- 1School of Pharmaceutical Sciences, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 2School of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, China
- 3The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Research Center for Clinical Application of Chinese Medicine Classics, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 6Department of Nephrology, The Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Background: Diabetic kidney disease (DKD) is a common pathway to End-stage renal disease (ESRD). Podocytes are crucial due to their dual barrier functions in kidney diseases. Their role in renal fibrosis and DKD regulatory mechanisms is increasingly studied. However, bibliometric research in this field has not been explored.
Methods: 1,250 publications from Jan. 1, 2000, to Feb. 16, 2024, were retrieved from the WoSCC database and analyzed by the Web of Science results analysis tool, VOSviewer, and CiteSpace.
Results: Our scrutiny reveals that authors Liu Youhua, Fogo Agnes B, and Zhao Yingyong have made substantial contributions to this domain. Notably, “Kidney International” has the highest volume of publications in this area. Furthermore, our analysis identifies ten co-citation clusters: DKD, IncRNA, reactive oxygen species, glomerulosclerosis, Poria cocos, glomerular diseases, fibroblasts, connective tissue growth factor, coagulation, and Wnt. Recent research accentuates keywords such as autophagy, TRPC6, ERS, epigenetics, and NLRP3 inflammasome as frequently occurring terms in this field. The prevailing research hotspot keywords include autophagy, biomarker, and exosomes.
Conclusion: Through the utilization of bibliometric tools and knowledge graph analysis, we have undertaken a comprehensive review of the intricate nexus between podocytes in DKD and renal fibrosis. This study imparts valuable insights to scholars regarding the dynamic evolution of this association and delineates prospective research avenues in this pivotal realm.
Highlights
• This is the first bibliometric analysis of 1,250 articles on podocytes, DKD, and renal fibrosis (2000–2024).
• TCM active compound Poricoic acid A (from Poria cocos) can reduce blood glucose and relieve DKD.
• Current hotspots: autophagy, exosomes, AMPK, Wnt/β-Catenin, MALAT1, biomarkers, epigenetics, and epigenomics.
• The research on podocytes, the progression of DKD, and renal fibrosis has seen an explosion in the past decade.
• Holistic integrated medicine (HIM) is a prospective DKD and Renal fibrosis direction.
1 Introduction
Globally, Diabetic kidney disease (DKD) (Thomas et al., 2015; KDOQI, 2007) stands as the primary cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD), accounting for 50% of all cases (Mohandes et al., 2023; Pereira et al., 2022). DKD is characterized by a gradual and progressive decline in kidney function (Calle and Hotter, 2020), culminating in renal fibrosis and eventual organ failure (Ketteler et al., 2018). Renal fibrosis is a common pathological consequence of DKD (Humphreys, 2018). In the glomerulus, capillary lumens are generally observed to be extended, the basement membrane is thickened, the extracellular matrix (ECM) is expanded, podocyte injury is present, and fibrosis is detected. Podocytes are highly specialized epithelial cells, also known as “octopus-like” highly specialized cells in the glomerulus. As part of the kidney filter (Liu et al., 2022; Rosenberg and Kopp, 2017), there is a key component of the glomerular filtration barrier which plays an indispensable role in maintaining the structure and function of the glomerulus. These cells are uniquely positioned at the outer layer of the glomerular capillaries which are critical in preventing the leakage of proteins into the urine. Podocytes extend into the basement membrane via primary, secondary, and tertiary foot processes, maintaining structural stability through an actin cytoskeleton. The approximately ∼200-nanometer-wide space between adjacent podocytes is bridged by the slit diaphragm, a structure that not only acts as an ∼60 kDa size-selective filter for both molecular size and electric charge. To prevent the passage of large molecules but also exhibits charge selectivity, tending to repel negatively charged macromolecules such as plasma proteins, thus ensuring the effectiveness of a dual size and charge-selective filtration mechanism. In cases of disease or injury, podocytes may fail to effectively compensate for physiological stresses such as circumferential stress and shear forces, leading to podocyte loss or increased mechanical load on the remaining podocytes. This can result in ECM deposition and further podocyte loss, accelerating the progression of kidney disease. Podocytes not only maintain the integrity of the glomerular filtration barrier but also play a crucial role in the regulatory mechanisms of renal fibrosis and DKD (Kopp et al., 2020).
Emerging evidence suggests that podocyte dysfunction and loss contribute significantly to the development and progression of renal fibrosis in DKD (Rosenberg and Kopp, 2017). Among the intricate interplay of various cellular and molecular mechanisms underlying DKD pathogenesis, the role of podocytes and their involvement in renal fibrosis has garnered substantial attention in recent years (Jiang et al., 2022). Podocytopathies (Rosenberg and Kopp, 2017; Wiggins, 2007) are kidney diseases that cause direct or indirect injury to the podocytes. Podocytopathies can lead to a decrease in the number of renal units and increase the risk of CKD (Drawz and Rahman, 2015). New evidence shows that podocyte dysfunction and loss have a significant impact on the occurrence and progress of renal fibrosis in DKD (Roccatello et al., 2023). Their injuries resulted in CKD and high risk (Johansen et al., 2023; System USRD, 2022). This condition has a global impact, with an estimated burden of 10% or greater (GBD Chronic Kidney Disease Collaboration, 2020; Murray and Lopez, 2013). While therapeutic interventions can significantly delay the progression of CKD, the long-term outlook remains guarded (Levin et al., 2017; Lv and Zhang, 2019). In academic inquiry, bibliometrics emerges as an indispensable analytical tool, wielding mathematical and statistical techniques to systematically scrutinize the corpus of scholarly literature and various forms of media.
This approach serves as a linchpin in the qualitative and quantitative evaluation of academic domains, encompassing examinations of countries or regions, academic institutions, authors, co-cited authors, journals, references, and keywords. Furthermore, bibliometrics demonstrates its capability to delineate and forecast pivotal research themes and emergent trends within specific disciplinary contexts (Zhang et al., 2023).
Despite the substantial knowledge accumulated in renal fibrosis associated with DKD, the intricate molecular mechanisms underlying podocyte injury and their implications within the context of renal fibrosis remain elusive. In the present study, we utilize bibliometric methodologies to illuminate the landscape of podocyte-related research within the domain of renal fibrosis in kidney disease, with a particular emphasis on the trends in this progressing field of inquiry.
2 Methods
2.1 Data acquisition and search strategy
Web of Science, renowned for its status as a preeminent and all-encompassing database platform, stands as a paragon of authority and academic excellence, housing an extensive array of scholarly journals. It currently reigns as the most frequently employed database for the purpose of bibliometric analysis (Zhang et al., 2023; Zhang et al., 2022). Consequently, we leveraged the Web of Science Core Collection (WoSCC) as the primary reservoir of data for our research endeavor.
In our quest for meticulousness and precision in data retrieval, we opted for the SCI-Expanded citation index. To ensure the inclusiveness and fidelity of our dataset, we executed a search query encompassing the keywords: “[(TS = (renal fibrosis)) OR TS = (kidney fibrosis)] AND TS = (podocyte*) AND Document types = (Article OR Review Article OR Early Access) AND Language = (English)”. This search was conducted over a defined temporal scope spanning from 1 January 2000 to 16 February 2024.
Moreover, it is imperative to note that all pertinent bibliographic information, publication year, title, authors, countries, institutional affiliations, abstracts, keywords, and publishing journals, was exported as a plain text file from the WoSCC database.
2.2 Bibliometric study and visual representation
Bibliometrics, constituting an autonomous scholarly discipline, furnishes quantitative methodologies essential for thoroughly scrutinizing and investigating extant literature within a specific academic domain (Zhang et al., 2023; Meng et al., 2022). Through this analytical process, a wealth of granular information encompassing authorship, keywords, journal outlets, geographical origins, institutional affiliations, references, and the like can be meticulously gleaned.
The application of visualization techniques serves as a potent tool in elucidating the inherent interrelationships among this multifaceted information landscape. This encompasses revelations such as distinct authors converging on a common research trajectory, divergent research emphases emanating from disparate institutions, novel theoretical paradigms emerging within established academic bastions, and more.
We conducted a comprehensive analysis of the articles from the perspectives of publication trends, citations, countries/regions, institutions, authors, journals, references, keywords, cooperative relationships, etc., using VOSviewer (Song et al., 2022) and CiteSpace (Sabe et al., 2022) software.
Furthermore, within this analysis, Taiwan has been categorized under the rubric of the People’s Republic of China; similarly, England, Scotland, North Ireland, and Wales have been collectively classified under the aegis of the United Kingdom.
3 Results
3.1 Global publication and citation trends
In the time spanning from 2000 to 2024, our study meticulously gathered a corpus of 1,250 scholarly articles from the esteemed WoSCC. This dataset encompassed 951 research articles, 266 reviews, and 18 early-access articles, all uniformly published in English. Notably, these 1,250 contributions, citing 29,902 articles pivotal to our investigation, bore the intellectual imprint of 6,342 distinct authors from 1,462 academic institutions distributed across 62 countries/regions, emanating from 366 journals.
