- Department of General Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, China
Introduction: Gemcitabine and cisplatin remain the cornerstone for the treatment of advanced or unresectable biliary tract cancers, but the incidence rate of the grade 3 or 4 toxic effects is high (70.7%). In recent years, significant progress has been achieved in the systemic treatment of cholangiocarcinoma with immune checkpoint inhibitors (ICIs), targeted therapy, and hepatic artery infusion chemotherapy (HAIC). HAIC may elevate the local drug concentration in the liver to 10–100 times the drug plasma concentration; therefore, it may enhance tumor cytotoxicity while minimizing systemic adverse effects. HAIC combined with immunotherapy and targeted therapy resulted in acceptable tumor responses and tolerable toxic effects in the treatment of hepatocellular carcinoma (HCC). However, whether this combination strategy can benefit patients with unresectable intrahepatic cholangiocarcinoma remains unclear.
Methods and Analysis: We describe a single-arm, open label, prospective clinical trial of HAIC sequential transcatheter arterial embolization (TAE) combined with tislelizumab and surufatinib in patients with unresectable intrahepatic cholangiocarcinoma. TAE + HAIC was performed at an interval of at least 3 weeks, and oxaliplatin (85 mg/m2) and rituximab (3 mg/m2) were infused. TAE was performed using undrugged microspheres. Tislelizumab was infused every 3 weeks and surufatinib was administered orally once a day, with 3-5 capsules (50 mg/capsule) each time. We plan to enroll 28 participants in this study. The primary study endpoint was objective response rate (ORR). The secondary endpoints were progression-free survival (PFS), conversion to surgical resection rate, overall survival (OS), 1-year OS rate, disease control rate (DCR), quality of life (QoL), and incidence of adverse events.
Trial registration number: NCT06239532.
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is a malignant tumor that originates from the epithelial cells of the intrahepatic bile duct (Shi et al., 2024; Shu et al., 2024; Zhao et al., 2024). The incidence rate of iCCA ranks second after hepatocellular carcinoma, comprising approximately 10%–15% of primary liver cancer (Zhou et al., 2022). In recent years, the global incidence of iCCA has increased significantly (Li et al., 2022). Because of its insidious onset, high heterogeneity, and lack of specific clinical manifestations, iCCA is typically diagnosed at a late stage, leading to a poor prognosis (Song et al., 2024; Shi A. D. et al., 2023; Liu et al., 2022). The 5-year survival rate for advanced iCCA is less than 10% (Moris et al., 2023).
Comprehensive treatment of iCCA includes local tumor treatment (surgical methods, radiotherapy, ablation, arterial infusion embolization) and systemic treatment (chemotherapy, targeted therapy, and immunotherapy) (Wang et al., 2021a). For advanced iCCA patients who are not suitable for surgical resection, local and systemic treatments are alternative treatment methods (Wang et al., 2021b). The combination of gemcitabine and cisplatin is the first-line standard treatment for unresectable iCCA (Valle et al., 2010). In the ABC-02 study, the median survival of gemcitabine combined with cisplatin for all biliary tumors was 11.7 months, while the median survival of gemcitabine alone was 8.1 months, with an objective response rate of 26.1%. Local treatment is also a commonly used treatment method for patients with locally advanced iCCA who are unsuitable for surgical resection. The commonly used local treatment methods for iCCA include arterial chemoembolization, arterial infusion chemotherapy, and arterial radiation embolization (Wei et al., 2023; Huang et al., 2024).
Immunotherapy has greatly changed the first-line treatment pattern of liver cancer and has also introduced new treatment options for advanced biliary tract tumors (Rimini et al., 2023; Oh et al., 2022; Kelley et al., 2023). The results of an immunological joint study on intrahepatic cholangiocarcinoma were announced at the 2021 ASCO of Clinical Oncology conference. The study involved GEMOX chemotherapy combined with tripolyimumab and lenvatinib as the first-line treatment for advanced intrahepatic cholangiocarcinoma, with stunning data (Shi G. M. et al., 2023). The objective response rate was as high as 80% (RECIST v1.1), the disease control rate (DCR) was 93.3%, the median progression-free survival (PFS) was 10.0 months, the median DOR was 9.8 months, and the 12-month overall survival (OS) rate was 73.3%.
