
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 19 December 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1450120
This article is part of the Research Topic Clinical Pharmacist Service Promotes the Improvement of Medical Quality Volume II View all 51 articles
Background: Posaconazole is a potent antifungal agent widely used to manage invasive fungal infections, especially in immunocompromised individuals. Achieving optimal therapeutic concentrations of posaconazole can be challenging due to interpatient variability, the availability of multiple formulations, and various dosing strategies.
Methods: We conducted a systematic search of PubMed, EMBASE, and the Cochrane Library to identify studies evaluating factors that influence blood concentrations of posaconazole. The primary outcome was the assessment of posaconazole concentrations in relation to various influencing factors, including age, sex, drug interactions, disease state, administered dose, and formulation.
Results: Our analysis included 46 studies involving a total of 8,505 patients. Co-administration of drugs that affect posaconazole metabolism significantly reduced its concentrations. High-fat meals, age, and sex did not have a significant impact on posaconazole oral suspension (POS) concentrations. Diarrhea substantially decreased concentrations of both delayed-release tablets (DRT) and POS. Neither vomiting nor mucositis significantly affected POS concentrations. Acid-suppressing agents, such as H2 receptor antagonists and proton pump inhibitors, notably decreased POS concentrations but had no significant effect on DRT. Comparative studies of different dosage forms revealed significantly higher concentrations with DRT compared to POS.
Conclusion: DRT maintain more stable concentrations than POS and are not affected by acid-suppressing drugs. Given the significant fluctuations in posaconazole concentrations, patients experiencing diarrhea require close monitoring.
Systematic Review Registration: PROSPERO, Identifier CRD42023428822 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023428822).
Invasive fungal infections (IFIs) pose significant challenges in clinical practice, particularly among immunocompromised patients, such as those undergoing hematopoietic stem cell transplantation (HSCT), solid organ transplantation (SOT), or those suffering from hematologic malignancies or HIV/AIDS (von Lilienfeld-Toal et al., 2019). Posaconazole, a second-generation triazole antifungal agent, exhibits broad-spectrum activity against various clinically relevant fungal pathogens, including Aspergillus spp., Candida spp., and Zygomycetes (Chen et al., 2020). Its efficacy and favorable safety profile have led to its widespread use in the prophylaxis and treatment of IFIs (Van Daele et al., 2020). Research has shown a correlation between low posaconazole concentrations and the occurrence of breakthrough invasive fungal infections (bIFIs) (Dolton et al., 2012). Recommended concentrations exceed 700 ng/mL for prophylaxis and 1,000 ng/mL for treatment (Kably et al., 2022; McCreary et al., 2023; Gómez-López, 2020). Additionally, a meta-analysis suggests that a concentration of 500 ng/mL is effective for prevention, while the toxicity threshold for trough concentrations is set at 3,750 ng/mL (Chen et al., 2018).
Achieving optimal posaconazole exposure remains challenging due to significant interpatient variability in pharmacokinetics, which arises from individual differences in drug absorption, distribution, metabolism, and elimination. Posaconazole is available in several formulations, including posaconazole oral suspension (POS), delayed-release tablets (DRT), and intravenous solutions, each with distinct pharmacokinetic characteristics. Patient-specific factors, such as age, concomitant medications, renal and hepatic function, and underlying disease conditions, can significantly influence posaconazole exposure.
This meta-analysis and systematic review aimed to investigate factors influencing posaconazole concentrations and to provide insights for optimizing antifungal therapy in clinical practice.
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review was registered with PROSPERO (registration number CRD42023428822). Quantitative data synthesis was performed using meta-analytic techniques. Analyses of posaconazole concentrations considered various formulations, dosing regimens, renal function, concomitant medications, and patient populations.
We conducted a comprehensive search of articles published before 29 December 2023, in three electronic databases: the Cochrane Library, EMBASE, and PubMed. The search strategy was developed using MeSH/EMTREE terms and free-text keywords to target relevant populations, outcomes, and study types. The following search terms in the PubMed were used in the search queries: ((((((“Plasma” [MeSH]) OR (plasma [Title/Abstract])) OR (“Blood” [MeSH]) OR (blood [Title/Abstract])) OR (“Serum” [MeSH]) OR (serum [Title/Abstract])) OR ((“Drug Monitoring” [MeSH]) OR (“Monitoring, Drug” [Title/Abstract])) OR (“therapeutic drug monitoring” [Title/Abstract])) OR (concentration [Title/Abstract])) AND ((“posaconazole” [Supplementary Concept]) OR (“Noxafil” [Title/Abstract]) OR (posaconazole [Title/Abstract])).
Two methodologically trained reviewers independently screened the titles and abstracts to determine whether the articles met the inclusion criteria. Discrepancies were resolved through consensus or, when necessary, arbitration by a third reviewer. Full-text articles were then reviewed, and relevant data were extracted. The reasons for inclusion or exclusion were documented. Studies published in non-English languages, case reports, letters, and meeting minutes were excluded.
The inclusion criteria were studies involving patients or healthy volunteers using posaconazole. Studies without available concentration data were excluded. We included randomized controlled trials (RCTs) that assigned patients to groups based on different influencing factors, as well as observational studies, prospective cohorts, retrospective cohorts, case-control studies, and intervention studies. We excluded case reports, comments, editorials, reviews, studies lacking concentration data, studies that did not investigate factors affecting concentration levels, and studies that lacked a control group.
