- 1Department of Burn and Plastic Surgery, Shenzhen Longhua District Central Hospital, Shenzhen, Guangdong, China
- 2Faculty of Geosciences and Environmental Engineering, Southwest Jiaotong University, Chengdu, Sichuan, China
- 3Hongqiao International Institute of Medicine, Shanghai Tongren Hospital/Faculty of Basic Medicine, Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of the Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Yusuf Hamied Department of Chemistry, University of Cambridge, London, United Kingdom
- 5School of Public Health, Fudan University, Shanghai, China
- 6Department of Histology and Embryology, Shanghai Key Laboratory of Cell Engineering, Naval Medical University, Shanghai, China
Background: Ovarian cancer is a highly aggressive malignancy with limited therapeutic options and a poor prognosis. Deubiquitinating enzymes (DUBs) have emerged as critical regulators of protein ubiquitination and proteasomal degradation, influencing various cellular processes relevant to cancer pathogenesis. In this study, the research progress between ovarian cancer and DUBs was mapped and visualized using bibliometrics, and the expression patterns and biological roles of DUBs in ovarian cancer were summarized.
Methods: Studies related to DUBs in ovarian cancer were extracted from the Web of Science Core Collection (WoSCC) database. VOSviewer 1.6.20, CiteSpace 6.3.R1, and R4.3.3 were used for bibliometric analysis and visualization.
Results: For analysis 243 articles were included in this study. The number of publications on DUBs in ovarian cancer has gradually increased each year. China, the United States, and the United Kingdom are at the center of this field of research. The Johns Hopkins University, Genentech, and Roche Holding are the main research institutions. David Komander, Zhihua Liu, and Richard Roden are the top authors in this field. The top five journals with the largest publication volumes in this field are Biochemical and Biophysical Research Communications, Journal of Biological Chemistry, PLOS One, Nature Communications, and Oncotarget. Keyword burst analysis identified five research areas: “deubiquitinating enzyme,” “expression,” “activation,” “degradation,” and “ubiquitin.” In addition, we summarized the expression profiles and biological roles of DUBs in ovarian cancer, highlighting their roles in tumor initiation, growth, chemoresistance, and metastasis.
Conclusion: An overview of the research progress is provided in this study on DUBs in ovarian cancer over the last three decades. It offers insight into the most cited papers and authors, core journals, and identified new trends.
Introduction
Ovarian cancer, which is the fifth most prevalent cancer among women, significantly contributes to global cancer-related mortalities in women (Siegel et al., 2023). Due to the non-specific or subtle symptoms associated with this disease, early detection and diagnosis remain challenging. Consequently, it is frequently diagnosed at advanced stages, leading to undesirable outcomes. Previous studies have identified various risk factors for ovarian cancer, including family history, age, obesity, genetic mutations, and early onset of menstruation (Wang et al., 2023a; Sung et al., 2023; Sandvei et al., 2023; Matan et al., 2022; Fortner et al., 2019; Arora et al., 2024). However, more efforts are still required to establish effective screening strategies, such as protein biomarkers, for the early diagnosis of ovarian cancer.
Post-translational modification plays an important role in regulating target protein activity, stability, interaction, and/or localization (Singh and Ostwal, 2019; Lee et al., 2023; Wang et al., 2022a; Li et al., 2023). Acetylation, sumoylation, ubiquitination, and phosphorylation are the most common types of protein post-translational modification (Wang et al., 2014a). Specifically, ubiquitination is a process in which an ubiquitin (Ub) protein, or a chain of Ub proteins, is covalently attached to the target substrate, ultimately leading to the proteasomal degradation or localization alteration of the target protein (Damgaard, 2021). This process can be reversed by deubiquitinases (DUBs), which cleave ubiquitin from targeted proteins (Snyder and Silva, 2021). The dynamic balance between ubiquitination and deubiquitination plays critical roles in biological activities, such as cell-signaling transduction, apoptosis, and drug resistance. To date, six classes of DUBs have been identified, namely, ovarian tumor proteases (OTUs), ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), and Josephin domain-containing proteins, MINDYs, and JAMMs (Harrigan et al., 2018). Among them, USPs form the largest family of DUBs. Accumulating evidence suggests that the dysregulation of USPs is involved in various diseases, including cancer. For example, we previously found that targeting USP47 could decrease tyrosine kinase inhibitor resistance and eradicate leukemia stem/progenitor cells in chronic myelogenous leukemia (Lei et al., 2021a). We and others have suggested that USP7 plays essential biological roles in the pathogenesis of multiple myelomas (Jing et al., 2018; Wang et al., 2022b; Chauhan et al., 2012). Importantly, USP7 has also been revealed as a promising target for ovarian cancer treatment (Ma and Yu, 2016; Zhang et al., 2016; Qin et al., 2016). Thus, DUBs, especially USPs, may serve as promising biomarkers for the early detection and diagnosis of ovarian cancer.
In this study, we performed a bibliometric analysis of the scientific articles published on DUBs in ovarian cancer to evaluate the study trends on this topic. Although several bibliometric analyses have been published on various topics in ovarian cancer (Song et al., 2024; Lin et al., 2024; Meng et al., 2024; Wang et al., 2024; Leng et al., 2023; Giles et al., 2023; Duan et al., 2023; Liu et al., 2023a), this is the first study to identify the most influential literature in this field. We also summarized the expression and biological roles of DUBs in ovarian cancer and explored their potential as biomarkers.
Methods
Data sources and search strategy
The literature search was conducted to retrieve related articles from inception to May 2024 from the Web of Science Core Collection (WoSCC). The search strategy is presented in Supplementary Table S1. This study included only “articles” and considered only documents written in English. As all data were obtained from a public database, ethical declarations or approvals are not applicable.
Data analysis and visualization
We extracted relevant data from the retrieved literature titles and used Microsoft Excel 16.0 to identify and calculate bibliometric parameters. These metrics cover key aspects of publications, including the number of publications per year, citation frequency, average citation frequency, journal title, journal impact factor, country/region of publication, publishing organization, and authors.
The visualization and analysis process involved the use of three powerful bibliometric analysis tools to fully analyze the academic data: VOSviewer (version 1.6.20), CiteSpace (version 6.3.R1), and R4.3.3. VOSviewer is a versatile software tool that plays a key role in mapping institutional collaborations, co-authorships, citations, and co-citations (van Eck and Waltman, 2010). It was used for keyword co-occurrence analysis. CiteSpace 6.3.R1 was used for keyword emergence detection and co-occurrence analysis, with the parameters set to time slicing: from January 1996 to May 2024 (research in this field was originally published in 1996). The time slicing was set to 1 year, and the node types were set to keywords. When nodes are keywords, the threshold (top N per segment) was set to 5, and pruning was set to the pathfinder + pruning merged network. Based on the parameter settings for each node, a visual analysis was performed to generate a timeline graph of deubiquitinating enzymes with keywords in the field of ovarian cancer research.
Results
Overview of the main information
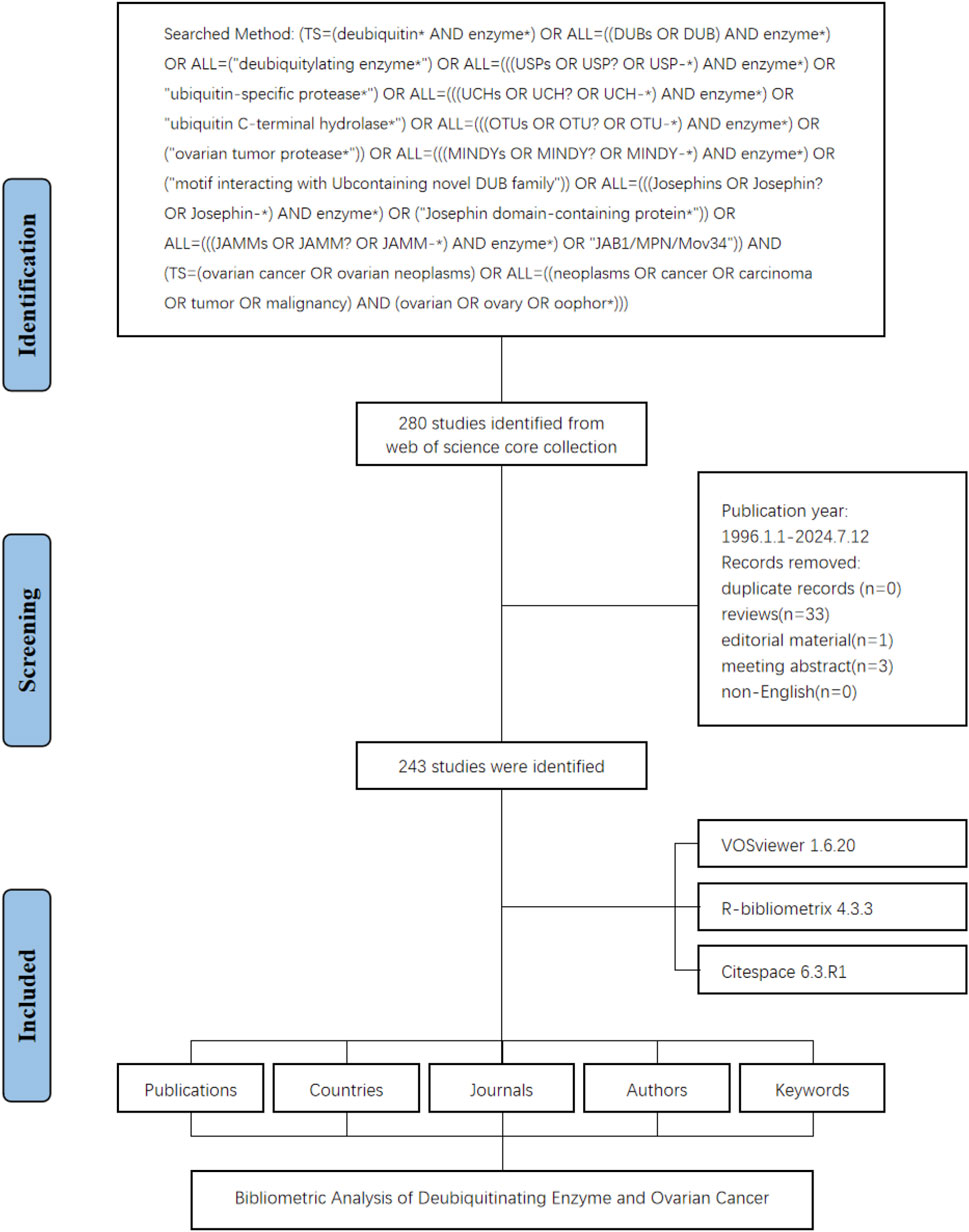
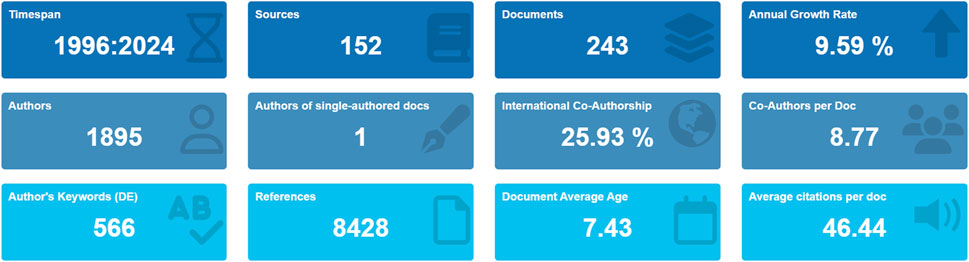
The study flowchart is presented in Figure 1. A total of 243 articles were identified in this study on DUBs in ovarian cancer over the last three decades. Our investigation showed that 1,895 authors from 926 institutions across 135 countries contributed to the production of these 243 manuscripts. These works were published in 152 journals, citing 8,428 references, with an average of 46.44 citations per article (Figure 2).
