- 1Department of Cardiothoracic Surgery, The First Affifiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 2Shanghai Yinji Technology Co., Ltd., Shanghai, China
- 3Shanghai Zhongyou Inspection & Medical Co., Ltd., Shanghai, China
Lung cancer is a prevalent malignancy, with the rearrangement of the anaplastic lymphoma kinase (ALK) gene being responsible for a minority of cases of non-small cell lung cancer (NSCLC). NSCLC patients harboring ALK fusion proteins demonstrate sensitivity to ALK tyrosine kinase inhibitors (TKIs). In this report, we describe the case of a female patient with metastatic lung adenocarcinoma, identified through NGS to carry a rare inverted SQSTM1-ALK (S5, A20) fusion. The patient received ensartinib as first-line therapy, resulting in a partial response (PR). At the time of publication, the patient’s condition remained favorable. We have, for the first time, identified the presence of SQSTM1-ALK fusion in pericardial effusion, with the favorable response to ensartinib validating the oncogenic potential of SQSTM1-ALK fusion. The substantial advancements and extensive utilization of NGS have facilitated the identification of rare fusion variants.
Introduction
Lung cancer is a prevalent malignancy, and those with specific molecular genetic alterations in the ALK, BRAF, or EGFR genes have been endorsed by the US Food and Drug Administration (FDA) as first-line targeted therapies. Rearrangement of the anaplastic lymphoma kinase (ALK) gene is observed in 2%–7% of NSCLC patients, and is particularly frequent in younger individuals, non-smokers, and those with adenocarcinoma (Kwak et al., 2010). NSCLC patients harboring ALK fusion proteins demonstrate sensitivity to ALK tyrosine kinase inhibitors (TKIs).
Approximately half of the cases are diagnosed at an advanced, metastatic stage. Advanced lung cancer may result in the occurrence of pericardial effusion. Due to the close relationship between the cardiovascular and pulmonary systems, it is common for patients with advanced lung cancer to exhibit pericardial effusion, bloody pericardial effusion, and metastasis of cancer cells. Typically, only a restricted amount of biopsy material is available for companion diagnosis using NGS panels. In this report, we depict a case study of a patient with metastatic lung adenocarcinoma who declined direct tumor biopsy sampling and instead opted for pericardial effusion extraction for diagnostic purposes. Subsequent testing revealed the presence of SQSTM1-ALK fusion. Subsequent to treatment with ensartinib, the ailment was accurately diagnosed via initial limited samplings, thereby obviating the need for multiple rebiopsies.
Case description
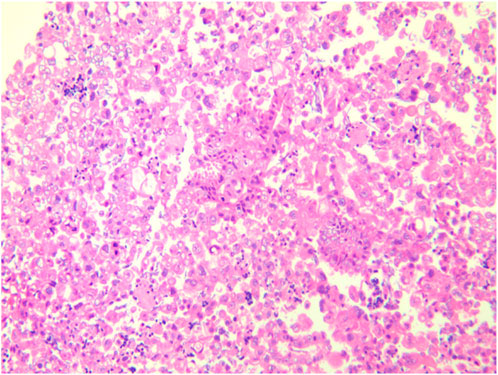
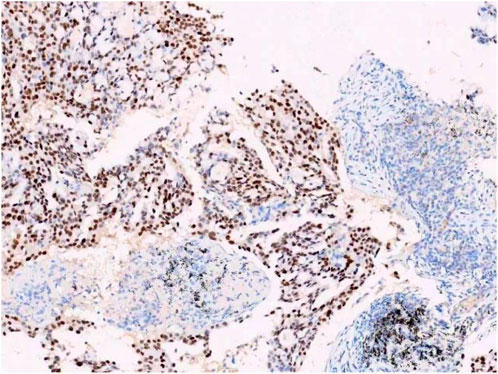
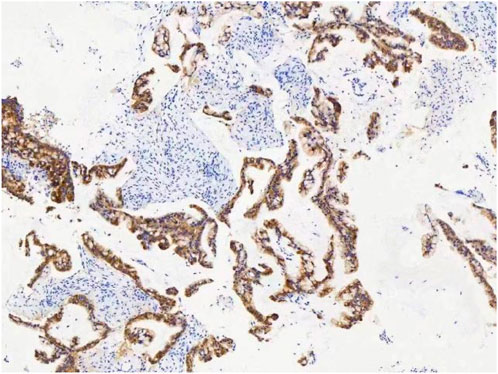
A female patient in her 60s, who is Chinese and of Zhuang nationality, with a junior high school education level, married status, a farmer by occupation and in poor economic condition, presented with chest pain accompanied by dyspnea and was diagnosed with advanced metastatic lung adenocarcinoma upon examination. The patient had no history of smoking and alcohol consumption. Neither her parents nor her siblings have a history of lung cancer. The clinical stage was T2bN2M1a, with no extrathoracic metastases detected upon radiological follow-up. Due to the patient’s decision to decline a lung tumor biopsy, pericardial effusion was obtained for HE staining, which revealed that tumor cells comprised 85% of the sample (Figure 1). TTF1 immunohistochemistry results confirmed that the malignant pericardial effusion originated from metastasis of lung adenocarcinoma (Figure 2). The ALK immunohistochemistry test also showed positive results (Figure 3). Following the confirmation of pericardial effusion as tumor metastasis, DNA of tumor cells was extracted from the effusion for genetic testing.
