- 1College of Ethnic Medicine, Yunnan University of Chinese Medicine, Kunming, Yunnan, China
- 2Yunnan Key Laboratory of Dai and Yi Medicines, Yunnan University of Chinese Medicine, Kunming, Yunnan, China
- 3Department of Thoracic Surgery, The Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
Hypertension is a common disease; however, it is more prevalent in older adults, and its prevalence is increasing in younger populations. Numerous studies have revealed that hypertension and the composition and functionality of the intestinal flora are closely correlated. The balance of the intestinal flora, intestinal barrier integrity, and metabolite content of the intestinal flora play significant roles in the occurrence and progression of hypertension. Therefore, we performed a comprehensive review of Traditional Chinese medicine (TCM) for hypertension, focusing on the role of the intestinal flora to understand the mechanism by which TCM regulates hypertension through its effects on the intestinal flora. We analyzed the findings using the terms “traditional Chinese medicine,” “hypertension,” “high blood pressure,” “blood pressure,” “intestinal flora,” “intestinal barrier function,” “intestinal flora metabolites,” and other keywords from the China National Knowledge Infrastructure, VIP Chinese Science and Technology, Wanfang Data, PubMed, and ScienceDirect databases. We found that TCM treats hypertension by regulating the balance of the intestinal microbiota, increasing the abundance of beneficial bacteria, reducing the abundance of harmful bacteria, improving intestinal barrier function, increasing compact proteins, reducing intestinal permeability, and regulating the content of intestinal flora metabolites. The use of TCM to treat hypertension by regulating the intestinal flora is a promising therapeutic strategy. However, most studies are limited by small sample sizes and there is a lack of large-scale randomized controlled trials. In the future, multi-center controlled clinical trials are needed to verify the efficacy and safety of TCM, optimize therapeutic protocols, and establish a foundation for the standardized and personalized application of TCM in hypertension management.
1 Introduction
Hypertension is characterized by a persistent rise in blood vessel pressure, which increases the risk of injury to the heart, brain, kidneys, and other organs (World Health Organization, 2021). Its pathogenesis includes sympathetic nervous system hyperactivity, renin-angiotensin-aldosterone system activation, vascular endothelial dysfunction, insulin resistance, and neurohumoral factor dysregulation (Yang et al., 2023a). Hypertension is a major cause of premature death worldwide (World Health Organization, 2023). In China, approximately 2.7 million people suffer from hypertension, with only 13.8% of patients achieving adequate control (World Health Organization, 2019). Hypertension in the Chinese population is mainly due to unhealthy lifestyles, such as high-salt diets, overweight and obesity, smoking, alcohol consumption, and insufficient physical activity. Moreover, vasospasm and atherosclerosis occur when blood vessel wall elasticity decreases with age. This also causes diminished function of the blood pressure regulation center, which is an important factor in the development of hypertension. The incidence of hypertension is relatively high in individuals with work pressure, high psychological pressure, chronic tension, and anxiety (Wu and Zhou, 2023). Currently, hypertension management in China relies primarily on Western medicine, including the use of diuretics, angiotensin-converting enzyme inhibitors, β-blockers, angiotensin Il (Ang Il) receptor antagonists, and calcium channel blockers (Zhang and Li, 2023). However, this conventional treatment often requires patients to take two or more antihypertensive drugs simultaneously, and long-term use of these drugs causes drug resistance, adverse effects, and an increased risk of cancer (Wang et al., 2023). TCM can be used to treat hypertension by targeting disease symptoms. It can effectively lower blood pressure, improve accompanying symptoms, reduce side effects, and enhance the therapeutic effects of Western medicine when used in combination. This approach helps protect target organs that are easily damaged by hypertension, such as the heart, brain, and kidneys, thereby improving the quality of life of patients and making them suitable for long-term use.
The human gut, which contains more than 100 trillion microbial cells, significantly influences metabolism. Alterations in the gut flora are associated with factors including diet, the environment, and drug use (Illiano et al., 2020). Gut microbes belong to five main groups: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Cerrucomicrobia (Tang et al., 2017; Rahman et al., 2022). Scientific studies have revealed an association between gut flora and hypertension. Additionally, the structure of the intestinal flora, intestinal barrier function, and intestinal flora metabolites are closely associated with hypertension.
Therefore, in this study, we analyzed the findings using the terms “traditional Chinese medicine,” “hypertension,” “high blood pressure,” “blood pressure, “intestinal flora, “intestinal barrier function, “intestinal flora metabolites” and other keywords from the China National Knowledge Infrastructure, VIP Chinese Science and Technology, Wanfang Data, PubMed, and ScienceDirect databases. During the literature screening process, 32 eligible studies were ultimately selected from an initial pool of 350 articles. Inclusion criteria required that the studies explored the relationship between TCM and hypertension, specifically focusing on the role of gut microbiota. The studies encompassed TCM monomers,single-flavor TCM,TCM pairs, and TCM compounding, providing data on the effects of TCM interventions on gut microbiota. All selected literature was published in peer-reviewed scientific journals in English or Chinese. Exclusion criteria included studies that did not focus on the impact of TCM on the gut microbiota in hypertensive patients, non-experimental studies (such as reviews, case reports, and opinion articles), studies lacking detailed data on TCM interventions and gut microbiota changes, and studies with data insufficient to evaluate TCM’s effect on blood pressure regulation. We searched relevant literature in the past 10 years to review the mechanism and current research status of regulating intestinal flora using TCM in the treatment of hypertension.
2 Relationship between hypertension and gut flora
2.1 Relationship between hypertension and intestinal flora
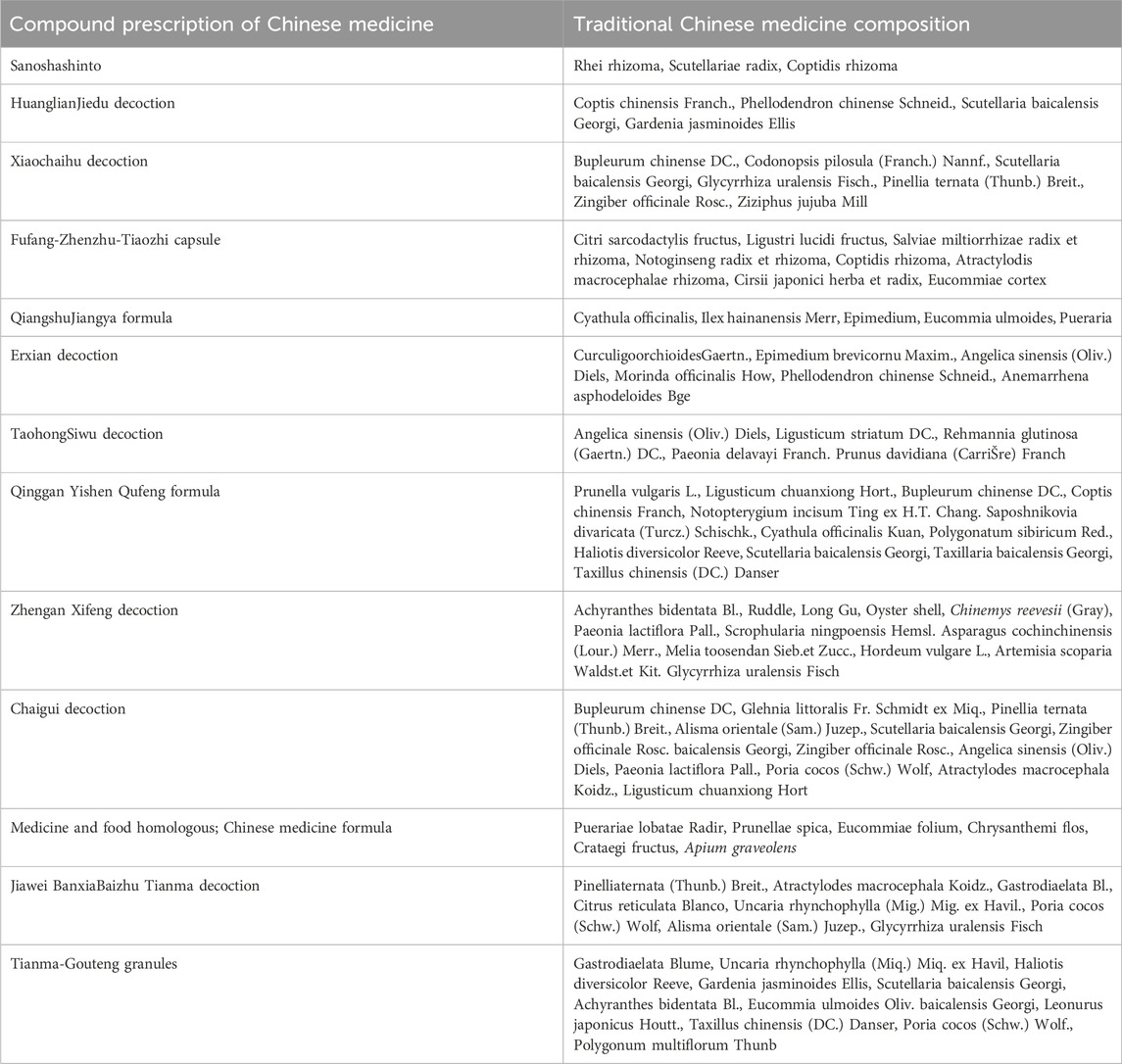
Specific structural changes in the intestinal flora, such as a decrease in beneficial bacteria and an increase in harmful bacteria, may activate signaling pathways associated with blood pressure regulation, thereby affecting blood pressure. Flora diversity and abundance are usually expressed using Chao1, Abundance-based Coverage Estimator, Operational Taxonomic Units, Shannon, and Simpson indices. Fecal transplantation from human donors with hypertension to germ-free mice showed that elevated blood pressure could be transmitted through the gut flora, revealing the direct effect of gut flora on host blood pressure (Li et al., 2017). Firmicutes and Bacteroidetes account for more than 90% of the total bacterial phyla (Zhang et al., 2021), and their ratio (F/B) is a biomarker of intestinal flora imbalance. F/B was significantly higher in patients with hypertension than in healthy individuals (Cai et al., 2023), and the F/B ratio of spontaneously hypertensive rats (SHRs) was 5-fold higher than that of Wistar rats (Yang et al., 2015). Additionally, hypertension is accompanied by a decrease and increase in beneficial and pathogenic flora, respectively. Beneficial bacteria, such as Bifidobacteria and Lactobacilli, which help maintain gut health