- 1School of Pharmacy, Henan University of Chinese Medicine, Zhengzhou, China
- 2Collaborative Innovation Center of Research and Development on the Whole Industry Chain of Yu-Yao, Henan Province, Zhengzhou, China
- 3Henan Engineering Technology Research Center for Integrated Traditional Chinese Medicine Production, Zhengzhou, China
- 4Henan Engineering Research Center of Traditional Chinese Medicine Characteristic Processing Technology, Zhengzhou, China
Introduction: Non-small cell lung cancer (NSCLC) is a prominent lung cancer disease worldwide. Currently, commonly used methods, such as surgery and radiotherapy, have significant side effects. Traditional Chinese medicine (TCM) has become a research hotspot because of its safe and effective characteristics. The branches and leaves of Taxus media are abundant in antitumor active compounds, and there has been no research conducted as yet regarding its anti–lung cancer molecular mechanism.
Objective: The aim of this study is to investigate the antitumor activity of two samples before and after fermentation of T. media, and to research the molecular mechanism of its inhibitory effect on NSCLC.
Methods: The chemical composition of pre-fermentation T. media (TM) and post-fermentation T. media qu (TMQ) were investigated using UHPLC-Q-Qrbitrap HRMS and high-performance liquid chromatography (HPLC). The anti-lung cancer activities of TM and TMQ were compared using an A549-induced tumor mouse model. An enzyme-linked immunosorbent assay (ELISA), hematoxylin and eosin (H&E) staining, immunohistochemistry, and immunofluorescence were used to determine the of TMQ mechanism of action.
Results: The results indicated that TM and TMQ contained 83 compounds, consisting primarily of flavonoids, organic acids, and taxanes. Both taxanes and flavonoids in TMQ were higher than that in TM. Both TM and TMQ effectively inhibited the tumor growth in non-small cell lung cancer (NSCLC), and the inhibition rate was greater in TMQ (57.24%) than in TM (49.62%). TMQ administration downregulated the tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and the glutathione (GSH) level and upregulated interferon-γ (IFN-γ), reactive oxygen species (ROS), and malondialdehyde (MDA) levels in the serum of tumor mice. TMQ treatment also increased the protein expression of Bax, Caspase-3, and Beclin-1 in tumor tissues. In contrast, the bcl-2, PI3K, Ki67, ULK1, and mTOR protein levels were suppressed by TMQ. Protein assay analyses reemphasized the superior antitumor effect of TMQ over TM. These cumulative findings demonstrated that the mechanism of action of TMQ was closely related to the activation of transcriptional misregulation in the cancer pathway that inhibited the cholinergic synaptic, AMPK, and PI3K/Akt/mTOR signaling pathways.
Conclusion: This study demonstrated that fermentation increased the active ingredient contents and antitumor effects of T. media. In addition, post-fermentation TMQ was superior to TM as a herbal medicine for NSCLC treatment.
1 Introduction
Lung cancer is the most lethal cancer in China, and the types include non-small cell lung cancer (NSCLC) and small cell lung cancer. NSCLC accounts for approximately 80% of lung cancer cases (Lei et al., 2024). The primary treatment options for lung cancer include surgery, radiotherapy, and chemotherapy (Zheng et al., 2023). However, all these treatment options have certain adverse reactions or side effects (Housman et al., 2014; Bukowski et al., 2020), while traditional Chinese medicine (TCM) has become a research focus for treating lung cancer diseases because of its safe and effective advantages (Niu et al., 2024; Arjsri et al., 2023). Therefore, Taxus monodorous medicine, alone or in combination with other TCMs, is often used for the clinical treatment of lung cancer (Ma et al., 2020).
Taxus media (Taxus media) is slightly sweet, bitter, and belongs to the lung, stomach, and large intestine. It possesses efficacy in detoxifying and dispersing toxins, invigorating collaterals, relieving pain, inducing diuresis, and subduing swellings (Lei and Zhang, 2023, p. 172–179). Taxus media is also known as yew and was first mentioned by Li SZ in his book “De Materia Medica” as a treatment for colds and cholera (Wang et al., 2012). Taxus media are abundant in terpenes, phenols, lignans, and polysaccharides (Li et al., 2017; Zhou et al., 2019). The plant’s multiple active ingredients, exhibit various medicinal properties, such as an antitumor, antipyretic, analgesic, immune-regulating, and anti-inflammatory properties (Wang and Huang, 2018). Taxanes and flavonoids are the principal active phytoconstituents in Taxus media, and these can treat a variety of tumors, such as lung cancer, liver cancer, and ovarian cancer (Zhu and Chen, 2019; Sharma and Garg, 2015). Therefore, some Taxus plants and the Fuzheng Quxie decoction are used in hospitals as clinical applications for the treatment of lung cancer (Wang et al., 2007).
Lung cancer, belongs to the category of “lung stagnation” in Chinese medicine, and is primarily characterized by deficiency, toxicity, and stasis, i.e., internal deficiency in the body, invasion of external evils leading to an abnormal flow of fluids in the body, and blockage of qi and poor blood circulation, that in turn leads to stagnation of qi and blood stasis by blocking the lungs (Tang et al., 2016). The anti-cancer and anti-inflammatory effects of Taxus plants depend on their efficacy of detoxification, dissipation of stagnation, and in the induction of diuresis and swelling (Lou et al., 2015; Sharma and Garg, 2015; Dai et al., 2022). Studies have shown that the methanol extract, ethanol extract, and aqueous extract of Taxus plants can inhibit the proliferation of tumor cells after treatment. Another clinical study showed that the ratio of CD3+ and CD4+ cells and the CD4+/CD8+ ratio of non-small cell lung cancer patients increased after administering a Taxus decoction, suggesting that Taxus may boost the immunity of patients, thus prolonging their survival time (Ma et al., 2020). However, the precise mechanism through which it combats non-small cell lung cancer remains to be fully investigated.
Quji is a TCM with fermentation characteristics, that apparently originated more than 3,000 years ago. Quji is a solid preparation made by mixing a powder of the medicine and flour, maintaining an appropriate temperature and humidity, and allowing it to ferment naturally, or a preparation made by mixing fermented medicinal materials with other medicinal materials (Gong, 2016, p. 416) After fermentation, the chemical composition of traditional Chinese medicines will change. Fermentation and processing can increase the concentrations of active components in Chinese herbal medicines. In addition, macromolecular substances that are difficult to be absorbed by the human body will be converted into small molecules by microorganisms during the fermentation process. Moreover, the fermentation process may produce new compounds that may enhance the efficacy of the drug.
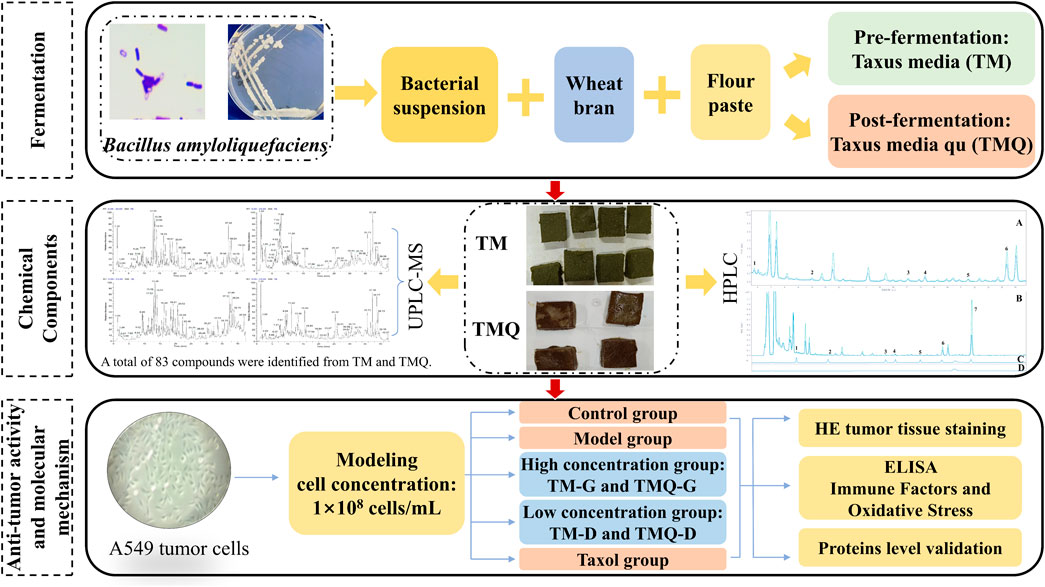
In this study, the fermentation method was used for the T. media. In addition, the pre-fermentation T. media (TM) and post-fermentation T. media qu (TMQ) were prepared. The antitumor effects of TM and TMQ were then compared using an animal model of the A549 tumor mice, and the mechanism of action of TM and TMQ against NSCLC was elucidated. The results showed that TMQ activated modulating immune factors, and oxidative stress, activated the cancer transcriptional deregulation pathways, inhibited the cholinergic synapses, the AMPK and PI3K/Akt/mTOR signaling pathways, thus exhibiting antitumor activity. These findings demonstrated that fermentation enhanced the efficacy of TM and TMQ in the treatment of NSCLC and provided a pharmacological rationale for the application of TMQ in NSCLC therapy. The technical flow chart design of our study is shown in Figure 1.