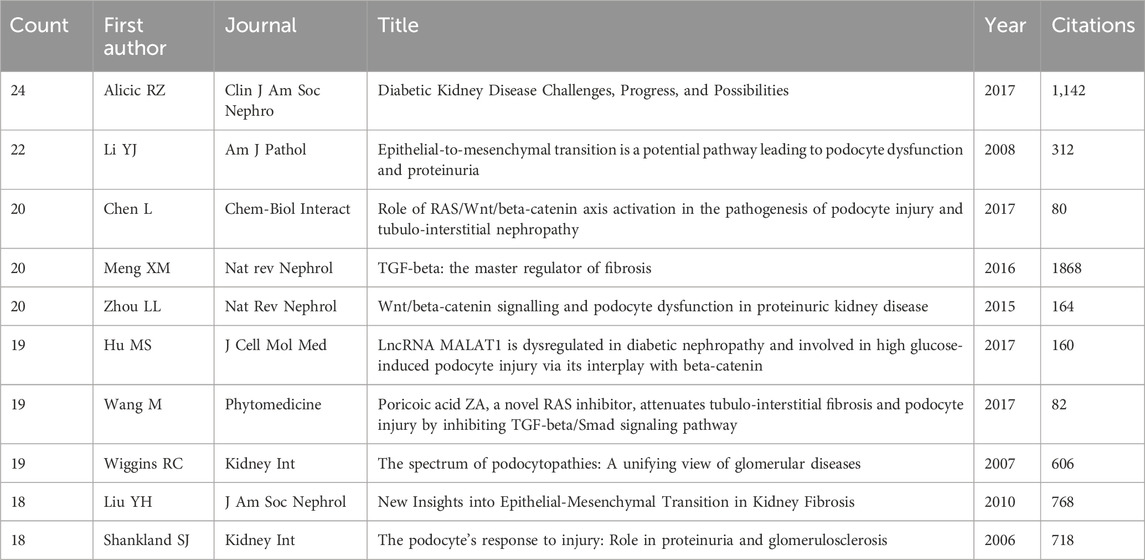
Noteworthy is the trajectory of research interest for the past 24 years (Jan. 1, 2000 to Feb. 16, 2024), as delineated in Figure 1A. The examination of podocyte injury’s role in precipitating renal fibrosis has witnessed a sustained and remarkable ascent in scholarly output, indicative of the burgeoning importance of this field.
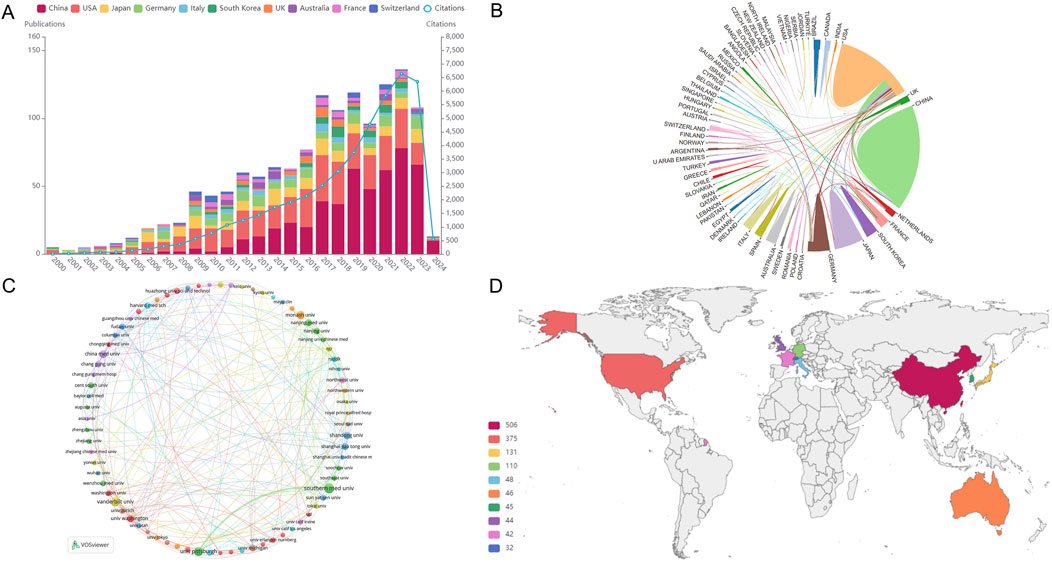
Figure 1. Analysis of publications, countries/regions and institutions. (A) Visually encapsulates the annual evolution and cumulative publication trends pertinent to this domain, spotlighting the top 10 countries at the forefront of this research endeavor. (B) Collaborative academic endeavors among countries/regions. (C) Institutional collaborative network via VOSviewer. (D) Global publication landscape.
Further, these scholarly contributions boasted an average citation rate of 36.03 per paper, amassing an impressive aggregate of 43,852 citations.
3.2 Analysis of countries/regions and institutions
In consonance with the global distribution of research contributions, our investigation, as delineated in Figure 1D, unveils a tapestry of scholarly engagement emanating from 62 countries and regions. The comprehensive insights into the quantity of publications and their corresponding citation metrics for each country/region are meticulously cataloged in Table 1. Notably, China (506), the United States (375), Japan (131), Germany (110), Italy (48), Australia (46), Korea (45), United Kingdom (44), France (42), and Switzerland (32) emerge as the foremost contributors to this discourse. China has the highest number of publications, as exemplified in Table 1.
For a vivid portrayal of collaborative dynamics among nations, Figure 1B presents an interactive collaboration map, wherein the thickness of segments signifies the frequency of intercountry cooperation.
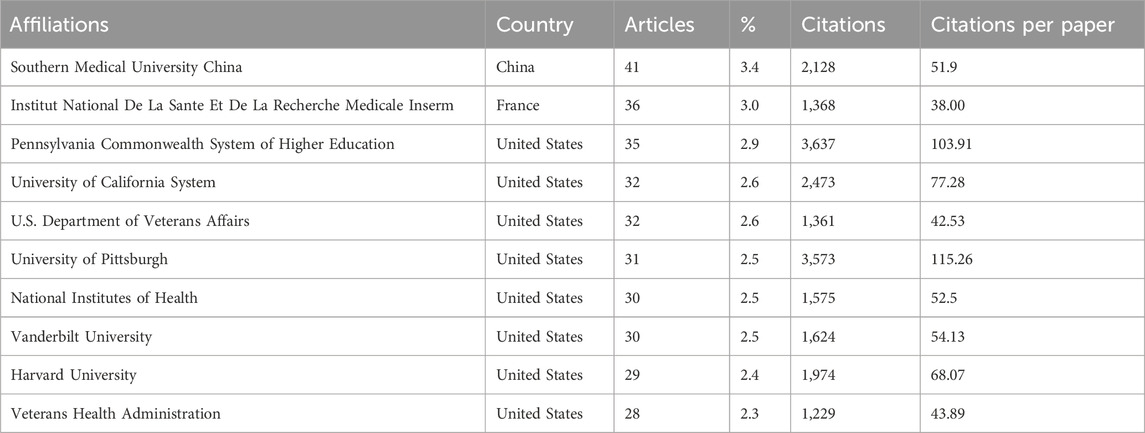
A confluence of scholarly endeavors encompassing 1,250 papers emanates from a diverse tapestry of 1,462 distinct institutions. Table 2 meticulously outlines the preeminent institutions spearheading this research with an intriguing observation. The top 10 institutions are predominantly located in the United States, China, and France, a trend that mirrors the country-level distribution of research output.
Within institutional cooperation, as visualized in Figure 1C using VOSviewer, a more pronounced network of collaboration is discernible among United States institutions, particularly gravitating around the University of Washington, compared to Chinese institutions.
In delving into the citation analysis of institutions, Table 2 unfurls the top three institutions commanding the highest citation counts: the Pennsylvania Commonwealth System of Higher Education (3,637 citations), the University of Pittsburgh (3,573 citations), and the University of California System (2,473 citations). It is also worth noting that the research emanating from the University of Pittsburgh wielded a particularly robust impact within this scholarly discourse.
Furthermore, the University of Pittsburgh has made noteworthy contributions to renal fibrosis research, boasting the highest average citation count per paper at 115.26. The Chinese research institution Southern Medical University has the highest volume of publications in this domain, amassing a total of 41.
3.3 Analysis of authors and co-cited authorships
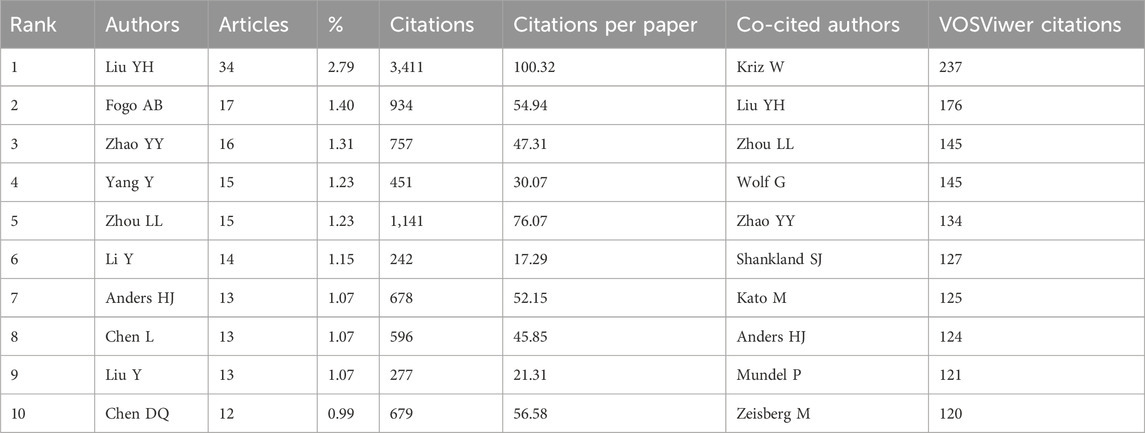
Within the ambit of our investigation, a cadre of 6,014 authors actively explored this subject matter. Table 3 provides a concise enumeration of the top 10 most prolific authors in this domain, bearing testimony to the profound contributions of these scholarly luminaries. Notably, seven authors (Liu Youhua, Zhao Yingyong, Yang Yang, Zhou Lili, Li Yan, Chen Lin, and Chen Danqian) are from China, underscoring the nation’s substantive scholarly presence in this field.
