant roles (Figure 6B). Altogether, these results suggest that the gut microbes exhibit alterations after EEO treatment.
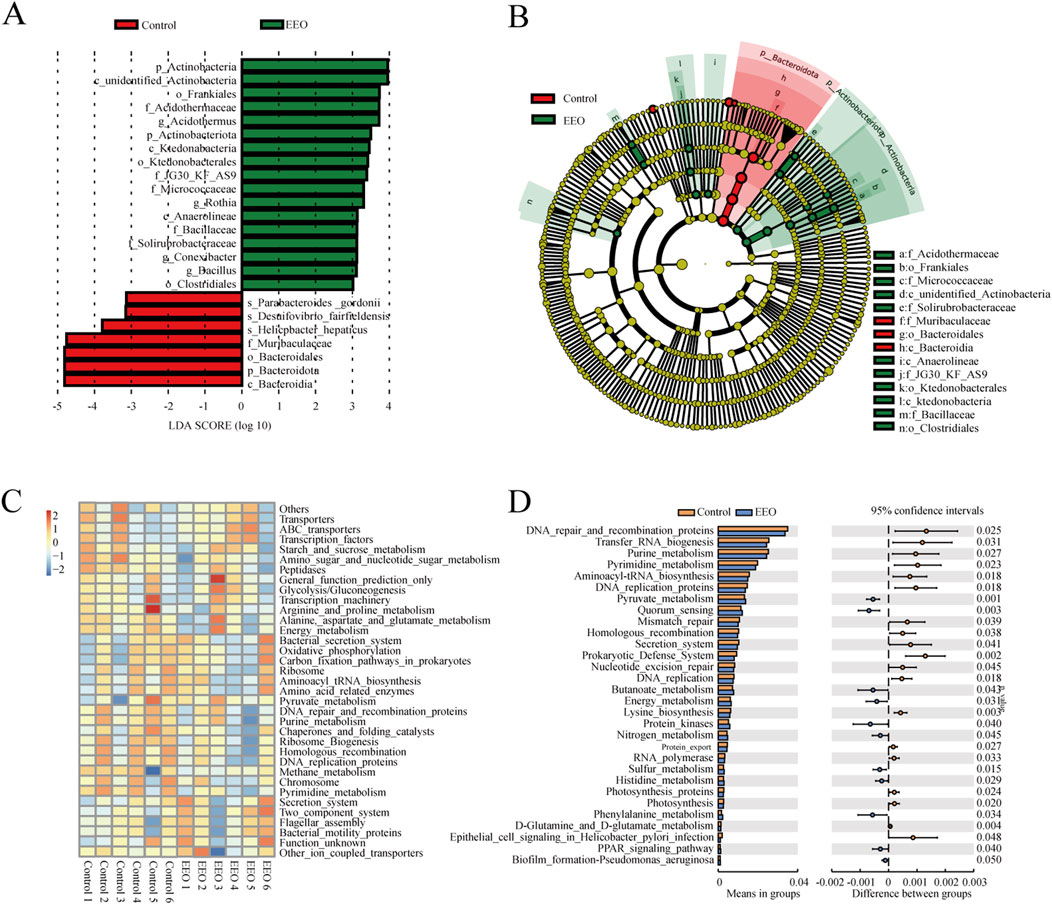
Figure 6. Distinct taxa with greater contributions at different taxonomic levels and the predicted functional changes in the gut microbes. (A) Linear discriminant analysis (LDA) effect size (LEfSe) identified microbial biomarkers from phylum to species levels that discriminate the control and EEO groups. (B) Cladogram constructed using the LEfSe method to display the phylogenetic distribution of bacteria that were most highly enriched in the control and EEO groups. (C) For predicting flora function, heat map of PICRUST analysis identified the KEGG pathway among the control and EEO groups. (D) The KEGG analysis showed the potential pathways associated with changes in the intestinal microbes after the EEO treatment. n = 6.
A KEGG functional prediction was subsequently performed by means of the PICRUST approach. The heat map showed that the results of KEGG three-level classification enrichment analysis (Figure 6C). The results showed that these differential microbes might be closely related with purine and pyrimidine metabolism, aminoacyl tRNA biosynthesis, energy metabolism and glutamine and glutamate metabolism (Figure 6D).
The correlation and significance between gut microbes and sedation behaviors in mice was investigated by Pearson’s correlation analysis (Figure 7A). The observed species, chao1 index showed significantly negative correlation (P < 0.05) with total distance and movement speed of mice. Shannon, simpson index showed significantly negative correlation (P < 0.05) with movement speed and total distance travelled respectively. Sleep duration was positively correlated with the abundance of Proteobacteria at the phylum level (Figure 7B), and positively correlated with Enterococcus, Pseudomonas, Cupriavidus, Prevotella_9 and Ralstonia at the genus level (Figure 7C). It suggested that the length of sleep duration was related to the composition of the gut microbes.