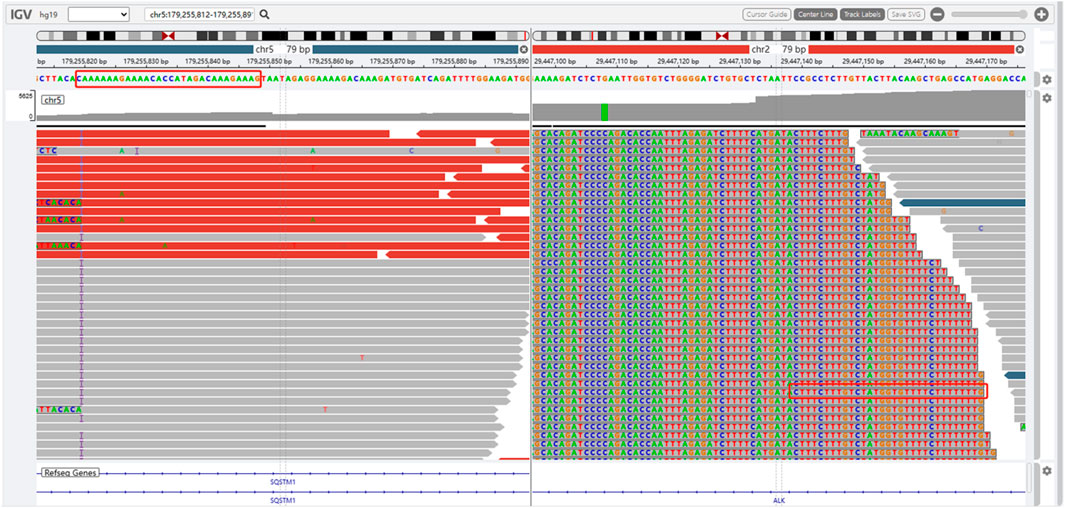
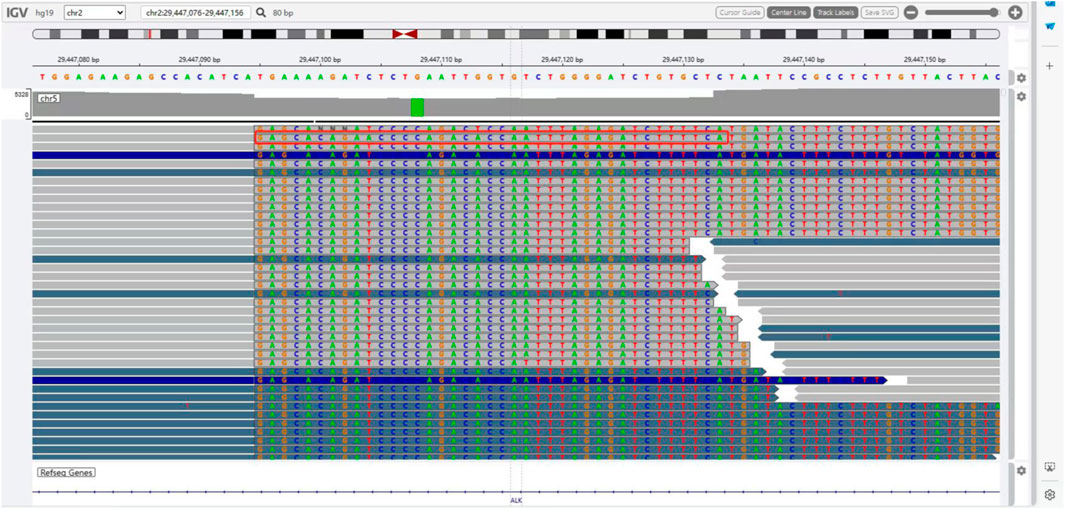
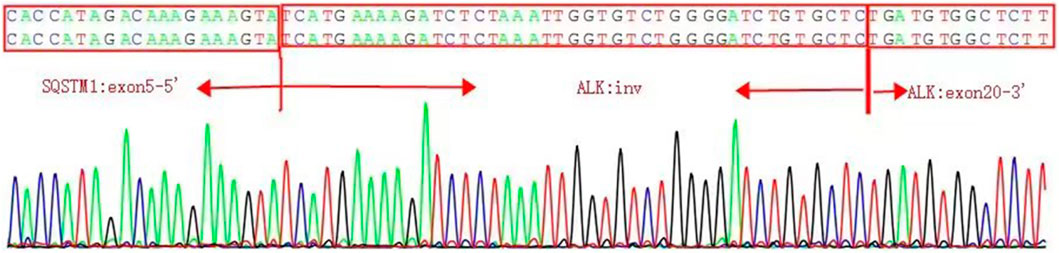
The presence of SQSTM1-ALK in the pericardial effusion sample was validated through next-generation DNA sequencing (NGS) using a 160-gene panel provided by Shanghai Zhongyou Inspection & Medical Co., Ltd. The genetic testing report (using the GenarsR system from Shanghai Yinji Technology Co., Ltd) revealed the location of the SQSTM1 breakpoint at Exon5 chr5:179255851, and the ALK breakpoint at Exon20 chr2:29447094. A 39-base pair inversion of the ALK gene was observed at the breakpoint location, as illustrated in Figures 4, 5. The patient was also found to harbor an unidentified MSH6 S346C mutation. This fusion has not previously been documented in cases of lung adenocarcinoma, and the Sanger verification results are provided in Figure 6.
