
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 21 October 2024
Sec. Inflammation Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1445708
 Nageh A. El-Mahdy1†
Nageh A. El-Mahdy1† Mariam G. Tadros2†
Mariam G. Tadros2† Thanaa A. El-Masry1
Thanaa A. El-Masry1 Ammena Y. Binsaleh3
Ammena Y. Binsaleh3 Nawal Alsubaie3*
Nawal Alsubaie3* Amani Alrossies3
Amani Alrossies3 Medhat I. Abd Elhamid4
Medhat I. Abd Elhamid4 Enas Y. Osman1
Enas Y. Osman1 Hadeel M. Shalaby5
Hadeel M. Shalaby5 Dalia S. Saif6
Dalia S. Saif6Background: Inflammation and angiogenesis are two main mechanisms that act as mutual pathways in rheumatoid arthritis (RA). This work aimed to study the efficacy of digoxin as an adjunct therapy to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) in active RA patients.
Methods: In a randomized, double-blinded, placebo-controlled study, 60 adult patients with active RA received a placebo or digoxin (0.25 mg every other day) combined with csDMARDs for 6 months. The American College of Rheumatology (ACR) 20, ACR50, and ACR70 response rates and the disease activity score (DAS28) were assessed for patients. Flow cytometric analysis of Th17 cells and serum concentrations of IL-17A, IL-23, HIF-1α, and VEGF were evaluated before and after three and 6 months of therapy.
Results: Following three and 6 months of digoxin therapy combined with csDMARDs, significant differences were detected in laboratory and clinical parameters relative to the control group. After 6 months, 83.3% of patients in the digoxin group, compared to 56.7% in the control group, achieved an ACR20 response (p = 0.024). The digoxin group had a significantly higher percentage of patients who achieved DAS28 remission after 6 months (p = 0.024). Notable improvements in the Health Assessment Questionnaire Disability Index, ACR50, and ACR70 were detected in the digoxin group.
Conclusion: Digoxin was well tolerated and exerted profound immunomodulatory and anti-inflammatory effects in RA patients, and may also exhibit anti-angiogenic properties, indicating that it might be an effective adjunct to csDMARDs in treating RA.
Clinical Trial Registration: clinicaltrials.gov, identifier NCT04834557.
Rheumatoid arthritis (RA) is a chronic, debilitating, progressive, autoimmune disease affecting synovial joints, inducing synovial inflammation, leukocyte infiltration, excessive release of various inflammatory mediators and autoantibodies, pannus formation, bone erosion, and cartilage destruction, with resultant joint deformity (Guo and Chen, 2020; Schinocca et al., 2021; Scott et al., 2010).
Many lines of evidence have shown that inflammation and angiogenesis are the two main mechanisms of RA, which act as mutual pathways rather than individual processes (Elshabrawy et al., 2015; Guo et al., 2018).
T helper 17 (Th17) cells have potential and direct involvement in RA’s pathogenesis. Th17 cells selectively secrete interleukin-17A (IL-17A), which triggers cytokine mediators of the inflammatory response, contributing to a persistent inflammatory response and subsequent tissue destruction (Alunno et al., 2015).
Th17 cell differentiation and activation are induced by the inflammatory cytokine mediator IL-23, with subsequent IL-17A production, which is preferentially implicated in RA’s pathogenesis. Therefore, the association between IL-23/IL-17A signaling and inflammatory arthritis development represents a crucial immunological pathway and a promising target for therapeutic intervention in various arthritic conditions (Schinocca et al., 2021).
Vascular endothelial growth factor (VEGF) stands out as the first identified vasculogenic factor, which induces new blood vessel formation through migration and proliferation of endothelial cells (Elshabrawy et al., 2015; Zisman et al., 2021). VEGF expression is amplified by hypoxia-inducible factor-1α (HIF-1α), a transcription factor that controls cell responses to low oxygen levels (George et al., 2020; Gong et al., 2019; Saponaro et al., 2013). Therefore, HIF-1α and VEGF are potential therapeutic targets for angiogenesis in RA.
Peripheral blood biomarkers, including neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI), are promising biomarkers for immune activation and inflammation that are strongly associated with the disease activity of several rheumatic diseases (Hao et al., 2017; Kumarasamy et al., 2019; Li et al., 2021; Mirestean et al., 2023; Sejópoles et al., 2023; Su et al., 2021; Targonska-Stepniak and Grzechnik, 2023; Urbanowicz et al., 2021; Zhou et al., 2023).
The effectiveness of conventional synthetic (cs) DMARDs (disease-modifying anti-rheumatic drugs) has acquired considerable attention as they can attenuate disease activity and delay joint deformation. However, even now, many patients either do not respond or respond only partially to these compounds, and complete disease remission in the long term is not achieved for many patients, which requires the development of new therapeutic options (Grennan et al., 2001; Guo et al., 2018).
Digoxin is a cardiovascular glycoside used for the management of heart failure and other cardiac complications (Jordaens et al., 1997). Its usage in the cardiac area has accounted for the majority of our knowledge; however, recent studies showed that digoxin has beneficial clinical therapeutic utilization in non-cardiac conditions, including viral infection, cancer, steatohepatitis, and autoimmune diseases (Jamshed et al., 2023). Intriguingly, reports propose that digoxin reduced the severity of arthritis and autoimmune encephalomyelitis in the experimental model and suppressed Th17 differentiation by inhibiting the retinoic acid-related orphan receptor γt (RORγt) transcriptional activity (Peters et al., 2011; Seki and Nishizawa, 2016). In this regard, digoxin-mediated inhibition of RORγt may be valuable in treating RA (Saeed et al., 2020).
Based on the aforementioned observations, we proposed to investigate the immunomodulatory, anti-inflammatory, and anti-angiogenic properties of digoxin when co-administered with csDMARDs in patients with RA who exhibit moderate to high disease activity with no other problems and its association with therapeutic response and improvement of disease activity to provide worthy insights for RA treatment.
This study was a prospective, randomized, double-blinded, placebo-controlled clinical trial performed on Egyptian RA patients. The Institutional Review Board of the Faculty of Medicine, Menoufia University, approved the study protocol under the IRB# PMRR2A. The study adhered to the ethical basics and procedures established in the 1964 Declaration of Helsinki and any later changes. Eligible participants were comprehensively informed about the study protocol before participation and were required to provide written confirmation of their informed consent that followed the guidelines declared by the local ethics committee. The clinical study was conducted at the Physical Medicine, Rheumatology, and Rehabilitation Department from November 2021 to September 2022 and was recorded at “ClinicalTrials.gov” with a unique identifier number of NCT04834557. The present article focused on analyzing and examining data, and reporting results from only two of the three intervention groups enrolled in this clinical trial.
