- 1College of Medicine and Health Sciences, China Three Gorges University, Yichang, China
- 2The First College of Clinical Medical Sciences, Institute of Rheumatology, China Three Gorges University, Yichang, China
- 3The Second Clinical Medical School, Guangzhou University of Chinese Medicine, Guangzhou, China
- 4The Second People’s Hospital of Yichang, The Second Clinical Hospital of Three Gorges University, Yichang, Hubei, China
Objective: To evaluate the methodological, reporting and evidence quality of systematic reviews or meta-analyses of Janus kinases (JAK) inhibitors for the treatment of rheumatoid arthritis (RA).
Methods: Our study systematically retrieved reviews from various databases, spanning from inception to June 2024. Two evaluators independently assessed the methodological, reporting, and evidence quality of each review using the AMSTAR-2 and PRIAMA2020 tools. The evidence quality was evaluated according to GRADE criteria. Six aspects were evaluated: publication year, study type, homogeneity, risk of publication bias, AMSTAR-2 methodology, and PRIAMA2020 reporting quality. Excel 2016 facilitated conversion of scores into radar plots.
Results: Following stringent selection criteria, a total of 18 relevant studies were identified. The AMSTAR-2 scores ranged from 4 to 13 points, with five studies rated as low quality and the remaining 13 as critically low quality. All studies encompassed populations, interventions, controls, and outcome measures, demonstrating commendable integrity. However, there is room for improvement in study protocol development and registration, comprehensive search strategies, inclusion and exclusion criteria, conflict of interest disclosure, and discussion of heterogeneity. PRIAMA2020 assessments ranged from 14.5 to 21 points, with two studies scoring below 15 points due to increased bias risk from data transformation and sensitivity analysis. Notably, all reviews (100%) adhered to PRIAMA2020 guidelines for certain items but none met all criteria. GRADE evaluation included 446 outcome measures, with 158 of moderate, 156 of low, and 132 of very low quality, indicating JAK inhibitors is effective in improving RA. According to radar chart, the average rank score was 13.13. One study achieved a balanced score across all dimensions, while 11 exceeded the average, five showed significant differences in PRIAMA2020 scores, and four in AMSTAR two scores.
Conclusion: Despite summarizing the efficacy and safety of JAK inhibitors in treating RA, the included studies exhibited poor methodological and reporting quality, along with low-quality evidence overall. Therefore, caution is warranted among decision-makers regarding the use of JAK inhibitors in RA treatment. Urgent requirements include high-quality, multicenter studies investigating JAK inhibitors for RA.
Systematic Review registration: https://www.crd.york.ac.uk/PROSPERO, identifier 413415.
1 Introduction
Rheumatoid arthritis (RA) is the most general chronic autoimmune disease, which is characterized by symmetrical polyarthritis that may cause bone and cartilage destruction, affecting the synovial tissue of the facet joints of the hands and feet, thereby causing tenderness, swelling and destruction of the joints (Mengdi et al., 2024; Díaz-González and Hernández-Hernández, 2023; Szekanecz et al., 2024). The current prevalence of RA is about 0.5%–1% (Brown et al., 2024). In addition, it was found that patients diagnosed with RA who had not received effective treatment within 2 years will have a 10-year disability rate of 50% according to the previous studies (Xiang et al., 2023). At present, the drugs for the treatment of RA are mainly nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, biological and targeted small-molecule drugs, etc (Singh, 2022; Guo et al., 2020). Those played an important role in reducing pain, joint tenderness and improving the function of joints (Xu et al., 2019; Radu and Bungau, 2021). However, a considerable number of patients had no response to the current treatment drugs, especially for joint pain and swelling, its application alone cannot fully achieve the effect of delaying the disease process and joint destruction (Guo et al., 2021; Zhu et al., 2020). Therefore, it is considered necessary to find a new or complementary treatment. According to the preceding research, we discovered that JAK inhibitors have been continuously developed for the treatment of RA and other autoimmune diseases in recent years (Szekanecz et al., 2024; Bonelli et al., 2024).
Janus kinase (JAK) inhibitors are small molecular biologic agents belonging to the intracellular tyrosine kinase family, which play a pivotal role in the signaling of many cytokine receptors, mediating inflammation and autoimmune diseases (Roskoski, 2022; Ipek et al., 2023). In recent years, JAK inhibitors have been widely used to treat RA and proved to have an obvious curative effect (Smolen et al., 2020; Langbour et al., 2023). However, the efficacy and safety of the clinical use of JAK inhibitors need to be further verified (Clarke et al., 2021; Cohen and Reddy, 2023). In recent years, several articles have reported on the systematic reviews and meta-analyses of JAK inhibitors in the treatment of RA (20, 21). However, JAK inhibitors still face a series of deficiencies such as rigorous scientific design, widely varied outcomes as well as lack of clinical sample size.
Therefore, the objective of this study is to comprehensively assess the methodological and evidence quality of JAK inhibitors in the treatment of rheumatoid arthritis, with the aim of providing valuable evidence for clinical decision-making. The assessment will be carried out using the Aid of the Measurement Tool for Evaluation of Systematic Reviews (AMSTAR-2), the Preferred Reporting Item for Systematic Reviews and Meta-Analyses 2020 (PRIAMA 2020), and the Grading of Evaluation (GRADE) system for assessment (Shea et al., 2017; Page et al., 2021; Guyatt et al., 2011). It is expected to provide valuable information for the implementation in the field of JAK inhibitors for RA through this study.
2 Material and methods
2.1 Search strategy
This study conducted a comprehensive search of the PubMed, Web of Science, Scopus, EMBASE, Livivo, China Scientific Journal Database (VIP), China National Knowledge Infrastructure (CNKI), and Wan Fang Database from inception to June 2024. Additionally, searches were also performed in PubMed Central and Open Grey to acquire gray literature. There were no language or publication time limitations. The search terms included (“rheumatoid arthritis” OR “RA”) AND (“Janus Kinase inhibitor” OR “JAK inhibitor” OR “Ruxolitinib” OR “Baricitinib” OR “Tofacitinib” OR “Fedratinib” OR “Momelotinib” OR “Pacritinib” OR “Fligotinib” OR “Upadacitinib” OR “Itacitinib” OR “Decernotinib” OR “Peficitinib” OR Abrocitinb” OR Ritlecitnibi) AND (“meta-analyses” or “systematic review”). Search strategies in various databases are shown in Supplementary Tables 1, 2.
2.2 Inclusion and exclusion criteria
The evaluation criteria are as follows: (i) Randomized controlled trial (RCT) or Non-randomized controlled trial; (ii) patients diagnosed with rheumatoid arthritis according to the 1987 American College of Rheumatology guidelines, aged 18–60 years; (iii) RA patients treated with JAK inhibitors in combination with leflunomide (LEF), methotrexate (MTX), hydroxychloroquine or other DMARDs, and controls treated with LEF, MTX, hydroxychloroquine, or other DMARDs alone.
The following articles will be excluded: (i) Reviews not related to rheumatoid arthritis or other complications of rheumatoid arthritis; (ii) Outside of a system review or meta-analysis or network meta-analysis; (iii) Evaluation of treatment without JAK inhibitors; (iv) Repeated comments; (v) Review of data, results and full text with obvious defects.
2.3 Literature screening and data extraction
The retrieved literature was imported into the literature management software Endnote X7 to remove duplicates. The two evaluators (Xiaolan Shen and Xiaoman Liu) completed literature screening, data sorting and scoring utilizing the evaluation tool independently. If the evaluation results were inconsistent, the two evaluators made a decision through consultation; if there was still exist a disagreement, a third person would be consulted (Zhitao Feng). The review that met the criteria was extracted: journals, publication time, authors, number of case, intervention methods, treatment groups, control groups, and outcome indicators.