Two reviewers independently screened the titles and abstracts of all studies, and they retrieved through the search strategies based on the predefined inclusion criteria. The following information was extracted: (a) publication details, including authors, year of publication, and country of study; (b) study design, specifying whether it was an RCT or an observational study; (c) patient demographics, including the number of participants, their ages, and genders; (d) diagnosis, dose administered, frequency of administration, and route of administration; (e) posaconazole concentrations, including means, medians, ranges, and interquartile ranges. Discrepancies in data extraction were resolved through discussion.
The quality of the studies was assessed using the Newcastle-Ottawa Scale (NOS). Each study could receive a maximum of nine stars, with one star awarded per item, except for comparability, which could receive up to two stars. Studies scoring 0–3 stars were considered to have a high risk of bias, 4–6 stars indicated a moderate risk, and 7–9 stars suggested a low risk of bias.
This review evaluated the impact of various factors, including age, sex, drug interactions, disease states, administered doses, and formulations, on posaconazole blood concentrations.
Data were analyzed using Review Manager version 5.4 (Cochrane Collaboration, Oxford, England). Continuous outcomes were measured by mean difference (MD) and reported with 95% confidence intervals (CIs). Results were presented descriptively for outcomes that were unsuitable for pooled effect estimates. For studies providing only individual patient data, the mean ± standard deviation was calculated. If studies did not directly report means and standard deviations, these were estimated using formulas from previous studies (Luo et al., 2018; Shi et al., 2020). Cochran’s Q test and I2 statistics were used to assess statistical heterogeneity and inconsistent treatment effects across studies. If there was no significant heterogeneity between studies, we analyzed using a fixed-effects model and vice versa using a random-effects model.
A total of 46 studies published between 2007 and 2020 met the inclusion criteria and were included in the analysis. The study selection process isdepicted in Figure 1. These studies had 8,505 patients, with individual study sample sizes ranging from 2 to 513 (Table 1). The patient populations were diverse, covering various indications for both prophylaxis and treatment of invasive fungal infections. Among the included studies, 8 were RCTs, 20 were retrospective, 7 were prospective, and 11 were parallel-group studies. Data on posaconazole concentrations in patients included in the quantitative analysis are detailed in Supplemental Table 1.
Two studies (Krishna et al., 2007c; Krishna et al., 2007b) involving 92 patients evaluated the impact of concurrent medication use on posaconazole concentrations in DRT form. The results indicated that the combined use of rifabutin and phenytoin significantly reduced drug blood levels in healthy volunteers [mean difference [MD] −251.16 ng/mL; 95% confidence interval [CI], −334.66 to −167.66; p < 0.001; Figure 2].
Three studies (Heinz et al., 2016; Lai et al., 2020; Sansone-Parsons et al., 2006) involving 145 patients assessed the effect of high-fat nutrition on POS concentrations. Quantitative analysis from two of these studies (Heinz et al., 2016; Lai et al., 2020) found no significant impact of high-fat diets on drug concentrations [mean difference [MD] −299.21 ng/mL; 95% confidence interval [CI], −877.78 to 279.35; p = 0.310; Figure 3]. However, in healthy volunteers, a single 400 mg dose of POS taken with a nutritional supplement resulted in a 3.4-fold increase in the maximum serum concentration of posaconazole from 0 to 72 h (Sansone-Parsons et al., 2006).
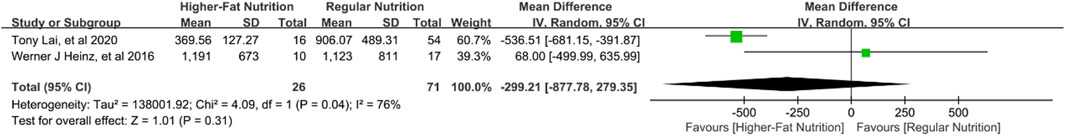
Figure 3. Forest plot of the effect of high-fat nutrition on the concentration of posaconazole oral suspension.
Five studies (Krishna et al., 2007d; Krishna et al., 2008; Sansone-Parsons et al., 2007; Krishna et al., 2007a; Bernardo et al., 2013) involving 594 patients explored the effect of patient age on posaconazole concentrations. Quantitative analysis of four studies (Krishna et al., 2007d; Krishna et al., 2008; Sansone-Parsons et al., 2007; Krishna et al., 2007a) found no significant differences in concentrations between patients younger and older than 18 years [mean difference [MD] 0.37 ng/mL; 95% confidence interval [CI], −268.39 to 269.14; p = 1.000; Figure 4) or between those younger and older than 45 years (MD −357.78 ng/mL; 95% CI, −986.90 to 271.35; p = 0.270; Figure 5]. Higher blood levels were observed in pediatric patients under 13 years of age who were dosed based on body weight (Bernardo et al., 2013).
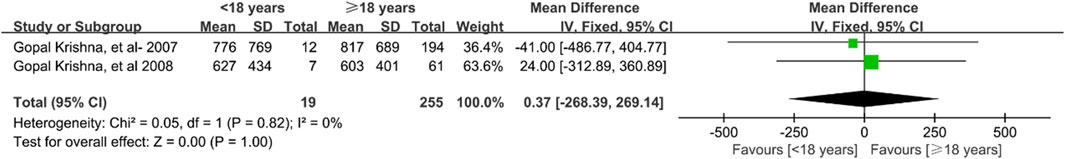
Figure 4. Forest plot of the effect of age 18 years up and down on the concentration of posaconazole oral suspension.