Seventy patients with a clinically established diagnosis of RA fulfilling the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria were recruited (Aletaha et al., 2010) (Figure 1).
RA disease activity status was identified by measuring the disease activity score-28 based on the levels of C-reactive protein (CRP) (DAS28-CRP) > 3.2. Patients who exhibited moderate to high disease activity and were treated with csDMARDs were eligible to participate in the study.
Exclusion criteria included; hypersensitivity to digoxin, other autoimmune diseases, patients on biologic DMARDs or refusing to give informed consent, cases of impaired liver functions and kidney functions, oral prednisolone >10 mg/day, pregnancy and lactation, as well as the existence of other comorbidities, including malignancies, any cardiovascular, psychiatric or neurological diseases, diabetes mellitus, active infections, and other inflammatory diseases.
Demographic data were reported for all RA patients, including age, gender, smoking, height, weight, body mass index (BMI), disease duration, and medications received (Table 1).
Each patient underwent a comprehensive clinical and thorough assessment by a rheumatologist and was given a blood sample at baseline, three and 6 months after treatment. Medical history was reported to assert that there was no interfering or interacting diseases or drugs. Comprehensive clinical evaluation of disease activity was done at the beginning of the study and after three and 6 months of treatment, including tender and swollen 28-joint counts (TJC28 and SJC28), joint movement assessment, the patient’s as well as physician’s global assessment (PtGA, PhGA; visual analog score from 0 to 100 mm), patient’s assessment of pain (0–100 mm, VAS), disease activity status assessed using DAS28-CRP and DAS28-ESR, morning stiffness, and the quality of life (QOL) of the patients was assessed using the Health Assessment Questionnaire Disability Index (HAQ-DI) and given a score from 0 (without any difficulty) to 3 (unable to do) where higher scores indicate poor QOL (Fries et al., 1980). Blood pressure was also assessed, with both systolic and diastolic values (mmHg) for all patients (Table 1).
Laboratory investigations were also performed, including measurements of CRP (mg/dL), ESR (mm/h), rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPAs), complete blood count (CBC), and differential. In addition, lipid profile, liver, and renal function tests were performed to monitor drug tolerability and toxicity (Table 1).
Based on the study reported by Saeed et al. (2020), the sample size was calculated on the assumption that a two-sided significance is 0.05 and a power of 90% is used with a 95% confidence interval; therefore, 30 participants were required for each treatment group.
A simple randomization process was employed according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines to shuffle the patients who fulfilled the inclusion criteria into either the control group or the digoxin group based on a 1:1 ratio. Patients, the physician who referred them, the data collectors, and the statistician were blinded to avoid bias in the study. During the research, if the patient’s study drug caused any adverse effect requiring immediate emergency care, the rheumatologist involved was not blinded, and after breaking the blinding, the patient was excluded from the study.
Thirty participants in the active control group were treated with csDMARDs, including methotrexate 7.5–15 mg/week with or without hydroxychloroquine 400 mg/day or leflunomide 10 mg/day plus one placebo tablet every other day for 6 months, whereas the other 30 participants in the digoxin group were treated with csDMARDs, including methotrexate 7.5–15 mg/week with or without hydroxychloroquine 400 mg/day or leflunomide 10 mg/day plus digoxin 0.25 mg every other day for 6 months. Oral glucocorticoids such as prednisolone (≤10 mg/day) were allowed. The csDMARDs were initiated prior to study enrollment and maintained at a stable dose throughout the six-month trial for both the active control and digoxin groups. The medication refill rate was used to assess the patient’s compliance with the prescribed treatment regimen, and non-adherent participants were excluded from the study.
The primary efficacy outcome focused on a clinically significant improvement in ACR20 response rates which correspond to a minimum of 20% improvement in tender and swollen 28-joint counts (TJC28 and SJC28), and at least three of the five parameters: HAQ-DI, patient’s and physician’s global assessment of arthritis, pain assessment, and CRP or ESR levels (Felson et al., 1995), with complementary evaluation of disease activity using the clinical disease activity index (CDAI) (Greenberg et al., 2009) and changes in acute phase reactants CRP and ESR levels that indicate inflammatory activity, and their reduction alongside improvement in ACR20 strengthens the evidence for the treatment’s effectiveness in reducing inflammation after three and 6 months of treatment. The CDAI, calculated by summing tender and swollen 28-joint counts, as well as patient’s and physician’s global assessments, offers a comprehensive, readily applicable assessment for evaluating disease activity and treatment response in RA patients with scores defined as: remission (≤2.8), low disease activity (>2.8 to ≤10.0), moderate disease activity (>10.0 to ≤22.0), and high disease activity (>22.0) (Aletaha and Smolen, 2005). The secondary efficacy outcomes, evaluated at the same time points, were defined as the assessed improvement in disease activity, which included ACR50 and ACR70 response rates, which correspond to a minimum of 50% and 70% improvement, respectively, in tender and swollen 28-joint counts, and in three of the following: HAQ-DI, patient’s and physician’s global assessment of arthritis, pain assessment, and CRP or ESR levels (Felson et al., 1995). Also, the study considered the EULAR response criteria that depend on the DAS28-CRP disease activity index and the change that occurred in it through the treatment period (Verhoeven et al., 2000). The other assessment indicators include DAS28-ESR, morning stiffness, simplified disease activity index (SDAI) (Smolen et al., 2003), and hematologic parameters calculated from total and differential complete blood counts (CBC), including NLR, PLR, LMR, SII, and SIRI, which are correlated with disease activity and prognosis.
Other outcomes involved the quantification of IL-17A, IL-23, HIF-1α, and VEGF serum levels together with the proportions of Th17 cells (IL-17A+ CD4+ T cells) at the beginning of the study and after the treatment period to investigate the potential biological changes induced by the trial medications.
Safety outcome measures were primarily evaluated throughout the study by recording the experienced adverse effects and their severity (mild, moderate, or severe) in both the control and digoxin groups. The patient’s medical history was carefully assessed, and they were comprehensively educated about the potential adverse effects of digoxin and were required to note any adverse events they encountered throughout the treatment course. In addition, clinical and physical examinations, as well as electrocardiograms (ECG), were carried out.
Venous samples were obtained from RA patients before as well as three and 6 months post-treatment initiation, and the samples were categorized into two parts: the whole blood portion, which was used to analyze Th17 cells by flow cytometry, and the serum portion, which was used for ELISA analysis.