and immune system balance, are often reduced in the hypertensive population. Probiotic yogurt reduces blood pressure in SHRs by improving the structure of the intestinal flora, increasing intestinal microbial diversity, and increasing the abundance of short-chain fatty acid (SCFA)-producing bacteria and fecal SCFAs levels (Kong et al., 2021). Intestinal pathogens and their metabolites enter the bloodstream through the mesentery, triggering chronic inflammation and vascular endothelial damage, leading to a decrease in vasodilatory factors, an increase in constrictive factors, and peripheral resistance, ultimately leading to an increase in blood pressure (Yang et al., 2023b). Moreover, probiotics can improve inflammation and lower blood pressure. For example, kefir treatment reduced interleukin (IL)-6 and tissue necrotic factor (TNF)-α protein densities and abolished microglial activation in the hypothalamic paraventricular nucleus and rostral ventrolateral medulla of SHRs (de Almeida et al., 2020). Hence, an imbalance in the gut flora changes metabolites, such as SCFAs, which stimulate the production of 5-hydroxytryptamine, which acts on the vagal nerve and vascular system, causing vasoconstriction and affecting cardiac regulatory regions of the brain through the blood-brain barrier. Furthermore, norepinephrine depresses parasympathetic nerves and, together with 5-hydroxytryptamine, increases blood pressure (Zubcevic et al., 2019). Yan and colleagues (Yan et al., 2020) found that a high-salt diet reduced Bacteroides and arachidonic acid levels in the gut of Wistar rats and increased gut-derived corticosterone production and serum and intestinal corticosterone levels, thereby promoting elevated blood pressure. Gamma-aminobutyric acid (GABA) is a neurotransmitter produced by Bacteroides via the glutamic acid decarboxylase system. GABA salt may reduce hypertension by decreasing endothelial cell dysfunction and M1 polarization. Moreover, GABA is significantly downregulated in high-salt diet-induced hypertensive rats (Otaru et al., 2021; Son et al., 2021). Thus, the intestinal flora may regulate blood pressure through GABA production. In summary, flora imbalance is closely associated with blood pressure regulation mechanisms, involving changes in flora structure, activation of inflammatory pathways, production of neurotransmitters, and changes in hormone levels, which when combined, contribute to blood pressure regulation.
2.2 Relationship between hypertension and barrier function of intestinal
The intestinal barrier is the sum of the structure and functions of the intestine that prevents harmful substances, such as bacteria and toxins, from passing through the intestinal mucosa, entering other tissues and organs, and circulating in the body. The intestinal barrier comprises microbial, chemical, physical, and immune barriers. The microbiological barrier comprises the normal intestinal flora of the host, in which beneficial bacteria support biological defenses through antagonism and immune functions. The chemical barrier includes secretions such as gastric acid, mucus, bile, glycoproteins, and enzymes, which are protective. Columnar epithelial cells and intercellular junctions, such as tight junctions, which separate the intestinal lumen from the internal environment and contribute to protection, constitute a physical barrier. The immune barrier comprises intestinal epithelial cells (iECs), intraepithelial lymphoid tissue (ilEL), lymphocytes, Peyer’s patches, mesenteric lymph nodes, and immunoglobulin A (slgA) from plasma cells (Cui et al., 2019). Gut barrier dysfunction is also associated with hypertension, and various factors, such as intestinal flora imbalance, diet, medications, genetic factors, and diseases, can influence the functioning of the intestinal barrier. Under physiological conditions, intestinal barrier function relies on tight junctions between epithelial cells, the mucus layer, and the effective functioning of the mucosal immune system to maintain intestinal homeostasis (Luissint et al., 2016). However, when these tight junctions are disrupted and mucosal defense mechanisms are impaired, intestinal permeability is enhanced, allowing inflammatory mediators, such as bacteria and endotoxins, to escape into the circulation, which further triggers systemic inflammation. This systemic release of inflammatory mediators leads to vascular endothelial dysfunction and inflammation, which promotes persistent hypertension, exacerbates cardiovascular target organ damage, and promotes the development of refractory hypertension (Yang et al., 2023a; Ge et al., 2024). Approximately one-third of the healthy population is salt-sensitive, and salt-sensitive hypertension accounts for more than 50% of patients with hypertension (Bailey and Dhaun, 2024). The absorption of sodium (Na+) and potassium (K+) associated with hypertension occurs in the upper ileum; however, the intestinal flora may indirectly influence the absorption and metabolism of these nutrients by modulating the permeability of the intestinal epithelium and the activity of sodium and potassium transporter proteins, which in turn influence blood pressure (Li and Ren, 2023).
2.3 Relationship between hypertension and gut flora metabolites
Gut flora metabolites, such as SCFAs, trimethylamine N-oxide (TMAO), lipopolysaccharide (LPS), hydrogen sulfide (H2S), and bile acids (BAs), are involved in blood pressure regulation (Ge et al., 2024). SCFAs are produced when gut bacteria ferment dietary fiber, primarily in the cecum and distal colon. It primarily comprises carboxylic acids with fewer than six carbon atoms. The most common s produced include acetate, propionate, and butyrate, which account for 95% of the total SCFAs content (Gao et al., 2024). They can lower blood pressure by dilating blood vessels. Fewer bacteria produce SCFAs when the gut flora is imbalanced, leading to the loss of epithelial barrier function, inflammation, and dysfunction of blood pressure regulation, leading to an increase in blood pressure (Felizardo et al., 2019). Furthermore, SCFAs play a pivotal role in the microbiota-gut-brain axis, influencing the integrity of the blood-brain barrier and the functionality of cells within the brain. Notably, acetate can exert antihypertensive effects by modulating microglia and astrocytes and suppressing neuroinflammation and sympathetic nerve output (Yin et al., 2024). Patients with recalcitrant hypertension have lower levels of propionate in their SCFAs than the healthy population (Ward et al., 2022). Propionate significantly inhibits the hypertensive inflammatory response via CD4+ T cell expression in mice (Bartolomaeus et al., 2019). Moreover, treatment with oral butyrate or acetate inhibited the increase in the F/B ratio and blood pressure in spontaneously hypertensive rats (Robles-Vera et al., 2020).
TMAO is a metabolite produced by intestinal microorganisms that metabolizes choline and levulinic acid to trimethylamine (TMA), which is subsequently oxidized in the liver by flavin monooxygenase (FMO) (Gao et al., 2024). TMAO negatively affects the cardiovascular system, especially blood pressure regulation. It enhances the vasoconstrictive effects of Ang II, leading to vascular smooth muscle contraction and increased peripheral vascular resistance, thereby increasing blood pressure (Jiang et al., 2021). Second, TMAO triggers oxidative stress and excessive reactive oxygen species (ROS) damage in vascular endothelial cells and impairs the endothelial ability to release nitric oxide (NO), leading to increased vascular stiffness and uncontrolled blood pressure. Additionally, TMAO promotes the accumulation of advanced glycosylation end products (AGEs), activates the receptor for AGEs (RAGE), triggers inflammation and oxidative stress, damages vascular elasticity and function, and contributes to atherosclerosis and increased blood pressure (Jiang and Duan, 2019; Han et al., 2024).