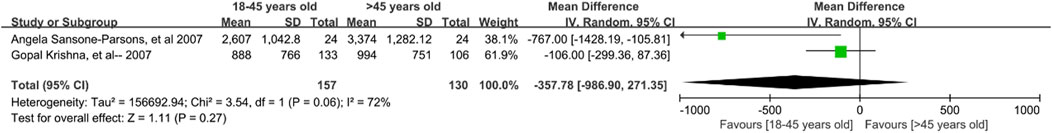
Figure 5. Forest plot of the effect of age 45 years up and down on the concentration of posaconazole oral suspension.
Three studies (Krishna et al., 2008; Krishna et al., 2007a; Bryant et al., 2011) involving 456 patients investigated the effect of gender on posaconazole concentrations. The results revealed no significant differences between male and female patients [mean difference [MD] −5.77 ng/mL; 95% confidence interval [CI], −76.57 to 88.11; p = 0.890; Supplemental Figure S1].
Six studies (Krishna et al., 2008; Krishna et al., 2007a; Bryant et al., 2011; Lebeaux et al., 2009; Miceli et al., 2015; Leclerc et al., 2018) involving 568 patients assessed the impact of diarrhea on posaconazole concentrations. Analysis of four studies (Krishna et al., 2008; Krishna et al., 2007a; Bryant et al., 2011; Lebeaux et al., 2009) on POS and two (Miceli et al., 2015; Leclerc et al., 2018) on DRT showed that diarrhea significantly reduced drug concentrations regardless of formulation [POS: mean difference [MD] −252.14 ng/mL; 95% confidence interval [CI], −332.26 to −172.02, p < 0.001; Supplemental Figure S2; DRT: MD -670.27 ng/mL; 95% CI, −756.86 to −583.67, p < 0.001; Supplemental Figure S3].
Two studies (Krishna et al., 2008; Bryant et al., 2011) involving 214 patients explored the impact of vomiting on posaconazole concentrations. The analysis found no significant effect [mean difference [MD] −15.43 ng/mL; 95% confidence interval [CI], −148.76 to 117.90; p = 0.820; Supplemental Figure S4].
Five studies (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Miceli et al., 2015; Pham et al., 2016) involving 526 patients examined the effect of H2 receptor antagonists (H2A) on posaconazole concentrations. Four studies (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Miceli et al., 2015; Pham et al., 2016) focused on POS, while two (Miceli et al., 2015; Pham et al., 2016) focused on DRT. The results indicated that co-administration of H2A did not significantly affect DRT concentrations [mean difference [MD] −285.74 ng/mL; 95% confidence interval [CI], −847.06 to 275.58; p = 0.320; Supplemental Figure S5]. However, H2A significantly reduced POS concentrations (MD -197.83 ng/mL; 95% CI, −377.64 to −18.02; p = 0.030; Supplemental Figure S6).
Nine studies (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Miceli et al., 2015; Pham et al., 2016; Crombag et al., 2012; Cojutti et al., 2013; Launay et al., 2018; Li et al., 2020) involving 600 patients investigated the impact of proton pump inhibitors (PPIs) on posaconazole concentrations. Three studies (Miceli et al., 2015; Pham et al., 2016; Launay et al., 2018) focused on DRTs, while seven (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Pham et al., 2016; Crombag et al., 2012; Cojutti et al., 2013; Li et al., 2020) focused on POS. The findings showed that co-administration of PPIs did not significantly affect DRT concentrations [mean difference [MD] −261.65 ng/mL; 95% confidence interval [CI], −638.21 to 114.92; p = 0.170; Supplemental Figure S7], but significantly reduced POS concentrations (MD -179.99 ng/mL; 95% CI, −246.83 to −113.14; p < 0.001; Supplemental Figure S8).
Nine studies (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Miceli et al., 2015; Pham et al., 2016; Crombag et al., 2012; Cojutti et al., 2013; Launay et al., 2018; Li et al., 2020) involving 600 patients investigated the impact of proton pump inhibitors (PPIs) on posaconazole concentrations. Three studies (Miceli et al., 2015; Pham et al., 2016; Launay et al., 2018) focused on DRTs, while seven (Lai et al., 2020; Krishna et al., 2008; Bryant et al., 2011; Pham et al., 2016; Crombag et al., 2012; Cojutti et al., 2013; Li et al., 2020) focused on POS. The findings showed that co-administration of PPIs did not significantly affect DRT concentrations [mean difference [MD] −261.65 ng/mL; 95% confidence interval [CI], −638.21 to 114.92; p = 0.170; Supplemental Figure S7], but significantly reduced POS concentrations (MD -179.99 ng/mL; 95% CI, −246.83 to −113.14; p < 0.001; Supplemental Figure S8).
Thirteen studies (Leclerc et al., 2018; Pham et al., 2016; Durani et al., 2015; Cumpston et al., 2015; Suh et al., 2017; AuthorAnonymous et al., 2017; Stelzer et al., 2017; Jeong et al., 2018; Liebenstein et al., 2018; Stelzer et al., 2018; Gautier-Veyret et al., 2019; Oh et al., 2020; Chae et al., 2020) involving 2,343 patients assessed differences in posaconazole concentrations between the oral suspension (POS) and DRT formulations. The analysis revealed significantly higher blood concentrations in patients using DRTs compared to those using POS [mean difference [MD] 845.86 ng/mL; 95% confidence interval [CI], 675.10 to 1,016.63; p < 0.001; Supplemental Figure S10].