2 Methods and materials
2.1 Plant material and chemical reagents
Six to ten year-old T. media branches and leaves were provided from the Zhiyuan planting base (Zhiyuan Biotechnology Co., Ltd., Pingdingshan, China). The fresh plant samples were dried in an oven at 60°C and powdered for later use. The flour was purchased from the Jinsha River Flour Industry (Jinsha River Flour Industry Co., Ltd., Hebei, China). The wheat bran was purchased from the New Garden Agricultural Products Industry (New Garden Agricultural Products Industry Co., Ltd., Nanyang, China).
2.2 Preparation of TM and TMQ
A thin batter of T. media was boiled and placed at room temperature. A fine powder of T. media and wheat bran was mixed with the above, and a certain amount of bacterial suspension was added to soften the materials so it could easily be kneaded into balls by hand and dispersed upon tossing. Squares of a uniform size and thickness were then made, and some of them were dried in a drying oven at 60°C as the pre-fermentation samples (0 h sample or the TM sample). The rest were placed under a constant temperature and humidity (HMS-250, Tianjin Leiboterry Equipment Co., Ltd., Tianjin, China) fermentation box at a temperature of 32.7°C, humidity of 75%, and fermented for 60 h as the post-fermentation samples (TMQ samples). These were then dried and stored in a refrigerator at 4°C.
2.3 Chemical composition analysis of TM and TMQ
2.3.1 Sample extraction
Each powder sample was accurately weighed to 1 g, placed in a tapered bottle, and mixed with 50 mL of methanol (Thermo Fisher Scientific China Co., Ltd, Shanghai, China). All samples were then weighed together for the total weight. Each sample was then extracted at 30°C for 30 min in an ultrasonic bath (power: 500 W; frequency: 40 kHz). The samples were then cooled it to room temperature, the lost weight was compensated using methanol, they were then shaken well. The supernatant was filtered, and 50 mL of methanol was added for a repeated extraction. The supernatant was extracted and combined with the filtrate. The filtrate was evaporated to dryness in a double-row six-hole instrument water bath (Shanghai ShuLi Instrument Co., Ltd., Shanghai, China) at 60°C, dissolved in methanol at a fixed volume of 10 mL, filtered through a 0.22 μm microporous membrane, placed in 1 mL of a solution in a 4°C centrifuge concentrator, concentrated in a vacuum for 5 h to dry, redissolved in 1 mL of methanol, and centrifuged using a high-speed refrigerated centrifuge. The supernatant was separated and filtered through a 0.22 μm microporous membrane.
2.3.2 HPLC analysis
Qualitative and quantitative analyses were then performed on a Waters Symmetry C18 column (4.6 mm × 250 mm, 5 μm) using an Agilent 1260 high performance liquid chromatograph. The mobile phase were solution A (methanol) and solution B (0.1% phosphoric acid aqueous solution). The gradient elution procedure was as follows: 0–15 min, 65%–45% B; 15–22.5 min, 45%–30% B; 22.5–32.5 min, 30%–10% B; 32.5–35 min, and 10%–65% B. The flow rate, injection volume, and column temperature were 1 mL/min, 10 μL, and 30°C, respectively. The diode array detection wavelength was 232 nm. A qualitative analysis based on the peak area and retention time of the mixed standard compounds compared to sample peaks was performed. Appropriate amounts of 10-deacetylbaccatin III (10-DAB), baccatin III (DAB), cephalomannine, taxol, 7-epitaxol, ginkgetin and sciadopitysin were accurately weighed and added to methanol to prepare the concentrations of 0.6067 mg/mL, 0.2800 mg/mL, 0.2500 mg/mL, 0.1633 mg/mL, 0.0667 mg/mL, 0.3800 mg/mL, and 0.4400 mg/mL mixed control solutions. A six-point curve was constructed, and the regression equations and R2 values were calculated for each standard.
2.3.3 UHPLC-Q-qrbitrap HRMS analysis
The compounds in TM and TMQ were qualitatively identified using the Ultimate 3000-Orbitrap Exploris 240 LMS with Hypersil GOLD (100 × 2.1 mm, 1.9 μm) with a mobile phase flow rate of 0.2 mL/min. Aqueous formic acid (0.1%) was used as solvent A, and pure acetonitrile was used as solvent B. A gradient elution was applied under the following conditions: mobile phase A was kept at a slope of 95% for 0–0.5 min of the run, then decreased to 75% over 8 min, 50% over 24 min, 5% over 38 min, 5% over 40 min, 95% over 40.1 min, then kept at 95% until the end of the run. The volume of the sample analyzed was 4 μL. The scanning range for the positive and negative ion detection modes, and spray voltages were m/z 100–1200, and 3.5–3.0 kV, respectively. The sheath gas flow rate, and auxiliary gas flow rate were 25 arbitrary units (arb) and 10 arb, respectively, at 350°C for both the auxiliary and ion transfer tubes. Xcalibur 3.0 software, combined with the reference products and online databases such as PubChem, was used to analyze the mass spectrum data. The parent ions and fragment ions of the compounds were accurately compared, and the compounds in the samples before and after the fermentation of T. media were identified.
2.4 In vitro studies
2.4.1 Cell line and cell culture
We obtained A549 cell lines, which are human pancreatic cancer cells, from the Service Biotechnology Company (Service Biotechnology Co., Ltd., Wuhan, China). The A549 cells were cultured in an RPMI1640 medium (Solar Biotechnology Technology Co., Ltd., Beijing, China) with a sterile incubator at 37°C with 5% CO2. Cells were resuscitated and passaged two to three times for the next step of the analysis when the cells were viable.
2.4.2 Cell inhibition assay
The concentration of A549 cells was adjusted to 25,000 cells/mL and added to 96-well plates at 200 μL per well. They were then cultured in a 5% CO2 incubator at 37°C. The cells were cultured in a 5% CO2 incubator at 37°C and treated with medicine after the cells were attached to the wall. The preparation method of the drug was the same as that of the MS test solution. The methanol extract was concentrated to dry using a centrifugal concentrator, placed on an ultra-clean workbench for ultraviolet (UV) sterilization for 36 h, a complete medium was then added to dissolve, and the drug concentration was adjusted to 64 mg/mL.
The cell proliferation inhibition experiment dosing operation: The complete culture medium was used in a 5-mL Eppendorf (EP) tube for drug allocation. Each group was allocated 4 mL of the drug, and three compound pores were established. The corresponding final drug concentrations were 64, 32, 16, 8, 2, and 0.5 mg/mL. The 96-well plate medium was discarded, and 200 μL of the drug concentration medium of the corresponding group was added to each well. After the drug was administered for the specified time, the supernatant was discarded, 100 μL of a medium containing 10% CCK-8 was added, and incubation was continued for 1 h. The absorbance at 450 nm (optical density (OD) value) was measured using an enzyme marker, and the cell survival rate was calculated.
Cell inhibition rate (%) = 1 - Cell viability rate.
A (drug): OD values of wells with cells, CCK-8 solution and drug solution.
A (control): OD value with cells, and CCK-8 solution but no drug solution.
A (blank): OD value without cells.
2.5 In vivo studies
2.5.1 Animals and treatments
Male Balb/c-nu mice (18–20 g) were housed at the Animal Center with free access to food and water. The animals were acclimatized and fed for approximately 1 week prior to the experiments. All experiments were conducted in accordance with the guidelines of the Laboratory Animal Welfare Ethics Committee of the Department of Laboratory Animals, the Henan University of Chinese Medicine (Permit Number: DWLL202203316). A549 cells were cultured in large numbers, and cells were collected by centrifugation. Their concentration was adjusted to 1×108 cells/mL, and each mouse was inoculated subcutaneously in the right anterior axilla with 0.2 mL. In approximately 4–7 days, a mung bean-sized hard mass was seen under the skin of the mice, and this was considered successful modeling. Tumor-bearing mice were randomly divided into six groups (n = 6): Model (M), TM high dose (TM-G), TM low dose (TM-D), TMQ high dose (TMQ-G), TMQ low dose (TMQ-D), and Taxol (T). The doses for TM and TMQ administration were calculated according to pharmacological experimental methodology, i.e., a clinical dose of 6 g/d for a 60 kg adult, with the low dose being two times the human clinical dose and the high dose being eight times the human clinical dose. The administered dose of the positive drug was equal to the human clinical dose. The high-dose group (9.872 g/kg) and the low-dose group (2.468 g/kg) were gavaged once a day, and the taxol group (7.62 mg/kg) was intraperitoneally injected once every 3 days. The mice were sacrificed on day 45.
2.5.2 General status and tumor growth of the mice
The mice in each group were observed daily for diet, water intake, mental status, and activity. The body weights were measured every 4 days and the tumors were measured every 2 days. The tumor volume (mm3) of each mouse was calculated [V = a × b2 × 0.50, with a = the long side and b = the short side]. After 45 days of treatment, the mice were executed, and the tumor weights, tumor inhibition rates, and splenic indices were weighed and calculated. The tumor suppression rate = [tumor mass (M/g) – the tumor mass in treatment group (g)]/tumor mass (M/g) × 100%, spleen index = spleen mass (mg)/body mass (g).
2.5.3 Evaluation of the serum oxidative stress and inflammatory factors
The levels of reactive oxygen species (ROS), malondialdehyde (MDA), glutathione (GSH), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-6 (IL-6) in the mouse serum were analyzed using enzyme-linked immunosorbent assay (ELISA) kits provided by Jiangsu Meimian Industrial Co., Ltd.
2.5.4 Morphology of the tumor tissue section
The fresh tumor tissue was immersed with 4% paraformaldehyde, embedded, paraffin sectioned, and the cell morphology of the tumor tissue section was visualized using hematoxylin and eosin (H&E) staining.
