- 1School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 2Department of Medical Oncology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 3Department for Infectious Disease Control and Prevention, Jinan Municipal Center for Disease Control and Prevention, Jinan, Shandong, China
- 4Department of Medical Oncology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, Shandong, China
- 5Department of Pharmacy, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
- 6Clinical Trial Center, NMPA Key Laboratory for Clinical Research and Evaluation of Innovative Drugs, Shandong University, Jinan, Shandong, China
Background: Aumolertinib demonstrated superior progression-free survival (PFS) and a well-tolerated toxicity profile compared to gefitinib in front-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) in the AENEAS trial. However, patient-reported outcomes (PROs) of aumolertinib have not been published.
Methods: In this real-world study, the efficacy was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0. PROs were evaluated using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (QLQ-C30) and the EORTC Quality of Life lung cancer-specific module (QLQ-LC13) in advanced NSCLC patients receiving aumolertinib as initial therapy. Pre-specified key symptoms were cough, hemoptysis, dyspnea, sore mouth or tongue, dysphagia, hair loss, tingling in hands or feet, chest pain, arm or shoulder pain, and pain at other sites.
Results: A total of 33 patients were included, 23 of whom had efficacy information up to January 2024. The median follow-up time was 264 days (interval: 36–491 days). The objective response rate and disease control rate were 65.2% and 91.3%, respectively. The EORTC QLQ-LC30 general health status scale showed that functional scales increased and symptom scales decreased during aumolertinib treatment. Symptom scales assessed by the EORTC QLQ-LC13 showed that improvements in cough, sore mouth or tongue, tingling in hands or feet, chest pain, arm or shoulder pain, and other pain sites were both clinically and statistically significant after 6 months of aumolertinib treatment (p < 0.05).
Conclusion: In this real-world study, aumolertinib showed comparable disease control and objective response rates as reported in the AENEAS trial for advanced NSCLC patients with EGFR-sensitizing mutations. Aumolertinib treatment improved PROs, further supporting it in first-line clinical practice.
1 Introduction
Worldwide, lung cancer ranks first in cancer-related deaths, of which non-small cell lung cancer (NSCLC) accounts for approximately 85% (Travis et al., 2015). The discovery of an epidermal growth factor receptor (EGFR)-sensitive mutation and the development of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) pioneered targeted therapy for NSCLC. EGFR-TKIs, including the first, second, and third-generation drugs, significantly prolonged progression-free survival (PFS) and overall survival (OS) for advanced NSCLC patients with sensitive EGFR mutations as first-line treatment (Zhou et al., 2011; Maemondo et al., 2010; Fukuoka et al., 2011; Wu et al., 2014; Yang et al., 2015; Wu et al., 2017; Mok et al., 2017).
Advanced NSCLC is characterized by a high symptom burden (Iyer et al., 2014). At least 90% of patients experience fatigue, appetite loss, dyspnea, and pain, which significantly negatively impact disease-specific health-related quality of life (HRQoL) (Iyer et al., 2013; Polanski et al., 2016). Knowledge of the effects of new therapies on patient experiences, when combined with survival data, can provide crucial information to assist physicians and patients in making informed treatment decisions (Bottomley et al., 2005; Fallowfield and Fleissig, 2011). Compared with chemotherapy, the first-generation EGFR-TKIs, including gefitinib and erlotinib, significantly improved symptom control and HRQoL. Afatinib, a representative of the second generation EGFR-TKIs, exhibited similar results (Chen et al., 2013; Geater et al., 2015; Oizumi et al., 2012). In the ARCHER 1050 trial, dacomitinib, when used as first-line treatment for NSCLC, demonstrated superior survival compared to gefitinib. However, global HRQoL improvements were observed only with gefitinib (Wu et al., 2017). Osimertinib, a third-generation EGFR-TKI, demonstrated superior survival outcomes compared to first-generation EGFR-TKIs (Soria et al., 2018). Investigators sought to determine whether osimertinib provided better patient-reported outcomes (PROs) in addition to its longer survival benefits. However, PRO results from the FLAURA trial revealed that key symptoms improved significantly and were clinically relevant in both the osimertinib and erlotinib/gefitinib arms (Leighl et al., 2020). These findings suggest that the efficacy data reported by investigators may not fully align with PROs. It is, therefore, recommended that PROs and HRQoL be assessed in all prospective clinical comparative effectiveness research studies (Bottomley et al., 2005).
Aumolertinib (HS-10296) is a novel, irreversible, third-generation EGFR-TKI targeting both EGFR-sensitizing and T790M mutations while sparing wild-type EGFR. In the APOLLO registrational trial, patients with EGFR T790M-positive advanced NSCLC after disease progression on a first- or second-generation EGFR-TKI achieved a median PFS of 12.4 months, and the toxicity profile was tolerable (Lu et al., 2022a). ANEAS, a randomized, double-blind, phase-III trial, evaluated the efficacy and safety of aumolertinib compared with gefitinib as a first-line treatment of locally advanced or metastatic EGFR-mutated NSCLC. Aumolertinib achieved better survival than gefitinib, with a median PFS of 19.3 months versus 9.9 months (hazard ratio, 0.46; 95% CI, 0.36 to 0.60; p < 0.0001) (Lu et al., 2022b). Based on these results, aumolertinib was approved in China to treat advanced NSCLC with EGFR-sensitizing and T790M mutations.