The serum levels of cytokines IL-17A (Sun Red International trade company, Shanghai, China; 201-12–0048) and IL-23 (Cloud-Clone Corp Co., United States; SEA384Hu), as well as the angiogenic factors HIF-1α (Sun Red International trade company, Shanghai, China; 201-12–0423) and VEGF (Cloud-Clone Corp Co., United States; SEA143Hu), were measured by specific human enzyme-linked immunosorbent assay (ELISA) kits based on the manufacturer’s instructions utilizing a Biotek ELx800 UV-Vis microplate reader. The values were presented in picograms per milliliter (pg/mL) with standard deviation (SD).
The peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque density gradient centrifugation (GE Healthcare Bio-Sciences, Uppsala, Sweden), and then the cells were washed in phosphate-buffered saline (PBS). Following staining with FITC-conjugated anti-CD4 (Beckman Coulter Life Sciences, Indianapolis, United States; IM0448U), fixation and permeabilization of cells were done using the Fixation/IntraPrep Permeabilization Kit (Beckman Coulter Company, France; A07803) and then stained intracellularly with PE-conjugated anti-human IL-17A (BD Biosciences Pharmingen, United States; 560436) according to the manufacturer’s protocol. Cell analysis was conducted utilizing the CYTOFLEX Flow Cytometer (Beckman Coulter Life Sciences, Indianapolis, United States) and CytExpert Software version 2.5 (Figure 2).
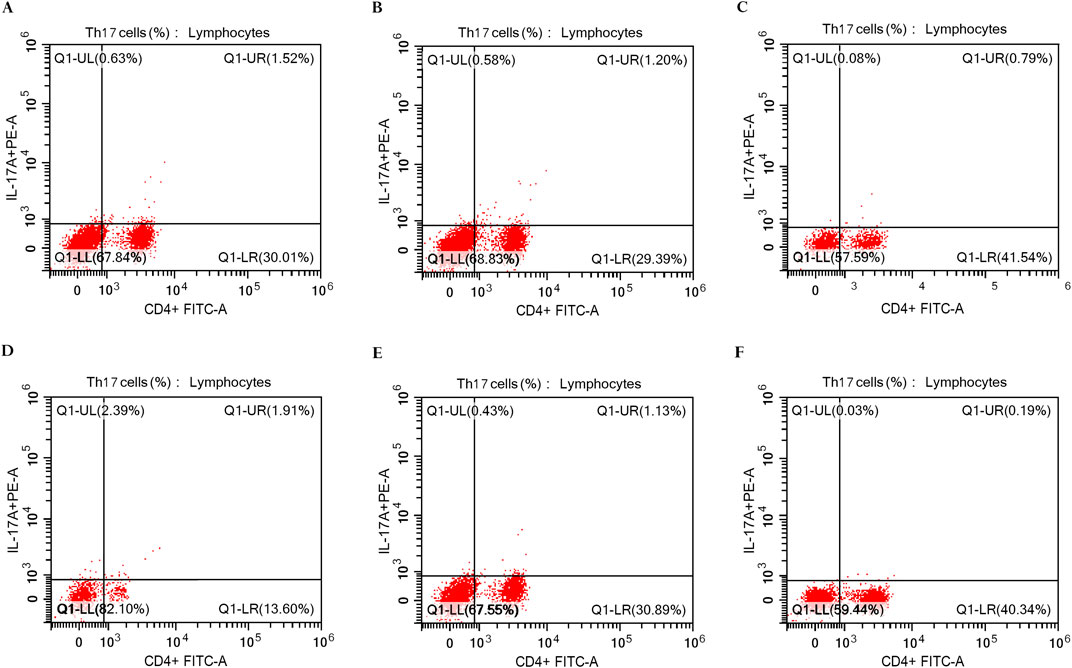
Figure 2. Flow cytometric analysis of Th17 cells in PBMCs of rheumatoid arthritis patients. (A) The percentage of IL-17A+ CD4+ T cells in RA patients (1.52%) at baseline in the control group (n = 30). (B) The percentage of IL-17A+ CD4+ T cells in RA patients (1.20%) after 3 months of treatment in the control group (n = 30). (C) The percentage of IL-17A+ CD4+ T cells in RA patients (0.79%) after 6 months of treatment in the control group (n = 30). (D) The percentage of IL-17A+ CD4+ T cells in RA patients (1.91%) at baseline in the digoxin group (n = 30). (E) The percentage of IL-17A+ CD4+ T cells in RA patients (1.13%) after 3 months of treatment in the digoxin group (n = 30). (F) The percentage of IL-17A+ CD4+ T cells in RA patients (0.19%) after 6 months of treatment in the digoxin group (n = 30).
Statistical analyses were conducted using IBM SPSS Statistics version 27.0 software (IBM Corporation, Armonk, New York, United States). Quantitative data were tested for normality using the Shapiro-Wilk test. Quantitative data were exhibited as means and standard deviations (SD) or medians and inter-quartile range (IQR), while qualitative data were displayed as frequencies (n) and percentages (%). Paired and unpaired Student's t-tests were employed for quantitative comparisons of parametric data, while Mann-Whitney U and Wilcoxon signed-rank tests were employed for quantitative comparisons of nonparametric data. Chi-Square (χ2) and Fisher’s exact tests were utilized for analyzing qualitative variables. Pearson’s correlation coefficient (r) for parametric data and Spearman’s rank correlation coefficient (r) for nonparametric data, evaluated the relationships between measured variables. Multivariate logistic and linear regression models were used to describe the associations of predictors (baseline demographic characteristics) with the outcomes. The results were expressed as odds ratios (ORs) in logistic regression models and as regression coefficients in linear regression models. The 95% confidence interval (95% CI) was calculated, and the statistical significance was determined at p < 0.05.
Out of the 94 encountered patients, 24 subjects were excluded: 17 patients due to disease comorbidities including cardiac diseases, endocrinal disorders, and impaired renal function (n = 17); 7 patients declined participation in the study (n = 7); therefore, the remaining 70 patients were enrolled and randomized to both groups in the study. Ten participants (5 in each group) were dropped out and their initial data were omitted from the final analysis which included 30 participants in each group. Only participants who adhered to the study protocol throughout the trial were included, while data from participants who deviated from the protocol were excluded from the analysis.
Baseline assessment showed that the two study groups displayed no significant differences concerning demographic features, clinical and laboratory variables, or csDMARDs received in each group (p > 0.05), as presented in Table 1.
