- 1Department of Neurology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Institute for Brain Disorders, Beijing University of Chinese Medicine, Beijing, China
- 3Beijing University of Chinese Medicine, Beijing, China
- 4Chinese Medicine Key Research Room of Brain Disorders Syndrome and Treatment of the National Administration of Traditional Chinese Medicine, Beijing, China
Background: Stroke is a serious health issue that can result in death or disability, leading to a significant economic strain on society and families. A growing number of studies have shown that the Naoshuantong capsule (NSTC) is beneficial as a treatment for ischemic stroke (IS) in recent years. Our study aims to provide an update on the safety and efficacy of the NSTC in IS patients.
Methods: We thoroughly searched eight databases to identify suitable randomized controlled trials (RCTs) assessing the effectiveness of the NSTC in the treatment of IS. The National Institute of Health Stroke Scale (NIHSS) for an acute period and modified Rankin Scale (mRS) at 3 months for a non-acute period were considered the primary outcome, and secondary outcomes included the NIHSS for a non-acute period, mRS, Barthel Index (BI), modified Barthel Index (MBI), Stroke-specific Quality of life (SS-QOL), and the recurrence rate of cerebrovascular events. Subsequently, its quality was assessed using the Cochrane risk assessment scale. Statistical analysis was conducted using RevMan 5.3 and Stata 14.0.
Results: A total of 27 RCTs were included, which involved 3,139 patients. The results showed that the NSTC improved neurological function not only in the acute period (MD = −2.53; 95% CI: −2.91, −2.15; p < 0.00001) but also in the non-acute period (MD = −3.70; 95% CI: −5.82, −1.58; p = 0.0006) and improved the long-term functional outcomes with lower mRS scores (MD = −0.68; 95% CI: −1.09, −0.26; p = 0.001). At the same time, the NSTC decreased the risk of cerebrovascular disease recurrence (RR = 0.43; 95% CI: 0.27, 0.70; p = 0.0006) and increased the quality of life in the acute period (MD = 23.88; 95% CI: 16.63, 31.13; p < 0.00001). Significant disparities in the incidence of adverse events between the NSTC and control groups were not observed. The certainty of evidence was estimated as moderate to very low.
Conclusion: The NSTC emerges as a potentially efficacious and safe treatment option for IS. NSTC could improve neurological function in different period of IS, and it has certain clinical value in secondary prevention. As a result of the poor quality and heterogeneity of the included trials, larger and standardized RCTs are needed to validate NSTC in IS treatment.
Systematic Review Registration:: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=482981, identifier CRD42023482981.
1 Introduction
Ischemic stroke (IS) morbidity continually increases, causing a huge burden to individuals and the society (Saini et al., 2021). Statistical results showed that the morbidity of IS in China is 1,256/100,000 individuals (Feigin et al., 2021). It has been confirmed that IS cause a series of pathophysiological changes, including excitatory toxicity, oxidative stress, blood–brain barrier permeability, and inflammatory reactions, ultimately leading to neuron necrosis and apoptosis (Feske, 2021). At present, antithrombotic therapy, intensive lipid-lowering therapy, and antihypertensive therapy are widely implemented in clinical care and recommended as important secondary prevention strategies by stroke guidelines in different countries (Kleindorfer et al., 2021; Li et al., 2022; Dawson et al., 2022). The role of neuroprotective agents in clinical practice has received increasing attention with the continuous research on the pathological mechanisms of IS. Regrettably, despite the promising efficacy demonstrated in animal studies, many antioxidants have not shown statistically significant differences in effectiveness between intervention and control groups in clinical trials (Goenka et al., 2019). To sum up, there is a lack of accepted treatments to combine treatment and prevention. Thus, it is worthy to figure out a new therapy taking effect in both the acute and recovery periods of IS.
The Naoshuantong capsule (NSTC) is a Chinese patent medicine based on the theory of stroke etiology and pathogenesis which is “toxin damaging brain collaterals” for detail. This theory was innovated by Yongyan Wang, an academician of the Academician of the Chinese Academy of Engineering. It has been approved to treat acute and convalescent IS in China by the State Food and Drug Administration since 2001. The NSTC is composed of five Chinese herbs, which are Typhae Pollen (Pu Huang, the dry pollen of Typha angustifolia L.), Paeoniae Radix Rubra (Chi Shao, the root of Paeonia lactiflora Pall.), Curcumae Radix (Yu Jin, the root of Curcuma wenyujin Y. H. Chen et C. Ling), Gastrodiae Rhizoma (Tian Ma, the root of Gastrodia elata B1.), and Rhapontici Radix [Lou Lu, the radix of Rhaponticum uniflorum (L.) DC.]. Detailed information is given in Supplementary Material Section I. Research in network pharmacology has revealed that the NSTC exhibits preventive and protective effects against ischemic stroke. This is attributed to the anti-inflammatory properties of its active ingredients and their role in enhancing the expression of TGF-β1 levels, which contribute to the repair of ischemic brain tissue (Luo et al., 2020). A trial of quality of life after 6 months of follow-up in patients with IS showed that those taking NSTC in combination with aspirin recovered better in self-care and daily work ability than those taking aspirin alone (Ye et al., 2015). Furthermore, meta-analysis studies showed that the curative effect of the NSTC and chemical medicine for acute IS was significantly better than that of chemical medicine alone (Zhang et al., 2019; Chang et al., 2019).
After reviewing the guidelines on integrated traditional Chinese and modern medicine for IS (Gao et al., 2023), the shortcomings of existing studies were identified: is there a drug that can be effective in both the acute and non-acute periods of IS? On the other hand, published meta-analyses of the NSTC focused on the efficacy analysis of the NSTC in the acute phase of cerebral infarction. Thus, it is necessary to accomplish a new meta-analysis and update clinical evidence of the NSTC.
2 Methods
This meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021) (Supplementary Table S2). Additionally, registration was completed in PROSPERO (CRD42023482981).
2.1 Search strategy
Two authors (CQ and YW) independently searched PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Internet (CNKI), Wanfang Data, China Science and Technology Journal Database (VIP), and Chinese Biomedical Literature Service System (SINOMED) databases from inception up to 1 May 2024. We used both Medical Subject Heading (MeSH) terms and free-text keywords for our search. The comprehensive search strategies are outlined in Supplementary Table S3. Study eligibility was determined through the participants, interventions, comparators, outcomes, and study design (PICOS) approach.
2.2 Eligibility criteria
The inclusion criteria are as follows: 1) adult patients diagnosed according to guidelines with IS; 2) the intervention must be a combination of basic treatment and NSTC; 3) comparing a placebo, standard care, neuroprotective drugs, or other ongoing interventions; 4) the outcome includes at least one of the following: i) the National Institute of Health Stroke Scale (NIHSS) for measuring neurological impairment; ii) Barthel Index (BI), modified Barthel Index (MBI), or modified Rankin Scale (mRS) used to evaluate daily life abilities; iii) Stroke-specific Quality of life (SS-QOL) for appraising the quality of life; and iv) recurrence of cerebrovascular events or other adverse events (AEs) used for safety assessment. When analyzing data, investigators plan to choose the NIHSS as the primary outcome for studies involving patients with acute IS as the research population, while mRS will be identified as the primary outcome for trials targeting non-acute period patients; and (5) study designed in the randomized controlled trial (RCT).
The gray literature was excluded because of its uncommon influence on meta-analytic findings (Hartling et al., 2017). Other excluded categories include 1) duplicate publication; 2) reviews, case reports, conference abstracts, animal experiments, correspondences, and expert insights; and 3) inadequate outcomes for meta-analysis.
2.3 Study selection
Abstract and full-text screening procedures were conducted simultaneously by two researchers (CQ and YW), with any discrepancies resolved through discussion. The corresponding author (XD) would be consulted when disagreements had not been resolved.
2.4 Data extraction
Four researchers (CQ, YW, GY, and CZ) independently extracted the following data from each trial using a standardized spreadsheet: writers, research site, number of participants, percentage of females, age range, kind of intervention, number of weeks of intervention, and results.
