- 1PharmaEssentia Corporation, Taipei, Taiwan
- 2PharmaEssentia Biotech (Beijing) Limited, Beijing, China
- 3Pharmaron Clinical Services Co., Ltd., Chengdu, China
- 4PharmaEssentia Japan KK, Tokyo, Japan
Ropeginterferon alfa-2b (Ropeg) is approved for the treatment of adults with polycythemia vera (PV). This report aims to analyze the ethnic sensitivity of Ropeg for the treatment of PV, comparing the pharmacokinetics (PK), efficacy, and safety profiles across diverse ethnic groups. We conducted a relevant review of PV and analysis of data obtained from clinical studies involving Ropeg. The PK behavior of ropeg showed no significant differences between Chinese and overseas populations. Their efficacy and safety profiles were similar across the ethnic groups. The analyses indicated that the dose-exposure-response profile of Ropeg was consistent irrespective of ethnic variations. The results suggest that Ropeg exhibits a consistent PK and pharmacodynamics profile and a similar therapeutic effect across different ethnic groups, confirming its efficacy and safety in the global treatment of PV. More generally, these findings support the broader application of Ropeg in diverse patient populations and emphasize the need for an inclusive clinical practice.
1 Introduction
Polycythemia vera (PV) is a common type of Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), which also include essential thrombocythemia (ET) and myelofibrosis (MF) (Guglielmelli and Vannucchi, 2020). It is characterized by the clonal proliferation of hematopoietic stem or progenitor cells, leading to the overproduction of red blood cells often accompanied by increased white blood cell (WBC) and platelet counts (Benevolo et al., 2023; Tefferi and Barbui, 2023). In the majority of cases, PV is associated with a gain-of-function mutation in the gene encoding Janus Kinase 2 (JAK2), termed JAK2V617F (Baxter et al., 2005; James et al., 2005; Levine et al., 2005; Kralovics et al., 2005). This mutation leads to the constitutive activation of the JAK-signal transducer and activator of transcription (STAT) signaling pathway, leading to the uncontrolled hematopoietic cell proliferation and increased blood counts (Chen and Mullally, 2014; Aruch and Mascarenhas, 2016).
Patients with PV manifest a wide range of clinical signs and symptoms, including fatigue, pruritus, night sweats, bone pain, and splenomegaly (Mesa et al., 2018; Mesa et al., 2021). Due to the increased blood volume and viscosity, patients are at an increased risk of both thrombotic and hemorrhagic events, which are the leading causes of morbidity and mortality in PV (Finazzi and Barbui, 2008; Marchioli et al., 2013; Griesshammer et al., 2019; Iurlo et al., 2020). Furthermore, PV has an inherent propensity of transformation to MF and acute myeloid leukemia (AML) over the long term (Cerquozzi and Tefferi, 2015; Stein et al., 2014). PV is associated with the risk of arterial and venous thrombosis and can be conventionally classified into two risk categories: high-risk (age >60 years or thrombosis history) and low-risk (age ≤60 years and no history of thrombosis) (Tefferi et al., 2021). The management of low-risk PV usually involves controlling hematocrit levels to reduce thrombotic risk, typically through phlebotomy and low-dose aspirin therapy, for patients who do not require cytoreductive therapy (Tefferi and Barbui, 2020). Hydroxyurea (HU) and polyethylene glycol-conjugated (PEGylated) interferon alpha (IFN-α) have often been used for cytoreduction in the management of high-risk patients (Bose and Verstovsek, 2019; Guglielmelli and Vannucchi, 2020). It has been shown that elevated WBC and platelet counts (>11×109/L and >400×109/L respectively) are correlated with increased thromboembolic (TE) risk (Bose and Verstovsek, 2019; Gerds et al., 2022). Therefore, the complete hematologic response (CHR) defined as a hematocrit <45% without phlebotomy, platelet count ≤400 × 109/L, and WBC count <10 × 109/L) is an important indicator for the efficacy of PV treatment and is used as an endpoint for the regulatory approvals (Gisslinger et al., 2020; Jin et al., 2023; Qin et al., 2024). Cytoreductive treatment has also been assessed in low-risk patients and has shown clinical benefits, including a reduced need of phlebotomy in the absence of thrombotic events and progression of leukocytosis or thrombocytosis (Barbui et al., 2023; Tefferi and Barbui, 2023).
IFN-α-based therapies have been shown to result in therapeutic cytoreduction and have been suggested to exhibit disease modifying potential in PV treatment (Masarova et al., 2017; Tashi et al., 2018; Abu-Zeinah et al., 2021; Kiladjian et al., 2022). IFN-α works together with its possible prototype IFN-β (Wittling et al., 2021), an important part of a network of tumor suppressors or their related proteins for the cell cycle-based anti-cancer surveillance (Qin et al., 1997; Kaynor et al., 2002; Qin, 2023a; Qin, 2024a). Furthermore, IFN-β/IFN-α induces tumor cell apoptosis if overexpressed, and can elicit natural killer cell and CD8+ lymphocytes-mediated anti-tumor activities (Qin et al., 1998; Qin et al., 2001; Brown et al., 2002; Zitvogel et al., 2015; Qin, 2023b).
Ropeginterferon alfa-2b (Ropeg) is a novel, site-selective, mono-PEGylated recombinant proline-IFN-α with a favorable pharmacokinetics (PK) profile (Huang et al., 2021; Huang et al., 2022; Miyachi et al., 2021). It showed robust CHRs, and safety in adult patients with PV across several clinical studies (Gisslinger et al., 2015; Gisslinger et al., 2020; Edahiro et al., 2022; Jin et al., 2023; Suo et al., 2024). Ropeg treatment has also been found to induce molecular responses by reducing JAK2V617F allele burden or variant allele frequency (VAF) under two different dosing regimens (Gisslinger et al., 2015; Gisslinger et al., 2020; Edahiro et al., 2022; Jin et al., 2023; Suo et al., 2024). It appears that Ropeg treatment at a higher starting dose and a simpler dose-titration regimen could lead to a greater molecular response (Jin et al., 2023; Suo et al., 2024). Ropeg is the only IFN-based therapy approved by the US Food and Drug Administration (FDA) for the treatment of patients with PV (FDA, 2019). The National Comprehensive Cancer Network (NCCN) has recently recommended Ropeg as the preferred cytoreductive treatment for patients with low-risk PV (NCCN. National Comprehensive Cancer Network, 2024). Understanding the ethnic sensitivity of Ropeg is vital for clinical practice. Ethnic sensitivity analysis addresses the genetic and physiological differences among populations that can influence drug metabolism and responses (ICH, 1998; Huang and Temple, 2008; Yasuda et al., 2008). This analysis is crucial for ensuring the global efficacy and safety of Ropeg, as it helps tailor treatment to diverse patient populations and aligns with the goals of personalized medicine (Bernal and Domenech Rodríguez, 2013; Vannucchi, 2017; Hong, 2023).
In this study, a comprehensive ethnic sensitivity analysis of Ropeg for the treatment of PV was performed. By comparing the global diagnostic and treatment criteria for PV and examining the PK, efficacy, and safety of Ropeg across different ethnic groups, we sought to understand its global applicability and effectiveness, contributing to the advancement of inclusive healthcare in managing PV.
