
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 18 September 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1450733
This article is part of the Research Topic Reviews in Ethnopharmacology: 2023 View all 31 articles
A correction has been applied to this article in:
Corrigendum: A review of the botany, metabolites, pharmacology, toxicity, industrial applications, and processing of Polygalae Radix: the “key medicine for nourishing life”
Polygalae radix (PR) is the dried root of Polygala tenuifolia Willd. and Polygala sibirica L. and enjoys the reputation as the “key medicine for nourishing life.” In this study, information about “Polygala tenuifolia Willd.,” “Polygala sibirica L.,” and “Yuanzhi” was retrieved from scientific databases, including Google Scholar, Baidu Scholar, Web of Science, PubMed, CNKI, and Wan Fang Data. Information from Chinese herbal medicine classics, Yaozhi Data, and the Gaide Chemical Network was also collected. Information related to botany, phytochemistry, pharmacology, toxicity, industrial applications, and processing is summarized in this paper to tap its potentialities and promote its further development and clinical application. More than 320 metabolites have been isolated from PR; saponins, xanthones, and oligosaccharide esters are the main functional metabolites. Pharmacological research shows that its pharmacological action mainly focuses on resisting nervous system diseases, and it also has the functions of anti-oxidation, anti-inflammation, anti-tumor, anti-pathogenic microorganisms and others. The gastrointestinal irritation of its saponins impeded its application, but this irritation can be reduced by controlling the dosage, compatibility with other herbs, or processing. The future progress of PR faces opportunities and challenges. More attention should be paid to the traditional application and processing methods of PR recorded in ancient books. The lack of safety and clinical studies has limited its application and transformation of achievements. Moreover, it is one-sided to take the content of only a few metabolites as the index of processing optimization and quality control, which cannot reflect the full pharmacological and toxicological activities of PR.
Polygalae Radix (PR), the dried root of Polygala tenuifolia Willd. or Polygala sibirica L., is mainly distributed in China, South Korea, Mongolia, and Russia (Jiang et al., 2016). PR is widely used in Japanese Kampo medicine (Tsukada et al., 2021), traditional Korean medicine (Kumar et al., 2013), traditional Chinese medicine (TCM), and Mongolian medicine as a tonic, expectorant, sedative, and antasthmatic. It is known as “Onji” in Japan (Kuroda et al., 2014) and “Wonji” in South Korea, and these terms refer only to the root or root bark of Polygala tenuifolia Willd. In China, PR is called “Yuanzhi” and ranks as the third most frequently used single herb in the clinical application of cognitive enhancement prescriptions (Peng et al., 2020a). According to the 2020 Chinese Pharmacopoeia, PR is usually harvested in spring and autumn, the fibrous root and silt are removed, and the product is dried directly or after the wood heart is removed. It possesses bitterness and pungency in flavor and warmth in nature, enters the heart and lung meridian, and holds the effects of calming the nerves and increasing intelligence, coordinating the heart and kidney, eliminating phlegm, and relieving swelling. It is usually used for treating insomnia and dreamful sleep, amnesia, palpitation, and obnubilation caused by heart-kidney imbalance, as well as ungratifying coughing of phlegm, swelling and sores, and breast pain. Other traditional applications of PR are shown in Supplementary Material.
According to Yaozhi Data, more than 800 prescriptions contain PR in diverse dosage forms, especially decoctions, pills, and powders. In 2018, three classic prescriptions containing PR, Kai-Xin-San, Di-Huang-Yin-Zi, and Gu-Yin-Jian, were included in the Catalogue of Ancient Classic Prescriptions (the First Batch) by the state administration of TCM. PR is praised as “the key medicine for nourishing life” by Shennong’s Herbal Classic. As a homologous plant of medicine and food, it shows great health-preserving potential in the fields of healthcare products and medicated diets. Owing to the continuous discovery of pharmacological activities, its application scope in the daily chemical industry tends to deepen and expand, giving it increasing commercial value.
More than 320 metabolites have been isolated from PR, including its main active ingredients—saponins, xanthones, and oligosaccharide esters, as well as other metabolites such as alkaloids, coumarins, lignins, and flavonoids (Wang et al., 2020a). Abundant research on the pharmacological activities of PR mainly focuses on its anti-neurological disease effects (Zhao et al., 2020) such as neurodegenerative diseases (NDD, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), aging, learning, and memory impairment, spinal cord injury), cardio-cerebrovascular diseases (such as hypoxia-reoxygenation injury, cerebral ischemia-reperfusion, myocardial ischemia, cerebral hemorrhage, arrhythmia) and mental disorders (such as depression, insomnia, anxiety, post-traumatic stress disorder, social disorder). In addition, it has antioxidant, anti-inflammatory, anti-fatigue, anti-tumor, anti-pathogenic microorganisms and other functions (Zheng et al., 2016; Lei et al., 2020; Yu, 2020; Huang and Lin, 2021; Wang et al., 2021a; Zeng et al., 2021a; Gao, 2022; Yang et al., 2022). According to literature reports, it also has certain biological activities in eliminating phlegm, relieving cough, killing sperm, lowering blood pressure, resisting diabetes, contracting the uterus, protecting the stomach, resisting bone loss, obesity, resisting fatty liver, promoting blood coagulation, and resisting radiation.
A comprehensive review is necessary to deepen the understanding of PR, facilitate further study, and guide its rational application. In this study, we reviewed PR from the aspects of botany, phytochemistry, pharmacology, toxicology, industrial applications, and processing (Graphic Abstract). In addition, the limitations to current research and the possible directions of future research were also discussed. This review is of great significance for propelling the deep ongoing research and expanding the application scope of PR.
PR (Figure 1) is the dried root of two perennials, Polygala tenuifolia Willd. and Polygala sibirica L. According to the Higher Plants of China (中国高等植物) and Flora of China (中国植物志), their botanical characteristics are shown in Supplementary Table 1. Saponins, PR’s main effective metabolite, mostly accumulate in its roots, stems, and leaves, with root bark having dozens of times higher content than xylem and aerial parts (Teng et al., 2009a). The root or root bark of PR is often used medicinally, and aerial parts are used in a few areas and are mainly discarded. The saponin content begins to increase at the flowering stage and peaks at the fruiting stage. The plant should be harvested in April and May, and the total saponin yield of a three-year-old PR is the highest (Teng et al., 2009b).
According to the Commercial Grades for Traditional Chinese Medicine distributed by the Chinese Traditional Medicine Association, the medicinal pieces of PR can be generally divided into three specifications: “Yuanzhi tong,” “Yuanzhi rou,” and “Yuanzhi gun.” “Yuanzhi tong” refers to the root bark of the thicker PR with a hollow cylinder shape, which has been loosened by kneading or other means, and its wood heart has been removed. According to the different removal rates of wood core and diameters, the “Yuanzhi tong” is subdivided into five grades, in which the diameter and removal rate of the wood core of the super ones exceed 0.4 cm and 95%, respectively, and the removal rate of the wood core of the lowest grade is ≥ 80%. “Yuanzhirou” is the ruptured root bark of narrower PR that has been smashed with a wood stick and had its wood heart removed. PR, which is too slender to remove the wood heart, is called “Yuanzhigun.” Among them, “Yuanzhitong” is considered to be the main type with superior quality; it is more expensive and is the opposite of “Yuanzhigun.”
Note that counterfeit products are rampant in the market; they are cheaper and similar in appearance to PR but are very different in flavor and efficacy. These adulterants not only degrade the quality of PR but also compromise the efficacy and safety of related compositions and formulations (Li et al., 2019). To distinguish PR from its adulterated products, we summarized common adulterants and their characteristics in Supplementary Table 2.
PR is mainly distributed in the temperate regions of East Asia and Europe (Figure 2), such as China, South Korea, Mongolia, the Korean Peninsula, and the Russian Federation. In China, Heilongjiang, Jilin, Liaoning, Inner Mongolia, Shanxi, Hebei, Shandong, Henan, Anhui, Jiangsu, Jiangxi, Hubei, Hunan, Sichuan, Qinghai, Gansu, Ningxia, and Shaanxi are all its producing areas. Shanxi and Shaanxi have the largest yield (Fan et al., 2020). On 2 April 2022, PR was included in the Catalogue of Genuine Medicinal Materials in Heilongjiang Province. Although it is one of the bulk medicinal materials and 85 traditional export herbs, the slow growth rate and finite production of wild PR cannot meet the huge market demands. As a result of over-exploitation, vegetation destruction, and global warming, wild PR resources are increasingly scarce (Liu et al., 2021). The relevant departments of the Chinese government have brought PR into the national third-class protected wild plants list and issued a policy to prohibit its harvest and trafficking. Apart from setting up nature reserves, improving the in-situ protection system, taking ex situ protection, and in vitro preservation when necessary, it is also a feasible measure to study the suitable growth conditions of PR and replace wild products with cultivated products (Peng et al., 2018; Deng and Jin, 2020). PR is drought-tolerant and likes cool temperatures, which are common on hillsides facing the sun, forest edges, roadsides, and ridges. PR is favorable to be grown where the warmest seasonal and annual precipitation range from 148 mm to 512 mm and 300 mm to 500 mm, respectively, the annual average temperature is between 8.4°C and 15.4°C, the total solar radiation is 502.42∼586.15 J/cm, and the altitude is between 100 m and 2,000 m.
As early as 1947, researchers from Shanghai, China, isolated an amorphous acidic saponin from PR, which was hydrolyzed to obtain Tenuigenin A and B (CHOU et al., 1947). In 1964, two kinds of sugar, 5-anhydro-D-sorbitol and N-acetyl-D-glucosamine, were isolated from PR (Takiura and Honda, 1964). In 1977, 3,4,5-trimethoxycinnamic acid (TMCA), 1,2,3,7-tetramethoxyxanthone, 1,2,3,6,7-pentamethoxyxanthone, and 6-hydroxy-1,2,3,7-tetramethoxyxanthone were isolated from PR (Ito et al., 1977). At present, more than 320 metabolites have been isolated and identified from PR, including saponins, xanthones, oligosaccharides, and other metabolites. In this section, we summarize these metabolites and list them in Tables 1–3, and their structures can be seen in Figures 3–7.
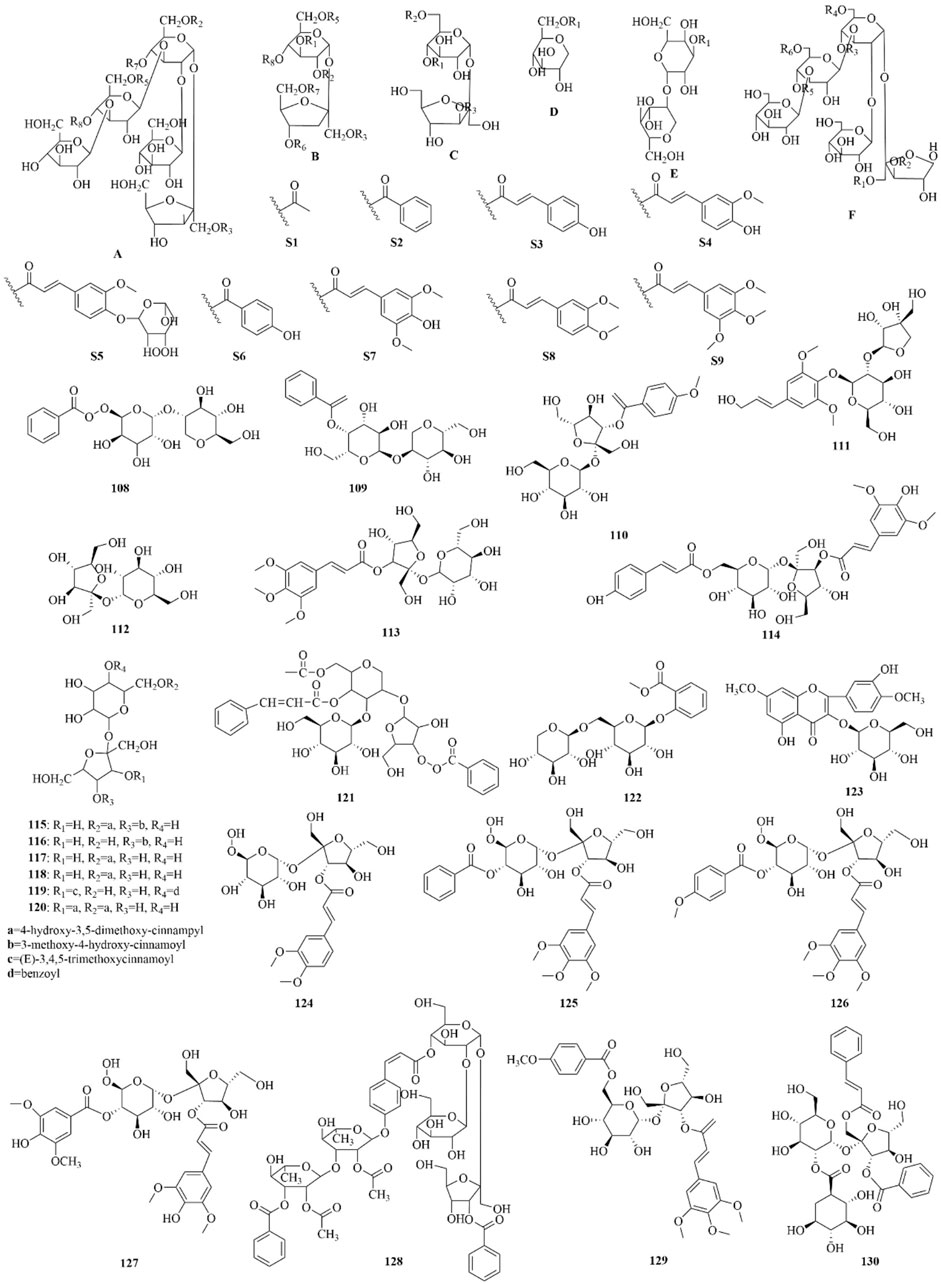
Figure 4. Structures of oligosaccharide esters [parent nucleus (A-F), substituent group (S1-S9), 108–130] in PR.
Saponins are the most important active metabolites of PR and are associated with a wide range of potent pharmacological effects. To date, 70 saponins with oleanane-type pentacyclic triterpene saponins as the parent nucleus, usually linked to glucose, rhamnose, lactose, xylose, galactose, and other sugar types, have been isolated from PR (As shown in Table 1; Figure 3) (Chen and Zhang, 2019). Saponins are unstable and can easily decompose into senegenin, polygalacic acid, tenuifolin, and polygalaxanthone III (PX3) when meeting acid (Gao et al., 2022), possibly explaining the few reports of the monomer. Modern research proves that PR saponins have multiple pharmacological effects, including enhancing immunity, protecting vision, anti-tumor, anti-cardiovascular, and anti-AD (Gao et al., 2022). TLC identification and content determination based on tenuifolin are important methods for the quality control of PR.