In summary, hepatic artery infusion chemotherapy (HAIC) combined with immunotherapy and targeted therapy has shown therapeutic effects in iCCA. However, most patients with unresectable intrahepatic cholangiocarcinoma treated with HAIC in combination with checkpoint inhibitors and targeted drugs are still in the clinical trial stage. This project aimed to conduct a prospective exploratory clinical study on the first-line treatment of unresectable intrahepatic cholangiocarcinoma with arterial infusion chemotherapy combined with tislelizumab and surufatinib. This study aimed to explore the effectiveness and safety of arterial infusion chemotherapy combined with tislelizumab and surufatinib for unresectable intrahepatic cholangiocarcinoma.
Methods and design
Study objectives and overall design
REACH-01 is a single-arm, open label, phase II study evaluating the safety and effectiveness of HAIC combined with transcatheter arterial embolization (TAE) plus an immune checkpoint inhibitor (ICI) and TKI in adult patients (aged ≥ 18 years) with unresectable intrahepatic cholangiocarcinoma. Twenty-five participants were enrolled in the study. The study started on 27 September 2022, and is anticipated to be completed on 31 December 2025. Tislelizumab (BGB-A317) is a human monoclonal antibody (HuMAb; IgG4) manufactured by BeiGene that combines with the PD-1 receptor and blocks the interaction between PD-1 and PD-L1.
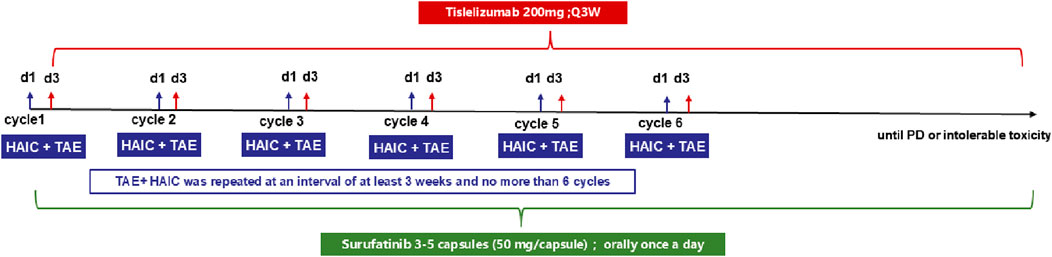
Eligible patients will receive TAE (undrugged microspheres–300–500 μm in diameter) and HAIC (Oxaliplatin (85 mg/m2), Raltitrexed (3 mg/m2), continuous infusion for 3 h) on day 1 and performed at an interval of at least 21 days. The first dose of tislelizumab was administered on day 3, and the second dose was administered on day 24 (the first day of week 4, ±3 days). Surufatinib was administered orally once a day, with 3-5 capsules (50 mg/capsule) administered each time (Figure 1).
This clinical trial was registered with clinicaltrials.gov (Registration Number for Clinical Trial: NCT06239532; Any), and any changes related to the protocol will be presented.
Patient and public involvement
No patients were involved.
Eligibility criteria
In brief, REACH-01 will recruit patients with unresectable iCCA with a confirmed diagnosis of cholangiocarcinoma by biopsy or by the non-invasive diagnostic criteria of the American Association for the Study of the Liver Diseases (AASLD).
Inclusion criteria
1. Written informed consent for the trial.
2. Aged ≥18 years.
3. Histologically confirmed intrahepatic cholangiocarcinoma.
4. No other previous systematic treatment for BTC.
5. At least one measurable lesion (RECIST 1.1).
6. Eastern Cooperative Oncology Group performance status 0 or 1.
7. Life expectancy of 3 months or greater.
8. Child-Pugh classification score ≤7.
9. The laboratory test values meet the following requirements:
1) Blood routine: Absolute neutrophil count (ANC) ≥ 1.5 × 109/L; Platelet count (PLT) ≥ 80 × 109/L; Hemoglobin (HGB) content ≥90 g/L;
2) Renal function: Serum creatinine (Cr) ≤ 1.5 × ULN, or for subjects with creatinine >1.5 × ULN, creatinine clearance rate (CCr) ≥ 45 mL/min (Cockcroft Gault formula);
3) Coagulation function: International Normalized Ratio (INR) or APTT ≤1.5 × ULN.