Annual publication trend
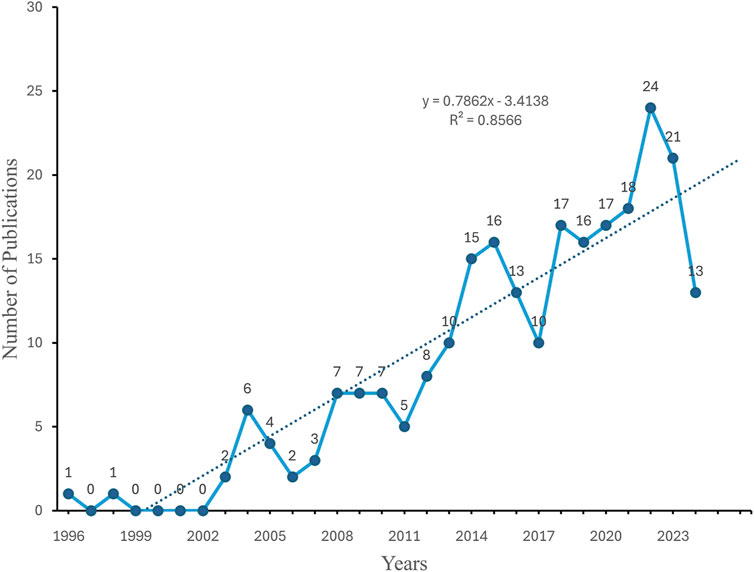
To gain insight into the evolution of related research in this field, we examined the annual publication trends. The study period exhibited a discernible upward trajectory in annual publications, particularly since 2003. The change in cumulative publications over time follows the trend line equation y = 0.7,862 × −3.4,138, with a correlation coefficient of 0.8566 and an annual growth rate of 9.59%. Additionally, 2022 witnessed the highest number of publications, accounting for 9.88% of the total (Figures 2, 3).
Analysis of countries
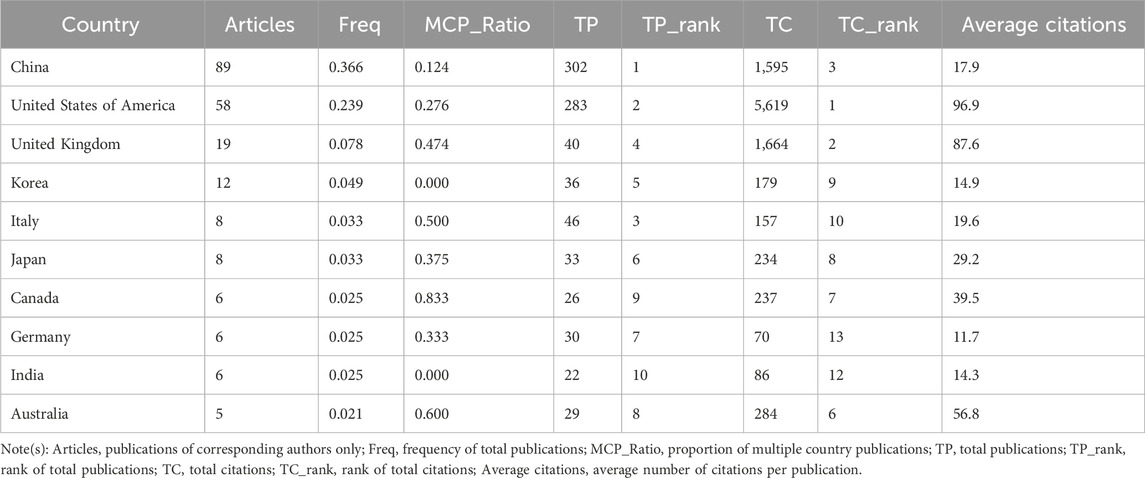
The identified publications came from 135 countries, with China leading in the number of studies (89 publications), constituting 36.62% of all documents. Other top contributors included the United States of America (58 publications), the United Kingdom (19 publications), Korea (12 publications), Japan (8 publications), and Italy (8 publications) (Figure 4A; Table 1). Despite China having the highest number of articles, the United States of America, France, and the United Kingdom had the highest average citations, that is, 96.9, 89.8, and 87.6, respectively. In addition, the collaboration among countries was visualized using VOSviewer. As shown in Figure 4B, the United States, the United Kingdom, and Germany were the top three countries with the strongest international collaboration network.
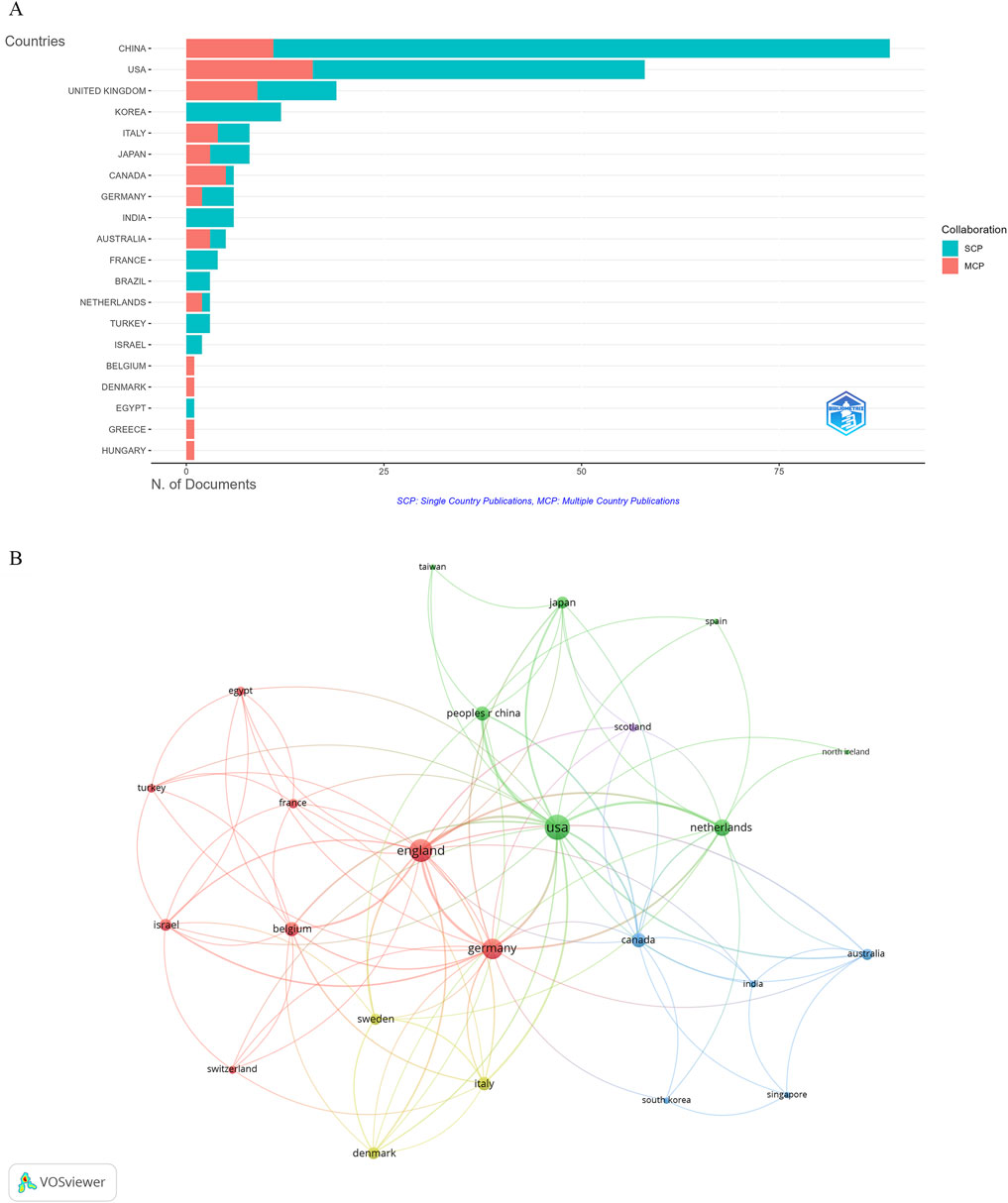
Figure 4. Visualization of countries. (A) Publications by country. (B) International collaboration network.
Analysis of institutions
Publications related to research on DUBs in ovarian cancer involved 926 institutions. The three institutions with the most publications were Johns Hopkins University (United States, 33 publications), Genentech (United States, 21 publications), and Roche Holding (United States, 21 publications) (Figure 5A). Institutions with at least two publications were included in the analysis of collaborative networks, which were visualized using VOSviewer. The clusters were arranged in different colors based on the frequency of collaboration between institutions (Figure 5B). Johns Hopkins University had the largest node, indicating the highest level of collaboration with other institutions.