LPS, or endotoxin, is present in the outer membrane of the most abundant bacteria in the intestinal microbiome. When transferred from the gut to the body, gram-negative bacteria induce inflammation and increase intestinal permeability (Verhaar et al., 2020). LPS binds to Toll-like receptor 4 (TLR4), activating inflammatory signaling pathways, which leads to the release of pro-inflammatory factors (TNF-α, IL-6, and IL-1β). These factors impair vascular endothelial function, inhibit NO production, and weaken vasodilatation, which in turn increases blood pressure (Lu et al., 2008; Zusso et al., 2019). Additionally, LPS induces oxidative stress and excessive damage due to reactive oxygen species (ROS), which affect endothelial cells, exacerbates vascular stiffness, and drives the progression of hypertension (Grylls et al., 2021). Moreover, endotoxins also stimulate the development of hypertension. Simultaneously, endotoxins exacerbate hypertension by stimulating the central nervous system and activating sympathetic nerves, leading to vasoconstriction and increased cardiac output (Dai et al., 2023). In summary, endotoxins mainly contribute to hypertension via inflammation and influence the development and progression of hypertension via inflammation, oxidative stress, and sympathetic activation.
H2S gas is reductive, has a high concentration in the colon, and is synthesized mainly by intestinal epithelial cells and intestinal flora through enzymatic reactions (Cirino et al., 2023). It promotes vasodilation by activating ATP-sensitive potassium channels in vascular smooth muscle cells, leading to hyperpolarization of the cell membrane and lowering of blood pressure (Kanagy et al., 2017). Additionally, H2S promotes the differentiation and proliferation of regulatory T cells (Tregs), attenuates vascular and renal immune inflammation, and inhibits blood pressure elevation through the sulfation of liver kinase B1 (LKB1) (Cui et al., 2020). The treatment of SHRs with sodium hydrosulfide (NaHS) as a donor of H2S resulted in a significant reduction in blood pressure compared with that in Wistar rats (Ni et al., 2018).
BAs are released into the small intestine during digestion. The intestinal flora further converts them into secondary BAs that promote the absorption of fats and fat-soluble molecules (Tang et al., 2017). BAs lower blood pressure by directly acting on vascular endothelial cells and reducing the vasoconstrictive response induced by norepinephrine. Specific BAs, such as lithocholic acid and taurine goose deoxycholate, promote NO production, which further promotes vasodilation (Tominaga et al., 1988; Guizoni et al., 2020). Additionally, BAs can activate calcium-activated K+ channels (BK(Ca) channels) in patients with hypertension and abnormal calcium metabolism. This condition leads to vasodilation and lowers blood pressure (Ling et al., 2023). BAs also increase vascular smoothness and blood pressure. These acids contribute to lowering blood pressure by activating the farnesylate X receptor (FXR) and G protein-coupled BA receptor in vascular smooth muscle cells and endothelial cells, increasing large-conductance calcium-activated potassium channel activity, and promoting vasodilation (Ishimwe et al., 2022). The intestinal flora influences the host’s metabolic and inflammatory responses by metabolizing BAs and dietary fiber, which may lead to alterations in blood pressure (Fan and Pedersen, 2021). Additionally, BAs may regulate the growth of flora through their antimicrobial effects, safeguarding the structural and functional integrity of the gut and maintaining homeostasis in the intestinal environment (Natividad et al., 2018) (Figure 1).
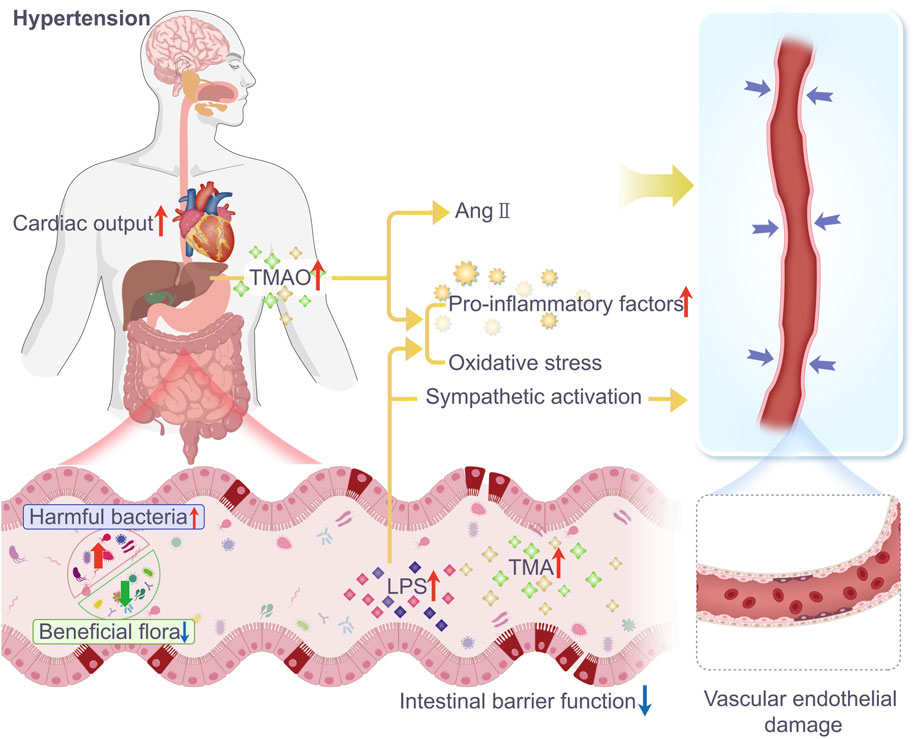
Figure 1. In patients with hypertension, the diversity and abundance of gut microbiota are significantly reduced, and there is a clear dysfunction of the intestinal barrier. The number of beneficial bacteria decreases, while the number of harmful bacteria and gut microbiota metabolites TMAO and LPS increase, leading to a reduction in tight junction proteins in the intestine and increased intestinal permeability. TMAO, a metabolite generated by intestinal microbes from TMA, is oxidized in the liver. It can cause hypertension by enhancing the vasoconstrictive effects of angiotensin II, increasing inflammatory factors, and inducing oxidative stress leading to vasoconstriction. LPS can exacerbate hypertension by releasing pro-inflammatory factors, inducing oxidative stress, and activating the sympathetic nervous system, leading to vasoconstriction and increased cardiac output.
3 Relationship between traditional Chinese medicine, intestinal flora, and hypertension
TCM can treat hypertension by regulating the balance between probiotics and pathogenic bacteria, restoring the balance of intestinal microorganisms, improving intestinal barrier function, and regulating metabolites of the intestinal flora (Yang et al., 2023b). Currently, an increasing number of reports describe how intestinal flora are modified by TCM for treating hypertension, including studies related to TCM monomers, single-flavor TCM, TCM pairs, and TCM combinations. Changes in the intestinal flora in the hypertension model induced by TCM intervention (comparison between the administered and model groups) are shown in Table 1.
Table 1. Changes in intestinal flora in a model of hypertension induced by TCM intervention (administered group vs model group).