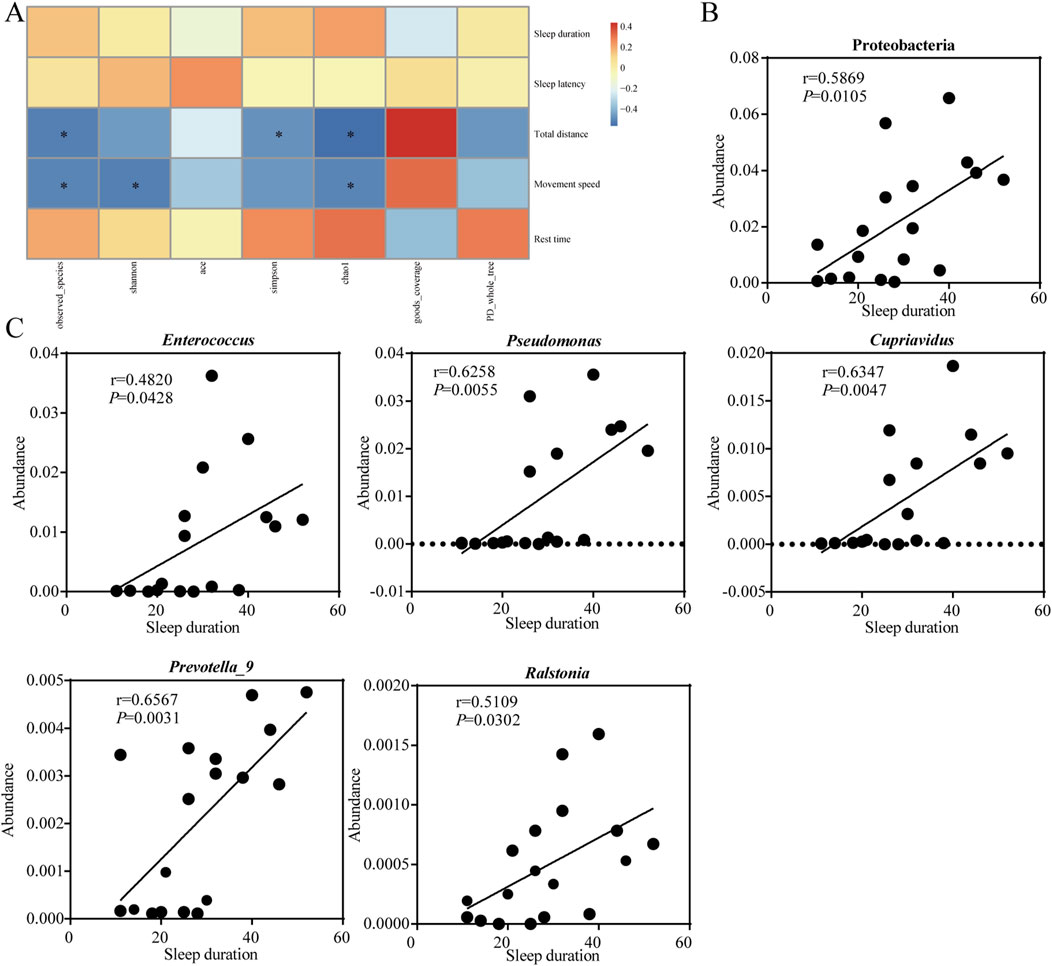
Figure 7. Sedative-hypnotic behaviors are associated with occupancy in the intestinal microbes. (A) Correlation analysis between mice behaviors with intestinal microbes α diversity. (B) Correlation analysis between sleep duration time and the abundance of gut microbes at the phylum level. (C) Correlation analysis between sleep duration time and the abundance of gut microbes at the genus level. Statistical significance is evaluated by Pearson correlation coefficient test. *p < 0.05; n = 18.
Through an extensive search, we obtained gut microbes associated with neurotransmitter synthesis (Supplementary Table 1). The total abundance of bacteria associated with dopamine, GABA, glycine, histamine, and serotonin (5-HT) synthesis were calculated. We found that microbes synthesize dopamine, GABA and glycine were considerably affected by EEO treatment. EEO significantly increased the abundance of GABA- and glycine-synthesize bacteria in the mouse gut (Figures 8B, C, P < 0.05), while it had no significant effect on the abundance of bacteria synthesize histamine and 5-HT (Figures 8D, E, P > 0.05). Diazepam treatment significantly increased the abundance of bacteria associated with the synthesis of dopamine, GABA and glycine (Figures 8A–C, P < 0.05).

Figure 8. Effects of EEO supplementation on the intestinal neurotransmitter-producing flora of mice. The abundance of functional microbes synthesizing dopamine (A), GABA (B), glycine (C), histamine (D), and serotonin (E) neurotransmitters. *p < 0.05, **p < 0.01; n = 6.