Liu Youhua, Fogo Agnes B, and Zhao Yingyong are the leading contributors, with 34, 17, and 16 publications. Furthermore, in co-cited authorships, Kriz W, Liu YH, and Zhou LL stand out as the top three, with 237, 176, and 145 citations each.
Professor Liu Youhua, a famous scholar in the State Key Laboratory of Organ Failure Research of the Southern Medical University of China, has been committed to the study of the pathological mechanism of renal fibrosis for a long time and has published more than 200 SCI papers and 2,300 influencing factors. He also serves as an assistant professor at Brown University in the United States and an associate professor at the University of Pittsburgh, demonstrating his key role and outstanding performance in this research field. Interestingly, Liu ranks in the top three among high-yield and co-cited authors, demonstrating academic strength and receiving high praise, as shown in Figure 2.
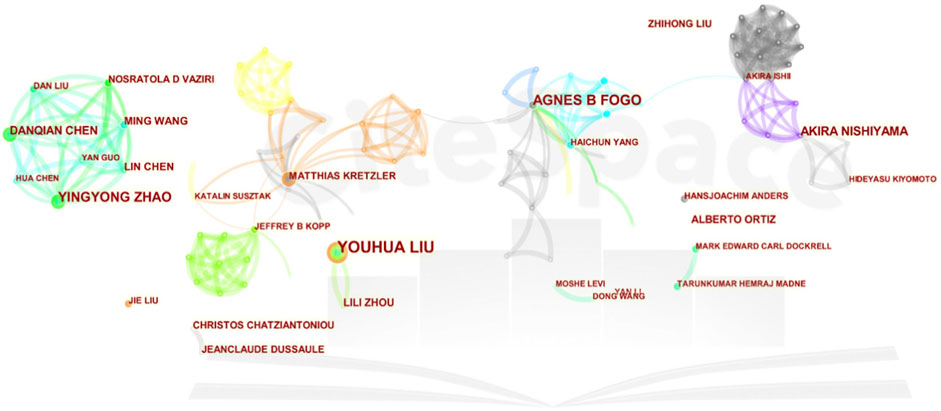
Figure 2. Collaborative network of authors. A visual representation delineating the cooperative networks and interactions among authors engaged in the study of this subject.
Liu research on factors related to renal fibrosis, in addition to his previous research on key factors related to epithelial-mesenchymal transition (EMT) and oxidative stress in the fibrotic microenvironment (Li et al., 2023), he believes that podocyte injury has a direct impact and leading cause on CKD and renal fibrosis. His latest research suggests that targeting Trim63 (an E3 ubiquitin ligase) may be a feasible therapeutic strategy for podocyte injury and proteinuria (Chen et al., 2023; Liu, 2010).
Fogo Agnes B from Vanderbilt University jointly participated in developing numerous guidelines; he believes that using high-dose angiotensin receptor blockers (ARBs) during urinary tract obstruction can protect podocytes and prevent glomerulosclerosis and renal fibrosis (Zhu et al., 2022).
As the academic leader of our team, Professor Zhao YY ranks third in overall research in this field. His research mainly focuses on the use of natural products for anti-fibrotic treatment. He believes that Poricoic acid A (Chen et al., 2019b) in Poria cocos serves as a regulator of TPH-1 expression (Chen et al., 2020a; Chen et al., 2019b), inhibiting renal fibrosis, stabilizing the regulatory protein of B-catenin, and mediating B-catenin transcription (Wang et al., 2018a) and natural products (including isolated compounds, crude extracts, and traditional Chinese herbal formulas) to regulate RAS (Yang et al., 2019) can inhibit the accumulation of ECM in HK-2 cells and alleviate podocyte damage and fibrosis (Chen et al., 2017).
3.4 Analysis of journals
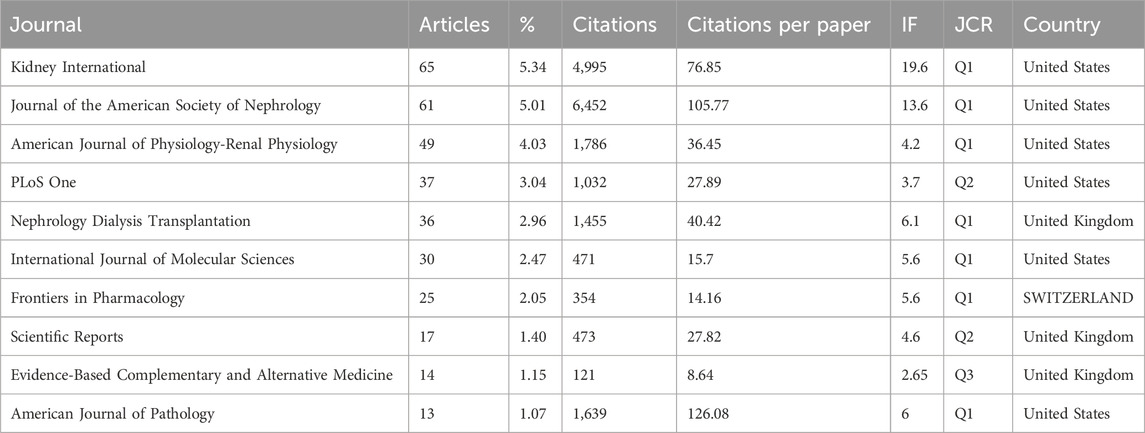
A comprehensive spectrum of 366 distinct journals has been instrumental in disseminating scholarly discourse pertaining to this subject matter, with 65 journals contributing substantial articles of more than five publications each. The delineation of the top 10 journals is elucidated in Table 4, collectively encapsulating 28.51% of the scholarly contributions, totaling 347 articles. Foremost among these, Kidney International stands out, having furnished the highest number of articles (n = 65).
Noteworthy is the scholarly prowess of these journals, as validated by the 2022 Journal Citation Report (JCR), wherein seven of the top 10 journals garner placement in the esteemed Q1 academic ranking tier. Of particular significance, two of these journals command an Impact Factor (IF) exceeding 10, with Kidney International reigning supreme with the highest IF at 19.6.
The intricate interplay of scholarly citation paths is elucidated through Figure 3, employing double-graph overlapping journals to elucidate the citation trajectory between source and cited journals. This visualization method bifurcates the citation pathway, commencing with the source journal on the left and culminating with the cited journal on the right while concurrently delineating the thematic foci of the journals. Notably, the terms “Molecular Biology and Immunology” and “Medicine, Medical, and Clinical” from cited journals evolved into one “Molecular Biology and Immunology”.
Figure 3. Dual-map overlap of journals in kidney repair research. A visual representation depicting the dual-mapping of journals engaged in the discourse surrounding kidney repair within the context of this research domain.
Our analysis of leading journals reveals that Kidney International, Journal of the American Society of Nephrology, and American Journal of Physiology Renal Physiology have emerged as the primary journals for kidney diseases. The remaining top ten journals are listed as follows (Table 4).
3.5 Analysis of co-cited references
Within the scope of this inquiry, collectively engendered the citation of a total of 29,902 references, all germane to the focal topic. Notably, Table 5 unveils the paramount co-cited references among the most cited (n = 24) and exhibiting substantial citation burst strength is the study titled “Diabetic Kidney Disease Challenges, Progress, and Possibilities,” authored by Alicic RZ (Alicic et al., 2017). This comprehensive investigation delves into the impact of intensive hyperglycemia treatment on microvascular outcomes in type 2 diabetes, in addition to offering evidence-based guidelines for the management of hypertension in adults. Furthermore, the study “Epithelial-to-mesenchymal Transition as a Potential Pathway Leading to Podocyte Dysfunction and Proteinuria,” authored by Li et al. (2008), explores the intricate pathways potentially culminating in podocyte dysfunction and proteinuria within chronic kidney diseases. This work emphasizes the role of epithelial-to-mesenchymal transition, underscoring the significance of key players such as P-cadherin, zonula occludens-1, and nephrin in podocyte functionality. The third most cited study, “The Spectrum of Podocytopathies: A Unifying View of Glomerular Diseases,” authored by RC Wiggins, presents a comprehensive review that offers a unified perspective on glomerular diseases. It underscores the pivotal role of the podocyte and underscores the importance of comprehending podocyte biology for both clinical and scientific strategies geared toward averting disease progression (Wiggins, 2007).
Intricacies of the co-citations are rendered comprehensible through Figure 4A, wherein CiteSpace facilitates the visual representation of the co-citation network of references. A meticulous examination delineates the emergence of ten distinct clusters, each tethered to pertinent keywords. These clusters are identified as follows: #1 IncRNA, #2 reactive oxygen species, #3 glomerulosclerosis, #4 Poria cocos, #5 glomerular diseases, #6 fibroblasts, #7 connective tissue growth factor, #8 coagulation, #9 Wnt. Moreover, Figure 4B furnishes a temporal perspective on these co-cited references, pivotal for discerning the evolving landscape of research hotspots over time. The clusters, characterized by keyword labels, are depicted at distinct positions and hues on the timeline, delineating variances in publication chronology.