Four studies (Lai et al., 2020; Chae et al., 2020; Vanstraelen et al., 2016; Peterlin et al., 2018) involving 693 patients explored the effects of hematopoietic stem cell transplantation (HSCT) on posaconazole concentrations. Two studies (Chae et al., 2020; Peterlin et al., 2018) specifically analyzed concentrations in patients undergoing HSCT and induction chemotherapy with dDRTs, finding no significant differences [mean difference [MD] 601.77 ng/mL; 95% confidence interval [CI], −355.53 to 1,559.08; p = 0.220; Supplemental Figure S11]. Additional findings from Lai et al. (2020) and Li et al. (2020) indicated lower plasma concentrations in hematologic patients receiving HSCT with POS compared to those not undergoing HSCT (288.46 ng/mL vs. 1,144.06 ng/mL). Similarly, pediatric patients under 13 years of age who received HSCT had lower posaconazole concentrations than those who did not undergo HSCT (569.11 ng/mL vs. 863.29 ng/mL) (Lai et al., 2020).
Ten studies (Cojutti et al., 2013; Gubbins et al., 2006; Krishna et al., 2011; Ray et al., 2011; Krishna et al., 2012; Ross et al., 2012; Heinz et al., 2013; Maertens et al., 2014; Kersemaekers et al., 2015; Kozuch et al., 2018) involving 372 patients assessed posaconazole concentrations across different formulations, including oral suspension (POS),DRT, and intravenous (IV) administration, at varying doses. For POS, five studies (Cojutti et al., 2013; Gubbins et al., 2006; Krishna et al., 2011; Ross et al., 2012; Heinz et al., 2013) found no significant differences in concentrations between daily doses of 200 mg and 400 mg [mean difference [MD] 3.24 ng/mL; 95% confidence interval [CI], −267.94 to 274.42; p = 0.980; Supplemental Figure S12] or between 600 mg and 800 mg (MD 152.64 ng/mL; 95% CI, −182.17 to 487.46; p = 0.370; Supplemental Figure S13). For DRTs, two studies (Krishna et al., 2012; Kozuch et al., 2018) showed significantly higher concentrations with a daily dose of 400 mg compared to 200 mg (MD 880.15 ng/mL; 95% CI, 266.65 to 1,493.64; p = 0.005; Supplemental Figure S14). For intravenous administration, two studies (Maertens et al., 2014; Kersemaekers et al., 2015) demonstrated that a daily dose of 300 mg resulted in significantly higher concentrations than 200 mg (MD 318.48 ng/mL; 95% CI, 3.82 to 633.15; p = 0.050; Supplemental Figure S15). In critically ill patients administered posaconazole via nasogastric tube, concentrations remained low with both 400 mg twice-daily and 200 mg four-times-daily regimens (Ray et al., 2011).
A study by Lai et al. (2020) reported that co-administration of the gastric stimulant metoclopramide decreased posaconazole concentrations in pediatric patients under 12 years of age receiving POS prophylactically (500.11 ng/mL vs. 887.52 ng/mL).
A study by Courtney et al. (2005) comparing healthy volunteers with patients experiencing renal impairment found that posaconazole concentrations were not affected by hemodialysis, suggesting that renal disease severity does not necessitate dosage adjustments. Another study by Moton et al. (2010) found no significant effect of varying degrees of hepatic impairment on posaconazole concentrations. The influence of elevated gamma-glutamyl transferase (γ-GT) levels was also considered clinically insignificant (Krishna et al., 2008).
In patients with digestive diseases, posaconazole concentrations were lower compared to those without such conditions (450 ng/mL vs. 1,035 ng/mL), although the difference was not statistically significant (p = 0.075) (Lebeaux et al., 2009).
A study by Miceli et al. (2015) observed that patients weighing ≥90 kg or with a BMI ≥30 had lower mean trough concentrations compared to lighter or less obese patients (740 ng/mL vs. 1,320 ng/mL; 890 ng/mL vs. 1,290 ng/mL, respectively).
Posaconazole exposure varied among patients with different hematologic malignancies. HSCT patients exhibited slightly higher concentrations than those with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) (1,870 ng/mL vs. 1,440 ng/mL) (Cornely et al., 2016).
Studies have shown that polymorphisms in the uridine diphosphate-glucuronosyltransferase (UGT)1A4 gene, which metabolizes posaconazole, contribute to variations in drug absorption. Patients with the UGT1A4*3 genotype exhibited lower POS steady-state concentrations compared to those with the wild-type genotype (Suh et al., 2018).
Posaconazole concentrations were lower in patients who developed acute GVHD compared to those with chronic GVHD (814 ng/mL vs. 1,413 ng/mL) (Krishna et al., 2007a). Concentrations were also lower in patients who developed gastrointestinal GVHD (1,080 ng/mL vs. 1,420 ng/mL) (Tonini et al., 2012).
Posaconazole is a triazole antifungal agent widely used for the prevention and treatment of various fungal infections. Maintaining therapeutic blood levels is crucial for achieving successful treatment outcomes (Van Daele et al., 2020). This study analyzes the impact of multiple factors on posaconazole blood concentrations, including patient-specific characteristics, drug interactions, formulation differences, and pharmacogenetic variations. Understanding these factors is essential for optimizing posaconazole therapy to ensure both efficacy and safety.
Factors such as age, sex, body weight, renal and hepatic function, vomiting, mucositis, and high-fat dietary intake did not significantly influence posaconazole concentrations. However, genetic variations in drug-metabolizing enzymes, such as UGT1A4, were found to affect posaconazole metabolism, highlighting the impact of individual genetic differences on drug concentrations. Diarrhea was found to significantly reduce posaconazole concentrations in this study, and another recent study on population pharmacokinetics also found that diarrhea resulted in underexposure to posaconazole extended-release tablets (Yamada et al., 2024).