After 3 months of treatment, digoxin administration led to a marked increase in the ACR20 response relative to the control group (66.7% vs. 36.7%, p = 0.020). Consistently, improved efficacy was observed at 6 months of treatment, with significantly higher rates of ACR50 (46.7% vs. 20%, p = 0.028) and ACR70 (26.7% vs. 6.7%, p = 0.038) responses in the digoxin group patients relative to patients in the control group (Table 2). Table 2 also demonstrates that after three and 6 months of treatment, patients in the digoxin group exhibited a statistically significant enhancement in their EULAR response criteria in contrast to the control group (p < 0.001 and p = 0.008, respectively).
Significant clinical improvements in the digoxin group were observed in multiple disease measures in contrast to the control group concerning DAS28-CRP, DAS28-ESR, tender as well as swollen joint counts, the patient’s as well as the physician’s global assessment, pain assessment, morning stiffness, HAQ-DI score, CDAI, and SDAI after three and 6 months of treatment initiation (Table 3). Serum levels of CRP and ESR were also significantly decreased in the digoxin group in contrast to the control group at both time points (Table 4).
Additionally, the inflammatory markers derived from hematological analysis or peripheral blood cell count ratio of RA patient samples, such as NLR, PLR, SII, and SIRI, which reflect systemic inflammation, significantly decreased after three and 6 months of treatment in the digoxin group in contrast to the control group (p = 0.028, p < 0.001; p = 0.021, p < 0.001; p = 0.013, p < 0.001; p = 0.004, p < 0.001, respectively). However, a significant elevation in the LMR in the digoxin group relative to the control group was observed at both measured time points (p = 0.002 and p < 0.001, respectively).
Baseline serum concentrations of the inflammatory markers IL-17A and IL-23 and the angiogenic factors HIF-1α and VEGF were similar at baseline in all study groups (p > 0.05). However, digoxin treatment led to a substantial decline in serum concentrations of these biomarkers following three and 6 months of treatment in contrast to the control group (p < 0.05) (Table 4).
In the digoxin group, analysis of peripheral blood elucidated significantly diminished proportions of Th17 cells at both measured time points in contrast to the control group (p = 0.003 and p < 0.001, respectively) (Table 4).
Pearson’s correlation analysis was employed for both groups to uncover the potential relationships between inflammatory biomarkers and clinical outcomes as well as laboratory variables. The study identified strong statistically significant positive correlations between Th17 cell proportions and serum concentrations of IL-17A, IL-23, HIF-1α, and VEGF (r = 0.843, p < 0.001; r = 0.817, p < 0.001; r = 0.850, p < 0.001; r = 0.759, p < 0.001, respectively). Similarly, Th17 cells, as well as serum concentrations of IL-17A, IL-23, HIF-1α, and VEGF, exhibited significant positive correlations with DAS28-CRP, DAS28-ESR, TJC, SJC, CRP, ESR, PtGA, PhGA, CDAI, SDAI, HAQ-DI, NLR, PLR, SII, and SIRI, and a significant negative correlation with LMR (Table 5).
To increase the robustness of the results, multivariate logistic and linear regression analyses were carried out to investigate the impact of baseline demographic factors and digoxin treatment on the clinical outcomes assessed for RA patients. Results from multivariate logistic regression (Table 6) for ACR20, ACR50, and ACR70 response rates as dependent variables, and multivariate linear regression (Table 7) for DAS28-CRP, CDAI, SDAI, and morning stiffness as dependent variables, demonstrated no significant associations between demographic factors and clinical outcomes in RA patients. While for digoxin treatment, there was a significant association between patients receiving digoxin and the clinical responses achieved according to the ACR20, ACR50, and ACR70 criteria, indicating that patients receiving digoxin are more likely to achieve clinical improvement compared to those not receiving it (Table 6). Additionally, digoxin treatment demonstrated significant associations with the clinical outcomes including DAS28-CRP, CDAI, SDAI, and morning stiffness, suggesting that it is associated with a reduction in disease activity and symptom severity, indicating its potential beneficial role in RA patients (Table 7).
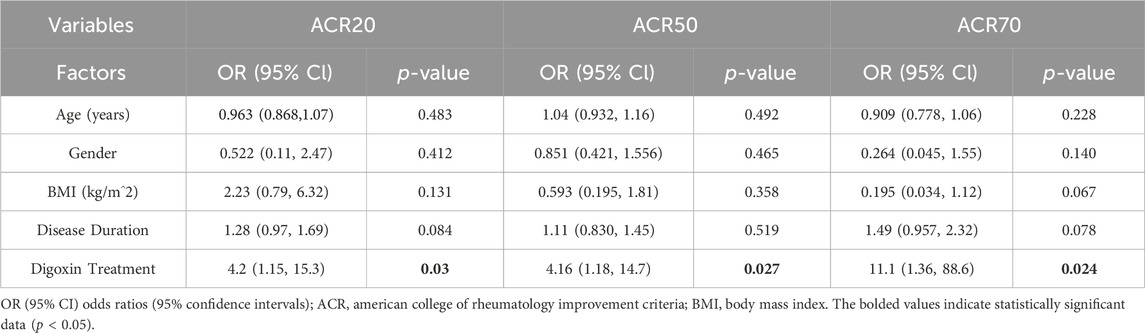
Table 6. Multivariate binary logistic regression analysis of independent predictors for clinical response in rheumatoid arthritis patients.
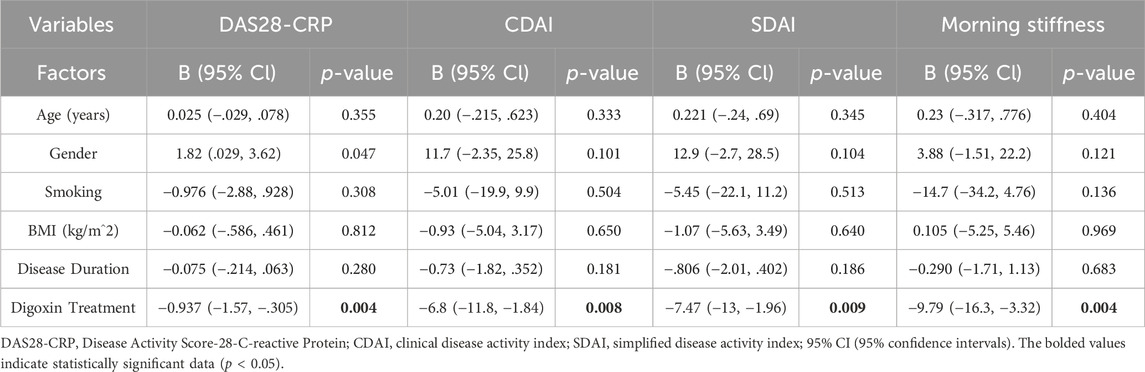
Table 7. Multivariate linear regression analysis of independent predictors for clinical outcomes in rheumatoid arthritis patients.
