- 1Dipartimento Promozione della Salute Materno-Infantile di Medicina Interna e Specialistica di Eccellenza “G. D’Alessandro” (PROMISE), Università degli Studi di Palermo, Palermo, Italy
- 2Institute of Translational Pharmacology (IFT), National Research Council of Italy (CNR), Palermo, Italy
- 3PhD National Program in One Health Approaches to Infectious Diseases and Life Science Research, Department of Public Health, Experimental and Forensic Medicine, University of Pavia, Pavia, Italy
- 4Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, Palermo, Italy
Background and Aim: Increased oxidative stress within the airways is associated to epithelial damage and amplification of inflammatory responses that in turn contribute to Chronic Obstructive Pulmonary Disease (COPD) progression. This study was aimed to identify whether a new formulation of N-acetylcisteine (NAC), carnitine, curcumin and B2 vitamin could counteract oxidative stress and downstream pro-inflammatory events promoted by cigarette smoke extract (CSE) exposure in primary bronchial epithelial cells (PBEC), both submerged/undifferentiated (S-PBEC) and cultured at the air-liquid interface (ALI-PBEC).
Methods: PBEC were exposed to CSE with/without the new formulation or NAC alone and ROS production, IL-8 and IL-6 gene expression and protein release were evaluated.
Results: CSE increased ROS, IL-8 and IL-6 gene expression and protein release and the new formulation counteracted these effects. NAC alone was not effective on IL-8 and IL-6 release. The effects of a similar nutraceutical formulation were evaluated in COPD patients treated for six months. The results showed that the treatment reduced the concentration of IL-8 in nasal wash and improved quality of life.
Conclusion: The tested formulation, exerting antioxidant and anti-inflammatory effects, can preserve airway epithelial homeostasis and improve clinical symptoms in COPD.
1 Introduction
COPD is a chronic inflammatory pulmonary disease, which affects the lungs and bronchi, has a multifactorial etiology and a variable entity depending on its severity (Mannion et al., 2022). It is a long-lasting chronic condition whose damage is often irreversible, is characterized by symptoms such as dyspnea, cough, wheezing, excessive mucus production, weakness, and has two main phenotypes, emphysema and chronic bronchitis (Labaki and Rosenberg, 2020). Exacerbations in COPD may be frequent, and are also major phenotypic traits (Hurst et al., 2010; Agustí et al., 2023). The best known and most common cause of COPD is cigarette smoke, which irritates the mucous membranes favoring the onset of inflammatory processes (Aghapour et al., 2018). Cigarette smoke is known to be a major agent inducing oxidative stress and chronic lung inflammation (Sun et al., 2019), also leading to accelerated lung aging (Fairley et al., 2023). Oxidative stress is a key factor in the pathogenesis of COPD, especially in the exacerbation phases (Barnes, 2020), also increases chronic inflammation, stimulates fibrosis and emphysema, and in some cases even causes corticosteroid resistance (Bodas et al., 2017). In recent years, the field of nutraceuticals has acquired increasing importance. In fact, it is now widely demonstrated that diet and the use of nutritional supplements have a beneficial impact on human health, in the context of various pathologies, including respiratory diseases (Scoditti et al., 2019; Rahman et al., 2022). N-Acetylcysteine (NAC) is widely employed as mucolytic and antioxidant agents in several human diseases, in particular respiratory diseases, such as COPD (Pedre et al., 2021; Tenório et al., 2021). In patients with COPD, reduced levels of GSH have been demonstrated, while NAC contributes to restore and maintain the concentration of GSH high, ensuring its antioxidant actions (Rushworth and Megson, 2014; Aldini et al., 2018; Raghu et al., 2021). Several in vitro studies demonstrated a protective role of NAC against the mitochondrial oxidative damage, reducing the amount of reactive oxygen species (ROS) and restoring the normal function of the mitochondrial respiratory chain (Xiao et al., 2016; Liu et al., 2021; Li et al., 2022). Another important role of NAC is related to its anti-inflammatory activity, widely shown both in vitro and in vivo studies (Mohanty et al., 2021). Patients with COPD show a typical inflammatory asset in which levels of interleukin-6 (IL-6) and interleukin-8 (IL-8/CXCL8) are high, especially during exacerbations. IL-8 is released by bronchial epithelial cells and is a crucial cytokine for the activation of neutrophils in lungs (Pace et al., 2011). NAC is able to reduce gene expression, and thus protein release, of inflammatory cytokines such as TNF-α, IL-6, IL-1β and chemokines such as IL-8, through the downregulation of the nuclear transcription factor kappa B (NF-κB) (Radomska-Leśniewska et al., 2010; Zhang et al., 2019; Chi et al., 2022; Le et al., 2022). Numerous natural substances, in addition to traditional pharmacological therapies, have demonstrated interesting antioxidant, anti-inflammatory and anticancer effects. One of the most studied categories is that of phytocompounds (Mucha et al., 2021; Ożarowski and Karpiński, 2021), among them Curcuma longa exerts a relevant role. The derivative compound, curcumin, showed interesting antioxidant and anti-inflammatory effects in the context of several diseases, counteracting the oxidative damage and by reducing the inflammatory mediators (Lin et al., 2019; Mrityunjaya et al., 2020; Ran et al., 2021). Several in vitro studies have also demonstrated an antioxidant role of L-carnitine (Li et al., 2023). L-Carnitine (beta-hydroxy-gamma N-trimethyl amino-butyric acid) is a quaternary amine that mediates as an essential transport cofactor that transports fatty acids into the mitochondrial matrix and plays a critical role in fatty acid energy metabolism (Hoang et al., 2023). L-Carnitine is a water-soluble vitamin-like substance that occurs naturally in the human body (Talenezhad et al., 2020; Carrillo-González et al., 2023). In COPD, cachexia and muscle weakness are common systemic clinical manifestations besides pulmonary symptoms. According to some studies, L-carnitine deficiency occurs in COPD patients, and use of L-carnitine as a potential therapeutic agent for the long-term management of COPD patients has been proposed (Hoang et al., 2023). Indeed, L-carnitine reduces oxidative stress and inflammation by modulating efficient fatty acid oxidation (Pekala et al., 2011), functions that may reduce cytokine production in the inflammatory process and decrease myocyte damage in COPD patients (Krajcovicová-Kudlácková et al., 2000). Vitamins also play a crucial role in the proper functioning of the organism. Disorders of their levels, both deficiency or excess, promote the development of various diseases, including those of the respiratory system (Cheng et al., 2023). Reduced level of soft B vitamins have been documented in COPD patients, and supplementation of B vitamins could have a beneficial effect on oxidative stress in COPD patients (Kırkıl and Muz, 2008). Interestingly, studies have shown a promising improvement in alleviating systemic oxidative stress and clinical symptoms, and an improvement in health-related quality of life in subjects with chronic lung complications after supplementation with certain curcuminoids and piperine (Panahi et al., 2016). Piperine also has excellent therapeutic capabilities. Studies show that piperine in mice with COPD suppresses airway obstruction, oxidative stress and mitigates the increase in inflammatory cells and inflammatory biochemicals mediated by cigarette smoke (Arora et al., 2022). In light of this, the aim of our work was to evaluate the antioxidant and anti-inflammatory efficacy on human primary bronchial epithelial cells of a new nutraceutical formulation that associates NAC, already widely recognized and employed for its beneficial effects, with other natural substances: curcumin, carnitine, vitamin B2. The degree of mitochondrial oxidative stress and the gene expression and release of inflammatory cytokines such as IL-6 and IL-8 was evaluated on submerged/undifferentiated primary bronchial epithelial cells (S-PBEC) and then the results were confirmed in a more complex model of differentiated cells (ALI-PBEC) that more closely mirrors the in vivo conditions. Finally, clinical benefits as well as anti-inflammatory effects were also evaluated in COPD patients before and after 6 months treatment with a nutraceutical drug with curcumin, carnitine, acetyl-N-cysteine-L, vitamin B2, piperine.
