- 1Department of Physical Medicine and Rehabilitation, The Affiliated Panyu Central Hospital, Guangzhou Medical University, Guangzhou, China
- 2Department of Rheumatology and Immunology, Nanjing Drum Tower Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Graduate School of Peking Union Medical College, Nanjing, China
- 3Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of Cardio-Cerebral Diseases, School of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
- 4Psychosomatic Laboratory, Department of Psychiatry, Daqing Hospital of Traditional Chinese Medicine, Daqing, China
- 5Department of Physiology, School of Medicine, University of Louisville, Louisville, KY, United States
- 6Tong Jiecheng Studio, Hunan University of Science and Technology, Xiangtan, China
- 7The Central Hospital of Shaoyang, Shaoyang, China
- 8Department of Oral and Maxillofacial Surgery, School of Dentistry, University of Maryland, Baltimore, MD, United States
- 9Fischell Department of Bioengineering, A.James Clark School of Engineering, University of Maryland, College Park, MD, United States
- 10People’s Hospital of Ningxiang City, Ningxiang, China
- 11Fudan University, Shanghai, China
- 12Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objective: To assess the efficacy and safety of iguratimod (IGU) in the treatment of inflammatory arthritis and degenerative arthritis.
Methods: Initially, randomized controlled trials (RCTs) on using IGU in treating inflammatory arthritis and degenerative arthritis were systematically gathered from various databases up to February 2024. Subsequently, two researchers independently screened the literature, extracted data, assessed the risk of bias in included studies, and conducted a meta-analysis using RevMan 5.4 software.
Results: Fifty-four RCTs involving three inflammatory arthritis were included, including ankylosing spondylitis (AS), osteoarthritis (OA), and rheumatoid arthritis (RA). For AS, the meta-analysis results showed that IGU may decrease BASDAI (SMD −1.68 [−2.32, −1.03], P < 0.00001) and BASFI (WMD −1.29 [−1.47, −1.11], P < 0.00001); IGU may also decrease inflammatory factor [ESR: (WMD −10.33 [−14.96, −5.70], P < 0.0001); CRP: (WMD −10.11 [−14.55, −5.66], P < 0.00001); TNF-α: (WMD −6.22 [−7.97, −4.47], P < 0.00001)]. For OA, the meta-analysis results showed that IGU may decrease VAS (WMD −2.20 [−2.38, −2.01], P < 0.00001) and WOMAC (WMD −7.27 [−12.31, −2.24], P = 0.005); IGU may also decrease IL-6 (WMD −8.72 [−10.00, −7.45], P < 0.00001). For RA, the meta-analysis results showed that IGU may improve RA remission rate [ACR20: (RR 1.18 [1.02, 1.35], P = 0.02); ACR50: (RR 1.32 [1.05, 1.64], P = 0.02); ACR70: (RR 1.44 [1.02, 2.04], P = 0.04)] and decrease DAS28 (WMD −0.92 [−1.20, −0.63], P < 0.00001); IGU may also decrease inflammatory factors [CRP: (SMD −1.36 [−1.75, −0.96], P < 0.00001); ESR: (WMD −9.09 [−11.80, −6.38], P < 0.00001); RF: (SMD −1.21 [−1.69, −0.73], P < 0.00001)]. Regarding safety, adding IGU will not increase the incidence of adverse events.
Conclusion: IGU might emerge as a promising and secure therapeutic modality for addressing AS, OA, and RA.
Systematic Review Registration: Identifier PROSPERO: CRD42021289249
1 Introduction
Arthritis encompasses various joint diseases and is associated with factors such as degenerative diseases and autoimmunity. Its hallmark features include chronic inflammation in one or more joints, often leading to pain and frequently resulting in disability. Primary clinical symptoms encompass joint pain, swelling, stiffness, and restricted mobility (Venetsanopoulou et al., 2023; Di Matteo et al., 2023). Epidemiological evidence indicates that arthritis is most prevalent among females, with an increasing incidence with age. Moreover, the prevalence of arthritis of different etiologies varies across populations (Syed et al., 2023; Katz and Bartels, 2024). Current research suggests the existence of over 100 distinct forms of arthritis, with osteoarthritis (OA) and rheumatoid arthritis (RA) being the most common; other types mainly involve arthritis linked to autoimmune diseases (Clark, 2023; Messina et al., 2023). Despite varying etiologies, these diseases are characterized by joint inflammation, resulting in pain and limited mobility (Messina et al., 2023). Presently, treatments for arthritis, both pharmacological and non-pharmacological, primarily address the progression of joint pain and the resolution of joint inflammation, especially with a common foundation in pain management (Marín et al., 2023). Osteoarthritis, a degenerative joint disease, is increasingly prevalent with the aging population (Gulati et al., 2023). According to the World Health Organization (WHO), there are over 400 million osteoarthritis patients globally (Minnig et al., 2024). In Asia, one in every six individuals is expected to develop OA at some stage (Minnig et al., 2024). Epidemiological investigations reveal that this growth is, in part, due to the rapid increase in the elderly and obese populations, resulting in a rise in osteoarthritis incidence (Wei et al., 2023; Scheuing et al., 2023; Perruccio et al., 2024). Rheumatoid arthritis (RA), characterized by primary synovial inflammation, is a chronic, disabling, autoimmune disease that can occur at any age, with a disability rate of up to 61.3% for a disease duration ≥1 year, significantly impacting patients’ physical function and quality of life (Lau, 2023; Burmester and Pope, 2017). Apart from joint pain, swelling, and restricted mobility, 40% of patients may also experience extra-articular manifestations (EAMs), among which interstitial lung disease (ILD) is a common EAM in RA and a pivotal factor contributing to the high mortality rate associated with RA (Gravallese and Firestein, 2023). RA remains challenging to cure currently; nevertheless, standardized diagnostic and therapeutic interventions can achieve optimal treatment outcomes. However, without consistent treatment, it may lead to joint deformities and functional loss (Gravallese and Firestein, 2023). Other forms of arthritis are also linked to inflammation and pain, posing significant burdens on patients, yet effective treatments addressing the root causes are still lacking.
Currently, the primary objective of arthritis treatment is to alleviate joint pain caused by arthritis inflammation, daily joint wear and tear, and muscle strains (Juma et al., 2023). Existing medications for managing arthritis encompass analgesics, steroids, non-steroidal anti-inflammatory drugs (NSAIDs), and biologic/targeted therapies aimed at alleviating severe pain and inflammation symptoms (Harmalkar et al., 2024). However, these medications entail numerous side effects that hinder their sustained ability to mitigate disease symptoms and progression over prolonged use. For instance, NSAIDs are linked to severe gastrointestinal complications and inadequate pain relief post-treatment, while biologic/targeted therapies present risks of immune disruption and adverse cardiovascular events (Di Matteo et al., 2023; Mohapatra et al., 2023; Taylor, 2023). Consequently, the treatment landscape for arthritis has evolved towards comprehensive management and therapy, with alternative modalities gradually becoming integral components of this holistic approach to management and treatment (Brown et al., 2024; Sarzi-Puttini et al., 2023). Disease-modifying antirheumatic drugs (DMARDs) serve as principal therapeutics for RA, and the emergence of novel conventional synthetic DMARDs (csDMARDs) and biologic/targeted DMARDs (b/tsDMARDs) in recent years has heralded groundbreaking advancements in the treatment of RA and RA-ILD (Brown et al., 2024; Sarzi-Puttini et al., 2023).
Iguratimod (IGU), regarded as a new type of csDMARDs, exhibits a diverse mechanism of action with comprehensive immune-regulatory effects (Ito, 2016). Studies indicate that IGU can modulate the immune balance mediated by T cells and associated inflammatory factors by regulating the quantities of helper T cells (e.g., Th1 and Th17), follicular helper T (Tfh) cells, and regulatory T (Treg) cells. Additionally, IGU can inhibit the differentiation of B cells into plasma cells, thereby suppressing the production of autoantibodies (Liu et al., 2021). In recent years, massive randomized controlled trials have been published, so there is an urgent need to summarize the efficacy and safety of IGU in treating inflammatory arthritis. This study provides future clinicians with better evidence for clinical practice, and it also offers more details for future clinical trial design by conducting a comprehensive systematic review and meta-analysis of these RCTs.
2 Materials and methods
2.1 Protocol
This systematic review and meta-analysis were conducted strictly in accordance with the protocol registered in PROSPERO (CRD42021289249) and PRISMA guidelines (see Supplementary Materials). There were not any significant deviations from the protocol.
2.2 Literature retrieval strategy
Chinese databases [VIP Database, China National Knowledge Infrastructure (CNKI), Wanfang Database and SINOMED] and English databases (Embase, PubMed, Medline Complete, Web of Science, Cochrane Library and ClinicalTrials.gov) were used for searching literature on IGU for the treatment of inflammatory arthritis. The retrieval period spans from the inception date to 1 February 2024. The search strategy is shown in Supplementary Table S1.
2.3 Search criteria
2.3.1 Inclusion criteria
1) Participants: Patients diagnosed with any type of inflammatory arthritis and degenerative arthritis by accepted criteria. 2) Intervention methods: The therapeutic approach in the experimental group involved the utilization of IGU, with unrestricted parameters in terms of dosage, formulation, and administration method. 3) Control: The therapeutic regimen in the control group encompassed interventions that did not include IGU, such as placebos and conventional therapies. 4) Outcomes: Disease-related therapeutic efficacy indicators, inflammation markers, and IGU-related adverse events. 5) Study design: randomized controlled trials (RCTs).
2.3.2 Exclusion criteria
1) Duplicate articles; 2) observational studies; 3) reviews, case reports, animal experiments, etc. ; 4) retracted articles.
2.4 Literature screening and data extraction
Initially, a preliminary literature search was conducted based on titles, abstracts, and keywords to select relevant literature initially. Subsequently, further literature inclusion was performed following established search criteria. Details regarding the study, including basic information, grouping methods, baseline conditions, treatment protocols, duration, and outcome measures, were extracted using predefined data extraction forms (Deeks et al., 2020a). Two researchers independently executed this process, with results cross-checked and any discrepancies resolved through discussion involving the entire team.
2.5 Risk of bias assessments
The quality assessment was conducted using the risk of bias assessment tools for RCTs recommended in the Cochrane Handbook (Deeks et al., 2020b). Each study was evaluated based on criteria, including random sequence generation, allocation concealment, blinding, attrition, and selective reporting risks. Two researchers independently performed bias risk assessments, with any inconsistencies resolved through discussion involving all researchers.
2.6 Data synthesis
Statistical analyses were performed using RevMan 5.4 software (Deeks et al., 2020c). Relative risk (RR) was utilized as the effect measure for dichotomous variables, while weighted mean difference (WMD) and standard mean difference (SMD) were employed for continuous variables. A 95% confidence interval (CI) was set for all analyses. Heterogeneity among results was assessed using the chi-square test, and if heterogeneity was minimal (P > 0.1, I2 < 50%), a fixed-effect model was employed for analysis; otherwise, a random-effects model was utilized.