10. If the subject is infected with HBV or HCV, the following criteria must be met:
1) HBV infected subjects (HBsAg or HBV-DNA positive): Prior to the first treatment, HBV infected subjects should receive antiviral therapy recommended by the guidelines for at least 3 days, and the HBV-DNA should decrease by at least 1 log value upon re examination. During the research period, it is necessary to continue receiving standardized antiviral treatment;
2) HCV infected subjects (HCVAb or HCV RNA positive): According to the researcher’s judgment, if they are in a stable state and receiving antiviral treatment, they will continue to receive approved anti HCV treatment during the study.
11. Female patients of childbearing potential should have a negative serum pregnancy test within 24 h of their first dose of Investigational Medicinal Product (IMP).
Exclusion criteria
1. Recurrent patients.
2. Eastern Cooperative Oncology Group performance status ≥two
3. Life expectancy of less than 3 months.
4. Child-Pugh classification score >8.
5. History of hepatic encephalopathy or liver transplantation.
6. Known hypersensitivity to any of the study drugs, study drug classes, or excipients in the formulation.
7. Symptomatic pleural effusion, ascites, and pericardial effusion that require drainage.
8. Portal vein tumor thrombus (PVTT) involves both the main trunk and contralateral branch or upper mesenteric vein. Inferior vena cava tumor thrombus.
9. Prior treatment with immunotherapy agents (including anti-PD-1, anti-PD-L1, anti-CTLA4, etc.).
10. History of (non-infectious) pneumonitis requiring steroids, evidence of interstitial lung disease, or active non-infectious pneumonitis.
11. The researcher considers it inappropriate to enter this study.
Withdrawal criteria
The withdrawal decided by the researcher
The researcher can decide to withdraw a subject from the study under the following circumstances.
1. During the study, the subjects may develop severe acute or chronic complications or special physiological changes that not appropriate for this study.
2. During the study, the subjects may have poor adherence to medications.
3. Other treatment drugs were added without following the researcher’s guidance during the entire study period.
Subjects withdrawal at their own will
Based on the informed consent form, the participants were free to withdraw from the trial at any point. Subjects who do not formally withdraw from the trial but no longer receive drugs and undergo testing or are lost to follow-up are also regarded as withdrawn. The reasons for withdrawing the subjects were ascertained and recorded whenever possible.
Study procedures
Written informed consent will be obtained by a liver surgeon with the participant or his delegate. Patients will undergo baseline tumor imaging, including CT scans of the chest and abdomen, and contrast-enhanced CT/MRI scans of the liver at screening. Tumor imaging will be repeated using contrast-enhanced CT/MRI every two cycles of TAE + HAIC treatment. During the follow-up period, contrast-enhanced CT/MRI was performed every 2–3 months.
Participants will require a full hepatitis serology screen prior to enrolment in the study, which includes HBV and HCV serology. In patients with positive serology for either virus, baseline HBV DNA and HCV RNA levels will be requested. Participants confirmed to have chronic and active hepatitis B and/or C (i.e., with detectable HBV DNA or HCV RNA at baseline) will have their viral load (HCV RNA and/or HBV DNA as appropriate) monitored regularly and at the end of the treatment follow-up visit.
A baseline core tumor biopsy will be performed and collected from participants during screening to confirm the diagnosis and for future relevant studies. The tumor tissue sample was frozen and stored at −80°C.
Procedure for TAE + HAIC: A 4F arterial sheath and RH tube were first inserted through the right femoral artery. Abdominal trunk and superior mesenteric artery angiography were performed, and the nutrient artery of the tumor was determined. A microcatheter was inserted into the nutrient artery of the tumor. Gastroduodenal artery and right gastric artery embolizations were performed if necessary. Two ml of 300–500 μm blank microspheres were injected through the microcatheter to reduce the tumor blood supply. The microcatheter was then fixed and the patient returned to the ward. Oxaliplatin and rituximab were injected through the microcatheters. The drug dosages were oxaliplatin (85 mg/m2) and rituximab (3 mg/m2). TAE + HAIC was repeated at an interval of at least 3 weeks and no more than 6 cycles for one patient.