2.4 Report quality and methodological evaluation
The methodological, reporting, and evidence quality of all included systematic reviews and meta-analyses were assessed using the AMSTAR-2, PRISMA 2020, and GRADE tools, respectively. (Shea et al., 2017; Page et al., 2021; Guyatt et al., 2011).
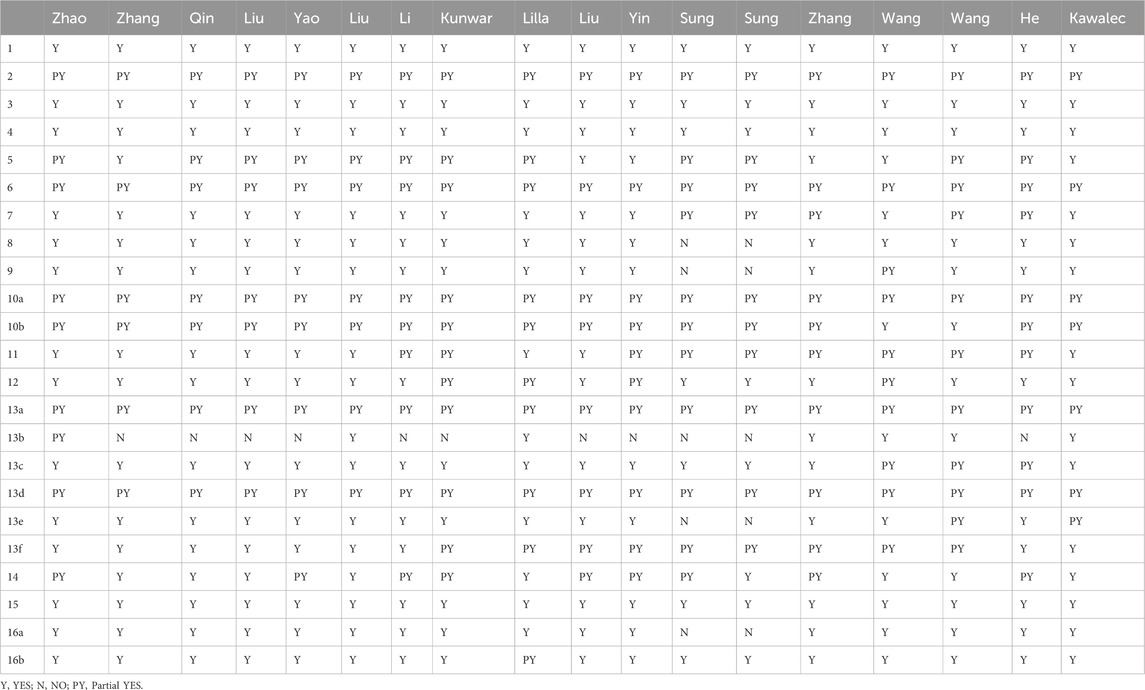
The AMSTAR-2 assessment consists of 16 items, items 2, 4, 7, 9, 11, 13, and 15 were identified as critical according to the guidelines. Items that fully meet the evaluation criteria are rated as “Yes”; those partially meeting the criteria are rated as “Partial Yes”; and items with no relevant information reported in the systematic review are rated as “No.” At the end of the assessment, reviews are categorized into four levels: high (one non-critical flaw), moderate (more than one non-critical flaw), low (one critical flaw, with or without non-critical flaws), and critically low (more than one critical flaw, with or without non-critical flaws) (Shea et al., 2017). The rationale of items of AMSTAR-2 is shown in Supplementary Table 3.
PRIAMA 2020 statement is designed to assess the completeness of information reporting in systematic reviews. It comprises a 27-item checklist organized into seven sections, each detailing reporting recommendations and providing examples for each item. The evaluation criteria include three categories: “Yes” (completely satisfies the criterion), “Partial Yes” (partially satisfies the criterion), and “No” (does not satisfy the criterion). Points are assigned as 1, 0.5, or 0, respectively, resulting in a total score ranging from 0 to 27 (23). The PRIAMA 2020 checklists are shown in Supplementary Table 4.
In this study, the GRADE tool was mainly used to assess the quality of evidence across all included reviews. The evidence was categorized as high (the true effect is likely very close to the estimated effect), moderate (the true effect is probably close to the estimated effect, but there is a possibility of a significant difference), low (the true effect may be considerably different from the estimate), and very low (the true effect is likely to be substantially different from the estimate). Additionally, five factors that could lead to downgrading the quality of evidence were considered: risk of bias, publication bias, imprecision, inconsistency, and indirectness. Each outcome measure was assessed, with downgrading factors rated as “not serious” or “serious” (resulting in a one-level downgrade, −1). Studies were also upgraded based on effect size and dose-response, with each upgrade resulting in a one-level increase (+1). The GRADE System tool checklists are shown in Supplementary Table 5.
The evaluation of reporting, methodological, and evidence quality evaluations were independently conducted by two appraisers using the same criteria to assess the quality of the included reviews. Any disagreements were resolved through consensus discussions, and if an agreement could not be reached, a third examiner was consulted to finalize the decision.