3 Results
3.1 Literature search results
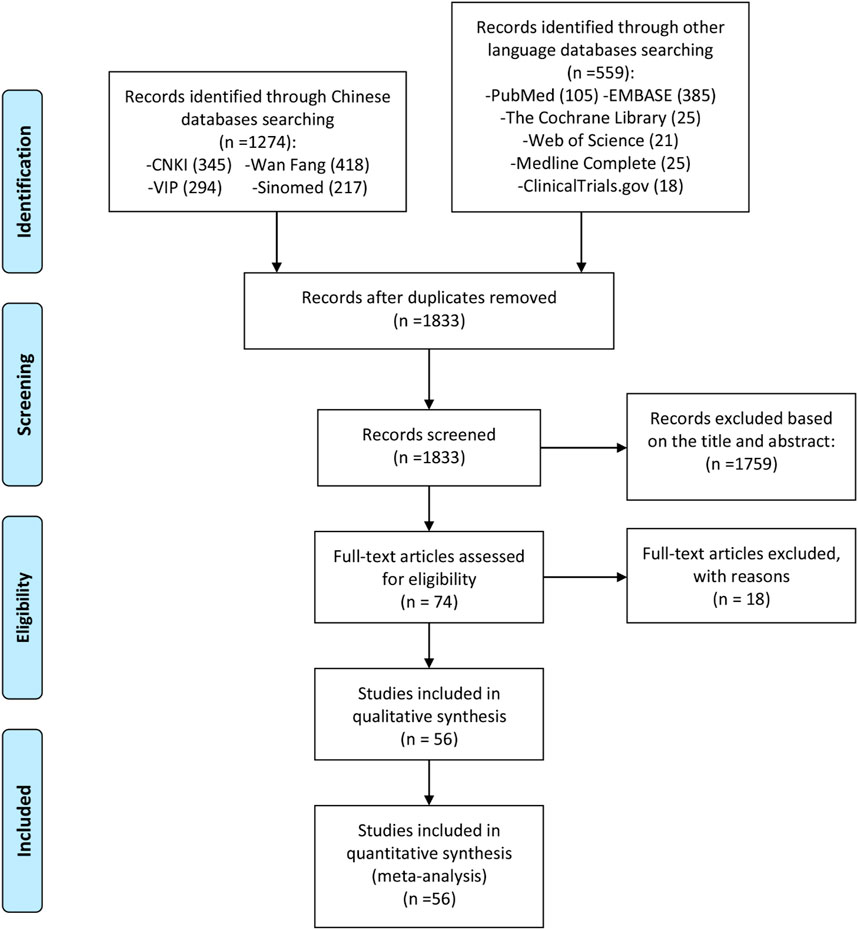
A total of 1,833 initial relevant articles were identified in this study, out of which 1,759 were excluded for mismeeting the research type and content criteria. Following a thorough review of the full texts, and based on the inclusion and exclusion criteria as well as the completeness of the literature information, 18 articles were excluded for not being RCTs (Gu et al., 2020; Guifeng and Yasong, 2014; He et al., 2015; Huang and Ma, 2018; Man and Yongxin, 2020; Lin, 2016; Luo et al., 2019; Luo et al., 2018; Meng et al., 2016a; Okamura et al., 2015; Shang et al., 2019; Suto et al., 2019; Wang et al., 2017; Wang et al., 2018; Wang, 2017; Xu et al., 2021; Yoshioka et al., 2016; Zhu et al., 2016). Consequently, 56 articles were included for quantitative and qualitative analysis (Li X. et al., 2021; Bai et al., 2021; Lin YP. et al., 2019; Xu BJ. et al., 2019; Zeng et al., 2016; Li Y. et al., 2021; Yuan et al., 2020; Pang et al., 2020; Zhang, 2022; Zeng et al., 2019; Zhang et al., 2023; Han et al., 2023; Wu et al., 2022; Lü et al., 2008; Hara et al., 2007; Mo and Ma, 2015; Xiong and Guanghui, 2020; Yan et al., 2022; Yi et al., 2022; Deng JX The effect of, 2017; Tian et al., 2020; Fan et al., 2020; Xie et al., 2018; Lianju et al., 2019; Zhao and Hao, 2018; Ma et al., 2019; Zhao et al., 2016; Meng et al., 2017; Xia et al., 2016; Lu, 2014; Qi et al., 2019; Zhao et al., 2017; Hu, 2014; Chen et al., 2018; Xia et al., 2020; Tian and Tao, 2017; Xu et al., 2017; Shi et al., 2015; Wang et al., 2019; Hara et al., 2014; Ishiguro et al., 2013; RAO et al., 2014; Xu et al., 2015; Bi, 2019; Sun and Li, 2022; Yan and Wang, 2018; Meng et al., 2016b; Duan et al., 2015; Xiaong et al., 2015; Lu et al., 2009; Ju et al., 2020; Meng et al., 2015; Li et al., 2016; Li and Huang, 2020; Mo et al., 2018; Shi et al., 2023). The literature screening process and results are shown in Figure 1.
3.2 Description of included trials
Two articles (Xia et al., 2016; Lu, 2014) originating from the same RCT were catalogued by Xia et al. (2016), Lu (2014). Similarly, two articles (Hara et al., 2014; Ishiguro et al., 2013) derived from the same RCT were documented by Hara et al. (2014), Ishiguro et al. (2013). Consequently, the 56 records pertain to 54 RCTs. In some randomized controlled trials with two experimental groups, the control group was divided into two equal portions to match them, each representing half of the population and labelled as Group A and Group B. Detailed characteristics of the studies are presented in Supplementary Information, Supplementary Table S2.
3.3 Risk of bias assessments
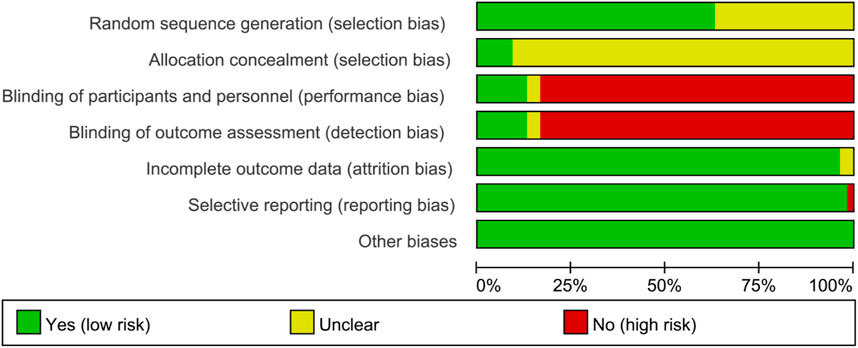
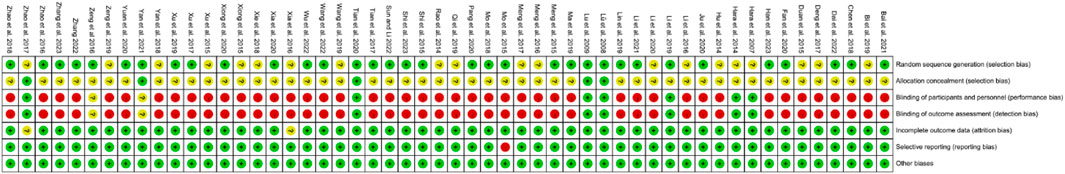
The graph and summary of bias risk are shown in Figures 2, 3, respectively.
3.4 The outcomes of IGU in the treatment of AS
3.4.1 The bath ankylosing spondylitis disease activity index (BASDAI)
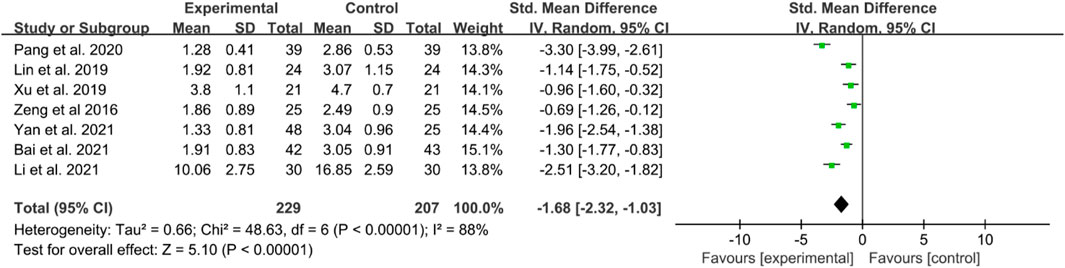
There are seven RCTs reporting BASDAI in their publication. The included studies showed high heterogeneity; thus, a random effects model was utilized. The IGU groups showed significantly lower BASDAI scores compared to the control groups (SMD −1.68 [−2.32, −1.03], P < 0.00001, Figure 4).
3.4.2 Bath ankylosing spondylitis functional index (BASFI)
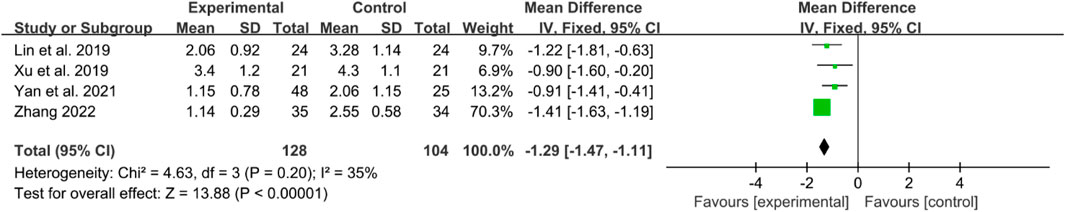
Four RCTs reported BASFI in their manuscripts. The heterogeneity test showed low heterogeneity, a fixed effects model was used. The IGU group had a significantly lower BASFI score compared to the control group (WMD −1.29 [−1.47, −1.11], P < 0.00001) (Figure 5).
3.4.3 Inflammatory factor
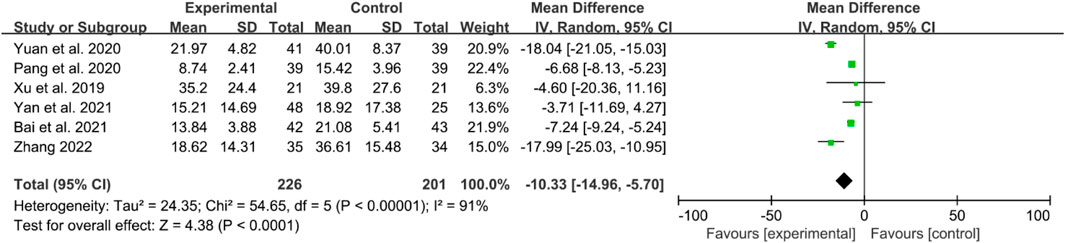
The inflammatory factors focused on in this part of the study include erythrocyte sedimentation rates (ESRs), C-reactive protein (CRP) levels, and tumor necrosis factor- α (TNF-α) levels. Here, six RCTs reported ESRs. High heterogeneity was observed, and a random effects model was used. The IGU group had significantly lower ESRs compared to the control group (WMD −10.33 [−14.96, −5.70], P < 0.0001, Figure 6).
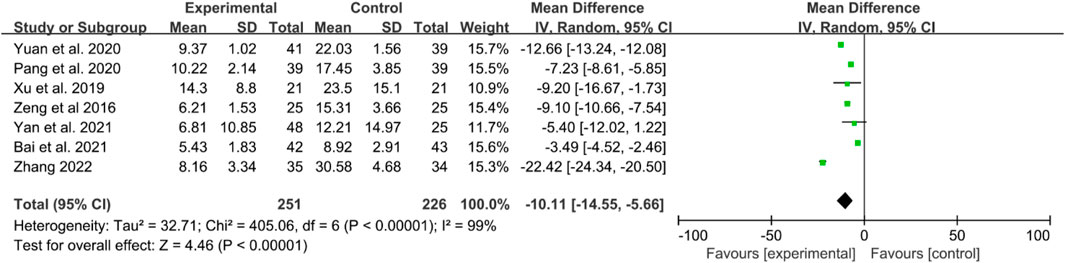
Seven RCTs reported CRP levels. The heterogeneity test indicated high heterogeneity, thus, a random effects model was utilized. The results demonstrated that IGU significantly decreased CRP levels compared to the control group (WMD −10.11 [−14.55, −5.66], P < 0.00001, Figure 7).
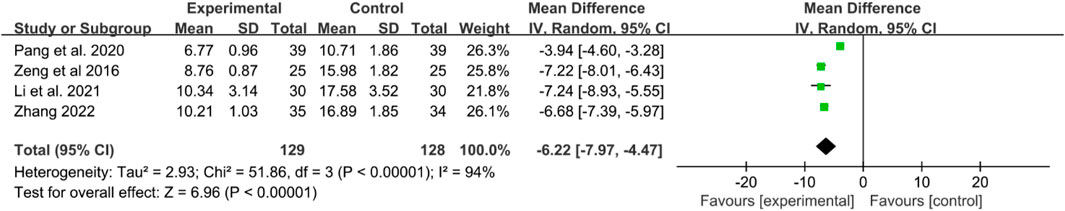
Four RCTs reported TNF-α levels. Significant heterogeneity was detected, and a random effects model was applied. The results indicated that TNF-α levels were significantly lower in the IGU group compared to the control group (WMD −6.22 [−7.97, −4.47], P < 0.00001, Figure 8).
3.4.4 Adverse events
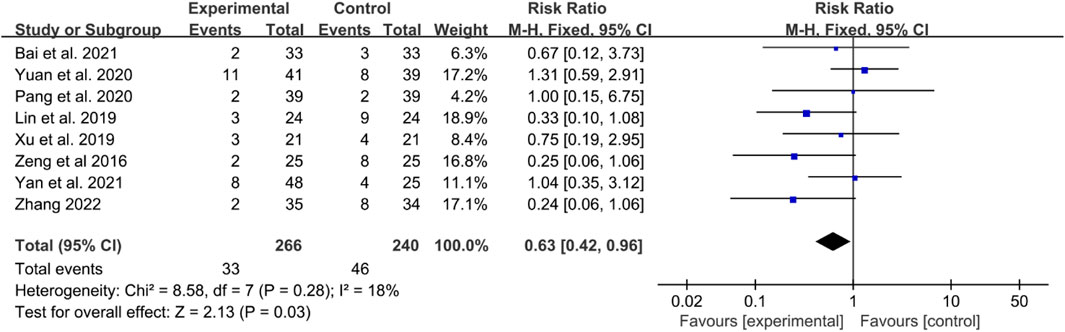
Eight RCTs reported adverse events. In these RCTs, Bai et al. reported that the main adverse events were rash, abnormal liver function, and gastrointestinal reactions in 2021 (Bai et al., 2021). Lin et al. also found that in the IGU group, two cases of upper abdominal discomfort and one case of oral ulcers were observed; in contrast, the control group experienced three cases of upper abdominal discomfort, five cases of liver function abnormalities, two cases of oral ulcers, two cases of anemia, and one case of leukopenia; some patients in both groups experienced two or more adverse reactions (Lin Y. P. et al., 2019). Xu et al. reported gastrointestinal discomfort and liver function abnormalities as adverse effects (Xu B. J. et al., 2019), and Zeng et al. mainly presented gastrointestinal reactions, leukopenia, and abnormal liver function (Zeng et al., 2016). Yan et al. primarily reported gastrointestinal discomfort (Li Y. et al., 2021), while Yuan et al. showed leukopenia, oral ulcers, nausea and vomiting, diarrhea, and abnormal liver function (Yuan et al., 2020). Pang et al. briefly reported gastrointestinal reactions, abnormal liver function and rash (Pang et al., 2020). Zhang mainly showed abnormal liver and kidney function, decreased leukocytosis, and gastrointestinal discomfort (Zhang, 2022).