Several studies also demonstrated the efficacy and safety profile of aumolertinib in real-world settings (Zhang et al., 2024; Ding et al., 2022; Zhang et al., 2022). However, all of these studies were retrospective, and some only reported individual cases. Importantly, PRO changes during aumolertinib treatment have not been reported. We prospectively collected European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) and EORTC Quality of Life lung cancer-specific module (QLQ-LC13) information and efficacy data. This showed that aumolertinib treatment significantly improved HRQoL.
2 Methods
2.1 Patients and study design
This prospective study was conducted between September 2022 and January 2024. Eligible patients were aged 18 years or older, with histologically/cytologically confirmed locally advanced or metastatic NSCLC, carrying an EGFR mutation, and having not received previous systemic anticancer therapy. The exclusion criteria were (i) previous receipt of any systemic therapy; (ii) concurrent presence of other malignancies requiring active treatment; and (iii) any other condition that, in the investigator’s judgment, rendered the patient unsuitable for participation in this study. Enrolled patients received oral aumolertinib 110 mg once daily until disease progression, intolerable toxicity, or a request to discontinue by the patient or physician.
The treatment response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 based on computed tomography (CT) imaging. HRQoL was assessed with the use of the self-administered cancer-specific European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire C30 (QLQ-C30) and its lung cancer-specific module, the QLQ-LC13. Patients were assessed monthly for the first half of the year from the start of treatment and then every 3 months until the 18th month. The primary endpoint was HRQoL. Secondary endpoints included objective tumor response (ORR) and disease control rate (DCR).
This study complied with the Ethical Guidelines for Medical and Health Research Involving Human Subjects. The study protocol received approval from the Ethical Review Boards and Institutional Review Boards of Qilu Hospital of Shandong University (KYLL-202308-041). All patients provided written, informed consent.
2.2 Assessment of tumor response and effectiveness
Tumor response was determined according to RECIST1.0 and was assessed every 2 months until disease progression. The objective response rate (ORR) was defined as the percentage of patients with a tumor-confirmed overall response of complete response (CR) or partial response (PR) in the total number of patients analyzed. The disease control rate (DCR) was defined as the percentage of patients with a tumor-confirmed overall response of complete CR, PR, or stable disease (SD). Progression-free survival (PFS) was followed up until the date of the first tumor progression or death for any reason, whichever occurred first. Overall survival (OS) was followed up until death for any reason.
2.3 EORTC QLQ-C30 and EORTC QLQ-LC13
The EORTC QLQ-C30 included five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, nausea, and vomiting), and the general health status scale. Multiple individual items on other common symptoms of cancer (dyspnea, loss of appetite, insomnia, constipation, and diarrhea) were also assessed, as were individual items measuring the economic impact of the disease. The majority of items were reported on verbal response scales of 1–4 with response options of “not at all,” “a little bit,” “quite a bit”, and “very much,” while the two general health status items were reported on numeric response scales of 1–7 with endings of “very poor” and “excellent”.
The EORTC QLQ-LC13 consists of 13 questions on a multi-item scale, including questions measuring lung-cancer-related symptoms (coughing, hemoptysis, and dyspnea) and treatment-related adverse effects (sore mouth or tongue, dysphagia, hair loss, tingling in the hands or feet, chest pain, arm or shoulder pain, other pain, and the usefulness of pain medication). The QLQ-LC 13 item uses the same 1–4 verbal response scale as the QLQ-C30 item.
For each scale or item, a linear transformation was applied to normalize the raw score to 0–100. Higher scores on the functional and general health status scales represented better health status, while the opposite was true for the symptom scales. Any score change of 10 points from baseline was considered to be clinically meaningful. Improvement in health status was defined as an increase of ≥10 points from baseline in functional scale scores and a decrease of ≥10 points in symptom scales/items. Deterioration was defined as a decrease of ≥10 points in functional scales and an increase of ≥10 points in symptom scales/items. Otherwise, they were considered stable.
2.4 Statistical analyses
Descriptive statistical analysis of demographic information and clinical characteristics was performed, and chi-square testing was used to verify whether the distribution of the parameters conformed to a normal distribution. Differences between groups were assessed by ANOVA and Kruskal–Wallis’s test. The Kaplan–Meier method was used to estimate the median PFS and OS with 95% confidence intervals (CIs). The questionnaire scales/items were scored according to the EORTC-published algorithm. Mean QLQ-C30 or QLQ-LC 13 scales or individual item scores and criteria were calculated at all time points to characterize patient efficacy after amitriptyline treatment (a 10-point difference between the score at each time point; the first month’s score was considered clinically significant). Statistical analysis and plotting were performed using SPSS version 27 (IBM, Chicago, IL, United States) and GraphPad Prism version 9.5.1 (San Diego, California, United States).