2.5 Create a radar map
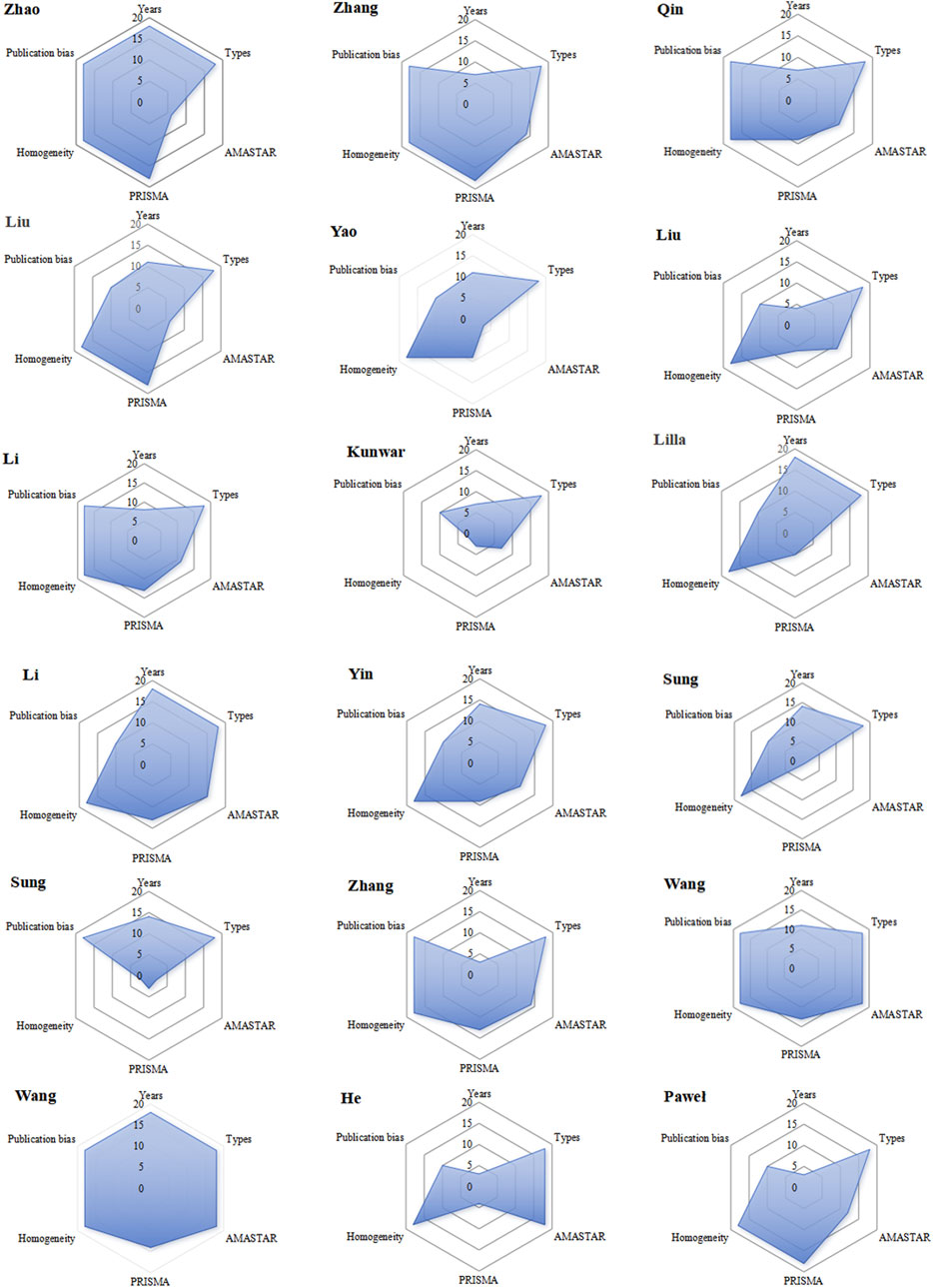
Extract the publication year, homogeneity level, research type, publication bias test, AMSTAR-2 and PRIAMA2020 evaluations from various system evaluations/meta analyses. Given the importance of timeliness in system evaluations, literature published more recently holds greater relevance. Therefore, literature with a publication year closer to the current date is assigned a higher rank. The distinction between high and low homogeneity in literature is determined by specific criteria: a Q test result of p ≥ 0.1 indicates high homogeneity, whereas a heterogeneity test result of I2 ≤ 50% signifies low homogeneity. In terms of research types, randomized controlled trials (RCTs) are considered to be of high rank due to their rigorous methodology, while quasi-randomized control trials (qRCTs) receive a lower rank due to their less stringent design. Furthermore, literature that has undergone publication bias testing is categorized as high rank, emphasizing its credibility, whereas literature lacking such testing is deemed low rank. Upon evaluating each piece of literature across various criteria, the cumulative rank is represented in a radar chart, providing a visual assessment of the literature’s quality. The literature is then sorted by rank, with a higher rank reflecting superior quality and evaluation results for the item.
Extract the publication year, homogeneity level, research type, publication bias test results, and AMSTAR-2 and PRISMA 2020 evaluations from various systematic reviews and meta-analyses. Given the importance of timeliness, more recent literature is ranked higher. Homogeneity is classified based on specific criteria: a Q test result of p ≥ 0.1 indicates high homogeneity, while an I2 ≤ 50% indicates low heterogeneity. Types of research are ranked based on methodological rigor, with randomized controlled trials (RCTs) being considered superior to quasi-randomized controlled trials (qRCTs) due to the latter’s less stringent design. Literature that includes a publication bias test is given higher credibility, while those lacking such testing are rated lower. After evaluating each study according to these criteria, the cumulative rank is presented in a radar chart, providing a clear assessment of the literature’s quality. Finally, the literature is sorted by rank, with higher ranks indicating superior quality and evaluation outcomes.
3 Results
3.1 Literature screening process and basic information
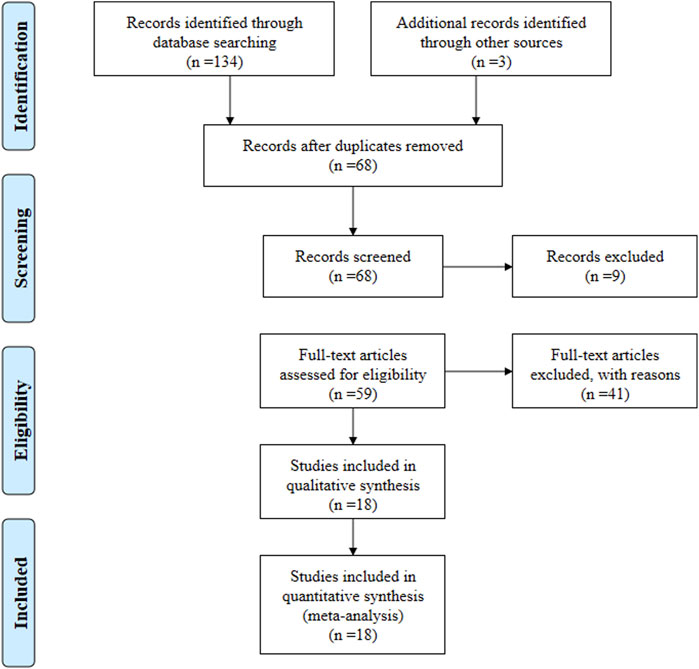
According to the search strategy, a total of 137 relevant articles were initially obtained. Aafter step-by-step screening, 18 systematic reviews from 2013 to 2023 (Liu et al., 2022; Tóth et al., 2022; Qian et al., 2022; Xin et al., 2020; Shiqin et al., 2019; Ya et al., 2015; Yuan and Fang, 2018; Chunyan et al., 2018; Lan et al., 2020; Kunwar et al., 2018; Wang et al., 2022; Yin et al., 2021; He et al., 2013; Wang et al., 2020; Sung and Lee, 2022; Sung and Lee, 2023; Kawalec et al., 2013; Zhang et al., 2014) were finally included, comprising of 11 in English and seven in Chinese. Figure 1. The characteristics of the included studies are summarized in Table 1.