2 Materials and methods
2.1 Human primary bronchial epithelial cells (PBECs) culture
Human primary bronchial epithelial cells (PBECs, ATCC-PCS-300-010) were purchased from American Type Culture Collection (ATCC, Rockville, MD, United States). PBECs were cultured submerged/undifferentiated (S-PBECs) to first evaluate markers and molecular mechanisms in a less complex in vitro model. PBECs were seeded on 6-well or 12-well plates previously coated with a collagen/fibronectin solution and grown in Bronchial Epithelial Cell Medium-basal (BEGM, LONZA Basel, Switzerland) and Dulbecco’s modified Eagle’s medium (DMEM) (Stemcell Technologies, Netherlands) mixture (1:1), supplemented with 12.5 mM Hepes, 1 mM Glutamax (Gibco, Thermo fisher scientific, Waltham, MA, United States), bronchial epithelial cell growth supplement (BEpiCGS; Sciencell, Sanbio, Netherlands), 100 U/mL penicillin and 100 μg/mL streptomycin (all from ScienCell, Sanbio, Netherlands) until confluence. To further investigate the same aspects in a more complex cellular model, PBECs were also cultured at the air–liquid interface (ALI-PBEC). In brief, 40,000 cells at passage two were seeded on 0.4 μm pore sized 12-well transwell membranes (Corning Costar, Glendale, AZ, United States), coated with a collagen/fibronectin solution. The cells were grown in the complete BEGM/DMEM medium (B/D), supplemented with 1 nM of the retinoic receptor agonist EC23 (Tocris, Bristol, UK) to support cultured submerged cells were until confluence; then, the cells were air-exposed by removing the apical medium and were differentiated by for 14 days in the same medium, with a higher concentration of EC23 (50 nM). Trans-epithelial electrical resistance [TEER >500 Ω∙cm2], cilia beating, and mucus secretion were assessed as markers of differentiation (Di Vincenzo et al., 2022). When the S-PBEC have reached the confluence and the ALI-PBEC completed the differentiation, cells were pre-treated with the NAC alone (1 mM) and with the antioxidant MIX (Curcumin 5μM, Vitamin B2 10 μM, NAC 1mM, Carnitin 1 mM) for 2 h, then was added CSE 20%. We used these concentrations based on previous publication (Vanella et al., 2017). Cells, basal medium and apical wash were collected after 24 h.
2.2 Preparation of cigarette smoke extract (CSE)
Cigarette smoke extract (CSE) was obtained using a peristaltic pump Watson-Marlow 323 E/D (Rotterdam, Netherlands) using two Kentucky 2R4F research-reference cigarettes (The Tobacco Research Institute, University of Kentucky) without filter. Briefly, each cigarette was smoked for 5 min in 20 mL of PBS to generate a CSE-PBS solution. The CSE solution was then filtered using a 0.22 μm pore filter to remove bacteria and large particles. This solution was considered 100% concentration. S-PBECs were stimulated with a concentration of 20% of CSE for 24h, while in the ALI-PBEC the 20% of CSE was added in the apical side for 30 min and then removed.
2.3 Reagents
Curcumin, Vitamin B2, L-Carnitin and N-acetyl-L-cysteine (NAC) were supplied, in powder form, by a pharmaceutical company (Nuova Farmaceutica, Catania) and solubilized in distilled water or a mix of water and DMSO (50% w/w) to obtain the working solutions. The new formulation was indicated as antioxidant mix (MIX).
2.4 Measure of mitochondrial superoxide (MitoSOX)
To evaluate the production of mitochondrial superoxide was used the MitoSOX™ Red mitochondrial superoxide indicator (Molecular Probes Waltham, MA, USA). After 24 h of stimulation, the cells were harvested, washed with PBS and stained with 3 μM MitoSOX Red probe for 15 min at 37°C. Then, PBECs were washed twice in PBS and then analyzed by flow cytometry using CytoFLEX (BeckmanCoulter). Analysis was done on 10,000 acquired events for each sample. Cell debris and dead cells were excluded from the analysis. Data were analyzed using the CytExpert software. The results were expressed as MFI (Mean Fluorescence Intensity).
2.5 Real time PCR analysis
The total RNA was isolated from PBECs using TRIzol Reagent (Life Technologies) after 24 h of stimulation, following the manufacturer’s instruction. 1 μg of RNA was reverse-transcribed to cDNA, using iScript cDNA Synthesis kit (Biorad). Gene expression of IL-8 and IL-6 was evaluated by qRT-PCR conducted at QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, United States) using specific FAM-labeled probe and primers (prevalidated TaqMan Gene expression assay for IL-8, Hs00174103_m1; for IL-6, Hs00985639_m1, Thermo Fisher Scientific). GAPDH (prevalidated TaqMan Gene expression assay for GAPDH, Hs03929097_g1) was used as endogenous control gene to normalize the expression of the genes. The relative quantitation of mRNA was carried out with the comparative Ct method (2^ΔΔCt) and was plotted as respective fold-change. Untreated cells (NT) were used as reference sample.
2.6 ELISA assay
The release of IL-8 and IL-6 was evaluated by enzyme-linked immunosorbent assays (ELISA) (DuoSet R&D Systems, Minneapolis, MN) on PBECs. The basal medium of both S-PBEC and ALI-PBEC was collected, centrifuged at 12,000 g for 5 min, and then transferred to clean microcentrifuge tubes to be stored at −20°C for the subsequent analysis. To obtain the apical supernatant of ALI-PBEC, 250 μL of PBS was dispensed to each well and incubated at 37°C for 30 min. The PBS was collected in microcentrifuge tubes and stored at −20°C until use.