3 Results
3.1 Patient characteristics
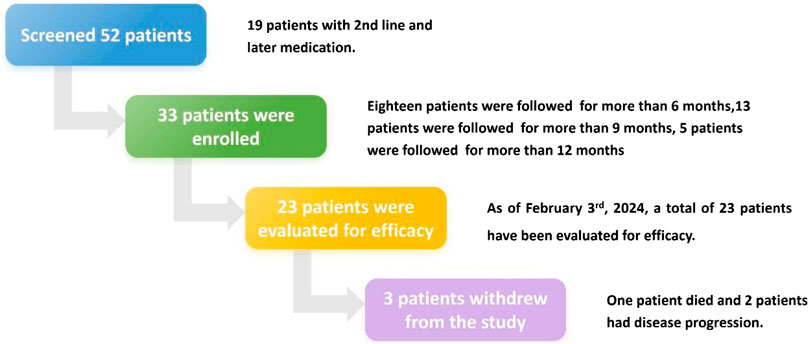
A total of 52 patients diagnosed with EGFR-mutated (Exon 19 deletion or Exon 21 L858R) locally advanced or metastatic NSCLC and receiving aumolertinib treatment were successively screened from September 2022 (Figure 1). Of the 52 patients screened, 19 received aumolertinib as second- or later-line treatment; finally, 33 patients were enrolled. During the course of the study, three cases dropped out. Of these, one patient passed away after 10 months of medication (it is unclear whether the death was related to the illness), and two withdrew from the study due to disease progression and switched to alternative treatments.
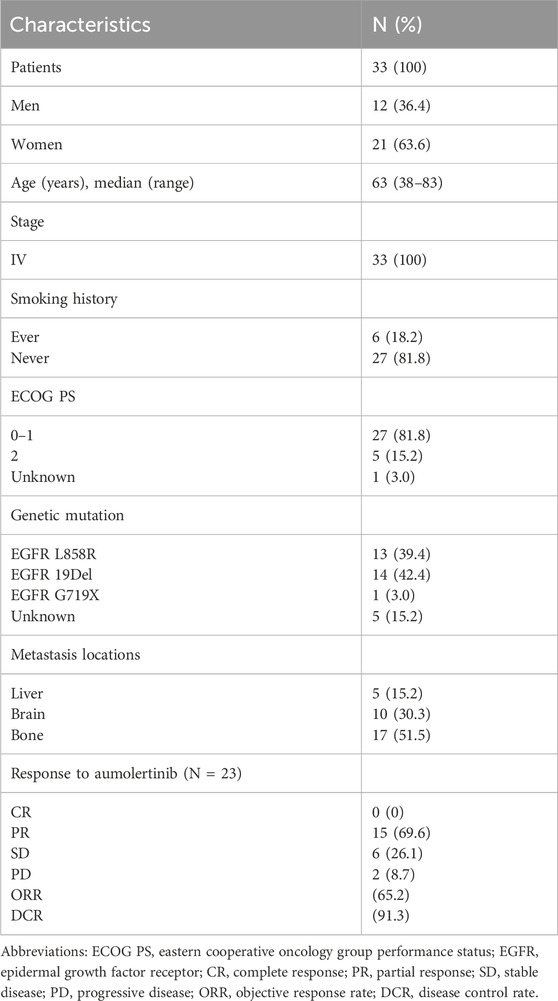
Patient demographics and clinicopathological characteristics are presented in Table 1. The median age at diagnosis was 63 years (range: 38–83). The cohort included 21 female patients (63.6%) and 12 males (36.4%). Among the patients, six (18.2%) were former smokers, while 27 (81.8%) had never smoked. The majority of patients (81.8%) had an ECOG-PS of 0 or 1. The proportions of EGFR L858R and EGFR 19DEL mutations were 42.4% and 39.4%, respectively. Liver metastasis, brain metastasis, and bone metastasis were observed in 15.2%, 30.3%, and 51.5% of patients, respectively.
3.2 Efficacy evaluation and safety profile
Before February 2024, 23 patients could be evaluated for treatment efficacy. The median follow-up time was 264 days (interval: 36–491 days). ORR and DCR were 65.2% and 91.3%, respectively. There was no significant difference in ORR between the major subgroups (Supplementary Table 1). In detail, 15 patients (65.2%) achieved PR, six (26.1%) achieved SD, and two patients (8.7%) experienced PD (Table 1). Due to the short follow-up period, median OS and PFS have not yet been reached (Supplementary Figure 1). The rate of treatment-related adverse events (TRAEs) and grade 3 or larger TRAEs was 87.9% and 12.1%, respectively. Detailed information is summarized in Supplementary Table 2.