Tislelizumab 200 mg was administered intravenously at an interval of 3 weeks. Surufatinib was administered orally once a day, with 3-5 capsules (50 mg/capsule) administered each time.
Patients will be reviewed to analyze the safety and tolerability following the completion of the first cycle of TAE + HAIC and tislelizumab treatment (follow-up visit 1; FU1). Safety FU2 will be conducted after the last cycle of TAE + HAIC and tislelizumab treatment. All adverse events (AEs) that occurred before the visit were recorded. Participants with ongoing AEs at the visit will be followed up by the PI or delegated until the resolution or stabilization of the event. Participants will be assessed every 2 months (±7 days) to collect information regarding disease status and survival. Long-term follow-up will continue for a total of 2 years for each patient. All personal information of the enrolled participants will be maintained and protected in the hospital information system and accessible only to the authorized medical staff to protect confidentiality. The PIs of the trial have access to the entire final trial dataset.
Outcome measures and endpoints
The primary study endpoints included objective response rate (ORR) according to the RECIST v1.1/mRECIST criteria and determination of safety and tolerability of the sequential HAIC + TAE/tislelizumab/surufatinib based on the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. The secondary endpoints were progression-free survival (PFS), conversion to surgical resection rate, overall survival (OS), 1-year overall survival rate, disease control rate (DCR), quality of life (QoL), and incidence rate of adverse events. The trial was designed to have a power of 80% to detect an increase in ORR from 26% in patients receiving cisplatin-gemcitabine to 50% in patients receiving sequential HAIC + TAE/tislelizumab/surufatinib. A total of 25 patients would be required, based on the use of the binomial exact test with a single-sided significance level of 5% and assuming that the trial would recruit for 2 years with at least 6 months of follow-up for each patient. To allow for dropouts (10%) and to ensure that we had sufficient evidence to meet the trial objectives, we aimed to recruit 28 patients. After the subjects sign the informed consent form and begin the study treatment, all adverse events and SAEs (regardless of whether they are related to the study treatment) will be reported until 30 days (AE) and 90 days (SAE) after the last study treatment, or other anti-tumor treatments will be initiated, whichever occurs first (regardless of whether observed by the researcher or reported by the subjects), and its severity will be evaluated according to CTCAE5.0. The reporting period for irAE (regardless of severity or non severity) is until 90 days after the last study treatment (regardless of whether other anti-tumor treatments have been initiated). After this period, researchers should report any SAEs evaluated as related to the investigational drug. Exploratory endpoints included the patient’s genetic background and immune response.
Statistical analysis
Statistical analyses will include an intent-to-treat analysis including all participants enrolled, and a per-protocol analysis including all participants who completed the study without major protocol violations. Baseline demographic and clinicopathological variables will be presented using descriptive statistics. Data analysis was performed after the study was completed. Interim analyses of the safety and tolerability data will be conducted at the end of FU1 and at the end of FU2. A comprehensive statistical analysis plan was finalized prior to the final analysis. Primary analysis of ORR will be conducted around 6 months after last patient in. For other secondary efficacy endpoints and safety endpoints, the final analysis will be conducted around 1 year after last patient in. All participants who received at least one dose of tislelizumab/surufatinib and one fraction of HAIC + TAE were included in the safety analysis. All participants who received at least one dose of tislelizumab/surufatinib and three cycles of HAIC + TAE were included in the efficacy analysis. The RECIST 1.1/mRECIST response rates (CR, PR, and ORR) are presented descriptively. Overall survival was calculated from the date of first cycle of TAE + HAIC until the date of death. Progression-free survival was measured from the date of first cycle of TAE + HAIC until the date of disease progression or death. Overall survival and progression-free survival were analyzed with the use of Kaplan - Meier curves and the log-rank test. A Cox proportional - hazards model was used to estimate the hazard ratios.
Ethics and dissemination
This study was approved by the Research Ethics Committee of Shandong University Qilu Hospital (KYLL-202208-043-1). An abstract of the interim results will be prepared for academic conferences such as the American Society of Clinical Oncology Annual Meeting. The results of this trial will be published in a peer-reviewed journal.