Sleep disorders can seriously affect people quality of life and increase socio-economic burden (Yuen and Pelayo, 2017; Wickwire et al., 2016). In recent years, many studies have explored the effects of natural products on sleep with a potential to replace conventional hypnotic drugs that may have serious side effects. In this study, we explored the sedative-hypnotic effects of EEO treatment in mice. To our knowledge, this is the first report of a depressant effect of EEO on the central nervous system in mice. The open field test was employed to assess whether EEO affected exploratory activity in mice, after the sleep induction test was performed, which was a specific test to assess sedative-hypnotic drugs. In the open field test, it was found that EEO treatment significantly reduced the total distance travelled and decreased the movement speed in mice, and the results of the barbiturate-induced sleep test also found that EEO significantly increased sleep duration. This was consistent with the effects induced by the positive control diazepam treatment group (Figure 1). This suggested that EEO had a significant sedative-hypnotic effect. Consistent with our study, one of the EEO constituents, 1,8-eudesmusin, was reported to reduce locomotion in mice with sedative effect (Santos and Rao, 2000). The main components of EEO were shown in Supplementary Table 2, and one of its main components, 1,8-eudesmusin, may be the main functional substance that exerted sedative-hypnotic effects. In terms of the safety of EEO, a study found that mice did not show significant changes in behavior, vital signs, and blood biochemical indicators when orally administered with 833 mg/kg B.W. of EEO (Kapnisis et al., 2022). According to the World Health Organization, this may indicate that EEO is classified as “unlikely to cause any acute or subacute hazard.” The good sedative-hypnotic effects of EEO make it a safe natural extract that has the potential to be used as an alternative to traditional hypnotic drugs for improving sleep.
Sleep regulation is a highly complex process that is finely regulated by changes in brain neurochemicals, and many neurotransmitters are involved in sleep regulation. Drugs that interfere or modulate these neurochemical systems can lead to changes in arousal and sleep, which in turn modulate sleep disorders. GABA is produced from glutamate by the enzyme glutamic acid decarboxylase. GABA is the main inhibitory neurotransmitter and it is well established that GABA inhibits nerve conduction and prevents neuronal over-excitation which in turn promotes sleep (Yamatsu et al., 2016; Kim et al., 2019). Glutamate is an excitatory neurotransmitter, and glutamate and glutamine can be interconverted, and glutamate can be inactivated by the glutamate-glutamine cycle, terminating the excitatory effect of glutamate (Chen et al., 2022). The results of our study showed that both EEO and diazepam groups significantly increased the levels of glutamine and GABA in the brain of mice compared to the control group (Figure 3). Similar to our study, many natural products such as Nelumbo nucifera Seed Extract and Valerian/Hop Mixture can improve sleep and prolong sleep duration in Drosophila by modulating GABA signal (Jo et al., 2018; Choi et al., 2017). Our study also found that EEO significantly increased glycine levels in the mouse brain (Figure 3). In the central nervous system, glycine acts as an inhibitory neurotransmitter by affecting glycine receptor GlyRs, and glycine has been reported to increase the duration of non-rapid eye movement sleep and reduce the number and mean duration of dark period awakening (Hondo et al., 2011). An increasing number of studies have found that 5-HT promotes sleep (Zhang et al., 2018), and tryptophan, as the sole substrate for 5-HT synthesis, have also been reported to have a hypnotic effect (Roth et al., 2021). N-acetylserotonin and 5-HIAA, as products of 5-HT acetylation and monoamine oxidation, have also been reported to improve sleep. The results of the study found that EEO could upregulate the levels of tryptophan, N-acetylserotonin and 5-HIAA in brain tissues (Figure 3). The study by Wang et al. (2020) found that Schisandrin B could reduce sleep latency in parachlorophenylalanine-induced insomnia in rats by increasing the levels of 5-HIAA and increasing sleep duration. Nyctinastic herbs decoction can also treat insomnia by increasing 5-HIAA levels (Yang et al., 2021). Supplementation with saffron extract increased N-acetylserotonin and tryptophan synthesis and improved sleep quality in rats (Munoz et al., 2023). Our studies illustrated the ability of EEO to affect the levels of sleep-related neurotransmitters in the brain, which in turn affected the sleep state.
Studies in recent years have found a strong association between gut microbes and sleep, and maintaining the homeostasis of the gut microbes is key to improving sleep (Neroni et al., 2021). Intake of Lactobacillus and Bacillus longum has been reported to improve sleep quality preventing sleep disorders (Matsuda et al., 2020). A study by Guo et al. (2019) found that Oolong Tea Polyphenols regulate circadian rhythms by enhancing beneficial gut microbes. All these findings suggested that gut microbes influenced sleep quality through GMBA. In our study, we found that EEO and the positive control diazepam significantly altered the gut microbes of mice and increased the α-diversity and β-diversity of the gut microbes (Figure 4). And observed-species and chao1 index showed significantly negative correlation with both total distance and movement speed. Shannon and simpson index showed significantly negative correlation with movement speed and total distance, respectively (Figure 7A). In addition, the gut microbes of mice in the EEO and diazepam groups possessed a higher number of repetitive OTUs and had a more similar flora structure. It is reported that gut microbial diversity is positively correlated with an increase in sleep efficiency and total sleep time, and that gut microbial diversity promotes healthier sleep (Smith et al., 2019). A study by Ogawa et al. (2020) found that treatment with a broad-spectrum antibiotic significantly reduced the duration of non-rapid eye movement sleep in mice while decreasing their gut microbes. These suggested that the diversity of gut microbes affected sleep quality. The diversity of gut microbes interacted with sleep conditions, and poorer sleep states reduced the diversity of gut microbes, leading to dysbiosis. A good gut microbial ecology promoted better sleep. This might be related to the fact that certain beneficial flora promoted sleep, or it might be due to the fact that gut microbes metabolism affected the synthesis of certain neurotransmitters and certain vitamins associated with sleep (El Aidy et al., 2020).