3.3 PROs
Patients with at least one quality-of-life questionnaire were included in this analysis, and 54.5% of patients completed the first half-year follow-up. Patients reported a tendency toward higher functional scale scores, indicating good physical, role, emotional, cognitive, and social functioning after aumolertinib treatment (Figure 2). The overall quality of life score increased from 67.17 at baseline to 70.37 at the 6-month follow-up and 75.0 at the 12-month follow-up (Figure 2). The mean scores of the symptom scales and items also showed decreasing trends (Figure 3), indicating that aumolertinib treatment controlled symptoms. The mean scores of the first-month symptom scores in the aumolertinib arm were 21.21 for fatigue, 21.72 for pain, 7.07 for nausea and vomiting, 30.3 for dyspnea, 20.2 for insomnia, 12.12 for appetite loss, 9.09 for constipation, 6.06 for diarrhea, and 31.31 for financial difficulties (Figure 3). Compared to baseline scores, aumolertinib showed a clinically meaningful improvement in mean scores for pain and dyspnea after 6 months of treatment (Figure 3). However, only a decrease in pain scores was statistically significantly meaningful (Figure 4). QLQ-LC13 showed that lung-cancer-related symptoms also improved a lot, of which coughing, sore mouth or tongue, tingling in the hands or feet, chest pain, arm or shoulder pain, and other pain improvements were clinically meaningful at 6 months (Figure 5). Coughing, sore mouth or tongue, chest pain, arm or shoulder pain, and other pain improvements were statistically significantly meaningful (Figure 6).
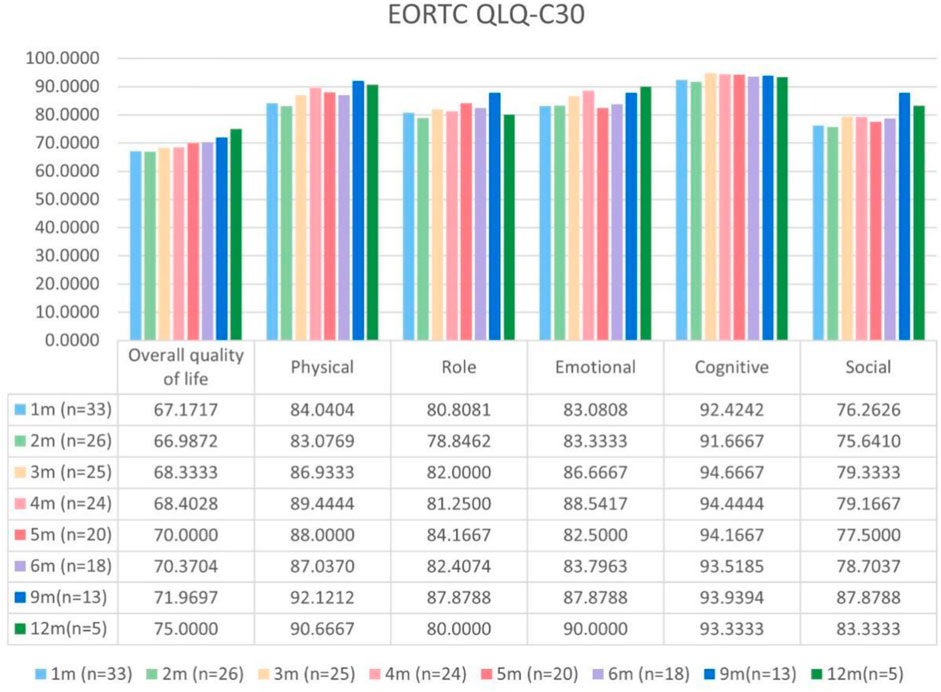
Figure 2. Mean scores of the general health status scale and functional scales from the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) with various time points.
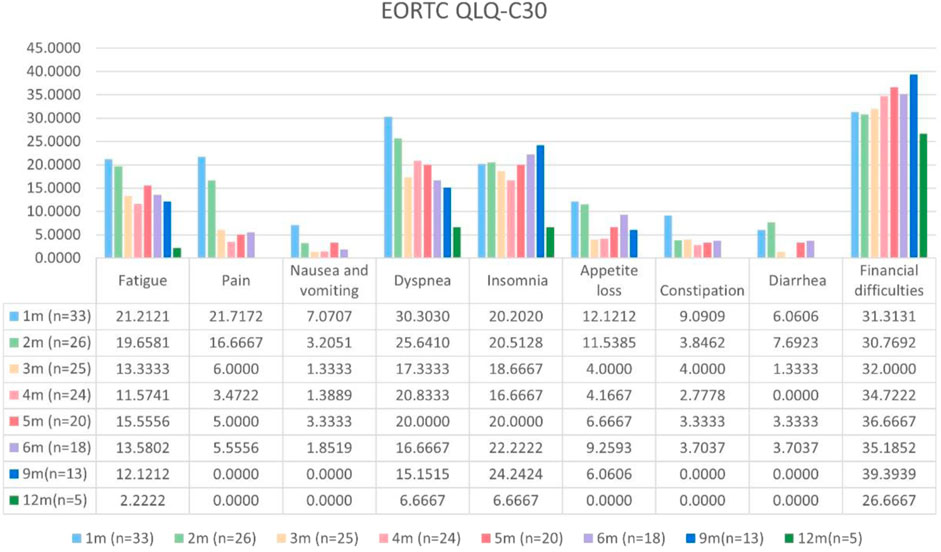
Figure 3. Mean scores of the symptom scales and items from the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) with various time points.