Discussion
Cholangiocarcinoma remains the most aggressive malignant tumor, with a poor prognosis and less treatment regime (Ilyas et al., 2023). Although surgical resection provides a chance for long-term survival, the recurrence rate after resection is high and the 3-year and 5-year overall survival is still poor (Xu et al., 2019). For advanced-stage patients, gemcitabine and cisplatin are the first-line recommendations, but patient survival is not satisfactory. The combination of chemotherapy and immunotherapy has recently achieved great progress and has prolonged patient survival. One phase II study evaluated toripalimab plus lenvatinib as first-line treatment for 31 patients with advanced iCCA. The ORR and DCR were 32.3% and 74.2%, and 6-months OS rate was 87.1% (Shi G. M. et al., 2023). In the TOPAZ-1 study, addition of durvalumab (mPFS 7.2 months, mOS 12.8 months, ORR 26.7%, and DCR 85.3%) significantly improved patient survival than GemCis group (mPFS 5.7 months, mOS 11.5 months, ORR 18.7%, and DCR 82.6%) (Rimini et al., 2023). Pembrolizumab plus lenvatinib as a subsequent-line therapy in 32 patients with BTC, has also demonstrated a mPFS of 4.9 months, a mOS of 11.0 months, an ORR of 25%, and a DCR of 78.1% (Lin et al., 2020). The phase II LEAP-005 study assessed lenvatinib plus pembrolizumab in a second-line setting for 31 patients with advanced BTC, demonstrating a mPFS of 6.1 months, a mOS of 8.6 months, an ORR of 10%, and a DCR of 68%. These results demonstrate the therapeutic potential of immunotherapy for the treatment of BTC.
HAIC is also considered an effective treatment option for patients with advanced intrahepatic cholangiocarcinoma. A study explored second-line and successive treatments using hepatic arterial infusion chemotherapy (HAIC) based on FOLFIRI after the failure of gemcitabine and platinum combined with target and immunotherapy in nine refractory CCAs (Huang et al., 2022). The objective response rate was 22.2%, the disease control rate was 55.5% (5/9), median progression-free survival was 5 months, and 6-month survival rate was 66.7% (6/9). Another study also evaluated the efficacy of HAIC with mFOLFOX in patients with unresectable intrahepatic cholangiocarcinoma and the 1-year overall survival (OS) rates was 60.2%, the 2-year OS rate was 38.7% (Cai et al., 2021). GEMOX protocol was also performed for HAIC combined with tyrosine kinase inhibitors and anti-PD-1 immunotherapy, and the 1-year PFS rate was 61.9% (Zhang et al., 2024). However, patients receiving HAIC treatment also experience long-term bed ridding and limited drug selection. The duration of FOLFIRI treatment is usually 44–46 h. The duration of the mFOLFOX therapy was 46 h. In our study, the treatment lasted for only 4–6 h and may be more tolerable for most patients.
In a single-arm, multicenter, open-label phase 2 trial, surufatinib monotherapy was evaluated as second-line VEGFR therapy in patients with BTC (Xu et al., 2021). 39 patients were enrolled, and the 16-week progression-free survival rate was 46.33% (95% CI, 24.38–65.73), with median progression-free survival of 3.7 months and median overall survival of 6.9 months. The overall safety of surufatinib is good with a low incidence of grade 3/4 adverse events. The main grade 3/4 adverse events were elevated bilirubin levels, proteinuria, and hypertension. The results of this study indicate that surufatinib offers moderate clinical efficacy and shows expected tolerability and safety profiles.
In this study, we aimed to evaluate the efficacy and safety of arterial infusion chemotherapy combined with tislelizumab and surufatinib as first-line treatment for unresectable iCCA. We hope that the results of this clinical trial will expand our knowledge about local treatment combined with immunotherapy and targeted therapy for unresectable iCCA, thus improving the outcome of iCCA.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the Shandong University Qilu Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
K-sL: Conceptualization, Writing–original draft, Writing–review and editing. YL: Conceptualization, Investigation, Writing–original draft. T-zZ: Investigation, Writing–original draft. Y-fX: Conceptualization, Investigation, Methodology, Writing–review and editing. Z-lZ: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Z-lZ received funding from the Key Research and Development Program of Shandong Province (Major Scientific Innovation Projects, 2021CXGC011105). The authors declare that this study received funding from BeiGene Co. Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, writing of this article, or the decision to submit it for publication.