Oligosaccharide esters in PR are a class of metabolites mainly based on sucrose, glucose, or other trisaccharide small molecules as the parent nucleus. They form oligosaccharides by linking with rhamnose or glucose through various glycosidic bonds and then further form esters with organic acid constituents such as acetic acid, benzoic acid, and phenylpropenoic acid. They have no more than five sugar molecules in their molecule. Modern research shows that oligosaccharide esters of PR have neuroprotective, antidepressant, anti-dementia, and other central pharmacological effects (Sang et al., 2017). 3′6-Disinapoyl sucrose is another quality control indicator of PR in the Chinese Pharmacopoeia. Oligosaccharide esters isolated and identified from PR are listed and shown in Supplementary Table 5; Figure 4.
Xanthone is a general term for secondary metabolites with a tricyclic aromatic hydrocarbon system structure, also known as benzophenanthrone and dibenzo-γ-pyrone. About 70 xanthones have been identified from PR, whose parent nucleus is composed of a benzene ring paralleled with a chromone and has eight substitution sites. Xanthones in PR are mainly simple ketones, with common substituents including hydroxyl, methoxy, and methylenedioxy groups (Wang et al., 2020b). PX3 is one of the index metabolites for content determination of PR in the Chinese Pharmacopoeia 2020 edition. The xanthones obtained from PR are shown in Table 2; Figure 5.
Alkaloids, organic acids, volatile oils, coumarins, phenylpropanoids, and steroidals are also present in PR (Zhao et al., 2020), as shown in Table 3; Figures 6, 7. PR also contains trace elements (Li, 2008), such as Zn, Cu, Fe, Mn, K, Ca, and Mg.
Nowadays, studies of metabolite separation from the roots of PR have gradually decreased, and the existing research mostly focuses on saponins and oligosaccharide esters. The attention to xanthones and other metabolites should be improved. This may be related to the relatively low content of these metabolites, and optimizing the technical of extraction, enrichment, and purification may improve the situation. The aerial parts of PR, which have been discarded as waste, are beginning to receive attention and show potential. Tan et al. adopted silica gel, a medium-pressure octadecylsilyl (ODS) chromatographic column, and high-performance liquid chromatography to separate and purify the n-butanol part of the 70% ethanol extract of the aerial part of PR from 13 metabolites of which nine metabolites, including saponin metabolite II, were isolated from plants of Polygalaceae for the first time (Tan et al., 2022). Further studies are needed to investigate the differences in chemical composition and content between the aerial parts and roots of PR to further guide their rational development and utilization. Furthermore, the structure-activity relationship of active substances in PR has not been fully revealed; future research is needed to guide the development of lead metabolites, which will help to synthesize or semi-synthesize new derivatives with high efficiency, low toxicity, and safety. In addition, the current extraction and separation technologies mainly use organic reagents such as n-butanol, ethyl acetate, petroleum, and so on, which are irritating to the human body and harmful to the environment. With the advancement of analytical methods and techniques, it is believed that more advanced and environmentally friendly technologies and instruments will be applied.
Modern pharmacological studies have shown that PR has significant pharmacological activity against nervous system diseases and has anti-oxidation, anti-inflammatory, anti-tumor, and anti-pathogenic microorganism functions, as well as other effects (Zhou et al., 2022), as shown in Supplementary Table 6.
PR can improve the transport and clearance of Aβ by up-regulating the protein expression of low-density lipoprotein receptor-related protein 1, promoting p21, and down-regulating the receptors for advanced glycation end products and cyclinD1 and E2F1 protein expressions. At the same time, it can inhibit caspase-3, caspase-8, and Bax expression and increase Bcl-2 expression (Yu et al., 2022). The expression of two main enzymes involved in Aβ degradation, insulin-degrading enzyme (IDE) and neprilysin (NEP), can be enhanced by a pectin polysaccharide named RP02-1. RP02-1 can also inhibit Aβ aggregation, and its effect on Aβ aggregation and production made it a candidate leading metabolite for AD (Zeng et al., 2020a). Polygala tenuifolia polysaccharide, another important polysaccharide from PR, reduces Aβ deposition, activates extracellular-signal-regulated kinase (ERK) pathway-related proteins, and enhances synaptic plasticity, which allows it to improve spatial cognitive deficits in AD mice to reduce cellular damage in the hippocampal CA3 region and to maintain the balance of the cholinergic system, thus exerting an anti-AD effect in vivo (Li et al., 2024). It is necessary to reveal their structure-activity relationship and study safety for structural modification and optimization as well as for the development of new drugs. 3,6′-Disinapoyl sucrose (DISS) is an oligosaccharide ester of PR that can regulate the mRNA expression of antioxidation and autophagy-related genes to reduce Aβ deposition and neurotoxicity to prolong the lifespan of Caenorhabditis elegans (C.elegans) (Tang et al., 2022). However, the lack of positive controls prevents us from determining whether DISS has an advantage over marketed drugs. Interestingly, it has been reported that the extract of PR can prevent axon growth cone collapse and axon atrophy caused by Aβ deposition by inhibiting endocytosis in transgenic mice and cultured neurons, but it has no effect on Aβ deposition. A study shows that significant clinical benefit can be observed in clinical trials only if the Aβ plaque is reduced to a low level (∼20 centiloids) (Karran and De Strooper, 2022). This finding reminds suggests making full use of advanced technology and equipment to quantify and monitor the magnitude and velocity of Aβ clearance in future experiments to screen the metabolites that are worthy of further clinical trials, which would help transform scientific research results into clinical application achievements.
It is well known that the presence of alpha-synuclein in the cytoplasm of residual neurons is one of the recognized pathological features of PD, and repeated amplification of more than 35 CAG trinucleotides leads to a long mutant polyQ region in the Huntington protein that causes HD. The therapeutic effect of ambroxol in PD is associated with a reduction in alpha-synuclein. It is more effective in combination with PR and is safe while not affecting its absorption (Yang et al., 2021). This evidence shows that PR and its metabolites can inhibit the secretion and production of Aβ, weaken its toxicity, promote its degradation and clearance, inhibit the hyperphosphorylation of Tau protein, and accelerate the clearance of the Huntington protein and α -synuclein to inhibit the deposition of all these proteins.
The synapse is the hub of neuronal signal transduction, and its function is influenced by neurotransmitters, calcium, presynaptic vesicle dynamics, and postsynaptic signal transduction. Its functional obstruction is one of the culprits of NDD. The therapeutic and relieving effects of PR on symptoms triggered by NDD are closely related to its effects of preventing axonal degeneration and improving synaptic plasticity. M2 microglia can inhibit inflammation and show a neuroprotective effect, and their relative preponderance over M1 can be induced by PR to protect the injured spinal cord from axonal degeneration. Sibiricose A5, DISS, PX3, sibiricose A6, and sibiricaxanthone A were detected in the spinal cord extract, and sibiricose A5 and DISS were found to be the active ingredients (Kuboyama et al., 2021). RP01-1 is a pectin from PR that showed a neurite outgrowth-inducing effect by increasing brain-derived neurotrophic factor (BDNF) expression and promoting AKT, ERK, and cAMP-response element-binding protein (CREB) and phosphorylation (Zeng et al., 2020b). Oligosaccharide esters (OEs) of PR can also promote BDNF, pAkt/Akt, and pCREB/CREB expressions (Niu et al., 2022). The dendritic length and dreblin cluster density were quantified by an in vitro high connotation imaging analysis system and immunocytochemical analysis, and the results showed that PR and tenuifolin could reduce dreblin cluster density by N-methyl-D-aspartic acid-type glutamate receptors, directly improving synaptic plasticity, and PR had a better effect than tenuifolin alone (Koganezawa et al., 2021). The synergistic effects of complex metabolites in PR and other monomers with this effect need further exploration and screening.
Due to the close polarity and mutual transformation of saponins, it is difficult to separate and purify the effective metabolites, and fully clarifying the synergistic effect of each metabolite in the extract and the influence of other metabolites on the pharmacokinetics of onjisaponin B. requires further work. Tenuifolin (TEN) can remove damaged mitochondria by up-regulating the mRNA expression of LC3, cathepsin D, Rab7, PTEN-induced putative kinase 1 (PINK1), Parkin, and the protein expression of LC3, PINK1, Parkin, and down-regulating the expression of p62 in vivo (Lu et al., 2021). When it was used to treat PC12 cells induced by Aβ25-35, it upregulated p62 expression (Li et al., 2023a). This divergence needs to be further verified and explained. A 2022 study found that TEN could remove damaged mitochondria via activating the mitochondrion phagocytosis mediated by PINK1/Parkin as well. It induced the formation of mitochondrial phagocytes and mitochondrial lysosomes, promoted the transformation from LC3 I to LC3 II, and downregulated p62 expression, and the transfer of Parkin to mitochondria was also promoted (Tian et al., 2022). In addition, PR can also inhibit autophagy protection-related proteins to treat spinal cord injury. TEN upregulated growth-associated protein 43 and neurofilament 200, decreased the Beclin-1 and LC3B-II/LC3B-I levels in the spinal cord, and suppressed the level of the PTPN1 protein, which can suppress IRS1 protein to reduce the pAkt and mTOR levels. What is more, the promoting effect of TEN on the functional recovery from spinal cord injury (SCI) rats was blocked by overexpression of PTPN1, an Akt/mTOR antagonist LY294002, and an autophagy inhibitor 3-MA. This indicates that TEN inhibits autophagy by blocking PTPN1 and modulating IRS1/Akt/mTOR signal transduction to promote the functional recovery of SCI rats (Zang et al., 2023).
Energy metabolism defects are involved in the pathological process of NDD, and maintaining the steady state of energy metabolism is a necessary condition for the normal function of neurons. Mitochondria, the basic organelles of almost all cells in the human body, supply energy, synthesize reactive oxygen species, maintain calcium homeostasis, and affect cell death. In addition to improving glucose utilization, PR can also regulate mitochondrial dysfunction to improve brain energy metabolism and play an important role in treating NDD. It can reverse the phenomena of decreased numbers of mitochondria, structural swelling, vacuole degeneration, distortion or disappearance of the mitochondrial crest, decreased mitochondrial membrane potential (MMP), and enhanced Cyt c expression, apoptosis, and oxidative stress. What is more, GSH, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) expression were increased, caspase-3 expression was downregulated, and tyrosine hydroxylase expression was upregulated. Tenuifolin has a mitochondrial protective function as well, which has been verified in many in vitro and in vivo studies. It can prevent the loss of MMP, reduce the positive rate of β-galactosidase, increase intracellular ATPase activity, inhibit the increase of inflammatory factors, downregulate the expression of NF-κB and cyclooxygenase-2 (COX-2), upregulate the expression of PPARγ and PGC-1α, and inhibit the activation of caspase-3 and caspase-9 (Wang et al., 2019; Jin et al., 2022; Li et al., 2023b). In recent years, significant changes in lipid metabolism have been observed in the brains of AD models, which may be a new target of NDD (Zhang et al., 2021), but it is not clear whether PR has an effect on this target. In order to further reveal the active mechanism of PR, laser microdissection, in combination with high-performance liquid chromatography-mass spectrometry, can be used to probe the probable lipid changes in the micro area in the brains of AD models before and after treatment.
PR has played an anti-AD role in mouse and PC 12 cell models induced by Aβ1-42, which improved the cognitive impairment of mice, increased cell viability, and inhibited apoptosis. A mechanism study showed that PR reduced the contents of reactive oxygen species (ROS) 8-hydroxy-deoxy-guanosine (8-OHdG) and 3-nitrotyrosine (3-NT), activated the PI3K/Akt signaling pathway, and increased the ratios of P-PI3K/PI3K, pAkt/Akt and Bax, enhanced heme oxygenase 1 (HO-1), and promoted nuclear factor E2-related factor 2 (Nrf2) nuclear translocation (Ren et al., 2022). The protective effect of onjisaponin B on a 1-methyl-4-phenyl-1.2.3.6-tetrahydropyridine (MPTP)-induced PD mouse model is achieved through the antioxidant and anti-inflammatory activities mediated by the RhoA/ROCK2 signaling pathway (Peng et al., 2020b). Tenuifolin can prevent the upstream activating factor of NF-κB and the migration of NF-κB to the nucleus and inhibit the release of inflammatory factors and the expression of inducible nitric oxide synthase (iNOS) and COX-2, thus antagonizing nitric oxide (NO)-induced oxidative stress and protecting BV2 and SH-SY5Y cells from Aβ42-induced inflammatory and oxidative stress (Chen and Jia, 2020). It can also improve the behavioral performance of sleep-disordered rats induced by sleep deprivation in a Y maze, object recognition, and an avoidance test, stimulate the production of anti-inflammatory factor IL-10, reduce the production of pro-inflammatory factors IL-1β, IL-6, and IL-18, and activate microglia and increase the expression of 2-related factor 2 and heme oxygenase-1 (HMOX1). At the same time, the expressions of NLRP 3 and caspase-1 p20 were inhibited, the signal cascade of BDNF was downregulated, and the damage to the hippocampal nerve was alleviated (Jiang et al., 2023).
The binding of tenuifolin and onjisaponin B with ChEs was studied using various spectral techniques (Gao and Du, 2022). It was found that there was mainly hydrophobic force between tenuifolin and acetylcholinesterase (AChE), which did not affect its structure, quenched its fluorescence dynamically by affecting tryptophan, and had no inhibitory activity on ChEs. The inhibitory effect of tenuifolin on AChE in these two experiments is the opposite, which may be due to the difference between the internal and external environments. More experimental evidence is needed. Onjisaponin B mainly binds ChEs by van der Waals forces and hydrogen bonds and changes their structures, which has cholinesterase (ChE) inhibitory activity by influencing the static quenching of endogenous fluorescence of tyrosine residues. Similarly, due to the difference in the internal and external environments, the enhancement potential of onjisaponin B on the central cholinergic system needs to be verified in more disease models. The aerial part of PR improved learning and memory impairment induced by D-galactose/NaNO2 and scopolamine in mice, increased the levels of ACh, choline acetyl-transferase (ChAT), BDNF, IL-10, glutathione, and SOD, and decreased the levels of IL-1β, AChE, and malondialdehyde (MDA) (Wang et al., 2020a). In addition, it also increased the protein and mRNA expressions of BDNF and tropomyosin receptor kinase B in the hippocampus (Zhang et al., 2020). These inspiring results show that scholars should compare the pharmacological effects and mechanisms of the aerial part of PR with its root. Expanding its application scope would be beneficial to reducing waste.