We found that EEO treatment altered the relative abundance of gut microbes, significantly increased the relative abundance of Firmicutes, Proteobacteria, and Actinobacteria at the phylum level and decreased the Bacteroidota/Firmicutes abundance ratio. At the genus level, EEO treatments increased the abundance of Bacteroides, Dubosiella, Prevotella_9, and Lachnospiraceae_NK4A136_groups (Figure 5). Lower Bacteroidota/Firmicutes ratio was beneficial for minimising pathogens and for probiotic colonisation (Ge et al., 2023). It has also been previously found that higher Firmicutes abundance is associated with higher sleep quality in the host (Smith et al., 2019). Therefore, we speculated that EEO may affect the abundance of Firmicutes, promoted probiotic colonisation, maintain intestinal homeostasis and exerted a sedative-hypnotic effect. In addition to this, we found that most of the gut microbes altered by EEO were short-chain fatty acid-producing beneficial bacteria. The members of Firmicutes mainly included beneficial bacteria, and Firmicutes were considered to be the main gate of short-chain fatty acid production in the mouse gut (Li W. T. et al., 2023; Wongsurawat et al., 2023). Proteobacteria promoted propionic acid production, and Actinobacteria mainly produced acetic acid and butyric acid (Zhao et al., 2019; Haenen et al., 2013). Bacteroides have been shown to be carbohydrate degraders and short chain fatty acid (SCFA) producers (Fan et al., 2023). SCFA produced by gut microbes were thought to be endogenous circadian signal molecules and were essential for gut-brain axis communication (Fredericks et al., 2020). SCFA interacted with receptors on enteroendocrine cells by inducing the secretion of gut hormones, such as GABA and 5-HT, and facilitating indirect signals to the brain via the somatic or vagal pathways (Silva et al., 2020), which can in turn alter the organism’s sleep state. Therefore, EEO may improve sleep by enriching SCFA-producing microbes and thereby increasing the metabolite SCFA.
Compositions of gut microbes were closely related to sleep quality, and we found that sleep duration time showed a significant correlation with the relative abundance of certain gut microbes using the Pearson correlation coefficient test. At the phylum level, Proteobacteria showed a significantly positive correlation with sleep duration (Figure 7B). Similar to our study, Song et al. (2022) found that the shorter the sleep duration caused by caffeine, the lower the relative abundance of Proteobacteria. At the genus level, Enterococcus, Pseudomonas, Cupriavidus, Prevotella_9, and Ralstonia showed significantly positive correlation with sleep duration (Figure 7C). Insomnia caused a decrease in Prevotella abundance in the gut, while the Radix Ginseng and Semen Ziziphi Spinosae treatment improved insomnia and was able to increase Prevotella abundance in the gut (Qiao et al., 2022). All of these studies suggested a strong association between sleep and certain microbes. Although there was insufficient evidence to prove a clear causal relationship between sleep problems and microbiological composition, it is undeniable that modulation of the gut microbes can be a key target for the treatment of sleep disorders.
Studies have now demonstrated that the gut microbes can communicate with the brain through functional metabolites, which in turn control circadian rhythms and sleep quality (Ogawa et al., 2020). Differential microbes in the prediction of KEGG function in gut microbes may be related to glutamine and glutamate metabolism (Figure 6D), associated with this, while there are elevated levels of glutamine in the brain. Therefore, we speculated that there may be gut microbes-induced neurotransmitter differences in the brain. In addition, the effect of EEO on the neurotransmitter-producing flora of the gut microbes was explored. Gut microbes were directly involved in the synthesis of a variety of neurotransmitters and can influence the levels of neurotransmitters in the nervous system through metabolic pathways (Zhao et al., 2022). Olson et al. (2018) found that Parabacteroides produced large amounts of GABA. Oscillibacter, Dialister, and Coprococcus have been reported to be associated with GABA synthesis (Liu and Huang, 2019). Bacteroides, Prevotella, Parabacteriodes have been reported to be involved in glycine production (Liu and Huang, 2019; Lynch et al., 2017). GABA and glycine, as the main inhibitory neurotransmitters in the nervous system, played an important role in the regulation of the sleep-wake cycle. There was no direct evidence that gut-produced GABA can cross the blood-brain barrier (BBB), but studies have found that physiological and psychological stress can increase BBB permeability and cause GABA inward flow (Constans et al., 2020; Varanoske et al., 2022). In contrast, glycine readily crossed the BBB via glycine transporter proteins and enters the brain to act on NMDA receptors in the supraoptic nucleus, which in turn affected the sleep cycle (Epping et al., 2022). Our finding that EEO significantly increased the abundance of gut microbes associated with GABA and glycine synthesis (Figures 8B, C), and EEO significantly increased GABA and glycine levels in the mouse brain (Figure 3B). The mechanism by which EEO regulates neurotransmitters in the brain is unclear, whether it acts directly on the brain to affect neurotransmitter synthesis or regulates neurotransmitter levels via gut microbes will require further studies to elucidate the exact mechanism. However, it is well known that gut microbes produce hormones and neurotransmitters that are transmitted via the afferents of the vagus nervous system, which activate or inhibit the corresponding neurons, thereby promoting sleep (Lyte, 2014; Dinan et al., 2015).