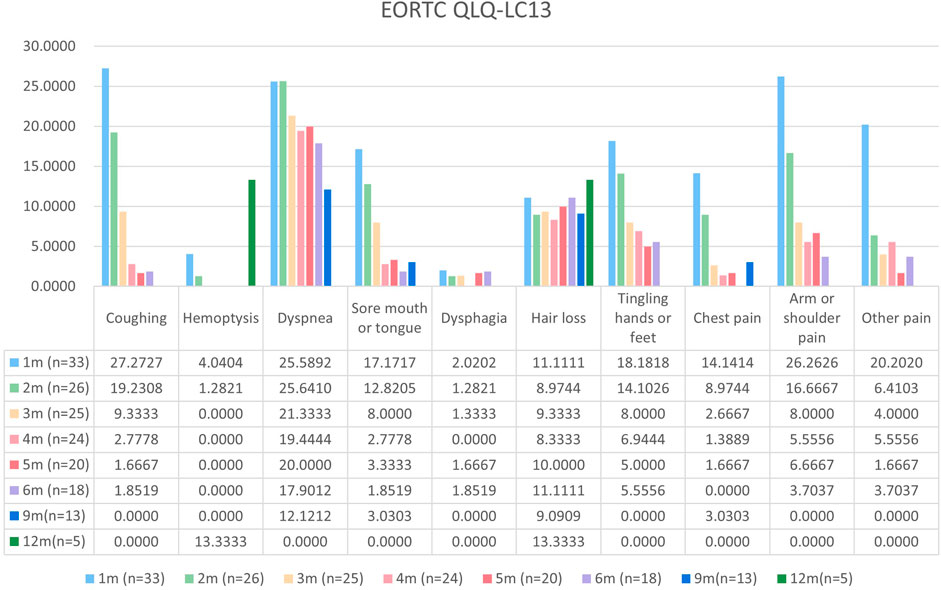
Figure 4. Mean scores of the symptom scales and items from the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life lung cancer-specific module QLQ-LC13 with various time points.
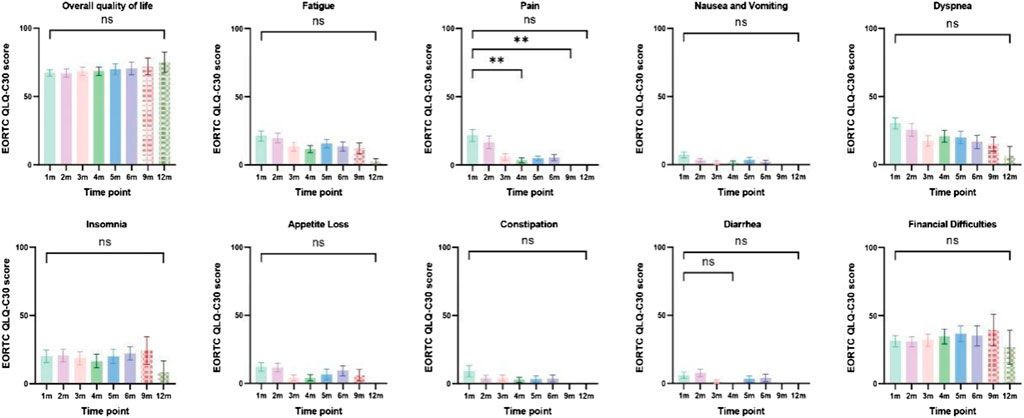
Figure 5. Column charts of general health status, three symptom scales, and multiple individual items. Statistical information: ns: non-significant, p > 0.05; "**", p < 0.01.