3.1 Traditional Chinese medicine monomers
TCM monomers are purified chemical compounds extracted from TCM and are an important part of the medicinal components of TCM. These monomeric compounds have high purity, well-defined chemical structures, and pharmacological activities, providing strong support for the modernization and development of TCM. Recently, it has been shown that some chemical components of TCM can regulate blood pressure by acting on the intestinal flora. For example, animal experiments have shown that baicalin can significantly inhibit Ang II-induced intestinal epithelial damage and barrier disruption in mice, inhibit inflammatory cell infiltration, and increase the expression of tight junction proteins (Zona Occludens 1 [ZO-1], cingulin, and occludin) and SCFA-producing flora in the intestinal tract (Aliceps and Butyricoccus). Therefore, it enhances intestinal mucosal barrier function, reduces intestinal permeability, protects the structural integrity of the intestine, and lowers blood pressure (Wu et al., 2019; Li B. et al., 2022). Rhynchophylline can optimize the intestinal flora structure by lowering the F/B ratio of SHRs, increasing and decreasing the abundance of beneficial and potentially pathogenic bacteria, respectively, thereby lowering blood pressure (Zhang et al., 2023). Quercetin reduces the F/B ratio, regulates gut flora balance, downregulates the TLR4/NF-κB inflammatory signaling pathway, attenuates myocardial fibrosis, and improves vascular dysfunction and vascular remodeling, thereby lowering blood pressure in SHRs and improving ventricular remodeling (Zhou et al., 2020). Moreover, resveratrol alters the intestinal flora of postnatal adult rats induced using a high-fat diet and NG-nitro-L-arginine-methyl ester. It also decreases the F/B ratio and increases the abundance of beneficial bacteria (Verrucomicrobia and Akkermansia), potentially preventing and reducing hypertension (Chen et al., 2019). Curcumin enhances the abundance of Lactobacillus muridarum in the intestines of hypertensive mice fed a high-salt diet. Lactobacillus muridarum prevents the exacerbation of salt-sensitive hypertension by regulating helper T cell 17 (TH17) (Han, 2021). Berberine reduces TMA production by modulating the abundance and activity of specific bacteria in the gut microbiota of patients with hypertension. This is achieved by inhibiting CutC/D-containing enzymes, thereby decreasing the plasma levels of TMAO. It also ameliorates vascular endothelial dysfunction by inhibiting the endoplasmic reticulum stress signaling pathway, thus regulating blood pressure (Wang et al., 2024). Dendrobium officinale polysaccharide regulates blood pressure by promoting the growth of beneficial flora (Lactobacillus and Lachnospiraceae_NK4A136_ group) and decreasing harmful flora (Desulfobacterota and Firmicutes) in the intestine, lowering the F/B ratio, modulating the production of SCFAs, activating the SCFA-GPCR43/41 pathway, improving vascular endothelial function and lipid levels, and enhancing intestinal barrier function. All these functions positively affect hypertension (Li M. et al., 2022). In summary, TCM monomers play a role in lowering blood pressure. This involves regulating the structure and function of the intestinal flora, altering the integrity of the intestinal barrier, decreasing inflammatory responses, and activating metabolite-related signaling pathways of the intestinal flora to reduce the production of harmful metabolites.
3.2 Single-flavor traditional Chinese medicines
Many studies have confirmed the efficacy of TCM in preventing and treating hypertension, and its mechanisms of action are closely related to the regulation of intestinal flora. For example, Digupi (Lycii Cortex) reduced the systolic and diastolic blood pressures of SHRs. The groups that received Digupi, including Elusimicrobia, Erysipelotrichia, Erysipelotrichales, Elusimicrobi-Ales, and Muribaculaceae, differed significantly from the model group (Shan et al., 2024). Theolatile oils from Danggui (Angelica sinensis (Oliv.) Diels) were used in SHR. The findings revealed that the abundance of Aspergillus spp. in the group that received high-dose Danggui volatile oil, which produces pro-inflammatory toxins, was significantly lower than that in the model group. Therefore, the volatile oil of Danggui may reduce blood pressure by decreasing the abundance of Aspergillus and the production of pro-inflammatory toxins (Shen et al., 2020). Gegen (Pinus lobata (Willd.) Ohwi.) and Xiakucao (Prunella vulgaris L.) significantly reduced elevated blood pressure induced by a high-salt diet in mice. This may partly restore the diversity of the intestinal flora and increase the abundance of the beneficial bacterium Clostridium by elevating the Shannon and Simpson indices. Additionally, it decreased the abundance of Lachnospiraceae, Anaerotruncus, Rhodobacter, Eubacteriaceae, and Streptococcus, which are harmful bacteria that are positively associated with hypertension (Li et al., 2020). Danshen (Salvia miltiorrhizage) can regulate blood pressure by improving the diversity and structure of intestinal microorganisms, increasing the abundance of beneficial flora, such as Prevotellaceae, decreasing the F/B ratio, modulating the immune response, and attenuating the inflammatory response and vascular damage induced by a high-salt diet (Qi et al., 2024). Papaya can lower blood pressure in SHRs through its rich dietary fiber content that regulates the intestinal flora, thus lowering the F/B ratio, increasing the activation of G protein-coupled receptor 41 (GPR41) by SCFAs, upregulating the expression of tight junction proteins, and restoring intestinal barrier function by reducing inflammatory factor release (Chen et al., 2023). The ultrafine powder of Dendrobium officinale enhances the gut microbiota and boosts the generation, transfer, and use of SCFAs, subsequently triggering the intestinal-vascular SCFA-GPCR43/41 signaling pathway, which enhances the endothelial function of the blood vessels and ultimately reduces blood pressure in rats with metabolic hypertension (Li et al., 2021). Huangjing (Polygonatum sibiricum Red. Superfine powder, PSP) enhances the integrity of the intestinal barrier by upregulating the expression of tight junction proteins (Claudin-1, occludin, and ZO-1), thereby reducing intestinal permeability and effectively reducing pathogens and harmful substances from LPS in the blood circulation. Additionally, PSP regulates intestinal flora by decreasing and increasing the abundance of harmful (Desulfobacter and Desulfovibrio) and beneficial (Streptococcus) bacteria, respectively. The combined effects improve blood pressure in rats with metabolic hypertension (MH) induced by a high-sugar, high-fat, and complex alcohol diet (Su et al., 2022). In summary, single-flavor TCM may exert blood pressure-lowering effects by improving the balance of intestinal flora, enhancing intestinal barrier function, promoting the metabolites produced by beneficial flora such as SCFAs, improving vascular endothelial function, and attenuating inflammatory responses.
3.3 Traditional Chinese medicine pairs
Some TCM pairs have been experimentally validated for the treatment of hypertension. Recently, the intestinal flora has received increasing attention as a novel therapeutic target for the treatment of hypertension, and studies on the effects of TCM pairs on intestinal flora have also increased. Duzhong (Eucommia ulmoides Oliv.) and Cijili (Tribulus terrestris) spontaneously reduced the abundance of actinomycetes in older rats with hypertension. The abundance of actinomycetes in these rats increases the level of SCFAs in feces and regulates blood pressure by reducing the production of inflammatory factors (Qi et al., 2019). Huangqin (Scutellaria baicalensis Georgi) and Huaihua (Sophora japonica L.) increased the biodiversity of the intestinal flora in SHRs, decreased the F/B ratio, and increased the abundance of beneficial bacteria. Moreover, Lactobacillaceae and Bifidobacteriaceae ameliorated intestinal damage, repaired intestinal villi, and increased mucin expression, which reduced blood pressure (Guan, 2020). Huangqi (Arabidopsis membranaceus (Fisch.) Bge. var. mongholicus (Bge) Hsiao) and Danshen may increase Akkermansia muciniphila by increasing the abundance of probiotics such as Lactobacillus spp., Bifidobacterium spp., Lactobacillus intestinalis, and Lactobacillus reuteri, which regulate the structure and diversity of the intestinal flora and decrease the F/B ratio, improving the intestinal microecology and further reducing the blood pressure of SHRs (Han et al., 2019).