Seventeen saponins, including onjisaponin B, can induce mitosis and inhibit NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory corpuscles by up-regulating SHP-2 to activate AMPK/mTOR and PINK1/parkin signaling pathways in Aβ1–42-, A53t-α-synuclein-, or Q74-induced BV-2 cells (Qiu et al., 2022). TEN can scavenge free radicals, downregulate NO, ROS, various inflammatory factors [TNF-α, IL-1β, IL-6, COX-2, prostaglandin E2(PGE2)], matrix metalloproteinases (MMP-9 and MMP-2) and other cytotoxic factors, and upregulate transcription factors related to oxidative stress (Nrf2 and HO-1) (Pi et al., 2020). The regulation of inflammatory and oxidative stress-related transcription factors is also one of the mechanisms by which tenuifolin improves the dyskinesia of the demyelinating mouse model induced by cuprizone (CPZ), increasing the myelin content in the corpus callosum, thus alleviating demyelination and treating multiple sclerosis (Li et al., 2023c). Through a combination of network pharmacological prediction and in vitro and in vivo experiments, it was found that the anti-inflammatory and anti-apoptosis effects of PA may be realized through PPARγ/NF-κB pathway because its effects can be reversed by PPARγ inhibitor GW9662 (Zhao and Jia, 2024). The development of network pharmacology and molecular docking technology makes elucidating the mechanism of action of drugs convenient. With this help, the complex pathways and targets of PR will become clear. Another polysaccharide from PR, PTP70-2, reduces ROS overproduction and MMP dissipation, which may be related to its downregulation of MyD88 and NF-κB signals through the toll-like receptor 4 (TLR4) pathway. Co-incubation with TLR4 inhibitor TAK242 also supports this possibility (Chen et al., 2022a). In addition, the heteropolysaccharide PTBP-1-3 isolated from the crude polysaccharide alkali extract of PR, which can inhibit the activation of microglia/astrocytes induced by lipopolysaccharide (LPS), reduces the production of NO, TNF-α, and IL-1β in a manner similar to minocycline (Zeng et al., 2022). The effective mechanisms of these new polysaccharides need to be further revealed and clarified, laying the foundation for them to be lead metabolites.
One study showed the effect of DISS was better than onjisaponin B and tenuifolin (Wang et al., 2021b). Bioinformatics methods and verification and verification based on in vitro experiments revealed that DISS can also prevent intracellular calcium overload and abnormal calpain system, increase synaptic protein expression, reduce m-calpain expression, inhibit oxidative stress and iron death, and upregulate BDNF/tropomyosin-related kinase B signal transduction with the help of the and d-galactose combined with Aβ1—42 (GCA) in an induced AD model (Li et al., 2023d).
In addition to the mechanisms mentioned above, PR can also prevent and treat NDD by exerting estrogen-like effects that regulate the intestinal microenvironment and the immune system. PR reversed these phenomena in a mouse model of estrogen exhaustion induced by 4-vinylcyclohexene diepoxide and increased the expression of Bcl-2-associated athanogene (BAG1) in the hippocampus without increasing the serum estradiol level (Han et al., 2021). The methanol metabolite of PR containing 17 saponins can reverse the abnormal increase of C3 complement protein, increase beneficial bacteria in the intestine, inhibit harmful bacteria to regulate the intestinal microenvironment, improve spatial memory of mice, and weaken neuroinflammation and aging and improve the behavior of C. elegans and prolong its life (Zeng et al., 2021b).
Modern research shows that TEN can significantly improve neurological function and reduce brain edema, hematoma volume, and hemoglobin level in an intracerebral hemorrhage (ICH) model 72 h after administration with the optimal dosage of 16 mg/kg by regulating 26 differentially expressed proteins mainly related to the complement and coagulation cascade (Wang et al., 2024). Both the root and aerial parts of PR can protect against myocardial injury induced by isoproterenol in rats by inhibiting oxidative stress. There was no significant difference between them, which once again proves that the aerial parts of PR have medicinal value (Fu et al., 2022). TEN has protective effects on brain diseases caused by hypoxia/reoxygenation and ischemia/reperfusion, and its mechanism is related to neuroprotection, inhibition of lipid peroxidation, apoptosis, and iron death. In the H/R model, it can increase the cell survival rate, enhance the expression of JNK and -c-Jun proteins, inhibit their phosphorylation, upregulate Bcl-2, SOD, GSH, GSH-Px, GPX4, SLC7A11 and MMP, inhibit Bax, caspase-3, NOX, LDH, MDA, ACSL4, and decrease intracellular ROS accumulation and Ca2+, Fe2+ levels (Liao et al., 2019; Qiu et al., 2021). Additionally, TEN could cooperate with endothelial progenitor cell transplantation to improve cardiac function in rats with acute myocardial infarction (Jin and Qiao, 2019). Generally speaking, PR has a strong therapeutic effect on cardiovascular and cerebrovascular diseases by regulating calcium channels, inhibiting cell death, regulating mitochondrial function, inhibiting oxidative stress, and inhibiting inflammation. TEN is its main effective metabolite.
Under the influence of COVID-19, in 2020, the number of patients with major depression and anxiety increased by 28% and 26%, respectively (Collaborators, 2021). According to the WHO World Mental Health Report 2022, nearly 1 billion people in the world suffer from mental illness. Statistics show that by December 2023, there were 6.988 million registered patients with severe mental disorders in China. Mental illnesses, such as depression, anxiety disorder, schizophrenia, bipolar disorder, and post-traumatic stress disorder, can not only lead to disability and suicide but can also lead to a significant reduction in life expectancy (Chan et al., 2023). Scholars are looking for and developing drugs to prevent and treat these diseases that bring heavy economic burdens and social problems to individuals, countries, and even the world. PR has attracted much attention because of its anti-mental disorder effect, and the related effects and mechanisms are summarized as follows.
Depression is the most common psychological disorder with high incidence and high mortality, and patients often have significant and persistent depression and pessimism. In addition to the effects of genetics and adverse life circumstances, the pathogenesis of depression involves multiple abnormalities of the monoamine neurotransmitter hypothalamic-pituitary-adrenal (HPA) axis, inflammatory cytokines, neuroplasticity and neurogenesis, and brain structure and function. Depression is triggered when there is a decrease in the level or function of neurotransmitters (e.g., 5-hydroxytryptamine (5-HT), DA, NE) and ACh in the synaptic gaps of the CNS that can affect the body’s sense of pleasure, wellbeing, transmission of emotions, and maintenance of learning and memory abilities. PR can inhibit the decreased levels or function of these neurotransmitters, exerting antidepressant effects similar to those of monoamine or triamine reuptake inhibitors. The effects of these two metabolites on animal models are unknown. In addition, polygalatenoside A compounds extracted from other plants have been reported to have antimelanoma potential (Kim et al., 2022). Wang et al. found that the antidepressant mechanism of tenuifolin was related to increasing the expression of 5-HT and decreasing the expression of indoleamine 2; 3-dioxygenase (IDO) in the cerebral cortex and inhibiting the AChE activity and increasing the activity of ChAT in the hippocampus of mice (Wang, 2020). PR can reverse abnormal behavior and enhance expression of LC3-II and beclin1 in chronic restraint stress (CRS) rats in the sucrose preference test, novelty inhibition feeding test, open-air test, and forced-swim test (FST), and it also reduces P62 levels in both in the cortex of mice and prefrontal cortex of rats and reverses microglia activation, astrocyte damage, NLRP3, ASC, caspase-1 protein and pro-inflammatory cytokine expression increases (Zhou et al., 2021).
The antidepressant effect of PR is also related to regulating intestinal flora, neuroprotection, and affecting metabolism in vivo. An extract and oligosaccharide ester of PR improved the depressive behavior of rats with chronic unpredictable mild stress (CUMS), increased the levels of NE, 5-HT, dihydroxyphenylacetic acid (DOPAC), and 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus, and decreased the levels of CRF, adrenocorticotropic hormone, corticosterone, IL-6, and LPS in serum. The results of transmission electron microscope observation show that PR can improve the structure of intestinal flora, restore the function of the intestinal barrier, reduce the release of intestinal endotoxin, and reduce the level of inflammation, thus playing an antidepressant role (Chen et al., 2021; Chen et al., 2023). Another study showed that the extract of PR could inhibit the activation of NF-κB-NLRP3 signal and intestinal inflammation, increase probiotics such as Lactobacillus and Bacteroides, regulate the content of metabolites and enzyme expression related to the metabolism of tryptophan-kynurenine in the colon, and increase the expression of tight junction protein to balance the gastrointestinal environment (Li et al., 2022a). However, it is necessary to screen the effective monomer metabolites in the extract and further explore whether the metabolites exert synergistic effects by influencing each other’s structures or generating co-assemblies. Yunfeng Zhou first discovered the disorder of metabolic pathways such as amino acids, energy, intestinal flora, and purine in the urine and hippocampus of olfactory bulbectomy rats by non-targeted metabonomics. This study confirmed that PR could enhance autophagy, correct the abnormality of the AMPK-mTOR signal pathway, inhibit neuroinflammation, improve neuroplasticity, play an antidepressant role, and improve the depressive behavior in a despair model, chronic unpredictable stress model, and an olfactory bulb resection model (Zhou, 2020). In a word, PR acts on depression through multiple channels and multiple targets, and it has great potential to be developed as an antidepressant.
Statistics from the WHO show that 27% of people in the world suffer from sleep disorders. Sleep disorder is not only an important pathogenic factor of NDD and mental diseases but also a complication. PR has sedative, hypnotic, antiepileptic, and anxiolytic effects and can be used to improve sleep disorders. PR plays a sedative role in zebrafish by inhibiting 5-HT reuptake, activating GABA, and improving the expression level of GABA transporter 1 (Chen et al., 2020). In the senile insomnia rat model, PR improved the animals’ weight, memory, and sleep time, increased the levels of 5-HT water and GABA, decreased Glu level, and downregulated Fuom and Pcp2, which indicated that the effects of Polygala tenuifolia on sleep disorders might be related to nerve and metabolic pathways in addition to the GABA signaling pathway (Ren et al., 2020). PR has been proven to regulate the disorder of circadian rhythm by regulating the CaMKII pathway and treating disorders caused by sleep delay syndrome, extreme nighttime activities, and social jet lag (Haraguchi et al., 2022). Zhang et al. (2023) used the selective antagonist of the GABA benzodiazepine (BZ) site and confirmed that TEN promoted a kind of non-rapid eye movement (NREM) sleep equivalent to physiological NREM sleep through the interaction with GABAA receptor and also confirmed the improvement of sleep disorder of PD mice by TEN. This suggests that animal models with two or more diseases could be used to explore the role of Polygala tenuifolia, which is conducive to further understanding its mechanism of treating different diseases with the same treatment. Recently, a study showed that two saponins of PR, YZ-I and YZ-II, had sedative and hypnotic effects, increased the concentration of 5-HT, NE, PGD2, IL-1β, and TNF-α, and inhibited its inflammation to GABAARα2, GABAARα3, GAD65/67, 5-HT1A, and 5-HT2A, as well as DPR, PGD2, iNOS, and TNF-α (Hao et al., 2024). Moreover, the purer YZ-II has a stronger effect on inflammatory factors and inflammatory mediators in a concentration-dependent manner, which suggests that it is necessary to further explore the differences in composition and mechanism of action between the two metabolites and that monomer metabolites also have important research value.
Note that PR plays a major role in Ren Shen Yang Rong Tang, a decoction used to treat several conditions, for improving the low sociality in NPY-KO zebrafish and increasing its time spent in the fish tank area by inhibiting the activity of HPA-, SAM-, and GABAergic neurons and down-regulating CREB signaling (Kawabe et al., 2022). Systematically screening the metabolites of PR that have therapeutic effects on social disorders, verifying their activities in vitro and in vivo, and exploring their mechanisms could prove useful in promoting their further development.
A study has shown that polysaccharides of PR can prolong the movement time of mice, increase the glycogen storage concentration in the liver and muscle of mice, enhance the activity of lactate dehydrogenase in vivo, and inhibit the production of serum urea nitrogen in tired mice, and these effects will be enhanced with the increase in dose (Xie, 2021). However, further studies on the related mechanisms and pathways are needed to reveal the connotation of polysaccharides in PR against exercise fatigue. In the weight-loaded swimming test, hypoxia tolerance under normal pressure, sodium nitrite poisoning survival test, and swimming with load and fatigue rotating rod test, the extracts of PR decreased serum CK, blood urea nitrogen (BUN) and LA, cleared sports metabolites, increased the levels of SOD, GSH, ATP, succinate dehydrogenase (SDH), and malate dehydrogenase (MDH), decreased MDA, and increased the protein expression levels of AMPK and Nrf-2, thus effectively relieving the fatigue and hypoxia of normal mice and hydrocortisone-induced kidney-yang deficiency mice (Wang, 2020c). However, the active monomers contained in PR need to be systematically screened. Overall, PR has the potential to be developed into food additives and health products because of its anti-aging and anti-fatigue effects.
Research has revealed that PR is composed of Ara, Gal, and Glc (molar ratio: 2.6: 1.8: 1.0) and has α and β configurations. It can induce apoptosis of lung cancer cells, upregulate the cascade reactions of FAS, FAS-L, and FADD to express the caspase family, induce apoptosis, upregulate LC 3B-II, and downregulate P62 to induce autophagy (Yu et al., 2020). In S180 sarcoma cells and S180 tumor-bearing mice, it increased the ratio of Bax and Bcl-2, promoted cytochrome c release and caspase-9/-3 expression, revised the immune organ indexes, the activities of NK cells and lymphocytes, and increased secretion of IL-2, interferon-γ (IFN-γ) and TNF-α (Yu et al., 2021). The RP02-1 also can inhibit the growth of pancreatic cancer cells, regulate Bcl-2 and Bax, and cleave caspase-3 to inhibit the proliferation, migration, and colony formation of cancer cells in vivo and in vitro with no toxicity (Bian et al., 2020). However, the safety of these polysaccharides needs further evaluation. In addition, targeted metabonomics technology can be used to study the pharmacokinetics of polysaccharides in target organs, revealing their absorption mechanisms and structure-activity relationships, which is helpful in guiding their structural modification.
Song et al. (2019) found the complex euxanthone with Cu (Ⅱ) has an inhibitory effect on esophagus cancer cells (ECA109), stomach cancer cells (SGC7901), and cervical cancer cells (HeLa) by intercalating and damaging their DNA to inhibit their growth. A study in 2021 used virtual screening, molecular docking, and molecular dynamics simulation to screen and verify that PX3 can inhibit an X-linked inhibitor of apoptosis protein and promote the release of caspases to induce apoptosis, thus treating cancer (Opo et al., 2021). Moreover, 4-O-benzoyl-3′-O-(O-methylsinapoyl)-sucrose from PR can inhibit glucuronidase to relieve side effects such as intestinal bleeding and diarrhea caused by anticancer drugs (Kim et al., 2021). However, these studies have only been verified at the in vitro level and lack in vivo data.