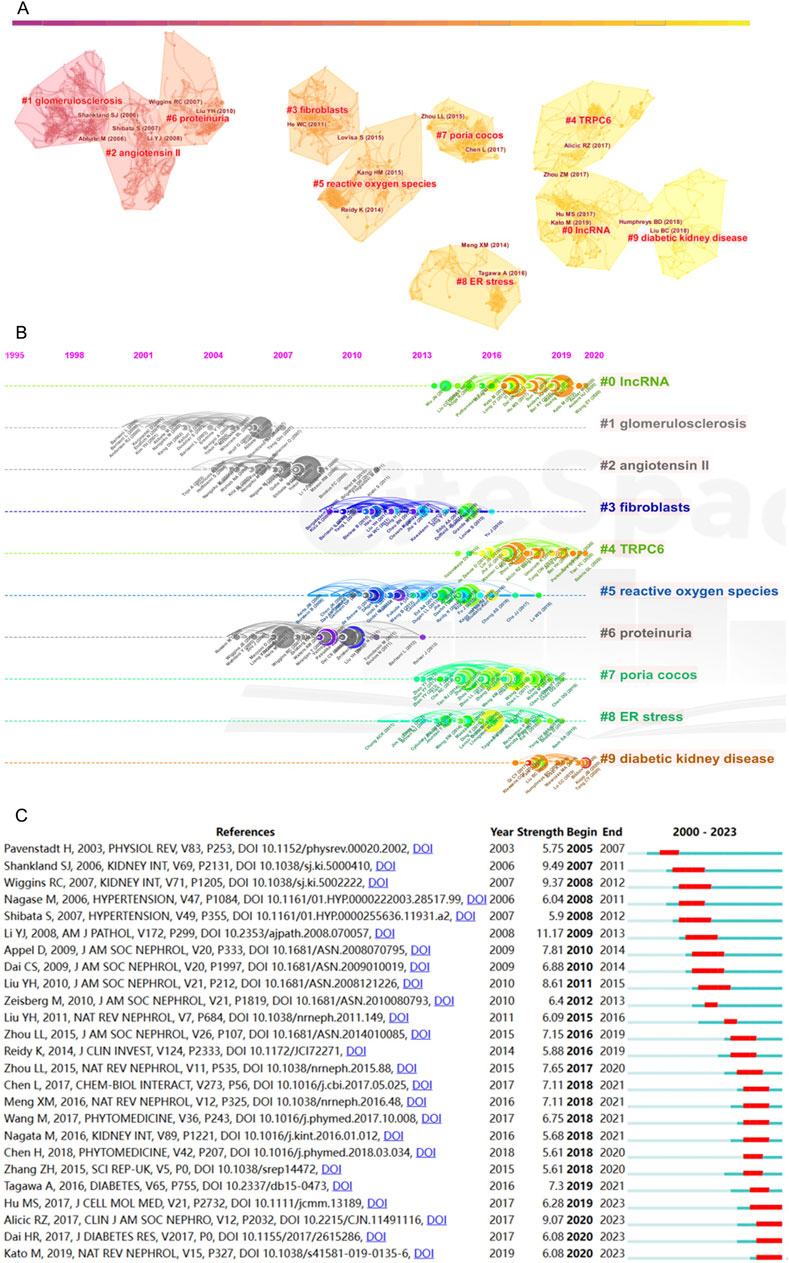
Figure 4. Analysis of co-cited references. (A) Cluster diagram of co-cited references with keywords as label source. (B) Temporal perspective on co-cited references through keywords. (C) Top 25 references with strongest citation bursts (red represents the outbreak time range).
The fourth cluster, “Poria cocos”, distinguishes itself among many mechanism-related articles. Notably, this particular Chinese herbal medicine occupies the foremost position in utilizing herbal formulations for treating kidney diseases in China (Cai et al., 2011). As a clinically effective drug for kidney protection, its mechanism of action is diverse and has not yet been fully explained. In the study, the herbal medicine Poria cocos emerges as a highly cited term, indicating that Eastern herbal medicines, as significant therapeutic agents for kidney diseases, are gradually garnering attention from scholars worldwide. Poricoic acid A (PAA), derived from Poria cocos, has been identified as a modulator of TPH-1 expression, demonstrating its efficacy in attenuating renal fibrosis (Chen et al., 2020b), as well as enhancing melatonin’s inhibitory effects on the transition from AKI to CKD (Chen et al., 2019a). Additionally, Poricoic acid ZC (PZC), Poricoic acid ZD (PZD), and Poricoic acid ZE (PZE) have exhibited renin-inhibiting properties and have shown promise in safeguarding against tubulo-interstitial fibrosis (Wang et al., 2018b).
CiteSpace’s citation burst analysis, a formidable analytical tool, unfurls the references that have garnered widespread scholarly attention. Figure 4C showcases the 25 references that have exhibited the most robust citation bursts, additionally disclosing the periods during which these references sustained peak citation intensity. Four of the most recent citation bursts merit particular attention. First, the article authored by Hu MS in 2017 (Hu et al., 2017), published in the Journal of Cellular and Molecular Medicine, explores the roles of MALAT1 and β-catenin in podocyte dysfunction and kidney fibrosis, spotlighting their potential as therapeutic targets for glomerular diseases. Secondly, Alicic RZ’s work (Alicic et al., 2017) is reiterated as a citation burst. Third, the review by Dai HR in 2017 (Dai et al., 2017), featured in the Journal of Diabetes Research, dissects the mechanisms underpinning podocyte injury in DKD while illuminating novel molecular insights that hold promise as therapeutic avenues for managing this condition. Lastly, Kato M’s study in 2019 (Kato and Natarajan, 2019), published in Nature Reviews Nephrology, explores the role of epigenetic mechanisms in the occurrence and progression of DKD, explores the concept of metabolic memory in-depth, and considers that group association studies identifying epigenetic signatures of DKD may also provide information for precision medicine methods.
3.6 Analysis of keywords
To unravel the intricate dynamics underpinning the distribution and temporal evolution of author keywords, both a co-occurrence network map and a cluster map were employed.
Figure 5A employs a distinctive color scheme to signify author keywords based on their average year of occurrence, effectively conveying the evolving temporal nuances within the research landscape. Notably, recent years have witnessed a surge in keywords such as “autophagy”, “TRPC6”, “endoplasmic reticulum stress”, “epigenetics” and “NLRP3 inflammasome”. These emergent keywords signify the current trends and areas of heightened interest.
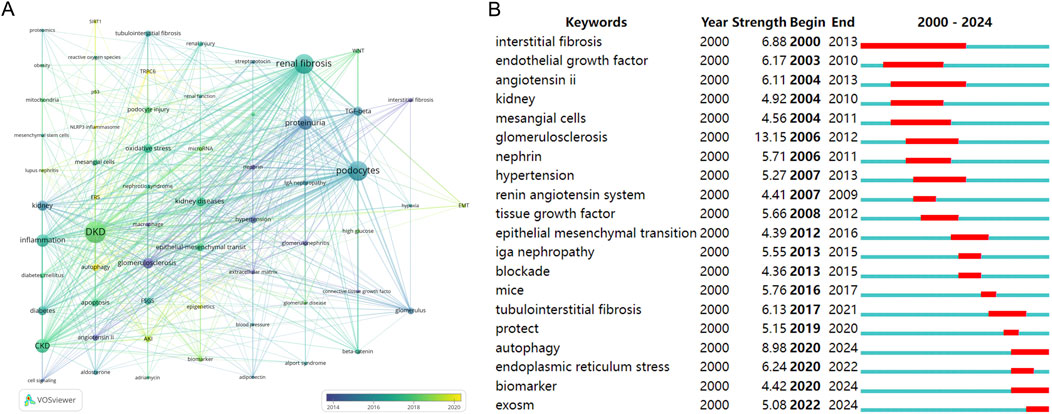
Figure 5. Analysis of keywords. (A) Cluster network of co-occurring author keywords and their temporal evolution. (B) Top 20 keywords with the strongest citation bursts (red represents the outbreak time range).
Figure 5B, utilizing CiteSpace to analyze keyword citation bursts, reveals that “autophagy”, “biomarker” and “exosomes” are presently considered focal points of research interest and activity.
As shown in Figure 5, whether it is hot words or keywords, we can see that autophagy has significant research interests in diabetic renal fibrosis. Autophagy, a highly conserved lysosomal degradation pathway governed by signaling pathways like mTOR, AMPK, and sirtuins, assumes a pivotal role in the maintenance of cellular homeostasis across various kidney cell types, encompassing renal tubular cells, podocytes, mesangial cells, and glomerular endothelial cells (Tang et al., 2020). Dysregulation of autophagy has been implicated in the pathogenesis of diverse renal pathologies. Zhao et al. (2019), for instance, conducted an in-depth analysis of the pathological implications and regulatory mechanisms of autophagy in renal fibrosis and associated kidney ailments, spanning both glomerular and tubulointerstitial compartments. Moreover, Liang et al. (2022) reported findings indicating that Qidan Dihuang decoction effectively mitigates diabetic renal injuries and fibrosis by modulating the PERK-eIF2 alpha-ATF4 pathway and promoting autophagy in DKD.