2.5.5 Immunohistochemical (IHC) staining
Tumor sections were processed using the primary antibodies Bax (GB114122), bcl-2 (GB114830), Caspase-3 (GB11532), and PI3K (GB11769) obtained from Servicebio Ltd. and then incubated with goat anti-rabbit IgG secondary antibodies. The specimens were stained with 3,3′-diaminobenzidine (DAB) and visualized under a microscope. The positive immunoreactivity revealed by DAB was brownish yellow. Images were quantitatively analyzed using Image Pro Plus software (6.0 version).
2.5.6 Immunofluorescence (IF) staining
Six groups of tumor specimens were fixed, paraffin-embedded, and microtome-sectioned. These sections were incubated with primary antibodies targeting Ki67(GB121141, Servicebio, Wuhan, China), mTOR (GB111839, Servicebio, Wu-han, China), Beclin-1 (GB11228, Servicebio, Wuhan, China), and ULK1 (GB11580, Service-bio, Wuhan, China). The groups were then incubated with CY3-labeled goat anti-mouse IgG secondary antibody. 4′,6-diamidino-2-phenylindole (DAPI) was used to re-stain the nucleus, quench the tissue autofluorescence, seal the film, collect the image, and the positive immuno-reactivity was red. Images were quantitatively analyzed using Image Pro Plus software (6.0 version).
2.6 Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). The statistical significance of the differences between groups was analyzed using a one-way analysis of variance (ANOVA). When analyzing the differences between groups, the least significant difference (LSD) multiple comparison method was used for the homogeneity of data variances, and the Dunnett’s T3 method was used for the heterogeneity of data variances. A value of p < 0.05 was considered statistically significant.
3 Results
3.1 Compositional studies of TM and TMQ
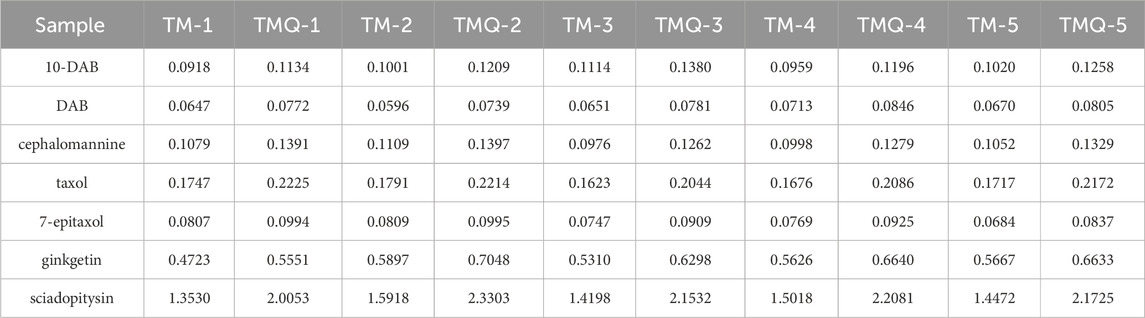
The regression equations for 10-DAB, DAB, cephalomannine, taxol, 7-epitaxol, ginkgetin, and sciadopitysin are shown in Supplementary Table S1. The contents of the selected taxanes and flavonoids in the samples are presented in Table 1. A quantitative analysis showed that the contents of 10-DAB, DAB, cephalomannine, taxol, 7-epitaxol, ginkgetin, and sci-adopitysin in TMQ were 23.25%, 20.42%, 27.74%, 25.58%, 22.10%, 18.15%, and 48.68% higher than in TM, respectively (Supplementary Figure S1). Chromatograms obtained using HPLC are presented in Supplementary Figure S2.
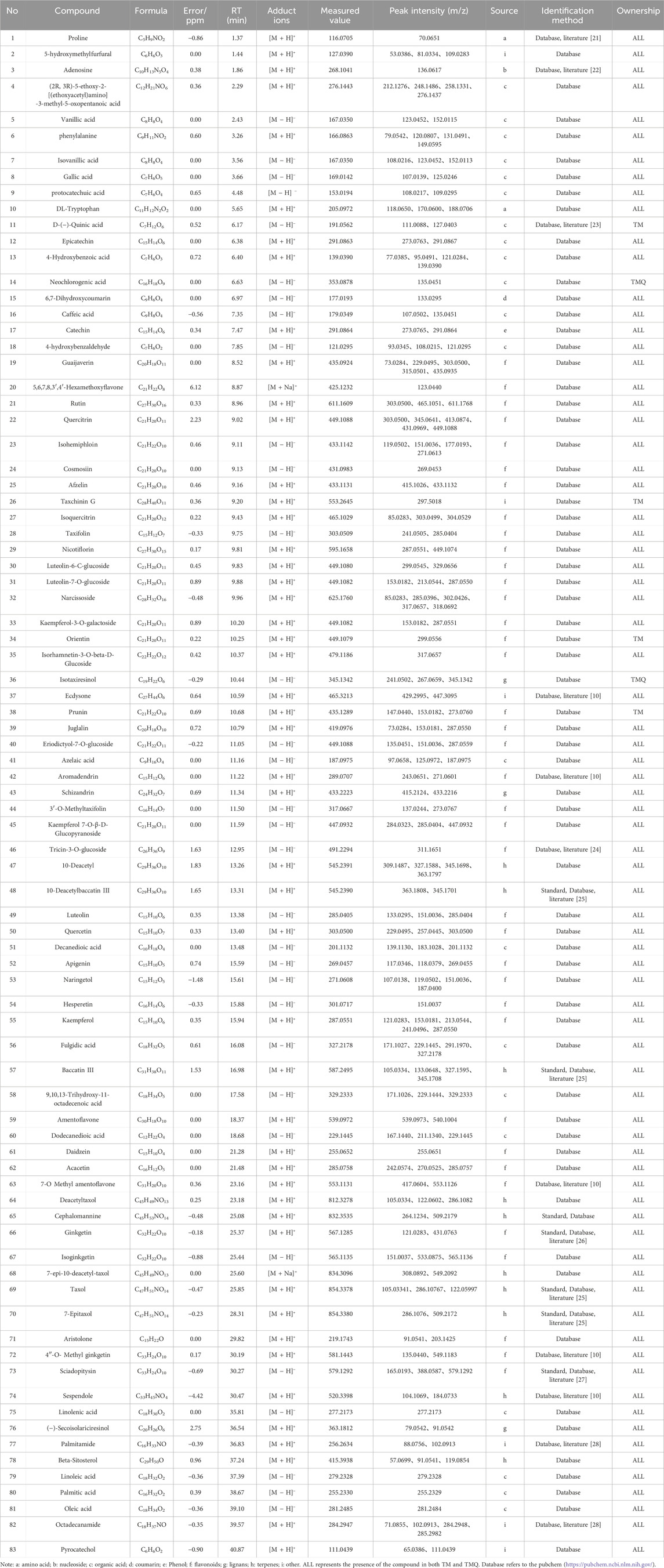
The positive and negative ion chromatograms of TM and TMQ are shown as Figure 2. The UHPLC-Q-Orbitrap HRMS identification results of the compounds in TM and TMQ are shown in Table 2. A total of 83 compounds, including two amino acids, one nucleoside, 21 organic acids, one phenol, one coumarin, 38 flavonoids, three lignans, 10 terpenes and six other types, were identified by reference materials, the literature, and the database of T. media. As shown in Figure 2 and Table 2, there were differences in the compound types and contents between TM and TMQ, with 81 compounds in TM and 79 compounds in TMQ. D-(−)-Quinic acid, taxchinin G, orientin, and prunin were detected only in TM, whereas isotaxiresinol, and neochlorogenic acid were detected only in TMQ.
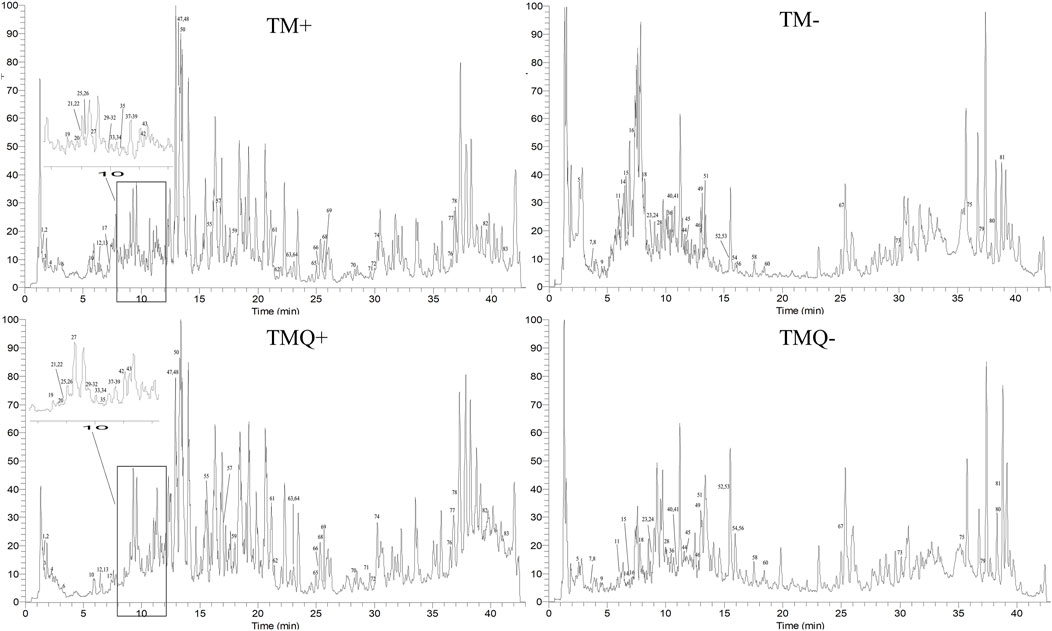
Figure 2. Total ion chromatograms (TICs) of TM and TMQ. (TM+) TIC of TM in positive ion mode; (TM-) TIC of TM in negative ion mode; (TMQ+) TIC of TMQ in positive ion mode; (TMQ-) TIC of TMQ in negative ion mode.