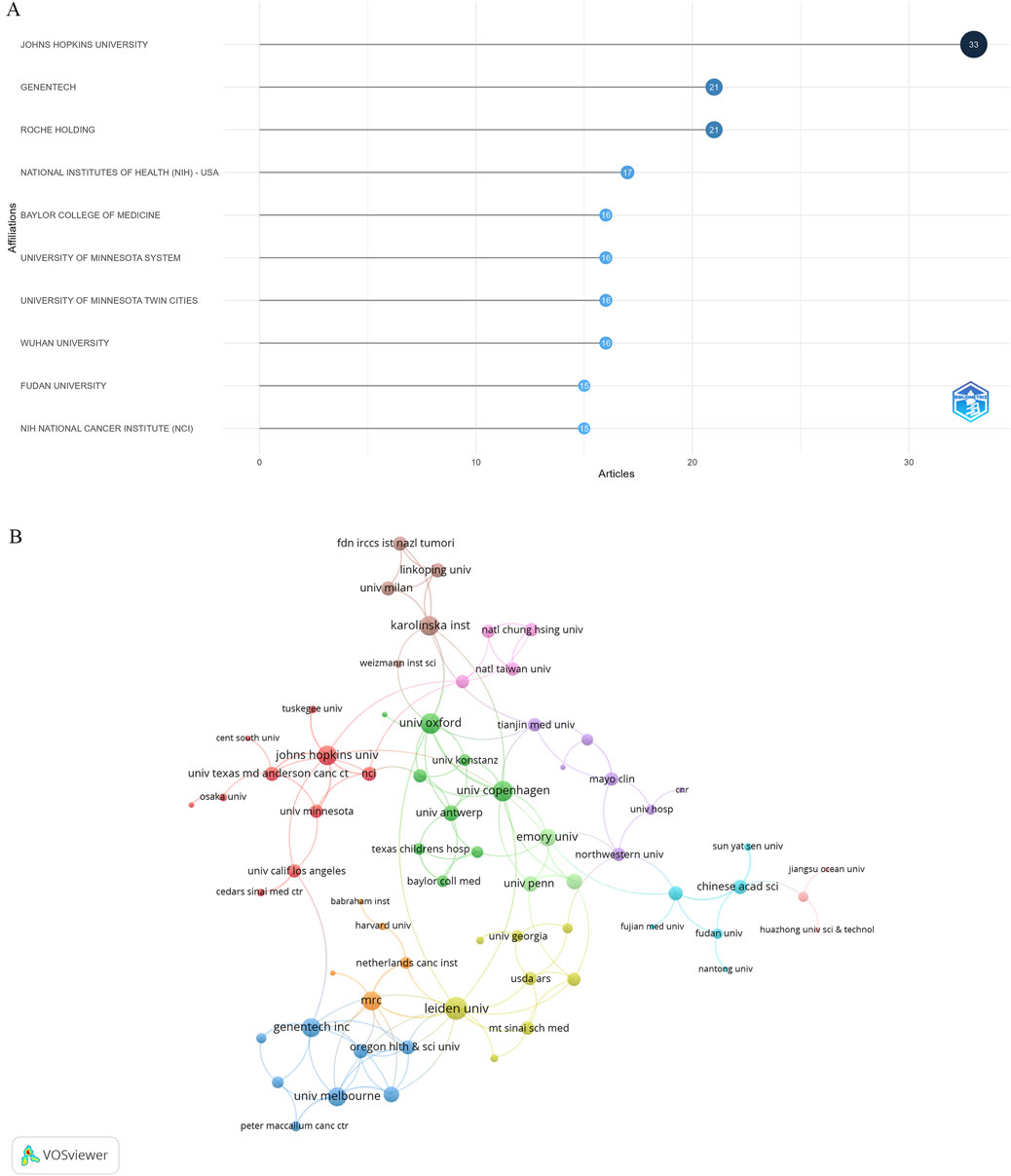
Figure 5. Visualization of institutions. (A) Publications by institution. (B) Collaborative networks of institutions.
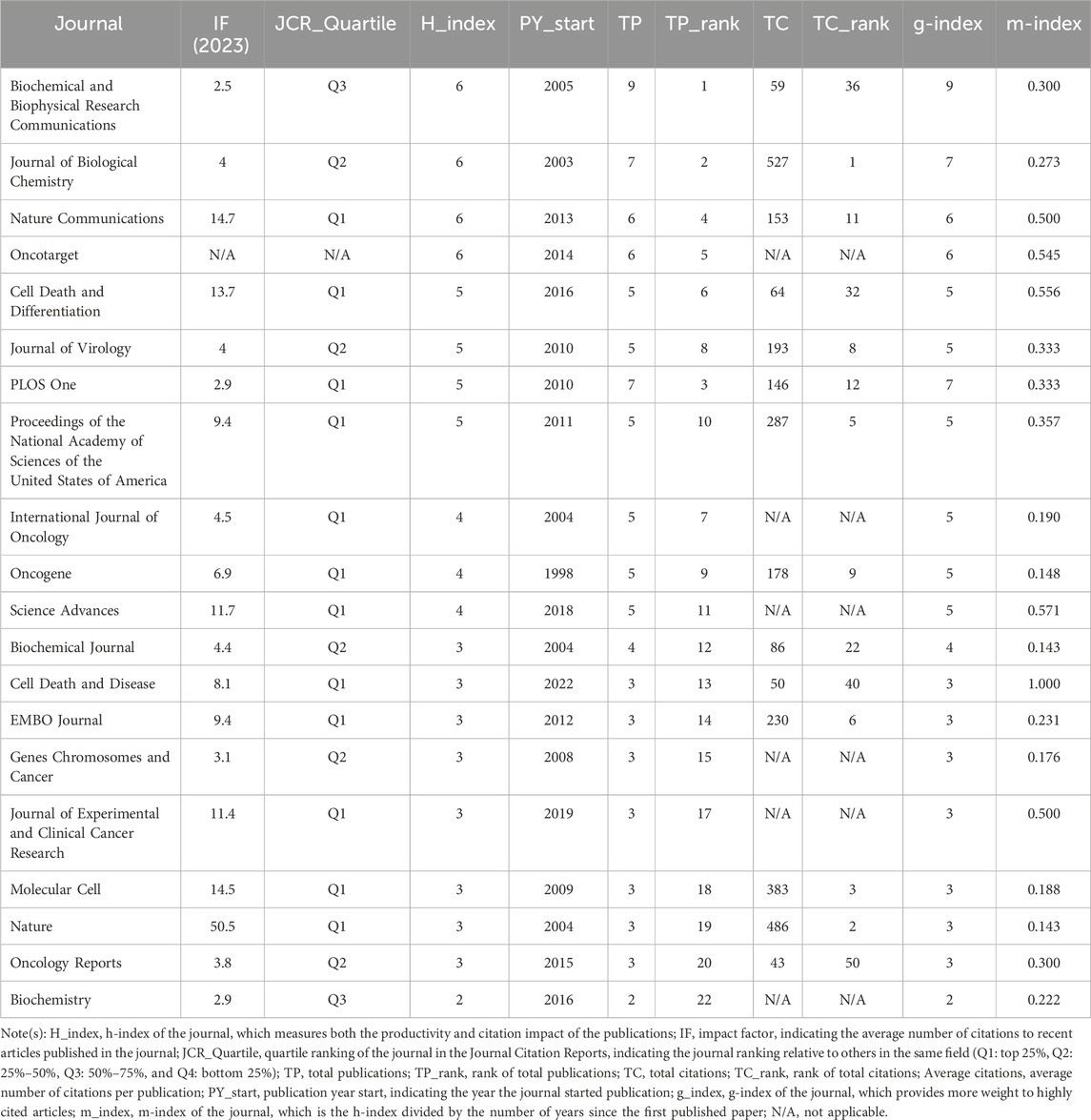
Analysis of journals and co-cited journals
Research on DUBs in ovarian cancer prominently features in 152 journals. Biochemical and Biophysical Research Communications leads with nine publications, accounting for 3.70% of the total, followed by the Journal of Biological Chemistry and PLOS One, each with seven papers, accounting for 2.88% each (Table 2). Co-citation analysis revealed that the five key journals with the highest total link strength were the Journal of Biological Chemistry (56), Proceedings of the National Academy of Sciences of the United States of America (54), PLOS One (48), Cell (47), and EMBO Reports (40) (Figure 6A). Bibliographic coupling analysis indicated that the five key journals with the highest total link strength were PLOS One (1,110), Proceedings of the National Academy of Sciences of the United States of America (1,053), Journal of Biological Chemistry (1,049), EMBO Journal (901), and Nature Communications (818) (Figure 6B).
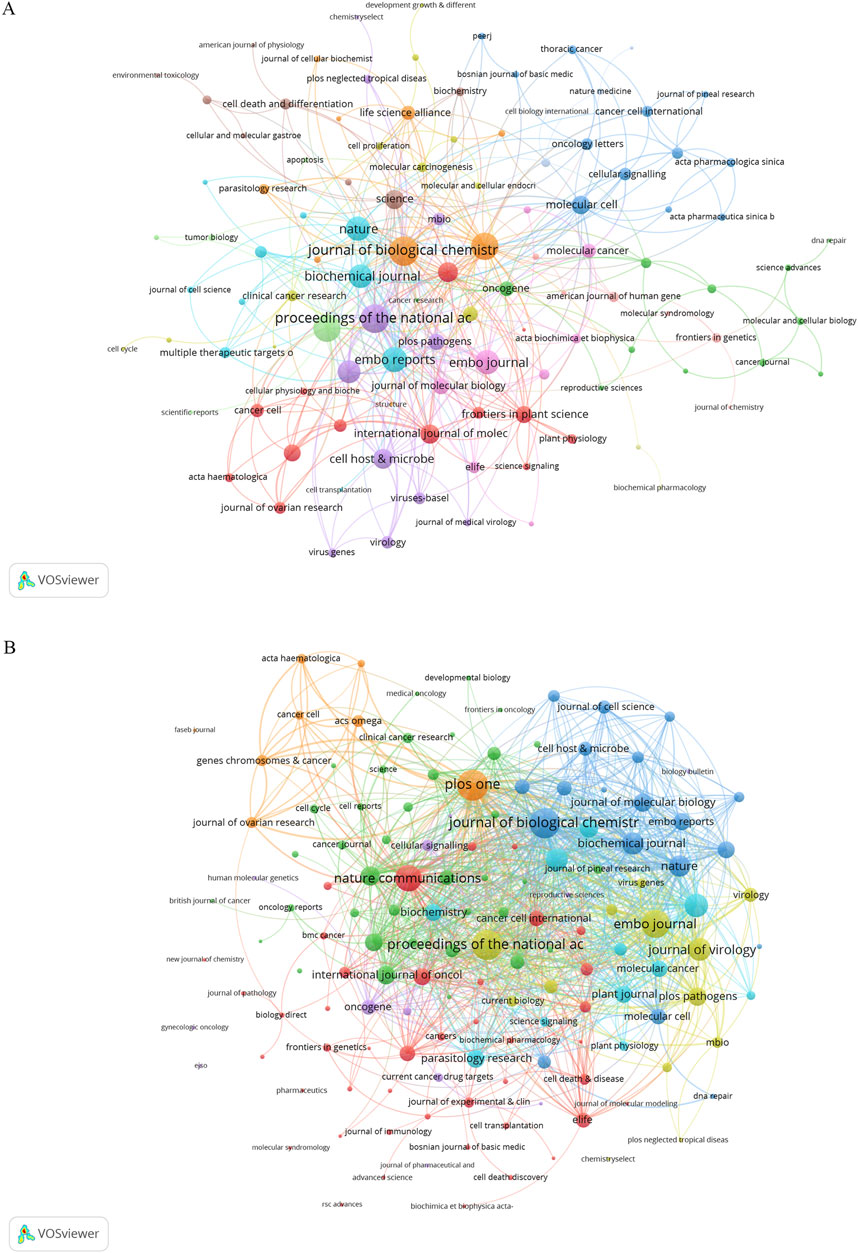
Figure 6. Co-citation and bibliographic coupling analysis. (A) Co-occurrence networks: journal link strength in co-occurrence networks measures the frequency with which two journals are cited together within the same articles or references. This metric reflects how often the publications from two different journals are associated in the bibliographies of scholarly articles. High link strength implies that the journals are often cited in tandem, indicating a thematic or topical connection between the research they publish. (B) Coupling networks: journal link strength in coupling networks assesses the extent to which journals are linked based on the common references cited in their articles. This metric captures the degree to which the research published in two different journals relies on the same body of prior work. Strong link strength in this context signifies that the journals share a substantial number of references, highlighting a shared intellectual foundation or research focus.