PR has an inhibitory effect on severe acute respiratory syndrome coronavirus 2 in vitro, with an IC50 value of 9.5 g/mL (Ngwe Tun et al., 2022). This shows that it has a potential therapeutic effect on COVID-19. More in vitro and in vivo experiments are needed to reveal its mechanisms and targets.
A chloroform extract of PR could inhibit the main target of diabetes—protein tyrosine phosphatase 1B (PTP1B), and xanthones, sterols, and fatty acids were speculated as the main active substances (Mei et al., 2021). The seed oil of PR can inhibit total cholesterol (TC) and triglyceride levels in plasma and the liver, reduce IL-6 and TNF-α levels, inhibit the NF-κB signaling pathway, and promote the inactivation of steroid regulatory element-binding protein-1 (SREBP1) and SREBP2 involved in lipogenesis (Xin et al., 2023). Therefore, it can potentially be developed as a dietary supplement for inhibiting metabolic-associated fatty liver disease.
PR can shorten the clotting time of mice, and the activity of ethyl acetate and n-butanol extracts is stronger than that of water extracts. The anticoagulant substances are mainly xanthones and OEs, and oleic acid and linoleic acid play an auxiliary role (Li et al., 2020). However, it is not clear whether PR acts on endogenous, exogenous, or common coagulation pathways, and it is necessary to further study its influence on coagulation factors. Onjisaponin B can target and regulate p65/Cas3 to resist radiation (Wang et al., 2022).
PR also has anti-inflammatory activities in vitro and has inhibitory effects on pro-inflammatory cytokines IL-12, p40, IL-6, and TNF-α. What is more, the sugar units at C-3 and/or C-28 of the aglycon in triterpenoid saponins and substitute groups in molecules of phenolic glycosides, especially –OH, –OCH3, and –COOH, were considered to be critical to their anti-inflammatory effect (Vinh et al., 2020). 3-O-(3,4,5-trimethoxy-cinnamoyl), 6′-O-(p-methoxybenzoyl) sucrose ester (TCMB), onjisaponin Fg, and 3,4,5-trimethoxycinnamic acid methyl ester can significantly inhibit NO and PGE2. TCMB, 3,4,5-trimethoxyxanthone, hydrocotoin, and 3,4,5-trimethoxycinnamic acid methyl ester can inhibit PGE2 most significantly. TCMB can decrease the protein levels of iNOS and COX-2 and the mRNA levels of TNF-α, IL-1β, and IL-6. In addition, the results of molecular docking show that it has an affinity for iNOS and COX-2 (Son et al., 2022). Sun et al. (2023) developed an ultra-high performance liquid chromatography-photo-diode array-electrospray ionization-quadrupole-time-of-flight-mass spectrometry-lipoxygenase-fluorescence detector (UPLC-PDA-ESI-Q-TOF-MS-LOX-FLD) method that can rapidly screen small molecular inhibitors of LOX and other enzymes and determined for the first time that tenuifoliside A, sibiricaxanthone B, 7-O-methylmangiferin, polygalaxanthone Ⅲ, onjisaponin B, onjisaponin F, and onjisaponin Z are the active anti-inflammatory metabolites. These experiments brought new anti-inflammatory metabolites of PR into our view, although they have only been verified in vitro. More animal experiments and clinical trials are needed to further validate these anti-inflammatory effects and provide a scientific basis for its selection as a natural anti-inflammatory metabolite for clinical treatment. Its structure can be optimized to enhance its targeting and stability, making it better applied to the development of anti-inflammatory drugs.
The extract of PR (1.2 mg/mL), consisting of eight OEs, two xanthones, and two saponins, had good 2,2-diphenyl-1-picrylhydrazyl (DPPH)·and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate (ABTS+)·scavenging capacity with a scavenging rate over 80%, and the synergistic effect of oligosaccharide esters and xanthones was presumed to be the material basis (Guo et al., 2019). Future scholars will be able to study the effects of different extraction methods and processes on the antioxidant activities of these polysaccharides. The aerial part of PR can improve the learning and memory ability of model mice, reduce the MDA content in serum, heart, brain, liver, and kidney tissues, and increase SOD, CAT, GSH-Px, and total antioxidant capacity in serum and tissues (Zhang et al., 2019).
In summary, PR has broad-spectrum pharmacological activity, and its related pharmacological research has achieved fruitful results, which provides a sufficient scientific basis for its application. Its further development offers many opportunities and challenges. First, the pathogenesis of various diseases, especially nervous system diseases and cancers, is extremely complicated, and the molecular mechanisms and material basis of PR are still not clear. Advanced technologies, such as spatial chemoproteomics and the combination of deep learning and ultra-high resolution confocal laser scanning microscopy, can be used to observe the changes in cell morphology and function of PR, which is helpful to further understand its pharmacological process and find its targets. In addition, Qualcomm quantitative metabolomics can be combined with LC-MS to screen and identify effective monomer metabolites and small molecular metabolites in the extracts and effective parts of PR. Second, in recent years, with the iteration of science and technology and the development of analytical methods, an increasing number of active metabolites have been separated from PR, especially those with anti-inflammatory activity, further revealing the material basis of pharmacological activities of PR. However, the effects of some metabolites are only reflected in vitro; in view of the different metabolic environments in vivo and in vitro, their activities need to be verified in vivo in animal models. Third, disease models based on nematodes, cells, fruit flies, rats, and mice are used to study pharmacological activities at present. In the future, rhesus monkeys, domestic dogs, and other animal disease models with genetic relationships and metabolic pathways that are more similar to humans can be used for the pharmacological study of PR, which will be conducive to improving the success rate of clinical transformation. Fourth, more toxicological and clinical experiments should be carried out on some metabolites that have shown good pharmacological activities in vitro and in vivo to provide a scientific basis for ensuring their safety and effectiveness and promoting their further development and rational application. Finally, the aerial parts of PR, which are always discarded, also have rich metabolites and similar pharmacological effects. Future research can systematically compare the differences in activity and metabolites between these parts and the roots of PR and provide scientific support for their medicinal value.
Although PR is rich in active ingredients and has systemic pharmacological effects, its toxic and side effects have been reported in ancient and modern literature, which limits its application to some extent. Modern pharmacological studies have found that the toxicity of PR is mainly aimed at the gastrointestinal tract. PR shows irritation to the gastrointestinal tract in rat and mouse models, which can reduce their activities and food intake, affect their hair, inhibit small intestine movement and gastrointestinal emptying, cause gastrointestinal flatulence, stomach volume expansion, intestinal wall thinning, necrosis, mucosal defects, inflammatory cell infiltration, reverse peristalsis, direction, blood vessel congestion, endothelial cell swelling, and other pathological tissue changes and deaths. The results of biochemical indexes showed that PR could inhibit the levels of gastrin (GAS), motilin, substance P (SP), PGE2, Ca2+-ATPase, pepsin activity, and intermittent cells of Cajal (ICC), cause slow waves in gastric electrogram amplitude, and increase the levels of vasoactive intestinal peptide (VIP), somatostatin (SS) and NO (Cui et al., 2021). Fortunately, the toxicity of PR can be reduced or eliminated by compatibility with other herbs or processing to promote the transformation of toxic substances or introduce new substances to counter its irritation to the gastrointestinal tract. For example, the content of magnolol in gastrointestinal fluid increases after the compatibility of PR and Magnolia officinalis cortex, which can inhibit the increase of NO content and VIP expression, regulate the metabolism of energy, amino acids, and fat, and reduce its gastrointestinal toxicity (Ma et al., 2019; Ma et al., 2021). Licorice-simmered PR can improve its irritation to zebrafish and rabbits by reducing DISS and PA, inhibiting the increase of inflammatory factors, and relieving the congestion and swelling of rabbit eyes as well as the damage of iris, cornea, and conjunctiva caused by raw PR (Chen et al., 2024). The aerial parts of PR have no acute toxicity (Sun et al., 2020). The existing toxicological research on PR and its active metabolites is not enough to completely prove its safety. It is necessary to carry out a series of systematic toxicological experiments, including but not limited to skin irritation experiments, mutagenic experiments, and teratogenic experiments.
PR is one of the medicinal and edible species approved by the Chinese government for use as a raw material in health foods. More than 70 health products contain PR or its extracts in the Yaozhi Data, most claiming to improve sleep quality. Other products purport to increase bone density, enhance immunity, delay aging, combat fatigue, clear throat, and improve memory. Yue. et al. developed a metabolite beverage with flavedo and polysaccharides of PR that has a good effect on exercise-induced fatigue (Yue et al., 2020). PR is also utilized for medicated diets with high nutritional value, such as Yuanzhi Lianfen Zhou, which is made of PR, polished japonica rice, and lotus seed. It has efficacy in nourishing the heart, improving intelligence, hearing, and eyesight, and relieving amnesia and insomnia.
According to Gaide Chemical Network statistics, Polygala tenuifolia extract and Polygala tenuifolia root extract are used as skin conditioners in about 207 and 394 cosmetics, respectively, with top safety and no acne risk. These products are various, including liquid foundation, toning lotion, cream, essence, hair tonic, cleansers, shampoo, and foot bath powder. For example, Polygala tenuifolia root extract acts as a skin conditioning agent in “The History of Whoo Cheongidan Hwa Hyun Lotion (后天气丹花献滋养乳).”
In addition, PR was made into an antibacterial sleep-aiding bamboo cellulose fiber for home textiles with the effect of helping sleep [CN202211232475.4] (Hu et al., 2023), an atomized liquid with sedative and soothing effects [CN202211087989.5] (Zhu and Liu, 2022), a composition for promoting skin regeneration and improving skin wound surface [CN202180020964.0] (Pu, 2022), a sleep-assisting tea capable of improving sleep [CN 202180020964.0] (Zhao, 2022), pills for improving intelligence and height [CN202210494926.5] (Zhou, 2022), a mask capable of whitening and removing freckles [CN202210505643.6] (Wang, 2022), a composition capable of curing the effects of drink [CN202211063577.8] (Zhang et al., 2022), and other products. Interestingly, PR can also be used as an additive in pig feed to increase the food intake and body weight of growing-finishing pigs [CN202210343455.8] (Zhan et al., 2022).
To sum up, PR has important practical and economic value and can be used to produce many products. We offer the following suggestions to maximize PR applications: First, with the awakening of public awareness of healthcare, a medicated diet shows great potential. Future research could mine medicinal diet data containing Yuanzhi from ancient and modern literature, explore effects and elucidate mechanisms via in vivo, in vitro, and clinical experiments, summarize differentiated effects across populations, and establish a unified quality control system. These efforts would solidify the foundations for industrialized mass production and market launch. In the second place, the application of PR in cosmetics is still in a marginalized position at present, and most products only utilize the cleaning effect of saponins; future development could address its antioxidant, anti-aging, and anti-inflammation effects and increase its use in products such as maintaining skin barrier stability and improving complexion. The nude mouse model can be used to investigate the effects of these metabolites on skin conditions. Next, effective metabolites and plant additives can be extracted from the aerial and other nonmedicinal parts of PR, reducing waste. Last but not least, the safety of the active ingredients and effective parts of PR should be investigated through the evaluation of the tolerance, toxicological safety, metabolism, and residue of the target animals, which can promote its application in the form of additives in health food, cosmetics, and food.
After processing, the chemical composition and structure and the properties of TCM have changed, and the toxicity is suppressed, which is more in line with the needs of TCM to adapt to the time, local conditions, and people. As a traditional Chinese medicine with superior efficacy and obvious toxic side effects, PR should be processed before clinical application. The ancient processing methods of PR include purification processing methods of removing the wood heart, pounding or grinding into powder, plain stir-frying methods of stir-frying slightly, stir-frying to yellow, baking, and stir-frying to scorch, as well as stir-frying with many different adjuvants (Gao et al., 2020). According to many ancient books, licorice, ginger juice, wine, wheat, black bean juice, bile, rice-washed water, honey, and other auxiliary materials are often used in PR processing. Ancient processing methods of PR are listed in Supplementary Table 3. In addition, there are records of processing PR with two or three of the above adjuvants; the method of decocting PR with black beans and licorice together was recorded in “Jing Yue’s Complete Work” (景岳全书). The common processed products of PR in modern times mainly include stir-fried PR, deep-fried PR, stewed PR, PR stir-fried with licorice juice, Magnolia officinalis cortex juice or honey stir-fried PR and, cinnabar mixed PR (Wu et al., 2024). Among them, PR stir-fried with Magnolia officinalis cortex juice and stewed PR are not included in the processing standards of Chinese herbal pieces, and few studies have examined PR mixed with cinnabar. The modern PR processing methods are shown in Supplementary Table 4.
Modern scholars differ on whether to remove the xylem of PR. On the one hand, they think that although the content of effective metabolites is low in xylem, it also has medicinal value (Liu et al., 2012) and should not be removed. On the other hand, they think that the xylem should be removed because the concentrations of its effective metabolites are several times lower than the phloem (Yang et al., 2018). The amino acids and small molecular organic acids contained in the xylem act on NMDA receptors by acting on 91 potential targets such as SLC6A1, GRM5, GRIA1, and SLC1A1, which makes people feel depressed or anxious (Lou, 2023). At present, the various Chinese herbal medicine quality standards have no compulsory requirement to remove the xylem, and more experimental data are required to reach a consensus on this dispute.
The increase of extractive substances after PR processing may be due to the introduction of liquiritin, glycyrrhizic acid, glucose, and other metabolites that increase with the addition of adjuvants, which can be used as a quality control index (Zhu et al., 2019; Cui et al., 2020). Saponins and oligosaccharide esters in PR are unstable and can be hydrolyzed during the processing. One is deglycosylated, and its glycosidic bonds in C3 or C28 were broken, and the other has its ester bonds broken, resulting in the generation of the second-level glycosides and (or) glycosides with less toxicity (Gao et al., 2021). In addition, licorice can promote the hydrolysis of some saponins in PR into onjisaponin Z and tenuifolin, and stewing PR with licorice can promote the absorption and distribution of oligosaccharides and increase their bioavailability (He et al., 2023; Xiong et al., 2023). The changes in the material basis after processing have important implications for reducing toxicity and increasing the efficiency of PR (Zhao et al., 2021). However, the potential co-assembly phenomena, regularity, and supramolecular mechanisms between various excipients and chemical metabolites of PR need further study.
The Chinese Pharmacopoeia and local processing standards do not specify processing parameters of different PR processed products, and adjuvant dosages and terminal points are not uniform. Currently, the terminal points are judged subjectively by color or whether products are sticky to hands. With the development of analytical technology and understanding of effective substances of PR, its technological optimization indexes have evolved from single extraction content to comprehensive indexes. For example, Yuan et al. (2021) optimized the technological parameters of PR stir-frying with licorice or honey based on seven metabolites. Song et al. (2023) optimized the water addition, licorice dosage, and drying process parameters based on the comprehensive score of five metabolites and established the relationship between the color and metabolites of stewed PR, which provided an important reference for determining the terminal point. However, it is necessary to carry out pre-production tests and scale-up verifications on the optimized processing technology to adapt to modern production and form a standardized operation flow for each processing technology of PR.