3.2 Anticancer activity
To evaluate the bioactivity of TM and TMQ, we performed a CCK8 assay using methanolic extracts. The results showed that TM had a cytotoxic effect on A549 cells, with a IC50 values of 37.87 mg/mL and 26.83 mg/mL after incubation for 12 h and 48 h, respectively. In contrast, the IC50 values of TMQ were even lower at 27.71 mg/mL and 19.43 mg/mL after 12 h and 48 h of incubation, respectively. The figures indicate that the cytotoxicity was higher than that of the TM. The antitumor abilities of TM and TMQ were evaluated based on the inhibitory rate of the drug on A549 lung cancer cells (Supplementary Figure S3). The inhibitory abilities of the TMQ samples in each drug group were better than those of TM (Figure 3). When the drug acted on A549 lung cancer cells for 12 h, there was no significant difference in the inhibitory ability between TM and TMQ of the drug group below 16 mg/mL. However, at 16 mg/mL and above, the inhibitory ability of the administration group was significantly higher than that of TM. When acting for 48 h, the inhibitory abilities of the six groups were significantly higher than those of TM.
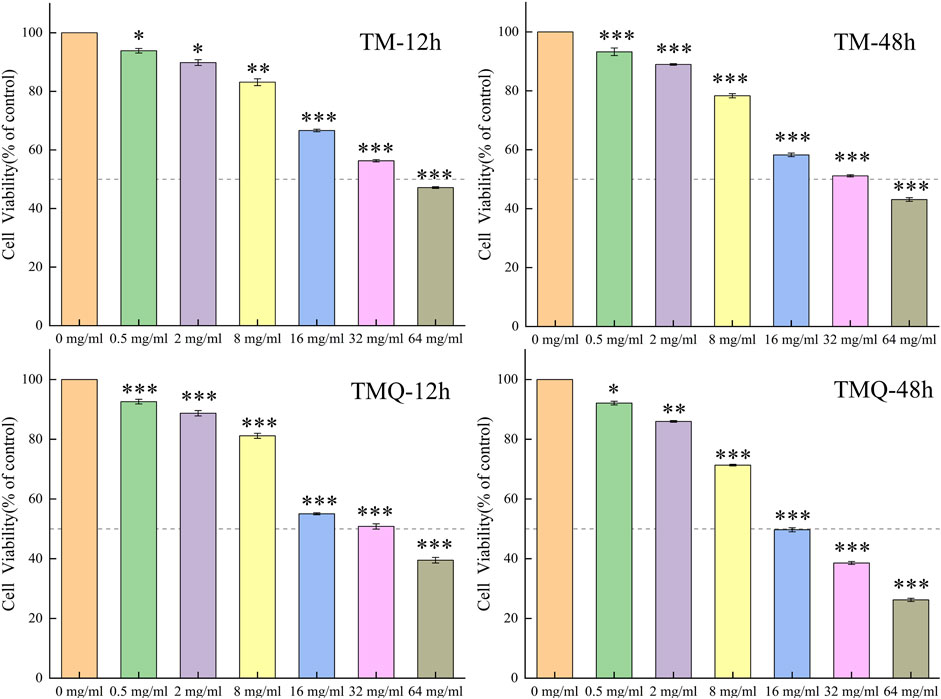
Figure 3. The effects of TM and TMQ on A549 cells viability were determined by CCK8 assay. The A549 cells were treated with 0–64 mg/mL of TM and 0–64 mg/mL of TMQ for 12, and 48 h. Data are presented as mean ± S.D. values of three experiments. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the control (0 mg/mL).
3.3 Influence of TM and TMQ on the body weights and tumors in the tumor mice
The results showed that the mice body weights in the model group were significantly higher than that in the administration groups, and the spleen indices were significantly lower than that in the administration groups. The tumor volumes and mass of the model group were the largest among the six groups of tumor-bearing mice, and all the administration groups effectively inhibited tumor growth (p < 0.05). The tumor volumes in the TMQ group were smaller than those in the TM group, while the tumor inhibition rate in the TMQ group was higher than that in the TM group (Figure 4). Moreover, after fermentation, the tumor inhibition rate of the TMQ-G group reached 57.24% (Table 3), which was very close to that of the positive drug group. The above results showed that the antitumor activity of TMQ was higher than that of TM, and the antitumor activity of the high-dose-group was stronger than that of the low-dose group.
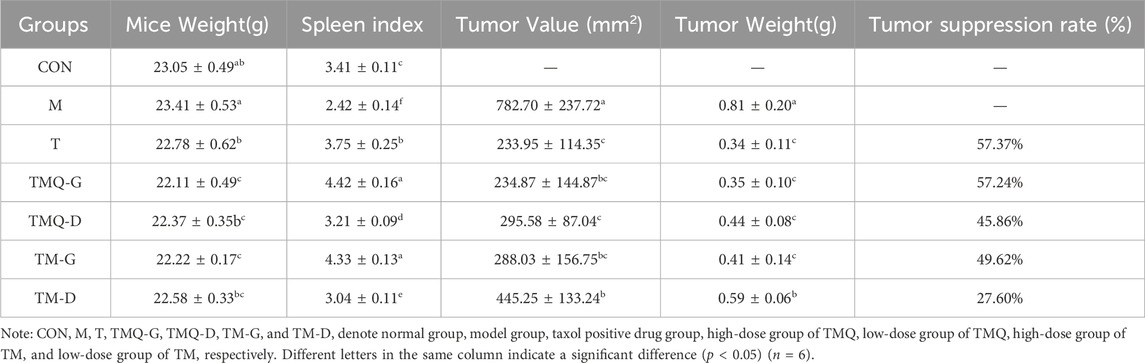
Table 3. The result of body mass, splenic index, tumor volume, tumor mass, and tumor inhibition rate of mice in each group after drug administration.
3.4 Effects of TM and TMQ on tumor tissue in mice
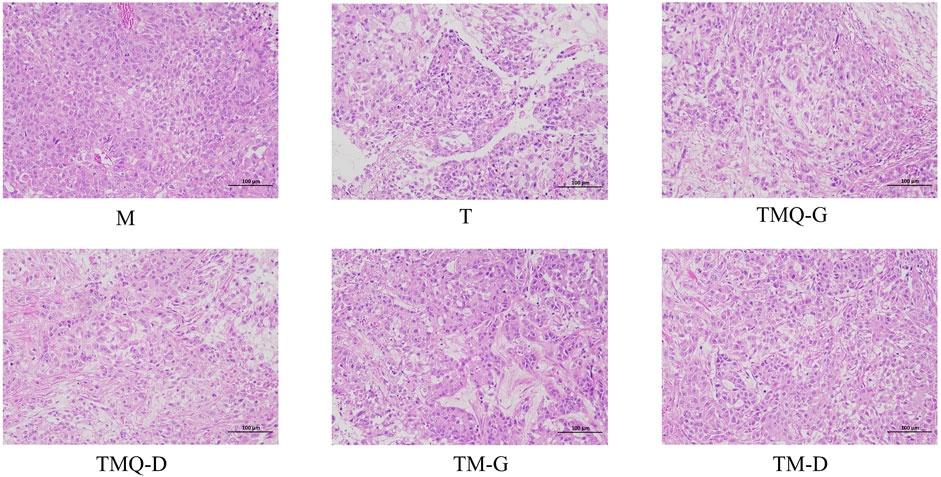
The results of the H&E staining showed that the tumor tissues of mice in the model group were characterized by abundant blood vessels, tight cell arrangements, irregular shapes, mostly round or short fusiform, sparse cytoplasm, unclear boundaries between cells, asymmetric chromosomes in the nucleus, and generally asymmetric division and multinucleation (Figure 5). In the same field of view, blood vessels and tumor cells were greatly reduced in the T group, with large cellular gaps in the tumor tissues and a large number of tumor cells with broken nuclei. The tumor tissue cell gap was larger and the number of tumor cells was reduced in the mice of each TCM group. Compared with TM, TMQ greatly increased the inhibition of tumor cell proliferation.
3.5 Effect of TM and TMQ on immune factors and oxidative stress in the mice serum
Inflammation is closely related to cancer development and progression, and inflammation is primarily associated with changes in the levels of IL-6, TNF-α, and IFN-γ (Wang et al., 2024; Abidi et al., 2024). Additionally, inflammation and oxidative stress are two interrelated processes, and it has been shown that increasing ROS and MDA and decreasing GSH can help inhibit tumor cell proliferation (Wang et al., 2024). The serum levels of the above indicators in mice are shown in Figure 6. Compared to the M group, the expression of IFN-γ, ROS, and MDA in each Chinese medicine group was significantly upregulated (p < 0.05), while the expression of TNF-α, IL-6, and GSH significantly decreased, indicating that the four Chinese medicine groups could inhibit the proliferation of tumor cells by reducing inflammatory factors, regulating immune function, and promoting oxidative stress. In addition, IFN-γ, ROS, and MDA in the TMQ-G group were significantly higher than in the TM-G group, and TNF-α and IL-6 in the TMQ-G group were significantly lower than in the TM-G group (p < 0.05). IFN-γ and MDA in the TMQ-D group were significantly higher than in the TM-D group, and TNF-α and IL-6 were significantly lower than the levels prior to fermentation (p < 0.05), which again indicated that the antitumor ability of the TMQ samples was stronger than that of the TM samples.