EEO supplementation reduced activity and improved sleep quantity in mice, consistent with the effects of diazepam. Through brain neurotransmitter-targeting histological assays, EEO was shown to increase brain levels of sleep-promoting neurotransmitters such as glutamine, GABA, glycine, tryptophan, N-acetylserotonin and 5-HIAA, and improve sleep in mice. EEO increased the abundance of intestinal flora, short-chain fatty acid-producing flora, and promoted probiotic colonisation, as well as the abundance of functional flora synthesize GABA and glycine neurotransmitters. EEO also increased the abundance of short-chain fatty acid-producing flora and the abundance of functional flora synthesizing GABA and glycine neurotransmitters. EEO may exert sedative-hypnotic effects by improving the gut microbiota and thereby altering brain neurotransmitters. Our findings suggested that gut microbes might serve as a target of action for EEO in the treatment of sleep deprivation, EEO as a natural product alternative for the treatment of insomnia and sleep disorders, and the potential for the development of new drugs for the treatment of sleep disorders.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was approved by All animal testing procedures were conducted in accordance with the guidelines of the Animal Ethics Committee of Zhejiang University (ethical programme code ZJU 20160403). The study was conducted in accordance with the local legislation and institutional requirements.
XL: Writing–original draft, Writing–review and editing. YZ: Writing–original draft. QZ: Writing–review and editing. AC: Writing–review and editing. JF: Writing–review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study has been supported by the Zhejiang Province Key R&D Program (grant number 2024C02004) and the National R&D Program of China (grant number 2022YFD1300504).
We thank Biotechnology R&D Center of Shandong Longchang Animal Health Products Co., Ltd. for providing reagent raw material support for the experiment. We also thank Wuhan Maiwei Metabolic Biotechnology Co., Ltd. for providing technical support.
Author AC was employed by Biotechnology R&D Center of Shandong Longchang Animal Health Products Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1464654/full#supplementary-material
Aziz, Z. A. A., Nasir, H. M., Ahmad, A., Setapar, S. H. M., Ahmad, H., Noor, M. H. M., et al. (2019). Enrichment of eucalyptus oil nanoemulsion by micellar nanotechnology: transdermal analgesic activity using hot plate test in rats’ assay. Sci. Rep. 9, e13678. doi:10.1038/s41598-019-50134-y
Bampidis, V., Azimonti, G., Bastos, M. D., Christensen, H., Durjava, M., Kouba, M., et al. (2023). Safety and efficacy of a feed additive consisting of an essential oil derived from Eucalyptus globulus Labill. (eucalyptus oil) for all animal species (FEFANA asbl). Efsa J. 7 (21), e08178. doi:10.2903/j.efsa.2023.8178
Barbosa, K. T., Acosta, A. P., Schulz, H. R., De Santi, I. I., Delucis, R. D., Beltrame, R., et al. (2021). Biochemical features of organic extractives from eucalyptus and corymbia woods using ethanol as a solvent. Maderas-Ciencia Y Tecnol. 23, 58–69. doi:10.4067/s0718-221x2021000100458
Broussard, J. L., Wroblewski, K., Kilkus, J. M., and Tasali, E. (2016). Two nights of recovery sleep reverses the effects of short-term sleep restriction on diabetes risk. Diabetes Care 39 (3), 40–e41. doi:10.2337/dc15-2214
Caceres, A. I., Liu, B. Y., Jabba, S. V., Achanta, S., Morris, J. B., and Jordt, S. E. (2017). Transient receptor potential cation channel subfamily m member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 174 (9), 867–879. doi:10.1111/bph.13760
Chen, T. C., Zhang, F. Y., Chen, J. Q., Zhong, Q. G., Hu, Y. X., Wu, R. R., et al. (2022). Effects of alcohol extracts from ganoderma resinaceum on sleep in mice using combined transcriptome and metabolome analysis. Front. Nutr. 9, e745624. doi:10.3389/fnut.2022.745624
Choi, H. S., Ko, B. S., Kim, H. D., Hong, K. B., and Suh, H. J. (2017). Effect of valerian/hop mixture on sleep-related behaviors in Drosophila melanogaster. Biol. Pharm. Bull. 40 (7), 1101–1110. doi:10.1248/bpb.b17-00262
Constans, C., Ahnine, H., Santin, M., Lehericy, S., Tanter, M., Pouget, P., et al. (2020). Non-invasive ultrasonic modulation of visual evoked response by GABA delivery through the blood brain barrier. J. Control. Release. 318, 223–231. doi:10.1016/j.jconrel.2019.12.006
Dasdelen, M. F., Er, S., Kaplan, B., Celik, S., Beker, M. C., Orhan, C., et al. (2022). A novel theanine complex, mg-l-theanine improves sleep quality via regulating brain electrochemical activity. Front. Nutr. 9, 874254. doi:10.3389/fnut.2022.874254
Dinan, T. G., Stilling, R. M., Stanton, C., and Cryan, J. F. (2015). Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res. 63, 1–9. doi:10.1016/j.jpsychires.2015.02.021
El Aidy, S., Bolsius, Y. G., Raven, F., and Havekes, R. (2020). A brief period of sleep deprivation leads to subtle changes in mouse gut microbiota. J. Sleep. Res. 29 (6), e12920. doi:10.1111/jsr.12920