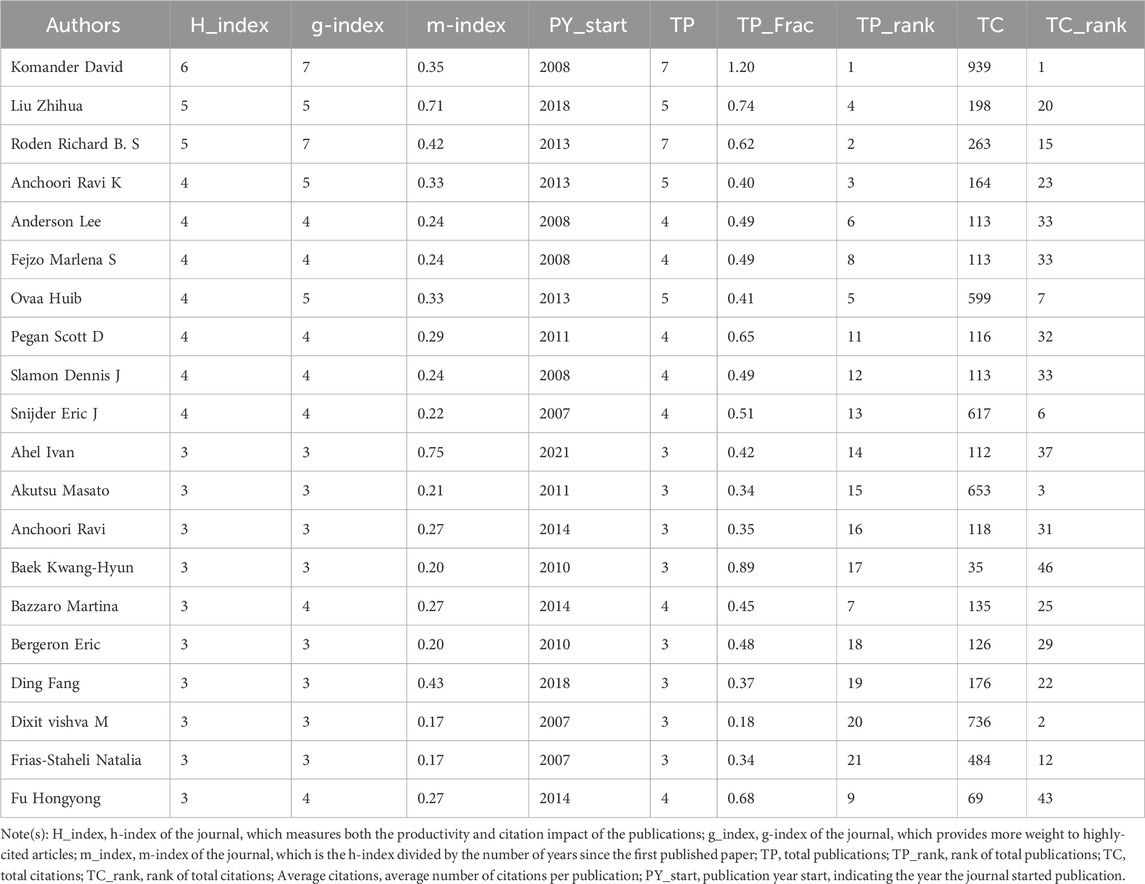
Analysis of authors and collaborations
The 243 articles were contributed by 1,895 authors. The distribution of authors was relatively concentrated, and a high degree of collaboration strength was observed. David Komander, Zhihua Liu, and Richard Roden contributed the highest number of publications, with total citations of 939, 198, and 263, respectively (Table 3). Using VOSviewer, a collaborative network analysis was conducted on authors with publication volumes of three or more. Among the 170 authors involved in international collaborations, Richard Roden had the highest number of collaborations with other countries (total link strength = 48), followed by Ravik Anchoori (total link strength = 35) and David Komander (total link strength = 27) (Figure 7).
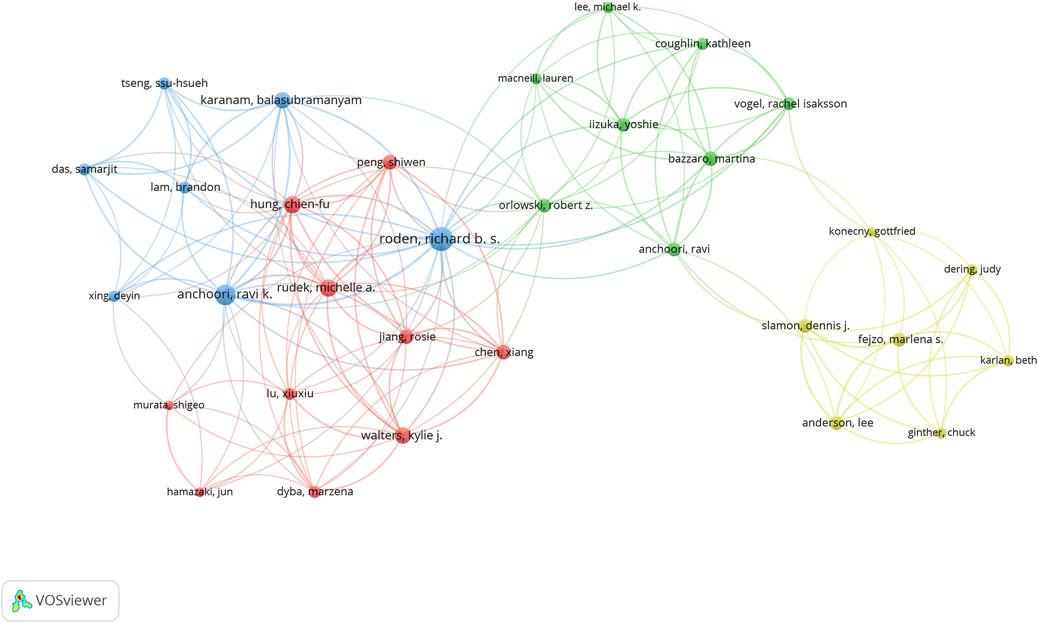
Figure 7. Visualization map depicting the collaboration among different authors. Nodes represent authors, with size indicating the publication count. Links represent co-authorships, with thickness showing collaboration strength. Colors indicate different research clusters. The total link strength in collaboration networks measures the frequency of co-authorship between authors, indicating the level of collaborative research.
Analysis of research hotspots and frontiers
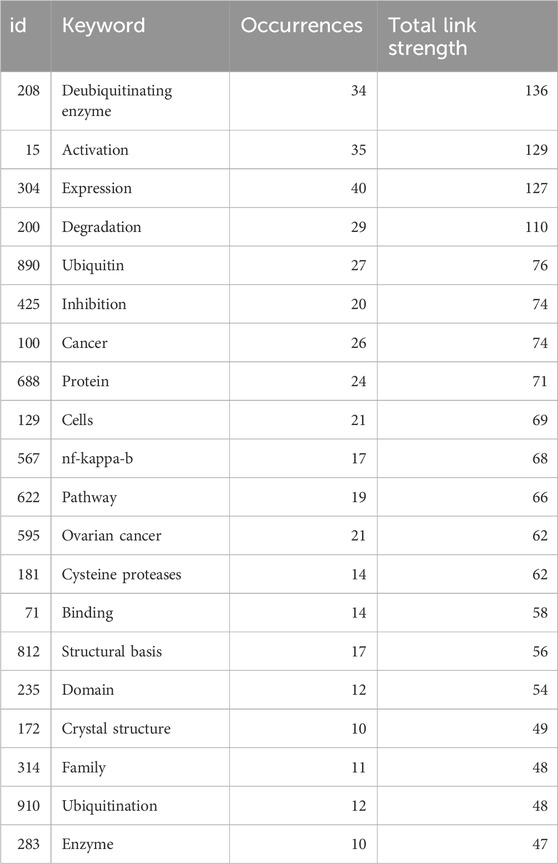
Keywords succinctly encapsulate the fundamental concepts of a paper, outlining the key areas of research interest. A comprehensive keyword analysis of the selected 243 articles related to DUBs was performed using “Author Keywords” from the Biblioshiny application and “Keywords Plus” provided by the VOSviewer application. In total, 566 keywords were identified. A network visualization map demonstrating the connections among these keyword co-occurrences was generated using VOSviewer. The sizes of the circles correspond to the frequency of occurrence of the keywords. A co-word analysis revealed that “deubiquitinating enzyme,” “degradation,” “expression,” “activation,” and “ubiquitin” were the most frequently co-occurring keywords (Figure 8). The top 20 co-occurring keywords are given in Table 4.
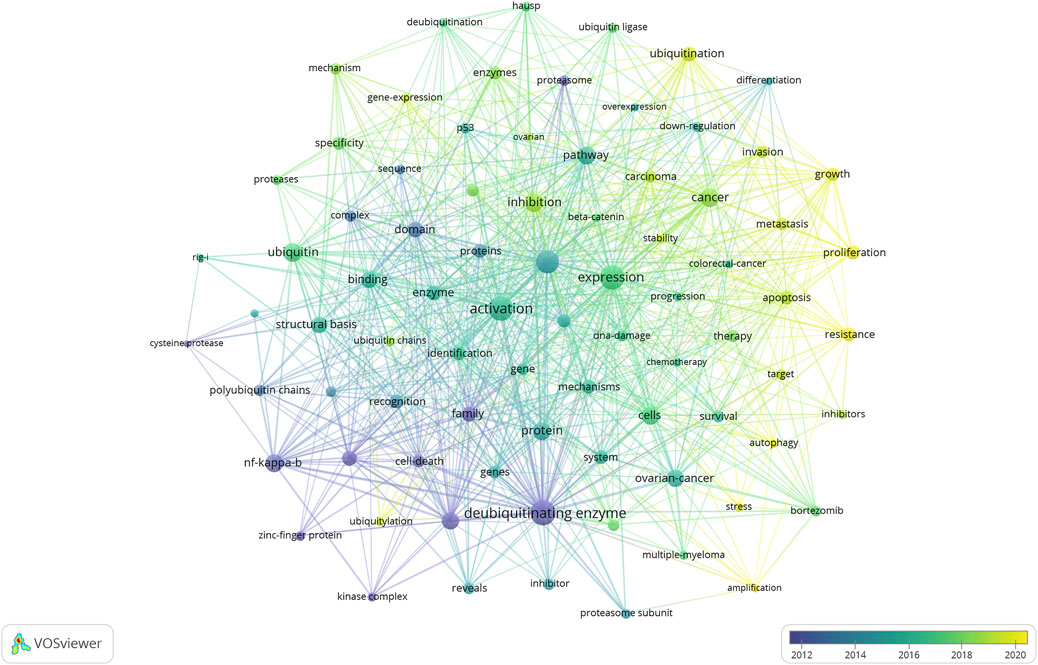
Figure 8. Visualization of keyword co-occurrence. This network visualization displays the co-occurrence of keywords in selected literature. Each node represents a keyword, with size indicating its frequency of occurrence. Links between nodes represent co-occurrence in the same documents, with thicker lines showing stronger associations. Colors reflect the average publication year of the articles, as indicated by the color gradient at the bottom right.