2 Materials and methods
2.1 Clinical trial design and participants
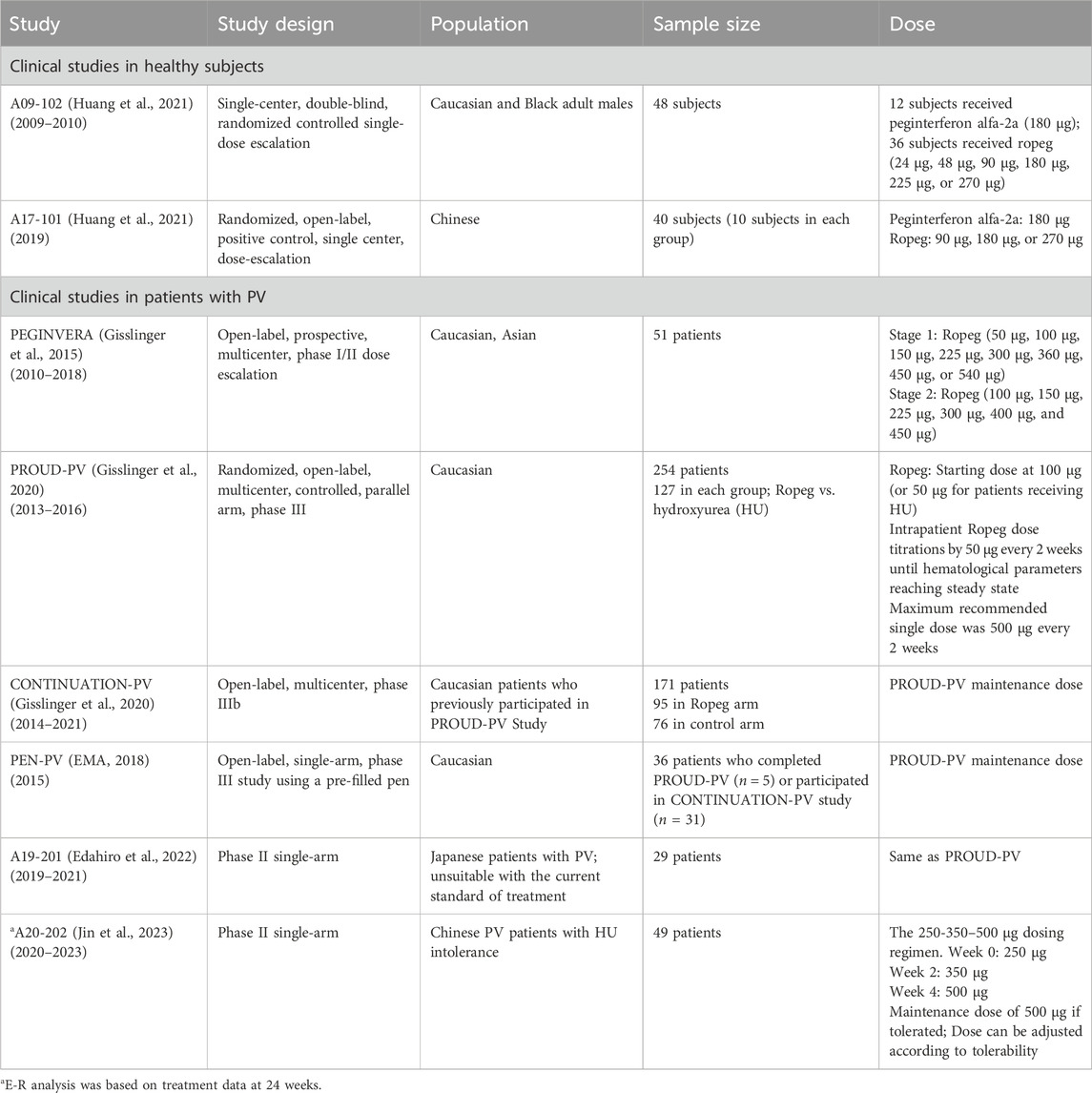
This study encompassed a comprehensive analysis of data derived from phase I-III clinical trials of Ropeg, focusing on its application in the treatment of PV. The relevant clinical trial information is summarized in Table 1. The analysis was designed to assess the PK, efficacy, and safety of Ropeg across diverse ethnic groups with a particular emphasis on comparing the responses between Chinese and overseas populations of different ethnic backgrounds. The participant pool included adult patients diagnosed with PV, ensuring a broad representation of demographic variables such as age, sex, and ethnicity.
Data collection encompassed a wide array of parameters, including patient demographics, specific Ropeg dosing regimens, PK measurements, efficacy outcomes, and adverse events (AEs). The PK data focused on capturing the temporal profile of Ropeg plasma concentrations after administration, providing a dataset for subsequent analyses.
2.2 PK analysis
A population PK (PopPK) model was used to analyze the PK behavior of Ropeg. This model is instrumental in identifying and quantifying the effects of various covariates, including ethnic background, on drug metabolism and distribution. The PopPK model was calibrated and validated using PK data to ensure robustness and reliability in assessing ethnic variations.
2.3 Exposure-response analyses
The efficacy of Ropeg was evaluated against the established clinical response criteria for PV. These criteria encompassed a range of hematological and clinical parameters, providing a comprehensive view of the therapeutic impact. The safety assessment focused on the incidence, severity, and pattern of AEs across different ethnic groups. The dual focus on efficacy and safety is pivotal for providing a balanced view of the therapeutic profile of Ropeg.
Logistic regression models are a key component of this framework, enabling the examination of the relationship between drug exposure and clinical response. These models were complemented by additional statistical tests and comparative assessments to elucidate any significant differences in PK and safety profiles across ethnic groups. Statistical significance was determined using predetermined alpha levels and confidence intervals were used to gauge the precision of the estimates.
2.4 Ethical considerations
All clinical trials included in the analyses adhered to ethical standards, in line with the principles of the 1,964 Helsinki Declaration and its subsequent amendments. Informed consent was obtained from all participants involved in the studies. Ethical rigor ensured the integrity and soundness of the study.
3 Results
3.1 Diagnosis, treatment, and efficacy assessment in PV
For the diagnosis of PV, the Chinese Guidelines for the Diagnosis and Treatment of Polycythemia Vera (Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association, 2022) recommend adhering to the diagnostic criteria set forth by the World Health Organization (WHO) (Barbui et al., 2018). To evaluate the efficacy of PV treatment in Chinese patients, Chinese guidelines recommend referring to the revised criteria of the European Leukemia Net (ELN) and the International Working Group for Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) (Barosi et al., 2013). This dual-reference framework integrates international standards into the Chinese clinical context, ensuring a rigorous and globally informed approach to both diagnosis and response assessment in PV management.