Posaconazole is metabolized partly by liver enzymes, including UGT1A4 and P-glycoprotein (P-gp). The concurrent use of drugs that induce or inhibit these enzymes can significantly alter posaconazole metabolism and blood levels. Co-administration of phenytoin and rifabutin, both inducers of the UGT enzyme system (Anderson, 2004; Reinach et al., 1999), significantly decreased posaconazole concentrations in healthy volunteers using DRTs. However, the specific UGT isoforms induced by these drugs remain unidentified.
Absorption plays a crucial role in determining blood concentrations of posaconazole. Drugs such as H2 receptor antagonists (H2A) and PPIs can affect posaconazole absorption by altering gastric pH. Although these drugs did not significantly affect concentrations in DRT formulations, they considerably reduced concentrations in POS formulations. This suggests that DRTs provide more stable posaconazole levels and are less susceptible to variations in gastrointestinal absorption conditions.
Posaconazole is available in various formulations, each with distinct bioavailability, absorption kinetics, and drug exposure profiles. DRTs achieved significantly higher concentrations than oral suspensions when administered at the recommended doses, suggesting that switching between formulations could influence therapeutic efficacy. Although this study did not compare intravenous formulations with others, intravenous forms are generally designated for treatment-refractory invasive fungal infections, typically in critically ill patients (Sime et al., 2019), with a recommendation to switch to oral administration as soon as clinically feasible.
The study revealed no significant differences in concentrations between 600 mg and 800 mg daily doses of POS for prophylactic and therapeutic use, respectively. However, for DRTs, concentrations were significantly higher at a 400 mg daily dose compared to 200 mg. In the intravenous form, a 300 mg dose resulted in higher drug concentrations compared to a 200 mg dose. These findings reveal variations in posaconazole concentrations across different formulations and dosing strategies. Oral suspension formulations demonstrated more significant variability compared to DRTs.
The results of this study emphasize the importance of individualized administration of posaconazole. Physicians need to make timely adjustments to the dosing regimen based on the patient’s TDM results. Factors such as formulation, dosing regimen, and drug interactions play critical roles in influencing posaconazole exposure, emphasizing the need for individualized approaches in antifungal therapy.
The findings of this research offer a guide for the clinical use of posaconazole in the prevention or treatment of fungal infections in patients with compromised immune systems. This can standardize posaconazole administration, increase treatment efficacy, and lessen adverse effects. Giving immunocompromised individuals DRTs could assist patients in maintaining a more constant level of posaconazole and prevent H2A or PPIs from affecting that concentration. Additionally, patients should refrain from taking medications that interfere with the action of enzymes like P-gp and UGT1A4, which metabolize posaconazole. TDM-based dosing of posaconazole should be a part of posaconazole prophylaxis.
This analysis has several limitations. It primarily focused on POS and DRT concentrations rather than intravenous formulations. The lack of randomized controlled trials and the low quality of the included studies could reduce the reliability of the results of this study and lead to publication bias. Pediatric patients require individualized dosing based on body weight. DRT has now been found to have high concentrations in pediatric patients as well (Weerdenburg et al., 2024). However, although we did not exclude pediatric patients from our exclusion criteria, the articles that met the inclusion criteria did not have studies that examined changes in posaconazole concentrations in pediatric patients. This resulted in our inability to explore the factors influencing concentrations after posaconazole administration in pediatric patients. ECMO-related data were not included in this study, which may have led us to omit certain factors affecting concentrations. And finally, this study only focused on the factors affecting the concentration of posaconazole and did not explore the factors affecting the AUC, which may lead to a more one-sided result. However, since there is already a strong correlation between concentration and efficacy, we believe that the efficacy of posaconazole can be judged by exploring the factors that interfere with concentration.