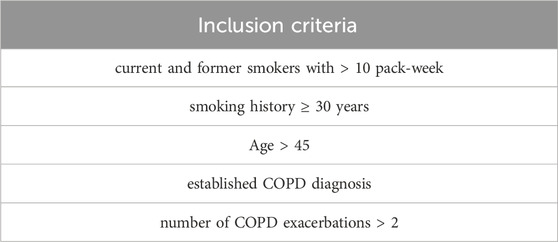
2.7 Observational clinical study
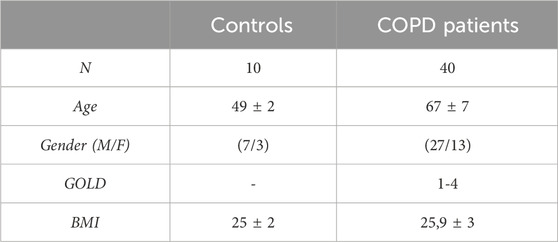
Fifty subjects were recruited at “Ospedali Riuniti Villa Sofia–Cervello”. The study was approved by the reference “Ospedali Riuniti Villa Sofia–Cervello” Ethic Committee (n. 221 AOR 2022) in accordance with the Declaration of Helsinki. Informed written consent was obtained from each subject. The COPD patients (N = 40) who meet the inclusion criteria, listed in Table 1, were recruited for the initial transversal study. A control group (N = 10) of healthy people was also included. Exclusion criteria were considered exacerbated COPD patients requiring systemic corticosteroids and/or antibiotic therapy, positive skin tests for common aeroallergen extracts and history of asthma and/or allergic rhinitis. Regarding comorbidities in COPD patients, the most prevalent (75%) was arterial hypertension under treatment, followed by dyslipidemia (25%), chronic ischemic heart disease (12.4%) and Type 2 diabetes mellitus (6.2%). Among forty COPD patients, only sixteen chose to carry out the 6 months observational study No significant differences were found for cigarette pack/year, Forced Expiratory Volume in 1 s (FEV1) L/%, Forced Vital Capacity (FVC) L/%, Body Mass Index (BMI), within the COPD patient group (Table 2).
2.8 Study design
Open prospective observational study, to assess the effects of a new nutraceutical formulation based on curcumin, carnitine, acetyl-N-cysteine-L, vitamin B2, piperine added to traditional therapy for patients with COPD. Sixteen patients with COPD agreed to take the new nutraceutical formulation every day for 6 months in addition to background therapy (LAMA + LABA + ICS). The subjects recruited for the study were evaluated at visit 1 (V1) (before administering the new nutraceutical formulation) and at visit 2 (V2) (after 6 month) for clinical and functional assessment (COPD Assessment Test (CAT) questionnaire, spirometry and walking test) and for nasal wash evaluations (IL-8).
2.9 Nasal wash sampling
Nasal washes were obtained from healthy controls and from COPD patients. Samples were obtained by irrigation of each naris with 9 mL of saline through a rinowash (Air Liquide Medical Systems). The fluid was collected in the external tank of the instrument, then transferred in a conical polypropylene tube and stored at 4°C until the processing in laboratory. The volumes of nasal washes collected were measured and Dithiothreitol (DTT) (Sputolysin, Calbiochem Corp., San Diego, CA, United States), freshly prepared in a 10% dilution with distilled water, was added to each sample in the equivalent volume to one-10th. Samples were vortexed to homogenization and centrifuged at 500 g for 10 min at 4°C. After centrifugation, the supernatant was collected and stored at −80°C for the subsequent analysis.
2.10 Evaluation of clinical and functional parameters
The recruited COPD patients treated with new nutraceutical formulation were evaluated at V1 and V2 for symptoms and functional parameters. The functional parameters, Forced Expiratory Volume in 1 s (FEV1) and Forced Vital Capacity (FVC), were evaluated by (SensorMedics Vmax) spirometry. Spirometry was performed in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (Graham et al., 2019). The functional parameters of this subjects at V1 were: FEV1 L: 1.33 ± 1.05; FEV1%: 63 ± 31, FVC L: 2.32 ± 1.33; FVC %: 85 ± 24.5; tiff: 0.6 ± 0.18. After 6 months of treatment, at V2, the functional parameters did not undergo any significant changes (FEV1 L: 1.27 ± 1.17; FEV1%: 63 ± 31, FVC L: 2.35 ± 1.6; FVC %: 82 ± 31.5; tiff: 0.6 ± 0.14). Furthermore, patient symptoms were collected using the COPD Assessment Test (CAT) questionnaire.
2.11 Six-minute walking test (6MWT)
The 6MWT was performed during the first visit and at the end of the 6 months observation period, according to the American Thoracic Society (ATS) guidelines (Enright, 2003). Briefly, patients were asked to walk for 6 min back and forth between two cones for a total of 30 m. Saturation was detected during the test. Data of the distances covered were recorded in percentage of meters walked by the patients.
2.12 Statistical analysis
Data from PBEC culture were expressed as mean ± SD and analyzed by using ANOVA test (Fisher’s). At p-value < 0.05, differences were considered to be statistically significant. Data from patients were analyzed by using Mann–Whitney test or Wilcoxon test. At p-value < 0.05, differences were considered to be statistically significant.
3 Results
3.1 Release of mitochondrial superoxide
To understand whether the new formulation of N-acetylcisteine (NAC), carnitine, curcumin and B2 vitamin could exert beneficial effects against cigarette smoke damage, we assessed the production of mitochondrial superoxide, the main ROS produced by the damage to the respiratory chain, in S-PBEC and ALI-PBEC. The results showed that CSE significantly increased the production of mitochondrial superoxide in both cellular models (Figures 1A, B). Pre-treatment with the new formulation, indicated as MIX, significantly counteracted the effect of CSE on oxidative stress (Figures 1A, B) in both types of PBEC culture. Pre-treatment with NAC alone significantly counteracted the effect of CSE on oxidative stress (Figures 1A, B) in S-PBEC.
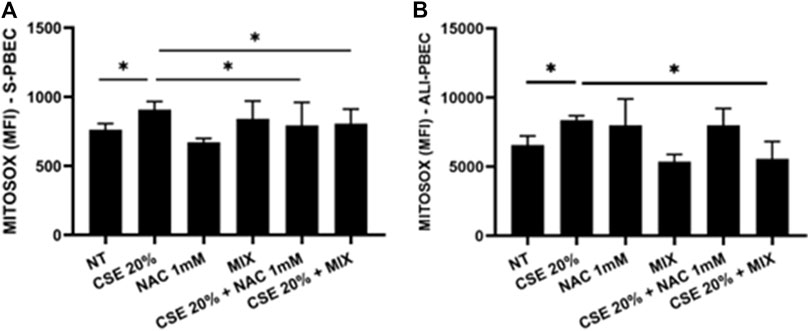
Figure 1. Production of mitochondrial superoxide in S-PBEC and in ALI-PBEC. S-PBEC (A) and ALI-PBEC (B) were pre-treated with NAC or MIX for 2 h and then stimulated with CSE 20%. After 24 h, mitochondrial superoxide production was evaluated by flow cytometry. Data are showed as Mean Fluorescence Intensity ±SD (S-PBEC, N = 6; ALI-PBEC, N = 6), *p < 0.05. ANOVA (Fisher’s) test.