The incidence rates of these adverse events were combined for meta-analysis. The heterogeneity test indicated low heterogeneity, suggesting that a fixed effects model was appropriate for analysis. The meta-analysis indicated that the incidence of adverse events in the IGU group was lower [RR 0.63 (0.24, 0.96), P = 0.03, Figure 9].
3.5 The outcomes of IGU in the treatment of OA
3.5.1 Visual analog scale (VAS)
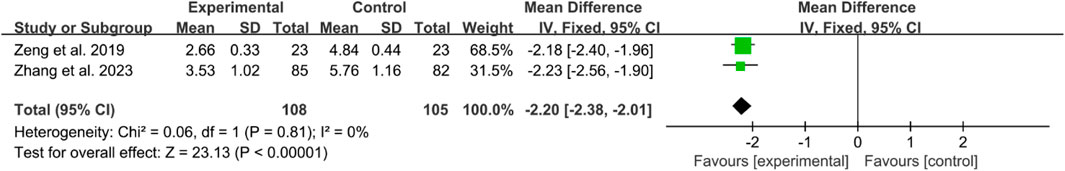
Two RCTs reported the VAS scores of OA. The heterogeneity test indicated low heterogeneity, suggesting that a fixed effects model was appropriate for analysis. The meta-analysis revealed that the VAS in IGU group was lower (WMD −2.20 [−2.38, −2.01], p < 0.00001, Figure 10).
3.5.2 The Western Ontario and McMaster universities osteoarthritis index (WOMAC)
Two RCTs reported WOMAC. The heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis indicated that the WOMAC in the IGU group was lower (WMD −7.27 [−12.31, −2.24], P = 0.005, Figure 11).
3.5.3 Inflammation factors
The inflammatory factors in this part of the study include TNF-α and interleukin (IL)-6.
Two RCTs reported TNF-α. The heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis indicated that the difference in TNF-α between the two groups was of no statistical significance (WMD −9.21 [−20.89, 2.47], P = 0.12, Figure 12).
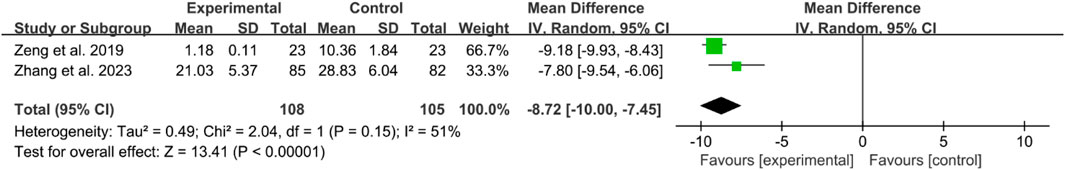
Two RCTs reported IL-6. The heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis indicated that the WOMAC in IGU group was lower (WMD −8.72 [−10.00, −7.45], P < 0.00001, Figure 13).
3.5.4 Adverse events
In the RCT conducted by Zeng et al. in 2019, the IGU group exhibited 1 case of mild abdominal discomfort post-treatment. In contrast, the control group experienced 1 case of gastrointestinal reaction and 1 case of rash (Zeng et al., 2019). In the study by Zhang et al. (2023), it was discovered that both groups of patients did not experience any drug-related adverse reactions, indicating that the medication is relatively safe.
3.6 The outcomes of IGU in the treatment of RA
3.6.1 RA remission rate
American College of Rheumatology (ACR)20, ACR50 and ACR70 were used to represent RA remission rate.
For ACR20, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the ACR20 in the IGU group is higher than the control group (RR 1.18 [1.02, 1.35], P = 0.02, Figure 14).
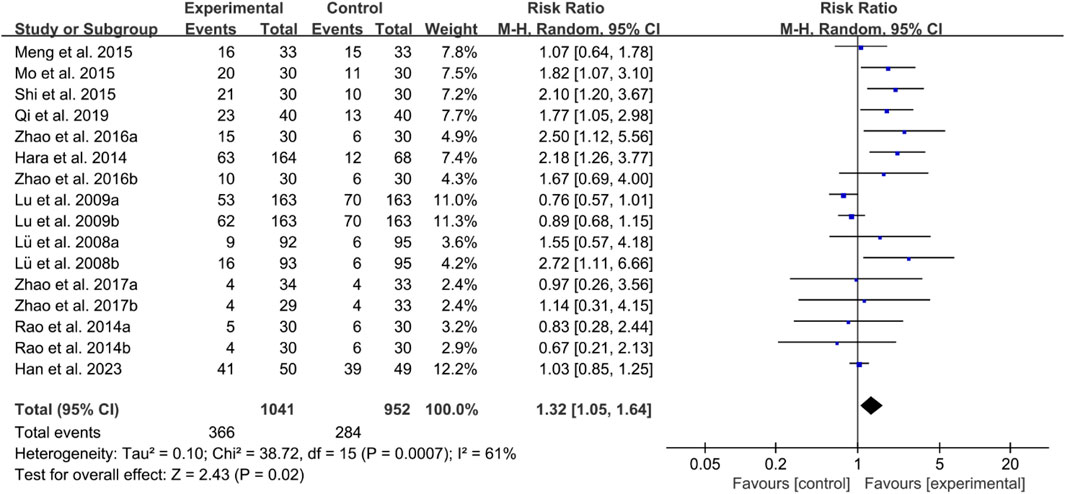
For ACR50, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the ACR50 in the IGU group is higher than the control group (RR 1.32 [1.05, 1.64], P = 0.02, Figure 15).
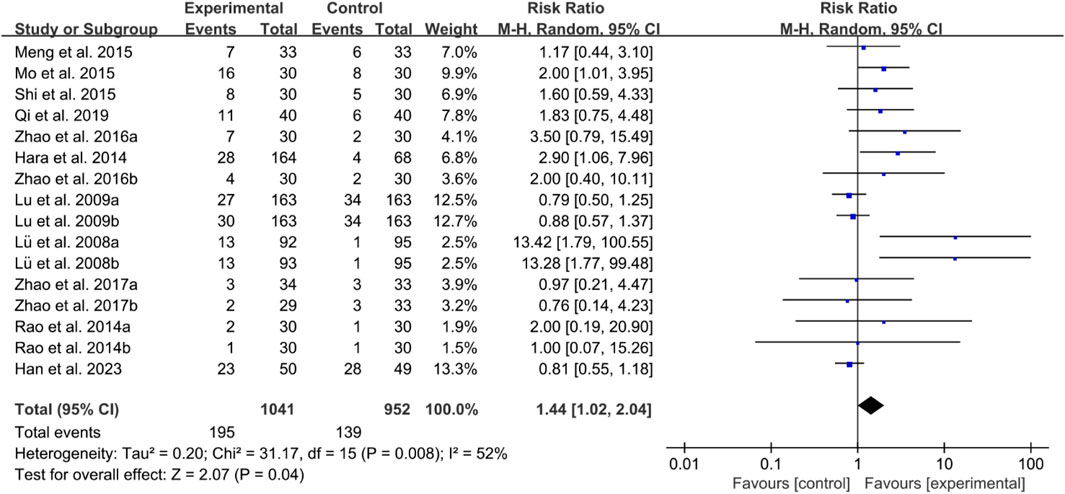
For ACR70, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the ACR70 in the IGU group is higher than the control group (RR 1.44 [1.02, 2.04], P = 0.04, Figure 16).
3.6.2 Disease activity score 28 (DAS28)
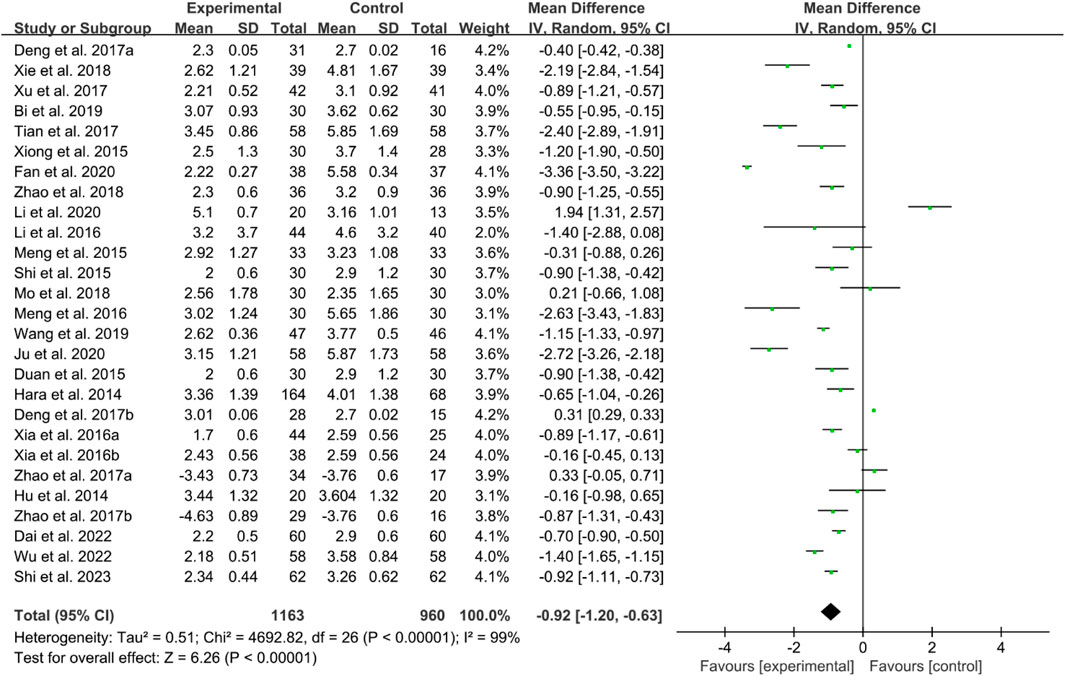
Twenty-four RCTs reported DAS28. The heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the DAS28 in IGU group is lower than control group (WMD −0.92 [−1.20, −0.63], P < 0.00001, Figure 17).
3.6.3 Inflammatory factor
Inflammatory factors focused in this section include CRP, ESR and rheumatoid factor (RF).
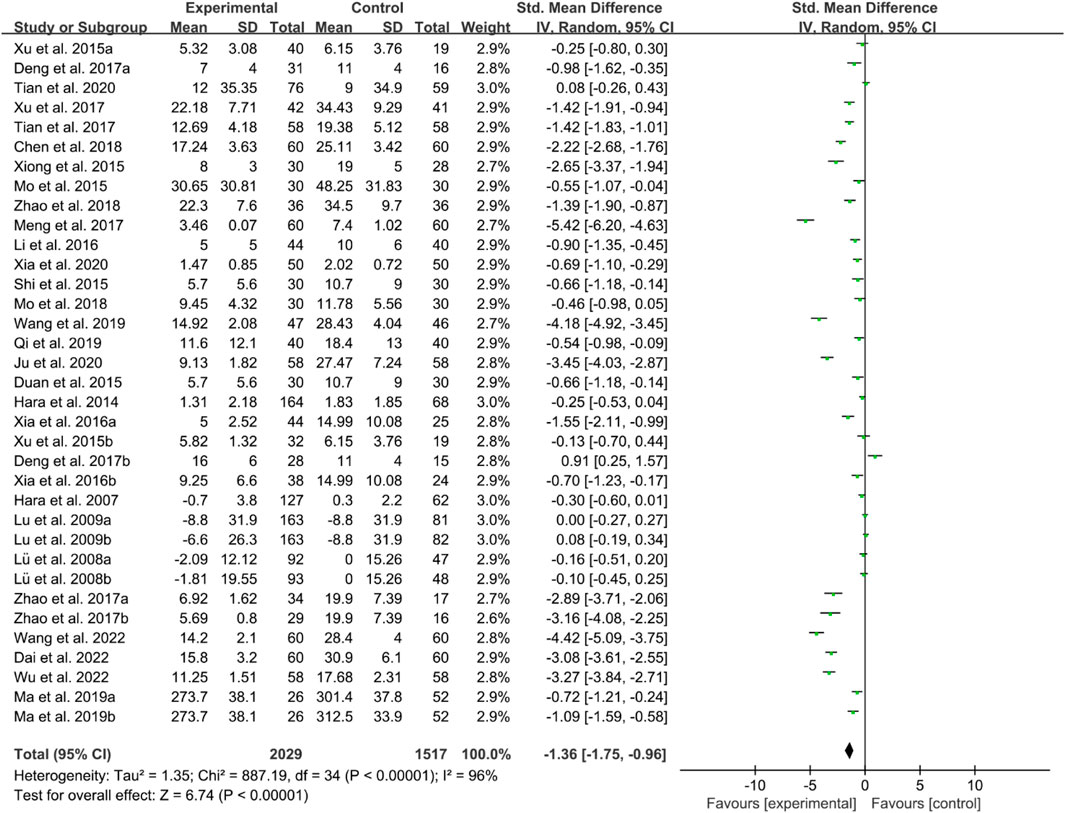
For CRP, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the CRP in the IGU group is higher than the control group (SMD −1.36 [−1.75, −0.96], P < 0.00001, Figure 18).