Conflict of interest
K-sL and Z-lZ is on the speakers’ bureau for BeiGene.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cai, Z., He, C., Zhao, C., and Lin, X. (2021). Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front. Oncol. 11, 611118. doi:10.3389/fonc.2021.611118
Huang, P., Huang, X., Zhou, Y., Yang, G., Sun, Q., Shi, G., et al. (2022). The efficacy and safety of hepatic arterial infusion chemotherapy based on FOLFIRI for advanced intrahepatic cholangiocarcinoma as second-line and successive treatment: a real-world study. Can. J. Gastroenterol. Hepatol. 2022, 9680933. doi:10.1155/2022/9680933
Huang, Y., Du, Z., Kan, A., He, M., Li, H., Lai, Z., et al. (2024). Clinical and biomarker analyses of hepatic arterial infusion chemotherapy plus lenvatinib and PD-1 inhibitor for patients with advanced intrahepatic cholangiocarcinoma. Front. Immunol. 15, 1260191. doi:10.3389/fimmu.2024.1260191
Ilyas, S. I., Affo, S., Goyal, L., Lamarca, A., Sapisochin, G., Yang, J. D., et al. (2023). Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 20 (7), 470–486. doi:10.1038/s41571-023-00770-1
Kelley, R. K., Ueno, M., Yoo, C., Finn, R. S., Furuse, J., Ren, Z., et al. (2023). Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401 (10391), 1853–1865. doi:10.1016/S0140-6736(23)00727-4
Li, Y. C., Li, K. S., Liu, Z. L., Tang, Y. C., Hu, X. Q., Li, X. Y., et al. (2022). Research progress of bile biomarkers and their immunoregulatory role in biliary tract cancers. Front. Immunol. 13, 1049812. doi:10.3389/fimmu.2022.1049812
Lin, J., Yang, X., Long, J., Zhao, S., Mao, J., Wang, D., et al. (2020). Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg. Nutr. 9 (4), 414–424. doi:10.21037/hbsn-20-338
Liu, Z., Liu, J., Chen, T., Wang, Y., Shi, A., Li, K., et al. (2022). Wnt-TCF7-SOX9 axis promotes cholangiocarcinoma proliferation and pemigatinib resistance in a FGF7-FGFR2 autocrine pathway. Oncogene 41 (20), 2885–2896. doi:10.1038/s41388-022-02313-x
Moris, D., Palta, M., Kim, C., Allen, P. J., Morse, M. A., and Lidsky, M. E. (2023). Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 73 (2), 198–222. doi:10.3322/caac.21759
Oh, D. Y., Lee, K. H., Lee, D. W., Yoon, J., Kim, T. Y., Bang, J. H., et al. (2022). Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 7 (6), 522–532. doi:10.1016/S2468-1253(22)00043-7
Rimini, M., Fornaro, L., Lonardi, S., Niger, M., Lavacchi, D., Pressiani, T., et al. (2023). Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int. 43 (8), 1803–1812. doi:10.1111/liv.15641
Shi, A., Liu, Z., Fan, Z., Li, K., Liu, X., Tang, Y., et al. (2024). Function of mast cell and bile-cholangiocarcinoma interplay in cholangiocarcinoma microenvironment. Gut 73, 1350–1363. doi:10.1136/gutjnl-2023-331715
Shi, A. D., Zhao, L. M., Sheng, G. L., Zhang, G. N., Tang, Y. C., Li, K. S., et al. (2023). SMAD4 regulates the progression of cholangiocarcinoma by modulating the expression of STING1. J. Cell Mol. Med. 27 (17), 2547–2561. doi:10.1111/jcmm.17857
Shi, G. M., Huang, X. Y., Wu, D., Sun, H. C., Liang, F., Ji, Y., et al. (2023). Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct. Target Ther. 8 (1), 106. doi:10.1038/s41392-023-01317-7