2.5 Risk of bias and quality assessment
Two investigators (GY and CZ) independently evaluated the methodological quality of the eligible studies using the standards listed in the Cochrane Risk of Bias 2 (RoB 2) (Sterne et al., 2019). RoB 2 assesses the risk of bias from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and in the selection of the reported result. Each domain was classified as a low risk of bias, high risk of bias, or some concerns. For each outcome, an overall summary RoB was derived; and an overall RoB for each study was determined based on the highest RoB level in any of the domains. Disagreements between the reviewers were resolved by discussion or arbitrated with a third coauthor (YG). Furthermore, we performed Egger’s statistical tests and visually inspected the funnel plot symmetry to identify potential publication bias.
2.6 Certainty of evidence
Grading of evidence is such a fundamental step for a meta-analysis. Two researchers (CQ and GY) applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework (Balshem et al., 2011) to categorize evidence as “very low,” “low,” “moderate,” or “high.” Disagreements were resolved by the corresponding author (XD).
2.7 Statistical analysis
The study used RevMan 5.3 and Stata 14.0 software applications for analysis. Relative risk (RR) and 95% confidence interval (CI) were used as the effect size for count data such as the recurrence rate of cerebrovascular events. Measurement data such as the NIHSS score were expressed as the mean difference (MD) and 95% CI. The selection of the data pooling model hinges on the presence and magnitude of heterogeneity. When the I2 statistics were <50%, indicating acceptable heterogeneity, the fixed-effects model was used. The random-effects model will be selected if the I2 statistics are ≥50%, indicating significant statistical heterogeneity. The random-effects model was also utilized when conducting subgroup analysis and when notable heterogeneity among the studies was apparent. Additionally, a funnel plot was used to investigate potential publication bias if 10 or more trials were included in the meta-analysis.
3 Results
3.1 Literature search
During our literature screening, a total of 659 eligible studies were initially identified in the databases. Following the removal of 303 duplicate entries, we conducted additional screening, eliminating 306 studies based on title and abstract assessment. Subsequently, a total of 23 studies were excluded due to criteria such as non-RCTs, outcomes not aligned with our interests, intervention methods inconsistent with review requirements, or other factors. Ultimately, a total of 27 studies were deemed suitable for inclusion and underwent pooled analysis. The flowchart of the literature screening process is shown in Figure 1.
3.2 Characteristics of the included studies
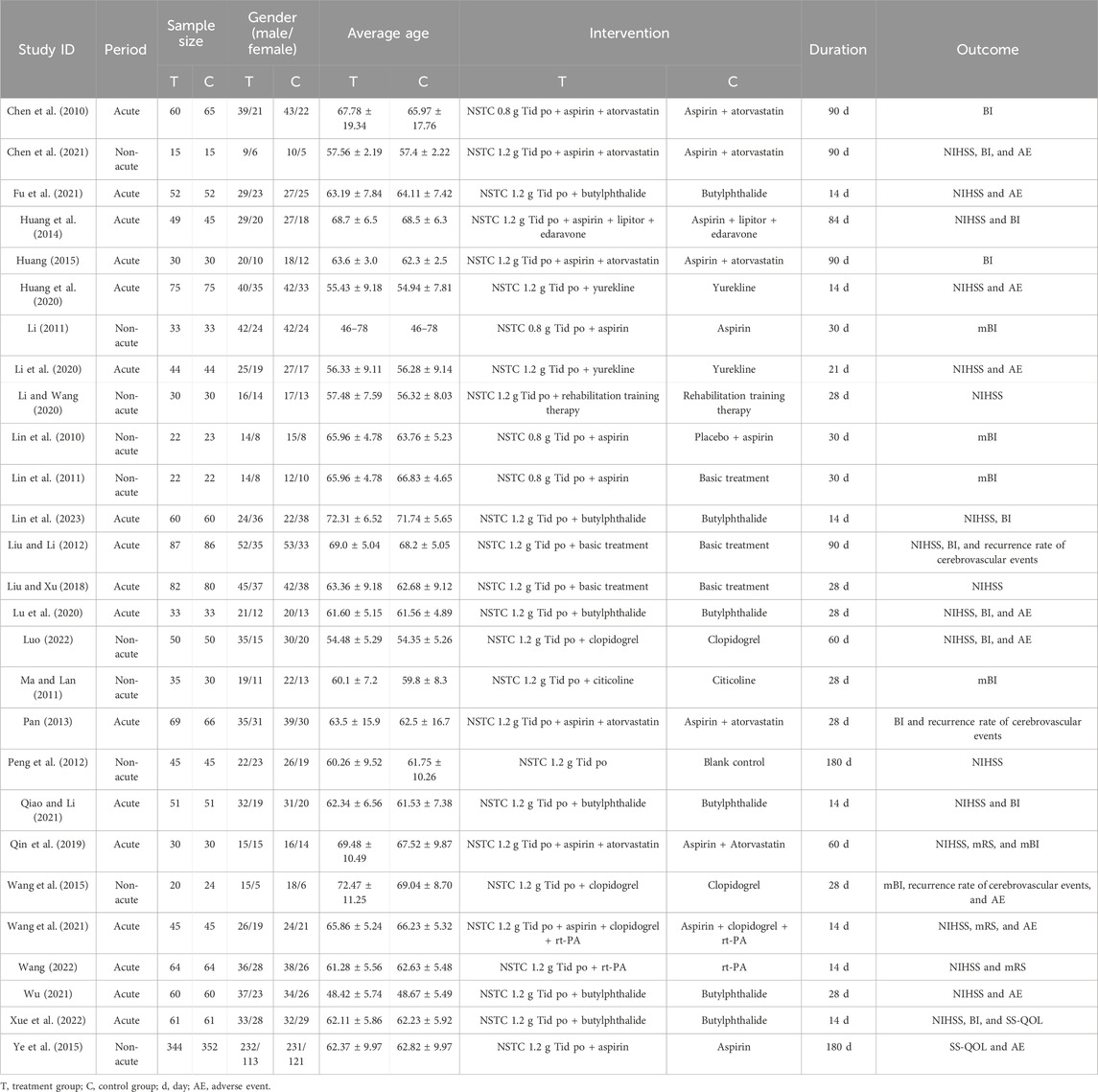
We encompassed 27 trials, enrolling a combined total of 3,139 patients. The maximum sample size in the NSTC group was up to 344, while it reached 352 in the control group. The minimum sample size was recorded at 15 in both groups. Participant ages across the studies ranged from 46 to 78 years on average. All selected articles were written in Chinese, published from 2010 to 2023. There were 15 trials including patients with acute IS, 4 trials including patients with convalescent IS, 2 trials including patients with sequelae IS, and 6 trials including patients with acute and convalescent IS. The duration of treatment ranged from 14 to 180 days. Twenty-four trials documented combination therapy alongside NSTC. Neurological impairment was evaluated by the NIHSS scale in 18 studies. Activity of daily life was assessed by the mRS in 3 studies, BI in 11 studies, and mBI in 6 studies. The quality of life was assessed by SS-QOL in two studies. Three studies mentioned the recurrence rate of cerebrovascular events, and 10 studies covered the safety outcome assessments about adverse events. The baseline characteristics of all included studies are shown in Table 1.
3.3 Risk of bias assessment
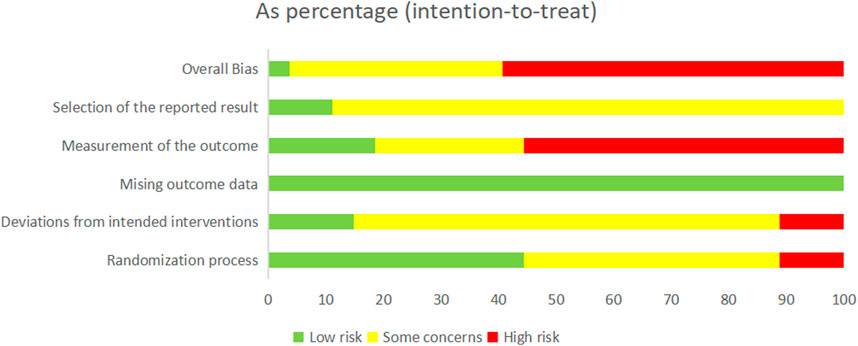
We rated the overall bias as “low risk of bias” in 1 study (Fu et al., 2021), while 10 of the remaining studies were judged to be “some concerns” and 16 were judged to be “high risk of bias,” suggesting the poor quality of the selected RCTs.