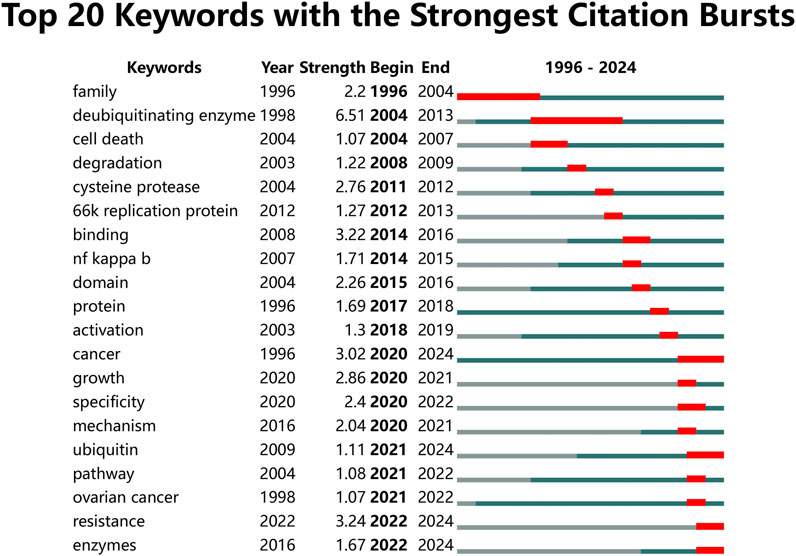
Figure 9 presents the top 20 keywords with the highest burst strengths. The most significant citation burst belongs to “deubiquitinating enzyme.” Particularly noteworthy is the concentration of keywords such as “cancer,” “growth,” “specificity,” “mechanism,” “ubiquitin,” “pathway,” “ovarian cancer,” “resistance,” and “enzymes” since 2020, indicating promising developments.
Discussion
Since 1996, studies on DUBs in ovarian cancer have experienced rapid growth, particularly after 2002, driven by their pivotal biological roles in cancer research. It is evident that DUBs have gradually emerged as a hotspot in ovarian cancer, indicated by an average citation of 47.41 per article. Additionally, the number of articles on DUBs in ovarian cancer has steadily increased, with an annual growth rate of 8.57%. Since 2020, keyword concentrations have focused on “cancer,” “growth,” “specificity,” “mechanism,” “ubiquitin,” “pathway,” “ovarian cancer,” “resistance,” and “enzymes,” highlighting future research directions for DUBs in ovarian cancer. Additionally, the most frequently co-occurring keywords are “deubiquitinating enzyme,” “degradation,” “expression,” “activation,” and “ubiquitin,” indicating that a deep understanding of the mechanisms of DUBs in ovarian cancer is a critical medical need. Interestingly, these keywords are centered around the critical regulatory functions of DUBs, suggesting that DUBs are widely entangled with the classic signaling pathways that have been well understood in ovarian cancer development. These findings highlight that DUBs may be of equal importance as the key regulatory proteins in cell division, growth, and proliferation, encouraging research workers to include DUBs as a part of the cellular regulatory network rather than as a simple tool for protein degradation and recycling. Therefore, based on this bibliometric analysis, studies of DUBs on ovarian cancer are likely to continue advancing by understanding their roles in cancer development and their potential as therapeutic targets.
The countries with the highest publication volume are primarily China, the United States, and the United Kingdom. China ranks the first in terms of publication quantity, whereas the United States and the United Kingdom have the highest average citations (all higher than 100) and intermediary centrality, highlighting their active and prominent roles in this field. However, the average citation frequency per paper in China is low, indicating that Chinese authors have lower citation frequencies, highlighting the need of high-quality paper publication. Notably, the top three institutions contributing to the publication volume were all from the United States, indicating a pioneering role in driving DUB-related research in ovarian cancer. Johns Hopkins University, Roche Holding, and Genentech had the highest intermediary centrality, serving as crucial contributors to fundamental DUB research in this disease. The top three cited articles had 1,509, 573, and 429 citations, respectively, and were published in Nature (impact factor = 50.5), Oncogene (impact factor = 6.9), and Cell (impact factor = 45.5) (Wertz et al., 2004; Jensen et al., 1998; Mevissen et al., 2013). All three articles focused on the mechanism of DUBs, highlighting the critical need of the mechanical analysis of this malignant disease.
We summarized the expression profile and biological roles of DUBs in ovarian cancer. Specifically, the following terms were used for the database search without language and regional restrictions: “ovarian cancer” or “ovarian neoplasms” AND “deubiquitinating enzymes” or “deubiquitinases” or “ovarian tumor proteases” or “ubiquitin-specific proteases” or “ubiquitin C-terminal hydrolases” or “Josephin domain-containing proteins” or “motif interacting with Ubcontaining novel DUB family” or “JAB1/MPN/Mov34 metalloenzyme.” Other eligible studies were also reviewed from the references of each article. As we retrieved zero results for Josephin domain-containing proteins in ovarian cancer, we mainly focused on the expression and functional role of OTUs, USPs, and UCHs in ovarian cancer (Table 5). Research workers may utilize this information to develop treatments against important molecular targets, such as mutant p53 and PTEN, or explore DUBs as potential therapeutic targets. For instance, USP7 is one of the representative DUBs that have been widely studied in cancer research. It exerts fine-tuned control over diverse protein levels and functions, impacting cell fate decisions and maintaining cellular homeostasis. USP7 is a critical regulator of many cancer-related proteins, including p53, MDM2, PTEN, and FOXO4. Zhang et al. (2016) suggested that USP7 expression is associated with poor prognosis in ovarian cancer, supported by cellular experiments. Ma and Yu (2016) found that USP7 is highly expressed in epithelial ovarian cancer patients, positively correlated with lymphatic invasion, and independently associated with poor overall survival. They concluded that the modulation of USP7 expression could affect ovarian cancer cell viability and invasion (Ma and Yu, 2016). Wang et al. (2017) reported that the inhibition of USP7 could induce cell death in ovarian cancers, regardless of the P53 status. This finding is consistent with that of previous research, showing that USP7 was highly expressed in ovarian cancer and inversely correlated with the differentiation level, and that inhibition of USP7 could lead to cell apoptosis (Qin et al., 2016). Furthermore, Wang et al. (2023b) found that USP7 deubiquitinases TRAF4, and the knockdown of USP7 suppressed ovarian cancer both in vitro and in vivo. A recent meta-analysis concluded that USP7 promotes ovarian cancer progression and predicts unfavorable clinical outcomes (Kisaï and Koji, 2021). These findings suggest that USP7 may act as an oncoprotein highly expressed in ovarian cancer cells and patients, and may be associated with poor clinical outcomes. In addition, USP14 may be another promising target in ovarian cancer treatment, with the earliest research traced back to 2007 (Yang et al., 2007). Subsequent studies have revealed the critical involvement of USP14 in various pathways, especially in tumor proliferation and chemoresistance (Wang et al., 2015; Wada et al., 2009; Shen et al., 2020; Huang et al., 2017; Luo et al., 2019; Ji et al., 2023). It can thus be hypothesized that targeting USP14 may be an effective strategy for second- and third-line therapies, during which chemoresistance is the major challenge. Moreover, UCHL1 is another interesting target for its broad implications in various ovarian cancer cell lines, as well as animal models and patient samples (Tangri et al., 2021; Okochi-Takada et al., 2006; Jin et al., 2013). Understanding its roles in different cell lines and signaling pathways may reveal common mechanisms in ovarian cancer development. It should be emphasized that although most DUBs are not direct executers in signaling pathways, they may be equally important as they essentially modulate the concentrations of the key regulators. This can be utilized to create novel therapeutic strategies against certain oncoproteins, especially against those with various mutations or thought to be “undruggable” (Lei et al., 2021b). For example, KRAS mutation is known to promote ovarian cancer development (Therachiyil et al., 2022), yet only a few drugs are proven effective against certain mutations of KRAS. Instead of directly inhibiting KRAS, inducing KRAS degradation by activating its DUB(s) may be a promising approach; furthermore, this strategy may be a “one-size-fits-all” solution that is robust against various KRAS mutations (Fraile et al., 2017), which may also be extended to other critical targets in cancer therapy.
Keywords reflect the primary content of publications and encapsulate the main topics covered in the literature. Analyzing keywords can offer insights into current study hotspots and future directions in the research field. By examining the frequency and co-occurrence of keywords, research workers can identify prevailing themes and emerging trends that shape the field trajectory. In this study, “deubiquitinating enzyme,” “degradation,” “expression,” and “activation” were the most frequently co-occurring keywords. These keywords highlight the central themes of current research, emphasizing the role of DUBs in cellular processes. DUBs are known for their ability to remove ubiquitin from target proteins, thereby preventing their degradation. This stabilization affects the activation and localization of various proteins, triggering cascades of biological processes that are crucial for maintaining cellular homeostasis and function. A timeline viewer for keyword analysis reveals the evolution of hotspots in the field over time, showing how the focus within the field has shifted and expanded. This tool helps visualize the progression of key research topics and provides a historical perspective on how the field has developed. For instance, the consistent appearance of terms like “degradation,” “expression,” and “activation” underscores the ongoing interest in understanding the fundamental mechanisms of DUBs and their broader biological implications. Regarding keywords with the strongest citation bursts, “cancer,” “ubiquitin,” “resistance,” and “enzymes” have been the latest hotspots in ovarian cancer research since 2020, and the focus on “ubiquitin” and “resistance” as future directions highlights the need for more research into how ubiquitin signaling pathways contribute to cancer progression and treatment outcomes. Understanding these pathways could lead to the development of novel interventions that target specific DUBs or their substrates, potentially overcoming resistance to current therapies and improving patient outcomes.
This bibliometric analysis provides a comprehensive and visual analysis of DUBs in ovarian cancer; however, several limitations should be acknowledged. This study only included articles indexed in the WoSCC, and the language was restricted to English. Therefore, publications in other databases or languages were not included in the analysis. Nevertheless, the WoSCC is a well-recognized database, and given its prominence, the impact of such omissions on the overall findings is expected to be low. Further studies are needed to include additional databases and languages to provide a more accurate and comprehensive analysis. Based on the narrative review and the bibliometric analysis, future studies may need to focus on the potential of DUBs as drug targets for the treatment and management of this disease.
Conclusion and outlook
In summary, a visual analysis of DUBs is presented in this study in the field of ovarian cancer research, facilitated by the use of CiteSpace, VOSviewer, and R4.3.3. The essential functions of DUBs in ovarian cancer biology include DNA repair, cell cycle regulation, apoptosis, oncogenic signaling, chemotherapy response, and chemoresistance. However, the precise functions and mechanisms of DUBs in ovarian cancer remain largely unexplored. Moreover, the expression levels and functions of some DUBs are still under debate; whether these DUBs serve as oncogenic proteins, tumor suppressors, or double-edged swords in ovarian cancer requires further investigation. Understanding the intricate interplay between DUBs and ovarian cancer biology offers promising prospects for developing innovative and more effective treatment strategies, ultimately improving outcomes for patients with this challenging disease. Future efforts are expected to decipher the specific roles of individual DUBs in ovarian cancer, identify potential therapeutic targets, and explore the feasibility of targeting DUBs as a novel approach to treating ovarian cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
FQ: writing–original draft, writing–review and editing, funding acquisition, and data curation. YL: data curation, writing–review and editing, resources, and investigation. LZ: resources, writing–review and editing, and funding acquisition. YiW: supervision and writing–review and editing. YuW: software and writing–review and editing. ZF: writing–review and editing, resources, and software. YWa: methodology and writing–review and editing. DQ: software, supervision, validation, and writing–review and editing. CL: project administration, supervision, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82402533), the Shenzhen Science and Technology Program (JCYJ20220531092609020), the Guangdong Basic and Applied Basic Research Foundation (2022A1515111152), the Scientific Research Projects of Medical and Health Institutions of Longhua District, Shenzhen (2023001 and 2024037), and the Postdoctoral Research Foundation of China (2023M733657).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1445037/full#supplementary-material
References
Alur, V. C., Raju, V., Vastrad, B., and Vastrad, C. (2019). Mining featured biomarkers linked with epithelial ovarian CancerBased on bioinformatics. Diagn. Basel, Switz. 9, 39. doi:10.3390/diagnostics9020039
Arora, T., Mullangi, S., Vadakekut, E. S., and Lekkala, M. R. (2024). in StatPearls (StatPearls Publishing).