Shu, L., Li, X., Liu, Z., Li, K., Shi, A., Tang, Y., et al. (2024). Bile exosomal miR-182/183-5p increases cholangiocarcinoma stemness and progression by targeting HPGD and increasing PGE2 generation. Hepatology 79 (2), 307–322. doi:10.1097/HEP.0000000000000437
Song, Y., Lian, S., Fan, H., Ma, C., Zheng, L., Huang, F., et al. (2024). Bmi1 facilitates the progression of cholangiocarcinoma by inhibiting Foxn2 expression dependent on a histone H2A ubiquitination manner. Cancer Lett. 592, 216921. doi:10.1016/j.canlet.2024.216921
Valle, J., Wasan, H., Palmer, D. H., Cunningham, D., Anthoney, A., Maraveyas, A., et al. (2010). Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362 (14), 1273–1281. doi:10.1056/NEJMoa0908721
Wang, Y., Chen, T., Li, K., Mu, W., Liu, Z., Shi, A., et al. (2021a). Recent advances in the mechanism research and clinical treatment of anti-angiogenesis in biliary tract cancer. Front. Oncol. 11, 777617. doi:10.3389/fonc.2021.777617
Wang, Y., Li, K., Zhao, W., Liu, Z., Liu, J., Shi, A., et al. (2021b). Aldehyde dehydrogenase 3B2 promotes the proliferation and invasion of cholangiocarcinoma by increasing Integrin Beta 1 expression. Cell Death Dis. 12 (12), 1158. doi:10.1038/s41419-021-04451-8
Wei, Z., Wang, Y., Wu, B., Liu, Y., Wang, Y., Ren, Z., et al. (2023). Hepatic arterial infusion chemotherapy plus lenvatinib with or without programmed cell death protein-1 inhibitors for advanced cholangiocarcinoma. Front. Immunol. 14, 1235724. doi:10.3389/fimmu.2023.1235724
Xu, J., Bai, Y., Sun, H., Bai, C., Jia, R., Li, Y., et al. (2021). A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. Cancer 127 (21), 3975–3984. doi:10.1002/cncr.33803
Xu, Y. F., Liu, Z. L., Pan, C., Yang, X. Q., Ning, S. L., Liu, H. D., et al. (2019). HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene 38 (6), 868–880. doi:10.1038/s41388-018-0485-8
Zhang, T., Zhu, C., Zhang, N., Zhang, L., Wang, S., Xun, Z., et al. (2024). Lenvatinib combined with PD-1 inhibitor plus Gemox chemotherapy versus plus HAIC for advanced biliary tract cancer. Int. Immunopharmacol. 129, 111642. doi:10.1016/j.intimp.2024.111642
Zhao, L., Liu, J., Li, K., Zhang, C., Chen, T., Liu, Z., et al. (2024). PTPN9 dephosphorylates FGFR2 pY656/657 through interaction with ACAP1 and ameliorates pemigatinib effect in cholangiocarcinoma. Hepatology 79 (4), 798–812. doi:10.1097/HEP.0000000000000552
Keywords: HAIC, TACE, tislelizumab, surufatinib, cholangiocarcinoma
Citation: Li K-s, Liu Y, Zhang T-z, Xu Y-f and Zhang Z-l (2024) Protocol of REACH-01: a single-arm, open label, prospective study of HAIC sequential TAE combined with tislelizumab and surufatinib in unresectable intrahepatic cholangiocarcinoma. Front. Pharmacol. 15:1435639. doi: 10.3389/fphar.2024.1435639
Received: 23 May 2024; Accepted: 05 November 2024;
Published: 18 November 2024.
Edited by:
Marko Jakopovic, University of Zagreb, CroatiaReviewed by:
Juhong Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaYan-Jun Xiang, Second Military Medical University, China
Copyright © 2024 Li, Liu, Zhang, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zong-li Zhang, enpsenpsMTkwMEAxNjMuY29t
 Kang-shuai Li
Kang-shuai Li Yi Liu
Yi Liu Yun-fei Xu
Yun-fei Xu Zong-li Zhang
Zong-li Zhang