In three studies, the “randomization process” was assessed to have a “high risk of bias” due to the improper concealment of allocation methods. Conversely, a total of 12 studies were rated as having “some concerns” for not providing a detailed and explicit description of their random sequence despite mentioning it. Regarding bias risk due to “deviations from the intended interventions,” three studies were assessed as having a “high risk of bias” because they did not use blinding. In contrast, four studies were judged to have a “low risk of bias” due to their double-blind design and appropriate intention-to-treat analysis. Furthermore, all studies were judged to have a “low risk of bias” in terms of “missing outcome data” as no reports of missing data were found or the missing rate could be ignored, and the missing data were balanced between the experimental and control groups. Regarding the bias risk due to “measurement of the outcome,” 15 studies were assessed as having a “high risk of bias” because of inappropriate measurement methods or because there were gaps in outcome measures between groups. Regarding the bias risk due to the “selection of the reported result,” three studies were rated as having a “low risk of bias” due to their transparent observational reports, while 24 studies raised “some concerns” due to the lack of relevant reporting. In conclusion, the detailed methodological quality assessment of the 27 RCTs is summarized in Figure 2 and Supplementary Figure S1.
3.4 Primary outcomes
3.4.1 Acute period of the NIHSS
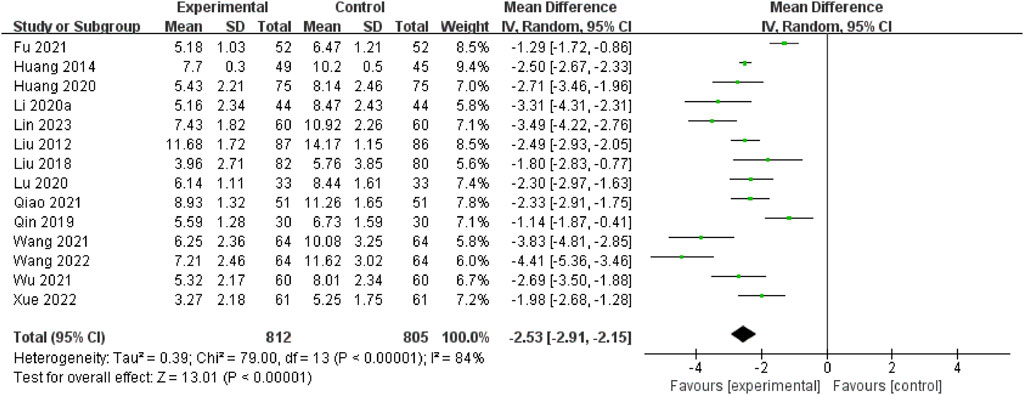
A total of 14 RCTs (Lin et al., 2023; Wang, 2022; Xue et al., 2022; Qiao and Li, 2021; Wu, 2021; Wang et al., 2021; Fu et al., 2021; Li et al., 2020; Lu et al., 2020; Huang et al., 2020; Qin et al., 2019; Liu and Xu, 2018; Huang et al., 2014; Liu and Li, 2012) assessed this outcome through the acute period of the NIHSS. The pooled data demonstrated that the NSTC group exhibited a reduction in NIHSS scores during the acute period (MD = −2.53; 95% CI: −2.91, −2.15; p < 0.00001). Because of the substantial heterogeneity (I2=84%, p < 0.00001), a random-effects model was used (Figure 3). The sensitivity analysis showed that even after deleting each study one at a time, the statistical heterogeneity was not altered (see Supplementary Figure S2). Subgroup analyses were subsequently conducted based on the intervention types (NSTC 1.2 g Tid po + butylphthalide vs butylphthalide, MD = −2.32; 95% CI: −2.95, −1.68; NSTC 1.2 g Tid po + aspirin + atorvastatin vs aspirin + atorvastatin, MD = −1.14; 95% CI: −1.87, −0.41; NSTC 1.2 g Tid po + yurekline vs yurekline, MD = −2.93; 95% CI: −3.52, −2.33) (Supplementary Figure S3). The results indicated that the NSTC combined with butylphthalide, yurekline, aspirin, and atorvastatin led to a statistically apparent reduction in NIHSS scores.
3.4.2 Modified Rankin Scale at 3 months
There was no literature that both meets the eligibility criteria and uses the mRS at 3 months as the outcome. Three out of the 27 included articles used the mRS as the outcome, but they focused on 14 days or 60 days from the day of onset. Details are discussed in the following sections.
3.5 Secondary outcomes
3.5.1 Non-acute period of the NIHSS
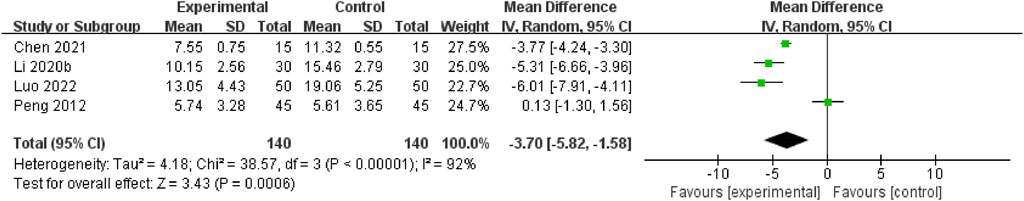
A total of four RCTs (Luo, 2022; Chen et al., 2021; Li and Wang, 2020; Peng et al., 2012) assessed this outcome through the non-acute period of the NIHSS. The pooled data demonstrated that the NSTC group exhibited a reduction in NIHSS scores during the non-acute period (MD = −3.70; 95% CI: −5.82, −1.58; p = 0.0006). Because of the substantial heterogeneity (I2=92%, p < 0.00001), a random-effects model was used (Figure 4). The sensitivity analysis showed that even after deleting each study one at a time, the statistical heterogeneity was not altered (see Supplementary Figure S4).
3.5.2 Modified Rankin scale
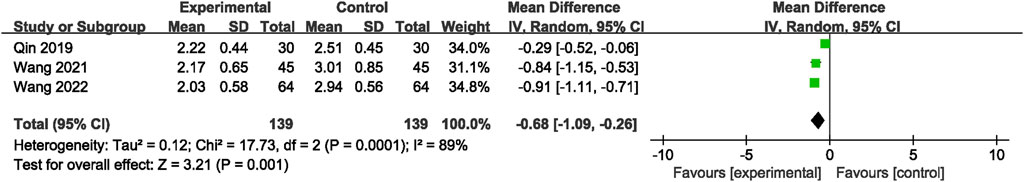
Three RCTs (Qin et al., 2019; Wang et al., 2021; Wang, 2022) reported mRS grading. The analytic results showed that adding the NSTC led to a reduction in mRS scores (MD = −0.68; 95% CI: −1.09, −0.26; p = 0.001) (Figure 5). A random-effects model was chosen due to significant heterogeneity (I2=89%, p = 0.001). The sensitivity analysis revealed that after the study by Qin et al. (2019) was screened out, the NSTC group demonstrated superior effectiveness over the control group in terms of neurological recovery (MD=−0.89; 95% CI: −1.06, −0.72) with no heterogeneity (Supplementary Figure S5).