3.2 Methodological quality assessment
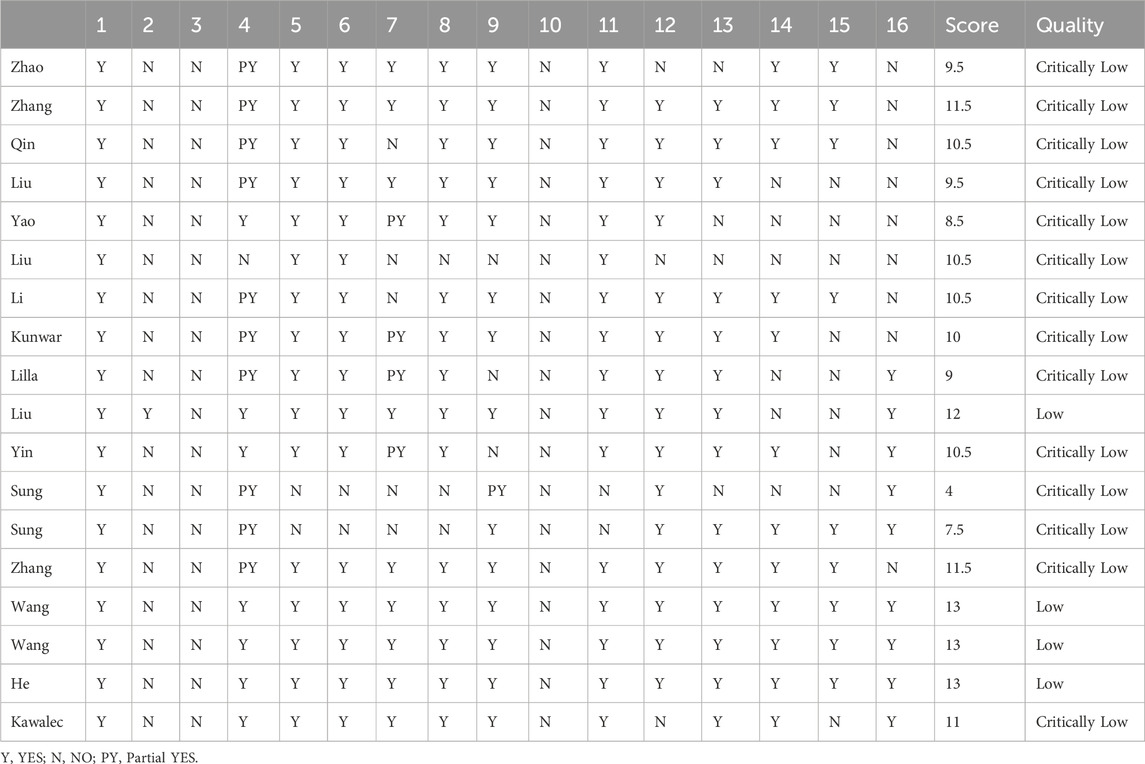
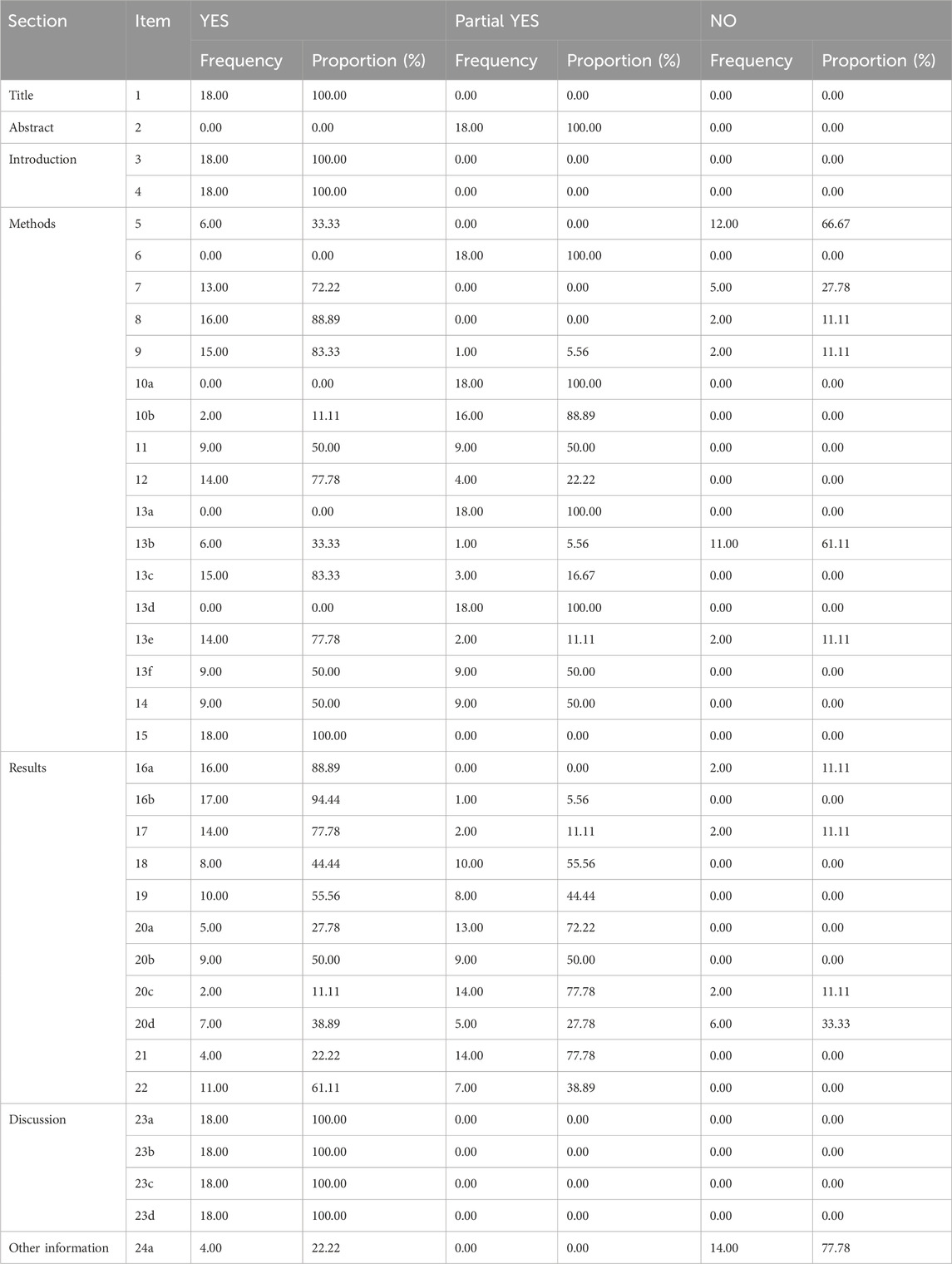
The AMSTAR-2 assessment included seven key evaluation areas: items 2, 4, 7, 9, 11, 13, and 15. Only one article met the second criterion; one article failed to meet the fourth criterion; five articles did not meet the seventh criterion; one article failed the ninth criterion; two articles did not meet the 11th criterion; three articles fell short of the 13th criterion, and seven articles did not meet the 15th criterion. The 18 studies included in AMSTAR-2 exhibited scores ranging from four to 13. Of these, 13 were classified as critically low quality, while five were categorized as low quality. None of them met the criteria for medium or high quality Table 2.
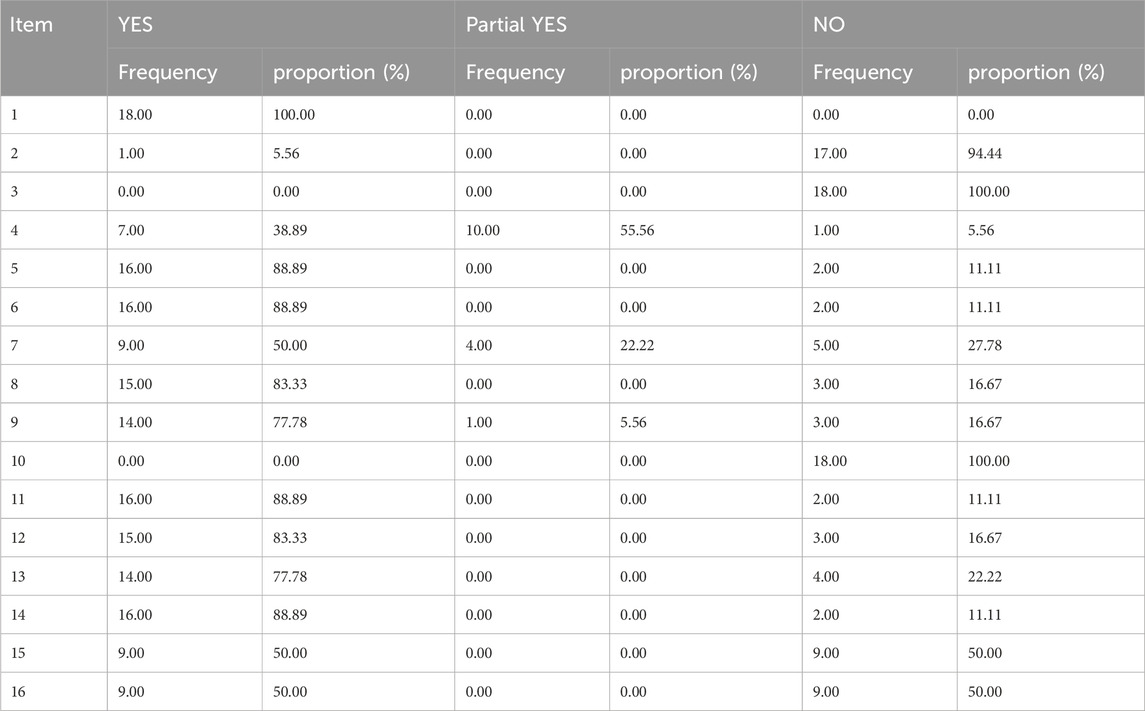
All eighteen reviews (100%) adhered to the PICO principle,covering population, intervention, control group, and outcome. Thirteen studies (72%) explicitly stated their inclusion and exclusion criteria. In addition, eleven studies (61%) employed a rigorous method to assess the risk of bias in the randomized controlled trials, considering their potential impacts on the meta-analyses results or evidence integration. These studies used appropriate statistical models, predicted the likely incidence of review outcomes, investigated sources of heterogeneity, and discussed their influence on the findings. Furthermore, sixteen studies (89%) involved multiple investigators who independently conducted screening and evaluation. Finally, eine studies (50%) demonstrated no conflicts of interest. The methodological quality evaluation results of the included reviews of AMSTAR-2 in Table 3.