Epping, L., Schroeter, C. B., Nelke, C., Bock, S., Gola, L., Ritter, N., et al. (2022). Activation of non-classical NMDA receptors by glycine impairs barrier function of brain endothelial cells. Cell. Mol. Life Sci. 79 (9), 479–488. doi:10.1007/s00018-022-04502-z
Fan, Y., Ju, T. T., Bhardwaj, T., Korver, D. R., and Willing, B. P. (2023). Week-old chicks with high bacteroides abundance have increased short-chain fatty acids and reduced markers of gut inflammation. Microbiol. Spectr. 11 (2), 345–356. doi:10.1128/spectrum.03616-22
Fredericks, E., Theunissen, R., and Roux, S. (2020). Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turk. J. Gastroenterol. 31 (12), 840–847. doi:10.5152/tjg.2020.19856
Galland, L. (2014). The gut microbiome and the brain. J. Med. Food. 17 (12), 1261–1272. doi:10.1089/jmf.2014.7000
Ge, C. Y., Luo, X. Y., Wu, L. C., Lv, Y. J., Hu, Z. Y., Yu, D. Y., et al. (2023). Plant essential oils improve growth performance by increasing antioxidative capacity, enhancing intestinal barrier function, and modulating gut microbiota in Muscovy ducks. Poult. Sci. 102 (8), e102813. doi:10.1016/j.psj.2023.102813
Golubeva, A. V., Joyce, S. A., Moloney, G., Burokas, A., Sherwin, E., Arboleya, S., et al. (2017). Microbiota-related changes in bile acid and tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. Ebiomedicine 24, 166–178. doi:10.1016/j.ebiom.2017.09.020
Guo, T. T., Song, D., Ho, C. T., Zhang, X., Zhang, C. D., Cao, J. X., et al. (2019). Omics analyses of gut microbiota in a circadian rhythm disorder mouse model fed with oolong tea polyphenols. J. Agric. Food Chem. 67 (32), 8847–8854. doi:10.1021/acs.jafc.9b03000
Haenen, D., Zhang, J., da Silva, C. S., Bosch, G., van der Meer, I. M., van Arkel, J., et al. (2013). A diet high in resistant starch modulates microbiota composition, scf concentrations, and gene expression in pig intestine. J. Nutr. 143, 274–283. doi:10.3945/jn.112.169672
Harding, K., and Feldman, M. (2008). Sleep disorders and sleep deprivation: an unmet public health problem. J. Am. Acad. Child. Adolesc. Psychiatry 47 (4), 473–474. doi:10.1097/01.CHI.0000270812.55636.3b
Hondo, M., Furutani, N., Yamasaki, M., Watanabe, M., and Sakurai, T. (2011). Orexin neurons receive glycinergic innervations. PloS One 6 (9), e25076. doi:10.1371/journal.pone.0025076
Horvath, T. D., Ihekweazu, F. D., Haidacher, S. J., Ruan, W., Engevik, K. A., Fultz, R., et al. (2022). Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. Iscience 25 (5), e104158. doi:10.1016/j.isci.2022.104158
Ieri, F., Cecchi, L., Giannini, E., Clemente, C., and Romani, A. (2019). GC-MS and HS-SPME-GC×GC-TOFMS determination of the volatile composition of essential oils and hydrosols (By-Products) from four Eucalyptus species cultivated in tuscany. Molecules 24 (2), 226–239. doi:10.3390/molecules24020226
Jo, K., Choi, H. S., Jeon, S., Ahn, C. W., and Suh, H. J. (2018). Nelumbo nucifera seed extract promotes sleep in drosophila melanogaster. Biol. Pharm. Bull. 41 (3), 399–408. doi:10.1248/bpb.b17-00763
Kapnisis, K., Chrysargyris, A., Prokopi, M., Varda, E., Kokkinidou, D., Samourides, A., et al. (2022). Effects on lettuce yield parameters and toxicological safety assessment of a plant-derived formulation based on rosemary and eucalyptus essential oils. Agronomy-Basel. 12, 2861. doi:10.3390/agronomy12112861
Kim, S., Jo, K., Hong, K. B., Han, S. H., and Suh, H. J. (2019). GABA and L-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 57, 65–73. doi:10.1080/13880209.2018.1557698
Leone, V., Gibbons, S. M., Martinez, K., Hutchison, A. L., Huang, E. Y., Cham, C. M., et al. (2015). Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17 (5), 681–689. doi:10.1016/j.chom.2015.03.006
Li, N., Tan, S. W., Wang, Y., Deng, J., Wang, N., Zhu, S., et al. (2023). Akkermansia muciniphila supplementation prevents cognitive impairment in sleep-deprived mice by modulating microglial engulfment of synapses. Gut Microbes 15 (2), e2252764. doi:10.1080/19490976.2023.2252764
Li, W. T., Wang, Z. X., Cao, J., Dong, Y. L., and Chen, Y. X. (2023). Melatonin improves skin barrier damage caused by sleep restriction through gut microbiota. J. Pineal. Res. 75 (1), e12874. doi:10.1111/jpi.12874
Liu, T., and Huang, Z. S. (2019). Evidence-based analysis of neurotransmitter modulation by gut microbiota. Lect. Notes Artif. Intell. 11837, 238–249. doi:10.1007/978-3-030-32962-4_22
Lynch, A., Crowley, E., Casey, E., Cano, R., Shanahan, R., McGlacken, G., et al. (2017). The Bacteroidales produce an N-acylated derivative of glycine with both cholesterol-solubilising and hemolytic activity. Sci. Rep. 7, e13270. doi:10.1038/s41598-017-13774-6
Lyte, M. (2014). Microbial endocrinology Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 5 (3), 381–389. doi:10.4161/gmic.28682
Matheson, E., and Hainer, B. L. (2017). Insomnia: pharmacologic therapy. Am. Fam. Physician 96 (1), 29–35.