The clinical phenotypes or manifestations of PV can be heterogeneous. The use of phlebotomy, cytoreductive agents such as HU, IFN-α-based treatment, and second-line therapy with the JAK inhibitor ruxolitinib can also vary depending on individual physicians providing the treatment (Palumbo et al., 2023). In addition, clonal heterogeneity with JAK2 mutations and heterogeneous gene expression have been observed in patients with PV (Li et al., 2008; Spivak et al., 2014). However, the overall treatment principles, particularly regarding the use of IFN-α-based therapies, were not observed to have major discrepancies among different ethnic groups. IFN-α-based therapies have been shown to exert disease-modifying effect by prolonging progression-free survival, event-free survival, and potentially, overall survival in patients with PV (Abu-Zeinah et al., 2021; Gisslinger et al., 2023). The Chinese Guidelines for the Diagnosis and Treatment of Polycythemia Vera, NCCN guidelines, and Austrian Treatment Recommendations for Polycythemia Vera 2018 (Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association, 2022; NCCN. National Comprehensive Cancer Network, 2024; Burgstaller et al., 2018) all endorse the IFN-α-based treatment as a first-line therapy for PV. This consensus highlights a global alignment in the therapeutic approach for PV treatment regarding the use of disease modifying therapies.
Therefore, the clinical standards for the diagnosis and treatment of PV are consistent across Chinese and overseas populations with different ethnic backgrounds. In clinical studies using Ropeg for PV treatment, similar diagnostic and efficacy evaluation criteria were applied. There were no significant ethnic differences in PV pathophysiology, diagnosis, or efficacy evaluations between Chinese and overseas populations with different ethnic backgrounds.
3.2 PK profiles in Chinese and Caucasian subjects
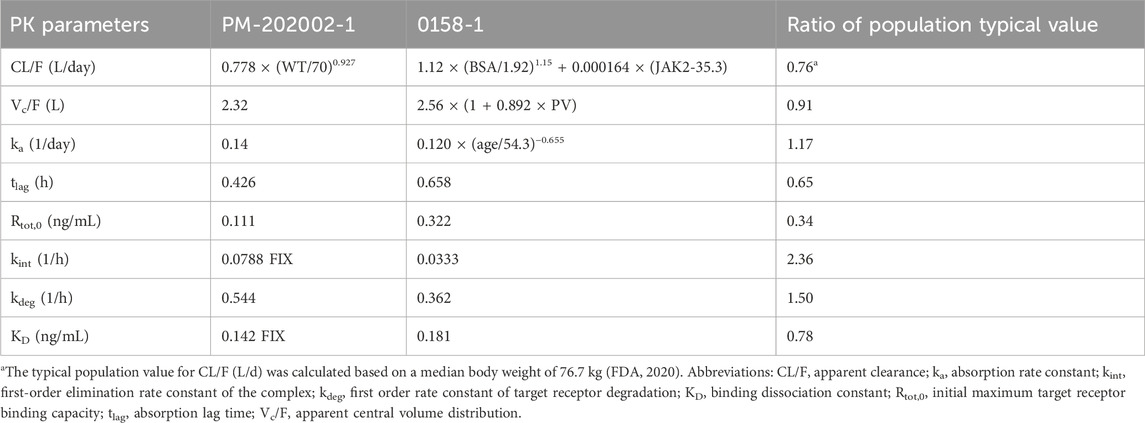
Data from phase I clinical studies A09-102 (Huang et al., 2021) and A17-101 (Huang et al., 2022) conducted on healthy subjects were utilized to construct the PopPK model PM-202002 (Zhu et al., 2021). A09-102 was conducted mostly in Caucasian subjects, while A17-101 was conducted in Chinese subjects. This model aimed to analyze the PK variance of Ropeg in healthy Chinese and Caucasian adult subjects. The analysis incorporated data from 57 participants (894 measurements). Covariate inclusion in the analysis was performed, highlighting a demographic composition of 52.63% Caucasians, and 47.37% Chinese. Age in the two ethnic groups was comparable (32.3 ± 6.9 vs. 31.6 ± 6.19), whereas the body weight was 14% lower in the Chinese population than the Caucasian population (68.1 ± 10 vs. 79.4 ± 9.41 kg) (Zhu et al., 2021). In the groups receiving identical doses (90 μg, 180 μg, or 270 μg), the plasma concentration-time profiles for subjects included in the analysis were demonstrated. Notably, there was a high degree of similarity between the concentration-time curves of the Chinese and Caucasian populations, indicating comparable PK behaviors between these groups. Furthermore, PopPK analysis identified ethnicity as a categorical variable representing Chinese and Caucasian populations in the covariate modeling analysis. Ethnicity showed no significant impact on the typical values for clearance (CL/F), volume of distribution (Vc/F), and absorption rate constant (ka) (Zhu et al., 2021), confirming the absence of a substantial difference in PK parameters between the Chinese and Caucasian populations.
Data from three clinical studies, A09-102, PEGINVERA, and PROUD-PV, were utilized in PopPK model 0158-1 (FDA, 2020). The PEGINVERA was conducted mostly in Caucasian patients with PV, whereas the PROUD-PV was conducted in Caucasian patients with PV. The model was designed to describe the plasma concentration-time profile of Ropeg following subcutaneous (SC) administration.
A comparative analysis of the parameter estimations from the two PopPK studies PM-202002 and 0158-1 is further summarized in Table 2. The results indicate that the CL/F and Vc/F were similar between the studies with the population typical values of Vc/F being 2.32 and 2.56 L, a difference of not higher than 10%; and those for CL/F being 0.85 and 1.12 L/day at 76.7 kg, a difference of not higher than 25%. This similarity suggests a comparable PK behavior between Chinese and Caucasian subjects. Furthermore, both PopPK analyses demonstrated that a non-linear increase in clearance was correlated with an increase in body weight. The findings from the PopPK analyses suggest that the PK behavior of Ropeg is similar between Chinese and Caucasian subjects, indicating that no significant ethnic differences exist in PK.
3.3 Exposure-response analyses in Chinese and Caucasian patients
3.3.1 Exposure-efficacy analysis in Chinese and Caucasian patients
Data from a phase II clinical study, A20-202, in Chinese patients with PV were utilized for an exposure-efficacy analysis (Qin et al., 2024), leading to the development of a logistic regression model correlating exposure (Cavg,0-24w) with efficacy, i.e., CHR. The analysis included 48 patients with PV, after excluding one patient due to early withdrawal from the study. To calculate the mean plasma concentrations over 24 weeks (Cavg,0-24w), the exposures were simulated based on the actual doses administered to each patient. The exposure levels categorized by quartiles are listed in Supplementary Table S1. They were then compared with exposure over 24 weeks in simulated patients in the PROUD-PV study (Gisslinger et al., 2020). Patients in A20-202 were administered Ropeg at a higher starting-dose regimen with faster dose-titrations (i.e. the 250-350–500 µg schema), while patients in PROUD-PV received the approved slow dose-titration schema as shown in Table 1.