Thirteen reviews were classified as critically low quality due to the presence of multiple key flaws, while the remaining five reviews were categorized as low quality based on the AMSTAR-2 evaluation criteria.
3.3 Evaluation of report quality
The PRIAMA2020 scores ranged from 14.5 to 21, with four of the 18 studies analyzed scoring between 21 and 27, fourteen scoring between 15 and 21, and only two studies receiving scores between 0 and 15 Table 4. All the reviews (100%) conform to the specification of item 1, 3, 4, 15, 16a, and 23 (a-d) in the PRIAMA2020 structure. However, none of the reviews (0%) conform to the specification of items 24 (b-c). The title, background, theoretical basis, purpose, evaluation method, outcome indicators, individual study results, and inter-study bias risk were adequately reported. Nevertheless, there was insufficient reporting on the causes of bias risk as well as on methods such as combining effect size, data transformation and conducting sensitivity analysis to ensure study stability. Additionally, details regarding study registration, protocol adherence along with public information were rarely provided. Partial reporting was observed for other components of the PRIAMA2020 statement as depicted in Table 5.
3.4 Evidence quality evaluation
The evidence quality of the 18 included studies was assessed, encompassing a total of 446 outcome indicators. Among these, 158 indicators (35.42%) were classified as medium quality, 156 indicators (34.98%) were categorized as low quality, and 132 indicators (29.60%) were deemed to have extremely low quality. The quality evidence of included reviews is shown in Supplementary Table 6.
3.5 Efficacy evaluation results reported in the systematic review
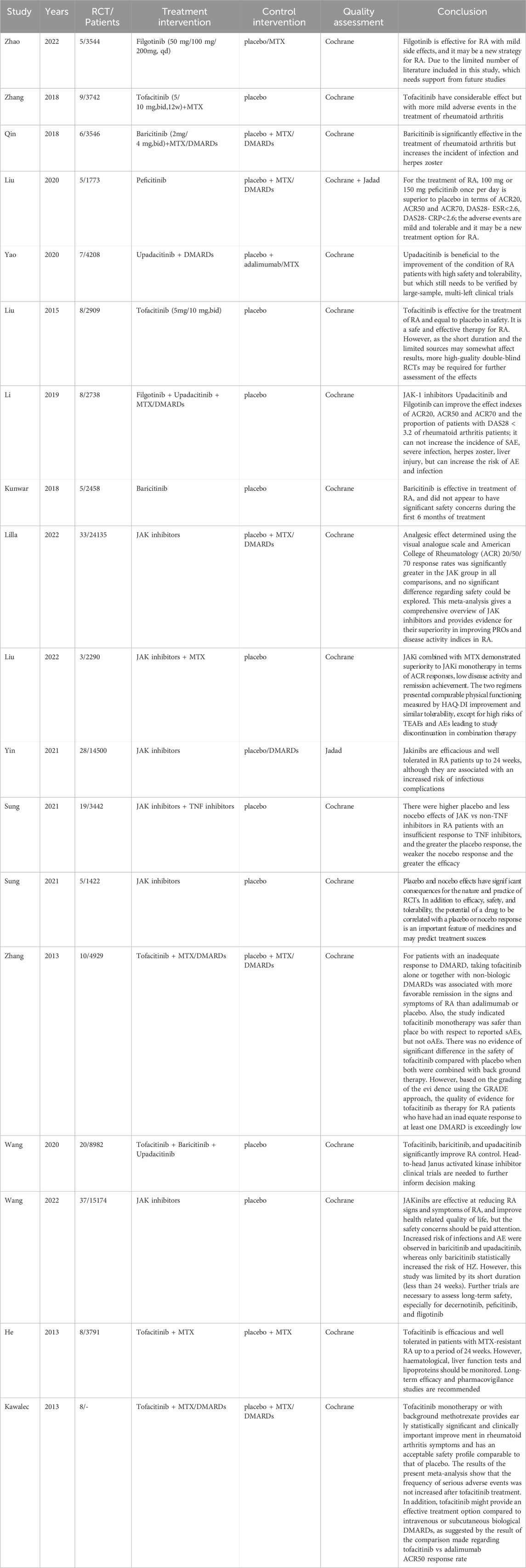
In comparison to placebo, treatment with Filgotinib 200 mg resulted in improved ACR50/70 index scores among patients, and the study was assessed as having moderate quality. Similarly, Baricitinib 2 mg demonstrated a medium-quality improvement in ACR50/70 index scores, while Upadacitinib 15 mg significantly improved ACR20/50/70 index scores with a medium-quality evaluation. Furthermore, in comparison to monotherapy with JAK inhibitors alone, the combination of Baricitinib and Filgotinib with MTX demonstrated significant efficacy and received a medium-quality assessment according to the GRADE scale. These findings provide evidence supporting the effectiveness and scientific rationale for selecting JAK inhibitors in RA treatment, Supplementary Table 5.
3.6 The safety evaluation results of the reports included in the systematic review
In the assessment of adverse reactions caused by JAK inhibitors, Filgotinib demonstrated a relatively low risk profile for severe adverse reactions, herpes zoster, and upper respiratory tract infection. This suggests that Filgotinib exhibits a comparatively favorable safety profile, with its evidence quality being evaluated as moderate. Baricitinib 4 mg also exhibited relatively low risks of adverse reactions and infection, with its evidence quality being assessed as moderate. Similarly, Upadacitinib 15 mg displayed relatively low risks of adverse reactions, severe adverse reactions, and infection while maintaining a moderate level of evidence quality. These findings suggest that different JAK inhibitors possess distinct advantages in terms of their side effect profiles, including adverse reactions, infections and herpes zoster. This indicates varying levels of safety among the different JAK inhibitors, Supplementary Table 5.