Matsuda, Y., Ozawa, N., Shinozaki, T., Wakabayashi, K., Suzuki, K., Kawano, Y., et al. (2020). Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl. Psychiatry. 10 (1), 170–179. doi:10.1038/s41398-020-0855-1
Medeiros, K., Dos Santos, J. R., Melo, T. C. D., De Souza, M. F., Santos, L. D., de Gois, A. M., et al. (2018). Depressant effect of geraniol on the central nervous system of rats: behavior and ecog power spectra. Biomed. J. 41 (5), 298–305. doi:10.1016/j.bj.2018.08.008
Muñoz, M. D., Román-Carmena, M., Amor, S., García-Villalón, A. L., Espinel, A. E., González-Hedström, D., et al. (2023). Effects of supplementation with the standardized extract of saffron (affron®) on the kynurenine pathway and melatonin synthesis in rats. Antioxidants 12 (8), 1619–1627. doi:10.3390/antiox12081619
Neroni, B., Evangelisti, M., Radocchia, G., Di Nardo, G., Pantanella, F., Villa, M. P., et al. (2021). Relationship between sleep disorders and gut dysbiosis: what affects what? Sleep. Med. 87, 1–7. doi:10.1016/j.sleep.2021.08.003
Ngo, D. H., and Vo, T. S. (2019). An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 24 (15), 2678. doi:10.3390/molecules24152678
Niu, Z. F., Wu, L., Kang, J., Bai, J. B., Chen, Y. J., and Xia, H. C. (2022). Regulation of sleep disorders in patients with traumatic brain injury by intestinal flora based on the background of brain-gut axis. Front. Neurosci. 16, e934822. doi:10.3389/fnins.2022.934822
Ogawa, Y., Miyoshi, C., Obana, N., Yajima, K., Hotta-Hirashima, N., Ikkyu, A., et al. (2020). Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 10 (1), e19554. doi:10.1038/s41598-020-76562-9
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. X. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 174 (2), 497–508. doi:10.1016/j.cell.2018.06.051
Pesarico, A. P., Vieira, A. T., and Rosa, S. G. (2023). Editorial: gut-microbiota-brain axis in depression: mechanisms and possible therapies. Front. Behav. Neurosci. 17, e1221141. doi:10.3389/fnbeh.2023.1221141
Poroyko, V. A., Carreras, A., Khalyfa, A., Khalyfa, A. A., Leone, V., Peris, E., et al. (2016). Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 6, e35405. doi:10.1038/srep35405
Qiao, T., Wang, Y., Liang, K., Zheng, B. Y., Ma, J., Li, F. X., et al. (2022). Effects of the Radix Ginseng and Semen Ziziphi Spinosae drug pair on the GLU/GABA-GLN metabolic cycle and the intestinal microflora of insomniac rats based on the brain-gut axis. Front. Pharmacol. 13, e1094507. doi:10.3389/fphar.2022.1094507
Regulation (EC) (2003). No 1831/2003 of the European Parliament and of the council of 22 September 2003 on the additives for use in animal nutrition. OJ L 268 (18.10), 29.
