- 1National Institute of Sensory Organ, National Hospital Organization of Tokyo Medical Center, Meguro City, Japan
- 2Faculty of Pharmaceutical Sciences, Setsunan University, Hirakata, Japan
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul, Republic of Korea
- 4Sensory Organ Research Institute, Seoul National University Medical Research Center, Seoul, Republic of Korea
Editorial on the Research Topic
Identifying novel drug delivery systems and treatments for hearing loss and related ear disorders, volume II
Development of drug delivery system (DDS) for the middle ear is a useful way to treat otitis media, which is still a very common disease.
Furthermore, the development of DDS for the inner ear is a very important, as is drug development for the inner ear. No matter how good a drug is, if it does not reach the inner ear, it has no clinical significance. On the other hand, even drugs with weak efficacy may be clinically effective if they reach the inner ear in large doses.
Most therapeutic drugs are administered orally or by injection, but the inner ear contains blood labyrinth barriers (BLBs), which are often difficult to reach by administration through the blood. Local administration or intratympanic injection (ITI) of drug to the inner ear is therefore considered, but the inner ear is anatomically surrounded by bone and has a barrier in the inner ear membrane. Additionally, because administering drugs through a hole in the inner ear membrane can cause mechanical damage, we also consider whether the drug can penetrate the barrier. Furthermore, we administer the drug slowly over a long period because the volume of inner ear is very small.
In this Research Topic, we have six following articles addressing the above issues.
1) Two articles discussing methods of inner ear administration, 2) two articles on intra-tympanic steroid administration, which is currently used in clinical practice, and 3) two articles attempting to improve age-related hearing loss (ARHL) or presbycusis (Figure 1).
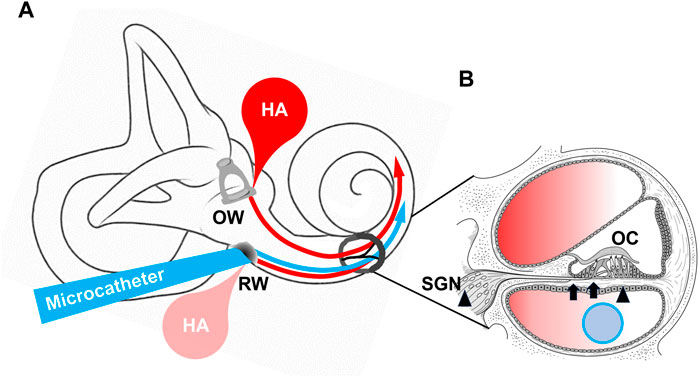
Figure 1. A. Drug delivery into cochlea Arrows indicate microcatheter and hyaluronic acid target inner ear. RW; round window OW; oval window B. Section of cochlea ELZC treatment target (arrows), and resveratrol treatment target (arrowheads) in presbycusis, respectively. OC; organ of Corti, SGN; spiral ganglion neuron.
The novel developments in drug delivery for middle and inner ear administration including novel controlled release therapeutics such as hydrogel and nanotechnology and finally, novel device delivery approaches such as microfluidic systems and cochlear prosthesis-mediated delivery (Delaney et al.).
Because the size of the inner ear differs between humans and other animals, it is questionable whether the results of nonclinical studies can be applied to humans, however, pharmacokinetics in catheterized piglets were evaluated using fluorescent dyes. Fluorescent dyes concentrations were significantly lower 6 h and 24 h compared to 2 h after administration (Yildiz et al.). We may need constant administration or vehicles that are designed to resist drainage through the Eutachian tube and enable sustained-release.
Intratympanic injection (ITI) of steroids is also used clinically, mainly for sudden senosorineural hearing loss, to reduce the side effects of systemic steroid administration, but ITI with multiple tympanostomy carries the risk of perforation of the tympanic membrane. Two strategies were therefore proposed on this Research Topic.
High-molecular-weight hyaluronic acid (HHA) is prolongs residence time, enhances drug concentration in target tissues, and ensures safety, highlighting the potential advantages of HHA + Dexamethasone over existing standard therapies (Hwang et al.).
ITI of steroid with embedded dual viscosity mixture vehicle (DVV) increases efficacy, and this activity is reflected in reduced ROS activity, inflammatory response, and actin filament-mediated wound healing processes (Jung et al.).
Finally, this Research Topic also included prevention of ARHL which is the most common form of inner ear diseases. The protective effects of ErLong ZuoC were predicted to be associated with cellular senescence, inflammatory response, and synaptic connections in vitro (Yang et al.). The other is an oral treatment in mice, low-dose resveratrol inhibited RIPK3-mediated necroptosis in aging cochlea and delayed the onset of ARHL, which was a promising therapeutic strategy for ARHL (Liu et al.).
In the future, many of the newer therapies for inner ear diseases will be topically administered. New drugs that can prevent ARHL without long-term medication are also expected.
Author contributions
SK: Writing–original draft, Writing–review and editing. TY: Writing–review and editing, M-WS: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Seoul National University Hospital Research Funding (1120235030, 2023-2676) and this is also supported by Grant-in-Aid for Scientific Research(C)23K08952(SK) and Grant-in-Aid for Scientific Research(C) 22K06641.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: inner ear, drug delivery, therapy, hearing loss, presbycusis
Citation: Kanzaki S, Yamaguchi T and Suh M-W (2024) Editorial: Identifying novel drug delivery systems and treatments for hearing loss and related ear disorders, volume II. Front. Pharmacol. 15:1464254. doi: 10.3389/fphar.2024.1464254
Received: 13 July 2024; Accepted: 15 July 2024;
Published: 29 July 2024.
Edited and reviewed by:
Nicholas M. Barnes, University of Birmingham, United KingdomCopyright © 2024 Kanzaki, Yamaguchi and Suh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sho Kanzaki, shokanza@yahoo.co.jp
 Sho Kanzaki
Sho Kanzaki