3.7 Radar chart for system evaluation
The publication years range from 2013 to 2022, with more recent publications ranked higher. The highest rank of 18 is held by the 2022 publication, while the lowest rank of three belongs to one from 2013. In terms of study types, pure RCTs are given the highest rank, while those including qRCTs receive the lowest. All 18 publications are classified as RCTs, each with a rank of 18. Two publications were classified as having low homogeneity, while the remaining ones were considered highly homogeneous. Eight articles used funnel plots to test for publication bias, while the remaining ten did not, resulting in a rank of 10 for thosethat did not use funnel plots. In PRIAMA2020 scoring, the highest rank is associated with a score of 21 points and is given to publications with a ranking of 18; whereas the lowest rank receives a score of only14.5 points and is assigned to those with a ranking of 1. The sum ranks for six entries were ordered, with higher ranks indicating better quality literature. Radar charts based on these ranks were drawn, with 18 charts arranged chronologically. A larger radar chart area indicates higher quality and greater reference value of the literature, Figure 2. The average rank score of the included studies was 13. Combined with the intuitive radar map, Wang Faping’s study demonstrated the highest quality and more balanced scores in all dimensions, while Sumit Kunwar’s study had the lowest quality, with low scores in AMSTAR-2, PRIAMA2020 and homogeneity. Eleven studies scored above the average across all dimensions. It is worth noting that the PRISMA 2020 scores varied significantly in five studies, and the AMSTAR-2 scores varied significantly in four studies. Of particular concern are two studies by Sung, which had notably low AMSTAR-2 and PRISMA scores, directly impacting their average rank. However, most of the included studies were relatively recent, focused on RCTs, and exhibited low publication bias, contributing to their overall credibility.
4 Discussion
4.1 The methodological rigor requires enhancement
The purpose of this study was to appraise the methodological and reporting quality, along with the quality of evidence, from published systematic reviews/meta-analyses of the efficacy and safety of JAK inhibitors for RA. High-quality systematic reviews and meta-analyses are used to summarize related research and estimating the benefits and harms of interventions for patients and clinicians. Unfortunately, the AMSTAR two scale in this study showed that of the 18 included articles, five were evaluated as low quality and 13 were evaluated as critically low quality, indicating that the quality of the AMSTAR method needs to be improved.
According to the quality assessment results from the AMSTAR-2 methodology, several aspects require improvement, especially item 2 (pre-study protocol), item 3 (justification of study inclusion criteria), item 4 (comprehensive search strategy), item seven (exclusion criteria and justifications), item 10 (source of funding), item 14 (discussion of heterogeneity), item 15 (investigation of publication bias), and item 16 (reporting of conflicts of interest). The main reason for the overall low quality assessment is that 94.44% of the research did not adequately provide preliminary study protocols for the included systematic reviews. Additionally, none of the research explained the reasons for inclusion criteria, which may decrease consistency, increase selection bias risk, and reduce rigor. Furthermore, most included studies did not conduct comprehensive literature searches and did not provide detailed search strategies or explanations of their inclusion/exclusion criteria, which also increases the risk of publication bias. Moreover, although most studies assessed the risk of bias in their study design or execution process, they overlooked analyzing the potential causes and impacts of these biases on the results, resulting in decreased assessment quality. We strongly recommend that researchers register their studies on the international registry platform PROSPERO before conducting future research. However, it is noteworthy that despite these limitations, the included literature still provided detailed descriptions of study population characteristics, as well as intervention measures adopted in conjunction with control measures and outcome indicators, thereby enhancing the comprehensiveness and integrity of the articles. Additionally, by assessing result heterogeneity and bias risk, most studies also employed appropriate methods to partially mitigate the risks associated with selection and publication bias.
To enhance comprehensiveness and reduce publication bias, it is recommended to plan and conduct comprehensive searches using professional databases, journals, and grey literature. Authors have a responsibility to disclose funding sources and conflicts of interest to avoid biasing the study towards sponsored products, and should conclude their reports with convincing results.
4.2 The quality of literature reports need to be improved
The PRIAMA2020 assessment results indicated that four papers achieved scores ranging from 21 to 27, reflecting a more comprehensive research methodology. Furthermore, 13 papers scored between 15 and 21, suggesting some flaws in the research method. Only one paper scored below 15, highlighting serious deficiencies in the conducted research. These findings imply the need for improving the quality of the included literature.
The limitations contributing to these issues are that all literature sources lacked complete structured abstracts with missing data source information, detailed interventions, and comprehensive data analysis methods; thereby compromising study integrity. Additionally, a number of studies inadequately described essential methods required for presenting or synthesizing data which may introduce subjectivity and ambiguity while increasing selection bias. What’s more, most of the included studies lacked registration information, leading to inconsistencies before and after implementing the research protocol, which significantly undermined study credibility due to subjective inclusion/exclusion of data. Finally, certain studies had incomplete literature retrieval by only considering a few databases without accounting for gray literature or manual supplementary methods; this affected study integrity.
In order to improve the quality of research, we strongly appeal that researchers should strictly adhere to the PRIAMA2020 checklist, and editors should require authors to adhere to updated assessment tools in addition to AMSTAR-2, PRIAMA 2020, or GRADE before accepting manuscripts. Most importantly, clinical trials are crucial in systematic reviews, and researchers should undergo rigorous training in conducting clinical trials to ensure high-quality systematic reviews.
4.3 Effectiveness of JAK inhibitors
In terms of clinical efficacy, firstly, JAK inhibitors demonstrated significant improvements in ACR20/50/70 indicators compared to placebo, indicating their advantageous role in treating RA. Filgotinib exhibited superior efficacy at 200 mg with medium-quality evaluations for ACR50/70 indicators. Baricitinib at 2mg and 4 mg showed certain advantages with medium-quality evaluations for ACR50/70 indicators. Upadacitinib treatment at 15 mg resulted in significantly improved ACR20/50/70 indicators with a medium-quality level evaluation. Tofacitinib treatment at 5 mg for 12 weeks led to significant improvements in ACR20 indicators with a medium-quality evaluation. Secondly, the combination of Baricitinib and Filgotinib with methotrexate treatment also demonstrated considerable efficacy compared to JAK inhibitor monotherapy, evaluated as medium quality using the GEADE scale. However, there is no discernible advantage among various types of JAK inhibitors due to the variability in efficacy and safety across different dosage regimens, rendering it challenging to ascertain drug superiority.
The observed outcomes can be attributed to several factors. Firstly, a scarcity of systematic reviews and research literature pertaining to JAK inhibitors exists, accompanied by notable methodological deficiencies, such as selection bias, publication bias, and others, consequently leading to a low level of evidence in the study. These deficiencies may encompass inadequacies in study design, data collection, and analysis methodologies. Consequently, these shortcomings have the potential to introduce biases such as selection bias and publication bias, thereby diminishing the reliability and validity of the study findings and leading to a diminished level of evidence. Secondly, the variability in pain tolerance levels among subjects introduces subjectivity in the assessment of outcome indicators. This subjectivity contributes to the generation of diverse and heterogeneous results, which in turn compromise the robustness and generalizability of the study outcomes. Finally, the plethora of available JAK inhibitors, coupled with the infrequent overlap in intervention doses selected by included studies, contributes to result heterogeneity. This heterogeneity not only complicates data interpretation but also undermines the comparability and consistency of study outcomes, thereby compromising the overall quality of the investigation.
4.4 Safety of JAK inhibitors
In terms of safety, GRADE scale evidence demonstrated that the clinical use of Filgotinib exhibited a lower incidence of adverse reactions such as herpes zoster, upper respiratory tract infection, and nasopharyngitis. Similarly, low-dose Baricitinib also resulted in fewer infections and cases of herpes zoster, indicating a certain level of safety. The selection of 15 mg dose of Upadacitinib showed a reduced occurrence of serious adverse events and infections, suggesting favorable safety profiles. However, several studies have also reported occurrences of infections, herpes zoster, and cardiovascular events subsequent to the administration of JAK inhibitors such as Tofacitinib, thereby diminishing the strength of supporting evidence.