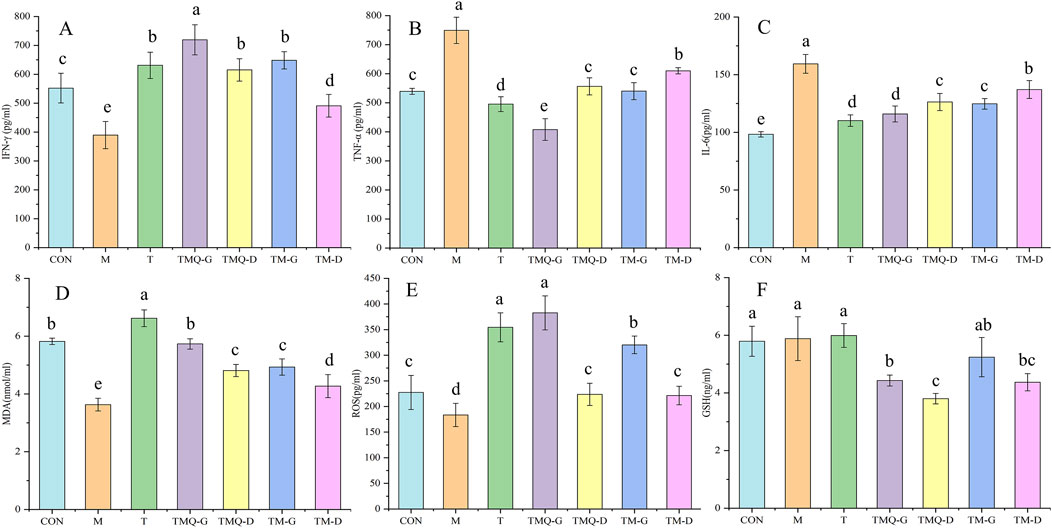
Figure 6. Results of immune factors and oxidative stress indexes. (A) IFN-γ, (B) TNF-α, (C) IL-6, (D) MDA, (E) ROS and (F) GSH were determined by ELISA. Different letters in the same column indicate a significant difference (p < 0.05) (n = 6).
3.6 Effects of TM and TMQ on the transcriptional misregulation in cancer pathway and tholinergic synapse pathway in A549-induced mice tumors
As shown in Figure 7, it was inferred that Bax and the caspase-3 protein expression levels were significantly higher (p < 0.05) and bcl-2 and the PI3K protein were significantly lower (p < 0.05) in the group treated with TM and TMQ than in the model group. Furthermore, the tumors of mice under same dose of mice treated with TMQ had considerably high (p < 0.05) expression levels of Bax compared with the TM group. Additionally, the expression levels of bcl-2 and the PI3K protein were significantly lower (p < 0.05) than those in the TM group.
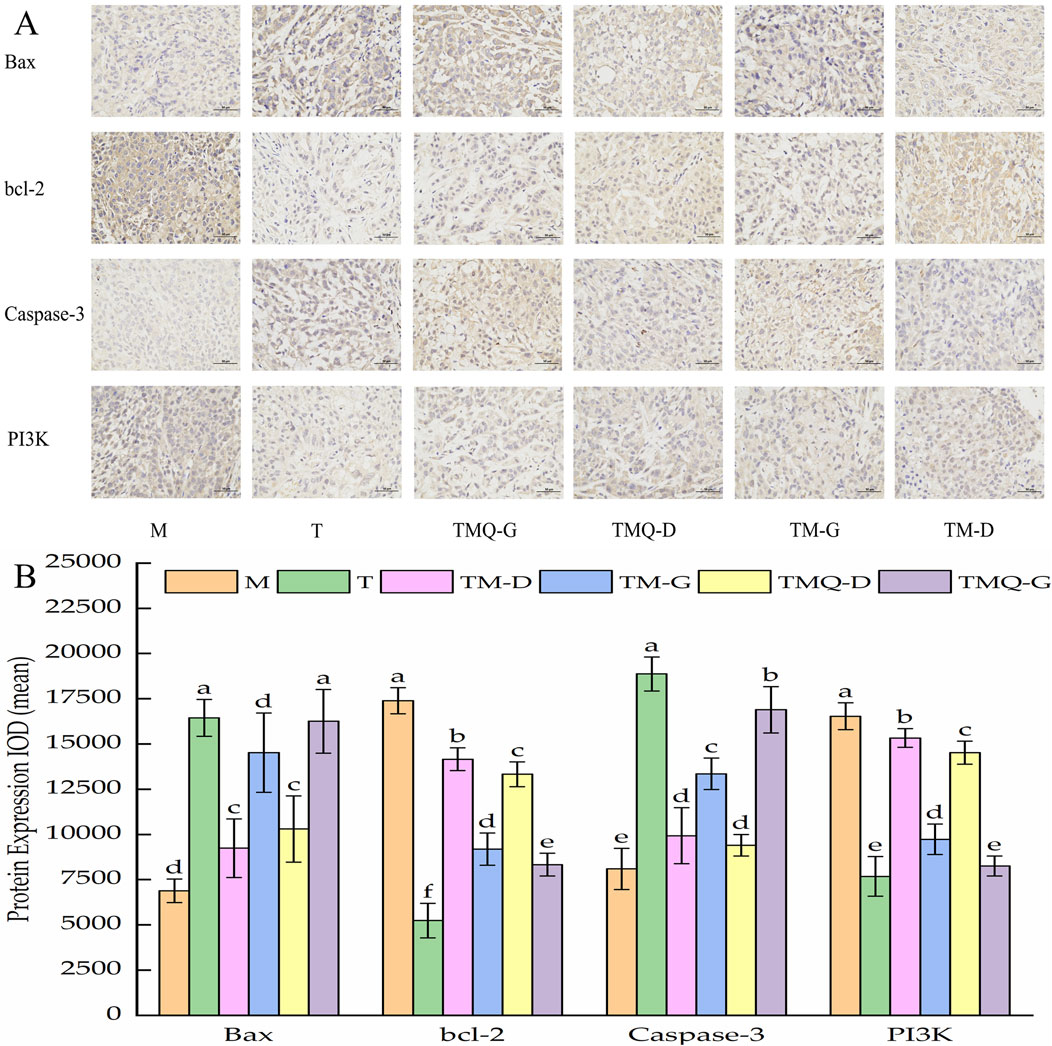
Figure 7. Immunohistochemical staining sections of Bax, bcl-2, Caspase-3 and PI3K in mouse tumor tissues (A) and their expression results (B).
3.7 Effects of TM and TMQ on the AMPK signaling pathway and PI3K/Akt/mTOR signaling pathway in A549-induced mice tumors
The results in Figure 8 show that the administration of TCM significantly reduced the expression levels of mTOR and the ULK1 proteins in the mice tumors (p < 0.05), but the levels were higher than those in the T group. In addition, TCM significantly increased the expression level of the Beclin-1 protein in mice tumors (p < 0.05), but the levels were lower than those in the T group. This was in contrast to the levels in the model groups.
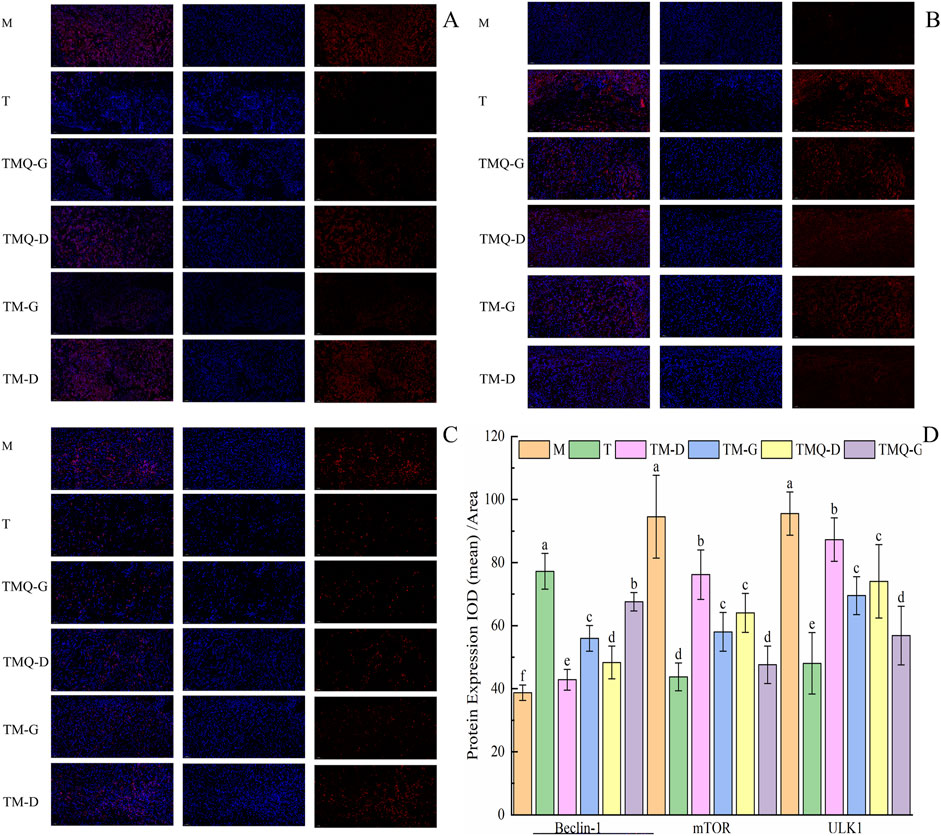
Figure 8. Expression of mTOR (A), Beclin-1 (B), ULK1 (C) and quantitative analysis (D) in mouse tumor tissues. a–f The different letters denote statistically significant differences (p < 0.05) in the data’s mean values between the ex-perimental groups (n = 3).