PR is a natural product with a long medicinal history, rich chemical metabolites, wide pharmacological effects, and high medicinal value. Research has achieved fruitful results and provides a scientific basis for its wide application in medicine, healthcare products, and cosmetics. However, some shortcomings need to be solved in follow-up studies.
As a popular best-selling herb at home and abroad, the scarcity of wild resources and the long artificial planting cycle put PR in short supply. The aerial parts and wood heart of PR, which were often discarded in the past, have been shown to have medicinal potential, which suggests that their chemical constituents and pharmacological effects should be studied and compared with the root bark of Polygala tenuifolia to expand the source of PR and rationally utilize resources.
PR is widely used in medicine pairs, composition, compound prescriptions, healthcare products, and cosmetics. However, the compatibility mechanisms of related drug pairs, compositions, and compound prescriptions have not been fully revealed, and the interaction between the chemical metabolites of the prescriptions is still unclear and needs further study. In addition, its active ingredients need to be evaluated for safety in order to expand its applications in cosmetics and health products.
With the awakening of modern health awareness, the related medicated diets recorded in ancient books and documents show great potential. Future research can study the compatibility principle, proportion, and applicable populations and promote the emergence of innovative products.
PR contains more than 320 metabolites, of which saponins, oligosaccharide esters, and xanthones are the main metabolites. The low content of xanthones may be the reason for less research into these compounds, so more advanced technologies could be used to extract, enrich, separate, and purify them, and more attention should be paid to this kind of metabolite. Few studies report on the structure-function relationships of the active metabolites in PR, limiting the understanding of the potential modifications and optimizations of the structure of its lead metabolites and hindering the artificial synthesis of its active metabolites and the development of related drugs and health products.
The pharmacological effects of PR, especially its effects against nervous system diseases, have been widely studied, but the transformation from scientific research results to clinical results is rarely seen. Future research could reveal the monomer composition of its active sites, explore its synergistic effects, and conduct safety inspections and clinical research on its active metabolites.
Limited toxicological studies show that PR has no toxicity, but it causes gastrointestinal irritation, probably due to its saponins. Studying the metabolism of saponins in blood, urine, feces, bile, cerebrospinal fluid, and target organs is helpful in clarifying its toxicological mechanism. Furthermore, strengthening the research and safety analysis of the safe range of PR and its active ingredients is conducive to its development and application in drugs, health products, and cosmetics.
Only a few of the many PR processing methods reported in ancient books have been used and supported by modern research. Comparing the chemical composition and pharmacological effects of different processed products of PR recorded in ancient books is beneficial to the inheritance and innovation and provides a reference for its application. In addition, optimizing the processing technology of PR by selecting pharmacological activity indexes in vivo based on different medicinal purposes helps obtain targeted processed products.
To sum up, this study reviews and analyzes the botany, phytochemistry, pharmacological action, toxicity, industrial applications, and processing research of PR and looks forward to its future development, aiming at providing a reference for its sustainable development and rational application.
HK: writing–original draft. LK: investigation, writing–review and editing. AH: investigation, validation, and writing–review and editing. AY: investigation and writing–review and editing. HJ: investigation, validation, and writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The project was funded by Heilongjiang Touyan Innovation Team Program, the National Famous Old Traditional Chinese Medicine Experts Inheritance Studio Construction Program of the National Administration of TCM [No. (2022) No. 75], The Seventh Batch of National Old Traditional Chinese Medicine Experts Academic Experience Inheritance Program [No. (2022) No. 76], the National Training Program for Traditional Chinese Medicine Characteristic Technology Inheritance Talents [No. (2023)], Construction of key disciplines of Chinese medicine processing [No. (2023) No. 14061230012], the Traditional Chinese medicine Processing technology inheritance base project. The stomach and intestine icons utilized in the GRAPHICAL ABSTRACT were generated using Servier Medical Art, provided by Servier (https://smart.servier.com/), and were licensed under https://creativecommons.org/licenses/by/4.0/. Some small icons were added on them to convey information. In addition, the texts in this paper were polished and significantly improved by Stork’s Writing Assistant (https://www.storkapp.me/writeassistant/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1450733/full#supplementary-material
Bian, Y., Zeng, H., Tao, H., Huang, L., Du, Z., Wang, J., et al. (2020). A pectin-like polysaccharide from Polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy. Int. J. Biol. Macromol. 162, 107–115. doi:10.1016/j.ijbiomac.2020.06.054
Chai, S., Yang, F., Yu, H., and Wang, Y. (2018). Analysis of chemical constituents of radix Polygalae by UPLC/ESI-Q-TOF MS. Tianjin J. Tradit. Chin. Med. 35 (01), 60–64. doi:10.11656/j.issn.1672-1519.2018.01.16
Chan, J. K. N., Correll, C. U., Wong, C. S. M., Chu, R. S. T., Fung, V. S. C., Wong, G. H. S., et al. (2023). Life expectancy and years of potential life lost in people with mental disorders: a systematic review and meta-analysis. EClinicalMedicine 65, 102294. doi:10.1016/j.eclinm.2023.102294
Chen, H., Zhong, J., Li, J., Zeng, Z., Yu, Q., and Yan, C. (2022a). PTP70-2, a novel polysaccharide from Polygala tenuifolia, prevents neuroinflammation and protects neurons by suppressing the TLR4-mediated MyD88/NF-κB signaling pathway. Int. J. Biol. Macromol. 194, 546–555. doi:10.1016/j.ijbiomac.2021.11.097
Chen, Q., Jia, T., Wu, X., Chen, X., Wang, J., and Ba, Y. (2023). Polygalae radix oligosaccharide esters may relieve depressive-like behavior in rats with chronic unpredictable mild stress via modulation of gut microbiota. Int. J. Mol. Sci. 24 (18), 13877. doi:10.3390/ijms241813877
Chen, Q., Yu, L., Zhao, W., Xv, R., Chen, X., Wu, X., et al. (2021). Effect of Polygalae radix extract on gut microbiota of depression rat. Chin. Tradit. Herb. Drugs 52 (08), 2313–2323. doi:10.7501/j.issn.0253-2670.2021.08.014
Chen, Q., and Zhang, X. (2019). Triterpenoid constituents and pharmacological activities of Polygalae. Chin. J. Ethnomed Ethnopharm. 28 (19), 49–56. doi:10.3969/j.issn.1007-8517.2019.19.zgmzmjyyzz201919015
Chen, S., and Jia, J. (2020). Tenuifolin attenuates amyloid-β42-induced neuroinflammation in microglia through the NF-κB signaling pathway. J. Alzheimers Dis. 76 (1), 195–205. doi:10.3233/JAD-200077
Chen, S., Jiang, Q., Yang, S., Lv, B., Ma, Z., Li, P., et al. (2024). Glycyrrhizae radix et rhizoma processing ameliorates adverse reactions of polygalae radix in zebra fish and rabbit models. J. Ethnopharmacol. 327, 118020. doi:10.1016/j.jep.2024.118020
Chen, Y., Tan, J., Li, J., Liu, K., Wang, J., Wang, Y., et al. (2022b). A new lignanamide from aerial part of Polygala tenuifolia. Chin. Tradit. Pat. Med. 44 (02), 435–438. doi:10.3969/j.issn.1001-1528.2022.02.018
Chen, Z., Peng, C., Pei, Z., Zhang, M., Yun, T., Yang, Z., et al. (2020). Effects of tenuifolin on rest/wake behaviour in zebrafish. Exp. Ther. Med. 19 (3), 2326–2334. doi:10.3892/etm.2020.8476
Cheng, M., Li, C., Ko, H., Ko, F., Lin, Y., and Wu, T. (2006). Antidepressant principles of the roots of Polygala tenuifolia. J. Nat. Prod. 69 (9), 1305–1309. doi:10.1021/np060207r
Cheng, Y., Tan, J., Li, J., Wang, J., Liu, K., Wang, S., et al. (2022a). Chemical constituents from the ethyl acetate layer of aerial parts of polygala tenuifolia. J. Chin. Med. Mater 45 (02), 331–334. doi:10.1080/14786419.2021.2013838
Cheng, Y., Tan, J., Li, J., Wang, S., Liu, K., Wang, J., et al. (2022b). Chemical constituents from the aerial part of Polygala tenuifolia. Nat. Prod. Res. 36 (21), 5449–5454. doi:10.1080/14786419.2021.2013838
Cho, N., Huh, J., Yang, H., Jeong, E., Kim, Y., Kim, J., et al. (2012). Chemical constituents of Polygala tenuifolia roots and their inhibitory activity on lipopolysaccharide-induced nitric oxide production in BV2 microglia. J. Enzym Inhib. Med. Chem. 27 (1), 1–4. doi:10.3109/14756366.2011.562203
Chou, T., Chu, J., and Mei, P. (1947). The sapogenins of the Chinese drug, yüan chih, Polygala tennuifolia, Willd. J. Am. Pharm. Assoc. 36 (8), 241. doi:10.1002/jps.3030360805
Collaborators, C. M. D. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet London, Engl. 398 (10312), 1700–1712. doi:10.1016/S0140-6736(21)02143-7
Cui, Y., Wu, P., Zhang, D., Zhou, Q., Zhen, Z., Zhao, X., et al. (2020). Comparison of HPLC fingerprints between Polygala tenuifolia and stewed Polygala tenuifolia. J. Chin. Med. Mater. 43 (03), 575–581. doi:10.13863/j.issn1001-4454.2020.03.012
Cui, Y., Zhao, X., Tang, Y., Zhang, Y., Sun, L., and Zhang, X. (2021). Comparative study on the chemical components and gastrointestinal function on rats of the raw product and Licorice-smmered product of Polygala tenuifolia. Evid-Based Compl Alt. Med. doi:10.1155/2021/8855536
Deng, X., and Jin, G. (2020). Suggestions for the artificial cultivation and developmentof Polygala radix. J. Shandong For. Sci. Technol. 50 (03), 102–106+176.
Fan, X., He, Y., Tian, H., Chen, X., and Ji, X. (2020). Polygalae radix industry development in Shanxi province from the perspective of industry chain based on SWOT analysis. Anhui Agr Sci. Bull. 26 (20), 30–33+73. doi:10.3969/j.issn.1008-0805.2020.10.029
Feng, G., Liu, S., Pi, Z., Song, F., and Liu, Z. (2019a). Comprehensive characterization of in vivo metabolic profile of Polygalae radix based on ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 165, 173–181. doi:10.1016/j.jpba.2018.12.005
Fu, Q., Sun, Y., Shi, W., Zheng, Z., Wang, S., and Fang, M. (2022). Protective effect of Polygala tenuifolia root and Polygala tenuifolia seedling on myocardial injury in rats. Chin. Tradit. Pat. Med. 44 (12), 4033–4036. doi:10.3969/j.issn.1001-1528.2022.12.050
Fujita, T., Da-you, L., Ueda, S., and Takeda, Y. (1992). Xanthones from polygala tenuifolia. Phytochemistry 31, 3997–4000. doi:10.1016/s0031-9422(00)97571-x
Gao, C., and Du, H. (2022). The interaction between two metabolites of polygala tenuifolia and cholinesterases. Protein Pept. Lett. 29 (12), 1051–1060. doi:10.2174/0929866529666220825143136
Gao, H., Huang, W., Xiong, Z., Zhang, X., Ye, J., Xiong, Y., et al. (2020). Research progress on processing of Polygalae radix. Chin. J. Exp. Tradit. Med. Form. 26 (23), 209–218. doi:10.13422/j.cnki.syfjx.20201558
Gao, H., Xiong, X., Zhang, Q., Cui, J., Ye, X., and Peng, L. (2021). Analysis of composition changes of Polygalae radix before and after processing based on UPLC-LTQOrbittrap. Trad. Chin. Drug Res. Clin. Pharmacol. 32 (12), 1845–1854. doi:10.19378/j.issn.1003-9783.2021.12.015
Gao, L., Zhou, C., Liu, Q., and Wan, X. (2022). Research progress on Polygala saponins and their pharmacological effects. J. Beijing Union Univ. 36 (03), 58–64. doi:10.27253/d.cnki.gnjzu.2022.000421
Gao, M. (2022). Mechanism of treatment of cognitive disorder in mice with Polygala and Senegenin. Nanjing, China: Nanjing Univ Tradit Chin Med.
Guo, L., Guo, R., Qin, N., Wang, X., and Zhang, X. (2019). Preparation process,composition and antioxidant activity of the Polygala tenuifolia extract. North Hortic. (13), 121–129. doi:10.11937/bfyy.20184422
Han, G., Choi, J., Cha, S., Kim, B., Kho, H., Jang, M., et al. (2021). Effects of radix Polygalae on cognitive decline and depression in estradiol depletion mouse model of menopause. Curr. Issues Mol. Biol. 43 (3), 1669–1684. doi:10.3390/cimb43030118
Hao, K. X., Shen, C. Y., and Jiang, J. G. (2024). Sedative and hypnotic effects of Polygala tenuifolia willd. saponins on insomnia mice and their targets. J. Ethnopharmacol. 323, 117618. doi:10.1016/j.jep.2023.117618
Haraguchi, A., Saito, K., Tahara, Y., and Shibata, S. (2022). Polygalae Radix shortens the circadian period through activation of the CaMKII pathway. Pharm. Biol. 60 (1), 689–698. doi:10.1080/13880209.2022.2048863
He, M., Zhuang, Z., Xing, Y., Li, H., Zhang, X., and Zhao, X. (2023). Exploring of transformation rule of saponins in Polygalae radix before and after processing based on simulated processing technology. Chin. J. Exp. Tradit. Med. Form. 29 (24), 169–176. doi:10.13422/j.cnki.syfjx.20230867
Hu, N., Li, J., Guo, W., Lu, Z., Wang, Y., Li, X., et al. (2023). An antibacterial sleep-aiding bamboo cellulose fiber for home textiles and preparation method thereof.