Changes in outcome measures can be ascribed to several factors. Firstly, the utilization of a wide array of JAK inhibitors in clinical practice, developed by different pharmaceutical companies, has led to inconsistent clinical responses. Secondly, the variance in administered doses across the studies included in the analysis has resulted in divergent adverse reactions. Lastly, the incorporation of studies with small sample sizes and wide confidence intervals may introduce publication bias, potentially skewing the overall interpretation of the findings.
4.5 Analysis of the contradiction between low score quality and clinical efficacy
The dosage of JAK inhibitor utilized in the study may differ from the clinical dosage, and the pharmaceutical company involved may also vary, leading to significant discrepancies in potential side effects. 2. Individuals of varying ages and physical conditions may exhibit different tolerances to the side effects of JAK inhibitors. Given the wide age range of participants in the study, it is challenging to definitively determine the safety profile of JAK inhibitors. Future research could consider conducting subgroup analyses based on age and health status to more accurately assess the safety profile of JAK inhibitors at specific levels.3. The score results of the outcome indicators included in the study are still satisfactory; however, the low final score can be attributed to methodological defects. In future studies, it is imperative to enhance the rigor of researchers, provide a reasonable and comprehensive explanation for any bias, clearly demonstrate the methods of inclusion and exclusion, and minimize researcher subjectivity.
4.6 Limitations
This study also has certain limitations. Firstly, only electronic literature was retrieved, resulting in a reduced number of included literature and potential missed detections, thereby compromising the comprehensiveness of the study. Secondly, although the aforementioned three scales for systematic review/meta-analysis of the evaluation process are relatively detailed and clear, there still exists a subjective judgment by researchers that can influence the final evaluation results. In addition, included non-randomized controlled trials alongside randomized controlled trials may introduce a higher risk of bias, potentially affecting the overall evaluation. Lastly, the quality of the included reviews is generally low and sample sizes are relatively small, which may somewhat undermine the reliability and authenticity of the evaluations.
5 Conclusion
In general, the key findings of this study indicate that JAK inhibitors have significant therapeutic effects on RA and are generally safe for clinical practice, while there is room for improvement in the methodological and reporting quality of research on JAK inhibitors for treating RA. Specifically, deficiencies were observed in the formulation and registration of study protocols, lack of justification for inclusion criteria, inadequate comprehensive search strategies leading to insufficient study completeness and consistency, and subjectivity in the criteria for inclusion and exclusion, which decreased the credibility of the research. Therefore, we call for future researchers to conduct deeper analyses in areas and to improve the quality of evidence by reducing the risk of bias in clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XS: Data curation, Writing – original draft, Writing - review and editing. XL: Data curation, Writing - review and editing. XG: Writing - review and editing. XH: Writing - review and editing. HH: Writing - review and editing. ZF: Writing - review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was supported by grants from the National Natural Science Foundation of China (Nos. 81703783 and 82274333); the Hubei Provincial Natural Science Foundation (No. 2017CFB126).
Acknowledgments
This study thanks to the platform support provided by the third-level Laboratory of the State Administration of Traditional Chinese Medicine, China Three Gorges University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1459511/full#supplementary-material
References
Bonelli, M., Kerschbaumer, A., Kastrati, K., Ghoreschi, K., Gadina, M., Heinz, L. X., et al. (2024). Selectivity, efficacy and safety of jakinibs: new evidence for a still evolving story. Ann. Rheum. Dis. 83 (2), 139–160. doi:10.1136/ard-2023-223850
Brown, P., Pratt, A. G., and Hyrich, K. L. (2024). Therapeutic advances in rheumatoid arthritis. Bmj 384 (12), e070856. doi:10.1136/bmj-2022-070856
Chunyan, Z., Xiaodong, F., Yuan, Q., Wenqiang, K., Chunyang, Z., and DU, B. (2018). Efficacy of janus kinase inhibitor tofacitinib in treatment of rheumatoid arthritis: a meta-analysis. J. Third Mil. Med. Univ. 40 (06), 543–550. doi:10.16016/j.1000-5404.201710056
Clarke, B., Yates, M., Adas, M., Bechman, K., and Galloway, J. (2021). The safety of jak-1 inhibitors. Rheumatol. Oxf. 60 (Suppl. 2), ii24–30. doi:10.1093/rheumatology/keaa895
Cohen, S., and Reddy, V. (2023). Janus kinase inhibitors: efficacy and safety. Curr. Opin. Rheumatol. 35 (6), 429–434. doi:10.1097/BOR.0000000000000972
Díaz-González, F., and Hernández-Hernández, M. V. (2023). Rheumatoid arthritis. Med. Clin. Barc. 161 (12), 533–542. doi:10.1016/j.medcli.2023.07.014
Guo, X., Ji, J., Feng, Z., Hou, X., Luo, Y., and Mei, Z. (2020). A network pharmacology approach to explore the potential targets underlying the effect of sinomenine on rheumatoid arthritis. Int. Immunopharmacol. 80, 106201. doi:10.1016/j.intimp.2020.106201
Guo, X., Ji, J., Zhang, J., Hou, X., Fu, X., Luo, Y., et al. (2021). Anti-inflammatory and osteoprotective effects of chikusetsusaponin ⅳa on rheumatoid arthritis via the jak/stat signaling pathway. Phytomedicine 93 (1), 153801. doi:10.1016/j.phymed.2021.153801
Guyatt, G. H., Oxman, A. D., Schünemann, H. J., Tugwell, P., and Knottnerus, A. (2011). Grade guidelines: a new series of articles in the journal of clinical epidemiology. J. Clin. Epidemiol. 64 (4), 380–382. doi:10.1016/j.jclinepi.2010.09.011
He, Y., Wong, A. Y., Chan, E. W., Lau, W. C., Man, K. K., Chui, C. S., et al. (2013). Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Bmc Musculoskelet. Disord. 14 (08), 298. doi:10.1186/1471-2474-14-298
Ipek, Y., Kilic, B., Gunay, U. B., and Eskazan, A. E. (2023). Novel janus-kinase (jak) inhibitors in myelofibrosis. Expert Opin. Investig. Drugs 32 (10), 931–940. doi:10.1080/13543784.2023.2269078
Kawalec, P., Mikrut, A., Wiśniewska, N., and Pilc, A. (2013). The effectiveness of tofacitinib, a novel janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 32 (10), 1415–1424. doi:10.1007/s10067-013-2329-9
Kunwar, S., Collins, C. E., and Constantinescu, F. (2018). Baricitinib, a janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Clin. Rheumatol. 37 (10), 2611–2620. doi:10.1007/s10067-018-4199-7