3.2 IL-8 and IL-6 gene expression and release
Since increased oxidative stress leads to amplified inflammatory responses (Bodas et al., 2017), we evaluated IL-8 gene expression and release in PBECs in both S-PBEC (Figures 2A, B) and in ALI-PBEC (Figures 3A, B). In S-PBEC, CSE increased the IL-8 gene expression and release and the MIX significantly counteracted CSE mediated effects on both IL-8 gene expression and release (Figures 2A, B) while NAC exerted counteracting activity only on IL-8 gene expression (Figure 2B). In ALI-PBEC, CSE increased the IL-8 gene expression and basal and apical IL-8 release and the MIX significantly counteracted CSE effects on both IL-8 gene expression and apical and basal release (Figures 3A–C) while NAC exerted counteracting activity only on IL-8 gene expression (Figure 3C). IL-6 gene expression and release was also evaluated in ALI-PBEC (Figures 4A, B). The results showed that CSE in-creased IL-6 gene expression and basal release (Figures 4A, B). No apical IL-6 release was detected. MIX significantly reduced IL-6 gene expression and basal release (Figures 4A, B). NAC alone reduced only the IL-6 gene expression. No significant results were obtained for the gene expression and release of IL-6 in S-PBEC (data not shown).

Figure 2. IL-8 release and gene expression in S-PBEC. PBECs were pre-treated with NAC or MIX for 2 h and then stimulated with CSE 20%. After 24 h, the medium was collected and total RNA was extracted to evaluate IL-8 protein release and gene expression, respectively. (A) IL-8 release measured by ELISA. Results are reported as pg/mL (N = 9). *p < 0.05 ANOVA (Fisher’s) test. (B) IL-8 gene expression measured by RT-PCR. Results are reported as mean fold change compared to control ±SD (N = 8). *p < 0.05 ANOVA (Fisher’s) test.
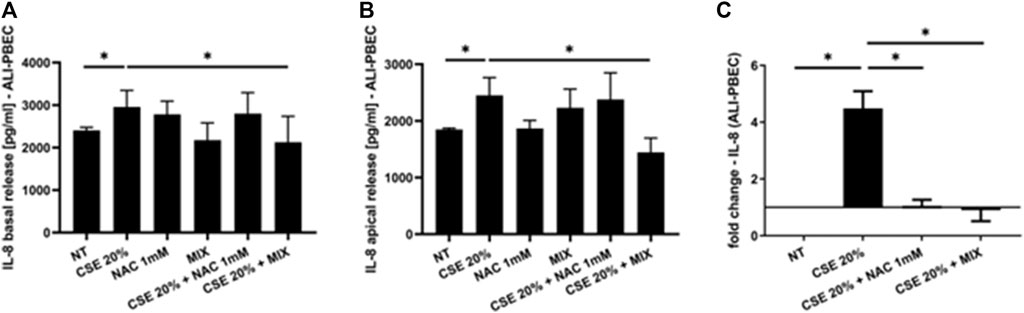
Figure 3. IL-8 basal and apical release and gene expression in ALI-PBEC. PBECs differentiated using ALI-culture were pre-treated with NAC or MIX for 2 h and then stimulated with CSE 20%. After 24 h, basal medium and apical wash were collected and total RNA was extracted to evaluate IL-8 protein release and gene expression. (A) IL-8 basal release and (B) apical release measured by ELISA. Results are reported as pg/mL (N = 9). *p < 0.05 ANOVA (Fisher’s) test. (C) IL-8 gene ex-pression measured by RT-PCR. Results are reported as mean fold change compared to control ±SD (N = 8). *p < 0.05 ANOVA (Fisher’s) test.
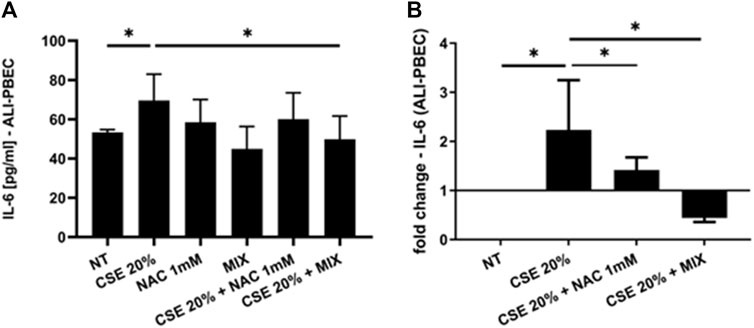
Figure 4. IL-6 basal release and gene expression in ALI-PBEC. PBECs differentiated using ALI-culture were pre-treated with NAC or MIX for 2 h and then stimulated with CSE 20%. After 24 h, basal medium was collected and total RNA was extracted to evaluate IL-6 protein release and gene expression. (A) IL-6 protein release measured by ELISA. Results are reported as pg/mL (N = 9). *p < 0.05 ANOVA (Fisher’s) test. (B) IL-6 gene expression measured by RT-PCR. Results are reported as mean fold change compared to control ±SD (N = 8). *p < 0.05 ANOVA (Fisher’s) test.
3.3 IL-8 in nasal wash from control subjects and COPD patients pre- and post-therapy with the nutraceutical drug
IL-8 levels were assessed in nasal wash from controls (n = 10) and COPD patients (n = 40). As shown in Figure 5A, IL-8 nasal wash was significantly higher in COPD than in Controls. To establish whether the new nutraceutical formulation was effective in vivo in modulating IL-8 nasal wash levels, an observational prospective study on COPD patients (n = 16), treated or not with the new nutraceutical formulation for 6 months, was conducted. Obtained data demonstrated that 6 months treatment with the new formulation significantly reduced nasal wash IL-8 levels (Figure 5B). Moreover, in COPD treated patients a significant improvement in walking test (Figure 6A) as well as in CAT score data (Figure 6B) was observed. The results of the study power calculations on these three outcomes to evaluate the effectiveness of the tested MIX are reported on Table 3.

Figure 5. IL-8 in nasal wash from control subjects and COPD patients pre- and post-therapy with antioxidant drug. IL-8 protein measured by ELISA in nasal wash from Controls and COPD patients. Results are reported as pg/mL. *p < 0.05 Mann-Whitney test (A). Comparison of IL-8 levels pre-and post-nutraceutical treatment intra-patient. Results are reported as pg/mL. *p-value < 0.05 Wilcoxon test (B).
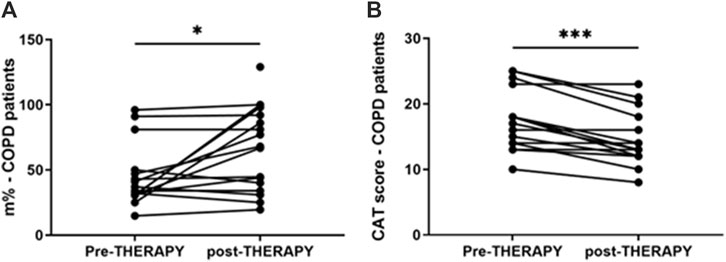
Figure 6. Walking test and CAT in COPD patients pre- and post-therapy with antioxidant drug. Walking test on COPD patients was performed at V1 and V2, after 6 months. Results are reported as distance in percentage of meters that patients quickly walked on a flat surface in 6 min *p-value < 0.05 Wilcoxon test (A). CAT score in COPD patients at V1 and V2, after 6 months. CAT values reported for individual patients. ***p-value = 0.001 Wilcoxon test (B).