Aziz, D., Etemadmoghadam, D., Caldon, C. E., Au-Yeung, G., Deng, N., Hutchinson, R., et al. (2018). 19q12 amplified and non-amplified subsets of high grade serous ovarian cancer with overexpression of cyclin E1 differ in their molecular drivers and clinical outcomes. Gynecol. Oncol. 151, 327–336. doi:10.1016/j.ygyno.2018.08.039
Chapel, D. B., Husain, A. N., Krausz, T., and McGregor, S. M. (2017). PAX8 expression in a subset of malignant peritoneal mesotheliomas and benign mesothelium has diagnostic implications in the differential diagnosis of ovarian serous carcinoma. Am. J. Surg. pathology 41, 1675–1682. doi:10.1097/pas.0000000000000935
Chauhan, D., Tian, Z., Nicholson, B., Kumar, K. G. S., Zhou, B., Carrasco, R., et al. (2012). A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 22, 345–358. doi:10.1016/j.ccr.2012.08.007
Chui, M. H., and Grisham, R. N. (2023). Somatic BAP1 loss in ovarian serous borderline tumor and recurrent low-grade serous carcinoma from a germline BAP1 mutation carrier. Int. J. Gynecol. pathology official J. Int. Soc. Gynecol. Pathologists 42, 432–433. doi:10.1097/pgp.0000000000000932
Corno, C., D'Arcy, P., Bagnoli, M., Paolini, B., Costantino, M., Carenini, N., et al. (2022). The deubiquitinase USP8 regulates ovarian cancer cell response to cisplatin by suppressing apoptosis. Front. Cell Dev. Biol. 10, 1055067. doi:10.3389/fcell.2022.1055067
Damgaard, R. B. (2021). The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities. Cell Death and Differ. 28, 423–426. doi:10.1038/s41418-020-00703-w
Davidson, B., Tötsch, M., Wohlschlaeger, J., Hager, T., and Pinamonti, M. (2018). The diagnostic role of BAP1 in serous effusions. Hum. Pathol. 79, 122–126. doi:10.1016/j.humpath.2018.05.012
Devins, K. M., Zukerberg, L., Watkins, J. C., Hung, Y. P., and Oliva, E. (2023). BAP1 and claudin-4, but not MTAP, reliably distinguish borderline and low-grade serous ovarian tumors from peritoneal mesothelioma. Int. J. Gynecol. pathology official J. Int. Soc. Gynecol. Pathologists 42, 159–166. doi:10.1097/pgp.0000000000000877
Du, Y., Lin, J., Zhang, R., Yang, W., Quan, H., Zang, L., et al. (2019). Ubiquitin specific peptidase 5 promotes ovarian cancer cell proliferation through deubiquitinating HDAC2. Aging 11, 9778–9793. doi:10.18632/aging.102425
Duan, Y., Zhang, P., Zhang, T., Zhou, L., and Yin, R. (2023). Characterization of global research trends and prospects on platinum-resistant ovarian cancer: a bibliometric analysis. Front. Oncol. 13, 1151871. doi:10.3389/fonc.2023.1151871
Eichhorn, P. J., Rodón, L., Gonzàlez-Juncà, A., Dirac, A., Gili, M., Martínez-Sáez, E., et al. (2012). USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat. Med. 18, 429–435. doi:10.1038/nm.2619
Fortner, R. T., Poole, E. M., Wentzensen, N. A., Trabert, B., White, E., Arslan, A. A., et al. (2019). Ovarian cancer risk factors by tumor aggressiveness: an analysis from the Ovarian Cancer Cohort Consortium. Int. J. cancer 145, 58–69. doi:10.1002/ijc.32075
Fraile, J. M., Manchado, E., Lujambio, A., Quesada, V., Campos-Iglesias, D., Webb, T. R., et al. (2017). USP39 deubiquitinase is essential for KRAS oncogene-driven cancer. J. Biol. Chem. 292, 4164–4175. doi:10.1074/jbc.M116.762757
Fukui, S., Nagasaka, K., Miyagawa, Y., Kikuchi-Koike, R., Kawata, Y., Kanda, R., et al. (2019). The proteasome deubiquitinase inhibitor bAP15 downregulates TGF-β/Smad signaling and induces apoptosis via UCHL5 inhibition in ovarian cancer. Oncotarget 10, 5932–5948. doi:10.18632/oncotarget.27219
Gao, D., Zhang, Z., Xu, R., He, Z., Li, F., Hu, Y., et al. (2022). The prognostic value and immune infiltration of USP10 in pan-cancer: a potential therapeutic target. Front. Oncol. 12, 829705. doi:10.3389/fonc.2022.829705
Gennaro, V. J., Stanek, T. J., Peck, A. R., Sun, Y., Wang, F., Qie, S., et al. (2018). Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc. Natl. Acad. Sci. U. S. A. 115, E9298–e9307. doi:10.1073/pnas.1807704115
Giles, E. D., Purcell, S. A., Olson, J., Vrieling, A., Hirko, K. A., Woodruff, K., et al. (2023). Trends in diet and cancer research: a bibliometric and visualization analysis. Cancers 15, 3761. doi:10.3390/cancers15153761
Guo, T., Tang, H., Yuan, Z., Zhang, E., and Wang, X. (2022). The dual role of USP11 in cancer. J. Oncol. 2022, 9963905. doi:10.1155/2022/9963905
Gutkin, D. W., Shurin, M. R., El Azher, M. A., Shurin, G. V., Velikokhatnaya, L., Prosser, D., et al. (2019). Novel protein and immune response markers of human serous tubal intraepithelial carcinoma of the ovary. Cancer biomarkers Sect. A Dis. markers 26, 471–479. doi:10.3233/cbm-190528
Habata, S., Iwasaki, M., Sugio, A., Suzuki, M., Tamate, M., Satohisa, S., et al. (2016). BAG3-mediated Mcl-1 stabilization contributes to drug resistance via interaction with USP9X in ovarian cancer. Int. J. Oncol. 49, 402–410. doi:10.3892/ijo.2016.3494
Han, C., Yang, L., Choi, H. H., Baddour, J., Achreja, A., Liu, Y., et al. (2016). Amplification of USP13 drives ovarian cancer metabolism. Nat. Commun. 7, 13525. doi:10.1038/ncomms13525
Han, G. H., Chay, D. B., Yi, J. M., Cho, H., Chung, J. Y., and Kim, J. H. (2019). Loss of both USP10 and p14ARF protein expression is an independent prognostic biomarker for poor prognosis in patients with epithelial ovarian cancer. Cancer genomics and proteomics 16, 553–562. doi:10.21873/cgp.20157
Harrigan, J. A., Jacq, X., Martin, N. M., and Jackson, S. P. (2018). Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat. Rev. Drug Discov. 17, 57–78. doi:10.1038/nrd.2017.152
Hu, L., Kolibaba, H., Zhang, S., Cao, M., Niu, H., Mei, H., et al. (2019). MicroRNA-204-5p inhibits ovarian cancer cell proliferation by down-regulating USP47. Cell Transplant. 28, 51S–58s. doi:10.1177/0963689719877372
Huang, H., Liu, N., Liao, Y., Liu, N., Cai, J., Xia, X., et al. (2017). Platinum-containing compound platinum pyrithione suppresses ovarian tumor proliferation through proteasome inhibition. J. Exp. and Clin. cancer Res. CR 36, 79. doi:10.1186/s13046-017-0547-8
Hunter, S. M., Anglesio, M. S., Ryland, G. L., Sharma, R., Chiew, Y. E., Rowley, S. M., et al. (2015). Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 6, 37663–37677. doi:10.18632/oncotarget.5438
Ito, F., Yoshimoto, C., Yamada, Y., Sudo, T., and Kobayashi, H. (2018). The HNF-1β-USP28-Claspin pathway upregulates DNA damage-induced Chk1 activation in ovarian clear cell carcinoma. Oncotarget 9, 17512–17522. doi:10.18632/oncotarget.24776
Jensen, D. E., Proctor, M., Marquis, S. T., Gardner, H. P., Ha, S. I., Chodosh, L. A., et al. (1998). BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 16, 1097–1112. doi:10.1038/sj.onc.1201861
Ji, J., Lv, J., Lv, M., Jing, A., Xu, M., Yuan, Q., et al. (2023). USP14 regulates heme metabolism and ovarian cancer invasion through BACH1 deubiquitination and stabilization. Biochem. biophysical Res. Commun. 667, 186–193. doi:10.1016/j.bbrc.2023.04.082
Ji, M., Shi, H., Xie, Y., Zhao, Z., Li, S., Chang, C., et al. (2015). Ubiquitin specific protease 22 promotes cell proliferation and tumor growth of epithelial ovarian cancer through synergy with transforming growth factor β1. Oncol. Rep. 33, 133–140. doi:10.3892/or.2014.3580
Jin, C., Yu, W., Lou, X., Zhou, F., Han, X., Zhao, N., et al. (2013). UCHL1 is a putative tumor suppressor in ovarian cancer cells and contributes to cisplatin resistance. J. Cancer 4, 662–670. doi:10.7150/jca.6641
Jing, B., Liu, M., Yang, L., Cai, H. Y., Chen, J. B., Li, Z. X., et al. (2018). Characterization of naturally occurring pentacyclic triterpenes as novel inhibitors of deubiquitinating protease USP7 with anticancer activity in vitro. Acta Pharmacol. Sin. 39, 492–498. doi:10.1038/aps.2017.119
Johnson, J., Bales, E. C., Bitler, B. G., and Watson, Z. L. (2020). Eliciting OTUD3/RIPK-dependent necroptosis to prevent epithelial ovarian cancer. bioRxiv 2004.2029, 069021. doi:10.1101/2020.04.29.069021
Kang, H., Choi, M. C., Kim, S., Jeong, J. Y., Kwon, A. Y., Kim, T. H., et al. (2021). USP19 and RPL23 as candidate prognostic markers for advanced-stage high-grade serous ovarian carcinoma. Cancers 13, 3976. doi:10.3390/cancers13163976
Kisaï, K., and Koji, S. (2021). Prognostic role of USP7 expression in cancer patients: a systematic review and meta-analysis. Pathology, Res. Pract. 227, 153621. doi:10.1016/j.prp.2021.153621
Kwon, J., Choi, H., Ware, A. D., Morillo, B. C., Wang, H., Bouker, K. B., et al. (2022a). USP13 promotes development and metastasis of high-grade serous ovarian carcinoma in a novel mouse model. Oncogene 41, 1974–1985. doi:10.1038/s41388-022-02224-x
Kwon, J., Zhang, J., Mok, B., and Han, C. (2022b). CK2-Mediated phosphorylation upregulates the stability of USP13 and promotes ovarian cancer cell proliferation. Cancers 15, 200. doi:10.3390/cancers15010200
Lee, J. M., Hammarén, H. M., Savitski, M. M., and Baek, S. H. (2023). Control of protein stability by post-translational modifications. Nat. Commun. 14, 201. doi:10.1038/s41467-023-35795-8
Lei, H., Wang, J., Hu, J., Zhu, Q., and Wu, Y. (2021b). Deubiquitinases in hematological malignancies. Biomark. Res. 9, 66. doi:10.1186/s40364-021-00320-w
Lei, H., Xu, H. Z., Shan, H. Z., Liu, M., Lu, Y., Fang, Z. X., et al. (2021a). Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia. Nat. Commun. 12, 51. doi:10.1038/s41467-020-20259-0
Lei, X., Li, X., Chen, H., and Liu, Z. (2020). USP48 sustains chemoresistance and metastasis in ovarian cancer. Curr. cancer drug targets 20, 689–699. doi:10.2174/1568009620666200503045400
Leng, Y., Li, S., Zhu, J., Wang, X., Luo, F., Wang, Y., et al. (2023). Application of medical imaging in ovarian cancer: a bibliometric analysis from 2000 to 2022. Front. Oncol. 13, 1326297. doi:10.3389/fonc.2023.1326297
Li, G., Shi, W., Xu, Y., Li, K., Chen, Z., Lv, M., et al. (2022b). The USP18-FBXO6 axis maintains the malignancy of ovarian cancer. Biochem. biophysical Res. Commun. 593, 101–107. doi:10.1016/j.bbrc.2022.01.020
Li, J., Olson, L. M., Zhang, Z., Li, L., Bidder, M., Nguyen, L., et al. (2008). Differential display identifies overexpression of the USP36 gene, encoding a deubiquitinating enzyme, in ovarian cancer. Int. J. Med. Sci. 5, 133–142. doi:10.7150/ijms.5.133
Li, M., Tang, Y., Zuo, X., Meng, S., and Yi, P. (2022a). Loss of Ras GTPase-activating protein SH3 domain-binding protein 1 (G3BP1) inhibits the progression of ovarian cancer in coordination with ubiquitin-specific protease 10 (USP10). Bioengineered 13, 721–734. doi:10.1080/21655979.2021.2012624
Li, T. T., and Wang, H. J. (2019). UCH-L3 expression in epithelial ovarian cancer and its clinical significance. Sichuan Da Xue Xue Bao Yi Xue Ban. 50, 556–560.