Huang, Q., and Lin, L. (2021). Research progress of sedative Chinese medicine Polygala tenuifolia. World J. Sleep. Med. 8 (01), 183–184. doi:10.3969/j.issn.2095-7130.2021.01.078
Ikeya, Y., Sugama, K., and Maruno, M. (1994). Xanthone C-glycoside and acylated sugar from Polygala tenuifolia. Chem. Pharm. Bull. 42 (11), 2305–2308. doi:10.1248/cpb.42.2305
Ikeya, Y., Sugama, K., Okada, M., and Mitsuhashi, H. (2008). Four new phenolic glycosides from polygala tenuifolia. Chem. Pharm. Bull. 39 (10), 2600–2605. doi:10.1248/cpb.39.2600
Ito, H., Taniguchi, H., Kita, T., Matsuki, Y., Tachikawa, E., and Fujita, T. (1977). Xanthones and a cinnamic acid derivatives from Polygala tenuifolia. Phytochemistry 16 (10), 1614–1616. doi:10.1016/0031-9422(77)84043-0
Jia, Y., Liu, L., Yang, X., Wang, C., and Ren, H. (2023). A new xanthone from the Polygala tenuifolia Wild of northern Shaanxi. Acta Pharm. Sin. B, 1–13. doi:10.16438/j.0513-4870.2023-0919
Jiang, H., Liu, T., Lin, L., Yao, Z., and Zhao, J. (2016). Predicting the potential distribution of Polygala tenuifolia Willd. under climate change in China. Plos One 11 (9), e0163718. doi:10.1371/journal.pone.0163718
Jiang, N., Zhang, Y., Yao, C., Liu, Y., Chen, Y., Chen, F., et al. (2023). Tenuifolin ameliorates the sleep deprivation-induced cognitive deficits. Phytother. Res. 37 (2), 464–476. doi:10.1002/ptr.7627
Jiang, Y., Duan, Y., Liu, Y., Fang, M., and Shi, R. (2011). Isolation and structure identification of chemical constituent of Yuanzhi(Radix Polygalae). J. Beijing Univ. Tradit. Chin. Med. 34 (02), 122–125.
Jiang, Y., Liu, L., and Tu, P. (2003). Study on chemical constituents of Polygala tenuifolia Ⅲ. Chin. J. Nat. Med. (03), 15–18.
Jiang, Y., and Tu, P. (2003). Tenuifoliose Q, a new oligosaccharide ester from the root of Polygala tenuifolia Willd. J. Asian Nat. Prod. Res. 5 (4), 279–283. doi:10.1080/1028602031000111987
Jiang, Y., and Tu, P. (2004). Structural characteristics and spectroscopic profiles of xanthones from Polygala. J. Peking. Univ. Heal. Sci(01) 36, 94–98. doi:10.3321/j.issn:1671-167X.2004.01.022
Jiang, Y., and Tu, P. (2005). Four new phenones from the cortexes of Polygala tenuifolia. Chem. Pharm. Bull. 53 (9), 1164–1166. doi:10.1248/cpb.53.1164
Jiang, Y., Zhang, W., Tu, P., and Xu, X. (2005). Xanthone glycosides from Polygala tenuifolia and their conformational analyses. J. Nat. Prod. 68 (6), 875–879. doi:10.1021/np050026+
Jin, B., and Park, J. (1993). Studies on the alkaloidal components of Polygala tenuifolia willd. China J. Chin. Mater Med. 18 (11), 675–677.
Jin, G., Yu, H., Lu, X., Huang, Z., and Yang, H. (2022). Protective effects of tenuifolin on hippocampus neurons and neuronal mitochondria in APP/PS1 double transgenic mice. Chin. J. Geriatr. Heart Brain Ves Dis. 24 (04), 426–429. doi:10.3969/j.issn.1009-0126.2022.04.023
Jin, W., and Qiao, X. (2019). Effect of senegenin on cardiac function in rats with acute myocardial infarction treated by transplantation of EPCs. Chin. J. Gerontol. 39 (02), 413–418. doi:10.3969/j.issn.1005-9202.2019.02.053
Karran, E., and De Strooper, B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21 (4), 306–318. doi:10.1038/s41573-022-00391-w
Kawabe, M., Nishida, T., Horita, C., Ikeda, A., Takahashi, R., Inui, A., et al. (2022). Ninjinyoeito improves social behavior disorder in neuropeptide Y deficient zebrafish. Front. Pharmacol. 13, 905711. doi:10.3389/fphar.2022.905711
Kim, H., Hwang, E., Park, B., and Kim, S. (2022). Novel potential NOX2 inhibitors, Dudleya brittonii water extract and polygalatenoside A inhibit intracellular ROS generation and growth of melanoma. Biomed. Pharmacother. 150, 112967. doi:10.1016/j.biopha.2022.112967
Kim, J., Vinh, L., Hur, M., Koo, S., Park, W., Moon, Y., et al. (2021). Inhibitory activity of 4-O-Benzoyl-3'-O-(OMethylsinapoyl) sucrose from Polygala tenuifolia on Escherichia coliβ-glucuronidase. J. Microbiol. Biotechn 31 (11), 1576–1582. doi:10.4014/jmb.2108.08004
Koganezawa, N., Sekino, Y., Kawakami, H., Fuchino, H., Kawahara, N., and Shirao, T. (2021). NMDA receptor-dependent and -independent effects of natural compounds and crude drugs on synaptic states as revealed by drebrin imaging analysis. Eur. J. Neurosci. 53 (11), 3548–3560. doi:10.1111/ejn.15231
Kuboyama, T., Kominato, S., Nagumo, M., and Tohda, C. (2021). Recovery from spinal cord injury via M2 microglial polarization induced by Polygalae Radix. Phytomedicine 82, 153452. doi:10.1016/j.phymed.2020.153452
Kumar, H., Song, S., More, S., Kang, S., Kim, B., Kim, I., et al. (2013). Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules 18 (12), 14670–14693. doi:10.3390/molecules181214670
Kuroda, M., Shizume, T., and Mimaki, Y. (2014). New acylated triterpene glycosides from the roots of Polygala tenuifolia. Nat. Prod. Commun. 9 (3), 1934578X1400900–382. doi:10.1177/1934578x1400900326
Le, T., Jeong, J., and Kim, D. (2012). Clionosterol and ethyl cholestan-22-enol isolated from the rhizome of Polygala tenuifolia inhibit phosphatidylinositol 3-kinase/Akt pathway. Biol. Pharm. Bull. 35 (8), 1379–1383. doi:10.1248/bpb.b12-00426
Lei, R., Yang, B., Gao, J., Wei, Y., Hu, B., and Peng, L. (2020). Effects of drought stress on secondary metabolite contents and antioxidant enzyme activities in callus of Polygala tenuifolia Willd. North Hortic. (22), 109–116. doi:10.11937/bfyy.20194366
Li, C. (2008). Studies on chemical constituents and biological activities of Polygala tenuifolia Willd and Polygala glomerata Lour. Beijing, China: Peking Union Med Coll.
Li, C., Gao, F., Qu, Y., Zhao, P., Wang, X., and Zhu, G. (2023a). Tenuifolin in the prevention of Alzheimer's disease-like phenotypes: investigation of the mechanisms from the perspectives of calpain system, ferroptosis, and apoptosis. Phytother. Res. PTR, 4621–4638. doi:10.1002/ptr.7930
Li, C., Yang, J., Yu, S., Chen, N., Xue, W., Hu, J., et al. (2008a). Triterpenoid saponins with neuroprotective effects from the roots of Polygala tenuifolia. Planta Med. 74 (2), 133–141. doi:10.1055/s-2008-1034296
Li, C., Yang, J., Yu, S., Zhang, D., Xue, W., Yuan, Y., et al. (2011). Triterpenoid saponins and oligosaccharides from the roots of Polygala tenuifolia Willd. Chin. J. Nat. Med. 9 (05), 321–328. doi:10.3724/SP.J.1009.2011.00321
Li, C., Ye, F., Xu, C., Chang, Q., Liu, X., and Pan, R. (2022a). Effect of radix Polygalae extract on the colonic dysfunction in rats induced by chronic restraint stress. J. Ethnopharmacol. 294, 115349. doi:10.1016/j.jep.2022.115349
Li, C., Zhu, G., Chen, Y., Qu, Y., Bian, Z., and Wang, X. (2023b). Protective effect of tenuifolin on HT-22 cells damaged by D-galactose synergistically with Aβ1-42 via PPARγ/PGC-1α signaling pathway. J. Biol. 40 (03), 16–21. doi:10.3969/j.issn.2095-1736.2023.03.016
Li, J., Jiang, Y., and Tu, P. (2006). New acylated triterpene saponins from Polygala tenuifolia Willd. J. Asian Nat. Prod. Res. 8 (6), 499–503. doi:10.1080/10286020500173358
Li, J., Wang, D., Kong, R., Tian, H., Zhang, F., Qin, X., et al. (2019). Identification of Polygala tenuifolia No.2 by PCR allele-specific primers of rDNA ITS sequence. J. Shanxi Med. Univ. 50 (08), 1143–1148. doi:10.3969/j.issn.1001-1528.2019.05.044
Li, J., Wu, S., Li, Y., Yu, L., and Li, Q. (2008b). Determination of trace elements in flos sophorae immaturus and radix polygalae. Lishizhen Med. Mater Med. Res. (02), 332–333. doi:10.3969/j.issn.1008-0805.2008.02.037
Li, J., Zhang, X., Chen, T., Wang, Q., Du, C., Qin, X., et al. (2020). Study on the pharmacodynamic material basis of anticoagulant effect of Polygala tenuifolia. J. Shanxi Univ. Chin. Med. 21 (04), 260–263+275. doi:10.19763/j.cnki.2096-7403.2020.04.08
Li, P., Yan, M., and Li, P. (2005). Isolation and identification of the chemical constituents from Polygala tenuifolia Wild. Chin. J. Med. Chem. (01), 42–45+45. doi:10.3969/j.issn.1005-0108.2005.01.008
Li, X., Chen, S., Chen, W., Song, J., and Zhang, Y. (2022b). Research progress on chemical constituents of Polygala tenuifolia and prevention and treatment of Alzheimer′s Disease. J. Chin. Pharm. 57 (01), 15–23. doi:10.11669/cpj.2022.01.003
Li, Y., Liang, X., and Zhou, Y. (2023c). Effect of Tenuifolin on PINK1/Parkin mediated mitochondrial autophagy in Aβ25-35-induced PC12 cells damage. Liaoning J. Tradit. Chin. Med. 50 (02), 149–152+224-225. doi:10.3964/j.issn.1000-0593(2023)04-1103-09
Li, Y., Wu, H., Liu, M., Zhang, Z., Ji, Y., Xu, L., et al. (2024). Polysaccharide from Polygala tenuifolia alleviates cognitive decline in Alzheimer's disease mice by alleviating Aβ damage and targeting the ERK pathway. J. Ethnopharmacol. 321, 117564. doi:10.1016/j.jep.2023.117564
Li, Y., Yang, Z., Wang, H., Yang, L., Song, L., Xiao, B., et al. (2023d). Tenuifolin reduces CPZ-induced demyelination in mice by inhibiting inflammation and oxidative stress. Lishizhen Med. Mater Med. Res. 34 (11), 2599–2605. doi:10.3969/j.issn.1008-0805.2023.11.09
Liao, D., Zhang, L., Lan, Y., Wang, J., and Zhang, J. (2019). Protective effect of senegenin on the injury of rat hippocampal neural stem cells induced by oxygen-glucose deprivation/reoxygenation culture in vitro. China J. TraditChin Med. Pharm. 34 (07), 2990–2993.
Ling, Y., Li, Z., Chen, M., Sun, Z., Fan, M., and Huang, C. (2013). Analysis of multiple constituents in Cong-Ming-Tang, a Chinese herbal formula for the treatment of amnesia, by high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. Phytochem. Anal. 24 (6), 677–688. doi:10.1002/pca.2454
Liu, J. (2004). Effective constituents of Kai-Xin-San, a basic TCM prescription for a therapy of Alzheimer's Disease. Peking. Union Med. Coll. [Doctoral thesis].
Liu, J., Yang, X., He, J., Xia, M., Xu, L., and Yang, S. (2007). Structure analysis of triterpene saponins in Polygala tenuifolia by electrospray ionization ion trap multiple-stage mass spectrometry. J. Mass Spectrom. 42 (7), 861–873. doi:10.1002/jms.1210
Liu, L., Feng, W., Liu, X., Liang, Y., Li, C., and Wang, Z. (2021). Research progress on Polygalae radix. China J. Chin. Mater Med. 46 (22), 5744–5759. doi:10.19540/j.cnki.cjcmm.20210518.601
Liu, M., Xu, W., Liang, N., Fang, J., and Li, Y. (2010). Studiy on chemical constituents of Polygala tenuifolia. Mod. Chin. Med. 12 (09), 18–21. doi:10.13313/j.issn.1673-4890.2010.09.004
Liu, Y., Li, Z., Hu, H., Xu, S., Chang, Q., Liao, Y., et al. (2015). Tenuifolin, a secondary saponin from hydrolysates of polygalasaponins, counteracts the neurotoxicity induced by Aβ25-35 peptides in vitro and in vivo. Pharmacol. Biochem. Be 128, 14–22. doi:10.1016/j.pbb.2014.11.010
Liu, Y., Peng, D., Yang, X., Shi, T., Jiang, Y., and Tu, P. (2012). Comparison of the chemical constituents and pharmacological activities between the cortexes and the roots of Polygala tenuifolia. J. Chin. Pharm. 47 (24), 1975–1979.
Lou, Y. (2023). Chemical characterization of aqueous extracts of Polygala tenuifolia and study on the biological effect of "MEN. Taiyuan, China: Shanxi University.
Lu, X., Jin, G., Yu, H., and Yang, H. (2021). Study on the effects of Tenuifolin on brain mitochondrial autophagy in AD model mice based on PINKI/Parkin signaling pathway. China Pharm. 32 (22), 2748–2754. doi:10.6039/j.issn.1001-0408.2021.22.11
Lv, G. (2007). A study of the chemical composition of Polygalae radix. Changchun, China: Changchun Univ of Tradit Chin Med.
Ma, R., Xie, Q., Wang, J., Huang, L., Guo, X., and Fan, Y. (2021). Combination of urine and faeces metabolomics to reveal the intervention mechanism of Polygala tenuifolia compatibility with Magnolia officinalis on gastrointestinal motility disorders. J. Pharm. Pharmacol. 73 (2), 247–262. doi:10.1093/jpp/rgaa022
Ma, R., Xie, Q., Wang, J., Huang, L., Tang, J., Li, W., et al. (2019). Metabolomics study on effects of compatibility of alcohol extracts of Magnolia officinalis and Polygala tenuifolin on urine metabolites in rats. Chin. Pharmacol. Bull. 35 (06), 870–877. doi:10.3969/j.issn.1001-1978.2019.06.026
Mei, Y., Pu, Y., Chen, T., Xv, X., Zhang, X., Zhang, F., et al. (2021). Inhibitory effect of Polygala tenuifolia extracts on PTP1B activity. J. Shanxi Univ. Nat. Sci. Ed. 44 (04), 824–831. doi:10.13451/j.sxu.ns.2019091
Miyase, T., Iwata, Y., and Ueno, A. (1992). Tenuifolioses G-P, oligosaccharide multi-esters from the roots of polygala tenuifolia WILLD. Chem. Pharm. Bull. 40 (10), 2741–2748. doi:10.1248/cpb.40.2741
Miyase, T., Noguchi, H., and Chen, X. (1999). Sucrose esters and xanthone C-glycosides from the roots of Polygala sibirica. J. Nat. Prod. 62 (7), 993–996. doi:10.1021/np990084t
Miyase, T., and Ueno, A. (1993). Sucrose derivatives from the roots of Polygala tenuifolia. Jpn. J. Pharm. 47 (3), 267–278.