3.5.3 Barthel Index
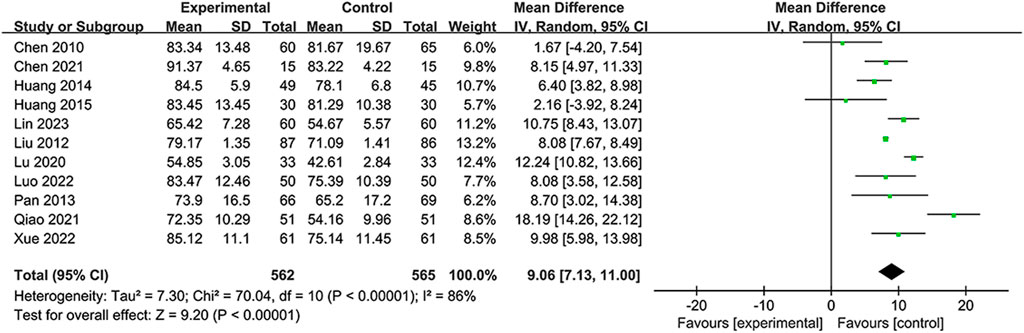
A total of 11 RCTS reported BI to demonstrate the ability to perform activities of daily life (Chen et al., 2010; Liu and Li, 2012; Pan, 2013; Huang et al., 2014; Huang, 2015; Lu et al., 2020; Chen et al., 2021; Qiao and Li, 2021; Luo, 2022; Xue et al., 2022; Lin et al., 2023). Due to substantial heterogeneity in the BI (I2 = 86%, p < 0.00001), the random-effects model was selected. The data (Figure 6) showed that the NSTC group showed an improved BI compared to only using conventional treatment such as aspirin or neuroprotective agents (MD = 9.06; 95% CI: 7.13, 11.00; p < 0.00001). Additional sensitivity analysis showed that when individual studies were sequentially excluded, statistical heterogeneity was not altered (Supplementary Figure S6). Subgroup analyses were developed focusing on the period, intervention, and duration time. The results revealed dramatic differences in mean values between the NSTC and control groups during the acute period (MD = 11.04; 95% CI: 8.26, –13.82) and non-acute period (MD = 8.13; 95% CI: 5.53, –10.72) (Supplementary Figure S7), while at the intervention, the NSTC combined with aspirin and atorvastatin or butylphthalide could improve the BI (NSTC combined with aspirin and atorvastatin vs aspirin and atorvastatin: MD = 6.86; 95% CI: 3.33, 10.39; NSTC combined with butylphthalide vs butylphthalide: MD = 12.56; 95% CI: 9.94, –15.18) (Supplementary Figure S8). By duration, the BI score was also statistically significant when the treatment course of the NSTC was 14 days, 28 days, and 90 days (14 days: MD = 12.86; 95% CI: 8.17, 17.56; 28 days: MD = 11.58; 95% CI: 8.89, 14.28; 90 days: MD = 6.34; 95% CI: 3.61, –9.08) (Supplementary Figure S9).
3.5.4 Modified Barthel Index
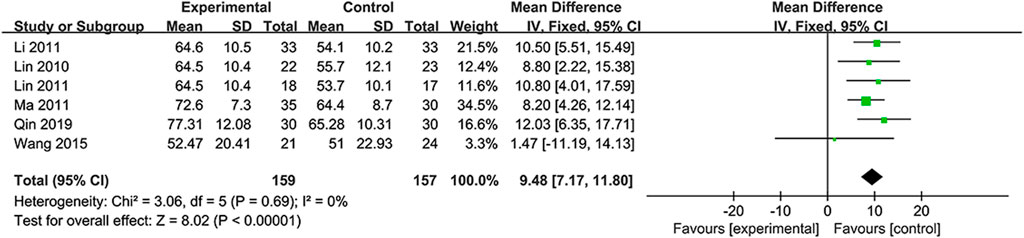
The independence was assessed by MBI scores in six RCTs (Lin et al., 2010; Li, 2011; Lin et al., 2011; Ma and Lan, 2011; Wang et al., 2015; Qin et al., 2019). Considering the absence of heterogeneity (I2=0%, p = 0.69), the fixed-effects model was selected. Based on the data, the NSTC group outperformed in terms of the mBI (MD = 9.48; 95% CI: 7.17, 11.80; p < 0.00001) (Figure 7). Subgroup analyses were developed focusing on different treatment periods and durations. The mean differences in the mBI were significantly different between the NSTC and control groups during both the acute period (MD=11.17; 95% CI: 7.42 –14.92) and non-acute period (MD=7.61; 95% CI: 3.84, 11.37) (Supplementary Figure S110). Additionally, the mBI of the NSTC group showed improvement after 28 and 30 days of treatment, with statistically significant differences observed (MD = 8.97; 95% CI: 6.44, 11.51) (Supplementary Figure S11).
3.5.5 SS-QOL
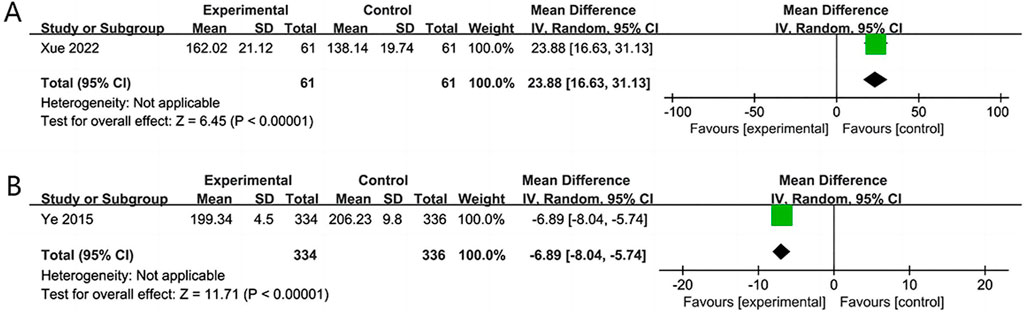
Two RCTs (Ye et al., 2015; Xue et al., 2022) assessed the quality of life over 14 and 180 days. The results revealed that the NSTC group showed improvement in SS-QOL at 14 days (MD = 23.88; 95% CI: 16.63, 31.13; p < 0.00001). However, at 180 days, the NSTC group did not exhibit improvement in SS-QOL (MD = −6.89; 95% CI: −8.04, −5.74; p < 0.00001) (Figure 8).
3.5.6 Recurrence rate of cerebrovascular events
Three RCTs (Liu and Li, 2012; Pan, 2013; Wang et al., 2015) involving 390 patients reported the recurrence rate of cerebrovascular events, revealing a great difference between the NSTC and control groups (RR = 0.43; 95% CI: 0.27, 0.70; p = 0.0006; I2 = 0%) (Figure 9). Thus, there is evidence safely suggesting that the NSTC may reduce the recurrence rate of cerebrovascular events.
3.5.7 Adverse events
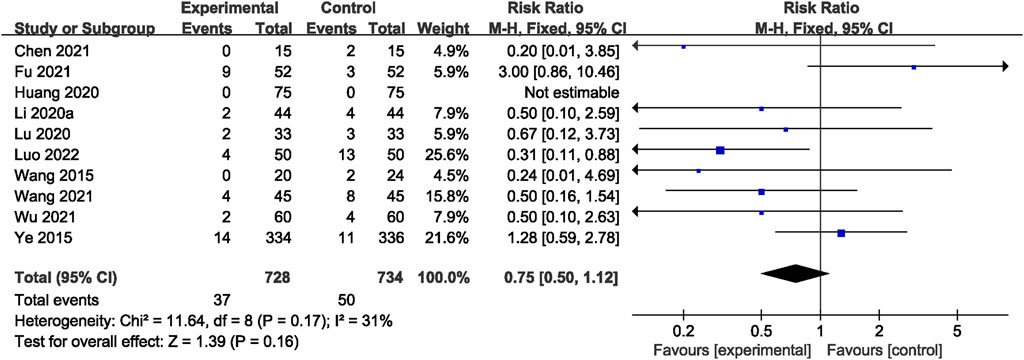
Across all studies, no related AEs were reported. A total of 10 RCTs (Ye et al., 2015; Wang et al., 2015; Li et al., 2020; Lu et al., 2020; Huang et al., 2020; Chen et al., 2021; Wu, 2021; Wang et al., 2021; Fu et al., 2021; Luo, 2022) reported adverse reactions. However, there was no significant difference observed between the two groups (RR = 0.75; 95% CI: 0.50, 1.12; p = 0.16; I2 = 31%) (Figure 10). The most frequently reported adverse reactions were nausea, vomiting, abnormal liver function, gastrointestinal reaction, and skin rash. Specific adverse events are given in Supplementary Table S4.