Both paths can be seen in Figures 5A, B. Endoplasmic reticulum emergency has significant research interest in diabetic renal fibrosis and podocytes. The delicate balance between the cytoprotective and cytotoxic effects of endoplasmic reticulum stress (ERS) activation is a subject of paramount significance. Pharmacological interventions that restore ERS to homeostatic levels hold immense therapeutic promise in preventing or arresting the progression of kidney-related pathologies (Cybulsky, 2017). Recent investigations have notably underscored the pivotal role ERS plays in both acute and chronic kidney diseases, particularly in renal fibrosis (Ke et al., 2017). ERS modulation emerges as a promising therapeutic avenue, potentially influencing renal fibrosis through a multitude of signaling pathways, culminating in podocyte injury. The seminal work by Bai XY further advances our understanding by elucidating the role of long intergenic non-coding RNA (LINC01619) in functioning as a competing endogenous RNA, orchestrating miR-27a/FOXO1-mediated ERS modulation and subsequent podocyte injury, as observed in DKD (Bai et al., 2018).
Transient receptor potential cation channel 6 (TRPC6) is a glomerular cleft diaphragm-associated channel required for normal renal function. DKD is associated with impaired podocyte autophagy and subsequent podocyte damage. However, the regulation of podocyte autophagy is unique and is related to the loss of calcium regulation homeostasis, leading to podocyte damage (Ilatovskaya et al., 2018). Diabetes increases the expression of TRPC6 in podocytes in vivo while reducing podocyte autophagy flux (Staruschenko et al., 2019) TRPC6 gene knockout can reduce the progression of DKD (Salemkour et al., 2023; Ma et al., 2019). Kim et al. (2018) discovery elucidates a causal link between mutations within the classic TRPC6 and manifestations of rare familial cases of focal and segmental glomerulosclerosis (FSGS). Substantial research has been diligently conducted, particularly on comprehending the intricate regulatory mechanisms governing TRPC6 channels, particularly within the context of podocyte function (Dryer et al., 2019). However, some scholars believe that fibrosis is not caused by the influx or outflow of calcium ions in podocytes. Electron microscopy shows that defective mutations in this gene do not cause FSGS changes. Rather than through TRPC6 increasing rather than decreasing calcium influx for podocyte death (Batool et al., 2023).
Epigenetics, an encompassing term for the study of heritable changes in gene function that are not rooted in alterations to the DNA sequence itself, has assumed pivotal importance in the context of renal fibrosis associated with podocyte dysfunction. These epigenetic modifications exert regulatory control over various genes implicated in the pathogenesis of renal diseases, including fibrosis and inflammation (Kato and Natarajan, 2019). Notably, recent research has unveiled the intricate interplay between epigenetic machinery and microRNA (miRNA) expression patterns, particularly in various disorders, including DKD (Sankrityayan et al., 2019). Furthermore, Rai et al.’s study underscores the significance of the epigenetic regulator FATp300 in fibrogenesis, identifying it as a prospective therapeutic target for mitigating pathological matrix remodeling and associated pathologies. Additionally, L002 emerges as a novel therapeutic candidate, potentially ameliorating hypertension-induced cardio-renal fibrosis while impeding pro-fibrogenic responses in fibroblasts, podocytes, and mesangial cells (Rai et al., 2017).
The kidney, characterized by heightened energy requirements, is replete with an abundance of mitochondria. Quadri’s investigative endeavors have underscored the pivotal involvement of mitochondrial dysfunction in the physiological progression of renal fibrosis (Quadri et al., 2019). The perturbation of mitochondria culminates in the release of dangerous molecules, including reactive oxygen species, DNA, and cardiolipin, which subsequently trigger the NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and the upregulation of interleukin-18 (IL-18) and interleukin-1 beta (IL-1β) (Szeto et al., 2017). Pharmacological inhibition of the NLRP3 inflammasome has proven effective in ameliorating renal injury across diverse animal models (Chang et al., 2014). Moreover, the activation of purinergic 2X7 receptors (P2X7R) in conjunction with the NLRP3 inflammasome has been identified as a significant contributor to renal inflammation and injury within the context of metabolic syndrome-related renal ailments. Notably, studies involving mice devoid of P2X7R have demonstrated reduced inflammation, diminished oxidative stress, and attenuated fibrosis, underscoring the therapeutic potential of these molecular targets in mitigating renal inflammation and injury associated with type 2 diabetes and metabolic syndrome (Solini et al., 2013; Li et al., 2022).
Furthermore, Figure 5B discerns research hotspots by identifying keywords that exhibit robust citation bursts. Keywords such as “autophagy”, “biomarker” and “exosomes” continue to experience sustained citation bursts, suggesting their potential to evolve into enduring research focal points.
4 Discussion
4.1 The trend analysis and contributions of countries over two-decade
From the development history of DKD, in the 1950s, the United States and Europe began to have the first record of in vivo renal biopsy technology and recorded the glomerular fibrosis of diabetic patients (Cameron, 2006). The subspace structure of podocytes, first described in 2005, was studied by Neal et al. (2005) on how it affects the microenvironment of podocytes, as well as filtration and lateral shear stress. The importance of podocytes as target cells in the pathogenesis of kidney diseases is self-evident. The main characteristics of podocytes as molecular sieves are their regularly intersecting foot processes and bridge structures used for kidney filtration. The dedifferentiation response factor of immortalized human podocytes in response to TGF-β and other TGF-dependent stimuli leads to dedifferentiation leading to the disappearance of foot processes, hypertrophic morphology and increased formation of intercellular tight junctions, which leads to the formation of fibrosis (Herman-Edelstein et al., 2011). Current anatomical studies indicate that approximately 60% of the glomerular filtration surface is covered by this space. Agnes B Fogo believes that podocyte loss has a severe impact on renal fibrosis (Fogo, 2021). From early this century, a notable escalation in annual publications about podocyte-associated diabetes and renal fibrosis is evident, which has a substantial spike in publications occurred from 2017 to 2023. It is extremely displaying that the research on renal fiber and chronic kidney disease caused by diabetes is still under in-depth exploration as a prevailing subject of research inquiry on podocytes. This research shows that the United States, China, and Japan are the top three countries with the most significant publications. The research field of diabetes and nephrology in China has made rapid progress, especially in the past 10 years. Seven of the ten authors form an essential team from China, indicating substantial academic influence in this field.
4.2 PAA as an effective complementary therapy for DKD and renal fibrosis
As to new therapies, Professor Zhao Yingyong mainly studied the protective mechanism of natural molecular compounds of Poria cocos on the kidneys and its anti-renal fibrosis effect (Chen et al., 2020b; Chen et al., 2019a; Wang et al., 2018b; Wang et al., 2023). In preliminary research, he has identified a small molecular component, PAA. It potentially holds considerable potential in mitigating renal damage caused by DKD, such as the fibrotic differentiation of podocytes and renal epithelial cells into fibroblasts, as well as in inhibiting renal fibrosis. He is also the third globally cited author.
PAA is a component isolated from the traditional Chinese medicine Poria cocos, which has hypoglycemic and anti-fibrotic effects. As a clinically effective renal protective drug, its mechanism of action is diverse and not yet fully explained. It has been confirmed that is related to improving podocytes. In fact, China, with a history of more than 5,000 years, has believed that diabetes is closely related to the kidney since ancient times, and Traditional Chinese Medicine (TCM) believes that the kidney is the key. Based on the academic theories of traditional Chinese medicine (as described in Classic medical books, Ling Shu (Unschuld, 2016), published for thousands of years, covering kidney Qi and blood energy and ion exchange), it provided more theoretical background for treatment. At the beginning of the third century AD, Zhang Zhongjing, a famous physician of the Eastern Han Dynasty, wrote in his book “Synopsis of Prescriptions of the Golden Chamber” (Zhang and Luo, 1987), “Men with kidney diseases may experience abnormal urination, sweetness in urine and frequent urination. At this time, it is necessary to take Shenqi pills.” A solution to this problem was proposed, and the famous formula “Bawei Shenqi Pills” (Liuwei Dihuang Pills, containing Poria cocos) was given in the “Xiao Ke Disease” chapter, which has been used for thousands of years.
At present, classic Chinese formulas have excellent vitality, which is also supported by co-citation literature analysis. In addition, PAA significantly reduced blood glucose and urinary protein levels in DKD mice and inhibited renal fibrosis. PAA can promote mitochondrial autophagy by downregulating FUNDC1, thereby having a beneficial effect on podocyte damage in DKD renal fibrosis. This indicates that traditional Chinese medicine molecular compounds, as important therapeutic drugs for kidney diseases, are receiving attention from scholars worldwide for their therapeutic effects.
4.3 Emerging prospective drugs and mechanism research hotspots
DKD development is characterized by intricate cellular and molecular interactions involving podocytes, glomerular endothelial cells, and mesangial cells. These core glomerular components are vital in their reciprocal impacts and pathological changes. Communication among these cells occurs via diverse signaling cascades, including those governed by angiopoietins, VEGF, TGF-β, Krüppel-like factors, RARRES1 (Hu et al., 2024), and extracellular vesicles, fostering essential intercellular communication for preserving glomerular filtration barrier function and renal homeostasis. Oxidative stress is a driving force behind podocyte hypertrophy, with elevated TGF-β1, Ang II, and mTORC1 expression contributing to this phenomenon under hyperglycemic conditions. Podocyte epithelial-to-mesenchymal transition is implicated in proteinuria onset, with Wnt/β-catenin, SDF-1α, and PI3K/AKT pathways triggering this process. Apoptosis, via both extrinsic and intrinsic mitochondrial pathways, contributes to proteinuria and glomerulosclerosis in DKD. Additionally, a newly developed drug treatments, Finerenone, the non-steroidal mineralocorticoid receptor antagonist, has demonstrated significant promise in the treatment of DKD (Fujii and Shibata, 2023). Research findings suggest that it improves mitochondrial dysfunction in renal tubular cells, mitigates inflammation and fibrosis, reduces proteinuria, and retards the progression of CKD (Barrera-Chimal et al., 2022).