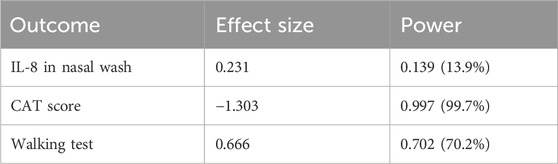
Table 3. Power Study Results: Effect Size and Statistical Power for the outcomes IL-8, CAT Scores e walking test.
4 Discussion
Cigarette smoking is the main cause of increased oxidative stress in the airways of COPD patients. The aim of our work was to assess whether a new formulation containing a MIX of substances, N-acetylcisteine (NAC), carnitine, curcumin and B2 vitamin, with recognized antioxidant properties would be more effective in counteracting the effects of cigarette smoke on epithelial cells. In details, we used a complex in vitro model of human primary bronchial epithelial cells differentiated to form a pseudostratified epithelium like the real airway epithelium. Our experimental models are based on the use of both a 2D model of submerged/undifferentiated primary bronchial epithelial cells (S-PBEC) and a 3D model differentiated cells (ALI-PBEC). Treatment exposures in S-PBECs are significant but experimentally simpler. The use of ALI-PBECs could physiologically represent a more realistic model and thus potentially more meaningful from a biological point of view. Furthermore, this model represents a fundamental resource that likely reflects inherent biological heterogeneity in terms of function (Lenz et al., 2013). Indeed, ALI-PBEC models represent a fully differentiated epithelium with more than 1 cell type (ciliated cells, goblet cells, and basal cells, etc.) that can also be combined with immunocompetent cells (dendritic cells, macrophages, neutrophils) (Upadhyay and Palmberg, 2018). However, in our study the use of ALI-PBEC as experimental model provides further insight into the pathogenesis of COPD, since they more closely approximate the physiological cell state in vivo compared to stable cell lines. Exposure to cigarette smoke causes the structural and functional alteration of the mitochondria at the level of the epithelium, with a consequent increase in oxidative stress (Hoffmann et al., 2013). The evaluation of mitochondrial ROS, compared with untreated cells, was an indication of the mitochondria functionality and homeostasis. Our results showed an increased production of mitochondrial ROS induced by CSE compared to untreated cells. In ALI-PBECs, pre-treatment with the MIX of antioxidants but not with NAC significantly reduced the amount of mitochondrial superoxide induced by CSE. NAC alone pretreatment was able to counteract the effects of CSE in mitochondrial superoxide production in S-PBEC confirming recent studies (Son et al., 2020; Hur et al., 2022) that have highlighted antioxidant properties of NAC, thanks to its ability to regenerate glutathione in similar submerged models. However, the use of additional substances such as curcumin, carnitine and vitamin B2, already widely used as a supplement (Patel et al., 2020; Suwannasom et al., 2020), can have a synergistic effect and greater therapeutic efficacy, restoring the mitochondrial homeostasis in a model closer to real airway mucosa. Notably, a previous report tested the similar antioxidant MIX in the cell line 16HBE (Vanella et al., 2017). Furthermore, our study evaluated the potential anti-inflammatory effects of the mixture in relation to both the gene expression and release of inflammatory cytokines and chemokines (IL-6 and IL-8, respectively) by the ALI-PBEC, following the exposure to CSE. The use of ALI-PBECs, compared to the continuous immortalized cell lines, allowed the analysis of the two different cellular compartments, basal and apical. Of particular importance are the data relating to the expression and release of IL-8, which had not been evaluated previously. It is worth noting that cigarette smoke causes abnormal airway inflammation, triggering the release of chemokines and promoting the infiltration of neutrophils and other inflammatory cells into the airways (Ma et al., 2023). Several studies reported an increase of IL-8 levels induced by cigarette smoke (Pace et al., 2008; Moon et al., 2013). IL-8 is a key mediator of inflammation response, with its chemoattractant capability, and it is released by several cell types, including epithelial cells (Brennan and Zheng, 2007). In particular, it is well known that IL-8 is a potent neutrophil chemoattractant chemokine. Neutrophil accumulation in the lung is a prominent feature of COPD. The accumulation occurs in the lung tissue of COPD patients and it is associated with alveolar damage and lung dysfunction (Williams and Jose, 2000). Some studies have found a correlation between IL-8 levels, neutrophil counts, markers of neutrophil activation, such as myeloperoxidase activity, and the degree of lung dysfunction (Jasper et al., 2019). Our results showed that ALI-PBEC, when exposed to CSE, released more IL-8 in both apical and basal side. These effects were well-counteracted by the pre-treatment with the antioxidant MIX, which led to an important reduction of IL-8 in both compartments. Also in S-PBEC, CSE increased IL-8 release, and MIX significantly counteracted the CSE-mediated effects of IL-8. This confirms that cigarette smoking could play a role in the airway and systemic neutrophilia characteristic of COPD in fact the release of IL-8 is elevated both towards the lumen of the respiratory tract (apical side) and towards the underlying tissue (basal side). Accordingly, here, the concentration of IL-8 in nasal lavage is significantly higher in COPD patients than in control subjects, highlighting the increased airways inflammatory state of COPD patients. Since COPD patients often experience infective based exacerbation, in ALI-PBEC the effects of both CSE and MIX on IL-6 gene expression and protein release were also assessed. IL-6 is a cytokine produced in the acute phase of inflammation in response to infections and other inflammatory stimuli, including CSE (Dawson et al., 2021; Krick et al., 2021). Several studies reported elevated levels of IL-6 in patients with respiratory diseases, such as asthma and COPD, in which the inflammatory status and the lung epithelial damage are well known (Rincon and Irvin, 2012; Bradford et al., 2017). Furthermore, an interesting association between high levels of IL-6 and increased mortality or poor prognosis of patients was found, since IL-6 plays a key role in the pathogenesis of these diseases and heavily alters lung homeostasis (Agustí et al., 2012; Ilmarinen et al., 2016). Our results in ALI-PBEC showed an increase of IL-6 basal release, when stimulated with CSE and pre-treatment with the antioxidant MIX led to a significant reduction of the gene expression levels and the release of the inflammatory cytokine, indicating a potential restoring of airway homeostasis. The emergence of Precision Medicine revolutionized the approach of translational research. Reason being, with our study we have sought to support and contribute to the development of patient-centered translational medicine by translating what has been basic research into what could be called clinical pre-experimentation. The clinical benefits and anti-inflammatory effects of a new nutraceutical formulation, containing curcumin, carnitine, acetyl-N-cysteine-L, vitamin B2, and piperine, were also evaluated in patients with COPD in an observational study before and after 6 months of treatment with this nutraceutical drug, similar to the MIX used in the in vitro models. An interesting result was obtained from the comparison between control subjects and COPD patients in nasal lavage. Indeed, it was shown that the concentration of IL-8 is significantly higher in patients, highlighting the normal inflammatory state that characterizes the latter. The concentration of IL-8 in the nasal washes of COPD patients after 6 months of treatment with the new nutraceutical formulation is reduced compared to before the therapy. The progressive obstruction of the airways typical of COPD patients leads to dyspnea, i.e., shortness of breath during exertion and, therefore, can cause patients limitations of physical exercise (Kerti et al., 2018). In light of this, during our observational study the evaluation of patients’ physical effort tolerance through the walking test were performed. The results showed a significant increase in the percentage of meters walked by patients during the test, demonstrating an effective improvement of the patient’s physical abilities. These results are also in agreement with those obtained from the COPD Assessment Test or CAT score. This test easily assesses the impact of COPD symptoms such as cough, dyspnea, sputum, and chest tightness on patients’ health status. The CAT score ranges from 0 to 40 and the higher the score, the more severe the impact of COPD on the patient’s life. Our intra-patient results evidenced a significant reduction of the CAT score after 6 months of the treatment with the nutraceutical formulation, reflecting an effective general improvement in the patient’s health conditions (Gil et al., 2021). The power study results showed that the evaluation of the IL-8 in nasal washes of patients, pre- and post-therapy, has a low power level (13.9%). This means that to achieve an acceptable power level, the sample size or effect size needs to be increased. However, for two of the outcomes of interest (i.e., the CAT score and walking test improvements) we obtained, respectively, a high (99.7%) and moderate (70,2%) power level, indicating that the study is very likely to detect a true effect. Thus, for the first time, this study demonstrated and further confirmed the beneficial effect and the protective role of the antioxidant and anti-inflammatory MIX in a complex model and could be employed as supportive therapy that could help prevent any relapses in COPD patients.