A visualization of the exposure-efficacy relationship model is illustrated in Figure 1. Based on the model simulation, patients in A20-202 were estimated to achieve a median CHR rate of approximately 63% at Week 24. In contrast, PROUD-PV, in which Caucasian patients treated with a slow dose-titration regimen (indicated in green), was estimated to have a CHR rate of approximately 35% in the same interval. This is consistent with the clinically observed CHR rates of 61.2% at Week 24 for A20-202 and 27% in PROUD-PV, respectively (Jin et al., 2023; Gisslinger et al., 2020). The fact that the model-simulated CHR rates were similar to the clinically realistic rates indicates that there is a similar exposure-response relationship between Chinese and Caucasian populations. The median predicted CHR rate was notably higher in A20-202 than in PROUD-PV because of the higher exposure resulting from the higher starting-dose regimen with faster dose-titrations used in A20-202.
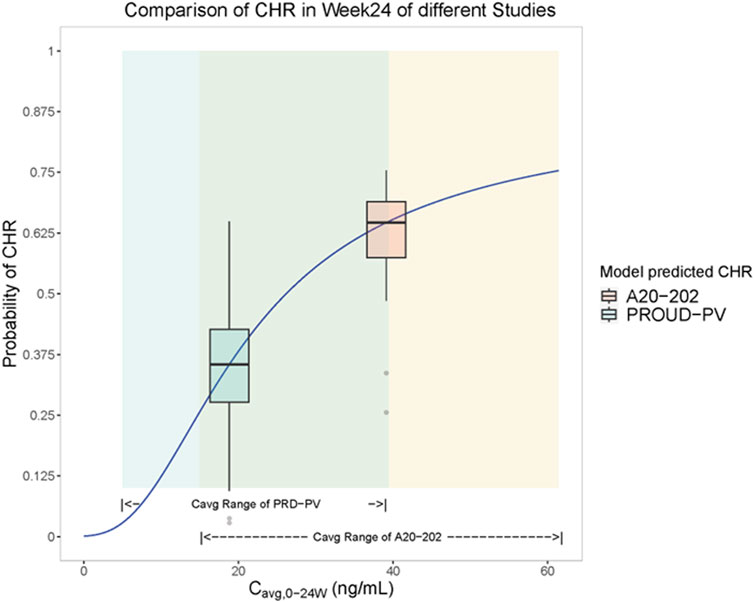
Figure 1. Visualization of the model simulation for the exposure-efficacy relationship: Cavg,0-24w - complete hematologic response (CHR) rate logistic regression model analysis (Solid blue lines are fitted to Cavg, 0-24w-CHR rate logistic regression models). Yellow shading represents exposure (Cavg, 0-24w) range over 24 weeks for subjects in A20-202 (n = 48) and green shading represents simulated exposure (Cavg, 0-24w) range over 24 weeks of PROUD-PV based on the available population typical values of PK parameters (FDA, 2020). Yellow box plots represent the predicted range of CHR rates in A20-202 study, the middle horizontal black line of the yellow box represents the median CHR rate over the exposure range in A20-202, and the vertical line of the yellow box corresponds to the abscissa of the median A20-202 trial exposure. The green box plot represents the predicted range of CHR rates in the PROUD-PV study, the middle horizontal black line of the green box represents the median CHR rate over the exposure range in PROUD-PV conducted in Europe, and the horizontal line corresponds to the median of PROUD-PV trial exposure.
JAK2V617F is a driver mutation that is present in most PV cases (Baxter et al., 2005; Tefferi et al., 2021). The reduction of JAK2V617 allele burden has emerged as an important indicator of treatment effect in PV as it appears to be associated with decreased risk of thrombosis, progression-free survival, and event-free survival (Stein et al., 2014; Harrison et al., 2023; Gisslinger et al., 2023; Moliterno et al., 2023). Ropeg treatment has been shown to reduce the JAK2V617F allele burden in patients with PV across multiple studies conducted in Europe, Japan, and China (Gisslinger et al., 2015; Gisslinger et al., 2020; Edahiro et al., 2022; Jin et al., 2023; Suo et al., 2024). Consistent with the dose-exposure-CHR data, greater reduction of the JAK2V617F allele burden was also observed in patients treated with Ropeg at the higher starting dose regimen and the burden reduction increased with increasing exposure (Jin et al., 2023; Suo et al., 2024; Qin et al., 2024). Ropeg represents a new-generation IFN-α-based therapy. IFN-α exerts its biological activities by binding to its the receptors IFNAR1 and 2 located on cell membranes. The mechanism of action of Ropeg in PV treatment is likely to be at least due to the activation of downstream tumor suppressors or their related proteins, and the inhibition of the relevant proto-oncogenes (Qin, 2024b). Therefore, the mechanism of action and effect of Ropeg on the JAK2V617F allele burden do not appear to differ with regards to ethnic variations.
3.3.2 Exposure-safety analysis in Chinese and Caucasian patients with PV
To analyze exposure-safety correlations, a logistic regression model was developed using data from the phase II clinical study A20-202 up to Week 24 in Chinese patients with PV (Qin et al., 2024). This study had exposure data categorized into different dose phases including 250 μg, 350 μg, and 500 μg, based on the actual doses administered. The average concentration of each phase (Cavg,250 μg, Cavg,350 μg, and Cavg,500 μg) was calculated. The analyzed safety indices included drug-related AEs, Grade 3 or higher treatment-related AEs, and AEs with higher incidence rates. These include increases in gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and asthenia and decreases in WBC and neutrophil counts (Qin al., 2024). No apparent correlation between the high frequency AEs and exposure to Ropeg was observed, except that increases in ALT and AST appeared to be associated with the average Ropeg exposure in the 500 µg phase. Other safety indices, including increased GGT, WBC, and neutrophil count decrease, and asthenia were not affected by the exposure levels, as shown in Supplementary Figures S1-S8. The ALT and AST levels did not increase with significant increases in bilirubin, clinical symptoms, or signs, such as jaundice (Jin et al., 2023; Suo et al., 2024). These results suggest that the safety risks associated with Ropeg treatment are acceptable.
Next, an exposure-safety analysis was performed using data from four clinical studies involving European patients with PV: PEGINVERA, PROUD-PV, CONTINUATION-PV, and PEN-PV (Table 1). This analysis included 178 patients who had been treated with a slow-dose titration regimen of Ropeg. The safety indices assessed included drug-related AEs, drug-related AEs of Grade 3 and higher, serious adverse events (SAEs), and drug-related SAEs. The analysis concluded that the exposure-safety relationships were acceptable, and the side effects were tolerated by most patients.
The exposure-safety analysis results in Chinese patients with PV align with those observed in Caucasian patient populations, indicating a similar safety profile for Ropeg in both Chinese and Caucasian individuals. These results suggest that the risk of AEs related to transaminases has increased. However, most cases were mild or moderate and did not require treatment discontinuation, reflecting an acceptable safety profile. The results indicated that Ropeg were consistently well-tolerated by the two ethnic groups.
4 Discussion
The comprehensive review and analyses in this study support the global applicability of Ropeg and highlight the effectiveness as a preferred first line cytoreductive intervention for patients with low- or high-risk PV among different ethnicities. The convergence of the diagnostic and efficacy evaluation criteria, in line with the WHO and ELN criteria, further validated the uniformity of diagnostic and response assessment practices.