The absence of statistically significant differences in the adverse effects encountered by the digoxin and control groups is evident from the data presented in Table 8.
Arrhythmia and other cardiac manifestations were not observed in any of the patients in either 302 group. No toxicities attributable to digoxin were observed, and the other adverse effects experienced 303 by the patients in both groups were mild, tolerable, temporary, and resolved spontaneously without 304 needing any specific intervention or treatment discontinuation. Routine evaluation of ECG, CBC, serum electrolytes (Na+, K+, total Ca+2, ionized Ca+2 305 ), and kidney as well as liver function tests 306 confirmed that neither digoxin nor csDMARDs revealed any serious adverse events in either of the 307 two groups.
This randomized, prospective, double-blinded, and placebo-controlled clinical study is the first to evaluate the potential therapeutic effect of digoxin as an adjunctive therapy to csDMARDs on RA disease activity. Beyond their recognized therapeutic effect in cardiac diseases, cardiac glycosides are gaining significant attention for their established anti-inflammatory properties and the interesting possibility of immunomodulatory effects. Recent research suggests that digoxin has a promising therapeutic potential in treating non-cardiac diseases, such as gastrointestinal diseases, including steatohepatitis, as well as viral infections, cancer, and other metabolic disorders. Intriguingly, studies have shown that small doses of digoxin are safe and can exert effective biological activity as they selectively inhibit Th17 cell differentiation (Jamshed et al., 2023).
In the present study, we evaluated the effect of digoxin on Th17 cell proportions in PBMCs of RA patients, as well as other inflammatory and angiogenic markers involved in RA’s pathogenesis and their association with treatment response and improved disease activity.
We found a significant decline in the DAS28-CRP and CDAI with an average of −2.03 and −17.20 in the digoxin group compared to a decline of −0.94 and −9.41 in the control group following 6 months of treatment. Furthermore, active RA patients in the digoxin group exhibited a significantly improved response based on ACR and EULAR response criteria and improved patients’ QOL, offering a potential advantage over patients who received csDMARDs alone.
The observed clinical improvement in the digoxin group could be an outcome of its anti-inflammatory properties, as it suppresses the key cytokine mediators of the inflammatory response involving IL-1β, IL-6, IL-17A, and IL-23; it also inhibits IL-23-induced Th17 cell differentiation in murine models (Huh et al., 2011; Saeed et al., 2020). Our study also revealed the down-regulating effect of digoxin on the inflammatory cytokine mediators IL-17A and IL-23, which, in turn, can effectively suppress Th17 cell proliferation.
It has been demonstrated that the high percentages of Th17 cells were correlated with elevated DAS28 scores and CRP levels (Schinocca et al., 2021; Zizzo et al., 2011). In the present study, we found that flow cytometric analysis of Th17 cells in the digoxin group showed a substantial decrease in the Th17 cell proportions in contrast to the control group.
In agreement with our results, Saeed et al. have found that digoxin treatment suppresses differentiation of the Th17 cell in the cultured PBMCs treated with digoxin and obtained from RA patients whose sample size was comparable to ours (Saeed et al., 2020). Also, Zaczkiewicz et al. have shown that digoxin treatment could significantly reduce CRP plasma levels in decompensated heart failure patients (Zaczkiewicz et al., 2022). The observed improvement of CRP within the digoxin group likely arises from its inherent anti-inflammatory activity, as it reduced the levels of IL-1β and IL-6 since these cytokine mediators are known to stimulate CRP synthesis in hepatocytes through NF-κB and STAT3 signaling pathways (Kleemann et al., 2003). Despite our sample size, the findings of this study are consistent with those reported in previous investigations with comparable participant numbers that provide evidence suggesting a potential benefit of digoxin for RA. However, larger-scale randomized controlled trials are warranted to confirm these observations and to assess the long-term efficacy and safety profile of the digoxin.
Several data indicate that the inflammatory cytokine mediator IL-23 is included in Th17 cell activation and differentiation with subsequent IL-17A production that actively participates in tissue inflammation and destruction, confirming its pivotal role in RA’s pathogenesis (Schinocca et al., 2021; Tsukazaki and Kaito, 2020). In light of these data, it has been shown that digoxin’s immunomodulatory and anti-inflammatory outcomes in RA patients are potentially mediated by downregulating Th17 cell populations with a subsequent reduction of serum IL-23 and IL-17A levels.
Therefore, augmenting csDMARD therapy with digoxin in RA patients can effectively downregulate Th17 cell populations, which is pivotal in RA’s inflammatory cascade and disease progression. This effect can be explained by digoxin’s potent inhibition of the transcriptional activity of RORγt, the master regulator of Th17 cell development (Ma et al., 2017; Saeed et al., 2020; Zhenjiang Hou and Wang, 2019).
Our results are consistent with the observations of Huh et al. who exhibited that digoxin suppresses Th17 differentiation in murine models by inhibiting the transcriptional activity of RORγt (Huh et al., 2011). In addition, Fujita-Sato et al. have demonstrated that digoxin is a RORγt antagonist with promising therapeutic benefits against Th17-driven autoimmune diseases (Fujita-Sato et al., 2011). Also, it was found that digoxin treatment could exert an anti-inflammatory effect and reduce the severity of autoimmune encephalomyelitis (EAE) and Crohn’s disease in an experimental model by selectively reducing the proportion of Th17 cells and through the downregulation of mRNAs associated with Th17-related cytokines (Huh et al., 2011; Tani et al., 2017).