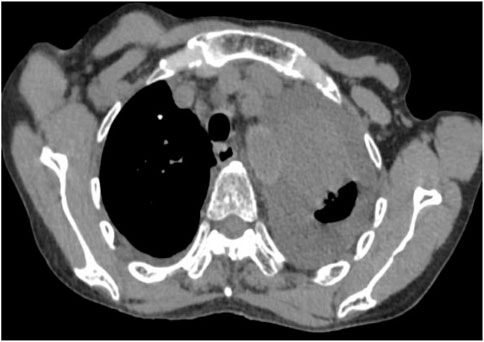
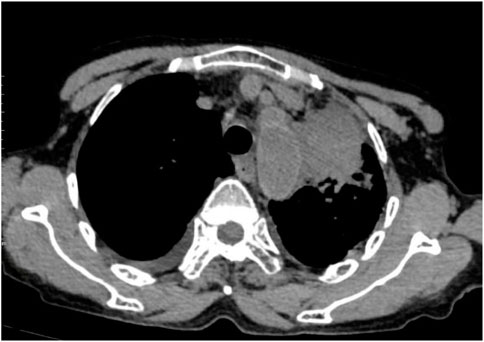
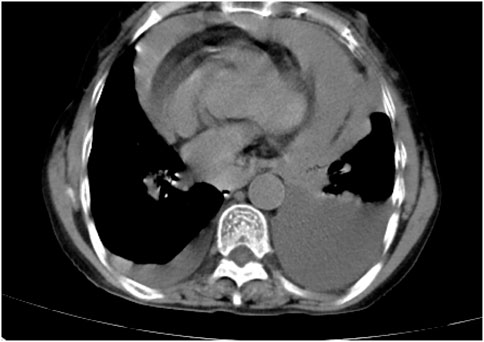
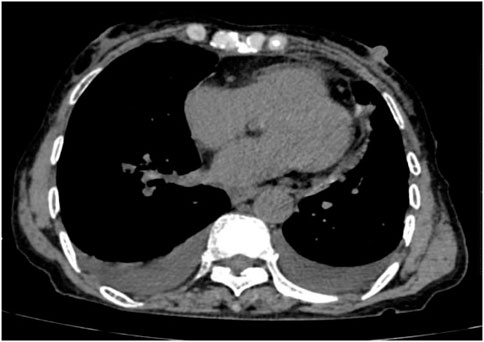
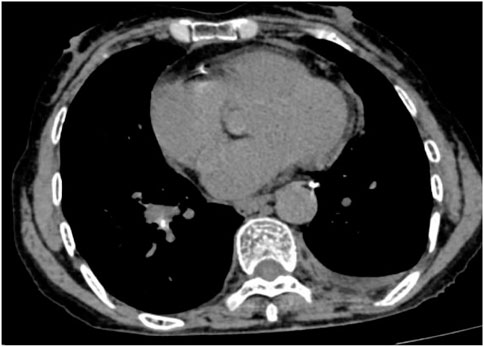
Following treatment with ensartinib at 225 mg QD, the patient exhibited a decrease in CEA level from 104.97 ng/mL to 5.25 ng/mL, and a reduction in carbohydrate antigen 125 level from 312.50 μg/mL to 43.84 μg/mL after 1 month. Additionally, the chest CT scan revealed a reduction in tumor size (Figures 7, 8) as well as pericardial effusion (Figures 9, 10). After 6 months of treatment, the tumor size remained the same as after 1 month of treatment (Figure 11). After 6 months of treatment, the condition of pericardial effusion and pleural effusion (Figure 12).