Roth, W., Zadeh, K., Vekariya, R., Ge, Y., and Mohamadzadeh, M. (2021). Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 22 (6), e2973. doi:10.3390/ijms22062973
Santos, F. A., and Rao, V. S. N. (2000). Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 14 (4), 240–244. doi:10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x
Sathantriphop, S., Thanispong, K., Sanguanpong, U., Achee, N. L., Bangs, M. J., and Chareonviriyaphap, T. (2014). Comparative behavioral responses of pyrethroid-susceptible and -resistant aedes aegypti (diptera: culicidae) populations to citronella and eucalyptus oils. J. Med. Entomol. 51 (6), 1182–1191. doi:10.1603/ME13191
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11, 25–36. doi:10.3389/fendo.2020.00025
Smith, R. P., Easson, C., Lyle, S. M., Kapoor, R., Donnelly, C. P., Davidson, E. J., et al. (2019). Gut microbiome diversity is associated with sleep physiology in humans. Plos One 14 (10), e0222394. doi:10.1371/journal.pone.0222394
Song, Z., Liu, L., Xu, Y. Y., Cao, R. F., Lan, X. Y., Pan, C. Y., et al. (2022). Caffeine-induced sleep restriction alters the gut microbiome and fecal metabolic profiles in mice. Int. J. Mol. Sci. 23 (23), e14837. doi:10.3390/ijms232314837
Takaishi, M., Fujita, F., Uchida, K., Yamamoto, S., Sawada, M., Hatai, C., et al. (2012). 1,8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain. 8, 86–98. doi:10.1186/1744-8069-8-86
Torres-Fuentes, C., Schellekens, H., Dinan, T. G., and Cryan, J. F. (2017). The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2 (10), 747–756. doi:10.1016/S2468-1253(17)30147-4
Varanoske, A. N., McClung, H. L., Sepowitz, J. J., Halagarda, C. J., Farina, E. K., Berryman, C. E., et al. (2022). Stress and the gut-brain axis: cognitive performance, mood state, and biomarkers of blood-brain barrier and intestinal permeability following severe physical and psychological stress. Brain Behav. Immun. 101, 383–393. doi:10.1016/j.bbi.2022.02.002
Walia, H. K., and Mehra, R. (2016). Overview of common sleep disorders and intersection with dermatologic conditions. Int. J. Mol. Sci. 17 (5), 654–663. doi:10.3390/ijms17050654
Wang, M. Y., Li, N., Jing, S., Wang, C. M., Sun, J. H., Li, H., et al. (2020). Schisandrin B exerts hypnotic effects in pcpa-treated rats by increasing hypothalamic 5-HT and γ-aminobutyric acid levels. Exp. Ther. Med. 20 (6), 142–156. doi:10.3892/etm.2020.9271
Wickwire, E. M., Shaya, F. T., and Scharf, S. M. (2016). Health economics of insomnia treatments: the return on investment for a good night's sleep. Sleep. Med. Rev. 30, 72–82. doi:10.1016/j.smrv.2015.11.004
Wongsurawat, T., Sutheeworapong, S., Jenjaroenpun, P., Charoensiddhi, S., Khoiri, A. N., Topanurak, S., et al. (2023). Microbiome analysis of Thai traditional fermented soybeans reveals short-chain fatty acid-associated bacterial taxa. Sci. Rep. 13 (1), 7573–7767. doi:10.1038/s41598-023-34818-0
Yamatsu, A., Yamashita, Y., Pandharipande, T., Maru, I., and Kim, M. (2016). Effect of oral γ-aminobutyric acid (gaba) administration on sleep and its absorption in humans. Food Sci. Biotechnol. 25 (2), 547–551. doi:10.1007/s10068-016-0076-9
Yang, Y. W., Wu, Y., Xu, P. Q., Guo, F., Guo, F., and Yang, B. C. (2021). Nyctinastic herbs decoction improves para-chlorophenylalanine-induced insomnia by regulating the expression level of neurotransmitters. Ann. Transl. Med. 9 (20), 1524–1532. doi:10.21037/atm-21-4462
Yuen, K. M., and Pelayo, R. (2017). Socioeconomic impact of pediatric sleep disorders. Sleep. Med. Clin. 12 (1), 23–30. doi:10.1016/j.jsmc.2016.10.005
Zhang, X., Yan, H. M., Luo, Y. J., Huang, Z. L., and Rao, Y. (2018). Thermoregulation-independent regulation of sleep by serotonin revealed in mice defective in serotonin synthesis. Mol. Pharmacol. 93 (6), 657–664. doi:10.1124/mol.117.111229
Zhao, C. H., Dong, H. J., Zhang, Y. P., and Li, Y. (2019). Discovery of potential genes contributing to the biosynthesis of short-chain fatty acids and lactate in gut microbiota from systematic investigation in E. coli. Npj Biofilms Microbiomes 5 (1), 19–28. doi:10.1038/s41522-019-0092-7
Keywords: eucalyptus essential oil, sedative-hypnotic, neurotransmitter, gut microbes, gut microbiota-brain axis
Citation: Li X, Zhang Y, Zhang Q, Cao A and Feng J (2024) Eucalyptus essential oil exerted a sedative-hypnotic effect by influencing brain neurotransmitters and gut microbes via the gut microbiota-brain axis. Front. Pharmacol. 15:1464654. doi: 10.3389/fphar.2024.1464654
Received: 14 July 2024; Accepted: 11 September 2024;
Published: 25 September 2024.
Edited by:
Ping Zheng, The University of Melbourne, AustraliaReviewed by:
Lourdes Franco, University of Extremadura, SpainCopyright © 2024 Li, Zhang, Zhang, Cao and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Feng, ZmVuZ2pAemp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.