3.6 Publication bias
The visual inspection of funnel plots [Figure 11A shows the funnel plots of the NIHSS (acute period), Figure 11B shows the funnel plots of the BI, and Figure 11C shows the funnel plots of AEs] and statistical tests identified substantial symmetry and revealed no significant publication bias among the involved trials concerning the acute period of the NIHSS score (Egger’s test, p = 0.303; Supplementary Figure S12), the BI score (Egger’s test, p = 0.448, Supplementary Figure S13), and adverse events (Egger’s test, p = 0.510, Supplementary Figure S14). The publication bias of the mRS, mBI, recurrence rate of cerebrovascular events, and SS-QOL score could not be estimated for only less than 10 RCTs.
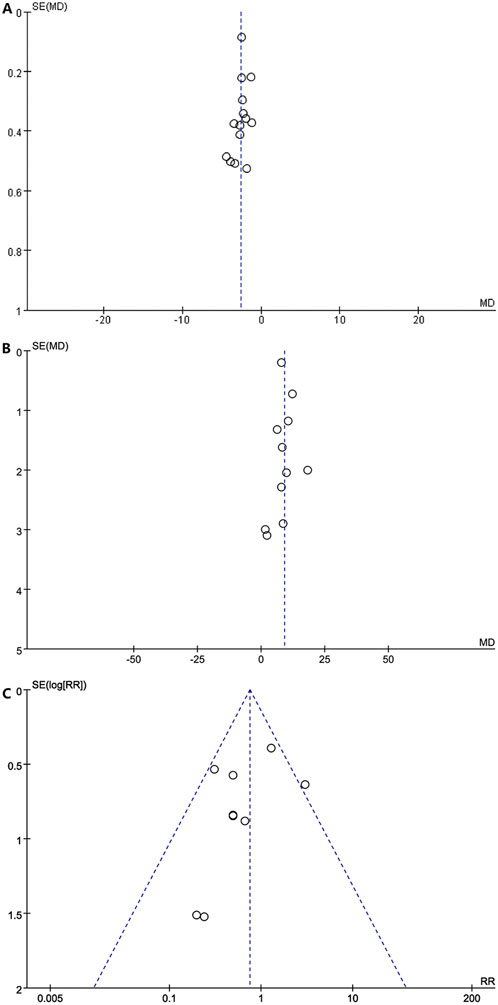
Figure 11. (A) Funnel plots of the NlHSS (acute period). (B) Funnel plots of the Bl. (C) Funnel plots of the AE.
3.7 Certainty of evidence
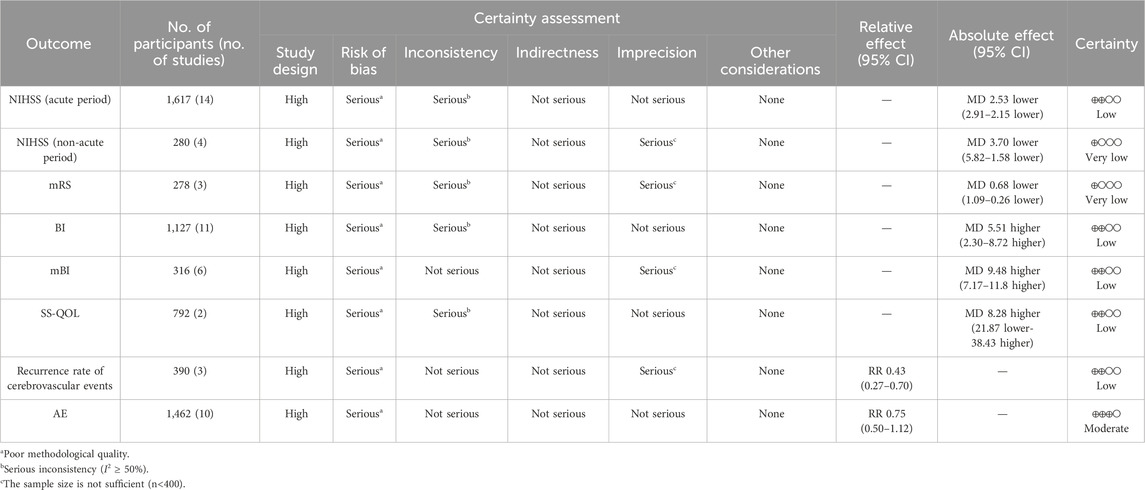
The certainty of evidence regarding the impact of the NSTC on adverse events was rated “moderate;” for the NIHSS (acute period), BI, mBI, SS-QOL, and the recurrence rate of cerebrovascular events, it was rated as “low;” and for the NIHSS (non-acute period) and mRS score, it was rated “very low” (refer to Table 2). The quality of evidence was assessed from moderate to very low, primarily because of poor methodological quality, significant inconsistency, and limited sample size.
4 Discussion
4.1 Summary of the findings
To the best of our knowledge, this study represents the first systematic review and meta-analysis to innovatively pay more attention to the clinical efficacy of the NSTC in the acute and non-acute phases of IS patients. The staging of stroke is a key problem in the daily practice of neurology. In addition, this study also focused on the safety of the NSTC in secondary prevention. Following the purpose, the study encompassed 27 RCTs involving 3,139 participants, suggesting potential improvements in the BI and mBI alongside reductions in the NIHSS, mRS scores, and the risk of recurrent cerebrovascular events. NSTC treatment demonstrated enhancements in SS-QOL at the 14-day mark. However, over the extended 180-day period, the patients’ quality of life decreased. Notably, there was no increase in adverse reactions. NSTC therapy emerges as a safe and effective approach, potentially valuable for the secondary prevention of IS.
Regarding neurological deficits, the findings suggest that incorporating NSTC prescriptions may accelerate the recovery process of neurological function in IS patients, as assessed by the NIHSS. Additionally, when combined with yurekline and butylphthalide capsules, the NSTC demonstrated a significant reduction in NIHSS scores. Three RCTs indicated that the NSTC could depress the disability rate assessed by the mRS. Moreover, treatment with the NSTC significantly improved the activities of daily life, as reflected in the BI and mBI scores. The subgroup analysis of BI scores revealed a significant difference between the NSTC and control groups in both acute and non-acute stages of stroke. NSTC combined with aspirin and atorvastatin calcium tablets or butylphthalide demonstrated improvements in BI scores, with statistically significant differences observed. Furthermore, compared to the control group, the NSTC group exhibited significant BI score improvements at 14, 28, and 90 days of treatment. In the subgroup analysis of mBI scores, the NSTC group played a better role in both acute and non-acute periods of IS, with statistically significant differences noted. Additionally, the mBI scores in the NSTC group improved after 28 and 30 days of treatment, with statistically significant differences observed. The results of the two RCTs indicated that adding NSTC prescriptions improved patient SS-QOL scores at 14 days. However, no improvement was observed in the NSTC group compared to the control group at 180 days. This may be due to a lack of rigorous design, resulting in insufficient sample size and bias in the results. On the other hand, it suggests that we may need more long-term follow-up studies for further evaluation. The NSTC group exhibited a statistically significant reduction in the recurrence rate of cerebrovascular events compared to the control group. Among the 27 including RCTs, 6 reported the incidence of adverse effects and clinical features, with no significant distinctions observed between groups.