Discussion
In this report, we present the case of a 60-year-old female diagnosed with advanced lung adenocarcinoma harboring the rare SQSTM1-ALK fusion, which had metastasized to the pericardium. While several ALK inhibitors have been approved for ALK-rearranged non-small cell lung cancer (NSCLC), this is the first documented case of using Ensartinib to treat a patient with this specific fusion. Our findings show that Ensartinib demonstrated significant efficacy, After 1 month of treatment, the patient achieved partial response (PR), and the effect was sustained for at least 6 months without any observable toxicity. This suggests that Ensartinib may be a promising therapeutic option for lung adenocarcinoma patients with the SQSTM1-ALK fusion, though complete remission (CR) was not achieved during the observation period.
Ensartinib was chosen over other ALK inhibitors such as Crizotinib and Alectinib based on its demonstrated superiority in preclinical and early clinical trials. Ensartinib has shown a broader inhibitory profile, effectively targeting ALK mutations, including those resistant to first- and second-generation inhibitors. This makes it a suitable first-line treatment, especially for patients with brain metastases, as Ensartinib crosses the blood-brain barrier more efficiently (Wang et al., 2023). Additionally, the patient’s overall health status and multiple metastases influenced the decision to use Ensartinib, as a more comprehensive systemic control was needed. Given the limited data on the effectiveness of other ALK inhibitors in patients with SQSTM1-ALK fusion, Ensartinib presented a novel approach for this case.
Although the patient did not experience any drug-related toxicity, Ensartinib’s potential side effects should still be considered, especially in comparison to other ALK inhibitors such as Alectinib and Crizotinib. Common side effects of Ensartinib include skin rash, elevated liver enzymes, and fatigue (Zhou et al., 2023). The absence of any significant side effects in this patient is particularly noteworthy, given the advanced stage and the presence of multiple metastases. In contrast, Alectinib and Crizotinib are often associated with gastrointestinal disturbances and muscle pain, with Crizotinib also known to cause higher incidences of vision problems (Zhou et al., 2023). The good tolerability of Ensartinib in this case suggests that it could be an effective treatment for patients unable to endure the side effects of other ALK inhibitors.
The SQSTM1-ALK fusion has been identified in other cancers, such as inflammatory myofibroblastic tumors (IMT), epithelioid fibrous histiocytoma (Dickson et al., 2018), and large B-cell lymphoma (d’Amore et al., 2013). However, the disease course and treatment responses in these cancers differ from those in lung adenocarcinoma. For example, a 46-year-old male with SQSTM1-ALK-positive IMT responded significantly to Alectinib, with a 17-month progression-free survival (Honda et al., 2019). In our patient, the presence of cardiac and brain metastases at diagnosis suggested a more aggressive disease progression. This suggests that the presence of the SQSTM1-ALK fusion in lung adenocarcinoma may lead to rapid progression, emphasizing the need for prompt and aggressive treatment strategies.
The role of STAT3 inhibitors in treating SQSTM1-ALK fusion-positive cancers, as well as the combination of ALK and STAT3 inhibitors, warrants further investigation. In lymphoma cases involving SQSTM1-ALK fusion, STAT3 phosphorylation plays a key role in pathogenesis (d’Amore et al., 2013). Exploring whether this mechanism similarly contributes to lung adenocarcinoma could open new therapeutic avenues, particularly for combined treatment strategies with ALK and STAT3 inhibitors. Moreover, resistance to second-generation ALK inhibitors highlights the need to assess third-generation inhibitors such as Lorlatinib, which has shown efficacy in overcoming resistance mutations (Lin et al., 2024). Clinical trials investigating Lorlatinib in SQSTM1-ALK fusion-positive patients could provide valuable insights into the long-term management of resistant cases.