3.4 Traditional Chinese medicine compounding
TCM compounding involves the combination of two or more TCMs, following certain compounding principles. Some TCM compounds developed to manage hypertension based on their action on intestinal flora have been effective and have been studied more extensively than monomers, single-flavored TCM, and TCM pairs. For example, Sanoshashinto can increase the number of lactobacilli in the intestines of SHRs, thereby regulating blood pressure (Wu et al., 2020). The Huanglian Jiedu decoction may relieve high blood pressure by increasing the intestinal flora of SHR, reducing and increasing the relative abundance of Firmicutes based on the relative abundance of the probiotic lactobacillus (Ma et al., 2020). Xiaochaihu decoction combined with irbesartan is more effective than irbesartan alone in lowering blood pressure. This combination reduces the relative abundance of enterococci, yeasts, and Enterobacteriaceae and increases the relative abundance of probiotic lactobacilli (Wu et al., 2022). Fufang-Zhenzhu-Tiaozhi capsule (FTZ)-treated HFS-fed rats with hypertension showed improved intestinal microbial abundance and diversity and increased abundance of Proteobacteria, Verrucomicrobia, and Fusobacteria compared with the model group. Transplantation with FTZ-modulated gut microbiota decreased blood pressure in HFS-fed rats, highlighting that FTZ modulates the intestinal flora and decreases blood pressure (Chen et al., 2022). The Qiangshu Jiangya formula reduces the F/B ratio in NG-nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced hypertension, increases the abundance of SCFA-producing Ruminococcus, and improves oxidative stress in vivo (Huang, 2022). Erxian decoction can decrease the relative abundance of TMAO-related Firmicutes and Ruminococcaceae, improve the metabolism of TMAO and its related precursors in circulation, affect the TXNIP/NLRP3 inflammatory pathway, reduce the inflammatory response, and decrease blood pressure elevation in ovariectomized rats (Hu, 2023). The mechanism of action of Taohong Siwu decoction combined with Dubosiella newyorkensis in the treatment of hypertension is the regulation of intestinal microecology, especially the increase in the beneficial bacteria Lactobacillus and Allobaculum, the improvement of serum BA metabolism, and the improvement of vascular endothelial function through this action, leading to the effective control of blood pressure (Liu et al., 2023). The compound Qinggan Yishen Qufeng inhibits pathological changes in the ileum and colon, protects the intestinal barrier structure, and regulates the positive correlation with blood pressure in a mouse model of Ang II-induced hypertension. It achieves this by positively influencing the abundance of specific bacterial groups (Actinobacteria, Acidobacteria, Myxococcales, Bacteroidaceae, g_Bacteroidaceae, and g_Tyzzerella, among others). Conversely, it negatively affects bacterial groups, such as Enterobacteriaceae and Rikenellaceae, including g_Alistipes and g_Rikenellaceae_RC9_gut_group, with other abundant specific bacterial groups and blood pressure-related metabolite (DPAn-6, desmethyldeoxycholic acid, and taurocholic acid) levels, thereby reducing blood pressure (Zhen, 2020). Zhengan Xifeng decoction significantly reduces blood pressure in SHRs, regulates the structure of the intestinal flora, reduces the F/B ratio, increases the number of SCFA-producing bacteria, promotes the conversion of lactic acid to butyric acid in the intestinal tract, reduces the levels of d-lactic acid and diamine oxidase (DAO) in the intestinal tract, and maintains the integrity of the intestinal barrier, thus lowering blood pressure (Yu et al., 2019; Xu et al., 2022). Moreover, Chaigui decoction can increase angiotensin-converting enzyme (ACE) two levels in the plasma and decrease renin levels in renal tissues, thereby decreasing the renin to ACE2 ratio. It also improves the intestinal flora by increasing the abundance of beneficial Bacteroides and decreasing the abundance of harmful Clostridia. Additionally, Chaigui decoction reduces systolic and diastolic blood pressure, modulates the renin-angiotensin-aldosterone system, affects serum levels of lysophosphatidylcholine, and may further reduce blood pressure by increasing the abundance of S24-7 Bacteroidia, which is negatively associated with blood pressure regulation; this effect has been validated in a hypertensive rat model (Zhu et al., 2023a; Zhu et al., 2023b). The medicinal and food homologous TCM compounding reduces the F/B ratio, increases the abundance of Lactobacillus, and regulates serum metabolites and their related metabolic pathways by modulating the intestinal flora structure of two kidneys and one clip (2K1C) rats with hypertension. This compounding reduced blood pressure in a hypertensive rat model, reduced metabolites and their related metabolic pathways, repaired vascular and organ damage, and exerted comprehensive therapeutic effects on hypertension (Guo et al., 2023). The Jiawei Banxia Baizhu Tianma Decoction (MBTD) regulates the structure of the intestinal microbial community by increasing the level of Streptococcus species, decreasing the level of Desulfovibrio desulfuricans and Vibrio desulfuricans, increasing the expression of short SCFAs and their receptors GPCR41 and GPCR43, enhancing intestinal barrier function, and decreasing the level of LPS in the serum. MBTD inhibits the vascular TLR4/MyD88 pathway, regulates the balance between NO and ET-1, and improves vascular endothelial function, thus effectively improving blood pressure and lipid metabolism disorders in hypertensive rats (Wu et al., 2024). Tianma-Gouteng granules increase the relative abundance of Desulfovibrio, Lachnoclostridium, and Turicibacter and decrease the relative abundance of Allobaculum and Monoglobus by regulating the balance of the intestinal flora in the hypertensive rat model. It further regulates BA metabolism through the gut–hepatic axis, affects the FXR-FGF15-CYP7A1 signaling pathway, and promotes the synthesis and secretion of BAs to comprehensively regulate blood pressure, thus playing an important role in the treatment of hypertension (Yu et al., 2024). The composition of each TCM compounding is listed in Table 2. TCM compounds show therapeutic effects on hypertension by regulating the balance of intestinal flora, increasing the abundance of beneficial bacteria, decreasing the abundance of harmful bacteria, improving the intestinal barrier function, promoting the production of short-chain fatty acids, regulating the level of metabolites related to blood pressure, and influencing the metabolism of BAs through the intestinal–hepatic axis, which in turn integrally regulates blood pressure (Figure 2).
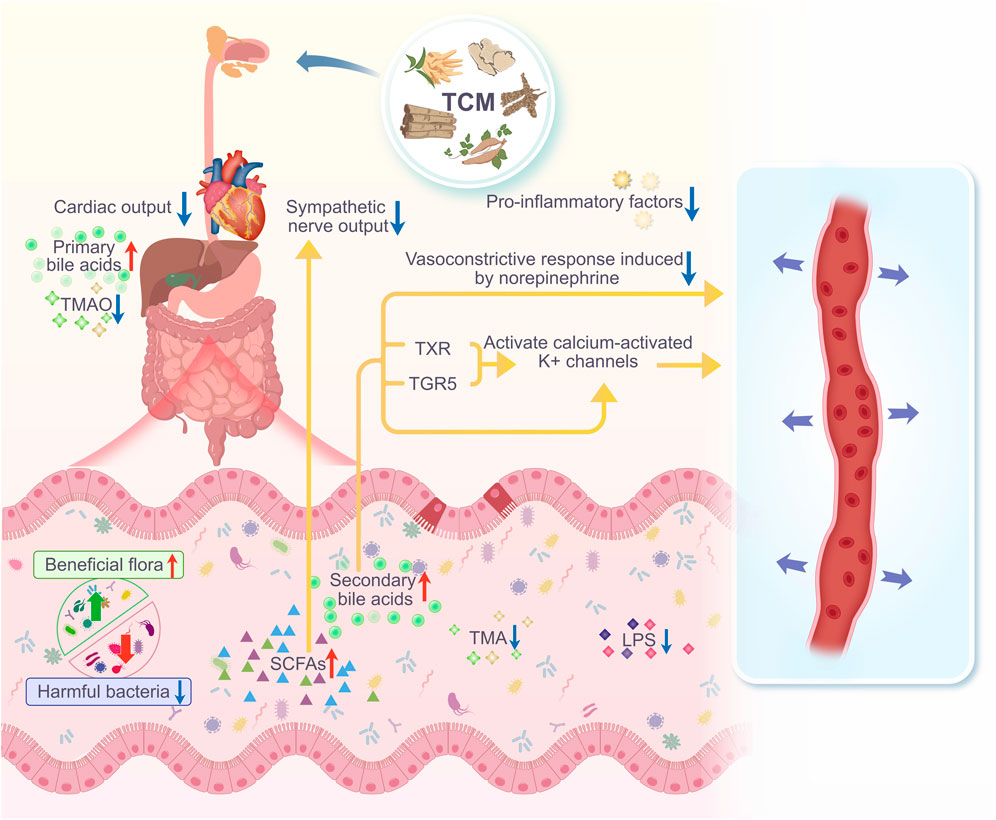
Figure 2. After taking TCM, the diversity and abundance of gut microbiota in hypertensive individuals/animals increase. The number of harmful bacteria decreases, while the number of beneficial bacteria increases, leading to a significant improvement in intestinal barrier function, and an increase in gut microbiota metabolites such as SCFAs and BAs. SCFAs can reduce inflammatory responses and inhibit sympathetic nerve output, effectively lowering blood pressure. Primary BAs are stored in the gallbladder and released into the intestine during digestion, where gut microbiota convert them into secondary BAs. These secondary BAs can also reduce vasoconstriction caused by norepinephrine, and can promote vasodilation by directly activating BK(Ca) channels, or by activating FXR and TGR5 to increase the activity of large-conductance BK(Ca) channels, thereby lowering blood pressure.
4 Conclusion
This study systematically reviewed current research on TCM regulation of gut microbiota to control hypertension, and demonstrated consistent findings. Various TCM approaches impacted blood pressure through multiple mechanisms, primarily in modulating the composition of gut microbiota, enhancing intestinal barrier function, regulating gut-derived metabolites, and suppressing inflammatory responses. First, TCM reshapes the microbiota by increasing beneficial bacteria, such as bifidobacterium and lactobacillus, and inhibiting harmful bacteria. Second, TCM reduces intestinal permeability by upregulating tight junction proteins, such as ZO-1 and occludin, thus preventing gut-derived toxins (e.g., LPS) from entering the bloodstream, thereby protecting the vascular endothelium. Additionally, TCM influences the production of gut metabolites, including SCFAs and TMAO. SCFAs contribute to vasodilation, whereas TMAO, associated with hypertension, increases vascular resistance and induces endothelial damage. TCM also reduces the expression of inflammatory factors such as IL-6 and TNF-α, mitigating vascular injury caused by hypertension. Several animal studies have shown that TCM significantly lowers systolic and diastolic blood pressure in hypertensive models, with reductions generally ranging from 10 to 50 mmHg, which is clinically meaningful. However, most existing studies are limited by small sample sizes and there is a lack of large-scale randomized controlled trials. Future multi-center clinical trials are essential to verify TCM’s efficacy and safety, optimize therapeutic protocols, and establish a basis for standardized and personalized applications in hypertension management.