4.2 Possible therapeutic mechanisms of the NSTC
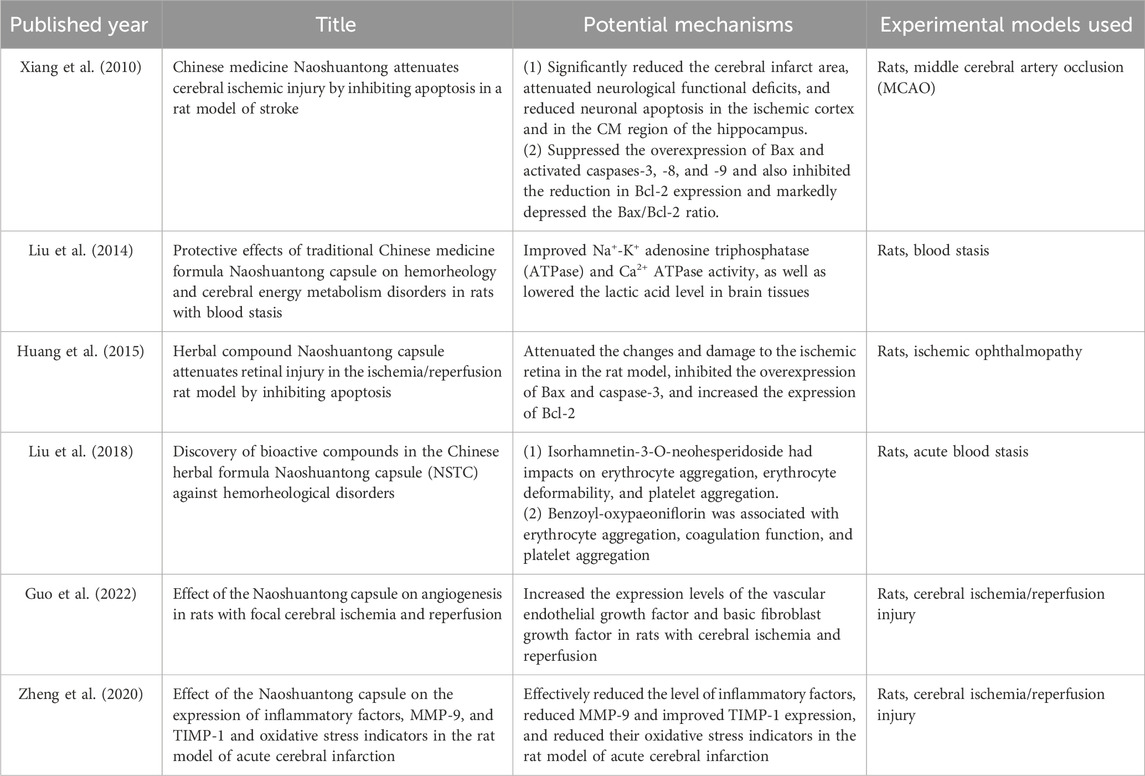
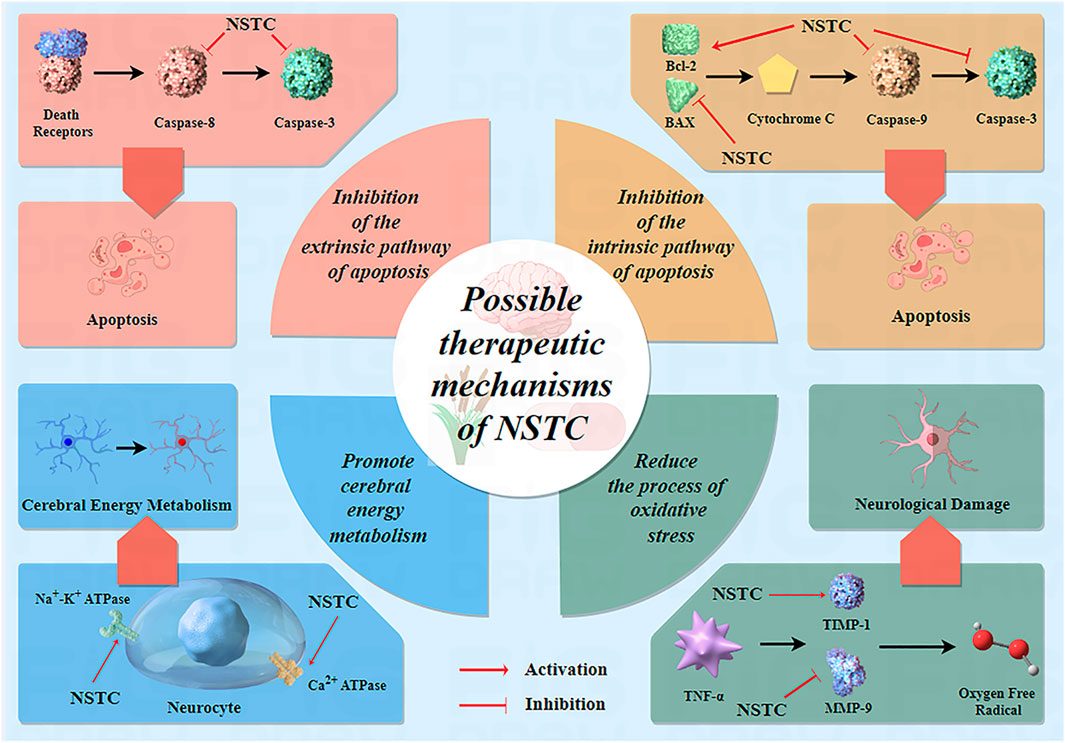
Over the past 15 years, numerous experimental studies on NSTC treatment for IS have been published globally, revealing its mechanisms of action at different levels, including at the molecule, cell, tissue, and organ levels. These studies have provided evidence of its effectiveness. In the earlier literature dating back to 2010, Xiang et al. (2010) conducted a study on focal cerebral ischemia in rats and discovered that the NSTC significantly decreased the area of cerebral infarction, alleviated neurological functional damage, and reduced neuronal apoptosis. The results of this basic research further support the results of the meta-analysis of this study, and NSTC can improve the degree of neurological deficit in patients with acute and non-acute IS in clinical practice. In addition, Xiang et al. (2010) and Huang et al. (2015) found that the NSTC could inhibit the overexpression of Bax and caspase-3 and enhance the production of Bcl-2. These results indicate that the NSTC can inhibit neuronal apoptosis and has a neuroprotective effect on cerebral ischemia. Furthermore, at the molecular level, experimental investigations by Liu et al. (2014) proved that the NSTC enhanced the activity of Na+-K+ ATPase and Ca2+ ATPase, reduced lactic acid levels in brain tissues, and ultimately facilitated brain tissue metabolism. At the genetic level, Zheng et al. (2020) proposed that the NSTC could apparently reduce the inflammatory factors, improve the expression of MMP-9 and TIMP-1, and decrease its oxidative stress index in a rat model of acute cerebral infarction. Recently, using experimental observations in rats (cerebral ischemia/reperfusion injury), Guo et al. (2022) found that the NSTC could significantly increase the expression level of the vascular endothelial growth factor and basic fibroblast growth factor, significantly increase the amount of angiogenesis during cerebral ischemia and reperfusion, and also significantly lessen the area of cerebral infarction during cerebral ischemia and reperfusion. Angiogenesis is essential for the recovery of ischemic areas. In clinical patients, angiogenesis not only contributes to short-term recovery but also helps improve long-term outcomes, including reduced recurrence rates. This also supports the results of our meta-analysis. More specific details of related studies on the mechanisms of NSTC treatment are shown in Table 3. Based on all evidence above, we can safely draw a conclusion that the NSTC has a significant therapeutic effect and improvement effect on the brain injury caused by IS. More specific mechanisms of drug action of the NSTC are given in Figure 12.
4.3 Strengths and limitations
This study focuses on the efficacy and safety of the NSTC for different stages of ischemic stroke. In contrast, previous studies (Chang et al., 2019) were merely focused on patients in the acute period. Considering that the NSTC is a kind of patent medicine based on toxins damaging brain collaterals theory in traditional Chinese medicine, which emphasizes the entire process of the disease, we included studies on stroke in the acute period, recovery period, and sequelae period in this study. The analytic results showed that using the NSTC could enhance the neurological function of patients. Furthermore, the meta-analysis showed that the NSTC could reduce the recurrence of cerebrovascular events. Lastly, our study produced the GRADE evidence-level evaluation, which is conducive to the revision of guidelines and consensus for better service and can also guide clinical practice. The NSTC might, therefore, prove to be an effective therapy choice in the future. Moreover, it is hoped that this systematic review can provide some assistance to future research.
However, this meta-analysis exhibited several shortcomings. First, certain RCTs exhibited a lower methodological grade as they were not able to adequately elucidate the procedures in randomizing, allocating, concealing, and blinding. Moreover, variations were observed in the average age of participants, intervention duration, types of adjunctive pharmacotherapy utilized, and outcome assessment criteria across the studies included in this analysis. Furthermore, the reports of adverse events in the included literature are not comprehensive, and more rigorous studies are needed in the future to monitor the relevant outcome indicators to fully demonstrate the safety of the NSTC. Finally, despite conducting database searches without language restrictions, all included studies originated from China, thus lacking geographical diversity. These limitations collectively influence the reliability of the findings, thereby restricting the universality of the analytic results of the study.