The development of renal fibrosis in patients with DKD is closely tied to abnormalities in multiple signaling pathways, with the overactivation of the TGF-β pathway emerging as a principal mechanism contributing to fibrosis. Researchers are actively investigating means to suppress this pathway, along with the expression and activity of other pertinent inflammatory factors, in an effort to potentially retard or reverse the fibrotic process (Liu et al., 2024). During EMT, podocytes should lose their epithelial polarity, intercellular junctions would be altered, and the actin cytoskeleton will be rearranged (Shankland, 2006). After stimulation with TGF-B1, podocytes lose renin and ZO-1 expression (Sun et al., 2023). As important slit diaphragm proteins, loss of expression of renin and ZO-1 is detrimental to podocyte function (Zhao et al., 2022). Podocyte autophagy, a type II programmed death, initiation is crucial in the progression of podocyte loss and massive proteinuria.
Research on gene regulation and epigenetics, the research team led by Professors Zhang DS and Dong Z have elucidated the role of the PRDM16 gene during the relatively inconspicuous stage of tubulointerstitial fibrosis in the early phase of DKD (Wang et al., 2017). Their findings show that the introduction of a viral vector encoding PRDM16 or the use of the small-molecule compound Formononetin can attenuate renal fibrosis in DKD.
GLP-1 receptor agonists (GLP-1RAs) (Sourris et al., 2024) have been found to exhibit not only potent glycemic-lowering effects, but also marked reno protective properties. Multiple clinical trials, including the LEADER study, have provided compelling evidence that these agents confer noticeable renal benefits in individuals with DKD.
Scientists are currently exploring integrated therapeutic interventions targeting multiple key aspects of the fibrosis cascade, encompassing but not restricted to the inhibition of fibroblast activation, modulation of ECM homeostasis, counteracting oxidative stress, and improving microcirculatory dysfunction (Cotas et al., 2024). Moreover, TCM characterized by their multitarget nature, hold great promise in treating DKD, making their effects highly anticipated in disease management (Wang et al., 2024).
In summary, a deeper understanding and exploration of these mechanisms hold promise for the development of novel therapeutic strategies aimed at protecting podocytes in DKD and treating renal fibrosis.
4.4 Will HIM diagnosis and treatment coming?
A multidisciplinary development trend is evident within the top 10 journals dedicated to this subject. The critical researches span various domains, including Nephrology, Cell research, Pharmacology and Molecular Biology, which illustrate the multifaceted and complex research in this field.
Clinicians may be familiar with the fact that some patients with diabetes have high blood sugar, which will cause their kidneys to suffer multiple blows for a long time, promote renal fibrosis, and then it is lead to the decline of renal function until that progresses to renal failure and even renal replacement therapy. This is a common clinical disease transformation and bad outcome. Here, the treatment and diagnosis process covers the responsibilities of the Endocrinology, Nephrology, and Urology departments, etc. However, is this really beneficial for patients?
In response to this dilemma, it is worth that trying the combination of HIM. Chinese academician Fan Daiming. Has already proposed the concept of Holistic Integrative Medicine (Fan, 2017), and he has proposed that the growth of medical knowledge, drug varieties, and technological advancements, coupled with rising health demands, needs a more comprehensive approach. Moreover, the medicine revolution offers opportunities for HIM, such as “Doctor Cloud”, an intelligent platform that expands doctors’ knowledge, accumulates experience, and provides precise patient services. Fogo AB, another expert in this field, his latest article on JAMA aims to demonstrate that marking should done by experts, allowing the accuracy of computer diagnostics to meet the highest professional standards. The combination of AI and medicine is worth thinking in depth, using the field of renal pathology as an example to illustrate its development, emphasizing that this field is only a model for other medical fields (Fogo et al., 2024). Experts have been discussing this point of view. However, it seems that the revolution in computing is integrating medicine. By leveraging AI and data bank, one platform forms a “medical brain”, allowing doctors nationwide to seek solutions for challenging clinical cases in real-time just around the corner. Internet technologies enable personalized integration plans tailored to individual patient needs, preliminary describing the future of HIM medical development.
Furthermore, HIM medicine model is used to provide personalized intervention to the research subjects. To Integrated medicine model: Endocrinologists, cardiologists, general medicine physicians, traditional Chinese medicine practitioners, nutritionists, psychological counselors, exercise managers, pharmacists, health managers, and basic medical professors jointly participate in on-site education and discussions. To develop a comprehensive intervention plan participate in one-to-one personalized management offline and online, and implement personalized anti-diabetes treatment from seven dimensions: Western medicine prevention and treatment, psychological counseling, nutritional intervention, exercise intervention, traditional Chinese medicine conditioning, health monitoring, and rehabilitation guidance plan. A team of senior professional title experts and excellent professional and technical qualifications from high-level hospitals, medical universities, and diabetes-related research institutions conduct offline and online on-site health education and discussion venues and clinics, and try to integrate them into a more complete system as much as possible. The environment is bound to be conducive to the development of doctors, patients and related research as a whole. Standing on the shoulders of many giants and rethinking the course and related progress of this disease is a feasible treatment strategy worthy of further research and thinking.
5 Conclusion
This study applied bibliometric to visualize the interplay between podocytes, DKD, and renal fibrosis from 2000 to 2024. Diabetes pose an inevitable challenge for society’s development. Despite a steady growth on podocytes’ role in DKD and renal fibrosis, the global scientific community has yet to fully elucidate their mechanisms and functions. This bibliometric analysis proposed key terms and novel therapies, including molecule compounds derived from TCM, which may offer valuable insights into the elusive mechanisms of podocytes. These findings deserve significant attention from nephrologists and related researchers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
D-YA: Conceptualization, Investigation, Writing–original draft. JT: Conceptualization, Writing–original draft. Y-DL: Conceptualization, Writing–review and editing. Z-HW: Conceptualization, Writing–original draft. Y-NB: Writing–original draft. Z-HY: Conceptualization, Writing–review and editing. Z-YC: Writing–original draft, Data curation. P-PW: Investigation, Writing–original draft. WZ: Writing–review and editing, Project administration. QY: Funding acquisition, Writing–review and editing. MH: Writing–review and editing, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This article was sponsored by the National Natural Science Foundation of China (No. 82204625 and No. 82404988), the Natural Science Foundation of Zhejiang Province (No. LQ23H280013 and No. LQ23H280004), and the Fundamental Research Funds for the Central Universities (No. 226-2023-00056).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alicic, R. Z., Rooney, M. T., and Tuttle, K. R. (2017). Diabetic kidney disease: challenges, progress, and Possibilities. Clin. J. Am. Soc. Nephrol. CJASN 12 (12), 2032–2045. doi:10.2215/CJN.11491116
Bai, X. Y., Geng, J., Li, X., Wan, J., Liu, J. X., Zhou, Z. M., et al. (2018). Long noncoding RNA LINC01619 regulates MicroRNA-27a/forkhead box protein O1 and endoplasmic reticulum stress-mediated podocyte injury in diabetic nephropathy. Antioxidants and Redox Signal. 29 (4), 355–376. doi:10.1089/ars.2017.7278
Barrera-Chimal, J., Lima-Posada, I., Bakris, G. L., and Jaisser, F. (2022). Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat. Rev. Nephrol. 18 (1), 56–70. doi:10.1038/s41581-021-00490-8