Author contributions
WC: Writing–review and editing, Writing–original draft, Conceptualization, Data curation, Formal analysis, Methodology. LX: Writing–original draft, Conceptualization, Software, Methodology. WG: Writing–original draft, Investigation, Data curation. HL: Writing–original draft, Methodology, Data curation. RC: Writing–review and editing, Methodology, Funding acquisition, Investigation. ZD: Writing–review and editing, Methodology, Investigation. QC: Writing–review and editing, Software, Project administration, Investigation, Visualization. QL: Writing–review and editing, Validation, Resources, Project administration, Methodology.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. 12th Five-year Key Construction Discipline of State Administration of Traditional Chinese Medicine “Dai Pharmacy”. Educational scientific research project of Yunnan University of Chinese Medicine (YB240326). The construction project of the National Public Employment Service Capacity Improvement Demonstration Project “Yi Medicine Master Studio”. Yunnan Province First-Class Discipline Provincial Key Support Construction Discipline - Traditional Chinese Medicine (TCM), Yunnan University of Chinese Medicine “Gu Ben Pei Yuan” Discipline Team Construction Project “Ethnic Characteristic Diagnosis and Treatment Research Discipline Team” (10171100600BK). Key Research and Development Plan of Yunnan Province Science and Technology Department Social Development Special Plan (202403AC100017). Yunnan Province Dai Medicine and Yi Medicine Key Laboratory Development Project (2024JS2409). Yunnan University of Chinese Medicine Young Talents Project. National Administration of Traditional Chinese Medicine Science and Technology Department Research on the Key Information Verification of Ancient Classic Prescriptions in Minority Medicines. Higher Education “121” Project Special Program - Traditional Chinese Medicine (TCM) - Ethnic Characteristic Diagnosis and Treatment Research Discipline Team (31271100200BK).
Acknowledgments
The authors gratefully acknowledge the support of the 12th Five-year Key Construction Discipline of State Administration of Traditional Chinese Medicine “Dai Pharmacy”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bailey, M. A., and Dhaun, N. (2024). Salt sensitivity: causes, consequences, and recent advances. Hypertension 81 (3), 476–489. doi:10.1161/HYPERTENSIONAHA.123.17959
Bartolomaeus, H., Balogh, A., Yakoub, M., Homann, S., Markó, L., Höges, S., et al. (2019). Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139 (11), 1407–1421. doi:10.1161/CIRCULATIONAHA.118.036652
Cai, M., Lin, L., Jiang, F., Peng, Y., Li, S., Chen, L., et al. (2023). Gut microbiota changes in patients with hypertension: a systematic review and meta-analysis. J. Clin. Hypertens. (Greenwich) 25 (12), 1053–1068. doi:10.1111/jch.14722
Chen, H. E., Lin, Y. J., Lin, I. C., Yu, H. R., Sheen, J. M., Tsai, C. C., et al. (2019). Resveratrol prevents combined prenatal N(G)-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 70, 28–37. doi:10.1016/j.jnutbio.2019.04.002
Chen, K., Wu, S., Guan, Y., Ma, Y., Huang, Y., Liu, X., et al. (2023). Changes in gut microbiota linked to a prevention of cardiac remodeling induced by hypertension in spontaneously hypertensive rats fed a pawpaw fruit diet. Heliyon 9 (5), e15576. doi:10.1016/j.heliyon.2023.e15576
Chen, Z., Yang, B., Wang, Z., Rong, X., Zhu, Q., and Guo, J. (2022). Modulation of the gut microbiota by Fufang-Zhenzhu-Tiaozhi capsule attenuates hypertension induced by a high-fructose and high-salt diet. Front. Cell. Infect. Microbiol. 12, 854849. doi:10.3389/fcimb.2022.854849
Cirino, G., Szabo, C., and Papapetropoulos, A. (2023). Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol. Rev. 103 (1), 31–276. doi:10.1152/physrev.00028.2021
Cui, C., Fan, J., Zeng, Q., Cai, J., Chen, Y., Chen, Z., et al. (2020). CD4(+) T-cell endogenous cystathionine γ lyase-hydrogen sulfide attenuates hypertension by Sulfhydrating liver kinase B1 to promote T regulatory cell differentiation and proliferation. Circulation 142 (18), 1752–1769. doi:10.1161/CIRCULATIONAHA.119.045344
Cui, Y., Wang, Q., Chang, R., Zhou, X., and Xu, C. (2019). Intestinal barrier function-non-alcoholic fatty liver disease interactions and possible role of gut microbiota. J. Agric. Food Chem. 67 (10), 2754–2762. doi:10.1021/acs.jafc.9b00080
Dai, Y., Shen, Z., Khachatryan, L. G., Vadiyan, D. E., Karampoor, S., and Mirzaei, R. (2023). Unraveling mechanistic insights into the role of microbiome in neurogenic hypertension: a comprehensive review. Pathol. Res. Pract. 249, 154740. doi:10.1016/j.prp.2023.154740
de Almeida Silva, M., Mowry, F. E., Peaden, S. C., Andrade, T. U., and Biancardi, V. C. (2020). Kefir ameliorates hypertension via gut-brain mechanisms in spontaneously hypertensive rats. J. Nutr. Biochem. 77, 108318. doi:10.1016/j.jnutbio.2019.108318
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 (1), 55–71. doi:10.1038/s41579-020-0433-9
Felizardo, R. J., Watanabe, I. K., Dardi, P., Rossoni, L. V., and Câmara, N. O. (2019). The interplay among gut microbiota, hypertension and kidney diseases: the role of short-chain fatty acids. Pharmacol. Res. 141, 366–377. doi:10.1016/j.phrs.2019.01.019
Gao, K., Wang, P. X., Mei, X., Yang, T., and Yu, K. (2024). Untapped potential of gut microbiome for hypertension management. Gut Microbes 16 (1), 2356278. doi:10.1080/19490976.2024.2356278
Ge, Y., Wang, J., Wu, L., and Wu, J. (2024). Gut microbiota: a potential new regulator of hypertension. Front. Cardiovasc. Med. 11, 1333005. doi:10.3389/fcvm.2024.1333005
Grylls, A., Seidler, K., and Neil, J. (2021). Link between microbiota and hypertension: focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed. Pharmacother. 137, 111334. doi:10.1016/j.biopha.2021.111334
Guan, Y. (2020). Study on the effect and mechanism of the matched pair of Scutellariae Radix and Sophora japonica L. ameliorate hypertension and hypertensive renal injury. China: Southern Medical University.
Guizoni, D. M., Vettorazzi, J. F., Carneiro, E. M., and Davel, A. P. (2020). Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 94, 48–53. doi:10.1016/j.niox.2019.10.008
Guo, L., Sheng, W., He, Y., Xu, X., Lin, S., and Chen, W. (2023). Effect of medicine and food homologous Chinese medicine on hypertensive rats by affecting intestinal flora structure and regulating serum metabolites. Chin. Trad. Herb. Drugs 54 (20), 6743–6752. doi:10.7501/j.issn.0253-2670.2023.20.018
Han, C., Jiang, Y., Li, W., Liu, Y., Qi, Z., and Bai, W. (2019). Study on the mechanism of Astragalus and Salvia miltiorrhiza on intestinal flora of spontaneously hypertensive rats based on 16S rDNA sequencing technology. China J. Trad. Chin. Med. Pharm. 34 (05), 2233–2237.
Han, J. (2021). Curcumin by activating TRPV4 channels in vascular endothelial cells improves hypertension. China: Jiangnan University.
Han, J. M., Guo, L., Chen, X. H., Xie, Q., Song, X. Y., and Ma, Y. L. (2024). Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: a meta-analysis and dose-response relationship analysis. Med. (Baltim.) 103 (1), e36784. doi:10.1097/MD.0000000000036784
Hu, J. (2023). Exploring the myocardial protective effects of EXD on OVX rats based on TMAO-NLRP3 pathway. Univ. China Acad. Chin. Med. Sci. doi:10.27658/d.cnki.gzzyy.2023.000153
Huang, X. (2022). Study on Pharmacodynamic material basis and mechanism of Qiangshu Jiangya formula in hypertensive rats. China: Guangdong Pharmaceutical University.