Multiple PopPK analyses were conducted using data from healthy Chinese and Caucasian individuals and patient populations. The results showed that the drug concentration-time profiles in the different populations were similar, suggesting comparable PK behaviors. Ethnicity showed no significant impact on the PK parameters, confirming the absence of substantial differences in PK characteristics between the Chinese and Caucasian populations. The slight difference in the PK of Ropeg between Caucasian and Chinese subjects might be caused by differences in body weight. This is consistent with our previous data that PK exposure was observed to be higher in Japanese than in Caucasian subjects, largely due to differences in the body weight, without exerting an overt effect on pharmacodynamic parameters, safety, and tolerability (Miyachi et al., 2021). Our model simulation analysis further suggested that the Chinese and Caucasian populations exhibited a similar dose-effect relationship, and the exposure-response profiles for Ropeg were comparable, suggesting a uniform therapeutic effect irrespective of ethnic variations. Similarly, the exposure-safety analysis indicated that Ropeg is well-tolerated among different ethnicities. The increased ALT and AST levels observed in Chinese patients with PV were most likely due to the use of a higher starting-dose regimen with simpler and faster dose titrations. However, transaminase increases are reversible and do not appear to be associated with significant increases in bilirubin levels or clinical signs and symptoms (Jin et al., 2023; Suo et al., 2024). Therefore, the positive benefit-over-risk balance with Ropeg treatment in patients with PV did not appear to change with a higher starting-dose regimen as a treatment option.
The results revealed no significant ethnic variation in the PK, efficacy, or safety profiles of Ropeg, which encompassed multiple clinical trials and PK analyses across ethnic cohorts, including Chinese and Caucasian populations. These findings are pivotal considering the slight PK differences that could be attributed to variations in body weight rather than ethnicity. The exposure-efficacy and exposure-safety analyses demonstrated analogous dose-effect relationships and safety profiles across different ethnic groups. This consistency is helpful in confirming the universal applicability of Ropeg and supporting its use in diverse populations. Finally, although we did not observe overt differences in pharmacodynamic parameters, safety and tolerability due to higher PK exposure in subjects with low body weight (Miyachi et al., 2021), it is reasonable to take caution when titrating the dose, with close monitoring in patients with very low body weights to avoid unwanted side effects caused by a higher PK exposure.
Our analyses had some limitations. PV is known to be heterogeneously associated with unique gene mutations or patterns regarding JAK2V617F, JAK2 exon 12 mutations and/or others (Vannucchi et al., 2007; Patnaik and Tefferi, 2009; Passamonti et al., 2011; Kanduła et al., 2023). Variations in non-driver mutations, including ASXL1, SRSF2, and IDH2, and differential blood counts can also lead to phenotypic heterogeneity, such as the risk of thrombosis and disease progression (Landolfi et al., 2007; Gangat et al., 2007; De Stefano et al., 2010; Tefferi et al., 2016; Gerds et al., 2022). Although most of the patients in the analyzed studies were known to have JAK2V617F, there is a lack of sufficient data or thorough analyses regarding the effect of Ropeg in patients with other JAK2 mutations, or with JAK2V617F but carrying different non-driver mutations, or with other relevant gene variations. There is also a lack of integrated analyses across various clinical studies to assess the effect of Ropeg in patients with different levels of leukocytes and platelets. However, Ropeg treatment as an IFN-α-based therapy can induce the type 1 IFN signaling leading to the activation of downstream, cell cycle- and senescence-regulatory tumor suppressor gene products or related proteins, which could impose gene and epigenetic regulations to inhibit the neoplastic cells that drive PV (Qin, 2023a; Qin, 2024b). Ropeg may be efficacious in these patients, although subtle differences in PK, efficacy, and safety might be present. Our study also did not analyze the data of patients with non-PV erythrocytosis, such as those with congenital polycythemia, who have an increased risk of thrombosis due to other mutations (Gordeuk et al., 2004; Tomasic et al., 2013; Gangat et al., 2021). However, Ropeg treatment was recently shown to induce remission in a patient carrying JAK2R715T from a germline origin (Song et al., 2024), supporting the broad therapeutic effect of Ropeg. Finally, our study did not include an overall pharmacoeconomic analysis to assess the global affordability of Ropeg as a frontline treatment of PV. Ropeg was previously found to be a cost-effective treatment option for a broad range of patients with PV in the US (Gerds et al., 2023). Further analyses of pharmacoeconomics in other countries or regions will be needed to evaluate the clinical application of Ropeg in diverse patient populations.
5 Conclusion
Our results support the use of Ropeg as an effective and tolerable first-line treatment for PV regardless of ethnic variations. The findings have implications for the clinical management of PV in patients of various ethnic backgrounds receiving Ropeg treatment. Future research may extend to explore any differences in treatment responses or side effects in patients with other JAK2 or non-JAK2 driver mutations besides JAK2V617F, differential non-driver mutations, or other relevant genetic variations to enhance personalized medical approaches in the PV treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The study involving patients in China was approved by The First Affiliated Hospital, Zhejiang University School of Medicine; Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College; The Second Hospital of Tianjin Medical University; The First Affiliated Hospital of Soochow University; Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College; Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital; Nanfang Hospital of Southern Medical University; Ruijin Hospital, Shanghai Jiaotong University School of Medicine; Zhongnan Hospital, Wuhan University; Shenzhen Second People’s Hospital; The First Affiliated Hospital of Chongqing Medical University; Huashan Hospital of Fudan University; The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China. Additionally, other studies were approved as indicated in the corresponding references. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent.
Author contributions
AQ: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. DW: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–original draft. JL: Conceptualization, Formal Analysis, Methodology, Writing–review and editing. SX: Formal Analysis, Methodology, Writing–review and editing. HC: Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. YG: Formal Analysis, Investigation, Methodology, Writing–original draft. JC: Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. XS: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. NM: Data curation, Formal Analysis, Investigation, Methodology, Writing–review and editing. TS: Data curation, Investigation, Methodology, Writing–review and editing. YL: Data curation, Formal Analysis, Methodology, Project administration, Writing–review and editing. JZ: Project administration, Writing–review and editing, Data curation, Formal Analysis, Methodology. WS: Data curation, Methodology, Project administration, Writing–review and editing. WW: Data curation, Investigation, Methodology, Project administration, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by PharmaEssentia Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, and the writing of this article.
Acknowledgments
We would like to thank our colleagues at PharmaEssentia and Pharmaron Clinical Services for their discussion and support. We are grateful to patients and their families, and all the investigators, study nurses, and other participants in the clinical studies analyzed in this report. English editing is provided by Editage.