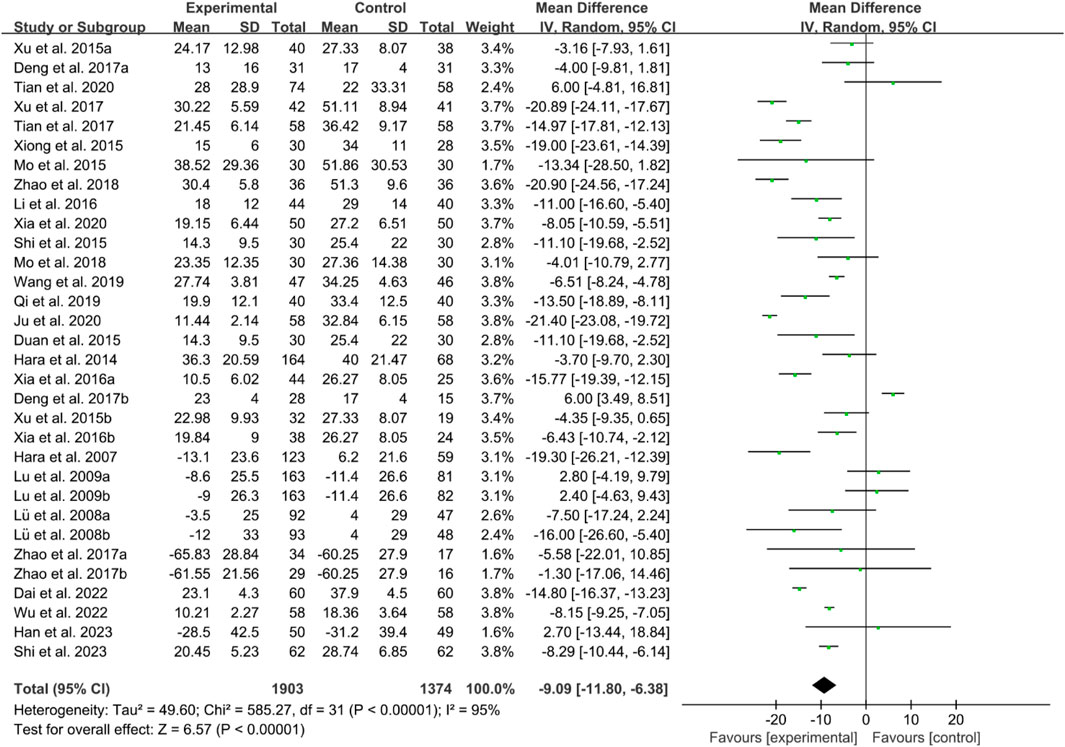
For ESR, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the ESR in the IGU group is higher than the control group (WMD −9.09 [−11.80, −6.38], P < 0.00001, Figure 19).
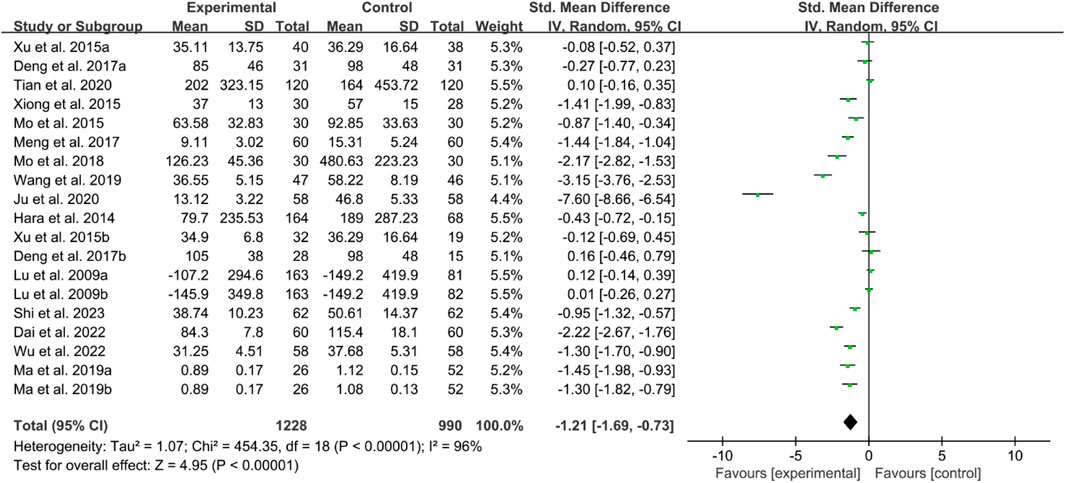
For RF, the heterogeneity test indicated high heterogeneity, suggesting that a random effects model was appropriate for analysis. The meta-analysis findings indicate that the RF in the IGU group is higher than the control group (SMD −1.21 [−1.69, −0.73], P < 0.00001, Figure 20).
3.6.4 Adverse events
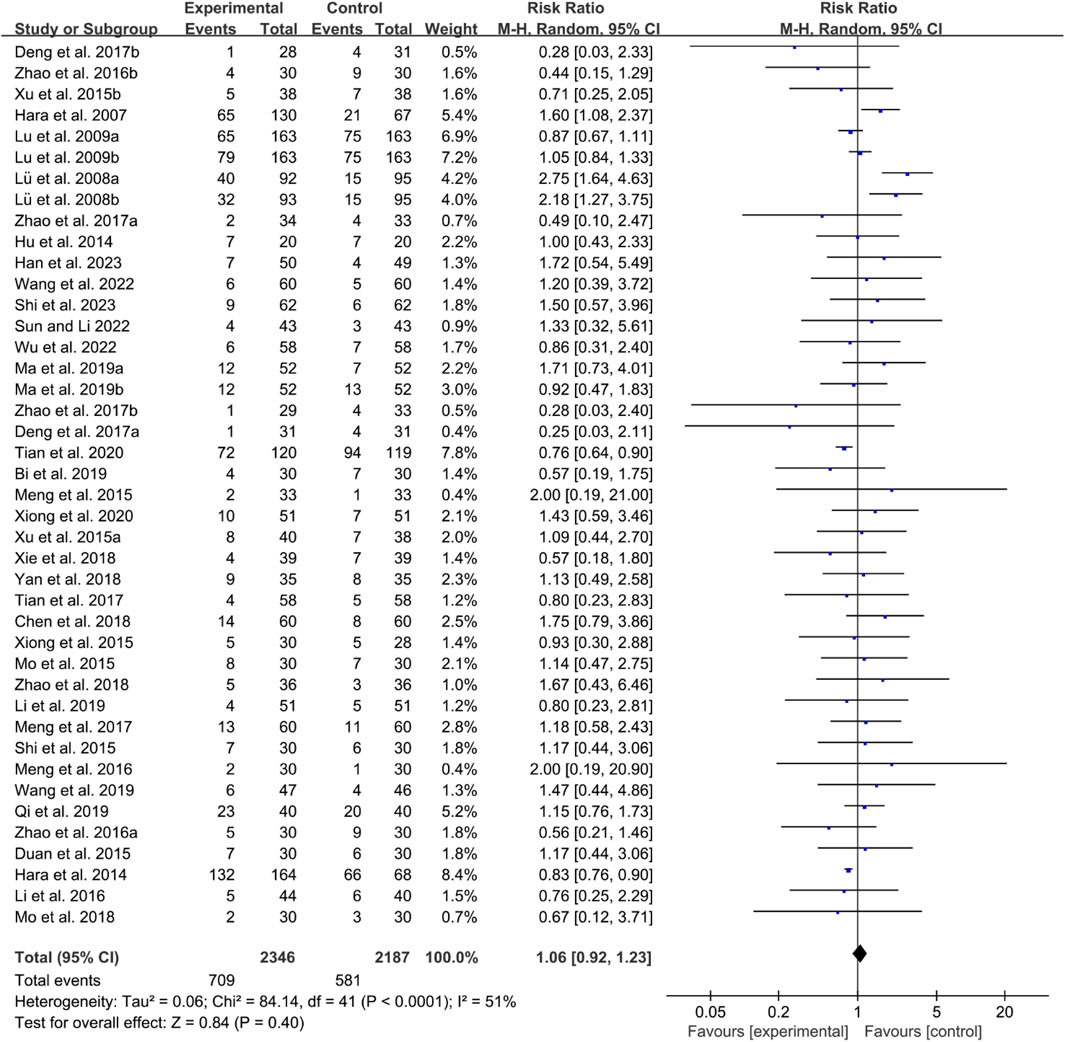
Thirty-five RCTs reported adverse events. The heterogeneity test indicated low heterogeneity with P < 0.0001 and I2 = 51%, suggesting that a fixed effects model was appropriate for analysis. The meta-analysis showed that the incidence of adverse events between the two groups was of no statistical significance (RR 1.06 [0.92, 1.23], P = 0.40, Figure 21).
4 Discussion
4.1 The molecular mechanism of IGU in treating inflammatory arthritis
IGU is a novel DMARD that offers anti-inflammatory, antifibrotic, anti-resorptive, immunomodulatory, and bone metabolism-regulating effects (Shen et al., 2024; Zeng et al., 2023; Zou et al., 2023). As research on IGU has advanced in recent years, its therapeutic applications have broadened. Current evidence indicates that IGU provides significant immunomodulatory benefits and comprehensive bone protection, balancing efficacy and safety, making it well-suited for combination therapy and long-term maintenance in the treatment of rheumatoid arthritis (Hu et al., 2024; Sun et al., 2023; Long et al., 2023). Compared to traditional DMARDs, IGU has been shown to reduce rheumatoid factor significantly and anti-CCP antibody levels, effectively preventing bone destruction and reducing the risk of disability and deformity associated with arthritis (Hu et al., 2024; Sun et al., 2023; Long et al., 2023).
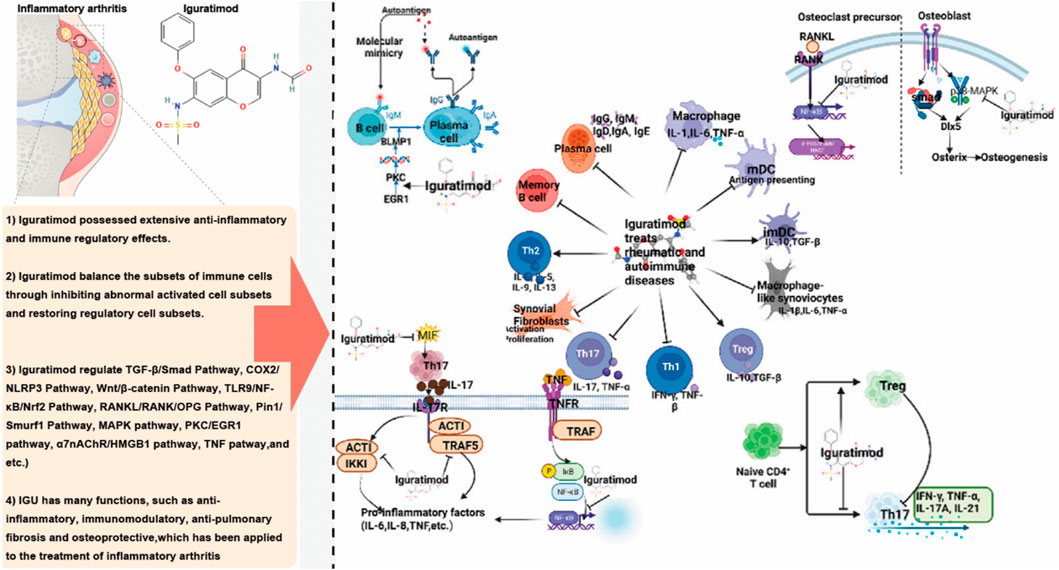
Regarding its anti-inflammatory effect, IGU exerts its anti-inflammatory effects by inhibiting the proliferation of inflammatory cells and reducing the release of pro-inflammatory cytokines, thereby mediating key anti-inflammatory signaling pathways (Figure 22). Specifically, IGU at lower concentrations primarily inhibits the migration of fibroblast-like synoviocytes (FLS), with higher concentrations leading to the suppression of FLS proliferation and even inducing apoptosis. In animal models of RA, OA and AS, IGU significantly reduces the expression of pro-inflammatory cytokines while increasing the expression of anti-inflammatory cytokines. This dual modulation reduces the infiltration of inflammatory cells in the bloodstream and affected tissues, enhancing its anti-inflammatory effect. In addition, IGU protects against inflammatory arthritis by activating the nuclear factor-κB (NF-κB) signaling pathway and downregulating sodium bicarbonate cotransporter e2 (NBCe2) in RA patients to inhibit protein citrullination and inflammation. In both acute and chronic inflammation models, such as carrageenan-induced paw edema and adjuvant-induced arthritis in rats, IGU demonstrated anti-inflammatory and analgesic effects. Unlike traditional NSAIDs, IGU does not cause gastrointestinal ulcer-like side effects in fasting rats and can inhibit kininogen in kaolin-induced inflammatory responses (Peng et al., 2024). IGU significantly reduces IgM production in mouse B cell cultures and promotes the switch to IgG1 under lipopolysaccharide and/or IL-4 induction (Chen et al., 2023; Nozaki, 2021). In human multiple myeloma cell cultures (ARH-77 cell line), IGU inhibits spontaneous IgG antibody production without affecting cell proliferation, and in human peripheral blood B cells induced by autologous T cells and anti-CD3 antibodies, IGU suppresses the production of both IgM and IgG, effectively inhibiting immunoglobulin production in B cells without causing blockage (Zeng et al., 2022a).