Illiano, P., Brambilla, R., and Parolini, C. (2020). The mutual interplay of gut microbiota, diet and human disease. FEBS J. 287 (5), 833–855. doi:10.1111/febs.15217
Ishimwe, J. A., Dola, T., Ertuglu, L. A., and Kirabo, A. (2022). Bile acids and salt-sensitive hypertension: a role of the gut-liver axis. Am. J. Physiol. Heart Circ. Physiol. 322 (4), H636–H646. doi:10.1152/ajpheart.00027.2022
Jiang, S., Shui, Y., Cui, Y., Tang, C., Wang, X., Qiu, X., et al. (2021). Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 46, 102115. doi:10.1016/j.redox.2021.102115
Jiang, X., and Duan, J. (2019). Research progress on mechanism of atherosclerosis induced by trimethylamine-N-oxide. Chin. J. Mult. Organ Dis. Elder. 18 (02), 157–160. doi:10.11915/j.issn.1671-5403.2019.02.031
Kanagy, N. L., Szabo, C., and Papapetropoulos, A. (2017). Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 312 (5), C537–C549. doi:10.1152/ajpcell.00329.2016
Kong, C. Y., Li, Z. M., Mao, Y. Q., Chen, H. L., Hu, W., Han, B., et al. (2021). Probiotic yogurt blunts the increase of blood pressure in spontaneously hypertensive rats via remodeling of the gut microbiota. Food Funct. 12 (20), 9773–9783. doi:10.1039/d1fo01836a
Li, B., He, X., Jin, H. Y., Wang, H. Y., Zhou, F. C., Zhang, N. Y., et al. (2021). Beneficial effects of Dendrobium officinale on metabolic hypertensive rats by triggering the enteric-origin SCFA-GPCR43/41 pathway. Food Funct. 12 (12), 5524–5538. doi:10.1039/d0fo02890h
Li, B., Wang, H. Y., Huang, J. H., Xu, W. F., Feng, X. J., Xiong, Z. P., et al. (2022). Polysaccharide, the active component of Dendrobium officinale, ameliorates metabolic hypertension in rats via regulating intestinal flora-SCFAs-vascular Axis. Front. Pharmacol. 13, 935714. doi:10.3389/fphar.2022.935714
Li, H., and Ren, M. (2023). Advance in the study on the relationship among salt, intestinal microbiota and its metabolites, and blood pressure. Chin. J. Arterioscler. 31 (11), 1007–1012. doi:10.20039/j.cnki.1007-3949.2023.11.012
Li, J., Zhao, F., Wang, Y., Chen, J., Tao, J., Tian, G., et al. (2017). Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5 (1), 14. doi:10.1186/s40168-016-0222-x
Li, M., Wu, D., Chen, Y., and Zhang, T. (2022). Effects of baicalin on the intestinal barrier impairment of hypertensive mice induced by angiotensin Ⅱ. China J. Trad. Chin. Med. Pharm. 37 (09), 5375–5379.
Li, X., Min, E., Wang, B., and Sun, L. (2020). Effect of Pueraria extract on salt-sensitive hypertension and gut MicrobiotaI in mice. Acta Nutr. Sin. 42 (05), 491–496 + 504. doi:10.13325/j.cnki.acta.nutr.sin.20201027.002
Ling, B., Jin, H., Zhang, Q., Wang, Y., Qi, S., Liu, S., et al. (2023). The role of the intestinal flora-bile acid axis in the pathogenesis of hypertension. Chin. J. Hypertens. 31 (11), 1043–1051. doi:10.16439/j.issn.1673-7245.2023.11.009
Liu, T., Li, X., Zhang, C., Zhao, L., Li, X., Yu, Y., et al. (2023). Lactobacillus and Allobaculum mediates the improvement of vascular endothelial dysfunction during hypertension with TaohongSiwu decoction combined with Dubosiella newyorkensis. Heliyon 9 (12), e22572. doi:10.1016/j.heliyon.2023.e22572
Lu, Y. C., Yeh, W. C., and Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine 42 (2), 145–151. doi:10.1016/j.cyto.2008.01.006
Luissint, A. C., Parkos, C. A., and Nusrat, A. (2016). Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 151 (4), 616–632. doi:10.1053/j.gastro.2016.07.008
Ma, X., Xiong, X., Mo, Y., Yue, G., Cai, T., and Huang, J. (2020). Study on changes of intestinal microflora in spontaneously hypertensive rats based on 16S rDNA sequencing and intervention of traditional Chinese medicine. Chin. Arch. Trad. Chin. Med. 38 (08), 71-74 + 265–266. doi:10.13193/j.issn.1673-7717.2020.08.017
Natividad, J. M., Lamas, B., Pham, H. P., Michel, M. L., Rainteau, D., Bridonneau, C., et al. (2018). Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 9 (1), 2802. doi:10.1038/s41467-018-05249-7
Ni, X., Zhang, L., Peng, M., Shen, T. W., Yu, X. S., Shan, L. Y., et al. (2018). Hydrogen sulfide attenuates hypertensive inflammation via regulating connexin expression in spontaneously hypertensive rats. Med. Sci. Monit. 24, 1205–1218. doi:10.12659/msm.908761
Otaru, N., Ye, K., Mujezinovic, D., Berchtold, L., Constancias, F., Cornejo, F. A., et al. (2021). GABA production by human intestinal Bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 12, 656895. doi:10.3389/fmicb.2021.656895
Qi, L., Wu, S., Liu, N., Zhang, X., Ping, L., and Xia, L. (2024). Salvia miltiorrhiza bunge extract improves the Th17/Treg imbalance and modulates gut microbiota of hypertensive rats induced by high-salt diet. J. Funct. Foods 117, 106211. doi:10.1016/j.jff.2024.106211
Qi, Y., Jiang, Y., Jiang, L., Shao, L., Liu, Z., Wang, Y., et al. (2019). Effects of Eucommia ulmoides-Tribulus terrestris on intestinal microbiota in aged spontaneously hypertensive rats. Chin. J. Hypertens. 27 (05), 454–462. doi:10.16439/j.cnki.1673-7245.2019.05.017
Rahman, M. M., Islam, F., Or-Rashid, M. H., Mamun, A. A., Rahaman, M. S., Islam, M. M., et al. (2022). The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell. Infect. Microbiol. 12, 903570. doi:10.3389/fcimb.2022.903570
Robles-Vera, I., Toral, M., de la Visitación, N., Sánchez, M., Gómez-Guzmán, M., Romero, M., et al. (2020). Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Mol. Nutr. Food Res. 64 (6), e1900616. doi:10.1002/mnfr.201900616
Shan, Y., Pang, T., Yuan, Y., and Chen, H. (2024). Effect of Digupi(Lycii cortex) on intestinal flora in rats with spontaneous hypertension based on 16S rDNA sequencing. J. Pract. Trad. Chin. Intern. Med. 38 (04), 143–144. doi:10.13729/j.issn.1671-7813.Z20232383
Shen, J., Yi, L., Yang, R., Mao, Y., Jiang, H., and He, Y. (2020). Analysis of the effect of Angelica sinensis oil on the diversity of intestinal flora in hypertensive rats by 16S rDNA technique. Lishizhen Med. Mater. Med. Res. 31 (10), 2332–2335. doi:10.3969/j.issn.1008-0805.2020.10.008
Son, M., Oh, S., Lee, H. S., Choi, J., Lee, B. J., Park, J. H., et al. (2021). Gamma-aminobutyric acid-salt attenuated high cholesterol/high salt diet induced hypertension in mice. Korean J. Physiol. Pharmacol. 25 (1), 27–38. doi:10.4196/kjpp.2021.25.1.27
Su, J., Wang, Y., Yan, M., He, Z., Zhou, Y., Xu, J., et al. (2022). The beneficial effects of Polygonatum sibiricum Red. superfine powder on metabolic hypertensive rats via gut-derived LPS/TLR4 pathway inhibition. Phytomedicine 106, 154404. doi:10.1016/j.phymed.2022.154404
Tang, W. H., Kitai, T., and Hazen, S. L. (2017). Gut microbiota in cardiovascular health and disease. Circ. Res. 120 (7), 1183–1196. doi:10.1161/CIRCRESAHA.117.309715
Tominaga, T., Suzuki, H., Ogata, Y., Imafuku, T., and Saruta, T. (1988). Bile acids are able to reduce blood pressure by attenuating the vascular reactivity in spontaneously hypertensive rats. Life Sci. 42 (19), 1861–1868. doi:10.1016/0024-3205(88)90025-2
Verhaar, B. J., Prodan, A., Nieuwdorp, M., and Muller, M. (2020). Gut microbiota in hypertension and atherosclerosis: a review. Nutrients 12 (10), 2982. doi:10.3390/nu12102982
Wang, S., Xie, L., Zhuang, J., Qian, Y., Zhang, G., Quan, X., et al. (2023). Association between use of antihypertensive drugs and the risk of cancer: a population-based cohort study in Shanghai. BMC Cancer 23 (1), 425. doi:10.1186/s12885-023-10849-8
Wang, Z., Shao, Y., Wu, F., Luo, D., He, G., Liang, J., et al. (2024). Berberine ameliorates vascular dysfunction by downregulating TMAO-endoplasmic reticulum stress pathway via gut microbiota in hypertension. Microbiol. Res. 287, 127824. doi:10.1016/j.micres.2024.127824
Ward, N. C., Carnagarin, R., Nolde, J. M., Lugo-Gavidia, L. M., Chan, J., Jose, A., et al. (2022). Circulating short-chain fatty acids in hypertension: a reflection of various hypertensive phenotypes. J. Hypertens. 40 (8), 1589–1596. doi:10.1097/HJH.0000000000003190
World Health Organization (2019). “Hypertension - China,” in Guideline for the pharmacological treatment of hypertension in adults. World Health Organization, 48. Available at: https://www.who.int/china/health-topics/hypertension (Accessed September 26, 2024).