Ngwe Tun, M., Toume, K., Luvai, E., Nwe, K., Mizukami, S., Hirayama, K., et al. (2022). The discovery of herbal drugs and natural compounds as inhibitors of SARS-CoV-2 infection in vitro. J. Nat. Med. 76 (2), 402–409. doi:10.1007/s11418-021-01596-w
Niu, F., Sang, X., Yang, Y., Tang, X., Liu, Y., and Fang, F. (2022). Effects of oligosaccharide esters of polygala tenuifolin on β-Amyloid protein 25∼35 induced SH-SY5Y cell injury and AKT/CREB/BDNF signal pathway. J. Beijing Univ. Tradit. Chin. Med. 45 (04), 414–420. doi:10.3969/j.issn.1006-2157.2022.04.015
Opo, F., Rahman, M., Ahammad, F., Ahmed, I., Bhuiyan, M., and Asiri, A. (2021). Structure based pharmacophore modeling, virtual screening, molecular docking and ADMET approaches for identification of natural anti-cancer agents targeting XIAP protein. Sci. Rep. 11 (1), 4049. doi:10.1038/s41598-021-83626-x
Pei, J., Wan, D., and Yang, L. (2005). Determining the polysaccharides from Polygala tenuifolia by Phenol-sulphate colorimetry. West China J. Pharm. Sci. (04), 337–339. doi:10.13375/j.cnki.wcjps.2005.04.023
Peng, F., Lu, L., Wei, F., Wu, D., Wang, K., and Tang, J. (2020a). The onjisaponin B metabolite tenuifolin ameliorates dopaminergic neurodegeneration in a mouse model of Parkinson's disease. Neuroreport 31 (6), 456–465. doi:10.1097/WNR.0000000000001428
Peng, L., Yan, Y., Chen, Y., Shen, X., Gao, J., Li, Y., et al. (2020b). Transcriptome analysis of Polygala tenuifolia seedlings induced by methyl jasmonate and key genes mining for triterpenoid biosynthetic pathway. Chin. Tradit. Herb. Drugs 51 (09), 2517–2529. doi:10.7501/j.issn.0253-2670.2020.09.029
Peng, Y., Fan, L., Mao, F., Zhao, Y., Xu, R., Yin, Y., et al. (2018). Genetic diversity and population structure of a protected species: polygala tenuifolia Willd. Cr Biol. 341 (3), 152–159. doi:10.1016/j.crvi.2018.01.007
Pi, T., Liang, Y., Ou, W., Zhu, H., Tao, Y., and Jin, X. (2020). Senegenin protects against lipopolysaccharide-induced neurite toxicity in a nerve cell model. Chin. J. Comp. Med. 30 (11), 52–58. doi:10.3969/j.issn.1671-7856.2020.11.009
Pu, Y. (2022). A composition for topical use including a raw medicine extract containing a combination of longan arillus for skin regeneration and treatment or improvement of skin wounds and its use.
Qiu, W. Q., Ai, W., Zhu, F. D., Zhang, Y., Guo, M. S., Law, B. Y., et al. (2022). Polygala saponins inhibit NLRP3 inflammasome-mediated neuroinflammation via SHP-2-Mediated mitophagy. Free Radic. Biol. Med. 179, 76–94. doi:10.1016/j.freeradbiomed.2021.12.263
Qiu, Z., Zhang, H., Li, J., Wang, H., Lu, D., and Qi, R. (2021). Effects of senegenin on hypoxia/reoxygenation-induced ferroptosis in PC12 cells. Chin. J. Pathophysiol. 37 (06), 988–997. doi:10.3969/j.issn.1000-4718.2021.06.004
Ren, X., Wang, G., Zhang, X., Wang, Q., and Peng, Z. (2020). Sedative and hypnotic effects and transcriptome analysis of Polygala tenuifolia in aged insomnia rats. Chin. J. Integr. Med. 26 (6), 434–441. doi:10.1007/s11655-020-3087-6
Ren, X., Zhang, J., Zhao, Y., and Sun, L. (2022). Senegenin inhibits aβ1-42-induced PC12 cells apoptosis and oxidative stress via activation of the PI3K/Akt signaling pathway. Neuropsych Dis. Treat. 18, 513–524. doi:10.2147/NDT.S346238
Sakuma, S., and Shoji, J. (1981). Studies on the constituents of the root of polygala tenuifolia WILLDENOW. II. On the structures of onjisaponins G and F. Chem. Pharm. Bull. 29 (3), 810–821.
Sakuma, S., and Shoji, J. (1982). Studies on the constituents of the root of polygala tenuifolia WILLDENOW. II. On the structures of onjisaponins A, B and E. Chem. Pharm. Bull. 30, 810–821. doi:10.1248/cpb.30.810
Sang, X., Yang, Y., and Fang, F. (2017). Research progress in pharmacological activities of oligosaccharide esters from Polygala tenuifolia. J. Chin. Pharm. 52 (18), 1576–1579. doi:10.11669/cpj.2017.18.003
Shen, X., Witt, M. R., Dekemendjian, K., and Nielsen, M. (2004). Isolation and identification of tetrahydrocolumba-mine as a dopamine receptor ligand from Polygala tenuifolia Willd. J. Hainan Med. Coll. (03), 133–135. doi:10.3969/j.issn.1007-1237.2004.03.002
Shi, T., Li, Y., Jiang, Y., and Tu, P. (2013a). Isolation of flavonoids from the aerial parts of Polygala tenuifolia Willd.and their antioxidant activities. J. Chin. Pharm. Sci. 22 (01), 36–39. doi:10.5246/jcps.2013.01.004
Shi, T., Wang, S., Zeng, K., Tu, P., and Jiang, Y. (2013b). Inhibitory constituents from the aerial parts of Polygala tenuifolia on LPS-induced NO production in BV2 microglia cells. Bioorg Med. Chem. Lett. 23 (21), 5904–5908. doi:10.1016/j.bmcl.2013.08.085
Son, S., Yoon, Y., Hong, J., Kim, J., Lee, K., and Jang, D. (2022). Chemical constituents of the roots of Polygala tenuifolia and their anti-inflammatory effects. Plants 11 (23), 3307. doi:10.3390/plants11233307
Song, J., Zhong, L., Xue, X., Xie, Y., Li, J., Wang, Z., et al. (2023). Optimization of processing technology of stewed Polygala tenuifolia and correlation analysis of its composition and color. Chin. Tradit. Pat. Med. 45 (12), 4085–4090. doi:10.3969/j.issn.1001-1528.2023.12.039
Song, L., Yu, Q., Qi, X., Yang, A., Zhang, X., Shen, R., et al. (2019). Antitumor activity of Cu(Ⅱ) complex with bioactive constituent Euxanthone from Polygala. Tianjin J. Tradit. Chin. Med. 36 (07), 701–704. doi:10.11656/j.issn.1672-1519.2019.07.19
Song, M., Wu, P., Zhang, X., Li, H., Liu, J., Meng, Y., et al. (2016). Comparison of 8 organic acids in three processed products of Polygalae radix. Chin. Tradit. Pat. Med. 38 (07), 1565–1569. doi:10.3969/j.issn.1001-1528.2016.07.027
Song, Y., Jiang, Y., Bi, D., Tian, X., Liang, L., and Tu, P. (2012a). Chemical constituents from n-butanol extract of aerial part of Polygala sibirica. China J. Chin. Mater Med. 37 (4), 471–474. doi:10.4268/cjcmm20120412
Song, Y., Jiang, Y., Zhou, S., Bi, D., and Tu, P. (2009). Studies on xanthones from aerial parts of Polygala sibirica. China J. Chin. Mater Med. 34 (5), 574–576. doi:10.3321/j.issn:1001-5302.2009.05.020
Song, Y., Zeng, K., Shi, T., Jiang, Y., and Tu, P. (2013a). Sibiricasaponins A–E, five new triterpenoid saponins from the aerial parts of Polygala sibirica L. Fitoterapia 84, 295–301. doi:10.1016/j.fitote.2012.12.017
Song, Y., Zhou, G., Zhou, S., Jiang, Y., and Tu, P. (2013b). Polygalins D-G, four new flavonol glycosides from the aerial parts of Polygala sibirica L. (Polygalaceae). Nat. Prod. Res. 27 (13), 1220–1227. doi:10.1080/14786419.2012.724412
Song, Y., Zhou, S., Wei, H., Jiang, Y., and Tu, P. (2012b). A novel sterol sulfate and new oligosaccharide polyester from the aerial parts of Polygala sibirica. Nat. Prod. Commun. 7 (9), 1934578X1200700–1168. doi:10.1177/1934578x1200700914
Sun, H. (2005). Isolation and evaluation of immunological adjuvant active saponins from Polygala tenuifolia Willd. Hangzhou, China: Zhejiang Univ.
Sun, M., Yuan, M., Wang, H., Yin, R., Yan, C., Yin, M., et al. (2023). Development of an online UPLC-PDA-ESI-Q-TOF-MS-LOX-FLD system for rapid screening of anti-inflammatory compounds in Polygala tenuifolia Willd. J. Pharm. Biomed. Anal. 229, 115353. doi:10.1016/j.jpba.2023.115353
Sun, X., Shi, S., and Yang, G. (2000). Studies on chemical constituents of fat oil of Polygala tenuifolia. J. Chin. Med. Mater 23 (1), 35–37. doi:10.3321/j.issn:1001-4454.2000.01.017
Sun, Y., Zhang, D., Wang, Y., Wang, X., Wang, L., Li, R., et al. (2020). “Experimental study on acute toxicity of aerial part of Polygalae radix,” in Shaanxi toxicology society symposium on prevention and control of epidemic infectious disease and toxic biohazard, Shaanxi and Shanxi. China.
Takiura, K., and Honda, S. (1964). Sugar components of the root of Polygala tenuifolia. Yakugaku zasshi J. Pharm. Soc. Jpn. 84, 1223–1224. doi:10.1248/yakushi1947.84.12_1223
Tan, J., Li, J., Su, Q., Cheng, Y., Chai, Z., Ma, C., et al. (2022). Chemical constituents of n-butanol fraction from aerial part of Polygala tenuifolia. Mod. Chin. Med., 1–5. doi:10.13313/j.issn.1673-4890.20211221006
Tang, X., Zhao, Y., Liu, Y., Liu, Y., Liu, Y., Niu, F., et al. (2022). 3,6'-disinapoyl sucrose attenuates Aβ1-42 - induced neurotoxicity in Caenorhabditis elegans by enhancing antioxidation and regulating autophagy. J. Cell Mol. Med. 26 (4), 1024–1033. doi:10.1111/jcmm.17153
Teng, H., Fang, M., Cai, X., and Hu, Z. (2009a). Localization and dynamic change of saponin in vegetative organs of Polygala tenuifolia. J. Integr. Plant Biol. 51 (6), 529–536. doi:10.1111/j.1744-7909.2009.00830.x
Teng, H., Fang, M., and Hu, Z. (2009b). The structure of vegetative organs, and saponins histochemical localization and content comparization in Polygala sibirica L. J. Mol. Cell Biol. 42 (1), 61–69.
Tian, Y., Qi, Y., Cai, H., Xu, M., and Zhang, Y. (2022). Senegenin alleviates Aβ1-42 induced cell damage through triggering mitophagy. J. Ethnopharmacol. 295, 115409. doi:10.1016/j.jep.2022.115409
Tsukada, M., Ikemoto, H., Lee, X., Takaki, T., Tsuchiya, N., Mizuno, K., et al. (2021). Kamikihito, a traditional Japanese Kampo medicine, increases the secretion of oxytocin in rats with acute stress. J. Ethnopharmacol. 276, 114218. doi:10.1016/j.jep.2021.114218
Vinh, L., Heo, M., Phong, N., Ali, I., Koh, Y., Kim, Y., et al. (2020). Bioactive compounds from Polygala tenuifolia and their inhibitory effects on lipopolysaccharide-stimulated pro-inflammatory cytokine production in bone marrow-derived dendritic cells. Plants 9 (9), 1240. doi:10.3390/plants9091240
Wang, B., Qiao, P., Wang, W., Song, W., Liu, C., Wang, X., et al. (2021a). Effect of Albiziae Flos and Polygalae radix alone and their combination on depression-like behavior and CREB and NOX2 expression in Hippocampus of chronic unpredictable stress-induced rats. Chin. J. Exp. Tradit. Med. Form. 27 (17), 32–39. doi:10.13422/j.cnki.syfjx.20211604
Wang, H., Tong, Y., Ye, W., and Zhao, S. (2003). Studies on chemical constituents from the root of Polygala tenuifolia. China J. Chin. Mater Med. (09), 38–40. doi:10.3321/j.issn:0253-2670.2002.10.004
Wang, L. (2020). Studies on the anti-exercise fatigue and hypoxia tolerance effects of Polygala herb extract Master's Theses. Xian, China: Northeastern Univ.
