Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect
- 1Endocannabinoid Research Group, Institute of Biomolecular Chemistry, CNR, Napoli, Italy
- 2Epitech Group SpA, Padova, Italy
- 3Department of Chemical, Biological, Pharmaceutical and Environmental Science University of Messina, Messina, Italy
- 4Innovative Statistical Research SRL, Padova, Italy
A Corrigendum on
Oral ultramicronized palmitoylethanolamide: plasma and tissue levels and spinal antihyperalgesic effect
by Petrosino S, Cordaro M, Verde R, Schiano Moriello A, Marcolongo G, Schievano C, Siracusa R, Piscitelli F, Peritore AF, Crupi R, Impellizzeri D, Esposito E, Cuzzocrea S and Di Marzo V (2018). Front. Pharmacol. 9:249. doi: 10.3389/fphar.2018.00249
In the published article, there was a problem in Figure 9B as published. As a result, we have provided another representative image from our database, belonging to the antibody in question. The corrected Figure 9 and its caption appear below.
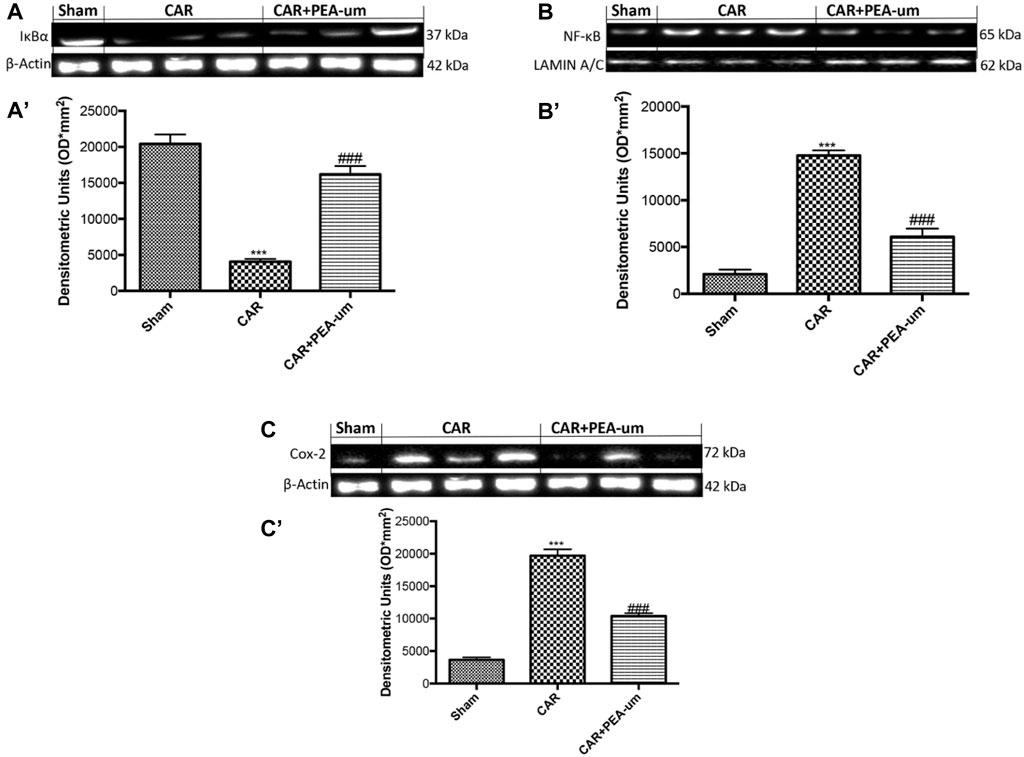
Figure 9. Effect of oral PEA-um on COX-2 expression, IκB-α degradation and NF-κB p65 nuclear translocation in rat paw tissue. The levels of IκB-α decreased in paw tissue homogenates from CAR-injected rats, as compared to sham-treated rats (A,A′). Oral treatment with PEA-um (10 mg/kg) significantly recovered IκB-α levels (A,A′). NF-κB p-65 translocation significantly increased in paw tissue homogenates from CAR-injected rats, as compared to sham-treated rats (B,B′), and PEA-um significantly decreased this effect. CAR-injected rats also displayed increased COX-2 expression as compared to sham-treated rats (C,C′) which was significantly limited by oral PEA-um treatment. A representative blot of lysates obtained from five animals for each group is shown, and densitometric analysis of all animals is reported. Values are means ± SEM of five animals for each group. ###p < 0.001 vs. CAR; ***p < 0.001 vs. sham.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: absorption, hyperalgesia, inflammation, micronization, palmitoylethanolamide
Citation: Petrosino S, Cordaro M, Verde R, Moriello AS, Marcolongo G, Schievano C, Siracusa R, Piscitelli F, Peritore AF, Crupi R, Impellizzeri D, Esposito E, Cuzzocrea S and Di Marzo V (2024) Corrigendum: Oral ultramicronized palmitoylethanolamide: plasma and tissue levels and spinal antihyperalgesic effect. Front. Pharmacol. 15:1441203. doi: 10.3389/fphar.2024.1441203
Received: 30 May 2024; Accepted: 03 June 2024;
Published: 18 June 2024.
Edited and reviewed by:
Annalisa Bruno, University of Studies G. d’Annunzio Chieti and Pescara, ItalyCopyright © 2024 Petrosino, Cordaro, Verde, Moriello, Marcolongo, Schievano, Siracusa, Piscitelli, Peritore, Crupi, Impellizzeri, Esposito, Cuzzocrea and Di Marzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Petrosino, c3BldHJvc2lub0BpY2IuY25yLml0
†These authors have contributed equally to this work
 Stefania Petrosino
Stefania Petrosino Marika Cordaro
Marika Cordaro Roberta Verde1
Roberta Verde1 Rosalba Siracusa
Rosalba Siracusa Fabiana Piscitelli
Fabiana Piscitelli Alessio F. Peritore
Alessio F. Peritore Rosalia Crupi
Rosalia Crupi Daniela Impellizzeri
Daniela Impellizzeri Emanuela Esposito
Emanuela Esposito Salvatore Cuzzocrea
Salvatore Cuzzocrea Vincenzo Di Marzo
Vincenzo Di Marzo