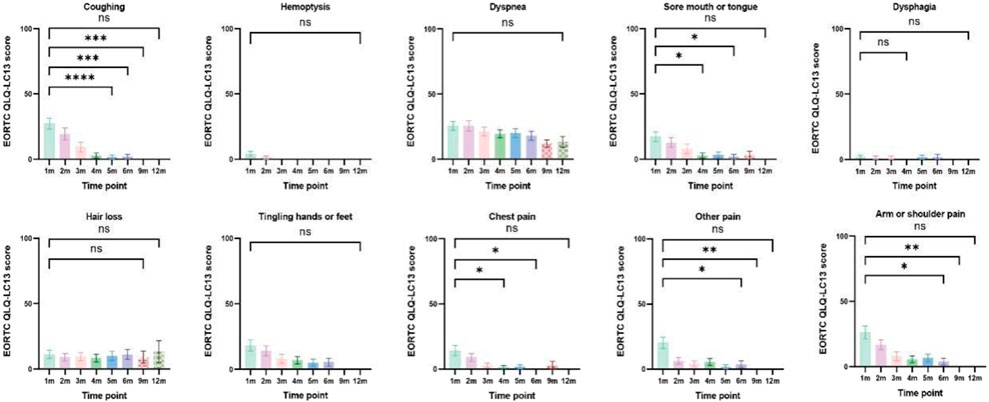
Figure 6. Column charts of lung-cancer-related symptoms and treatment-related adverse effects. Statistical information: ns: non-significant, p > 0.05; "*", p < 0.05; "**", p < 0.01; "***", p < 0.001; "****", and p < 0.0001.
4 Discussion
In the AENEAS trial, first-line treatment with aumolertinib demonstrated superior efficacy to gefitinib in advanced NSCLC patients with an activating EGFR mutation (Lu et al., 2022b). However, PROs have not been reported until now. In this real-world study, we evaluated the efficacy of aumolertinib with a particular focus on PROs. Our findings were consistent with those of the results reported in the AENEAS trial; importantly, we observed improvements in key lung cancer symptoms from baseline. Improvements in symptoms such as cough, sore mouth or tongue, tingling in the hands or feet, chest pain, arm or shoulder pain, and other types of pain were both clinically and statistically significant.
In the management of advanced NSCLC patients, incremental gains in PFS or OS are thought of as clinically meaningful only if they are achieved without a marked negative effect on HRQoL (Yang et al., 2015). Therefore, it is of great significance to record PROs in trials and real-world studies. The AENEAS trial showed a significant improvement in PFS of aumolertinib compared with gefitinib (19.3 months vs. 9.9 months) and a similar ORR of aumolertinib compared with gefitinib (73.8% vs. 72.1%) (Lu et al., 2022b). In this real-world study, the ORR of aumolertinib was 65.2%, which is comparable to that reported in clinical trials. The median PFS and OS were not reached because of a relatively shorter follow-up, and we will continue to track them. The similar short-term efficacy and demographics in our study support the following PRO analysis and may also reflect the results in AENEAS.
The EORTC QLQ-LC13 and QLQ-C30 questionnaires are well-established and are widely used in advanced NSCLC treatment trials (Geater et al., 2015; Bezjak et al., 2006; Blackhall et al., 2014; Brahmer et al., 2017; Yang et al., 2013), and have been thoroughly validated (Bergman et al., 1994; Aaronson et al., 1993; Sprangers et al., 1996). In this prospective and real-world study, questionnaire completion rates were high, with 54.5% of patients completing it during the first half year of aumolertinib treatment. Patients receiving first-line EGFR-TKI treatment usually have good performance status and low symptom burden, resulting in low symptom scores at baseline and difficulty in improvement measures. In practice, a score change equal to or greater than 10 points on the EORTC QLQ-LC13 and QLQ-C30 questionnaires is commonly deemed clinically significant (Fiteni et al., 2016). However, it has been shown that a lower, 5-point cut-off could also be clinically relevant. When the 5-point cut-off was administered here, fatigue, nausea and vomiting, dyspnea, appetite loss, and constipation score improvement at 6 months were clinically relevant.
There were several limitations in this real-world study. Firstly, it was a single-center study and the sample size was relatively small. With the development of EGFR-TKIs, advanced NSCLC patients with sensitive EGFR mutations have many choices, including gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, furmonertinib, and befotertinib, which restricts the number of people receiving a specific drug. Secondly, the mPFS and mOS were not reached, and these patients are still in follow-up.
In conclusion, the PRO results from this real-world study showed improvements from baseline in key lung cancer symptoms in advanced NSCLC patients receiving aumolertinib as first-line therapy. Further follow-up of survival and symptom scores is ongoing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethical Review Boards and Institutional Review Boards of Qilu Hospital of Shandong University. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: data curation, formal analysis, investigation, methodology, software, and writing–original draft. WZ: data curation, formal analysis, funding acquisition, investigation, methodology, software, and writing–original draft. CC: data curation, resources, validation, and writing–original draft. TX: formal analysis, methodology, validation, and writing–original draft. CW: data curation, formal analysis, investigation, and writing–original draft. RZ: data curation, formal analysis, investigation, and writing–original draft. ChY: data curation, formal analysis, investigation, and writing–original draft. JW: formal analysis, methodology, validation, and writing–original draft. CuY: formal analysis, methodology, validation, and writing–original draft. XW: methodology, resources, validation, and writing–original draft. SY: conceptualization, project administration, resources, supervision, and writing–review and editing. JL: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82303749), Hui Lan Public Welfare Foundation Project (HLZY-20231128001), and Beijing Science and Technology Innovation Medical Development Foundation (KC2023-JX-0186-PZ091).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1444707/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Kaplan–Meier survival curves of (A) overall survival in all evaluated patients and (B) progression-free survival in all evaluated patients.