Our in vitro study demonstrated the efficacy of a novel antioxidant formulation against the effects of CSE in both S-PBECs and ALI-PBECs. Specifically, pretreatment with the antioxidant MIX counteracts the effects of CSE on oxidation and markers of inflammation, restoring cellular homeostasis. Moreover, inflammatory cytokines such as Thymic stromal lymphopoietin (TSLP), IL-33 and Transforming growth factor beta (TGF-β) were also investigated in our in vitro study, but we did not obtain significant results (data not shown). However, further inflammatory cytokines (i.e., Tumor necrosis factor alpha (TNF-α) and antioxidant mechanisms could be explored in future studies. It would be also interesting to study the effect of the antioxidant MIX on the activation of damaged respiratory epithelium on cells of the innate and adaptive immune system. Interestingly, the observational study showed interesting preliminary data on the use of MIX antioxidant. The case is very interesting in the context of translational medicine that is increasingly focused on patient wellbeing. This study could provide the interesting basis for larger clinical studies and to further validate the antioxidant and anti-inflammatory effect of this nutraceutical on symptoms and on the prevention of periodic exacerbation in COPD patients. However, this study presents limitations. Since it is designed as a pilot study and we observed a small number of patients, there is the lack of a control group (placebo) to which compare the effectiveness of the MIX regarding the outcomes of our interest, such as changes in inflammatory cytokines. Furthermore, only 16 patients completed the study. The reasons for patient dropout were varied, including difficulty in dissolving the MIX power in water, poor palatability of the nutraceutical, and, in some cases, side effects such as abdominal pain and diarrhea. However, these aspects will certainly be addressed and improved in future large-scale clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of “Ospedali Riuniti Villa Sofia–Cervello” (n. 221 AOR 2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VL: Data curation, Formal Analysis, Investigation, Validation, Writing–original draft. PaP: Data curation, Formal Analysis, Investigation, Validation, Writing–original draft. SD: Data curation, Formal Analysis, Investigation, Validation, Writing–review and editing. MF: Data curation, Formal Analysis, Investigation, Validation, Writing–review and editing. FC: Investigation, Writing–review and editing. PiP: Investigation, Writing–review and editing. EP: Conceptualization, Resources, Supervision, Writing–review and editing. MB: Conceptualization, Funding acquisition, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research has been financed by the Project “Alimenti Nutraceutica e Salute” (Acronimo: TRIAL) - Azione 1.1.5 - Codice Progetto n. 08TP1041100162 - CUP: G48I18001120007. This research was also supported by Italian National Research Council, University of Palermo and by the project “One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases” Acronimo INF-ACT - codice identificativo PE00000007- CUP B53C20040570005.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1439835/full#supplementary-material
References
Aghapour, M., Raee, P., Moghaddam, S. J., Hiemstra, P. S., and Heijink, I. H. (2018). Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 58, 157–169. doi:10.1165/rcmb.2017-0200TR
Agustí, A., Celli, B. R., Criner, G. J., Halpin, D., Anzueto, A., Barnes, P., et al. (2023). Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am. J. Respir. Crit. Care Med. 207, 819–837. doi:10.1164/rccm.202301-0106PP
Agustí, A., Edwards, L. D., Rennard, S. I., MacNee, W., Tal-Singer, R., Miller, B. E., et al. (2012). Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS ONE 7, e37483. doi:10.1371/journal.pone.0037483
Aldini, G., Altomare, A., Baron, G., Vistoli, G., Carini, M., Borsani, L., et al. (2018). N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res. 52, 751–762. doi:10.1080/10715762.2018.1468564
Arora, P., Athari, S. S., and Nainwal, L. M. (2022). Piperine attenuates production of inflammatory biomarkers, oxidative stress and neutrophils in lungs of cigarette smoke-exposed experimental mice. Food Biosci. 49, 101909. doi:10.1016/j.fbio.2022.101909
Barnes, P. J. (2020). Oxidative stress-based therapeutics in COPD. Redox Biol. 33, 101544. doi:10.1016/j.redox.2020.101544
Bodas, M., Silverberg, D., Walworth, K., Brucia, K., and Vij, N. (2017). Augmentation of S-nitrosoglutathione controls cigarette smoke-induced inflammatory–oxidative stress and chronic obstructive pulmonary disease-emphysema pathogenesis by restoring cystic fibrosis transmembrane conductance regulator function. Antioxidants and Redox Signal. 27, 433–451. doi:10.1089/ars.2016.6895
Bradford, E., Jacobson, S., Varasteh, J., Comellas, A. P., Woodruff, P., O’Neal, W., et al. (2017). The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 18, 180. doi:10.1186/s12931-017-0662-2
Brennan, K., and Zheng, J. (2007). “Interleukin 8,” in xPharm: the comprehensive pharmacology reference (Elsevier), 1–4. doi:10.1016/B978-008055232-3.61916-6
Carrillo-González, D. F., Hernández-Herrera, D. Y., and Maldonado-Estrada, J. G. (2023). The role of L-carnitine in bovine embryo metabolism. A review of the effect of supplementation with a metabolic modulator on in vitro embryo production. Anim. Biotechnol. 34, 413–423. doi:10.1080/10495398.2021.1938593
Cheng, X., Hu, Y., Ruan, Z., Zang, G., Chen, X., and Qiu, Z. (2023). Association between B-vitamins intake and frailty among patients with chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 35, 793–801. doi:10.1007/s40520-023-02353-7
Chi, L., Shan, Y., and Cui, Z. (2022). N-Acetyl-L-Cysteine protects airway epithelial cells during respiratory syncytial virus infection against mucin Synthesis, oxidative stress, and inflammatory response and inhibits HSPA6 expression. Anal. Cell. Pathol. 2022, 4846336–4846339. doi:10.1155/2022/4846336