Conflict of interest
Authors AQ, JL, are employed by PharmaEssentia Corporation. Authors DW, YL, JZ, WS, WW are employed by PharmaEssentia Biotech (Beijing) Limited. Authors SX, HC, YG, JC, XS are employees of Pharmaron Clinical Services Co., Ltd. Authors NM, TS are employed by PharmaEssentia Japan KK.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1455979/full#supplementary-material
References
Abu-Zeinah, G. S., Krichevsky, T., Cruz, G., Hoberman, D., Jaber, N., Savage, N., et al. (2021). Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia 35 (9), 2592–2601. doi:10.1038/s41375-021-01183-8
Aruch, D., and Mascarenhas, J. (2016). Contemporary approach to essential thrombocythemia and polycythemia vera. Curr. Opin. Hematol. 23 (2), 150–160. doi:10.1097/MOH.0000000000000216
Barbui, T., Thiele, J., Gisslinger, H., Kvasnicka, H. M., Vannucchi, A. M., Guglielmelli, P., et al. (2018). The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 8 (2), 15. doi:10.1038/s41408-018-0054-y
Barbui, T., Vannucchi, A. M., De Stefano, V., Carobbio, A., Ghirardi, A., Carioli, G., et al. (2023). Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid. 2 (6), EVIDoa2200335. doi:10.1056/EVIDoa2200335
Barosi, G., Mesa, R., Finazzi, G., Harrison, C., Kiladjian, J. J., Lengfelder, E., et al. (2013). Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 121 (23), 4778–4781. doi:10.1182/blood-2013-01-478891
Baxter, E. J., Scott, L. M., Campbell, P. J., East, C., Fourouclas, N., Swanton, S., et al. (2005). Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet , 365(9464), 1054–1061.
Benevolo, G., Marchetti, M., Melchio, R., Beggiato, E., Sartori, C., Biolé, C. A., et al. (2023). Diagnosis and management of cardiovascular risk in patients with polycythemia vera. Vasc. Health Risk Manag. 19, 765–778. doi:10.2147/VHRM.S429995
Bernal, G., and Domenech Rodríguez, M. M. (2013). Tailoring treatment to the patient’s race and ethnicity. Psychologists' Desk Ref. 64, 310–313. doi:10.1093/med:psych/9780199845491.003.0064
Bose, P., and Verstovsek, S. (2019). Updates in the management of polycythemia vera and essential thrombocythemia. Ther. Adv. Hematol. 10, 2040620719870052–13. doi:10.1177/2040620719870052
Brown, J. L., Barsoum, J., and Qin, X. Q. (2002). CD4+ T helper cell-independent antitumor response mediated by murine IFN-beta gene delivery in immunocompetent mice. J. Interferon cytokine Res. 22, 719–728. doi:10.1089/10799900260100222
Burgstaller, S., Buxhofer-Ausch, V., Sliwa, T., Beham-Schmid, C., Gastl, G., Geissler, K., et al. (2018). Austrian recommendations for the management of polycythemia vera. Wien. Klin. Wochenschr. 130, 535–542. doi:10.1007/s00508-018-1359-3
Cerquozzi, S., and Tefferi, A. (2015). Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 5 (11), e366. doi:10.1038/bcj.2015.95
Chen, E., and Mullally, A. (2014). How does JAK2V617F contribute to the pathogenesis of myeloproliferative neoplasms? Hematology 1, 268–276. doi:10.1182/asheducation-2014.1.268
De Stefano, V., Za, T., Rossi, E., Vannucchi, A. M., Ruggeri, M., Elli, E., et al. (2010). Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am. J. Hematol. 85 (2), 97–100. doi:10.1002/AJH.21593
Edahiro, Y., Ohishi, K., Gotoh, A., Takenaka, K., Shibayama, H., Shimizu, T., et al. (2022). Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. Int. J. Hematol. 116 (2), 215–227. doi:10.1007/s12185-022-03341-9
EMA (2018). European medicines agency. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/besremi (Accessed May 15, 2024).
FDA (2019). United States Food and drug administration. FDA news release: FDA approves treatment for rare blood disease. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-rare-blood-disease (Accessed December 21, 2021).
FDA (2020). Center for drug evaluation and research. Application number:761166orig1s000, clinical. Pharmacol. Rev.
Finazzi, G., and Barbui, T. (2008). Evidence and expertise in the management of polycythemia vera and essential thrombocythemia. Leukemia 22 (8), 1494–1502. doi:10.1038/leu.2008.177
Gangat, N., Strand, J., Li, C. Y., Wu, W., Pardanani, A., and Tefferi, A. (2007). Leucocytosis in polycythaemia vera predicts both inferior survival and leukaemic transformation. Br. J. Haematol. 138 (3), 354–358. doi:10.1111/J.1365-2141.2007.06674.X
Gangat, N., Szuber, N., Pardanani, A., and Tefferi, A. (2021). JAK2 unmutated erythrocytosis: current diagnostic approach and therapeutic views. Leukemia 35 (8), 2166–2181. doi:10.1038/s41375-021-01290-6
Gerds, A. T., Castro, C., Snopek, F., Flynn, M. M., Ellis, A. G., Manning, M., et al. (2023). Cost-effectiveness of ropeginterferon alfa-2b-njft for the treatment of polycythemia vera. J. Comp. Eff. Res. 12 (9), e230066. doi:10.57264/cer-2023-0066
Gerds, A. T., Mesa, R., Burke, J. M., Grunwald, M. R., Scherber, R., Yu, J., et al. (2022). P1062: a real-world evaluation of the association between elevated blood counts and thrombotic events in polycythemia vera: an analysis of data from the REVEAL study. Hemasphere 6 (Suppl. l), 952–953. doi:10.1097/01.HS9.0000847116.74895.d8
Gisslinger, H., Klade, C., Georgiev, P., Krochmalczyk, D., Gercheva-Kyuchukova, L., Egyed, M., et al. (2020). Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 7 (3), e196–208. doi:10.1016/S2352-3026(19)30236-4
Gisslinger, H., Klade, C., Georgiev, P., Krochmalczyk, D., Gercheva-Kyuchukova, L., Egyed, M., et al. (2023). Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia 37, 2129–2132. doi:10.1038/s41375-023-02008-6
Gisslinger, H., Zagrijtschuk, O., Buxhofer-Ausch, V., Thaler, J., Schloegl, E., Gastl, G. A., et al. (2015). Ropeginterferon alfa-2b, a novel IFNα-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 126 (15), 1762–1769. doi:10.1182/blood-2015-04-637280
Gordeuk, V. R., Sergueeva, A. I., Miasnikova, G. Y., Okhotin, D., Voloshin, Y., Choyke, P. L., et al. (2004). Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103 (10), 3924–3932. doi:10.1182/blood-2003-07-2535
Griesshammer, M., Kiladjian, J. J., and Besses, C. (2019). Thromboembolic events in polycythemia vera. Ann. Hematol. 98, 1071–1082. doi:10.1007/s00277-019-03625-x
Guglielmelli, P., and Vannucchi, A. M. (2020). Current management strategies for polycythemia vera and essential thrombocythemia. Blood Rev. 42, 100714. doi:10.1016/j.blre.2020.100714
Harrison, C. N., Nangalia, J., Boucher, R., Jackson, A., Yap, C., O'Sullivan, J., et al. (2023). Ruxolitinib versus best available therapy for polycythemia vera intolerant or resistant to hydroxycarbamide in a randomized trial. J. Clin. Oncol. 41, 3534–3544. doi:10.1200/JCO.22.01935
Hong, J. (2023). Prognostication in myeloproliferative neoplasms, including mutational abnormalities. Blood Res. 58 (S1), S37–S45. doi:10.5045/br.2023.2023038
Huang, S. M., and Temple, R. (2008). Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin. Pharmacol. Ther. 84 (3), 287–294. doi:10.1038/clpt.2008.144
Huang, Y. W., Qin, A., Fang, J., Wang, T. F., Tsai, C. W., Lin, K. C., et al. (2022). Novel long-acting ropeginterferon alfa-2b: pharmacokinetics, pharmacodynamics and safety in a phase I clinical trial. Br. J. Clin. Pharmacol. 88 (5), 2396–2407. doi:10.1111/bcp.15176
Huang, Y. W., Tsai, C. Y., Tsai, C. W., Wang, W., Zhang, J., Qin, A., et al. (2021). Pharmacokinetics and pharmacodynamics of novel long-acting ropeginterferon alfa-2b in healthy Chinese subjects. Adv. Ther. 38 (9), 4756–4770. doi:10.1007/s12325-021-01863-y
ICH (1998). Harmonised tripartite guideline e5(r1) ethnic factors in the acceptability of foreign clinical data. Available at: https://database.ich.org/sites/default/files/E5R1Guideline.pdf.1998 (Accessed June 09, 2024).