A study found that in chronic rheumatoid arthritis models, such as adjuvant-induced arthritis (AIA) rats and Murphy Roths large lymphoproliferation (MRL/lpr) mice, IGU not only improved arthritis lesions but also reduced hypergammaglobulinemia (Jiang et al., 2020). Regarding cytokine inhibition, IGU suppressed the production of IL-1β, TNF-α, IL-6, IL-8, and monocyte chemoattractant protein (MCP)-1. In synovial cell cultures derived from patients with rheumatic diseases, IGU significantly reduced the production of IL-6, IL-8, and colony-stimulating factors. Additionally, IGU inhibited the upregulation of costimulatory molecules, including CD54, CD58, and CD106, in synovial cells stimulated by IFN-γ. In a mouse model of subcutaneous air pouch inflammation, oral administration of IGU at doses of 30–100 mg/kg significantly suppressed TNFα-induced MCP-1 production (Jiang et al., 2020; Li et al., 2019). Pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α play a crucial role in bone resorption and are key pathological factors in RA, closely associated with disease activity (Xie et al., 2020). These cytokines activate osteoclasts, increasing bone resorption and subsequent loss. Inhibiting IL-1, IL-6, and TNF-α can effectively control RA and prevent related bone degradation. MRI results in the collagen-induced arthritis (CIA) rat model showed that IGU nearly completely suppressed inflammation and bone marrow edema associated with CIA. X-ray and CT scans revealed significant inhibition of bone resorption and joint destruction in rats treated with IGU (Zeng et al., 2022b).
IGU also plays a significant role in promoting bone formation and regulating bone metabolism. In vitro studies have shown that IGU enhances osteoblast differentiation. At the same time, in vivo experiments demonstrate its ability to augment bone morphogenetic protein (BMP)-2-mediated bone formation, which is believed to be associated with increased Osterix (Osx) expression. Osx is crucial for bone differentiation and formation (Sun et al., 2023). Recent research has revealed that IGU improves disuse osteoporosis in mice by inhibiting sclerostin and the receptor activator of NF-κB ligand (RANKL) through the extracellular signal-regulated kinase/early growth response protein 1/TNF-α pathway in osteocytes (Miura et al., 2024). In the ST2 bone marrow stromal cell line, Osx expression is minimal in the absence of recombinant human BMP (rhBMP)-2, but IGU can stimulate Osx expression by more than threefold when rhBMP-2 is present. In the pre-osteoblast cell line MC3T3-E1, IGU directly stimulates Osx expression, independent of rhBMP-2, thereby promoting osteoblast differentiation. Further studies have shown that IGU dose-dependently stimulates the secretion of osteocalcin in both ST2 and MC3T3-E1 cells; in the presence of rhBMP-2, IGU increases calcium content in ST2 cells by 14-fold, leading to the formation of mineralized nodules. In mouse models, IGU increased calcium content in the ossicles by 1.7-fold (Hou et al., 2021).
4.2 IGU in the treatment of AS
AS is a chronic inflammatory disease that affects the spine and joints, and its pathogenesis is still not entirely understood. Current understanding suggests that AS results from interactions among genes, microbes, and other factors, leading to an imbalance where osteogenesis by osteoblasts surpasses bone resorption by osteoclasts, ultimately culminating in joint ankylosis (Rodolfi et al., 2024). Throughout the inflammatory process of AS, cellular factors like TNF-α and IL-1 play pivotal roles (Liu et al., 2023). Current guidelines for AS treatment still recommend the use of NSAIDs and TNF antagonists, while drugs like sulfasalazine (SSZ) and methotrexate (MTX) are recommended for those with peripheral joint involvement (Mysler et al., 2024; van de Sande and Elewaut, 2023). Studies have shown that IGU can inhibit the production of inflammatory cytokines such as interleukin-1 (IL-1), IL-6, IL-17, and TNF-α. Here, TNF-α is a crucial inflammatory factor in the pathogenesis of AS, and the IL-23/IL-17 pathway has been proven to be important in the mechanism underlying AS (Li et al., 2020; Ishikawa and Ishikawa, 2019; Macleod et al., 2023). Consequently, in recent years, multiple studies have been progressively examining the efficacy of IGU in treating AS.
This systematic review and meta-analysis showed that IGU can reduce disease activity (reduce BASDAI and BASFI) and improve patients’ inflammatory response (reduce ESR, CRP and TNF-α) in patients with AS. In terms of safety, compared with the control group, the incidence of adverse events with the addition of IGU was lower. However, given the high risk of bias in blinding and the unknown risks associated with allocation concealment and random sequence generation in most RCTs, the stability of the conclusions may be compromised. Therefore, the findings should be interpreted with caution.
4.3 IGU in the treatment of OA
OA is a disease that affects all joints. The increasing prevalence of OA is attributed to the accelerated aging of the population, escalating rates of obesity, and subsequent joint injuries (Knights et al., 2023; Kim, 2022). Furthermore, recent studies indicate a trend towards a younger age of onset for OA. In the early stages of OA, the primary manifestation is joint pain during activity, with relief experienced at rest. As the disease progresses, continuous pain may develop, potentially leading to joint deformity, impairing joint function, and, in severe cases, resulting in disability (Zhou et al., 2024). Research has confirmed that IGU can inhibit the production of inflammatory factors such as IL-17, TNF-α, and IL-6, exhibiting anti-inflammatory effects, while simultaneously acting as an NSAID. The mechanism of action of this drug aligns closely with the therapeutic goals of treating OA (Horváth et al., 2023). It has been found that IGU has clear chondroprotective effects in rheumatoid arthritis currently (Cong et al., 2021), and new research similarly suggests that IGU assists in protecting cartilage in OA (Xu B. et al., 2019; Xu et al., 2023). Studies have shown that in rats with IGU administered orally, there is an increase in the expression of transient receptor potential cation channel subfamily V member 4 (TRPV4) in cartilage, resulting in significant pain reduction and notable inhibition of cartilage destruction. Following in vitro experiments involving the cultivation of cartilage cells post-IGU intervention, it is observed that in rats receiving IGU treatment, the differentiation, activity, and migratory capabilities of rat cartilage cells are significantly enhanced. Hence, based on preliminary results, IGU appears to delay cartilage degradation and promote differentiation and migration, possibly acting through the TRPV4 ion channel (Xu et al., 2023).
This systematic review and meta-analysis showed that IGU can reduce pain caused by OA (reduce VAS and WOMAC) and improve patients’ inflammatory response (reduce IL-6). Regarding safety, adding IGU does not increase the incidence of adverse events. However, as the number of RCTs was only two and there was a high risk of bias in blinding and an unknown risk of bias in allocation concealment, the conclusions need to be interpreted with caution.
4.4 IGU in the treatment of RA
RA is an autoimmune disease primarily characterized by symmetrical damage to small joints. Research indicates that IGU can selectively inhibit cyclooxygenase-2 (COX-2) and NF-κB to alleviate inflammatory responses, particularly in cases of primary or secondary drug resistance in RA (Deng et al., 2022). IGU primarily functions by suppressing inflammatory cytokines to inhibit the occurrence and progression of synovitis. Recent studies demonstrate that IGU can effectively restrain the proliferation of RA-FLS. Wang et al. substantiated that IGU can selectively repress the expression of COX-2 mRNA and c-fos mRNA, subsequently inhibiting RA-FLS proliferation, with the inhibitory effects conforming to a dose-response relationship (WANG and SHEN, 2015). Du et al. revealed that IGU decreases the expression of matrix metalloproteinase (MMP)-1 and MMP-3, thereby suppressing excessive proliferation of FLS (Du et al., 2012). Additionally, Meng et al. demonstrated that IGU reduces vascular endothelial growth factor release, enhances endothelin production, and reduces synovial vascular neogenesis consequently (Meng D. Z. et al., 2016).
Lin J. et al. (2019) showed that through cell migration experiments, IGU significantly inhibits the invasive behavior of RA-FLS via the mitogen-activated protein kinase (MAPK) signaling pathway and promotes apoptosis. Pathological changes in bone loss in RA joints are closely associated with the activation of pro-inflammatory factors leading to osteoclast activation and bone resorption. Osteoprotegerin (OPG) competes with the receptor activator of NF-κB ligand (RANKL) for binding to the activator of NF-κB receptor (RANK). Clinical studies illustrate that IGU can lower serum IL-17 levels to attenuate the expression of inflammatory factors like IL-9 and IL-8, reduce RANKL levels, and directly modulate the OPG/RANKL/RANK axis system, consequently delaying bone destruction. Combining with MTX can significantly increase OPG levels, yielding better therapeutic outcomes (Luo et al., 2013). Feng et al. indicated that IGU plays a pivotal role in inhibiting the expression of genes essential for osteoclast differentiation and activation, such as RANK, acidic phosphatase, tissue protease K, and MMP-1, thereby inhibiting osteoclast proliferation and differentiation, and showing a dose-dependent relationship with efficacy (Feng et al., 2019). Positive anti-citrullinated protein antibody status is closely associated with bone loss in RA, and IGU can dose-dependently downregulate peptidyl arginine deiminase (PADI) 2 and PADI4 in neutrophils, thereby suppressing protein citrullination and alleviating bone loss (Li et al., 2020).
MTX serves as an anchor drug for treating RA and is commonly used in combination with IGU. A meta-analysis by Shrestha et al. suggested that at 24 weeks, the therapeutic effects, disease status, and adverse reactions exhibited by IGU and MTX are similar, indicating the potential of IGU as a substitute for MTX (Shrestha et al., 2020). Additionally, another meta-analysis by Wu et al. revealed that the combination of IGU and MTX in the treatment of RA leads to superior efficacy in increasing ACR20/50/70 response rates, reducing ESR, CRP, assessing the activity of 28 joint diseases, and VAS scores compared to individual use, without a significant increase in adverse reactions (Wu et al., 2018). A study by Ren et al. (2017) suggested that combination therapy can significantly reduce abnormally elevated platelet counts and decrease serum immunoglobulins (IgA, IgG, IgM) and T lymphocyte subsets (CD3+, CD4+ and CD8+ T cells).
The lungs are one of the most frequently affected extra-articular organs in RA, primarily manifesting as interstitial lung disease. Prolonged, low-dose use of MTX can lead to adverse reactions, causing interstitial lung disease. Han et al. found in animal experiments that IGU improves bleomycin-induced spontaneous pulmonary fibrosis by suppressing inflammation (Han et al., 2018). Short-term clinical observations by Hao et al. indicate that IGU effectively treats RA combined with chronic interstitial pneumonia, with a lower incidence of adverse reactions and no concomitant infections (L and i, 2014). Zhao et al. demonstrated that IGU can ameliorate lung tissue fibrosis by inhibiting the expression of factors like MMP-9, IL-1, and IL-6 (Zhao et al., 2019). Therefore, in patients with lung complications, consider prioritizing IGU. RA specifically impacts the cardiovascular system, including the cardiac conduction system. An essential factor contributing to heart function impairment in RA patients is the imbalance between oxidation and the antioxidant system. A clinical study on refractory RA revealed that the combination of IGU and MTX can increase superoxide dismutase, reduce total antioxidant capacity, and, in controlling oxidative stress, suppress cardiovascular diseases associated with RA (Lai, 2018).
This systematic review and meta-analysis showed that IGU can reduce disease activity (increase RA remission rate and reduce DAS28) and improve patients’ inflammatory response (reduce ESR, CRP and RF) in patients with RA. Regarding safety, adding IGU does not increase the incidence of adverse events. However, considering that most RCTs have a high risk of bias in blind implementation and an unknown risk of bias in allocation concealment and random sequence generation, especially Mo et al. (2018), which has a high risk of bias in selective reporting, the stability of the conclusions has been affected to a certain extent, and the conclusions need to be interpreted with caution.
4.5 Possible sources of heterogeneity
The heterogeneity of BASDAI, WOMAC, ACR and some inflammatory factors was high. We consider that this may be related to the following reasons: 1) basic characteristics of patients (such as age, gender, severity of disease, etc.); 2) different IGU preparations, or different combination therapies or basic treatments for each patient and RCT; 3) The heterogeneity of subjective outcome indicators (BASDAI, WOMAC and ACR) may be related to the high risk of bias of the blinding method.
4.6 Limitations and future prospects
This systematic review and meta-analysis has several limitations. First, most of the included RCTs were conducted in China and Japan, which may limit the applicability of the findings to the East Asian populations. As a result, the conclusions drawn from this analysis may primarily reflect the effectiveness of IGU in treating RA, AS, and OA in East Asian individuals. Additionally, the limited number of RCTs focusing on IGU for OA patients underscores the need for more studies to strengthen the evidence base. Furthermore, since IGU has only been recently introduced, its mechanisms of action and interactions with other medications, such as MTX and leflunomide, require further exploration. In summary, IGU demonstrates superior efficacy in treating RA, OA, and AS compared to control groups without increasing the incidence of adverse reactions. This suggests that IGU offers a promising new treatment option for these conditions. However, further multicenter, large-sample, high-quality randomized controlled trials are needed to provide more robust evidence.