Dawson, R. E., Jenkins, B. J., and Saad, M. I. (2021). IL-6 family cytokines in respiratory health and disease. Cytokine 143, 155520. doi:10.1016/j.cyto.2021.155520
Di Vincenzo, S., Ninaber, D. K., Cipollina, C., Ferraro, M., Hiemstra, P. S., and Pace, E. (2022). Cigarette smoke impairs airway epithelial wound repair: role of modulation of epithelial-mesenchymal transition processes and notch-1 signaling. Antioxidants 11, 2018. doi:10.3390/antiox11102018
Fairley, L. H., Das, S., Dharwal, V., Amorim, N., Hegarty, K. J., Wadhwa, R., et al. (2023). Mitochondria-targeted antioxidants as a therapeutic strategy for chronic obstructive pulmonary disease. Antioxidants 12, 973. doi:10.3390/antiox12040973
Gil, H.-I., Zo, S., Jones, P. W., Kim, B.-G., Kang, N., Choi, Y., et al. (2021). Clinical characteristics of COPD patients according to COPD assessment test (CAT) score level: cross-sectional study. COPD 16, 1509–1517. doi:10.2147/COPD.S297089
Graham, B. L., Steenbruggen, I., Miller, M. R., Barjaktarevic, I. Z., Cooper, B. G., Hall, G. L., et al. (2019). Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am. J. Respir. Crit. Care Med. 200, e70–e88. doi:10.1164/rccm.201908-1590ST
Hoang, B. X., Han, B., Fang, W. H., Nguyen, A. K., Shaw, D. G., Hoang, C., et al. (2023). Targeting skeletal muscle dysfunction with L-carnitine for the treatment of patients with chronic obstructive pulmonary disease. Vivo 37, 1399–1411. doi:10.21873/invivo.13224
Hoffmann, R. F., Zarrintan, S., Brandenburg, S. M., Kol, A., De Bruin, H. G., Jafari, S., et al. (2013). Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 14, 97. doi:10.1186/1465-9921-14-97
Hur, J., Rhee, C. K., and Jo, Y. S. (2022). Effects of antioxidant on oxidative stress and autophagy in bronchial epithelial cells exposed to particulate matter and cigarette smoke extract. Tuberc. Respir. Dis. 85, 237–248. doi:10.4046/trd.2021.0152
Hurst, J. R., Vestbo, J., Anzueto, A., Locantore, N., Müllerova, H., Tal-Singer, R., et al. (2010). Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 363, 1128–1138. doi:10.1056/NEJMoa0909883
Ilmarinen, P., Tuomisto, L. E., Niemelä, O., Danielsson, J., Haanpää, J., Kankaanranta, T., et al. (2016). Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur. Respir. J. 48, 1052–1062. doi:10.1183/13993003.02198-2015
Jasper, A. E., McIver, W. J., Sapey, E., and Walton, G. M. (2019). Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res 8, F1000 Faculty Rev-557. doi:10.12688/f1000research.18411.1
Kerti, M., Balogh, Z., Kelemen, K., and Varga, J. (2018). The relationship between exercise capacity and different functional markers in pulmonary rehabilitation for COPD. COPD 13, 717–724. doi:10.2147/COPD.S153525
Kırkıl, G., and Muz, M. H. (2008). B group vitamin levels in patients with chronic obstructive pulmonary disease and the relation between pulmonary functions. Turkish Thorac. J. 9, 88–92.
Krajcovicová-Kudlácková, M., Simoncic, R., Béderová, A., Babinská, K., and Béder, I. (2000). Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 49, 399–402.
Krick, S., Helton, E. S., Easter, M., Bollenbecker, S., Denson, R., Zaharias, R., et al. (2021). ST6GAL1 and α2-6 sialylation regulates IL-6 expression and secretion in chronic obstructive pulmonary disease. Front. Immunol. 12, 693149. doi:10.3389/fimmu.2021.693149
Labaki, W. W., and Rosenberg, S. R. (2020). Chronic obstructive pulmonary disease. Ann. Intern. Med. 173, ITC17-ITC32–ITC32. doi:10.7326/AITC202008040
Le, J., Sun, M., Zhu, J., and Fan, H. (2022). Protective effect of N-acetylcysteine on acute lung injury in septic rats by inhibiting inflammation, oxidation, and apoptosis. Iran. J. Basic Med. Sci. 25, 859–864. doi:10.22038/ijbms.2022.65350.14384
Lenz, A.-G., Karg, E., Brendel, E., Hinze-Heyn, H., Maier, K. L., Eickelberg, O., et al. (2013). Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: a comparison with conventional, submerged cell-culture conditions. BioMed Res. Int. 2013, 652632. doi:10.1155/2013/652632
Li, Q., Liao, J., Chen, W., Zhang, K., Li, H., Ma, F., et al. (2022). NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic. Biol. Med. 187, 158–170. doi:10.1016/j.freeradbiomed.2022.05.024
Li, X., Wu, X., Ma, T., Zhang, Y., Sun, P., Qi, D., et al. (2023). Protective effect of L-carnitine against oxidative stress injury in human ovarian granulosa cells. Exp. Ther. Med. 25, 161. doi:10.3892/etm.2023.11860
Lin, X., Bai, D., Wei, Z., Zhang, Y., Huang, Y., Deng, H., et al. (2019). Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 14, e0216711. doi:10.1371/journal.pone.0216711
Liu, J., Liu, Q., Han, J., Feng, J., Guo, T., Li, Z., et al. (2021). N-acetylcysteine inhibits patulin-induced apoptosis by affecting ROS-mediated oxidative damage pathway. Toxins 13, 595. doi:10.3390/toxins13090595
Ma, R., Su, H., Jiao, K., and Liu, J. (2023). Association between IL-17 and chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD 18, 1681–1690. doi:10.2147/COPD.S412626
Mannion, J. M., McLoughlin, R. M., and Lalor, S. J. (2022). The airway microbiome-IL-17 Axis: a critical regulator of chronic inflammatory disease. Clin. Rev. Allerg. Immunol. 64, 161–178. doi:10.1007/s12016-022-08928-y
Mohanty, R. R., Padhy, B. M., Das, S., and Meher, B. R. (2021). Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence. Eur. Rev. Med. Pharmacol. Sci. 25, 2802–2807. doi:10.26355/eurrev_202103_25442
Moon, H.-G., Zheng, Y., An, C. H., Kim, Y.-K., and Jin, Y. (2013). CCN1 secretion induced by cigarette smoking extracts augments IL-8 release from bronchial epithelial cells. PLoS ONE 8, e68199. doi:10.1371/journal.pone.0068199
Mrityunjaya, M., Pavithra, V., Neelam, R., Janhavi, P., Halami, P. M., and Ravindra, P. V. (2020). Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 11, 570122. doi:10.3389/fimmu.2020.570122
Mucha, P., Skoczyńska, A., Małecka, M., Hikisz, P., and Budzisz, E. (2021). Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 26, 4886. doi:10.3390/molecules26164886
Ożarowski, M., and Karpiński, T. M. (2021). Extracts and flavonoids of passiflora species as promising anti-inflammatory and antioxidant substances. CPD 27, 2582–2604. doi:10.2174/1381612826666200526150113