World Health Organization (2021). Guideline for the pharmacological treatment of hypertension in adults. World Health Organization. 48.
World Health Organization (2023). Hypertension. Available at: https://www.who.int/news-room/fact-sheets/detail/hypertension (Accessed September 26, 2024).
Wu, D., Ding, L., Tang, X., Wang, W., Chen, Y., and Zhang, T. (2019). Baicalin protects against hypertension-associated intestinal barrier impairment in part through enhanced microbial production of short-chain fatty acids. Front. Pharmacol. 10, 1271. doi:10.3389/fphar.2019.01271
Wu, J., Nakashima, S., Nakamura, S., and Matsuda, H. (2020). Effects of Sanoshashinto on left ventricular hypertrophy and gut microbiota in spontaneously hypertensive rats. J. Nat. Med. 74 (2), 482–486. doi:10.1007/s11418-020-01387-9
Wu, P., and Zhou, X. (2023). Research progress on hypertension status and influencing factors in China. Adv. Clin. Med. 13 (12), 18604–18609. doi:10.12677/acm.2023.13122615
Wu, S., Jiang, N., Shi, Y., Zhao, X., Guo, Z., Liu, J., et al. (2022). Effects of Xiaochaihu decoction combined with lrbesartan on intestinal flora and lipid metabolism in patients with hypertension. Chin. Arch. Trad. Chin. Med. 40 (01), 169–172. doi:10.13193/j.issn.1673-7717.2022.01.040
Wu, S., Yu, G., Hu, X., and Chi, W. (2024). The beneficial effect and related mechanism of Jiawei Banxia Baizhu Tianma Decoction on metabolic hypertension (MH) rats. J. Pathog. Biol 19 (02), 149–156. doi:10.13350/j.cjpb.240205
Xu, X., Yu, X., Jin, H., Yan, C., and Zhang, Q. (2022). Influence of Zhengan Xifeng decoction on blood pressure and cecal and colonic flora in spontaneously hypertensive rats. Pharmacol. Clin. Chin. Mater. Med. 38 (04), 9–14. doi:10.13412/j.cnki.zyyl.2022.04.015
Yan, X., Jin, J., Su, X., Yin, X., Gao, J., Wang, X., et al. (2020). Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ. Res. 126 (7), 839–853. doi:10.1161/CIRCRESAHA.119.316394
Yang, T., Santisteban, M. M., Rodriguez, V., Li, E., Ahmari, N., Carvajal, J. M., et al. (2015). Gut dysbiosis is linked to hypertension. Hypertension 65 (6), 1331–1340. doi:10.1161/HYPERTENSIONAHA.115.05315
Yang, Z., Lin, S., Liu, Y., Song, Z., Ge, Z., Fan, Y., et al. (2023a). Targeting intestinal microecology: potential intervention strategies of traditional Chinese medicine for managing hypertension. Front. Pharmacol. 14, 1171119. doi:10.3389/fphar.2023.1171119
Yang, Z., Wang, Q., Liu, Y., Wang, L., Ge, Z., Li, Z., et al. (2023b). Gut microbiota and hypertension: association, mechanisms and treatment. Clin. Exp. Hypertens. 45 (1), 2195135. doi:10.1080/10641963.2023.2195135
Yin, X., Duan, C., Zhang, L., Zhu, Y., Qiu, Y., Shi, K., et al. (2024). Microbiota-derived acetate attenuates neuroinflammation in rostral ventrolateral medulla of spontaneously hypertensive rats. J. Neuroinflammation 21 (1), 101. doi:10.1186/s12974-024-03061-3
Yu, J., Zhu, Q., Zhou, M., Huang, X., Le, Y., Ouyang, H., et al. (2024). Mechanism of Tianma-Gouteng granules lowering blood pressure based on the bile acid-regulated farnesoid X receptor-fibroblast growth factor 15- cholesterol 7α-hydroxylase pathway. J. Ethnopharmacol. 328, 118091. doi:10.1016/j.jep.2024.118091
Yu, X., Zhang, X., Jin, H., Wu, Z., Yan, C., Liu, Z., et al. (2019). Zhengganxifeng decoction affects gut microbiota and reduces blood pressure via renin-angiotensin system. Biol. Pharm. Bull. 42 (9), 1482–1490. doi:10.1248/bpb.b19-00057
Zhang, G., Chen, Y., Wang, M., and Zheng, G. (2023). Influence of rhynchophylline on blood pressure and gut microbiota of spontaneously hypertensive rats based on 16S rDNA sequencing. Chin. J. Clin. Pharmacol. 39 (01), 57–60. doi:10.13699/j.cnki.1001-6821.2023.01.012
Zhang, G. X., Jin, L., Jin, H., and Zheng, G. S. (2021). Influence of dietary components and traditional Chinese medicine on hypertension: a potential role for gut microbiota. Evid. Based Complement. Altern. Med. 2021, 5563073. doi:10.1155/2021/5563073
Zhang, J., and Li, G. (2023). Interpretation of the 2021 World Health Organization’s guidelines for drug treatment of adult hypertension. Chin. J. Hypertens. 31 (01), 18–20. doi:10.16439/j.issn.1673-7245.2023.01.007
Zhen, X. (2020). Study on the effect of Qinggan Yishen Qufeng formula on the changes of brain-gut axis related to hypertension induced by angiotensin II. China: Shanghai University of Traditional Chinese Medicine.
Zhou, X., Liu, J., and Li, J. (2020). Effects and mechanism of quercetin on blood pressure,intestinal flora and ventricular remodeling in spontaneously hypertensive rats. Nat. Prod. Res. Dev. 32 (09), 1449–1455. doi:10.16333/j.1001-6880.2020.9.001
Zhu, H., Xu, C., Dong, Y., Lu, S., and Guo, L. (2023a). Chai-Gui decoction and its representative components ameliorate spontaneous hypertension rats by modulating lipid metabolism and gut microbiota. J. Ethnopharmacol. 305, 116116. doi:10.1016/j.jep.2022.116116
Zhu, H., Xu, C., Gong, S., Mao, A., Zhou, C., and Lu, S. (2023b). Intervention of Chaigui decoction on angiotensin-converting enzyme 2 and intestinal flora in hypertensive mice. World Chin. Med. 18 (11), 1547–1550 + 1557. doi:10.3969/j.issn.1673-7202.2023.11.010
Zubcevic, J., Richards, E. M., Yang, T., Kim, S., Sumners, C., Pepine, C. J., et al. (2019). Impaired autonomic nervous system-microbiome circuit in hypertension. Circ. Res. 125 (1), 104–116. doi:10.1161/CIRCRESAHA.119.313965
Keywords: hypertension, intestinal flora, traditional Chinese medicine (TCM), intestinal flora metabolites, intestinal barrier function
Citation: Chen W, Xiao L, Guo W, Li H, Chen R, Duan Z, Chen Q and Lei Q (2024) Research progress of traditional Chinese medicine regulating intestinal flora in the treatment of hypertension. Front. Pharmacol. 15:1449972. doi: 10.3389/fphar.2024.1449972
Received: 20 June 2024; Accepted: 22 November 2024;
Published: 09 December 2024.
Edited by:
Wenzhi Hao, Jinan University, ChinaReviewed by:
Luoyang Wang, Qingdao University, ChinaClaire Elizabeth Robertson, University of Westminster, United Kingdom
Copyright © 2024 Chen, Xiao, Guo, Li, Chen, Duan, Chen and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Lei, MTI0MjgxMjYxQHFxLmNvbQ==; Qinghua Chen, MTM3MjgwNDYxQHFxLmNvbQ==; Zhongyu Duan, OTgxMDM5Mzc2QHFxLmNvbQ==; Rong Chen, MTg3MjUwOTIwMzRAMTYzLmNvbQ==
†These authors share first authorship
 Wenjun Chen
Wenjun Chen Longfei Xiao1†
Longfei Xiao1† Rong Chen
Rong Chen