5 Conclusion
Given the existing data, IGU might emerge as a promising and secure therapeutic modality for addressing AS, OA, and RA. Nevertheless, additional RCTs are imperative to assess its effectiveness across other inflammatory joint disorders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
ZL: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. LZ: Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. KY: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. JC: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. YL: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. CD: Data curation, Formal Analysis, Writing–original draft. QH: Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. YD: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. AG: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. XZ: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. WH: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. LS: Data curation, Formal Analysis, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge all the institutes, universities and hospitals affiliated for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1440584/full#supplementary-material
References
Bai, Y. J., Wang, X. Y., and Yao, Y. J. (2021). Observation on the clinical effect of iguratimod in the treatment of axial spondyloarthritis. Chin. Med. Innov. 18 (2), 44–47. doi:10.3969/j.issn.1674-4985.2021.02.011
Bi, W. H. (2019). The effect of Iguratimod combined with methotrexate on serum VEGF levels in patients with rheumatoid arthritis and evaluation of the efficacy[D]. Inner Mongolia Medical University.
Brown, P., Pratt, A. G., and Hyrich, K. L. (2024). Therapeutic advances in rheumatoid arthritis. BMJ 384, e070856. doi:10.1136/bmj-2022-070856
Burmester, G. R., and Pope, J. E. (2017). Novel treatment strategies in rheumatoid arthritis. Lancet 389 (10086), 2338–2348. doi:10.1016/S0140-6736(17)31491-5
Chen, J., Che, Q., Kou, Y., Rong, X., Zhang, X., Li, M., et al. (2023). A novel drug combination of Tofacitinib and Iguratimod alleviates rheumatoid arthritis and secondary osteoporosis. Int. Immunopharmacol. 124 (Pt B), 110913. doi:10.1016/j.intimp.2023.110913
Chen, J., Ding, Z. H., and Liu, J. (2018). Effects of Iguratimod combined with methotrexate on serum inflammatory factors and bone metabolism in patients with rheumatoid arthritis. Zhejiang J. Integr. Traditional Chin. West. Med. 28, 552–555.
Clark, G. P. (2023). Treatment options for symptomatic knee osteoarthritis in adults. JAAPA 36 (11), 1–6. doi:10.1097/01.JAA.0000979536.73946.98
Cong, S., Meng, Y., Wang, L., Sun, J., Shi Nu Er Xia Ti, T. B., and Luo, L. (2021). T-614 attenuates knee osteoarthritis via regulating Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 16 (1), 403. doi:10.1186/s13018-021-02530-2
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020a). “Chapter 16: special topics in statistics,” in Cochrane Handbook for systematic reviews of interventions. Editors J. P. Higgins, and S. Green (UK: The Cochrane Collaboration).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020b). “Chapter 8: assessing risk of bias in included studies,” in Cochrane Handbook or systematic reviews of interventions. Editors J. P. Higgins, and S. Green (UK: The Cochrane Collaboration).
Deeks, J. J., Higgins, J. P., and Altman, D. G. (2020c). “Chapter 9: analyzing data and undertaking meta-analyses,” in Cochrane Handbook for systematic reviews of interventions. Editors J. P. Higgins, and S. Green (UK: The Cochrane Collaboration).
Deng JX the effect of Iguratimod on the proliferation and migration of fibroblast-like synovial cells in rheumatoid arthritis and the clinical observation. Southern Medical University,2017.
Deng, L., Yao, F., Tian, F., Luo, X., Yu, S., and Wen, Z. (2022). Influence of iguratimod on bone metabolism in patients with rheumatoid arthritis: a meta-analysis. Int. J. Clin. Pract. 2022, 5684293. doi:10.1155/2022/5684293
Di Matteo, A., Bathon, J. M., and Emery, P. (2023). Rheumatoid arthritis. Lancet 402 (10416), 2019–2033. doi:10.1016/S0140-6736(23)01525-8
Du, F., Lü, L. J., Teng, J. L., Shen, N., Ye, P., and Bao, C. D. (2012). T-614 alters the production of matrix metalloproteinases (MMP-1 and MMP-3) and inhibits the migratory expansion of rheumatoid synovial fibroblasts, in vitro. Int. Immunopharmacol. 13 (1), 54–60. doi:10.1016/j.intimp.2012.03.003
Duan, X. W., Zhang, X. L., Mao, S. Y., Shang, J. J., and Shi, X. D. (2015). Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin. Rheumatol. 34, 1513–1519. doi:10.1007/s10067-015-2999-6
Fan, X., Wu, P., Song, M., and Tang, J. (2020). The clinical efficacy of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. J. Clin. Ration. Use 13, 81–83. doi:10.15887/j.cnki.13-1389/r.2020.33.031
Feng, J., Gong, S., Xia, Y., Tian, R., Yang, N., Zhao, X., et al. (2019). Effects of Iguratimod on peripheral blood osteoclast differentiation and osteoclast-related gene expression in rheumatoid arthritis. Chin. J. Osteoporos. 25 (1), 97–102. doi:10.3969/j.issn.1006-7108.2019.01.018
Gravallese, E. M., and Firestein, G. S. (2023). Rheumatoid arthritis - common origins, divergent mechanisms. N. Engl. J. Med. 388 (6), 529–542. doi:10.1056/NEJMra2103726
Gu, X. J., Chen, H., Li, R. P., Gan, F. Y., and Guo, D. B. (2020). The clinical efficacy of iguratimod combined with methotrexate in the treatment of early rheumatoid arthritis. Contemp. Med. 26, 138–139.
Guifeng, H., and Yasong, Li (2014). Observation on the short-term clinical effect of iguratimod on rheumatoid arthritis complicated with chronic interstitial pneumonia. China Mod. Appl. Pharm. 31 (10), 1275–1278. doi:10.13748/j.cnki.issn1007-7693.2014.10.029
Gulati, M., Dursun, E., Vincent, K., and Watt, F. E. (2023). The influence of sex hormones on musculoskeletal pain and osteoarthritis. Lancet Rheumatol. 5 (4), e225–e238. doi:10.1016/S2665-9913(23)00060-7
Han, Q., Fang, C., Wang, Z., Tuo, B., Liang, Q., Wu, Z., et al. (2018). Protective effects of Iguratimod on lung tissue of mice with pulmonary fibrosis. Chin. J. Clin. Immun. Allergy 12 (2), 151–155. doi:10.3969/j.issn.1673-8705.2018.02.003
Han, Y., Huang, L., Yang, M., Huang, S., Xiao, Y., Huang, Y., et al. (2023). 104 cases of active elderly rheumatoid arthritis treated with Iguratimod or methotrexate combined with leflunomide. Chin. J. New Drugs 32 (17), 1749–1755.
Hara, M., Abe, T., Sugawara, S., Mizushima, Y., Hoshi, K., Irimajiri, S., et al. (2007). Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod. Rheumatol. 17 (1), 1–9. doi:10.1007/s10165-006-0542-y
Hara, M., Ishiguro, N., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. Iguratimod-Clinical Study Group (2014). Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: an open-label extension of a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 24, 410–418. doi:10.3109/14397595.2013.843756
Harmalkar, D. S., Sivaraman, A., Nada, H., Lee, J., Kang, H., Choi, Y., et al. (2024). Natural products as IL-6 inhibitors for inflammatory diseases: synthetic and SAR perspective. Med. Res. Rev. 44, 1683–1726. doi:10.1002/med.22022
He, Y., Yang, G., Zheng, Y., Xiaofang, Xu, Pan, X., and Wu, H. (2015). Clinical observation of iguratimod in the treatment of rheumatoid arthritis complicated with interstitial lung disease. J. Pract. Clin. Med. 19 (23), 152–154.
Horváth, E., Sólyom, Á., Székely, J., Nagy, E. E., and Popoviciu, H. (2023). Inflammatory and metabolic signaling interfaces of the hypertrophic and senescent chondrocyte phenotypes associated with osteoarthritis. Int. J. Mol. Sci. 24 (22), 16468. doi:10.3390/ijms242216468
Hou, N., Liu, L., and Ma, X. (2021). Iguratimod alleviates systemic lupus erythematosus-induced renal injury by inhibiting IL-33/ST2 pathway. Minerva Med. doi:10.23736/S0026-4806.20.07227-4
Hu, H. (2014). Observation on the efficacy and safety of Iguratimod in the treatment of rheumatoid arthritis. Suzhou University. doi:10.7666/d.D506769
Hu, P., Cai, J., Yang, C., Xu, L., Ma, S., Song, H., et al. (2024). SLAMF3 promotes Th17 differentiation and is reversed by iguratimod through JAK1/STAT3 pathway in primary Sjögren's syndrome. Int. Immunopharmacol. 126, 111282. doi:10.1016/j.intimp.2023.111282
Huang, B. J., and Ma, J. X. (2018). Observation on the short-term curative effect of iguratimod in the treatment of ankylosing spondylitis. Chin. Community Physician 34 (13), 92–93. doi:10.3969/j.issn.1007-614x.2018.13.052
Ishiguro, N., Yamamoto, K., Katayama, K., Kondo, M., Sumida, T., Mimori, T., et al. Iguratimod-Clinical Study Group (2013). Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 23, 430–439. doi:10.1007/s10165-012-0724-8
Ishikawa, K., and Ishikawa, J. (2019). Iguratimod, a synthetic disease modifying anti-rheumatic drug inhibiting the activation of NF-κB and production of RANKL: its efficacy, radiographic changes, safety and predictors over two years' treatment for Japanese rheumatoid arthritis patients. Mod. Rheumatol. 29 (3), 418–429. doi:10.1080/14397595.2018.1481565
Jiang, H., Gao, H., Wang, Q., Wang, M., and Wu, B. (2020). Molecular mechanisms and clinical application of Iguratimod: a review. Biomed. Pharmacother. 122, 109704. doi:10.1016/j.biopha.2019.109704
Ju, Y., Guo, D., Chen, H., and Li, W. (2020). Evaluation of the clinical efficacy of methotrexate and iguratimod in the treatment of refractory rheumatoid arthritis. China Mod. Dr. 058, 106–109.
Juma, S. N., Liao, J., Huang, Y., Vlashi, R., Wang, Q., Wu, B., et al. (2023). Osteoarthritis versus psoriasis arthritis: physiopathology, cellular signaling, and therapeutic strategies. Genes Dis. 11 (3), 100986. doi:10.1016/j.gendis.2023.04.021
Katz, J., and Bartels, C. M. (2024). Multimorbidity in rheumatoid arthritis: literature review and future directions. Curr. Rheumatol. Rep. 26 (1), 24–35. doi:10.1007/s11926-023-01121-w
Kim, H. A. (2022). Osteoarthritis - insights from recent research. J. Rheum. Dis. 29 (3), 132–139. doi:10.4078/jrd.2022.29.3.132
Knights, A. J., Redding, S. J., and Maerz, T. (2023). Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr. Opin. Rheumatol. 35 (2), 128–134. doi:10.1097/BOR.0000000000000923
Lai, S. F. (2018). Efficacy of Iguratimod and bisacodyl ryanodine in patients with refractory rheumatoid arthritis and their effects on the levels of inflammatory factors and superoxide dismutase and other indicators. Anti-infective Pharmacol. 15 (1), 153–155. doi:10.13493/j.issn.1672-7878.2018.01-057
Lau, C. S. (2023). Burden of rheumatoid arthritis and forecasted prevalence to 2050. Lancet Rheumatol. 5 (10), e567–e568. doi:10.1016/S2665-9913(23)00240-0
Li, B., Li, P., and Bi, L. (2020). Iguratimod dose dependently inhibits the expression of citrullinated proteins and peptidylarginine deiminases 2 and 4 in neutrophils from rheumatoid arthritis patients. Clin. Rheumatol. 39 (3), 899–907. doi:10.1007/s10067-019-04835-4
Li, C., and Huang, W. (2020). The effect of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis with peripheral blood nuclear factor kappa B receptor activator ligand and bone protective factor. Chin. Med. Clin. 20, 2981–2983. doi:10.11655/zgywylc2020.18.001
Li, J., Bao, J., Zeng, J., Yan, A., Zhao, C., and Shu, Q. (2019). Iguratimod: a valuable remedy from the Asia Pacific region for ameliorating autoimmune diseases and protecting bone physiology. Bone Res. 7, 27. doi:10.1038/s41413-019-0067-6
Li, J., Chen, Q., Liu, J., Wang, X., and Liu, D. (2016). The treatment of 84 cases of senile rheumatoid arthritis with the combination of iguratimod and methotrexate. Shaanxi Med. J. 45, 120–121. doi:10.3969/j.issn.1000-7377.2016.01.049
Li, X., Pan, T., and Chen, M. P. (2021a). Observation of the curative effect of iguratimod in the treatment of ankylosing spondylitis. J Med Theor Prac 34 (17), 3009–3011.