Several studies have demonstrated that HIF-1α regulates Th17 signature genes by directly activating the transcription of RORγt, which promotes Th17 development (Dang et al., 2011; Paradowska-Gorycka et al., 2020). Additionally, research has demonstrated that digoxin inhibits inflammasome activity maintained by the HIF-1α pathway in a nonalcoholic steatohepatitis (NASH) mouse model, thereby validating its role as an inhibitor of HIF-1α activation (Ouyang et al., 2018; Zhang et al., 2008; Zhao et al., 2019). Also, it was noted that digoxin inhibited the growth of prostate cancer cells in a murine model by downregulating HIF-1α-dependent gene expression and suppressing its protein synthesis (Platz et al., 2011). Furthermore, Wei et al. showed that digoxin inhibited non-small-cell lung cancer cell growth as it acts as a potent inhibitor of HIF-1α synthesis in A549 cells, causing downregulation of hypoxia-induced VEGF and NDRG1 overexpression (Wei et al., 2013). While this is an area of ongoing research and there is currently a lack of clinical studies specifically assessing the antiangiogenic effects of digoxin. This study is the first to evaluate the potential anti-angiogenic effects of digoxin in RA patients. Our results may be consistent with the previous experimental studies, which revealed that digoxin may possess anti-angiogenic properties through suppression of the angiogenic markers HIF-1α and VEGF, suggesting that digoxin may have potential antiangiogenic properties in RA patients and exert its therapeutic effects through HIF-1α/VEGF axis suppression.
We observed that the NLR, PLR, and the novel immune-inflammatory markers SII and SIRI were significantly reduced, and LMR was markedly elevated in the digoxin group in contrast to the control group after three and 6 months of treatment, which confirmed digoxin’s anti-inflammatory outcomes in RA patients. In addition, positive relationships were observed between these markers and both laboratory and clinical indicators of disease activity in the studied groups, indicating their prognostic value in assessing RA disease activity.
Zhou et al. (2023) showed that the elevation of NLR contributed independently to higher risks of cardiovascular mortality in RA patients. Furthermore, studies revealed that high neutrophil and monocyte levels, along with reduced lymphocytes, are independent risk factors for cardiovascular disease (Çırakoğlu Ö and Yılmaz, 2021; Targonska-Stepniak and Grzechnik, 2023; Walzik et al., 2021). Therefore, we can expect that digoxin may have protective effects against cardiovascular risks among RA patients, as it inhibits NLR, which is considered an independent predictor of cardiovascular risks among those patients, so further future clinical studies regarding the protective effects of digoxin for cardiovascular risk prevention are required in RA patients.
Baseline demographic characteristics, including age, sex, body mass index (BMI) and disease duration, were not significant predictors of clinical response to various treatments in RA as assessed by both logistic and linear regression models. These findings suggest that these factors did not influence the treatment outcomes in our study population. In consistent with our results (Jassim and Redha, 2021; Mirpourian et al., 2014; Narvaez et al., 2011; Pers et al., 2015) have indicated that demographic factors do not significantly influence treatment outcomes in rheumatoid arthritis patients. However, patients receiving digoxin, when adjusted for other variables such as age, sex, BMI, and disease duration, experienced significant improvements in various clinical measures and increased the likelihood of achieving clinically meaningful improvements in response criteria, suggesting that digoxin may play a beneficial role in the management of RA.
Overall, no major safety concerns arising from digoxin use in RA patients were identified during the study period. Furthermore, no clinically relevant pharmacokinetic interactions between digoxin and csDMARDs were reported.
Despite the encouraging results regarding the usage of digoxin as an adjuvant therapy in managing RA, our trial had certain limitations: the relatively small sample size and the recruitment of RA patients from a single medical center; therefore, we cannot fully generalize our findings until further research is conducted at multiple centers. Furthermore, we did not perform a radiological evaluation to ascertain the extent of radiological changes during treatment and correlate this with laboratory and clinical improvement; however, significant radiographic changes may not have been detected due to the brief study period. Therefore, we can describe the contribution of digoxin in treating RA as an adjuvant or complementary therapy, and we still need to learn more about its effectiveness through comprehensive, large-scale, multi-center clinical trials carried out for extended follow-up periods to provide deeper insights into digoxin’s therapeutic action in RA.
In conclusion, our results suggest that the co-administration of digoxin can improve and strengthen the effect of conventional treatment in RA patients, as indicated by better patient response and lower disease activity, supporting previous studies implying that drugs with anti-inflammatory properties might exhibit an enhanced anti-rheumatic impact.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The present study was performed in accordance with the Guidelines of the Declaration of Helsinki and approved by the ethical committee in the faculty of medicine, Menoufia University under the IRB# PMRR2A. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
NE-M: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing, Resources. TE-M: Writing–original draft, Writing–review and editing, Conceptualization, Project administration, Supervision. AB: Conceptualization, Writing–original draft, Writing–review and editing. NA: Conceptualization, Formal Analysis, Resources, Software, Writing–original draft, Writing–review and editing. AA: Conceptualization, Validation, Visualization, Writing–original draft, Writing–review and editing. MA: Conceptualization, Data curation, Resources, Writing–original draft, Writing–review and editing. EO: Conceptualization, Resources, Validation, Writing–original draft, Writing–review and editing. HS: Writing–original draft, Writing–review and editing. MT: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing, Formal Analysis, Investigation, Resources. DS: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors express their gratitude to all the health professionals, the study team, and patients who participated in the registers. Authors are thankful to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R420), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aletaha, D., Neogi, T., Silman, A. J., Funovits, J., Felson, D. T., Bingham, C. O., et al. (2010). 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 62 (9), 2569–2581. doi:10.1002/art.27584
Aletaha, D., and Smolen, J. (2005). The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 23 (5 Suppl. 39), S100–S108.