5 Conclusion
This meta-analysis study revealed the difference in the efficacy of the NSTC in different periods of stroke and the value of secondary prevention, which helps further optimize the stroke treatment process and guide doctors to provide appropriate treatment within the best time window, thus expanding the scope of choice for doctors in clinical practice. At the same time, this study may promote the updating and improvement of relevant treatment guidelines and promote the standardization and standardization of stroke treatment. Given the low quality of the evidence so far, in the future, further large-scale, high-quality, and meticulously designed RCTs are warranted to validate these findings and make more efforts to confirm the contribution of the NSTC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
CQ: data curation, formal analysis, project administration, software, visualization, writing–original draft, and writing–review and editing. YW: data curation, project administration, writing–original draft, and writing–review and editing. GY: data curation, visualization, writing–original draft, and writing–review and editing. CZ: data curation and writing–review and editing. ZL: writing–review and editing. XL: writing–review and editing. MQ: writing–review and editing. XX: writing–review and editing. XZ: writing–review and editing. XD: conceptualization, methodology, supervision, validation, and writing–review and editing. YG: conceptualization, methodology, supervision, validation, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the 2019 Major Difficult Diseases Clinical Collaboration Capacity Building Project of Traditional Chinese and Western Medicine—Cerebral Infarction (YW082); the Construction Project of the Cerebral Vascular Disease Prevention and Treatment Office of Traditional Chinese Medicine in Beijing (YW083); and the Pilot Project of Clinical Research and Achievement Transformation Capacity Improvement (DZMG-MLZY-23002).
Acknowledgments
The authors acknowledge contributions from all the included studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1434764/full#supplementary-material
Abbreviations
AE, adverse event; BI, Barthel Index; CNKI, China National Knowledge Internet; CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; IS, ischemic stroke; MD, mean difference; MBI, modified Barthel Index; mRS, modified Rankin Scale; NSTC, Naoshuantong capsule; NIHSS, National Institutes of Health Stroke Scale; PRISMA, Preferred Reporting Items for Systematic Reviews; RCTs, randomized controlled trials; RR, relative risk; SS-QOL, Stroke-specific Quality of life; VIP, Chinese VIP information.
References
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64 (4), 401–406. doi:10.1016/j.jclinepi.2010.07.015
Chang, Z., Lin, J., Gao, Q., Tlan, D., Zhang, D., Wang, Y., et al. (2019). Efficacy of Naoshuantong capsule in the treatment of acute cerebral infarction:a meta-analysis. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 17 (20), 3097–3103. doi:10.12102/j.issn.1672-1349.2019.20.006
Chen, J., Zhang, X., and Li, Q. (2021). Study on the pharmacological effect and safety of Naoshuantong capsule in the treatment of speech disorder after ischemic stroke. J. Med. Aesthetice Cosmetol. 21, 15–16.
Chen, Y., Wu, Z., Yu, Z., Yang, D., He, X., and Tan, T. (2010). Effect of Naoshuantong capsule on acute ischemic stroke. Chin. Tradit. Pat. Med. 32 (06), 903–905.
Dawson, J., Béjot, Y., Christensen, L. M., De Marchis, G. M., Dichgans, M., Hagberg, G., et al. (2022). European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur. Stroke J. 7 (3), I–II. doi:10.1177/23969873221100032
Feigin, V. L., Stark, B. A., Johnson, C. O., Roth, G. A., Bisignano, C., Abady, G. G., et al. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. Neurology 20 (10), 795–820. doi:10.1016/S1474-4422(21)00252-0
Feske, S. K. (2021). Ischemic stroke. Am. J. Med. 134 (12), 1457–1464. doi:10.1016/j.amjmed.2021.07.027
Fu, Q., Lin, X., Zhang, C., Fu, S., and Huang, L. (2021). Analysis of clinical value of Naoshuantong combined with butylphthalide in the treatment of lschemic stroke. Prog. Mod. Biomed. 21 (03), 553–556+595. doi:10.13241/j.cnki.pmb.2021.03.033
Gao, Y., Zhao, X., Zhou, L., Li, Z., Chen, W., Cui, F., et al. (2023). Guideline for the diagnosis and treatment of cerebral infarction with the integrated traditional Chinese and western medicine. Available at: http://www.caim.org.cn/info_content.jsp?id=10322.
Goenka, L., Uppugunduri, S. C., S, S. K., and George, M. (2019). Neuroprotective agents in acute ischemic stroke-A reality check. Biomed. and Pharmacother = Biomedecine and Pharmacother. 109, 2539–2547. doi:10.1016/j.biopha.2018.11.041
Guo, M., Liu, T., Wu, Z., Tlan, X., Zhu, Z., Zheng, W., et al. (2022). Effect of Naoshuantong capsule on angiogenesis in rats with focal cerebral lschemia reperfusion. Laboratory Animal Sci. 39 (05), 20–25. doi:10.3969/i.issn.1006-6179.2022.05.004
Hartling, L., Featherstone, R., Nuspl, M., Shave, K., Dryden, D. M., and Vandermeer, B. (2017). Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med. Res. Methodol. 17 (1), 64. doi:10.1186/s12874-017-0347-z
Huang, C., Gao, Y., Yu, Q., and Feng, L. (2015). Herbal compound Naoshuantong capsule attenuates retinal injury in ischemia/reperfusion rat model by inhibiting apoptosis. Int. J. Clin. Exp. Med. 8 (8), 12252–12263.
Huang, H., Ding, Y., Deng, W., and Pan, X. (2014). Application research on aspirin resistance in patients with cerebral infarction treated by Naoshuantong. Guide China Med. 12 (5), 19–20. doi:10.15912/j.cnki.gocm.2014.05.159
Huang, L., Chen, J., and Song, X. (2020). Clinical study on Naoshuantong Capsules combined with uriklin in treatment of acute cerebral infarction. Drugs and Clin. 35 (01), 61–64. doi:10.7501/j.issn.1674-5515.2020.01.013
Huang, S. (2015). Effect of Naoshuantong capsule on acute ischemic stroke. Prev. Treat. Cardiovasc. Dis. 12, 19–20.
Kleindorfer, D. O., Towfighi, A., Chaturvedi, S., Cockroft, K. M., Gutierrez, J., Lombardi-Hill, D., et al. (2021). 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke 52 (7), e364–e467. doi:10.1161/STR.0000000000000375
Li, D. (2011). Long-term efficacy analysis of Naoshuantong capsule in the treatment of patients with cerebral infarction. J. China Traditional Chin. Med. Inf. 3 (23), 380.
Li, J., Ji, Y., and Zuo, Y. (2020). Clinical effect of Naoshuantong capsule combined with urinary kallidinogenase in the treatment of acute cerebral infarction. Clin. Res. Pract. 5 (36), 22–24. doi:10.19347/j.cnki.2096-1413.202036009
Li, N., and Wang, X. (2020). Clinical observation of Naoshuantong combined with auricular acupoint pressing beans in the treatment of dysphagia after cerebral infarction. J. Pract. Traditional Chin. Med. 36 (07), 892–894.
Li, Z., Peng, B., Zhao, X., Wang, Y., and Zeng, J. (2022). Chinese guideline for the secondary prevention of ischemic stroke and transient ischemic attack 2022. Chin. J. Neurology 55 (10), 1071–1110. doi:10.3760/cma.j.cn113694-20220714-00548
Lin, R., Gao, M., Li, Q., and Li, J. (2023). Observation on the therapeutic effect of Naoshuantong capsule combined with edaravone on patients with acute cerebral infarction. Tianjin Pharm. 35 (02), 51–55. doi:10.3969/j.issn.1006-5687.2023.02.013
Lin, Z., Chen, Y., and Zhou, W. (2010). The effects of Naoshuantong capsule on neurological deficits and motor function in pations with cerebral infarction. China Pract. Med. 5 (23), 25–27. doi:10.14163/j.cnki.11-5547/r.2010.23.169
Lin, Z., Guo, Y., and Lai, J. (2011). Long-term effect of Naoshuantong capsule on patients with cerebral infarction. Chin. Manip. and Rehabilitation Med. 2 (10), 17–18.