Iurlo, A., Cattaneo, D., Bucelli, C., and Baldini, L. (2020). New perspectives on polycythemia vera: from diagnosis to therapy. Int. J. Mol. Sci. 21 (16), 5805. doi:10.3390/ijms21165805
James, C., Ugo, V., Le Couédic, J. P., Staerk, J., Delhommeau, F., Lacout, C., et al. (2005). A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148. doi:10.1038/nature03546
Jin, J., Qin, A., Zhang, L., Shen, W., Wang, W., Zhang, J., et al. (2023). A phase II trial to assess the efficacy and safety of ropeginterferon α-2b in Chinese patients with polycythemia vera. Future Oncol. 19 (11), 753–761. doi:10.2217/FON-2022-1141
Jin, J., Zhang, L., Qin, A., Wu, D., Shao, Z., Bai, J., et al. (2023). A new dosing regimen of ropeginterferon alfa-2b is highly effective and tolerable: findings from a phase 2 study in Chinese patients with polycythemia vera. Exp. Hematol. Oncol. 12 (1), 55. doi:10.1186/s40164-023-00415-0
Kanduła, Z., Janowski, M., Więckowska, B., Paczkowska, E., and Lewandowski, K. (2023). JAK2V617F variant allele frequency, non-driver mutations, single-nucleotide variants and polycythemia vera outcome. J. Cancer Res. Clin. Oncol. 149 (8), 4789–4803. doi:10.1007/s00432-022-04327-0
Kaynor, C., Xin, M., Wakefield, J., Barsoum, J., and Qin, X. Q. (2002). Direct evidence that IFN-beta functions as a tumor-suppressor protein. J. Interf. cytokine Res. 22 (11), 1089–1098. doi:10.1089/10799900260442511
Kiladjian, J. J., Klade, C., Georgiev, P., Krochmalczyk, D., Gercheva-Kyuchukova, L., Egyed, M., et al. (2022). Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia 36 (5), 1408–1411. doi:10.1038/s41375-022-01528-x
Kralovics, R., Passamonti, F., Buser, A. S., Teo, S.-S., Tiedt, R., Passweg, J. R., et al. (2005). A gain-of-function mutation of JAK2 in myeloproliferative disorder. N. Engl. J. Med. 352, 1779–1790. doi:10.1056/NEJMoa051113
Landolfi, R., Di Gennaro, L., Barbui, T., De Stefano, V., Finazzi, G., Marfisi, R., et al. (2007). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109 (6), 2446–52. doi:10.1182/blood-2006-08-042515
Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association (2022). Chinese guideline for the diagnosis and treatment of polycythemia vera. Zhonghua Xue Ye Xue Za Zhi 43 (7), 537–541. doi:10.3760/cma.j.issn.0253-2727.2022.07.002
Levine, R. L., Wadleigh, M., Cools, J., Ebert, B. L., Wernig, G., Huntly, B. J. P., et al. (2005). Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell 7 (4), 387–397. doi:10.1016/j.ccr.2005.03.023
Li, S., Kralovics, R., De Libero, G., Theocharides, A., Gisslinger, H., and Skoda, R. C. (2008). Clonal heterogeneity in polycythemia vera patients with JAK2 exon12 and JAK2-V617F mutations. Blood 111 (7), 3863–3866. doi:10.1182/blood-2007-09-111971
Marchioli, R., Finazzi, G., Specchia, G., Cacciola, R., Cavazzina, R., Cilloni, D., et al. (2013). Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl. J. Med. 368 (1), 22–33. doi:10.1056/NEJMoa1208500
Masarova, L., Yin, C. C., Cortes, J. E., Konopleva, M., Borthakur, G., Newberry, K. J., et al. (2017). Histomorphological responses after therapy with pegylated interferon α-2a in patients with essential thrombocythemia (ET) and polycythemia vera (PV). Exp. Hematol. Oncol. 6, 30. doi:10.1186/s40164-017-0090-5
Mesa, R., Boccia, R. V., Grunwald, M. R., Oh, S. T., Colucci, P., Paranagama, D., et al. (2018). Patient-reported outcomes data from REVEAL at the time of enrollment (baseline): a prospective observational study of patients with polycythemia vera in the United States. Clin. Lymphoma Myeloma Leuk. 18 (9), 590–596. doi:10.1016/j.clml.2018.05.020
Mesa, R., Palmer, J., Eckert, R., and Huberty, J. (2021). Quality of life in myeloproliferative neoplasms: symptoms and management implications. Hematol. Oncol. Clin. North Am. 35 (2), 375–390. doi:10.1016/j.hoc.2020.12.006
Miyachi, N., Zagrijtschuk, O., Kang, L., Yonezu, K., and Qin, A. (2021). Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin. Drug Investig. 41 (4), 391–404. doi:10.1007/s40261-021-01026-5
Moliterno, A. R., Kaizer, H., and Reeves, B. N. (2023). JAK2V617F allele burden in polycythemia vera: burden of proof. Blood 141, 1934–1942. doi:10.1182/blood.2022017697
NCCN. National Comprehensive Cancer Network. (2024). NCCN clinical practice guidelines in oncology: myeloproliferative neoplasms. Accessed 29May2024.