Li, Y., Luo, K., Yin, Y., Wu, C., Deng, M., Li, L., et al. (2017). USP13 regulates the RAP80-BRCA1 complex dependent DNA damage response. Nat. Commun. 8, 15752. doi:10.1038/ncomms15752
Li, Y., Zhang, R., and Hei, H. (2023). Advances in post-translational modifications of proteins and cancer immunotherapy. Front. Immunol. 14, 1229397. doi:10.3389/fimmu.2023.1229397
Lin, F. T., Lin, V. Y., Lin, V. T., and Lin, W. C. (2016). TRIP6 antagonizes the recruitment of A20 and CYLD to TRAF6 to promote the LPA2 receptor-mediated TRAF6 activation. Cell Discov. 2, 15048. doi:10.1038/celldisc.2015.48
Lin, X., Zheng, W., Zhao, X., Zeng, M., Li, S., Peng, S., et al. (2024). Microbiome in gynecologic malignancies: a bibliometric analysis from 2012 to 2022. Transl. cancer Res. 13, 1980–1996. doi:10.21037/tcr-23-1769
Liu, J., Ma, J., Zhang, J., Li, C., Yu, B., Choe, H. C., et al. (2023a). Corrigendum: bibliometric and visualized analysis of drug resistance in ovarian cancer from 2013 to 2022. Front. Oncol. 13, 1228879. doi:10.3389/fonc.2023.1228879
Liu, L., Chen, C., Liu, P., Li, J., Pang, Z., Zhu, J., et al. (2023b). MYH10 combines with MYH9 to recruit USP45 by deubiquitinating snail and promotes serous ovarian cancer carcinogenesis, progression, and cisplatin resistance. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 10, e2203423. doi:10.1002/advs.202203423
Liu, M., Gao, S., Xu, X., Zhang, L., Xu, B., and Lu, D. (2022). USP18 contributes to the proliferation and migration of ovarian cancer cells by regulating the AKT/mTOR signaling pathway. Acta biochim. Pol. 69, 417–422. doi:10.18388/abp.2020_5871
Lu, H., Wang, X., Urvalek, A. M., Xie, H., Yu, L., et al. (2014). Transformation of human ovarian surface epithelial cells by Krüppel-like factor 8. Oncogene 33, 10–18. doi:10.1038/onc.2012.545
Luo, H., Wang, X., Ge, H., Zheng, N., Peng, F., Fu, Y., et al. (2019). Inhibition of ubiquitin-specific protease 14 promotes connexin 32 internalization and counteracts cisplatin cytotoxicity in human ovarian cancer cells. Oncol. Rep. 42, 1237–1247. doi:10.3892/or.2019.7232
Ma, M., and Yu, N. (2016). Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. OncoTargets Ther. 9, 1559–1569. doi:10.2147/ott.s100050
Maresca, L., Spugnesi, L., Lodovichi, S., Cozzani, C., Naccarato, A. G., Tancredi, M., et al. (2015). MSH2 role in BRCA1-driven tumorigenesis: a preliminary study in yeast and in human tumors from BRCA1-VUS carriers. Eur. J. Med. Genet. 58, 531–539. doi:10.1016/j.ejmg.2015.09.005
Matan, L. S., Perri, T., Kogan, L., Brandt, B., Meyer, R., and Levin, G. (2022). Ovarian cancer risk management in BRCA-mutation carriers: a comparison of six international and national guidelines. Eur. J. obstetrics, Gynecol. reproductive Biol. 278, 166–171. doi:10.1016/j.ejogrb.2022.09.035
Meng, S., Song, S., and Yin, R. (2024). Bibliometric and visual analysis of immune checkpoint inhibitors for ovarian cancer. Asian J. Surg. 47, 3205–3206. doi:10.1016/j.asjsur.2024.03.056
Mevissen, T. E., Hospenthal, M. K., Geurink, P. P., Elliott, P. R., Akutsu, M., Arnaudo, N., et al. (2013). OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154, 169–184. doi:10.1016/j.cell.2013.05.046
Nakae, A., Kodama, M., Okamoto, T., Tokunaga, M., Shimura, H., Hashimoto, K., et al. (2021). Ubiquitin specific peptidase 32 acts as an oncogene in epithelial ovarian cancer by deubiquitylating farnesyl-diphosphate farnesyltransferase 1. Biochem. biophysical Res. Commun. 552, 120–127. doi:10.1016/j.bbrc.2021.03.049
Okochi-Takada, E., Nakazawa, K., Wakabayashi, M., Mori, A., Ichimura, S., Yasugi, T., et al. (2006). Silencing of the UCHL1 gene in human colorectal and ovarian cancers. Int. J. cancer 119, 1338–1344. doi:10.1002/ijc.22025
Padmanabhan, A., Candelaria, N., Wong, K. K., Nikolai, B. C., Lonard, D. M., O'Malley, B. W., et al. (2018). USP15-dependent lysosomal pathway controls p53-R175H turnover in ovarian cancer cells. Nat. Commun. 9, 1270. doi:10.1038/s41467-018-03599-w
Qin, D., Wang, W., Lei, H., Luo, H., Cai, H., Tang, C., et al. (2016). CDDO-Me reveals USP7 as a novel target in ovarian cancer cells. Oncotarget 7, 77096–77109. doi:10.18632/oncotarget.12801
Sandvei, M. S., Pinborg, A., Gissler, M., Bergh, C., Romundstad, L. B., van Leeuwen, F. E., et al. (2023). Risk of ovarian cancer in women who give birth after assisted reproductive technology (ART)-a registry-based Nordic cohort study with follow-up from first pregnancy. Br. J. cancer 128, 825–832. doi:10.1038/s41416-022-02097-7
Shen, J., Hong, L., and Chen, L. (2020). Ubiquitin-specific protease 14 regulates ovarian cancer cisplatin-resistance by stabilizing BCL6 oncoprotein. Biochem. biophysical Res. Commun. 524, 683–688. doi:10.1016/j.bbrc.2020.01.150
Shen, J., Xie, M., Xu, Y., Qian, Q., Qiu, T., Shi, W., et al. (2023). Identification of the deubiquitinase USP28 as a novel molecular therapeutic target of ovarian cancer. Biochem. biophysical Res. Commun. 638, 184–191. doi:10.1016/j.bbrc.2022.11.055
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics. CA a cancer J. Clin. 73, 17–48. doi:10.3322/caac.21763
Simoneau, A., Engel, J. L., Bandi, M., Lazarides, K., Liu, S., Meier, S. R., et al. (2023). Ubiquitinated PCNA drives USP1 synthetic lethality in cancer. Mol. cancer Ther. 22, 215–226. doi:10.1158/1535-7163.mct-22-0409
Singh, H. R., and Ostwal, Y. B. (2019). Post-translational modification, phase separation, and robust gene transcription. Trends Genet. TIG 35, 89–92. doi:10.1016/j.tig.2018.11.002
Snyder, N. A., and Silva, G. M. (2021). Deubiquitinating enzymes (DUBs): regulation, homeostasis, and oxidative stress response. J. Biol. Chem. 297, 101077. doi:10.1016/j.jbc.2021.101077
Sonego, M., Pellarin, I., Costa, A., Vinciguerra, G. L. R., Coan, M., Kraut, A., et al. (2019). USP1 links platinum resistance to cancer cell dissemination by regulating Snail stability. Sci. Adv. 5, eaav3235. doi:10.1126/sciadv.aav3235
Song, B., Jiang, Y., Jiang, Y., Lin, Y., and Liu, J. (2022). ML323 suppresses the progression of ovarian cancer via regulating USP1-mediated cell cycle. Front. Genet. 13, 917481. doi:10.3389/fgene.2022.917481
Song, S., Meng, S., and Yin, R. (2024). Application of anti-angiogenic drugs in ovarian cancer from the perspective of bibliometrics. Asian J. Surg. 47, 2228–2229. doi:10.1016/j.asjsur.2024.01.110
Stiff, P. J., McKenzie, R. S., Alberts, D. S., Sosman, J. A., Dolan, J. R., Rad, N., et al. (1994). Phase I clinical and pharmacokinetic study of high-dose mitoxantrone combined with carboplatin, cyclophosphamide, and autologous bone marrow rescue: high response rate for refractory ovarian carcinoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 12, 176–183. doi:10.1200/jco.1994.12.1.176
Sung, S., Hong, Y., Kim, B. G., Choi, J. Y., Kim, J. W., Park, S. Y., et al. (2023). Stratifying the risk of ovarian cancer incidence by histologic subtypes in the Korean Epithelial Ovarian Cancer Study (Ko-EVE). Cancer Med. 12, 8742–8753. doi:10.1002/cam4.5612
Tangri, A., Lighty, K., Loganathan, J., Mesmar, F., Podicheti, R., Zhang, C., et al. (2021). Deubiquitinase UCHL1 maintains protein homeostasis through the PSMA7-APEH-proteasome Axis in high-grade serous ovarian carcinoma. Mol. cancer Res. MCR 19, 1168–1181. doi:10.1158/1541-7786.mcr-20-0883
Tavares, V., Pinto, R., Assis, J., Coelho, S., Brandão, M., Alves, S., et al. (2021). Implications of venous thromboembolism GWAS reported genetic makeup in the clinical outcome of ovarian cancer patients. pharmacogenomics J. 21, 222–232. doi:10.1038/s41397-020-00201-9
Therachiyil, L., Anand, A., Azmi, A., Bhat, A., Korashy, H. M., and Uddin, S. (2022). Role of RAS signaling in ovarian cancer. F1000Research 11, 1253. doi:10.12688/f1000research.126337.1
Tserpeli, V., Stergiopoulou, D., Londra, D., Giannopoulou, L., Buderath, P., Balgkouranidou, I., et al. (2021). Prognostic significance of SLFN11 methylation in plasma cell-free DNA in advanced high-grade serous ovarian cancer. Cancers 14, 4. doi:10.3390/cancers14010004
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi:10.1007/s11192-009-0146-3