Conclusion
This study presents the inaugural successful treatment of lung adenocarcinoma patients positive for SQSTM1-ALK with pericardial effusion using ensartinib. The outcomes indicate that ensartinib may be considered as an effective therapeutic option in the presence of SQSTM1-ALK. These findings hold significant implications for clinical practice, emphasizing the necessity of genetic testing and tailored treatment approaches for lung adenocarcinoma.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the First Affifiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PW: Conceptualization, Investigation, Supervision, Writing–original draft, Writing–review and editing. ZJ: Investigation, Writing–original draft, Writing–review and editing. JG: Data curation, Investigation, Writing–review and editing. TL: Data curation, Resources, Writing–review and editing. ZL: Formal Analysis, Writing–review and editing. DW: Formal Analysis, Writing–review and editing. HL: Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Health Commission of Guangxi Zhuang Autonomous Region self-finances research projects, and Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project, National Clinical Key Specialty Construction Project, the Clinical Key Specialty Construction Project in Guangxi Zhuang Autonomous Region and the Clinical Key Discipline Construction Project in Guangxi Zhuang Autonomous Region.
Conflict of interest
Authors ZJ and ZL were employed by Shanghai Yinji Technology Co., Ltd. Authors DW and HL were employed by Shanghai Zhongyou Inspection & Medical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALK, Anaplastic lymphoma kinase; CEA, Carcinoembryonic Antigen; IMFT, Inflammatory Myofibroblastic Tumor; TTF1, Thyroid Transcription Factor-1; IMT, Inflammatory Myofibroblastic Tumor; SQSTM1, Sequestosome 1; TPS, Tumor Proportion Score.
References
d’Amore, E. S., Visco, C., Menin, A., Famengo, B., Bonvini, P., and Lazzari, E. (2013). STAT3 pathway is activated in ALK-positive large B-cell lymphoma carrying SQSTM1-ALK rearrangement and provides a possible therapeutic target. Am. J. Surg. pathology 37 (5), 780–786. doi:10.1097/PAS.0b013e318287791f
Dickson, B. C., Swanson, D., Charames, G. S., Fletcher, C. D., and Hornick, J. L. (2018). Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod. pathology official J. U. S. Can. Acad. Pathology 31 (5), 753–762. doi:10.1038/modpathol.2017.191
Honda, K., Kadowaki, S., Kato, K., Hanai, N., Hasegawa, Y., Yatabe, Y., et al. (2019). Durable response to the ALK inhibitor alectinib in inflammatory myofibroblastic tumor of the head and neck with a novel SQSTM1-ALK fusion: a case report. Investig. new drugs 37 (4), 791–795. doi:10.1007/s10637-019-00742-2
Kwak, E. L., Bang, Y. J., Camidge, D. R., Shaw, A. T., Solomon, B., Maki, R. G., et al. (2010). Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363 (18), 1693–1703. doi:10.1056/NEJMoa1006448
Lin, J. J., Horan, J. C., Tangpeerachaikul, A., Swalduz, A., Valdivia, A., Johnson, M. L., et al. (2024). NVL-655 is a selective and brain-penetrant inhibitor of diverse ALK-mutant oncoproteins, including lorlatinib-resistant compound mutations. Cancer Discov. 13, OF1–OF20. Published online September. doi:10.1158/2159-8290.CD-24-0231
Wang, W. Q., Xu, T., Zhang, J. J., Wang, Y., Wang, Y., Zhang, W., et al. (2023). A comprehensive clinical evaluation of first-line drugs for ALK-positive advanced non-small cell lung cancer. J. Thorac. Dis. 15 (4), 1935–1947. doi:10.21037/jtd-23-380
Keywords: SQSTM1-ALK, rearrangement, lung cancer, targeted therapy, ensartinib
Citation: Wang P, Jiang Z, Guo J, Liu T, Liu Z, Wang D and Li H (2024) Case report: Treatment with ensartinib shows good response to SQSTM1-ALK fusion in lung adenocarcinoma. Front. Pharmacol. 15:1433894. doi: 10.3389/fphar.2024.1433894
Received: 16 May 2024; Accepted: 14 October 2024;
Published: 01 November 2024.
Edited by:
Hardeep Singh Tuli, Maharishi Markandeshwar University, IndiaReviewed by:
Luigi De Petris, Karolinska Institutet (KI), SwedenIsha Rani, Maharishi Markandeshwar University, India
Copyright © 2024 Wang, Jiang, Guo, Liu, Liu, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Liu, ZHJsaXVfY3R2c0AxNjMuY29t; Jianji Guo, Z3VvamlhbmppQDE2My5jb20=
 Pandeng Wang
Pandeng Wang Zhuo Jiang
Zhuo Jiang Jianji Guo1*
Jianji Guo1*