Palumbo, G. A., Breccia, M., Baratè, C., Bonifacio, M., Elli, E. M., Iurlo, A., et al. (2023). Management of polycythemia vera: a survey of treatment patterns in Italy. Eur. J. Haematol. 110 (2), 161–167. doi:10.1111/ejh.13889
Passamonti, F., Elena, C., Schnittger, S., Skoda, R. C., Green, A. R., Girodon, F., et al. (2011). Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 117 (10), 2813–2816. doi:10.1182/BLOOD-2010-11-316810
Patnaik, M. M., and Tefferi, A. (2009). The complete evaluation of erythrocytosis: congenital and acquired. Leukemia 23 (5), 834–44. doi:10.1038/leu.2009.54
Qin, A. (2023a). An anti-cancer surveillance by the interplay between interferon-beta and retinoblastoma protein RB1. Front. Oncol. 13, 1173467. doi:10.3389/fonc.2023.1173467
Qin, A. (2023b). Letter to the editor: a favorable benefit-risk balance maybe expected with replication-defective adenovirus-mediated interferon gene therapy for cancer treatment. Hum. Gene Ther. 34 (7-8), 339–340. doi:10.1089/hum.2023.028
Qin, A. (2024a). A plain language summary about a cell cycle-based, new surveillance mechanism against cancer. Future Oncol. in press. doi:10.1080/14796694.2024.2402649
Qin, A. (2024b). Mechanism of action of ropeginterferon alfa-2b in polycythemia vera treatment. Clin. Thera. 46, 439–440. doi:10.1016/j.clinthera.2024.03.005
Qin, A., Wu, D., Li, Y., Zhang, J., Wang, W., Shen, W., et al. (2024). Exposure-efficacy and exposure-safety analyses of ropeginterferon alfa-2b treatment in patients with polycythaemia vera. Br. J. Clin. Pharmacol. 90 (6), 1493–1502. doi:10.1111/bcp.16043
Qin, X. Q., Beckham, C., Brown, J. L., Lukashev, M., and Barsoum, J. (2001). Human and mouse IFN-beta gene therapy exhibits different anti-tumor mechanisms in mouse models. Mol. Ther. 4 (4), 356–364. doi:10.1006/mthe.2001.0464
Qin, X. Q., Runkel, L., Deck, C., DeDios, C., and Barsoum, J. (1997). Interferon-beta induces S phase accumulation selectively in human transformed cells. J. Interf. Cytokine Res. 17, 355–367. doi:10.1089/jir.1997.17.355
Qin, X. Q., Tao, N., Dergay, A., Moy, P., Fawell, S., Davis, A., et al. (1998). Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc. Natl. Acad. Sci. USA. 95 (24), 14411–6. doi:10.1073/pnas.95.24.14411
Song, J., Lanikova, L., Kim, S. J., Papadopoulos, N., Meznarich, J., Constantinescu, S. N., et al. (2024). Novel germline JAK2R715T mutation causing PV-like erythrocytosis in 3 generations. Amelioration by Ropeg-Interferon. Amer. J. Hematol. 99 (7), 1220–1229. doi:10.1002/ajh.27311
Spivak, J. L., Considine, M., Williams, D. M., Talbot, Jr. C. C., Rogers, O., Moliterno, A. R., et al. (2014). Two clinical phenotypes in polycythemia vera. N. Engl. J. Med. 371, 808–817. d I. doi:10.1056/NEJMoa1403141
Stein, B. L., Moliterno, A. R., and Tiu, R. V. (2014). Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann. Hematol. 93 (12), 1965–76. doi:10.1007/s00277-014-2205-y
Suo, S. S., Fu, R. F., Qin, A., Shao, Z. H., Bai, J., Chen, S. N., et al. (2024). Effective management of polycythemia vera with ropeginterferon alfa-2b treatment. J. Hematol. 13 (1-2), 12–22. doi:10.14740/jh1245
Tashi, T., Swierczek, S., Kim, S. J., Salama, M. E., Song, J., Heikal, N., et al. (2018). Pegylated interferon Alfa-2a and hydroxyurea in polycythemia vera and essential thrombocythemia: differential cellular and molecular responses. Leukemia 32 (8), 1830–1833. doi:10.1038/s41375-018-0080-6
Tefferi, A., and Barbui, T. (2020). Polycythemia vera and essential thrombocythemia:: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 95 (12), 1599–1613. doi:10.1002/ajh.26008
Tefferi, A., and Barbui, T. (2023). Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 98 (9), 1465–1487. doi:10.1002/ajh.27002
Tefferi, A., Vannucchi, A. M., and Barbui, T. (2021). Polycythemia vera: historical oversights, diagnostic details, and therapeutic views. Leukemia 35, 3339–3351. doi:10.1038/s41375-021-01401-3
Tefferi, A., Lasho, T. L., Guglielmelli, P., Finke, C. M., Rotunno, G., Elala, Y., et al. (2016). Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 1 (1), 21–30. doi:10.1182/bloodadvances.2016000216
Tomasic, N. L., Piterkova, L., Huff, C., Bilic, E., Yoon, D., Miasnikova, G. Y., et al. (2013). The phenotype of polycythemia due to Croatian homozygous VHL (571C>G:H191D) mutation is different from that of Chuvash polycythemia (VHL 598C>T:R200W). Haematologica 98 (4), 560–567. doi:10.3324/haematol.2012.070508
Vannucchi, A. M. (2017). From leeches to personalized medicine: evolving concepts in the management of polycythemia vera. Haematologica 102 (1), 18–29. doi:10.3324/haematol.2015.129155
Vannucchi, A. M., Antonioli, E., Guglielmelli, P., Rambaldi, A., Barosi, G., Marchioli, R., et al. (2007). Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 110 (3), 840–846. doi:10.1182/blood-2006-12-064287
Wittling, M. C., Cahalan, S. R., Levenson, E. A., and Rabin, R. L. (2021). Shared and unique features of human interferon-beta and interferon-alpha subtypes. Front. Immunol. 11, 605673. doi:10.3389/fimmu.2020.605673
Yasuda, S. U., Zhang, L., and Huang, S. M. (2008). The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin. Pharmacol. Ther. 84 (3), 417–23. doi:10.1038/clpt.2008.141
Zhu, M., Wang, M. X., Li, Z. R., Wang, W., Su, X., and Jiao, Z. (2021). Population pharmacokinetics of ropeginterferon alfa-2b: a comparison between healthy Caucasian and Chinese subjects. Front. Pharmacol. 12, 673492. doi:10.3389/fphar.2021.673492
Keywords: ropeginterferon alfa-2b, ethnic sensitivity analyses, polycythemia vera, pharmacokinetics, safety
Citation: Qin A, Wu D, Liao J, Xie S, Chen H, Gao Y, Cui J, Su X, Miyachi N, Sato T, Li Y, Zhang J, Shen W and Wang W (2024) Ethnic sensitivity analyses of pharmacokinetics, efficacy and safety in polycythemia vera treatment with ropeginterferon alfa-2b. Front. Pharmacol. 15:1455979. doi: 10.3389/fphar.2024.1455979
Received: 27 June 2024; Accepted: 27 August 2024;
Published: 24 September 2024.
Edited by:
Jianxiang Wang, Institute of Hematology, ChinaReviewed by:
Richard T. Silver, Weill Cornell Medical Center, United StatesCristian Sandoval, Santo Tomás University, Chile
Copyright © 2024 Qin, Wu, Liao, Xie, Chen, Gao, Cui, Su, Miyachi, Sato, Li, Zhang, Shen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Albert Qin, YWxiZXJ0X3FpbkBwaGFybWFlc3NlbnRpYS5jb20=
 Albert Qin
Albert Qin Daoxiang Wu2
Daoxiang Wu2 Haoqi Chen
Haoqi Chen Yucheng Gao
Yucheng Gao Narihisa Miyachi
Narihisa Miyachi Wei Wang
Wei Wang