3.8 Effects of TM and TMQ inhibition of cell proliferation in mice tumors
Figure 9 shows that the expression levels of the Ki67 protein in the mice tumors were significantly reduced in each administration group compared with the model group (p < 0.05). The T group showed the lowest Ki67 protein expression, followed by the TMQ-G group. Additionally, post-fermentation TMQ significantly reduced the expression level of the Ki67 protein in the mice tumors (p < 0.05).
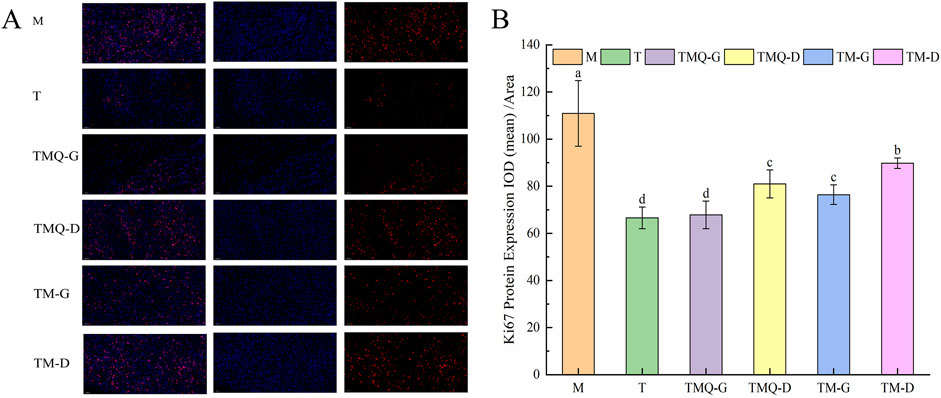
Figure 9. Expression of Ki67 (A) and quantitative analysis (B) in mouse tumor tissues. a–d The different letters denote statistically significant differences (p < 0.05) in the data’s mean values between the experimental groups (n = 3).
4 Discussion
Lung cancer is one of the three major cancers and poses the greatest threat to life (de-Medeiros et al., 2024). Although many studies have been conducted on the treatment of lung cancer in recent decades, these methods have not significantly improved the survival rate of lung cancer patients (Lin et al., 2016). Many chemotherapy methods are expensive and unaffordable for patients and have serious adverse reactions and side effects that affect the quality of life of patients (Housman et al., 2014; Bukowski et al., 2020), Chinese herbal medicine is safe and effective, with little side effects, and has a moderate price; hence, it has gradually become a significant research subject for the treatment of lung cancer. Studies have shown that after solid fermentation, traditional Chinese medicine can not only inhibit the proliferation of tumor cells, but also kill tumor cells by improving immunity, and the inhibition effect after fermentation is stronger than that prior to fermentation (Chen et al., 2020; Liu et al., 2023; Zhao, 2021).
The objective of our study was to investigate the effects of fermentation on the chemical composition and antitumor activity of T. media and the molecular mechanism of its treatment of NSCLC. The enhancement of antitumor activity after fermentation cannot be separated from specific active components of TCM. Therefore, HPLC and UPLC-MS experiments were first conducted to study the effects of fermentation on the chemical components of T. media. The HPLC results showed that the contents of 10-DAB, DAB, cephalomannine, taxol, 7-epitaxol, ginkgetin, and sciadopitysin in TMQ were 23.25%, 20.42%, 27.74%, 25.58%, 22.10%, 18.15%, and 48.68% higher than in TM, respectively. A total of 83 compounds were identified by UHPLC-Q-Qrbitrap HRMS, most of which were important terpenoids, flavonoids and organic acids. The composition study showed that the types of compounds in T. media were basically the same before and after fermentation, and the difference was primarily in content. The contents of antitumor components in TMQ were higher than that in TM; therefore, we speculate that its antitumor effect was also better than TM. We also used A549 lung cancer cells for preliminary verification of our speculation. The results of the CCK8 experiment confirmed that the inhibition rate of high concentration TMQ was 73.78%, which was significantly higher than that of the TM sample. We confirmed that this was consistent with the results obtained by Shu Q and colleagues (Shu et al., 2014), whose inhibition rate was the highest at 42.23%. In this study, the inhibition rate of T. media on A549 lung cancer cells was 60.49%, which may have been due to the longer effect of the experimental drug on cells.
Subsequently, in vivo experiments were conducted using mouse models to explore the antitumor activities of TMQ and TM and their molecular mechanisms in treating non-small cell lung cancer. The mice were divided into a control group, a model group, a positive drug group and TMQ and TM groups with different doses. We measured the body mass and tumor related parameters in the mice. The results of this study showed that, with the administration of TM and TMQ, the body weights, tumor volumes, and mass were lower, and the splenic indices were higher than in M group, indicating that TM and TMQ promoted the development of the mice spleens, improved the immune ability of the mice, and inhibited the proliferation of tumor cells. The inhibition rate of the TMQ-G group (57.24%) was close to that of the T group (57.37%). The diet and water consumption of the mice in the TMQ-G group were higher than that of the T group, and the vitality of the mice was more vigorous, suggesting that the TMQ drug better improved the quality of life of the mice. We then used an ELISA kit to detect the inflammatory factors and markers of oxidative stress in the serum.
Studies have confirmed that chronic inflammation, such as in pneumonia, hepatitis, and colitis, may promote the development of cancers such as lung, liver, and colon cancer (Remila et al., 2015; Metwally et al., 2024; Tan et al., 2024). IFN-γ is a cellular interferon that can regulate immune function and inhibit tumor cell proliferation, TNF-α is related to inflammatory response and immune response (Lei et al., 2024), and IL-6 is the most central inflammatory factor that links inflammation and tumors (Liu et al., 2024). The results showed that the IFN-γ, TNF-α, and IL-6 levels significantly increased in all administration groups (p < 0.05) vis-à-vis the M group. The effects of the TMQ-G group and T group on the levels of inflammatory factors were basically the same. Studies have shown that reducing TNF-α and IL-6 levels and increasing IFN-γ can help inhibit inflammation and tumor cell proliferation (Situmorang et al., 2024). In addition, oxidative stress plays an important role in cancer occurrence and development. Excessive ROS can cause oxidative stress damage and induce apoptosis in tumor cells. GSH can scavenge free oxygen, and GSH depletion can induce tumor cell apoptosis (Abidi et al., 2024; Özbeyli et al., 2017). MDA is a product of lipid peroxidation, and it can cause lipid peroxidation in cell membranes and apoptosis (Shokrian-Zeini et al., 2024; Xie et al., 2022). Compared with the M group, the TMQ-G group exhibited significantly increased, ROS and MDA levels, while the GSH level in the TMQ-G group significantly decreased (p < 0.05). Increases in the ROS and MDA levels and decreases in the GSH level help to aggravate the oxidative damage to cell membranes, and this leads to impaired functions of tumor cells and inhibits tumor growth. Compared with the same dose prior to fermentation, the IFN-γ, ROS, and MDA levels significantly increased, and the TNF-α and IL-6 levels significantly decreased after fermentation (p < 0.05). In conclusion, TMQ and TM inhibited tumor proliferation by regulating the related indices of inflammatory factors and oxidative stress, and TMQ had a significantly stronger inhibitory ability on tumor proliferation than TM.
To investigate the mechanism of TMQ and TM in inhibiting non-small cell lung cancer, we performed a tumor biopsy analysis. The apoptosis of tumor cells is closely related to Bax and bcl-2, and Bax and bcl-2 are proapoptotic factors and antiapoptotic factors. Caspase-3 is also an apoptotic factor (Wu et al., 2014). These three factors jointly regulate the transcriptional dysregulation of tumor pathways. The expression of Bax and caspase-3 significantly increased in the four Chinese medicine groups compared with the M group, while the expression of bcl-2 significantly decreased (p < 0.05). In addition, the expression of Bax and the caspase-3 protein in the TMQ-G group was significantly higher than that in the TM-G group, and the expression of bcl-2 was significantly lower than that in the TM-G group (p < 0.05). These data suggested that TMQ may induce tumor cell apoptosis by activating the tumor transcriptional dysregulation pathway. PI3K and bcl-2 proteins are located downstream of the cholinergic synaptic pathway and are closely related to cell proliferation and differentiation. PI3K and ULK1 proteins are located downstream of the AMPK signaling pathway. Compared with the M group, the expression levels of bcl-2 and the PI3K and ULK1 proteins in the TMQ group and TM group significantly decreased (p < 0.05), indicating that the Chinese medicine inhibited the cholinergic synaptic pathway and AMPK signaling pathway by downregulating the PI3K, Bcl-2, and ULK1 proteins and thus inhibiting the proliferation of tumor cells. According to our results, TMQ showed more potent antitumor activity than TM. The occurrence and development of tumors are closely related to tumor cell proliferation, apoptosis, and autophagy (Li et al., 2021; Li et al., 2024). The PI3K/AKT/mTOR pathway is one of the most common dysregulated pathways in cancer (Janku et al., 2018), and the PI3K/AKT/mTOR signaling pathway is closely related to tumor cell proliferation and angiogenesis. Therefore, we examined the levels of beclin-1 and mTOR proteins. The results showed that TMQ increased the expression of the beclin-1 protein and activated autophagy. It also downregulates PI3K and mTOR proteins (Rossetti et al., 2024), inhibits the PI3K/Akt/mTOR signaling pathway, and enhances autophagy. In addition, Ki67 is closely related to cell mitosis, and higher expression indicates more active cell proliferation (Groen-van-Schooten et al., 2024). The expression of the Ki67 protein in the TMQ group was significantly lower than that in the TM and M groups (p < 0.05). In summary, these data indicated that TMQ enhanced tumor autophagy and effectively inhibited tumor growth in mice by activating the cancer transcriptional dysregulation pathway, and inhibiting the cholinergic synaptic pathway, the AMPK signaling pathway and the PI3K/Akt/mTOR signaling pathway, thus playing a role in the treatment of non-small cell lung cancer. The therapeutic effect of TMQ on non-small cell lung cancer was significantly stronger than that of TM. The limitations of our study require further exploration regarding the mechanism by which TMQ simultaneously activates the tumor transcriptional dysregulation pathway, and inhibits the cholinergic synaptic pathway, the AMPK signaling pathway, and the PI3K/Akt/mTOR signaling pathway, and the clinical relevance of these mechanisms. In addition, safety studies on the clinical use of TMQ are insufficient and further in vitro and in vivo studies are necessary.