Li, Y. (2014). Recent clinical efficacy observation of Iguratimod on rheumatoid arthritis combined with chronic interstitial pneumonia. China Mod. Appl. Pharm. 31 (10), 1275–1278. doi:10.13748/j.cnki.issn1007-7693.2014.10.029
Li, Y., Li, K., Zhao, Z., Wang, Y., Jin, J., Guo, J., et al. (2021b). Randomised, double-blind, placebo-controlled study of iguratimod in the treatment of active spondyloarthritis. Front. Med. (Lausanne) 8, 678864. doi:10.3389/fmed.2021.678864
Li, L., Wang, J., and Li, X. (2019). A clinical study on the treatment of early rheumatoid arthritis by iguratimod combined with methotrexate. China Pharm. 28, 63–65.
Lin, J., Yu, Y., Wang, X., Ke, Y., Sun, C., Yue, L., et al. (2019b). Iguratimod inhibits the aggressiveness of rheumatoid fibroblast-like synoviocytes. J. Immunol. Res. 2019, 6929286. doi:10.1155/2019/6929286
Lin, Q. (2016). A clinical study on the treatment of rheumatoid arthritis complicated with pulmonary interstitium by iguratimod. North. Pharm. 13 (08), 124–125.
Lin, Y. P., Liu, H., and Gao, J. T. (2019a). Preliminary observation on the treatment of ankylosing spondylitis with iguratimod. J. Clin. Ration. Use 012 (014), 9–13.
Liu, S., Cui, Y., and Zhang, X. (2021). Molecular mechanisms and clinical studies of iguratimod for the treatment of ankylosing spondylitis. Clin. Rheumatol. 40 (1), 25–32. doi:10.1007/s10067-020-05207-z
Liu, Z., Cai, M., Ke, H., Deng, H., Ye, W., Wang, T., et al. (2023). Fibroblast insights into the pathogenesis of ankylosing spondylitis. J. Inflamm. Res. 16, 6301–6317. doi:10.2147/JIR.S439604
Long, Z., Zeng, L., He, Q., Yang, K., Xiang, W., Ren, X., et al. (2023). Research progress on the clinical application and mechanism of iguratimod in the treatment of autoimmune diseases and rheumatic diseases. Front. Immunol. 14, 1150661. doi:10.3389/fimmu.2023.1150661
Lu, J. (2014). The therapeutic effect of combined application of iguratimod and methotrexate on active rheumatoid arthritis. Shandong University.
Lu, L. J., Bao, C. D., Dai, M., Teng, J. L., Fan, W., Du, F., et al. (2009). Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum. 61 (7), 979–987. doi:10.1002/art.24643
Lü, L. J., Teng, J. L., Bao, C. D., Han, X. H., Sun, L. Y., Xu, J. H., et al. (2008). Safety and efficacy of T-614 in the treatment of patients with active rheumatoid arthritis: a double blind, randomized, placebo-controlled and multicenter trial. Chin. Med. J. Engl. 121 (7), 615–619. doi:10.1097/00029330-200804010-00008
Luo, C., Shi, Y., Chen, X., and Wu, L. (2019). Clinical analysis of 46 cases of PrimarySjogren’s syndrome treated by iguratimod. Chin. J. Pract. Diagn. Ther. 33 (12), 1232–1235. doi:10.13507/j.issn.1674-3474.2019.12.024
Luo, Q., Sun, Y., Liu, W., Qian, C., Jin, B., Tao, F., et al. (2013). A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J. Immunol. 191 (10), 4969–4978. doi:10.4049/jimmunol.1300832
Luo, Y., Zheng, N., and Wu, R. (2018). Is iguratimod effective in refractory axial spondyloarthritis? Scand. J. Rheumatol. 47 (6), 518–520. doi:10.1080/03009742.2017.1390150
Ma, D., Zhang, Z., Wang, D., and Wang, S. (2019). Efficacy and effect on serum HIF-1α and IL-22 levels of radicicic acid polyside combined with iguratimod in the treatment of RA combined with interstitial lung disease. J. Guangxi Med. Univ. 36 (1), 45–48. doi:10.16190/j.cnki.45-1211/r.2019.01.011
Macleod, T., Bridgewood, C., and McGonagle, D. (2023). Role of neutrophil interleukin-23 in spondyloarthropathy spectrum disorders. Lancet Rheumatol. 5 (1), e47–e57. doi:10.1016/S2665-9913(22)00334-4
Man, Li, and Yongxin, X. (2020). Clinical efficacy of iguratimod in the treatment of inflammatory myopathy complicated with interstitial pneumonia. J. Clin. Ration. Med. 13 (21), 12–14. doi:10.15887/j.cnki.13-1389/r.2020.21.004
Marín, J. S., Mazenett-Granados, E. A., Salazar-Uribe, J. C., Sarmiento, M., Suárez, J. F., Rojas, M., et al. (2023). Increased incidence of rheumatoid arthritis after COVID-19. Autoimmun. Rev. 22 (10), 103409. doi:10.1016/j.autrev.2023.103409
Meng, D. Y., Pan, W. Y., Li, J., Li, H., Li, F., Liu, S. S., et al. (2016b). The effect of methotrexate combined with iguratimod in the treatment of refractory rheumatoid arthritis. China Med. Her. 13, 137–141.
Meng, D. Y., Pan, W. Y., Liu, Y., Jiang, Z., Li, J., Li, H., et al. (2016a). Iguratimod combined with methotrexate on angiogenesis-related cytokines in patients with refractory rheumatoid arthritis the influence of. Med. Her. 35, 148–151.
Meng, D., Wang, G., Pan, W., Li, H., Liu, S., Li, Y., et al. (2015). Short-term clinical efficacy of methotrexate combined with Iguratimod on refractory rheumatoid arthritis. Chin. J. Clin. Res. 28, 40–42.
Meng, D., Pan, W., Liu, Y., Jiang, Z., Li, J., Li, H., et al. (2016c). Effect of Iguratimod combined with methotrexate on angiogenesis-related cytokines in patients with refractory rheumatoid arthritis. Med. Her. (2), 153–156. doi:10.3870/j.issn.1004-0781.2016.02.011
Meng, Y., Li, M. Y., Rode, M., Zhang, X. Y., and Luo, L. (2017). Clinical study on the treatment of senile rheumatoid arthritis with Iguratimod tablets combined with methotrexate tablets. Chin. J. Clin. Pharmacol. 33, 1098–1101.
Messina, O. D., Vidal, M., Adami, G., Vidal, L. F., Clark, P., Torres, J. A. M., et al. (2023). Chronic arthritides and bone structure: focus on rheumatoid arthritis-an update. Aging Clin. Exp. Res. 35 (7), 1405–1416. doi:10.1007/s40520-023-02432-9
Minnig, M. C. C., Golightly, Y. M., and Nelson, A. E. (2024). Epidemiology of osteoarthritis: literature update 2022-2023. Curr. Opin. Rheumatol. 36 (2), 108–112. doi:10.1097/BOR.0000000000000985
Miura, T., Etani, Y., Noguchi, T., Hirao, M., Takami, K., Goshima, A., et al. (2024). Iguratimod suppresses sclerostin and receptor activator of NF-κB ligand production via the extracellular signal-regulated kinase/early growth response protein 1/tumor necrosis factor alpha pathway in osteocytes and ameliorates disuse osteoporosis in mice. Bone 181, 117026. doi:10.1016/j.bone.2024.117026
Mo, H., and Ma, S. B. (2015). Clinical study on the treatment of active rheumatoid arthritis with Iguratimod combined with methotrexate. Intern. Med. 10, 156–159. doi:10.16121/j.cnki.cn45-1347/r.2015.02.06
Mo, M., Tang, D., Zhang, J., Liu, Y., and Xie, L. (2018). A randomized controlled trial of methotrexate combined with iguratimod in the treatment of active rheumatoid arthritis. J. Fujian Med. Univ. 52, 40–43.
Mohapatra, A., Patwari, S., Pansari, M., and Padhan, S. (2023). Navigating pain in Rheumatology: a physiotherapy-centric review on non-pharmacological pain management strategies. Cureus 15 (12), e51416. doi:10.7759/cureus.51416
Mysler, E., Burmester, G. R., Saffore, C. D., Liu, J., Wegrzyn, L., Yang, C., et al. (2024). Safety of upadacitinib in immune-mediated inflammatory diseases: systematic literature review of indirect and direct treatment comparisons of randomized controlled trials. Adv. Ther. 41 (2), 567–597. doi:10.1007/s12325-023-02732-6
Nozaki, Y. (2021). Iguratimod: novel molecular insights and a new csDMARD for rheumatoid arthritis, from Japan to the World. Life (Basel) 11 (5), 457. doi:10.3390/life11050457
Okamura, K., Yonemoto, Y., Suto, T., Okura, C., and Takagishi, K. (2015). Efficacy at 52 weeks of daily clinical use of iguratimod in patients with rheumatoid arthritis. Mod. Rheumatol. 25, 534–539. doi:10.3109/14397595.2014.998361
Pang, L., Zheng, Z., Li, Z., Li, Y., and Wu, Z. (2020). Curative effect of iguratimod combined with etanercept on ankylosing spondylitis. J. Trop. Med. 20 (04), 538–541. doi:10.3969/j.issn.1672-3619.2020.04.027
Peng, T., Li, B., Bi, L., and Zhang, F. (2024). Iguratimod inhibits protein citrullination and inflammation by downregulating NBCe2 in patients with rheumatoid arthritis. Biomed. Pharmacother. 174, 116551. doi:10.1016/j.biopha.2024.116551
Perruccio, A. V., Young, J. J., Wilfong, J. M., Denise Power, J., Canizares, M., and Badley, E. M. (2024). Osteoarthritis year in review 2023: epidemiology and therapy. Osteoarthr. Cartil. 32 (2), 159–165. doi:10.1016/j.joca.2023.11.012
Qi, D. X., Liu, Y., and Huang, D. H. (2019). Study on the efficacy and safety of methotrexate and isilamod in the treatment of rheumatoid arthritis. Chin. J. Drug Eval. 036, 217–220.
Rao, Y., Jun, T. A. N., and Dianchen, W. U. (2014). Observation on the clinical efficacy of Iguratimod in the treatment of rheumatoid arthritis. China Med. Guide 12 (29), 41–42. doi:10.15912/j.cnki.gocm.2014.29.027
Ren, Y., Dong, W., Liu, H., and Liu, L. (2017). Clinical observation of Iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. China Pharm. 28 (32), 4530–4533. doi:10.6039/j.issn.1001-0408.2017.32.18
Rodolfi, S., Davidson, C., and Vecellio, M. (2024). Regulatory T cells in spondyloarthropathies: genetic evidence, functional role, and therapeutic possibilities. Front. Immunol. 14, 1303640. doi:10.3389/fimmu.2023.1303640
Sarzi-Puttini, P., Zen, M., Arru, F., Giorgi, V., and Choy, E. A. (2023). Residual pain in rheumatoid arthritis: is it a real problem? Autoimmun. Rev. 22 (11), 103423. doi:10.1016/j.autrev.2023.103423
Scheuing, W. J., Reginato, A. M., Deeb, M., and Acer Kasman, S. (2023). The burden of osteoarthritis: is it a rising problem? Best. Pract. Res. Clin. Rheumatol. 37 (2), 101836. doi:10.1016/j.berh.2023.101836
Shang, K, Jiang, L., and Wang, Y. (2019). Efficacy of iguratimod tablet monotherapy in the treatment of active rheumatoid arthritis and its effect on bone metabolism. Jiangxi Med. 54 (06), 593–595+619. doi:10.3969/j.issn.1006-2238.2019.6.004
Shen, L., Yin, H., Sun, L., Zhang, Z., Jin, Y., Cao, S., et al. (2024). Correction: iguratimod attenuated fbrosis in systemic sclerosis via targeting early growth response 1 expression. Arthritis Res. Ther. 26 (1), 34. doi:10.1186/s13075-024-03268-y
Shi, W., Liu, B., and Wang, W. (2023). Analysis of the efficacy of iguratimod combined with disease-improving antirheumatic drugs in the treatment of rheumatoid arthritis combined with osteoporosis. J. Clin. Pharmacother. 21 (02), 31–35.
Shi, X. D., Zhang, X. L., and Duan, X. W. (2015). Efficacy and safety of methotrexate combined with iguratimod in the treatment of active rheumatoid arthritis. J. Nanjing Med. Univ. 55, 5.
Shrestha, S., Zhao, J., Yang, C., and Zhang, J. (2020). Relative efficacy and safety of iguratimod monotherapy for the treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 39 (7), 2139–2150. doi:10.1007/s10067-020-04986-9
Sun, L., Tao, J., Han, Z., Chen, H., Huang, Z., Wang, Z., et al. (2023). Efficacy of iguratimod on mineral and bone disorders after kidney transplantation: a preliminary study. Ren. Fail 45 (2), 2256418. doi:10.1080/0886022X.2023.2256418
Sun, P., and Li, R. (2022). Clinical efficacy of Iguratimod combined with methotrexate in the treatment of rheumatoid arthritis and its effect on bone metabolism in patients. Clin. Med. Eng. 29 (01), 41–42.