Posaconazole concentrations exhibit considerable variability depending on the formulation, dosing regimen, and patient population. DRTs provide more stable drug concentrations than oral suspensions and are less susceptible to changes in gastrointestinal absorption conditions. To optimize therapy, patients should avoid medications that affect UGT enzymes whenever possible and carefully monitor posaconazole levels. This is particularly important in cases of diarrhea, which can significantly reduce drug concentrations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RQ: Data curation, Investigation, Software, Visualization, Writing–original draft. YL: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. YZ: Data curation, Validation, Writing–original draft. ZW: Data curation, Methodology, Writing–original draft. SY: Data curation, Investigation, Writing–original draft. SL: Data curation, Methodology, Writing–original draft. JY: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded through the Medical Science Research Project of the Health Commission of Hebei Province (No. 20210368), the Department of Finance of Hebei, and the Health Commission of Hebei Province’s Funding for Outstanding Talent Cultivation Program in Clinical Medicine (No. ZF2024130).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1450120/full#supplementary-material
Anderson, G. D. (2004). Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63, S3–S8. doi:10.1212/wnl.63.10_suppl_4.s3
Bernardo, V. A., Cross, S. J., Crews, K. R., Flynn, P. M., Hoffman, J. M., Knapp, K. M., et al. (2013). Posaconazole therapeutic drug monitoring in pediatric patients and young adults with cancer. Ann. Pharmacother. 47, 976–983. doi:10.1345/aph.1R775
Bryant, A. M., Slain, D., Cumpston, A., and Craig, M. (2011). A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int. J. Antimicrob. Agents 37, 266–269. doi:10.1016/j.ijantimicag.2010.11.021
Chae, H., Cho, S.-Y., Yi, Y., Lee, J. J., Cha, K., Kim, M., et al. (2020). Evaluation of posaconazole plasma concentrations achieved with the delayed-release tablets in Korean high-risk patients with haematologic malignancy. Mycoses 63, 131–138. doi:10.1111/myc.13031
Chen, L., Krekels, E. H. J., Verweij, P. E., Buil, J. B., Knibbe, C. A. J., and Brüggemann, R. J. M. (2020). Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 80, 671–695. doi:10.1007/s40265-020-01306-y
Chen, L., Wang, Y., Zhang, T., Li, Y., Meng, T., Liu, L., et al. (2018). Utility of posaconazole therapeutic drug monitoring and assessment of plasma concentration threshold for effective prophylaxis of invasive fungal infections: a meta-analysis with trial sequential analysis. BMC Infect. Dis. 18, 155. doi:10.1186/s12879-018-3055-3
Cojutti, P., Candoni, A., Simeone, E., Franceschi, L., Fanin, R., and Pea, F. (2013). Antifungal prophylaxis with posaconazole in patients with acute myeloid leukemia: dose intensification coupled with avoidance of proton pump inhibitors is beneficial in shortening time to effective concentrations. Antimicrob. Agents Chemother. 57, 6081–6084. doi:10.1128/AAC.01586-13
Cornely, O. A., Duarte, R. F., Haider, S., Chandrasekar, P., Helfgott, D., Jiménez, J. L., et al. (2016). Phase 3 pharmacokinetics and safety study of aposaconazole tablet formulation inpatients at risk for invasive fungal disease. J. Antimicrob. Chemother. 71, 718–726. doi:10.1093/jac/dkv380
Courtney, R., Sansone, A., Smith, W., Marbury, T., Statkevich, P., Martinho, M., et al. (2005). Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J. Clin. Pharmacol. 45, 185–192. doi:10.1177/0091270004271402
Crombag, M.-R. B. S., Huisman, C., Kemper, E. M., Brüggemann, R. J. M., and Bijleveld, Y. A. (2012). Posaconazole treatment in hematology patients: a pilot study of therapeutic drug monitoring. Ther. Drug Monit. 34, 320–325. doi:10.1097/FTD.0b013e31824d135c
Cumpston, A., Caddell, R., Shillingburg, A., Lu, X., Wen, S., Hamadani, M., et al. (2015). Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob. Agents Chemother. 59, 4424–4428. doi:10.1128/AAC.00581-15
Dolton, M. J., Ray, J. E., Chen, S. C.-A., Ng, K., Pont, L., and McLachlan, A. J. (2012). Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 56, 5503–5510. doi:10.1128/AAC.00802-12
Durani, U., Tosh, P. K., Barreto, J. N., Estes, L. L., Jannetto, P. J., and Tande, A. J. (2015). Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob. Agents Chemother. 59, 4914–4918. doi:10.1128/AAC.00496-15
Gautier-Veyret, E., Bolcato, L., Roustit, M., Weiss, S., Tonini, J., Brenier-Pinchart, M.-P., et al. (2019). Treatment by posaconazole tablets, compared to posaconazole suspension, does not reduce variability of posaconazole trough concentrations. Antimicrob. Agents Chemother. 63, 004844-19. doi:10.1128/AAC.00484-19
Gómez-López, A. (2020). Antifungal therapeutic drug monitoring: focus on drugs without a clear recommendation. Clin. Microbiol. Infect. 26, 1481–1487. doi:10.1016/j.cmi.2020.05.037
Gubbins, P. O., Krishna, G., Sansone-Parsons, A., Penzak, S. R., Dong, L., Martinho, M., et al. (2006). Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50, 1993–1999. doi:10.1128/AAC.00157-06
Heinz, W. J., Cabanillas Stanchi, K. M., Klinker, H., Blume, O., Feucht, J., Hartmann, U., et al. (2016). Posaconazole plasma concentration in pediatric patients receiving antifungal prophylaxis after allogeneic hematopoietic stem cell transplantation. Med. Mycol. 54, 128–137. doi:10.1093/mmy/myv087
Heinz, W. J., Einsele, H., Helle-Beyersdorf, A., Zirkel, J., Grau, A., Schirmer, D., et al. (2013). Posaconazole concentrations after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 15, 449–456. doi:10.1111/tid.12108
Jeong, W., Snell, G. I., Levvey, B. J., Westall, G. P., Morrissey, C. O., Wolfe, R., et al. (2018). Single-centre study of therapeutic drug monitoring of posaconazole in lung transplant recipients: factors affecting trough plasma concentrations. J. Antimicrob. Chemother. 73, 748–756. doi:10.1093/jac/dkx440