5 Conclusion
This study confirmed that TM and TMQ had positive effects on A549 cell-induced lung cancer. TMQ showed superior therapeutic effects to TM in non-small cell lung cancer, including an increased splenic index, IFN-γ, ROS, and MDA levels, and decreased TNF-α and IL-6 levels. In addition, the therapeutic mechanisms of TM and TMQ were related to the activation of transcriptional misregulation in the cancer pathway, inhibition of the cholinergic synapse pathway, the AMPK signaling pathway and the PI3K/Akt/mTOR signaling pathway, and inhibition of tumor cell proliferation. This study demonstrated that fermentation enhanced the efficacy of the TCMs in the treatment of NSCLC, elucidated a portion of the molecular mechanism of TM and TMQ in the treatment of NSCLC, and provided pharmacological data for the application of TMQ in the treatment of NSCLC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by Ethics Committee of Experimental Animals in Hennan Academy of Chinese Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XG: Writing–review and editing, Writing–original draft, Software, Methodology, Investigation, Formal Analysis. R-SW: Writing–review and editing, Validation, Supervision, Software, Resources, Methodology. Z-LZ: Writing–review and editing, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization. H-WZ: Writing–review and editing, Validation, Methodology. S-CW: Writing–review and editing, Validation, Project administration. SZ: Writing–review and editing, Validation. Y-NW: Writing–review and editing, Data curation. Y-JL: Writing–review and editing, Data curation. JY: Writing–review and editing, Data curation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the 2023 Zhengzhou City Collaborative Innovation Special Project (2023XTCX041), and the 2022 Post-graduate Research Innovation Category Project of Henan University of Traditional Chinese Medicine (Grant No. 2022KYCX032).
Acknowledgments
We would like to thank Shu-Ding Sun for technical support from Public Service Platform for Scientific Re-search of Academy of Chinese Medicine Sciences, Henan University of Chinese Medicine. We also thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1449498/full#supplementary-material
Abbreviations
NSCLC, Non-small cell lung cancer; TCM, traditional Chinese medicine; TM, pre-fermentation Taxus media; TMQ, post-fermentation Taxus media qu; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; GSH, Glutathione; IFN-γ, interferon-γ; ROS, reactive oxygen species; MDA, malondialdehyde; T. media, Taxus media; 10-DAB, 10-deacetylbaccatin III; DAB, baccatin III; M, Model group; TM-G, TM high dose group; TM-D, TM low dose group; TMQ-G, TMQ high dose group; TMQ-D, TMQ low dose group; T, Taxol group.
References
Abidi, O., Abdelkafi-Koubaa, Z., Bettaieb-Dridi, I., Toumi, L., Marzouki, L., and Souilem, O. (2024). Pistacia lentiscus L. revealed in vitro anti-proliferative activity on MCF-7 breast cancer cells and in vivo anti-mammary cancer effect on C57BL/6 mice through necrosis, anti-inflammatory and antioxidant enhancements. PLoS One 19, e0301524. doi:10.1371/journal.pone.0301524
Arjsri, P., Srisawad, K., Semmarath, W., Umsumarng, S., Rueankham, L., Saiai, A., et al. (2023). Suppression of inflammation-induced lung cancer cells proliferation and metastasis by exiguaflavanone A and exiguaflavanone B from Sophora exigua root extract through NLRP3 inflammasome pathway inhibition. Front. Pharmacol. 14, 1243727. doi:10.3389/fphar.2023.1243727
Bukowski, K., Kciuk, M., and Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21, 3233. doi:10.3390/ijms21093233
Carazzone, C., Mascherpa, D., Gazzani, G., and Papetti, A. (2013). Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 138, 1062–1071. doi:10.1016/j.foodchem.2012.11.060
Chen, H. Y., Liu, H., Lou, T. T., He, R., He, D. T., Liu, R., et al. (2020). Study on antitumor activity of total saponins from Dioscorea Aspergillus oryzae solid fermentation. Jilin J. Chin. Med. 40, 526–529+545. doi:10.13463/j.cnki.jlzyy.2020.04.030
Dai, S. Y., Zhang, G. C. X., Liu, Y., and Shu, Q. J. (2022). Study on the antitumor effect of IFN-γ in vitro promoting by aqueous extract of Taxus Chinese var.mairei via the modulation and down-regulating PD-L1 expression. China J. Traditional Chin. Med. Pharm. 37, 5114–5119.
de-Medeiros, J. P., Rodrigues, S. A., Sakumoto, K., Ruiz, S. P., Faria, M. G. I., Gonçalves, J. E., et al. (2024). Bioactives of the essential oil from the leaves of Eugenia pyriformis Cambess (Myrtaceae) on the effects of tobacco. Front. Pharmacol. 15, 1415659. doi:10.3389/fphar.2024.1415659
Gong, Q. (2016). Traditional Chinese medicine processing: new century. 4th ed., Beijing, China: China Press of Traditional Chinese Medicine, 416.
Groen-van-Schooten, T. S., Harrasser, M., Seidel, J., Bos, E. N., Fleitas, T., van-Mourik, M., et al. (2024). Phenotypic immune characterization of gastric and esophageal adenocarcinomas reveals profound immune suppression in esophageal tumor locations. Front. Immunol. 15, 1372272. doi:10.3389/fimmu.2024.1372272
He, X. G. (2000). On-line identification of phytochemical constituents in botanical extracts by combined high-performance liquid chromatographic-diode array detection-mass spectrometric techniques. J. Chromatogr. A 880, 203–232. doi:10.1016/s0021-9673(00)00059-5
Housman, G., Byler, S., Heerboth, S., Lapinska, K., Longacre, M., Snyder, N., et al. (2014). Drug resistance in cancer: an overview. Cancers (Basel) 6, 1769–1792. doi:10.3390/cancers6031769
Janku, F., Yap, T. A., and Meric-Bernstam, F. (2018). Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291. doi:10.1038/nrclinonc.2018.28
Lei, M., Liu, J., Gao, Y., Dai, W., Huang, H., Jiang, Q., et al. (2024). DPP inhibition enhances the efficacy of PD-1 blockade by remodeling the tumor microenvironment in lewis lung carcinoma model. Biomolecules 14, 391. doi:10.3390/biom14040391
Lei, S. Y., and Zhang, J. L. (2023). Henan province Chinese Materia Medica standard. Zheng Zhou, China: Henan Science and Technology Press, 172–179.