Alunno, A., Manetti, M., Caterbi, S., Ibba-Manneschi, L., Bistoni, O., Bartoloni, E., et al. (2015). Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediat. Inflamm. 2015, 751793. doi:10.1155/2015/751793
Çırakoğlu Ö, F., and Yılmaz, A. S. (2021). Systemic immune-inflammation index is associated with increased carotid intima-media thickness in hypertensive patients. Clin. Exp. Hypertens. 43 (6), 565–571. doi:10.1080/10641963.2021.1916944
Dang, E. V., Barbi, J., Yang, H. Y., Jinasena, D., Yu, H., Zheng, Y., et al. (2011). Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 146 (5), 772–784. doi:10.1016/j.cell.2011.07.033
Elshabrawy, H. A., Chen, Z., Volin, M. V., Ravella, S., Virupannavar, S., and Shahrara, S. (2015). The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 18 (4), 433–448. doi:10.1007/s10456-015-9477-2
Felson, D. T., Anderson, J. J., Boers, M., Bombardier, C., Furst, D., Goldsmith, C., et al. (1995). American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 38 (6), 727–735. doi:10.1002/art.1780380602
Fries, J. F., Spitz, P., Kraines, R. G., and Holman, H. R. (1980). Measurement of patient outcome in arthritis. Arthritis Rheum. 23 (2), 137–145. doi:10.1002/art.1780230202
Fujita-Sato, S., Ito, S., Isobe, T., Ohyama, T., Wakabayashi, K., Morishita, K., et al. (2011). Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J. Biol. Chem. 286 (36), 31409–31417. doi:10.1074/jbc.M111.254003
George, G., Shyni, G. L., and Raghu, K. G. (2020). Current and novel therapeutic targets in the treatment of rheumatoid arthritis. Inflammopharmacology 28 (6), 1457–1476. doi:10.1007/s10787-020-00757-9
Gong, Y., Yu, Z., Wang, Y., Xiong, Y., Zhou, Y., Liao, C. X., et al. (2019). Effect of moxibustion on HIF-1α and VEGF levels in patients with rheumatoid arthritis. Pain Res. Manag. 2019, 4705247. doi:10.1155/2019/4705247
Greenberg, J. D., Harrold, L. R., Bentley, M. J., Kremer, J., Reed, G., and Strand, V. (2009). Evaluation of composite measures of treatment response without acute-phase reactants in patients with rheumatoid arthritis. Rheumatology 48 (6), 686–690. doi:10.1093/rheumatology/kep054
Grennan, D. M., Gray, J., Loudon, J., and Fear, S. (2001). Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann. Rheum. Dis. 60 (3), 214–217. doi:10.1136/ard.60.3.214
Guo, Q., Wang, Y., Xu, D., Nossent, J., Pavlos, N. J., and Xu, J. (2018). Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15. doi:10.1038/s41413-018-0016-9
Guo, X., and Chen, G. (2020). Hypoxia-inducible factor is critical for pathogenesis and regulation of immune cell functions in rheumatoid arthritis. Front. Immunol. 11, 1668. doi:10.3389/fimmu.2020.01668
Hao, X., Li, D., Wu, D., and Zhang, N. (2017). The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Sci. Rep. 7 (1), 10833. doi:10.1038/s41598-017-11398-4
Huh, J. R., Leung, M. W., Huang, P., Ryan, D. A., Krout, M. R., Malapaka, R. R., et al. (2011). Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472 (7344), 486–490. doi:10.1038/nature09978
Jamshed, F., Dashti, F., Ouyang, X., Mehal, W. Z., and Banini, B. A. (2023). New uses for an old remedy: digoxin as a potential treatment for steatohepatitis and other disorders. World J. Gastroenterol. 29 (12), 1824–1837. doi:10.3748/wjg.v29.i12.1824
Jassim, N. A., and Redha, A. A. (2021). Challenges in applying treat to target strategy in sample of Iraqi patients with rheumatoid arthritis. Mediterr. J. Rheumatol. 32 (4), 331–337. doi:10.31138/mjr.32.4.331
Jordaens, L., Trouerbach, J., Calle, P., Tavernier, R., Derycke, E., Vertongen, P., et al. (1997). Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur. Heart J. 18 (4), 643–648. doi:10.1093/oxfordjournals.eurheartj.a015310
Kleemann, R., Gervois, P. P., Verschuren, L., Staels, B., Princen, H. M., and Kooistra, T. (2003). Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood 101 (2), 545–551. doi:10.1182/blood-2002-06-1762
Kumarasamy, C., Sabarimurugan, S., Madurantakam, R. M., Lakhotiya, K., Samiappan, S., Baxi, S., et al. (2019). Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Med. Baltim. 98 (24), e14834. doi:10.1097/md.0000000000014834
Li, X., Gu, L., Chen, Y., Chong, Y., Wang, X., Guo, P., et al. (2021). Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann. Med. 53 (1), 1827–1838. doi:10.1080/07853890.2021.1991591
Ma, A., Yang, Y., Wang, Q., Wang, Y., Wen, J., and Zhang, Y. (2017). Antiinflammatory effects of oxymatrine on rheumatoid arthritis in rats via regulating the imbalance between Treg and Th17 cells. Mol. Med. Rep. 15 (6), 3615–3622. doi:10.3892/mmr.2017.6484
Mirestean, C. C., Stan, M. C., Iancu, R. I., Iancu, D. P. T., and Bădulescu, F. (2023). The prognostic value of platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and monocyte-lymphocyte ratio in head and neck squamous cell carcinoma (HNSCC)-A retrospective single center study and a literature review. Diagn. (Basel) 13 (22), 3396. doi:10.3390/diagnostics13223396
Mirpourian, M., Salesi, M., Abdolahi, H., Farajzadegan, Z., and Karimzadeh, H. (2014). The association of body mass index with disease activity and clinical response to combination therapy in patients with rheumatoid arthritis. J. Res. Med. Sci. 19 (6), 509–514.
Narvaez, J., Díaz-Torné, C., Ruiz, J. M., Hernandez, M. V., Torrente-Segarra, V., Ros, S., et al. (2011). Predictors of response to rituximab in patients with active rheumatoid arthritis and inadequate response to anti-TNF agents or traditional DMARDs. Clin. Exp. Rheumatol. 29 (6), 991–997.