Kably, B., Launay, M., Derobertmasure, A., Lefeuvre, S., Dannaoui, E., and Billaud, E. M. (2022). Antifungal drugs TDM: trends and update. Ther. Drug Monit. 44, 166–197. doi:10.1097/FTD.0000000000000952
Kersemaekers, W. M., van Iersel, T., Nassander, U., O’Mara, E., Waskin, H., Caceres, M., et al. (2015). Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects. Antimicrob. Agents Chemother. 59, 1246–1251. doi:10.1128/AAC.04223-14
Kozuch, J. M., Feist, A., Yung, G., Awdishu, L., Hays, S., Singer, J. P., et al. (2018). Low dose posaconazole delayed release tablets for fungal prophylaxis in lung transplant recipients. Clin. Transpl. 32, e13300. doi:10.1111/ctr.13300
Krishna, G., AbuTarif, M., Xuan, F., Martinho, M., Angulo, D., and Cornely, O. A. (2008). Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28, 1223–1232. doi:10.1592/phco.28.10.1223
Krishna, G., Ma, L., Martinho, M., Prasad, P., Wahl, J., and Tavakkol, A. (2011). Determination of posaconazole levels in toenails of adults with onychomycosis following oral treatment with four regimens of posaconazole for 12 or 24 weeks. Antimicrob. Agents Chemother. 55, 4424–4426. doi:10.1128/aac.01302-10
Krishna, G., Ma, L., Martinho, M., Preston, R. A., and O’Mara, E. (2012). A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J. Antimicrob. Chemother. 67, 2725–2730. doi:10.1093/jac/dks268
Krishna, G., Martinho, M., Chandrasekar, P., Ullmann, A. J., and Patino, H. (2007a). Pharmacokinetics of oral posaconazole in allogeneichematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27, 1627–1636. doi:10.1592/phco.27.12.1627
Krishna, G., Parsons, A., Kantesaria, B., and Mant, T. (2007b). Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr. Med. Res. Opin. 23, 545–552. doi:10.1185/030079906X167507
Krishna, G., Sansone-Parsons, A., and Kantesaria, B. (2007c). Drug interaction assessment following concomitant administration of posaconazole and phenytoin in healthy men. Curr. Med. Res. Opin. 23, 1415–1422. doi:10.1185/030079907X187937
Krishna, G., Sansone-Parsons, A., Martinho, M., Kantesaria, B., and Pedicone, L. (2007d). Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob. Agents Chemother. 51, 812–818. doi:10.1128/AAC.00454-06
Lai, T., Alffenaar, J.-W., Kesson, A., Bandodkar, S., and Roberts, J. A. (2020). Evaluation of target attainment of oral posaconazole suspension in immunocompromised children. J. Antimicrob. Chemother. 75, 726–729. doi:10.1093/jac/dkz481
Launay, M., Roux, A., Beaumont, L., Douvry, B., Lecuyer, L., Douez, E., et al. (2018). Posaconazole tablets in real-life lung transplantation: impact on exposure, drug-drug interactions, and drug management in lung transplant patients, including those with cystic fibrosis. Antimicrob. Agents Chemother. 62. doi:10.1128/AAC.02061-17
Lebeaux, D., Lanternier, F., Elie, C., Suarez, F., Buzyn, A., Viard, J.-P., et al. (2009). Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob. Agents Chemother. 53, 5224–5229. doi:10.1128/AAC.00939-09
Leclerc, E., Combarel, D., Uzunov, M., Leblond, V., Funck-Brentano, C., and Zahr, N. (2018). Prevention of invasive Aspergillus fungal infections with the suspension and delayed-release tablet formulations of posaconazole in patients with haematologic malignancies. Sci. Rep. 8, 1681. doi:10.1038/s41598-018-20136-3
Li, W., Xia, F., Zhou, H., Qiu, H., Wu, D., Ma, X., et al. (2020). Efficacy of posaconazole prophylaxis for fungal disease in hematology patients treated with chemotherapy and transplantation: an open-label, prospective, observational study. Front. Microbiol. 11, 349. doi:10.3389/fmicb.2020.00349
Liebenstein, T. K., Widmer, K. M., and Fallon, M. J. (2018). Retrospective analysis of goal drug level attainment of posaconazole for invasive fungal infection prophylaxis in patients with acute myeloid leukemia pre- and post-switch to tablet formulation. J. Oncol. Pharm. Pract. 24, 599–603. doi:10.1177/1078155217722405
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27, 1785–1805. doi:10.1177/0962280216669183
Maertens, J., Cornely, O. A., Ullmann, A. J., Heinz, W. J., Krishna, G., Patino, H., et al. (2014). Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution inpatients at risk for invasive fungal disease. Antimicrob. Agents Chemother. 58, 3610–3617. doi:10.1128/AAC.02686-13
Belling, M., Kanate, A. S., Shillingburg, A., Lu, X., Wen, S., Shah, N., et al. (2017). Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leukemia Res. Treat. 2017, 3460892. doi:10.1155/2017/3460892
McCreary, E. K., Davis, M. R., Narayanan, N., Andes, D. R., Cattaneo, D., Christian, R., et al. (2023). Utility of triazole antifungal therapeutic drug monitoring: insights from the society of infectious diseases pharmacists: endorsed by the mycoses study group education and research consortium. Pharmacotherapy 43, 1043–1050. doi:10.1002/phar.2850
Miceli, M. H., Perissinotti, A. J., Kauffman, C. A., and Couriel, D. R. (2015). Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58, 432–436. doi:10.1111/myc.12339
Moton, A., Krishna, G., Ma, L., O’Mara, E., Prasad, P., McLeod, J., et al. (2010). Pharmacokinetics of a single dose of the antifungal posaconazole as oral suspension in subjects with hepatic impairment. Curr. Med. Res. Opin. 26, 1–7. doi:10.1185/03007990903364657