Li, G., Zhong, Y., Wang, W., Jia, X., Zhu, H., Jiang, W., et al. (2021). Sempervirine mediates autophagy and apoptosis via the akt/mTOR signaling pathways in glioma cells. Front. Pharmacol. 12, 770667. doi:10.3389/fphar.2021.770667
Li, N., Pan, Z., Zhang, D., Wang, H. X., Yu, B., Zhao, S. P., et al. (2017). Chemical components, biological activities, and toxicological evaluation of the fruit (aril) of two precious plant species from genus Taxus. Chem. Biodivers. 14. doi:10.1002/cbdv.201700305
Li, N., Yu, Y., Chen, Q., Niu, J., Gao, C., Qu, X., et al. (2024). A gene delivery system with autophagy blockade for enhanced anti-angiogenic therapy against Fusobacterium nucleatum-associated colorectal cancer. Acta Biomater. 3 (24), 278–291. doi:10.1016/j.actbio.2024.05.051
Li, Z., Dong, F., Sun, Y., Sun, Z., Song, X., Dong, Y., et al. (2022). Qualitative and quantitative analysis of six fatty acid amides in 11 edible vegetable oils using liquid chromatography-mass spectrometry. Front. Nutr. 9, 857858. doi:10.3389/fnut.2022.857858
Lin, J. J., Cardarella, S., Lydon, C. A., Dahlberg, S. E., Jackman, D. M., Jänne, P. A., et al. (2016). Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J. Thorac. Oncol. 11, 556–565. doi:10.1016/j.jtho.2015.12.103
Liu, H., Yan, S., Yang, R., Huang, C., Guo, K., Wang, S., et al. (2024). Jiedu xiaozheng yin inhibits the progression of colitis associated colorectal cancer by stimulating macrophage polarization towards an M1 phenotype via the TLR4 pathway. Integr. Cancer Ther. 23, 15347354241247061. doi:10.1177/15347354241247061
Liu, J., Wei, B. H., Bi, W. H., Yang, W. Z., Yang, X., Li, X., et al. (2023). Chemical components and antitumor activity of inonotus-eckloniae thallus bidirectional fermentation product. Food Res. Dev. 44, 61–67. doi:10.12161/j.issn.1005-6521.2023.19.009
Lou, P. T., Xia, A. P., and Ying, Y. (2015). Experimental study on the analgesic and anti-inflammatory effects of stem and leaf extracts of the Taxus Chinese var.mairei via. Zhejiang J. Traditional Chin. Med. 50, 566–567. doi:10.13633/j.cnki.zjtcm.2015.08.011
Ma, X. J., Zhang, H., and Wang, C. D. (2020). Clinical study on the effect of treating non-small cell lung cancer by adding Taxus chinensis in anti-cancer body-resistance-strengthening de-coction. Strait Pharm. J. 32, 85–87. doi:10.3969/j.issn.1006-3765.2020.10.027
Metwally, E. M., Lund, J. L., Drummond, M. B., Peacock-Hinton, S., Poole, C., and Thompson, C. A. (2024). COPD with lung cancer among older United States adults: prevalence, diagnostic timeliness, and association with earlier stage tumors. Chronic Obstr. Pulm. Dis. 5, 382–385. doi:10.15326/jcopdf.2024.0489
Niu, J., Jia, X., Yang, N., Ran, Y., Wu, X., Ding, F., et al. (2024). Phytochemical analysis and anticancer effect of Camellia oleifera bud ethanol extract in non-small cell lung cancer A549 cells. Front. Pharmacol. 15, 1359632. doi:10.3389/fphar.2024.1359632
Özbeyli, D., Berberoglu, A. C., Özen, A., Erkan, O., Başar, Y., Şen, T., et al. (2017). Protective effect of alpha-lipoic acid, aerobic or resistance exercise from colitis in second hand smoke exposed young rats. Clin. Exp. Pharmacol. Physiol. 44, 62–70. doi:10.1111/1440-1681.12682
Qin, W. H., Yang, Y., Li, Q., Wang, Y. H., Guo, Y. L., Hua, L., et al. (2019). UPLC-Q-TOF-MS based analysis of chemical constituents of Cordyceps sinensis from Nepal. Chin. J. New Drugs 28, 1574–1581. doi:10.3969/j.issn.1003-3734.2019.13.006
Remila, S., Atmanti-Kilani, D., Delemasure, S., Connat, J. L., Azib, L., Richard, T., et al. (2015). Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. 7, 274–286. doi:10.1016/j.eujim.2015.03.009
Rossetti, S., Broege, A., Sen, A., Khan, S., MacNeil, I., Molden, J., et al. (2024). Gedatolisib shows superior potency and efficacy versus single-node PI3K/AKT/mTOR inhibitors in breast cancer models. NPJ Breast Cancer 10, 40. doi:10.1038/s41523-024-00648-0
Sharma, H., and Garg, M. (2015). Neuropharmacological activities of Taxus wallichiana bark in Swiss albino mice. Indian J. Pharmacol. 47, 299–303. doi:10.4103/0253-7613.157128
Sharma, H., and Garg, M. (2015). A review of traditional use, phytoconstituents and biological activities of Himalayan yew, Taxus wallichiana. Integr. Med. 13, 80–90. doi:10.1016/S2095-4964(15)60161-3
Shokrian-Zeini, M., Pakravesh, S. M., Jalili-Kolour, S. M., Soghala, S., Dabbagh-Ohadi, M. A., Ghanbar-Ali-Akhavan, H., et al. (2024). Astaxanthin as an anticancer agent against breast cancer: an in vivo and in vitro investigation. Curr. Med. Chem. 17. doi:10.2174/0109298673288774240406053607
Shu, Q. J., Shen, M. H., Wang, B. B., Cui, Q. L., Zhou, X. Y., and Zhu, L. M. (2014). Aqueous extract of Taxus chinensis (Pilger) Rehd inhibits lung carcinoma A549 cells through the epidermal growth factor receptor/mitogen-activated protein kinase pathway in vitro and in vivo. J. Tradit. Chin. Med. 34, 293–301. doi:10.1016/s0254-6272(14)60093-5
Situmorang, P. C., Ilyas, S., Nugraha, S. E., Syahputra, R. A., and Nik-Abd-Rahman, N. M. A. (2024). Prospects of compounds of herbal plants as anticancer agents: a comprehensive review from molecular pathways. Front. Pharmacol. 15, 1387866. doi:10.3389/fphar.2024.1387866
Tan, F., Zhou, X., Ren, L., and Kong, C. S. (2024). Effect of lactiplantibacillus plantatum HFY11 on colitis in mice. Foods 13, 1496. doi:10.3390/foods13101496
Tang, D. X., Yang, Z., and Liu, S. Y. (2016). LIU Shangyi’s academic viewpoints of treating tumors as Ulcer and treating tumors from membrane in diagnosis and treatment of cancer. J. Traditional Chin. Med. 57, 1732–1734. doi:10.13288/j.11-2166/r.2016.20.006.169-174
Tiwary, E., Berryhill, T. F., Wilson, L., Barnes, S., Prasain, J. K., and Wells, J. M. (2023). LC-MS/MS method for proline-glycine-proline and acetylated proline-glycine-proline in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1228, 123815. doi:10.1016/j.jchromb.2023.123815
Wang, N., Ding, C., Xie, Y., Meng, J., Fan, X., Fan, D., et al. (2024). Characteristics of citrate-esterified starch and enzymatically debranched starch and their effects on diabetic mice. Foods 13, 1486. doi:10.3390/foods13101486
Wang, N. N., and Huang, F. H. (2018). Research progress on effective components and pharmacological effects of Taxus chinensis. Zhejiang J. Traditional Chin. Med. 53, 621–623. doi:10.13633/j.cnki.zjtcm.2018.08.050
Wang, S. W., Ma, X., Ping, W. X., and Zhou, D. P. (2007). Research advances on taxol producion by microbe fermentation. Microbiology 34, 561–565. doi:10.13344/j.microbiol.china.2007.03.041
Wang, Y. F., Wang, Q., Ruan, X., and Zhang, Y. Y. (2012). Research status and utilization strategies of rare medicinal plants in Taxus. Sci. Silvae Sin. 48, 116–125. doi:10.11707/j.1001-7488.20120518
Wu, W., Wang, X., Xiang, Q., Meng, X., Peng, Y., Du, N., et al. (2014). Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 5, 158–166. doi:10.1039/c3fo60400d
Xie, J., Liu, L., Li, H., Che, H., and Xie, W. (2022). Ink melanin from Sepiapharaonis ameliorates colitis in mice via reducing oxidative stress, andprotecting the intestinal mucosal barrier. Food. Res. Int. 151, 110888. doi:10.1016/j.foodres.2021.110888
Yin, C. Y., Zhang, C., Sun, M. Q., Lin, L., and Liu, J. X. (2020). Mass spectrometric fragmentation pathways of three ginkgo biloba flavonoids using HPLC-Q-TOF MS. J. Chin. Mass Spectrom. Soc. 41, 57–65. doi:10.7538/zpxb.2019.0011
Zhang, C. Y., Li, J., Gao, J. M., Zhang, Q. M., and Shi, Y. Q. (2016). The impurity profiling of paclitaxel and its injection by UPLC-MS/MS. Acta Pharm. Sin. 51, 965–971. doi:10.16438/j.0513-4870.2015-0750
Zhao, X. L. (2021). Study on anti-tumor activity and mechanism of solid fermentation products of leucocalocybe mongolica on H22 tumor-bearing mice. Jilin Province: Jilin Agricultural University. doi:10.27163/d.cnki.gjlnu.2021.000060
Zheng, X., Lu, X., and Hu, Y. (2023). Distinct respiratory microbiota associates with lung cancer clinicopathological characteristics. Front. Oncol. 13, 847182. doi:10.3389/fonc.2023.847182
Zhou, T., Luo, X., Zhang, C., Xu, X., Yu, C., Jiang, Z., et al. (2019). Comparative metabolomic analysis reveals the variations in taxoids and flavonoids among three Taxus species. BMC Plant Biol. 19, 529. doi:10.1186/s12870-019-2146-7
Zhu, L., and Chen, L. (2019). Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 24, 40. doi:10.1186/s11658-019-0164-y
Keywords: non-small cell lung cancer1, Taxus media2, fermentation3, antitumor activity4, herbal medicine5
Citation: Guo X, Wang R-S, Zhang Z-L, Zhang H-W, Wang S-C, Zhang S, Wu Y-N, Li Y-J and Yuan J (2024) Effect of fermentation on the constituents in the branches and leaves of Taxus media and non-small cell lung cancer. Front. Pharmacol. 15:1449498. doi: 10.3389/fphar.2024.1449498
Received: 15 June 2024; Accepted: 01 October 2024;
Published: 23 October 2024.
Edited by:
Haishu Lin, Shenzhen Technology University, ChinaReviewed by:
SOROMOU Lanan Wassy, Other, GuineaYue Ding, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2024 Guo, Wang, Zhang, Zhang, Wang, Zhang, Wu, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Ling Zhang, emx6aGFuZ0BoYWN0Y20uZWR1LmNu
 Xing Guo
Xing Guo Rui-Sheng Wang1
Rui-Sheng Wang1