Wang, L., Jin, G., Yu, H., Lu, X., Zou, Z., Liang, J., et al. (2019). Protective effects of tenuifolin isolated from Polygala tenuifolia Willd roots on neuronal apoptosis and learning and memory deficits in mice with Alzheimer's disease. Food Funct. 10 (11), 7453–7460. doi:10.1039/c9fo00994a
Wang, L., and Xia, J. (2012). Plant resources and traditional curative effects of medicinal Polygala tenuifolia. Heilongjiang Sci. Technol. Inf. (01), 16. doi:10.3969/j.issn.1673-1328.2012.01.036
Wang, L., Zhu, S., and Li, D. (2020a). Effect of tenuifolin on mental behavior in deprssion model mice. Chin. J. Public Health 36 (04), 570–573. doi:10.11942/j.issn1002-2767.2020.10.0093
Wang, P., Shen, Y., Manaenko, A., Liu, F., Yang, W., Xiao, Z., et al. (2024). TMT-based quantitative proteomics reveals the protective mechanism of tenuigenin after experimental intracerebral hemorrhage in mice. J. Ethnopharmacol. 319, 117213. doi:10.1016/j.jep.2023.117213
Wang, T., Hu, Y., Wang, X., Li, Y., Zhang, F., Yan, Y., et al. (2022). Targeting p65 to inhibit Cas3 transcription by Onjisaponin B for radiation damage therapy in p65+/- mice. Phytomedicine 104, 154317. doi:10.1016/j.phymed.2022.154317
Wang, X., Liu, C., Zhou, J., Li, M., Zhou, Y., Jiang, W., et al. (2020b). Research advances in the chemical constituents and pharmacological effects of Polygalae radix(PR),a traditional Chinese medicine,and a predictive analysis of potential PR quality-makers. J. Int. Pharm. Res. 47 (07), 483–495+513. doi:10.27405/d.cnki.gxbdu.2020.000944
Wang, X., Xiao, H., Wu, Y., Kong, L., Chen, J., Yang, J., et al. (2021b). Active constituent of Polygala tenuifolia attenuates cognitive deficits by rescuing hippocampal neurogenesis in APP/PS1 transgenic mice. BMC Complement. Med. 21 (1), 267. doi:10.1186/s12906-021-03437-5
Wang, X., Zhang, D., Song, W., Cai, C., Zhou, Z., Fu, Q., et al. (2020c). Neuroprotective effects of the aerial parts of Polygala tenuifolia Willd extract on scopolamine-induced learning and memory impairments in mice. Biomed. Rep. 13 (5), 37. doi:10.3892/br.2020.1344
Wang, Y., Yang, J., Zhang, J., and Chen, J. (2005). Studies on chemical constituents of polygala tenuifolia. Chin. Tradit. Herb. Drugs (09), 15–17. doi:10.3321/j.issn:0253-2670.2005.09.003
Wu, H., Xv, R., Zhang, Q., and Huang, Y. (2024). Research progress on processing of Polygalae radix. Glob. Tradit. Chin. Med. 17 (02), 373–376. doi:10.3969/j.issn.1674-1749.2024.02.036
Wu, Z. (2010). Analysis of the volatile oil components of Polygala tenuifolia Willd. by GC-MS. J. Anhui Agr Sci. 38 (09), 4562+4574. doi:10.3969/j.issn.0517-6611.2010.09.055
Xie, F. (2021). Effects of anti-fatigue in vivo and anti-oxidant in vitro of polysaccharides from Polygala tenuifolia Willd.on exhaustive exercise mice. Sci. Technol. Food Ind. 42 (06), 332–336. doi:10.13386/j.issn1002-0306.2020050292
Xin, M., Wang, H., Wang, M., Yang, B., Liang, S., Xu, X., et al. (2023). Attenuating effect of Polygala tenuifolia Willd. seed oil on progression of MAFLD. Front. Pharmacol. 14, 1253715. doi:10.3389/fphar.2023.1253715
Xiong, X., Lu, M., Shi, L., Zhong, L., and Ye, X. (2023). Effect of Jianchang stewing method on the pharmacokinetics and tissue distribution of 4 components in Polygala tenuifolia in rats. Cent. South Pharm. 21 (05), 1149–1156. doi:10.7539/j.issn.1672-2981.2023.05.006
Xu, L., Li, C., Yang, J., Luo, Y., and Zhang, D. (2014). Chemical constituents of Polygala tenuifolia root. J. Chin. Med. Mater. 37 (9), 1594–1596. doi:10.13863/j.issn1001-4454.2014.09.02
Yan, D. (2004). Studies on extraction of saponin and quality standard of radix Polygalae (Yuanzhi). Chengdu Univ. Tradit. Chin. Med. doi:10.7666/d.y623536
Yang, B., Li, X., Yu, K., Jiang, X., Wang, L., Li, F., et al. (2022a). Sugar easters and xanthones from the roots of Polygala tenuifolia Willd. and their cytoprotective activity. Fitoterapia 161, 105256. doi:10.1016/j.fitote.2022.105256
Yang, C., Peng, W., Lin, L., and Tsai, T. (2021). Protein unbound pharmacokinetics of ambroxol in the blood and brains of rats and the interaction of ambroxol with Polygala tenuifolia by multiple microdialysis. J. Ethnopharmacol. 269, 113764. doi:10.1016/j.jep.2020.113764
Yang, F., Yu, H., Chai, X., Peng, S., Yang, J., Wu, D., et al. (2018). Illumination on "reserving phloem and discarding xylem" and quality evaluation of radix polygalae by determining oligosaccharide esters, saponins, and xanthones. Molecules 23 (4), 836. doi:10.3390/molecules23040836
Yang, S., Liu, Y., Xiu, M., Yang, X., Yang, F., and He, J. (2022b). Research progress on anti-aging mechanism of saponins of traditional Chinese medicine. Chin. J. Gerontol. 42 (13), 3327–3335. doi:10.3969/j.issn.1008-0104.2022.03.001
Yang, X., Xu, L., and Yang, S. (2000). Advances in the research of chemistry and pharmacology of xanthones extracted from polygala L. Nat. Prod. Res. Dev. (05), 88–94. doi:10.16333/j.1001-6880.2000.05.020
Yoo, S., Le, T., Jeong, J., and Kim, D. (2014). Poligapolide, a PI3K/Akt inhibitor in immunodeficiency virus type 1 TAT-transduced CHME5 cells, isolated from the rhizome of Polygala tenuifolia. Chem. Pharm. Bull. 62 (5), 467–471. doi:10.1248/cpb.c13-00958
Yu, H., Lu, X., Jin, G., and Yang, H. (2022). The effect of Tenuigenin on inhibiting neuronal cell cycle activation and apoptosis by regulating Aβ transport. Chin. J. Gerontol. 42 (17), 4310–4314. doi:10.3969/j.issn.1005-9202.2022.17.046
Yu, S. (2020). Preparation,structure and antitumor activity of polysaccharides from the Polygala tenuifolia Master's Theses. Tianjin Univ. Sci. doi:10.27359/d.cnki.gtqgu.2020.000345
Yu, S., Dong, X., Ji, H., Yu, J., and Liu, A. (2021). Antitumor activity and immunomodulation mechanism of a novel polysaccharide extracted from Polygala tenuifolia Willd. evaluated by S180 cells and S180 tumor-bearing mice. Int. J. Biol. Macromol. 192, 546–556. doi:10.1016/j.ijbiomac.2021.10.025
Yu, S., Ji, H., Dong, X., Liu, A., and Yu, J. (2020). FAS/FAS-L-mediated apoptosis and autophagy of SPC-A-1 cells induced by water-soluble polysaccharide from Polygala tenuifolia. Int. J. Biol. Macromol. 150, 449–458. doi:10.1016/j.ijbiomac.2020.02.010
Yu, X., Liu, G., Zhu, B., Hao, K., Ling, F., and Wang, G. (2014). In vitro immunocompetence of two compounds isolated from Polygala tenuifolia and development of resistance against grass carp reovirus (GCRV) and Dactylogyrus intermedius in respective host. Fish. Shellfish Immun. 41 (2), 541–548. doi:10.1016/j.fsi.2014.10.004
Yuan, M., Yin, M., Tang, Z., Xv, H., and Chen, S. (2021). Processing technology of Glycyrrhiza Polygala tenuifolia and honey-stir-baked Polygala tenuifolia by Box-Behnken response surface methodology. Cent. South Pharm. 19 (07), 1310–1315. doi:10.7539/j.issn.1672-2981.2021.07.008
Yue, T., Huang, D., and Deng, Y. (2020). Study on preparation and resistant exercise fatigue of compound beverage of Polygala tenuifolia Willd.and Flavedo. Food Sci. Technol. 45 (12), 73–79. doi:10.13684/j.cnki.spkj.2020.12.011
Yukinobu, I., Kô, S., Minoru, O., and Hiroshi, M. (1991). Two xanthones from Polygala tenuifolia. Phytochemistry 30 (6), 2061–2065. doi:10.1016/0031-9422(91)85067-a
Zang, L., Fu, D., Zhang, F., Li, N., and Ma, X. (2023). Tenuigenin activates the IRS1/Akt/mTOR signaling by blocking PTPN1 to inhibit autophagy and improve locomotor recovery in spinal cord injury. J. Ethnopharmacol. 317, 116841. doi:10.1016/j.jep.2023.116841
Zeng, H., Huang, L., Tao, H., Zhang, Y., and Ding, K. (2020a). Structural elucidation of a pectin from roots of Polygala tenuifolia and its neuritogenesis inducing activity in PC12 cells. Carbohyd Polym. 236, 116048. doi:10.1016/j.carbpol.2020.116048
Zeng, H., Li, P., Zhou, L., and Ding, K. (2020b). A novel pectin from Polygala tenuifolia blocks Aβ42 aggregation and production by enhancing insulin-degradation enzyme and neprilysin. Int. J. Biol. Macromol. 161, 35–43. doi:10.1016/j.ijbiomac.2020.05.212
Zeng, L., Wang, C., Wang, S., Chen, Y., Huang, W., Wang, Z., et al. (2021a). Study progress on chemical constituents and biological activities of Polygala genus. For. Environ. Sci. 37 (04), 160–175. doi:10.3969/j.issn.1006-4427.2021.04.023
Zeng, W., Wu, A., Zhou, X., Khan, I., Zhang, R., Lo, H., et al. (2021b). Saponins isolated from Radix polygalae extent lifespan by modulating complement C3 and gut microbiota. Pharmacol. Res. 170, 105697. doi:10.1016/j.phrs.2021.105697
Zeng, Z., Chang, X., Zhang, D., Chen, H., Zhong, X., Xie, Y., et al. (2022). Structural elucidation and anti-neuroinflammatory activity of Polygala tenuifolia polysaccharide. Int. J. Biol. Macromol. 219, 1284–1296. doi:10.1016/j.ijbiomac.2022.08.161
Zhan, N., Liu, X., and Ren, H. (2022). A herbal feed additive for growing and fattening pigs and its preparation method.
Zhang, D., Miyase, T., Kuroyanagi, M., Umehara, K., and Ueno, A. (1996). Five new triterpene saponins, polygalasaponins XXVIII-XXXII from the root of Polygala japonica Houtt. Chem. Pharm. Bull. 44 (4), 810–815. doi:10.1248/cpb.44.810
Zhang, D., Wang, X., Li, R., Wang, L., Zhou, Z., Fu, Q., et al. (2020). Extract of the aerial part of Polygala tenuifolia attenuates d-Galactose/NaNO2-induced learning and memory impairment in mice. Planta Med. 86 (18), 1389–1399. doi:10.1055/a-1212-3212
Zhang, D., Wang, Y., Wang, X., Wang, L., Li, R., and Fang, M. (2019). Antioxidant effects of aqueous extract from Polygala tenuifolia Willd. seedlings on D-galactose induced aging mice. China J. Tradit. Chin. Med. Pharm. 34 (09), 4322–4326.
Zhang, D., Zhang, W., Deng, S., Liu, L., Wei, H., Xue, F., et al. (2023). Tenuigenin promotes non-rapid eye movement sleep via the GABAA receptor and exerts somnogenic effect in a MPTP mouse model of Parkinson's disease. Biomed. Pharmacoth 165, 115259. doi:10.1016/j.biopha.2023.115259
Zhang, J., Han, B., Wang, X., and Liu, Z. (2022). An anti-alcoholic composition and its preparation method and application.
Zhang, T., Rong, W., Li, Q., and Bi, K. (2016). Research progress on Polygalae radix. Chin. Tradit. Herb. Drugs 47 (13), 2381–2389. doi:10.7501/j.issn.0253-2670.2016.13.029
Zhang, X., Wu, C., and Tan, W. (2021). Brain lipid dynamics in amyloid precursor protein/presenilin 1 mouse model of early Alzheimer's disease by desorption electrospray ionization and matrix assisted laser desorption ionization-mass spectrometry imaging techniques. J. Prot. Res. 20 (5), 2643–2650. doi:10.1021/acs.jproteome.0c01050
Zhao, T., and Jia, J. (2024). Polygalacic acid attenuates cognitive impairment by regulating inflammation through PPARγ/NF-κB signaling pathway. CNS Neurosci. Ther. 30 (2), e14581. doi:10.1111/cns.14581
Zhao, X., Cui, Y., Wu, P., Zhang, Z., Wang, Y., and Zhang, X. (2021). Regulatory effect of Polygala tenuifolia and Licorice-simmered P. tenuifolia on learning and memory, HPA axis function and neurotransimitters in rats with heart-kidney lmbalance insomnia. Chin. J. Exp. Tradit. Med. Form. 27 (11), 147–154. doi:10.3969/j.issn.1001-9677.2021.01.014
Zhao, X., Cui, Y., Wu, P., Zhao, P., Zhou, Q., Zhang, Z., et al. (2020). Polygalae Radix: a review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 147, 104759. doi:10.1016/j.fitote.2020.104759
Zheng, Y., Chen, J., You, F., and Yang, H. (2016). Research progress on neuroprotective effect and mechanisms of Polygala tenuifolia. Med. Rec. 22 (08), 1561–1564. doi:10.3969/j.issn.1006-2084.2016.08.031
Zhou, H., Zhang, L., Zeng, J., Wang, Z., Liu, L., Huang, J., et al. (2022). CiteSpace knowledge map of research hotspots and frontiers of Polygalae radix. China J. Chin. Mater Med., 1–12.
Zhou, Y. (2020). Research on the antidepressant effects and mechanisms of radix Polygalae Doctoral Dissertations. Beijing, China: Peking Union Med Coll.
Zhou, Y., Jiang, Y., Wen, J., Chen, Y., and Tu, P. (2008). Chemical constituents from the roots of Polygala sibirica L. J. Chin. Pharm. Sci. (02), 148–152.
Zhou, Y., Yan, M., Pan, R., Wang, Z., Tao, X., Li, C., et al. (2021). Radix Polygalae extract exerts antidepressant effects in behavioral despair mice and chronic restraint stress-induced rats probably by promoting autophagy and inhibiting neuroinflammation. J. Ethnopharmacol. 265, 113317. doi:10.1016/j.jep.2020.113317
Zhou, Y., Zhang, S., Guo, Q., Chai, X., Jiang, Y., and Tu, P. (2014). Chemical investigation of the roots of Polygala sibirica L. Chin. J. Nat. Med. 12 (03), 225–228. doi:10.1016/S1875-5364(14)60038-8
Zhu, J., and Liu, X. (2022). A atomized liquid with calming and sleep-aiding effect and its preparation method.
Keywords: Polygalae Radix, pharmacological effects, metabolites, applications, toxicity, processing
Citation: Kuang H, Kong L, Hou A, Yang A and Jiang H (2024) A review of the botany, metabolites, pharmacology, toxicity, industrial applications, and processing of Polygalae Radix: the “key medicine for nourishing life”. Front. Pharmacol. 15:1450733. doi: 10.3389/fphar.2024.1450733
Received: 18 June 2024; Accepted: 29 July 2024;
Published: 18 September 2024.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Guangxin Yuan, Beihua University, ChinaCopyright © 2024 Kuang, Kong, Hou, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Jiang, amlhbmdoYWlfNzc3QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.