SUPPLEMENTARY FIGURE S2 | Subgroup analysis of significantly changed factors in EORTC QLQ-C30 and EORTC QLQ-LC13.
SUPPLEMENTARY TABLE S1 | Subgroup analysis of ORR.
SUPPLEMENTARY TABLE S2 | Summary of safety profiles in all patients receiving aumolertinib.
References
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., et al. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 85 (5), 365–376. doi:10.1093/jnci/85.5.365
Bergman, B., Aaronson, N. K., Ahmedzai, S., Kaasa, S., and Sullivan, M. (1994). The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC study group on quality of life. Eur. J. Cancer 30a (5), 635–642. doi:10.1016/0959-8049(94)90535-5
Bezjak, A., Tu, D., Seymour, L., Clark, G., Trajkovic, A., Zukin, M., et al. (2006). Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J. Clin. Oncol. 24 (24), 3831–3837. doi:10.1200/JCO.2006.05.8073
Blackhall, F., Kim, D. W., Besse, B., Nokihara, H., Han, J. Y., Wilner, K. D., et al. (2014). Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J. Thorac. Oncol. 9 (11), 1625–1633. doi:10.1097/JTO.0000000000000318
Bottomley, A., Flechtner, H., Efficace, F., Vanvoorden, V., Coens, C., Therasse, P., et al. (2005). Health related quality of life outcomes in cancer clinical trials. Eur. J. Cancer 41 (12), 1697–1709. doi:10.1016/j.ejca.2005.05.007
Brahmer, J. R., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2017). Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 18 (12), 1600–1609. doi:10.1016/S1470-2045(17)30690-3
Chen, H. J., Yan, H. H., Yang, J. J., Chen, Z. H., Su, J., Zhang, X. C., et al. (2013). Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol. Oncol. Res. 19 (4), 833–838. doi:10.1007/s12253-013-9651-z
Ding, X., Ding, J., Leng, Z., and Song, Y. (2022). Aumolertinib challenge as an optional treatment in advanced non small-cell lung cancer after osimertinib failure with epidermal growth factor receptor-sensitive mutation: a case series. Oncol. Lett. 24 (5), 400. doi:10.3892/ol.2022.13520
Fallowfield, L. J., and Fleissig, A. (2011). The value of progression-free survival to patients with advanced-stage cancer. Nat. Rev. Clin. Oncol. 9 (1), 41–47. doi:10.1038/nrclinonc.2011.156
Fiteni, F., Anota, A., Westeel, V., and Bonnetain, F. (2016). Methodology of health-related quality of life analysis in phase III advanced non-small-cell lung cancer clinical trials: a critical review. BMC Cancer 16, 122. doi:10.1186/s12885-016-2152-1
Fukuoka, M., Wu, Y. L., Thongprasert, S., Sunpaweravong, P., Leong, S. S., Sriuranpong, V., et al. (2011). Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29 (21), 2866–2874. doi:10.1200/JCO.2010.33.4235
Geater, S. L., Xu, C. R., Zhou, C., Hu, C. P., Feng, J., Lu, S., et al. (2015). Symptom and quality of life improvement in LUX-lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J. Thorac. Oncol. 10 (6), 883–889. doi:10.1097/JTO.0000000000000517
Iyer, S., Roughley, A., Rider, A., and Taylor-Stokes, G. (2014). The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 22 (1), 181–187. doi:10.1007/s00520-013-1959-4
Iyer, S., Taylor-Stokes, G., and Roughley, A. (2013). Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 81 (2), 288–293. doi:10.1016/j.lungcan.2013.03.008
Leighl, N. B., Karaseva, N., Nakagawa, K., Cho, B. C., Gray, J. E., Hovey, T., et al. (2020). Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur. J. Cancer 125, 49–57. doi:10.1016/j.ejca.2019.11.006
Lu, S., Dong, X., Jian, H., Chen, J., Chen, G., Sun, Y., et al. (2022b). AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or MetastaticNon-small-cell lung cancer with EGFR Exon 19 deletion or L858R mutations. J. Clin. Oncol. 40 (27), 3162–3171. doi:10.1200/JCO.21.02641
Lu, S., Wang, Q., Zhang, G., Dong, X., Yang, C. T., Song, Y., et al. (2022a). Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M+ NSCLC: updated post-national medical products administration approval results from the APOLLO registrational trial. J. Thorac. Oncol. 17 (3), 411–422. doi:10.1016/j.jtho.2021.10.024