Ouyang, X., Han, S. N., Zhang, J. Y., Dioletis, E., Nemeth, B. T., Pacher, P., et al. (2018). Digoxin suppresses pyruvate kinase M2-promoted HIF-1α transactivation in steatohepatitis. Cell Metab. 27 (2), 1156–1350.e3. doi:10.1016/j.cmet.2018.04.007
Paradowska-Gorycka, A., Wajda, A., Romanowska-Prochnicka, K., Walczuk, E., Kuca-Warnawin, E., Kmiolek, T., et al. (2020). Th17/Treg-Related transcriptional factor expression and cytokine profile in patients with rheumatoid arthritis. Front. Immunol. 11, 572858. doi:10.3389/fimmu.2020.572858
Pers, Y. M., Godfrin-Valnet, M., Lambert, J., Fortunet, C., Constant, E., Mura, T., et al. (2015). Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J. Rheumatol. 42 (4), 580–584. doi:10.3899/jrheum.140673
Peters, A., Lee, Y., and Kuchroo, V. K. (2011). The many faces of Th17 cells. Curr. Opin. Immunol. 23 (6), 702–706. doi:10.1016/j.coi.2011.08.007
Platz, E. A., Yegnasubramanian, S., Liu, J. O., Chong, C. R., Shim, J. S., Kenfield, S. A., et al. (2011). A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer Discov. 1 (1), 68–77. doi:10.1158/2159-8274.cd-10-0020
Saeed, H., Mateen, S., Moin, S., Khan, A. Q., and Owais, M. (2020). Cardiac glycoside digoxin ameliorates pro-inflammatory cytokines in PBMCs of rheumatoid arthritis patients in vitro. Int. Immunopharmacol. 82, 106331. doi:10.1016/j.intimp.2020.106331
Saponaro, C., Malfettone, A., Ranieri, G., Danza, K., Simone, G., Paradiso, A., et al. (2013). VEGF, HIF-1α expression and MVD as an angiogenic network in familial breast cancer. PLoS One 8 (1), e53070. doi:10.1371/journal.pone.0053070
Schinocca, C., Rizzo, C., Fasano, S., Grasso, G., La Barbera, L., Ciccia, F., et al. (2021) Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview, Front. Immunol., 12. 637829, doi:10.3389/fimmu.2021.637829
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid arthritis. Lancet 376 (9746), 1094–1108. doi:10.1016/s0140-6736(10)60826-4
Sejópoles, M. D., Souza-Silva, J. P., Silva-Santos, C., Paula-Duarte, M. M., Fontes, C. J., and Gomes, L. T. (2023). Prognostic value of neutrophil and lymphocyte counts and neutrophil/lymphocyte ratio for predicting death in patients hospitalized for COVID-19. Heliyon 9 (6), e16964. doi:10.1016/j.heliyon.2023.e16964
Seki, R., and Nishizawa, K. (2016). Factors regulating Th17 cells: a review, Biomed. Res. Clin. Pract., 1. doi:10.15761/BRCP.1000122
Smolen, J. S., Breedveld, F. C., Schiff, M. H., Kalden, J. R., Emery, P., Eberl, G., et al. (2003). A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatol. Oxf. 42 (2), 244–257. doi:10.1093/rheumatology/keg072
Su, G., Zhang, Y., Xiao, R., Zhang, T., and Gong, B. (2021). Systemic immune-inflammation index as a promising predictor of mortality in patients with acute coronary syndrome: a real-world study. J. Int. Med. Res. 49 (5), 3000605211016274. doi:10.1177/03000605211016274
Tani, S., Takano, R., Tamura, S., Oishi, S., Iwaizumi, M., Hamaya, Y., et al. (2017). Digoxin attenuates murine experimental colitis by downregulating Th17-related cytokines. Inflamm. Bowel Dis. 23 (5), 728–738. doi:10.1097/mib.0000000000001096
Targonska-Stepniak, B., and Grzechnik, K. (2023). The usefulness of cellular immune inflammation markers and ultrasound evaluation in the assessment of disease activity in patients with spondyloarthritis. J. Clin. Med. 12 (17), 5463. doi:10.3390/jcm12175463
Tsukazaki, H., and Kaito, T. (2020). The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int. J. Mol. Sci. 21 (17), 6401. doi:10.3390/ijms21176401
Urbanowicz, T., Olasińska-Wiśniewska, A., Michalak, M., Rodzki, M., Witkowska, A., Straburzyńska-Migaj, E., et al. (2021). The prognostic significance of neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR) and platelet to lymphocyte ratio (PLR) on long-term survival in off-pump coronary artery bypass grafting (OPCAB) procedures. Biol. (Basel) 11 (1), 34. doi:10.3390/biology11010034
Verhoeven, A. C., Boers, M., and Linden, S. v. d. (2000). Responsiveness of the core set, response criteria, and utilities in early rheumatoid arthritis. Ann. Rheum. Dis. 59 (12), 966–974. doi:10.1136/ard.59.12.966
Walzik, D., Joisten, N., Zacher, J., and Zimmer, P. (2021). Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur. J. Appl. Physiol. 121 (7), 1803–1814. doi:10.1007/s00421-021-04668-7
Wei, D., Peng, J. J., Gao, H., Li, H., Li, D., Tan, Y., et al. (2013). Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells. Int. J. Mol. Sci. 14 (4), 7273–7285. doi:10.3390/ijms14047273
Zaczkiewicz, M., Kostenzer, K., Graf, M., Mayer, B., Zimmermann, O., and Torzewski, J. (2022). Cardiac glycosides lower C-reactive protein plasma levels in patients with decompensated heart failure: results from the single-center C-reactive protein-digoxin observational study (C-dos). J. Clin. Med. 11 (7), 1762. doi:10.3390/jcm11071762
Zhang, H., Qian, D. Z., Tan, Y. S., Lee, K., Gao, P., Ren, Y. R., et al. (2008). Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. U. S. A. 105 (50), 19579–19586. doi:10.1073/pnas.0809763105
Zhao, P., Han, S. N., Arumugam, S., Yousaf, M. N., Qin, Y., Jiang, J. X., et al. (2019). Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am. J. Physiol. Gastrointest. Liver Physiol. 317 (4), G387–G397. doi:10.1152/ajpgi.00054.2019
Zhenjiang Hou, Z. M., and Wang, C. (2019). Research progress of Th17/treg cells and their transcription factors in autoimmune diseases. Am. J. Clin. Exp. Med. 7 (4), 83–92. doi:10.11648/j.ajcem.20190704.12
Zhou, E., Wu, J., Zhou, X., and Yin, Y. (2023). The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: results from NHANES 1999-2020. Front. Immunol. 14, 1309835. doi:10.3389/fimmu.2023.1309835
Zisman, D., Safieh, M., Simanovich, E., Feld, J., Kinarty, A., Zisman, L., et al. (2021). Tocilizumab (TCZ) decreases angiogenesis in rheumatoid arthritis through its regulatory effect on miR 146a-5p and EMMPRIN/CD147. Front. Immunol. 12, 739592. doi:10.3389/fimmu.2021.739592
Keywords: rheumatoid arthritis, digoxin, inflammatory markers, angiogenesis, ACR
Citation: El-Mahdy NA, Tadros MG, El-Masry TA, Binsaleh AY, Alsubaie N, Alrossies A, Abd Elhamid MI, Osman EY, Shalaby HM and Saif DS (2024) Efficacy of the cardiac glycoside digoxin as an adjunct to csDMARDs in rheumatoid arthritis patients: a randomized, double-blind, placebo-controlled trial. Front. Pharmacol. 15:1445708. doi: 10.3389/fphar.2024.1445708
Received: 07 June 2024; Accepted: 17 September 2024;
Published: 21 October 2024.
Edited by:
Chiara Bolego, University of Padua, ItalyReviewed by:
Melania Dovizio, University of Studies G.d’Annunzio Chieti and Pescara, ItalyCopyright © 2024 El-Mahdy, Tadros, El-Masry, Binsaleh, Alsubaie, Alrossies, Abd Elhamid, Osman, Shalaby and Saif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nawal Alsubaie, bmhhbHN1YmFpZUBwbnUuZWR1LnNh
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.