Batool, L., Hariharan, K., Xu, Y., Kaßmann, M., Tsvetkov, D., Gohlke, B. O., et al. (2023). An inactivating human TRPC6 channel mutation without focal segmental glomerulosclerosis. Cell. Mol. life Sci. 80 (9), 265. doi:10.1007/s00018-023-04901-w
Cai, Y. M., He, Y., Qiu, T., Zou, J., Sun, D. P., Peng, Q. H., et al. (2011). Research on frequency of application with modern Chinese herbal medicine. Chin. J. Integr. Med. 17 (1), 64–70. doi:10.1007/s11655-011-0609-2
Calle, P., and Hotter, G. (2020). Macrophage phenotype and fibrosis in diabetic nephropathy. Int. J. Mol. Sci. 21 (8), 2806. doi:10.3390/ijms21082806
Cameron, J. S. (2006). The discovery of diabetic nephropathy: from small print to centre stage. J. Nephrol. 19 (Suppl. 10), S75–S87. Available at: https://pubmed.ncbi.nlm.nih.gov/16874718/
Chang, A., Ko, K., and Clark, M. R. (2014). The emerging role of the inflammasome in kidney diseases. Curr. Opin. Nephrol. Hypertens. 23 (3), 204–210. doi:10.1097/01.mnh.0000444814.49755.90
Chen, D. Q., Feng, Y. L., Chen, L., Liu, J. R., Wang, M., Vaziri, N. D., et al. (2019a). Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2 axis. Free Radic. Biol. and Med. 134, 484–497. doi:10.1016/j.freeradbiomed.2019.01.046
Chen, D. Q., Wu, X. Q., Chen, L., Hu, H. H., Wang, Y. N., and Zhao, Y. Y. (2020a). Poricoic acid A as a modulator of TPH-1 expression inhibits renal fibrosis via modulating protein stability of β-catenin and β-catenin-mediated transcription. Ther. Adv. Chronic Dis. 11, 2040622320962648. doi:10.1177/2040622320962648
Chen, D. Q., Wu, X. Q., Chen, L., Hu, H. H., Wang, Y. N., and Zhao, Y. Y. (2020b). Poricoic acid A as a modulator of TPH-1 expression inhibits renal fibrosis via modulating protein stability of β-catenin and β-catenin-mediated transcription. Ther. Adv. Chronic Dis. 11. doi:10.1177/2040622320962648
Chen, L., Cao, G., Wang, M., Feng, Y. L., Chen, D. Q., Vaziri, N. D., et al. (2019b). The matrix metalloproteinase-13 inhibitor poricoic acid ZI ameliorates renal fibrosis by mitigating epithelial-mesenchymal transition. Mol. Nutr. Food Res. 63 (13), e1900132. doi:10.1002/mnfr.201900132
Chen, L., Chen, D. Q., Wang, M., Liu, D., Chen, H., Dou, F., et al. (2017). Role of RAS/Wnt/β-catenin axis activation in the pathogenesis of podocyte injury and tubulo-interstitial nephropathy. Chemico-biological Interact. 273, 56–72. doi:10.1016/j.cbi.2017.05.025
Chen, Q., Xie, C., Tang, K., Luo, M., Zhang, Z., Jin, Y., et al. (2023). The E3 ligase Trim63 promotes podocyte injury and proteinuria by targeting PPARα to inhibit fatty acid oxidation. Free Radic. Biol. and Med. 209 (Pt 1), 40–54. doi:10.1016/j.freeradbiomed.2023.09.039
Cotas, J., Lomartire, S., Pereira, L., Valado, A., Marques, J. C., and Gonçalves, A. M. M. (2024). Seaweeds as nutraceutical elements and drugs for diabetes mellitus: future perspectives. Mar. drugs 22 (4), 168. doi:10.3390/md22040168
Cybulsky, A. V. (2017). Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 13 (11), 681–696. doi:10.1038/nrneph.2017.129
Dai, H., Liu, Q., and Liu, B. (2017). Research progress on mechanism of podocyte depletion in diabetic nephropathy. J. Diabetes Res. 2017, 2615286. doi:10.1155/2017/2615286
Drawz, P., and Rahman, M. (2015). Chronic kidney disease. Ann. Intern. Med. 162 (11), ITC1–ITC16. doi:10.7326/AITC201506020
Dryer, S. E., Roshanravan, H., and Kim, E. Y. (2019). TRPC channels: regulation, dysregulation and contributions to chronic kidney disease. Biochimica Biophysica Acta-Molecular Basis Dis. 1865 (6), 1041–1066. doi:10.1016/j.bbadis.2019.04.001
Fan, D. (2017). Holistic integrative medicine: toward a new era of medical advancement. Front. Med. 11 (1), 152–159. doi:10.1007/s11684-017-0499-6
Fogo, A. B. (2021). Gains in understanding of podocyte loss. Kidney Int. 100 (5), 978–980. doi:10.1016/j.kint.2021.08.003
Fogo, A. B., Kronbichler, A., and Bajema, I. M. (2024). AI's threat to the medical profession. Jama 331 (6), 471–472. doi:10.1001/jama.2024.0018
Fujii, W., and Shibata, S. (2023). Mineralocorticoid receptor antagonists for preventing chronic kidney disease progression: current evidence and future challenges. Int. J. Mol. Sci. 24 (9), 7719. doi:10.3390/ijms24097719
GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395 (10225), 709–733. doi:10.1016/S0140-6736(20)30045-3
Herman-Edelstein, M., Thomas, M. C., Thallas-Bonke, V., Saleem, M., Cooper, M. E., and Kantharidis, P. (2011). Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: a model for diabetic podocytopathy. Diabetes 60 (6), 1779–1788. doi:10.2337/db10-1110
Hu, M., Wang, R., Li, X., Fan, M., Lin, J., Zhen, J., et al. (2017). LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with β-catenin. J. Cell Mol. Med. 21 (11), 2732–2747. doi:10.1111/jcmm.13189
Hu, S., Hang, X., Wei, Y., Wang, H., Zhang, L., and Zhao, L. (2024). Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review. Cell Commun. Signal 22 (1), 136. doi:10.1186/s12964-024-01502-3
Humphreys, B. D. (2018). Mechanisms of renal fibrosis. Annu. Rev. physiology 80, 309–326. doi:10.1146/annurev-physiol-022516-034227
Ilatovskaya, D. V., Blass, G., Palygin, O., Levchenko, V., Pavlov, T. S., Grzybowski, M. N., et al. (2018). A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J. Am. Soc. Nephrol. JASN 29 (7), 1917–1927. doi:10.1681/ASN.2018030280
Jiang, A., Song, A., and Zhang, C. (2022). Modes of podocyte death in diabetic kidney disease: an update. J. Nephrol. 35 (6), 1571–1584. doi:10.1007/s40620-022-01269-1
Johansen, K. L., Chertow, G. M., Gilbertson, D. T., Ishani, A., Israni, A., Ku, E., et al. (2023). US renal data system 2022 annual data Report: epidemiology of kidney disease in the United States. Am. J. kidney Dis. Official J. Natl. Kidney Found. 81 (3 Suppl. 1), A8–a11. doi:10.1053/j.ajkd.2022.12.001
Kato, M., and Natarajan, R. (2019). Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 15 (6), 327–345. doi:10.1038/s41581-019-0135-6
KDOQI (2007). KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. kidney Dis. Official J. Natl. Kidney Found. 49 (2 Suppl. 2), S12–S154. doi:10.1053/j.ajkd.2006.12.005
Ke, B., Zhu, N., Luo, F. L., Xu, Y., and Fang, X. D. (2017). Targeted inhibition of endoplasmic reticulum stress: new hope for renal fibrosis (Review). Mol. Med. Rep. 16 (2), 1014–1020. doi:10.3892/mmr.2017.6762
Ketteler, M., Block, G. A., Evenepoel, P., Fukagawa, M., Herzog, C. A., McCann, L., et al. (2018). Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann. Intern Med. 168 (6), 422–430. doi:10.7326/M17-2640
Kim, E. Y., Shotorbani, P. Y., and Dryer, S. E. (2018). Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J. Mol. Medicine-Jmm 96 (7), 631–644.
Kopp, J. B., Anders, H.-J., Susztak, K., Podestà, M. A., Remuzzi, G., Hildebrandt, F., et al. (2020). Podocytopathies. Nat. Rev. Dis. Prim. 6 (1), 68. doi:10.1038/s41572-020-0196-7
Levin, A., Tonelli, M., Bonventre, J., Coresh, J., Donner, J. A., Fogo, A. B., et al. (2017). Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390 (10105), 1888–1917. doi:10.1016/S0140-6736(17)30788-2
Li, L., Fu, H. Y., and Liu, Y. H. (2022). The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat. Rev. Nephrol. 18 (9), 545–557. doi:10.1038/s41581-022-00590-z
Li, L., He, M., Tang, X., Huang, J., Li, J., Hong, X., et al. (2023). Proteomic landscape of the extracellular matrix in the fibrotic kidney. Kidney Int. 103 (6), 1063–1076. doi:10.1016/j.kint.2023.01.021
Li, Y., Kang, Y. S., Dai, C., Kiss, L. P., Wen, X., and Liu, Y. (2008). Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. pathology 172 (2), 299–308. doi:10.2353/ajpath.2008.070057
Liang, Q. E., Bai, Z. Y., Xie, T., Lu, H. Q., Xiang, L., Ma, K., et al. (2022). Deciphering the pharmacological mechanisms of qidan Dihuang decoction in ameliorating renal fibrosis in diabetic nephropathy through experimental validation in vitro and in vivo. Evidence-Based Complementary Altern. Med. 2022, 4137578. doi:10.1155/2022/4137578
Liu, F., Chen, J., Luo, C., and Meng, X. (2022). Pathogenic role of MicroRNA dysregulation in podocytopathies. Front. Physiol. 13, 948094. doi:10.3389/fphys.2022.948094
Liu, F., Zhao, L., Wu, T., Yu, W., Li, J., Wang, W., et al. (2024). Targeting autophagy with natural products as a potential therapeutic approach for diabetic microangiopathy. Front. Pharmacol. 15, 1364616. doi:10.3389/fphar.2024.1364616
Liu, Y. (2010). New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21 (2), 212–222. doi:10.1681/ASN.2008121226