Maemondo, M., Inoue, A., Kobayashi, K., Sugawara, S., Oizumi, S., Isobe, H., et al. (2010). Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362 (25), 2380–2388. doi:10.1056/NEJMoa0909530
Mok, T. S., Wu, Y. L., Ahn, M. J., Garassino, M. C., Kim, H. R., Ramalingam, S. S., et al. (2017). Osimertinib or platinum-pemetrexed in EGFR t790m-positive lung cancer. N. Engl. J. Med. 376 (7), 629–640. doi:10.1056/NEJMoa1612674
Oizumi, S., Kobayashi, K., Inoue, A., Maemondo, M., Sugawara, S., Yoshizawa, H., et al. (2012). Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 17 (6), 863–870. doi:10.1634/theoncologist.2011-0426
Polanski, J., Jankowska-Polanska, B., Rosinczuk, J., Chabowski, M., and Szymanska-Chabowska, A. (2016). Quality of life of patients with lung cancer. Onco Targets Ther. 9, 1023–1028. doi:10.2147/OTT.S100685
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378 (2), 113–125. doi:10.1056/NEJMoa1713137
Sprangers, M. A., Groenvold, M., Arraras, J. I., Franklin, J., te Velde, A., Muller, M., et al. (1996). The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J. Clin. Oncol. 14 (10), 2756–2768. doi:10.1200/JCO.1996.14.10.2756
Travis, W. D., Brambilla, E., Nicholson, A. G., Yatabe, Y., Austin, J. H. M., Beasley, M. B., et al. (2015). The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 10 (9), 1243–1260. doi:10.1097/JTO.0000000000000630
Wu, Y. L., Cheng, Y., Zhou, X., Lee, K. H., Nakagawa, K., Niho, S., et al. (2017). Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 18 (11), 1454–1466. doi:10.1016/S1470-2045(17)30608-3
Wu, Y. L., Zhou, C., Hu, C. P., Feng, J., Lu, S., Huang, Y., et al. (2014). Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15 (2), 213–222. doi:10.1016/S1470-2045(13)70604-1
Yang, J. C., Hirsh, V., Schuler, M., Yamamoto, N., O'Byrne, K. J., Mok, T. S. K., et al. (2013). Symptom control and quality of life in LUX-Lung 3: a phase III study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31 (27), 3342–3350. doi:10.1200/JCO.2012.46.1764
Yang, J. C., Wu, Y. L., Schuler, M., Sebastian, M., Popat, S., Yamamoto, N., et al. (2015). Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 16 (2), 141–151. doi:10.1016/S1470-2045(14)71173-8
Zhang, Q., Liu, H., and Yang, J. (2022). Aumolertinib effectively reduces clinical symptoms of an EGFR l858r-mutant non-small cell lung cancer case coupled with osimertinib-induced cardiotoxicity: case report and review. Front. Endocrinol. (Lausanne) 13, 833929. doi:10.3389/fendo.2022.833929
Zhang, X., Zhang, M., Du, X., Zhang, G., Niu, Y., Wei, C., et al. (2024). Clinical efficacy and safety analysis of aumolertinib in real-world treatment of EGFR-mutated advanced non-small-cell lung cancer. Front. Pharmacol. 15, 1331138. doi:10.3389/fphar.2024.1331138
Zhou, C., Wu, Y. L., Chen, G., Feng, J., Liu, X. Q., Wang, C., et al. (2011). Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12 (8), 735–742. doi:10.1016/S1470-2045(11)70184-X
Keywords: non-small cell lung cancer, epidermal growth factor receptor, aumolertinib, patient-reported outcomes, efficacy
Citation: Li H, Zhao W, Chang C, Xuan T, Wang C, Zhang R, Yang C, Wang J, Yi C, Wang X, Yu S and Li J (2024) Efficacy and patient-reported outcomes in advanced non-small cell lung cancer patients receiving aumolertinib as first-line therapy: a real-world study. Front. Pharmacol. 15:1444707. doi: 10.3389/fphar.2024.1444707
Received: 06 June 2024; Accepted: 12 August 2024;
Published: 06 September 2024.
Edited by:
Qinglin Shen, Jiangxi Provincial People’s Hospital, ChinaReviewed by:
Jincheng Song, The Second Affiliated Hospital of Dalian Medical University, ChinaHaiyong Wang, Shandong Cancer Hospital, Shandong University, China
Copyright © 2024 Li, Zhao, Chang, Xuan, Wang, Zhang, Yang, Wang, Yi, Wang, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jisheng Li, bGlqaXNoZW5nQHNkdS5lZHUuY24=; Shuwen Yu, eXVzaHV3ZW5Ac2R1LmVkdS5jbg==
†These authors have contributed equally to this work
 Hongxin Li
Hongxin Li Wen Zhao
Wen Zhao Caiyun Chang3
Caiyun Chang3 Tiantian Xuan
Tiantian Xuan Chengjun Wang
Chengjun Wang Rongyu Zhang
Rongyu Zhang Jisheng Li
Jisheng Li