Lan, Y., Shen-kui, F., Xiang-dong, M., Shan, G., Shi-qin, L., Jun-feng, Y., et al. (2020). Systematic review of efficacy and safety of upadacitinib for rheumatoid arthritis. China Acad. J. Electron. Publ. House. 39 (10), 623–630. doi:10.14109/j.cnki.xyylc.2020.10.11
Langbour, C., Rene, J., Goupille, P., and Carvajal, A. G. (2023). Efficacy of janus kinase inhibitors in rheumatoid arthritis. Inflamm. Res. 72 (5), 1121–1132. doi:10.1007/s00011-023-01717-z
Liu, L., Yan, Y. D., Shi, F. H., Lin, H. W., Gu, Z. C., and Li, J. (2022). Comparative efficacy and safety of jak inhibitors as monotherapy and in combination with methotrexate in patients with active rheumatoid arthritis: a systematic review and meta-analysis. Front. Immunol. 13 (03), 977265. doi:10.3389/fimmu.2022.977265
Mengdi, H., Zhipeng, H., and Maoyi, Y. (2024). Effects of total glucosides of paeony on serum inflammatory cytokines in animal models of rheumatoid arthritis: a systematic review and meta-analysis. Front. Pharmacol. 15 (15), 233–241. doi:10.3389/fphar.2024.1349259
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Qian, Z., Jin, W., Rui-xiang, C., Wei, D., Xiao-bo, L., Qing-shu, Z., et al. (2022). Efficacy and safety of filgotinib for rheumatoid arthritis: a meta analysis. Cent. South Pharm. 20 (02), 421–428. doi:10.7539/j.issn.1672-2981.2022.02.032
Radu, A. F., and Bungau, S. G. (2021). Management of rheumatoid arthritis: an overview. Cells 10 (11), 2857. doi:10.3390/cells10112857
Roskoski, R. J. (2022). Janus kinase (jak) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharmacol. Res. 183, 106362. doi:10.1016/j.phrs.2022.106362
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj 358, j4008. doi:10.1136/bmj.j4008
Shiqin, L., Yaling, L., Yilan, H., Yun, Y., Shan, G., Zhirong, Z., et al. (2019). Efficacy and safety of selective janus kinase 1 inhibitor upadacitinib and filgotinib in the treatment of rheumatoid arthritis: a meta-analysis. China Acad. J. Electron. Publ. House. 30 (15), 2130–2135. doi:10.6039/j.issn.1001-0408.2019.15.22
Singh, J. A. (2022). Treatment guidelines in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 48 (3), 679–689. doi:10.1016/j.rdc.2022.03.005
Smolen, J. S., Landewé, R., Bijlsma, J., Burmester, G. R., Dougados, M., Kerschbaumer, A., et al. (2020). Eular recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79 (6), 685–699. doi:10.1136/annrheumdis-2019-216655
Sung, Y. K., and Lee, Y. H. (2022). Placebo and nocebo responses in randomized controlled trials of janus kinase inhibitor monotherapy for rheumatoid arthritis: a meta-analysis. Z Rheumatol. 81 (5), 430–437. doi:10.1007/s00393-021-00969-6
Sung, Y. K., and Lee, Y. H. (2023). Placebo and nocebo responses in randomized controlled trials of non-tumor necrosis factor biologics and janus kinase inhibitors in patients with active rheumatoid arthritis showing insufficient response to tumor necrosis factor inhibitors: a meta-analysis. Z Rheumatol. 82 (Suppl. 1), 59–67. doi:10.1007/s00393-021-01047-7
Szekanecz, Z., Buch, M. H., Charles-Schoeman, C., Galloway, J., Karpouzas, G. A., Kristensen, L. E., et al. (2024). Efficacy and safety of jak inhibitors in rheumatoid arthritis: update for the practising clinician. Nat. Rev. Rheumatol. 20 (2), 101–115. doi:10.1038/s41584-023-01062-9
Tóth, L., Juhász, M. F., Szabó, L., Abada, A., Kiss, F., Hegyi, P., et al. (2022). Janus kinase inhibitors improve disease activity and patient-reported outcomes in rheumatoid arthritis: a systematic review and meta-analysis of 24,135 patients. Int. J. Mol. Sci. 23 (03), 1246. doi:10.3390/ijms23031246
Wang, F., Sun, L., Wang, S., Davis, J. R., Matteson, E. L., Murad, M. H., et al. (2020). Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin. Proc. 95 (7), 1404–1419. doi:10.1016/j.mayocp.2020.01.039
Wang, F., Tang, X., Zhu, M., Mao, H., Wan, H., and Luo, F. (2022). Efficacy and safety of jak inhibitors for rheumatoid arthritis: a meta-analysis. J. Clin. Med. 11 (15), 4459. doi:10.3390/jcm11154459
Xiang, G., Gao, M., Qin, H., Shen, X., Huang, H., Hou, X., et al. (2023). Benefit-risk assessment of traditional Chinese medicine preparations of sinomenine using multicriteria decision analysis (mcda) for patients with rheumatoid arthritis. Bmc Complement. Med. Ther. 23 (1), 37. doi:10.1186/s12906-023-03864-6
Xin, L., Changjing, X., Xiaoyan, Z., Danjie, Z., Bin, Y., and Yilan, H. (2020). Efficacy and safety of peficitinib for treating rheumatoid arthritis: a systematic review. China Acad. J. Electron. Publ. House. 31 (07), 859–864. doi:10.6039/j.issn.1001-0408.2020.07.18
Xu, J., Feng, Z., Chen, S., Zhu, J., Wu, X., Chen, X., et al. (2019). Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels. Clin. Rheumatol. 38 (1), 19–27. doi:10.1007/s10067-017-3646-1
Ya, L., Kecheng, Z., Yanzhou, C., Weinei, Y., Yanwen, P., Lizhao, W., et al. (2015). Efficacy and safety of tofacitinib in treating rheumatoid arthritis: a meta-analysis. Chin. J. Rheumatology 19 (10), 674–677. doi:10.3760/cma.j.issn.1007-7480.2015.010.006
Yin, Y., Liu, M., Zhou, E., Chang, X., He, M., Wang, M., et al. (2021). Efficacy and safety of jakinibs in rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 40 (10), 3989–4005. doi:10.1007/s10067-021-05686-8
Yuan, Q., and Fang, P. (2018). Efficacy of janus kinase inhibitor baricitinib for patients with rheumatoid arthritis: a meta-analysis. Chin. J. New Drugs Clin. Rem. 37 (08), 477–483. doi:10.14109/j.cnki.xyylc.2018.08.010
Zhang, X., Liang, F., Yin, X., Xiao, X., Shi, P., Wei, D., et al. (2014). Tofacitinib for acute rheumatoid arthritis patients who have had an inadequate response to disease-modifying antirheumatic drug (dmard): a systematic review and meta-analysis. Clin. Rheumatol. 33 (2), 165–173. doi:10.1007/s10067-013-2452-7
Keywords: JAK inhibitors, rheumatoid arthritis, AMSTAR-2, PRIAMA 2020, GRADE system
Citation: Shen X, Liu X, Guo X, Hou X, Huang H and Feng Z (2024) Systematic review of Janus kinases inhibitors for rheumatoid arthritis: methodology, reporting, and quality of evidence evaluation. Front. Pharmacol. 15:1459511. doi: 10.3389/fphar.2024.1459511
Received: 04 July 2024; Accepted: 10 September 2024;
Published: 25 September 2024.
Edited by:
Salahuddin Ahmed, Washington State University College of Pharmacy and Pharmaceutical Sciences, United StatesReviewed by:
Paul Panipinto, Washington State University, United StatesFarheen Shaikh, Washington State University, United States
Sadik Khuder, University of Toledo Medical Center, United States
Copyright © 2024 Shen, Liu, Guo, Hou, Huang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhitao Feng, emhpdGFvLmZlbmdAY3RndS5lZHUuY24=; Huiliang Huang, YW5uMTk4MzA0MThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaolan Shen
Xiaolan Shen Xiaoman Liu
Xiaoman Liu Xiang Guo
Xiang Guo Xiaoqiang Hou
Xiaoqiang Hou Huiliang Huang
Huiliang Huang Zhitao Feng
Zhitao Feng