Wada, T., Yamashita, Y., Saga, Y., Takahashi, K., Koinuma, K., Choi, Y. L., et al. (2009). Screening for genetic abnormalities involved in ovarian carcinogenesis using retroviral expression libraries. Int. J. Oncol. 35, 973–976. doi:10.3892/ijo_00000410
Wang, H., Song, Y., Liu, C., Qian, X., Zhang, D., et al. (2022b). Overexpression of Foxc1 ameliorates sepsis-associated encephalopathy by inhibiting microglial migration and neuroinflammation through the IκBα/NF-κB pathway. Mol. Med. Rep. 25, 107. doi:10.3892/mmr.2022.12623
Wang, L. (2021). A novel glycosyltransferase-related gene signature for overall survival prediction in patients with ovarian cancer. Int. J. general Med. 14, 10337–10350. doi:10.2147/ijgm.s332945
Wang, L., Chen, T., Li, X., Yan, W., Lou, Y., Liu, Z., et al. (2019b). USP39 promotes ovarian cancer malignant phenotypes and carboplatin chemoresistance. Int. J. Oncol. 55, 277–288. doi:10.3892/ijo.2019.4818
Wang, L., Chen, Y. J., Xu, K., Wang, Y. Y., and Shen, X. Z. (2014b). High expression of UCH37 is significantly associated with poor prognosis in human epithelial ovarian cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 35, 11427–11433. doi:10.1007/s13277-014-2446-3
Wang, M., Zhang, Y., Wang, T., Zhang, J., Zhou, Z., Sun, Y., et al. (2017). The USP7 inhibitor P5091 induces cell death in ovarian cancers with different P53 status. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol. 43, 1755–1766. doi:10.1159/000484062
Wang, S., Osgood, A. O., and Chatterjee, A. (2022a). Uncovering post-translational modification-associated protein-protein interactions. Curr. Opin. Struct. Biol. 74, 102352. doi:10.1016/j.sbi.2022.102352
Wang, S., Wang, Z., Li, J., Qin, J., Song, J., Li, Y., et al. (2021). Splicing factor USP39 promotes ovarian cancer malignancy through maintaining efficient splicing of oncogenic HMGA2. Cell death and Dis. 12, 294. doi:10.1038/s41419-021-03581-3
Wang, T., Zhu, T., Zhang, Y., Bai, J., Xue, Y., Xu, G., et al. (2022c). Pan-cancer analysis of the prognostic and immunological role of BRCA1-associated protein 1 gene (BAP1): friend or foe? Gene 840, 146765. doi:10.1016/j.gene.2022.146765
Wang, W., Wang, J., Yan, H., Zhang, K., and Liu, Y. (2019a). Upregulation of USP11 promotes epithelial-to-mesenchymal transition by deubiquitinating Snail in ovarian cancer. Oncol. Rep. 41, 1739–1748. doi:10.3892/or.2018.6924
Wang, W., Wei, J., Feng, D., and Ling, B. (2024). Current trends and emerging patterns in the application of nanomaterials for ovarian cancer research: a bibliometric analysis. Front. Pharmacol. 15, 1344855. doi:10.3389/fphar.2024.1344855
Wang, Y., Luo, X., Wu, N., Liao, Q., and Wang, J. (2023b). USP7 mediates TRAF4 deubiquitination to facilitate the malignant phenotype of ovarian cancer via the RSK4/PI3K/AKT axis. J. cancer Res. Ther. 19, 97–107. doi:10.4103/jcrt.jcrt_517_22
Wang, Y., Wang, J., Zhong, J., Deng, Y., Xi, Q., He, S., et al. (2015). Ubiquitin-specific protease 14 (USP14) regulates cellular proliferation and apoptosis in epithelial ovarian cancer. Med. Oncol. N. Lond. Engl. 32, 379. doi:10.1007/s12032-014-0379-8
Wang, Y., Wang, Z., Zhang, Z., Wang, H., Peng, J., and Hong, L. (2023a). Burden of ovarian cancer in China from 1990 to 2030: a systematic analysis and comparison with the global level. Front. public health 11, 1136596. doi:10.3389/fpubh.2023.1136596
Wang, Y., Zhou, X., Xu, M., Weng, W., Zhang, Q., Yang, Y., et al. (2016). OTUB1-catalyzed deubiquitination of FOXM1 facilitates tumor progression and predicts a poor prognosis in ovarian cancer. Oncotarget 7, 36681–36697. doi:10.18632/oncotarget.9160
Wang, Y.-C., Peterson, S. E., and Loring, J. F. (2014a). Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 24, 143–160. doi:10.1038/cr.2013.151
Wertz, I. E., O'Rourke, K. M., Zhou, H., Eby, M., Aravind, L., Seshagiri, S., et al. (2004). De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430, 694–699. doi:10.1038/nature02794
Wu, Q., Huang, Y., Gu, L., Chang, Z., and Li, G. M. (2021). OTUB1 stabilizes mismatch repair protein MSH2 by blocking ubiquitination. J. Biol. Chem. 296, 100466. doi:10.1016/j.jbc.2021.100466
Xu, L., Zhang, B., and Li, W. (2021). Downregulated expression levels of USP46 promote the resistance of ovarian cancer to cisplatin and are regulated by PUM2. Mol. Med. Rep. 23, 263. doi:10.3892/mmr.2021.11902
Xu, M., Takanashi, M., Oikawa, K., Tanaka, M., Nishi, H., Isaka, K., et al. (2009). USP15 plays an essential role for caspase-3 activation during Paclitaxel-induced apoptosis. Biochem. biophysical Res. Commun. 388, 366–371. doi:10.1016/j.bbrc.2009.08.015
Yan, C., Yuan, J., Xu, J., Zhang, G., Li, X., Zhang, B., et al. (2019). Ubiquitin-specific peptidase 39 regulates the process of proliferation and migration of human ovarian cancer via p53/p21 pathway and EMT. Med. Oncol. N. Lond. Engl. 36, 95. doi:10.1007/s12032-019-1308-7
Yan, Y., Xu, Z., Huang, J., Guo, G., Gao, M., Kim, W., et al. (2020). The deubiquitinase USP36 Regulates DNA replication stress and confers therapeutic resistance through PrimPol stabilization. Nucleic acids Res. 48, 12711–12726. doi:10.1093/nar/gkaa1090
Yang, Y., Hou, J. q., Qu, L. y., Wang, G. q., Ju, H. w., Zhao, Z. w., et al. (2007). Differential expression of USP2, USP14 and UBE4A between ovarian serous cystadenocarcinoma and adjacent normal tissues. Xi bao yu fen zi mian yi xue za zhi = Chin. J. Cell. Mol. Immunol. 23, 504–506.
Yildirim, N., Kocal, G. C., Isik, Z., Saatli, B., Saygili, U., Uysal, T., et al. (2019). Ubiquitin-Proteasome Axis, especially ubiquitin-specific protease-17 (USP17) gene family, is a potential target for epithelial-mesenchymal transition in high-grade serous ovarian cancer. Reprod. Sci. (Thousand Oaks, Calif.) 26, 794–805. doi:10.1177/1933719118799189
Zhang, J., Chen, Y., Chen, X., Zhang, W., Zhao, L., Weng, L., et al. (2021). Deubiquitinase USP35 restrains STING-mediated interferon signaling in ovarian cancer. Cell death Differ. 28, 139–155. doi:10.1038/s41418-020-0588-y
Zhang, L., Wang, H., Tian, L., and Li, H. (2016). Expression of USP7 and MARCH7 is correlated with poor prognosis in epithelial ovarian cancer. Tohoku J. Exp. Med. 239, 165–175. doi:10.1620/tjem.239.165
Zhang, M. H., Zhang, H. H., Du, X. H., Gao, J., Li, C., Shi, H. R., et al. (2020). UCHL3 promotes ovarian cancer progression by stabilizing TRAF2 to activate the NF-κB pathway. Oncogene 39, 322–333. doi:10.1038/s41388-019-0987-z
Zhang, S., Zhang, M., Jing, Y., Yin, X., Ma, P., Zhang, Z., et al. (2018). Deubiquitinase USP13 dictates MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat. Commun. 9, 215. doi:10.1038/s41467-017-02693-9
Zhao, L., Tang, Y., Yang, J., Lin, F., Liu, X., Zhang, Y., et al. (2023). Integrative analysis of circadian clock with prognostic and immunological biomarker identification in ovarian cancer. Front. Mol. Biosci. 10, 1208132. doi:10.3389/fmolb.2023.1208132
Zhu, X., Zhang, Y., Luo, Q., Wu, X., Huang, F., Shu, T., et al. (2021). The deubiquitinase USP11 promotes ovarian cancer chemoresistance by stabilizing BIP. Signal Transduct. Target. Ther. 6, 264. doi:10.1038/s41392-021-00580-w
Keywords: ovarian cancer, deubiquitinating enzyme, bibliometric analysis, biologic role, systematic review
Citation: Qiu F, Li Y, Zhou L, Wu Y, Wu Y, Fan Z, Wang Y, Qin D and Li C (2024) Mapping and visualization of global research progress on deubiquitinases in ovarian cancer: a bibliometric analysis. Front. Pharmacol. 15:1445037. doi: 10.3389/fphar.2024.1445037
Received: 06 June 2024; Accepted: 27 August 2024;
Published: 12 September 2024.
Edited by:
Zhi-Bin Wang, Central South University, ChinaReviewed by:
Adán Pinto Fernández, University of Oxford, United KingdomYanfeng Wang, Beijing Institute of Technology, China
Copyright © 2024 Qiu, Li, Zhou, Wu, Wu, Fan, Wang, Qin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoqun Li, MTUxMTE1MjAwMTVAZnVkYW4uZWR1LmNu; Dongjun Qin, cWluZG9uZ2p1bjE0QDE2My5jb20=
†These authors have contributed equally to this work
 Fang Qiu
Fang Qiu Yuntong Li2†
Yuntong Li2† Yingli Wu
Yingli Wu Zhilei Fan
Zhilei Fan Chaoqun Li
Chaoqun Li