Liu, H., Long, C., Peng, Y., Zhang, W., Li, P., Wang, Y., et al. (2018). Discovery of bioactive compounds in the Chinese herbal formula NaoShuanTong Capsule (NSTC) against hemorheological disorders. Biotechnology 32 (6), 1598–1605. doi:10.1080/13102818.2018.1519378
Liu, H., Peng, Y., Liang, F., Chen, S., Li, P., Peng, W., et al. (2014). Protective effects of traditional Chinese medicine formula NaoShuanTong capsule on haemorheology and cerebral energy metabolism disorders in rats with blood stasis. Biotechnol. Biotechnol. Equip. 28 (1), 140–146. doi:10.1080/13102818.2014.901678
Liu, J., and Li, X. (2012). The therapy and prevention of Naoshuantong capsule for cerebral infarction. Guide China Med. 10 (18), 5–6. doi:10.15912/j.cnki.gocm.2012.18.467
Liu, T., and Xu, Z. (2018). Clinical observation of naoshuantong capsule in treating 162 cases of cerebral ischemic stroke. Front. Med. 8 (12), 219–220. doi:10.3969/j.issn.2095-1752.2018.12.184
Lu, X., Zhang, F., and Zhang, S. (2020). Clinical efficacy of Naoshuantong capsule combined with clopidogrel bisulfate tablets in the treatment of sequelae of stroke. Mod. Med. Health Res. Electron. J. 4 (11), 81–83.
Luo, L., Wu, S., Chen, R., Rao, H., Peng, W., and Su, W. (2020). The study of neuroprotective effects and underlying mechanism of Naoshuantong capsule on ischemia stroke mice. Chin. Med. 15 (1), 119. doi:10.1186/s13020-020-00399-7
Luo, T. (2022). Clinical efficacy of Naoshuantong capsule combined with clopidogrel bisulfate tablets in the treatment of sequelae of stroke. Spec. Health (7), 139–141.
Ma, Y., and Lan, R. (2011). Clinical observation of Naoshuantong capsule combined with rehabilitation training in the treatment of 35 patients with ischemic stroke in recovery stage. Liaoning J. Traditional Chin. Med. 38 (03), 394–395. doi:10.13192/j.ljtcm.2011.03.15.mayzh.002
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 18 (3), e1003583. doi:10.1371/journal.pmed.1003583
Pan, J. (2013). Effects of Naoshuantong capsule on neurological function and activities of daily living in patients with acute ischemic stroke. Asia-Pacific Tradit. Med. 9 (11), 169–170.
Peng, W., Guo, J., and Yang, Y. (2012). Effect on cerebral blood flow reserve in cerebral infarction patients by Naoshuantong capsules. Clin. J. Chin. Med. 4 (24), 93–94.
Qiao, Y., and Li, Z. (2021). Therapeutic effect of Naoshuantong combined with Edaravone on serum MMP-10, NSE, S100B, cytokines and oxidative stress of stroke patients in acute stage. Chin. J. Gerontology 41 (22), 4902–4905. doi:10.3969/j.issn.1005-9202.2021.22.009
Qin, H., Liu, H., and Zhang, X. (2019). Clinical study on naoshuangtong capsule for ischemic stroke. New Chin. Med. 51 (6), 124–127. doi:10.13457/j.cnki.jncm.2019.06.037
Saini, V., Guada, L., and Yavagal, D. R. (2021). Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 97 (20 Suppl. 2), S6–S16. doi:10.1212/WNL.0000000000012781
Sterne, J., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin. Res. ed. 366, l4898. doi:10.1136/bmj.l4898
Wang, B., Cao, Q., Feng, S., Wang, Y., and Zhang, Y. (2021). Eficacy of Naoshuantong capsule combined with alteplase for injection in treatment of acute cerebral infarction and lts influence on the levels of serum NT-prOBNPTNF-α. Chin. J. Pharmacoepidemiol. 30 (09), 586–590. doi:10.19960/j.cnki.issn1005-0698.2021.09.003
Wang, K. (2022). Efficacy of Naoshuantong capsule combined with alteplase for injection in patients with acute cerebral infarction. Yishou Baodian (29), 0023–0025.
Wang, Z., Zhan, L., Pan, R., Guo, Y., Yang, Z., Li, M., et al. (2015). Effects of Naoshuantong capsule and clopidogrel tablets on motor function and daily living ability in patients with cerebral infarction in recovery stage. New Chin. Med. 47 (08), 21–23. doi:10.13457/j.cnki.jncm.2015.08.010
Wu, X. (2021). Clinical study on Naoshuantong capsules combined with butylphthalide for cerebral infarction. New Chin. Med. 53 (21), 84–87. doi:10.13457/j.cnki.jncm.2021.21.020
Xiang, J., Tang, Y. P., Wu, P., Gao, J. P., and Cai, D. F. (2010). Chinese medicine Nao-Shuan-Tong attenuates cerebral ischemic injury by inhibiting apoptosis in a rat model of stroke. J. Ethnopharmacol. 131 (1), 174–181. doi:10.1016/j.jep.2010.06.021
Xue, F., Zhang, Y., Zhang, J., and Jia, X. (2022). Effect of Naoshuantong capsule combined with butylphthalide injection on acute cerebral infarction and lts effect on hemodynamics. Chin. Archives Traditional Chin. Med. 40 (02), 215–218. doi:10.13193/j.issn.1673-7717.2022.02.050
Ye, X., Xle, Y., Zou, Y., Zhao, X., Han, J., Wang, X., et al. (2015). Effect of Naoshuantong capsule on change of SSQOL index in patients with ischemic stroke in six months follow-up. China J. Chin. Materia Medica 40 (21), 4297–4300. doi:10.4268/cjcmm20152132
Zhang, H., Xing, Y., Chang, J., Wang, L., An, N., Tian, C., et al. (2019). Efficacy and safety of NaoShuanTong capsule in the treatment of ischemic stroke: a meta-analysis. Front. Pharmacol. 10, 1133. doi:10.3389/fphar.2019.01133
Zheng, Y., Xu, Z., Li, R., Lin, C., Ma, S., and Liu, Y. (2020). Effects of Naoshuantong capsule on expressions of infammatory Factors,MlMP-9 and TlMP-1 and oxidative stress lndicators in rats with acute cerebral infarction. J. Emerg. Traditional Chin. Med. 29 (06), 1027–1030+1044. doi:10.3969/j.issn.1004-745X.2020.06.023
Keywords: ischemic stroke, Naoshuantong capsule, meta-analysis, systematic review, Chinese patent medicine
Citation: Que C, Wei Y, Yin G, Zhou C, Liu Z, Lai X, Qin M, Xiong X, Zheng X, Dong X and Gao Y (2024) Updated evidence of the Naoshuantong capsule against ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 15:1434764. doi: 10.3389/fphar.2024.1434764
Received: 21 May 2024; Accepted: 11 September 2024;
Published: 26 September 2024.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Johanna Mahwahwatse Bapela, University of Pretoria, South AfricaLuo Lvkeng, Sun Yat-sen University, China
Copyright © 2024 Que, Wei, Yin, Zhou, Liu, Lai, Qin, Xiong, Zheng, Dong and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Gao, Z2FveWluZzk3M0AxNjMuY29t; Xinglu Dong, YXJ0aGFzZHhsQDE2My5jb20=
†ORCID: Cuilin Que, orcid.org/0009-0003-3255-5959, Yupeng Wei, orcid.org/0009-0009-2541-3534, Guanxiang Yin, orcid.org/0009-0009-0707-3740, Xinglu Dong, orcid.org/0000-0003-3071-295X, Ying Gao, orcid.org/0000-0001-6972-3846
‡These authors have contributed equally to this work
 Cuilin Que
Cuilin Que Yupeng Wei
Yupeng Wei Guanxiang Yin
Guanxiang Yin Congren Zhou
Congren Zhou Zhenhong Liu
Zhenhong Liu Xinxing Lai
Xinxing Lai Mingzhen Qin
Mingzhen Qin Xuejiao Xiong
Xuejiao Xiong Xiangyi Zheng
Xiangyi Zheng Xinglu Dong
Xinglu Dong Ying Gao
Ying Gao