Oh, J., Kang, C.-I., Kim, S.-H., Huh, K., Cho, S. Y., Chung, D. R., et al. (2020). Antifungal prophylaxis with posaconazole tablet and oral suspension in patients with haematologic malignancy: therapeutic drug monitoring, efficacy and risk factors for the suboptimal level. Mycoses 63, 89–94. doi:10.1111/myc.13020
Peterlin, P., Chauvin, C., Le Gouill, S., Pere, M., Dalichampt, M., Guillaume, T., et al. (2018). Fungal prophylaxis with a gastro-resistant posaconazole tablet for patients with hematological malignancies in the POSANANTES study. Antimicrob. Agents Chemother. 62. doi:10.1128/AAC.01746-17
Pham, A. N., Bubalo, J. S., and Lewis, J. S. (2016). Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 59, 226–233. doi:10.1111/myc.12452
Ray, J., Campbell, L., Rudham, S., Nguyen, Q., and Marriott, D. (2011). Posaconazole plasma concentrations in critically ill patients. Ther. Drug Monit. 33, 387–392. doi:10.1097/FTD.0b013e31821fb197
Reinach, B., de Sousa, G., Dostert, P., Ings, R., Gugenheim, J., and Rahmani, R. (1999). Comparative effects of rifabutin and rifampicin on cytochromes P450 and UDP-glucuronosyl- transferases expression in fresh and cryopreserved human hepatocytes. Chem. Biol. Interact. 121, 37–48. doi:10.1016/s0009-2797(99)00089-7
Ross, A. L., Slain, D., Cumpston, A., Bryant, A. M., Hamadani, M., and Craig, M. (2012). Evaluation of an alternative posaconazole prophylaxis regimen in haematological malignancy patients receiving concomitant stress ulcer prophylaxis. Int. J. Antimicrob. Agents 40, 557–561. doi:10.1016/j.ijantimicag.2012.09.001
Sansone-Parsons, A., Krishna, G., Calzetta, A., Wexler, D., Kantesaria, B., Rosenberg, M. A., et al. (2006). Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 50, 1881–1883. doi:10.1128/AAC.50.5.1881-1883.2006
Sansone-Parsons, A., Krishna, G., Simon, J., Soni, P., Kantesaria, B., Herron, J., et al. (2007). Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 51, 495–502. doi:10.1128/AAC.00472-06
Shi, J., Luo, D., Weng, H., Zeng, X.-T., Lin, L., Chu, H., et al. (2020). Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods 11, 641–654. doi:10.1002/jrsm.1429
Sime, F. B., Byrne, C. J., Parker, S., Stuart, J., Butler, J., Starr, T., et al. (2019). Population pharmacokinetics of total and unbound concentrations of intravenous posaconazole in adult critically ill patients. Crit. Care 23, 205. doi:10.1186/s13054-019-2483-9
Stelzer, D., Weber, A., Ihle, F., Matthes, S., Ceelen, F., Zimmermann, G., et al. (2017). Comparing azole plasma trough levels in lung transplant recipients: percentage of therapeutic levels and intrapatient variability. Ther. Drug Monit. 39, 93–101. doi:10.1097/FTD.0000000000000371
Stelzer, D., Weber, A., Ihle, F., Matthes, S., Ceelen, F., Zimmermann, G., et al. (2018). Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses 61, 186–194. doi:10.1111/myc.12724
Suh, H. J., Kim, I., Cho, J. Y., Park, S. I., Yoon, S. H., Lee, J. O., et al. (2017). Comparison of plasma concentrations of posaconazole with the oral suspension and tablet in Korean patients with hematologic malignancies. Infect. Chemother. 49, 135–139. doi:10.3947/ic.2017.49.2.135
Suh, H. J., Yoon, S. H., Yu, K.-S., Cho, J.-Y., Park, S.-I., Lee, E., et al. (2018). The genetic polymorphism UGT1A4*3 is associated with low posaconazole plasma concentrations in hematological malignancy patients receiving the oral suspension. Antimicrob. Agents Chemother. 62. doi:10.1128/AAC.02230-17
Tonini, J., Thiébaut, A., Jourdil, J. F., Berruyer, A. S., Bulabois, C. E., Cahn, J. Y., et al. (2012). Therapeutic drug monitoring of posaconazole in allogeneic hematopoietic stem cell transplantation patients who develop gastrointestinal graft-versus-host disease. Antimicrob. Agents Chemother. 56, 5247–5252. doi:10.1128/AAC.00815-12
Van Daele, R., Spriet, I., and Maertens, J. (2020). Posaconazole in prophylaxis and treatment of invasive fungal infections: apharmacokinetic, pharmacodynamic and clinical evaluation. Expert Opin. Drug Metab. Toxicol. 16, 539–550. doi:10.1080/17425255.2020.1764939
Vanstraelen, K., Prattes, J., Maertens, J., Lagrou, K., Schoemans, H., Peersman, N., et al. (2016). Posaconazole plasma exposure correlated to intestinal mucositis in allogeneic stem cell transplant patients. Eur. J. Clin. Pharmacol. 72, 953–963. doi:10.1007/s00228-016-2057-6
von Lilienfeld-Toal, M., Wagener, J., Einsele, H., Cornely, O. A., and Kurzai, O. (2019). Invasive fungal infection. Dtsch. ArzteblInt 116, 271–278. doi:10.3238/arztebl.2019.0271
Weerdenburg, H., Walker, H., Curtis, N., Duffull, S., Haeusler, G., Cole, T., et al. (2024). Posaconazole in paediatric malignancy and haematopoietic stem cell transplant: dosing to achieve therapeutic concentration. J. Antimicrob. Chemother. 79 (7), 1493–1507. doi:10.1093/jac/dkae099
Keywords: posaconazole oral suspension, posaconazole delayed-release tablets, concentration, plasma, meta-analysis
Citation: Qu R, Liu Y, Zhao Y, Wang Z, Yuan S, Liu S and Yu J (2024) Factors affecting posaconazole plasma concentrations: a meta-analysis and systematic review. Front. Pharmacol. 15:1450120. doi: 10.3389/fphar.2024.1450120
Received: 17 June 2024; Accepted: 28 November 2024;
Published: 19 December 2024.
Edited by:
Shusen Sun, Western New England University, United StatesReviewed by:
Xiao Li, Shandong Provincial Qianfoshan Hospital, ChinaCopyright © 2024 Qu, Liu, Zhao, Wang, Yuan, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yu, eXVqaW5nQGhlYm11LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.