Suto, T., Yonemoto, Y., Okamura, K., Sakane, H., Takeuchi, K., Tamura, Y., et al. (2019). The three-year efficacy of iguratimod in clinical daily practice in patients with rheumatoid arthritis. Mod. Rheumatol. 29, 775–781. doi:10.1080/14397595.2018.1510879
Syed, N. H., Mussa, A., Elmi, A. H., Jamal Al-Khreisat, M., Ahmad, M., Zain, M. R., et al. (2023). Role of MicroRNAs in inflammatory joint diseases: a review. Immunol. Invest. 53, 185–209. doi:10.1080/08820139.2023.2293095
Taylor, P. C. (2023). Pain in the joints and beyond; the challenge of rheumatoid arthritis. Lancet Rheumatol. 5 (6), e351–e360. doi:10.1016/S2665-9913(23)00094-2
Tian, J. W., and Tao, P. F. (2017). The effect of Iguratimod combined with methotrexate on serum M-CSF, IL-6, IL-8 and bone metabolism in patients with rheumatoid arthritis. Hainan Med. 28, 391–394.
Tian, X. P., Liu, S. Y., Li, Q., Bi, L. Q., Kong, X. D., Zhao, D. B., et al. (2020). Efficacy and safety of iguratimod or leflunomide combined with methotrexate in the treatment of active rheumatoid arthritis Sexual comparison: a multicenter randomized double-blind double-simulation controlled clinical study. Chin. J. Rheumatology 24, 148–158. doi:10.3760/cma.j.issn.1007-7480.2020.03.002
van de Sande, M. G. H., and Elewaut, D. (2023). Pathophysiology and immunolgical basis of axial spondyloarthritis. Best. Pract. Res. Clin. Rheumatol. 37 (3), 101897. doi:10.1016/j.berh.2023.101897
Venetsanopoulou, A. I., Alamanos, Y., Voulgari, P. V., and Drosos, A. A. (2023). Epidemiology and risk factors for rheumatoid arthritis development. Mediterr. J. Rheumatol. 34 (4), 404–413. doi:10.31138/mjr.301223.eaf
Wang, L., Liu, S., Chen, R., and Fu, K. (2019). Clinical study on the treatment of rheumatoid arthritis with methotrexate tablets combined with Iguratimod tablets. Chin. J. Clin. Pharmacol. 35, 231–234.
Wang, X., Ma, C., Li, P., Zhao, F., and Bi, L. (2017). Effects of iguratimod on the levels of circulating regulators of bone remodeling and bone remodeling markers in patients with rheumatoid arthritis. Clin. Rheumatol. 36, 1369–1377. doi:10.1007/s10067-017-3668-8
Wang, X., Yuan, X., Wang, Q., Zhou, Y., Li, X., Wang, G., et al. (2018). Clinical study of effectiveness and safety of iguratimod in treating primary sjogren’s syndrome. Chin. J. Dis. Control Prev. 22 (1), 75–78. doi:10.16462/j.cnki.zhjbkz.2018.01.017
Wang, X. T. (2017). Study on the effect of iguratimod on bone metabolism in patients with rheumatoid arthritis[D]. Jilin, China: Jilin University.
Wang, X., and Shen, H. (2015). Experimental study on the in vitro intervention of Iguratimod on synovial cells in rheumatoid arthritis. Shaanxi Med. J. (3), 292–293. doi:10.3969/j.issn.1000-7377.2015.03.011
Wei, G., Lu, K., Umar, M., Zhu, Z., Lu, W. W., Speakman, J. R., et al. (2023). Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone Res. 11 (1), 63. doi:10.1038/s41413-023-00301-9
Wu, J., Liang, Y., Li, M., Sha, H., Wang, C., Wang, L., et al. (2022). Repairing effect and safety analysis of Iguratimod combined with methotrexate and tretinoin on articular chondrocyte damage in rheumatoid arthritis. Anhui Med. 26 (01), 183–187.
Wu, J., Yang, X., Chen, X., Huang, R., Huang, Q., Zhao, Y., et al. (2018). Systematic evaluation and meta-analysis of the efficacy and safety of iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. Chin. Clin. Pharmacol. Ther. 23 (10), 1132–1140. doi:10.12092/j.issn.1009-2501.2018.10.008
Xia, N. N., Chen, Z. F., and Zhang, W. F. (2020). Observation on the effects of Iguratimod and Tripterygium Glycosides in the treatment of rheumatoid arthritis. Pract. Integr. Traditional Chin. West. Med. 20, 76–77.
Xia, Z., Lyu, J., Hou, N., Song, L., Li, X., and Liu, H. (2016). Iguratimod in combination with methotrexate in active rheumatoid arthritis: therapeutic effects. Z Rheumatol. 75, 828–833. English. doi:10.1007/s00393-015-1641-y
Xie, Li, Zou, Q. H., Shi, Y., Cheng, X., and Fang, Y. F. (2018). The effect of Iguratimod combined with MTX on IL-1, serum TNF-α and VEGF levels in patients with refractory rheumatoid arthritis. Guizhou Med. 42, 831–832.
Xie, S., Li, S., Tian, J., and Li, F. (2020). Iguratimod as a new drug for rheumatoid arthritis: current landscape. Front. Pharmacol. 11, 73. doi:10.3389/fphar.2020.00073
Xiong, M., and Guanghui, G. (2020). The clinical efficacy of methotrexate combined with iguratimod on active rheumatoid arthritis. Henan Med. Res. 29 (2020), 93–95.
Xiong, Y., Fan, S., and Zou, L. (2015). Study on the effect of iguratimod combined with methotrexate treatment on anti-cyclic guanidine peptide antibodies and other indicators in patients with rheumatoid arthritis. J. Clin. Intern. Med. 32, 833–835. doi:10.3969/j.issn.1001-9057.2015.12.014
Xu, B., Mo, S., and Xue, X. (2015). Clinical study of methotrexate combined with iguratimod in the treatment of rheumatoid arthritis. J. Clin. Med. Pract. 19, 120–122.
Xu, B., Xing, R., and Huang, Z. (2023). The ion channel TRPV4 participates in the cartilage protection of Iguratimod on knee osteoarthritis. EULAR, POS0399.
Xu, B., Xing, R., Huang, Z., Yin, S., Li, X., Zhang, L., et al. (2019b). Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 228, 158–166. doi:10.1016/j.lfs.2019.05.003
Xu, B. J., Mo, S. Q., and Xue Xq Wu, Y. (2019a). Study on the efficacy and safety of iguratimod in the treatment of ankylosing spondylitis. New Med. 50 (12), 915–918.
Xu, L. M., Yuan, M., Liu, Y. F., Sun, H. X., Liu, L. N., Shi, Y. J., et al. (2017). Clinical observation on the treatment of rheumatoid arthritis with Iguratimod combined with methotrexate. Guide Chin. Med. 15, 47–48.
Xu, Y. W., Tao, Y. L., Zhang, H., and Dai, S. M. (2021). Observation on the clinical efficacy of iguratimod in the treatment of axial spondyloarthritis. Shanghai Med. J. 44 (06), 421–424. doi:10.19842/j.cnki.issn.0253-9934.2021.06.013
Wang, Y., Chen, C., Gao, W., and Guo, F. (2022). Evaluation of the efficacy of high-frequency ultrasound in the treatment of rheumatoid arthritis with Iguratimod. Med. Rev. 28 (10), 2055–2059.
Yan, X. Z., and Wang, F. L. (2018). Effect of Iguratimod combined with methotrexate on serum-related cytokines and bone metabolism in patients with rheumatoid arthritis. J. Changchun Univ. Traditional Chin. Med. 34, 369–372. doi:10.13463/j.cnki.cczyy.2018.02.054
Yi, D., Deng, S., Zou, S., and Dou, T. (2022). Observation on the efficacy of methotrexate combined with iguratimod in the treatment of rheumatoid arthritis. Mod. Pract. Med. 34 (01), 114–116.
Yoshioka, Y., Takahashi, N., Kaneko, A., Hirano, Y., Kanayama, Y., Kanda, H., et al. (2016). Disease activity early in treatment as a predictor of future low disease activity in RA patients treated with iguratimod. Mod. Rheumatol. 26, 169–174. doi:10.3109/14397595.2015.1069475
Yuan, F. F., Chen, Y. H., Lin, J. X., and Luo, J. (2020). Efficacy of iguratimod combined with methotrexate in the treatment of refractory ankylosing spondylitis and its effect on serum SOD and CTX-I levels in patients. J. Pharm. Epidemiol. 29 (03), 163–165+205.
Zeng, H. Q., Kong, W. H., Zhuang, P., Dong, H. J., Yin, Z. H., Chen, X., et al. (2016). Observation on the efficacy of iguratimod in the treatment of ankylosing spondylitis. Hainan Med. 27 (01), 118–120.
Zeng, H., Luo, G., Lu, X., Huang, X., Zhang, Y., and Ye, Z. (2019). A preliminary study on the efficacy and mechanism of action of Iguratimod on knee osteoarthritis. Chin. J. Biomed. Eng. (02), 216–219. doi:10.3760/cma.j.issn.1674?1927.2019.02.016
Zeng, L., He, Q., Deng, Y., Li, Y., Chen, J., Yang, K., et al. (2023). Efficacy and safety of iguratimod in the treatment of rheumatic and autoimmune diseases: a meta-analysis and systematic review of 84 randomized controlled trials. Front. Pharmacol. 14, 1189142. doi:10.3389/fphar.2023.1189142
Zeng, L., He, Q., Yang, K., Hao, W., Yu, G., and Chen, H. (2022a). A systematic review and meta-analysis of 19 randomized controlled trials of iguratimod combined with other therapies for sjogren's syndrome. Front. Immunol. 13, 924730. doi:10.3389/fimmu.2022.924730
Zeng, L., Yu, G., Yang, K., Hao, W., and Chen, H. (2022b). The effect and safety of iguratimod combined with methotrexate on rheumatoid arthritis: a systematic review and meta-analysis based on a randomized controlled trial. Front. Pharmacol. 12, 780154. doi:10.3389/fphar.2021.780154
Zhang, W. (2022). Efficacy of Iguratimod combined with celecoxib in the treatment of ankylosing spondylitis. Med. Inf. 35 (15), 114–116. doi:10.3969/j.issn.1006-1959.2022.15.026
Zhang, Y., Chen, L., and Chen, J. (2023). Clinical efficacy of Iguratimod combined with glucosamine sulfate in the treatment of osteoarthritis and its effect on patients' cartilage metabolism. China Hosp. Drug Eval. Analysis 23 (04), 404–406+411. doi:10.14009/j.issn.1672-2124.2023.04.004
Zhao, H. N., and Hao, X. J. (2018). The clinical effect of Iguratimod combined with methotrexate in the treatment of rheumatoid arthritis. Clin. Med. Res. Pract. 003, 44–45.
Zhao, L., Jiang, Z., Zhang, Y., Ma, H., and Cai, C. (2017). Analysis of efficacy and safety of treatment of active rheumatoid arthritis with iguratimod and methotrexate. Biomed. Res. 28 (5), 2353–2359.
Zhao, L., Mu, B., Zhou, R., Cheng, Y., and Huang, C. (2019). Iguratimod ameliorates bleomycin-induced alveolar inflammation and pulmonary fibrosis in mice by suppressing expression of matrix metalloproteinase-9. Int. J. Rheum. Dis. 22 (4), 686–694. doi:10.1111/1756-185X.13463
Zhao, W. M., Yao, D. Y., Huo, H. S., Qin, C. M., Wei, Q. J., and Sun, K. (2016). Clinical study of Iguratimod in the treatment of active rheumatoid arthritis. Chin. J. Postgraduates Med. 39, 450–452.
Zhou, S., Zhao, G., Chen, R., Li, Y., Huang, J., Kuang, L., et al. (2024). Lymphatic vessels: roles and potential therapeutic intervention in rheumatoid arthritis and osteoarthritis. Theranostics 14 (1), 265–282. doi:10.7150/thno.90940
Zhu, Qi, Song, J., Yunzhi, Xu, Liu, H., Miao, Y., Fan, Y., et al. (2016). A study on the clinical efficacy of iguratimod on rheumatoid arthritis and the regulation of T helper cell subsets. Chin. Rheumatism J. Sci. 20 (02), 93–99.
Keywords: inflammatory arthritis, iguratimod, systematic review, meta-analysis, degenerative arthritis
Citation: Long Z, Zeng L, Yang K, Chen J, Luo Y, Dai CC, He Q, Deng Y, Ge A, Zhu X, Hao W and Sun L (2024) A systematic review and meta-analysis of the efficacy and safety of iguratimod in the treatment of inflammatory arthritis and degenerative arthritis. Front. Pharmacol. 15:1440584. doi: 10.3389/fphar.2024.1440584
Received: 29 May 2024; Accepted: 26 August 2024;
Published: 10 October 2024.
Edited by:
Luciane Cruz Lopes, University of Sorocaba, BrazilReviewed by:
Ruina Kong, The First Affiliated Hospital of Naval Medical University, ChinaQiang Shu, Shandong University, China
Copyright © 2024 Long, Zeng, Yang, Chen, Luo, Dai, He, Deng, Ge, Zhu, Hao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingyun Sun, lingyunsun@nju.edu.cn; Zhiyong Long, 2212471438@qq.com
†These authors share first authorship
 Zhiyong Long1*†
Zhiyong Long1*†