Lv, J. C., and Zhang, L. X. (2019). Prevalence and disease burden of chronic kidney disease. Adv. Exp. Med. Biol. 1165, 3–15. doi:10.1007/978-981-13-8871-2_1
Ma, R., Xu, Y., Zhou, H., Zhang, D., Yao, D., Song, L., et al. (2019). Participation of the AngII/TRPC6/NFAT axis in the pathogenesis of podocyte injury in rats with type 2 diabetes. Mol. Med. Rep. 19 (3), 2421–2430. doi:10.3892/mmr.2019.9871
Meng, T., Wang, P., Ding, J., Du, R., Gao, J., Li, A., et al. (2022). Global research trends on ventricular remodeling: a bibliometric analysis from 2012 to 2022. Curr. Problems Cardiol. 47 (11), 101332. doi:10.1016/j.cpcardiol.2022.101332
Mohandes, S., Doke, T., Hu, H., Mukhi, D., Dhillon, P., and Susztak, K. (2023). Molecular pathways that drive diabetic kidney disease. J. Clin. Investigation 133 (4), e165654. doi:10.1172/JCI165654
Murray, C. J., and Lopez, A. D. (2013). Measuring the global burden of disease. N. Engl. J. Med. 369 (5), 448–457. doi:10.1056/NEJMra1201534
Neal, C. R., Crook, H., Bell, E., Harper, S. J., and Bates, D. O. (2005). Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J. Am. Soc. Nephrol. 16 (5), 1223–1235. doi:10.1681/ASN.2004100822
Pereira, P. R., Carrageta, D. F., Oliveira, P. F., Rodrigues, A., Alves, M. G., and Monteiro, M. P. (2022). Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med. Res. Rev. 42 (4), 1518–1544. doi:10.1002/med.21883
Quadri, M. M., Fatima, S. S., Che, R. C., and Zhang, A. H. (2019). Mitochondria and renal fibrosis. Adv. Exp. Med. Biol. 1165, 501–524. doi:10.1007/978-981-13-8871-2_25
Rai, R., Verma, S. K., Kim, D., Ramirez, V., Lux, E., Li, C. J., et al. (2017). A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis. Epigenetics 12 (11), 1004–1013. doi:10.1080/15592294.2017.1370173
Roccatello, D., Lan, H. Y., Sciascia, S., Sethi, S., Fornoni, A., and Glassock, R. (2023). From inflammation to renal fibrosis: a one-way road in autoimmunity? Autoimmun. Rev. 23 (4), 103466. doi:10.1016/j.autrev.2023.103466
Rosenberg, A. Z., and Kopp, J. B. (2017). Focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 12 (3), 502–517. doi:10.2215/CJN.05960616
Sabe, M., Pillinger, T., Kaiser, S., Chen, C., Taipale, H., Tanskanen, A., et al. (2022). Half a century of research on antipsychotics and schizophrenia: a scientometric study of hotspots, nodes, bursts, and trends. Neurosci. and Biobehav. Rev. 136, 104608. doi:10.1016/j.neubiorev.2022.104608
Salemkour, Y., Yildiz, D., Dionet, L., t Hart, D. C., Verheijden, K. A. T., Saito, R., et al. (2023). Podocyte injury in diabetic kidney disease in mouse models involves TRPC6-mediated calpain activation impairing autophagy. J. Am. Soc. Nephrol. 34 (11), 1823–1842. doi:10.1681/ASN.0000000000000212
Sankrityayan, H., Kulkarni, Y. A., and Gaikwad, A. B. (2019). Diabetic nephropathy: the regulatory interplay between epigenetics and microRNAs. Pharmacol. Res. 141, 574–585. doi:10.1016/j.phrs.2019.01.043
Shankland, S. J. (2006). The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69 (12), 2131–2147. doi:10.1038/sj.ki.5000410
Solini, A., Menini, S., Rossi, C., Ricci, C., Santini, E., Fantauzzi, C. B., et al. (2013). The purinergic 2X(7) receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J. Pathology 231 (3), 342–353. doi:10.1002/path.4237
Song, L., Zhang, J., Ma, D., Fan, Y., Lai, R., Tian, W., et al. (2022). A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front. Immunol. 13, 910444. doi:10.3389/fimmu.2022.910444
Sourris, K. C., Ding, Y., Maxwell, S. S., Al-Sharea, A., Kantharidis, P., Mohan, M., et al. (2024). Glucagon-like peptide-1 receptor signaling modifies the extent of diabetic kidney disease through dampening the receptor for advanced glycation end products-induced inflammation. Kidney Int. 105 (1), 132–149. doi:10.1016/j.kint.2023.09.029
Staruschenko, A., Spires, D., and Palygin, O. (2019). Role of TRPC6 in progression of diabetic kidney disease. Curr. Hypertens. Rep. 21 (7), 48. doi:10.1007/s11906-019-0960-9
Sun, X., Xi, Y., Yan, M., Sun, C., Tang, J., Dong, X., et al. (2023). Lactiplantibacillus plantarum NKK20 increases intestinal butyrate production and inhibits type 2 diabetic kidney injury through PI3K/akt pathway. J. Diabetes Res. 2023, 8810106. doi:10.1155/2023/8810106
Szeto, H. H., Liu, S. Y., Soong, Y., Seshan, S. V., Cohen-Gould, L., Manichev, V., et al. (2017). Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J. Am. Soc. Nephrol. 28 (5), 1437–1449. doi:10.1681/ASN.2016070761
Tang, C. Y., Livingston, M. J., Liu, Z. W., and Dong, Z. (2020). Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16 (9), 489–508. doi:10.1038/s41581-020-0309-2
Thomas, M. C., Brownlee, M., Susztak, K., Sharma, K., Jandeleit-Dahm, K. A., Zoungas, S., et al. (2015). Diabetic kidney disease. Nat. Rev. Dis. Prim. 1, 15018. doi:10.1038/nrdp.2015.18
Unschuld, P. U. (2016). Huang di nei jing ling Shu: the ancient classic on needle therapy. Oakland, California, USA: Univ of California Press.
Wang, L., Tao, T., Su, W., Yu, H., Yu, Y., and Qin, J. (2017). A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab. Chip 17 (10), 1749–1760. doi:10.1039/c7lc00134g
Wang, M., Chen, D. Q., Chen, L., Liu, D., Zhao, H., Zhang, Z. H., et al. (2018a). Novel RAS inhibitors poricoic acid ZG and poricoic acid ZH attenuate renal fibrosis via a wnt/β-catenin pathway and targeted phosphorylation of smad3 signaling. J. Agric. Food Chem. 66 (8), 1828–1842. doi:10.1021/acs.jafc.8b00099
Wang, M., Chen, D. Q., Chen, L., Cao, G., Zhao, H., Liu, D., et al. (2018b). Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br. J. Pharmacol. 175 (13), 2689–2708. doi:10.1111/bph.14333
Wang, M., Yin, F., Kong, L., Yang, L., Sun, H., Sun, Y., et al. (2024). Chinmedomics: a potent tool for the evaluation of traditional Chinese medicine efficacy and identification of its active components. Chin. Med. 19 (1), 47. doi:10.1186/s13020-024-00917-x
Wang, Y. N., Miao, H., Yu, X. Y., Guo, Y., Su, W., Liu, F., et al. (2023). Oxidative stress and inflammation are mediated via aryl hydrocarbon receptor signalling in idiopathic membranous nephropathy. Free Radic. Biol. and Med. 207, 89–106. doi:10.1016/j.freeradbiomed.2023.07.014
Wiggins, R. C. (2007). The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71 (12), 1205–1214. doi:10.1038/sj.ki.5002222
Yang, T., Chen, Y. Y., Liu, J. R., Zhao, H., Vaziri, N. D., Guo, Y., et al. (2019). Natural products against renin-angiotensin system for antifibrosis therapy. Eur. J. Med. Chem. 179, 623–633. doi:10.1016/j.ejmech.2019.06.091
Zhang, D., Liu, B. W., Liang, X. Q., and Liu, F. Q. (2023). Immunological factors in cirrhosis diseases from a bibliometric point of view. World J. Gastroenterol. 29 (24), 3899–3921. doi:10.3748/wjg.v29.i24.3899
Zhang, Y., Jin, D., Duan, Y., Zhang, Y., Duan, L., Lian, F., et al. (2022). Bibliometric analysis of renal fibrosis in diabetic kidney disease from 1985 to 2020. Front. Public Health 10, 767591. doi:10.3389/fpubh.2022.767591
Zhang, Z., and Luo, X. (1987). Synopsis of Prescriptions of the golden chamber. Beijing, China: New World Press.
Zhao, H., Chen, X., Zhang, L., Meng, F., Zhou, L., Pang, X., et al. (2022). Lacticaseibacillus rhamnosus Fmb14 prevents purine induced hyperuricemia and alleviate renal fibrosis through gut-kidney axis. Pharmacol. Res. 182, 106350. doi:10.1016/j.phrs.2022.106350
Zhao, X. C., Livingston, M. J., Liang, X. L., and Dong, Z. (2019). Cell apoptosis and autophagy in renal fibrosis. Adv. Exp. Med. Biol. 1165, 557–584. doi:10.1007/978-981-13-8871-2_28
Keywords: podocytes, DKD, renal fibrosis, Poria cocos, holistic integrated medicine
Citation: An D-Y, Tan J, Lu Y-D, Wen Z-H, Bao Y-N, Yao Z-H, Chen Z-Y, Wang P-P, Zhou W, Yang Q and Hao M (2024) Focus on podocytes: diabetic kidney disease and renal fibrosis — a global bibliometric analysis (2000–2024). Front. Pharmacol. 15:1454586. doi: 10.3389/fphar.2024.1454586
Received: 25 June 2024; Accepted: 29 October 2024;
Published: 15 November 2024.
Edited by:
Min Chen, Peking University, ChinaReviewed by:
Huangjin Tong, Nanjing University of Chinese Medicine, ChinaHongyan Zhang, University of Chinese Academy of Sciences, China
Xianjin Zhang, Guangzhou University of Chinese Medicine, China
Copyright © 2024 An, Tan, Lu, Wen, Bao, Yao, Chen, Wang, Zhou, Yang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Hao, aGFvX21pbjA1MDlAMTYzLmNvbQ==; Qiao Yang, eXFAemNtdS5lZHUuY24=; Wei Zhou, ZHJhY296aG91QHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Dong-Yang An
Dong-Yang An Jun Tan
Jun Tan Yan-Dan Lu1†
Yan-Dan Lu1† Ze-Huai Wen
Ze-Huai Wen Wei Zhou
Wei Zhou Min Hao
Min Hao