Pace, E., Ferraro, M., Siena, L., Melis, M., Montalbano, A. M., Johnson, M., et al. (2008). Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 124, 401–411. doi:10.1111/j.1365-2567.2007.02788.x
Pace, E., Giarratano, A., Ferraro, M., Bruno, A., Siena, L., Mangione, S., et al. (2011). TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum. Immunol. 72, 54–62. doi:10.1016/j.humimm.2010.09.009
Panahi, Y., Ghanei, M., Hajhashemi, A., and Sahebkar, A. (2016). Effects of curcuminoids-piperine combination on systemic oxidative stress, clinical symptoms and quality of life in subjects with chronic pulmonary complications due to sulfur mustard: a randomized controlled trial. J. Diet. Suppl. 13, 93–105. doi:10.3109/19390211.2014.952865
Patel, S. S., Acharya, A., Ray, R. S., Agrawal, R., Raghuwanshi, R., and Jain, P. (2020). Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 60, 887–939. doi:10.1080/10408398.2018.1552244
Pedre, B., Barayeu, U., Ezeriņa, D., and Dick, T. P. (2021). The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol. and Ther. 228, 107916. doi:10.1016/j.pharmthera.2021.107916
Pekala, J., Patkowska-Sokola, B., Bodkowski, R., Jamroz, D., Nowakowski, P., Lochynski, S., et al. (2011). L-carnitine - metabolic functions and meaning in humans life. CDM 12, 667–678. doi:10.2174/138920011796504536
Radomska-Leśniewska, D. M., Skopińska-Różewska, E., Jankowska-Steifer, E., Sobiecka, M., Sadowska, A. M., Hevelke, A., et al. (2010). N-acetylcysteine inhibits IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol. Rep. 62, 131–138. doi:10.1016/S1734-1140(10)70250-4
Raghu, G., Berk, M., Campochiaro, P. A., Jaeschke, H., Marenzi, G., Richeldi, L., et al. (2021). The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr. Neuropharmacol. 19, 1202–1224. doi:10.2174/1570159X19666201230144109
Rahman, Md.M., Bibi, S., Rahaman, Md.S., Rahman, F., Islam, F., Khan, M. S., et al. (2022). Natural therapeutics and nutraceuticals for lung diseases: traditional significance, phytochemistry, and pharmacology. Biomed. and Pharmacother. 150, 113041. doi:10.1016/j.biopha.2022.113041
Ran, Y., Su, W., Gao, F., Ding, Z., Yang, S., Ye, L., et al. (2021). Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxidative Med. Cell. Longev. 2021, 1552127–1552225. doi:10.1155/2021/1552127
Rincon, M., and Irvin, C. G. (2012). Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 8, 1281–1290. doi:10.7150/ijbs.4874
Rushworth, G. F., and Megson, I. L. (2014). Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. and Ther. 141, 150–159. doi:10.1016/j.pharmthera.2013.09.006
Scoditti, E., Massaro, M., Garbarino, S., and Toraldo, D. M. (2019). Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients 11, 1357. doi:10.3390/nu11061357
Son, E. S., Park, J.-W., Kim, Y. J., Jeong, S. H., Hong, J. H., Kim, S.-H., et al. (2020). Effects of antioxidants on oxidative stress and inflammatory responses of human bronchial epithelial cells exposed to particulate matter and cigarette smoke extract. Toxicol. Vitro 67, 104883. doi:10.1016/j.tiv.2020.104883
Sun, X., Feng, X., Zheng, D., Li, A., Li, C., Li, S., et al. (2019). Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin. Sci. 133, 1523–1536. doi:10.1042/CS20190331
Suwannasom, N., Kao, I., Pruß, A., Georgieva, R., and Bäumler, H. (2020). Riboflavin: the health benefits of a forgotten natural vitamin. IJMS 21, 950. doi:10.3390/ijms21030950
Talenezhad, N., Mohammadi, M., Ramezani-Jolfaie, N., Mozaffari-Khosravi, H., and Salehi-Abargouei, A. (2020). Effects of l-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin. Nutr. ESPEN 37, 9–23. doi:10.1016/j.clnesp.2020.03.008
Tenório, M. C., dos, S., Graciliano, N. G., Moura, F. A., Oliveira, A. C. M. de, and Goulart, M. O. F. (2021). N-acetylcysteine (NAC): impacts on human health. Antioxidants 10, 967. doi:10.3390/antiox10060967
Upadhyay, S., and Palmberg, L. (2018). Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 164, 21–30. doi:10.1093/toxsci/kfy053
Vanella, L., Li Volti, G., Distefano, A., Raffaele, M., Zingales, V., Avola, R., et al. (2017). A new antioxidant formulation reduces the apoptotic and damaging effect of cigarette smoke extract on human bronchial epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 21, 5478–5484. doi:10.26355/eurrev_201712_13938
Williams, T. J., and Jose, P. J. (2000). “Neutrophils in chronic obstructive pulmonary disease,” in Novartis foundation symposia. Editors D. Chadwick, and J. A. Goode (Wiley), 136–148. doi:10.1002/0470868678.ch9
Xiao, H., Wu, M., Shao, F., Guan, G., Huang, B., Tan, B., et al. (2016). N-Acetyl-L-cysteine protects the enterocyte against oxidative damage by modulation of mitochondrial function. Mediat. Inflamm. 2016, 8364279–9. doi:10.1155/2016/8364279
Keywords: airway epithelial cells, cigarette smoke, inflammation, oxidative stress, antioxidant, nutraceutical formulation
Citation: Lazzara V, Pinto P, Di Vincenzo S, Ferraro M, Catalano F, Provinzano P, Pace E and Bonsignore MR (2024) In vitro evidence of antioxidant and anti-inflammatory effects of a new nutraceutical formulation explains benefits in a clinical setting of COPD patients. Front. Pharmacol. 15:1439835. doi: 10.3389/fphar.2024.1439835
Received: 28 May 2024; Accepted: 09 August 2024;
Published: 20 August 2024.
Edited by:
Achuthan Raghavamenon, Amala Cancer Research Centre, IndiaReviewed by:
Prathyusha Bagam, National Center for Toxicological Research (FDA), United StatesLin Lin, Guangdong Provincial Hospital of Chinese Medicine, China
Copyright © 2024 Lazzara, Pinto, Di Vincenzo, Ferraro, Catalano, Provinzano, Pace and Bonsignore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Di Vincenzo, c2VyZW5hLmRpdmluY2Vuem9AY25yLml0
†These authors have contributed equally to this work and share first authorship
 Valentina Lazzara
Valentina Lazzara Paola Pinto1,2,3†
Paola Pinto1,2,3† Serena Di Vincenzo
Serena Di Vincenzo Maria Ferraro
Maria Ferraro Elisabetta Pace
Elisabetta Pace Maria Rosaria Bonsignore
Maria Rosaria Bonsignore